- Mental Health Centre Copenhagen, Gentofte Hospital, Copenhagen University Hospital, Hellerup, Denmark
The notion of immunological pathways playing a role in the etiology of a subset of psychotic disorders has received increased interest in the last decades. One of the findings that has spiked interest herein, is an apparent link between autoimmune diseases and psychotic disorders. This is supported by genetic findings associating immune-related genetic markers with schizophrenia and clinical studies finding increased levels of inflammatory markers in patients with psychosis. Several large-scale epidemiologic studies have found positive associations between autoimmune diseases and psychosis. Particularly, autoimmune diseases as multiple sclerosis and lupus are known to have higher frequencies of neuropsychiatric symptoms, including psychosis, compared to healthy controls. Cross sectional studies have found higher prevalence of psychiatric diagnoses among those with autoimmune diseases, and longitudinal studies have shown bidirectional associations between several autoimmune diseases and increased risks associated with schizophrenia. Moreover, a family history of autoimmune diseases has been shown to be associated with an increased risk of psychotic disorders and vice versa. In this review we will summarize the epidemiologic evidence on associations between autoimmune diseases and psychosis. Possible mechanisms accountable for the association will be discussed, amongst others the probable role of shared genetic risk factors, the impact of infections on both autoimmunity and the development of psychotic disorders, and the potential role of the microbiome. We discuss the findings on and influence of autoantibodies and dysregulation of T- and B-cells in both disease categories, and why further research hereon is needed. In addition to the potential importance of autoimmunity in etiological mechanisms of psychotic disorders, the association also brings important attention to somatic comorbidity in patients with psychotic disorders.
Introduction
The association between immunological processes and mental disorders was observed by doctors centuries before the immune system was discovered. Psychosis arising either with the occurrence or disappearance of acute fever has been described by many scientists from Hippocrates around 400 BC to Kraepelin around 1900. In the 1930s it was first hypothesized by Hermann Lehmann-Facius that schizophrenia was the product of an autoimmune reaction with antibodies attacking brain tissue (1). In the 1950s and 1960s it was noticed that celiac disease seemed to occur more often within those suffering from schizophrenia than in the general population (2), and conversely, that schizophrenia occurred less frequently within patients with rheumatoid arthritis (3, 4). Additionally, autoantibodies cross-reacting with brain antigens were found in patients with schizophrenia back in the 1960s (5, 6), and interest in anti-neuronal antibodies in psychotic disorders has increased during the last couple of decades, with an increasing number of reports on previously unknown antibodies with brain reactivity in patients suffering from psychosis (7–9).
The amount of evidence supporting the notion of a link between immunological processes and psychotic disorders has increased. Elevated levels of inflammatory markers have been found both in the blood (10, 11) and CSF (12–14) of patients with psychosis, with even higher levels in patients in first episode psychosis or acute relapse. Furthermore, some have found association between higher levels of inflammation in childhood and adolescence and increased risk of psychotic disorders (15, 16), elevated inflammatory biomarkers has been associated with lack of treatment response (17), and anti-inflammatory treatment has been found to have especially beneficial effect in an inflamed subgroup of patients (18–21). Moreover, it has been suggested that schizophrenia could be an autoimmune disease, based on similarities such as the remitting-relapsing phenotype of the illness, as well as the above-mentioned immunological processes (22).
Research in the field of psychoneuroimmunology is still evolving, with many different aspects being investigated. The notion of a role of the immune system in psychotic disorders seems evident, and understanding the link between autoimmune diseases and mental disorders may shed light on possible etiological mechanisms herein. Understanding how the immune system and psychotic illness interact can improve our understanding of psychosis and give rise to a wide range of new treatment options in psychiatry; amongst other the possibility to identify subgroups of patients with psychotic disorders and ongoing inflammatory processes that could benefit from more targeted treatment. Additionally, it is very important for clinicians to be aware of somatic comorbidities, particularly in patients with psychotic disorders, in order to improve detection and treatment, and thus the course of illness.
Epidemiological Associations
The world-wide prevalence of schizophrenia is known to be around 1% (23) and the prevalence of autoimmune diseases have been found in a Danish nationwide study to be 4% (24). The vast majority of epidemiological studies have found a general association between autoimmunity and psychotic disorders (24–29). In large-scale register-based studies from Denmark, 6% of those diagnosed with schizophrenia also had a hospital contact related to an autoimmune disease during follow-up (25, 26), and a Taiwanese study found that 3.4% of persons with a hospital contact for autoimmune diseases also had a hospital contact related to schizophrenia (29). A Danish study based on 7704 patients with schizophrenia, found an increased prevalence by about 45% of the occurrence of an autoimmune disease (28), which was later confirmed in a Taiwanese population-based study (27). Regarding the risk of psychosis after an autoimmune disease diagnosis, a Danish nationwide study found this to be increased by 45%, which diminished to a 29% increased risk when excluding the effect of infections (26), and a very recent meta-analysis by Cullen et al. (30) found that a diagnosis of a non-neurological autoimmune disease increased the risk of later being diagnosed with a psychotic disorder by 43%.
Additionally, being diagnosed with schizophrenia increases the lifetime prevalence of autoimmune diseases. Two Danish register-based studies found that individuals with psychotic disorders had a subsequently elevated risk for autoimmune diseases by around 50% (25, 28). Supporting this, the recent meta-analysis found that the risk of having an autoimmune disease was 55% higher among those with a prior diagnosis of a psychotic disorder (30).
Autoimmune diseases and psychosis are not only associated on an individual level. Having a first degree relative with schizophrenia has also been found to increase the risk of autoimmune diseases with 6% (25), and a family history of autoimmunity has been found to increase risk of both schizophrenia and non-affective psychoses with 10% (24).
