- 1Department Basic and Applied Neurobiology, V. Serbsky Federal Medical Research Centre of Psychiatry and Narcology, Moscow, Russia
- 2N.A. Alekseev Psychiatric Clinical Hospital № 1, Moscow, Russia
- 3Department of Medical Nanobiotechnology, Pirogov Russian National Research Medical University, Moscow, Russia
We investigated the associations of rs4680 COMT, rs6280 DRD3, and rs7322347 5HT2A with youth-onset schizophrenia in the Russian population in a case–control study, and the role of the genotype in the severity of clinical features. The association between rs7322347 and schizophrenia (p = 0.0001) is described for the first time. Furthermore, we found a link with rs6280 and rs4680 in females (p = 0.001 and p = 0.02 respectively) and with rs7322347 in males (p = 0.002). Clinical symptoms were assessed on three scales: the Clinician-Rated Dimensions of Psychosis Symptom Severity scale, Positive and Negative Syndrome Scale, and Frontal Assessment Battery. Gender differences in clinical features are of particular interest. In our study we found gender differences in the severity of clinical features—higher scores for delusions (Positive and Negative Syndrome Scale and Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition) in males and higher scores for depression, delusions, somatic concern, motor retardation, poor attention were found in females.
Introduction
Schizophrenia, as a chronic progressive disease, is a socially significant problem, primarily due to the fact that the unemployment rate among people diagnosed with schizophrenia reaches 80–90% (1). Most often, schizophrenia affects young people of working age. According to statistics, the prevalence of the disease ranges from 0.8–1% of the population (2). In 40% of cases, a relapse of psychotic symptoms is observed within a year after the initial hospitalization. In 40–80% of patients, the course of the disease becomes chronic (3). The life expectancy of schizophrenic patients is on average 15–20 years shorter than in the general population (4). A genome-wide association study has established independent associations between schizophrenia and single-nucleotide polymorphisms (SNPs) in 145 loci called “schizophrenia risk loci” (5). However, none of them can be considered as an absolute predictor of schizophrenia.
In addition, with respect to the majority of genes associated with schizophrenia, one cannot confidently say that a particular gene is responsible for a specific phenotypic trait. Candidate genes may occur in only 2–5% of patients, and the odds ratio rarely reaches 1:20. There is also no evidence of a stable association of genomic variations with forms of schizophrenia (6). Data on the association of certain genetic polymorphisms with particular manifestations of schizophrenia are scarce. Examples are links: between polymorphism in solute carrier family 6 member 4 (SLC6A4) gene with defect in facial emotion recognition in schizophrenia (7), between polymorphism in 2’3’-cyclonucleotide 3’-phosphodiesterase (CNP) gene with psychopathological symptoms including catatonia, depression, anxiety, and autism manifestations (8) and polymorphism in a brain-derived neurotrophic factor (BDNF) gene with symptoms of insertion and withdrawal of thoughts, verbal hallucinations, and delusions (9).
Selection of a homogeneous cohort of patients in studies of genetic associations with phenotypic manifestations is a key factor in this type of research. The age of onset has been considered the important characteristic of schizophrenia that may yield clues to its origin, and there is strong evidence for an underlying genetic contribution to the age of onset in schizophrenia (10). Comparatively there are not enough studies to understand clearly how genetic risk factors influence the development of youth-onset schizophrenia, especially gender differences in expression of the disease.
Epidemiological studies exploring geographical variation in the incidence of psychotic disorders have highlighted various characteristics of environments that are typically associated with an increased risk, including high population density, urbanicity, income deprivation, income inequality and low social cohesion.
The empirical findings on the association between income inequality and mental health are not clear cut. The extant literature in the field is characterised by small effect sizes and a high level of heterogeneity in findings (11). Socioeconomic status alone does not increase the likelihood of youth-onset schizophrenia but it somehow magnifies the deleterious effect of early onset schizophrenia on negative symptoms (12).
In our opinion genetic factors play a larger role in the development of schizophrenia with youth-onset. We consider that neuropathology, neurodegenerative and environmental factors are more important than genetic factors for the development of schizophrenia in later-onset groups of patients (13–15). Stress produces altered neural processing and durability of gray-matter changes (16, 17). According to the literature, age at the onset of schizophrenia is defined as the age when positive symptoms first become apparent (18). Youth-onset schizophrenia presents most commonly during early adulthood (late teens to age 26) (19). Middle-onset (between 26 and 40 years) and late-onset schizophrenia (after 40 years of age) involve a different interplay of risk factors compared with the youth-onset form of the disease (18). The association of some SNPs with age of onset of the disease has been shown, as well as the association with symptoms resistant to therapy (20, 21). Most studies find that the age of onset for women is several years later than for men (22).
There are insufficient genetic studies of youth-onset schizophrenia in ethnically and socio-economic homogenous samples. We decide to use in our study a homogenous groups of men and women with positive symptoms of schizophrenia that first become apparent in the age period 18 to 26.
Single-Nucleotide Polymorphisms Selection
Catechol-O-methyltransferase (COMT) is an enzyme that is involved in the metabolism of catecholamines (epinephrine, norepinephrine, and dopamine). The COMT gene is located in the chromosomal region 22q11.1–q11.2. It contains a single-nucleotide substitution of G472A in exon 4, which results in the replacement of the amino acid valine with methionine (Val158Met polymorphism). This replacement (Met allele) leads to a decrease in enzyme activity (23) that can influence the catecholamines turnover in the prefrontal cortex. COMT Val158Met is widely regarded as potentially important for understanding the genetic etiology of many different psychiatric disorders, and the results of numerous studies suggest pleiotropy and the importance of examining subgroups defined by variables such as age of onset, sex, and ethnicity (24).
The dopamine receptor D3 (DRD3) is a D2-like receptor and is expressed in the ventral striatum. The DRD3 gene is located on chromosome 3 (3q13.31) and has 12 exons and five introns. The rs6280 polymorphic locus is a C/T single-nucleotide substitution that leads to the replacement of serine with a glycine residue at position 113890815 of the N-terminal domain of the extracellular receptor (Ser9Gly) (25). The DRD3 gene is considered a candidate gene for schizophrenia: in patients with this disease, an increase in the density of DRD3 in the striatum region has been found, together with a relative accumulation of “truncated forms” of receptor protein formed during abnormal splicing. DRD3 receptors are involved in the mechanisms of affective reactions and cognitive processes (26).
The 5-hydroxytryptamine 2A receptors (5HT2A) are class A heptahelical receptors activated by serotonin (5-hydroxytryptamine, 5-HT) that signal, at least in part, by activating heterotrimeric G proteins of the Gαq-11 subtype (27). The 5-HT2A receptors are expressed at high levels on pyramidal cells of the prefrontal cortex, where they are ideally positioned to modulate both cognitive functions, such as working memory or executive control, and also emotions, through dynamic interactions with the amygdala (28). Serotonin receptors are also distributed along the midbrain periaqueductal gray (PAG) and the hypothalamus (29). The 5-HT2A receptors, especially those located in the prefrontal cortex, play an important role in the pathophysiology and therapeutics of neuropsychiatric disorders, including schizophrenia and depression (30–33).
During the last decade, several groups have investigated SNPs of the HTR2A gene in connection with psychiatric and personality disorders (34–39). Most of the observations have been made with regard to the number of variations located in the promoter or the coding region; however, literature data also indicate that intronic variant rs7322347 might well be of interest with regard to behavioural aspects, as it has shown marked association with the combined subtype of childhood attention-deficit hyperactivity disorder and with suicide attempts in females subjected to physical assault at a younger age (40, 41). In a recent study, a robust contribution of the rs7322347 variation within the gene encoding serotonin receptor 2A to aggressive traits, was demonstrated (42).
