- 1Beijing Hui-Long-Guan Hospital, Peking University, Beijing, China
- 2Department of Psychiatry, The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 3Illawarra Health and Medical Research Institute and School of Medicine, University of Wollongong, Wollongong, NSW, Australia
Background: Evidence indicates that the serum concentration of uric acid (UA) in patients may relate both to the pathophysiology and therapeutics of bipolar disorder (BPD). The purpose of this study was to examine the changes and clinical significance of serum UA concentrations in first-episode manic patients suffering from BPD.
Methods: Seventy-six drug-naive patients with first-episode bipolar mania and 76 age- and gender-matched healthy subjects were recruited. Young Mania Rating Scale and Hamilton Depression Rating Scale were used to assess clinical symptoms. We tested serum UA concentrations by sandwich enzyme-linked immunosorbent assay at baseline and at the end of 8-week treatment in BPD patients and in the control group.
Results: After 8-week quetiapine and sodium valproate treatment, this study revealed that the serum UA concentrations in remitted patients were significantly lower than nonremitted patients; however, those remitted patients still had higher serum UA than healthy controls. We discovered that the baseline UA concentration was higher in nonremitted than remitted patients after 8 weeks of treatment. Finally, a positive association was found between serum UA and symptom relief in the first episode of manic disorder patients.
Conclusion: Patients with first-episode BPD had high levels of serum UA, which responds to treatment mainly in remitted patients. Our results suggest that serum UA concentrations might present potentially a trait marker in bipolar patients.
Introduction
Bipolar disorder (BPD) is a severe and chronic mental disorder with a lifetime prevalence of approximately 1% to 2% in the general population (1). The disease is associated with a potentially devastating impact on individual well-being including social, occupational, and general functioning (2–4). Drugs such as mood stabilizers, atypical antipsychotics, and nondrug therapy, including electroconvulsive therapy, transcranial magnetic stimulation, and psychotherapy, are shown to have limited efficacy; the outcomes in a large number of patients are unsatisfactory (5–7). Therefore, the therapeutics of BPD remains an important challenge in psychiatry. This may be due to the underlying neurobiology of the diseases being still largely unclear. Improved understanding of the pathophysiological basis of BPD is required for the development of more targeted therapeutics.
Uric acid (UA) and purine regulate a number of brain activities such as cognition, memory, and mood and highly exist in the limbic regions and basal ganglia of brain (8). These brain regions have been reported to have some abnormalities in BPD through neuroimaging studies (9, 10). Growing evidence from genetic and clinical studies suggested the purinergic system might be a contributing factor in the pathophysiology of BPD (8). The purinergic system includes signaling pathways of the neurotransmitter adenosine triphosphate and the neuromodulator adenosine. Agonists of adenosine are considered to have sedative, anticonvulsant, antiaggressive, and antipsychotic properties, whereas its antagonists, for example, caffeine, may cause irritability and disrupt the sleep–wake cycle, which is a common precipitant for manic relapse (11). In addition, adenosinergic dysfunction is found in euthymic patients with BPD, and there is a significant association between higher functioning impairment and lower concentrations of adenosine in blood (12).
UA is a purine metabolite produced by the xanthine oxidoreductase from xanthine or hypoxanthine. Peripheral and central UA concentrations are highly correlated (13). An increased serum UA concentration could be due to the result of amplified purinergic turnover or reduced adenosine transmission (14, 15). Over the past decades, several studies have shown that BPD patients in all phases (manic/hypomanic, mixed depressed, or euthymic) could have an increased serum UA concentration, as compared with healthy or other mental disorder subjects (16–20). These studies have indicated altered regulation of purinergic system in BPD (21). However, no significant differences in serum UA levels were also reported between euthymic BPD and healthy subjects (12, 20, 22, 23). Also, no significant differences were found in serum UA between the patients with manic episodes and euthymic status (20, 24, 25). Therefore, whether an elevated UA concentration might be a trait marker for BPD remains to be determined.
A positive correlation was reported between serum UA levels and symptom severity in mania patients (18, 19, 26). In line with their study, a combinational therapy of valproate together with allopurinol (hyperuricemia-lowering drug) results in significant improvements in patients suffering from acute mania (27, 28). In contrast, no improvement was also reported by using allopurinol in treating manic symptoms (29, 30). Also, similar UA levels between manic state and remitted state were reported (20, 24).
In consideration of controversial data from literature, we carried out a cohort study on first-episode and drug-naive manic patients and investigated whether (1) serum UA concentrations differ among manic episode, remission after treatment, and healthy subjects, matched for age and gender; (2) treatment with an atypical antipsychotic drug (quetiapine) and mood stabilizer (sodium valproate) affects the serum UA concentrations in manic patients; and (3) there is any relationship between serum UA concentration and the therapeutic efficacy.
Methods
Subjects
Seventy-six inpatients were recruited from inpatients suffering BPD. The study was conducted from January 2015 to December 2017 in Beijing Hui-Long-Guan Hospital, affiliated with Clinical Medical College of Peking University, China. The inclusion criteria were subjects between 18 and 45 years old, diagnosis of a manic/hypomanic episode by the consensus of two independent senior psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria (31), and first-episode and never-treated history, which was confirmed by collateral histories from family members. All subjects were Han Chinese. The patients with a history of affective disorder and other Axis I diagnoses such as schizophrenia and neurological illness were excluded from this study.
The control group had 76 healthy subjects and was recruited from the local community, matched with the patients for age and gender. Healthy subjects were 18 to 45 years of age without any record of personal or family history of psychiatric disorders and were Han Chinese. All participants were without any record of organic brain diseases, cardiovascular disease, chronic inflammatory disease, diabetes, nephropathy, and gout or other diseases associated with changes in serum UA concentrations, as determined by a complete medical history, physical examination, and laboratory tests. Neither bipolar patients nor control subjects were taking drugs or medications, none had alcohol abuse/dependence, and none were pregnant/breastfeeding, which could influence UA concentrations.
All subjects gave signed and informed consent that was approved by the institutional review board of the Beijing Hui-Long-Guan Hospital, Peking University.
All patients received the combination quetiapine (606.58 ± 150.85 mg, daily) plus sodium valproate (1,077.97 ± 338.77 mg, daily) treatment for 8 weeks, which was in agreement with the first-line treatment of mania in international evidence-based guidelines (32).
