- 1III Department of Psychiatry, Institute of Psychiatry and Neurology, Warsaw, Poland
- 2Department of Psychiatry, Centre of Postgraduate Medical Education, Warsaw, Poland
Background: People suffering from schizophrenia are notably vulnerable to cardiometabolic risk factors (CMRF), such as obesity, high blood pressure, hyperglycemia and insulin resistance, high serum triglycerides, and low serum high-density lipoprotein (HDL), which are related to increased mortality and decreased quality of life. The increased risk of “metabolic syndrome” (MS) is related to low physical activity, an unhealthy diet, and side effects of antipsychotic drugs. Nonpharmacological interventions seem to be important in the prevention and therapy of MS.
Aim: This paper provides an overview of published studies and a critical analysis of pilot programs involving nonpharmacological measures aimed at prevention and treatment of CMRF in patients with schizophrenia.
Material and Method: We searched the PubMed, PsycARTICLES, and Cochrane Library databases to identify clinical trials. We included full-text studies that met the following criteria: age > 18 years, a diagnosis of schizophrenia or schizoaffective disorder, and monitored parameters associated with MS.
Results: All 1,555 references were evaluated for inclusion in the review, and 20 met the inclusion criteria. Nonpharmacological interventions led to improvement in physical health and showed a promising potential for implementation in treatment programs dedicated to this particular group of patients. However, a critical analysis revealed limitations, which have implications for the direction of future research.
Conclusions: Patients suffering from schizophrenia can benefit from nonpharmacological interventions aimed at counteracting CMRF, improving either metabolic parameters, cardiovascular fitness, or their health perception. Notwithstanding, to achieve long-term effects, future studies should comprise appropriate follow-up procedures.
Introduction
Schizophrenia is associated with significant reduction in health-related quality of life. Meta-analyses reveal significant health differences between people suffering from schizophrenia and the general population. Patients with schizophrenia (PwS) are two to three times more likely to have a higher mortality rate and a reduced lifespan expectancy of 13–30 years. This also applies in countries where the quality of the medical care system is generally considered to be good. Sixty percent of mortality among PwS is due to complications of metabolic and cardiovascular diseases. Weight gain applies to 72% of patients taking APs, whereas 42–60% of PwS are obese. Metabolic syndrome affects 19.4–68% of patients treated for schizophrenia (differences depend on the country, gender, age, ancestry, and medication). The risk of developing type 2 diabetes, one of the components of MS, is estimated to be two to three times higher than in the general population and nearly half of patients remain untreated (1). A higher risk of cardiovascular complications is commonly related to a high frequency of diabetes, hypertension, smoking, poor nutrition, obesity, impaired lipid profile, and low physical activity (2, 3), but also with the use of antipsychotics (APs). It has been established that treatment with APs, particularly the second generation, largely contribute to metabolic complications. However, even before the introduction of new medications, Henry Maudsley noted a relationship between diabetes and schizophrenia (4). Many studies have shown that a large proportion of untreated patients are characterized by impaired glucose tolerance (and the same applies to siblings of patients with schizophrenia), as well as increased distribution of visceral fat or insulin resistance (5–7). Recent studies have indicated that diabetes and schizophrenia may share a common genetic background (8). It has also been shown that people suffering from schizophrenia are significantly less active compared to a control group (2, 3). In addition, it is important to note that metabolic risk factors are often present in patients with a first-episode psychosis (FEP) (9–11). This may be related to specific symptoms of schizophrenia: negative symptoms, depressive symptoms, and impaired cognitive function (2). Importantly, there is significant disparity in access to health-care services in this group of patients. It seems reasonable to state that the problem of comorbidities and premature mortality among PwS requires interventions by representatives of mental health care, as this is often the only medical care used by this group of patients. Clinical guidelines underline the importance of regular monitoring of metabolic and cardiovascular parameters and recommend nonpharmacological interventions, such as physical activity, diet, or psychotherapy. Recently published reviews evaluated the quality of guidelines for cardiovascular risk in people with schizophrenia. The authors acknowledged a significant variability in the guidelines and a need for further investigation (12, 13). Most of the guidelines recommended monitoring the following measurements (in order of frequency): fasting glucose, body mass index, triglycerides, total cholesterol, waist, high-/low-density lipoprotein, blood pressure, and symptoms of diabetes (12). Moreover, in terms of nonpharmacological interventions, most guidelines recommended regular physical activity, diet, psychoeducation, treatment of metabolic abnormalities, and smoking cessation (12, 13). Authors commonly claim that assessments should be repeated 6 and 12 weeks after initiation of a new AP drug treatment (12). The intention of the guidelines is to improve cooperation and shared care between different health-care professionals and to increase awareness among psychiatrists and primary care physicians about the need to screen and treat cardiovascular risk factors and diabetes (14). Research conducted both among the general population and people with mental disorders has consistently shown the impact of nonpharmacological interventions in reducing the risk of cardiovascular disease (15). People suffering from schizophrenia may require a greater effort in the prevention of obesity and related comorbidities.
The aim of this review was to analyze the published data on nonpharmacological treatments of the components of metabolic syndrome among PwS. We focused on the three most commonly recommended and used treatments in clinical practice interventions: physical activity, diet, and psychotherapy, as well as the relationship between the type of intervention and the improvement of metabolic parameters.