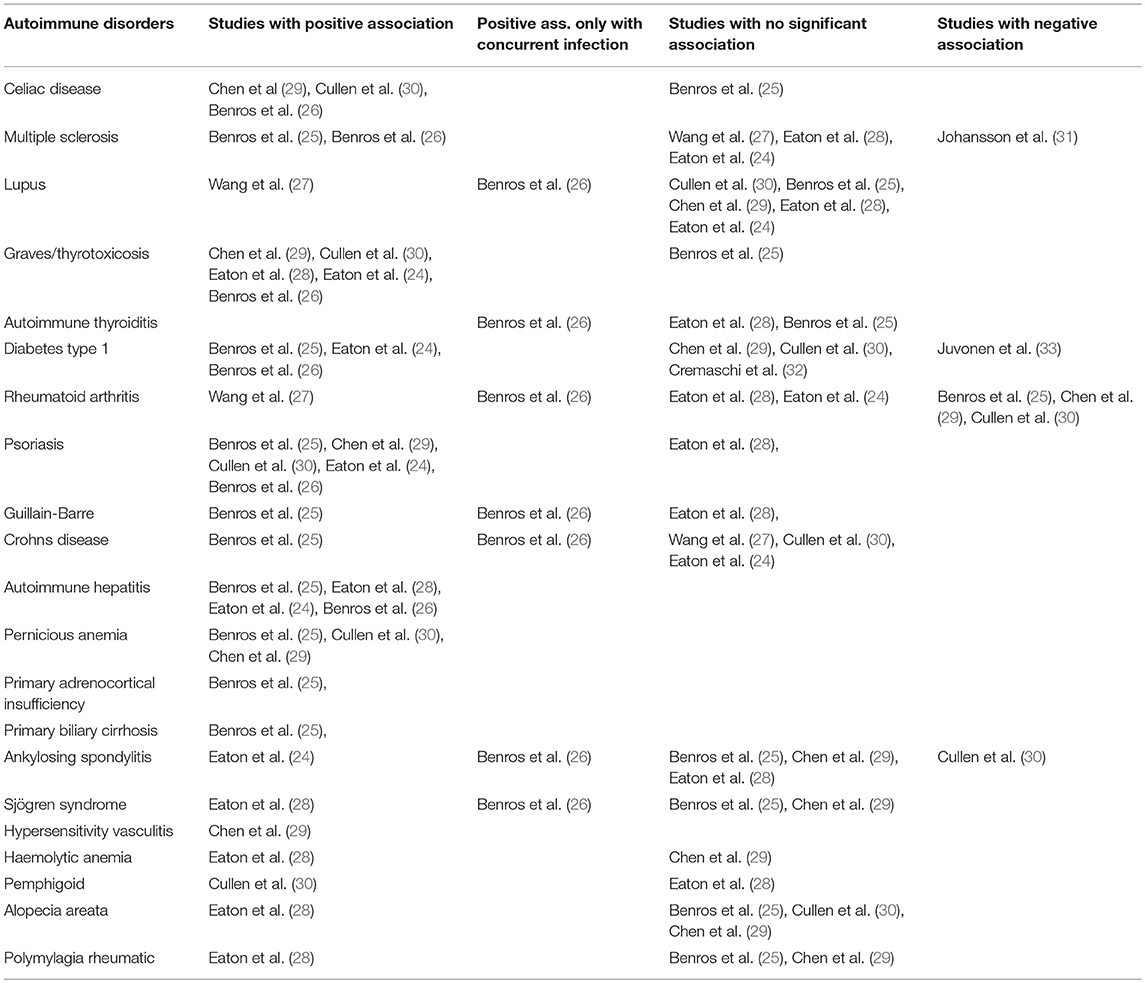
The associations with psychotic disorders have been found for a broad range of autoimmune diseases. For an overview of the associations between specific autoimmune diseases with psychotic disorders, please see Table 1 and the below sections.
Celiac Disease
The original findings from the 1950s of an association between celiac disease and schizophrenia has since been explored further. During the next decades it was noticed that populations with lower consumption of wheat had lower incidence rates of schizophrenia (34–36), and small studies have since found beneficial effect on psychotic symptoms of a gluten-free diet in patients suffering from both celiac disease and schizophrenia (37, 38). One epidemiological study found no significant correlation between celiac disease and psychosis (24). However, another large-scale study found a 2.11-times increased risk of schizophrenia (26) and the recent meta-analysis also found an association with an elevated risk of schizophrenia with 53% (30). Additionally, it has been found in a Taiwanese population, that the risk of celiac disease is increased when suffering from schizophrenia (29). When discussing epidemiological studies using health records, it is important to note that celiac disease might be majorly underdiagnosed particularly within those who have already debuted with psychotic symptoms. In summary, most studies found a positive association between celiac disease and psychotic disorders.
Multiple Sclerosis
Multiple sclerosis (MS) is an autoimmune disease associated with many neuropsychiatric symptoms, such as depression and anxiety (39). It has been found that 4% of patients with MS experiences psychosis (40), a prevalence much higher than that of the general population. Danish register-based studies have found that having MS increases the risk of schizophrenia with up to 44% (24, 26), with an even further increase in risk when having both MS and a prior hospital contact due to infection (26). Two studies found increased risk of schizophrenia with up to 30% in individuals with a family history of MS; however, they found no associations on an individual level (24, 28), and a study from Taiwan only found a trend toward an increased risk of schizophrenia in those with a diagnosis of MS (27). On the risk of a subsequent MS diagnosis in patients with schizophrenia, contradictory results have been found between a Danish and a Swedish nationwide study, finding the risk to be respectively increased by 57% (25) and decreased by 40% (31). Current evidence of an association between MS and psychotic disorders is limited with studies showing conflicting results. Many, especially sensory, symptoms of multiple sclerosis might be misinterpreted as part of the patients' psychotic disorders, complicating the diagnostic process, and psychotic symptoms in people with MS might not be diagnosed since they are considered to be delirium in relation to acute MS exacerbations.
Lupus
Systemic Lupus Erythematosus (SLE) is another autoimmune disease known to have a high degree of neuropsychiatric problems, such as depression and anxiety, occurring in between 21 and 95% of patients (41). However, it has been estimated that only 13–38%, are directly attributable to SLE, whereas the remaining is suggested to be due to for example treatment complications (41). Regarding psychosis in SLE, the prevalence ranges from 2.3 to 11% in studies (42–44). A study from England comprising 458 patients with SLE, found that only 2.3% experienced psychosis (42), while a higher prevalence of psychosis have been found in a black Caribbean study population (366 patients, 7% with psychosis) (43) and in a Brazilian population (520 patients, 11% with psychosis) (44). In those experiencing psychosis, this was one of the initial symptoms of SLE in up to 60% of these patients (42). In population-based studies, a nationwide Taiwanese study found an increased risk of schizophrenia among those with SLE (27), and in one Danish study the presence of both SLE and a prior hospital contact with infection resulted in an increased risk of schizophrenia (26). None of the other epidemiological studies have found significant association between psychotic disorders and SLE (24, 25, 28, 30), but noteworthy, the number of cases available was very small in all studies, limiting the significance of possible findings. In summary, large scale studies with a greater number of cases have been able to find positive associations between SLE and psychotic disorders, while smaller studies have failed to do so.
Autoimmune Thyroid Disorders
Graves' disease, the most common cause of hyperthyroidism, is also known to be linked to neuropsychiatric issues, and some even present with psychotic disorders (45). A German study found that in a cohort of 100 patients with a schizophreniform illness, 19 had increases antithyroid autoantibodies in sera, and 13 showed signs of intrathecal synthesis hereof (46). In epidemiological studies, both Graves' disease and thyrotoxicosis have been linked with an increased risk of schizophrenia (24, 26, 30). Additionally, the prevalence hereof has been found to be increased among individuals with schizophrenia (28, 29), though this finding has not been replicated in all studies (25, 32). Hence, most studies indicate a positive association between Graves' disease/thyrotoxicosis and psychotic disorders.
No studies has been able to show a significant association between autoimmune thyroiditis and schizophrenia on an individual level (24, 28), but one Danish study found an increased incidence among parents and siblings of patients with schizophrenia (28).
Diabetes Type 1
Diabetes mellitus type 1 is a disease characterized by the presence of glutamic acid decarboxylase (GAD) antibodies. These autoantibodies have been linked with neurological problems (47), and thus have shown ability to cross the blood brain barrier, making them an interesting topic in the discussion of pathophysiological mechanisms. However, conflicting results have been found regarding the association of type 1 diabetes and psychotic disorders. Two Danish studies found an increased risk of schizophrenia when suffering from type 1 diabetes (24, 26), and one found an increased risk of type 1 diabetes after having been diagnosed with schizophrenia (25). This, however, was not replicated neither in a Swedish cohort (32), a Taiwanese cohort (29) nor in the recent meta-analysis (30), and a Finnish study even found a negative association (33). In summary, there does not seem to be a clear association between type 1 Diabetes and psychosis.