Assessment
Psychopathological symptoms of patients were assessed on the day of blood sampling by four psychiatrists who were blind to the clinical status and treatment conditions. Young Mania Rating Scale (YMRS) (33) was used to measure the severity of manic symptoms before and after treatment. YMRS and the 17-item version of the Hamilton Depression Rating Scale (HDRS) (34) were used to confirm the remission during the recovery period. The patients in remission were defined by both YMRS and HDRS scores being ≤7. To ensure consistency and reliability of ratings, before undertaking the study, four psychiatrists who worked over 5 years in clinical practice simultaneously received a training session on how to use YMRS and HDRS, ensuring a correlation coefficient maintained greater than 0.8 by repeated assessments across the study.
The blood samples from all subjects were taken between 6 and 7 after overnight fasting as baseline and were taken again after 8 weeks of treatment between 6 and 7. Serum UA concentrations were assessed using sandwich enzyme-linked immunosorbent assay, which was by a commercially available kit (DRG kit cat. no. EIA-2925, Germany). The parameters potentially affecting serum UA concentrations such as metabolic parameters (triglycerides, high-density lipoprotein cholesterol, and fasting blood glucose) and kidney function (creatinine, urea) were also measured. The height, body weight, and blood pressure of all subjects were measured. Body mass index (BMI) was calculated as body weight in kilograms divided by height in meters squared. Hyperuricemia was defined as serum level of UA >420 µmol/l (7.0 mg/dl) for men and >360 µmol/l (6.0 mg/dl) for women (17). Current smoking referred to smoking at least one cigarette per day over the last 1 month before enrollment.
Statistical Analysis
The differences of demographic data between patients and controls were analyzed by χ2 test or independent-samples t test, wherever it is appropriate. The paired t test was used to compare serum UA concentrations, YMRS scores, and HDRS scores between the baseline and posttreatment in the patient group. The paired t test was also used to compare serum UA levels in drug-naive manic patients and patients in remission after treatments. Pearson correlation coefficient was used to evaluate the relationship between serum UA levels and YMRS scores when data were in normal distribution. Independent-samples t test was applied to single-time-point data. When significant differences were detected, covariant analysis controlling for BMI, age, and sex and Bonferroni post hoc analyses for multiple comparisons were applied. Results are expressed as mean ± SD. All statistical tests were two-tailed, and p < 0.05 was considered as statistically significant. Statistical analyses were performed using SPSS (version 17.0).
Results
Demographic and clinical characteristics of the study population are shown in Table 1. Patients and controls were matched with respect to age and gender. There were no significant differences in demographic and clinical characteristics (Table 1). Sixty-eight cases were mania (39 men and 29 women), and the other eight cases were hypomania (five men and three women). No differences in the levels of serum UA between mania and hypomania patients (379.74 ± 99.25 vs 368.50 ± 103.70 µmol/l, t = 0.34, p = 0.74).
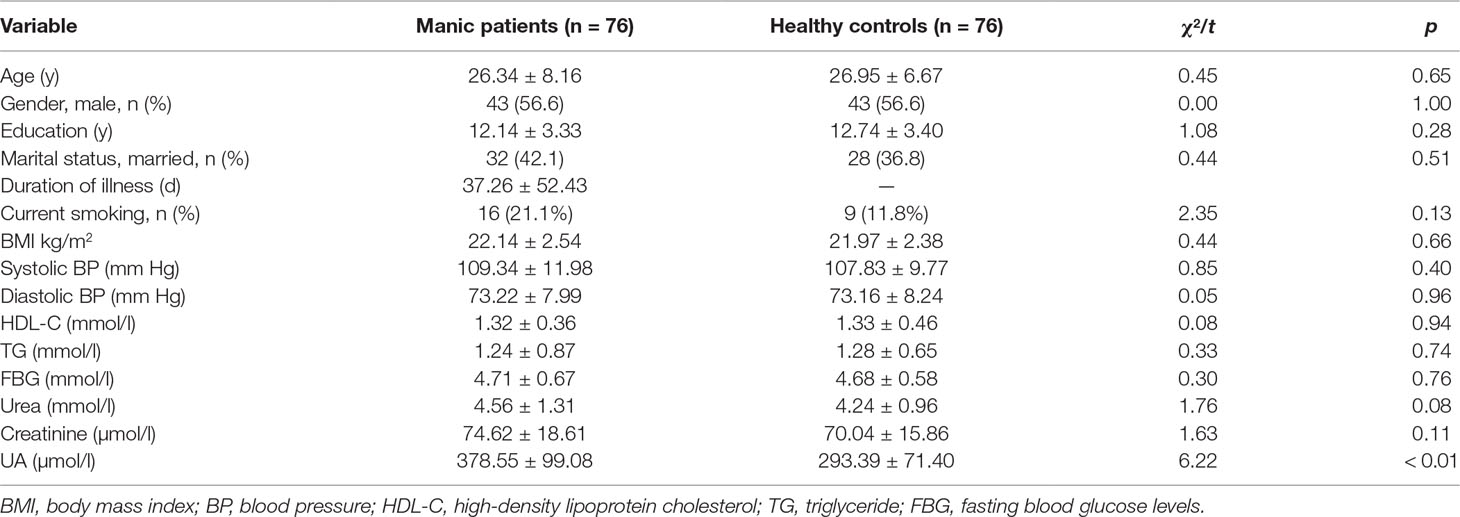
Table 1 Demographic and clinical characteristics of drug-naive patients with first-episode manic patients and healthy controls.
