
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Psychiatry , 16 May 2019
Sec. Psychopathology
Volume 10 - 2019 | https://doi.org/10.3389/fpsyt.2019.00334
 Anna Sierawska1*
Anna Sierawska1* Alexander Prehn-Kristensen2
Alexander Prehn-Kristensen2 Vera Moliadze3
Vera Moliadze3 Kerstin Krauel4
Kerstin Krauel4 Rafal Nowak5
Rafal Nowak5 Christine M. Freitag6
Christine M. Freitag6 Michael Siniatchkin3,7
Michael Siniatchkin3,7 Alena Buyx8 on behalf of the STIPED Consortium
Alena Buyx8 on behalf of the STIPED ConsortiumAttention deficit hyperactivity disorder (ADHD) is a disorder most frequently diagnosed in children and adolescents. Although ADHD can be effectively treated with psychostimulants, a significant proportion of patients discontinue treatment because of adverse events or insufficient improvement of symptoms. In addition, cognitive abilities that are frequently impaired in ADHD are not directly targeted by medication. Therefore, additional treatment options, especially to improve cognitive abilities, are needed. Because of its relatively easy application, well-established safety, and low cost, transcranial direct current stimulation (tDCS) is a promising additional treatment option. Further research is needed to establish efficacy and to integrate this treatment into the clinical routine. In particular, limited evidence regarding the use of tDCS in children, lack of clear translational guidelines, and general challenges in conducting research with vulnerable populations pose a number of practical and ethical challenges to tDCS intervention studies. In this paper, we identify and discuss ethical issues related to research on tDCS and its potential therapeutic use for ADHD in children and adolescents. Relevant ethical issues in the tDCS research for pediatric ADHD center on safety, risk/benefit ratio, information and consent, labeling problems, and nonmedical use. Following an analysis of these issues, we developed a list of recommendations that can guide clinicians and researchers in conducting ethically sound research on tDCS in pediatric ADHD.
Attention deficit hyperactivity disorder (ADHD) is a neuropsychiatric disorder characterized by developmentally inappropriate levels of hyperactivity, impulsivity, and inattention (1). With a prevalence of about 5%, ADHD is one of the most prevalent childhood disorders, often associated with oppositional-defiant conduct, tics, anxiety, and mood disorders (2). Furthermore, a range of adverse long-term outcomes, including increased rates of accidents and injury, poor educational achievements, increased risk of substance abuse, and criminal activity, emphasize the individual and societal burden of ADHD (2, 3). Treatment mainly involves a combination of stimulant medication (4) and behavioral therapy (5). While the treatment with stimulants is characterized by high effect sizes (>1), it comes with a number of adverse events (6). These include a decreased appetite, sleep problems, headaches, nausea, and delayed growth. Patients are known to discontinue treatment because of these adverse events (7). In addition, in some children and adolescents, the medication shows an insufficient improvement of symptoms, especially of cognitive and motor inhibition or inattention symptoms (8). Alternative treatment options such as behavioral therapy, neurofeedback, cognitive training, as well as dietary interventions have been studied (9). However, these are characterized by small effect sizes and are time consuming, requiring high motivation and compliance of patients and their families (5).
There is thus a need to develop additional treatment options for children and adolescents with ADHD. Transcranial direct current stimulation (tDCS) is a new treatment option that aims at improving cognitive as well as behavioral symptoms. TDCS is a brain stimulation technique. It applies a weak constant direct current between head-mounted electrodes, which affects relatively large cortical areas. The mechanisms are electrode-dependent and involve either membrane depolarization or hyperpolarization (10, 11) [for a review, see Ref. (12)]. TDCS is capable of inducing changes in neuronal membrane potentials in a polarity-dependent manner. It has been shown that the effects of tDCS are induced by membrane depolarization (anodal) and hyperpolarization (cathodal) (10, 13). Therefore, tDCS may prime the system by increasing or decreasing the excitability of the system, or by increasing or decreasing the threshold response [see Ref. (14)]. TDCS is regarded as a noninvasive brain stimulation in comparison to, e.g., deep brain stimulation (DBS), which involves neurosurgical procedures (15).
However, as with many novel medical interventions, it also comes with a number of ethical challenges.
Building on a short description of current evidence of tDCS as a treatment option for pediatric ADHD, we discuss the ethical implications of researching this technology for clinical translation. Based on the results of this analysis, we present preliminary guidelines to enable high ethical standards for future tDCS studies in children and adolescents.
A systematic literature search was conducted, based on the methodology from systematic reviews of reasons (14). This included a broad database selection (PubMed/MEDLINE, PsycInfo, PhilIndex) and database-specific search strings with relevant keywords. Keywords were derived from the areas of “ethics,” “social,” “pediatric research,” “ADHD,” “neuropsychiatric disorders,” “brain stimulation,” and “tDCS.” Papers of all types were included in the analysis, ranging from articles providing a purely theoretical reflection to reports of empirical studies of ethical aspects, to policy proposals. Papers were excluded if they did not discuss any ethical or social issues specifically, such as when these where only mentioned in passing.
