
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 24 October 2019
Sec. Psychological Therapy and Psychosomatics
Volume 10 - 2019 | https://doi.org/10.3389/fpsyt.2019.00717
 Constance H. Fung1,2*
Constance H. Fung1,2* Jennifer L. Martin1,2
Jennifer L. Martin1,2 Cathy Alessi1,2
Cathy Alessi1,2 Joseph M. Dzierzewski3
Joseph M. Dzierzewski3 Ian A. Cook4,5
Ian A. Cook4,5 Alison Moore6
Alison Moore6 Austin Grinberg1,2
Austin Grinberg1,2 Michelle Zeidler1,2
Michelle Zeidler1,2 Lara Kierlin7
Lara Kierlin7Chronic use of hypnotic medications such as benzodiazepines is associated with adverse consequences including increased risk of falls. Efforts to help patients discontinue these medications have had varying levels of success. We developed a blinded (masked) tapering protocol to help patients taper off hypnotics. In this blinded protocol, patients consented to a drug taper but agreed to forego knowledge about the specific tapering schedule or the actual dose each night until the end of the taper. Blinded tapering aims to reduce negative expectancies for withdrawal effects that may impair a patient’s successful discontinuation of hypnotics. In preparation for a randomized trial, we tested the feasibility of adding a blinded tapering component to current best evidence practice [supervised hypnotic taper combined with cognitive behavioral therapy for insomnia (CBTI)] in 5 adult patients recruited from an outpatient mental health practice in Oregon. A compounding pharmacy prepared the blinded capsules for each patient. During the gradual blinded taper, each participant completed CBTI. Measures collected included feasibility/process (e.g., recruitment barriers), hypnotic use, the Dysfunctional Beliefs and Attitudes about Sleep Scale, Insomnia Severity Index, Epworth Sleepiness Scale, and Patient Health Questionnaire-9 (depressive symptoms). The intervention was feasible, and participants reported high satisfaction with the protocol and willingness to follow the same treatment again. All five participants successfully discontinued their hypnotic medication use post-treatment. Dysfunctional beliefs/attitudes about sleep and insomnia severity improved. Blinded tapering is a promising new method for improving hypnotic discontinuation among patients treated with a combination of hypnotic tapering plus CBTI.
Clinical guidelines recommend non-pharmacological therapy such as cognitive behavioural therapy for insomnia (CBTI) as first-line treatment of chronic insomnia disorder in all adults (1). Pharmacological therapy for insomnia, however, is common (2). Chronic use of hypnotic medications such as benzodiazepines is associated with adverse consequences such as increased risk of falls (3, 4). Discontinuation of hypnotics such as benzodiazepines and benzodiazepine receptor agonists is recommended, particularly in older adults (5) and patients who have not tried non-pharmacological therapy. Published studies have employed a variety of discontinuation strategies, including supervised dose reduction, and have established the safety of supervised reduction approaches (6). The efficacy of supervised dose reduction alone, however, is modest, and therefore, approaches that combine supervised dose reduction with psychological interventions such as CBTI have been recommended. Unfortunately, studies examining the combination of these two treatment methods have yielded mixed results (7).
One factor that may interfere with dose reduction of a hypnotic may be discomfort related to withdrawal symptoms from pharmacological effects. However, pharmacological effects likely do not entirely explain the symptoms that patients report when tapering a hypnotic medication or similar agent (8). Negative expectations about stopping an agent have the potential to produce withdrawal symptoms (9). A strategy to overcome these expectations is blinded (or masked) tapering of a drug; this entails gradually tapering the drug without providing the patient with explicit knowledge of the taper schedule.
This study was designed to test the feasibility of adding a blinded tapering component to an existing protocol that combines supervised tapering of hypnotics with CBTI. Our primary aim was to gather feasibility/process information needed to design a randomized trial testing the benefits of blinded versus standard tapering. Our secondary aim was to measure differences in hypnotic use and attitudes about sleep and hypnotics from baseline to post-treatment. Our a priori hypotheses were that hypnotic use would decrease from baseline to post-treatment and that attitudes and beliefs about sleep and hypnotics would improve from baseline to post-treatment.
Our study design was a case series intended to assess the feasibility of adding a blinded tapering component to a protocol involving a supervised gradual hypnotic taper plus CBTI. Between February 2014 and October 2014, patients were recruited from a community outpatient mental health practice. Adult patients with chronic hypnotic use who were referred for treatment of chronic insomnia were provided a study information sheet at their initial appointment and given the opportunity to ask questions about the study. Interested patients were invited to give full informed consent to the study protocol that included CBTI concurrent with blinded tapering of their current sleep medication. Patients were fully informed that during the study they would not know the amount of hypnotic they would be taking each night, but would be informed of the tapering schedule at the end of the study. Follow-up was limited to a single post-treatment assessment because the purpose of the study was to assess feasibility of the intervention and to test blinding procedures. No financial incentives were provided to participants. Participants were responsible for usual treatment costs (e.g., co-payments) associated with usual care, including office visits and medications. All research activities were approved by the Providence Health and Services Institutional Review Board.
