- 1Department of Psychiatry, China Medical University, Shenyang, China
- 2Department of Psychiatry, First Affiliated Hospital, China Medical University, Shenyang, China
- 3Brain Function Research Section, First Affiliated Hospital, China Medical University, Shenyang, China
- 4Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 5Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, United States
- 6Department of Radiology, First Affiliated Hospital, China Medical University, Shenyang, China
- 7Department of Geriatric Medicine, First Affiliated Hospital, China Medical University, Shenyang, China
Finding neural features of suicide attempts (SA) in major depressive disorder (MDD) may be helpful in preventing suicidal behavior. The ventral and medial prefrontal cortex (PFC), as well as the amygdala form a circuit implicated in emotion regulation and the pathogenesis of MDD. The aim of this study was to identify whether patients with MDD who had a history of SA show structural and functional connectivity abnormalities in the amygdala and PFC relative to MDD patients without a history of SA. We measured gray matter volume in the amygdala and PFC and amygdala-PFC functional connectivity using structural and functional magnetic resonance imaging (MRI) in 158 participants [38 MDD patients with a history of SA, 60 MDD patients without a history of SA, and 60 healthy control (HC)]. MDD patients with a history of SA had decreased gray matter volume in the right and left amygdala (F = 30.270, P = 0.000), ventral/medial/dorsal PFC (F = 15.349, P = 0.000), and diminished functional connectivity between the bilateral amygdala and ventral and medial PFC regions (F = 22.467, P = 0.000), compared with individuals who had MDD without a history of SA, and the HC group. These findings provide evidence that the amygdala and PFC may be closely related to the pathogenesis of suicidal behavior in MDD and implicate the amygdala-ventral/medial PFC circuit as a potential target for suicide intervention.
Introduction
Major depressive disorder (MDD) is one of the most common mental disorders (1), which is commonly associated with a higher suicide risk (2, 3). Previous studies have reported that patients with MDD had a 2%–12% lifetime risk of dying by suicide (4, 5), and the new study reported that the rated of suicide attempts (SA) was greater 4.78% in MDD by assessments of 3,284 adults with/without suicidal acts (6). Therefore, finding neurological features relating to SA in MDD patients could be a major achievement in preventing suicidal behavior (7).
The amygdala and prefrontal cortex (PFC) are two key brain regions which are responsible for processing emotional and cognitive information, especially emotional stimulation and executive function (8, 9). Recent structural and functional magnetic resonance imaging (MRI) studies demonstrate that the amygdala and PFC have been strongly implicated in MDD (10–17). For example, previous studies in MDD patients provided evidence of increased activation in the amygdala, and enlarged amygdala volume or reduced amygdala volume (10–14), which are mixed results due to different sample and method, so we will continue to explore the amygdala volume issue in this study. Regarding the PFC, morphological and functional alterations have been shown primarily in the orbitofrontal cortex, dorsolateral and ventrolateral PFC in MDD patients (12, 15–17). Depressed individuals also display decreased relationships between amygdala and dorsolateral PFC activity (12).
Neuroimaging studies suggest that abnormalities of the amygdala and PFC are closely related with SA in patients with MDD. For example, patients with a history of suicidal attempt have larger right amygdala volumes than nonsuicidal (18). Wagner and Blumberg and their colleagues found that MDD patients with prior SA showed significantly thinner cortex in the left dorsolateral, ventrolateral and prefrontal cortex in contrast to nonsuicide attempted patients (19, 20). MDD patients with a history of SA had lower orbitofrontal cortex gray matter volumes compared with those with no SA, or healthy comparison subjects (18, 21–23). A recent diffusion tensor imaging study showed that abnormal projections to the orbitofrontal cortex may disrupt affective and cognitive function, thereby conferring a heightened vulnerability for SA in MDD (24). However, few structural MRI studies have examined the gray matter volumes in amygdala and PFC in MDD with SA.
In addition to structural anomalies, there is also evidence for functional alterations in MDD patients with a history of suicidal behavior. Functional MRI studies found that MDD might be associated with a disturbed amygdala-PFC functional connectivity (FC). For example, MDD patients display decreased coupling between the amygdala and dorsolateral PFC (12, 25). Work from our lab has also shown altered amygdala-rostral, ventral, and dorsolateral PFC FC in MDD patients (26–28). Furthermore, patients who have attempted suicide show significant reductions in amygdala-prefrontal FC compared with a nonattempter patient group (29). With regard to MDD patients with SA, Kang and colleagues identified greater resting state FC in the amygdala in suicide attempters with MDD versus nonattempters, and there was greater connectivity of the left amygdala with the left superior OFC (30). So alterations in amygdala-PFC FC may be very important feature for MDD patients with SA. Understanding the PFC-amygdala neural circuitry with SA in MDD may be more beneficial to prevent suicide behavior.
The MRI literatures on suicide attempt in MDD suggested alterations of both structure and function in the amygdala-PFC neural circuitry. Compared with single neuroimaging analysis, the multiple neuroimaging analyses such as combining functional connectivity, regional gray matter volume, as well as combining amplitude of low-frequency fluctuations and white matter connectivity, could be used not only to explore the local functional and structural abnormalities, but also to identify more precisely the key neural circuitry in the mental disorders, providing the evidence to better understand the the mechanism of mental disorders in-depth (29, 31–33). Additionally, the latest studies suggested that data from multimodal fusion of structural and functional brain imaging analyses may be helpful to specifically predict symptoms and treatment effects in mental diseases (34, 35). Therefore, in the present study, we applied a multivariate model, which evaluate to more thoroughly characterize brain structural and functional abnormalities in MDD patient with SA. We hypothesized that patients with MDD who had a history of SA would demonstrate volumetric and FC differences in the amygdala and PFC relative to both HC and MDD patients without a history of SA, and we would perform to explore the relationships between the structural and functional findings across modalities.
Materials and Methods
Subjects
The study included 158 subjects aged 18–59 years: 38 MDD patients with a history of SA (mean age: 27.61 ± 10.536 years; 11 males), 60 MDD patients without a history of SA (mean age: 28.13 ± 7.617 years; 13 males), and 60 HC individuals (mean age: 25.83 ± 5.898 years; 17 males). Patients were divided into two groups: those with a history of SA [at least one attempt defined as a self-destructive act with some degree of intent to die (36)] and patients without a history of SA (nonattempters; no such history). Subjects were not considered suicide attempters if their self-injurious behavior was determined to have no suicidal intent. All patients were from Shenyang Mental Health Center and the Department of Psychiatry, First Affiliated Hospital of China Medical University, Shenyang, China. HC were recruited by advertising within the community. This study was approved by the Institutional Review Board of China Medical University. All participants provided written informed consent after receiving a detailed description of the study. All participants were evaluated by two trained psychiatrists to determine the presence or absence of Axis I psychiatric diagnoses using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Axis I Disorders (SCID). MDD participants met DSM-IV diagnostic criteria for MDD and did not meet the criteria for any other Axis I disorder. HC participants did not have a current or lifetime Axis I disorder, or a history of psychosis, other Axis I disorders, or a history of SA in first-degree relatives as determined by a detailed family history. Participants were excluded if they had substance/alcohol abuse/dependence or a concomitant major medical disorder, any MRI contraindications, a history of head trauma with loss of consciousness for ≥5 min, or any neurological disorder. All subjects were evaluated using the Hamilton Depression Rating Scale (HAM-D) and Young Mania Rating Scale (YMRS).