Ethical and social issues were then grouped according to the normative principles from established frameworks of research ethics (16, 17) and approaches to ethical innovation (18). Issues were compared to mainstream interpretations of the ethical and social principles from these frameworks and discussed in detail. The ethical analysis was thus inspired by the background ethical theory of reflective equilibrium, which seeks to establish coherence between a variety of established ethical principles (19, 20).
Several studies on tDCS have been performed in adults, but only ∼5% of all published papers described effects of tDCS in children and adolescents (21). Recent results suggest that tDCS is a promising additional treatment for pediatric ADHD (23, 24) [for review, see Ref. (25)], with some evidence that anodal tDCS can reduce clinical symptoms in children and adults suffering from ADHD. In particular, symptoms of inattention were reduced by tDCS (22, 23).
Additionally, in pediatric ADHD, tDCS did not only influence connectivity of neuronal networks that are involved in cognitive performance (24, 25) but also improved cognitive performance itself, such as inhibitory control (26–28) and attentional processes (26).
TDCS has been reported to show only limited adverse events in children, adolescents, and adults (21, 29, 30). The fact that the technology is inexpensive further renders tDCS an attractive tool to explore in research and ultimately, to use in treatment in children with neuropsychological disorders, including ADHD. It also has the potential of being used as a portable device at home by patients (31, 32).
In addition to research interest, tDCS has been gaining attention within the general population (33). The interest in nonmedical application of tDCS could be explained by media hype around this technique (34), by emerging online forums that discuss the use of “do it yourself” (DIY) devices, and by easy access to information about the application of tDCS (35). Some concerns about the unregulated, nonclinical use of tDCS, especially regarding cognitive enhancement, have been raised within the scientific community (36). However, there is still little guidance for tDCS researchers and, eventually, healthcare professionals on how to approach this issue.
Experts in the field have acknowledged the need of further regulations when applying tDCS in order to minimize ethical challenges for researchers and clinicians working with tDCS and to enable effective clinical trials (37). Some guidelines were formulated to support facilitating better clinical applications, including calls for ensuring safety by the monitoring and reporting of adverse effects and by acknowledging the differences between age groups (29). Similar issues were presented in another document, which stressed the importance of education of healthcare professionals and patients about tDCS (38). However, available recommendations mainly focus on the technical and practical issues when conducting clinical trials, or on information for manufacturers (39); their focus is also on studies with tDCS in general, and in adult populations (29). No clear guidance exists on how to conduct trials with tDCS in pediatric populations with neuropsychiatric disorders such as ADHD, and how to make sure that these trials help translate results into practice in an ethical manner.
The need to protect vulnerable groups in general and children in particular in research can sometimes lead to a vicious circle: for many treatments, evidence does not exist to initially establish, e.g., relevant safety thresholds. However, in order for research on a vulnerable group to be conducted, any relevant risks must be specified and ideally minimized, to satisfy the usual standards of minimal risk and minimal burden when conducting research in children and adolescents (16, 17). Because long-term evidence in tDCS is not available, this can be difficult to do, leading to difficulties in getting study approval, and thus, fewer studies, which then leads to a lack of evidence, and so on. This situation is compounded in children with ADHD, who are not only minors but also vulnerable due to the disorder itself, making the design and approval of studies particularly difficult (this also applies to pharmacological studies in pediatric ADHD) (40). However, in view of the significant health needs of children with ADHD that are not met, and the general importance of providing robust clinical evidence in pediatrics (41), it is urgently required to design studies that consider the complexities of research in such vulnerable study populations. In the following sections, we examine the ethical challenges of conducting research with tDCS in children with ADHD based on established frameworks of research ethics (16, 17) and ethical innovation [e.g., Ref. (18)], in order to develop recommendations on how to design studies that are ethical.
The issue of safety in tDCS is often regarded to be marginal, since the applied electrical current is low when it comes to the potential of causing neuronal injury (21). Associated unintended effects of the procedure are mild headaches, itching, or weak burning on the spots where electrodes are placed (29). More severe harm might occur as a result of a failure of the equipment, although this is more likely to happen when used in nonclinical environments and especially from DIY systems outside of any medical oversight, which, unlike research-grade stimulation devices, do not provide built-in safety features (29).
The type and magnitude of reported adverse effects in the tDCS studies do not differ between children/adolescents and adults, and available evidence delivers no established risks specific to tDCS apart from those mentioned above. A recent German guideline includes tDCS for children and adolescents, thus mirroring recommendations for adult populations (42).
However, this assessment might be premature. Due to increased excitatory activity in young children, they could be more prone to seizures due to increased glutamate sensitivity, reduced glutamate clearance, and incomplete GABA-mediated inhibition in the developing brain (43), and this has to be monitored carefully.
Moreover, many studies in children and adolescents have used adult parameters without adjustment. Recently, it was shown that contrary to effects in adults (10), in a study with healthy children and adolescents, corticospinal excitability was found to increase, rather than decrease, following 1 mA cathodal tDCS. Various anatomic parameters change with age: thickness of the scalp and calvarium, scalp-to-brain distance, cerebrospinal fluid volume, and developmental changes in tissue architecture. Previous studies that performed current flow modeling in children suggested that a reduced tDCS current intensity is enough to produce the same peak brain electric fields in children compared with adults (44). Using realistic head modeling, Kessler and colleagues (45) demonstrated that lower applied current intensity (ca. 1 mA) may achieve brain current densities in young participants on average comparable to densities seen in adults to 2 mA current. Therefore, future studies have to consider that neuroanatomical differences of children’s brains might impact their stimulation protocols based on an adult population. Hence, the same tDCS parameters could have a larger impact in children and adolescents (46). This could lead to unexpected or even dangerous results if it misdirects plasticity or increases the likelihood of brain tissue lesions due to a thinner skull, resulting in higher peak current density in the child’s brain (47, 48).