Patients who expressed interest in participating were screened for eligibility criteria through a clinical assessment interview with the study principal investigator. The eligibility criteria were: age ≥18 years old, current insomnia disorder according to insomnia diagnostic criteria of the International Classification of Sleep Disorders, 2nd edition (10) (which were the criteria in place at the time), current use of benzodiazepine or non-benzodiazepine benzodiazepine receptor agonist or trazodone for sleep on more than 50% of nights for at least 3 months, and availability to attend 6 weeks of in-person CBTI. Patients were excluded if they: had a history of sleep apnea of moderate or severe severity (apnea–hypopnea index ≥ 15) or periodic limb movements during sleep [periodic limb movement with arousal ≥ 15 (10)] based on a prior sleep study, were currently participating in psychotherapy with a provider other than the study therapist, were using psychotropic drugs other than the study medications or over the counter sleep agents, had severe mental illness including major depressive disorder with active suicidal ideation, bipolar disorder, current psychosis, or current alcohol/substance abuse, or allergy or intolerance to both lactose and corn (which were in the inert fillers used by the compounding pharmacy to prepared the study medication capsules).
Demographic measures included age, gender, living arrangement, level of education, and employment status. The duration of hypnotic use and number of prior attempts to discontinue sedative-hypnotic medications was also collected. Expectations and credibility of the study program (11) were collected at baseline and post-treatment (immediately after the treatment was completed). Sleep onset latency, wake after sleep onset, time in bed, and total sleep time were collected daily in a sleep diary during intervention. Participants were also asked to record on their sleep diary the dose they believed they were taking each night (“guessed dose”). At post-treatment, satisfaction was assessed with the following items: “I was satisfied with the medication reduction method” and “I would be willing to follow the same treatment again if necessary” [with response options ranging from “Strongly agree” (0) to “Strongly disagree” (3)].
Several structured questionnaires were performed at baseline and post-treatment. The Epworth Sleepiness Scale measured hypersomnolence (8 items; score range 0–24; higher scores suggest more daytime sleepiness) (12). The Insomnia Severity Index measured insomnia severity (13). The Dysfunctional Beliefs and Attitudes Scale-16 (DBAS-16; 16 items; score range 0–160; higher scores suggest more dysfunctional beliefs) (14) was also administered, with a mean score ≥ 4 indicating unrealistic expectations about sleep (15). The Patient Health Questionnaire-9 was collected to measure depressed mood (9 items; score range 0–27; higher scores are associated with worse mood) (16). During the treatment, the Clinical Institute Withdrawal Assessment Scale-Benzodiazepine (17) was administered to test for symptoms of withdrawal (20 items; score range 0-80; higher scores are associated with more withdrawal symptoms).
Table 1 summarizes the intervention, which was received by all participants. Participants were informed they would receive a supervised hypnotic tapering protocol, with the goal of eliminating hypnotic use by the sixth week of treatment (18–20). Participants were not informed of either their particular tapering protocol or the actual dose of hypnotic they would receive each night. Each participant’s tapering schedule was individualized according to the type of hypnotic, baseline dose, and frequency of use. Several principles were employed during intervention development, including: 1) assisting participant in setting goals, 2) (if applicable) stabilization of hypnotic use to one hypnotic agent if more than one hypnotic in use (in this case series, no participants were on more than one type of hypnotic at baseline), 3) (if applicable) conversion of hypnotic use from as-needed to a schedule of nightly capsule use, and 4) reduction of the initial dose by roughly 25% every 1 to 2 weeks until the lowest available dose of active drug was reached. The actual capsules were provided by a compounding pharmacy, were not marked with dose information, and contained a step-wise decrease in the dose of active hypnotic medication plus a step-wise increase in the amount of inactive filler (either lactose or corn starch, depending upon whether the patient reported a history of allergy or intolerance to either substance). In addition, the protocol included the introduction of an increasing number of reduced drug nights each week through blinded tapering (3). The actual dose reductions in the individualized tapering protocol schedules varied based upon each patient’s readiness to discontinue the hypnotic (reported at baseline) and adjusted (if necessary) based on findings from weekly monitoring. At the end of treatment, all participants would expect that the dose in the final capsule was zero milligrams. For participants taking a benzodiazepine or benzodiazepine receptor agonist, we monitored for the presence or absence of withdrawal symptoms (based on Clinical Institute Withdrawal Assessment Scale-Benzodiazepine (17) for participants taking a benzodiazepine or benzodiazepine receptor agonist). The capsules that were prepared by a local licensed compounding pharmacy were given to participants in a blister pack to ensure the correct capsule ingested on the correct date and that no information on dosage was revealed to the participant.
Participants received six sessions of CBTI delivered by a licensed psychiatrist/sleep medicine physician with specific specialty training in CBTI.
Six patients were provided detailed information about the study. Of these six patients, one declined due to perceived “psychological dependence” on the hypnotic. Five patients (4 female; 1 male) were consented and enrolled. Table 2 summarizes the cases. The mean age was 55.9 years (SD 19.6; range 49–72 years). Four patients lived with their spouse. One patient lived with his/her spouse and other family. The mean years of formal education was 17.2 years (SD 4.1; range 14 to 22 years). No participants were currently employed at the time of the study.
Table 3 summarizes results related to the feasibility of the intervention (in preparation for a future randomized trial). Patients were willing to participate in the study. The main challenge to recruitment was financial—namely, the co-payment associated with 6 weekly sessions. Providing information on recruitment feasibility, one prospective patient who declined participation voiced “attachment” to his/her zolpidem prescription. Participants were very satisfied with the protocol, although they wished they could have received all study medications at the initial session. The participants’ health insurances covered a maximum of 30 days per prescription, which limited the supply that could be dispensed at the initial study visit. No participant disclosed using “extra” doses of hypnotics that were not part of their blister packs. No issues were encountered with preparation of the study medications. Participants’ satisfaction at post-treatment with the method of medication reduction was 0.4, indicating strong satisfaction (where 0 is strong satisfaction). Participants’ willingness to follow the same treatment again was 0.4 (where 0 is strong willingness). Finally, the participants’ assessment at post-treatment that they would follow the treatment for getting better sleep was 0.4, which indicates strong agreement.
