
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry , 15 February 2019
Sec. Molecular Psychiatry
Volume 10 - 2019 | https://doi.org/10.3389/fpsyt.2019.00058
This article is part of the Research Topic Precision Psychiatry from a Pharmacogenetics Perspective View all 10 articles
There is conflicting evidence for the association between genetic polymorphisms in the serotonin (5-HT)2C receptor (HTR2C) and response to antipsychotic drugs (APD) in schizophrenic patients. We tested the association between the HTR2C polymorphisms, Cys23Ser, −759C/T, and −697G/C, and response to APDs (mainly clozapine) in a 6 month prospective study in 171 patients with schizophrenia. Ser23 was significantly associated with treatment response (positive symptoms, X2 = 7.540, p = 0.01; negative symptoms, X2 = 4.796, p = 0.03) in male patients only. A −759C-Ser23 haplotype was similar associated with positive (X2 = 6.648, p = 0.01) and negative (X2 = 6.702, p = 0.01) symptom improvement. Logistic regression, after controlling for covariates, also showed significant haplotypic associations. A meta-analysis of six studies for Ser23 and treatment response showed an overall odds ratio of 2.00 (95%CI, 1.38–2.91, p = 0.0003) or 1.94 (95%CI, 1.27–2.99, p = 0.0024) under fixed or random effect models. These results provide additional evidence that HTR2C polymorphisms are associated with treatment response to APD with HTR2C antagonism or inverse agonism, in male schizophrenic patients.
The serotonin (5-HT)2C receptor (HTR2C), located at Xq24, belongs to the seven-transmembrane-spanning G protein–coupled receptor superfamily. It is widely distributed in brain regions which are relevant to schizophrenia. HTR2C receptors exert a tonic inhibitory effect on dorsal and ventral striatal, limbic, hippocampal, and cortical dopamine (DA) release (1, 2), modulate serotonergic activity in the dorsal raphe (3), and regulate 5-HT and glutamate efflux in rat cortex (4).
HTR2C is involved in the neurobiology of schizophrenia and the efficacy and side effects of some APDs. It is one of the key regulators of dopaminergic activity in the limbic system. Stimulation of DA D2 receptors in the ventral and dorsal striatum can lead to delusions and hallucinations (5). Activation of HTR2C receptors might lead to decreases in DA release in key brain regions for schizophrenia (6). HTR2C are expressed on principal neurons and GABAergic interneurons in the prefrontal cortex (7) and, thus, may be relevant to the hypoglutamatergic basis for various components of the schizophrenia syndrome (8). The ability of 5-HT- stimulated and constitutively active HTR2C receptors to inhibit DA release in limbic brain areas has been postulated to cause psychosis and to modulate the efficacy of APDs that act by blocking DA D2 receptors (9–12). Blockade of the constitutive activity of HTR2C receptors enhances cortical and limbic DA release by some APDs (12). The ability of HTR2C agonists to reduce DA release from terminals of VTA neurons in mesolimbic areas is consistent with the antipsychotic effect of the HTR2C agonist, vabicaserin (13, 14). Vabicaserin is effective in reversal of phencyclidine and amphetamine-induced hyperactivity (15).
Evidence from genetic association studies also implicates HTR2C in a variety of neuropsychiatric diseases. The HTR2C has a well-characterized promoter region harboring multiple polymorphisms (Figure 1A), suggesting their potential impact on CpG methylation and putative transcription factor binding, resulting in alteration of HTR2C expression. −759C/T and −697G/C are the most widely investigated promoter polymorphisms. −759C/T polymorphism is associated with antipsychotic induced weight gain (17). −759C/T or −697G/C, has also been linked to therapeutic response to APDs (18–20). However, these results are contradictory to each other with regard to gender and risk allele.