We decided to investigate the association of rs4680 COMT, rs6280 DRD3, and rs7322347 5HT2A with youth-onset schizophrenia in a homogenous Russian population, because of their prominence in the literature. Associations between schizophrenia and SNPs in COMT and DRD3 genes are still controversial, there are studies both confirming and refuting these relationships (43, 46). SNP in 5HT2A gene associated with aggression and suicidal behavior (42, 41), so we have a hypothesis about the link between this SNP and schizophrenia.
Due to genetic heterogeneity between different populations, it is necessary to conduct separate studies for each one and results obtained in one population may not apply elsewhere.
The aim of our study is to find which of these SNPs is most strongly associated with our sample of youth onset schizophrenia patients. In the future, we would like to find a panel of objective markers that would help doctors predict the type and character of pathology and make decisions accordingly.
Materials and Methods
Participants
The protocol of this study was approved by the Interdisciplinary Ethics Committee, Moscow (22/07/2017).
A total of 462 (186 male, 276 female, mean age 32.6 ± 11.6) healthy volunteer controls and 212 (100 male, 112 female, mean age 29 ± 8.4) patients who gave their consent to participating and met the inclusion criteria of youth-onset schizophrenia (from 18 to 26 years old), participated in the study. All participants (patients and control) were Slavs from the central part of Russia. This was clarified using questions about the nationality of the participants, their parents and grandparents. Patients were recruited from N. A. Alekseev Psychiatric Hospital No. 1, Moscow, Russia. Inclusion criteria were inpatients, diagnosed with schizophrenia. Next, we selected the patients with the onset of prodromal events prior to age 26 years old and after age 18. After inclusion, all patients underwent complete diagnostic evaluation. Exclusion criteria were intellectual disabilities, substance abuse and dependence and any comorbid severe somatic or neurological disorder. Patients were diagnosed by at least two psychiatrists according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition, DSM-V). (47, 48). Patients were evaluated using a structured interview for the Positive and Negative Syndrome Scale (PANSS), including the PANSS positive, PANSS negative, and PANSS general psychopathology subscales (49). The Russian version of PANSS, with acceptable validity and reliability, was used to evaluate the severity of psychosis symptoms of schizophrenia patients in this study (50). The mean sum of PANSS scores was 104.15 ± 30.67. Cognitive conditions were examined using the Frontal Assessment Battery (FAB) (51).