All 5 participants had complete cessation of hypnotic use at post-treatment compared with baseline (5/5 were using hypnotic at baseline versus 0/5 at post-treatment). Table 4 summarizes the changes in patient-reported sleep and mood measures. For the DBAS-16, 4/5 participants at baseline had a mean DBAS item score of ≥4 (threshold for unrealistic expectations about sleep) compared with 0/5 at post-treatment. For the Insomnia Severity Index, 4/5 of participants had “clinical insomnia” (ISI > 14) at baseline compared with 0/5 at post-treatment. For the Epworth Sleepiness Scale, 1/5 of participants had hypersomnolence (ESS > 10) at baseline compared with 0/5 post-treatment. For the Patient Health Questionnaire-9 (PHQ-9), 2/5 participants had at least mild depressive symptoms (≥5) at baseline compared with 0/5 at post-treatment. Two participants were assessed for benzodiazepine withdrawal (the other 3 were not taking benzodiazepine receptor agonists) and neither had concerning CIWA scores (both were in the low end of the “mild: 1–20” category).
Figures 1–5 show diary-reported sleep onset latency, wake after sleep onset, total sleep time, time in bed, and sleep efficiency during CBTI and blinded hypnotic tapering. Overall, sleep efficiency improved from the treatment day 1 to the end of treatment. Participants’ sleep onset latency and wake after sleep onset remained the same or improved during treatment. Figure 1 also shows the nightly guessed dose of medication for each participant. Figure 6 shows the participants’ estimates of their nightly doses.
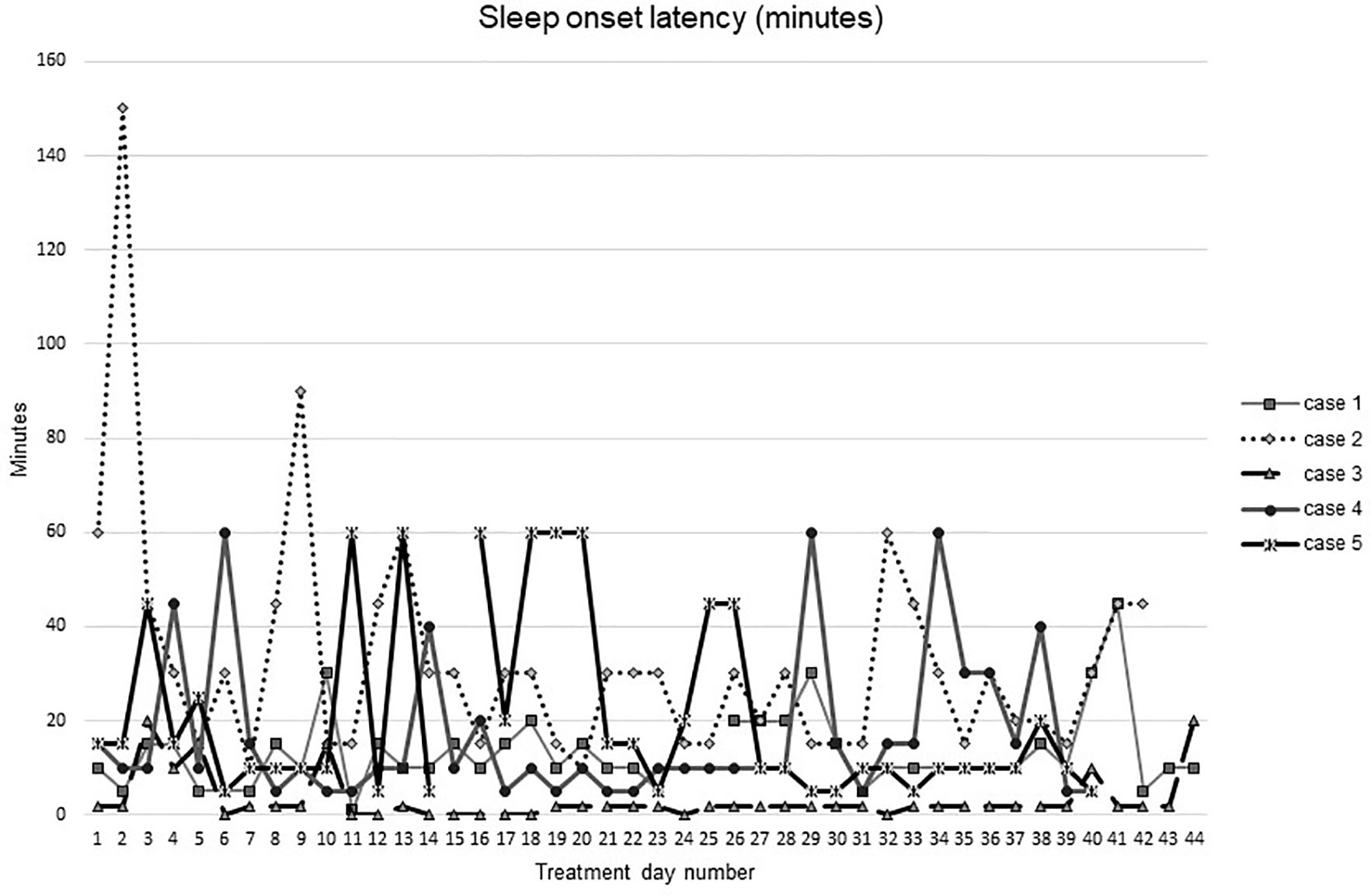
Figure 1 Sleep onset latency during intervention (cognitive behavioral therapy for insomnia and hypnotic taper).