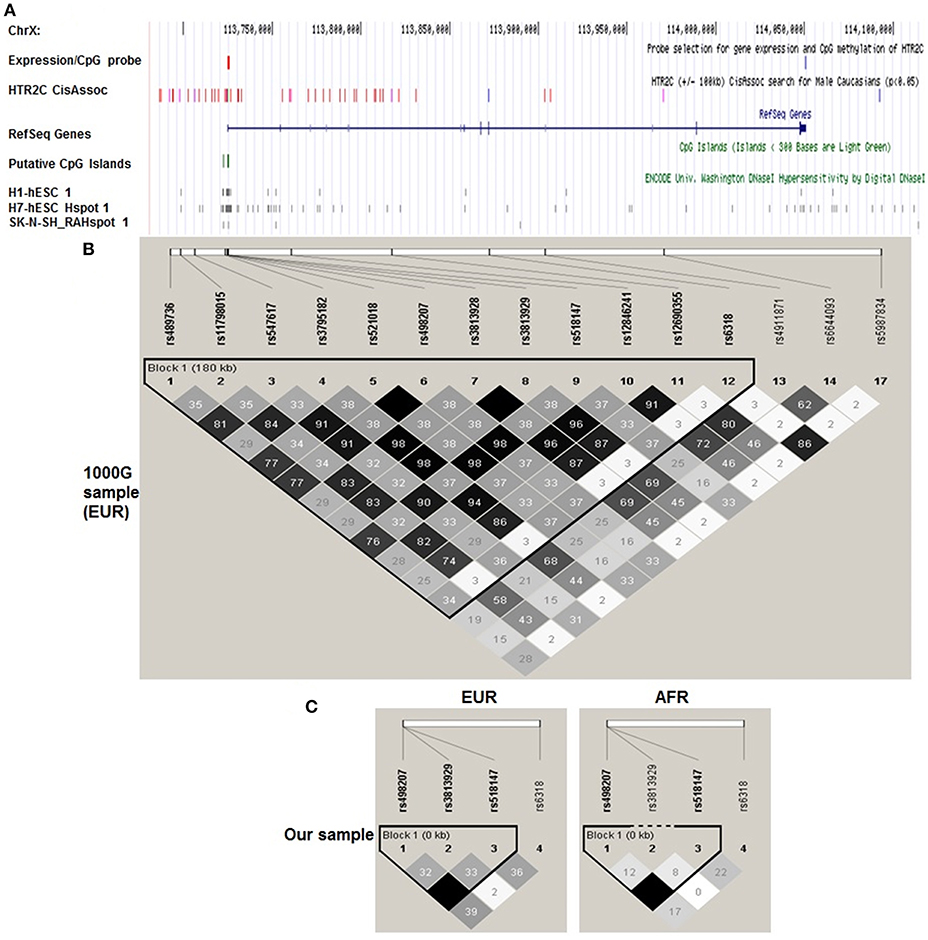
Figure 1. Haploview plot of cis-eQTLs identified by Braincloud for HTR2C in EUR. A. Multiple custom annotation tracks were created at UCSC Genome Browser to show the cis eQTLs for HTR2C. “expression/CpG Probe” track represents the probe location for gene expression (blue) and CpG island (red); (A) Single expression probe selected for HTR2C is located at the 3′ end of mRNA which represents the major form of HTR2C cloned from multiple brain regions according to NCBI Aceview. “HTR2C CisAssoc” track represents identified cis-eQTLs (p < 0.05) by Braincloud data (purple) and all other SNPs in LD (r2 > 0.8) with cis-eQTLs (red). We successfully identified the tag SNPs for the candidate SNPs (r2 > 0.85) by SNAP, based on earlier Hapmap data (16) in EUR. rs12846241 (r2 = 0.96), rs498207 (r2 = 0.98), and rs5987834 (r2 = 0.86) are the tag SNPs, genotyped in Braincloud, for −759C/T, −697G/C, and Cys23Ser, respectively, in EUR. Most of cis-eQTLs are aggregated at the promoter or 5′ UTR regions as expected. −759C/T, tagged by rs12846241, has been confirmed as cis-eQTL for HTR2C. The T genotype has a lower mRNA level than C genotype (p = 0.006). −697G/C, tagged by rs498207, also suggests a potential impact on gene expression (p = 0.043), but not as strong as −759C/T. Cys23Ser and its tag SNP rs5987834 were negative controls (blue); “CpG Islands” track represents putative CpG Islands for HTR2C. We also include putative cis regulatory elements identified by DNase I hypersensitivity analysis on several cell lines with potential neuronal lineage. (B) A haploview plot of those SNPs in EUR from 1,000 Genome data. Grayscale from black to white is correlated with r2 from high to low in each box. (C) Haploview plot of genotyped HTR2C SNPs in our sample separated by ethnicity. The LD and haplotype pattern of genotyped SNPs from our EUR samples matched those from 1,000 Genome EUR sample.
Cys23Ser, is a non-synonymous SNP which results in an amino acid substitution of cysteine to serine at position 23. This substitution can disrupt a disulfide bridge and potentially alter the structure or stability of the HTR2C protein (21). Although this functional polymorphism has been found to be associated with numerous neuropsychiatric diseases, including anorexia nervosa (22), unipolar, and bipolar depression (16, 23), psychotic symptoms in late-onset Alzheimer's disease (24), vulnerability to cocaine cue reactivity (25), migraine with aura (26), and stress-related cortisol levels (27), it's association with schizophrenia is less clear (28). An association between the Cys23Ser and visual hallucinations and depression in schizophrenia patients has been reported (24), but has not been replicated by others (29, 30). Cys23Ser is associated with chronic hospitalization in schizophrenia patients (31) and APD-induce extrapyramidal side effects (32) suggests it is more related to the impact of treatment on the disease process, possibly through effects on dopaminergic activity. Most importantly for this study, Cys23Ser has also been linked in some (33, 34) but not all studies (35–37) to the extent of response to clozapine. These studies will be the subject of a meta-analysis included in the Results section. Clozapine response in treatment-resistant schizophrenia patients does not occur within the conventional 6 week clinical trial period in many patients (38, 39). Due to the inconsistent relationship between HTR2C polymorphisms and the psychopathology of schizophrenia, the response to clozapine, and functional activity assays, we examine all three widely-investigated HTR2C SNPs as possible predictors of response of positive and negative symptoms to APD treatment in schizophrenia. Male and female subjects were analyzed separately in order to determine a possible association with gender. Finally, a meta-analysis was performed to determine the overall association between HTR2C polymorphisms and response to clozapine and other APDs.