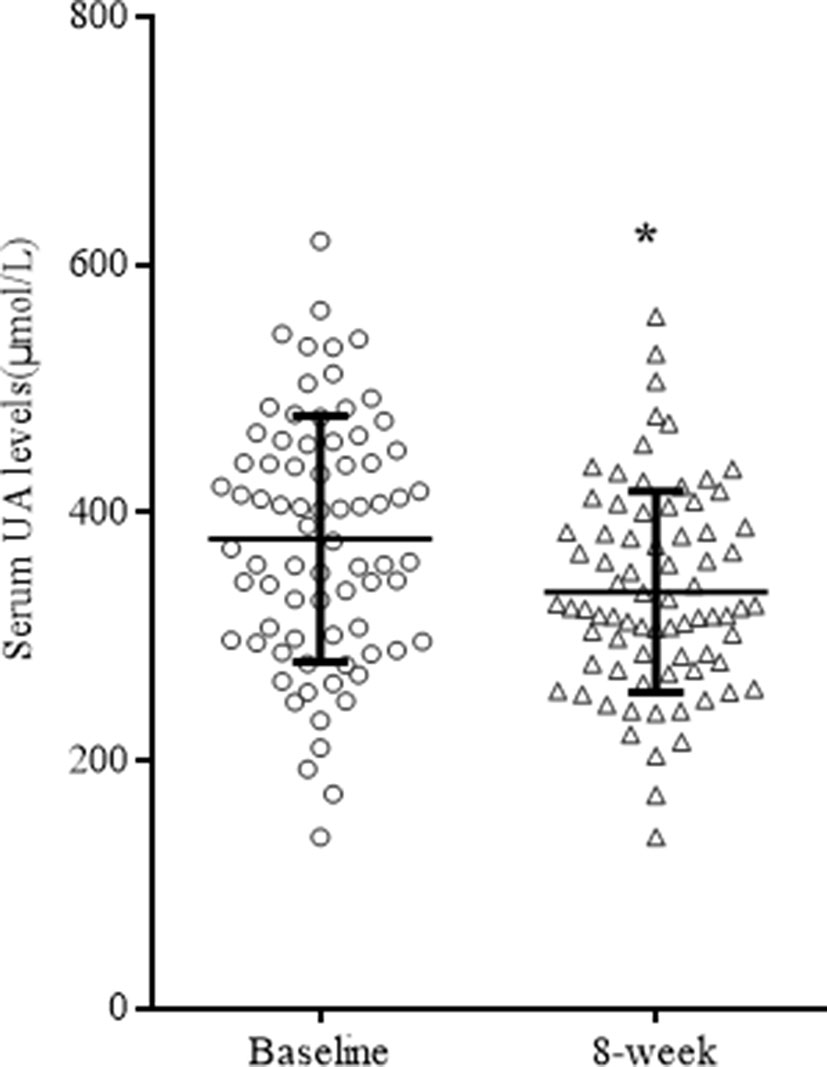
After 8 weeks of quetiapine and sodium valproate treatment, YMRS scores (t = 25.76 p < 0.01) and serum UA concentrations (t = 3.36, p = 0.01) were lower compared to baselines (Table 2, Figure 1). Fifty-three patients reached remission status according to the scores of YMRS and HDRS. We detected that the serum UA concentrations were significantly lower in the remission group compared to nonremission group both at baseline (361.89 ± 92.36 vs 416.96 ± 105.31 µmol/l, t = 2.29, p = 0.03) and after 8 weeks of treatment (323.57 ± 73.53 vs 364.57 ± 91.98 µmol/l, t = 2.07, p = 0.04) (Figure 2). These differences remained after controlling for age, gender, and BMI (all p < 0.05).
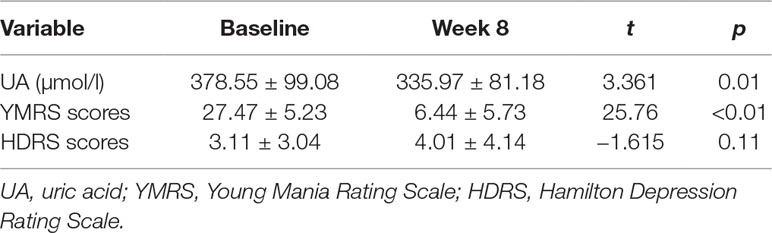
Table 2 Serum UA levels, YMRS scores, and HDRS scores among bipolar patients receiving treatment for 8 weeks.
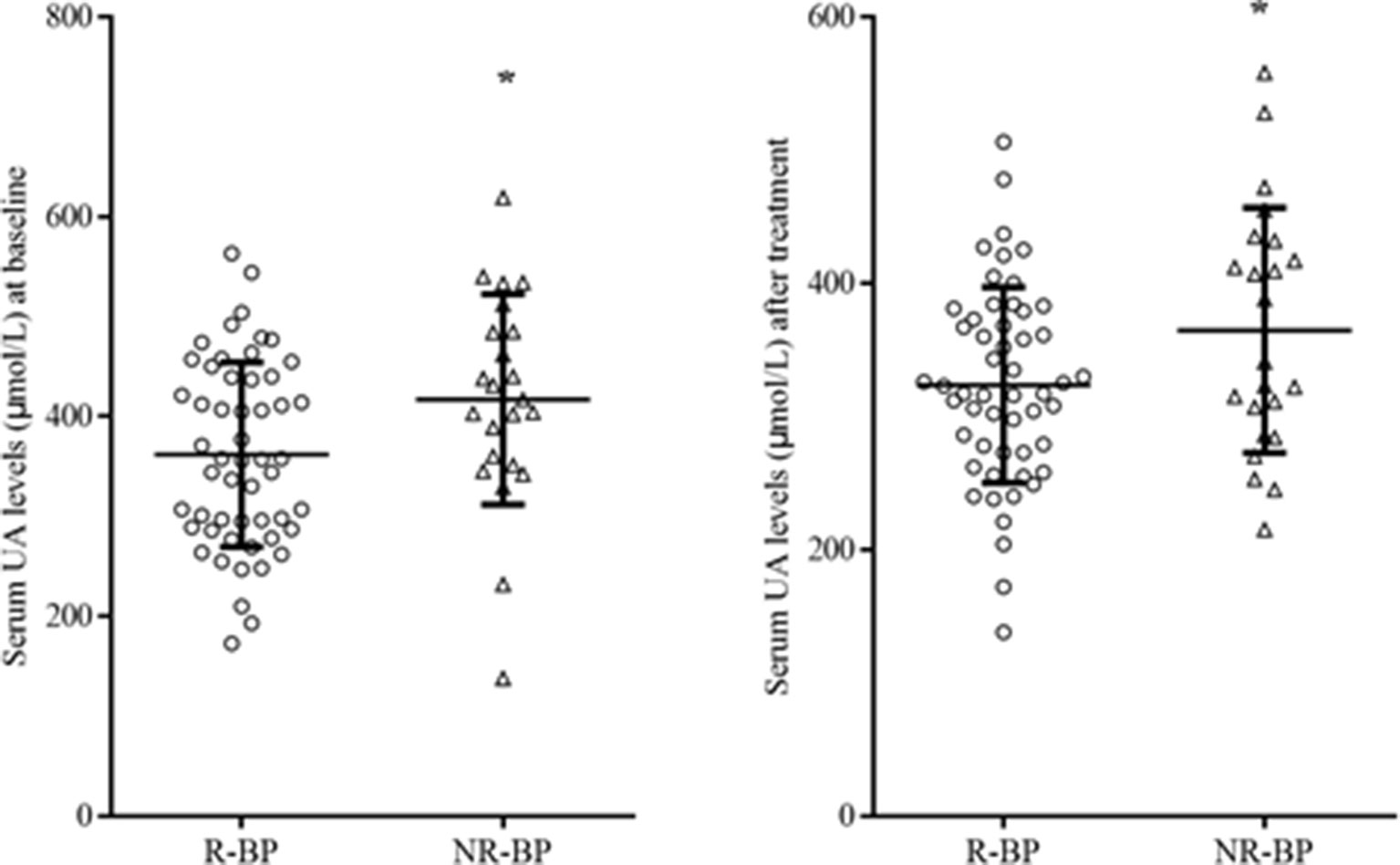
Figure 2 Serum UA levels R-BP (n = 53) and NR-BP (n = 23) at baseline (left panel) and after treatment (right panel). R-BP, remitted bipolar patients; NR-BP, non-remitted bipolar patients. *p < 0.05.