Another concern of safety is the potential of tDCS to alter cognition in unintended ways. There is still limited evidence on this issue, but available findings suggest that stimulation of a certain part of the brain might improve some of the targeted brain activities, while impairing others at the same time (49). In ADHD, where the treatment is focused on, i.e., improving working memory, this could lead to a deterioration of functioning in other parts of the brain. The concern has thus been raised that stimulation aiming to enhance attention or working memory could have detrimental effects for cognition associated with creativity (50). Moreover, even within the construct of working memory, different parameters may be either improved or worsened by tDCS. For example, it has been demonstrated that some types of anodal tDCS can improve reaction time and performance variability but worsen error processing in adolescents with ADHD (24). Furthermore, it cannot be fully established whether changes induced by tDCS will be temporary or permanent. In the developing brain, there is a risk that changes might impact cognitive developmental trajectories in an undesirable way, particularly in view of the high plasticity of the developing brain. Some researchers have also raised concerns that the use of tDCS might be detrimental to a developing brain because it might alter cognitive functions, worsen the brain’s overall development or lead to interactions with pharmacological treatment (47).
Ethical acceptability of research studies in vulnerable groups rests on an assessment of the real benefits and risks. First of all, according to established ethical guidelines on biomedical research, the exposure to risk in research is ethically justifiable only if there is a social and scientific value of the research (17). This is the case in tDCS because there is a clear need that has not been met—current treatment alternatives for ADHD in children are not sufficient for treating all patients successfully. In addition, there might also be direct benefits to individual study participants who might find tDCS an effective treatment with fewer side effects compared to medication.
However, the assessment of potential benefits is complicated by the necessity to factor in the environmental, emotional, and other factors that might influence the tDCS data during a stimulation. Available research shows the significance of measuring the environmental and emotional factors—so-called state-dependent tDCS (51, 52); nevertheless, little is known about the extent to which those are considered during actual data collection. Another such factor is the brain’s state “wake vs. sleep,” since in children with ADHD, the application of tDCS during sleep had a beneficial impact on cognitive performance (27, 53). Documenting these factors systematically might require a more in-depth assessment prior to every stimulation session.
For any study on a vulnerable population to be ethically acceptable, usually, a positive ratio of benefits to risks is required, which is also difficult to assess in tDCS. To examine whether tDCS is an effective treatment, at least some study participants will have to forgo pharmacological treatment. In early stages of research, the assessment of an add-on effect (e.g., using designs such as “medication+tDCS vs. medication+sham”) could be sufficient. However, in the search for alternatives to standard treatments, research involving groups where the concomitant standard treatment is omitted is desirable.
ADHD medication might have side effects, but it has overall been proven to be effective and its mechanisms are well known. Thanks to long-term studies (54) and the knowledge of pharmacokinetics, it is much more feasible to anticipate possible adverse events. In contrast, the mechanisms of tDCS are less well explored, contributing to the list of “unknowns” in tDCS. Lack of evidence regarding the long-term effects of stimulation also complicates assessing the risk–benefit ratio.
Finally, the question of risk assessment should include both temporary and potentially permanent (unwanted) changes in cognition. For instance, if changes occurred only for a limited time, this might be ethically acceptable; however, any risk of permanent changes would affect the ethical acceptability of the risk–benefit ratio significantly.
Overall, in addition to considering standard inclusion/exclusion criteria, the “unknowns” in tDCS in pediatric ADHD mandate the individual risk assessment of every participant at the time of inclusion, for example, regarding the potential withdrawal of medication. It is also important to minimize and monitor any findings regarding unwanted changes in cognition closely, and to conduct longer follow-up. Finally, it must be clearly communicated, especially to study participants and their parents, that some risks have not yet been fully mapped/understood.
A long-standing principle of research ethics requires that research is only performed if informed consent has been given. In case of pediatric ADHD, research needs to be conducted with a study group that is vulnerable in two respects.
First, research occurs in minors, a group that by definition lacks both full functional and full legal capacity to provide informed consent. For this reason alone, children as research participants are regarded as a group that warrant special protection (55).
Second, subjects in pediatric ADHD research are vulnerable in that they have a neuropsychiatric disorder, which might contribute to a lower or fluctuating capacity to understand and consent to research, thus impacting on their autonomy and decision-making. Furthermore, the high comorbidity of ADHD and learning difficulties (56) might impact patients’ comprehension, ability to read a written material, and to understand the assent to treatment. This is compounded by the fact that the onset of ADHD is usually at a young age, making it particularly difficult to gain an adequate understanding of the research and providing adequate consent.