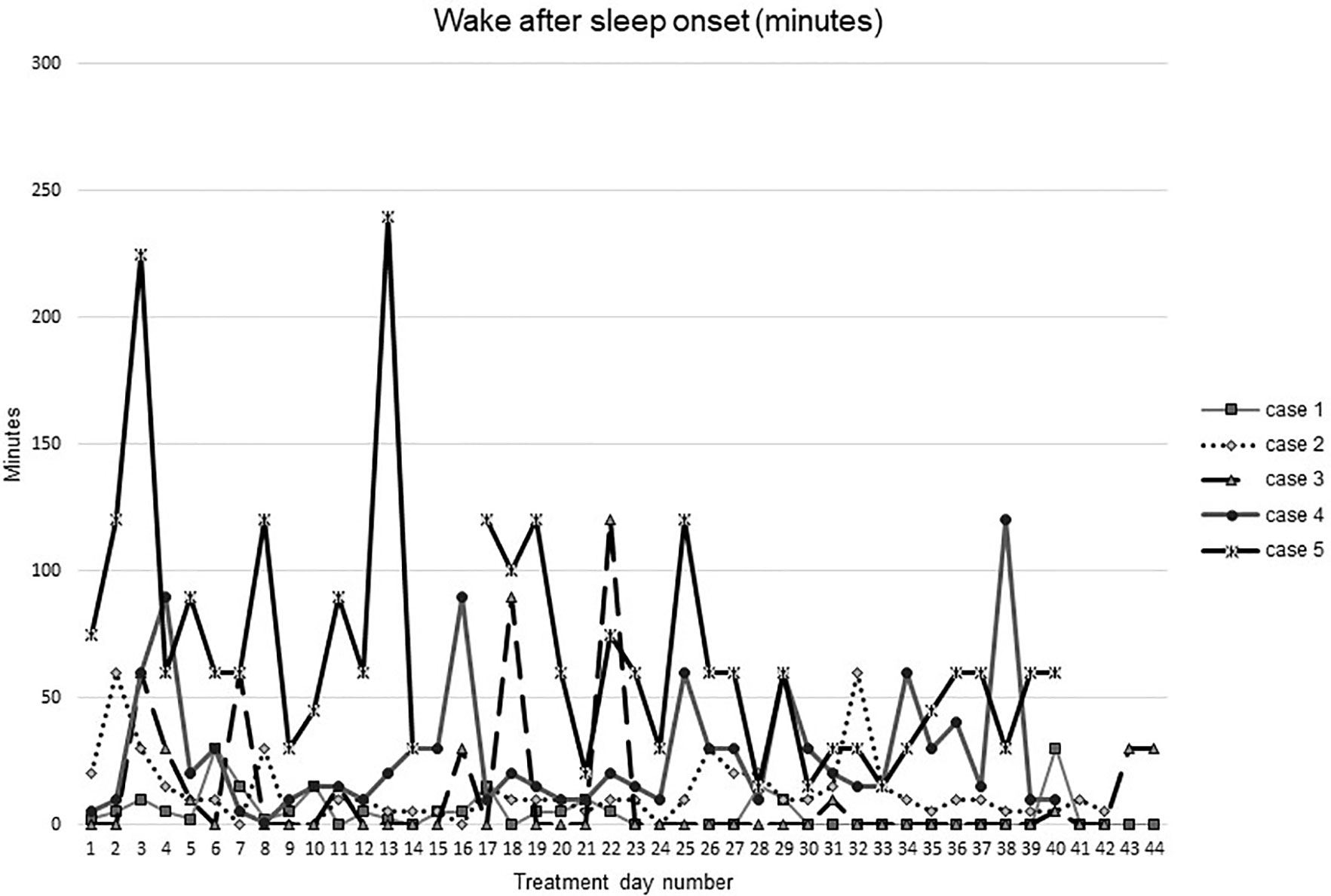
Figure 2 Wake after sleep onset during intervention (cognitive behavioral therapy for insomnia and hypnotic taper).