The 171 (male/female, 115/56) patients with schizophrenia or schizoaffective disorder who participated in this study were part of an NIMH-sponsored extramural clinical research center at Case Western Reserve University School of Medicine and Vanderbilt University School of Medicine. Details about recruitment and assessment of subjects have been reported previously (40). Categorical treatment response was evaluated at 6 week and 6 months, using the criteria based upon Kane et al. (41). Subjects with a reduction of >20% in the Brief Psychiatric Rating Scale (BPRS) total score or subcategories, BPRS Positive and Negative, was defined as a responder. Patients were treated with standard doses of the following atypical antipsychotic drugs: clozapine, 550 mg(400–900 mg), 78%; melperone, 250 mg(100–400 mg), 7.0%; risperidone, 6 mg(4–8 mg), 3.8%; or olanzapine, 20 mg(15–40 mg), 2.1%, or typical antipsychotic drugs, mainly haloperidol (10 mg, 9.0%). Antidepressants (14%) and mood stabilizers (5%) were used sparingly.
Taqman® assay for three SNPs, −759C/T(rs3813929), −697G/C(rs518147), and Cys23Ser(rs6318) was performed at Northwestern University Genomic Core. Call rates are 95.32, 98.83, and 97.66%, respectively. The linkage disequilibrium (LD) and haplotype pattern of genotyped SNPs from our EUR samples (Figure 1C) matched those from 1,000 Genome EUR sample (Figure 1B).
We analyzed the males and females separately. The relationship between genotypes and demographic variables was analyzed using chi-square (χ2) or ANOVA. Genotype or haplotype associated differential response to APD was initially evaluated by χ2 test and then ANCOVA (SPSS), adjusted for race, drugs, age of onset, and the corresponding baseline psychopathology or status of early response. Statistical significance was defined as p < 0.05. As all results were considered exploratory, there was no adjustment for multiple testing. Mapping cis eQTL or methylation QTL was performed using Braincloud data (42, 43). In order to review and elucidate the general relationship between HTR2C polymorphisms and drug response to APDs, a meta-analysis of six studies, including ours, with accessible genotyping data for Cys23Ser and binary outcome for symptom improvement, were conducted by R “meta” package. Heterogeneity among the studies was assessed by means of the I2 inconsistency test and Cochran's Q statistics under a null hypothesis test in which p < 0.05.
Cys23Ser, −759C/T, and −697G/C were genotyped for schizophrenic patients with European (EUR, n = 118) and African (AFR, n = 53) ancestry Table 1. In the male group, the age at onset for the Ser23 carriers was significantly older than that for non-carriers (p = 0.009; Table 1A). This difference was not observed in the females (p = 0.899; Table 1B). There was no significant difference in the proportions of patients who were treatment resistant or unmedicated at baseline between the genotypes for each SNP. Duration of illness and number of previous hospitalization also did not differ. Although Ser23 carriers had a higher total BRPS score in the male patients (p = 0.04), there was no significant difference with regard to the subcategories of psychopathology including positive, negative, and anxiety/depression subscales. Race, drug, age of onset, and baseline psychopathology were included as covariates in the following ANCOVA.

Table 1. Demographic information grouped by genotypes of three HTR2C SNPs and separated by gender (1A for Male; 1B for Female).
We successfully identified the tag SNPs for the candidate SNPs (r2 > 0.85) by SNAP and these tag SNPs are in LD with the three candidates by a haploview analysis of the genotype data from 1,000 Genome Figure 1, Supplementary Table 1. −759C/T, tagged by rs12846241, have been confirmed as cis-eQTL and methylation-QTL for HTR2C (Supplementary Table 2). Cys23Ser, tagged by rs5987834, has no impact on gene expression and % methylation. Based on the previous studies (genetic and functional) and our cis eQTL findings, −759C/T, −697G/C, Cys23Ser, and the combinations of two or all three, were targets for the subsequent genotype-phenotype association study. Since in vitro functional assays indicated that −759C/T and Cys23Ser have a significant impact on HTR2C activity through distinctive mechanisms, we further explored if −759C-Ser, “a super combination,” we propose it produces a greater expression of the constitutively more active form of HTR2C, may demonstrate an even stronger association with dichotomous symptom improvement in an additive mode, after treatment with the APDs, which are HTR2C inverse agonists or antagonists studied here.
A significant association between Cys23Ser and dichotomous treatment response was observed only in the male group for both positive symptoms, X2 = 7.540, p = 0.006; and negative symptoms, X2 = 4.796, p = 0.029, at 6 month (Table 2A). Haplotype analysis showed that −759C-Ser23 maintained the same level of significant association with positive symptom improvement (X2 = 6.648, p = 0.010) and negative symptom improvement (X2 = 6.702, p = 0.010) at 6 month (Table 2A). All of the above significant findings were only observed in the male patients, except for a borderline significance for Cys23Ser associated with negative symptom improvement in female (X2 = 3.9, p = 0.048) at 6 month (Supplementary Table 3).
ANCOVA test on absolute change (Table 2B) or % change in BPRS (data not shown) in symptom improvement after controlling for race, drugs, age of onset, and the corresponding baseline psychopathology indicated that male Ser23 carriers had a significant improvement in positive and negative symptoms (p = 0.025 and 0.019, respectively) after 6 months treatment (Table 2B). Neither −759C/T nor −697G/C alone were significantly associated with symptom improvement.
A similar significant association was observed between Cys23Ser and positive/negative symptom improvement in male subjects treated with clozapine only (Supplementary Table 4). Female Ser23 carriers also showed an association with negative symptom improvement at 6 week.