In populations that cannot (yet) consent themselves, surrogate consent by legal guardians—in the case of children, usually parents—must be issued. In this process of decision-making, depending on a child’s age and maturity, their perspective should also be considered (16, 17). Usually, there is a requirement of seeking the child’s assent (a form of approval below the standard of consent). Regulation varies across countries in establishing the age thresholds for assent [for the EU overviews, see Ref. (57)]. As an alternative to age thresholds, more personalized approaches to improving children’s understanding and gaining their approval for study participation have been proposed (58).
In tDCS research in children with ADHD, the provision of information to study participants and their parents is further complicated by the limited knowledge of potential risks of this technique. In addition to the aforementioned “unknowns” related to the overall mechanism and effect of stimulation, long-term risks, and the lack of translational studies from adults to children, there is also a need for clear dosing guidelines (59). All this results in several difficulties when designing tDCS studies for children with ADHD, including how to determine which doses are safe, how to develop a framework for establishing informed consent in children and their carers, and how to implement an efficient system for monitoring and reporting adverse effects during and following the brain stimulation in minors (see also the discussion above under Safety).
Communicating these complex considerations appropriately to parents and children is a major challenge for translational research on tDCS in pediatric ADHD. Guidance on how to enable an informed decision through clear and transparent information and communication that is also attuned to the particular needs of the population, should be developed.
TDCS is usually described as noninvasive. This relates to the fact that the technique is applied through the skin, without any surgical procedures. Furthermore, being a relatively safe procedure with minimal side effects in adults contributes to this characteristic [see Regulation (EU) 2017/745]. However, it has been argued that using the term “noninvasive” with regard to tDCS might be misleading (60). It should therefore be explained why applying a direct current across the skull with effects on brain function can be considered noninvasive in case of tDCS. Otherwise, there is the danger that participants and surrogate decision makers may underestimate or overestimate the effects of the stimulation. On the other hand, it is important that tDCS is distinguished from procedures that require, e.g., surgery, such as DBS, or general anesthesia, such as electroconvulsive therapy (ECT) (especially in view of the stigma of ECT still present, due to its history) (61). While both of these also involve electrical stimulation, they have significantly higher risks than tDCS.
One way around the challenge of labelling would be to avoid using the word “noninvasive” in study information materials altogether, as it might lead to confusion, which eventually might undermine research practices in this area. Alternatively, by presenting tDCS and its characteristics in comparison with other procedures, participants and carers could decide for themselves whether the degree of invasiveness of tDCS is acceptable to them.
The use of ADHD medications for nonmedical purposes has been discussed in the literature, especially with regard to so-called “cosmetic psychopharmacology” (62–64). This involves individuals without a diagnosis of ADHD using ADHD drugs for “enhancing” purposes, such as better concentration, focus, or alertness at work or in school (62, 63). There is some anecdotal evidence (e.g., YouTube videos and online communities) that people use brain stimulation—often through DIY tDCS kits—for similar purposes (33). This issue thus requires attention.
The ethical implications of applying tDCS for enhancement purposes in minors have not yet been sufficiently explored [but see Ref. (36)]. With other interventions, there is consensus that in children, the use of neuroenhancement should be strongly discouraged due to the fact that children’s’ brains are still developing and any treatment that is not medically necessary could pose risks of harm (65). And indeed, home tDCS use might result in a different current flow in pediatric brains (45, 66). This, in turn, leads to an important challenge when researching tDCS in children with ADHD, particularly when there is a goal to develop home application kits. Home devices might enable more successful therapeutic application and make the treatment available to a wider group of patients. Safety issues of home use of tDCS have been discussed in the literature (21, 29), however, there is a general scarcity of research on home-based tDCS (67). Additionally, the potential for misuse, e.g., for enhancement purposes, and whether researchers are obliged to curb this, should be further addressed.
Finally, as with any novel technology, there is commercial interest in developing devices and applications of tDCS for pediatric populations. This is not unethical as such, but it does come with potential conflicts of interest. As with all research, the latter must be avoided, and researchers should be transparent regarding the support of, e.g., manufacturers in developing stimulation applications, particularly for home settings. The low cost and relatively easy application of tDCS and the current lack of regulation might create a possibility for industry to market devices directly to consumers and healthcare professionals who lack any in-depth knowledge of tDCS.
The analysis of ethical issues above highlights the need for a more rigorous ethical framework for pediatric tDCS studies. So far, this has not developed beyond calls for researchers to be aware of their ethical responsibility (29), or to exert extreme caution (59). Here, based on established principles of research ethics as well as the ethical analysis above, we attempt to provide clinicians and researchers with some initial and practical recommendations on how to conduct ethical research with pediatric tDCS. These include recommendations on transparency, informed consent and assent, risk assessment, monitoring and reporting, public communication, and enhancement/home use. Our focus is on research with children with ADHD, as there are both an ethical imperative to address needs in this population that have not been met and a tension of “great needs matching great uncertainty” (68). The framework developed below, however, could be applied to a broader range of studies on tDCS and neuropediatric disorders (see Table 1). Obviously, all research on tDCS in pediatric ADHD must correspond with established principles of research ethics. Thus, in our recommendations, we do not repeat every requirement for research to be ethical [but see Refs. (16, 17)]. Instead, we focus on those issues that are particular to tDCS, or that warrant special attention from researchers working with this technology in children. We also do not claim that the guidelines derived from the framework are complete. We offer these recommendations to all colleagues involved with tDCS for neuropsychiatric disorders in children, to the professional associations involved in the field, and to the wider professional community as a starting point for discussing and developing more comprehensive guidelines for future practice. We hope that they can serve as a first step to ensure research in this promising area is ethical, and the translation into the clinic successful.