Healthy controls matched with the patients in sex, age, and birthplace at the same time were randomly recruited from the volunteers who came to give a blood donation (Blood Center named after O.K. Gavrilov—a medical institution specializing in transfusion of blood and its components). Subjects were excluded due to any of the following: positive family histories (first-degree relatives) of psychiatric illness; substance abuse; abnormal birth; febrile convulsions; juvenile adoption or residence with a single-parent family; pregnancy or lactation, in addition, the socio-economic status of the participants was approximately the same, all participants were either university student or had completed university and came from the middle income families.
Genotyping
Genomic DNA samples were obtained from peripheral blood lymphocytes using an automatic DNA extraction (QIAGEN QIAcube) system, according to the manufacturer’s recommendations. DNA concentration and sample quality were assessed spectrophotometrically (NanoVue, GE Healthcare). The obtained DNA samples were normalized in TE buffer to a final concentration of 4 ng/µl in the 384-well plate format. All SNPs were typed using predesigned TaqMan SNP genotyping assays (Applied Biosystems, Thermo Fisher, USA). PCR and allelic discrimination assays were run using a QuantStudio 5 Real-Time PCR system (Applied Biosystems, Thermo Fisher, USA).
Statistical Analysis
Statistical assessment of the reliability of differences in the frequency distribution of polymorphic alleles and genotypes between a group of patients with schizophrenia and a group of healthy individuals, and the identification of genotype associations with individual symptoms was performed using the standard χ2 method. The strength of associations was evaluated in terms of the odds ratio and its 95% confidence interval (95% CI).
We assessed the associations between the SNP genotype and scales performances using one-way ANOVA in Statistica 7.0. Bonferroni corrections were applied to correct the p-values of alleles (corrected α = 0.05/3 = 0.017), to control inflation of the type I error rate.
All of the scales (PANSS, Clinician-Rated Dimensions of Psychosis Symptom Severity, and FAB) were two-tailed, with statistical significances of p < 0.05 using a post hoc Fisher analysis.
Results
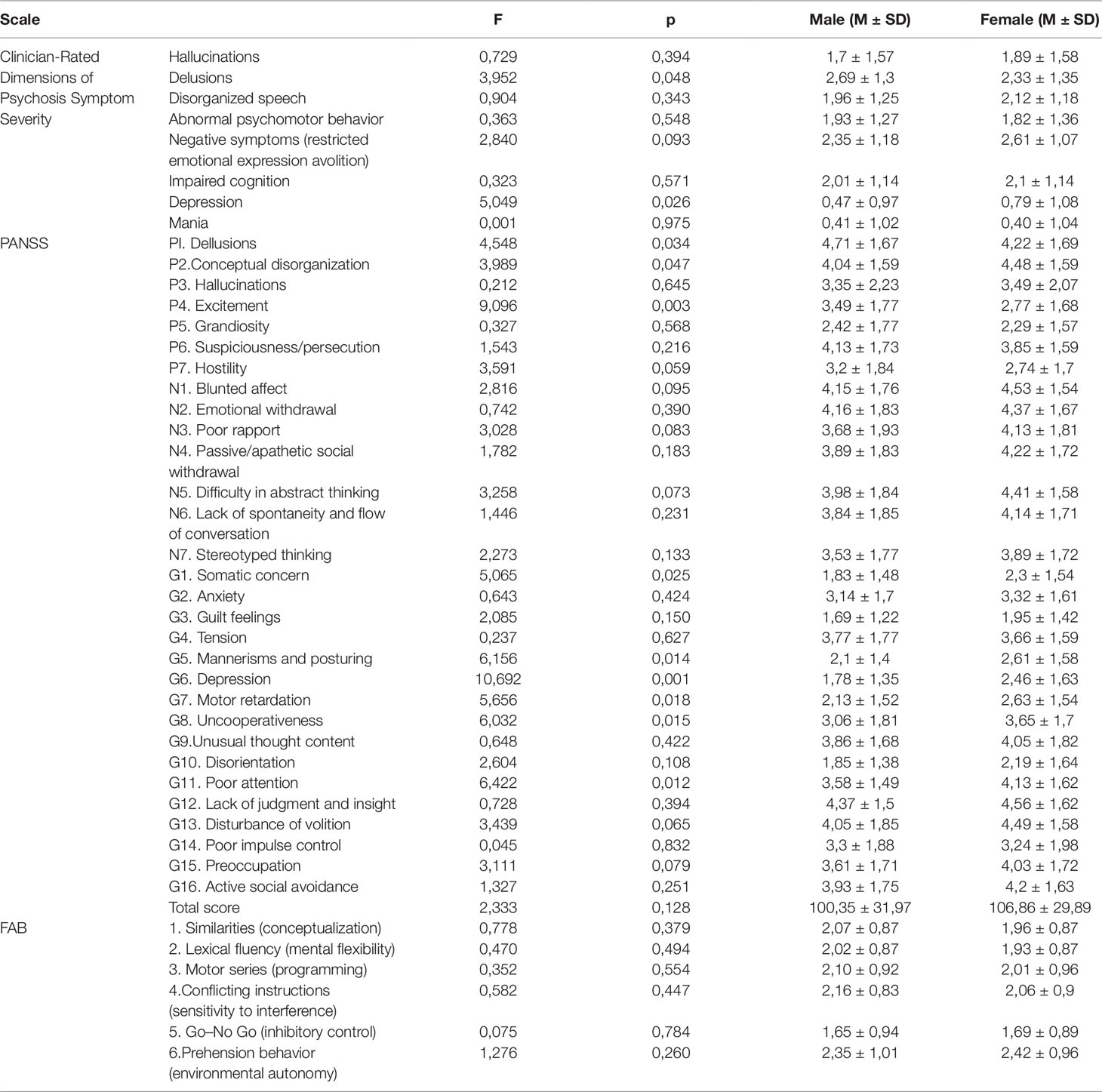
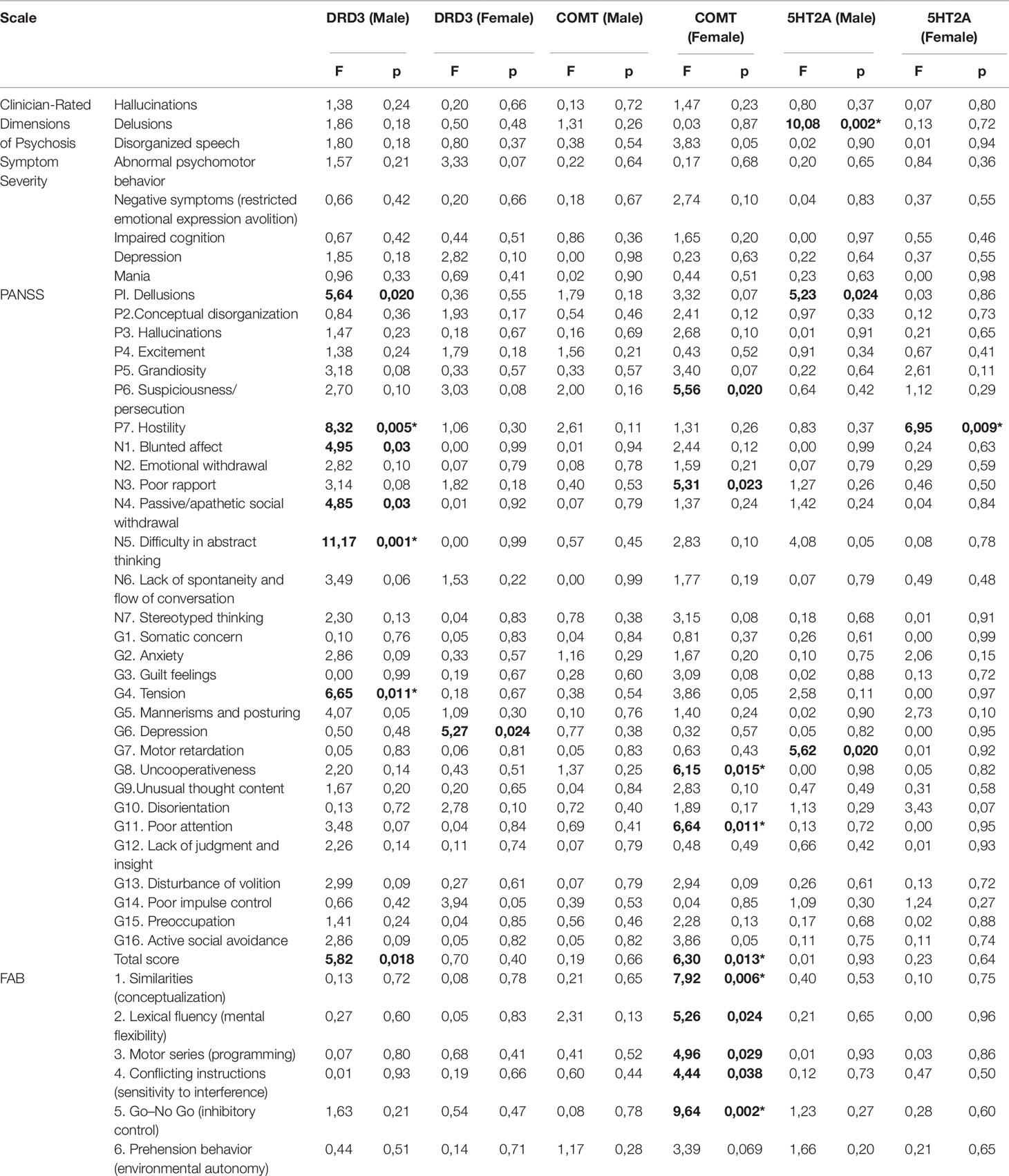
There were no significant differences between case and control groups in terms of gender, age, and years of education (p > 0.05). Genotype frequencies in all groups followed the Hardy–Weinberg equilibrium. Allele and genotype frequencies in the groups, as well as p-values of HWE, are shown in Table 1. Analysis revealed significant gender-specific associations between studied polymorphisms and youth-onset schizophrenia. Gender stratification showed that association with schizophrenia risk was significant for rs6280 and rs4680 in female subjects and for rs7322347 in male subjects (Table 2). That’s mean We found significant differences in clinical features between males and females on the Clinician-Rated Dimensions of Psychosis Symptom Severity and PANSS scales. The gender differences in the clinical characteristics of the patients are presented in Table 3. Analysis of the association between genotype and schizophrenia symptoms has been provided for male and female groups separately. The clinical characteristics of the patients across genotypes are presented in Table 4.
