- 1Research Center of Clinical Medicine, Affiliated Hospital of Nantong University, Nantong, China
- 2Center of Laboratory Medicine, Nantong Mental Health Center, Nantong, China
- 3Center of Laboratory Medicine, Affiliated Hospital of Nantong University, Nantong, China
Psychiatric disorders impose a huge burden on individuals, families, and society. The Alu repeat sequence is a member of the short interspersed nuclear element (SINE) family of mammalian genomes, however, its expression pattern and role in psychiatric disorders is unclear. The current paper aimed at determining the concentrations of Alu in patients with schizophrenia (SZ), major depressive disorder (MDD), and alcohol-induced psychotic disorder (AIPD), and to further define the role and value of Alu as a potential biomarker in psychiatric disorders. In this work, we found that the concentration of Alu was considerably incremented in patients with SZ, and a significant difference existed between patients diagnosed with SZ and MDD or AIPD. ROC analysis also indicated that Alu was effective in the complementary diagnosis of SZ, and differentially diagnosed between SZ patients and patients with MDD or AIPD. In addition, we found a positive relationship between the Alu concentrations and interleukin-1β (IL-1β) in patients with SZ, MDD, and AIPD, and between the concentrations of Alu and interleukin-18 (IL-18) in patients with SZ. Overall, the present work indicates that Alu might be an innovative biomarker for diagnosing psychiatric disorders, and provides the basis for hypotheses about the pathophysiology of psychiatric disorders.
Introduction
The development and progression of psychiatric disorders, whose clinical signs include disorders in emotion, cognition, and behavior, are related to genetic and environmental factors. The total prevalence of psychiatric disorders is 17% in China (1) and the prevalence of psychiatric disorders is increasing in each year (2). Huge burdens are imposed by psychiatric disorders on families, individuals, and society. The psychiatric disorders burden is about 13% in China, ranking third in the world after cardiovascular disease and cancer. Schizophrenia (SZ), major depressive disorder (MDD), and alcohol-induced psychotic disorder (AIPD) are the main types of psychiatric disorders in Nantong, Jiangsu, China. The underlying causes of psychiatric disorders are unidentified in 90% of the cases. Diagnosis mainly depends on clinical symptoms, prognosis, and course of the disease, however, they are not specific for psychiatric disorders. Some characteristics are applied to SZ, MDD, and AIPD. Difficulties exist in the diagnosis and treatment of psychiatric disorders, which are based merely on clinical indications. Serological biomarkers are precise, objective, and easy to apply, with wide applications in various diseases like inflammation, tumors, and cardiovascular diseases. Therefore, it is worthwhile to seek innovative molecular markers for psychiatric disorders (3–6).
The pathophysiology of psychiatric disorders is still mysterious. Presently, neuronal apoptosis is regarded as one of the mechanisms of psychiatric disorders, in general. The apoptosis levels incremented in patients suffering from psychiatric disorders and the succeeding neuronal, dendritic, and synaptic losses are caused by an incremented or imbalanced apoptotic mechanism (7–9). Glutamate excitotoxicity, oxidative stress, and decreased neurotrophic support that are linked to psychiatric disorders, also directly or indirectly related to the apoptosis (10–12). There are several studies reporting some genes associated with apoptosis, which were changed in patients with SZ, like Bcl-2 (13) and down-expressed GSK3 levels (14–16) and increased Bax/Bcl-2 ratios, caspase-3 activity (17), and the p53 expression level (18–21). The pathogenesis of neuropsychiatric diseases such as depression and anxiety is also associated with apoptosis (22–25).
The Alu repeat sequence is a member of the short interspersed nuclear element (SINE) family of mammalian genomes, and it is expressed exclusively by primates. It can be released from apoptotic cells. Several copies of unedited Alu repeats were found by Hardy et al., contained in exosome-like nanovesicles (ApoExos), which were released by apoptotic endothelial cells (26). Alu has been utilized as a biomarker in many kinds of diseases including cancer (27, 28). The level of Alu is increased significantly in patients with cancer, in comparison with healthy individuals, and after treatment, it is decreased considerably (29). High levels of Alu are related to a poor prognosis of patients. The Alu concentration is a promising molecular marker to assess cancer progression (30). Nevertheless, the concentrations and role of Alu in SZ, MDD, and AIPD are not clear.
The present work aimed at determining the concentrations of Alu in patients with SZ, MDD, and AIPD, and further exploring the value and role of Alu as a potential biomarker in psychiatric disorders. Based on our result, the concentration of Alu was considerably incremented in patients with SZ, and we found a considerable difference between SZ patients and MDD or AIPD patients. Moreover, ROC analysis also indicated that Alu was helpful in the complementary diagnosis of SZ, and differential identification between patients with SZ and patients with MDD or AIPD. Additionally, we found a positive relationship between interleukin-1β (IL-1β) and the concentrations of Alu in patients with SZ, MDD, and AIPD, and between the concentrations of Alu and interleukin-18 (IL-18) in patients with SZ. In sum, this work offers an innovative biomarker in diagnosing the psychiatric disorders and a promising hypothesis of the pathophysiology of psychiatric disorders.
Materials and Methods
Human Serum Samples
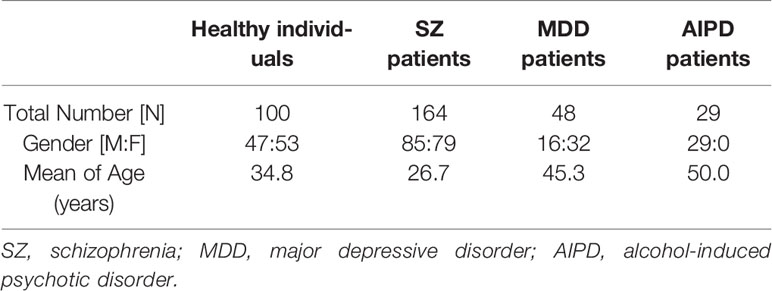
The samples were taken from 164 SZ patients, 48 MDD patients, and 29 AIPD patients from the Nantong Fourth Peoples Hospital (Nantong, China) between Dec 2017 to Aug 2018. All patients with psychiatric disorders confirmed in terms of the criteria of Diagnostic and Statistical Manual of Mental Disorders, Four Edition. One hundred healthy individuals were gathered from the Physical Examination Center of the Affiliated Hospital of Nantong University (Nantong, China) who had passed the physical test. Table 1 shows the features of the human samples. Before collecting the sample, informed consent was obtained. All samples were anonymous and the study was permitted by the Human Research Ethics Committee of the Affiliated Hospital of Nantong University. The disposable vacuum blood collection tubes and blood collection needles were used for blood specimen collection. The procedure is strictly in accordance with the Guidelines for the Collection of Adult Venous Blood Specimens (GB/T 1.1-2009). The specimen tube was erected and left to avoid oscillation, falling, and direct sunlight after blood collecting. During operation, protective clothing, safety goggles, or face shields, as well as clean disposable gloves and masks, were worn. Moreover, clean disposable tips and centrifuge tubes were used. The gloves were removed and the hands were washed or disinfected immediately after the operation. The clothes, gloves, and other items contaminated by blood were put into the medical waste garbage bag. The sharps were placed into a special anti-seepage and stab-resistant disposable sharp box. Both the garbage bags and the sharp boxes were sealed and treated by centralized medical waste treatment of our hospital. The contaminated tabletops were cleaned with disinfectant such as hypochlorous acid.
