
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry , 08 November 2019
Sec. Addictive Disorders
Volume 10 - 2019 | https://doi.org/10.3389/fpsyt.2019.00737
This article is part of the Research Topic Addiction and Attachment View all 23 articles
 Lane Strathearn1,2*†
Lane Strathearn1,2*† Carol E. Mertens1,2†
Carol E. Mertens1,2† Linda Mayes3
Linda Mayes3 Helena Rutherford3
Helena Rutherford3 Purva Rajhans1,4
Purva Rajhans1,4 Guifeng Xu1,2,5
Guifeng Xu1,2,5 Marc N. Potenza3,6
Marc N. Potenza3,6 Sohye Kim4,7,8
Sohye Kim4,7,8Substance use disorders constitute a significant public health problem in North America and worldwide. Specifically, substance addictions in women during pregnancy or in the postpartum period have adverse effects not only on the mother, but also on mother-infant attachment and the child’s subsequent development. Additionally, there is growing evidence suggesting that parental addiction may be transmitted intergenerationally, where the child of parents with addiction problems is more likely to experience addiction as an adult. The current review takes a developmental perspective and draws from animal and human studies to examine how compromised early experience, including insecure attachment, early abuse/neglect, and unresolved trauma, may influence the development of neurobiological pathways associated with addictions, ultimately increasing one’s susceptibility to addictions later in life. We approach this from three different levels: molecular, neuroendocrine and behavioral; and examine the oxytocin affiliation system, dopamine reward system, and glucocorticoid stress response system in this regard. Increased understanding of these underlying mechanisms may help identify key targets for early prevention efforts and inform needed intervention strategies related to both insecure attachment and addiction.
Within the United States and throughout the world, substance addiction is a significant problem with wide-ranging implications. Substance-use disorders in North America are a growing public health crisis with associated costs reported as reaching billions of dollars annually (1). According to the National Institute on Drug Abuse, the abuse of tobacco, alcohol, and illicit drugs in the United States tops more than $740 billion annually in costs related to crime, loss of work productivity, and health care (2). Aside from these monetary costs, the disruption to the development of secure attachment in children living in environments with substance abusing parent(s) may result in substantial risk to children, parents, and society. Of further concern is the paucity of effective treatment options, which are usually focused on the current addiction behavior rather than the underlying experiences and mechanisms that may have predisposed the individual to addiction (3).
Substance-abusing women who are either pregnant and/or have children face a significant challenge, with their addiction associated with many potential long-term adverse consequences impacting their children. Nearly 90% of women who struggle with substance-use disorders are of reproductive age (4). Although some women may abstain from substances during pregnancy, many resume substance use during the postpartum period, with adverse effects on their parenting capacities and their children’s developmental trajectories. Indeed, some have suggested that the stress associated with parenting may become a risk factor for relapse in substance-using parents (5).
Addictions in mothers are associated with a range of parenting difficulties and may sometimes involve child abuse and neglect (6, 7). Ultimately, the ramifications may include having their children placed in foster care, which may further compromise the quality of the parent-child attachment. Mothers with addictions are considerably more likely than those without addictions to lose custody of their children as a result of child neglect or abuse (8). Further, an expanding body of research supports the notion that parental addiction may be transmitted intergenerationally through several possible mechanisms, with the child more likely to experience addiction as an adult (3, 9).
Twenty years ago, based on the accumulation of brain imaging research, it was argued that addiction should be defined as a brain disease, rather than solely a social or societal problem (10). While still acknowledging crucial behavioral and social-context components, this model characterized addiction as the result of repeated exposure to drugs of abuse, causing changes in brain structure and function related to reward experience and anticipation, perception and memory, and cognitive control. This model accentuated the importance of treatments that incorporated biological, as well as behavioral and societal approaches.
More recently, however, this widely accepted model has been challenged (11–13), with some authors advocating a “developmental-learning model” which characterizes addiction as a product of cognitive and emotional development, particularly during early childhood and adolescence. Lewis (12, 13) provides a specific neurobiological account of how experience and learning—particularly in an environment of chronic stress—may alter neural development and connectivity leading to addiction via the normal mechanisms of neuroplasticity, producing a ventral-to-dorsal shift in striatal activation with more compulsive behavior, and diminished cortical control via the dorsolateral prefrontal cortex.
Investigating and understanding the relationship between attachment and substance addiction is of utmost importance. Here, the neural mechanisms underlying this relationship will be considered at three different levels: molecular, neuroendocrine, and behavioral, in terms of major biological systems associated with each. These include the dopamine-related reward/reinforcement/habit formation system, the oxytocin-related affiliation system, and the glucocorticoid-related stress response system (Figure 1).
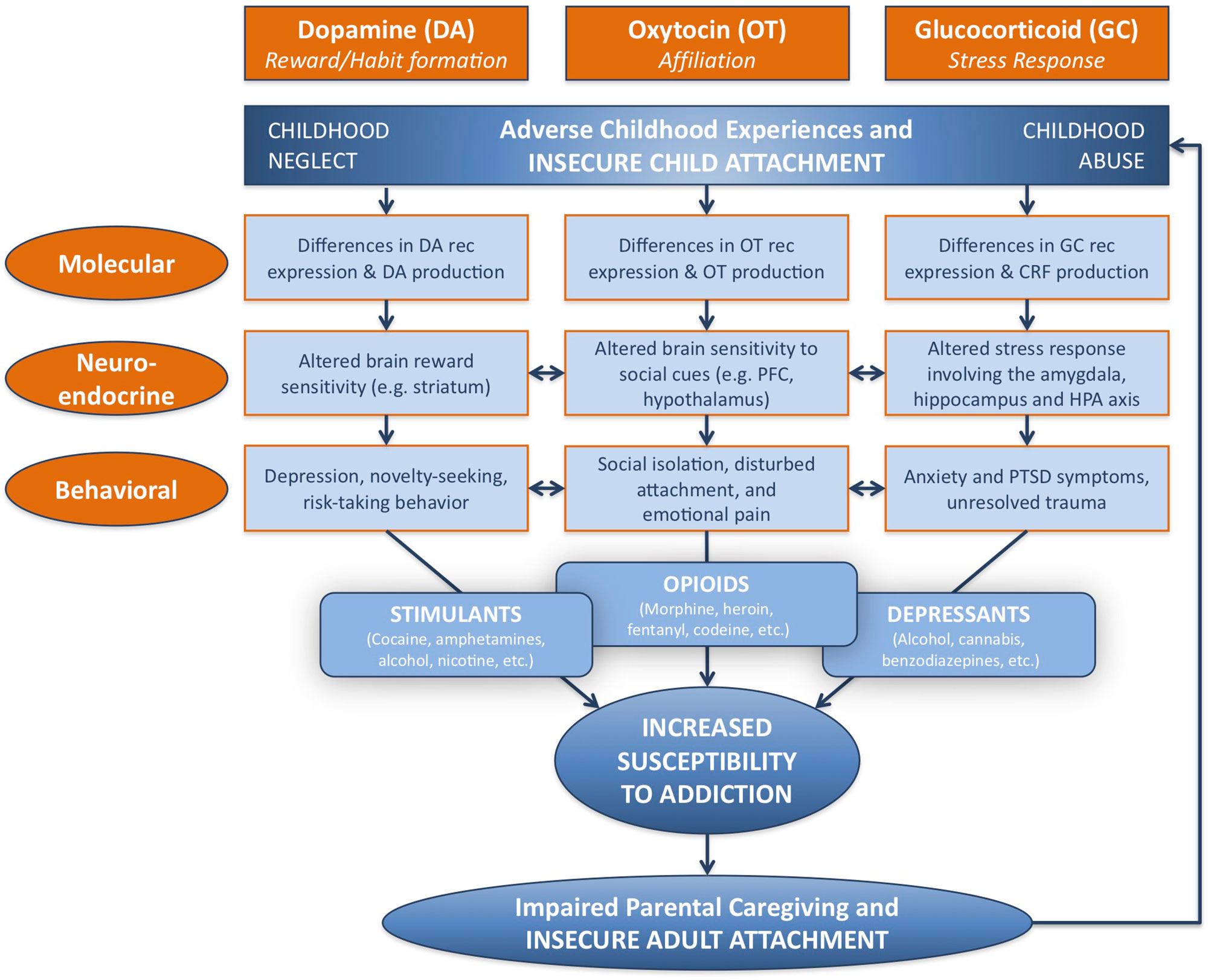
Figure 1 Developmental and neurobiological pathways linking adverse childhood experience to susceptibility to addiction, via modifications in dopamine-related, oxytocin-related, and glucocorticoid-related systems at molecular, neuroendocrine, and behavioral levels. Childhood adversity, including abuse and neglect, may be associated with insecure attachment, and lead to behavioral patterns linked with specific patterns of substance abuse. Parental addiction may impair parental caregiving capacity as a result of insecure patterns of adult attachment, and perpetuate the cycle of childhood adversity and addiction. DA, dopamine; OT, oxytocin; GC, glucocorticoid; rec, receptor; CRF, corticotropin-releasing factor; PFC, prefrontal cortex; HPA, hypothalamic–pituitary–adrenal; PTSD, post-traumatic stress disorder. Adapted from (3) © 2016 New York Academy of Sciences. Used with permission from John Wiley and Sons.
For the purposes of this manuscript, addiction will be restricted to substance-use disorders as defined by criteria in the fifth edition of the Diagnostic and Statistical Manual (DSM-5). It is important to note that the DSM-5 and the recently approved eleventh edition of the International Classification of Diseases (ICD-11) include non-substance or behavioral addictions (e.g., gambling disorder), and existing data indicate negative impacts of gambling disorder on children (14). Gambling disorder has also been associated with insecure attachment styles, suggesting that early life experiences relating to attachment are important to consider in behavioral addictions (15). Substance-use disorders, especially those at the more severe end of the spectrum, are psychiatric conditions characterized by habitual and pathological patterns of drug-seeking and drug-consuming behaviors (16). Habitual patterns of drug-seeking and drug consumption absorb a large amount of time and attention in drug addictions, leading to significant functional impairment in meeting responsibilities at home, school and work (16). When abstaining from the use of a drug or chemical substance, symptoms of distress and strong urges or cravings to use the substance again may emerge and become particularly salient (9). In addition to substance addiction, behavioral addictions to activities such as gambling, gaming and potentially other behaviors (sex, specific types and patterns of internet use) share parallel features with substance addictions: 1) continuing engagement in the addictive behavior despite adverse outcomes; 2) compulsive engagement in the addictive behavior; 3) a craving or appetitive urge state prior to the engagement in the addictive behavior; and 4) diminished control over engagement in the addictive behavior (17, 18). While less research has investigated the impact of behavioral addictions (as compared to substance addictions) on parenting and attachment, features of substance addictions parallel behavioral addictions, suggesting that behavioral addictions may also interfere with parent–child attachment.