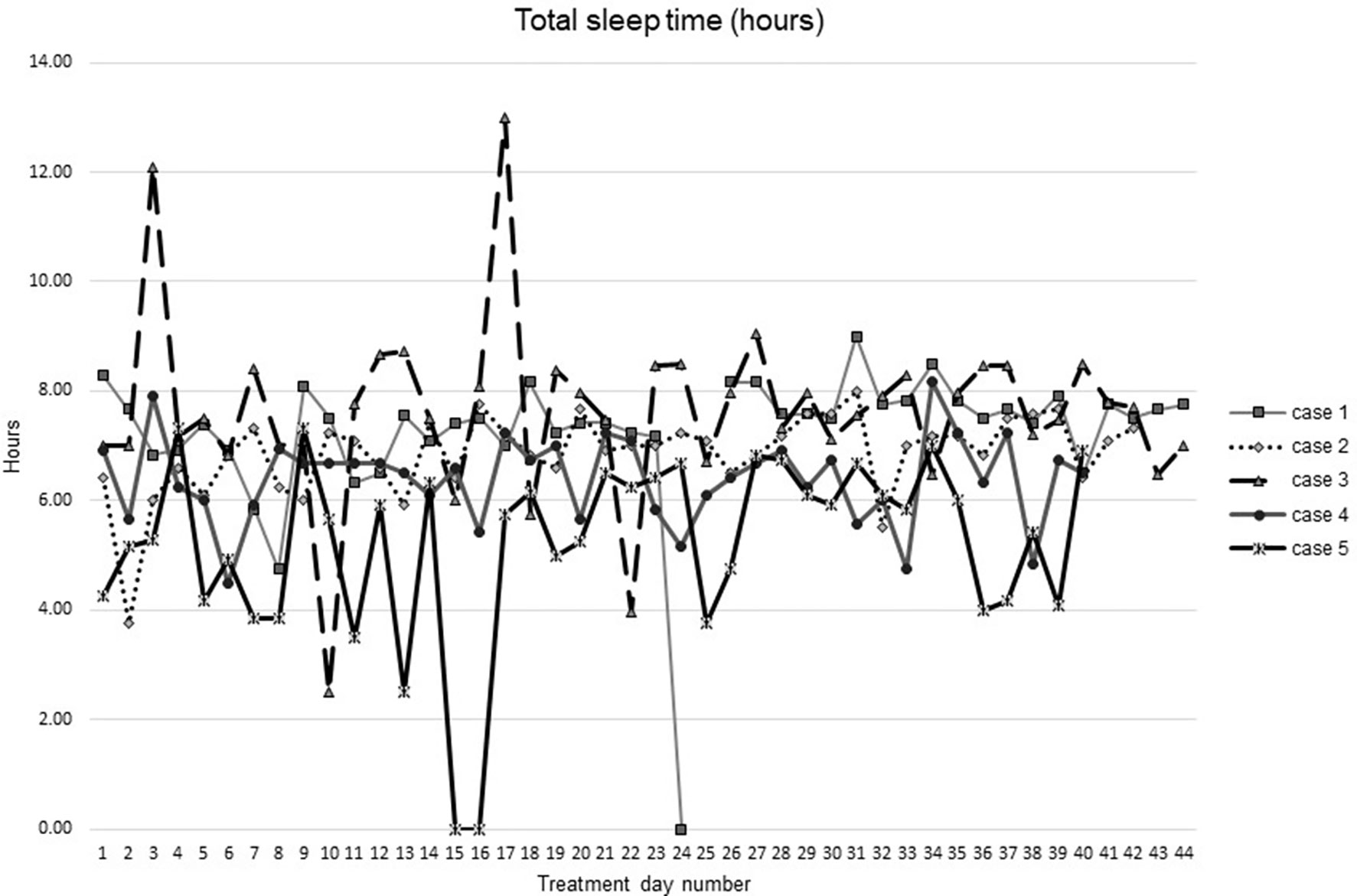
Figure 3 Total sleep time from during intervention (cognitive behavioral theraphy for insomia and hypnotic taper).
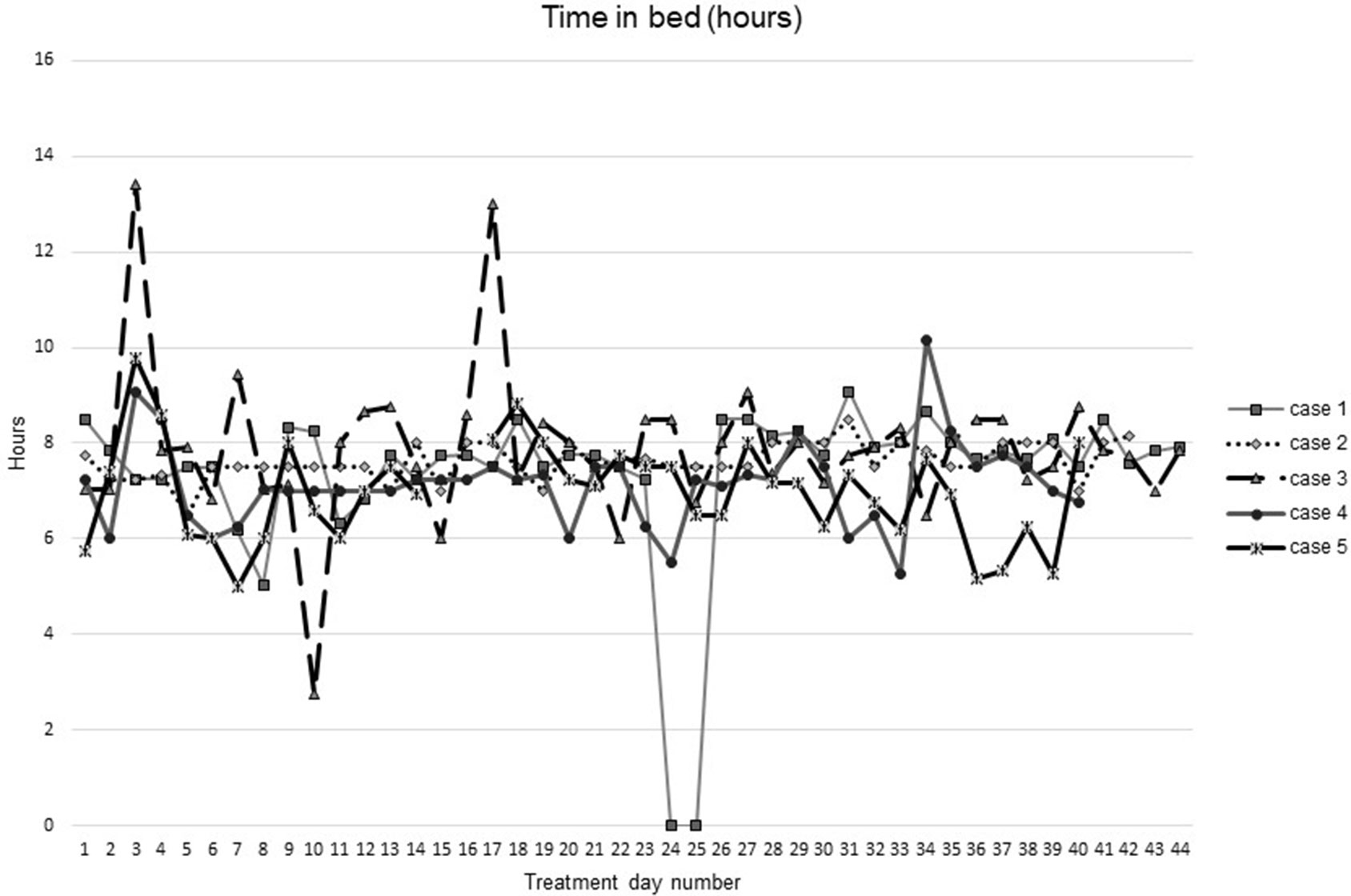
Figure 4 Time in bed during intervention (cognitive behavioral therapy for insomnia and hypnotic taper).
