- 1Faculty of Medicine, School of Clinical and Experimental Sciences, University of Southampton, Southampton, United Kingdom
- 2Department of Sleep Medicine, Southampton Children’s Hospital, Southampton, United Kingdom
- 3Department of Community Child Health, Solent NHS Trust, Portsmouth, United Kingdom
- 4Department of Respiratory and Sleep Medicine, Sheffield Children’s NHS Foundation Trust, Sheffield, United Kingdom
- 5Department of Sleep Medicine, Evelina London Children’s Hospital, Guys St Thomas’s NHS Trust, London, United Kingdom
- 6Southampton Centre for Biomedical Research, University Hospital Southampton NHS Trust, Southampton, United Kingdom
- 7School of Psychotherapy & Psychology, Faculty of Humanities, Arts and Social Sciences, Regent's University London, London, United Kingdom
- 8Laboratoire de Physiologie intégrée du système d’éveil CRNL‐ INSERM U1028‐CNRS UMR 5292, Université Claude Bernard Lyon 1, Hospices Civils de Lyon, Lyon, France
Study Objectives: Obstructive sleep apnea (OSA) is common in children with Down syndrome (DS) and is associated with adverse health and cognitive outcomes. Daytime clinical assessment is poorly predictive of OSA, so regular screening with sleep studies is recommended. However, sleep studies are costly and not available to all children worldwide. We aimed to evaluate the psychometric properties and predictive value of a newly developed screening questionnaire for OSA in this population.
Methods: 202 children aged 6 months to 6th birthday with DS were recruited, of whom 188 completed cardio-respiratory sleep studies to generate an obstructive apnea hypopnea index (OAHI). Parents completed the 14-item Down syndrome OSA screening questionnaire. Responses were screened, a factor analysis undertaken, internal consistency calculated and receiver operator characteristic (ROC) curves drawn to generate an area under the curve (AUC) to assess criterion related validity.
Results: Of 188 children who completed cardiorespiratory sleep studies; parents completed the screening questionnaire for 186. Of this study population 15.4% had moderate to severe OSA defined by an OAHI of ≥5/h. Sixty-three (33.9%) participants were excluded due to “unsure” responses or where questions were not answered. Using the remaining 123 questionnaires a four-factor solution was found, with the 1st factor representing breathing related symptoms, explaining a high proportion of the variance. Internal consistency was acceptable with a Cronbach alpha of 0.87. ROC curves for the total score generated an AUC statistic of 0.497 and for the breathing subscale an AUC of 0.603 for moderate to severe OSA.
Conclusion: A well designed questionnaire with good psychometric properties had limited predictive value to screen for moderate to severe OSA in young children with DS. The use of a screening questionnaire is not recommended. Screening for OSA in this population requires objective sleep study measures.
Introduction
Down syndrome (DS) is the commonest chromosomal abnormality affecting approximately 1:1,200 live births worldwide (1). Children with DS are at increased risk of obstructive sleep apnea (OSA) a condition characterized by repetitive partial (hypopnea) or complete (apnea) airway collapse in sleep, despite continued respiratory effort. OSA is estimated to affect 75%, of this population compared to 1.2% of typically developing (TD) children (2, 3). Risk factors are multifactorial including syndrome-specific characteristics such as hypotonia, macroglossia, craniofacial structure, and obesity, exacerbated by adenotonsillar hypertrophy in early childhood (4).
OSA causes nocturnal hypoxia and fragmented sleep with adverse health consequence that have been extensively studied in TD children including: hypertension (systemic and pulmonary) (5), cognitive deficits (impaired attention and executive function) leading to impaired learning and school performance (6), as well as reduced quality of life (7), and increased health care utilization (8). Similar findings are emerging in children with DS who arguably may be more at risk due to their limited cognitive reserve and underlying cardiovascular disease (9). Indeed, Breslin et al.. studied 38 school-aged children with DS and reported that co-occurring OSA was associated with a nine point reduction in verbal IQ and reduced cognitive flexibility (10). We have also recently reported that OSA predicts deficits in parent-reported executive function behaviors in very young children with Down syndrome (11). It has further been hypothesized that OSA in DS may be a risk factor for the development of Alzheimer’s disease (12). Prompt identification and treatment of OSA in DS is therefore an important goal.
Multiple studies have reported poor correlation between parental report of OSA symptoms and polysomnography (PSG) results (the gold standard for the diagnosis of OSA) (13, 14). This may be due to a lack of awareness of nocturnal symptoms or the presence of silent apnea which is difficult for parents to detect. Children with DS referred for PSG have more severe disease than TD children, suggesting that milder symptoms are overlooked or attributed to unmodifiable symptoms of DS.
Given the increased burden of disease and challenges in diagnosis in this population, the American Academy of Pediatrics recommends routine screening with PSG by the age of 4 years (15). There is evidence of limited compliance with these guidelines with one study reporting that only 47.7% of children had undergone a PSG (16). In the UK, screening is recommended annually from infancy to 3–5 years of age in DS, using a minimum of pulse oximetry (17). If there is any abnormality detected on pulse oximetry, or clinical suspicion of a false negative oximetry result, then further assessment with, as a minimum, cardiorespiratory polygraphy studies is recommended (17). We have published recommended oximetry screening thresholds that can be used to determine the need for further diagnostic evaluation in this population (18). This screening method has a high reported sensitivity (92%) with a specificity (63%) with one night of domiciliary Masimo pulse oximetry. While oximetry is a screening tool that is, on the whole, well tolerated, it has resource implications (19). Other groups have researched alternative screening methods, including urinary biomarkers, 3D photogrammetry, and combined measures including cephalometry and multiple clinical variables (20–22). All of these approaches have limitations of time and cost and therefore a screening questionnaire is an appealing alternate approach.
Screening questionnaires are used in clinical practice to identify sleep problems in TD children. There has been increasing work looking at the utility of these questionnaires in the DS population. Ebsensen et al.. studied the convergent validity of three questionnaires, the Behavioral Evaluation of Disorders of Sleep (BEDS), Children’s Sleep Habits Questionnaire (CSHQ), and Sleep Disturbances Scale for Children (SDSC) in a group of 30 children with DS aged 6–17 years. All three questionnaires have sub-scales relating to sleep disordered breathing and were previously validated (23–25). There were strong correlations between these sub-scales but, in the absence of an objective measure of OSA in this study, no conclusions could be drawn about the sub-scales’ ability to predict OSA (26). OSA screening questionnaires have been designed for TD children. The Pediatric Sleep Questionnaire (PSQ) had initial reported sensitivities and specificity of 0.85 and 0.87 respectively to predict moderate to severe OSA in TD children at 2–18 years (27, 28), however concerns have been raised about its specificity within TD populations. Sproson et al.. reported specificity of only 0.17 for an OAHI ≥5/h in a young UK population (29). It may have further limitations in the DS population as it includes questions that relate to child behavior and growth that may be due to underlying features of their DS, as opposed to co-occurring OSA. Cielo et al.. encountered this difficulty when testing the PSQ in children with cranio-facial abnormalities where sensitivity and specificity were only 0.57 and 0.48 respectively to predict moderate to severe OSA (30). Furthermore, Pabery et al.. reported an even lower sensitivity of 0.37 for the PSQ to predict moderate to severe OSA in 35 children with Down syndrome aged 2–16 years (31).