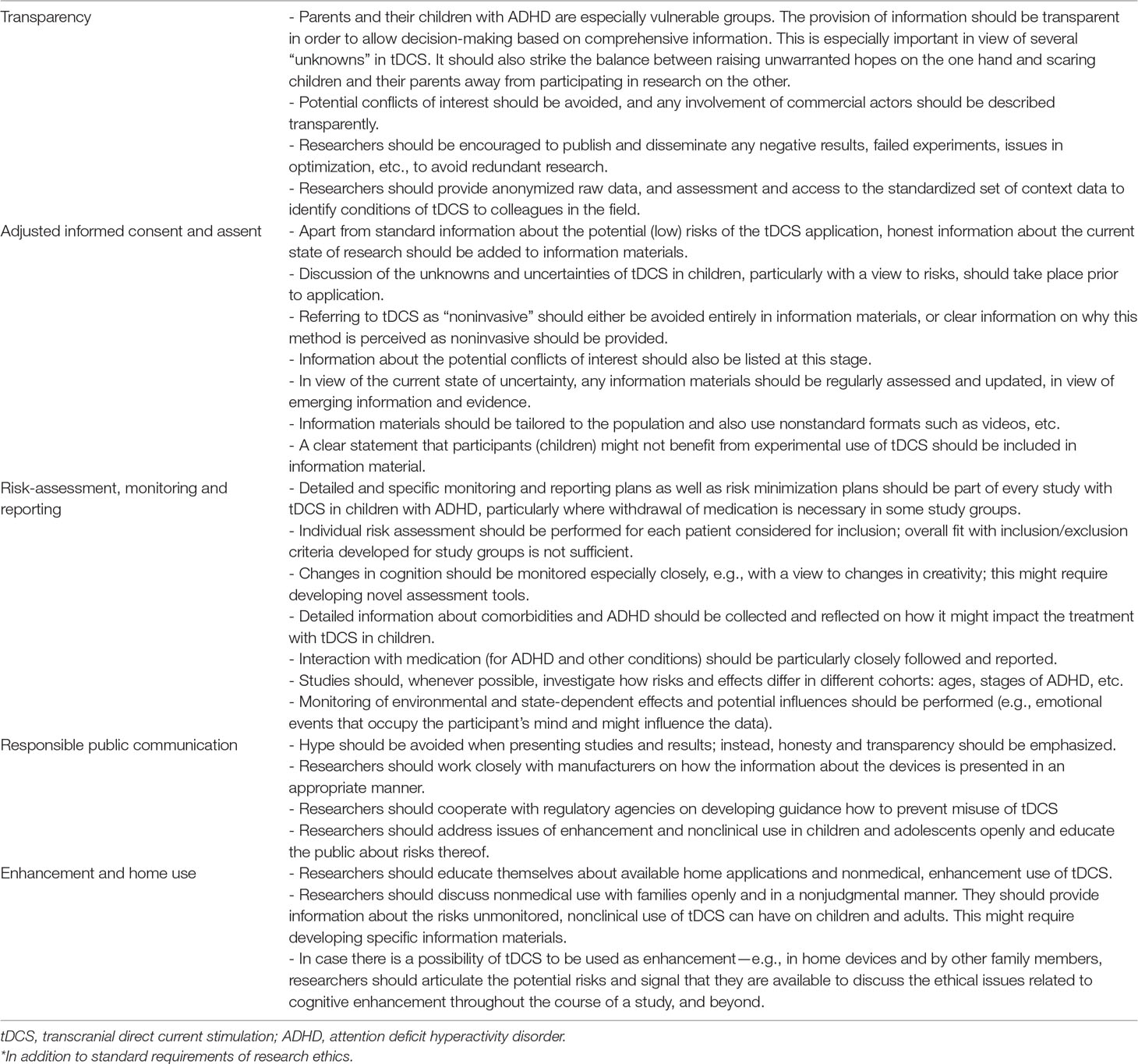
Table 1 Ethical framework and recommendations for ethical research on transcranial direct current stimulation (tDCS) in pediatric populations with attention deficit hyperactivity disorder (ADHD)*.
AS: substantial contributions to the conception or design of the work, drafting the work or revising it critically for important intellectual content. AP-K: substantial contributions to the content development. VM: substantial contributions to the content development. KK: substantial contributions to the content development. RN: substantial contributions to the content development. CF: substantial contributions to the content development, revising it critically for important intellectual content. MS: substantial contributions to the content development, revising it critically for important intellectual content. AB: substantial contributions to the conception or design of the work, revising it critically for important intellectual content.
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 731827. This text reflects only the authors’ views and the Commission is not liable for any use that may be made of the information contained therein.