MRI Acquisition
MRI data were acquired using a GE signa HDX 3.0T scanner with a standard 8-channel head coil at the First Affiliated Hospital of China Medical University, Shenyang, China. Three-dimensional, high-resolution, T1-weighted images was collected using a 3D fast spoiled gradient-echo (FSPGR) sequence with the following parameters: TR/TE = 7.1/3.2 ms, image matrix = 240 × 240, field of view (FOV) = 240 mm2 × 240 mm2, 176 contiguous slices of 1mm without gap, voxel size = 1.0 mm3. We acquired fMRI images using a spin echo planar imaging (EPI) sequence, parallel to the anterior-posterior commissure plane with the following scan parameters: repetition time (TR) = 2000 ms; echo time (TE) > = 40 ms; image matrix = 64×64; field of view (FOV) = 24 cm2 × 24 cm2; 35 contiguous slices of 3 mm without gap; scan time = 6 min 40 s (the 6 min 40 s scans included a total of 200 volumes). We acquired a high-resolution structural image using a three-dimensional fast spoiled gradient-echo T1-weighted sequence: TR = 7.1 ms, TE = 3.2 ms, FOV = 24 cm × 24 cm, matrix = 240 × 240, slice thickness = 1.0 mm without gap, and 176 slices.
Data Preprocessing
Structural brain images were processed using VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm8/), in Statistical Parametric Mapping 8 (SPM8; The Wellcome Department of Cognitive Neurology). VBM8 processing includes bias correction, tissue classification, and spatial normalization with Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) (37). Using the default parameters of VBM8, images were spatially normalized to the Montreal Neurological Institute (MNI) space to 1.5-mm3 voxel resolution. The modulation process was performed using nonlinear deformations employed for normalization that allowed for comparison of the absolute amount of tissue, corrected for individual brain sizes. Finally, all images were smoothed with an isotropic Gaussian kernel of 8-mm full width at half maximum (FWHM). These segmented, normalized, modulated, and spatially smoothed GM images were then used for subsequent VBM second-level statistical analysis.
Resting-state fMRI data preprocessing was carried out using Data Processing and Analysis for Brain Imaging software (DPABI; DPABI_V1.2_141101, http://rfmri.org/dpabi) (38), a toolbox for SPM8. The first ten volumes were discarded to allow for steady-state magnetization. Further data preprocessing included slice timing correction, head motion correction, spatial normalization, and smoothing. Spatial normalization was performed with the standard MNI EPI template. Spatial smoothing was completed with a 6-mm FWHM Gaussian filter. Data were then linearly detrended (39) and filtered using a band-pass temporal filter (0.01-0.08 Hz). We regressed for nuisance covariates, including the rigid-body 6 model, white matter signal, cerebrospinal fluid signal, and global signals. Subjects with head motion parameters exceeding 3 mm in displacement or 3° in rotation were excluded from the final analysis.
Definition of Region of Interest
The Wake Forest University PickAtlas (http://fmri.wfubmc.edu/software/PickAtlas) was used to define the regions of interest for the PFC and amygdala. The PFC included the superior frontal gyrus, dorsolateral (Frontal_Sup), superior frontal gyrus, orbital part (Frontal_Sup_Orb), middle frontal gyrus (Frontal_Mid), middle frontal gyrus, orbital part (Frontal_Mid_Orb), inferior frontal gyrus, opercular part (Frontal_Inf_Oper), inferior frontal gyrus, triangular part (Frontal_Inf_Tri), inferior frontal gyrus, orbital part (Frontal_Inf_Orb), superior frontal gyrus, medial (Frontal_Sup_Medial), and superior frontal gyrus, medial orbital (Frontal_Mid_Orb), in the left and right hemispheres. We used the bilateral Automated Anatomical Labeling atlas template to define the amygdala regions of interest (40).
FC Analysis
FC analysis was performed using correlation analysis between the seed amygdala ROI and PFC mask in a voxelwise manner using DPABI. The correlation coefficients were then transformed to z-values using the Fisher r-to-z transformation.
Statistical Analysis
The demographic and clinical characteristics of the subjects were analyzed using IBM SPSS Statistics for Windows, Version 22.0 (Armonk, NY, USA). Student's t-tests, one-way analyses of variance, or Chi-square tests were used depending on the normality of the distribution and type of data. Categorical variables were described using frequencies and proportions. Statistical significance was determined by P < 0.05. Continuous variables were presented as mean ± standard deviation (P < 0.05, Bonferroni test). Partial correlation analyses (two-tailed), controlling for age and sex, were performed to explore the relationships between GMVs, FC, and symptom scores in the patient group (P < 0.05, Bonferroni correction). Additionally, we performed correlation analyses between GMVs/FC and HAM-D scores in MDD with SA group or in MDD without SA group in order to explore the association between GMVs/FC and the severity of attempt suicide, which could be evaluated by the HAM-D scores in MDD [(6, 41)] (P < 0.05, Bonferroni correction).
The GMVs of the bilateral PFC and amygdala were compared among the groups using one-way analysis of variance (ANOVA), with age and gender as covariates, using the general linear model in SPM8 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Amygdala-PFC FC was compared among the groups by one-way analysis of variance (One-way ANOVA), with age and gender as covariates, using Data Processing and Analysis for Brain Imaging software (DPABI; DPABI_V1.2_141101, http://rfmri.org/dpabi). Group differences were considered significant for p values less than 0.001 (corrected, Gaussian random field [GRF] correction), and corresponding to a threshold of 69, 1, and 27 contiguous voxels for the GMVs of the bilateral PFC and amygdala and Z values of FC, respectively. We then extracted GMVs and Z values of FC for each cluster with significant differences for the three group comparison and conducted pairwise two sample t-tests, corrected for multiple comparisons (P < 0.05, Bonferroni test).
Results
Demographics and Clinical Characteristics
Table 1 shows detailed participant demographic and clinical data. There were no significant differences among the three groups in terms of age (F = 1.374, P = 0.256), or sex (χ2 = 0.928, P = 0.629). There was no significant difference in illness duration (T = 1.105, P = 0.275), or medication status (χ2 = 0.582, P = 0.445) between the two MDD groups. As expected, three groups showed significant differences in HAM-D scores and YMRS scores (HAM-D: F = 83.907, P = .000; YMRS: F = 5.449, P = .005). Post hoc analyses found the MDD with and without a history of SA groups showed significantly higher HAM-D scores and YMRS scores than the HC group, however, HAM-D and YMRS scores demonstrated no significant differences between the two MDD groups.