We have previously reported the methodology used to design a 14-item OSA screening questionnaire intended for children with DS aged up to 6 years (32, 33). Specifically, we used a content validity process to design a questionnaire specific to children with DS incorporating expertise from health care professional and parents into the design process. Details of this process are outlined elsewhere (28, 29). The present study aimed to evaluate the psychometric properties and predictive value of this questionnaire when tested in a population of young children with DS.
Materials and Methods
Participants
Children with a confirmed diagnosis of DS between the ages of 6 months to 6th birthday were recruited to one of three research centers in the UK at Southampton, Sheffield, and The Evelina London Children’s hospitals. Children were excluded if they had undergone a cardiorespiratory sleep study in the preceding 3 months, were receiving home oxygen therapy or non-invasive ventilation. Children were recruited through multiple approaches as previously described (19).
Measures
Demographics and Medical History
Parent/caregivers provided information on their child’s age, gender, relevant past medical history (use of prophylactic asthma treatment, upper airway surgery, epilepsy, congenital cardiac condition, home oxygen use and whether born prematurely under 37 weeks gestation) and socio-demographic characteristics including parental education levels and smoking status. Children were weighed and measured and a body mass index calculated.
Questionnaire
The DS OSA questionnaire, developed by Sanders et al. (32), comprises 14 items rated on a five point Likert scale: never (never in the past 6 months), Rarely (less than one night a week), Occasionally (1–3 nights a week), almost always (4–6 nights a week), always (every night). An additional “unsure” response was allowed for each item. The questionnaire was designed to be completed by the child’s primary caregiver. Details of the questions can be found in Tables 1 and 4.
Domiciliary Cardiorespiratory Polygraphy
OSA was assessed using the SOMNOtouch device (SOMNOmedics, Germany) comprising chest and abdominal respiratory inductance plethysmography (RIP) bands, internal pulse oximetry, nasal pressure flow with snore sensor, body position sensor, and actigraphy. We have previously reported our positive experience of domiciliary studies in this population (34). A sleep log recorded sleep onset, night waking’s, and morning wake up times.
Studies were scored by an experienced technologist (RNK), using Domino Light software (SOMNOmedics, Germany). Details of scoring criteria and quality assessment of studies have been published (19). Sleep and wake were estimated using parental sleep log and integrated actigraphy. As per AASM scoring criteria, where two or more signals were of poor quality, data were excluded. Respiratory events were scored according to standard pediatric scoring criteria for adapted sensors (35). Where nasal flow signal was lost an “undefined apnea” was scored, where RIP sum indicated paradoxical breathing in the presence of a minimum three percent oxyhemoglobin desaturation for at least two breaths. The obstructive apnea/hypopnea index (OAHI) was calculated by summing obstructive apnea, hypopnea, mixed and undefined apnea indices during the total sleep time. OSA was diagnosed if OAHI was ≥ 5/h representing both a meaningful threshold for clinical intervention and reflecting the sensitivity of domiciliary cardiorespiratory polygraphy in children (6).
Procedure
The study was approved by the UK National Research Ethics Committee (reference 13/SC/0106). Parents provided informed consent for their child to participate. Procedures for the full study are published (18).
Statistical Analysis
Analyses were performed using SPSS (IBM SPSS, version 22.00, Chicago, IL, USA). Questionnaire data were checked for entry error and 10% were double entered at random to check for data integrity.
Responses were initially screened for missing and unsure responses. Items at this point were considered for ongoing inclusion in further analysis. As principle component analysis and reliability analysis require continuous data entry, 63 participants who had given an “unsure” response or had failed to respond to any item were excluded.
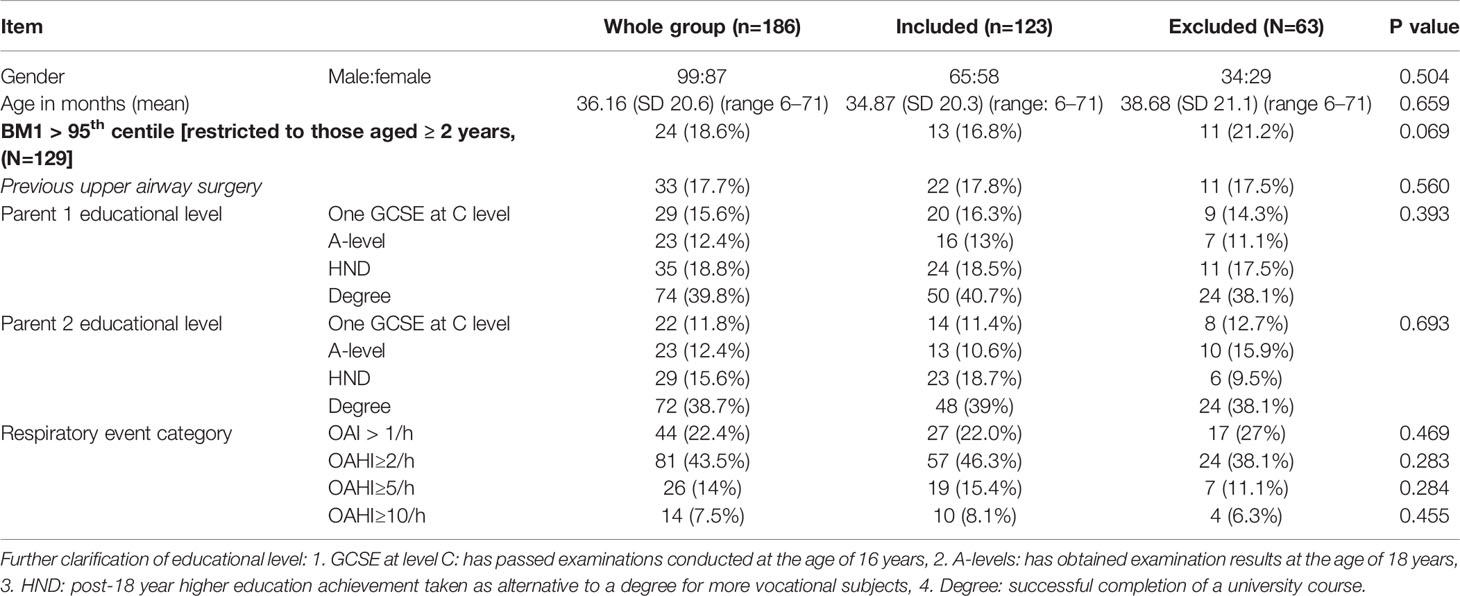
Demographics, past medical history and OAHI were compared between the excluded and included participants using either the chi-squared test or paired T tests to detect significant differences between the groups.
