- Shanghai Mental Health Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Objective: This study was aimed to explore the impact of fish oil (Omega-3 fatty acids) on hostility and psychopathology among patients with acute violent schizophrenia.
Method: Sixty seven acute hospitalized patients demonstrating violent behavior in the context of a schizophrenic illness, treated with antipsychotics, were randomly assigned to a supplement with either fish oil (N=32) or placebo (N=35) in a double-blind, placebo-controlled trial. Assessments were conducted at the baseline, week 4 and week 8.
Results: The symptoms and hostility decreased after treatment for 4 and 8 weeks in both groups, with no group differences.
Conclusions: The current study did not find improvements in symptoms or hostility from the Omega-3 fatty acid supplementation in patients with schizophrenia. The implication is that Omega-3 fatty acids do not reduce psychopathology and hostility in acute patients with schizophrenia.
Introduction
The hypothesis of the membrane phospholipid in schizophrenia is based on Horrobin's postulate (1, 2). Polyunsaturated fatty acids (PUFA), which are regarded as important components of the cell membrane, were found to decline in patients with schizophrenia (3). Lipid dysfunction may be part of the etiology (4, 5). Thus, it was suggested that supplementation of PUFA might improve symptoms of patients with schizophrenia. The mechanism of action of the PUFA is still unclear. Previous studies suggested that PUFAs may interact with the dopamine and serotonin system (4, 6, 7), while some studies implied that PUFAs induced antiapoptotic factors (8, 9).
Some studies testified this hypothesis. Animal studies showed that the administration of omega-3 fatty acids in adolescent rats prevented positive, negative and cognitive symptoms in a ketamine animal model of schizophrenia (10). A study in adolescents and young adults with subthreshold psychosis found that the transition rate to full-threshold psychosis in the Omega-3 PUFA group was much lower as compared to the placebo group. Omega-3 PUFA also significantly reduced positive, negative and general symptoms, alongside improving functioning (4). In first episode psychosis patients, supplementation of the eicosapentaenoic acid (EPA) as an augmented treatment needed 20% lesser antipsychotic medications and had lesser side effects than those treated with antipsychotic medication alone (11). In chronic, severe schizophrenic patients, the EPA supplementation group had significant reduction in their scores for the Positive and Negative Syndrome Scale (PANSS) and that of dyskinesia, than the placebo group (12). However, the results are inconsistent. While there have been several positive studies, a recent meta-analysis showed that Omega-3 had mixed results in patients with stable chronic schizophrenia, with only some patients experiencing significant benefits. Among patients with chronic schizophrenia, use of omega-3 fatty acids by both those experiencing acute exacerbations and those who had discontinued antipsychotic medications resulted in worsening of psychotic symptoms (13). As a consequence, the aim of this study was determine whether supplement PUFA, especially Omega-3 fatty acid PUFA, with acute violent patients could ameliorate symptoms in schizophrenia. We were particularly interested in the symptom of hostility.
Studies in mood disorders (14, 15), borderline personality disorder (16), young males in prison (17); also suggest that PUFAs may influence mood, impulsivity and aggression. Violence is one of the primary reasons for hospitalization currently (18). Blood levels of EPA alone or with docosahexaenoic acid (DHA) have reported being negatively correlated with psychometric measures of aggression (19–21). Dietary supplements of EPA have been associated with a reduction in violent behavior in British and Dutch forensic populations (17, 22). Zanarini's study among borderline personality disorder patients got similar results (16).
Hostility involves unfriendly attitudes including irritability, anger, resentment, or aggression. It can be assessed by PANSS, which defined hostility as a positive symptom (23). A cross-sectional observational study found that consumption of any fish rich in Omega-3 fatty acids, was independently associated with lower odds of high hostility (OR=0.82; 95% CI=0.69–0.97; P=0.02) in young adulthood (24). Some researchers found that concentrations of EPA and DHA in erythrocytes (RBC) showed significant negative correlations with the hostility score of PANSS in acute drug-free schizophrenia (25).
However, to date, there is relatively little research concerning the potential use of Omega-3 fatty acids as a treatment to reduce hostility in acute patients with schizophrenia. This study therefore aimed to explore the impact of Omega-3 fatty acids supplementation on hostility and psychotic symptomatology of acute schizophrenia inpatients.