While substance use may influence the brain and behavior, it is still unclear why some individuals struggle with addiction and others do not. Once a person has used a particular substance, it may or may not lead to addiction. The developmental-learning model of addiction proposes that early experience may alter susceptibilities to different types of addiction through changes in specific neural circuits. Along this line, Alvarez-Monjaras and colleagues (2018) (9) have integrated neurobiological and psychodynamic theories through the lens of attachment: the neurobiological approach centers on identifying biological mechanisms that may influence the development of substance use and addiction while the psychodynamic approach provides a framework for understanding relational and representational aspects of addiction within a developmental perspective.
Attachment has been defined by Ainsworth (19) as a tie that endures across time and space, to a particular person to whom one turns when feeling vulnerable or in need of protection from danger. John Bowlby (20–22) introduced attachment theory as a structural, systemic model focused on the function and development of human protective behavior. Bowlby’s attachment theory asserts that humans are inherently predisposed to form attachment relationships to their primary caregivers, particularly the mother. These attachment relationships serve to protect the child and occur in an organized form by the end of the first year of life (23). According to the Dynamic-Maturational Model of Attachment and Adaptation (23), the organization of attachment continues across one’s lifetime as a means of: 1) adapting to adverse environments and strategically protecting oneself from danger; and 2) ensuring reproductive success. Individuals who are exposed to danger, either from direct threat or absence of care, particularly during infancy, are more likely to distort or negate cognitive and affective information, and thus misinterpret crucial information from their environment. This may help to explain seemingly irrational decisions made by individuals with substance addictions as they attempt to secure a sensation of reward, gain social affiliation, or reduce stress by using substances.
More recently, with advances in neuroimaging, attachment has been systematically associated with neuroendocrine responses to salient attachment cues, such as a mother’s response to seeing her baby’s smiling or crying face. Such neural and endocrine responses involve activation of the dopamine-related reward system, oxytocin-related affiliation system, and glucocorticoid-related stress-response system (24), which pathways are also implicated in the neurobiology of drug addiction.
Since Bowlby first published his seminal work (20), numerous methods have been developed to systematically classify patterns of attachment, as observed from infancy to adulthood. Ainsworth (19) initially identified and empirically linked three major patterns of attachment with their origins in maternal responsiveness and sensitivity: Secure (Type B), Avoidant (Type A), and Ambivalent (Type C). This has led to the development of what has become one of the most accepted and empirically tested measures of attachment in adulthood: the Adult Attachment Interview (AAI) (25). The AAI is a semi-structured interview designed to identify differences in state of mind with regard to overall attachment history by examining participants’ abilities to describe attachment-related memories while simultaneously maintaining coherent, cooperative discourses (26). The results of a recent longitudinal meta-analysis of 34 samples (total N of 56,721) confirmed a significant association between insecure attachment and substance-abuse problems (27).
The Dynamic-Maturational Model of Attachment and Adaptation (23) involves a modification of the original AAI, extending Ainsworth’s childhood classifications into adulthood. This model, which has a broader focus on psychopathology and trauma, may be particularly suited for understanding high-risk populations including those of substance-using individuals (28). From an analysis of the transcribed discourse, four basic attachment patterns emerge among adults, summarized as secure (Type B1-3), “insecure/dismissing” (Type A1-6), “insecure/preoccupied” (Type C1-6), and “insecure/mixed” (Type A/C). Longitudinal studies have suggested the unique capacity of a caregiver’s AAI to predict attachment patterns in the infant offspring (29, 30). Understanding the neurobiological differences between attachment patterns among adults may help us better understand the mechanisms underlying the intergenerational transmission of addictions.
Crittenden has suggested that basic attachment patterns may, in fact, represent differences in how the brain processes sensory information (31). Accordingly, she proposed that sensory stimulation is transformed into one of two basic forms of information: 1) temporally ordered “cognitive” information and 2) intensity-based arousal or “affective” information. The first is proposed to be the predominant mechanism in “dismissing” attachment organization, whereas the second is proposed to be central in “preoccupied” attachment. “Secure” organization may involve a balanced integration of both sources of information. For example, “dismissing” adults tend to dismiss their own feelings, intentions and perspectives and rely more upon rules and learned temporal relations in predicting future rewards. “Preoccupied” adults, in contrast, may organize their behavior around affective information, such as fear, anger or desire for comfort. They tend to be preoccupied by their own feelings and perspectives, while omitting or distorting cognitive or temporally ordered information. Adults with “secure” or balanced patterns of attachment may be best able to integrate temporally ordered information regarding causal effects, as well as more affect-based information, such as emotional states and imaged memory, in order to form close relationships, make accurate decisions and predict future reward. The organization of attachment may also involve the differential development of specific memory systems within the brain, such as procedural and semantic memory, imaged memory, and episodic and working memory systems, each of which is specifically coded in the AAI as considered in the Dynamic-Maturational Model, and linked to the function of specific brain regions and networks (23).
Two neuroendocrine systems that appear to be related to Crittenden’s theory of cognitive and affective forms of information processing and attachment are the dopamine and oxytocin systems. These neurodevelopmental processes appear to be shaped by early-life experience, including variations in maternal behavior (32–35) (Figure 1). The dopamine system includes two main components: the mesocorticolimbic and nigrostriatal dopamine pathways (Figure 2). The former involves stimulus-reward learning and prospective decision-making based upon predicted reward (37); the latter is implicated in motoric behaviors and habit formation. The oxytocin system is important in prosocial affiliative behavior, the formation of social and spatial memories, and emotion regulation (38). Oxytocin neurons connect the hypothalamus with the mesocorticolimbic dopamine system, including the ventral tegmental area and the ventral striatum, and may facilitate reward responses and reinforcement to affective and social cues. Recent data have suggested interactive relationships between the mesocorticolimbic dopamine, oxytocin and glucocorticoid physiological stress systems (34, 39). Oxytocin receptor blockade gives rise to both an exaggerated adrenocorticotropic hormone (ACTH) as well as corticosterone stress hormone response in rats (40). Similar results are observed in oxytocin-deficient knock-out mice (41). Working alongside the oxytocin system, the mesocorticolimbic dopamine system similarly has a stress inhibitory effect on the amygdala (42) through the medial prefrontal cortex (43). Consequently, it appears that one important function of these inter-related neuroendocrine systems may be to modulate human stress responses and thereby facilitate optimal social bonding and attachment, through different but complementary mechanisms.
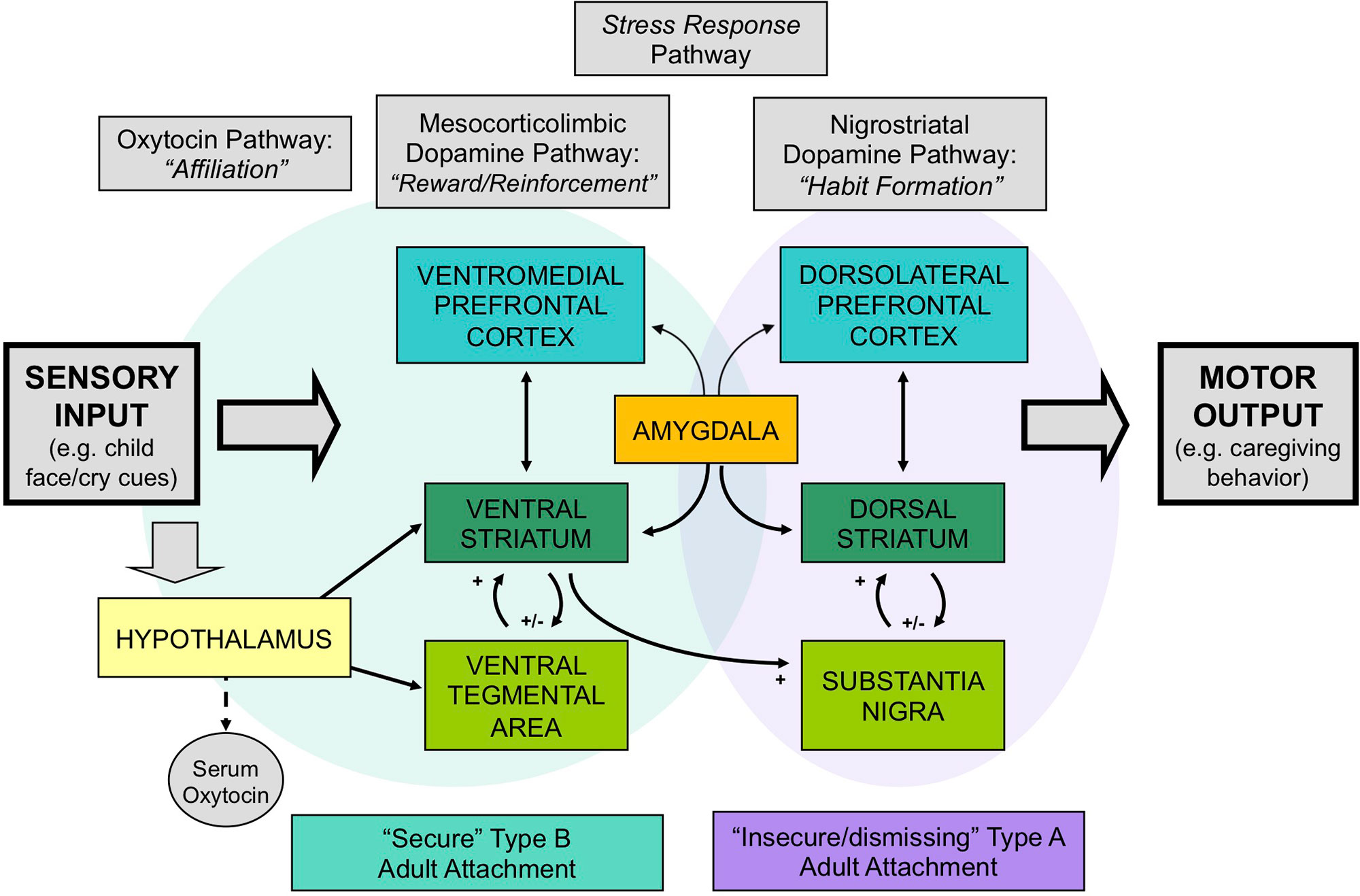
Figure 2 The neurobiology of attachment, incorporating 1) dopamine-related “reward/reinforcement” and “habit” pathways, 2) oxytocin-related “affiliation” pathways, and 3) glucocorticoid-related stress-response pathways. Secure patterns of attachment are associated with greater activation of the mesocorticolimbic and oxytocin-associated circuits, whereas insecure/dismissing attachment is associated with greater activation of nigrostriatal dopamine pathways. Adapted from (36) © 2011 The Author, Journal of Neuroendocrinology. © 2011 Blackwell Publishing Ltd. Used with permission from John Wiley and Sons.
Our research suggests that differences in human attachment strategies are associated with significant differences in these brain activation patterns, when mothers view powerful visual stimuli that may impact the attachment system: images of their own infant’s face (24) (Figure 2). Mothers with secure (Type B) patterns of attachment, compared with insecure/dismissing (Type A) mothers, showed greater activation of regions in the mesocorticolimbic dopamine pathway, including the ventral striatum and ventromedial prefrontal cortex, and the oxytocin-associated hypothalamic/pituitary region. Mothers with Type A attachment, in general, showed greater activation of the dorsolateral prefrontal cortex, which has been implicated in cognitive control and habit formation.