Question responses were split into positive or negative responses based on clinical significance. For example, in question 1 “how often does your child snore when they do not have a cold” a negative response was counted as “never, rarely, and occasionally” and a positive response as “almost always and always.” Demographics and past medical history of participants were compared for these dichotomized responses using a chi-squared test or paired T-test to identify any bias in response.
A principal component analysis was conducted to identify structure within the questionnaire and to determine the relationship of its underlying dimensions. A Kaiser-Meyer-Olkin (KMO) adequacy of sample measure was performed (36). To be an adequate sample a value of 0.5 is required. Factors were initially extracted using an Eigen-value greater than one. To aid extraction, scree plots and an approach with fixed number of factors were used. An orthogonal varimax rotation aids interpreting the factors to produce defined subscales. Factors were interpreted and assigned meaning by the authors SG-H, KS, and CH. Stability of the factors were checked by performing a split half method and ensuring similar results were achieved to those for the group as a whole. Cronbach alpha, a measure of internal consistency, was checked for the scale as a whole. Internal consistency of the subscales was then measured using a split half method.
Receiver operator characteristic (ROC) curves were generated for both the total score of the questionnaire and the underlying subscales as predicators of OSA status. Questionnaire responses were scored from 1 to 5, where 1=never and 5=always. Area under the curve (AUC) statistics were generated: An AUC of 0.5 indicates no predictive power and an AUC of 1 indicates perfect predictive power.
The study aimed to choose a point on the curve which maximized sensitivity over specificity to identify the maximal number of true positives based on the concept that the questionnaire would act as an initial screening tool rather than a diagnostic tool. This value would be out of total maximum possible points scored from the questionnaire.
Results
Two hundred two participants enrolled in the study, of whom 186 had both a completed DS OSA questionnaire and a calculated OAHI. Participant flow through the study is shown in Figure 1.
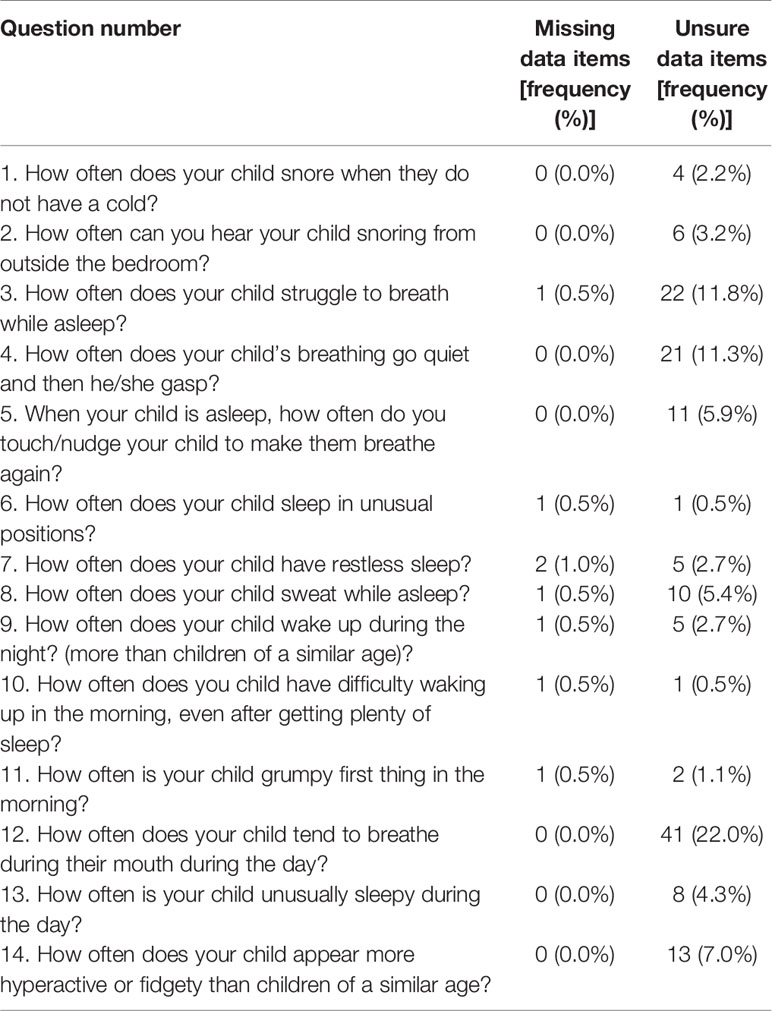
Missing and Unsure Data Items
The number of unsure and missing data items among this sample of 186 are shown in Table 1. Twenty-two percent of all parents completing the questionnaire answered “unsure” to question 12 “how often does your child tend to breathe through their mouth during the day.” It was therefore felt to be a poor question and excluded from further analysis.
Sixty-three (33.9%) parents answered randomly “unsure,” or data were missing, for one or more other question and these questionnaires were excluded from data analysis leaving a sample of 123 (66.1%) fully completed questionnaires for the final analysis.
Demographic and Clinical Characteristics
Demographics of the final sample are shown in Table 2. There were no significant differences in child’s age, gender, body mass index (BMI) category, relevant past medial history, parental socio-demographic features, or study center between the 123 (66.1%) participants included in the final analysis and 63 (33.9%) that were excluded. Significant difference in responses to questions are shown in Table 3A for when comparing “unsure response” to an alternative response and Table 3B when comparing dichotomized positive and negative responses to individual questions. Where significant differences in clinical or demographic characteristics were identified these were discussed by lead authors. It was agreed that these did not represent any systemic bias. No question items were rejected on the basis of these findings.

Table 3A Responses to question items where proportion answering unknown varied significantly according to demographic and clinical characteristics of the sample (n=186).