Pearson correlation analysis showed that serum UA concentrations were not correlated with severity of symptoms, which were assessed by YMRS at both baseline (r = 0.18, p = 0.12) and at the end of 8 weeks of treatment (r = 0.21, p = 0.07). However, there was a significant correlation between changes in serum UA concentrations and that of the change of the YMRS scores after treatments (pretreatment − posttreatment, r = 0.25, p = 0.03; Figure 3). The correlations remained significant after controlling for the baseline YMRS score and UA levels (r = 0.23, p = 0.047).
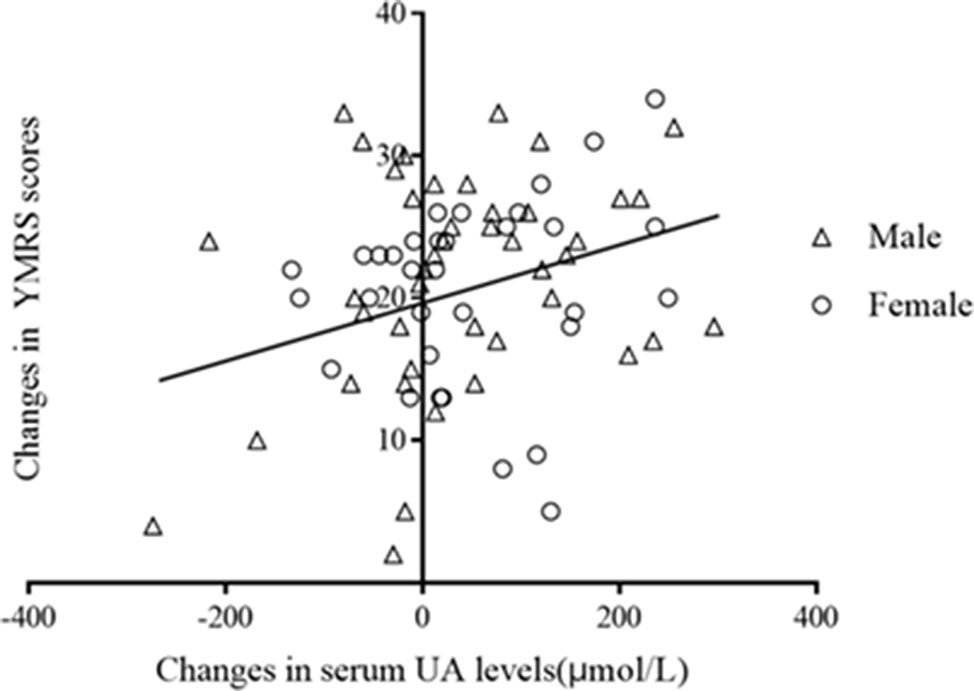
Figure 3 Correlation between changes in serum UA levels and that in YMRS scors after treatment (r = 0.25, p = 0.03).
The prevalence rate of hyperuricemia between the remitted patients and healthy controls did not differ (17.0% and 10.5%, χ2 = 1.14, p = 0.29). The prevalence rate of hyperuricemia in all patients at baseline was 40.9%, which was significantly higher than the remitted patients and healthy controls (χ2 = 18.25, p < 0.01; and χ2 = 8.27, p < 0.01, respectively). A paired-sample t test revealed that serum UA levels of the remitted patients were significantly higher than these patients in drug-naive phase (Tables 1 and 2, t = 2.71, p < 0.01). Independent-samples t test showed that serum UA levels in healthy controls were lower than first-episode drug-naive patients (Table 1, t = 6.22, p < 0.01) and the remitted patients after 8-week treatments (t = 2.49, p = 0.01; Figure 4). Covariance analysis showed that the serum UA differences between healthy controls and manic patients and remitted patients remained statistically significant after adjusting for gender, age, and BMI (all p < 0.01; Table 1 and Figure 4).
