
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 15 May 2019
Sec. Schizophrenia
Volume 10 - 2019 | https://doi.org/10.3389/fpsyt.2019.00333
This article is part of the Research Topic Early Intervention in Psychotic Disorders View all 13 articles
 Yuji Yamada1
Yuji Yamada1 Takuma Inagawa1
Takuma Inagawa1 Kazuki Sueyoshi2
Kazuki Sueyoshi2 Norio Sugawara3
Norio Sugawara3 Natsuki Ueda3
Natsuki Ueda3 Yoshie Omachi1
Yoshie Omachi1 Naotsugu Hirabayashi1
Naotsugu Hirabayashi1 Madoka Matsumoto2
Madoka Matsumoto2 Tomiki Sumiyoshi2*
Tomiki Sumiyoshi2*Backgrounds: Social cognition deficits are a core feature of schizophrenia and deteriorate functionality of patients. However, evidence is sparse for the treatment effect on social cognition impairments in the early stage of psychosis. Here, we provide a systematic review of the literature on social cognitive impairment in early psychosis in relation to its intervention.
Methods: A literature search was conducted on English articles identified by Web of Science and PubMed databases, according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.
Results: Five papers met the inclusion criteria. Results from two studies of cognitive training and one study of modafinil indicate positive results regarding social cognition outcomes in patients with early psychosis. On the other hand, two studies with oxytocin and modafinil did not suggest such effects.
Conclusions: Further research is warranted to explore the benefit of early intervention into disturbances of social cognition in psychoses.
Schizophrenia affects approximately 0.7% of the world’s population (1) and is characterized by positive (hallucinations, delusions), negative (apathy, anhedonia, social withdrawal, etc.), and cognitive symptoms. The first signs and symptoms usually appear between the end of adolescence and beginning of early adulthood. The disease has a chronic course with continual psychotic episodes that generally lead to deterioration in cognitive and social functioning (2, 3), as well as unemployment in more than 70% of patients at the chronic stage (4, 5).
Cognitive impairment is a core feature of schizophrenia and is present over the course of the illness (6). Research has shown that neurocognitive domains, such as memory, attention, executive functions, language, and intelligence, are most severely affected (7). Similar impairments are also found in social cognition (8), i.e., mental operations underlying social behavior. Social cognition is understood as a multidimensional construct that comprises emotional processing, social perspective and knowledge, attributional bias, and theory of mind (ToM). Some studies report that social cognition explains the variance of functional outcome more effectively than does neurocognition. Thus, social cognition has been considered an important treatment target for functional improvement in people with psychoses (9–12).
Impairment of social cognition, including emotional recognition (13, 14), ToM (15), and attributional biases (16), is evident before the onset of psychosis, continues throughout the early phase of illness, and may even worsen during the first episode (17–19). There have been attempts to determine the relationship between social cognition and social functioning in early psychosis (20). Available research suggests that deficits in social functioning due to social cognition deficits are present early in the course of psychotic disorders (21–23) and also in first-degree relatives of patients (24, 25).
Individuals in the early phase of psychosis exhibit a greater brain plasticity and milder structural and functional brain changes than those in patients with chronic illnesses, providing the rationale for early treatment (26, 27). So far, most published trials of cognitive remediation have used middle-aged, chronically ill patients (28), and its efficacy for those in the prodromal phase or first episode of psychotic illness is largely unknown. As data from current pharmacological interventions suggest limited effects on social cognition impairments of schizophrenia (29, 30), there is a clear need to develop effective therapeutics to target them.
Here, we provide a systematic review of the literature regarding intervention for social cognition deficits in individuals with early psychosis or high risk for developing psychosis.
This systematic review was performed based on the PRISMA guidelines (31). From inception to March 15, 2019, YY and TI independently examined the Web of Science and PubMed databases. The following search terms were used as keywords: (“early psychosis” OR “first-episode psychosis” OR “FEP” OR “first-episode schizophrenia” OR “ultra-high risk” OR “UHR” OR “psychosis prodrome” OR “at risk mental state” OR “ARMS” OR “clinical high risk”) AND (“social cognition” OR “theory of mind” OR “emotion recognition” OR “attributional style” OR “social knowledge” OR “social perception”) AND (“training” OR “rehabilitation” OR “remediation” OR “cognitive behavioral therapy” OR “CBT” OR “intervention” OR “pharma*” OR “drug” OR “antipsychotics” OR “antidepressant”) AND (“randomized controlled trial” OR “RCT”). Only studies with human participants and written in English were included. The senior reviewer (TS) approved the final list of the studies included.
Prespecified inclusion criteria were as follows: 1) randomized controlled trials (RCTs) comparing a social cognition intervention with treatment as usual, a minimal educational intervention, sham training, or placebo therapy; 2) participants were adults or adolescents between 10 and 40 years old diagnosed with early psychosis (i.e., schizophreniform disorder, schizophrenia, or schizoaffective disorder) (<5 years illness duration) without a) current substance dependence on alcohol or drugs, b) intellectual disability (intelligence quotient <70), c) a history of a significant neurological disorder, and d) florid psychotic or related symptoms likely to require immediate intervention (e.g., suicidality); 3) interventions were training or pharmacotherapy targeted to one or more social cognition domains; 4) comparisons were treatment as usual, a minimal educational intervention, sham training, or placebo therapy; and 5) outcomes were objective scales defined as ToM, emotion recognition, attributional style, social perception, and social knowledge.