Group Differences in GMV
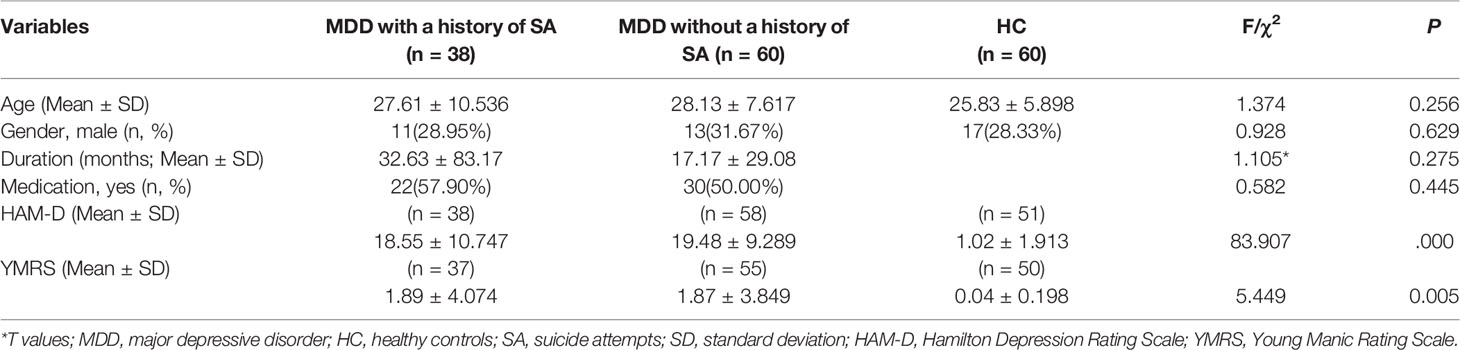
Significant group differences were found in grey matter volumes in the bilateral amygdala (F = 30.270, P = 0.000) and in PFC regions including the ventral, medial, and dorsal PFC (F = 15.349, P = 0.000) (Figure 1, Table 2). Post hoc comparisons showed significant differences in GMV across all 3 groups (P < 0.03): HC > MDD without history of SA > MDD with history of SA for bilateral amygdala and ventral/medial/dorsal PFC volumes (Figure 2). Findings were unchanged with intracranial volume (ICV) as a covariate.
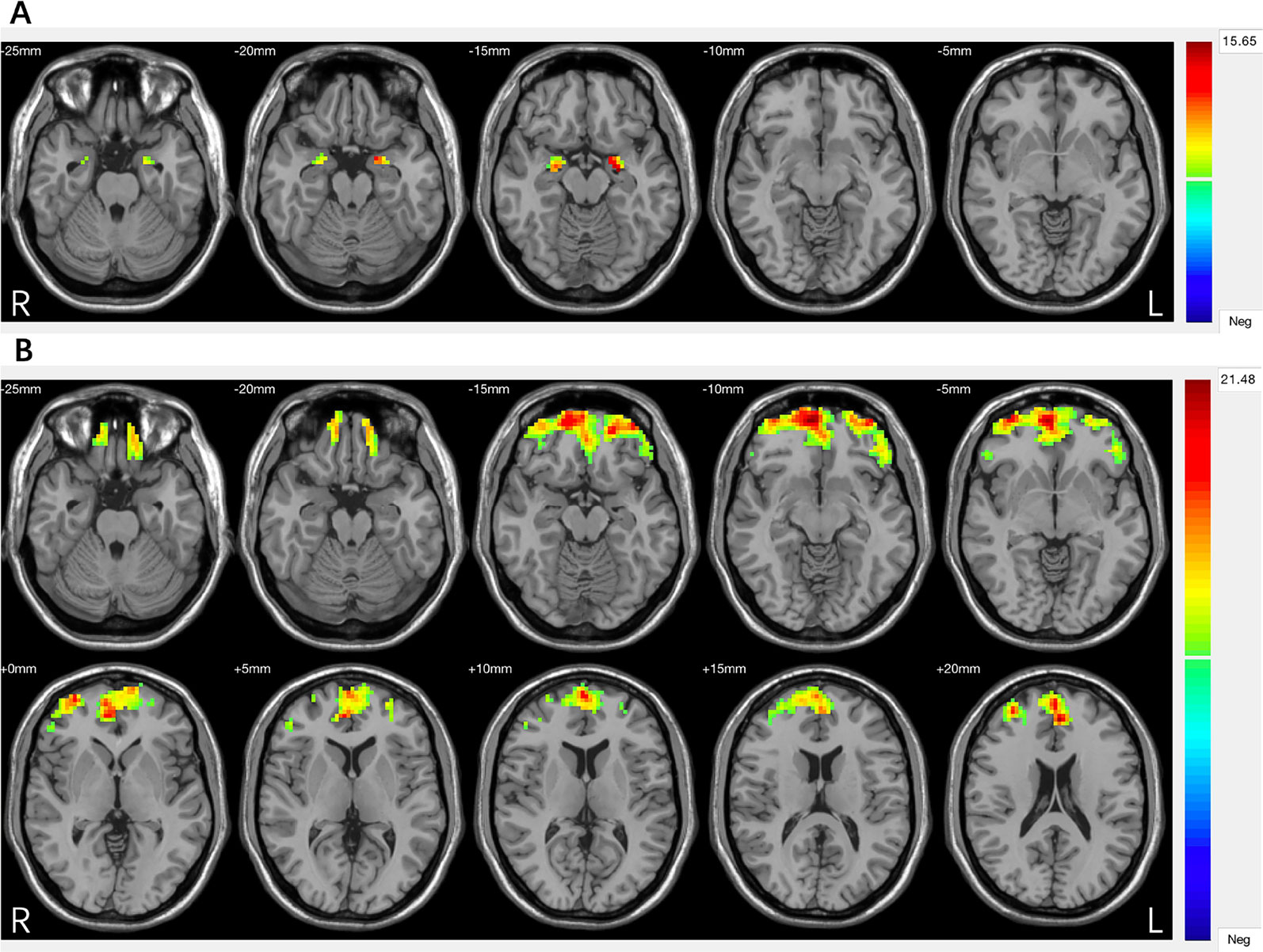
Figure 1 (A) Significant differences in grey matter volumes of the amygdala among the major depressive disorder (MDD) with a history of suicide attempts (SA), MDD without a history of SA, and healthy controls (HC) groups. (B) Significant differences in grey matter volumes of the prefrontal cortex (PFC) among the MDD with a history of SA, MDD without a history of SA, and HC groups. Significant at P < 0.001, corrected by Gaussian random field (GRF) correction.