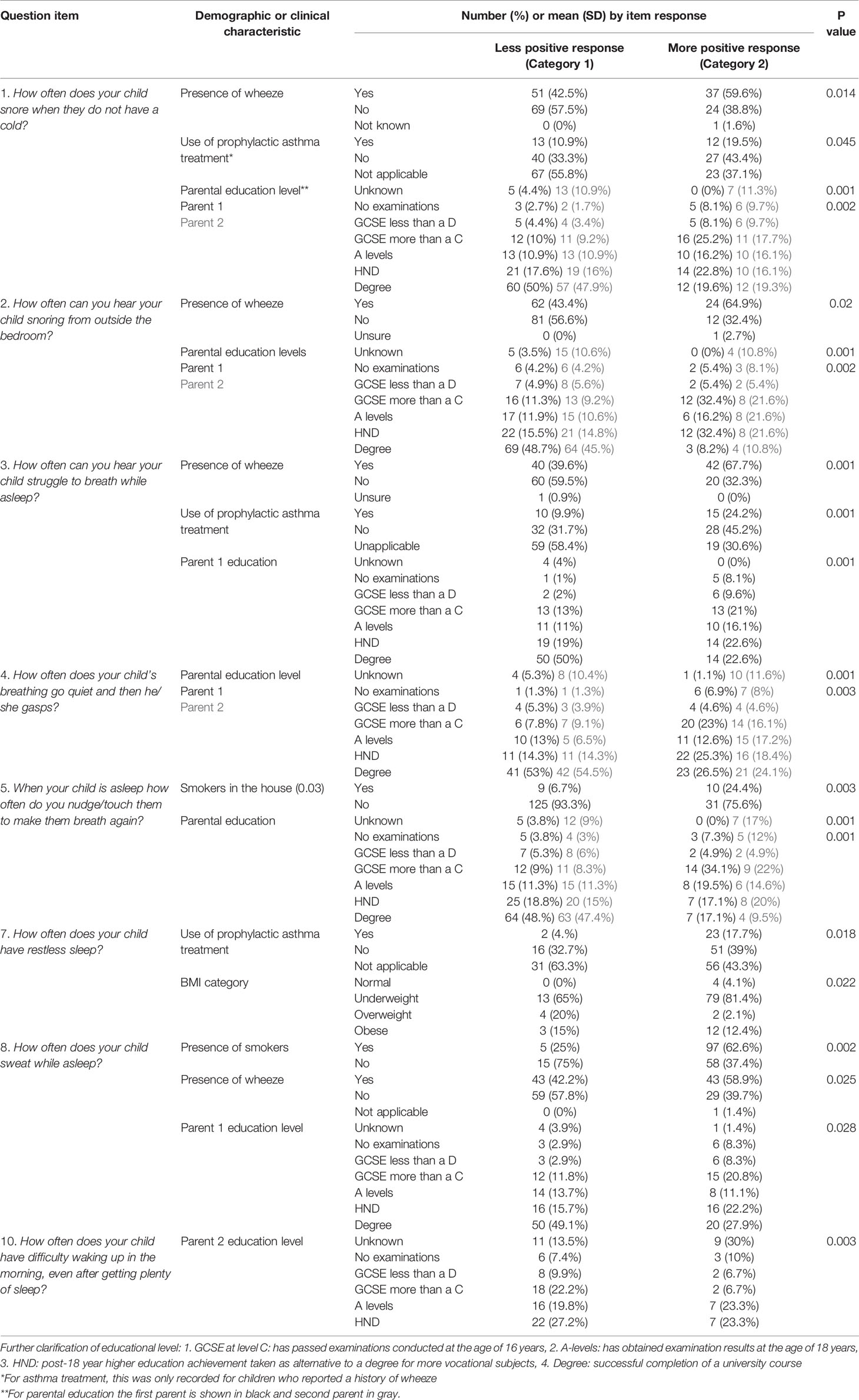
Table 3B Demographic and clinical characteristics of the sample that differed significantly in respondents answering positively versus negatively to specific questions.
Psychometric Properties
A principle component analysis was conducted on 13 items with an orthogonal rotation (varimax). The Kaiser-Mayer-Olkin (KMO) measure verified the sampling adequacy for the analysis with a KMO =0.843, additionally all individual KMO values were above the acceptable limit.
Initial analysis extracted three factors with Eigen-values greater than 1. This explained 61.7% of the variance, however most of the variance was accounted for by the first factor (41.3%). The screen plot showed inflexion both at the second factor and the 4th, with the 3rd and 4th factor contributing a similar amount of the total variance. To interpret the factors further it was forced to produce either a two, three, or four factor solution. These were reviewed by the authors and it was felt that a four-factor solution was the most appropriate and meanings were assigned to each factor, generating subscales where factor 1 represented breathing and physical related symptoms; factor 2 represented night time behavior; factor 3 represented morning behavior; and factor 4 represented the impact of poor sleep on the next day’s behavior. Items were considered to load on to a factor if they had a value of greater than 0.3 and substantially load if they had value greater than 0.7. If items loaded onto multiple factors they were assigned to the factor in which they had the highest loading. If they had similar loading, as was the case with item 5 (when your child is asleep, how often do you touch/nudge your child to make them breathe again), they were assigned to the factor which clinically matched the best item. Loading of the factors determined the sub-scale structure of the questionnaire which is shown in Table 4.
Reliability
A split half method was used to examine the internal consistency of the individual subscales. Spearman Brown coefficients were 0.8 for subscale 1, 0.79 for subscale 2, 0.75 for subscale 3, and 0.5 for subscale 4. An acceptable reliability was therefore achieved in subscales 1–3 but not subscale 4.
The reliability for the scale, as a whole, was assessed using Cronbach alpha which gave a value of 0.87
Receiver Operator Characteristic Analysis
The mean and standard deviation for the total score and subscale scores are shown in Table 5.
The AUC for the total questionnaire score was 0.497 (95% CI 0.352–0.642) for on OAHI > 5/h, and 0.569 (0.360–0.778) for an OAHI > 10/h. The breathing subscale gave an AUC of 0.542 (0.407–0.677) for an OAHI> 5/h, and 0.603 (0.409–0.796) for an OAHI> 10/h. AUC values for other subscales are shown in Table 6. ROC analysis was additionally performed for the other subscales and is shown in Table 6. Furthermore, the group was stratified by gender, age, and previous ear, nose, and throat (ENT) surgery for the total score and breathing subscale. No AUC greater than 0.7 was achieved.
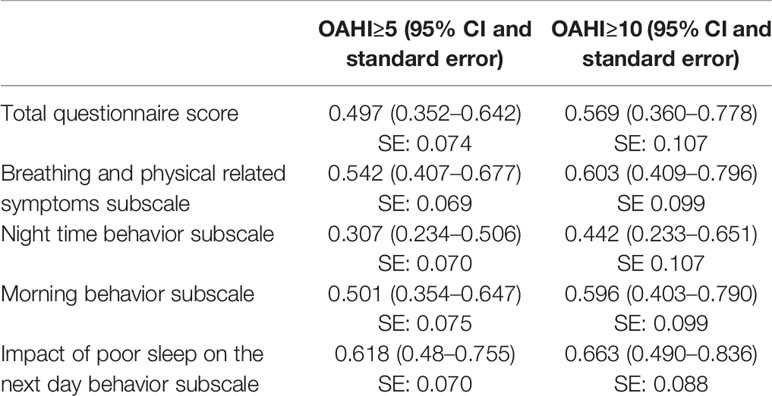
Table 6 Area under the curve values for total score of the questionnaire and subscales for obstructive apnea hypopnea index (OAHI).