Rheumatoid Arthritis
A disease which has consistently been found to be negatively associated with schizophrenia is rheumatoid arthritis (RA). This apparent “protective” effect of schizophrenia on the development of rheumatoid arthritis was investigated as early as the 1950s (48, 49). The negative association between the two has since been backed by epidemiological studies, finding decreased risk of schizophrenia in those with RA (30) and vice versa (25, 29, 50, 51). However, some studies did not find associations (24, 52), and regarding the association on the risk of psychosis after a RA diagnosis, more controversy exist, with a Danish study finding that a combined history of a hospital contact due to infection and RA increased the risk of schizophrenia (26) and a new Taiwanese study finding an increased risk of developing schizophrenia in individuals with a history of RA (27). Moreover, a Danish study found an increased prevalence of RA in the family of those with schizophrenia (28). One explanation of the consistent finding of negative association with subsequent RA diagnosis after a schizophrenia diagnosis could be that RA tends to be underdiagnosed in those suffering from psychotic disorder, and in concordance with this, both a Swedish and Danish nationwide study has shown that the same negative association can be found with other musculoskeletal diseases (50, 51).
Autoimmune Encephalitis
Something that really spiked the interest in autoimmunity as a player in mental illness, was the discovery of autoimmune encephalitis. As a group, these diseases are characterized by the presence of neuronal surface antibodies (NSAbs) and symptoms include psychiatric and cognitive alterations, seizures and movement disorders, with the most commonly affected part of the brain being the limbic system. The most discussed antibody in psychotic disorders at the moment is the N-methyl-D-aspartate receptor (NMDA-R) antibody. It has been reported that as many as 74% of patients suffering from NMDA-R encephalitis experience psychotic symptoms (53, 54), and a recent smaller study found that 13% were initially admitted to the hospital with a psychiatric diagnosis (55). Multiple studies have investigated the frequency of NMDA-R antibodies in schizophrenia, but so far most have only had access to serum not CSF, most have had no healthy control group, and results have varied markedly (56).
Other Autoimmune Diseases
Associations have been found between psychotic disorders and other autoimmune diseases as well. The incidence of psoriasis have been found to be significantly increased in individuals with schizophrenia (25, 29), but not in all studies (28). Increased incidence of psoriasis have also been found in individuals with a family history of schizophrenia (28). In addition, the risk of developing schizophrenia has been found in multiple studies to be increased in those with a history of psoriasis (24, 26, 30), with an additional increase when combined with a prior hospital contact due to an infection (26). The risk of developing Guillain-Barré syndrome, an autoimmune disease attacking peripheral nerves, has been found to be increased markedly in individuals with schizophrenia (25), and when having both a history of a hospital contact with an infection as wells as Guillain-Barré, the risk of developing schizophrenia has also been found to be increased (26). However, one other study found no association (28). Autoimmune hepatitis has been found to be greatly associated with psychotic disorders as well, with both individual history and family history hereof increasing the risk of schizophrenia (24, 26), and schizophrenia increasing the risk of autoimmune hepatitis (25). Some evidence of an association between schizophrenia and Crohn's disease has also been found (25, 26), though no significantly increased risk was shown in two other studies (24, 27) or the recent meta-analysis (30).
Possible Mechanisms
The potential etiological background and the many factors that can influence the association between autoimmune diseases and psychosis are numerous and not mutually exclusive as outlined in the following sections. For an overview hereof, see Figure 1.
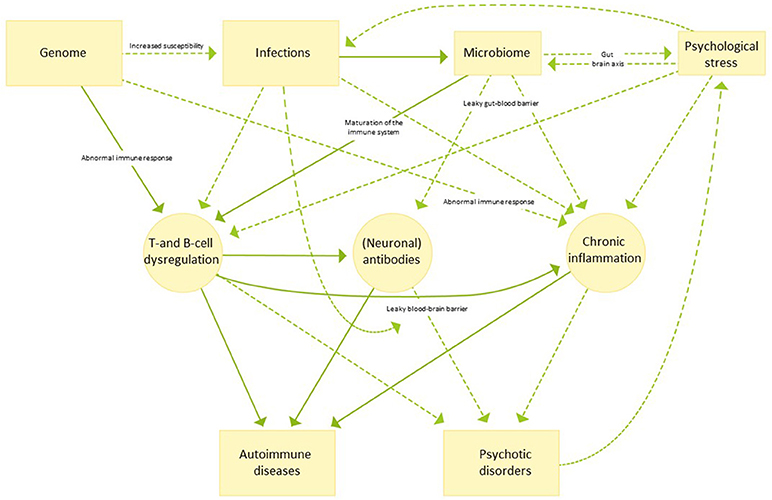
Figure 1. An illustrative overview of possible etiological mechanisms linking autoimmune disease and psychotic disorders. Continuous arrows indicate pathways for which evidence is strong, while dotted arrows indicate pathways which are currently not well understood or more speculative in nature.
Antibodies
One potential contributing factor to the link between some autoimmune diseases with mental illness, can be the presence of neuronal surface antibodies (NSAbs). GAD-antibodies have been linked with multiple neurological problems (47), and in neuropsychiatric lupus, an increased amount of antibodies was found both in serum and CSF compared to lupus with no neuropsychiatric manifestation (57). Furthermore, gliadin antibodies, associated with celiac disease, have been found to be increased in the serum of patients with recent onset schizophrenia (58). With the discovery of NMDA-receptor encephalitis, and its ability to mimic mental disorders, the interest spiked further, and with GAD-antibodies being able to induce limbic encephalitis (59, 60) and antibodies reacting with the NR2 subunit of NMDA being present in some cases of lupus (61), a possible link emerged.
Many studies have sought to evaluate the presence of multiple different NSAbs in mental illness, but so far, consistency in methods and assays have limited the generalization of the findings (56, 62). Many studies have lacked a healthy control group to compare their results with, and most studies have included serum but not CSF samples. The relevance of circulating NSAbs in serum is still unknown, and therefore comprehensive studies including healthy controls evaluating antibodies in both CSF and serum is needed to increase knowledge further.
Dysregulated Immune System
A dysregulated balance between regulatory T cells and Th17 cells have been described to be essential for immunological homeostasis and have been implicated in the development of several autoimmune disorders (63). Signs of a dysregulated immune system has also been found in mental illnesses and might play a role in the association found between the two.
A meta-analysis found that levels of several lymphocytes differed when examining patients with schizophrenia compared to healthy controls (64), and studies have linked decreased regulatory T cells with negative symptoms and cognitive deficits (65), as well as increased levels of Th17 with psychopathology (66).
In recent years, B cells have received increasing attention in the pathology of autoimmunity, and have been implicated to play a big role in for example MS, where it has also been found that anti B-cell antigen (anti-CD20) have great efficacy in the treatment hereof (67). It has been shown that oligoclonal bands (OCBs) in the CSF, something which is found in approximately 90% of patients with MS, is a sign of ongoing stimulation and maturation of antibody-expressing B-cells (68). Interestingly, a recent meta-analysis found that OCBs were found in the CSF of up to 12.5% of patients with schizophrenia (14).
Another frequent finding in patients with schizophrenia is increased levels of pro-inflammatory and decreased levels of anti-inflammatory cytokines in serum (10). Dysregulation of the anti-inflammatory cytokine IL-10 has been found to be linked with abnormal responses to common infections, and to increase the risk of developing autoimmune diseases (69).