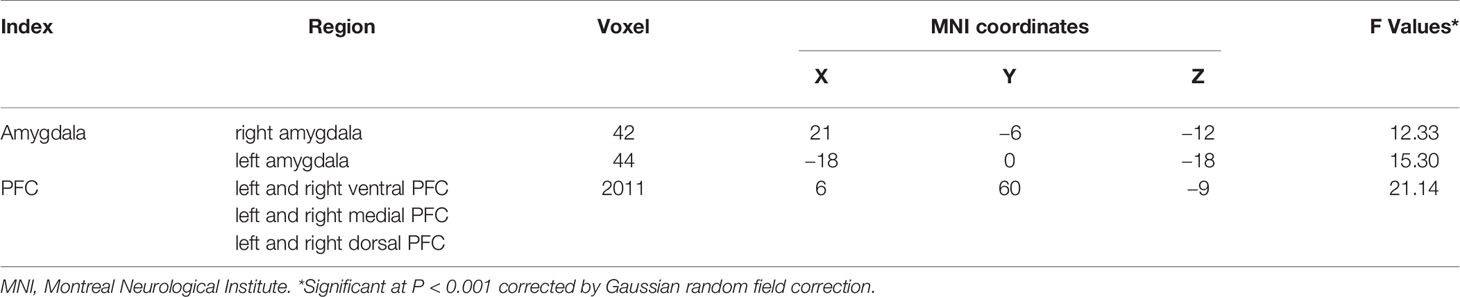
Table 2 Brain regions showing significant differences in amygdala and prefrontal cortex (PFC) between major depressive disorder (MDD) with and without a history of suicide attempts (SA) and healthy controls (HC) groups.
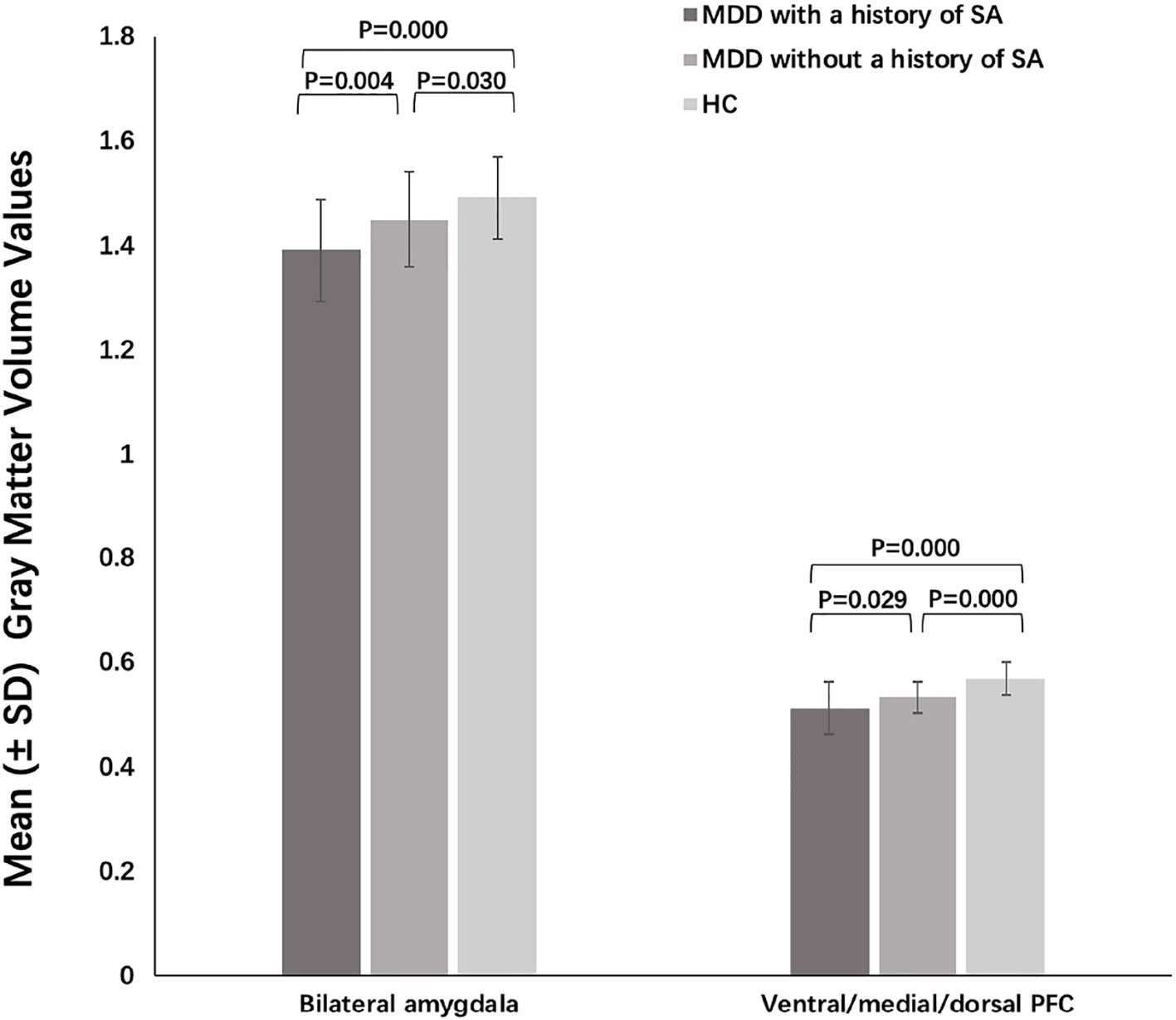
Figure 2 Post-hoc analysis of grey matter volumes of amygdala and prefrontal cortex (PFC) among the major depressive disorder (MDD) with a history of suicide attempts (SA), MDD without a history of SA, and HC groups.
Group Differences in FC
Three-group analysis of FC showed significant differences in bilateral amygdala-left and right ventral PFC and bilateral amygdala-left and right medial PFC (F = 22.467, P = 0.000) (Table 3, Figure 3). MDD with a history of SA had significantly decreased FC in bilateral amygdala-left and right ventral PFC and bilateral amygdala-left and right medial PFC, compared with the HC (P = 0.000), and the MDD without a history of SA (P = 0.000), but no difference was observed in FC between the MDD without a history of SA and the HC group (P = 1.000) (Figure 4). Findings were unchanged with ICV as a covariate.
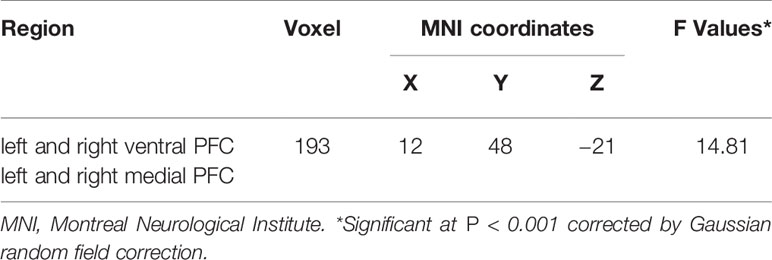
Table 3 Prefrontal cortex (PFC) regions showing significant changes from bilateral amygdala to PFC functional connectivity (FC) between major depressive disorder (MDD) with and without a history of suicide attempts (SA) and healthy controls (HC) groups.