Outcome measures identified by this search were discussed in relation to three domains of social cognition, i.e., emotion recognition, theory of mind (ToM), and attributional bias (see Table 1).
Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT) (37) measures the participant’s ability to perceive, use, understand, and regulate emotions, while Facial Expressions of Emotions Task (FEEST) (38) requires subjects to identify six basic emotions (happiness, sadness, anger, fear, surprise, and disgust) from facial expressions, although Cacciotti-Saija et al. (34) gave no information about whether they used morphing images of different emotional valences or varying degree of emotional intensities. Movie Stills Task (39) requires identification of emotions (happy, surprised, afraid, angry, disgusted, sad, or neutral) from a complex movie scene. On the other hand, Pictures of Facial Affect (POFA) (40) uses facial photos providing the morphing faces of different emotions, or emotional face of different emotional intensities (0% fearful, 10% fearful, 20% fearful, 30% fearful, … and 100% fearful). In this task, subjects are instructed to recognize basic emotions (happiness, sadness, anger, disgust, and surprise) in 60 faces. Furthermore, Emotion Recognition Task (ERT) consists of a series of mixed ethnic background faces photographs depicting seven emotions: happiness, surprise, neutral, fear, disgust, anger, and sadness (41). Finally, a subdomain of social cognition of the MATRICS Consensus Cognitive Battery (MCCB) (42, 43) was developed for use in schizophrenia.
False Belief Picture Sequencing Task (44) consists of arrangement of picture-cards into a logical sequence of events to test the ability to go beyond objective information to reason that a story protagonist is acting on the basis of a false belief. Reading the Mind in the Eyes Test (RMET) (45) assesses the ability to infer mental states from images of eye regions, and provides a sensitive measure of social cognition impairments in early psychosis (46). The modified Faux Pas Task (47) requires participants to respond when faux pas are present. The Empathy Quotient (48) is a self-report measure of cognitive and affective aspects of empathy.
ToM task consists of four classic false belief/deception stories; the “Sally & Anne” (49) and “Box of Chocolate” stories (50) are used to assess first-order ToM abilities, while “Burglar” (50) and “Ice-Cream Van” (50) are used to assess second-order ToM skills. These stories are read aloud by the examiner, and subjects are asked to listen and subsequently answer a ToM question and a control question. In order to avoid a possible learning effect, two homologous false belief/deception first-order ToM stories [“Cigarettes” (51) and “Piggy bank” (51)] and second-order ToM stories [“Train station” (52) and “Coke” (52)] are administered at baseline and posttreatment. Hinting Task (53) is also used, in which patients have to understand indirect speech and infer the mental state of one of the characters.
Ambiguous Intentions Hostility Questionnaire (54) contains five short vignettes describing negative interpersonal events with ambiguous causality. Internal, Personal, and Situational Attributions Questionnaire (IPSAQ) (55) is designed to assess the extent to which individuals attribute negative and positive events to different attributional loci. The task consists of 32 social items describing 16 positive and 16 negative events. Patients are asked to generate the most likely cause of each event and state whether the cause is due to self, other people, or circumstances. Six subscale scores are generated (number of positive events attributed to self, other people, and circumstances, and corresponding scores for negative events), which are used to calculate two composite scores: externalizing bias (EB) and personalizing bias (PB).
Initially, titles and abstracts were screened to identify eligible studies. Full-text articles were obtained for all the studies considered compatible based on the abstract screening and were further reviewed for eligibility.
We selected the Cochrane Collaboration’s risk of bias tool to evaluate risk of bias in each trial. Two independent reviewers (YY and TI) determined 1) if patients were correctly randomized, 2) if the randomization method was properly concealed, and 3) if subjects and/or investigators and/or raters were blinded. We assessed whether the authors collected and reported all results for all pre-specified outcomes. A senior reviewer (TS) approved the final decision of the assessment of risk of bias.
The initial search provided a total of 39 records. After removing duplicates, 32 articles were screened, of which 11 English full texts were available. Five articles found eligible for the systematic review. Articles describing studies that involved only secondary analysis of baseline data from RCT (n = 3), and no social cognition outcome measures (n = 3) were excluded. The PRISMA study selection flowchart is shown in Figure 1. The summary of risk of bias is presented in Figure 2.