Subjects and Methods
Subjects enrolled in this study were patients with an ICD-10 diagnosis of schizophrenia in the Shanghai Mental Health Center from June 2015 to November 2017. This study was approved by the Ethics Committee of the Shanghai Mental Health Center, (Clinic Trial No. NCT02552758). Patients participated in this study during their first 2 weeks of hospitalization. Symptomatology was measured by PANSS. The validity and reliability of the Chinese version of PANSS were acceptable (internal consistency reliability was 0.87, internal consistency reliability of the five dimensions ranged from 0.74 to 0.90) (26). Violent behavior was assessed by the Modified Overt Aggression Scale (MOAS). The MOAS is divided into four subscales: verbal aggression, physical aggression against objects, physical aggression against self and physical aggression against others. The reliability and validity of the Chinese version of the MOAS was modest, with Intra-class correlation coefficient (ICC=0.94, P < 0.001), Kendall's W coefficient of concordance (W=0.83, P= 0.001) and the Mann–Whitney U test (z=− 2.89, P= 0.002) (27). All subjects in this study were required to score higher than 4, which meant that they had severe verbal aggression for 6 months. Assessments were conducted at the baseline, 4 and 8 weeks after treatment. Patients were excluded if they met the criteria for other psychiatric disorders or serious physical diseases. All participants were treated with antipsychotic medication during the study.
Patients were randomly assigned to be treated with either the fish oil, containing 540 mg of EPA and 360 mg of DHA (GNC, USA); or a placebo (10 mg of Vitamin E) once a day, based on a randomization code that was not available to the investigators. The Vitamin E capsule was like the fish oil in color, shape and texture. Participants were unaware about the kind of supplement they received. The nurses were in charge of dispensing the fish oil or placebo to the patients, so that the clinicians and doctors were kept blind to the interventions. The measurement of the levels of DHA and EPA in plasma was taken at the baseline and the 8th week. These levels were analyzed by the gas chromatography-mass spectrometry. All patients or their authorized representatives signed the consent forms.
Chi-square tests, t-test, repeat measure ANOVA, ANCOVA and a correlation analysis were carried out using the Statistical Package for the Social Sciences (SPSS).
Results
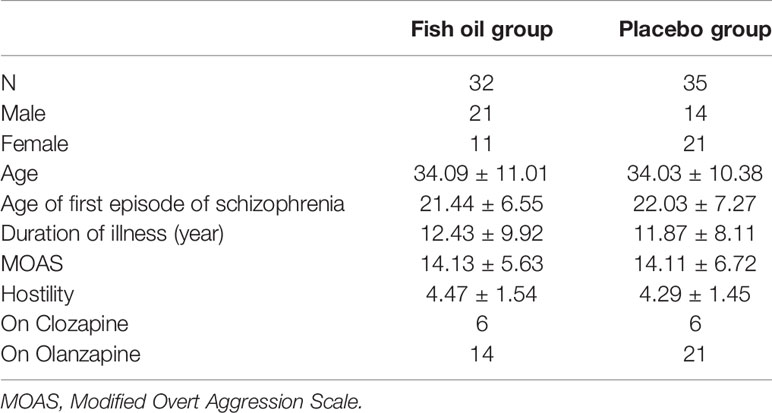
A total of 67 patients (35 males, 32 females) participated in this study. Among them, 32 patients received the intervention of the fish oil and 35 of the placebo. Forty-seven patients (70.1%) completed the 8-week intervention. There were no significant group differences in age, age of first episode of psychosis and the whole duration of illness (Table 1). However, gender was not equally distributed between the groups (P=0.036), that is, 21 males and 11 females were allocated in the fish oil group, 14 males and 21 females to the control group. The scores of the MOAS and hostility at the baseline were not statistically different. We documented the psychopharmacologic treatments of both the groups. Since olanzapine and clozapine are most likely to have anti-aggression effects (28, 29), we listed the number of patients who were on these two medications from both groups. There was no significant difference found between the groups (Table 1).