Infections as a Common Risk Factor
It is thought that one of the most important triggers for developing autoimmune diseases is infection (70), and it is known that infectious encephalitis, specifically with herpes-simplex virus, markedly increases the risk of developing NMDA-receptor encephalitis (71). As it was shown in a large Danish nationwide study, prior infection increased the risk of developing schizophrenia in a dose-response fashion (26), and this finding has been repeated in other large studies (72, 73). The effect of infection on risk of schizophrenia was present regardless of autoimmune diseases, but additionally, a significant synergy was found in those with both a history of autoimmunity and infections (26). For many of the individual autoimmune diseases, it was seen that the effect on the risk of schizophrenia increased when a prior hospital contact due to infection was also present (26).
Being exposed to viral or bacterial infection is known to increase the permeability of the blood-brain-barrier (BBB) (74), which allows the entering into the central nervous system of immune cells and pro-inflammatory cytokines. This in itself might allow an inflammatory state in the brain, which has been theorized to play a role in the development of psychotic disorders. It may also explain the synergistic effect on risk of schizophrenia of having both an autoimmune disease and prior infections, as BBB disruption might also allow the entering of circulating antibodies. Supporting the role hereof, signs of a disrupted BBB has been found in patient with schizophrenia with evidence of increased albumin CSF:plasma ratio (75, 76) and increased levels of circulating s100-b (77).
It has also been found that infections during pregnancy increases the risk of schizophrenia in the offspring (78). On the basis hereof, it has been considered whether infections during the prenatal phase might prime the immune system, making it more vulnerable and perhaps more likely to produce abnormal responses to later infections, resulting in increased inflammation. However, a new study have shown that even maternal infections before and after pregnancy increases the risk of mental illness (79), which could also indicate a genetic susceptibility for infections associated with mental illness.
Genetics
Both schizophrenia and autoimmune diseases are known to be highly hereditable. The most consistent finding in genetic studies of patients with schizophrenia, are differences in genes known to be linked to the immune system (80), and several genetic loci that increases the risk of autoimmune diseases has been located (81). As with schizophrenia, some of the discovered genetic loci in autoimmune diseases are located in the MHC region (82). However, while one study found significant overlap in genes between MS and schizophrenia (but not MS and bipolar disorder) (83), another study found no genetic association between 25 different autoimmune diseases and schizophrenia (84). Genetic pleiotropy has also been hypothesized to account for the negative association found between RA and schizophrenia, with genes found to be associated with schizophrenia possibly decreasing the risk of RA (85).
Another possible role of genetics in the association of autoimmune diseases and psychotic disorders could be a hereditary susceptibility for shared risk factors. It has been hypothesized that some of the genetic findings associated with schizophrenia might increase the risk of having infections (86, 87), that then subsequently increase the risk of both autoimmune diseases and psychotic disorders. Additionally, it has been theorized whether some individuals with schizophrenia, might have a genetic predisposition for an abnormal immune response to common infections and foreign pathogens, for example via differences in the HLA region and complement system (88, 89), which in turn could increase the risk of developing autoimmune reactions. The complement system has also been implicated to play a role in neurodevelopment and -maturation (90), and evidence of altered complement activity in patients with schizophrenia have been found (reviewed in (88)).
The Microbiome
The gastro-intestinal tract of humans contains vast amounts of bacteria, phyla and other microorganisms, their genes collectively known as the microbiome, containing at least 100 times more genetic material than the human genome (91). This area has received great attention in the research of many different illnesses in the last years and have been implicated as a possible etiological factor in both neuropsychiatric illnesses and autoimmune diseases. As early as 1953, interest in gastro-intestinal inflammation in psychosis was raised, when a group of researchers found in an autopsy study that out of 82 patients with schizophrenia, 50% had gastritis, 88% enteritis and 92% colitis (92). This study has not since been replicated, but other signs of microbiome dysbiosis in this group of patients has been found with significant difference between cases and controls in the presence of both bacteria and fungi (93), and bacteriophages (94). Studies so far have mainly focused on the oropharyngeal microbiome due to practical limitations. One study however, has looked into fecal microbiome, finding no significant difference between healthy controls and patients, but showing associations between microbiome composition and symptom severity and outcome (95).
The composition of the microbiome has been hypothesized to be very important in the development of both the central nervous system (96) and the immune system (97, 98). Dysbiosis of the microbiome has been shown to affect both the Th1/Th2 balance and the ratio of T regulatory and Th17 cells, impacting the immune response to foreign pathogens (99). Dysbiosis have been found to influence the T-cell mediated inflammation in MS patients (100, 101), and has also been suspected to play a part in the development of celiac disease (102), as well as non-gastro-intestinal autoimmune diseases (103). In rodents, disruption of the microbiome has been found to impair social functioning (104), behavior and cognition (105), and induce neurodevelopmental disorders (106).
An important function of the microbiome, seems to be its effect on the epithelial cells in the GI wall, with evidence implicating that the composition of the microbiome is important for the tightness of the gut-blood barrier (107). Severance et al. (108) found markers in the serum of patients with schizophrenia indicating increased permeability, also known as “leaky gut.” A leaky gut allows the entrance of foreign pathogens and antigens into the blood. It has been suspected to induce systemic inflammation, and in mice it has been found to even result in neuroinflammation (109), both of which might increase the risk of mental illness and autoimmune diseases.
Interestingly, both infections and the treatment hereof with antibiotics can modulate the microbiome, linking the previously mentioned epidemiological findings of the influence of infections (26) with the microbiome theory. Additionally, it has been theorized that maternal infection might alter both the maternal and fetal microbiome (110), possibly impacting the immune system and neuropsychiatric development of the offspring.
A few studies have tried probiotic treatment in patients with schizophrenia, but no evidence of effect hereof on psychopathology has yet been found (111, 112). However, further research on the actual composition of the microbiome in patients with mental illness as well as the possibility of using probiotics as treatment hereof is warranted.
Psychological Stress
Psychological stress such as sexual abuse, physical abuse, emotional/psychological abuse, neglect, parental death, and bullying, both in childhood and later on, has been associated with increased risk of psychotic disorders in multiple studies (113, 114). A Swedish register-based study found that stress-related disorders increased the risk of subsequent development of autoimmune disorders (115) and, accordingly, in many other studies, stress have been found to be associated with disease onset and disease exacerbations in several autoimmune conditions (116).
Stress can theoretically influence many of the above-mentioned possible etiological factors. Acute psychological stress, even in brief episodes, have been found in a meta-analysis to increase circulating proinflammatory cytokines such as IL-6, IL-1β, and TNF-α (117), possibly via the sympathetic nerve system and the HPA axis, and multiple adverse life events or stressful living conditions might therefore possibly contribute to a more chronic inflammatory state with dysregulation of immune response (118).
Psychological stress have been thought to influence composition of the microbiome and vice versa, as well as the microbiome's effect on peripheral inflammation (119). Additionally, it has been hypothesized to increase susceptibility to infections, with one study finding that healthy subjects with higher scores on questionnaires on psychological stress were more prone to developing clinical cold and respiratory infections after exposure to respiratory viruses (120). Acute stress, for example as a result of a psychiatric disorder or hospitalization, may also lead to exacerbation in symptoms of autoimmune diseases, leading to the discovery of a disease formerly undiagnosed.