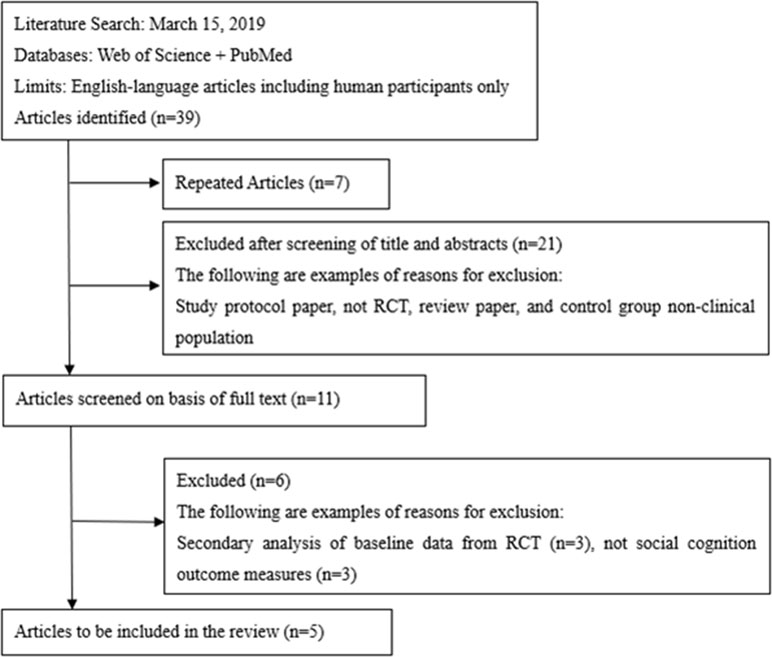
Figure 1 Study selection flowchart, following the guidelines of the PRISMA statement. Initially, titles and abstracts were screened to identify eligible studies. Full-text articles were obtained for all the studies considered compatible, based on the abstract screening, and were further reviewed for eligibility.
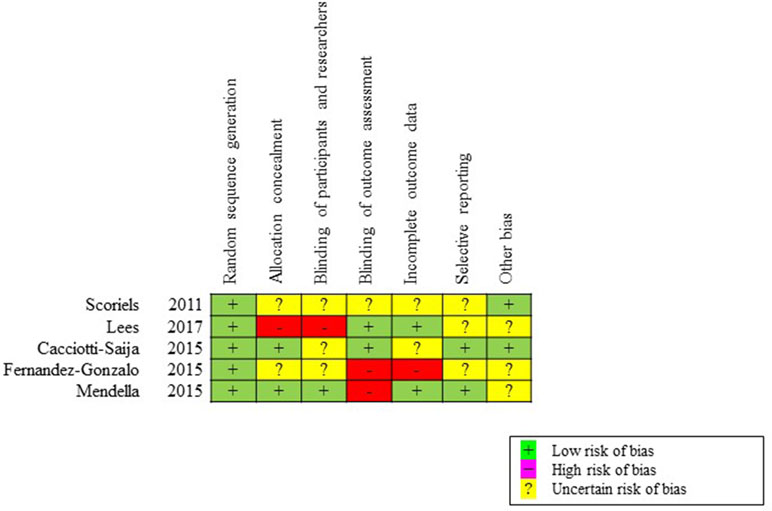
Figure 2 Assessment of risk of bias for included studies, based on the Cochrane Collaboration’s risk of bias tool. We determined whether each trial had a low, high, or uncertain risk of bias in terms of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.
The five studies included in the current review encompassed 212 subjects (151 men and 61 women). Characteristics of the selected studies are shown in Table 2. There were considerable differences between the studies in terms of demographics, intervention type, and outcome measures. Two studies (32, 36) targeted first-episode psychosis (FEP) subjects, while three (33–35) included early psychosis patients with less than 5-year illness duration. Two studies used cognition training or rehabilitation as their intervention (35, 36), while one concerned intervention with oxytocin (34), and two with modafinil (32, 33). For these studies, treatment as usual (35, 36) or placebo therapy (32–34) was used as a comparison group.
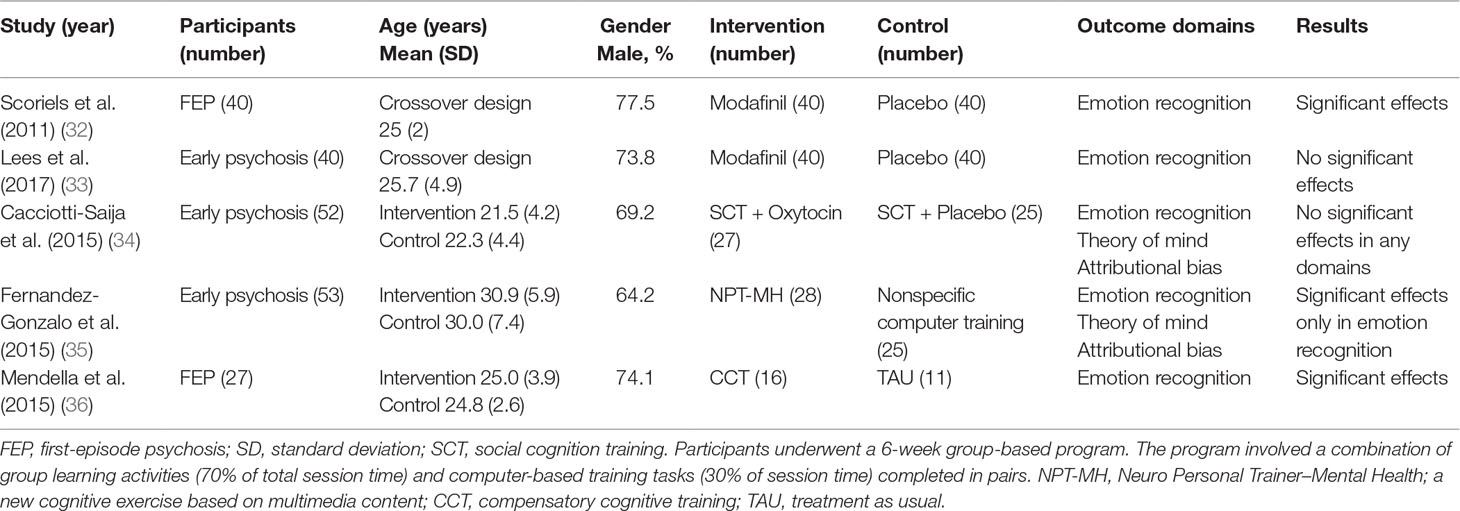
Table 2 Summary of studies comparing the performance on social cognition tasks in individuals with early psychosis.