Methods
This systematic review was performed in accordance with the PRISMA recommendations (16). A database search was performed in order to identify clinical trials. We included only full-text publications that met the following criteria: age of study participants ≥18 years, diagnosis of schizophrenia or schizoaffective disorder, and monitored parameters associated with metabolic syndrome. Two reviewers independently conducted literature searches using PubMed, PsycARTICLES, and the Cochrane Library databases. The keywords for the search were the following: “schizophrenia and metabolic syndrome,” “schizophrenia and physical exercise,” “schizophrenia and metabolic syndrome and exercise,” “schizophrenia and metabolic syndrome and diet,” “schizophrenia and metabolic syndrome and fitness,” and “schizophrenia and metabolic syndrome and psychotherapy.” The search was last updated on May 28th, 2018.
Results
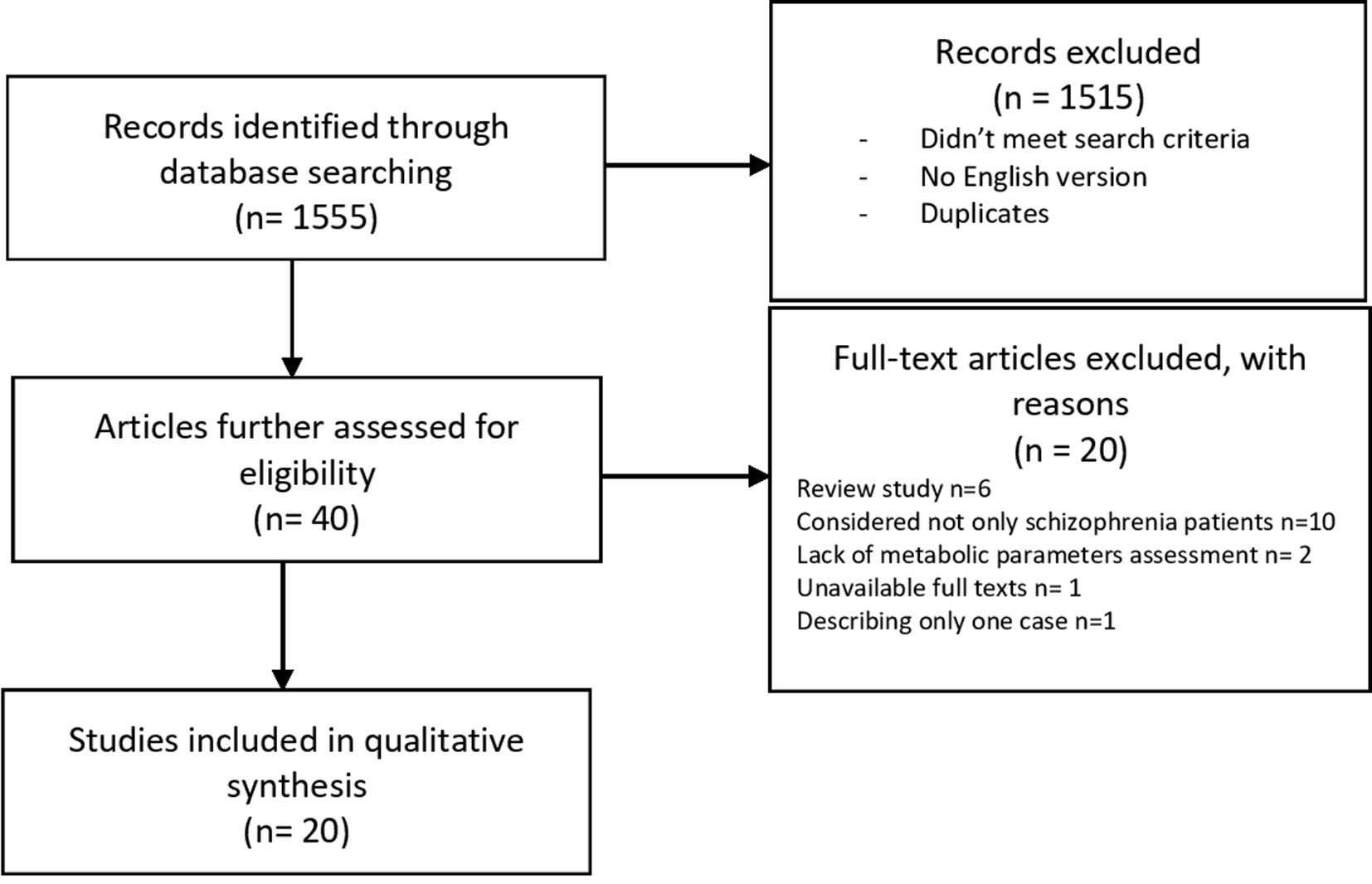
The selection process is shown in Figure 1. A total of 1,555 publications were found, and 40 of them met the initial criteria. Through the selection process, 20 were included in the analysis. Participants with ICD criteria for schizophrenia spectrum disorders were represented across the included studies. Studies that reported age identified the range from 18 to 65 years old. Weight (kg) or BMI (kg m−2) was identified as the primary outcome measure in 18 of the 20 included studies (17–34). Researchers used scales and questionnaires in order to report changes in the psychiatric domain of participants after applying interventions in nine programs (18, 23–27, 30, 32, 35). The analysis of the clinical research data presented in Table 1 shows that authors focused on physical activity as the main nonpharmacological intervention aimed at reducing metabolic abnormalities in patients with schizophrenia. Most authors described the length, frequency, and duration of the program and the types of exercise. The intensity of training was based primarily on the values of heart rate during exercise. However, in many of the articles there was no information about the recommended exercise intensity. Participants were recruited mostly from outpatient or daily departments (17, 18, 21, 23–25, 30, 34, 36), but in almost half of the cases, the authors did not specify these data (19, 20, 26, 27, 29, 31, 32, 35, 36). Analyses of the long-term effects were conducted in four studies (21, 23, 27, 29). One study shows a correlation between the number of steps and an increase in the distance of 6-MWT immediately after the test as well as 1 month from the end (23). In a program introduced by Hsu et al. (21), the authors observed changes in the heart rate variability (HRV) parameter and body weight reduction by an average of 2.3 kg after an 8-week program. However, only weight reduction remained in the exercise group after discontinuation of the exercise program, which implies that, in patients with schizophrenia, HRV parameters are more sensitive to the effects of exercise discontinuation than body weight (21). Eight programs included a control group. All participants received APs. Study groups were highly heterogeneous in terms of age, race and ethnicity, anthropometric parameters, length of illness, and medications used. Interestingly, most of the authors did not take into account metabolic parameters in the criteria for inclusion. Only in five studies were there patients with an elevated baseline body mass index (17–21), and only one trial included the waist circumference (WC) measures (20).