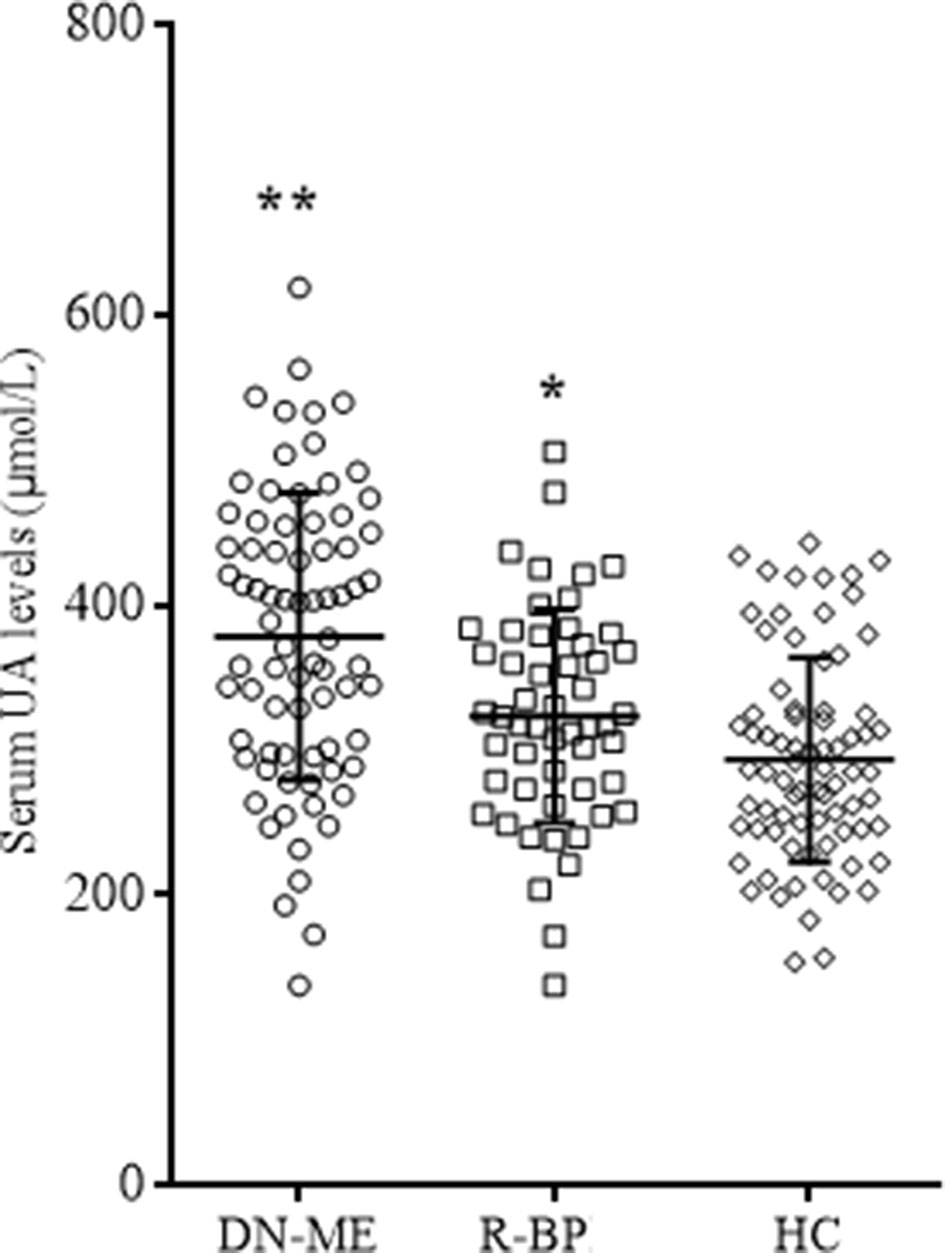
Figure 4 Serum UA levels in different groups. FE-MP, fist-episode manic patients (n = 76); R-BP, remitted bipolar disorder (n = 53); HC, healty controls (n = 76). *p < 0.05 comparing with HC; **p < 0.01 comparing with HC.
Discussion
This study analyzed the relationship of system levels of UA to measures of YMRS and HDRS in first-episode manic patients suffering from BPD prior and after 8-week quetiapine and sodium valproate treatment. Our results revealed that the serum UA levels in remitted patients were significantly lower than nonremitted patients, although they were still had higher than healthy controls. Furthermore, we showed that the baseline UA was significantly higher in nonremitted than remitted patients after 8 weeks of treatment. Also, a positive association was found between serum UA and symptom relief in these patients.
In the past few years, a number of studies have investigated the changes of UA in patients suffering BPDs; however, the data are inconsistent and even controversial (20, 22, 35, 36). This may be due to various confounders involved in studies because the nature of BPD is a complex, chronic, and heterogeneous psychiatric disease (37). Two studies have investigated serum UA levels in first-episode manic patients with the sample sizes of 20 based on the criteria of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition and 31 based on the criteria of International Classification of Diseases, 10th Revision (26, 38). Both studies have had relatively small sample size without taking into consideration of confounders such as the levels of creatinine, cholesterol, and glucose and BMI, which could have influenced UA levels (39, 40). We conducted a prospective study on a cohort of 68 first-episode manic patients controlling for potential confounders including metabolic and kidney function–related variables. From this study, we suggest that a possible contributing factor could be due to discrepancy of baseline levels of UA in the BPD patient cohort studied.
Overall, recent studies support that the patients suffering BPD at manic phase have significantly higher levels of serum UA during both acute and chronic phases compared with healthy controls (24, 28, 38, 41). Also, the serum UA levels were higher in mania phase than depression and remission phases in the same patients suffering from BPD (19, 25, 42). Consistent with these results, our study showed that first-episode drug-naive patients in manic phase had significantly higher serum UA than those of patients in remission and healthy controls. Therefore, we support that increased UA levels were associated with a state of manic episode, which may be used as a routine check in the manic phase of BPD (13).
Psychotropic and antipsychotic drugs could have different effects on serum UA levels. Lithium and carbamazepine reduce UA serum levels (43, 44). Zotepine reduces UA levels more than risperidone and haloperidol (45, 46). Conversely, it has been reported that olanzapine increases UA levels in moderate mania (47, 48). The present study observed that bipolar patients in mania phase treated with quetiapine and valproate decreased serum UA, which was in consistence with a report of a previous study (18). Previous study showed that adjunctive allopurinol reduces UA and increases remission rate in manic patients (49). Similarly, we found that decreased UA was in parallel with an improvement of YMRS scores in mania patients. It was possible that UA may have contributed to the severity of mania symptoms, while reduced UA helped relief of manic symptoms. One explainable mechanism was that alleviated oxidative stress by lowering UA may have led to the effect of antimanic drug because an elevated UA is known to induce oxidative stress, which may worsen the pathophysiology of manic disorder (50–53).