Most early investigations of parent–child attachment focused solely on the mother-child attachment relationship, overlooking the father as an essential element in the child’s attachment formation process. According to Scism and Cobb (2017), the importance of creating an immediate mother-infant bond overshadowed and deferred the efforts of researchers to document factors and interventions that influence father–infant bonding. In the late 1970s and early 1980s, investigators began to recognize the importance of paternal involvement during the immediate postpartum period (44, 45), leading to additional weight gain in preterm infants, reducing cognitive delays in offspring, and increasing breastfeeding rates in mothers (46, 47).
In recent years, the influence of father–infant interactions on attachment has become a focus of research within the field of child development, showing, for example, that fathers have a unique but complementary neuroendocrine response to infant interactions, compared with mothers (48). Much evidence has indicated that fathers are critical for the well-being of their children (49, 50) and that the absence of fathers is associated with numerous risk conditions (51). Observational investigations of the nature of parental involvement have found that father–child interaction patterns have a distinctive quality that is more dynamic and stimulating than mother-child interaction patterns, which may help promote exploration and risk-taking behaviors and ultimately facilitate cognitive development (49). Although some preliminary studies have explored the effect of parenting interventions in fathers with addiction problems (52), little is known about the direct role of fathers in preventing later addiction problems in their children (53).
Proposed pathways leading to addiction are numerous and multifaceted, involving expression of specific molecular and genetic entities, altered brain sensitivities to reward- and stress-related cues, environmental influences, and cognitive, behavioral, motivational and emotional constructs that include depression, risk-taking, social isolation, emotional pain and/or unresolved trauma (3) (Figure 1). Epidemiological studies have shown associations between adverse early childhood experience and addictions in adolescence and adulthood. For example, after adjusting for multiple sociodemographic and potential confounding variables, individuals who had experienced childhood abuse and/or neglect were more likely to use tobacco and alcohol in early adolescence, become dependent on cannabis, and smoke and inject drugs in early adulthood (54–57). Parental figures who abuse drugs have often experienced inadequate caregiving environments during their own childhoods (9). Furthermore, their own substance addiction increases the likelihood that they will provide neglectful or abusive care to their own children (58). This may also lead to children being placed in foster care, further impacting parent-child attachment.
Negative childhood experiences often have a profound and enduring influence upon the developing child’s quality of life and well-being, frequently well into adulthood. The Adverse Childhood Experiences (ACEs) Study has examined retrospectively recalled traumatic experiences that occurred during the first 18 years of life (59, 60). ACE categories include multiple forms of abuse (physical, emotional, and sexual), neglect (physical and emotional), parental separation or divorce, household violence, substance use, mental illness, and incarceration (59). An examination of the relationship between illicit drug use and ACEs found that each ACE experience increased the likelihood of early initiation of drug use 2- to 4-fold, while people with ≥5 ACEs were 7- to 10-fold more likely to report illicit drug use, addiction to illicit drugs, and drug use by parents (60, 61).
A substantial threat to healthy development is growing up in poverty. According to the KIDS COUNT Data Book (2018), a national average of 19% (14.1 million) of children in the United States lived in poverty in 2016, with some states reaching as high as 30%. Notably, poverty rates vary tremendously according to race, with African-American and American Indian children (both 34%) experiencing nearly three times the poverty rate for Caucasian and Asian and Pacific Islander (both 12%). Employment insecurity and high rates of job instability may disrupt daily living and relationships and compromise families’ abilities to invest in their children’s development. This may lead to diminished achievement in school and reduced likelihoods of future success. Secure employment is a significant pathway to financial equilibrium and well-being in families. However, Koball and Jiang (62) note that being a child in a low-income family does not happen by chance. Factors related to poverty include parental education, parental employment, race and ethnicity, family structure, region of residence, residential instability, and utility and housing insecurity. Children and parents who live in high-poverty neighborhoods face many challenges that may impact their lives on a daily basis including greater financial instability, poorer health, higher rates of violence and crime, poorer schools, and limited access to support systems and job opportunities. Each of these challenges may add to the stress level of both the child and the parent, which may interfere with attachment relationships and predispose the offspring to addiction (63).
Understanding the mechanisms by which early adverse experience may increase susceptibility to addiction is of critical importance to adequately formulating plans for prevention and treatment. Three neurobiological pathways have been identified that may link attachment and early experience with addiction at molecular, neuroendocrine and behavioral levels. These pathways include: 1) the dopamine-related reward system; 2) the oxytocin-related affiliation system; and, 3) the glucocorticoid-related stress response system (3). Each of these systems will be outlined below, summarizing what we understand about the neurobiology of attachment and how this may relate to addiction behaviors and susceptibility.
Oxytocin is a neuropeptide that functions as a neuro-regulator of social behavior within mammalian species (64). Considerable attention has been focused on oxytocin’s core roles in attachment formation and stress regulation, and there has been a surge of interest in the connection between dysregulated oxytocin systems and disorders of psychosocial functions (65–67). In particular, the developing oxytocin system has been implicated in increasing vulnerability to addiction across the lifespan (68) as well as serving a protective function against the development of addiction (69). Specifically, oxytocin may enhance the salience and familiarity of social cues and lessen novelty- and reward-seeking, which have been implicated in pathways to addiction (69). Drug use may also impact the maternal oxytocin system. In postpartum human mothers, cocaine exposure during pregnancy was associated with decreased oxytocin in plasma relative to mothers not using substances during pregnancy (70). In rodent dams, chronic cocaine exposure during pregnancy also resulted in decreased oxytocin levels in the maternal brain, including in the hippocampus, ventral tegmental area, and medial preoptic area (71). Therefore, the oxytocin system may be implicated in addiction susceptibility before the transition to motherhood, and may be modulated by drug exposure during pregnancy and the postpartum period.
The oxytocin system may be of particular significance in relation to addiction and attachment because of its neuroplasticity in response to an individual’s early social environment (69). Growing evidence suggests early life experiences may substantially impact long-term functioning of the oxytocin system (24, 72, 73). On the behavioral level, rat pups experiencing early-life deprivation show multiple social impairments including diminished social motivation (74), reduced affiliative behavior (75), impaired social learning (76) and increased aggressive behavior (74, 77). Rat pups who received low levels of care early in life tended to develop into adults that display similarly low levels of care with their own pups (78, 79). This intergenerational transmission appears to be at least partially mediated by oxytocin-related molecular and neuroendocrine alterations, including changes in oxytocin receptor expression and oxytocin production (80, 81) (Figure 1).
Empirical evidence from human research parallels findings from animal models. Extreme early deprivation in humans has been prospectively associated with severe long-term attachment disorders and social deficits (81). Numerous longitudinal and cross-sectional studies have linked early trauma and/or disrupted attachment to long-term social and attachment difficulties (82–85). Other studies have linked compromised oxytocin functioning with social deficits (65, 86, 87). In humans, social isolation and low social support may accelerate the emergence and recurrence of substance use and predict substance addiction (88).
A history of early childhood trauma or stress has been negatively correlated with levels of oxytocin as assessed in cerebrospinal fluid, urine, or plasma (89–92). A dose-dependent inverse relationship has been observed between the severity of trauma experiences and oxytocin concentration (65, 68). Among the different types of trauma, emotional abuse and neglect appear to have the strongest associations (90, 91). Since adverse experiences in childhood (rather than adolescence and adulthood) emerge as arguably the most robust predictors of long-term oxytocin functioning, the timing of the trauma and adversity appears critical to the impact that oxytocin may have upon individual functioning (92).
Infants or children who experience less synchronous, less sensitive, or less responsive caregiving show blunted salivary oxytocin levels, both at baseline and in response to social cues (72, 93), as well as disrupted patterns of attachment (94, 95). Adults with an insecure/dismissing (Type A) pattern of attachment often have a diminished peripheral oxytocin response when interacting with their infants (Figure 3A), which is correlated with reduced brain activation in oxytocin- and dopamine-associated brain regions, including the hypothalamus and ventral striatum (24) (Figure 3B). Peripheral oxytocin response (between a baseline of mother-infant separation and an interaction period) was also associated with differences in maternal behavior. A lower (or negative) oxytocin response was associated with diminished maternal gaze toward her infant, especially during heightened infant distress (86).
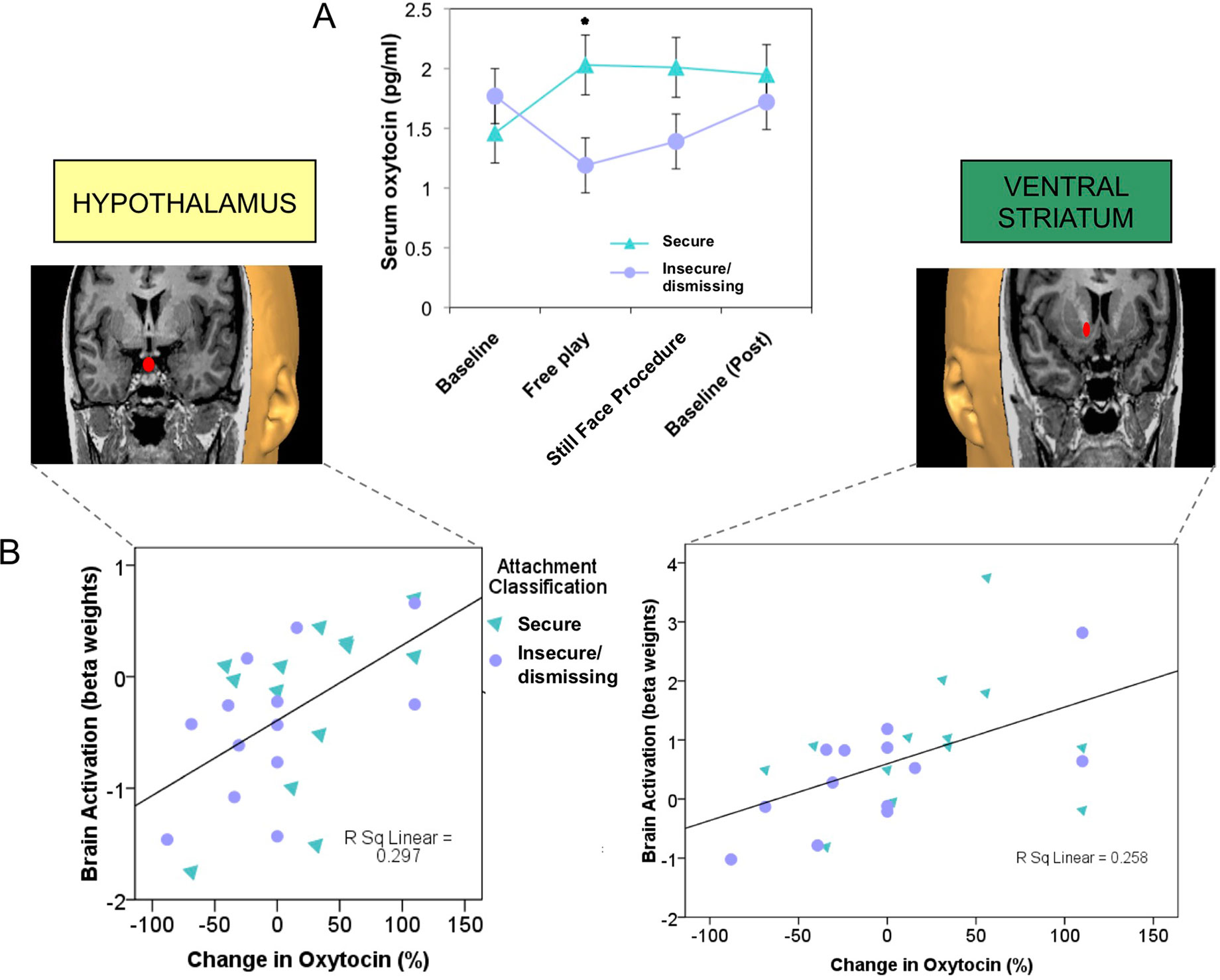
Figure 3 Plasma oxytocin response to mother–infant interaction is reduced in mothers with insecure/dismissing attachment, compared with securely attached mothers (A), and is correlated with activation of hypothalamus (rs = 0.6, p = 0.001) and ventral striatum (rs = 0.57, p = 0.001), in response to viewing own-infant faces in a functional MRI scanner (B). (36) Adapted from (24) © 2011 The Author, Journal of Neuroendocrinology. © 2011 Blackwell Publishing Ltd. Used with permission from John Wiley and Sons.