Serum Collection and Cell-Free DNA Isolation
Blood samples (4–5 ml) directly were gathered into serum separator tubes (Vacuette, Kresmunster, Austria). The complete blood was centrifuged at 3000×g for 10 min, and the serum was stored at −80°C till being used. Using the TIAN LONG DNA Kit (Suzhou, China) based on the protocol of the manufacturer, cell-free DNA was extracted from 0.2 ml of serum.
Quantitation of Alu
The concentration of Alu was quantitated by real-time PCR (RT-PCR) established by Hao TB et al. in our study team (31). RT-PCR was performed in triplicate with FastStart Universal SYBR Green Master Mix kit (Roche, Germany) by 7500 RT-PCR System (ABI, Abilene, TX, USA). A standard curve (from 0.222 to 22 200 ng/ml) of human genomic DNA (Promega, Madison, WI, USA) was used to quantify the absolute level of Alu. The sequence of Alu-115 forward primer was 5'-CCTGAGGTCAGGAGTTCGAG-3' and the reverse primer was 5'-CCCGAGTAGCTGGGATTACA-3'. A 20 µl PCR mixture contained 5 µl DNA template, 0.5 µl of the forward and reverse primer (10 μM), 10 µl SYBR Green Master Mix, and 4 µl ddH2O at 95°C for 10 min, followed by 35 cycles of denaturation at 95°C for 15 s, and annealing at 64°C for 1 min.
Quantizing the IL-18 and IL-1β
The IL-18 and IL-1β expression levels were found by the Human IL-18 ELISA kit (Fcmacs Technology, China) and Human IL-1β ELISA kit (Fcmacs Technology, China) based on the manufacturer protocols, respectively.
Statistical Analysis
Using GraphPad Prism v5.0 software, statistical analysis was conducted. The concentrations of Alu, IL-18, and IL-1β are provided as the median with the 25th and the 75th percentile values. The nonparametric quantitative information was compared in the form of inter-group through the Mann Whitney test. The nonparametric quantitative data of more than two groups were compared using the Kruskal-Wallis test. Via the Spearman test, the correlation was examined. To investigate the diagnostic accurateness of each parameter, receiver operating characteristic curves (ROC) were made, and the specificity and sensitivity of the optimum cut-off point were determined as values maximizing the ROC curve (AUC) area. The confidence interval of ROC was determined as 95%. All statistical examinations were two-sided, and the statistical significance implied a P-value less than 0.05.
Results
The Concentrations of Alu in Patients with SZ, MDD, AIPD, and Healthy Individuals
In this work, 164 patients with SZ, 48 patients with MDD, and 29 patients with AIPD were included from the Nantong Fourth Peoples Hospital (Nantong, China). One hundred healthy individuals were gathered from the Physical Examination Center of the Affiliated Hospital of Nantong University (Nantong, China). Table 1 represents the demographic details of the participants. The average concentrations of Alu in patients suffering from SZ, MDD, AIPD, and healthy people were 593.5 ng/ml (interquartile range 226.8–1,337.1 ng/ml), 225.2 ng/ml (interquartile range 139.8–655.3 ng/ml), 315.3 ng/ml (interquartile range 148.5–462.9 ng/ml), and 318.3 ng/ml (interquartile range 253.5–427.4 ng/ml), respectively. The significant difference was found between patients with SZ and healthy individuals (P < 0.0001), and also between SZ patients and patients with MDD (P < 0.001) or patients with AIPD (P < 0.01). However, no statistical difference was found among patients with MDD, AIPD, and healthy people (P > 0.05) (Figure 1).
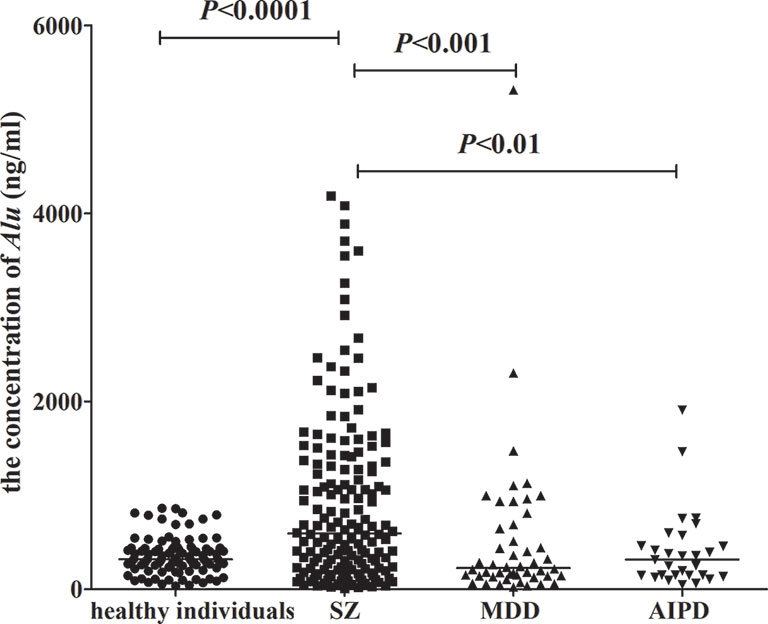
Figure 1 The concentrations of Alu in healthy individuals, patients with SZ, MDD, and AIPD. Mann Whitney test was used in this figure. The concentrations of Alu were measured in 164 SZ patients, 48 MDD patients, 29 AIPD patients, and 100 healthy individuals. The results for the concentrations of Alu are presented as the median with the 25th and the 75th percentile values. Horizontal lines indicate the median for each group. SZ, schizophrenia; MDD, major depressive disorder; AIPD, alcohol-induced psychotic disorder.