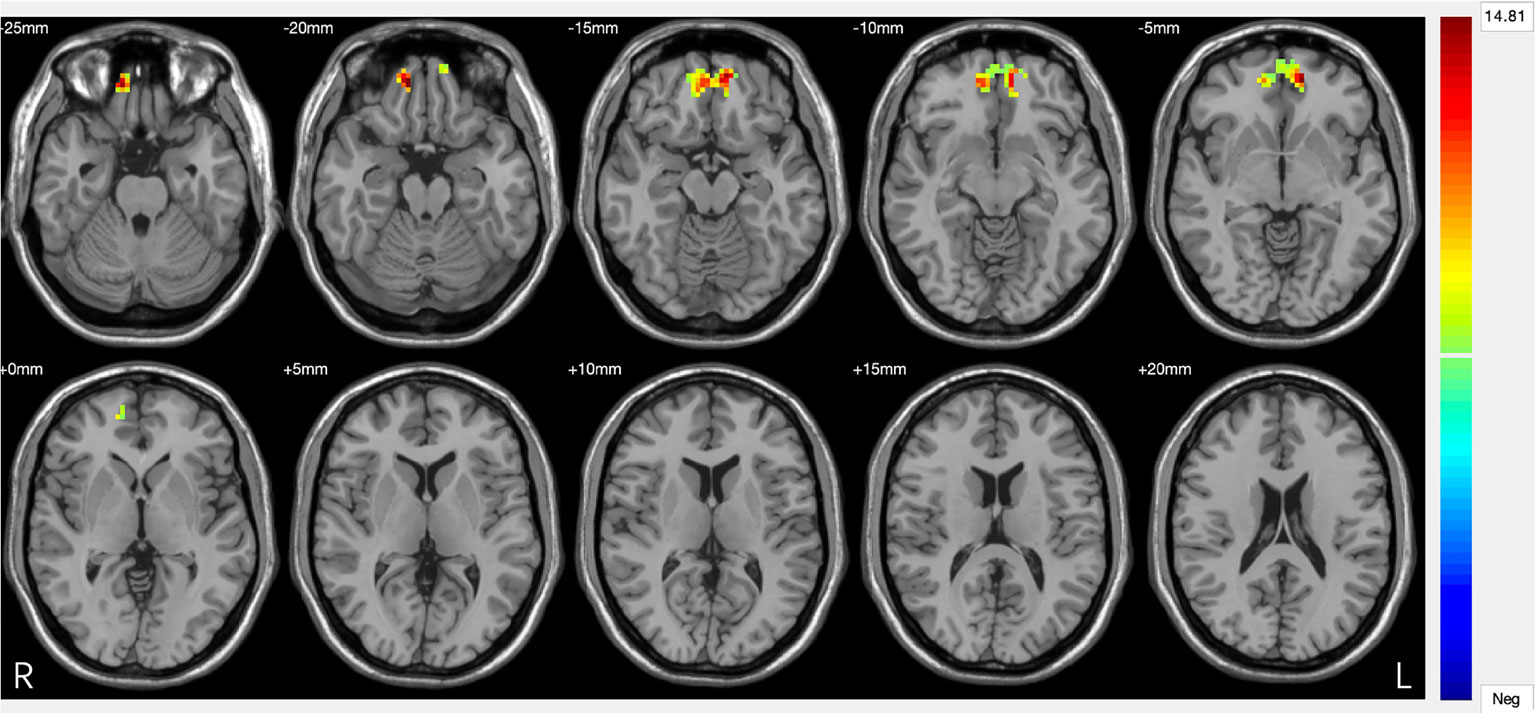
Figure 3 Significant differences in amygdala–prefrontal cortex (PFC) functional connectivity (FC) among the major depressive disorder (MDD) with a history of suicide attempts (SA), MDD without a history of SA, and HC groups. Significant at P < 0.001, corrected by Gaussian random field (GRF) correction.
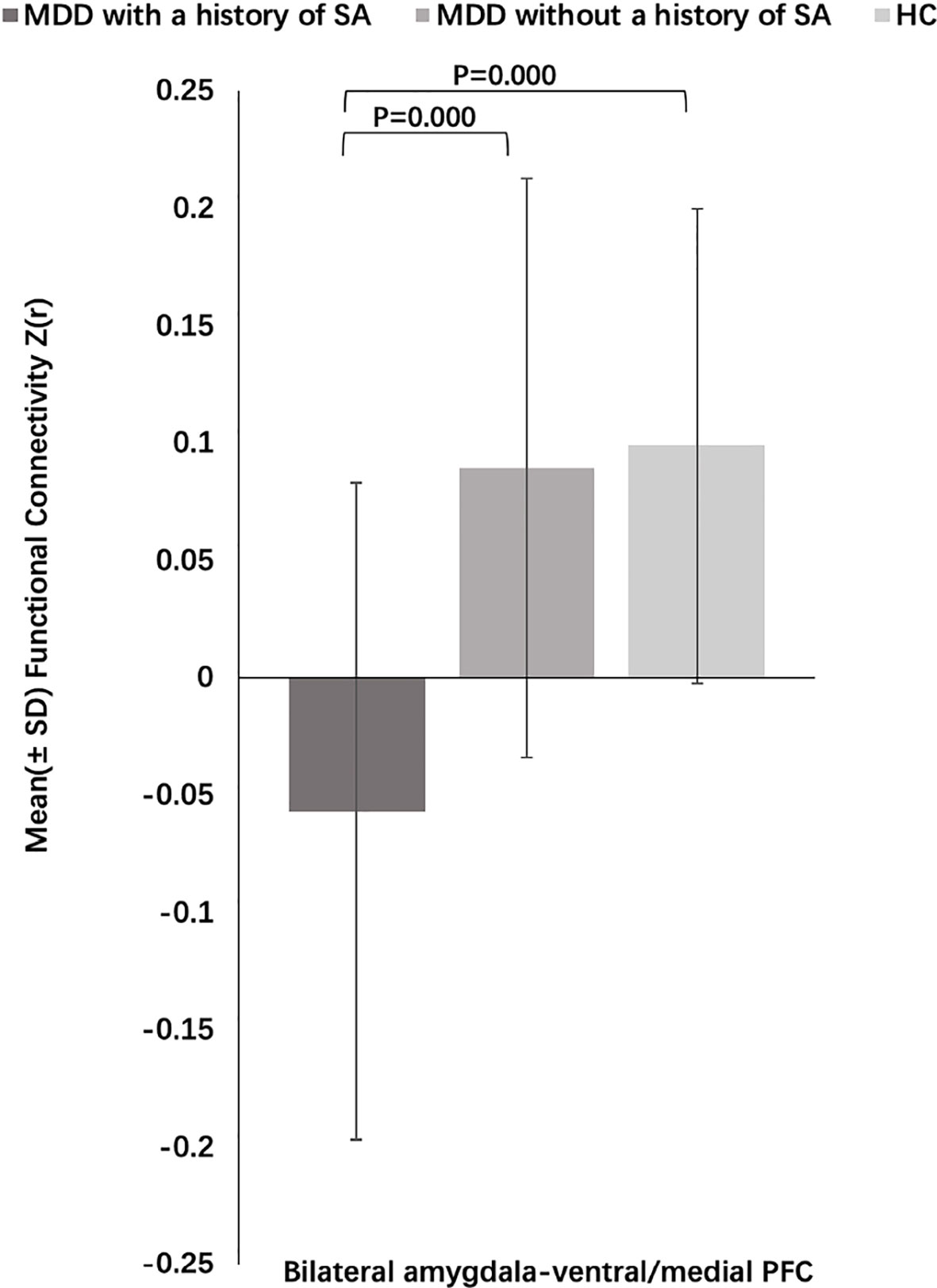
Figure 4 Post-hoc analysis of amygdala–prefrontal cortex (PFC) functional connectivity (FC) among the major depressive disorder (MDD) with a history of suicide attempts (SA), MDD without a history of SA, and HC groups.