Studies are currently underway to test whether oxytocin may be an adjunctive treatment alongside methadone maintenance therapy for opioid-use disorders (96, 97). According to the brain opioid theory of social attachment (98), endogenous opioids are released in response to social bonding experiences, including social touch and breastfeeding. Social isolation may lead to reduced opioid activity and subsequent feelings of distress and emotional pain relating to separation and loss. Rising rates of opioid abuse and overdose deaths may be one consequence of disrupted “social capital” in society (99), mediated via changes in the oxytocin affiliation system.
The dopamine-related reward system contributes to the regulation of reward, motivation, and decision-making. The majority of dopamine neurons are located in the ventral part of the midbrain (100), where the mesocorticolimbic and nigrostriatal dopamine systems originate (Figure 2). Dopamine-related dysfunction has been associated with the pathophysiology of many psychiatric disorders, including depression and substance addictions. While multiple studies have reported abnormal dopamine-related functioning in addiction (101, 102), including decreased striatal dopamine receptor availability and dopamine release, these differences are seen primarily in stimulant and alcohol abuse, rather than abuse of opioids and cannabis (103) (Figure 1).
A significant and growing body of research has established the role of early-life experience in shaping the development of dopamine-related systems. Animal models have shown that early adverse experience alters dopamine-related neuronal activity and synaptic functions. For example, rat pups that are reared in isolation with prolonged maternal separation, show reduced dopamine transporter binding in the ventral striatum, increased baseline dopamine levels, and exaggerated dopamine release in response to acute stress (34, 104). Rodent studies investigating naturally occurring variations in maternal behavior (i.e., licking/grooming in rats) have demonstrated that diminished dopamine release in the ventral striatum leads to decreased licking and grooming of the rat pups in response to pup vocalization (32). High levels of postnatal maternal care provided to rat pups have been associated with an increased density of dopaminergic cell bodies within the ventral tegmental area and increased dopamine receptor mRNA levels within the ventral striatum (Figure 2). This association appears to persist into adulthood (105).
Notably, early adverse experience may also impact levels of stimulus-evoked dopamine release. Rodents with early adversity show dampened dopamine release in the ventral striatum in response to pups (106), enhanced dopamine release in the ventral striatum and hypothalamus in response to stress (107), and enhanced dopamine release in the ventral striatum in response to the administration of amphetamine (108). More specifically, suboptimal early caregiving in humans has been correlated with elevated dopamine release in the ventral striatum in response to stress (35) and amphetamine administration (109–111).
Behaviorally, rat-pups subjected to early maternal deprivation are more sensitive to novel stimuli in adulthood, an indicator of their enhanced spontaneous locomotor activity in novel settings (111, 112). In humans, novelty-seeking, or sensation-seeking, is defined by an amplified tendency toward novel sensations and experiences, often leading to impulsive risk-taking and/or the active pursuit of rewards (113). The link between early adversity and novelty-seeking has been observed in both community (114) and high-risk samples (115). Among different classes of substances, individuals with high novelty-seeking behavior display a preference for stimulant drugs that activate dopamine-related pathways (116). In a study of a large sample presenting with a complex set of risk factors, novelty-seeking emerged as the strongest factor contributing to the development of substance-related disorders (115). The link between childhood adversity and the presence of substance-use disorders was found to be at least partially mediated by increased novelty-seeking in individuals with histories of adverse life events (115).
Depression is likewise associated with differences in function of dopamine-related regions. Both depressed adolescents, as well as non-depressed adolescents whose mothers were currently depressed, both showed diminished activation of the ventral striatum in a reward-based functional MRI task (117) (Figure 4). Furthermore, activation of the ventral striatum in the adolescents was inversely correlated with the mother’s—and not the adolescent’s—depression scores, suggesting that maternal depression may be contributing to an abnormal reward response in the offspring. Taken together, depression, novelty-seeking, and risk-taking behavior have been associated with increased susceptibility to addiction (Figure 1).
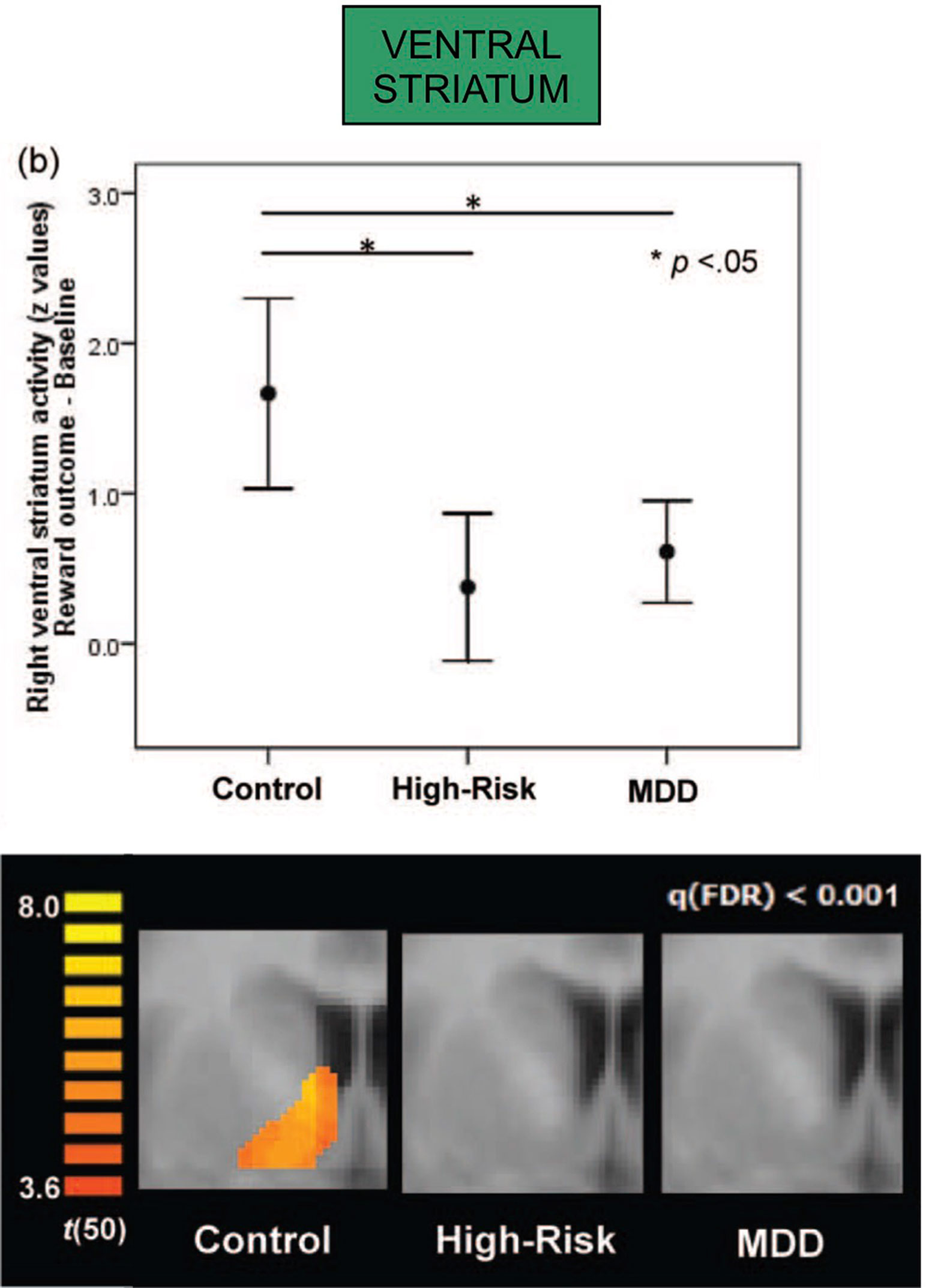
Figure 4 Both major depressive disorder (MDD) and high-risk groups (with current depression in mother but not in adolescent) show attenuated right ventral striatum activation in response to a standard reward outcome during functional MRI scanning (both *p < .05; error bars depict 95% confidence interval); activation for the reward outcome versus baseline contrast within the ventral striatum region of interest, presented at false-discovery-rate-corrected q < .001; the activations are shown at y = 5. Adapted from (117). Used with permission.
Glucocorticoids (cortisol in primates) are steroid hormones that contribute to the physiological stress response. A cascading set of neurotransmitters and hormones involved in this organization is collectively described as the hypothalamus–pituitary–adrenal (HPA) axis. Psychological or physical stress triggers the release of corticotrophin-releasing factor (CRF) in the hypothalamus, which binds to receptors in the pituitary to promote ACTH release. ACTH is then transported to the adrenal glands, resulting in secretion of the glucocorticoid stress hormone. Once released, glucocorticoids activate glucocorticoid receptors, which suppress further synthesis and release of CRF and ACTH, thereby providing negative feedback inhibition of the HPA system and restoring homeostasis.
In humans, decreased responsiveness, sensitivity, and synchrony in early caregiving have been correlated with prolonged or exaggerated increases in cortisol in response to stress (118, 119), whereas secure parental attachment has been associated with lower cortisol levels in response to stress (120) (Figure 1). However, in cases of more severe early deprivation or maltreatment, patterns of HPA responsiveness have been mixed, perhaps due to the complex nature of maltreatment and the co-occurrence with psychiatric disorders. Affected individuals may undergo a transition from early hypercortisolism to later hypocortisolism due to frequent and persistent adverse experiences (121, 122). This change may reflect adaptive down-regulation of the HPA system following chronic stress exposure, leading to flattened diurnal rhythms of cortisol secretion (with lower than normal daytime cortisol levels) (118, 123).
Rodents who experience diminished maternal care show increased DNA methylation of the glucocorticoid promoter region, which is associated with decreased glucocorticoid expression in the hippocampus and limited inhibitory feedback to stress (124). This results in elevated anxiety and fearfulness in adulthood and decreased exploratory behavior. These behavioral outcomes resemble signs and symptoms of anxiety, post-traumatic stress and addictive disorders in humans (125–127).