It was possible that some meaningful data were lost from over-stringent removal of questionnaires with unsure responses. Therefore, factor analysis was repeated including these questionnaires and replacing unsure responses with a mean imputation method. There was no change in the factors extracted. Next, including this complete data set, ROC curve analysis was repeated. The AUC for the total score to predict OAHI > 5/h was 0.515. Further analyses were not performed.
Predictive Value
Based on ROC curve analysis to maximize sensitivity an optimal total questionnaire score cut off score of 19.5 (out of a total of 65) was generated. This identified 18/19 of the true positives (sensitivity of 94.7%) and 6/104 of true negatives (specificity of 1.9%). The positive predictive value was 0.14 and negative predictive value was 0.86. This is illustrated in Figure 2, which highlights the failure of the questionnaire to screen out children with OAHI ≥ 5/h. The predictive value of the questionnaire did not improve when the 22 children with a past history of upper airway surgery were removed from the sample. In practice, therefore, for every 100 children screened, 94 would screen positive and require confirmatory diagnostics.
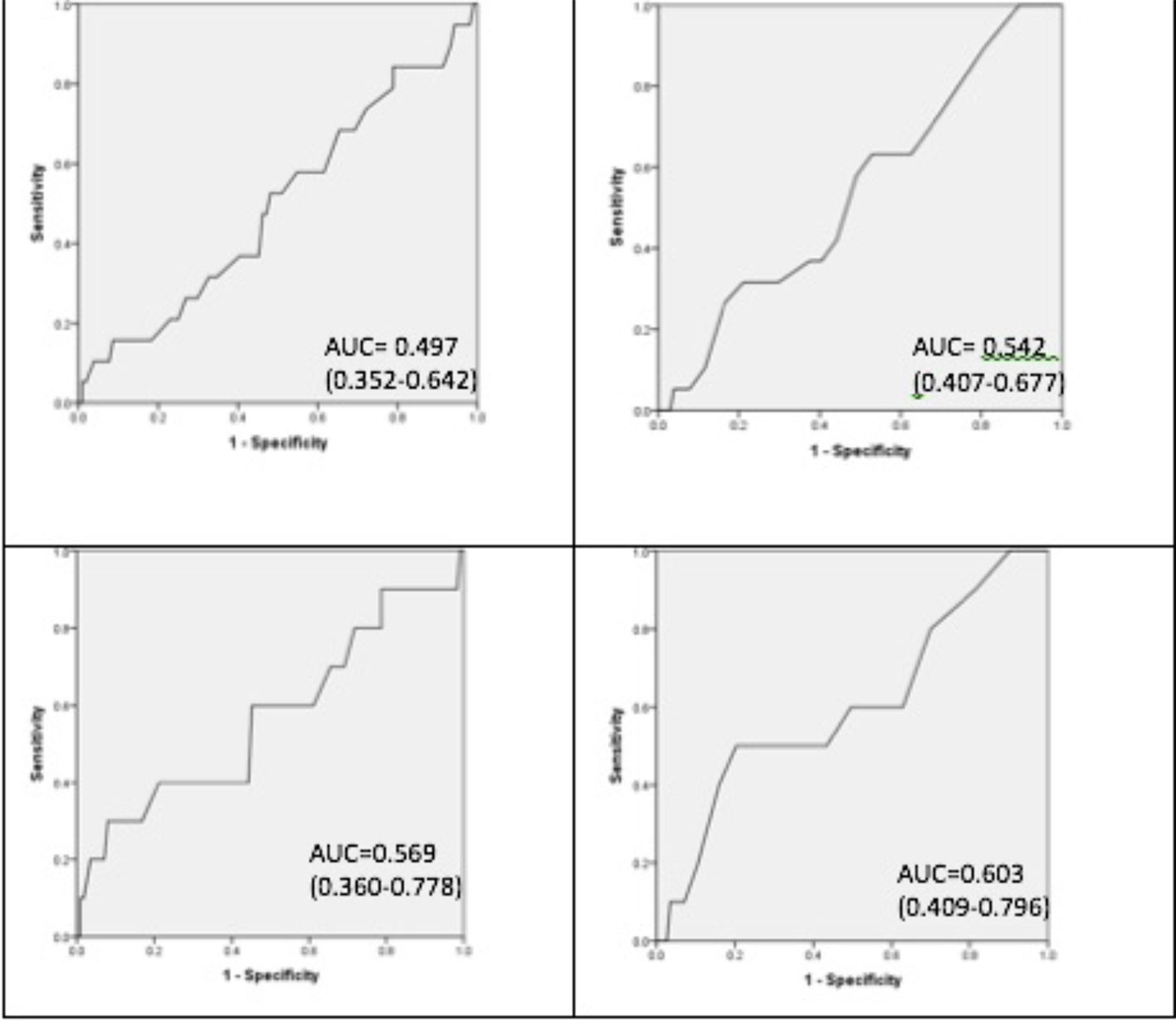
Figure 2 Receiver operating characteristic curves. From left to right. Top left obstructive apnea hypopnea index (OAHI)≥5/h and whole questionnaire score. Top right OAHI > 5/h and breathing subscale. Bottom left OAHI≥10/h and whole questionnaire score. Bottom right OAHI≥10/h and breathing subscale.
Discussion
We have demonstrated that the DS OSA questionnaire has poor positive predictive value for clinically relevant OSA in young children with DS, despite robust psychometric properties. This supports previous findings that parental report in children with DS is a poor predictor of OSA (14, 37–39).
Similarly, the literature indicates that health professionals struggle to diagnose OSA based on clinical findings in DS patients, even when supported by questionnaire items (13, 29, 40). Recent data from the UK support our findings by demonstrating that the PSQ questionnaire, which has established high sensitivity in TD children, performs very poorly in this group (31).
Other groups have combined simple objective measures, such as BMI, with questionnaire data and medical history to improve prediction of OSA. Skotko et al. developed a tool to identify OSA in the Down syndrome population using data from 130 patients aged 3–24 years (22). The model had 300 rules and 101 variables, including questions from the CSHQ and sleep-related breathing disorder subscale of the PSQ questionnaires, patients’ past medical history, physical examination, and BMI. This model had a negative predictive value of 90% and positive predictive value of 25% for an AHI ≥5/h. While this shows promise it has yet to be validated in another data set. Furthermore, given the large number of variables required for analysis, it may not be a simple tool to introduce into routine clinical practice unless technological aids are also established (22).
Development of the DS OSA questionnaire closely followed recommended methodology (33). The failure of this questionnaire to be a useful screen for OSA, despite a structured design process and good psychometric properties, reminds researchers of the importance of objective screening measures for OSA in clinical practice. It also serves to remind clinicians about the importance of only using questionnaire tools that have been robustly validated in the relevant population.