The present study was the first to investigate the serum UA levels in patients suffering from the first manic episode who have achieved remission after 8 weeks of treatment. We found that serum UA levels in remitted patients were higher than those in healthy controls after adjusting for age, gender, and BMI, which supported the idea that altered purinergic regulation may be involved in the genesis of BPD (19, 21, 24, 54). In addition, several studies have reported higher levels of UA in the depressive episodes of BPD compared with healthy controls (19, 24, 54). Therefore, an increased UA may be a predisposition in bipolar patients as a trait marker, which is independent of some current or past affective episodes. On the other hand, it was noted that some euthymic patents had similar UA levels as healthy controls particularly if the patients had a longer duration of illness, high number of affected mood episodes, and longer mood stabilizer exposure (12, 20, 22, 23).
The present study had several limitations. First, our data should be seen as a pilot study instead of generalized clinical evidence or relevance because our study had relatively small sample size, especially of female patients from one district of China. Second, bipolar patients with depressive episodes or recurrent episodes (possibly with years of pharmacological intervention) were not enrolled in this study. Third, the prevalence of cigarette smoking was normally higher in patients with BPD than healthy controls, but how smoking affects serum UA in BPD patients is still largely unknown (55, 56). Fourth, all inpatients had the same dietary profile; however, healthy subjects might have a different dietary profile, which could affect the comparison of serum UA between patients and healthy controls (57). Lastly, only one time point in remission was checked; thus, the time course of UA response to therapy remains unknown.
Conclusions
Bipolar patients at the first-episode drug-naive phase had significantly higher levels of serum UA. Patients in remission after 8 weeks of treatment had significantly reduced serum UA, while it was still higher than healthy controls. Patients in nonremission after 8 weeks of treatment had significantly less reduction in serum UA compared with those of remitted patients. Therefore, high levels of serum UA were a pathological condition of bipolar disease at manic phase, contributed to the severity of symptoms, and were reduced in remitted patients, but not in nonremitted patients. Furthermore, serum UA levels might be potentially a trait marker in bipolar patients.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the first author on reasonable request.
Ethics Statement
This research was approved by the Human Ethics Committee of Beijing Hui-Long-Guan Hospital, China. All patients were provided with written informed consent. Participation was voluntary and participants could withdraw at any time from the study.
Author Contributions
Conception of the study: J-XC, F-DY, and X-FH were involved in study design. J-XC, L-GZ, K-ZL, H-MC, and S-JZ were responsible for recruiting the patients, performing the clinical rating, collecting all samples, and helping with statistical analysis and manuscript preparation. X-FH, J-XC, AJ, Y-LT, and F-DY were involved in interpreting the data, intellectual input, and writing and editing the paper. All authors have read and approved the manuscript.
Funding
This study was supported by the Capital Foundation of Medicine Research and Development (2018–3–2132); the Special Foundation of Beijing Municipal Science & 258 Technology Commission, China (Z131107002213099); and Scientific Research Foundation of Sichuan Province (2017JY0322).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BMI, body mass index; BP, blood pressure; BPD, bipolar disorder; CI, confidence interval; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; FBG, fasting blood glucose; HDL-C, high density lipoprotein cholesterol; OR, odds ratio; SPSS, Statistical Package for the Social Sciences; UA, uric acid.
References
1. Fagiolini A, Forgione R, Maccari M, Cuomo A, Morana B, Dell’Osso MC, et al. Prevalence, chronicity, burden and borders of bipolar disorder. J Affect Disord (2013) 148:161–9. doi: 10.1016/j.jad.2013.02.001
2. Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet (2016) 387:1561–72. doi: 10.1016/S0140-6736(15)00241-X
3. Morselli PL, Elgie R, Cesana BM. GAMIAN-Europe/BEAM survey II: cross-national analysis of unemployment, family history, treatment satisfaction and impact of the bipolar disorder on life style. Bipolar Disord (2004) 6:487–97. doi: 10.1111/j.1399-5618.2004.00160.x
4. Revicki DA, Matza LS, Flood E, Lloyd A. Bipolar disorder and health-related quality of life: review of burden of disease and clinical trials. Pharmacoeconomics (2005) 23:583–94. doi: 10.2165/00019053-200523060-00005
5. Atkin T, Nunez N, Gobbi G. Practitioner review: the effects of atypical antipsychotics and mood stabilisers in the treatment of depressive symptoms in paediatric bipolar disorder. J Child Psychol Psychiatry (2017) 58:865–879. doi: 10.1111/jcpp.12735
6. Poon SH, Sim K, Sum MY, Kuswanto CN, Baldessarini RJ. Evidence-based options for treatment-resistant adult bipolar disorder patients. Bipolar Disord (2012) 14:573–84. doi: 10.1111/j.1399-5618.2012.01042.x
7. Schöttle D, Huber CG, Bock T, Meyer TD. Psychotherapy for bipolar disorder: a review of the most recent studies. Curr Opin Psychiatry (2011) 24:549–55. doi: 10.1097/YCO.0b013e32834b7c5f
8. Ortiz R, Ulrich H, Zarate CA Jr, Machado-Vieira R. Purinergic system dysfunction in mood disorders: a key target for developing improved therapeutics. Prog Neuropsychopharmacol Biol Psychiatry (2015) 57:117–31. doi: 10.1016/j.pnpbp.2014.10.016
9. Brambilla P, Harenski K, Nicoletti MA, Mallinger AG, Frank E, Kupfer DJ, et al. Anatomical MRI study of basal ganglia in bipolar disorder patients. Psychiatry Res (2001) 106:65–80. doi: 10.1016/S0925-4927(01)00073-7
10. Chepenik LG, Wang F, Spencer L, Spann M, Kalmar JH, Womer F, et al. Structure-function associations in hippocampus in bipolar disorder. Biol Psychol (2012) 90:18–22. doi: 10.1016/j.biopsycho.2012.01.008
11. Zarate CA Jr., Manji HK. Bipolar disorder: candidate drug targets. Mt Sinai J Med (2008) 75:226–47. doi: 10.1002/msj.20042
12. Gubert C, Jacintho Moritz CE, Vasconcelos-Moreno MP, Quadros Dos Santos BTM, Sartori J, Fijtman A, et al. Peripheral adenosine levels in euthymic patients with bipolar disorder. Psychiatry Res (2016) 246:421–6. doi: 10.1016/j.psychres.2016.10.007
13. Machado-Vieira R. Purinergic system in the treatment of bipolar disorder: uric acid levels as a screening test in mania. J Clin Psychopharmacol (2012) 32:735–6. doi: 10.1097/JCP.0b013e318268391d
14. Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov (2008) 7:575–90. doi: 10.1038/nrd2605
15. Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta (2011) 1808:1380–99. doi: 10.1016/j.bbamem.2010.12.001
16. Bartoli F, Crocamo C, Gennaro GM2, Castagna G, Trotta G, Clerici M, et al. Exploring the association between bipolar disorder and uric acid: A mediation analysis. J Psychosom Res (2016a) 84:56–9. doi: 10.1016/j.jpsychores.2016.03.014
17. Chen J, Chen H, Feng J, Zhang L, Li J, Li R, et al. Association between hyperuricemia and metabolic syndrome in patients suffering from bipolar disorder. BMC Psychiatry (2018) 18:390. doi: 10.1186/s12888-018-1952-z
18. Gültekin BK, Kesebir S, Kabak SG, Ergün FF, Tatlidil Yaylaci E. Are uric acid levels different from healthy subjects in bipolar affective disorder and schizophrenia?: relationship between clinical improvement and episode severity in male patients. Noro Psikiyatr Ars (2014) 51:229–32. doi: 10.4274/npa.y6827
19. Kesebir S, Süner O, Yaylaci ET, Bayrak A, Turan C. Increased uric acid levels in bipolar disorder: is it trait or state? J Biol Regul Homeost Agents. (2013) 27:981–8.