Early adverse experience is a potent pathway for the development of anxiety and trauma-related disorders later in life (90). Altered glucocorticoid and HPA responsiveness may contribute to the etiology of these disorders and mediate early adversity with later psychopathology and addiction (128, 129). Numerous studies connect stress dysregulation and HPA dysfunction to substance addiction (130, 131). Stress exposure may precipitate the onset of substance use, diminish the motivation to abstain, and heighten the risk for relapse, particularly in those with exaggerated HPA reactivity (131). This process may reflect the effects of a chronically activated HPA axis on enhanced striatal extracellular dopamine release, which may expose the reward system to the reinforcing properties of addictive substances (35, 132, 133). Disorders that are associated with HPA dysfunction, such as anxiety and trauma-related disorders, may serve as precursors to the development of substance addiction (128, 129, 134). These disorders may also modulate the progression of substance addiction, such that the illness course is typically more severe and persistent (135).
The amygdala, which contributes importantly to the processing and regulation of emotions, connects with the striatum and prefrontal cortex (Figure 2), and its development has been associated with early life stress and trauma. Enlarged amygdala volumes have been seen in children exposed to chronic maternal depression (136), and in those raised in orphanages (137). In a study of mothers with unresolved trauma, based on the AAI, amygdala activation appeared to be “turned off” when these mothers viewed their own infant’s distressed face (138), despite responding similarly to mothers without unresolved trauma when viewing unknown infant faces (Figure 5). This suggests that unresolved childhood trauma may alter amygdala reactivity to salient attachment-based cues.
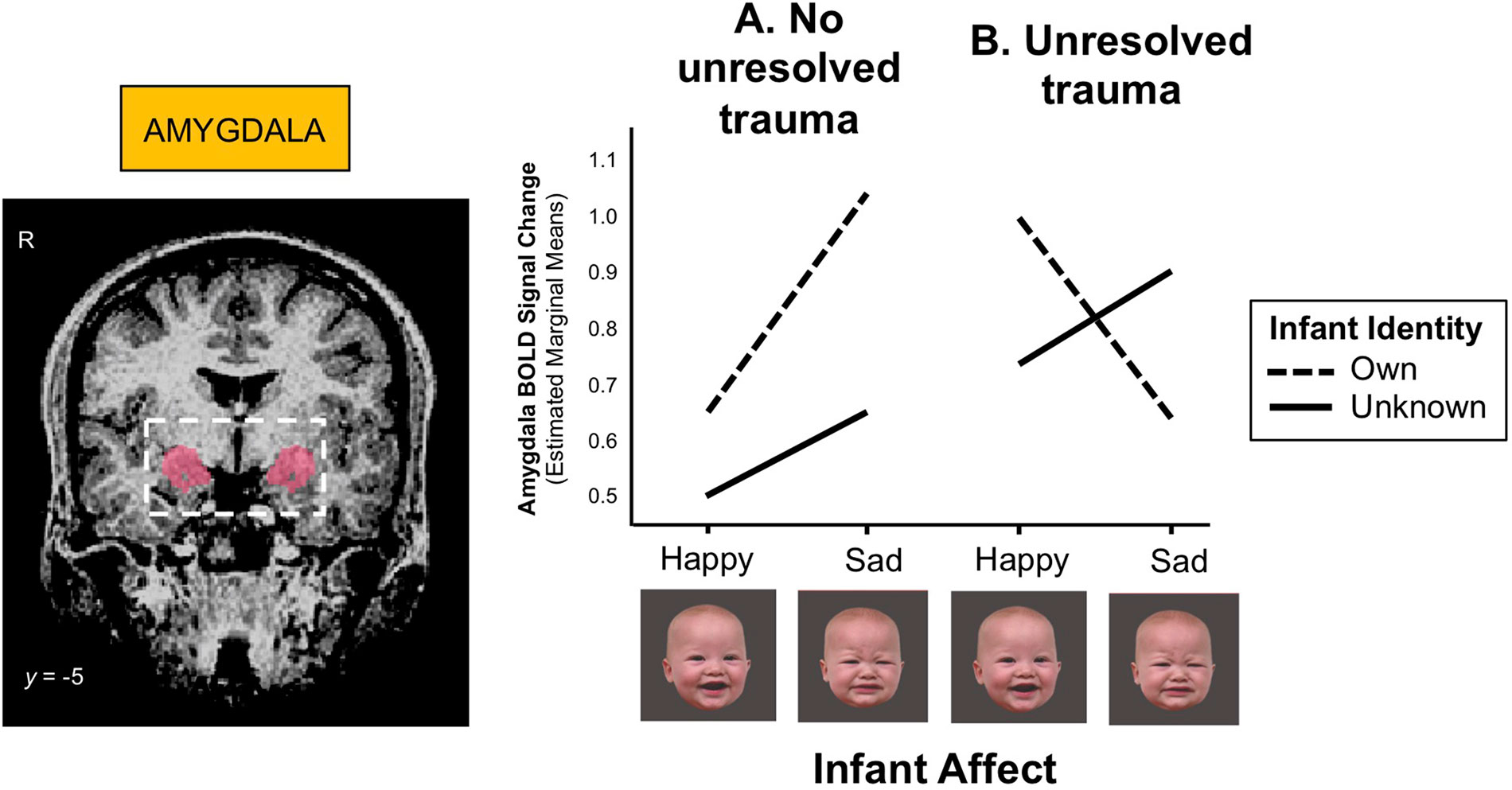
Figure 5 (A) Mothers with no unresolved trauma show a greater amygdala response to sad than happy infant faces (z = 3.00, p = 0.003), whereas (B) mothers with unresolved trauma show a blunted amygdala response to their own infant’s distress cues during functional MRI scanning (z = -2.38, p = 0.017). BOLD (blood-oxygen-level-dependent) signals were extracted from a bilateral amygdala mask (shown left) and submitted to mixed-effects linear regression analysis. Adapted from (138). Used with permission from Taylor & Francis Ltd.
Substances of abuse, particularly depressants such as alcohol, benzodiazepines and cannabis, may be used to diminish the physiological and psychological effects of chronic stress as a result of childhood abuse (Figure 1). For example, one effect of alcohol is to dampen the neuroendocrine stress system by reducing peripheral glucocorticoid levels (140, 141). The effectiveness by which this is accomplished may itself lead to an increased susceptibility to addiction.
Although the prior discussion has focused on individual neuroendocrine systems, each system is interconnected with the other, rather than acting in isolation (5). For example, oxytocinergic neurons connect the hypothalamus with key dopaminergic brain nuclei, including the ventral tegmental area and the ventral striatum (142), as well as the amygdala. These systems all appear to play an important role in maternal behavior, pair-bond formation and social attachment (143, 144).
In humans, intranasal oxytocin enhances brain reward activation in both the ventral tegmental area and the ventral striatum (145). The effect of individual differences in oxytocin functioning on dopamine and other neuroendocrine systems, as well as the stress axis, may underlie differences in susceptibility to addiction (68). Likewise, exposure to drugs such as amphetamines may impair bonding and attachment via changes in oxytocin and dopamine neurotransmission in areas such as the ventral striatum and medial prefrontal cortex (146).
Oxytocin also appears to have a stress inhibitory effect, attenuating symptoms of anxiety and activation of the hypothalamic–pituitary–adrenal axis, especially with regard to substance abuse and withdrawal (147–149). One model proposes that oxytocin may attenuate stress and addiction by shifting the preference for novelty and reward seeking toward a greater appreciation for familiarity and attachment (69). As noted previously, early life stress, such as via maternal separation, may also effect dopamine functioning, from neuronal development, dopamine signaling and receptor expression, to addiction behaviors (3).
Our own published work has demonstrated that mothers with drug addiction problems show a different brain response pattern in both dopamine reward and oxytocin-associated affiliation pathways, including the hypothalamus, ventral striatum and ventromedial prefrontal cortex, when viewing pictures of their own infant’s smiling face (139) (Figure 6). Instead of showing an increased response in these brain regions, as demonstrated in non-substance using mothers (150), the response in mothers with addiction problems was diminished, compared with the responses to unknown infant faces.
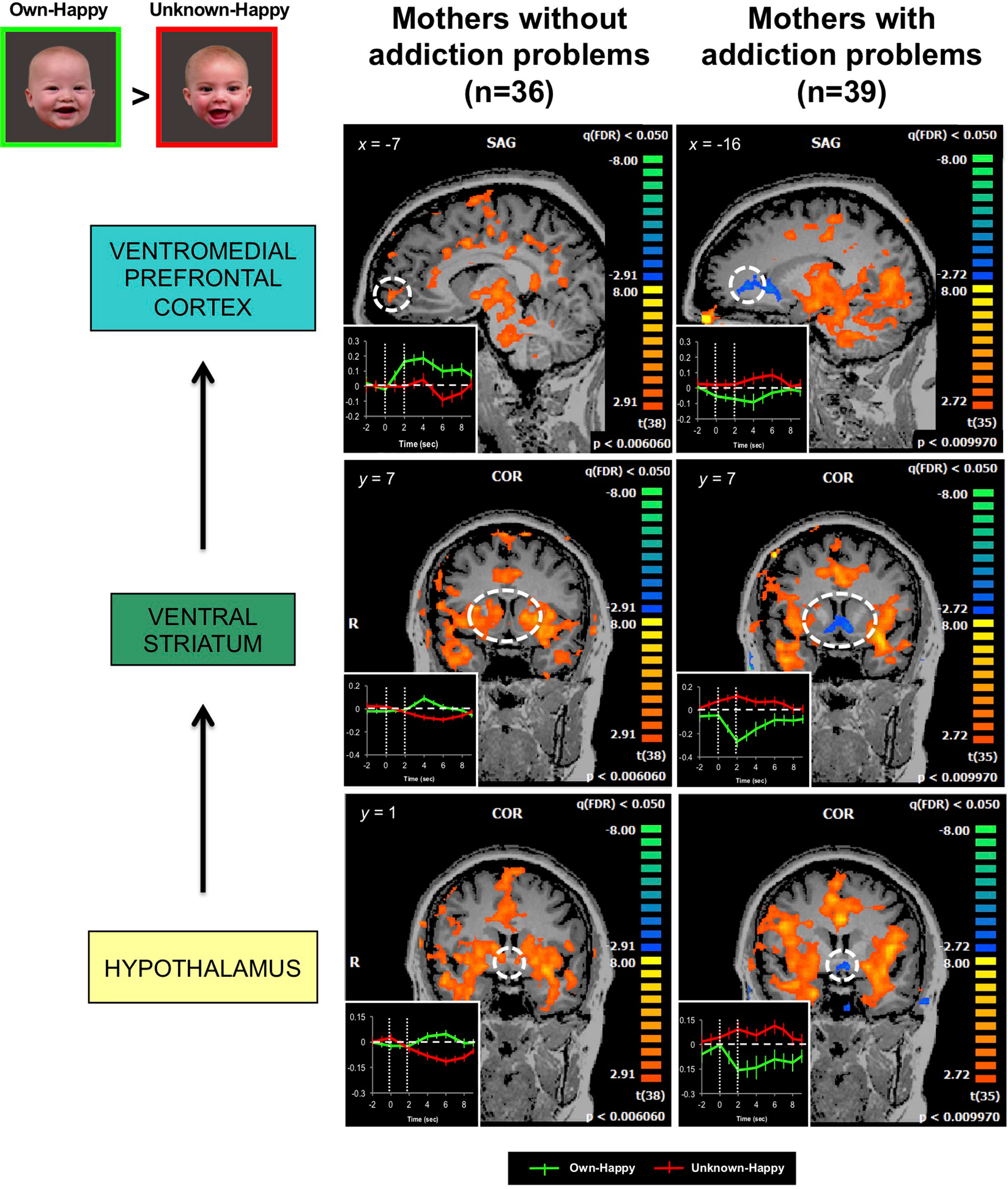
Figure 6 In response to own- vs. unknown-infant happy faces (OH > UH), mothers with addiction problems show deactivation in the hypothalamus, ventral striatum, and ventromedial prefrontal cortex, regions wherein strong activation has been observed in mothers without a history of substance use (random effects analysis; FDR-corrected p < 0.05). Inset shows brain response time courses extracted from the peak voxels in each specified region (hashed line circle), after presentation of own-happy (green plot) and unknown-happy (red plot) infant face cues between 0 and 2 seconds. Adapted from (139). © 2017 Wiley Periodicals, Inc. Used with permission.