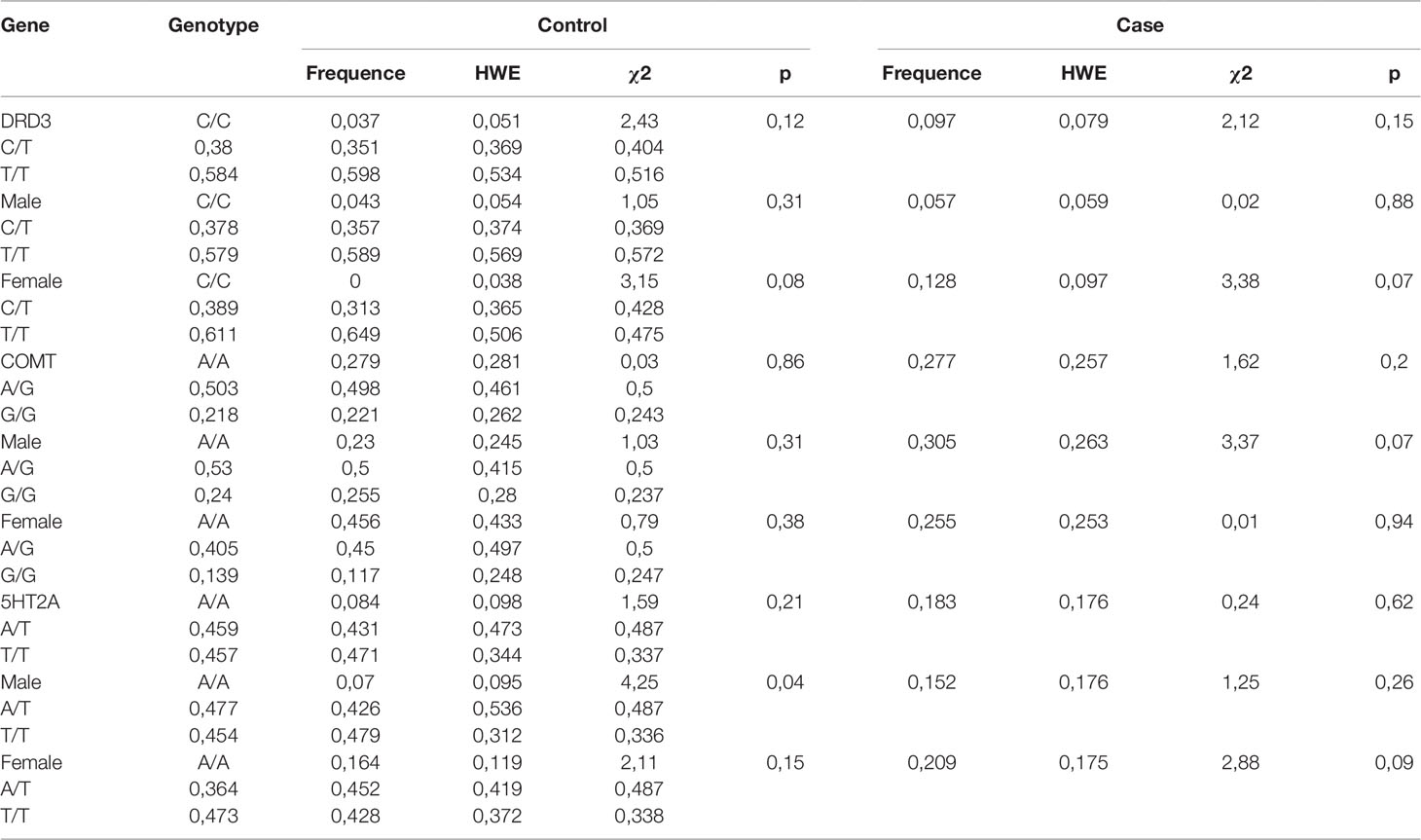
Table 1 Genotype frequencies of rs6280 (DRD3), rs4680 (COMT), and rs7322347 (5HT2A) in the control and youth-onset schizophrenia groups.
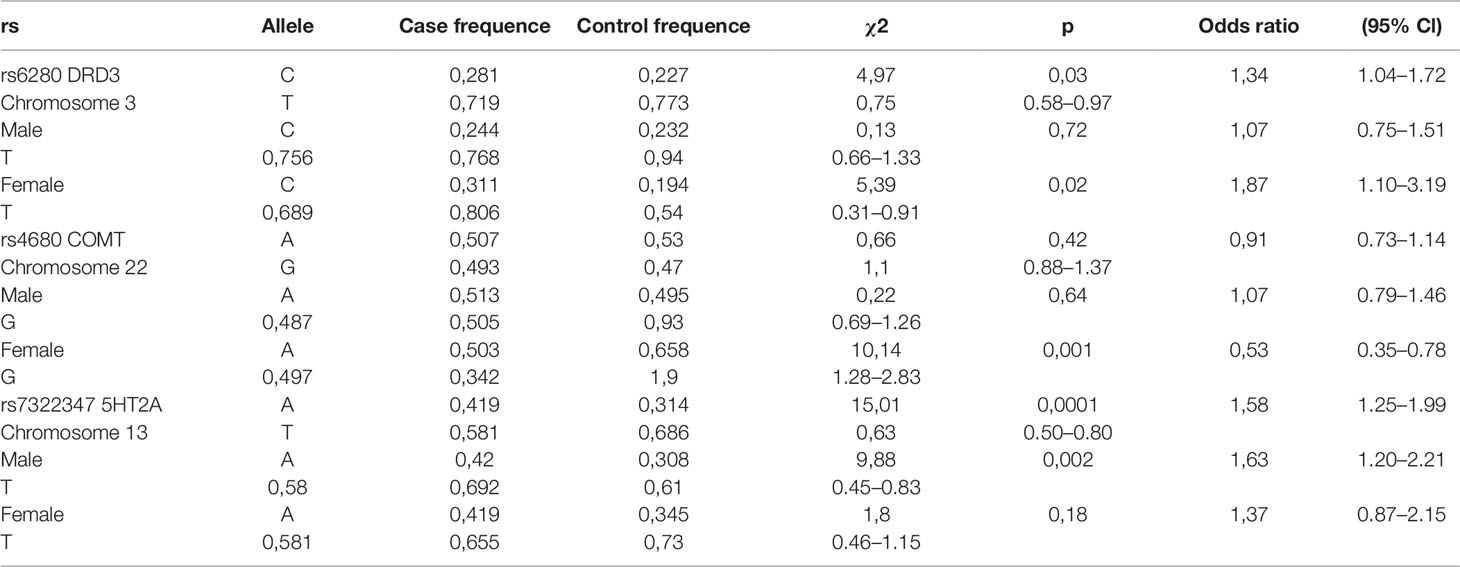
Table 2 Distribution of DRD3, COMT, and 5HT2A polymorphisms in youth-onset schizophrenia and control groups.
Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition; Clinician-Rated Dimensions of Psychosis Symptom Severity) for Rs7322347 Genotypes in Youth-Onset Schizophrenia Patients
In male patients (Table 4) ANOVA revealed a significant effect (F = 10.09; p = 0.002) of the 5HT2A genotype on the delusions score. After Bonferroni correction, differences still remained in delusion scores: homozygotes for the minor allele A had significantly lower scores than men who were homozygotes or heterozygotes for the T allele (2.86 ± 1.21 and 1.71 ± 1.44).
PANSS for Rs7322347 Genotypes in Youth-Onset Schizophrenia Patients
In men, differences were found on the PANSS scale for P1 (F = 5.23; p = 0.024) and G7 (F = 5.62; p = 0.02). In females, differences were found in the symptom P1 “hostility” (F = 6.95; p = 0.01). The association remained statistically reliable after Bonferroni correction. The presence of the homozygous genotype for the minor A allele is statistically significantly associated with an increase in the number of points for hostility in P7 (2.5 ± 1.64 and 3.6 ± 1.79).
PANSS for Rs4680 Genotypes in Youth-Onset Schizophrenia Patients
Significant differences were found in the COMT rs4680 genotypes with regard to PANSS scores. Groups were identified which were homozygous for allele A with the allele G (homozygous A/G and heterozygous G/G). In men, no genotype associations were found with signs, according to the three scales studied. In women, differences were found on the PANSS scale for P6 (F = 5.64; p = 0.02), N5 (1.17 ± 1.03; 4.14 ± 1.79), G8 (F = 6.65; p = 0.014), G11 (F = 6.65; p = 0.014) and the overall PANSS score (F = 5.82; p = 0.018). It should be noted that for this polymorphism, associations of the genotypes with schizophrenia in case–control studies were also found in women only, but not in the total sample. Therefore, after correction for multiple comparisons, the differences remained significant in terms of G8, G11 and the overall PANSS score. Thus, the presence of the G allele in a homozygote or heterozygote increases the severity of the G8 traits (3.07 ± 1.8; 3.84 ± 1.63), G11 traits (3.51 ± 1.42; 4.32 ± 1.64) and the total PANSS score (97 ± 30.13; 109.88 ± 29.34).