Six studies from Table 3, including ours, with accessible genotyping data for Cys23Ser and binary outcome for positive symptom improvement in EUR, were included in a meta-analysis. We reported the overall odds ratio is 2.00 (95%CI, 1.38–2.91, p = 0.0003) or 1.94 (95%CI, 1.27–2.99, p = 0.0024) under the fixed or random effect models, respectively (Figure 2). The heterogeneity between the studies was insignificant (Cochran's Q = 6.03, p = 0.30; I2 = 0.17 (95% CI, 0.00 to 0.62). QUANTO 1.2 was used to calculate the power of the test. The Ser23 carriers were found to have a frequency of 0.15 to 0.45 according to Table 3. As Ser23 carriers increased the odds of having treatment response by 2.0, population risk (Kp) = 0.30, dominant mode of inheritance, and 216 responders/738 non-responders were genotyped, the power (chance) to detect an association with significance p < 0.01 was over 90%.
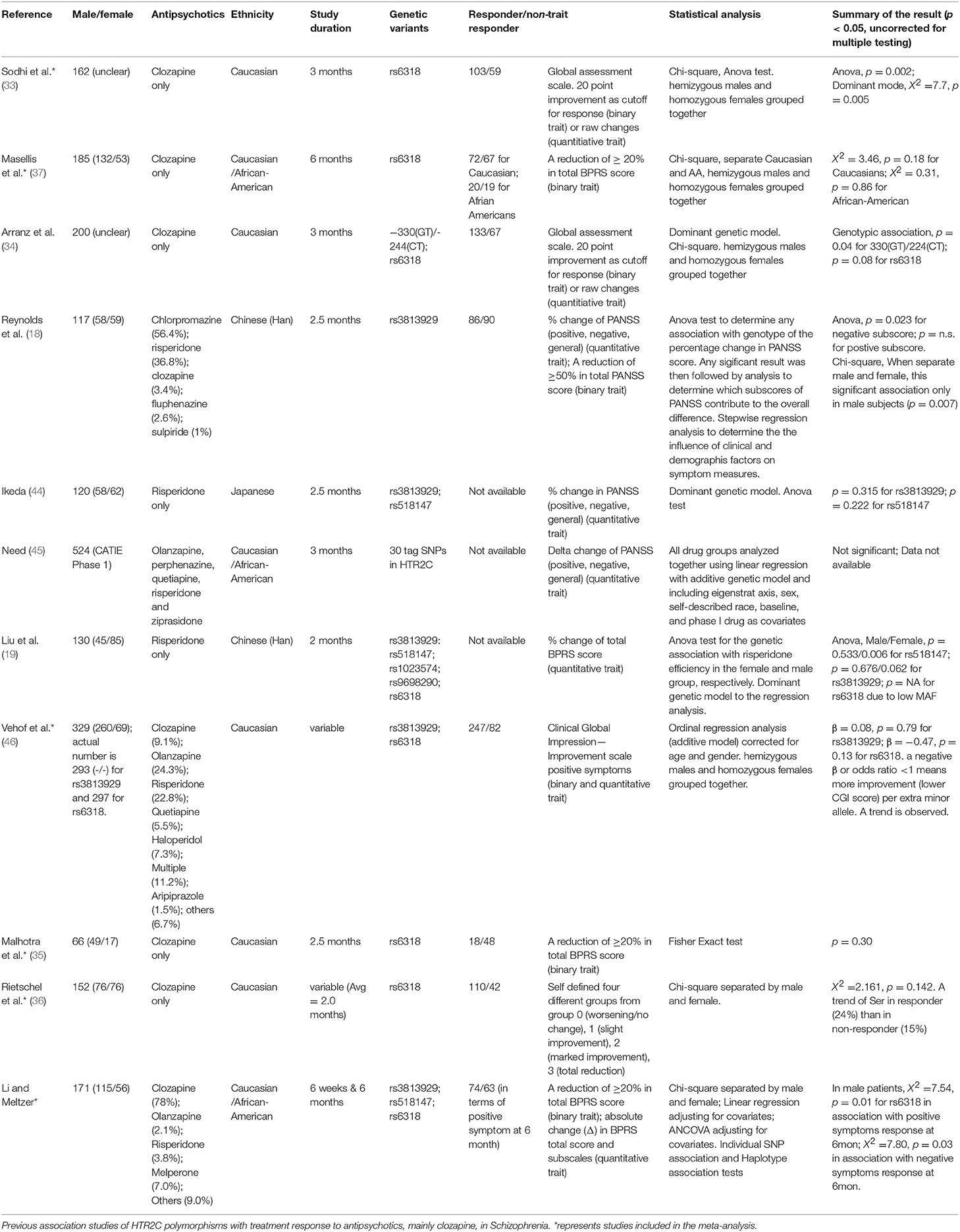
Table 3. A meta-analysis of six studies to determine the general relationship between HTR2C Cys23Ser and drug response to APDs.