The plasma concentration of DHA and EPA at the baseline was statistically not different for the two groups. The concentration of EPA increased after 8 weeks of intervention in the fish oil group (p < 0.01), while the concentration of DHA remained unchanged for the two groups.
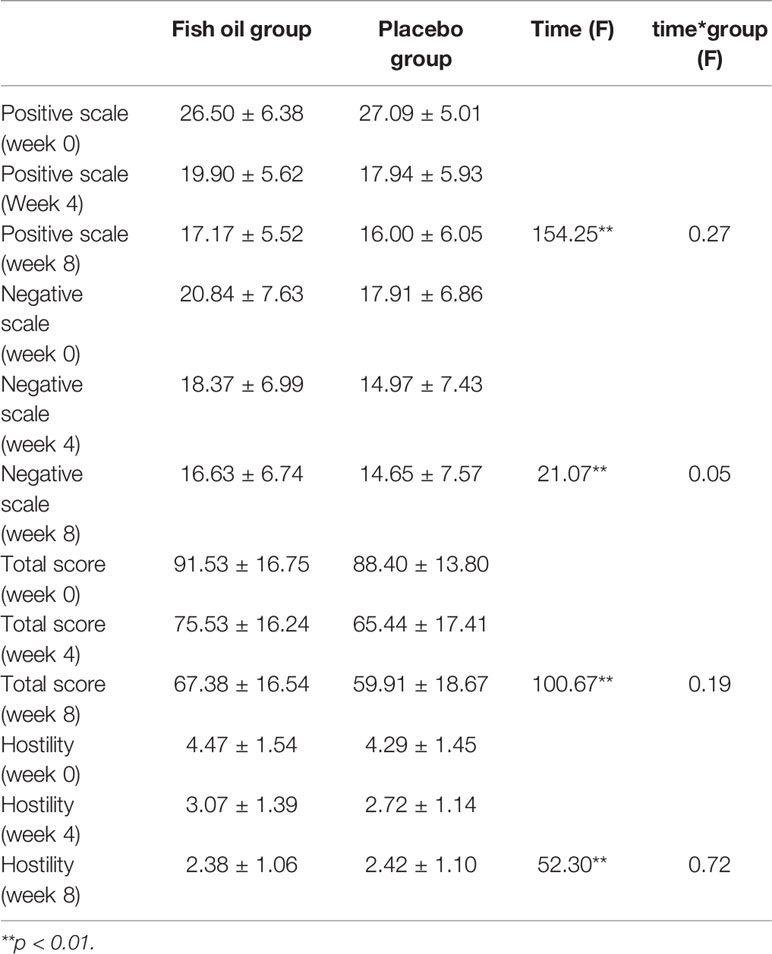
The scores for the positive scale, negative scale, total scale and hostility of PANSS declined significantly with the treatment going on in both groups at week 4 and week 8, but there were no group differences (Table 2). After controlling for gender as a covariate, the result remained same (P=0.524, 0.353, 0.165, and 0.884 for positive scale, negative scale, total scale, and hostility respectively).
There was a significant correlation between MOAS and hostility at the baseline (R=0.25, P < 0.05). But there was no correlation between hostility and the plasma level of DHA or EPA at the baseline and after treatment.
Discussion
Scores on the positive, negative and total scale of PANSS declined significantly in both groups at week 4 and week 8, but there were no group differences. This indicated that adjunctive supplementation of Omega-3 fatty acids provided no benefits on symptoms when compared to antipsychotics alone. This result was similar to previous studies among chronic patients with schizophrenia (30–32). One meta-analysis study found that studies in chronic schizophrenia had mixed results, with two studies showing no benefit from EPA, while one study showing that EPA alone led to a worse outcome (2). Even among first episode patients with schizophrenia, one study did not find the extra benefits of EPA on symptom ratings (33). In our study, some patients were not in their first episodes and the average age of the participants was about 34 years. Though all the participants were in the acute phase, some of them were chronic patients with schizophrenia. This may be a confound.