The Utility of Alu in Diagnosis and Differential Diagnosis
In patients with SZ, the concentrations of Alu were considerably increased. Whether or not Alu can contribute to the complementary diagnosing SZ and differential diagnoses between patients with SZ and MDD or AIPD was still undetermined. The diagnostic efficiency of Alu was assessed using Receiver Operating Characteristics (ROC) analysis. The (AUC) analysis curve area obtained a value of 0.6732 (95% CI: 0.6091–0.7372) between SZ patients and healthy people (Figure 2A). The sensitivity was 51.83% and the specificity was 90% when the cut-off value was 567.1 ng/ml. The AUC was 0.6690 in sorting out the SZ patients from MDD patients with a 60.42% sensitivity and a 70.73% specificity at the cut-off value (288 ng/ml) (Figure 2B), and 0.6684 in sorting out SZ patients from AIPD patients with a 75.86% sensitivity and a 57.93% specificity at the considered cut-off value (469.7 ng/ml) (Figure 2C).
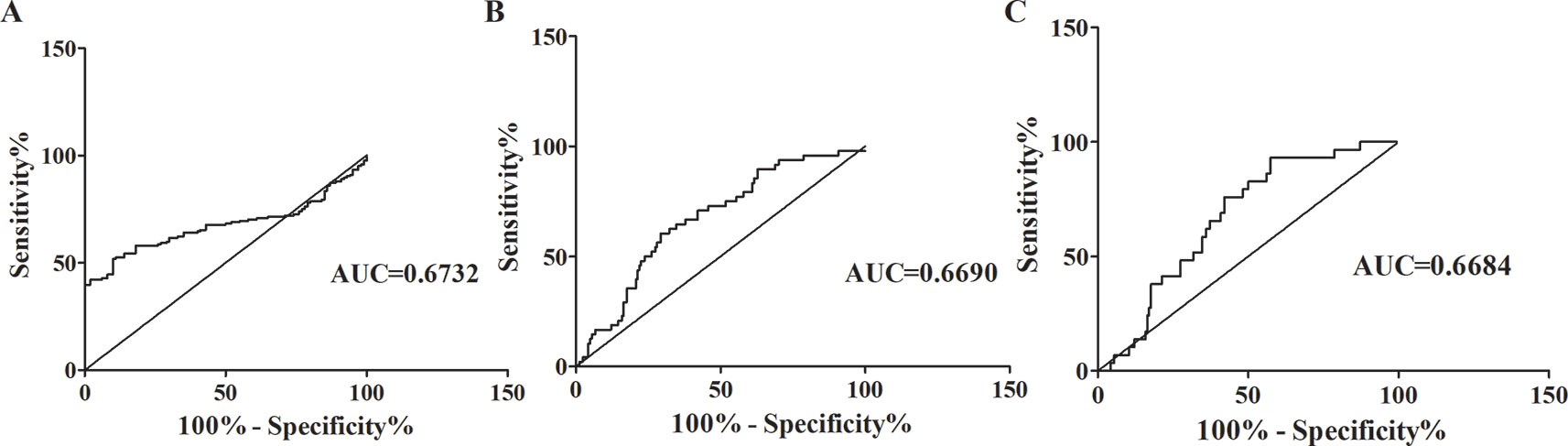
Figure 2 ROC curves analysis of Alu. (A) ROC curves analysis of Alu between SZ patients and healthy individuals. AUC was 0.6732 in separating patients with SZ from healthy individuals (95% confidence interval, 0.6091–0.7372). (B) ROC curves analysis of Alu between SZ patients and MDD patients. AUC was 0.6690 in separating patients with SZ from patients with MDD (95% confidence interval, 0.5860–0.7519). (C) ROC curves analysis of Alu between SZ patients and AIPD patients. AUC was 0.6684 in separating patients with SZ from patients with AIPD (95% confidence interval, 0.5783–0.7586). ROC, receiver operating characteristic; AUC, the area under the ROC curve; SZ, schizophrenia; MDD, major depressive disorder; AIPD, alcohol-induced psychotic disorder.
Analysis Between Alu and Clinical Characteristics of Patients with SZ, MDD, and AIPD
Tables 2–4 represent the concentrations of Alu in various subdivisions of patients suffering from SZ, MDD, and AIPD based on gender, current age, psychosis onset age, current treatment, duration of psychosis and pharmacological treatment, and family history. No statistically considerable difference was found in Alu in these various classes based on gender (P > 0.05), current age (P > 0.05), psychosis onset age (P > 0.05), duration of pharmacological treatment (P > 0.05), duration of psychosis (P > 0.05), current treatment (P > 0.05), and family history (P > 0.05).
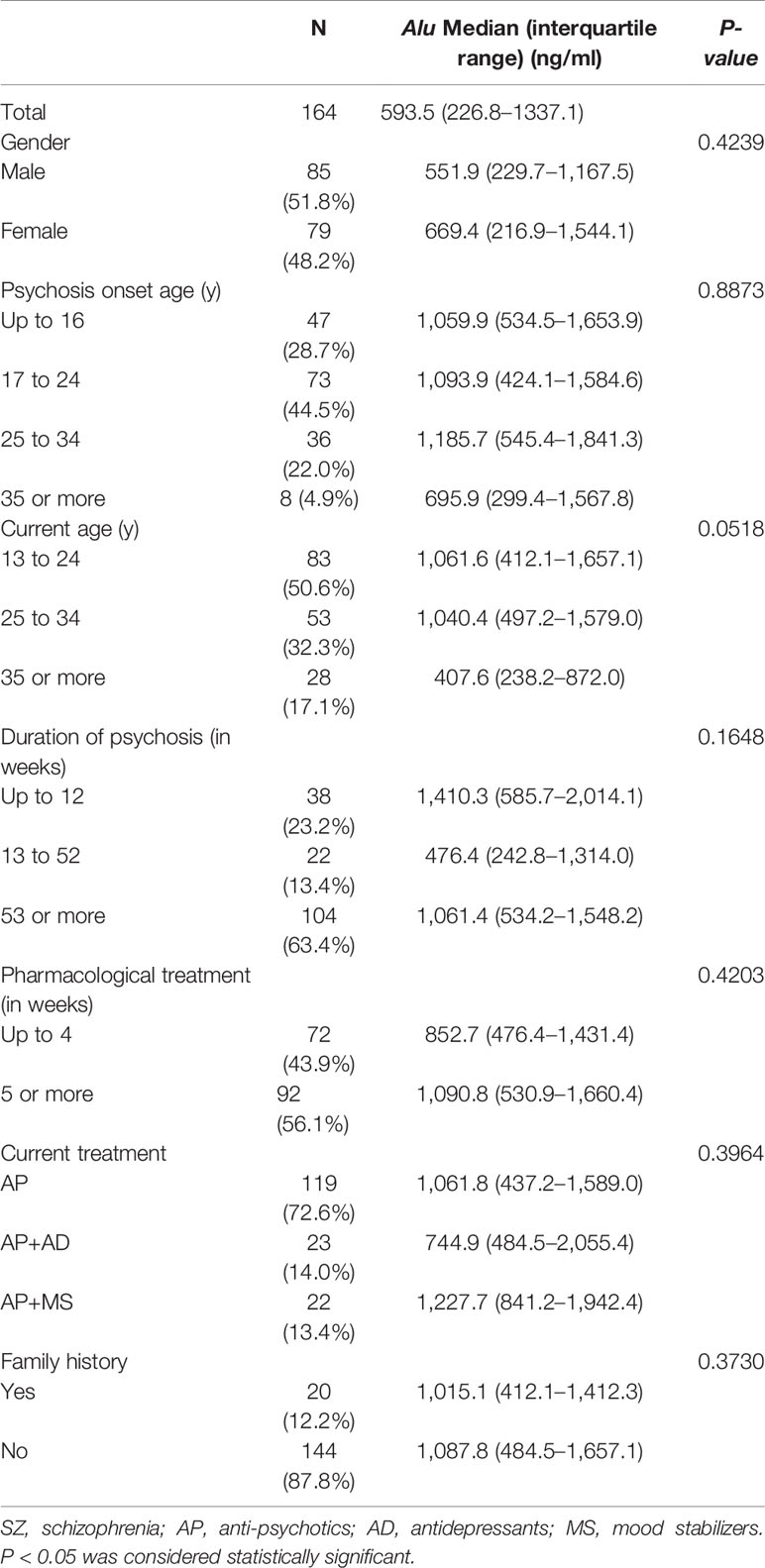
Table 2 Clinical features of patients of SZ (n = 164) according to the specific diagnostic categories.