Correlations Between GMV, FC, and Symptom Scores
No significant correlations between the GMVs, FC, and symptom measures were observed in the MDD patient group (P > 0.05, Bonferroni correction). There were also no significant correlations between GMVs/FC and HAM-D scores in MDD with SA group or MDD without SA group, separately (P > 0.05, Bonferroni correction). We used the left and right amygdala separately as the seed ROI, the GMVs of left and right amygdala and the left and right amygdala-PFC functional connectivity were compared among the groups. Additionally, the subjects were divided into males and females, and the structural and functional changes were compared among three groups separately. We have added these detailed analyses (Supplementary Material).
Discussion
We report that MDD patients with a history of SA have decreased gray matter volume in the amygdala and PFC, and reduced functional connectivity from the amygdala to PFC, compared to both HC and individuals with MDD without a history of SA. Structural differences occurred in a graded fashion, such that MDD with a history of SA showed the lowest volumes, then MDD without a history of SA, then the HC group. In contrast, functional connectivity differences were only present between the MDD SA group and the MDD without SA group; there were no significant differences between the MDD without SA and the HC groups. We discuss the implications of these findings below.
The amygdala has a well-documented role in emotion processing (8, 42). We found reduced amygdala volume in patients with a history of SA, consistent with 43). A recent meta-analysis found decreased GMV in subcortical structures in MDD with suicidal ideation and attempt compared with controls (44), also in line with our findings of decreased amygdala and PFC GM volumes. However, larger amygdala volumes have been shown in MDD females with suicidal ideation compared with nonsuicidal MDD (18). The inconsistency may relate to differences in patient characteristics (both males and females with MDD were included in this study). Future studies specifically examining sex differences in the amygdala in MDD and suicidal behavior are warranted. Several prior studies have found physiological differences in the amygdala in MDD patients who have attempted or died by suicide compared with individuals who have not, implicating the amygdala as a potential neurobiological substrate for suicidal behavior in MDD. Ballard et al. suggest that the amygdala may mediate fear-potentiated startle in MDD individuals with lifetime history of suicide attempt (45). In addition, reductions in the messenger RNA levels of multiple transcripts of QKI in the amygdala have been found in suicide victims compared with control subject (46), and significantly lower numbers of a 2A-adrenoceptors in the amygdala of depressed suicide completers compared with controls (47).
The PFC may be an important associated with suicidal behavior, particularly in the ventral PFC, medial PFC, and dorsal PFC (18, 48–51). The current evidence in adults who have attempted suicide supports ventral prefrontal volume decreases and dysfunction have been linked to lethality (52). Prefrontal localized hypofunction and impaired serotonergic responsivity are proportional to the lethality of the suicide attempt in depressed patients, especially in the ventral, medial, and lateral PFC (53). Suicide action was associated with abnormal activity in the medial PFC (54). In our study, significant volume decreases in the ventral, medial, and dorsal PFC regions were found in suicide attempters with MDD.
Prior studies have reduced GMV in the ventral PFC and medial PFC in MDD individuals with prior suicide attempters (18, 29, 43, 49, 50, 55, 56). Other studies have found reduced GVM in orbitofrontal cortex in MDD patients with a history of SA, compared with those without SA and HC subjects (18, 21–23). Thus, alterations in PFC GMV are implicated in suicidal behavior in MDD patients.
FC between the bilateral amygdala and ventral and medial PFC regions was decreased in MDD patients with a history of SA, compared to MDD patients with a history of SA and HC groups in this study. Our findings are supported by prior finding of decreased FC between the amygdala and ventral PFC in suicide attempters (29). However, increased FC between the left amygdala with left superior OFC has been observed in suicide attempters with MDD versus nonattempter (30). Differences in sample size (this study had larger sample size) and methodology (different amygdala seed regions) may contribute to differences in findings. Nevertheless, these findings altogether suggest that abnormalities in amygdala-ventral/medial PFC neural circuitry may relate to suicidal behavior and could provide insight into the mechanisms underlying suicidality in MDD. In this study, amygdala-ventral/medial PFC FC was lower in the MDD without a history of SA but not statistically different when compared to the HC group. Prior studies, including work from this group, has found alterations in FC between the amygdala and ventral/medial PFC in MDD (27, 28, 57). So whether the functional neural basis from the amygdala to the PFC is related to the pathophysiology of MDD need the further study.
There are several limitations to this study. Firstly, the sample size was not large enough, but we are continuing to collect relevant samples for future research. Secondly, some patients are prescribed psychotropic medications or demonstrate a longer duration of illness, which may affect the accuracy of the results. Thirdly, our study excluded the patients with other psychiatric comorbidities. While this provided sample homogeneity and likely improved our ability to detect significant effects, the generalizability of our findings is unclear as the majority MDD patients have other psychiatric comorbidities. Fourthly, we did not collect other information to assess severity of suicide ideation and suicide attempt (e.g., Suicide Intent Scale (SIS), and Columbia Suicide Severity Rating Scale (C-SSRS)), so we did not know what our findings are related to the severity of SA. Fourthly, in our study we did not collect information on the numbers of SA associated with the severity attempts. It is an important to record the times of SA and assess the severity of depression related to severity attempts to better understand the severity of SA. Finally, our study had no specific assessment of the impulse scale associated with suicidal behavior in our study. Further studies addressing these limitations are needed for more definitive understanding of complex relationship between neural structure and function and suicidal behavior in MDD.
Conclusions
MDD patients with a history of SA exhibited decreased GMV in the amygdala, and ventral/medial/dorsal PFC, as well as reduced FC between amygdala and ventral/medial PFC. These findings indicate structural and functional alterations in the amygdala in suicidal behavior in MDD. Moreover, the study highlights the importance of the amygdala and PFC circuitry in suicidal behavior in MDD and implicates the amygdala-ventral/medial PFC circuit as a potential target for suicide intervention.
Data Availability Statement
The datasets for this study are available on request to the corresponding authors.
Ethics Statement
This study was approved by the Institutional Review Board of the China Medical University and was performed according to the principles of the Declaration of Helsinki. All participants provided written informed consent after receiving a detailed description of the study.