The scores of hostility declined significantly in both groups at week 4 and week 8, but no group differences were found in this study. There was no correlation between hostility and the plasma level of DHA or EPA. In a previous study, significant correlations between hostility and PUFAs in erythrocyte were found in drug-free patients with schizophrenia (25). However, that was a cross-sectional study, so the direction was impossible to determine whether the PUFA supplement could ameliorate hostility in patients with schizophrenia. Another pilot study in which 12-violent treatment-resistant in patients with chronic schizophrenia received a supplement each of DHA, EPA, and vitamin E for 12 weeks and the index of agitation significantly decreased (34). However, it was not an RCT trial; the results might have been confounded by the effects of a pharmacologic treatment. Our study does not support the benefits from Omega-3 fatty acids supplementation on hostility in patients with schizophrenia. Some researchers (35) found that anger stems from the delusion, which in turn mediates the violent behavior in psychotic patients. Violence, aggression and hostility are regarded as symptoms of psychotic patients for some psychiatrists. This result coincides with our first finding that supplements of Omega-3 fatty acids did not show extra benefits on symptoms.
There are several other possible explanations for our negative results: 1) the dosages of DHA and EPA in this study were relatively low (540 mg EPA + 360 mg DHA) and may have been insufficient; 2) our sample size was relatively small, with the possibility of false-negative errors.
There were limitations in this study. First, since gender was not equally distributed among the two groups, we did an analysis of covariance. After controlling for gender as a covariate, the result remained the same. Secondly, we used Vitamin E (10 mg) as a placebo in this study. The reason for the choice of low doses of Vitamin E was due to the shape, texture and color similarities for keeping the blinding. Some studies have employed Vitamin E for the purpose of reducing violence. Gesch etal. (17) found that antisocial behavior in prisons, including violence, was reduced by vitamins, minerals and essential fatty acids. The dosage of Vitamin E was 10 mg in that study, but there were 25 kinds of vitamins and minerals in the tablets. Hence, it was hard to tell how much 10 mg of Vitamin E contributed to the result. Légaré etal. (34) also provided Vitamin E (400 IU) as a supplement together with Omega-3 (400 mg EPA and 200 mg DHA) to patients with schizophrenia and suggested that nutritional supplements might reduce agitation and psychopathology. However, in that study, the dosage of Vitamin E was much higher than in our study and it was not an RCT trial. Hence, the reduction may have resulted from the psychopharmacologic treatment. Though the doses of Vitamin E are low in our study, it is an active substance. Further studies should avoid using an active substance as a placebo to avoid the possible confounding.
Further studies could recruit more patients with schizophrenia who do not commit violent behavior and identify whether the level of Omega-3 PUFA is one of the biomarkers of schizophrenia. Future studies could prolong the intervention of Omega-3 fatty acids, trying to explore the long-term effects on the hostility, violence and psychopathology of schizophrenia.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai Mental Health Center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YQ and CL prepared for the manuscript. HH and FL helped to collect samples and do the blood test. YS helped to do the clinic evaluation. BX was responsible for the study proposal.
Funding
This work was supported by grants from The Three-Year Action Plan for The Construction of Public Health System in Shanghai (GWIV-5, PI: BX), the National Natural Science Foundation of China (81302624, PI: YQ; 81873909, PI: QL), and Quantitative evaluation based strategic research of interventions on relapse of schizophrenia (19411950800, PI: BX).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res (1998) 30(3):193–208. doi: 10.1016/S0920-9964(97)00151-5
2. Peet M. Omega-3 polyunsaturated fatty acids in the treatment of schizophrenia. Israel J Psychiatry Related Sci (2008) 45(1):19.
3. Yao J. Abnormalities of fatty acid metabolism in red cells, platelets and brain in schizophrenia. In: . Phospholipid Spectrum Disorders in Psychiatry and Neurology, 2nd edition. Lancashire, UK: Marius Press (2003). p. 193–212.
4. Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain ω-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry (2010) 67(2):146–54. doi: 10.1001/archgenpsychiatry.2009.192
5. Skosnik P, Yao J. From membrane phospholipid defects to altered neurotransmission: is arachidonic acid a nexus in the pathophysiology of schizophrenia? Prostaglandins Leukotrienes Essential Fatty Acids (2003) 69(6):367–84. doi: 10.1016/j.plefa.2003.08.008
6. Amminger GP, Patrick D, McGorry PD. Update on omega-3 polyunsaturated fatty acids in early-stage psychotic disorders. Neuropsychopharmacology (2012) 37(1 ):309. doi: 10.1038/npp.2011.187
7. Berger GE, Wood SJ, Wellard RM, Proffitt TM, McConchie M, Amminger GP, et al. Ethyl-eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study. Neuropsychopharmacology (2008) 33(10):2467. doi: 10.1038/sj.npp.1301628
8. Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience (2006) 139(3):991–7. doi: 10.1016/j.neuroscience.2006.01.021
9. Pfrommer CA, Erl W, Weber PC. Docosahexaenoic acid induces ciap1 mRNA and protects human endothelial cells from stress-induced apoptosis. Am J Physiology-Heart Circulatory Physiol (2006) 290(6):H2178–H86. doi: 10.1152/ajpheart.00933.2005
10. Gama CS, Canever L, Panizzutti B, Gubert C, Stertz L, Massuda R, et al. Effects of omega-3 dietary supplement in prevention of positive, negative and cognitive symptoms: a study in adolescent rats with ketamine-induced model of schizophrenia. Schizophr Res (2012) 141(2):162–7. doi: 10.1016/j.schres.2012.08.002
11. Berger GE, Proffitt T-M, McConchie M, Yuen H, Wood SJ, Amminger GP, et al. Ethyl-eicosapentaenoic acid in first-episode psychosis: a randomized, placebo-controlled trial. J Clin Psychiatry (2007) 68(12):1867–75. doi: 10.4088/JCP.v68n1206
12. Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry (2002) 159(9):1596–8. doi: 10.1176/appi.ajp.159.9.1596
13. Chen AT, Chibnall JT, Nasrallah HA. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: Possible stage-specific effects. Ann Clin Psychiatry : Off J Am Acad Clin Psychiatrists (2015) 27(4):289–96.
14. Nemets B, Stahl Z, Belmaker R. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry (2002) 159(3):477–9. doi: 10.1176/appi.ajp.159.3.477
15. Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry (2002) 59(10):913–9. doi: 10.1001/archpsyc.59.10.913
16. Zanarini MC, Frankenburg FR. Omega-3 fatty acid treatment of women with borderline personality disorder: a double-blind, placebo-controlled pilot study. Am J Psychiatry (2003) 160(1):167–9. doi: 10.1176/appi.ajp.160.1.167
17. Gesch CB, Hammond SM, Hampson SE, Eves A, Crowder MJ. Influence of supplementary vitamins, minerals and essential fatty acids on the antisocial behaviour of young adult prisoners. Br J Psychiatry (2002) 181(1):22–8. doi: 10.1192/bjp.181.1.22
18. Colasanti A, Natoli A, Moliterno D, Rossattini M, De Gaspari IF, Mauri MC. Psychiatric diagnosis and aggression before acute hospitalisation. Eur Psychiatry (2008) 23(6):441–8. doi: 10.1016/j.eurpsy.2007.09.005
19. Beier AM, Lauritzen L, Galfalvy HC, Cooper TB, Oquendo MA, Grunebaum MF, et al. Low plasma eicosapentaenoic acid levels are associated with elevated trait aggression and impulsivity in major depressive disorder with a history of comorbid substance use disorder. J Psychiatr Res (2014) 57(1):133–40. doi: 10.1016/j.jpsychires.2014.06.012
20. Meyer BJ, Byrne MK, Collier C, Parletta N, Crawford D, Winberg PC, et al. Baseline omega-3 index correlates with aggressive and attention deficit behaviours in adult prisoners. PloS One (2014) 1(3):9–. doi: 10.1016/j.jnim.2014.10.022