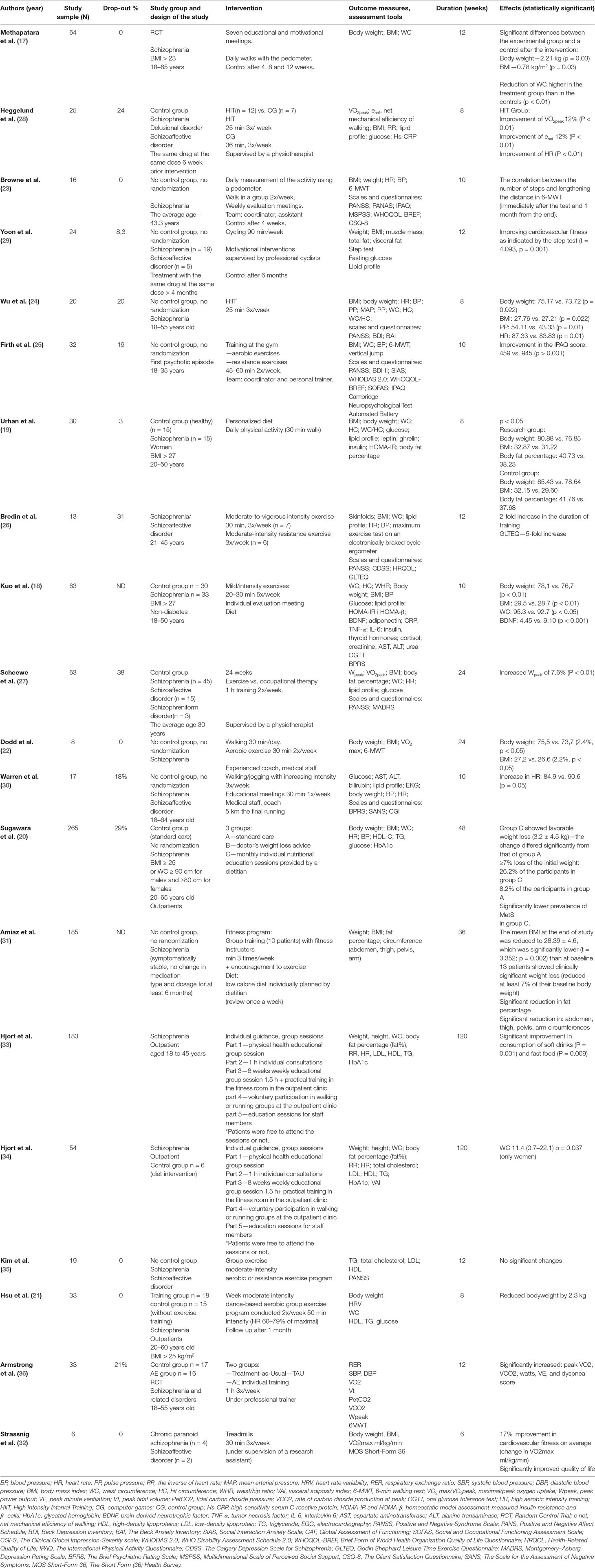
Table 1 Short-term non-pharmacological interventions for the treatment of cardiometabolic risk factors in people with schizophrenia.
Group and Individual Exercise
In eight programs, participants took part in only group exercises (21, 22, 24, 28, 30, 31, 33, 34). The analyzed studies show a reduction in BMI and body weight (21, 22, 24, 31), waist circumference (34), and an improvement in physical fitness (28).
In programs where patients were involved in only individual sessions (19, 32, 36), an improvement in physical fitness was noted (32, 36), and in one program, patients from the research group obtained a statistically significant reduction in BMI, body weight, and body fat percentage compared to the control group (19).