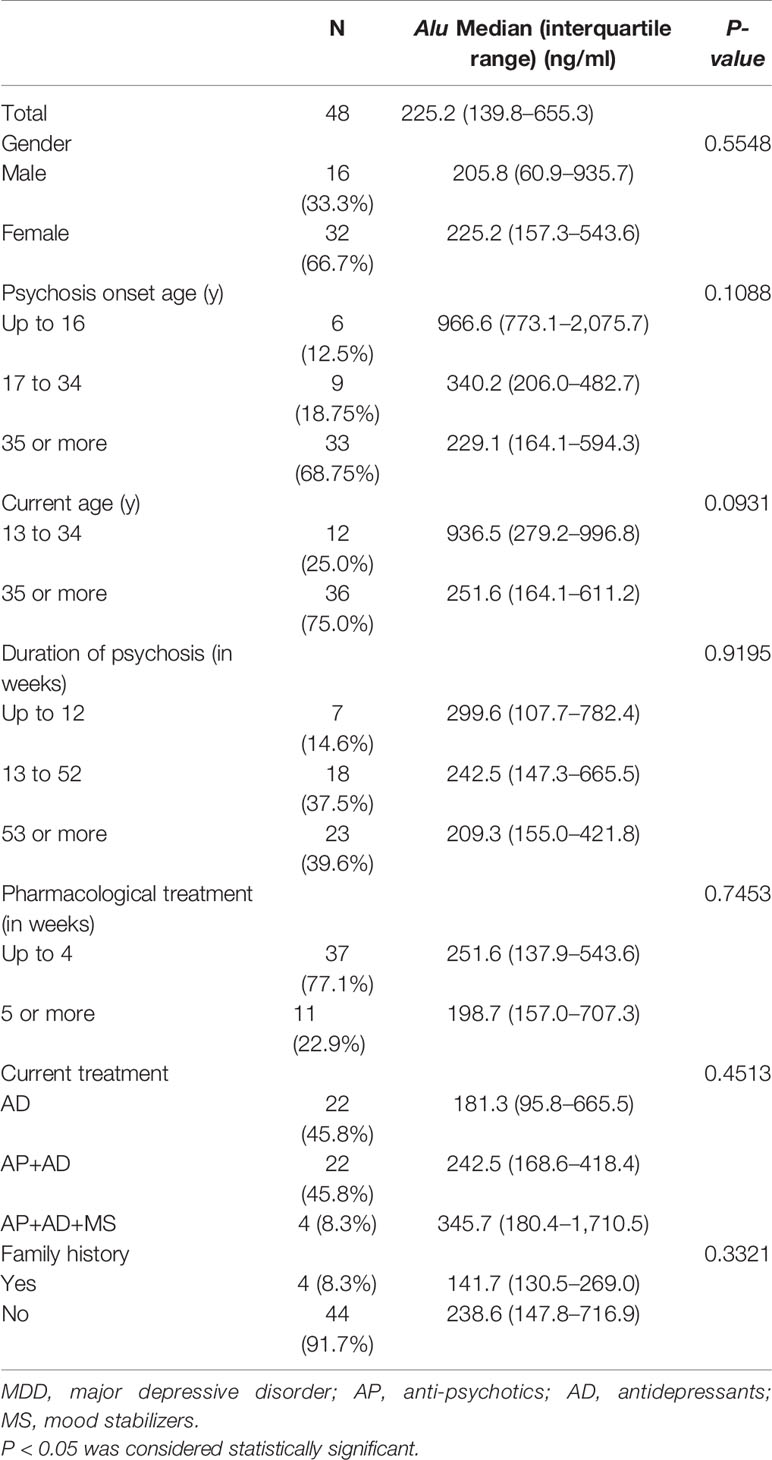
Table 3 Clinical features of patients of MDD (n = 48) according to the specific diagnostic categories.

Table 4 Clinical features of patients of AIPD (n = 29) according to the specific diagnostic categories.
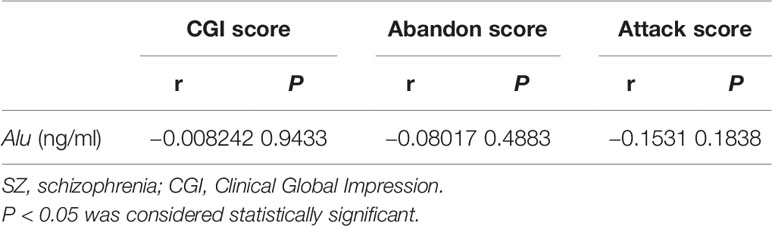
In the present work, the correlations between Alu and Clinical Global Impression (CGI) score, abandon score, and attack score of patients with SZ were also examined and no significant difference was found (Table 5).