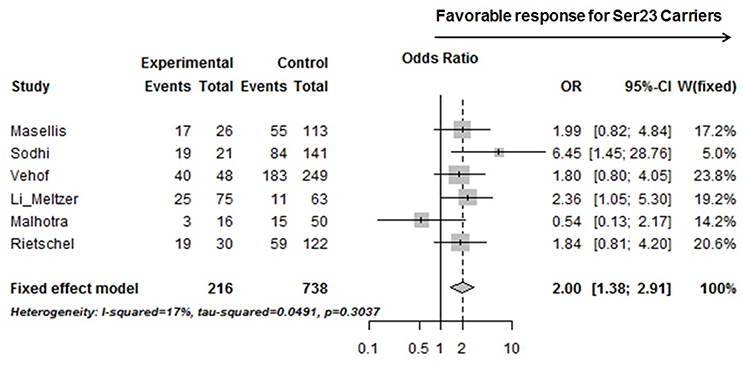
Figure 2. A forest plot showed a meta-analysis of the Ser23 carrier for rs6318 (Cys23Ser) associated with treatment response (binary data) in positive symptom across six studies on EUR samples. Experimental, Ser23 carrier; Control, Non-Ser23 carrier; Events, number of Responder; Total, number of subjects with that genotype. The squares represent odds ratio to have a favorable treatment response if it is over 1 and the horizontal lines show the 95% confidence intervals of the corresponding odds ratio. For Vehof's study, the raw data was imputed based on published improvement/no improvement ratio, genotyping data, and the corresponding odds ratio.
We tested the association between the HTR2C polymorphisms, Cys23Ser, −759C/T, and −697G/C, and treatment response in 171 schizophrenic patients after treatment with APDs, mainly clozapine, for 6 months. One of the strengths of this study was that the majority of the patients were unmedicated at the time of initial assessment. Both Ser23 and −759C–Ser23 haplotype were significantly associated with positive and negative symptom improvement in male patients and Cys23Ser, not the promoter polymorphisms, is the major genetic contributor of the HTR2C in modulating symptom improvement to clozapine. The previously published association studies (Table 3) no consistent results. Attempted replication studies (37, 46) for Sodhi et al.'(33) reported negative results, but the individual p-values as well as the p-value from a meta-analysis (47) were suggestive of a trend for association (Masellis, p = 0.18, Vehof, p = 0.13, and Gressier, p = 0.12). Our meta-analysis of six original studies (33, 35–37, 46) suggests that HTR2C Cys23Ser is associated with symptom improvement after treatment with clozapine. This is consistent with a previous meta-analysis that included several APDs (48).
Some atypical APDs, e.g., clozapine, olanzapine, risperidone, and sertindole, are potent inverse agonists of both HTR2C and HTR2A receptors (49, 50). On the other hand, some typical APDs, e.g., chlorpromazine, thioridazine, spiperone, and thiothixene, are HTR2C neutral antagonists, which would preclude their affecting the constitutive activity of HTR2Cs, although the combination of a neutral antagonist and inverse agonist, could lead to blockade of the neutral antagonist (51). The Ki (nM) for D2, HTR2A, and HTR2C are provided in Supplementary Table 5 for each APD (51). Studies based mainly on a single APD are more likely to generate positive results than those based on diverse drug treatments because APDs have variable effects on non-5-HT2C, receptors which can affect their actions as antipsychotics and cognitive enhancers (49, 50). However, the meta-analysis reported here suggests our findings may generalize to atypical APDs which are HTR2C antagonists or inverse agonists at clinically effective doses.
Many factors may contribute to the inconsistent results in pharmacogenetic studies of APDs response. These include the heterogeneity in patient populations, utilization of different rating scales, definition of response, frequency of genetic variants in distinct ethnic groups, APDs which differ with regard to HTR2C pharmacology and actions on other receptors which impact response, e.g., D2, 5-HT1A, and alpha 2 adrenoreceptors, duration of clinical assessment, proposed mode of inheritance, and statistical methods.
In vitro functional studies provide a partial explanation of why Cys23Ser has a main effect on response to APD treatment. Ser23 receptor displayed greater constitutive activity to mobilize calcium than Cys23 receptor (52). Ser23 receptor had greater cell surface expression and more rapid resensitization following exposure to SB206553, a mixed HTR2B antagonists and HTR2C inverse agonist (53). It may be concluded that prolonged exposure of both HTR2C isoreceptors to an inverse agonist increases receptor responsiveness to endogenous 5-HT or other HTR2C agonists, and cells or presumably individuals carrying Ser23 have prompter response to the stimuli than Cys23 carriers. Dopaminergic circuitry is more sensitive to pain stress in Ser23 carriers (54). Greater dopamine release in the nucleus accumbens, caudate nucleus, and putamen was observed in the Ser23 carriers during pain, suggesting mesoaccumbal stress sensitivity may mediate the effects of HTR2C variation on the risk of neuropsychiatric disorders. Significant differences in regional cerebral blood flow between Ser23 and Cys23 male carriers after treatment with serotonin agonist meta-Chlorophenylpiperazine suggests that this polymorphism does have distinct functional consequences (55).
In conclusion, these results provide additional evidence that HTR2C polymorphisms, particularly Cys23Ser, are associated with response to APD treatment, mainly clozapine with HTR2C antagonism or partial agonism, in male schizophrenic patients.
This study was carried out in accordance with the recommendations of Internal Review Board of Vanderbilt University and Case Western Reserve University with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Internal Review Board of Vanderbilt University and Case Western Reserve University.
JL mostly contributed to data analysis, interpretation of results, and manuscript writing. HH contributed to initial data analysis and manuscript writing. HM has designed the study and written the manuscript in close collaboration with JL. All authors have approved the final manuscript.