In five programs, combining both group exercise and individual training (17, 18, 23, 25, 29), the authors noted a reduction in metabolic or physical fitness parameters.
Professional Supervisor
Eleven pilot programs engaged professional coaches (personal trainers/fitness instructor/physiotherapist or dietitian) who motivated patients and/or provided information and monitored the participants’ progress (18, 20–22, 25, 26, 28, 29, 31, 35, 36). In 10 of the studies, researchers obtained a statistically significant improvement in metabolic parameters or physical fitness. However, the review shows that programs without any supervisor or those that only engage medical staff without a background in fitness training/exercise can lead to similar changes in the analyzed parameters.
Type of Exercise
Seven programs that implemented walking as a main intervention showed statistically significant changes in the tested parameters (17, 19, 22, 23, 30, 33, 34). In three of them, participants used a pedometer, which supported patients in setting individual goals and helped supervisor to track progress (17, 23, 30).
Eleven authors chose aerobic exercise to reduce metabolic syndrome risk factors and improve physical fitness in patients with schizophrenia (21, 22, 24–29, 31, 32, 35, 36). In most of them, improvement in cardiovascular fitness was achieved, and in four of them, there was also a reduction in weight and BMI. Importantly, these kinds of interventions usually require special sport facilities, such as heart rate monitors, gym equipment, and pedometers, as well as supervision by a professional trainer, which was provided in the described studies. In four programs, resistance exercise was added to basic aerobic exercises, which led to improved physical fitness but not parameters such as BMI, body fat percentage, waist circumferences, or lipid profile (25, 26, 31, 35). In nine studies, exercises were planned individually based on patient achievement, such as number of steps, maximum heart rate, or setting individual goals (17, 22–24, 29, 31, 32, 35, 36).
Diet Intervention
Sugawara et al. applied individual nutritional education provided by a dietitian as the only nonpharmacological intervention for obese people with schizophrenia (20). Four programs applied healthy diet education as an additional intervention (19, 31, 33, 34). One study provided daily healthy meals for participants but did not specify what kind of group activity patients were involved in. This study indicated a positive correlation between blood levels of brain-derived neurotrophic factor (BDNF) and body weight reduction. Moreover, a lower level of neuropeptides was observed in people with schizophrenia compared to the control group and a statistically significant increase in BDNF after successful weight reduction (18). Results show that healthy diet educational intervention, as well as physical exercise, could reduce weight in patients with schizophrenia.
Motivational and Educational Interventions
Thirteen authors applied a variety of motivational and educational interventions to increase compliance and hence improve outcomes (17–20, 22, 23, 25, 27, 29–31, 33, 34, 36). Six researchers conducted educational sessions where participants were introduced to healthy behavior, the importance of physical activity, and a healthy diet (17, 19, 20, 30, 33, 34). However, these interventions did not lead to better outcomes compared to those without any educational sessions. The authors encouraged patients to maintain a daily activity and diet logbook as a motivational method to maintain participation and improve metabolic parameters (18, 20, 22, 25, 27, 30, 31). Two programs offered financial incentives for participants (23, 36). In one of the studies, patients were encouraged to participate in a 10-week program, which ended with a 5-km running event (30). In two programs, one of the interventions involved increased awareness of physical health among all staff members (by education sessions for staff members). The staff’s physical health was monitored annually. Furthermore, staff members were encouraged to take part in walking and running groups on a weekly basis at the clinic. It seems that additional educational and motivational interventions did not improve results compared to programs involving only physical activity.
Discussion
In this systematic review, a total of 20 studies implemented nonpharmacological interventions such as physical activity, dietary modification, or psychoeducation that targeted physical health aspects of metabolic and physical fitness. We found that programs that were >12 and <12 weeks can be just as effective and led to modest but significant weight loss or improved cardiovascular fitness among patients with schizophrenia. The review provides information that nonpharmacological interventions are feasible to conduct in outpatient and inpatient environments where patients with schizophrenia are hospitalized.