Social cognitive impairment, including emotional recognition, ToM, and attributional biases, was evident during the early phase of psychosis, as shown in Table 3.
Social cognitive training exhibited significant effects in limited domains. The three studies included in the systematic review used social cognitive training, two of which found significant effects, as shown in Table 2. Effect sizes by means of Cohen’s d (56, 57) indicated large effects on emotional recognition domains in two studies (35, 36), while other domains were not affected (see Table 3).
There were no significant effects of oxytocin on any outcomes of social cognition (34). One study found that modafinil significantly improved the recognition of sad facial expressions (32), although there was no significant effect on social cognition performance, as measured by the MCCB, in another study of modafinil (33) (Table 3).
Five papers met the inclusion criteria for the current review. Two studies of cognitive training showed positive results in terms of social cognition. One study (32) of modafinil also reports improvement of recognition of sad facial expressions. On the other hand, two pharmacological studies (33, 34) on oxytocin or modafinil did not exhibit such effects.
Social cognition training was shown to improve emotional processing in early psychosis (35). Patients with first-episode schizophrenia present difficulties in identifying facial emotions, specifically negative ones (58), which have been related with functionality (9). Current reviews suggest that emotional processing may be improved by cognitive training even at early stages of the illness. On the other hand, efficacy of cognitive remediation was not evident in other domains of social cognition, which requires further investigations.
Social cognitive training programs aim to improve specific domains of social cognitive impairments that are related to social functioning and readily transferable to real-world situations (59). These cognitive models of early psychosis rest on aberrant salience and biased appraisal processes (60). These biological processes consist of increased striatal dopamine release, which is associated with aberrant salience. Aberrant salience opens the gates to consciousness for trivial stimuli to enter the center of attention, and the salient stimulus cries out for an appraisal (60, 61). The appraisal process elicited by aberrant salience is a key mechanism of developing delusions. A characteristic of individuals with early psychosis is that they are still open for multiple explanations for extraordinary experiences. Cognitive therapy targets appraisal processes that accompany perceptual aberrations and suspiciousness to normalize extraordinary experiences with education (61).
Although Cacciotti-Saija et al. (34) and Fernandez-Gonzalo et al. (35) used the same Ekman’s photos as dependent measure, their studies reported different intervention effects. This suggests that social cognitive training and oxytocin treatment may change different neurobiological substrates.
Although existing evidence indicates that oxytocin impacts favorably on domains of social cognition (62), its treatment effects, in comparison with placebo, were absent in young people with early psychosis. Oxytocin is a neuropeptide that interacts with a variety of neuromodulators, including serotonin and dopamine, in the nucleus accumbens and amygdala (63). Results from a previous study (64) suggest that genetic variants of oxytocin receptors may be responsible for social cognitive impairments of schizophrenia. The reliability of benefits of oxytocin and other neuropeptides, e.g., vasopressin, across population and contexts remains an ongoing issue.
Modafinil is a wake-promoting agent for the treatment of excessive daytime sleepiness. It activates monoamines and glutamate, and inhibits γ-aminobutyric acid neurotransmitters in several brain regions, including the prefrontal cortex, hippocampus, hypothalamus, thalamus, and basal ganglia. Modafinil also induces changes of neurotransmissions in the hippocampus and limbic regions, an action related to memory- and mood-enhancing properties (32).
Scoriels et al. (32) reported the efficacy of modafinil on emotional recognition using the Emotion Recognition Task (ERT). Critical nodes in the emotional face recognition circuitry include the amygdala, which is activated during performance on the ERT (65). Modafinil activates amygdala (66) and increases serotonin levels in it (67). These observations suggest that modafinil improves emotional face recognition in patients with FEP through serotonergic effects on the amygdala. On the other hand, Lees et al. (33) did not find the ability of modafinil to improve social cognition in early psychosis, as measured by the MCCB. These results indicate that the prosocial cognition effects of modafinil or other compounds depend on the type of cognitive tests used.
The neural network of social cognition consists of orbitofrontal cortex, medial prefrontal cortex, superior temporal sulcus, and amygdala, whose functional connectivity is decreased in psychotic patients (68, 69). Previous studies showed that the amygdala plays a key role in perception of facial emotional expression (39), while the prefrontal cortices are strongly associated with ToM (70). On the other hand, the superior temporal sulcus is related to both domains of social cognition (71). These lines of evidence may provide a clue to the development of novel therapeutics, including those of neuromodulation methods.