Author Contributions
SW and YT designed the experiment. RZ, PZ, XJ, LK, FW, and YMZ carried it out. LW, SW, and YMZ analyzed the data. LW, YFZ, and SW wrote the manuscript. EE and FYW edited the manuscript. All the authors discussed the results and reviewed the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81701336 to SW; 81271499 and 81571311 to YT), the Liaoning Education Foundation (L2015591 to SW), and National Key Research and Development Program (2016YFC1306900 to YT). We would like to thank the patients and family members who contributed so much to this study and the First Affiliated Hospital of China Medical University for its active support of the project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2019.00923/full#supplementary-material
References
1. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry (2005) 62:617–27. doi: 10.1016/j.schres.2013.08.026
2. Gomes AP, Soares ALG, Kieling C, Rohde LA, Goncalves H. Mental disorders and suicide risk in emerging adulthood: the 1993 Pelotas birth cohort. Rev Saude (2019) 53:96. doi: 10.1073/pnas.071043098
3. Salvo L, Ramirez J, Castro A. [Risk factors for suicide attempts in people with depressive disorders treated in secondary health care]. Rev Med Chil (2019) 147:181–9. doi: 10.1002/brb3.1384
4. Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry (2000) 157:1925–2. doi: 10.1176/appi.ajp.157.12.1925
5. Nock MK, Borges G, Bromet EJ, Alonso J, Angermeyer M, Beautrais A, et al. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. Br J Psychiatry (2008) 192:98–105. doi: 10.1176/appi.ajp.161.8.1433
6. Baldessarini RJ, Tondo L, Pinna M, Nunez N, Vazquez GH. Suicidal risk factors in major affective disorders. Br J Psychiatry (2019) 167:1–6. doi: 10.1192/bjp.2019.167
7. Lee BH, Kim YK. Potential peripheral biological predictors of suicidal behavior in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry (2011) 35:842–7. doi: 10.3389/fnins.2019.00971
8. Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev (2008) 58:57–70. doi: 10.1016/j.brainresrev.2007.10.012
9. Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev (2014) 42:180–92. doi: 10.3389/fpsyt.2018.00323
10. Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry (2005) 57:1079–88. doi: 10.1016/j.biopsych.2005.02.021
11. Weniger G, Lange C, Irle E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. J Affect Disord (2006) 94:219–9. doi: 10.1007/s12021-016-9299-4
12. Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry (2007) 61:198–209. doi: 10.1016/j.jpsychires.2012.01.003
13. Kronenberg G, Tebartz Van Elst L, Regen F, Deuschle M, Heuser I, Colla M. Reduced amygdala volume in newly admitted psychiatric in-patients with unipolar major depression. J Psychiatr Res (2009) 43:1112–7. doi: 10.1016/j.biopsych.2003.08.021
14. Arnone D, Mckie S, Elliott R, Thomas EJ, Downey D, Juhasz G, et al. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. Am J Psychiatry (2012) 169:841–50. doi: 10.1176/appi.ajp.2012.11121774
15. Kinou M, Takizawa R, Marumo K, Kawasaki S, Kawakubo Y, Fukuda M, et al. Differential spatiotemporal characteristics of the prefrontal hemodynamic response and their association with functional impairment in schizophrenia and major depression. Schizophr Res (2013) 150:459–67. doi: 10.1016/j.biopsych.2009.05.010
16. Shen T, Li C, Wang B, Yang WM, Zhang C, Wu Z, et al. Increased cognition connectivity network in major depression disorder: a FMRI study. Psychiatry Invest (2015) 12:227–34. doi: 10.1016/j.biopsych.2006.05.048
17. Zuo Z, Ran S, Wang Y, Li C, Han Q, Tang Q, et al. Altered structural covariance among the dorsolateral prefrontal cortex and amygdala in treatment-naive patients with major depressive disorder. Front Psychiatry (2018) 9:323. doi: 10.3389/fpsyt.2018.00323
18. Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry (2007) 12:360–66. doi: 10.1192/bjp.bp.107.040113
19. Wagner G, Schultz CC, Koch K, Schachtzabel C, Sauer H, Schlosser RG. Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. J Psychiatr Res (2012) 46:1449–55. doi: 10.1038/srep44316
20. Fan S, Lippard ETC, Sankar A, Wallace A, Johnston JAY, Wang F, et al. Gray and white matter differences in adolescents and young adults with prior suicide attempts across bipolar and major depressive disorders. J Affect Disord (2019) 245:1089–7. doi: 10.2147/NDT.S174015
21. Ahearn EP, Jamison KR, Steffens DC, Cassidy F, Provenzale JM, Lehman A, et al. MRI correlates of suicide attempt history in unipolar depression. Biol Psychiatry (2001) 50:266–70. doi: 10.1016/S0006-3223(01)01098-8
22. Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry (2002) 51:273–9. doi: 10.1016/S0006-3223(01)01336-1
23. Lacerda AL, Keshavan MS, Hardan AY, Yorbik O, Brambilla P, Sassi RB, et al. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol Psychiatry (2004) 55:353–8. doi: 10.1146/annurev.neuro.23.1.155
24. Jia Z, Wang Y, Huang X, Kuang W, Wu Q, Lui S, et al. Impaired frontothalamic circuitry in suicidal patients with depression revealed by diffusion tensor imaging at 3.0 T. J Psychiatry Neurosci (2014) 39:170–7. doi: 10.1176/appi.ajp.2016.15050652
25. Lu Q, Li H, Luo G, Wang Y, Tang H, Han L, et al. Impaired prefrontal-amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: a dynamic causal modeling study on MEG. Neurosci Lett (2012) 523:125–30. doi: 10.1109/EMBC.2018.8513313
26. Kong L, Chen K, Tang Y, Wu F, Driesen N, Womer F, et al. Functional connectivity between the amygdala and prefrontal cortex in medication-naive individuals with major depressive disorder. J Psychiatry Neurosci (2013) 38:417–22. doi: 10.1016/j.jpsychires.2009.03.007
27. Tang Y, Kong L, Wu F, Womer F, Jiang W, Cao Y, et al. Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study. Psychol Med (2013) 43:1921–7. doi: 10.1006/nimg.2001.0978
28. Wei S, Womer F, Geng H, Jiang X, Zhou Q, Chang M, et al. Similarities and differences of functional connectivity in drug-naive, first-episode adolescent and young adult with major depressive disorder and schizophrenia. Sci Rep (2017) 7:44316. doi: 10.1016/j.jad.2006.04.017
29. Johnston JAY, Wang F, Liu J, Blond BN, Wallace A, Liu J, et al. Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. Am J Psychiatry (2017) 174:667–75. doi: 10.1016/j.pnpbp.2017.04.029
30. Kang SG, Na KS, Choi JW, Kim JH, Son YD, Lee YJ. Resting-state functional connectivity of the amygdala in suicide attempters with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry (2017) 77:222–7. doi: 10.1001/archpsyc.62.6.617
31. Fang Y, Li M, Mei M, Sun X, Han D. Characteristics of brain functional and structural connectivity in alexithymic students. Neuropsychiatr Dis Treat (2018) 14:2609–15. doi: 10.11606/s1518-8787.20190530012356
32. Luo N, Tian L, Calhoun VD, Chen J, Lin D, Vergara VM, et al. Exploring different impaired speed of genetic-related brain function and structures in schizophrenic progress using multimodal analysis. Conf Proc IEEE Eng Med Biol Soc (2018) 2018:4126–9. doi: 10.1002/hbm.24802
33. Lei X, Zhong M, Zhang B, Yang H, Peng W, Liu Q, et al. Structural and functional connectivity of the anterior cingulate cortex in patients with borderline personality disorder. Front Neurosci (2019) 13:971. doi: 10.1016/j.neulet.2012.06.058
34. Maglanoc LA, Kaufmann T, Jonassen R, Hilland E, Beck D, Landro NI, et al. Multimodal fusion of structural and functional brain imaging in depression using linked independent component analysis. Hum Brain Mapp (2019) 41:241–55. doi: 10.1016/j.jad.2006.06.016
35. Schmitgen MM, Niedtfeld I, Schmitt R, Mancke F, Winter D, Schmahl C, et al. Individualized treatment response prediction of dialectical behavior therapy for borderline personality disorder using multimodal magnetic resonance imaging. Brain Behav (2019) 9:e01384. doi: 10.4306/pi.2015.12.2.227
36. Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, et al. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. Am J Psychiatry (2004) 161:1433–41. doi: 10.1001/archpsyc.60.1.14
37. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage (2007) 38:95–113. doi: 10.1016/j.neuroimage.2007.07.007
38. Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (Resting-State) brain imaging. Neuroinformatics (2016) 14:339–51. doi: 10.1016/j.neubiorev.2014.02.005
39. Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR Am J Neuroradiol (2001) 22:1326–33.
40. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage (2002) 15:273–89. doi: 10.1016/j.neuroimage.2010.08.082
41. Mcgirr A, Renaud J, Seguin M, Alda M, Benkelfat C, Lesage A, et al. An examination of DSM-IV depressive symptoms and risk for suicide completion in major depressive disorder: a psychological autopsy study. J Affect Disord (2007) 97:203–9. doi: 10.1038/sj.mp.4001919
42. Ledoux JE. Emotion circuits in the brain. Annu Rev Neurosci (2000) 23:155–84. doi: 10.1016/j.pnpbp.2010.08.001
43. Harenski CL, Harenski KA, Calhoun VD, Kiehl KA. Source-based morphometry reveals gray matter differences related to suicidal behavior in criminal offenders. Brain Imaging Behav (2018). doi: 10.1523/JNEUROSCI.2531-12.2012
44. Renteria ME, Schmaal L, Hibar DP, Couvy-Duchesne B, Strike LT, Mills NT, et al. Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Transl Psychiatry (2017) 7:e1116. doi: 10.4067/s0034-98872019000200181
45. Ballard ED, Ionescu DF, Vande Voort JL, Slonena EE, Franco-Chaves JA, Zarate CA Jr., et al. Increased fear-potentiated startle in major depressive disorder patients with lifetime history of suicide attempt. J Affect Disord (2014) 162:34–8. doi: 10.1016/j.jad.2014.03.027
46. Klempan TA, Ernst C, Deleva V, Labonte B, Turecki G. Characterization of QKI gene expression, genetics, and epigenetics in suicide victims with major depressive disorder. Biol Psychiatry (2009) 66:824–31. doi: 10.1503/jpn.120117
47. De Paermentier F, Mauger JM, Lowther S, Crompton MR, Katona CL, Horton RW. Brain alpha-adrenoceptors in depressed suicides. Brain Res (1997) 757:60–8. doi: 10.1038/tp.2015.1
48. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U.S.A. (2001) 98:4259–64. doi: 10.1007/s11682-018-9957-2
49. Hwang JP, Lee TW, Tsai SJ, Chen TJ, Yang CH, Lirng JF, et al. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. J Geriatr Psychiatry Neurol (2010) 23:171–84. doi: 10.1503/jpn.130023
50. Benedetti F, Radaelli D, Poletti S, Locatelli C, Falini A, Colombo C, et al. Opposite effects of suicidality and lithium on gray matter volumes in bipolar depression. J Affect Disord (2011) 135:139–47. doi: 10.1016/j.jad.2011.07.006
51. Ding Y, Lawrence N, Olie E, Cyprien F, Le Bars E, Bonafe A, et al. Prefrontal cortex markers of suicidal vulnerability in mood disorders: a model-based structural neuroimaging study with a translational perspective. Transl Psychiatry (2015) 5:e516. doi: 10.3389/fpsyt.2018.00500
52. Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA. Structural brain abnormalities and suicidal behavior in borderline personality disorder. J Psychiatr Res (2012) 46:516–25. doi: 10.1017/S0033291712002759
53. Oquendo MA, Placidi GP, Malone KM, Campbell C, Keilp J, Brodsky B, et al. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch Gen Psychiatry (2003) 60:14–22. doi: 10.1016/j.jad.2010.03.005
54. Reisch T, Seifritz E, Esposito F, Wiest R, Valach L, Michel K. An fMRI study on mental pain and suicidal behavior. J Affect Disord (2010) 126:321–5. doi: 10.1038/tp.2017.84
55. Wagner G, Koch K, Schachtzabel C, Schultz CC, Sauer H, Schlosser RG. Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? Neuroimage (2011) 54:1607–14. doi: 10.1016/j.jpsychires.2012.07.013
56. Dominguez-Baleon C, Gutierrez-Mondragon LF, Campos-Gonzalez AI, Renteria ME. Neuroimaging studies of suicidal behavior and non-suicidal self-injury in psychiatric patients: a systematic review. Front Psychiatry (2018) 9:500. doi: 10.1016/j.jad.2018.11.095
Keywords: major depressive disorder, suicide attempts, gray matter volume, functional connectivity, amygdala, prefrontal cortex
Citation: Wang L, Zhao Y, Edmiston EK, Womer FY, Zhang R, Zhao P, Jiang X, Wu F, Kong L, Zhou Y, Tang Y and Wei S (2020) Structural and Functional Abnormities of Amygdala and Prefrontal Cortex in Major Depressive Disorder With Suicide Attempts. Front. Psychiatry 10:923. doi: 10.3389/fpsyt.2019.00923
Received: 06 August 2019; Accepted: 20 November 2019;
Published: 08 January 2020.
Edited by:
Feng Liu, Tianjin Medical University General Hospital, ChinaReviewed by:
Liang Gong, Chengdu Second People's Hospital, ChinaChengxiang Qiu, University of Washington, United States
Copyright © 2020 Wang, Zhao, Edmiston, Womer, Zhang, Zhao, Jiang, Wu, Kong, Zhou, Tang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanqing Tang, yanqingtang@163.com; Shengnan Wei, weisn_1982@126.com
 Lifei Wang1,2,3
Lifei Wang1,2,3 Elliot K. Edmiston
Elliot K. Edmiston Shengnan Wei
Shengnan Wei