Nine reviews that were available as full-text articles published in English were also analyzed. Three of the nine reviews included only randomized studies (15, 37, 38). A systematic review by Bruins et al. shows that nonpharmacological interventions are more effective in the prevention of weight gain compared to weight reduction in those who already suffer from obesity. Research characterized by an individual approach to the patient was more effective than group intervention; furthermore, combined individual and group activities turned out to be the most effective in reducing obesity. Group interventions have many advantages, i.e., the imitation of behavior, mutual support, and motivation, but do not offer the opportunity to address the patient’s needs as is the case during individual sessions with a coach or therapist. Interestingly, it turned out that studies conducted in Asia were more effective than those in Central Europe, even though exercise intensity and patients’ baseline weight did not differ significantly. The authors were not able to explain these differences (15). Papanastasiou et al. analyzed 42 programs of nonpharmacological interventions targeting metabolic disturbances in schizophrenia and severe mental illness, 44 studies of pharmacological interventions, and 9 combining both methods. According to the review, a holistic approach—behavioral interventions and exercises—improved the subjective evaluation of physical health, physical fitness, and significantly increased patients’ participation in the program. The review by Papanastasiou also took into account the impact of nutritional interventions. Caloric restrictions and nutritional education seemed to be effective in weight gain prevention in several studies and mitigated weight gain in patients taking drugs such as olanzapine or clozapine. In most of the review papers, the authors failed to extract a particularly effective and distinctive intervention (39). Interestingly, Cynthia Noverand et al. suggested that there was huge potential in involving social workers due to their social skills and opportunities to work closely with patients in programs implementing nonpharmacological interventions (40). According to a meta-analysis by Chalfoun et al., in programs involving physical activity as the only intervention, aerobic interval training has a great advantage over continuous moderate exercise. Analyses of supervised programs have revealed that groups of patients with schizophrenia are able to regularly participate in such activities. However, there are some safety concerns about exercising in patients with cardiovascular disease, obesity, and low efficiency. Owing to numerous barriers and variable intensities during training, this type of physical activity in particular requires supervision by qualified personnel (41). Firth et al. in their review excluded studies involving alternative forms of activity, i.e., yoga and progressive muscle relaxation, as well as programs combining physical activity with psychosocial intervention. The average length of exercise time was 75 min per week (25–160 min/week). Of 11 experiments, 10 showed a statistically significant change in at least one of the parameters that were evaluated. Physical activity did not have a statistically significant effect on BMI; however, it significantly improved physical fitness and influenced other cardiovascular disease risk factors (42). Vancampfort et al. examined the effects of aerobic exercise on the parameters of cardiovascular performance. This study shows that an increase in VO2max/VO2peak is directly associated with clinical improvement and reduction in mortality. It has recently been agreed that poor physical fitness is a better predictor of mortality and morbidity than obesity. Body weight reduction is perceived as a big challenge in the general population. Therefore, improving parameters of physical fitness seems more realistic and easier to achieve even after a short-term intervention (43). The purpose of the review by Garcia et al. was to analyze the benefits of physiotherapy in a multidisciplinary approach in the care of people suffering from schizophrenia. The authors took into account only randomized studies and focused mainly on the impact of physical activity on psychiatric symptoms and quality of life. The results showed that aerobic exercise significantly reduced psychiatric symptoms, improved patients’ quality of life, and reduced the risk of metabolic disorders and weight gain. Interestingly, alternative forms of activity, i.e., yoga, tai-chi, and progressive muscle relaxation, significantly statistically improved patients’ mental health and quality of life. They all represent promising forms of intervention and an interesting alternative to the currently existing practices (37). In order to compare the effectiveness of various interventions, Hjorth et al. divided studies into categories (dietary interventions, n = 4; exercise, n = 5; cognitive behavioral therapy, n = 3; and the combination of these three interventions, n = 11). Reduction in body weight after the implementation of a diet was usually not maintained after the program as patients returned to their former eating habits. All tests including physical activity as the main intervention showed efficacy in term of weight reduction. Cognitive behavioral therapy also had positive effects on preventing drug-induced weight gain among patients treated with olanzapine. In all studies, the combination of three types of intervention noted a positive effect on weight reduction and some of the metabolic parameters. There was a relationship between the length of the program and its long-term effects (44). The purpose of the review by Stanton et al. was to describe the diversity of aerobic exercise and impact on the treatment of schizophrenia and schizoaffective disorder. Although the review does not include the impact on metabolic parameters and cardiovascular efficiency, it reveals the nature of the recent studies. The authors report that the variables defining aerobic exercise have not been thoroughly described. Exercise intensity was based solely on the age of the participants (38). Side effects of nonpharmacological interventions were considered to be rare and were not described in the studies.
Many studies were found to have low methodological quality, involving single-group pre–post, uncontrolled feasibility, or quasi-experimental designs. Studies predominantly used BMI as the primary outcome measure, which is a convenient and reliable measure that is predictive of health risk status (45). However, it does not reflect mass or fat distribution. Furthermore, it is likely that a decrease in fat mass and an increase in muscle mass will be less influential on BMI but more significant with regard to waist circumference. Therefore, measures of abdominal visceral adipose tissue or waist circumference should also be included as it is a measurement that indicates accumulation of abdominal fat and is associated with obesity. Very commonly, it was not clear who implemented individual interventions—the physician, therapist, nutritionist, trainer, or social worker—and who collected the data.
Caution should be taken while drawing firm conclusions from nonrandomized studies. Generally, applied interventions led to changes of metabolic and cardiovascular parameters, but most of the studies were carried out in outpatient settings where high levels of control over behavior and diet is difficult and may not be possible. Exercises were generally well described by the authors, while information on nutritional recommendations, educational and motivational interventions, or the type of implemented psychotherapy were scarce. The review suggests that adding behavioral interventions, such as educational and motivational meetings, to physical activity can bring positive results in the improvement of metabolic parameters. Walking is a form of exercise that can be performed in practically any environment, and a pedometer is a small, relatively cheap, and easy-to-use device that monitors daily activities. Patients were generally provided access to participate in exercise, but they were often encouraged to exercise on their own (17, 18, 23). Some studies suggest that medical personnel should set a good example for a healthy lifestyle, which is fundamental for “modeling” the patients (33, 34). Research shows that those medical personnel with a normal BMI are more reliable and willing to urge patients to maintain a healthy lifestyle (46).