A key limitation of the questionnaire was the inclusion of the unsure response item. The aim was to prevent respondents giving false response to questions. It also allowed us to identify questions which could potentially lack clarity. This did, however, result in the exclusion of 33.9% of the sample. Given that there were no significant demographic or clinical differences between the final sample and the excluded group it is unlikely that this led to any systematic bias. Furthermore, using mean imputation methods to replace these questions did not change the factor structure of the questionnaire.
A further limitation of our data was the use of cardiorespiratory polygraphy rather than gold standard polysomnography to generate the OAHI. Cardiorespiratory studies tend to underestimate the OAHI as this technique cannot detect hypopneas associated with arousal. Use of cardiorespiratory studies in our study was a pragmatic choice reflecting typical UK practice. Furthermore, recent data in children indicate that this technology predicts OSA (defined by OAHI ≥ 5.6/h from polysomnography) with a sensitivity of 90.9% (95% CI, 79.6–100%) and a specificity of 94.1% (95% CI, 80–100%) (41). For this reason, we selected an OAHI of >5/h as a threshold to define OSA resulting in a prevalence rate of OSA in the sample of only 15%.
An additional limitation was that measurements for height and weight were only taken once by trained research nurses. A single measure may have led to inaccuracy.
Higher prevalence rates of OSA have been reported in large sample of individuals with DS. However, prevalence rates are influenced by age, sampling strategy, and the threshold used to define OSA. For example, Maris et al. reported OSA in 66.4% of 122 children with DS aged 0–18 years (based on a threshold of OAHI of >2/h) (42). However, 57% of these children were clinically referred with concerns about apnea. In contrast Skoto et al. reported lower rates of 44.4% in 56 children aged 3–5 years randomly selected from a DS follow-up program at Boston Children’s Hospital (based on a threshold of OAHI of >2/h) (22). Due to the lack of state funded healthcare in the USA it is possible that children with access to regular care were from wealthier families. In the same way, however, social class can influence clinical research participants. Indeed in our study 42% of children had a parent who was a graduate suggesting a similar class bias in both studies (43). Our study population had a narrow age range (0.5–6 years), were largely community recruited and we used a threshold OAHI of > 5/h to reflect the sensitivity of cardiorespiratory polygraphy for the present analysis. Using a threshold of OAHI >2/h in our sample prevalence rates of OSA are 46.3%, almost identical to the Skoto figures, although there are difference as noted above in the population recruited. Also of note 17.8% of the final sample of 123 children had previously had adenotonsillectomy, potentially reducing their OAHI.
While in principle the low numbers with OSA as defined in this study may have reduced our ability to explore the validity of the questionnaire, as illustrated by Figure 3, there were no differences in responses between those with and without OSA.
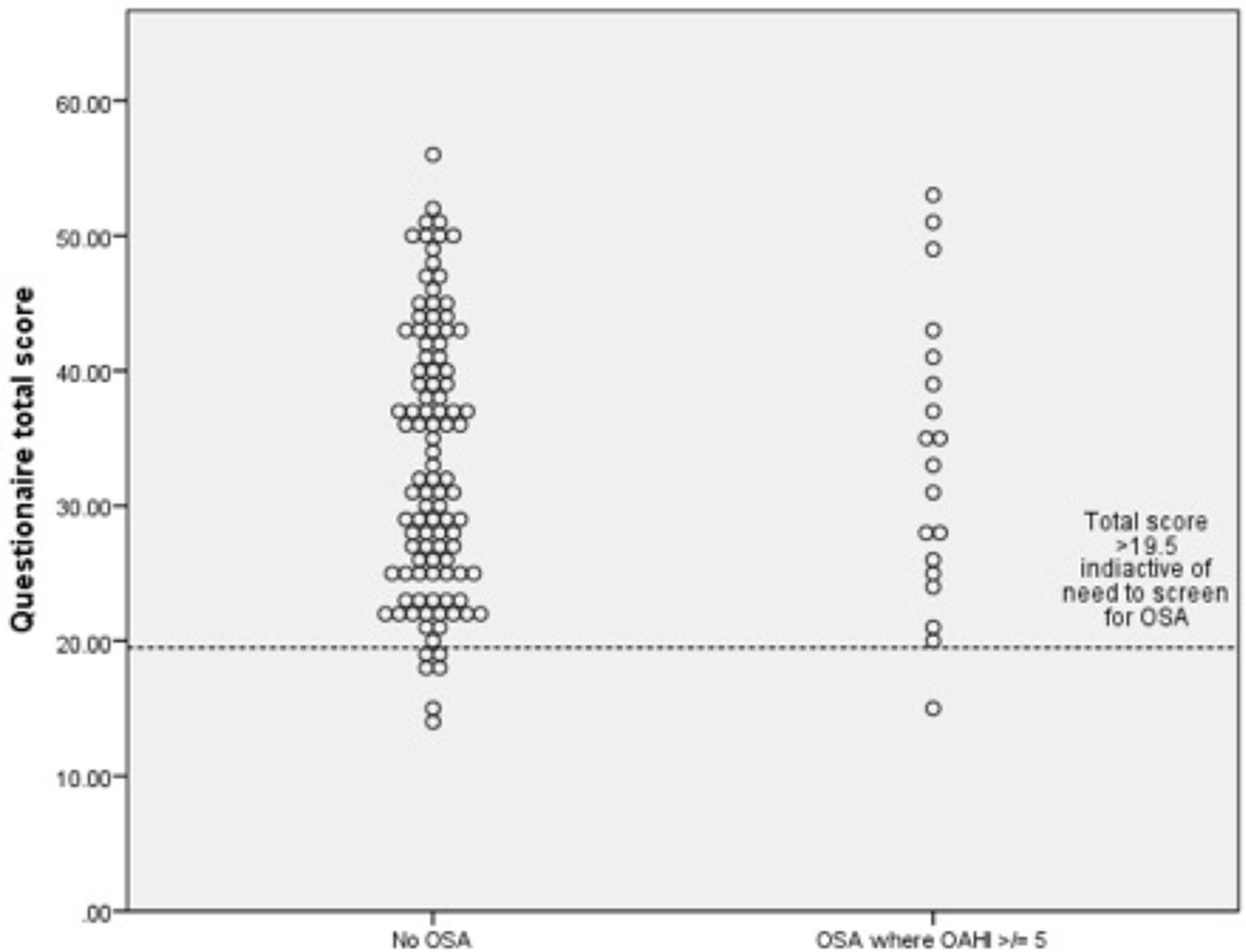
Figure 3 Dot plots for obstructive apnea hypopnea index (OAHI) for (n-123) for children with and without obstructive sleep apnea (OSA) with a questionnaire score cut off of 19.5.