This research was supported by donations from the Weisman Family, Mr. Michael Burke, and Mr. Michael Shmerling. Neither had any role in study design, data collection, analysis and interpretation, writing of the report, or the decision to submit the paper for publication.
HM is a stockholder in SureGene and ACADIA and receives additional grant support from Sunovion and Sumitomo Dainippon Pharma for other studies. HM also receives grant support from Alkermes, Auspex, Boehringer Mannheim, Eli Lilly, Janssen, Lundbeck, Mag T, Otsuka, and Reviva.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was first presented at the Annual Meeting of American Society of Human Genetics in 2014 as a poster presentation and the abstract was collected at www.ashg.org/2014meeting/abstracts/fulltext/f140120372.htm.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2019.00058/full#supplementary-material
1. Di Giovanni G, De Deurwaerdere P, Di Mascio M, Di Matteo V, Esposito E, Spampinato U. Selective blockade of serotonin-2C/2B receptors enhances mesolimbic and mesostriatal dopaminergic function: a combined in vivo electrophysiological and microdialysis study. Neuroscience (1999) 91:587–97. doi: 10.1016/S0306-4522(98)00655-1
2. Marquis KL, Sabb AL, Logue SF, Brennan JA, Piesla MJ, Comery TA, et al. WAY-163909 [(7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole]: a novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther. (2007) 320:486–96. doi: 10.1124/jpet.106.106989
3. Boothman L, Raley J, Denk F, Hirani E, Sharp T. In vivo evidence that 5-HT(2C) receptors inhibit 5-HT neuronal activity via a GABAergic mechanism. Br J Pharmacol. (2006) 149:861–9. doi: 10.1038/sj.bjp.0706935
4. Calcagno E, Carli M, Baviera M, Invernizzi RW. Endogenous serotonin and serotonin2C receptors are involved in the ability of M100907 to suppress cortical glutamate release induced by NMDA receptor blockade. J Neurochem. (2009) 108:521–32. doi: 10.1111/j.1471-4159.2008.05789.x
5. Howes OD, Williams M, Ibrahim K, Leung G, Egerton A, Mcguire PK, et al. Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain (2013) 136:3242–51. doi: 10.1093/brain/awt264
6. Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5-HT(2C) receptors in the control of central dopamine function. Trends Pharmacol Sci. (2001) 22:229–32. doi: 10.1016/S0165-6147(00)01688-6
7. Santana N, Artigas F. Laminar and cellular distribution of monoamine receptors in rat medial prefrontal cortex. Front Neuroanat. (2017) 11:87. doi: 10.3389/fnana.2017.00087
8. Thomas EHX, Bozaoglu K, Rossell SL, Gurvich C. The influence of the glutamatergic system on cognition in schizophrenia: a systematic review. Neurosci Biobehav Rev. (2017) 77:369–87. doi: 10.1016/j.neubiorev.2017.04.005
9. Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology (1999) 38:1083–152. doi: 10.1016/S0028-3908(99)00010-6
10. Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2003) 27:1159–72. doi: 10.1016/j.pnpbp.2003.09.010
11. Giorgetti M, Tecott LH. Contributions of 5-HT(2C) receptors to multiple actions of central serotonin systems. Eur J Pharmacol. (2004) 488:1–9. doi: 10.1016/j.ejphar.2004.01.036
12. Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res. (2008) 172:177–97. doi: 10.1016/S0079-6123(08)00909-6
13. Dunlop J, Watts SW, Barrett JE, Coupet J, Harrison B, Mazandarani H, et al. Characterization of vabicaserin (SCA-136), a selective 5-hydroxytryptamine 2C receptor agonist. J Pharmacol Exp Ther. (2011) 337:673–80. doi: 10.1124/jpet.111.179572
14. Shen JH, Zhao Y, Rosenzweig-Lipson S, Popp D, Williams JB, Giller E, et al. A 6-week randomized, double-blind, placebo-controlled, comparator referenced trial of vabicaserin in acute schizophrenia. J Psychiatr Res. (2014) 53:14–22. doi: 10.1016/j.jpsychires.2014.02.012
15. Rosenzweig-Lipson S, Dunlop J, Marquis KL. 5-HT2C receptor agonists as an innovative approach for psychiatric disorders. Drug News Perspect. (2007) 20:565–71. doi: 10.1358/dnp.2007.20.9.1162244
16. Gutierrez B, Fananas L, Arranz MJ, Valles V, Guillamat R, Van Os J, et al. Allelic association analysis of the 5-HT2C receptor gene in bipolar affective disorder. Neurosci Lett. (1996) 212:65–7. doi: 10.1016/0304-3940(96)12746-4
17. De Luca V, Muller DJ, Hwang R, Lieberman JA, Volavka J, Meltzer HY, et al. HTR2C haplotypes and antipsychotics-induced weight gain: X-linked multimarker analysis. Hum Psychopharmacol. (2007) 22:463–7. doi: 10.1002/hup.868