The present studies are highly varied, especially in terms of inclusion criteria, design, type of intervention, and outcome assessment. All the analyzed studies revealed significant limitations. A common problem for nonpharmacological interventions is the high dropout rate. This was clearly illustrated in a 6-month exercise program, where participants had constant and free access to a fitness center. In this study, the percentage of dropout from the study reached 90% at 6 months and 70% at 5 months. In contrast, in the general population, this percentage is 50% at 6 months (47). One reason for withdrawing from the training program was lack of support from medical staff and families. Other important factors limiting participation in the programs are coexisting substance abuse, difficulty in making an appointment, or transfer to another health-care facility. Another reason for dropout or lack of willingness to initiate participation in the program may be the lack of an individualized approach to each patient and too many demanding exercises. The majority of recent studies suggest a need for incentives to reduce dropout from nonpharmacological intervention programs and to improve patients’ compliance. Part of the study populations was heterogeneous in term of age, race and ethnicity, anthropometric parameters, length of illness, and taken medications, which affects the representativeness of the sample. Undoubtedly, groups should be carefully selected. Most programs lacked randomization and control groups.
Future studies should certainly focus on the long-term effects that require appropriate follow-up procedures that were missing in the above-analyzed clinical trials. Engagement of a multidisciplinary team seems to be the best idea in order to optimize participation and thus achieve significant improvement and achieve long-term effects. The task of the therapists and physiotherapists would be to inform patients about alternative forms of physical activity, to provide a thorough briefing of the exercises, as well as to provide support in the process of improving individual performance and efficiency. The task of the physician and the nursing staff is to motivate patients and control for metabolic and cardiovascular parameters. Moreover, the implementation of some of the training courses is problematic due to a lack of availability of proper equipment in psychiatric departments or a lack of access to sports facilities after the end of the program. Therefore, interventions that do not require specialized sports equipment should be promoted.
Conclusion
Patients suffering from schizophrenia can benefit from nonpharmacological interventions aimed at counteracting the CMRF. Almost all interventions appeared to have benefits for patients, either towards improving their metabolic parameters, cardiovascular fitness, or their health perception. Moreover, our systematic review provides evidence for the safety of described interventions. Future studies should comprise appropriate follow-up procedures in order to focus on the long-term effects. Study findings also show that engagement of a multidisciplinary team seems to be the best idea in order to optimize participation and thus achieve significant improvement and achieve long-term effects.
Data Availability
All datasets generated for this study are included in the manuscript and the supplementary files.
Author Contributions
ET and ML-S designed research; ET, ML-S, and AW were involved in data collection; ET and ML-S analyzed data; ET, ML-S, AW, and MJ participated in interpretation of findings; ET and ML-S wrote the first draft. All authors read, edited, and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hert M DE, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry (2011) 10(1):52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x
2. Stubbs B, Firth J, Berry A, Schuch FB, Rosenbaum S, Gaughran F, et al. How much physical activity do people with schizophrenia engage in? A systematic review, comparative meta-analysis and meta-regression. Schizophr Res (2016) 176(2–3):431–40. doi: 10.1016/j.schres.2016.05.017
3. Stubbs B, Koyanagi A, Schuch F, Firth J, Rosenbaum S, Gaughran F, et al. Physical activity levels and psychosis: a mediation analysis of factors influencing physical activity target achievement among 204 186 people across 46 low- and middle-income countries. Schizophr Bull (2017) 43(3):536–545. doi: 10.1093/schbul/sbw111
5. Thakore J, Mann J, Vlahos I, Martin A, Reznek R. Increased visceral fat distribution in drug-naive and drug-free patients with schizophrenia. Int J Obes Relat Metab Disord (2002) 26:137–41. doi: 10.1038/sj.ijo.0801840
6. Venkatasubramanian G, Chittiprol S, Neelakantachar N, Naveen M, Thirthall J, Gangadhar B, et al. Insulin and insulin-like growth factor-1 abnormalities in anti psychotic-naive schizophrenia. Am J Psychiatry (2007) 164:1557–60. doi: 10.1176/appi.ajp.2007.07020233