The differential effects of treatment on emotion recognition, ToM, and attribution styles deserve discussions. Emotion processing shows a consistent relationship with community functioning, which includes a wide range of activities and behaviors related to work functioning and independent living (72, 73). ToM relates to the capacity to interpret beliefs and feelings of others, i.e., predicting general psychotic symptoms, especially negative ones (74). Moreover, ToM is strongly associated with multiple dimensions of social functioning, including interpersonal communication, recreational activities, independence, and performance (73). On the other hand, attributional bias describes how individuals make sense the causes of the positive and negative social events and interactions encountered in life, providing a significant impact on behaviors (75). These findings support the roles for individual domains of social cognition in mediating neurocognition and functional outcomes, which may be relevant to early psychosis.
To conquer social cognition impairments in established schizophrenia, psychosocial approaches, e.g., social cognition and interaction training (SCIT), metacognitive training, training of affect recognition (TAR), emotion and ToM imitation training, emotion processing, and ToM video-based training, as well as pharmacological approaches, e.g., aripiprazole and risperidone, have been attempted. However, there is no such attempt targeting early psychosis, indicating a need for further efforts in this area.
Since no definite strategy has been established to treat social cognition deficits in early psychoses, some types of neuromodulation have been drawing attention. For example, repetitive transcranial magnetic stimulation (rTMS) has been shown to ameliorate facial affect recognition, assessed by “Picture of Facial Affect,” in patients with chronic schizophrenia (76). This result may indicate that noninvasive brain stimulations may improve social cognition in patients with psychosis. Transcranial direct current stimulation (tDCS) is another type of transcranial electrical stimulation procedures. So far, tDCS has been shown to improve neurocognition, as well as daily-living skills and depressive symptoms, in patients with schizophrenia (77). Of note, the effect on psychotic symptoms was associated with oxy-hemoglobin concentrations in cortical regions, as measured by near-infrared spectroscopy (78). Based on these considerations, efforts to evaluate the benefit of neuromodulation on social cognition in psychosis are warranted.
In the present review, we did not find any study exploring the influence of antipsychotic treatments on social cognitions, such as ToM, emotion recognition, and attributional style, in patients with early psychosis. This area also deserves further investigations.
The limitations of the present review should be noted here. Although 2006 workshop sponsored by the National Institute of Mental Health (NIMH) (11) recommended five domains (attributional style, emotion recognition, social knowledge, social perception, and ToM) for the evaluation of social cognition in psychotic disorders, no study to date has comprehensively examined these domains in the same sample; heterogeneity in terms of social cognitive domains across studies may have obscured findings on the efficacy of treatments. Further investigations circumventing these methodological issues deserve considerations.
As interventions into disturbances of social cognition in early psychosis provide an important issue, further studies, including those with novel paradigms, are warranted.
YY and TS planned the study. YY designed it and drafted the first manuscript. YY and TI independently searched and assessed the literature. TS approved the final list of included studies. TI, MM, KS, NS, NU, YO, NH, and TS critically reviewed the draft and revised it. All authors made substantial contributions and approved the final manuscript.
This study was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI No. 17K10321, Intramural Research Grant (29-1, 30-1, 30-8) for Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry (NCNP), and AMED under Grant Numbers 18dk0307069 and 18dk0307081.
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Drs. Kazuyuki Nakagome and Mitsutoshi Okazaki at the National Center of Neurology and Psychiatry for supporting our research activity.
1. MacDonald AW, Schulz SC. What we know: findings that every theory of schizophrenia should explain. Schizophr Bull (2009) 35(3):493–508. doi: 10.1093/schbul/sbp017
2. Andreasen NC. Schizophrenia: the fundamental questions. Brain Res Rev (2000) 31(2–3):106–12. doi: 10.1016/S0165-0173(99)00027-2
3. Mueser KT, McGurk SR. Schizophrenia. Lancet (2004) 363(9426):2063–72. doi: 10.1016/S0140-6736(04)16458-1