RN works as a full-time employee for Neuroelectrics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing (2013). doi: 10.1176/appi.books.9780890425596
2. Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry (2007) 164:942–8. doi: 10.1176/ajp.2007.164.6.942
3. Erskine HE, Moffitt TE, Copeland WE, Costello EJ, Ferrari AJ, Patton G, et al. A heavy burden on young minds: the global burden of mental and substance use disorders in children and youth. Psychol Med (2015) 45:1551–63. doi: 10.1017/S0033291714002888
4. Castle L, Aubert RE, Verbrugge RR, Khalid M, Epstein RS. Trends in Medication Treatment for ADHD. J Atten Disord (2007) 10:335–42. doi: 10.1177/1087054707299597
5. Watson SMR, Richels C, Michalek AP, Raymer A. Psychosocial Treatments for ADHD: a Systematic Appraisal of the Evidence. J Atten Disord (2015) 19:3–10. doi: 10.1177/1087054712447857
6. Padilha S, Virtuoso S, Tonin FS, Borba HHL, Pontarolo R. Efficacy and safety of drugs for attention deficit hyperactivity disorder in children and adolescents: a network meta-analysis. Eur Child Adolesc Psychiatry (2018) 27:1335–45. doi: 10.1007/s00787-018-1125-0
7. Clavenna A, Bonati M. Pediatric pharmacoepidemiology - safety and effectiveness of medicines for ADHD. Expert Opin Drug Saf (2017) 16:1335–45. doi: 10.1080/14740338.2017.1389894
8. Childress AC, Sallee FR. Attention-deficit/hyperactivity disorder with inadequate response to stimulants: approaches to management. CNS drugs (2014) 28:121–9. doi: 10.1007/s40263-013-0130-6
9. Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry (2013) 170:275–89. doi: 10.1176/appi.ajp.2012.12070991
10. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol (2000) 527(3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x
11. Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology (2001) 57:1899–901. doi: 10.1212/WNL.57.10.1899
12. Polania R, Nitsche MA, Ruff CC. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci (2018) 21:174–87. doi: 10.1038/s41593-017-0054-4
13. Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain (2002) 125:2238–47. doi: 10.1093/brain/awf238
14. Strech D, Sofaer N. How to write a systematic review of reasons. J Med Ethics (2012) 38:121–6. doi: 10.1136/medethics-2011-100096
15. Fitzgerald PB, Segrave RA. Deep brain stimulation in mental health: review of evidence for clinical efficacy. Aust N Z J Psychiatry (2015) 49:979–93. doi: 10.1177/0004867415598011
16. World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA (2013) 310(20):2191–4. doi: 10.1001/jama.2013.281053
17. Council for International Organizations of Medical Sciences. International ethical guidelines for health-related research involving humans. Geneva (2016).
18. Reijers W, Wright D, Brey P, Weber K, Rodrigues R, O’Sullivan D, et al. Methods for practising ethics in research and innovation: a literature Review, critical analysis and recommendations. Sci Eng Ethics (2018) 24:1437–81. doi: 10.1007/s11948-017-9961-8
19. Nichols P. Wide reflective equilibrium as a method of justification in bioethics. Theor Med Bioeth (2012) 33:325–41. doi: 10.1007/s11017-012-9223-3
20. Reuzel RP, van der Wilt GJ, Have HA, de Vries Robbe PF. Interactive technology assessment and wide reflective equilibrium. J Med Philosophy (2001) 26:245–61. doi: 10.1076/jmep.26.3.245.3015
21. Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul (2016) 9:641–61. doi: 10.1016/j.brs.2016.06.004
22. Soff C, Sotnikova A, Christiansen H, Becker K, Siniatchkin M. Transcranial direct current stimulation improves clinical symptoms in adolescents with attention deficit hyperactivity disorder. J Neural Transm (2017) 124:133–44. doi: 10.1007/s00702-016-1646-y
23. Cachoeira CT, Leffa DT, Mittelstadt SD, Mendes LST, Brunoni AR, Pinto JV, et al. Positive effects of transcranial direct current stimulation in adult patients with attention-deficit/hyperactivity disorder - A pilot randomized controlled study. Psychiatry Res (2017) 247:28–32. doi: 10.1016/j.psychres.2016.11.009
24. Sotnikova A, Soff C, Tagliazucchi E, Becker K, Siniatchkin M. Transcranial direct current stimulation modulates neuronal networks in attention deficit hyperactivity disorder. Brain Topogr (2017) 30:656–72. doi: 10.1007/s10548-017-0552-4
25. Cosmo C, Ferreira C, Miranda JG, Rosario RS, Baptista AF, Montoya P, et al. Spreading effect of tDCS in individuals with attention-deficit/hyperactivity disorder as shown by functional cortical networks: a randomized, double-blind, sham-controlled trial. Front Psychiatry (2015) 6:111. doi: 10.3389/fpsyt.2015.00111
26. Bandeira ID, Guimaraes RS, Jagersbacher JG, Barretto TL, de Jesus-Silva JR, Santos SN, et al. Transcranial direct current stimulation in children and adolescents with Attention-Deficit/Hyperactivity Disorder (ADHD): a pilot study. J Child Neurol (2016) 31:918–24. doi: 10.1177/0883073816630083
27. Munz M, Prehn-Kristensen A, Thielking F, Mölle M, GR, BL. Slow oscillating transcranial direct current stimulation during non-rapid eye movement sleep improves behavioral inhibition in attention-deficit/hyperactivity disorder. Front Cell Neurosci (2015) 9:1–8. doi: 10.3389/fncel.2015.00307
28. Soltaninejad Z, Nejati V, Ekhtiari H. Effect of anodal and cathodal Transcranial direct current stimulation on DLPFC on modulation of inhibitory control in ADHD. J Atten Disord (2015) 23(4):325–32. doi: 10.1177/1087054715618792
29. Antal A, Alekseichuk I, Bikson M, Brockmoller J, Brunoni AR, Chen R, et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol (2017) 128:1774–809. doi: 10.1016/j.clinph.2017.06.001
30. Krishnan C, Santos L, Peterson MD, Ehinger M. Safety of noninvasive brain stimulation in children and adolescents. Brain stimulation (2015) 8:76–87. doi: 10.1016/j.brs.2014.10.012
31. Shaw MT, Kasschau M, Dobbs B, Pawlak N, Pau W, Sherman K, et al. Remotely supervised transcranial direct current stimulation: an update on safety and tolerability. J Vis Exp (2017) (128):56211. doi: 10.3791/56211
32. Palm U, Kumpf U, Behler N, Wulf L, Kirsch B, Wörsching J, et al. Home use, remotely supervised, and remotely controlled transcranial direct current stimulation: a systematic review of the available evidence. Neuromodulation: Technol Neural Interface (2018) 21:323–33. doi: 10.1111/ner.12686.