7. Fernandez-Egea E, Bernardo M, Parellada E, Justicia A, Garcia-Rizo C, Esmatjes E, et al. Glucose abnormalities in the siblings of people with schizophrenia. Schizophr Res (2008) 103:110–3. doi: 10.1016/j.schres.2008.04.017
8. Hansen T, Ingason A, Djurovic S, Melle I, Fenger M, Gustafsson O, et al. At-risk variant in Tcf7l2 for type II diabetes increases risk of schizophrenia. Biol Psychiatry (2011) 70:59–63. doi: 10.1016/j.biopsych.2011.01.031
9. Misiak B, Stańczykiewicz B, Łaczmański Ł, Frydecka D. Lipid profile disturbances in antipsychotic-naive patients with first-episode non-affective psychosis: a systematic review and meta-analysis. Schizophr Res (2017) 190:18–27. doi: 10.1016/j.schres.2017.03.031
10. Perry BI, McIntosh G, Weich S, Singh S, Rees K. The association between first-episode psychosis and abnormal glycaemic control: systematic review and meta-analysis. Lancet Psychiatry (2016) 3(11):1049–58. doi: 10.1016/S2215-0366(16)30262-0
11. Pillinger T, Beck K, Stubbs B, Howes OD. Cholesterol and triglyceride levels in first-episode psychosis: systematic review and meta-analysis. Br J Psychiatry (2017) 211(6):339–49. doi: 10.1192/bjp.bp.117.200907
12. De Hert M, Vancampfort D, Correll CU, Mercken V, Peuskens J, Sweers K, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry (2011) 199(2):99–105. doi: 10.1192/bjp.bp.110.084665
13. Vancampfort D, Sweers K, Probst M, Mitchell AJ, Knapen J, De Hert M. Quality assessment of physical activity recommendations within clinical practice guidelines for the prevention and treatment of cardio-metabolic risk factors in people with schizophrenia. Community Ment Health J (2011) 47(6):703–10. doi: 10.1007/s10597-011-9431-8
14. De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Möller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry (2009) 24(6):412–24. doi: 10.1016/j.eurpsy.2009.01.005
15. Bruins J, Jörg F, Bruggeman R, Slooff C, Corpeleijn E, Pijnenborg M. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: a meta-analysis. PLoS One (2014) 9(12):e112276. doi: 10.1371/journal.pone.0112276
16. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
17. Methapatara W, Srisurapanont M. Pedometer walking plus motivational interviewing program for Thai schizophrenic patients with obesity or overweight: a 12-week, randomized, controlled trial. Psychiatry ClinNeurosci (2011) 65(4):374–80. doi: 10.1111/j.1440-1819.2011.02225.x
18. Kuo FC, Lee CH, Hsieh CH, Kuo P, Chen YC, Hung YJ. Lifestyle modification and behaviour therapy effectively reduce body weight and increase serum level of brain-derived neurotrophic factor in obese non-diabetic patients with schizophrenia. Psychiatry Res (2013) 209(2):150–4. doi: 10.1016/j.psychres.2012.11.020
19. Urhan M, Ergün C, Aksoy M, Ayer A. Effects of weight loss diet therapy on anthropometric measurements and biochemical variables in schizophrenic patients. Nord J Psychiatry (2015) 69(5):323–30. doi: 10.3109/08039488.2014.981288
20. Sugawara N, Sagae T, Yasui-Furukori N, Yamazaki M, Shimoda K, Mori T, et al. Effects of nutritional education on weight change and metabolic abnormalities among patients with schizophrenia in Japan: A randomized controlled trial. J Psychiatr Res (2018) 97:77–83. doi: 10.1016/j.jpsychires.2017.12.002
21. Hsu CC, Liang CS, Tai YM, Cheng SL. Incongruent changes in heart rate variability and body weight after discontinuing aerobic exercise in patients with schizophrenia. Int J Psychophysiol (2016) 109:132–7. doi: 10.1016/j.ijpsycho.2016.08.011
22. Dodd KJ, Duffy S, Stewart JA, Impey J, Taylor N. A small group aerobic exercise programme that reduces body weight is feasible in adults with severe chronic schizophrenia: a pilot study. Disabil Rehabil (2011) 33(13–14):1222–9. doi: 10.3109/09638288.2010.526162
23. Browne J, Penn DL, Battaglini CL, Ludwig K. Work out by walking: a pilot exercise program for individuals with schizophrenia spectrum disorders. J NervMent Dis (2016) 204(9):651–7. doi: 10.1097/NMD.0000000000000556
24. Wu MH, Lee CP, Hsu SC, Chang CM, Chen CY. Effectiveness of high-intensity interval training on the mental and physical health of people with chronic schizophrenia. Neuropsychiatr Dis Treat. (2015) 11:1255–63. doi: 10.2147/NDT.S81482
25. Firth J, Carney R, Elliott R, French P, Parker S, McIntyre R, et al. Exercise as an intervention for first-episode psychosis: a feasibility study. Early Interv Psychiatry (2018) 12(3):307–315. doi: 10.1111/eip.12329
26. Bredin SS, Warburton DE, Lang DJ. The health benefits and challenges of exercise training in persons living with schizophrenia: a pilot study. Brain Sci (2013) 3(2):821–48. doi: 10.3390/brainsci3020821
27. Scheewe TW, Backx FJ, Takken T, Jörg F, van Strater AC, Kroes AG, et al. Exercise therapy improves mental and physical health in schizophrenia: a randomised controlled trial. Acta Psychiatr Scand (2013) 127(6):464–73. doi: 10.1111/acps.12029
28. Heggelund J, Nilsberg GE, Hoff J, Morken G, Helgerud J. Effects of high aerobic intensity training in patients with schizophrenia: a controlled trial. Nord J Psychiatry (2011) 65(4):269–75. doi: 10.3109/08039488.2011.560278