4. Lehman AF, Goldberg R, Dixon LB, McNary S, Postrado L, Hackman A, et al. Improving employment outcomes for persons with severe mental illnesses. Arch Gen Psychiatry (2002) 59(2):165–72. doi: 10.1001/archpsyc.59.2.165
5. Marwaha S, Johnson S. Schizophrenia and employment—a review. Soc Psychiatry Psychiatr Epidemiol (2004) 39(5):337–49. doi: 10.1007/s00127-004-0762-4
6. Jahshan C, Heaton RK, Golshan S, Cadenhead KS. Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology (2010) 24(1):109–20. doi: 10.1037/a0016791
7. Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated meta-analysis of the scientific evidence. BMC Psychiatry (2012) 12:64. doi: 10.1186/1471-244X-12-64
8. Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull (2006) 32(Suppl 1):S44–63. doi: 10.1093/schbul/sbl029
9. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev (2011) 35(3):573–88. doi: 10.1016/j.neubiorev.2010.07.001
10. Green MF, Olivier B, Crawley JN, Penn DL, Silverstein S. Social cognition in schizophrenia: recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr Bull (2005) 31(4):882–7. doi: 10.1093/schbul/sbi049
11. Green MF, Penn DL, Bentall R, Carpenter WT, Gaebel W, Gur RC, et al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull (2008) 34(6):1211–20. doi: 10.1093/schbul/sbm145
12. Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophr Bull (2008) 34(3):408–11. doi: 10.1093/schbul/sbn014
13. Amminger GP, Schäfer MR, Klier CM, Schlögelhofer M, Mossaheb N, Thompson A, et al. Facial and vocal affect perception in people at ultra-high risk of psychosis, first-episode schizophrenia and healthy controls. Early Interv Psychiatry (2012) 6(4):450–4. doi: 10.1111/j.1751-7893.2012.00362.x
14. Horan WP, Green MF, DeGroot M, Fiske A, Hellemann G, Kee K, et al. Social cognition in schizophrenia, Part 2: 12-month stability and prediction of functional outcome in first-episode patients. Schizophr Bull (2012) 38(4):865–72. doi: 10.1093/schbul/sbr001
15. Bora E, Pantelis C. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: systematic review and meta-analysis. Schizophr Res (2013) 144(1–3):31–6. doi: 10.1016/j.schres.2012.12.013
16. Thompson A, Papas A, Bartholomeusz C, Nelson B, Yung A. Externalized attributional bias in the Ultra High Risk (UHR) for psychosis population. Psychiatry Res (2013) 206(2–3):200–5. doi: 10.1016/j.psychres.2012.10.017
17. Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry (1994) 51(2):124–31. doi: 10.1001/archpsyc.1994.03950020048005
18. Bilder RM, Reiter G, Bates J, Lencz T, Szeszko P, Goldman RS, et al. Cognitive development in schizophrenia: follow-back from the first episode. J Clin Exp Neuropsychol (2006) 28(2):270–82. doi: 10.1080/13803390500360554
19. Eastvold AD, Heaton RK, Cadenhead KS. Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophr Res (2007) 93(1–3):266–77. doi: 10.1016/j.schres.2007.03.013
20. Addington J, Saeedi H, Addington D. Influence of social perception and social knowledge on cognitive and social functioning in early psychosis. Br J Psychiatry (2006) 189:373–8. doi: 10.1192/bjp.bp.105.021022
21. Achim AM, Ouellet R, Roy MA, Jackson PL. Mentalizing in first-episode psychosis. Psychiatry Res (2012) 196(2–3):207–13. doi: 10.1016/j.psychres.2011.10.011
22. Bourdeau G, Masse M, Lecomte T. Social functioning in early psychosis: are all the domains predicted by the same variables? Early Interv Psychiatry (2012) 6(3):317–21. doi: 10.1111/j.1751-7893.2011.00337.x
23. Lecomte T, Corbière M, Ehmann T, Addington J, Abdel-Baki A, Macewan B. Development and preliminary validation of the First Episode Social Functioning Scale for early psychosis. Psychiatry Res (2014) 216(3):412–7. doi: 10.1016/j.psychres.2014.01.044
24. Glatt SJ, Stone WS, Faraone SV, Seidman LJ, Tsuang MT. Psychopathology, personality traits and social development of young first-degree relatives of patients with schizophrenia. Br J Psychiatry (2006) 189:337–45. doi: 10.1192/bjp.bp.105.016998
25. Lavoie MA, Plana I, Bédard Lacroix J, Godmaire-Duhaime F, Jackson PL, Achim AM. Social cognition in first-degree relatives of people with schizophrenia: a meta-analysis. Psychiatry Res (2013) 209(2):129–35. doi: 10.1016/j.psychres.2012.11.037
26. Keshavan MS, Hogarty GE. Brain maturational processes and delayed onset in schizophrenia. Dev Psychopathol (1999) 11(3):525–43. doi: 10.1017/S0954579499002199
27. Berger G, Dell’Olio M, Amminger P, Cornblatt B, Phillips L, Yung A, et al. Neuroprotection in emerging psychotic disorders. Early Interv Psychiatry (2007) 1:114–27. doi: 10.1111/j.1751-7893.2007.00021.x
28. Kim EJ, Bahk YC, Oh H, Lee WH, Lee JS, Choi KH. Current status of cognitive remediation for psychiatric disorders: a review. Front Psychiatry (2018) 9:461. doi: 10.3389/fpsyt.2018.00461
29. Hill SK, Bishop JR, Palumbo D, Sweeney JA. Effect of second-generation antipsychotics on cognition: current issues and future challenges. Expert Rev Neurother (2010) 10(1):43–57. doi: 10.1586/ern.09.143