20. Yang X, Tao H, Xiao L, Li C, Tang Y, Liu Y. Increased serum C3 and decreased UA in patients of bipolar disorder in chinese han population. Front Psychiatry (2018) 9:381. doi: 10.3389/fpsyt.2018.00381
21. Cheffer A, Castillo ARG, Corrêa-Velloso J, Gonçalves MCB, Naaldijk Y, Nascimento IC, et al. Purinergic system in psychiatric diseases. Mol Psychiatry (2018) 23:94–106. doi: 10.1038/mp.2017.188
22. Tatay-Manteiga A, Balanzá-Martínez V, Bristot G, Tabarés-Seisdedos R, Kapczinski F, Cauli O. Peripheral oxidative stress markers in patients with bipolar disorder during euthymia and siblings. Endocr Metab Immune Disord Drug Targets (2019) doi: 10.2174/1871530319666190307165355
23. Wiener C, Rassier GT, Kaster MP, Jansen K, Pinheiro RT, Klamt F, et al. Gender-based differences in oxidative stress parameters do not underlie the differences in mood disorders susceptibility between sexes. Eur Psychiatry (2014) 29:58–63. doi: 10.1016/j.eurpsy.2013.05.006
24. Albert U, De Cori D, Aguglia A, Barbaro F, Bogetto F, Maina G. Increased uric acid levels in bipolar disorder subjects during different phases of illness. J Affect Disord (2015) 173:170–5. doi: 10.1016/j.jad.2014.11.005
25. Bartoli F, Crocamo C, Mazza MG, Clerici M, Carrà G. Uric acid levels in subjects with bipolar disorder: a comparative meta-analysis. J Psychiatr Res (2016b) 81:133–9. doi: 10.1016/j.jpsychires.2016.07.007
26. Chatterjee SS, Ghosal S, Mitra S, Mallik N, Ghosal MK. Serum uric acid levels in first episode mania, effect on clinical presentation and treatment response: data from a case control study. Asian J Psychiatr. (2018) 35:15–7. doi: 10.1016/j.ajp.2018.04.030
27. Jahangard L, Soroush S, Haghighi M, Ghaleiha A, Bajoghli H, Holsboer-Trachsler E, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant allopurinol improved symptoms of mania in in-patients suffering from bipolar disorder. Eur Neuropsychopharmacol (2014) 24:1210–21. doi: 10.1016/j.euroneuro.2014.05.013
28. Machado-Vieira R, Soares JC, Lara DR, Luckenbaugh DA, Busnello JV, Marca G, et al. A double-blind, randomized, placebo-controlled 4-week study on the efficacy and safety of the purinergic agents allopurinol and dipyridamole adjunctive to lithium in acute bipolar mania. J Clin Psychiatry (2008) 69:1237–45. doi: 10.4088/JCP.v69n0806
29. Fan A, Berg A, Bresee C, Glassman LH, Rapaport MH. Allopurinol augmentation in the outpatient treatment of bipolar mania: a pilot study. Bipolar Disord (2012) 14:206–10. doi: 10.1111/j.1399-5618.2012.01001.x
30. Weiser M, Burshtein S, Gershon AA, Marian G, Vlad N, Grecu IG, et al. Allopurinol for mania: a randomized trial of allopurinol versus placebo as add-on treatment to mood stabilizers and/or antipsychotic agents in manic patients with bipolar disorder. Bipolar Disord (2014) 16:441–7. doi: 10.1111/bdi.12202
31. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association, (1994).
32. Parker GB, Graham RK, Tavella G. Is there consensus across international evidence-based guidelines for the management of bipolar disorder? Acta Psychiatr Scand (2017) 135:515–26. doi: 10.1111/acps.12717
33. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. (1978) 133:429–35. doi: 10.1192/bjp.133.5.429
34. Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry (1988) 45:742–7. doi: 10.1001/archpsyc.1988.01800320058007
35. Bartoli F, Carra G, Clerici M. Purinergic dysfunction in bipolar disorder: Any role for the antioxidant uric acid as a trait and state biomarker? Psychiatry Clin Neurosci (2017a) 71:417. doi: 10.1111/pcn.12518
36. Machado-Vieira R, Salem H, Frey BN4, Barbosa IG, Teixeira AL. Convergent lines of evidence support the role of uric acid levels as a potential biomarker in bipolar disorder. Expert Rev Mol Diagn (2017) 17:107–8. doi: 10.1080/14737159.2017.1270758
37. Martin DJ, Smith DJ. Is there a clinical prodrome of bipolar disorder? A review of the evidence. Expert Rev Neurother (2013) 13:89–98. doi: 10.1586/ern.12.149
38. Salvadore G, Viale CI, Luckenbaugh DA, Zanatto VC, Portela LV, Souza DO, et al. Increased uric acid levels in drug-naive subjects with bipolar disorder during a first manic episode. Prog Neuropsychopharmacol Biol Psychiatry (2010) 34:819–21. doi: 10.1016/j.pnpbp.2010.02.027
39. Godin O, Leboyer M, Gaman A, Aouizerate B, Berna F, Brunel L, et al. Metabolic syndrome, abdominal obesity and hyperuricemia in schizophrenia: results from the FACE-SZ cohort. Schizophr Res (2015) 168:388–94. doi: 10.1016/j.schres.2015.07.047
40. Xia X, Luo Q, Li B, Lin Z, Yu X, Huang F. Serum uric acid and mortality in chronic kidney disease: a systematic review and meta-analysis. Metabolism (2016) 65:1326–41. doi: 10.1016/j.metabol.2016.05.009
41. De Berardis D, Conti CM, Campanella D, Carano A, Di Giuseppe B, Valchera A, et al. Evaluation of plasma antioxidant levels during different phases of illness in adult patients with bipolar disorder. J Biol Regul Homeost Agents. (2008) 22:195–200.