Clinical Implications
The increasing knowledge on the potential involvement of inflammatory processes in mental disorders and the associations found between autoimmunity and psychotic disorders can help the expanding field of immuno-psychiatry and have impact on the outcome of patients. In the last couple of years, researchers have focused on the role of infections, autoantibodies and other immune components that plays a major role in autoimmune diseases. Potentially this might also be the case for mental disorders. Risk factors for both autoimmune diseases and schizophrenia includes an interaction between environmental factors, such as infections and stress, with genetic factors involving the HLA region. Autoimmune reactions with activation of immune components and the production of NSAbs can induce a broad spectrum of psychiatric symptoms, hereunder psychosis. The potential autoimmune-mediated psychosis group might only be a small part of a broader immune-related psychosis group, and an even smaller fraction of the overall psychosis group. However, identification of this subgroup might allow for precision medicine strategies where immune-based treatment could possibly improve the psychotic symptoms. A quick discovery and treatment of autoimmune encephalitis markedly reduces the neuropsychiatric sequelae, and intensive immunotherapy in lupus patients with psychosis massively benefits psychiatric symptoms (42, 121).
Focus on the association between autoimmunity and psychosis, regardless of etiology, is important, not only for researchers but also for the individual patient. It is known that patients suffering from schizophrenia have an excess early mortality, with a life expectancy up to 13.5 years shorter than the general population, primarily due to physical diseases (122). Bearing this in mind and considering that patients with psychotic disorders might struggle with reporting on somatic symptoms, it is important for clinicians to be aware of an increased prevalence of autoimmune disease in this group. Symptoms from a disease such as celiac disease or rheumatoid arthritis might very well be overlooked and cast aside as a part of the patient's psychosis, or possibly adverse events caused by their treatment. With increasingly sufficient treatment strategies in autoimmune diseases, overlooking and therefore not treating these diseases, increases the health gap between those with schizophrenia and the general population even further. Therefore, patients with a psychotic disorder need to be thoroughly and frequently examined when presenting with symptoms possibly related to autoimmunity or other health problems.
Author Contributions
RJ was responsible for literature search and wrote the first draft of the manuscript. MB contributed with supervision and expert advice and revised the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Funding
The work was funded by the Independent Research Fund Denmark (grant number 7025-00078B) and by an unrestricted grant from The Lundbeck Foundation (grant number R268-2016-3925). The sponsor had no role in the present work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Steinberg H, Kirkby K, Himmerich H. The historical development of immunoendocrine concepts of psychiatric disorders and their therapy. Int J Mol Sci. (2015) 16:28841–69. doi: 10.3390/ijms161226136
3. Pilkington TL. The coincidence of rheumatoid arthritis and schizophrenia. J Nerv Ment Dis. (1956) 124:604–6. doi: 10.1097/00005053-195612000-00007
4. Trevathan RD, Tatum JC. Rarity of concurrence of psychosis and rheumatoid arthritis in individual patients; report of a case. J Nerv Ment Dis. (1954) 120:83–4.
5. Fessel WJ. Autoimmunity and mental illness. Arch Gen Psychiatry. (1962) 6:320. doi: 10.1001/archpsyc.1962.01710220062008
6. Heath RG, Krupp IM. Schizophrenia as an immunologic disorder. Arch Gen Psychiatry. (1967) 16:1. doi: 10.1001/archpsyc.1967.01730190003001
7. Zandi MS, Irani SR, Lang B, Waters P, Jones PB, McKenna P, et al. Disease-relevant autoantibodies in first episode schizophrenia. J Neurol. (2011) 258:686–8. doi: 10.1007/s00415-010-5788-9
8. Steiner J, Walter M, Glanz W, Sarnyai Z, Bernstein H-G, Vielhaber S, et al. Increased prevalence of diverse N -Methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia. JAMA Psychiatry. (2013) 70:271. doi: 10.1001/2013.jamapsychiatry.86
9. Pollak TA, Rogers JP, Nagele RG, Peakman M, Stone JM, David AS, et al. Antibodies in the diagnosis, prognosis, and prediction of psychotic disorders. Schizophr Bull. (2018) 45:233–246. doi: 10.1093/schbul/sby021
10. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. (2011) 70:663–71. doi: 10.1016/j.biopsych.2011.04.013
11. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. (2016) 21:1696–709. doi: 10.1038/mp.2016.3
12. Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, Maxeiner HG. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood–CSF barrier dysfunction. J Psychiatr Res. (2010) 44:321–30. doi: 10.1016/j.jpsychires.2009.08.008
13. Cavanagh J, Klados MA, Tebartz Van Elst L, Endres D, Perlov E, Baumgartner A, et al. Immunological findings in psychotic syndromes: a tertiary care hospital's CSF sample of 180 patients. (2015) 9:476. doi: 10.3389/fnhum.2015.00476
14. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, Nordentoft M, Kondziella D, Krogh J, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. (2018) doi: 10.1038/s41380-018-0220-4. [Epub ahead of print].
15. Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA psychiatry. (2014) 71:1121–8. doi: 10.1001/jamapsychiatry.2014.1332
16. Metcalf SA, Jones PB, Nordstrom T, Timonen M, Mäki P, Miettunen J, et al. Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: a prospective birth cohort study. Brain Behav Immun. (2017) 59:253–9. doi: 10.1016/j.bbi.2016.09.008
17. Mondelli V, Ciufolini S, Belvederi Murri M, Bonaccorso S, Di Forti M, Giordano A, et al. Cortisol and inflammatory biomarkers predict poor treatment response in first episode psychosis. Schizophr Bull. (2015) 41:1162–70. doi: 10.1093/schbul/sbv028
18. Girgis RR, Ciarleglio A, Choo T, Haynes G, Bathon JM, Cremers S, et al. A randomized, double-blind, placebo-controlled clinical trial of tocilizumab, an interleukin-6 receptor antibody, for residual symptoms in schizophrenia. Neuropsychopharmacology. (2018) 43:1317–23. doi: 10.1038/npp.2017.258