The research field attests to a motivation of clinicians and researchers to offer a simpler screening alternative to cardiorespiratory or polysomnographic evaluation of OSA for children with DS. This motivation is understandable as sleep studies are expensive and may be poorly tolerated by children with learning disabilities. Alternative screening methods have been researched to offer a non-invasive alternative to polysomnography. Esbensen et al. investigated the potential of actigraphy to identify OSA in 27 children aged 5–17 years with DS. Actigraphy correlated with PSG for the total sleep time, wake after sleep onset, and sleep efficiency but not a clinical diagnosis of OSA (44). Elsharkawi et al. reported that a combination of four urinary biomarkers had a positive predictive value of 90% and negative predictive value of 68% to predict OSA at an AHI≥1/h (21). These techniques are expensive, not widely available and the authors noted that further studies were required in larger populations before this approach could be recommended as a screening tool. Imaging techniques have been studied. Three-dimensional photogrammetric measurements have been compared in DS children with and without OSA and with no differences established (20). Similarly, cephalometry was not found to usefully contribute to prediction of OAHI in a study of 130 children and young adults with DS (22). UK Royal College of Paediatrics and Child Health currently recommends screening children with DS annually for OSA from infancy to 5 years with a minimum of pulse oximetry (19). There has previously been little evidence to support this technique in this population but we have recently demonstrated a high sensitivity (92%) and specificity (63%) of one night of domiciliary Masimo pulse oximetry to predict OSA diagnosed by cardiorespiratory polygraphy (18).
Conclusion
A carefully constructed questionnaire with good content validity lacks criterion validity to make it a useful tool in clinical practice. This is in keeping with the literature that parental report and clinical evaluation in routine practice are poor predictors of OSA in DS. As such, objective screening methods should be adopted and our previous findings suggest that domiciliary pulse oximetry could offer an acceptable first-line screening approach, halving the number of children requiring more detailed sleep studies.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by UK National Research Ethics Committee (reference 13/SC/0106). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
CH and HJE conceived the idea for the study. CH, HEE, CT, and ES designed the questionnaire. CH, HJE, HEE, RK, JM, JR, AJ, and PG were involved in the recruitment of the subjects, conducting the questionnaire and PSG. SG-H, CH, and KS were involved in data analysis, data interpretation, and authoring the manuscript.
Funding
This study was supported by Action Medical Research and the Garfield Weston Foundation [grant reference 2040].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the UK Down Syndrome Medical Interest group as well as the Down Syndrome Association for their help with recruiting children to the study. Most importantly we thank the children and families for their enthusiasm to take part.
The authors would also like to acknowledge the Southampton NIHR Wellcome Trust Clinical Research Facility for their support of this work.
References
1. Cocchi G, Gualdi S, Bower C, Halliday J, Jonsson B, Myrelid A, et al. International trends of Down syndrome 1993-2004: Births in relation to maternal age and terminations of pregnancies. Birth Defects Res A Clin Mol Teratol (2010) 88(6):474–9. doi: 10.1002/bdra.20666
2. Lee CF, Lee CH, Hsueh WY, Lin MT, Kang KT. Prevalence of Obstructive Sleep Apnea in Children With Down Syndrome: A Meta-Analysis. J Clin Sleep Med (2018) 14(5):867–75. doi: 10.5664/jcsm.7126
3. Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep (2009) 32(6):731–6. doi: 10.1093/sleep/32.6.731
4. Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med (2001) 164(1):16–30. doi: 10.1164/ajrccm.164.1.2008171
5. Kaditis A. From obstructive sleep apnea in childhood to cardiovascular disease in adulthood: what is the evidence? Sleep (2010) 33(10):1279–80. doi: 10.1093/sleep/33.10.1279
6. Hunter SJ, Gozal D, Smith DL, Philby MF, Kaylegian J, Kheirandish-Gozal L. Effect of Sleep-disordered Breathing Severity on Cognitive Performance Measures in a Large Community Cohort of Young School-aged Children. Am J Respir Crit Care Med (2016) 194(6):739–47. doi: 10.1164/rccm.201510-2099OC
7. Mitchell RB, Kelly J. Behavior, neurocognition and quality-of-life in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol (2006) 70(3):395–406. doi: 10.1016/j.ijporl.2005.10.020
8. Tarasiuk A, Greenberg-Dotan S, Simon-Tuval T, Freidman B, Goldbart AD, Tal A, et al. Elevated morbidity and health care use in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med (2007) 175(1):55–61. doi: 10.1164/rccm.200604-577OC
9. Horne RS, Wijayaratne P, Nixon GM, Walter LM. Sleep and sleep disordered breathing in children with down syndrome: Effects on behaviour, neurocognition and the cardiovascular system. Sleep Med Rev (2019) 44:1–11. doi: 10.1016/j.smrv.2018.11.002
10. Breslin J, Spano G, Bootzin R, Anand P, Nadel L, Edgin J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev Med Child Neurol (2014) 56(7):657–64. doi: 10.1111/dmcn.12376
11. Joyce A, Elphick H, Farquhar M, Gringras P, Evans H, Bucks RS, et al. Obstructive Sleep Apnoea Contributes to Executive Function Impairment in Young Children with Down Syndrome. Behav Sleep Med (2019) 16:1–11. doi: 10.1080/15402002.2019.1641501
12. Fernandez F, Edgin JO. Poor Sleep as a Precursor to Cognitive Decline in Down Syndrome : A Hypothesis. J Alzheimers Dis Parkinsonism (2013) 3(2):124. doi: 10.4172/2161-0460.1000124
13. Brietzke SE, Katz ES, Roberson DW. Can history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypopnea syndrome? A systematic review of the literature. Otolaryngol Head Neck Surg (2004) 131(6):827–32. doi: 10.1016/j.otohns.2004.07.002
14. Lin SC, Davey MJ, Horne RS, Nixon GM. Screening for obstructive sleep apnea in children with Down syndrome. J Pediatr (2014) 165(1):117–22. doi: 10.1016/j.jpeds.2014.02.032
15. Bull MJ. Health supervision for children with Down syndrome. Pediatrics (2011) 128(2):393–406. doi: 10.1542/peds.2011-1605
16. Esbensen AJ, Beebe DW, Byars KC, Hoffman EK. Use of Sleep Evaluations and Treatments in Children with Down Syndrome. J Dev Behav Pediatr (2016) 37(8):629–36. doi: 10.1097/DBP.0000000000000333
17. Royal College of Paediatrics and Child Health. Working Party on Sleep Physiology and Respiratory Control Disorders in Childhood, Standards for Services for Children with Disorders of Sleep Physiology Report. London (2009).