Thus, all three of these neuroendocrine systems may have interactive effects on attachment and the subsequent susceptibility to addiction.
In this review paper, we have focused on three interconnected neuroendocrine pathways which, to some extent, may be programmed by early life experience and related to patterns of childhood attachment. Multiple overlapping adverse childhood experiences, ranging from traumatic abuse to absence of nurturant care and neglect, may have a profound impact on the development of secure attachment and on each of these three biological systems: the dopamine-related reward system, the oxytocin-related affiliation system, and the glucocorticoid-related stress response system. Other factors may also contribute to risk, including genetic differences, other neuroendocrine systems such as serotoninergic and glutamatergic pathways, and the effect that substance abuse itself may have on brain functioning and ongoing development.
We are currently working to determine whether differences in brain responses in mothers with addiction problems are related to drug use per se, as proposed in the brain disease model of addiction (10), or more fundamental underlying conditions, such as unresolved childhood trauma, insecure attachment, or other psychological or socio-demographic factors. A focus on attachment and developmental pathways may be important in delivering optimal treatment for drug-exposed mothers, as seen in some notable evidence-based recovery programs (151–153), as well as identifying key targets for early intervention and prevention efforts. By employing a lifespan developmental perspective, we may most appropriately address and target the intergenerational risk of substance use and addiction, and provide more hope for future generations.
All authors contributed to the formulation of the review paper topic. LS, CM, and SK drafted the original manuscript, and the other authors provided additional contributions and critical feedback.
MP has received financial support or compensation for the following: he has consulted for RiverMend Health, Game Day Data, the Addiction Policy Forum, and Opiant Pharmaceuticals; has received research support from Mohegan Sun Casino and the National Center for Responsible Gaming; and has consulted for gambling and legal entities on issues related to addictive disorders.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD065819 and R03 HD080998); National Institute on Drug Abuse (R01 DA026437, R01 DA06025, R01 DA02446 and R03 DA045289); the National Center for Responsible Gaming; the Connecticut Council on Problem Gambling; and the Connecticut Department of Mental Health and Addiction Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of these institutes or the National Institutes of Health.
1. Fletcher K, Nutton J, Brend D. Attachment, a matter of substance: the potential of attachment theory in the treatment of addictions. Clin Social Work J (2015) 43(1):109–17. doi: 10.1007/s10615-014-0502-5
3. Kim S, Kwok S, Mayes LC, Potenza MN, Rutherford HJV, Strathearn L. Early adverse experience and substance addiction: dopamine, oxytocin, and glucocorticoid pathways. Ann N Y Acad Sci (2017) 1394(1):74–91. doi: 10.1111/nyas.13140
4. Kuczkowski KM. The effects of drug abuse on pregnancy. Curr Opin Obstet Gynecol (2007) 19(6):578–85. doi: 10.1097/GCO.0b013e3282f1bf17
5. Rutherford HJV, Mayes LC. Parenting stress: a novel mechanism of addiction vulnerability. Neurobiol Stress (2019) 11:100172. doi: 10.1016/j.ynstr.2019.100172
6. Mayes LC, Feldman R, Granger R. The effects of polydrug use with and without cocaine on mother infant interaction at 3 and 6 months. Infant Behav Dev (1997) 20(4):489–502. doi: 10.1016/S0163-6383(97)90038-2
7. Strathearn L, Mayes LC. Cocaine addiction in mothers: potential effects on maternal care and infant development. Ann N Y Acad Sci (2010) 1187(1):1–183. doi: 10.1111/j.1749-6632.2009.05142.x
8. Minnes S, Singer LT, Humphrey-Wall R, Satayathum S. Psychosocial and behavioral factors related to the post-partum placements of infants born to cocaine-using women. Child Abuse Neglect (2008) 32(3):353–66. doi: 10.1016/j.chiabu.2007.12.002
9. Alvarez-Monjaras M, Mayes LC, Potenza MN, Rutherford HJ. A developmental model of addictions: integrating neurobiological and psychodynamic theories through the lens of attachment. Attach Hum Dev (2018) 21(6):616–37. doi: 10.1080/14616734.2018.1498113
10. Leshner AI. Addiction is a brain disease, and it matters. Science (1997) 278(5335):45–7. doi: 10.1126/science.278.5335.45
11. Levy N. Addiction is not a brain disease (and it matters). Front Psychiatry (2013) 4:24. doi: 10.3389/fpsyt.2013.00024
12. Lewis M. Addiction and the brain: development, not disease. Neuroethics (2017) 10(1):7–18. doi: 10.1007/s12152-016-9293-4
13. Lewis M. The biology of desire: why addiction is not a disease. New York: Public Affairs (2015).
14. Darbyshire P, Oster C, Carrig H. Children of parent(s) who have a gambling problem: a review of the literature and commentary on research approaches. Health Soc Care Commun (2001) 9(4):185–93. doi: 10.1046/j.0966-0410.2001.00302.x
15. Di Trani M, Renzi A, Vari C, Zavattini GC, Solano L. Gambling disorder and affect regulation: the role of alexithymia and attachment style. J Gambl Stud (2017) 33(2):649–59. doi: 10.1007/s10899-016-9637-3
16. APA. Diagnostic and statistical manual of mental disorders (DSM V). Washington, D.C: American Psychiatric Association (2013).
17. Potenza MN. Clinical neuropsychiatric considerations regarding nonsubstance or behavioral addictions. Dialogues Clin Neurosci (2017) 19(3):281–91.
18. Yau YH, Potenza MN. Gambling disorder and other behavioral addictions: recognition and treatment. Harv Rev Psychiatry (2015) 23(2):134–46. doi: 10.1097/HRP.0000000000000051
19. Ainsworth MDS. The development of infant-mother attachment. In: Caldwell BM, Ricciutti HN, editors. Review of child development research. University of Chicago Press (1973).
24. Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology (2009) 34(13):2655–66. doi: 10.1038/npp.2009.103
25. George C, Kaplan N, Main M. Adult Attachment Interview. In: Department of Psychology (unpublished manuscript), 3rd edition Berkley (1996).
26. Main M. The organized categories of infant, child, and adult attachment: flexible vs. inflexible attention under attachment-related stress. J Am Psychoanal Assoc (1997) 48(4): 1055–95. doi: 10.1177/00030651000480041801
27. Fairbairn CE, Briley DA, Kang D, Fraley RC, Hankin BL, Ariss T. A meta-analysis of longitudinal associations between substance use and interpersonal attachment security. Psychol Bull (2018) 144(5):532–55. doi: 10.1037/bul0000141
28. Crittenden M. Raising Parents. Attachment, parenting and child safety. Collumpton, UK: Willan Publishing (2008).
29. van Ijzendoorn MH. Adult attachment representations, parental responsiveness, and infant attachment: a meta-analysis on the predictive validity of the Adult Attachment Interview. Psychol Bull (1995) 117(3):387–403. doi: 10.1037/0033-2909.117.3.387
30. Shah PE, Fonagy P, Strathearn L. Is attachment transmitted across generations? The plot thickens. Clin Child Psychol Psychiatry (2010) 15(3):329–45. doi: 10.1177/1359104510365449
31. Crittenden PM. Special article: attachment, information processing, and psychiatric disorder. World Psychiatry (2002) 1(2):72–5.
32. Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci (2004) 24(17):4113–23. doi: 10.1523/JNEUROSCI.5322-03.2004
33. Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol (2002) 14(5):349–53. doi: 10.1046/j.0007-1331.2002.00776.x
34. Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology (2002) 27(1-2):127–38. doi: 10.1016/s0306-4530(01)00040-3
35. Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci (2004) 24(11):2825–31. doi: 10.1523/JNEUROSCI.3422-03.2004
36. Strathearn L. Maternal neglect: oxytocin, dopamine and the neurobiology of attachment. J Neuroendocrinol (2011) 23(11):1054–65. doi: 10.1111/j.1365-2826.2011.02228.x
37. McClure SM, Daw ND, Montague PR. A computational substrate for incentive salience. Trends Neurosci (2003) 26(8):423–8. doi: 10.1016/s0166-2236(03)00177-2
38. Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol (2002) 23(2):200–24. doi: 10.1006/frne.2002.0229
39. Insel TR. Is social attachment an addictive disorder? Physiol Behav (2003) 79(3):351–7. doi: 10.1016/s0031-9384(03)00148-3
40. Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience (2000) 95(2):567–75. doi: 10.1016/s0306-4522(99)00433-9
41. Amico JA, Mantella RC, Vollmer RR. Plasma corticosterone response of oxytocin deficient mice exposed to stress. Soc Neurosci (2002). Program No. 176.7.