PANSS for Rs6280 Genotypes in Youth-Onset Schizophrenia Patients
Groups were identified which were homozygotes in the minor allele C with the allele T (homozygote T/T and heterozygote C/T). In men, differences were found on the PANSS scale for P1 (F = 5.64; p = 0.02), P7 (F = 8.324; p = 0.005), N1 (F = 4.95; p = 0.03), N4 (F = 4.85; p = 0.03), N5 (F = 11.17; p = 0.001), G4 (F = 6.65; p = 0.014), and the total PANSS score (F = 5.82; p = 0.018). After Bonferroni correction, the differences remained significant for P7, N5, and G4. Thus, the presence of the T allele in a homozygote or heterozygote increases the severity of the signs of P7 (1.17 ± 0.4; 3.32 ± 1.82), N5 (1.17 ± 1.03; 4.14 ± 1.79), and G4 (2 ± 1.26; 3.88 ± 1.75). In women, differences were found in the symptoms of G6—”depression” (F = 5.27; p = 0.024), but these differences did not survive Bonferroni correction.
FAB for Rs4680 Genotypes in Youth-Onset Schizophrenia Patients
The genotype relationship was identified by five out of the six signs in the whole scale, i.e., all except the “prehension behavior (environmental autonomy)”, but after applying the Bonferroni correction, the differences remained significant only in sign 1—”similarities (conceptualization)” (F = 7.92; p = 0.006) and sign 5—”go–no go (inhibitory control)” (F = 9.64; p = 0.003). The G allele was associated with lower scores: sign 1—”similarities (conceptualization)” (2.44 ± 0.51; 1.83 ± 0.9) and sign 5—”go–no go (inhibitory control)” (2.22 ± 0.65; 1.56 ± 0.9). These features are common for women, but not for men.
Discussion
In this study we investigated the associations with youth-onset schizophrenia of rs4680 COMT and rs6280 DRD3, which had been previously described in literature, but never before in Russians, and explorer the hypothesis that rs7322347 5HT2A might also be associated with schizophrenia in this population.
The link between rs7322347 and schizophrenia is described for the first time. In addition, we found that association with disease risk was significant for rs6280 and rs4680 in females and for rs7322347 in males, in case–control study. Associations of the studied polymorphisms with individual symptoms were also found. It should be noted that we used a specific population group with youth-onset schizophrenia, and therefore the associations found here may differ from those found in other cohorts.
The effect of sex steroids on the dopamine and serotonin systems has been shown in the literature (52–54). Clinical and experimental data confirm the role of steroid–dopamine interactions in the pathophysiology of schizophrenia, depression, and Parkinson’s disease (55). One study showed that oestrogens have a psychoprotective action that appears to be mediated by central dopaminergic and serotoninergic mechanisms (56). Gender-dependent differences in the incidence and symptoms of schizophrenia have been shown (57–59). Oestrogens could play a beneficial role for brain dopamine activity (55).
In our study, we found gender differences in the severity of clinical features. Higher scores for delusions (PANSS and DSM-V) and excitement (P4) were found in males and higher scores for: depression (PANSS and DSM-V), P2, G1, G5, G7, G8, and G11 were found in females. We did not find gender differences in the FAB scale. Therefore, studies of the associations of SNP of dopamine and serotonin system genes with symptoms of schizophrenia should be carried out taking into account the patient’s gender and particularly considering the sex differences found in our study. The dopamine system involvement in the development of schizophrenia has long been known (60), although it is not sufficient to account for the pathogenesis of all schizophrenia symptoms. The enzyme that carries methionine (Met/Met) retains 25% of the activity of the wild-type Val/Val enzyme. The alleles are codominant, and the enzyme activity of Val/Met heterozygotes is midway between the activities of the homozygotes (43). In our study, Val158Met polymorphism showed associations only in women, but not in the total distribution including both men and women. According to Sazci et al., (61), this polymorphism was even more significantly distributed in women with schizophrenia (p = 0.044 compared to p = 0.005 in women). The COMT gene encodes the COMT enzyme, which degrades catecholamines, including dopamine, especially in the prefrontal cortex (62, 63). Furthermore, it modulates dopamine levels in the frontostriatal network and, in turn, affects executive function performance and cognitive functions (64). A functional SNP rs4680, which leads to the replacement of Val158Met, may cause individual differences in emotion processing and in performance of cognitive tasks (65). In our study, the impairment of functions associated with the frontal lobe is clearly seen in the association with SNP rs4680, for all parameters in FAB scale. The presence of at least one Val, worsened cognitive impairment. After applying Bonferroni’s correction, the differences remained significant for sign 1—”similarities (conceptualization)” and sign 5—”go–no go (inhibitory control)”. Poor attention (G11) also reflects cognitive impairment; the severity of this symptom depends on the presence of the allele G in the genotype. Emotional-motivational disturbances (G8 – “uncooperativeness” and N3 – “poor rapport”) (which did not survive Bonferroni’s correction in PANSS), were also more pronounced in women with the presence of Val, which reduces enzyme activity (63). Furthermore, a link between the genotype and the total PANSS score was observed. It should be noted that these associations were found only in women. The correlation between the COMT genotype and psychiatric health has sex differences (65). Compared to men, women have significantly more dopamine cells within the mesocortical pathway, a major dopaminergic pathway projecting to the prefrontal cortex (50 vs 30% respectively) (66).
The DRD3 gene is involved in the development of mental symptoms associated with the dopamine system. In our study, a weak association of SNP rs6280 with a diagnosis of schizophrenia (p = 0.03) was shown. In numerous studies, the link between rs6280 and schizophrenia (67, 68) and furthermore with youth-onset schizophrenia (69), has been described.
However, a meta-analysis conducted by Qi et al., (70) showed an absence of such correlations. In our study, the presence of the T allele (Ser/Gly and Gly/Gly) increased the severity of cognitive impairment (N5—”difficulty in abstract thinking” in PANSS) in men, by almost four times, compared to the Ser/Ser homozygotes. The DRD3 receptors is selectively localized in the limbic regions of the brain in rats, but is more widespread in humans, present in sensory, association, and motor areas, as well as limbic areas of basal ganglia. In our study, associations of rs6280 with hostility (P7 in PANSS) are also shown. The localization of DRD3 in the dopamine pathway also plays a role in the formation of emotional reactions. For example, the DRD3 receptors agonist cariprazine reduced hostility in patients with schizophrenia (71). In another study, the association between rs6280 and delusions was shown (72). The identified associations of rs6280 with G4—”tension” in PANSS in men, can be explained by the localization of the DRD3 in the nigrostriatal pathway. It should be noted that, in contrast to rs4680 COMT, the link between the genotype and the phenotype was detected only in men. The associations with G6 – “depression” become meaningless after correction for multiple comparisons.
Another polymorphism studied in our work is rs7322347. Until now, the available data have not shown associations with schizophrenia.
There is evidence of the possible role of rs6280 and rs7322347 interactions in the development of schizophrenia (73).