21. Zaalberg A, Wielders J, Bulten E, Staak CVD, Wouters A, Nijman H. Relationships of diet-related blood parameters and blood lead levels with psychopathology and aggression in forensic psychiatric inpatients. Criminal Behav Ment Health (2015) 26(3):196–211. doi: 10.1002/cbm.1954
22. Zaalberg A, Nijman H, Bulten E, Stroosma L, Staak CVD. Effects of nutritional supplements on aggression, rule-breaking, and psychopathology among young adult prisoners. Aggressive Behav (2010) 36(2):117–26. doi: 10.1002/ab.20335
23. Kay SR, Opler LA, Lindenmayer J-P. The positive and negative syndrome scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl (1989) 155: (S7):59–65. doi: 10.1192/S0007125000291514
24. Iribarren C, Markovitz J, Jacobs JRD, Schreiner P, Daviglus M. Hibbeln JJEJoCN. Dietary intake of n-3, n-6 fatty acids and fish: relationship with hostility in young adults—the CARDIA study. Eur J Clin Nutr (2004) 58(1):24. doi: 10.1038/sj.ejcn.1601739
25. Watari M, Hamazaki K, Hirata T, Hamazaki T, Okubo Y. Hostility of drug-free patients with schizophrenia and n– 3 polyunsaturated fatty acid levels in red blood cells. Psychiatry Res (2010) 177(1):22–6. doi: 10.1016/j.psychres.2010.02.016
26. Tianmei S, Yang J, Shu L. The reliability, validity of PANSS and its implication. Chin Ment Health J (1992). 18(1):45–7. doi: 10.1007/BF02911031
27. Huang HC, Wang Y-T, Chen KC, Yeh TL, Lee IH, Chen PS, et al. The reliability and validity of the Chinese version of the Modified Overt Aggression Scale. Int J Psychiatry Clin Pract (2009) 13(4):303–6. doi: 10.3109/1365150090305653
28. Maier GJ. The impact of clozapine on 25 forensic patients. Bull Am Acad Psychiatry Law (1992) 20(3):297–307. doi: 10.1177/009318539202000211
29. Volavka J, Czobor P, Nolan K, Sheitman B, Lindenmayer JP, Citrome L, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol (2004) 24(2):225–8. doi: 10.1097/01.jcp.0000117424.05703.29
30. Emsley R, Niehaus DJ, Koen L, Oosthuizen PP, Turner HJ, Carey P, et al. The effects of eicosapentaenoic acid in tardive dyskinesia: a randomized, placebo-controlled trial. Schizophr Res (2006) 84(1):112–20. doi: 10.1016/j.schres.2006.03.023
31. Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry (2001) 158(12):2071–4. doi: 10.1176/appi.ajp.158.12.2071
32. Peet M, Horrobin DF, Group IawtEMS. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res (2002) 36(1):7–18. doi: 10.1016/S0022-3956(01)00048-6
33. Berger G, Proffitt T, Wood S, McConchie M, Yuen H, Horrobin D, et al. editors. Ethyl-eicosapentaenoic acid (E-EPA) supplementation in early psychosis. A double blind randomized placebo-controlled add on study in 80 drug-naive first episode psychosis patients. International Journal of Neuropsychopharmacology. Cambridge Univ. Press: New York, NY (2004).
34. Légaré N, Brosseau É, Joyal CC. Omega-3 and violence in schizophrenia. Schizophr Res (2007) 96(1):269. doi: 10.1016/j.schres.2007.05.023
Keywords: schizophrenia, psychopathology, hostility, omega-3, randomized control trial
Citation: Qiao Y, Liu CP, Han HQ, Liu FJ, Shao Y and Xie B (2020) No Impact of Omega-3 Fatty Acid Supplementation on Symptoms or Hostility Among Patients With Schizophrenia. Front. Psychiatry 11:312. doi: 10.3389/fpsyt.2020.00312
Received: 09 January 2020; Accepted: 30 March 2020;
Published: 21 April 2020.
Edited by:
Kelly Anne Allott, University of Melbourne, AustraliaReviewed by:
Mary V. Seeman, University of Toronto, CanadaJoshua T. Kantrowitz, Columbia University, United States
Copyright © 2020 Qiao, Liu, Han, Liu, Shao and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Qiao, cWlhb3lpMjAwNEBtc24uY29t; Yang Shao, c2F3eWVyMjAwMkAxNjMubmV0; Bin Xie, eGllYmluQHNtaGMub3JnLmNu
†These authors have contributed equally to this work
 Yi Qiao
Yi Qiao Cai Ping Liu
Cai Ping Liu Hui Qin Han
Hui Qin Han Yang Shao
Yang Shao