42. Nestler EJ, Carlezon WA Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry (2006) 59(12):1151–9. doi: 10.1016/j.biopsych.2005.09.018
43. Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Hormone Res (2003) 59(4):161–79. doi: 10.1159/000069325
45. Greenberg M, Morris N. Engrossment: The newborn’s impact upon the father. Am J Orthopsychiatry (1974) 44(4):520–31. doi: 10.1111/j.1939-0025.1974.tb00906.x
46. Bronte-Tinkew J, Carrano J, Horowitz A, Kinukawa A. Involvement among resident fathers and links to infant cognitive outcomes. J Fam Issues (2008) 29(9):1211–44. doi: 10.1177/0192513X08318145
47. Garfield CF, Isacco A. Fathers and the well child visit. Pediatrics (2006) 117:637–45. doi: 10.1542/peds.2005-1612
48. Rajhans P, Goin-Kochel RP, Strathearn L, Kim S. It takes two! Exploring sex differences in parenting neurobiology and behavior. J Neuroendocrinol (2019) 31(9): e12721. doi: 10.1111/jne.12721
49. Sethna V, Perry E, Domoney J, Iles J, Psychogiou L, Rowbotham NEL, et al. Father–child interactions at 3 months and 24 months: contributions to children’s cognitive development at 24 months. Infant Ment Health J (2017) 38(3):378–90. doi: 10.1002/imhj.21642
50. Panter-Brick C, Burgess A, Eggerman M, McAllister F, Pruett K, Leckman JF. Practitioner review: engaging fathers—recommendations for a game change in parenting interventions based on a systematic review of the global evidence. J Child Psychol Psychiatry (2014) 55(11):1187–212. doi: 10.1111/jcpp.12280
51. Amato PR. Father–child relations, mother–child relations, and offspring psychological well-being in early adulthood. J Marriage Fam (1994) 56(4):1031–42. doi: 10.2307/353611
52. Stover CS, McMahon TJ, Moore K. A randomized pilot trial of two parenting interventions for fathers in residential substance use disorder treatment. J Subst Abuse Treat (2019) 104:116–27. doi: 10.1016/j.jsat.2019.07.003
53. McMahon TJ, Rounsaville BJ. Substance abuse and fathering: adding poppa to the research agenda. Addiction (2002) 97(9):1109–15. doi: 10.1046/j.1360-0443.2002.00159.x
54. Abajobir AA, Kisely S, Williams G, Clavarino A, Strathearn L, Najman JM. Gender-based differences in injecting drug use by young adults who experienced maltreatment in childhood: Findings from an Australian birth cohort study. Drug Alcohol Depend (2017) 173:163–9. doi: 10.1016/j.drugalcdep.2016.12.027
55. Abajobir AA, Najman JM, Williams G, Strathearn L, Clavarino A, Kisely S. Substantiated childhood maltreatment and young adulthood cannabis use disorders: a pre-birth cohort study. Psychiatry Res (2017) 256:21–31. doi: 10.1016/j.psychres.2017.06.017
56. Mills R, Alati R, Strathearn L, Najman JM. Alcohol and tobacco use among maltreated and non-maltreated adolescents in a birth cohort. Addiction (2014) 109(4):672–80. doi: 10.1111/add.12447
57. Mills R, Kisely S, Alati R, Strathearn L, Najman JM. Child maltreatment and cannabis use in young adulthood: a birth cohort study. Addiction (2017) 112(3):494–501. doi: 10.1111/add.13634
58. Tedgård E, Rastam M, Wirtberg I. Struggling with one’s own parenting after an upbringing with substance abusing parents. Int J Qual Studies Health Well-Being (2018) 13(1):1435100. doi: 10.1080/17482631.2018.1435100
59. Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards VE, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. Am J Preventive Med (2019) 56(6):774–86. doi: 10.1016/j.amepre.2019.04.001
60. Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics (2003) 111(3):564–72. doi: 10.1542/peds.111.3.564
61. Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci (2006) 256(3):174–86. doi: 10.1007/s00406-005-0624-4
62. Koball H, Jiang YH. Basic facts about low-income children: children under 18 years, 2016. In: National Center for Children in Poverty. Columbia University Mailman School of Public Health (2018).
63. Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci (2008) 1141:105–30. doi: 10.1196/annals.1441.030
64. Campbell A. Oxytocin and human social behavior. Pers Social Psychol Rev. (2010) 14:281–95. doi: 10.1177/1088868310363594
65. Kim S, Strathearn L. Trauma, mothering, and intergenerational transmission: a synthesis of behavioral and oxytocin research. Psychoanal Stud Child (2017) 70(1):200–23. doi: 10.1080/00797308.2016.1277897
66. Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci (2011) 12(9):524–38. doi: 10.1038/nrn3044
67. Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci (2012) 35(11):649–59. doi: 10.1016/j.tins.2012.08.004
68. Buisman-Pijlman FT, Sumracki NM, Gordon JJ, Hull PR, Carter CS, Tops M. Individual differences underlying susceptibility to addiction: Role for the endogenous oxytocin system. Pharmacol Biochem Behav (2014) 119:22–38. doi: 10.1016/j.pbb.2013.09.005
69. Tops M, Koole SL, IJzerman H, Buisman-Pijlman FT. Buisman-Pijlman: Why social attachment and oxytocin protect against addiction and stress: Insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacol Biochem Behav (2014) 119:39–48. doi: 10.1016/j.pbb.2013.07.015
70. Light KC, Grewen KM, Amico JA, Boccia M, Brownley KA, Johns JM. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict Behav (2004) 29(8):1541–64. doi: 10.1016/j.addbeh.2004.02.062
71. Johns JM, Lubin DA, Walker CH, Meter KE, Mason GA. Chronic gestational cocaine treatment decreases oxytocin levels in the medial preoptic area, ventral tegmental area and hippocampus in Sprague-Dawley rats. Neuropeptides (1997) 31(5):439–43. doi: 10.1016/S0143-4179(97)90037-8
72. Feldman R, Gordon I, Zagoory-Sharon O. The cross-generation transmission of oxytocin in humans. Horm Behav (2010) 58(4):669–76. doi: 10.1016/j.yhbeh.2010.06.005
73. Feldman R. Oxytocin and social affiliation in humans. Horm Behav (2012) 61(3):380–91. doi: 10.1016/j.yhbeh.2012.01.008
74. Todeschin AS, Winkelmann-Duarte EC, Jacob MH, Aranda BC, Jacobs S, Fernandes MC, et al. Effects of neonatal handling on social memory, social interaction, and number of oxytocin and vasopressin neurons in rats. Horm Behav (2009) 56(1):93–100. doi: 10.1016/j.yhbeh.2009.03.006
75. Bales KL, Boone E, Epperson P, Hoffman G, Carter CS. Are behavioral effects of early experience mediated by oxytocin? Front Psychiatry (2011) 2:24. doi: 10.3389/fpsyt.2011.00024
76. Levy F, Melo AI, Galef BG Jr., Madden M, Fleming AS. Complete maternal deprivation affects social, but not spatial, learning in adult rats. Dev Psychobiol (2003) 43(3):177–91. doi: 10.1002/dev.10131
77. Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology (2007) 32(5):437–50. doi: 10.1016/j.psyneuen.2007.02.008
78. Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (microtus ochrogaster). Front Behav Neurosci (2009) 3:17. doi: 10.3389/neuro.08.017.2009
79. Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science (1999) 286(5442):1155–8. doi: 10.1126/science.286.5442.1155
80. Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol (2000) 12(12):1145–8. doi: 10.1046/j.1365-2826.2000.00599.x
81. Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology (2003) 28(5):910–8. doi: 10.1038/sj.npp.1300128
82. Lyons-Ruth K, Block D. The disturbed caregiving system: relations among childhood trauma, maternal caregiving, and infant affect and attachment. Infant Ment Health J (1996) 17:257–75. doi: 10.1002/(SICI)1097-0355(199623)17:3<257::AID-IMHJ5>3.0.CO;2-L
83. Hesse E, Main M. Second-generation effects of unresolved trauma as observed in non-maltreating parents: dissociated, frightened, and threatening parental behavior. Psychoanal Inq (1999) 19:481–540. doi: 10.1080/07351699909534265
84. Iyengar U, Kim S, Martinez S, Fonagy P, Strathearn L. Unresolved trauma in mothers: intergenerational effects and the role of reorganization. Front Psychol (2014) 5:966. doi: 10.3389/fpsyg.2014.00966
85. Kim S, Fonagy P, Allen J, Martinez S, Iyengar U, Strathearn L. Mothers who are securely attached in pregnancy show more attuned infant mirroring 7 months postpartum. Infant Behav Dev (2014) 37(4):491–504. doi: 10.1016/j.infbeh.2014.06.002
86. Kim S, Fonagy P, Koos O, Dorsett K, Strathearn L. Maternal oxytocin response predicts mother-to-infant gaze. Brain Res (2014) 1580:133–42. doi: 10.1016/j.brainres.2013.10.050
87. Kim S. The mind in the making: Developmental and neurobiological origins of mentalizing. Pers Disord (2015) 6(4):356–65. doi: 10.1037/per0000102
88. Stockdale SE, Wells KB, Tang L, Belin TR, Zhang L, Sherbourne CD. The importance of social context: neighborhood stressors, stress-buffering mechanisms, and alcohol, drug, and mental health disorders. Soc Sci Med (2007) 65(9):1867–81. doi: 10.1016/j.socscimed.2007.05.045
89. Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci U S A (2005) 102(47):17237–40. doi: 10.1073/pnas.0504767102
90. Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry (2009) 14(10):954–8. doi: 10.1038/mp.2008.112
91. Bertsch K, Schmidinger I, Neumann ID, Herpertz SC. Reduced plasma oxytocin levels in female patients with borderline personality disorder. Horm Behav (2013) 63(3):424–9. doi: 10.1016/j.yhbeh.2012.11.013
92. Opacka-Juffry J, Mohiyeddini C. Experience of stress in childhood negatively correlates with plasma oxytocin concentration in adult men. Stress (2012) 15(1):1–10. doi: 10.3109/10253890.2011.560309
93. Feldman R, Golan O, Hirschler-Guttenberg Y, Ostfeld-Etzion S, Zagoory-Sharon O. Parent-child interaction and oxytocin production in pre-schoolers with autism spectrum disorder. Br J Psychiatry (2014) 205(2):107–12. doi: 10.1192/bjp.bp.113.137513
94. Pierrehumbert B, Torrisi R, Ansermet F, Borghini A, Halfon O. Adult attachment representations predict cortisol and oxytocin responses to stress. Attach Hum Dev (2012) 14(5):453–76. doi: 10.1080/14616734.2012.706394
95. Samuel S, Hayton B, Gold I, Feeley N, Carter CS, Zelkowitz P. Attachment security and recent stressful life events predict oxytocin levels: a pilot study of pregnant women with high levels of cumulative psychosocial adversity. Attach Hum Dev (2015) 17(3):272–87. doi: 10.1080/14616734.2015.1029951
96. Stauffer CS, Musinipally V, Suen A, Lynch KL, Shapiro B, Woolley JD. A two-week pilot study of intranasal oxytocin for cocaine-dependent individuals receiving methadone maintenance treatment for opioid use disorder. Addict Res Theor (2016) 24(6):490–8. doi: 10.3109/16066359.2016.1173682
97. Lin SH, Lee LT, Tsai HC, Chen KC, Chen WT, Lee IH, et al. Association between blood level of plasma oxytocin and novelty seeking among methadone-maintained heroin users. Neuropsychobiology (2015) 71(2):65–9. doi: 10.1159/000371637
98. Machin AJ, Dunbar RIM. The brain opioid theory of social attachment: a review of the evidence. Behavior (2011) 148:985–1025. doi: 10.1163/000579511X596624
99. Zoorob MJ, Salemi JL. Bowling alone, dying together: the role of social capital in mitigating the drug overdose epidemic in the United States. Drug Alcohol Depend (2017) 173:1–9. doi: 10.1016/j.drugalcdep.2016.12.011
100. Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci (2007) 30(5):194–202. doi: 10.1016/j.tins.2007.03.006
101. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology (2010) 35(1):217–38. doi: 10.1038/npp.2009.110
102. Grant JE, Brewer JA, Potenza MN. The neurobiology of substance and behavioral addictions. CNS Spectr (2006) 11(12):924–30. doi: 10.1017/s109285290001511x
103. Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci (2015) 16(5):305–12. doi: 10.1038/nrn3939
104. Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, et al. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav (1998) 59(4):859–72. doi: 10.1016/s0091-3057(97)00510-8
105. Pena CJ, Neugut YD, Calarco CA, Champagne FA. Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. Eur J Neurosci (2014) 39(6):946–56. doi: 10.1111/ejn.12479
106. Afonso VM, King SJ, Novakov M, Burton CL, Fleming AS. Accumbal dopamine function in postpartum rats that were raised without their mothers. Horm Behav (2011) 60(5):632–43. doi: 10.1016/j.yhbeh.2011.08.016
107. Arborelius L, Eklund MB. Both long and brief maternal separation produces persistent changes in tissue levels of brain monoamines in middle-aged female rats. Neuroscience (2007) 145(2):738–50. doi: 10.1016/j.neuroscience.2006.12.007
108. Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse (1999) 32(1):37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4
109. Oswald LM, Wand GS, Kuwabara H, Wong DF, Zhu S, Brasic JR. History of childhood adversity is positively associated with ventral striatal dopamine responses to amphetamine. Psychopharmacol (Berl) (2014) 231(12):2417–33. doi: 10.1007/s00213-013-3407-z
110. Chocyk A, Dudys D, Przyborowska A, Majcher I, Mackowiak M, Wedzony K. Maternal separation affects the number, proliferation and apoptosis of glia cells in the substantia nigra and ventral tegmental area of juvenile rats. Neuroscience (2011) 173:1–18. doi: 10.1016/j.neuroscience.2010.11.037
111. Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci (2004) 19(7):1863–74. doi: 10.1111/j.1460-9568.2004.03286.x
112. Rentesi G, Antoniou K, Marselos M, Syrrou M, Papadopoulou-Daifoti Z, Konstandi M. Early maternal deprivation-induced modifications in the neurobiological, neurochemical and behavioral profile of adult rats. Behav Brain Res (2013) 244:29–37. doi: 10.1016/j.bbr.2013.01.040
113. Zuckerman M, Link K. Construct validity for the sensation-seeking scale. J Consult Clin Psychol (1968) 32(4):420–6. doi: 10.1037/h0026047
114. Buchmann AF, Hohm E, Witt SH, Blomeyer D, Jennen-Steinmetz C, Schmidt MH, et al. Role of CNR1 polymorphisms in moderating the effects of psychosocial adversity on impulsivity in adolescents. J Neural Transm (Vienna) (2015) 122(3):455–63. doi: 10.1007/s00702-014-1266-3
115. Lukasiewicz M, Neveu X, Blecha L, Falissard B, Reynaud M, Gasquet I. Pathways to substance-related disorder: a structural model approach exploring the influence of temperament, character, and childhood adversity in a national cohort of prisoners. Alcohol (2008) 43(3):287–95. doi: 10.1093/alcalc/agm183
116. Adams JB, Heath AJ, Young SE, Hewitt JK, Corley RP, Stallings MC. Relationships between personality and preferred substance and motivations for use among adolescent substance abusers. Am J Drug Alcohol Abuse (2003) 29(3):691–712. doi: 10.1081/ADA-120023465
117. Sharp C, Kim S, Herman L, Pane H, Reuter T, Strathearn L. Major depression in mothers predicts reduced ventral striatum activation in adolescent female offspring with and without depression. J Abnorm. Psychol. (2014) 123(2):298–309. doi: 10.1037/a0036191
118. Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Dev Psychobiol (1996) 29(3):191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M
119. Albers EM, Riksen-Walraven JM, Sweep FC, de Weerth C. Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. J Child Psychol Psychiatry (2008) 49(1):97–103. doi: 10.1111/j.1469-7610.2007.01818.x
120. Kuo PX, Saini EK, Tengelitsch E, Volling BL. Is one secure attachment enough? Infant cortisol reactivity and the security of infant-mother and infant-father attachments at the end of the first year. Attach Hum Dev (2019) 21(5):1–19. doi: 10.1080/14616734.2019.1582595
121. Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav (2006) 50(4):623–31. doi: 10.1016/j.yhbeh.2006.06.012
122. McCrory E, De Brito SA, Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front Psychiatry (2011) 2:48. doi: 10.3389/fpsyt.2011.00048
123. Cicchetti DFAR. The impact of child maltreatment: a review of child maltreatment and psychopathology on neuroendocrine functioning. Dev Psychopathol (2001) 13:783–804. doi: 10.1017/s0954579401004035
124. Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci (2004) 7(8):847–54. doi: 10.1038/nn1276
125. Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, et al. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev (2005) 29(4-5):649–74. doi: 10.1016/j.neubiorev.2005.03.011
126. Marco EM, Adriani W, Llorente R, Laviola G, Viveros MP. Detrimental psychophysiological effects of early maternal deprivation in adolescent and adult rodents: altered responses to cannabinoid exposure. Neurosci Biobehav Rev (2009) 33(4):498–507. doi: 10.1016/j.neubiorev.2008.03.008
127. Faturi CB, Tiba PA, Kawakami SE, Catallani B, Kerstens M, Suchecki D. Disruptions of the mother–infant relationship and stress-related behaviours: altered corticosterone secretion does not explain everything. Neurosci Biobehav Rev (2010) 34(6):821–34. doi: 10.1016/j.neubiorev.2009.09.002
128. Rao U, Hammen CL, Poland RE. Mechanisms underlying the comorbidity between depressive and addictive disorders in adolescents: interactions between stress and HPA activity. Am J Psychiatry (2009) 166(3):361–9. doi: 10.1176/appi.ajp.2008.08030412
129. Kaplow JB, Curran PJ, Angold A, Costello EJ. The prospective relation between dimensions of anxiety and the initiation of adolescent alcohol use. J Clin Child Psychol (2001) 30(3):316–26. doi: 10.1207/S15374424JCCP3003_4
130. Rutherford HJ, Williams SK, Moy S, Mayes LC, Johns JM. Disruption of maternal parenting circuitry by addictive process: rewiring of reward and stress systems. Front Psychiatry (2011) doi: 10.3389/fpsyt.2011.00037
131. Lijffijt M, Hu K, Swann AC. Stress modulates illness-course of substance use disorders: a translational review. Front Psychiatry (2014) 5:83. doi: 10.3389/fpsyt.2014.00083
132. Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci (2005) 25(22):5389–96. doi: 10.1523/JNEUROSCI.0955-05.2005
133. Nikulina EM, Lacagnina MJ, Fanous S, Wang J, Hammer RP Jr. Intermittent social defeat stress enhances mesocorticolimbic DeltaFosB/BDNF co-expression and persistently activates corticotegmental neurons: implication for vulnerability to psychostimulants. Neuroscience (2012) 212:38–48. doi: 10.1016/j.neuroscience.2012.04.012
134. McKenzie M, Olsson CA, Jorm AF, Romaniuk H, Patton GC. Association of adolescent symptoms of depression and anxiety with daily smoking and nicotine dependence in young adulthood: findings from a 10-year longitudinal study. Addiction (2010) 105(9):1652–9. doi: 10.1111/j.1360-0443.2010.03002.x
135. Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ. The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. Am J Orthopsychiatry (1996) 66(1):17–31. doi: 10.1037/h0080151
136. Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci U S A (2011) 108(34):14324–9. doi: 10.1073/pnas.1105371108
137. Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci (2010) 13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x
138. Kim S, Fonagy P, Allen J, Strathearn L. Mothers’ unresolved trauma blunts amygdala response to infant distress. Soc Neurosci (2014) 9(4):352–63. doi: 10.1080/17470919.2014.896287
139. Kim S, Iyengar U, Mayes LC, Potenza MN, Rutherford HJV, Strathearn L. Mothers with substance addictions show reduced reward responses when viewing their own infant’s face. Hum Brain Mapp (2017) 38(11):5421–39. doi: 10.1002/hbm.23731
140. Lu YL, Richardson HN. Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience (2014) 277:139–51. doi: 10.1016/j.neuroscience.2014.06.053
141. Becker HC. Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology (2017) 122:115–26. doi: 10.1016/j.neuropharm.2017.04.028
142. Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology (2010) 151(5):2276–86. doi: 10.1210/en.2009-1271
143. Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience (2003) 121(3):537–44. doi: 10.1016/s0306-4522(03)00555-4
144. Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett (2006) 394(2):146–51. doi: 10.1016/j.neulet.2005.10.019
145. Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, et al. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci U S A (2013) 110(50):20308–13. doi: 10.1073/pnas.1314190110
146. Young KA, Liu Y, Gobrogge KL, Wang H, Wang Z. Oxytocin reverses amphetamine-induced deficits in social bonding: evidence for an interaction with nucleus accumbens dopamine. J Neurosci (2014) 34(25):8499–506. doi: 10.1523/jneurosci.4275-13.2014
147. Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry (2003) 54(12):1389–98. doi: 10.1016/s0006-3223(03)00465-7
148. MacDonald K, Feifel D. Oxytocin’s role in anxiety: a critical appraisal. Brain Res (2014) 1580:22–56. doi: 10.1016/j.brainres.2014.01.025
149. Cardoso C, Kingdon D, Ellenbogen MA. A meta-analytic review of the impact of intranasal oxytocin administration on cortisol concentrations during laboratory tasks: moderation by method and mental health. Psychoneuroendocrinology (2014) 49:161–70. doi: 10.1016/j.psyneuen.2014.07.014
150. Strathearn L, Kim S. Mothers’ amygdala response to positive or negative infant affect is modulated by personal relevance. Front Neurosci (2013) 7:176. doi: 10.3389/fnins.2013.00176
151. Lyden H, Suchman N. Transmission of parenting models at the level of representation: implications for mother–child dyads, affected by maternal substance abuse. In: Suchman N, Pajulo M, Mayes LC, editors. Parenting and substance abuse: Developmental approaches to intervention. Oxford University Press (2013).
152. Suchman NE. Mothering from the Inside Out: A mentalization-based therapy for mothers in treatment for drug addiction. Int J Birth Parent Educ (2016) 3(4):19–24. doi: 10.1093/med:psych/9780199743100.001.0001
Keywords: attachment, addiction, oxytocin, dopamine, glucocorticoid, adverse childhood experiences
Citation: Strathearn L, Mertens CE, Mayes L, Rutherford H, Rajhans P, Xu G, Potenza MN and Kim S (2019) Pathways Relating the Neurobiology of Attachment to Drug Addiction. Front. Psychiatry 10:737. doi: 10.3389/fpsyt.2019.00737
Received: 09 June 2019; Accepted: 16 September 2019;
Published: 08 November 2019.
Edited by:
Andrew J. Lewis, Murdoch University, AustraliaReviewed by:
Mark J. Ferris, Wake Forest School of Medicine, United StatesCopyright © 2019 Strathearn, Mertens, Mayes, Rutherford, Rajhans, Xu, Potenza and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lane Strathearn, bGFuZS1zdHJhdGhlYXJuQHVpb3dhLmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.