33. Wexler A. Who uses direct-to-consumer brain stimulation products, and why? A study of home users of tDCS devices. J Cogn Enhanc (2018) 2:114–34. doi: 10.1007/s41465-017-0062-z
34. Dubljević V, Saigle V, Racine E. The rising tide of tDCS in the media and academic literature. Neuron (2014) 82:731–6. doi: 10.1016/j.neuron.2014.05.003
35. DaSilva AF, Volz MS, Bikson M, Fregni F. Electrode positioning and montage in transcranial direct current stimulation. J Vis Exp (2011) (51):2744. doi: 10.3791/2744
36. Maslen H, Douglas T, Cohen Kadosh R, Levy N, Savulescu J. The regulation of cognitive enhancement devices: refining Maslen et al.’s model. J Law Biosci (2015) 2:754–67. doi: 10.1093/jlb/lsv029
37. Fregni F, Nitsche MA, Loo CK, Brunoni AR, Marangolo P, Leite J, et al. Regulatory considerations for the clinical and research use of Transcranial Direct Current Stimulation (tDCS): review and recommendations from an expert panel. Clin Res Regul Aff (2015) 32:22–35. doi: 10.3109/10601333.2015.980944
38. Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol (2017) 128:56–92. doi: 10.1016/j.clinph.2016.10.087
39. Bikson M, Paneri B, Mourdoukoutas A, Esmaeilpour Z, Badran BW, Azzam R, et al. Limited output transcranial electrical stimulation (LOTES-2017): engineering principles, regulatory statutes, and industry standards for wellness, over-the-counter, or prescription devices with low risk. Brain Stimul (2018) 11:134–57. doi: 10.1016/j.brs.2017.10.012
40. Bourgeois FT, Kim JM, Mandl KD. Premarket safety and efficacy studies for ADHD medications in children. PLoS One (2014) 9:e102249. doi: 10.1371/journal.pone.0102249
41. Klassen TP, Hartling L, Craig JC, Offringa M. Children are not just small adults: the urgent need for high-quality trial evidence in children. PLoS Med (2008) 5:e172. doi: 10.1371/journal.pmed.0050172
42. Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, S3-Leitlinie: ADHS bei Kindern, Jugendlichen und Erwachsenen. (2017).