19. Vincenzi B, Stock S, Borba CPC, Cleary SM, Oppenheim CE, Petruzzi LJ, et al. A randomized placebo-controlled pilot study of pravastatin as an adjunctive therapy in schizophrenia patients: Effect on inflammation, psychopathology, cognition and lipid metabolism. Schizophr Res. (2014) 159:395–403. doi: 10.1016/j.schres.2014.08.021
20. Müller N, Ulmschneider M, Scheppach C, Schwarz MJ, Ackenheil M, Möller H-J, et al. COX-2 inhibition as a treatment approach in schizophrenia: Immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci. (2004) 254:14–22. doi: 10.1007/s00406-004-0478-1
21. Laan W, Grobbee DE, Selten J-P, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders. J Clin Psychiatry. (2010) 71:520–7. doi: 10.4088/JCP.09m05117yel
22. Al-Diwani AAJ, Pollak TA, Irani SR, Lennox BR. Psychosis: an autoimmune disease? Immunology. (2017) 152:388–401. doi: 10.1111/imm.12795
23. Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. (2005) 2:e141. doi: 10.1371/journal.pmed.0020141
24. Eaton WW, Pedersen MG, Nielsen PR, Mortensen PB. Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord. (2010) 12:638–46. doi: 10.1111/j.1399-5618.2010.00853.x
25. Benros ME, Pedersen MG, Rasmussen H, Eaton WW, Nordentoft M, Mortensen PB. A nationwide study on the risk of autoimmune diseases in individuals with a personal or a family history of schizophrenia and related psychosis. Am J Psychiatry. (2014) 171:218–26. doi: 10.1176/appi.ajp.2013.13010086
26. Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. (2011) 168:1303–10. doi: 10.1176/appi.ajp.2011.11030516
27. Wang L-Y, Chen S-F, Chiang J-H, Hsu C-Y, Shen Y-C. Autoimmune diseases are associated with an increased risk of schizophrenia: a nationwide population-based cohort study. Schizophr Res. (2018). doi: 10.1016/j.schres.2018.06.033
28. Eaton WW, Byrne M, Ewald H, Mors O, Chen C-Y, Agerbo E, et al. Association of schizophrenia and autoimmune diseases: linkage of danish national registers. Am J Psychiatry. (2006) 163:521–8. doi: 10.1176/appi.ajp.163.3.521
29. Chen S-J, Chao Y-L, Chen C-Y, Chang C-M, Wu EC-H, Wu C-S, et al. Prevalence of autoimmune diseases in in-patients with schizophrenia: nationwide population-based study. Br J Psychiatry. (2012) 200:374–80. doi: 10.1192/bjp.bp.111.092098
30. Cullen AE, Holmes S, Pollak TA, Blackman G, Joyce DW, Kempton MJ, et al. Associations between non-neurological autoimmune disorders and psychosis: a meta-analysis. Biol Psychiatry. (2018) 85:35–48. doi: 10.1016/j.biopsych.2018.06.016
31. Johansson V, Lundholm C, Hillert J, Masterman T, Lichtenstein P, Landén M, et al. Multiple sclerosis and psychiatric disorders: comorbidity and sibling risk in a nationwide Swedish cohort. Mult Scler J. (2014) 20:1881–91. doi: 10.1177/1352458514540970
32. Cremaschi L, Kardell M, Johansson V, Isgren A, Sellgren CM, Altamura AC, et al. Prevalences of autoimmune diseases in schizophrenia, bipolar I and II disorder, and controls. Psychiatry Res. (2017) 258:9–14. doi: 10.1016/j.psychres.2017.09.071
33. Juvonen H, Reunanen A, Haukka J, Muhonen M, Suvisaari J, Arajärvi R, et al. Incidence of schizophrenia in a nationwide cohort of patients with type 1 diabetes mellitus. Arch Gen Psychiatry. (2007) 64:894–9. doi: 10.1001/archpsyc.64.8.894
34. Dohan FC. Wheat "consumption" and hospital admissions for schizophrenia during world war II. a preliminary report. Am J Clin Nutr. (1966) 18:7–10. doi: 10.1093/ajcn/18.1.7
35. Dohan F. CŒLIAC DISEASE AND SCHIZOPHRENIA. Lancet. (1970) 295:897–8. doi: 10.1016/S0140-6736(70)91729-0
36. Dohan FC, Harper EH, Clark MH, Rodrigue RB, Zigas V. Is schizophrenia rare if grain is rare? Biol Psychiatry. (1984) 19:385–99.
37. Singh MM, Kay SR. Wheat gluten as a pathogenic factor in schizophrenia. Science. (1976) 191:401–2.
38. Jackson J, Eaton W, Cascella N, Fasano A, Warfel D, Feldman S, et al. A gluten-free diet in people with schizophrenia and anti-tissue transglutaminase or anti-gliadin antibodies. Schizophr Res. (2012) 140:262–3. doi: 10.1016/j.schres.2012.06.011
39. Murphy R, O'Donoghue S, Counihan T, McDonald C, Calabresi PA, Ahmed MA, et al. Neuropsychiatric syndromes of multiple sclerosis. J Neurol Neurosurg Psychiatry. (2017) 88:697–708. doi: 10.1136/jnnp-2016-315367
40. Marrie RA, Fisk JD, Tremlett H, Wolfson C, Warren S, Tennakoon A, et al. CIHR team in the epidemiology and impact of comorbidity on multiple sclerosis. differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology. (2015) 85:1972–9. doi: 10.1212/WNL.0000000000002174
41. Tay SH, Mak A. Diagnosing and attributing neuropsychiatric events to systemic lupus erythematosus: time to untie the Gordian knot? Rheumatology. (2016) 56:i14–24. doi: 10.1093/rheumatology/kew338
42. Pego-Reigosa JM, Isenberg DA. Psychosis due to systemic lupus erythematosus: characteristics and long-term outcome of this rare manifestation of the disease. Rheumatology (Oxford). (2008) 47:1498–502. doi: 10.1093/rheumatology/ken260
43. Flower C, Hambleton I, Corbin D, Marquez S, Edghill R. The spectrum of neuropsychiatric lupus in a black caribbean population: a report of the Barbados National Lupus Registry. Lupus. (2017) 26:1034–41. doi: 10.1177/0961203317692431
44. Appenzeller S, Cendes F, Costallat LTL. Acute psychosis in systemic lupus erythematosus. Rheumatol Int. (2008) 28:237–43. doi: 10.1007/s00296-007-0410-x
45. Golub D, Rodack V. Antipsychotics in hyperthyroid-related psychosis: case report and systematic review. Neuro Endocrinol Lett. (2018) 39:65–74.
46. Endres D, Dersch R, Hochstuhl B, Fiebich B, Hottenrott T, Perlov E, et al. Intrathecal thyroid autoantibody synthesis in a subgroup of patients with schizophreniform syndromes. J Neuropsychiatry Clin Neurosci. (2017) 29:365–74. doi: 10.1176/appi.neuropsych.16110296
47. Baizabal-Carvallo JF, Alonso-Juarez M. Cerebellar disease associated with anti-glutamic acid decarboxylase antibodies: review. J Neural Transm. (2017) 124:1171–82. doi: 10.1007/s00702-017-1754-3
48. Trevathan R, Tatum JC. Rarity of concurrence of psychosis and rheumatoid arthritis in individual patients; report of a case. J Nerv Mental Dis. (1954) 120:83–4.
50. Sellgren C, Frisell T, Lichtenstein P, Landen M, Askling J. The association between schizophrenia and rheumatoid arthritis: a nationwide population-based swedish study on intraindividual and familial risks. Schizophr Bull. (2014) 40:1552–9. doi: 10.1093/schbul/sbu054
51. Mors O, Mortensen PB, Ewald H. A population-based register study of the association between schizophrenia and rheumatoid arthritis. Schizophr Res. (1999) 40:67–74.