29. Yoon S, Ryu JK, Kim CH, Chang JG, Lee HB, Kim DH, et al. Preliminary effectiveness and sustainability of group aerobic exercise program in patients with schizophrenia. J Nerv Ment Dis (2016) 204(9):644–50. doi: 10.1097/NMD.0000000000000534
30. Warren KR, Ball MP, Feldman S, Liu F, McMahon RP, Kelly DL. Exercise program adherence using a 5-kilometer (5K) event as an achievable goal in people with schizophrenia. Biol Res Nurs (2011) 13(4):383–90. doi: 10.1177/1099800410393272
31. Amiaz R, Rubinstein K, Czerniak E, Karni Y, Weiser M. A diet and fitness program similarly affects weight reduction in schizophrenia patients treated with typical or atypical medications. Pharmacopsychiatry (2016) 49(3):112–6. doi: 10.1055/s-0035-1569416
32. Strassnig MT, Newcomer JW, Harvey PD. Exercise improves physical capacity in obese patients with schizophrenia: pilot study. Schizophr Res (2012) 141(2–3):284–5. doi: 10.1016/j.schres.2012.08.011
33. Hjorth P, Espensen CH, Madsen NJ, Viuff AG, Munk-Jørgensen P. Reducing the risk of type 2 diabetes in nonselected outpatients with schizophrenia: a 30-month program. J Psychiatr Pract (2018) 24(1):21–31. doi: 10.1097/PRA.0000000000000278
34. Hjorth P, Juel A, Hansen MV, Madsen NJ, Viuff AG, Munk-Jørgensen P. Reducing the risk of cardiovascular diseases in non-selected outpatients with schizophrenia: a 30-month program conducted in a real-life setting. Arch Psychiatr Nurs (2017) 31(6):602–9. doi: 10.1016/j.apnu.2017.08.005
35. Kim DD, Lang DJ, Warburton DE, Barr AM, Smith GN, Thornton AE, et al. Effects of exercise on serum triglycerides and symptoms of schizophrenia. J Clin Psychopharmacol (2017) 37(2):273–4. doi: 10.1097/JCP.0000000000000648
36. Armstrong HF, Bartels MN, Paslavski O, Cain D, Shoval HA, Ballon JS, et al. The impact of aerobic exercise training on cardiopulmonary functioning in individuals with schizophrenia. Schizophr Res (2016) 173(1–2):116–7. doi: 10.1016/j.schres.2016.03.009
37. Vera-Garcia E, Mayoral-Cleries F, Vancampfort D, Stubbs B, Cuesta-Vargas AI. A systematic review of the benefits of physical therapy within a multidisciplinary care approach for people with schizophrenia: An update. Psychiatry Res (2015) 229(3):828–39. doi: 10.1016/j.psychres.2015.07.083
38. Stanton R, Happell B. A systematic review of the aerobic exercise program variables for people with schizophrenia. Curr Sports Med Rep (2014) 13(4):260–6. doi: 10.1249/JSR.0000000000000069
39. Papanastasiou E. Interventions for the metabolic syndrome in schizophrenia: a review. Ther Adv Endocrinol Metab (2012) 3(5):141–62. doi: 10.1177/2042018812458697
40. Nover C, Jackson SS. Primary care-based educational interventions to decrease risk factors for metabolic syndrome for adults with major psychotic and/or affective disorders: a systematic review. Syst Rev (2013) 2:116. doi: 10.1186/2046-4053-2-116
41. Chalfoun C, Karelis AD, Stip E, Abdel-Baki A. Running for your life: a review of physical activity and cardiovascular disease risk reduction in individuals with schizophrenia. J Sports Sci. (2016) 34(16):1500–15. doi: 10.1080/02640414.2015.1119875
42. Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med (2015) 45(7):1343–61. doi: 10.1017/S0033291714003110
43. Vancampfort, Rosenbaum S, Ward PB, Stubbs B. Exercise improves cardiorespiratory fitness in people with schizophrenia: a systematic review and meta-analysis. Schizophr Res (2015) 169(1–3):453–7. doi: 10.1016/j.schres.2015.09.029
44. Hjorth P, Davidsen AS, Kilian R, Skrubbeltrang C. A systematic review of controlled interventions to reduce overweight and obesity in people with schizophrenia. Acta Psychiatr Scand (2014) 130(4):279–89. doi: 10.1111/acps.12245
45. Després JP, Lemieux I, Prud’homme D. Treatment of obesity: need to focus on high risk abdominally obese patients. BMJ (2001) 322(7288):716–20. doi: 10.1136/bmj.322.7288.716
46. Bleich SN, Bennett WL, Gudzune KA, Cooper LA. Impact of physician BMI on obesity care and beliefs. Obesity (Silver Spring) (2012) 20(5):999–1005. doi: 10.1038/oby.2011.402
Keywords: schizophrenia, metabolic syndrome, cardiometabolic risk factors, exercise, diet, psychotherapy
Citation: Tumiel E, Wichniak A, Jarema M and Lew-Starowicz M (2019) Nonpharmacological Interventions for the Treatment of Cardiometabolic Risk Factors in People With Schizophrenia—A Systematic Review. Front. Psychiatry 10:566. doi: 10.3389/fpsyt.2019.00566
Received: 13 February 2019; Accepted: 19 July 2019;
Published: 16 August 2019.
Edited by:
Jerzy Samochowiec, Pomeranian Medical University, PolandReviewed by:
Agata Szulc, Medical University of Warsaw, PolandHubertus Himmerich, King’s College London, United Kingdom
Copyright © 2019 Tumiel, Wichniak, Jarema and Lew-Starowicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ewa Tumiel, ZXR1bWllbEBpcGluLmVkdS5wbA==; Adam Wichniak, d2ljaG5pYWtAaXBpbi5lZHUucGw=; Marek Jarema, amFyZW1hQGlwaW4uZWR1LnBs; Michał Lew-Starowicz, bWxldy1zdGFyb3dpY3pAaXBpbi5lZHUucGw=
 Ewa Tumiel
Ewa Tumiel Adam Wichniak
Adam Wichniak Marek Jarema1*
Marek Jarema1*