43. Hameed MQ, Dhamne SC, Gersner R, Kaye HL, Oberman LM, Pascual-Leone A, et al. Transcranial magnetic and direct current stimulation in children. Curr Neurol Neurosci Rep (2017) 17(2):11. doi: 10.1007/s11910-017-0719-0
44. Minhas P, Bikson M, Woods AJ, Rosen AR, Kessler SK. Transcranial direct current stimulation in pediatric brain: a computational modeling study. Conference proceedings:… Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference 2012 (2012) 859–62. doi: 10.1109/EMBC.2012.6346067
45. Kessler SK, Minhas P, Woods AJ, Rosen A, Gorman C, Bikson M. Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PLoS One (2013) 8:e76112. doi: 10.1371/journal.pone.0076112
46. Kolb B, Teskey GC. Age, experience, injury, and the changing brain. Dev Psychobiol (2012) 54:311–25. doi: 10.1002/dev.20515
47. Palm U, Segmiller FM, Epple AN, Freisleder FJ, Koutsouleris N, Schulte-Korne G, et al. Transcranial direct current stimulation in children and adolescents: a comprehensive review. J Neural Transm (2016) 123:1219–34. doi: 10.1007/s00702-016-1572-z
48. Lee JC, Kenney-Jung DL, Blacker CJ, Doruk Camsari D, Lewis CP. Transcranial direct current stimulation in child and adolescent psychiatric disorders. Child Adolesc Psychiatr Clin N Am (2019) 28:61–78. doi: 10.1016/j.chc.2018.07.009
49. Iuculano T, Kadosh R. The mental cost of cognitive enhancement. J Neurosci (2013) 33:4482–6. doi: 10.1523/JNEUROSCI.4927-12.2013
50. Wurzman RP, Hamilton RH. Ethical Aspects of tDCS Use in Neuropsychiatry and the Risk of Misuse. In: Brunoni A, Nitsche M, Loo C, editors. Transcranial Direct Current Stimulation in Neuropsychiatric Disorders: Clinical Principles and Management. Cham: Springer International Publishing, (2016). p. 363–82. doi: 10.1007/978-3-319-33967-2_24
51. Li LM, Violante IR, Leech R, Ross E, Hampshire A, Opitz A, et al. Brain state and polarity dependent modulation of brain networks by transcranial direct current stimulation. Hum Brain Mapp (2019) 40:904–15. doi: 10.1002/hbm.24420
52. Hsu TY, Juan CH, Tseng P. Individual differences and state-dependent responses in transcranial direct current stimulation. Front Hum Neurosci (2016) 10:643. doi: 10.3389/fnhum.2016.00643
53. Prehn-Kristensen A, Munz M, Goder R, Wilhelm I, Korr K, Vahl W, et al. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimul (2014) 7:793–9. doi: 10.1016/j.brs.2014.07.036
54. Schweren L, Hoekstra P, van Lieshout M, Oosterlaan J, Lambregts-Rommelse N, Buitelaar J, et al. Long-term effects of stimulant treatment on ADHD symptoms, social-emotional functioning, and cognition. Psychol Med (2019) 49(2):217–23. doi: 10.1017/S0033291718000545
56. DuPaul GJ, Gormley MJ, Laracy SD. Comorbidity of LD and ADHD: implications of DSM-5 for assessment and treatment. J Learn Disabil (2013) 46:43–51. doi: 10.1177/0022219412464351
57. European Medicines Agency. Informed Consent for Paediatric Clinical Trials in Europe 2015. London (2018).
58. Waligora M, Dranseika V, Piasecki J. Child’s assent in research: age threshold or personalisation? BMC Med Ethics (2014) 15:44. doi: 10.1186/1472-6939-15-44
59. Davis NJ. Transcranial stimulation of the developing brain: a plea for extreme caution. Front Hum Neurosci (2014) 8:600. doi: 10.3389/fnhum.2014.00600
60. Davis N, Koningsbruggen M. “Non-invasive” brain stimulation is not non-invasive. Front Syst Neurosci (2013) 7:76. doi: 10.3389/fnsys.2013.00076
61. Aoki Y, Yamaguchi S, Ando S, Sasaki N, Bernick PJ, Akiyama T. The experience of electroconvulsive therapy and its impact on associated stigma: a meta-analysis. Int J Social Psychiatry (2016) 62:708–18. doi: 10.1177/0020764016675379
62. Advokat CD, Guidry D, Martino L. Licit and illicit use of medications for attention-deficit hyperactivity disorder in undergraduate college students. J Am Coll Health (2008) 56:601–6. doi: 10.3200/JACH.56.6.601-606
63. Rabiner DL, Anastopoulos AD, Costello EJ, Hoyle RH, McCabe SE, Swartzwelder HS. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord (2009) 13:259–70. doi: 10.1177/1087054708320399
64. Buyx A. Smart drugs: ethical issues and suggestions for policy making. In: Clausen J, Levy N, editors. Handbook on neuroethics. Springer (2014). p. 1191–206.
65. Maslen H, Earp BD, Cohen Kadosh R, Savulescu J. Brain stimulation for treatment and enhancement in children: an ethical analysis. Front Hum Neurosci (2014) 8:953. doi: 10.3389/fnhum.2014.00953
66. Miranda PC, Mekonnen A, Salvador R, Ruffini G. The electric field in the cortex during transcranial current stimulation. Neuroimage (2013) 70:48–58. doi: 10.1016/j.neuroimage.2012.12.034
67. Palm U, Kumpf U, Behler N, Wulf L, Kirsch B, Wörsching J, et al. Remotely supervised, and remotely controlled transcranial direct current stimulation: a systematic review of the available evidence. Neuromodulation (2018) 21:323–33. doi: 10.1111/ner.12686
Keywords: tDCS—transcranial direct current stimulation, ethics, pediatric research, ADHD (attention deficit and hyperactivity disorder), noninvasive brain stimulation (NIBS)
Citation: Sierawska A, Prehn-Kristensen A, Moliadze V, Krauel K, Nowak R, Freitag CM, Siniatchkin M and Buyx A (2019) Unmet Needs in Children With Attention Deficit Hyperactivity Disorder—Can Transcranial Direct Current Stimulation Fill the Gap? Promises and Ethical Challenges. Front. Psychiatry 10:334. doi: 10.3389/fpsyt.2019.00334
Received: 04 December 2018; Accepted: 29 April 2019;
Published: 16 May 2019.
Edited by:
Xavier Noel, Free University of Brussels, BelgiumCopyright © 2019 Sierawska, Prehn-Kristensen, Moliadze, Krauel, Nowak, Freitag, Siniatchkin and Buyx. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Sierawska, YS5zaWVyYXdza2FAaWVtLnVuaS1raWVsLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.