Analysis Between Alu and IL-1β, IL-18 in Patients with SZ, MDD, and AIPD
It was reported that inflammatory cytokines IL-1β (32, 33) and IL-18 (34, 35) played a role in the development of psychiatric disorders. Some studies indicated that Alu RNA was capable of up-regulating IL-18 and IL-1β expression and secretion, and the apoptosis was caused subsequently (36, 37). However, enhancement in the expression of IL-1β and IL-18 in psychiatric disorders by Alu is still unclear. To clarify this matter, a correlation analysis was carried out in this work, between Alu and IL-1β or IL-18 in patients with SZ, MDD, and AIPD. We found that a positive correlation existed between the Alu and IL-1β concentrations in patients with SZ (P < 0.05), MDD (P < 0.05), and AIPD (P < 0.05), and between the concentration of Alu and IL-18 in patients with SZ (P < 0.05). However, there was no correlation between the concentrations of Alu and IL-18 in patients with MDD and AIPD (P > 0.05) (Table 6).
Based on these findings, our study indicates that the concentration of Alu might be useful biomarker for the diagnosis of psychiatric disorders. Further, the present results may suggest novel hypotheses for the pathogenesis of psychiatric disorders.
Discussion
Recent studies indicated numerous molecular changes in psychiatric disorders, and psychiatric disorders development and progression have a role in the abnormal expression pattern (38–40). In this study, we found that the concentration of Alu was considerably incremented in patients suffering SZ, and a significant difference was found between patients suffering from SZ and MDD or AIPD. Moreover, ROC analysis also showed that Alu was helpful in assist in the complementary diagnosis of SZ, and differentially diagnose between the patients with MDD or AIPD and patients with SZ. Furthermore, we also found a positive correlation between the Alu and IL-1β concentrations in patients with SZ, MDD, and AIPD, and between the concentrations of Alu and IL-18 in patients with SZ.
There are numerous kinds of psychiatric disorders, with diverse and overlapping clinical manifestations. It is difficult to distinguish the negative symptoms of SZ from MDD. Although the SZ and AIPD have different etiologies, they include some similar symptomatic and functional impairments. This causes challenges in diagnosing psychiatric disorders. The psychiatric disorders include some insignificant symptoms in the initial stages that are easily overlooked, however, it might represent a potential danger to individuals, families, and society. Hence, it is helpful to investigate the expression pattern and role of innovative molecular markers in psychiatric disorders.
Alu has been extensively utilized as a molecular biomarker in diagnosing various diseases (41, 42). Only a few studies exist on Alu in psychiatric disorders (43), moreover, the concentration of Alu was not investigated in patients and healthy controls. In addition, the correlations between the concentration of Alu and clinical characteristics were not studied. In the present study, we found that the Alu concentrations were incremented considerably in patients suffering from SZ, in comparison to healthy people. It was also found that the concentrations of Alu of patients with MDD and AIPD are significantly lower compared to the patients with SZ. In addition, ROC analysis showed the AUC 0.6732 between SZ patients and healthy individuals with a sensitivity of 51.83% and a specificity of 90%. Moreover, the AUC was 0.6690 between SZ patients and MDD patients with a 60.42% sensitivity and a 70.73% specificity, and the AUC was 0.6684 between SZ patients and AIPD patients with a 75.86% sensitivity and a 57.93% specificity. These findings show that Alu may be useful in the diagnosis of SZ, and may be a marker that distinguishes between SZ, MDD, and AIPD. However, the values of AUC are not yet ideal, and so the sensitivity and specificity of this potential biomarker would need further improvement. The progression and development of psychiatric disorders are caused by the interaction between genetic and environmental factors. Identifying diseases based on a single molecule is difficult. Future studies will increase the size and further characterize the patient group, and combine Alu with other indexes to enhance the sensitivity and accuracy of psychiatric disorders diagnosis.
Based on the analysis between Alu and clinical characteristics in patients with SZ, MDD, and AIPD, it was showed that no statistically significant difference exists in Alu in these various groups in terms of gender, current age, psychosis onset age, duration of psychosis, current treatment, family history, and duration of pharmacological treatment. There was no significant difference between Alu and CGI scores, abandon score, and attack score of patients with SZ. It indicated that Alu might be an independent indicator in relation to these indexes. Alu is released from cells, and the changes in the concentrations of Alu only represent changes at the cellular level. In addition to the variations in nerve cells, the clinical manifestations of patients with psychiatric disorders are also influenced by many other factors, including physique, character, and living environment of patients. This might be one of the reasons for the lack of a correlation between Alu and these indexes.
The apoptosis of neuronal takes place within developing psychiatric disorders. Large quantities of cellular contents are released by apoptotic cells into the interstitial space of cells while triggering the inflammatory reactions (44, 45). Neuroinflammation can further enhance the progression of psychiatric disorders. Some inflammatory cytokines, including interleukin 10 (IL-10) (46), IL-1β, IL-6 (47), and interferon (IFN)-λ, are considerably incremented in patients with SZ. These indicators are related to the worse functional outcomes of patients with SZ (48). High levels of inflammation markers are linked to the vulnerability to anxiety disorders (49). A therapeutic impact was imposed by anti-inflammatory drugs on patients with psychiatric disorders (50). In recent years, it has been reported that Alu played a role also in the process of inflammation (51, 52). The injection of ApoExos containing unedited Alu repeats resulted in inflammation in mice (26). Alu had an influence on the inflammatory response mechanisms of atherosclerosis and coronary artery disease (53). The formation of NLRP3 inflammasome and upregulation of IL-18 and IL-1β subsequently were activated by the accumulation of Alu transcripts (36, 37). This process might have a role in both neuroinflammation and neurodegeneration related to Alzheimer’s disease (AD) and other degenerative brain disorders (54). Hence, in the present work, the correlation of Alu and inflammatory factor IL-18 and IL-1β was investigated. We found a positive correlation between Alu and IL-1β in patients with SZ, MDD, and AIPD. We also found a positive correlation between Alu and IL-18 in patients with SZ. Kim et al. found that the accumulation of Alu RNA led to the elaboration of IL-18 in human eyes with geographic atrophy (36), consequently, the activation of NLRP3 inflammasome and the apoptosis in an IL-18-dependent mode were triggered (55, 56). It was also found that Alu up-regulated the expression level of IL-1β within the endothelial dysfunction process (37). According to these contents, we propose a hypothesis that apoptosis occurred in progressing the psychiatric disorders while releasing large quantities of Alu sequences from apoptotic cells. Later, the axis of Alu-IL-18/IL-1β was activated and inflammation was triggered aggravating the progression and development of psychiatric disorders, in turn (Figure 3). As the future work, we will focus on the mechanism of psychiatric disorders based on this hypothesis.