18. Reynolds GP, Yao Z, Zhang X, Sun J, Zhang Z. Pharmacogenetics of treatment in first-episode schizophrenia: D3 and 5-HT2C receptor polymorphisms separately associate with positive and negative symptom response. Eur Neuropsychopharmacol. (2005) 15:143–51. doi: 10.1016/j.euroneuro.2004.07.001
19. Liu BC, Zhang J, Wang L, Li XW, Wang Y, Wei ZY, et al. HTR2C promoter polymorphisms are associated with risperidone efficacy in Chinese female patients. Pharmacogenomics (2010) 11:685–92. doi: 10.2217/pgs.10.23
20. Xu Q, Wu X, Li M, Huang H, Minica C, Yi Z, et al. Association studies of genomic variants with treatment response to risperidone, clozapine, quetiapine and chlorpromazine in the Chinese Han population. Pharmacogenomics J. (2015) 16:357–65. doi: 10.1038/tpj.2015.61
21. Lappalainen J, Zhang L, Dean M, Oz M, Ozaki N, Yu DH, et al. Identification, expression, and pharmacology of a Cys23-Ser23 substitution in the human 5-HT2c receptor gene (HTR2C). Genomics (1995) 27:274–9. doi: 10.1006/geno.1995.1042
22. Hu X, Giotakis O, Li T, Karwautz A, Treasure J, Collier DA. Association of the 5-HT2c gene with susceptibility and minimum body mass index in anorexia nervosa. Neuroreport (2003) 14:781–3. doi: 10.1097/00001756-200305060-00001
23. Lerer B, Macciardi F, Segman RH, Adolfsson R, Blackwood D, Blairy S, et al. Variability of 5-HT2C receptor cys23ser polymorphism among European populations and vulnerability to affective disorder. Mol Psychiatry (2001) 6:579–85. doi: 10.1038/sj.mp.4000883
24. Holmes C, Arranz MJ, Powell JF, Collier DA, Lovestone S. 5-HT2A and 5-HT2C receptor polymorphisms and psychopathology in late onset Alzheimer's disease. Hum Mol Genet. (1998) 7:1507–9. doi: 10.1093/hmg/7.9.1507
25. Anastasio NC, Liu S, Maili L, Swinford SE, Lane SD, Fox RG, et al. Variation within the serotonin (5-HT) 5-HT(2)C receptor system aligns with vulnerability to cocaine cue reactivity. Transl Psychiatry (2014) 4:e369. doi: 10.1038/tp.2013.131
26. Kusumi M, Araki H, Ijiri T, Kowa H, Adachi Y, Takeshima T, et al. Serotonin 2C receptor gene Cys23Ser polymorphism: a candidate genetic risk factor of migraine with aura in Japanese population. Acta Neurol Scand. (2004) 109:407–9. doi: 10.1111/j.1600-0404.2004.00236.x
27. Brummett BH, Babyak MA, Jiang R, Shah SH, Becker RC, Haynes C, et al. A functional polymorphism in the 5HTR2C gene associated with stress responses also predicts incident cardiovascular events. PLoS ONE (2013) 8:e82781. doi: 10.1371/journal.pone.0082781
28. Murad I, Kremer I, Dobrusin M, Muhaheed M, Bannoura I, Muller DJ, et al. A family-based study of the Cys23Ser 5HT2C serotonin receptor polymorphism in schizophrenia. Am J Med Genet. (2001) 105:236–8. doi: 10.1002/ajmg.1260
29. Assal F, Alarcon M, Solomon EC, Masterman D, Geschwind DH, Cummings JL. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Arch Neurol. (2004) 61:1249–53. doi: 10.1001/archneur.61.8.1249
30. Pritchard AL, Harris J, Pritchard CW, Coates J, Haque S, Holder R, et al. Role of 5HT 2A and 5HT 2C polymorphisms in behavioural and psychological symptoms of Alzheimer's disease. Neurobiol Aging (2008) 29:341–7. doi: 10.1016/j.neurobiolaging.2006.10.011
31. Segman RH, Ebstein RP, Heresco-Levy U, Gorfine M, Avnon M, Gur E, et al. Schizophrenia, chronic hospitalization and the 5-HT2C receptor gene. Psychiatr Genet. (1997) 7:75–8. doi: 10.1097/00041444-199722000-00003
32. Gunes A, Dahl ML, Spina E, Scordo MG. Further evidence for the association between 5-HT2C receptor gene polymorphisms and extrapyramidal side effects in male schizophrenic patients. Eur J Clin Pharmacol. (2008) 64:477–82. doi: 10.1007/s00228-007-0450-x
33. Sodhi MS, Arranz MJ, Curtis D, Ball DM, Sham P, Roberts GW, et al. Association between clozapine response and allelic variation in the 5-HT2C receptor gene. Neuroreport (1995) 7:169–72. doi: 10.1097/00001756-199512000-00041
34. Arranz MJ, Munro J, Birkett J, Bolonna A, Mancama D, Sodhi M, et al. Pharmacogenetic prediction of clozapine response. Lancet (2000) 355:1615–6. doi: 10.1016/S0140-6736(00)02221-2
35. Malhotra AK, Goldman D, Ozaki N, Rooney W, Clifton A, Buchanan RW, et al. Clozapine response and the 5HT2C Cys23Ser polymorphism. Neuroreport (1996) 7:2100–2. doi: 10.1097/00001756-199609020-00007
36. Rietschel M, Naber D, Fimmers R, Moller HJ, Propping P, Nothen MM. Efficacy and side-effects of clozapine not associated with variation in the 5-HT2C receptor. Neuroreport (1997) 8:1999–2003. doi: 10.1097/00001756-199705260-00040