52. Marrie RA, Hitchon CA, Walld R, Patten SB, Bolton JM, Sareen J, et al. Increased burden of psychiatric disorders in rheumatoid arthritis. Arthritis Care Res. (2018) 70:970–8. doi: 10.1002/acr.23539
53. Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain J Neurol. (2010) 133:1655–67. doi: 10.1093/brain/awq113
54. Kayser MS, Titulaer MJ, Gresa-Arribas N, Dalmau J. Frequency and characteristics of isolated psychiatric episodes in anti– N -Methyl- d -aspartate receptor encephalitis. JAMA Neurol. (2013) 70:1133. doi: 10.1001/jamaneurol.2013.3216
55. Baumgartner A, Rauer S, Hottenrott T, Leypoldt F, Ufer F, Hegen H, et al. Admission diagnoses of patients later diagnosed with autoimmune encephalitis. J Neurol. (2018) 266:124–132. doi: 10.1007/s00415-018-9105-3
56. Pollak TA, McCormack R, Peakman M, Nicholson TR, David AS. Prevalence of anti-N-methyl-d-aspartate (NMDA) receptor antibodies in patients with schizophrenia and related psychoses: a systematic review and meta-analysis. Psychol Med. (2014) 44:2475–87. doi: 10.1017/S003329171300295X
57. Ho RC, Thiaghu C, Ong H, Lu Y, Ho CS, Tam WW, et al. A meta-analysis of serum and cerebrospinal fluid autoantibodies in neuropsychiatric systemic lupus erythematosus. Autoimmun Rev. (2016) 15:124–38. doi: 10.1016/j.autrev.2015.10.003
58. Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, et al. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. (2010) 68:100–4. doi: 10.1016/j.biopsych.2010.03.021
59. Matà S, Muscas GC, Naldi I, Rosati E, Paladini S, Cruciatti B, et al. Non-paraneoplastic limbic encephalitis associated with anti-glutamic acid decarboxylase antibodies. J Neuroimmunol. (2008) 199:155–9. doi: 10.1016/j.jneuroim.2008.05.015
60. Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. (2010) 67:470–8. doi: 10.1002/ana.21917
61. DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. (2001) 7:1189–93. doi: 10.1038/nm1101-1189
62. Al-Diwani A, Pollak TA, Langford AE, Lennox BR. Synaptic and neuronal autoantibody-associated psychiatric syndromes: controversies and hypotheses. Front psychiatry. (2017) 8:13. doi: 10.3389/fpsyt.2017.00013
63. Diller ML, Kudchadkar RR, Delman KA, Lawson DH, Ford ML. Balancing inflammation: the link between Th17 and regulatory T Cells. Mediators Inflamm. (2016) 2016:6309219. doi: 10.1155/2016/6309219
64. Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. (2013) 73:993–9. doi: 10.1016/j.biopsych.2012.09.007
65. Fernandez-Egea E, Vértes PE, Flint SM, Turner L, Mustafa S, Hatton A, et al. Peripheral immune cell populations associated with cognitive deficits and negative symptoms of treatment-resistant schizophrenia. PLoS ONE. (2016) 11:e0155631. doi: 10.1371/journal.pone.0155631
66. Ding M, Song X, Zhao J, Gao J, Li X, Yang G, et al. Activation of Th17 cells in drug naïve, first episode schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. (2014) 51:78–82. doi: 10.1016/j.pnpbp.2014.01.001
67. Disanto G, Morahan JM, Barnett MH, Giovannoni G, Ramagopalan S V. The evidence for a role of B cells in multiple sclerosis. Neurology. (2012) 78:823–32. doi: 10.1212/WNL.0b013e318249f6f0
68. Bankoti J, Apeltsin L, Hauser SL, Allen S, Albertolle ME, Witkowska HE, et al. In multiple sclerosis, oligoclonal bands connect to peripheral B-cell responses. Ann Neurol. (2014) 75:266–76. doi: 10.1002/ana.24088
69. Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. (2012) 32:23–63. doi: 10.1615/CritRevImmunol.v32.i1.30
70. Getts DR, Chastain EML, Terry RL, Miller SD. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev. (2013) 255:197–209. doi: 10.1111/imr.12091
71. Armangue T, Spatola M, Vlagea A, Mattozzi S, Cárceles-Cordon M, Martinez-Heras E, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. (2018) 17:760–72. doi: 10.1016/S1474-4422(18)30244-8
72. Nielsen PR, Benros ME, Mortensen PB. Hospital contacts with infection and risk of schizophrenia: a population-based cohort study with linkage of Danish national registers. Schizophr Bull. (2014) 40:1526–32. doi: 10.1093/schbul/sbt200
73. Nielsen PR, Laursen TM, Agerbo E. Comorbidity of schizophrenia and infection: a population-based cohort study. Soc Psychiatry Psychiatr Epidemiol. (2016) 51:1581–9. doi: 10.1007/s00127-016-1297-1
75. Melkersson K, Bensing S. Signs of impaired blood-brain barrier function and lower IgG synthesis within the central nervous system in patients with schizophrenia or related psychosis, compared to that in controls. Neuro Endocrinol Lett. (2018) 39:33–42.
76. Severance EG, Gressitt KL, Alaedini A, Rohleder C, Enning F, Bumb JM, et al. IgG dynamics of dietary antigens point to cerebrospinal fluid barrier or flow dysfunction in first-episode schizophrenia. Brain Behav Immun. (2015) 44:148–58. doi: 10.1016/j.bbi.2014.09.009
77. Aleksovska K, Leoncini E, Bonassi S, Cesario A, Boccia S, Frustaci A. Systematic review and meta-analysis of circulating S100B blood levels in schizophrenia. PLoS ONE. (2014) 9:e106342. doi: 10.1371/journal.pone.0106342
78. Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. (2013) 43:239–57. doi: 10.1017/S0033291712000736
79. Lydholm CN, Köhler-Forsberg O, Nordentoft M, Yolken RH, Mortensen PB, Petersen L, Benros ME. Parental infections before, during, and after pregnancy as risk factors for mental disorders in childhood and adolescence: a nationwide danish study. Biol Psychiatry. (2018) 85:317–25. doi: 10.1016/j.biopsych.2018.09.013
80. Biological insights from 108 schizophrenia-associated genetic loci. Nature. (2014) 511:421–7. doi: 10.1038/nature13595
81. Feero WG, Guttmacher AE, Cho JH, Gregersen PK. Genomic medicine genomics and the multifactorial nature of human autoimmune disease. N Engl J Med. (2011) 365:1612–23. doi: 10.1056/NEJMra1100030
82. Lie BA, Thorsby E. Several genes in the extended human MHC contribute to predisposition to autoimmune diseases. Curr Opin Immunol. (2005) 17:526–31. doi: 10.1016/J.COI.2005.07.001
83. Andreassen OA, Harbo HF, Wang Y, Thompson WK, Schork AJ, Mattingsdal M, et al. Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: differential involvement of immune-related gene loci. Mol Psychiatry. (2015) 20:207–14. doi: 10.1038/mp.2013.195
84. Hoeffding LK, Rosengren A, Thygesen JH, Schmock H, Werge T, Hansen T. Evaluation of shared genetic susceptibility loci between autoimmune diseases and schizophrenia based on genome-wide association studies. Nord J Psychiatry. (2017) 71:20–5. doi: 10.1080/08039488.2016.1198420
85. Malavia TA, Chaparala S, Wood J, Chowdari K, Prasad KM, McClain L, et al. Generating testable hypotheses for schizophrenia and rheumatoid arthritis pathogenesis by integrating epidemiological, genomic, and protein interaction data. NPJ Schizophr. (2017) 3:11. doi: 10.1038/s41537-017-0010-z
86. Avramopoulos D, Pearce BD, Mcgrath J, Wolyniec P, Wang R, Eckart N. Infection and inflammation in schizophrenia and bipolar disorder: a genome wide study for interactions with genetic variation. PLoS ONE. (2015) 10:116696. doi: 10.1371/journal.pone.0116696
87. Bamne M, Wood J, Chowdari K, Watson AM, Celik C, Mansour H, et al. Evaluation of HLA polymorphisms in relation to schizophrenia risk and infectious exposure. Schizophr Bull. (2012) 38:1149–54. doi: 10.1093/schbul/sbs087
88. Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. (2016) 530:177–83. doi: 10.1038/nature16549
89. Nimgaonkar VL, Prasad KM, Chowdari KV, Severance EG, Yolken RH. The complement system: a gateway to gene–environment interactions in schizophrenia pathogenesis. Mol Psychiatry. (2017) 22:1554–61. doi: 10.1038/mp.2017.151
90. Prasad KM, Chowdari KV, D'Aiuto LA, Iyengar S, Stanley JA, Nimgaonkar VL. Neuropil contraction in relation to complement C4 gene copy numbers in independent cohorts of adolescent-onset and young adult-onset schizophrenia patients–a pilot study. Transl Psychiatry. (2018) 8:134. doi: 10.1038/s41398-018-0181-z
91. Gill SR, Pop M, DeBoy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. (2006) 312:1355–9. doi: 10.1126/science.1124234
92. Buscaino VM. “Patologia extraneurale della schizofrenia : fegato, tubo digerente, sistema reticolo-endoteliale,” in Acta Neurologica VIII., 1–60.