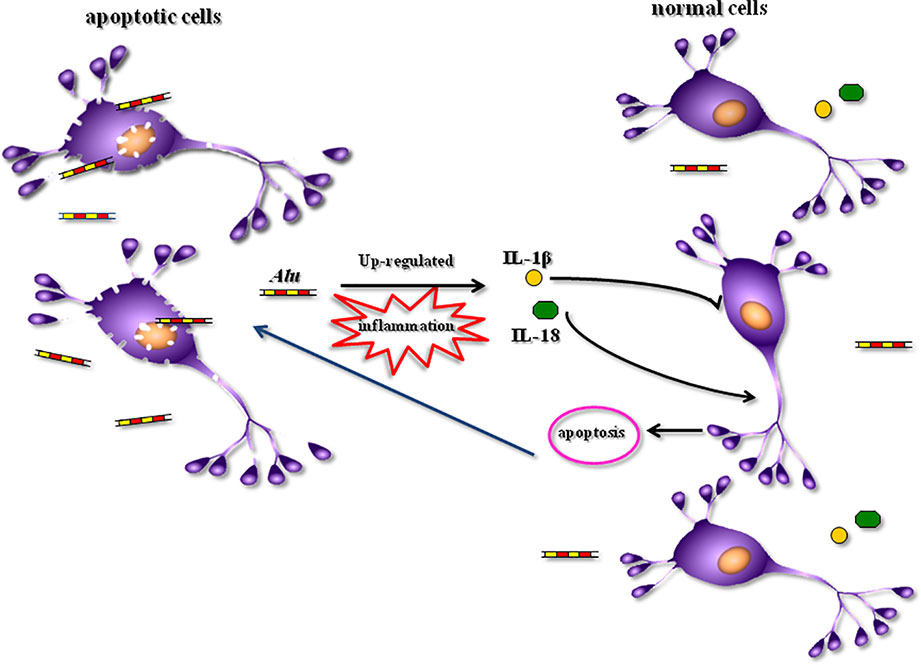
Figure 3 A hypothesis of the role of Alu sequences in the progression of psychiatric disorders. IL-1β, interleukin-1β; IL-18, interleukin-18.
In conclusion, the present study identified a considerable difference in Alu, between SZ patients and MDD, AIPD patients, and healthy individuals. Additionally, we also found a positive relation between IL-1β and the concentrations of Alu in patients with SZ, MDD, and AIPD, and between the concentrations of Alu and IL-18 in patients with SZ. It may indicate the Alu concentration might be helpful in the complementary diagnosis of psychiatric disorders, and Alu-IL-18/IL-1β might be involved in progressing the psychiatric disorders.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Human Research Ethics Committee of the Affiliated Hospital of Nantong University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
JQ analyzed the data and wrote the paper, L-YC performed experiments. X-JS collected the samples. S-QJ designed the study.
Funding
This work was supported by grants from The Six Talent Peaks Project of Jiangsu Province of China (2012-WS-119) and The Health and Planning Project of Nantong (QB201910).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported with the resources and the use of facilities at the Research Center of Clinical Medicine, Affiliated Hospital of Nantong University, Center of Laboratory Medicine, Nantong Mental Health Center and Center of Laboratory Medicine, Affiliated Hospital of Nantong University. The authors sincerely thank all the patients who participated in this study for making the research possible.
References
1. Tianmei S, Xunshen S. Minutes of the 16th national academic annual meeting of the chinese medical association psychiatric branch. Chin J Psychiatry (2019) 52:163–5. doi: 10.3760/cma.j.issn.1006-7884.2019.02.012
2. Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry (2019) 6:211–24. doi: 10.1016/S2215-0366(18)30511-X
3. Zhang Z, Cui J, Gao F, Li Y, Zhang G, Liu M, et al. Elevated cleavage of neuregulin-1 by beta-secretase 1 in plasma of schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry (2019) 90:161–8. doi: 10.1016/j.pnpbp.2018.11.018
4. Amoli MM, Khatami F, Arzaghi SM, Enayati S, Nejatisafa AA. Over-expression of TGF-β1 gene in medication free Schizophrenia. Psychoneuroendocrinology (2019) 99:265–70. doi: 10.1016/j.psyneuen.2018.10.009
5. Torres-Berrío A, Nouel D, Cuesta S, Parise EM, Restrepo-Lozano JM, Larochelle P, et al. MiR-218: a molecular switch and potential biomarker of susceptibility to stress. Mol Psychiatry (2019). doi: 10.1038/s41380-019-0421-5
6. Ritter P, Brandt M, Schrempf W, Brezan F, Krupka A, Storch A, et al. Role of the IL-6-Receptor expression in CD14+ monocytes in modulating sleep in patients with bipolar disorder. J Affect Disord (2018) 239:152–60. doi: 10.1016/j.jad.2018.06.037
7. Ganguly S, Seth S. A translational perspective on histone acetylation modulators in psychiatric disorders. Psychopharmacol (Berl) (2018) 235:1867–73. doi: 10.1007/S00213-018-4947-Z
8. Berk M, Post R, Ratheesh A, Gliddon E, Singh A, Vieta E, et al. Staging in bipolar disorder: from theoretical framework to clinical utility. World Psychiatry (2017) 16:236–44. doi: 10.1002/wps.20441
9. Scaini G, Fries GR, Valvassori SS, Zeni CP, Zunta-Soares G, Berk M, et al. Perturbations in the apoptotic pathway and mitochondrial network dynamics in peripheral blood mononuclear cells from bipolar disorder patients. Transl Psychiatry (2017) 7:e1111. doi: 10.1038/tp.2017.83
10. Jarskog LF, Glantz LA, Gilmore JH, Lieberman JA. Apoptotic mechanisms in the pathophysiology of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2005) 29:846–58. doi: 10.1016/j.pnpbp.2005.03.010
11. Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res (2006) 81:47–63. doi: 10.1016/j.schres.2005.08.014
12. Nunomura A, Lee HG, Zhu X, Perry G. Consequences of RNA oxidation on protein synthesis rate and fidelity: implications for the pathophysiology of neuropsychiatric disorders. Biochem Soc Trans (2017) 45:1053–66. doi: 10.1042/BST20160433
13. Tsai MC, Liou CW, Lin TK, Lin IM, Huang TL. Bcl-2 associated with positive symptoms of schizophrenic patients in an acute phase. Psychiatry Res (2013) 210:735–8. doi: 10.1016/j.psychres.2013.08.032
14. Benedetti F, Poletti S, Radaelli D, Bernasconi A, Cavallaro R, Falini A, et al. Temporal lobe grey matter volume in schizophrenia is associated with a genetic polymorphism influencing glycogen synthase kinase 3-β activity. Genes Brain Behav (2010) 9:365–71. doi: 10.1111/j.1601-183X.2010.00566.x
15. Chen X, Sun C, Chen Q, O'Neill FA, Walsh D, Fanous AH, et al. Apoptotic engulfment pathway and schizophrenia. PloS One (2009) 4:e6875. doi: 10.1371/journal.pone.0006875
16. Yang Y, Xiao Z, Chen W, Sang H, Guan Y, Peng Y, et al. Tumor suppressor gene TP53 is genetically associated with schizophrenia in the Chinese population. Neurosci Lett (2004) 369:126–31. doi: 10.1016/j.neulet.2004.07.068
17. Jarskog LF, Gilmore JH, Glantz LA, Gable KL, German TT, Tong RI, et al. Caspase-3 activation in rat frontal cortex following treatment with typical and atypical antipsychotics. Neuropsychopharmacology (2007) 32:95–102.