A significant link was shown with aggression in both men and women in a sample of 887 people (42). In our study, the minor allele of the 5HT2A gene (p = 0.0001) was significantly more common among patients with early schizophrenia in the total distribution, and in men (p = 0.002). The serotonergic system has a powerful role in the pathophysiology of schizophrenia. The 5HT2A receptors is the most important excitatory receptors, regulating serotonin transmission in the brain (74). In addition, the 5HT2A also interacts with dopamine systems and indirectly increases dopamine release in the prefrontal cortex (75). The association of 5HT2A SNP with schizophrenia may be associated with the fact that brexpiprazole, which is also an antagonist of this receptors, is effective in reducing schizophrenia relapses (76). Furthermore, antipsychotic drugs such as risperidone, olanzapine, and clozapine have high affinity for DRD2 and 5-HT2A receptors (77). Li et al., (75) showed dysregulation of COMT and 5-HTR2A gene expression in first-episode antipsychotic-naïve schizophrenia. Subsequent antipsychotic treatment has no significant effect on the mRNA expressions of COMT and 5-HTR2A. In our study, the case–control association of rs7322347 with schizophrenia was first demonstrated. Further identification of the link between the genotype and the symptoms of schizophrenia demonstrates differences in delusions in men and hostility (P7, PANSS) in women. Thus, the presence of a minor allele A significantly increases the scores for hostility but is associated with a decrease on the scale of delusions. Due to the fact that other researchers have not investigated the association of this SNP with schizophrenia, the interactions between the emotional–motivational spheres and rs7322347 have not yet been studied. Therefore, this requires further investigation.
Special features of this genetic study include the unique youth-onset schizophrenia group from the Russian population, which has not previously been investigated. The link between rs7322347 and schizophrenia was described for the first time. Gender differences in clinical features and the genetic association of rs4680 COMT, rs6280 DRD3, and rs7322347 5HT2A with symptoms, are of particular interest. Our assumption is that our findings hold true only for youth onset schizophrenia and that gene associations in middle and late onset schizophrenia may differ.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by Interdisciplinary Ethics Committee, Moscow (22/07/2017). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AM, KP, YZ, EZ, and OP prepared all experiments and AM and YZ wrote the manuscript. ZS: data processing. NZ, OK, AR: worked with patients. VC and GK: revised the manuscript.
Funding
This work was supported by grant No 17-29-02164 from Russian 911 Foundation for Basic Research (RFBR).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to all the participants for their valuable contributions to this study.
References
1. Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry (2014) 13(2):153–60. doi: 10.1002/wps.20128
2. Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr Bull (2018) 44(6):1195–203. doi: 10.1093/schbul/sby058
3. Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci (2001) 2(2):129–36. doi: 10.1038/35053579
4. Fleischhacker WW, Arango C, Arteel P, Barnes TR, Carpenter W, Duckworth K, et al. Schizophrenia–time to commit to policy change. Schizophr Bull (2014) 3:S165–94. doi: 10.1093/schbul/sbu006
5. Pardiñas AF, Holmans P, Pocklington AJ, Escott-Price V, Ripke S, Carrera N, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet (2018) 50(3):381–9. doi: 10.1038/s41588-018-0059-2. GERAD1 Consortium; CRESTAR Consortium; GERAD1 Consortium; CRESTAR Consortium.
6. Harrison PJ. Recent genetic findings in schizophrenia and their therapeutic relevance. J Psychopharmacol (2015) 29(2):85–96. doi: 10.1177/0269881114553647
7. Alfimova MV, Golimbet VE, Korovaitseva GI, Lezheiko TV, Abramova LI, Aksenova EV, et al. The effect of the serotonin transporter 5-HTTLPR polymorphism on the recognition of facial emotions in schizophrenia. Zh Nevrol Psikhiatr Im S Korsakova (2014) 114(1):42–8. doi: 10.1007/s11055-015-0119-3
8. Hagemeyer N, Goebbels S, Papiol S, Kästner A, Hofer S, Begemann M, et al. A myelin gene causative of a catatonia-depression syndrome upon aging. EMBO Mol Med (2012) 4(6):528–39. doi: 10.1002/emmm.201200230
9. Zhang XY, Chen DC, Tan YL, Tan SP, Luo X, Zuo L, et al. BDNF polymorphisms are associated with schizophrenia onset and positive symptoms. Schizophr Res (2015) 170(1):41–7. doi: 10.1016/j.schres.2015.11.009
10. Immonen J, Jääskeläinen E, Korpela H. Miettunen J Age at onset and the outcomes of schizophrenia: A systematic review and meta-analysis. Early Interv Psychiatry (2017) 11(6):453–60. doi: 10.1111/eip.12412
11. Tibber MS, Kirkbride JB, Mutsatsa S, Harrison I, Barnes TRE, Joyce EM, et al. Are socioenvironmental factors associated with psychotic symptoms in people with first-episode psychosis? A cross-sectional study of a West London clinical sample. BMJ Open (2019) 9(9):e030448. doi: 10.1136/bmjopen-2019-030448
12. Gallagher BJ, Jones BJ. Early-onset schizophrenia: Symptoms and social class of origin. Int J Soc Psychiatry (2017) 63(6):492–7. doi: 10.1177/0020764017719302
13. Castle DJ, Howard R. What do we know about the aetiology of late-onset schizophrenia?. Eur Psychiatry (1992) 7(3):99–108.
14. Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. J Psychiatr Res (1999) 33(6):513–21. doi: 10.1016/S0022-3956(99)00033-3
15. Aricioglu F, Ozkartal CS, Unal G, Dursun S, Cetin M, Müller N. Neuroinflammation in schizophrenia: a critical review and the future. Klinik Psikofarmakoloji Bulteni (2016) 26(4):429–37. doi: 10.5455/bcp.20161123044657
16. Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci (2012) 15(5):663–8. doi: 10.1038/nn.3083
17. Akdeniz C, Schäfer A, Streit F, Haller L, Wüst S, Kirsch P, et al. Sex-Dependent association of perigenual anterior cingulate cortex volume and migration background, an environmental risk factor for schizophrenia. Schizophr Bull (2017) 43(4):925–34. doi: 10.1093/schbul/sbw138
18. Chen L, Selvendra A, Stewart A, Castle D. Risk factors in early and late onset schizophrenia. Compr Psychiatry (2018) 80:155–62. doi: 10.1016/j.comppsych.2017.09.009
19. Yuana A, Yi Z, Suna J, Dua Y, Yub T, Zhanga C, et al. Effect of SOX10 gene polymorphism on early onset schizophrenia in Chinese Han population. Neurosci Lett (2012) 521:93–7. doi: 10.1016/j.neulet.2012.05.040
20. Numata S, Ueno S, Iga J, Yamauchi K, Hongwei S, Ohta K, et al. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism in schizophrenia is associated with age at onset and symptoms. Neurosci Lett (2006) 401(1-2):1–5. doi: 10.1016/j.neulet.2006.02.054
21. Addington AM, Gornick M, Duckworth J, Sporn A, Gogtay N, Bobb A, et al. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol Psychiatry (2005) 10:581–8. doi: 10.1038/sj.mp.4001599
22. Häfner H. From onset and prodromal stage to a life-long course of schizophrenia and its symptom dimensions: how sex, age, and other risk factors influence incidence and course of illness. Psychiatry J (2019) 2019:9804836. doi: 10.1155/2019/9804836 doi: 10.1155/2019/9804836
23. Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet (2004) 75:807–21. doi: 10.1086/425589
24. Taylor S. Association between COMT Val158Met and psychiatric disorders: A comprehensive meta-analysis. Am J Med Genet B Neuropsychiatr Genet (2018) 177(2):199–210. doi: 10.1002/ajmg.b.32556
25. Nunokawa A, Watanabe Y, Kaneko N, Sugai T, Yazaki S, Arinami T, et al. The dopamine D3 receptor (DRD3) gene and risk of schizophrenia: case-control studies and an updated meta-analysis. Schizophr Res (2010) 116(1):61–7. doi: 10.1016/j.schres.2009.10.016
26. Terzić M, Kastelic V, Dolžan T. Genetic polymorphisms in dopaminergic system and treatment-resistant schizophrenia. Psychiatria Danubina (2016) 28(2):127–31.
27. Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International union of pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev (1994) 46:157–203.
28. Weber ET, Andrade R. Htr2a Gene and 5-HT(2A) Receptor Expression in the cerebral cortex studied using genetically modified mice. Front Neurosci (2010) 4:36. doi: 10.3389/fnins.2010.00036
29. Siegel A, Bhatt S, Bhatt R, Zalcman SS. The neurobiological bases for development of pharmacological treatments of aggressive disorders. Curr Neuropharmacol (2007) 5:135–47. doi: 10.2174/157015907780866929
30. Dean B. The cortical serotonin2A receptor and the pathology of schizophrenia: a likely accomplice. J Neurochem (2003) 85:1–13. doi: 10.1046/j.1471-4159.2003.01693.x
31. Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Sci (2006) 313:536–40. doi: 10.1126/science.1123432
32. Gray JA, Roth BL. The pipeline and future of drug development in schizophrenia. Mol Psychiatry (2007) 12:904–22. doi: 10.1038/sj.mp.4002062
33. Serretti A, Drago A, De RD. HTR2A gene variants and psychiatric disorders: a review of current literature and selection of SNPs for future studies. Curr Med Chem (2007) 14:2053–69. doi: 10.2174/092986707781368450
34. Stoltenberg SF, Christ CC, Highland KB. Serotonin system gene polymorphisms are associated with impulsivity in a context dependent manner. Prog Neuropsychopharmacol Biol Psychiatry (2012) 39:182–91. doi: 10.1016/j.pnpbp.2012.06.012
35. Jakubczyk A, Wrzosek M, Lukaszkiewicz J, Sadowska-Mazuryk J, Matsumoto H. The CC genotype in HTR2A T102C polymorphism is associated with behavioral impulsivity in alcohol-dependent patients. J Psychiatr Res (2012) 46:44–9. doi: 10.1016/j.jpsychires.2011.09.001
36. Ni X, Bismil R, Chan K, Sicard T, Bulgin N. Serotonin 2A receptor gene is associated with personality traits, but not to disorder, in patients with borderline personality disorder. Neurosci Lett (2006) 408:214–9. doi: 10.1016/j.neulet.2006.09.002
37. Oades RD, Lasky-Su J, Christiansen H, Faraone SV, Sonuga-Barke EJ. The influence of serotonin- and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): Findings from a family-based association test (FBAT) analysis. Behav Brain Funct (2008) 4:48. doi: 10.1186/1744-9081-4-48
38. Wilkie MJ, Smith G, Day RK, Matthews K, Smith D. Polymorphisms in the SLC6A4 and HTR2A genes influence treatment outcome following antidepressant therapy. Pharmacogenomics J (2009) 9:61–70. doi: 10.1038/sj.tpj.6500491
39. Rubin DH, Althoff RR, Ehli EA, Davies GE, Rettew DC. Candidate gene associations with withdrawn behavior. J Child Psychol Psychiatry (2013) 54:1337–45. doi: 10.1111/jcpp.12108
40. Ribases M, Ramos-Quiroga JA, Hervas A, Bosch R, Bielsa A. Exploration of 19 serotoninergic candidate genes in adults and children with attention-deficit/hyperactivity disorder identifies association for 5HT2A, DDC and MAOB. Mol Psychiatry (2009) 14:71–85. doi: 10.1038/sj.mp.4002100
41. Ben-Efraim YJ, Wasserman D, Wasserman J, Sokolowski M. Family-based study of HTR2A in suicide attempts: observed gene, gene x environment and parent-of-origin associations. Mol Psychiatry (2013) 18:758–66. doi: 10.1038/mp.2012.86
42. Banlaki Z, Elek Z, Nanasi T, Szekely A, Nemoda Z, Sasvari-Szekely M, et al. Polymorphism in the Serotonin receptor 2a (HTR2A) gene as possible predisposal factor for aggressive traits. PloS One (2015) 10(2):e0117792. doi: 10.1371/journal.pone.0117792
43. Bombin I, Arango C, Mayoral M, Castro-Fornieles J, Gonzalez-Pinto A, Gonzalez-Gomez C, et al. DRD3, but not COMT or DRD2, genotype affects executive functions in healthy and first-episode psychosis adolescents. Am J Med Genet Part B (Neuropsychiatric Genetics) (2008) 147B:873–9. doi: 10.1002/ajmg.b.30710
44. Loch AA, de Bilt MT, Bio DS, Prado CM, de Sousa RT, Valiengo LL, et al. Epistasis between COMT Val158Met and DRD3 Ser9Gly polymorphisms and cognitive function in schizophrenia: genetic influence on dopamine transmission. Braz J Psychiatry (2015) 37(3):235–41. doi: 10.1590/1516-4446-2014-1553
45. Escamilla R, Camarena B, Saracco-Alvarez R, Fresán A, Hernández S, Aguilar-García A. Association study between COMT, DRD2, and DRD3 gene variants and antipsychotic treatment response in Mexican patients with schizophrenia. Neuropsychiatr Dis Treat (2018) 14:2981–7.
46. Benkovits J, Magyarosi S, Pulay AJ, Makkos Z, Egerhazi A, Balogh N, et al. [Investigation of CNTF, COMT, DDR1, DISC1, DRD2, DRD3, and DTNBP1 candidate genes in schizophrenia: results from the hungarian SCHIZOBANK Consortium]. Neuropsychopharmacol Hung (2016) 18(4):181–7.
47. Nam B, Bahk W-M, Lee S-Y, Lee K, Jon D-I, Lim E, et al. The clinical implication of clinician-rated dimensions of psychosis symptom severity (crdpss) for diagnosis by DSM-5. Schizophr Bull (2018) 44(Suppl 1):S367–8. doi: 10.1093/schbul/sby018.897
48. Regier DA, Kuhl EA, Kupfer DJ. The DSM-5: Classification and criteria changes. World Psychiatry (2013) 12(2):92–8. doi: 10.1002/wps.20050. official translation by Russian Psychiatric Association.
49. Opler GA, Yavorsky C, Daniel DG. positive and negative syndrome scale (PANSS) training challenges, solutions, and future directions. Innov Clin Neurosci (2017) 14(11-12):77–81.
50. Ivanova E, Khan A, Liharska L, Reznik A, Kuzmin S, Kushnir O, et al. Validation of the russian version of the positive and negative syndrome scale (PANSS-Ru) and normative data. Innov Clin Neurosci (2018) 15(9-10):32–48.
51. Dubois M, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurol (2000) 55:1621–6. doi: 10.1212/WNL.55.11.1621
52. Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci (1994) 5(1):27–41. doi: 10.1515/REVNEURO.1994.5.1.27
53. Purves-Tyson TD, Owens SJ, Double KL, Desai R, Handelsman DJ, Weickert CS. Testosterone induces molecular changes in dopamine signaling pathway molecules in the adolescent male rat nigrostriatal pathway. PloS One (2014) 9(3):e91151. doi: 10.1371/journal.pone.0091151
54. Botsakis K, Theodoritsi S, Grintzalis K, Angelatou F, Antonopoulos I, Georgiou CD, et al. 17β-Estradiol/N-acetylcysteine interaction enhances the neuroprotective effect on dopaminergic neurons in the weaver model of dopamine deficiency. Neuroscience (2016) 320:221–9. doi: 10.1016/j.neuroscience.2016.01.068
55. Sánchez MG, Bourque M, Morissette M, Di Paolo T. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther (2010) 16(3):e43–71. doi: 10.1111/j.1755-5949.2010.00163.x
56. Fink G. The psychoprotective action of strogen is mediated by central serotonergic as well dopaminergic mechanisms. In: Takada Curzon G editors. Serotonin in the central nervous system and periphery. Elsevier Science BV (1995). p. 175–87.
57. Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinol (2003) 28(Suppl 2):17–54. doi: 10.1016/S0306-4530(02)00125-7
58. Salokangas RK. Gender and the use of neuroleptics in schizophrenia. Schizophr Res (2004) 66:41–9. doi: 10.1016/S0920-9964(02)00530-3
59. Riecher-Rössler A, Hafner H. Gender aspects in schizophrenia: bridging the border between social and biological psychiatry. Acta Psychiatr Scand (2004) 407(Suppl 102):58–62. doi: 10.1034/j.1600-0447.2000.00011.x
60. Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol (2015) 29(2):97–115. doi: 10.1177/0269881114563634
61. Sazci A, Ergul E, Kucukali I, Kilic G, Kaya G, Kara I. Catechol-O-methyltransferase gene Val108/158Met polymorphism, and susceptibility to schizophrenia: association is more significant in women. Brain Res Mol Brain Res (2004) 132(1):51–6. doi: 10.1016/j.molbrainres.2004.09.005
62. Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-O-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci (2004) 24:5331–5. doi: 10.1523/JNEUROSCI.1124-04.2004
63. Huertas I, Jesús S, García-Gómez FJ, Lojo JA, Bernal-Bernal I, Bonilla-Toribio M, et al. Genetic factors influencing frontostriatal dysfunction and the development of dementia in Parkinson's disease. PloS One (2017) 12(4):e0175560. doi: 10.1371/journal.pone.0175560
64. Nombela C, Rowe JB, Winder-Rhodes SE, Hampshire A, Owen AM, Breen DP, et al. Genetic impact on cognition and brain function in newly diagnosed Parkinson's disease: ICICLE-PD study. Brain (2014) 137(Pt 10):2743–58. doi: 10.1093/brain/awu201. study group.
65. Hill LD, Lorenzetti MS, Lyle SM, Fins AI, Tartar A, Tartar JL. Catechol-O-methyltransferase Val158Met polymorphism associates with affect and cortisol levels in women. Brain Behav (2018) 8(2):e00883. doi: 10.1002/brb3.883
66. Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci (2008) 28(38):9525–35. doi: 10.1523/JNEUROSCI.2637-08.2008
67. Crocq MA, Mant R, Asherson P, Williams J, Hode Y, Mayerova A, et al. Association between schizophrenia and homozygosity at the dopamine D3 receptor gene, J. Med. Genet (1992) 29:858–60. doi: 10.1136/jmg.29.12.858
68. Ishiguro H, Okuyama Y, Toru M, Arinami T. Mutation and association analysis of the 5 region of the dopamine D3 receptor gene in schizophrenia patients: identification of the Ala38Thr polymorphism and suggested association between DRD3 haplotypes and schizophrenia, Mol. Psychiatry (2003) 5:433–8. doi: 10.1038/sj.mp.4000731
69. Iwata Y, Matsumoto H, Minabe Y, Osada N, Nakamura K, Sekizawa T, et al. Early-onset schizophrenia and dopaminerelated gene polymorphism. Am J Med Genet B-Neuropsychiatr Genet (2003) 116B:23–6. doi: 10.1002/ajmg.b.10759
70. Qi XL, Xuan JF, Xing JX, Wang BJ, Yao J. No association between dopamine D3 receptor gene Ser9Gly polymorphism (rs6280) and risk of schizophrenia: an updated meta-analysis. Neuropsychiatr Dis Treat (2017) 13:2855–65. doi: 10.2147/NDT.S152784
71. Citrome L, Durgam S, Lu K, Ferguson P, Laszlovszky I. The effect of cariprazine on hostility associated with schizophrenia: post hoc analyses from 3 randomized controlled trials. J Clin Psychiatry (2016) 77(1):109–15. doi: 10.4088/JCP.15m10192
72. Morimoto K, Miyatake R, Nakamura M, Watanabe T, Hirao T, Suwaki H. Delusional disorder: molecular genetic evidence for dopamine psychosis. Neuropsychopharmacol (2002) 26:794–801. doi: 10.1016/S0893-133X(01)00421-3
73. Dominguez E, Loza MI, Padin F, Gesteira A, Paz E, Paramo M, et al. Extensive linkage disequilibrium mapping at HTR2A and DRD3 for schizophrenia susceptibility genes in the Galician population. Schizophr Res (2007) 90:123–9. doi: 10.1016/j.schres.2006.09.022
74. Selvaraj S, Arnone D, Cappai A, Howes O. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev (2014) 45:233–45. doi: 10.1016/j.neubiorev.2014.06.005
75. Li Z, He Y, Han H, Zhou Y, Ma X, Wang D, et al. COMT, 5-HTR2A, and SLC6A4 mRNA Expressions in First-Episode Antipsychotic-Naïve Schizophrenia and Association With Treatment Outcomes. Front Psychiatry (2018) 9:577. doi: 10.3389/fpsyt.2018.00577
76. Ward K, Citrome L. Brexpiprazole for the maintenance treatment of adults with schizophrenia: an evidence-based review and place in therapy. Neuropsychiatr Dis Treat (2019) 15:247–57. doi: 10.2147/NDT.S169369
77. Meltzer HY. Serotonergic mechanisms as targets for existing and novel antipsychotics. Handb Exp Pharmacol (2012) 212:87–124. doi: 10.1007/978-3-642-25761-2_4
78. Domschke K, Freitag CM, Kuhlenbäumer G, Schirmacher A, Sand P, Nyhuis P, et al. Association of the functional V158M catechol-O-methyl-transferase polymorphism with panic disorder in women. Int J Neuropsychopharmacol (2004) 7(2):183–8. doi: 10.1017/S146114570400416X. https://doi.org/10.1017/S146114570400416X
Keywords: single-nucleotide polymorphism, schizophrenia, youth-onset, 5-hydroxytryptamine 2A receptors, dopamine receptor D3, catechol-O-methyltransferase
Citation: Morozova A, Zorkina Y, Pavlov K, Pavlova O, Storozheva Z, Zubkov E, Zakharova N, Karpenko O, Reznik A, Chekhonin V and Kostyuk G (2019) Association of rs4680 COMT, rs6280 DRD3, and rs7322347 5HT2A With Clinical Features of Youth-Onset Schizophrenia. Front. Psychiatry 10:830. doi: 10.3389/fpsyt.2019.00830
Received: 23 July 2019; Accepted: 21 October 2019;
Published: 12 November 2019.
Edited by:
Elizabeth A. Thomas, The Scripps Research Institute, United StatesReviewed by:
Barbara Jean Knowlton, University of California, Los Angeles, United StatesMary V. Seeman, University of Toronto, Canada
Copyright © 2019 Morozova, Zorkina, Pavlov, Pavlova, Storozheva, Zubkov, Zakharova, Karpenko, Reznik, Chekhonin and Kostyuk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Morozova, aGFrdXJhdGU3N0BnbWFpbC5jb20=
 Anna Morozova
Anna Morozova Yana Zorkina
Yana Zorkina Konstantin Pavlov
Konstantin Pavlov Olga Pavlova1
Olga Pavlova1 Olga Karpenko
Olga Karpenko