93. Castro-Nallar E, Bendall ML, Pérez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, et al. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. (2015) 3:e1140. doi: 10.7717/peerj.1140
94. Yolken RH, Severance EG, Sabunciyan S, Gressitt KL, Chen O, Stallings C, et al. Metagenomic sequencing indicates that the oropharyngeal phageome of individuals with schizophrenia differs from that of controls. Schizophr Bull. (2015) 41:1153–61. doi: 10.1093/schbul/sbu197
95. Schwarz E, Maukonen J, Hyytiäinen T, Kieseppä T, Orešič M, Sabunciyan S, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. (2018) 192:398–403. doi: 10.1016/j.schres.2017.04.017
96. Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. (2016) 167:915–32. doi: 10.1016/j.cell.2016.10.027
97. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. (2011) 474:327–36. doi: 10.1038/nature10213
98. Rook G, Bäckhed F, Levin BR, Mcfall-Ngai MJ, Mclean AR. Evolution, human-microbe interactions, and life history plasticity. Evolut Pub Health. (2017) 390:521–30. doi: 10.1016/S0140-6736(17)30566-4
99. Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. (2010) 330:1768–73. doi: 10.1126/science.1195568
100. Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA. (2017) 114:10713–18. doi: 10.1073/pnas.1711235114
101. Cekanaviciute E, Pröbstel A-K, Thomann A, Runia TF, Casaccia P, Katz Sand I, et al. Multiple sclerosis-associated changes in the composition and immune functions of spore-forming bacteria. mSystems. (2018) 3:e00083. doi: 10.1128/mSystems.00083-18
102. Elson CO, Alexander KL. Host-microbiota interactions in the intestine. Dig Dis. (2015) 33:131–6. doi: 10.1159/000369534
103. Opazo MC, Ortega-Rocha EM, Coronado-Arrázola I, Bonifaz LC, Boudin H, Neunlist M, et al. Intestinal microbiota influences non-intestinal related autoimmune diseases. Front Microbiol. (2018) 9:432. doi: 10.3389/fmicb.2018.00432
104. Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. (2014) 19:146–148. doi: 10.1038/mp.2013.65
105. Desbonnet L, Clarke G, Traplin A, O'sullivan O, Crispie F, Moloney RD, et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour.Brain Behav Immun. (2015) 48:165–73. doi: 10.1016/j.bbi.2015.04.004
106. Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. (2013) 155:1451–63. doi: 10.1016/j.cell.2013.11.024
107. Burger-Van Paassen N, Vincent A, Puiman PJ, Van Der Sluis M, Bouma J, Boehm G, et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. (2009) 420:211–9. doi: 10.1042/BJ20082222
108. Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. (2012) 138:48–53. doi: 10.1016/j.schres.2012.02.025
109. Alhasson F, Das S, Seth R, Dattaroy D, Chandrashekaran V, Ryan CN, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS ONE. (2017) 12:e0172914 doi: 10.1371/JOURNAL.PONE.0172914
110. Romano-Keeler J, Weitkamp J-H. Maternal influences on fetal microbial colonization and immune development. Pediatr Res. (2015) 77:189–95. doi: 10.1038/pr.2014.163
111. Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CLG, et al. Probiotic normalization of candida albicans in schizophrenia: a randomized, placebo-controlled, longitudinal pilot study. Brain Behav Immun. (2017) 62:41–5. doi: 10.1016/j.bbi.2016.11.019
112. Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CLG, Schweinfurth LAB, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning. Prim Care Companion CNS Disord. (2014) 16:13m01579. doi: 10.4088/PCC.13m01579
113. Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. (2012) 38:661–71. doi: 10.1093/schbul/sbs050
114. Lataster J, Myin-Germeys I, Lieb R, Wittchen H-U, van Os J. Adversity and psychosis: a 10-year prospective study investigating synergism between early and recent adversity in psychosis. Acta Psychiatr Scand. (2012) 125:388–99. doi: 10.1111/j.1600-0447.2011.01805.x
115. Song H, Fang F, Tomasson G, Arnberg FK, Mataix-Cols D, Fernández de la Cruz L, et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA. (2018) 319:2388. doi: 10.1001/jama.2018.7028
116. Sharif K, Watad A, Coplan L, Lichtbroun B, Krosser A, Lichtbroun M, et al. The role of stress in the mosaic of autoimmunity: an overlooked association. Autoimmun Rev. (2018) 17:967–83. doi: 10.1016/j.autrev.2018.04.005
117. Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav Immun. (2017) 64:208–19. doi: 10.1016/j.bbi.2017.01.011
118. Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. (2014) 58:193–210. doi: 10.1007/s12026-014-8517-0
119. Gur TL, Bailey MT. Effects of stress on commensal microbes and immune system activity. Adv Exp Med Biol. (2016) 874:289–300. doi: 10.1007/978-3-319-20215-0_14
120. Cohen S, Tyrrell DAJ, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. (1991) 325:606–12. doi: 10.1056/NEJM199108293250903
121. Paholpak P, Rangseekajee P, Foocharoen C. Characteristics, treatments and outcome of psychosis in Thai SLE patients. J Psychosom Res. (2012) 73:448–51. doi: 10.1016/j.jpsychores.2012.08.006
Keywords: autoimmune, immune system, psychosis, schizophrenia, mental illness
Citation: Jeppesen R and Benros ME (2019) Autoimmune Diseases and Psychotic Disorders. Front. Psychiatry 10:131. doi: 10.3389/fpsyt.2019.00131
Received: 29 November 2018; Accepted: 25 February 2019;
Published: 20 March 2019.
Edited by:
Marion Leboyer, Université Paris-Est Créteil Val de Marne, FranceReviewed by:
Stefania Schiavone, University of Foggia, ItalyKonrad Prasad, University of Pittsburgh, United States
Copyright © 2019 Jeppesen and Benros. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Eriksen Benros, YmVucm9zQGRhZGxuZXQuZGs=
 Rose Jeppesen
Rose Jeppesen Michael Eriksen Benros
Michael Eriksen Benros