42. De Berardis D, Conti CM, Campanella D, Carano A, Di Giuseppe B, Valchera A, et al. Serum uric acid levels and different phases of illness in bipolar I patients treated with lithium. Psychiatry Res (2015) 225:604–8. doi: 10.1016/j.psychres.2014.11.038
43. El-Mallakh RS, Jefferson JW. Prethymoleptic use of lithium. Am J Psychiatry (1999) 156:129. doi: 10.1176/ajp.156.1.129
44. Ring HA, Heller AJ, Marshall WJ, Johnson AL, Reynolds EH. Plasma uric acid in patients receiving anticonvulsant monotherapy. Epilepsy Res (1991) 8:241–4. doi: 10.1016/0920-1211(91)90070-V
45. Chan HY, Jou SH, Juang YY, Chang CJ, Chen JJ, Chen CH, et al. A single-blind, comparative study of zotepine versus haloperidol in combination with a mood stabilizer for patients with moderate-to-severe mania. Psychiatry Clin Neurosci (2010) 64:162–9. doi: 10.1111/j.1440-1819.2010.02066.x
46. Chan HY, Lin AS, Chen KP, Cheng JS, Chen YY, Tsai CJ. An open-label, randomized, controlled trial of zotepine and risperidone for acutely ill, hospitalized, schizophrenic patients with symptoms of agitation. J Clin Psychopharmacol (2013) 33:747–52. doi: 10.1097/JCP.0b013e31829e8168
47. Tohen M, Kryzhanovskaya L, Carlson G, Delbello M, Wozniak J, Kowatch R, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry (2007) 164:1547–56. doi: 10.1176/appi.ajp.2007.06111932
48. Tohen M, Vieta E, Goodwin GM, Sun B, Amsterdam JD, Banov M, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry (2008) 69:1776–89. doi: 10.4088/JCP.v69n1113
49. Bartoli F, Crocamo C, Clerici M, Carrà G. Allopurinol as add-on treatment for mania symptoms in bipolar disorder: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry. (2017b) 210:10–5. doi: 10.1192/bjp.bp.115.180281
50. Akarsu S, Bolu A, Aydemir E, Zincir SB, Kurt YG, Zincir S, et al. The relationship between the number of manic episodes and oxidative stress indicators in bipolar disorder. Psychiatry Investig (2018) 15:514–9. doi: 10.30773/pi.2016.12.31
51. Kang DH, Ha SK. Uric acid puzzle: dual role as anti-oxidantand pro-oxidant. Electrolyte Blood Press (2014) 12:1–6. doi: 10.5049/EBP.2014.12.1.1
52. Song C, Zhao X. Uric acid promotes oxidative stress and enhances vascular endothelial cell apoptosis in rats with middle cerebral artery occlusion. Biosci Rep (2018) 38:1–9. doi: 10.1042/BSR20170939
53. Steckert AV, Valvassori SS, Moretti M, Dal-Pizzol F, Quevedo J. Role of oxidative stress in the pathophysiology of bipolar disorder. Neurochem Res (2010) 35:1295–301. doi: 10.1007/s11064-010-0195-2
54. Kesebir S, Tatlıdil, Yaylacı E, Süner O, Gültekin BK. Uric acid levels may be a biological marker for the differentiation of unipolar and bipolar disorder: the role of affective temperament. J Affect Disord (2014) 165:131–4. doi: 10.1016/j.jad.2014.04.053
55. Dickerson F, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Schroeder J, et al. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv (2013) 64:44–50. doi: 10.1176/appi.ps.201200143
56. Haj Mouhamed D, Ezzaher A, Neffati F, Douki W, Gaha L, Najjar MF. Effect of cigarette smoking on plasma uric acid concentrations. Environ Health Prev Med (2011) 16:307–12. doi: 10.1007/s12199-010-0198-2
Keywords: bipolar disorder, hyperuricemia, mania, therapeutic efficacy, uric acid
Citation: Chen J-X, Zhang L-G, Liu K-Z, Chen H-M, Zhou S-J, Wang N, Tan Y-L, Wang S-L, Jones A, Yang F-D and Huang X-F (2019) Patients With Drug-Naive Bipolar Disorder in Remission After 8 Weeks of Treatment Had Decreased Serum Uric Acid Concentrations. Front. Psychiatry 10:767. doi: 10.3389/fpsyt.2019.00767
Received: 09 May 2019; Accepted: 24 September 2019;
Published: 31 October 2019.
Edited by:
Manit Srisurapanont, Chiang Mai University, ThailandReviewed by:
Natalia P. Rocha, University of Texas Health Science Center at Houston, United StatesAlessandra Maria Passarotti, University of Illinois at Chicago, United States
Copyright © 2019 Chen, Zhang, Liu, Chen, Zhou, Wang, Tan, Wang, Jones, Yang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu-Feng Huang, eGh1YW5nQHVvdy5lZHUuYXU=; Fude Yang, eWFuZ2ZkMjAwQDEyNi5jb20=
†These authors have contributed equally to this work
 Jing-Xu Chen1†
Jing-Xu Chen1†