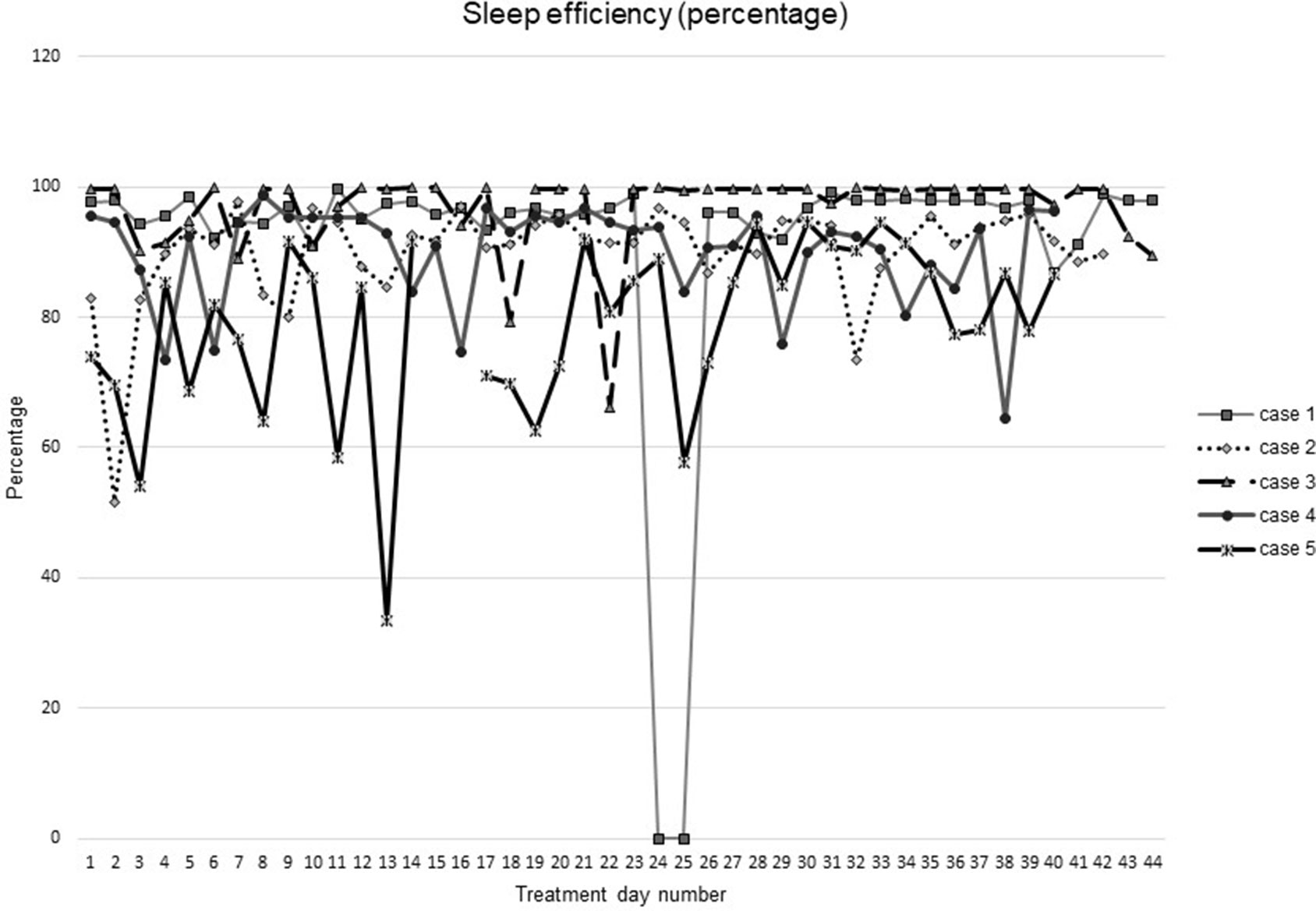
Figure 5 Sleep efficiency during intervention (cognitive behavioral therapy for insomnia and hypnotic taper).