30. Kucharska-Pietura K, Mortimer A. Can antipsychotics improve social cognition in patients with schizophrenia? CNS Drugs (2013) 27(5):335–43. doi: 10.1007/s40263-013-0047-0
31. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol (2009) 62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006
32. Scoriels L, Barnett JH, Murray GK, Cherukuru S, Fielding M, Cheng F, et al. Effects of modafinil on emotional processing in first episode psychosis. Biol Psychiatry (2011) 69(5):457–64. doi: 10.1016/j.biopsych.2010.09.043
33. Lees J, Michalopoulou PG, Lewis SW, Preston S, Bamford C, Collier T, et al. Modafinil and cognitive enhancement in schizophrenia and healthy volunteers: the effects of test battery in a randomized controlled trial. Psychol Med (2017) 47(13):2358–68. doi: 10.1017/S0033291717000885
34. Cacciotti-Saija C, Langdon R, Ward PB, Hickie IB, Scott EM, Naismith SL, et al. A double-blind randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis. Schizophr Bull (2015) 41(2):483–93. doi: 10.1093/schbul/sbu094
35. Fernandez-Gonzalo S, Turon M, Jodar M, Pousa E, Hernandez Rambla C, García R, et al. A new computerized cognitive and social cognition training specifically designed for patients with schizophrenia/schizoaffective disorder in early stages of illness: a pilot study. Psychiatry Res (2015) 228(3):501–9. doi: 10.1016/j.psychres.2015.06.007
36. Mendella PD, Burton CZ, Tasca GA, Roy P, St Louis L, Twamley EW. Compensatory cognitive training for people with first-episode schizophrenia: results from a pilot randomized controlled trial. Schizophr Res (2015) 162(1–3):108–11. doi: 10.1016/j.schres.2015.01.016
37. Caruso DR, Mayer JD, Salovey P. Relation of an ability measure of emotional intelligence to personality. J Pers Assess (2002) 79(2):306–20. doi: 10.1207/S15327752JPA7902_12
38. Young A, Perrett D, Calder A, Sprengelmeyer R, Ekman P. Facial expressions of emotion: stimuli and tests (FEEST). Edmunds, UK: Thames Valley Test Company (2002).
39. Adolphs R, Tranel D. Amygdala damage impairs emotion recognition from scenes only when they contain facial expressions. Neuropsychologia (2003) 41:1281–9. doi: 10.1016/S0028-3932(03)00064-2
40. Ekman P, Friesen W. Pictures of facial affect. Palo Alto, CA: Consulting Psychologist Press (1976).
41. Matsumoto D, Ekman P. Japanese and Caucasian facial expressions of emotion (JACFEE) and neutral Faces (JACNeuF) [slides and brochure]. San Francisco: San Francisco State University (1988).
42. Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry (2008) 165:203–13. doi: 10.1176/appi.ajp.2007.07010042
43. Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry (2008) 165:214–20. doi: 10.1176/appi.ajp.2007.07010043
44. Langdon R, Michie PT, Ward PB, McConaghy N, Catts SV, Coltheart M. Defective self and/or other mentalising in schizophrenia: a cognitive neuropsychological approach. Cogn Neuropsychiatry (1997) 2(3):167–93. doi: 10.1080/135468097396324
45. Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry (2001) 42:241–51. doi: 10.1017/S0021963001006643
46. Guastella AJ, Hermens DF, Van Zwieten A, Naismith SL, Lee RS, Cacciotti-Saija C, et al. Social cognitive performance as a marker of positive psychotic symptoms in young people seeking help for mental health problems. Schizophr Res (2013) 149:77–82. doi: 10.1016/j.schres.2013.06.006
47. Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci (1998) 10:640–56. doi: 10.1162/089892998562942
48. Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord (2004) 34:163–75. doi: 10.1023/B:JADD.0000022607.19833.00
49. Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition (1985) 21:37–46. doi: 10.1016/0010-0277(85)90022-8
50. Happe FG. An advanced test of theory of mind: understanding of story characters; thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord (1994) 24:129–54. doi: 10.1007/BF02172093
51. Baron-Cohen S. The autistic child’s theory of mind: a case of specific developmental delay. J Child Psychol Psychiatry (1989) 30:285–97. doi: 10.1111/j.1469-7610.1989.tb00241.x
52. Frith CD, Corcoran R. Exploring ‘theory of mind’ in people with schizophrenia. Psychol Med (1996) 26:521–30. doi: 10.1017/S0033291700035601
53. Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr Res (1995) 17(1):5–13. doi: 10.1016/0920-9964(95)00024-G
54. Combs DR, Penn DL, Wicher M, Waldheter E. The Ambiguous Intentions Hostility Questionnaire (AIHQ): a new measure for evaluating hostile social-cognitive biases in paranoia. Cogn Neuropsychiatry (2007) 12:128–43. doi: 10.1080/13546800600787854
55. Kinderman P, Bentall RP. A new measure of causal locus: the internal, personal and situational attributions questionnaire. Pers Individ Dif (1995) 20:261–4. doi: 10.1016/0191-8869(95)00186-7
56. Cohen J. Statistical power analysis for the behavioral sciences (2nd ed). Hillsdale, NJ: Lawrence Earlbaum associates (1988).
57. Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods (2002) 7:105–25. doi: 10.1037//1082-989X.7.1.105
58. Daros AR, Ruocco AC, Reilly JL, Harris MS, Sweeney JA. Facial emotion recognition in first-episode schizophrenia and bipolar disorder with psychosis. Schizophr Res (2014) 153(1–3):32–7. doi: 10.1016/j.schres.2014.01.009
59. Brown EC, Tas C, Brüne M. Potential therapeutic avenues to tackle social cognition problems in schizophrenia. Expert Rev Neurother (2012) 12(1):71–81. doi: 10.1586/ern.11.183
60. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry (2003) 160(1):13–23. doi: 10.1176/appi.ajp.160.1.13
61. van der Gaag M, van den Berg D, Ising H. CBT in the prevention of psychosis and other severe mental disorders in patients with an at risk mental state: a review and proposed next steps. Schizophr Res (2019) 203:88–93. doi: 10.1016/j.schres.2017.08.018
62. Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry (2010) 67(7):692–4. doi: 10.1016/j.biopsych.2009.09.020
63. Bukovskaya O, Shmukler A. Oxytocin and social cognitions in schizophrenia: a systematic review. Psychiatr Q (2016) 87(3):521–43. doi: 10.1007/s11126-015-9407-x
64. Davis MC, Horan WP, Nurmi EL, Rizzo S, Li W, Sugar CA, et al. Associations between oxytocin receptor genotypes and social cognitive performance in individuals with schizophrenia. Schizophr Res (2014) 159(2–3):353–7. doi: 10.1016/j.schres.2014.09.006
65. Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain (1999) 122(Pt 5): 883–93. doi: 10.1093/brain/122.5.883
66. Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci (2000) 20(22):8620–8. doi: 10.1523/JNEUROSCI.20-22-08620.2000
67. Ferraro L, Fuxe K, Tanganelli S, Tomasini MC, Rambert FA, Antonelli T. Differential enhancement of dialysate serotonin levels in distinct brain regions of the awake rat by modafinil: possible relevance for wakefulness and depression. J Neurosci Res (2002) 68(1):107–12. doi: 10.1002/jnr.10196
68. Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci (1990) 1:27–51.
69. Frith CD, Frith U. Social cognition in humans. Curr Biol (2007) 17(16):R724–32. doi: 10.1016/j.cub.2007.05.068
70. Baron-Cohen S, Ring H, Moriarty J, Schmitz B, Costa D, Ell P. Recognition of mental state terms. Br J Psychiatry (1994) 165(5):640–9. doi: 10.1192/bjp.165.5.640
71. Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res (2006) 148(2–3):75–92. doi: 10.1016/j.pscychresns.2006.05.001
72. Kee KS, Green MF, Mintz J, Brekke JS. Is emotion processing a predictor of functional outcome in schizophrenia? Schizophr Bull (2003) 29(3):487–97. doi: 10.1093/oxfordjournals.schbul.a007021
73. Javed A, Charles A. The importance of social cognition in improving functional outcomes in schizophrenia. Front Psychiatry (2018) 9:157. doi: 10.3389/fpsyt.2018.00157
74. Brown EC, Tas C, Can H, Esen-Danaci A, Brüne M. A closer look at the relationship between the subdomains of social functioning, social cognition and symptomatology in clinically stable patients with schizophrenia. Compr Psychiatry (2014) 55(1):25–32. doi: 10.1016/j.comppsych.2013.10.001
75. Pinkham AE. Social cognition in schizophrenia. J Clin Psychiatry (2014) 75(Suppl 2):14–9. doi: 10.4088/JCP.13065su1.04
76. Wölwer W, Lowe A, Brinkmeyer J, Streit M, Habakuck M, Agelink MW, et al. Repetitive transcranial magnetic stimulation (rTMS) improves facial affect recognition in schizophrenia. Brain Stimul (2014) 7(4):559–63. doi: 10.1016/j.brs.2014.04.011
77. Narita Z, Inagawa T, Sueyoshi K, Lin C, Sumiyoshi T. Possible facilitative effects of repeated anodal transcranial direct current stimulation on functional outcome 1 month later in schizophrenia: an open trial. Front Psychiatry (2017) 8:184. doi: 10.3389/fpsyt.2017.00184
78. Narita Z, Noda T, Setoyama S, Sueyoshi K, Inagawa T, Sumiyoshi T. The effect of transcranial direct current stimulation on psychotic symptoms of schizophrenia is associated with oxy-hemoglobin concentrations in the brain as measured by near-infrared spectroscopy: a pilot study. J Psychiatr Res (2018) 103:5–9. doi: 10.1016/j.jpsychires.2018.05.004
Keywords: first-episode psychosis, schizophrenia, ultra-high risk, at risk mental state, theory of mind, emotion recognition, randomized controlled trial
Citation: Yamada Y, Inagawa T, Sueyoshi K, Sugawara N, Ueda N, Omachi Y, Hirabayashi N, Matsumoto M and Sumiyoshi T (2019) Social Cognition Deficits as a Target of Early Intervention for Psychoses: A Systematic Review. Front. Psychiatry 10:333. doi: 10.3389/fpsyt.2019.00333
Received: 18 January 2019; Accepted: 29 April 2019;
Published: 15 May 2019.
Edited by:
Young-Chul Chung, Chonbuk National University, South KoreaReviewed by:
Suk Kyoon An, Yonsei University, South KoreaCopyright © 2019 Yamada, Inagawa, Sueyoshi, Sugawara, Ueda, Omachi, Hirabayashi, Matsumoto, and Sumiyoshi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomiki Sumiyoshi, c3VtaXlvdEBuY25wLmdvLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.