37. Masellis M, Basile V, Meltzer HY, Lieberman JA, Sevy S, Macciardi FM, et al. Serotonin subtype 2 receptor genes and clinical response to clozapine in schizophrenia patients. Neuropsychopharmacology (1998) 19:123–32. doi: 10.1016/S0893-133X(98)00007-4
38. Meltzer HY. Duration of a clozapine trial in neuroleptic-resistant schizophrenia. Arch Gen Psychiatry (1989) 46:672. doi: 10.1001/archpsyc.1989.01810070098017
39. Meltzer HY, Bobo WV, Roy A, Jayathilake K, Chen Y, Ertugrul A, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry (2008) 69:274–85. doi: 10.4088/JCP.v69n0214
40. Meltzer HY, Brennan MD, Woodward ND, Jayathilake K. Association of Sult4A1 SNPs with psychopathology and cognition in patients with schizophrenia or schizoaffective disorder. Schizophr Res. (2008) 106:258–64. doi: 10.1016/j.schres.2008.08.029
41. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry (1988) 45:789–96. doi: 10.1001/archpsyc.1988.01800330013001
42. Colantuoni C, Lipska BK, Ye T, Hyde TM, Tao R, Leek JT, et al. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature (2011) 478:519–23. doi: 10.1038/nature10524
43. Numata S, Ye T, Hyde TM, Guitart-Navarro X, Tao R, Wininger M, et al. DNA methylation signatures in development and aging of the human prefrontal cortex. Am J Hum Genet. (2012) 90:260–72. doi: 10.1016/j.ajhg.2011.12.020
44. Ikeda M, Yamanouchi Y, Kinoshita Y, Kitajima T, Yoshimura R, Hashimoto S, et al. Variants of dopamine and se rotonin candidate genes as predictors of response to risperidone treatment in first-episode schizophrenia. Pharmacogenomics (2008) 9:1437–43. doi: 10.2217/14622416.9.10.1437
45. Need AC, Keefe RS, Ge D, Grossman I, Dickson S, McEvoy JP, et al. Pharmacogenetics of antipsychotic response in the CATIE trial: a candidate gene analysis. Eur J Hum Genet. (2009) 17:946–57. doi: 10.1038/ejhg.2008.264
46. Vehof J, Burger H, Wilffert B, Al Hadithy A, Alizadeh BZ, Snieder H. Clinical response to antipsychotic drug treatment: association study of polymorphisms in six candidate genes. Eur Neuropsychopharmacol. (2012) 22:625–31. doi: 10.1016/j.euroneuro.2012.01.006
47. Gressier F, Porcelli S, Calati R, Serretti A. Pharmacogenetics of clozapine response and induced weight gain: a comprehensive review and meta-analysis. Eur Neuropsychopharmacol. (2016) 26:163–85. doi: 10.1016/j.euroneuro.2015.12.035
48. Kirchheiner J, Nickchen K, Bauer M, Wong ML, Licinio J, Roots I, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry (2004) 9:442–73. doi: 10.1038/sj.mp.4001494
49. Rauser L, Savage JE, Meltzer HY, Roth BL. Inverse agonist actions of typical and atypical antipsychotic drugs at the human 5-hydroxytryptamine(2C) receptor. J Pharmacol Exp Ther. (2001) 299:83–9.
50. Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology (2003) 28:519–26. doi: 10.1038/sj.npp.1300027
51. Herrick-Davis K, Grinde E, Teitler M. Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther. (2000) 295:226–32.
52. Okada M, Northup JK, Ozaki N, Russell JT, Linnoila M, Goldman D. Modification of human 5-HT(2C) receptor function by Cys23Ser, an abundant, naturally occurring amino-acid substitution. Mol Psychiatry (2004) 9:55–64. doi: 10.1038/sj.mp.4001357
53. Walstab J, Steinhagen F, Bruss M, Gothert M, Bonisch H. Differences between human wild-type and C23S variant 5-HT2C receptors in inverse agonist-induced resensitization. Pharmacol Rep. (2011) 63:45–53. doi: 10.1016/S1734-1140(11)70397-8
54. Mickey BJ, Sanford BJ, Love TM, Shen PH, Hodgkinson CA, Stohler CS, et al. Striatal dopamine release and genetic variation of the serotonin 2C receptor in humans. J Neurosci. (2012) 32:9344–50. doi: 10.1523/JNEUROSCI.1260-12.2012
Keywords: serotonin2C, schizophrenia, genetic, treatment response, antipsychotic agents, clozapine, meta-analysis, polymorphism
Citation: Li J, Hashimoto H and Meltzer HY (2019) Association of Serotonin2c Receptor Polymorphisms With Antipsychotic Drug Response in Schizophrenia. Front. Psychiatry 10:58. doi: 10.3389/fpsyt.2019.00058
Received: 04 October 2018; Accepted: 25 January 2019;
Published: 15 February 2019.
Edited by:
Chad A. Bousman, University of Calgary, CanadaReviewed by:
Chin B. Eap, Université de Lausanne, SwitzerlandCopyright © 2019 Li, Hashimoto and Meltzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Herbert Y. Meltzer, aC1tZWx0emVyQG5vcnRod2VzdGVybi5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.