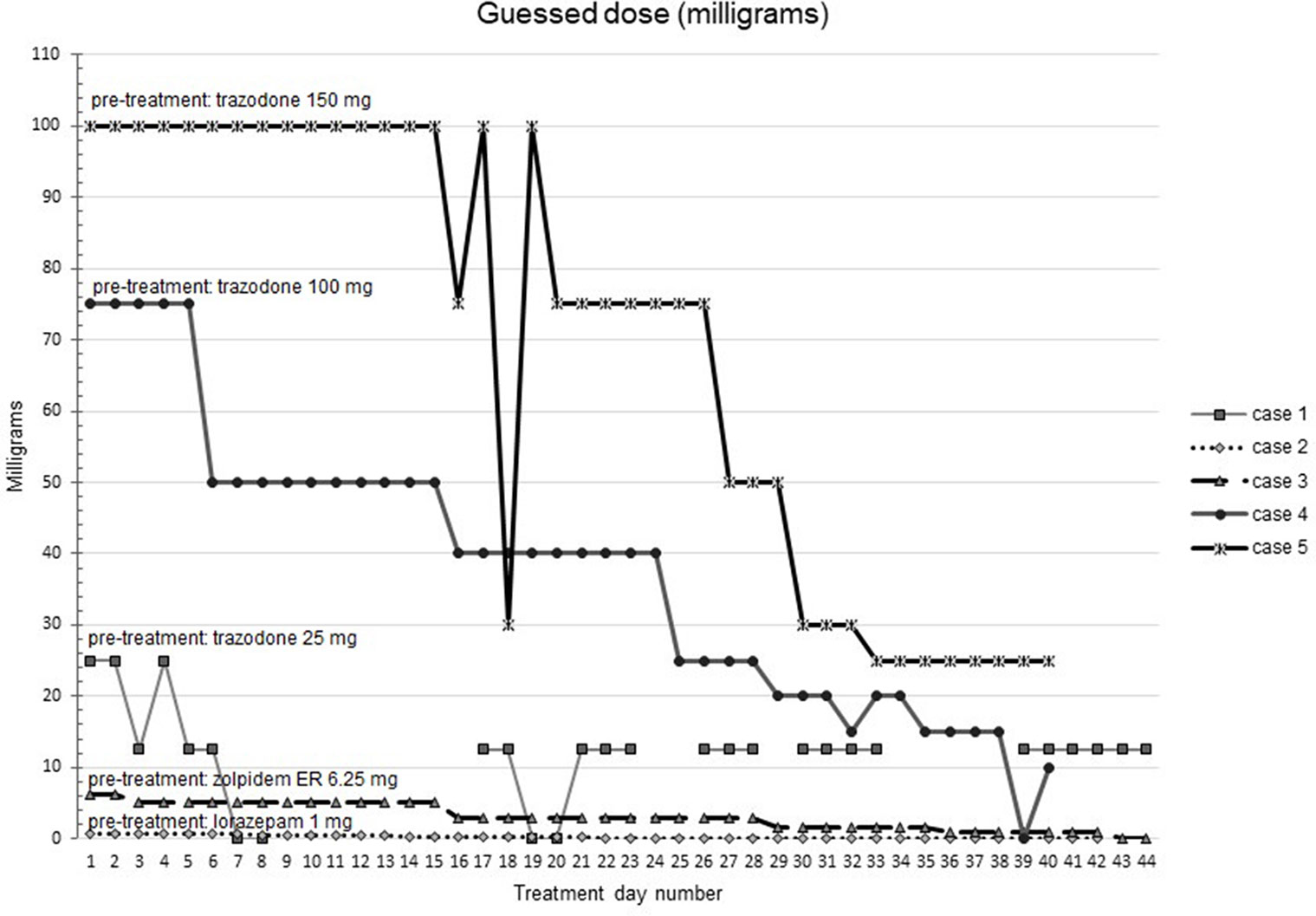
Figure 6 Participants’ beliefs about their hypnotic doses during intervention (cognitive behavioral therapy for insomnia and hypnotic taper). Participant 1 (baseline trazodone 25 mg) did not report any sleep on one day 24 during treatment due to an acute medical illness. Participant 2 took lorazepam 1 mg at baseline. Participant 3 took zolpidem extended release 6.25 mg at baseline. Participant 4 took trazodone 100 mg at baseline. Participant 5 took trazodone 150 mg at baseline.
These results indicate that the addition of blinded tapering to a CBTI plus hypnotic taper protocol is feasible and may be beneficial to patients who are interested in discontinuing their hypnotic medication. The program was well-received by study participants. All participants were successfully drug-free at the end of the program. Insomnia symptoms, dysfunctional beliefs about sleep, and depressive symptoms improved. No patients experienced clinical withdrawal symptoms.
We believe the blinded taper protocol may improve hypnotic discontinuation success by reducing patient’s beliefs that they will experience withdrawal symptoms during tapering, such as increased insomnia or anxiety. These beliefs, also sometimes called expectancies, may have been formed during prior attempts to taper medications (i.e., direct experience), but also may develop through observing or speaking with friends or family who have attempted hypnotic discontinuation (21). Negative expectancies that occur when patients have been provided information about a drug that has been administered have been demonstrated in many studies. For example, a study of adult smokers found that participants reported greater reduction in nicotine cravings when informed that the nicotine-containing inhaler contained nicotine compared with when they were informed that the inhaler was nicotine-free, irrespective of the actual nicotine content (22). In a recent study of caffeine tapering, individuals assigned to a blinded tapering protocol reported fewer withdrawal symptoms than individuals assigned to open tapering where individuals were told their daily caffeine dose (8).
This feasibility study identified several issues that should be addressed in planning for a larger study. Participants in this study were responsible for paying costs associated with their study medications. A future study should provide study medication at reduced or no cost to participants. We also identified several issues that would help with data analysis of a future study including biomarkers of medication use. Finally, a longer follow-up time period would help with comparisons to published studies of duration of effects of the intervention.