18. Hill CM, Elphick HE, Farquhar M, Gringras P, Pickering RM, Kingshott RN, et al. Home oximetry to screen for obstructive sleep apnoea in Down syndrome. Arch Dis Child (2018) 103(10):962–7. doi: 10.1136/archdischild-2017-314409
19. Hill CM, Evans HJ, Elphick H, Farquhar M, Pickering RM, Kingshott R, et al. Prevalence and predictors of obstructive sleep apnoea in young children with Down syndrome. Sleep Med (2016) 27-28:99–106. doi: 10.1016/j.sleep.2016.10.001
20. Jayaratne YSN, Elsharkawi I, Macklin EA, Voelz L, Weintraub G, Rosen D, et al. The facial morphology in Down syndrome: A 3D comparison of patients with and without obstructive sleep apnea. Am J Med Genet A (2017) 173(11):3013–21. doi: 10.1002/ajmg.a.38399
21. Elsharkawi I, Gozal D, Macklin EA, Voelz L, Weintraub G, Skotko BG. Urinary biomarkers and obstructive sleep apnea in patients with Down syndrome. Sleep Med (2017) 34:84–9. doi: 10.1016/j.sleep.2017.02.005
22. Skotko BG, Macklin EA, Muselli M, Voelz L, McDonough ME, Davidson E, et al. A predictive model for obstructive sleep apnea and Down syndrome. Am J Med Genet A (2017) 173(4):889–96. doi: 10.1002/ajmg.a.38137
23. Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep (2000) 23(8):1043–51. doi: 10.1093/sleep/23.8.1d
24. Schreck KA, Mulick JA, Rojahn J. Development of the behavioural evaluation of disorders of sleep scale. J Child Family Stud (2003) 12(3):349–59. doi: 10.1023/A:1023995912428
25. Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res (1996) 5(4):251–61. doi: 10.1111/j.1365-2869.1996.00251.x
26. Esbensen AJ, Hoffman EK. Reliability of parent report measures of sleep in children with Down syndrome. J Intellect Disabil Res (2017) 61(3):210–20. doi: 10.1111/jir.12315
27. Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep Med Rev (2011) 15(1):19–32. doi: 10.1016/j.smrv.2010.07.005
28. Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med (2000) 1(1):21–32. doi: 10.1016/S1389-9457(99)00009-X
29. Sproson EL, Hogan AM, Hill CM. Accuracy of clinical assessment of paediatric obstructive sleep apnoea in two English centres. J Laryngol Otol (2009) 123(9):1002–9. doi: 10.1017/S0022215109005532
30. Cielo CM, Silvestre J, Paliga JT, Maguire M, Gallagher PR, Marcus CL, et al. Utility of screening for obstructive sleep apnea syndrome in children with craniofacial disorders. Plast Reconstr Surg (2014) 134(3):434e–41e. doi: 10.1097/PRS.0000000000000484
31. Pabary R, Goubau C, Russo K, Laverty A, Abel F, Samuels M. Screening for sleep-disordered breathing with Pediatric Sleep Questionnaire in children with underlying conditions. J Sleep Res (2019) 28(5):e12826. doi: 10.1111/jsr.12826
32. Sanders E, Hill CM, Evans HJ, Tuffrey C. The Development of a Screening Questionnaire for Obstructive Sleep Apnea in Children with Down Syndrome. Front Psychiatry (2015) 6:147. doi: 10.3389/fpsyt.2015.00147
33. Spruyt K, Gozal D. Development of pediatric sleep questionnaires as diagnostic or epidemiological tools: a brief review of dos and don’ts. Sleep Med Rev (2011) 15(1):7–17. doi: 10.1016/j.smrv.2010.06.003
34. Kingshott RN, Gahleitner F, Elphick HE, Gringras P, Farquhar M, Pickering RM, et al. Cardiorespiratory sleep studies at home: experience in research and clinical cohorts. Arch Dis Child (2019) 104(5):476–81. doi: 10.1136/archdischild-2018-315676
35. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med (2012) 8(5):597–619. doi: 10.5664/jcsm.2172
37. Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med (2013) 368(25):2366–76. doi: 10.1056/NEJMoa1215881
38. Rosen D, Lombardo A, Skotko B, Davidson EJ. Parental perceptions of sleep disturbances and sleep-disordered breathing in children with Down syndrome. Clin Pediatr (Phila) (2011) 50(2):121–5. doi: 10.1177/0009922810384260
39. Friedman NR, Ruiz AG, Gao D, Ingram DG. Accuracy of Parental Perception of Nighttime Breathing in Children with Down Syndrome. Otolaryngol Head Neck Surg (2018) 158(2):364–7. doi: 10.1177/0194599817726286
40. Nehme J, LaBerge R, Pothos M, Barrowman N, Hoey L, Monsour A, et al. Predicting the presence of sleep-disordered breathing in children with Down syndrome. Sleep Med (2017) 36:104–8. doi: 10.1016/j.sleep.2017.03.032
41. Alonso-Alvarez ML, Teran-Santos J, Ordax Carbajo E, Cordero-Guevara JA, Navazo-Eguia AI, Kheirandish-Gozal L, et al. Reliability of home respiratory polygraphy for the diagnosis of sleep apnea in children. Chest (2015) 147(4):1020–8. doi: 10.1378/chest.14-1959
42. Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of Obstructive Sleep Apnea in Children with Down Syndrome. Sleep (2016) 39(3):699–704. doi: 10.5665/sleep.5554
43. Unger JM, Hershman DL, Albain KS, Moinpour CM, Petersen JA, Burg K, et al. Patient income level and cancer clinical trial participation. J Clin Oncol (2013) 31(5):536–42. doi: 10.1200/JCO.2012.45.4553
Keywords: Down syndrome, trisomy 21, screening, obstructive sleep apnea/apnea, psychometric properties
Citation: Grantham-Hill S, Evans HJ, Tuffrey C, Sanders E, Elphick HE, Gringras P, Kingshott RN, Martin J, Reynolds J, Joyce A, Hill CM and Spruyt K (2020) Psychometric Properties and Predictive Value of a Screening Questionnaire for Obstructive Sleep Apnea in Young Children With Down Syndrome. Front. Psychiatry 11:285. doi: 10.3389/fpsyt.2020.00285
Received: 29 July 2019; Accepted: 24 March 2020;
Published: 28 April 2020.
Edited by:
David Gozal, University of Missouri, United StatesReviewed by:
Irma Rukhadze, UCLA David Geffen School of Medicine, United StatesBrian Gary Skotko, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2020 Grantham-Hill, Evans, Tuffrey, Sanders, Elphick, Gringras, Kingshott, Martin, Reynolds, Joyce, Hill and Spruyt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah Grantham-Hill, c2FyYWguZ3JhbnRoYW0taGlsbEBuaHMubmV0; Catherine M. Hill, Qy5NLkhpbGxAc290b24uYWMudWs=
†These authors share last authorship
 Sarah Grantham-Hill
Sarah Grantham-Hill Hazel J. Evans
Hazel J. Evans Catherine Tuffrey3
Catherine Tuffrey3 Heather E. Elphick
Heather E. Elphick Paul Gringras
Paul Gringras Janine Reynolds
Janine Reynolds Catherine M. Hill
Catherine M. Hill Karen Spruyt
Karen Spruyt