18. Jarskog LF, Gilmore JH, Selinger ES, Lieberman JA. Cortical bcl-2 protein expression and apoptotic regulation in schizophrenia. Biol Psychiatry (2000) 48:641–50.
19. Jarskog LF, Selinger ES, Lieberman JA, Gilmore JH. Apoptotic proteins in the temporal cortex in schizophrenia: high Bax/Bcl-2 ratio without caspase-3 activation. Am J Psychiatry (2004) 161:109–15.
20. Kozlovsky N, Belmaker RH, Agam G. Low GSK-3beta immunoreactivity in postmortem frontal cortex of schizophrenic patients. Am J Psychiatry (2000) 157:831–3. doi: 10.1176/appi.ajp.157.5.831
21. Beyazyüz M, Küfeciler T, Bulut L, Ünsal C, Albayrak Y, Akyol ES, et al. Increased serum levels of apoptosis in deficit syndrome schizophrenia patients: a preliminary study. Neuropsychiatr Dis Treat (2016) 12:1261–8. doi: 10.2147/NDT.S106993
22. Shal B, Khan A, Naveed M, Ullah Khan N, Ihsan-Ul-Haq D, AlSharari S, et al. Effect of 25-methoxy hispidol a isolated from Poncirus trifoliate against bacteria-induced anxiety and depression by targeting neuroinflammation, oxidative stress and apoptosis in mice. BioMed Pharmacother (2019) 111:209–23. doi: 10.1016/j.biopha.2018.12.047
23. Huang GH, Chen K, Sun YY, Zhu L, Sun ZL, Feng DF. 4-Phenylbutyrate ameliorates anxiety disorder by inhibiting endoplasmic reticulum stress after diffuse axonal injury. J Neurotrauma (2019) 36:1856–68. doi: 10.1089/neu.2018.6048
24. Olianas MC, Dedoni S, Onali P. Inhibition of TNF-α-induced neuronal apoptosis by antidepressants acting through the lysophosphatidic acid receptor LPA1. Apoptosis (2019) 24:478–98. doi: 10.1007/s10495-019-01530-2
25. Amidfar M, Kim YK, Scaini G, Quevedo J. Evidence for additionally increased apoptosis in the peripheral blood mononuclear cells of major depressive patients with a high risk for suicide. Am J Med Genet B Neuropsychiatr Genet (2018) 177:388–96. doi: 10.1002/ajmg.b.32623
26. Hardy MP, Audemard É, Migneault F, Feghaly A, Brochu S, Gendron P, et al. Apoptotic endothelial cells release small extracellular vesicles loaded with immunostimulatoryviral-like RNAs. Sci Rep (2019) 9:7203. doi: 10.1038/s41598-019-43591-y
27. Vizza E, Corrado G, De Angeli M, Carosi M, Mancini E, Baiocco E, et al. Serum DNA integrity index as a potential molecular biomarker in endometrial cancer. J Exp Clin Cancer Res (2018) 37:16. doi: 10.1186/s13046-018-0688-4
28. Madhavan D, Wallwiener M, Bents K, Zucknick M, Nees J, Schott S, et al. Plasma DNA integrity as a biomarker for primary and metastatic breast cancer and potential marker for early diagnosis. Breast Cancer Res Treat (2014) 146:163–74. doi: 10.1007/s10549-014-2946-2
29. Cheng J, Holland-Letz T, Wallwiener M, Surowy H, Cuk K, Schott S, et al. Circulating free DNA integrity and concentration as independent prognostic markers in metastatic breast cancer. Breast Cancer Res Treat (2018) 169:69–82. doi: 10.1007/s10549-018-4666-5
30. Pu WY, Zhang R, Xiao L, Wu YY, Gong W, Lv XD, et al. Prediction of cancer progression in a group of 73 gastric cancer patients by circulating cell-free DNA. BMC Cancer (2016) 16:943. doi: 10.1186/s12885-016-2977-7
31. Hao TB, Shi W, Shen XJ, Qi J, Wu XH, Wu Y, et al. Circulating cell-free DNA in serum as a biomarker for diagnosis and prognostic prediction of colorectal cancer. Br J Cancer (2014) 111:1482–9. doi: 10.1038/bjc.2014.470
32. Munshi S, Parrilli V, Rosenkranz JA. Peripheral anti-inflammatory cytokine Interleukin-10 treatment mitigates interleukin-1β – induced anxiety and sickness behaviors in adult male rats. Behav Brain Res (2019) 372:112024. doi: 10.1016/j.bbr.2019.112024
33. Coleman LG Jr, Zou J, Qin L, Crews FT. HMGB1/IL-1β complexes regulate neuroimmune responses in alcoholism. Brain Behav Immun (2018) 72:61–77. doi: 10.1016/j.bbi.2017.10.027
34. Orhan F, Fatouros-Bergman H, Schwieler L, Cervenka S, Flyckt L, Sellgren CM, et al. First-episode psychosis patients display increased plasma IL-18 that correlates with cognitive dysfunction. Schizophr Res (2018) 195:406–8. doi: 10.1016/j.schres.2017.09.016
35. Wu JQ, Chen DC, Tan YL, Tan SP, Xiu MH, Wang ZR, et al. Altered interleukin-18 levels are associated with cognitive impairment in chronic schizophrenia. J Psychiatr Res (2016) 76:9–15. doi: 10.1016/j.jpsychires.2016.01.013
36. Kim Y, Tarallo V, Kerur N, Yasuma T, Gelfand BD, Bastos-Carvalho A, et al. DICER1/Alu RNA dysmetabolism induces Caspase-8-mediated cell death in age-related macular degeneration. Proc Natl Acad Sci U.S.A. (2014) 111:16082–7. doi: 10.1073/pnas.1403814111
37. Wang W, Wang WH, Azadzoi KM, Dai P, Wang Q, Sun JB, et al. Alu RNA accumulation in hyperglycemia augments oxidative stress and impairs eNOS and SOD2 expression in endothelial cells. Mol Cell Endocrinol (2016) 426:91–100. doi: 10.1016/j.mce.2016.02.008
38. Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G. Inflammatory biomarkers and risk of schizophrenia: a 2-sample mendelian randomization study. JAMA Psychiatry (2017) 74:1226–33. doi: 10.1001/jamapsychiatry.2017.3191
39. Graham BM, Zagic D, Richardson R. low endogenous fibroblast growth factor 2 levels are associated with heightened conditioned fear expression in rats and humans. Biol Psychiatry (2017) 82:601–7. doi: 10.1016/j.biopsych.2017.03.020
40. Conus P, Seidman LJ, Fournier M, Xin L, Cleusix M, Baumann PS, et al. N-acetylcysteine in a double-blind randomized placebo-controlled trial: toward biomarker-guided treatment in early psychosis. Schizophr Bull (2018) 44:317–27. doi: 10.1093/schbul/sbx093
41. Leng S, Zheng J, Jin Y, Zhang H, Zhu Y, Wu J, et al. Plasma cell-free DNA level and its integrity as biomarkers to distinguish non-small cell lung cancer from tuberculosis. Clin Chim Acta (2018) 477:160–5. doi: 10.1016/j.cca.2017.11.003
42. Thongsroy J, Patchsung M, Mutirangura A. The association between Alu hypomethylation and severity of type 2 diabetes mellitus. Clin Epigenet (2017) 9:93. doi: 10.1186/s13148-017-0395-6
43. Dannlowski U, Kugel H, Redlich R, Halik A, Schneider I, Opel N, et al. Serotonin transporter gene methylation is associated with hippocampal gray matter volume. Hum Brain Mapp (2014) 35:5356–67. doi: 10.1002/hbm.22555
44. Werfel TA, Cook RS. Efferocytosis in the tumor microenvironment. Semin Immunopathol (2018) 40:545–54. doi: 10.1007/s00281-018-0698-5
45. Paone S, Baxter AA, Hulett MD, Poon IKH. Endothelial cell apoptosis and the role of endothelial cell-derived extracellular vesicles in the progression of atherosclerosis. Cell Mol Life Sci (2019) 76:1093–106. doi: 10.1007/s00018-018-2983-9
46. Fu G, Zhang W, Dai J, Liu J, Li F, Wu D, et al. Increased peripheral interleukin 10 relate to white matter integrity in schizophrenia. Front Neurosci (2019) 13:52. doi: 10.3389/fnins.2019.00052
47. Francesconi LP, Victorino AT, Salah IA, Cordova VHS, Dias da Rosa E, Oliveira L, et al. Proinflammatory and anti-inflammatory biomarkers in schizophrenia and influence of simvastatin on the interleukin-6. Int Clin Psychopharmacol (2019) 34:84–8. doi: 10.1097/YIC.0000000000000241
48. Goldsmith DR, Haroon E, Miller AH, Addington J, Bearden C, Cadenhead K, et al. Association of baseline inflammatory markers and the development of negative symptoms in individuals at clinical high risk for psychosis. Brain Behav Immun (2019) 76:268–74. doi: 10.1016/j.bbi.2018.11.315
49. de Baumont A, Bortoluzzi A, Wollenhaupt de Aguiar B, Scotton E, Pinto Guimarães LS, Kapczinski F, et al. Anxiety disorders in childhood are associated with youth IL-6 levels: a mediation study including metabolic stress and childhood traumatic events. J Psychiatr Res (2019) 115:43–50. doi: 10.1016/j.jpsychires.2019.05.011
50. Ren Q. Soluble epoxide hydrolase inhibitor: a novel potential therapeutic or prophylactic drug for psychiatric disorders. Front Pharmacol (2019) 10:420. doi: 10.3389/fphar.2019.00420
51. Hou YQ, Liang DY, Lou XL, Zhang M, Zhang ZH, Zhang LR. Branched DNA-based Alu quantitative assay for cell-free plasma DNA levels in patients with sepsis or systemic inflammatory response syndrome. J Crit Care (2016) 31:90–5. doi: 10.1016/j.jcrc.2015.10.013
52. Jylhävä J, Nevalainen T, Marttila S, Jylhä M, Hervonen A, Hurme M. Characterization of the role of distinct plasma cell-free DNA species in age-associated inflammation and frailty. Aging Cell (2013) 12:388–97. doi: 10.1111/acel.12058
53. Hueso M, Cruzado JM, Torras J, Navarro E. ALUminating the path of atherosclerosis progression: chaos theory suggests a role for Alu repeats in the development of atherosclerotic vascular disease. Int J Mol Sci (2018) 19:E1734. doi: 10.3390/ijms19061734
54. Polesskaya O, Kananykhina E, Roy-Engel AM, Nazarenko O, Kulemzina I, Baranova A, et al. The role of Alu-derived RNAs in Alzheimer’s and other neurodegenerative conditions. Med Hypotheses (2018) 115:29–34. doi: 10.1016/j.mehy.2018.03.008
55. Mao X, Fang W, Liu Q. An emerging role of Alu RNA in geographic atrophy pathogenesis: the implication for novel therapeutic strategies. Discovery Med (2016) 22:337–49.
Keywords: biomarker, Alu, psychiatric disorders, interleukin-1β, interleukin-18
Citation: Qi J, Chen L-Y, Shen X-J and Ju S-Q (2020) Analytical Value of Cell-Free DNA Based on Alu in Psychiatric Disorders. Front. Psychiatry 10:992. doi: 10.3389/fpsyt.2019.00992
Received: 05 August 2019; Accepted: 13 December 2019;
Published: 21 January 2020.
Edited by:
Joseph F. Cubells, Emory University, United StatesReviewed by:
Bhaskar Roy, University of Alabama at Birmingham, United StatesKurt Leroy Hoffman, Autonomous University of Tlaxcala, Mexico
Copyright © 2020 Qi, Chen, Shen and Ju. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shao-Qing Ju, anNxODE0QGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Jing Qi
Jing Qi Ling-Yun Chen2†
Ling-Yun Chen2†