Blinded tapering of hypnotic medications is a promising component that could be added to existing strategies. We are currently conducting larger, controlled trials [CBTI plus blinded taper versus CBTI plus standard (open) taper; NCT 03687086] testing the addition of blinded tapering to currently recommended approaches.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by Providence Health and Services Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
CF, JM, CA, and LK contributed to the design of the study. LK contributed to data collection. CF and LK contributed to data analysis. CF, JM, CA, JD, IC, AM, AG, MZ, and LK contributed to interpretation of data and preparation of the manuscript.
Research reported in this publication was supported by the Department of Veterans Affairs [IIR 17-234], UCLA Department of Medicine, National Institute On Aging of the National Institutes of Health [K23AG045937, K23AG049955, and 1R01AG057929-01], The Beeson Career Development in Aging Research Award Program (supported by NIA, AFAR, The John A. Hartford Foundation, and The Atlantic Philanthropies), and National Institute of Heart, Lung and Blood [K24 HL143055]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, UCLA, or the Department of Veterans Affairs.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: A clinical practice guideline from the american college of physicians. Ann Intern Med (2016) 165:125–33. doi: 10.7326/M15-2175
2. Maust DT, Solway E, Clark SJ, Kirch M, Singer DC, Malani P. Prescription and nonprescription sleep product use among older adults in the United States. Am J Geriatr Psychiatry (2019) 27(1):32–41. doi: 10.1016/j.jagp.2018.09.004
3. Donnelly K, Bracchi R, Hewitt J, Routledge PA, Carter B. Benzodiazepines, Z-drugs and the risk of hip fracture: A systematic review and meta-analysis. PLoS One (2017) 12(4):e0174730. doi: 10.1371/journal.pone.0174730
4. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ (2000) 162(2):225–33.
5. Fick DM, Semia TP, Steinman M, Beizer J, Brandt M, Dombrowski R, et al. By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for potentially inappropriate medication use in older adults. J Am Geriatr Soc (2019) 67(4):674–94. doi: 10.1111/jgs.15767
6. Gould RL, Coulson MC, Patel N, Highton-Williamson E, Howard RJ. Interventions for reducing benzodiazepine use in older people: meta-analysis of randomised controlled trials. Br J Psychiatry (2014) 204(2):98–107. doi: 10.1192/bjp.bp.113.126003
7. Parr JM, Kavanagh DJ, Cahill L, Mitchell G, McD YR. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta-analysis. Addiction (2009) 104(1):13–24. doi: 10.1111/j.1360-0443.2008.02364.x
8. Mills L, Boakes RA, Colagiuri B. The effect of dose expectancies on caffeine withdrawal symptoms during tapered dose reduction. J Psychopharmacol (2018) 33:994–1002. doi: 10.1177/0269881118817158
9. Colloca L, Miller FG. The nocebo effect and its relevance for clinical practice. Psychosom Med (2011) 73(7):598–603. doi: 10.1097/PSY.0b013e3182294a50
10. Medicine AAoS. The international classification of sleep disorders. 2nd ed. Vol. 2005. Westchester, IL: American Academy of Sleep Medicine (2005).
11. Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry (2000) 31(2):73–86. doi: 10.1016/S0005-7916(00)00012-4
12. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep (1991) 14(6):540–5. doi: 10.1093/sleep/14.6.540
13. Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med (2001) 2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4
14. Morin CM, Vallieres A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16). Sleep (2007) 30(11):1547–54. doi: 10.1093/sleep/30.11.1547
15. Carney C. My beliefs about sleep 2013. http://drcolleencarney.com/wp-content/uploads/2013/05/My-beliefs-about-sleep.pdf
16. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
17. Busto UE, Sykora K, Sellers EM. A clinical scale to assess benzodiazepine withdrawal. J Clin Psychopharmacol (1989) 9(6):412–6. doi: 10.1097/00004714-198912000-00005
18. Morin CM, Bastien C, Guay B, Radouco-Thomas M, Leblanc J, Vallieres A. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry (2004) 161(2):332–42. doi: 10.1176/appi.ajp.161.2.332
19. Belleville G, Guay C, Guay B, Morin CM. Hypnotic taper with or without self-help treatment of insomnia: a randomized clinical trial. J Consult Clin Psychol (2007) 75(2):325–35. doi: 10.1037/0022-006X.75.2.325
20. O’Connor K, Marchand A, Brousseau L, Aardema F, Mainguy N, Landry P, et al. Cognitive-behavioural, pharmacological and psychosocial predictors of outcome during tapered discontinuation of benzodiazepine. Clin Psychol Psychother (2008) 15(1):1–14. doi: 10.1002/cpp.556
Keywords: benzodiazepine discontinuation, cognitive behavioral therapy for insomnia, placebo effect, nocebo effect, medication taper
Citation: Fung CH, Martin JL, Alessi C, Dzierzewski JM, Cook IA, Moore A, Grinberg A, Zeidler M and Kierlin L (2019) Hypnotic Discontinuation Using a Blinded (Masked) Tapering Approach: A Case Series. Front. Psychiatry 10:717. doi: 10.3389/fpsyt.2019.00717
Received: 25 June 2019; Accepted: 09 September 2019;
Published: 24 October 2019.
Edited by:
Michael Noll-Hussong, Saarland University Hospital, GermanyReviewed by:
Markku Partinen, Vitalmed Research Center, FinlandCopyright © 2019 Fung, Martin, Alessi, Dzierzewski, Cook, Moore, Grinberg, Zeidler and Kierlin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Constance H. Fung, Y29uc3RhbmNlLmZ1bmdAdmEuZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.