
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Psychiatry, 08 February 2019
Sec. Psychological Therapy and Psychosomatics
Volume 9 - 2018 | https://doi.org/10.3389/fpsyt.2018.00786
This article is part of the Research TopicReducing the Mortality Gap in People with Severe Mental Disorders: The Role of Lifestyle Psychosocial InterventionsView all 19 articles
 Arlene T. Dalcin1
Arlene T. Dalcin1 Gerald J. Jerome2
Gerald J. Jerome2 Lawrence J. Appel1
Lawrence J. Appel1 Faith B. Dickerson3
Faith B. Dickerson3 Nae-Yuh Wang1
Nae-Yuh Wang1 Edgar R. Miller1
Edgar R. Miller1 Deborah R. Young1
Deborah R. Young1 Jeanne B. Charleston1
Jeanne B. Charleston1 Joseph V. Gennusa1
Joseph V. Gennusa1 Stacy Goldsholl1
Stacy Goldsholl1 Ann Heller1
Ann Heller1 A. Eden Evins4
A. Eden Evins4 Corinne Cather4
Corinne Cather4 Emma E. McGinty5
Emma E. McGinty5 Rosa M. Crum6
Rosa M. Crum6 Gail L. Daumit1*
Gail L. Daumit1*Persons with serious mental illness (SMI) comprise a high-risk group for cardiovascular disease (CVD)-related mortality with rates at least twice those of the overall US. Potentially modifiable CVD risk behaviors (tobacco smoking, obesity, physical inactivity, unhealthy diet) and risk factors (hypertension, diabetes, dyslipidemia) are all markedly elevated in persons with SMI. Evaluations of programs implementing integrated medical care into specialty mental health settings have not shown meaningful effects on CVD risk factor reduction. Rigorously tested, innovative interventions are needed to address the large burden of CVD risk in populations with SMI. In this article, we describe the design of a comprehensive 18-month intervention to decrease CVD risk that we are studying in a randomized clinical trial in a community mental health organization with psychiatric rehabilitation programs. The individual-level intervention incorporated health behavior coaching and care coordination/care management to address all seven CVD risk behaviors and risk factors, and is delivered by a health coach and nurse. If successful, the intervention could be adopted within current integrated care models and significantly improve the physical health of persons with SMI.
Persons with serious mental illness (SMI) comprise a high-risk group for cardiovascular disease (CVD)-related mortality with rates at least twice those of the overall US (1–5). Potentially modifiable CVD risk behaviors (tobacco smoking, obesity, physical inactivity, unhealthy diet) and risk factors (hypertension, diabetes, dyslipidemia) are all markedly elevated in persons with SMI (6–10). The American Heart Association set strategic goals to improve cardiovascular (CV) health to ideal levels for each risk factor and reduce CVD mortality by 20% for all Americans by 2020 (11). However, persons with SMI often have challenges in everyday functioning due to cognitive impairment and ongoing psychiatric symptoms (12, 13). Together with high prevalence of substance use, unemployment, low-income and social isolation, these factors make implementing effective CVD risk reduction interventions challenging for this population (13–17). Adapted behavioral weight loss interventions and smoking cessation interventions tailored to persons with SMI have shown to be efficacious in clinical trials (18–21), however, evidenced-based interventions are needed that address more than one CVD risk factor. Without special efforts to develop interventions that promote CV health and control risk factors in persons with SMI, health disparities will persist and likely worsen.
While in the general population there is substantial evidence that non-physician interventionists (e.g., care managers) who facilitate and coordinate treatment with primary care physicians (PCPs) can improve multiple CVD risk factors (22–28), evidence for effective interventions in populations with SMI is needed. To address the premature mortality from CVD and other medical causes (3, 29–32) and need for improved access to quality care for physical health conditions (33, 34) among people with SMI, there has been a recent increase in implementation of programs integrating medical care into mental health settings (35–45).
In the US, one such model is the behavioral health home (BHH), which incorporates primary care coordination and management into the specialty mental healthcare setting (35–42). However, results from evaluations of BHHs and other integrated programs to-date have been mixed with either no or minimal effects on actual CVD risk factors (37, 46–51).
Thus, rigorously tested, innovative interventions are needed to address the large burden of CVD risk in populations with SMI. Here we describe the design and implementation of a comprehensive risk reduction intervention incorporating health behavior coaching and care coordination/care management to address all CVD risk factors in persons with SMI that we are studying in a randomized clinical trial.
This community organization-based two-arm randomized clinical trial (the IDEAL trial) tests an 18-month comprehensive, practical risk reduction program compared to control in reducing CVD risk as assessed by the global Framingham Risk score (52) in persons with SMI. Participants were randomized to receive a control condition or the active intervention, which incorporates health behavior coaching and care coordination/care management to improve CVD risk behaviors and factors. Individual CVD risk behaviors and factors comprise secondary outcomes.
Two hundred sixty-nine participants with SMI 18 and older and at least one CVD risk factor (hypertension, diabetes mellitus, dyslipidemia, current tobacco smoker, or BMI ≥ 25 kg/m2) were enrolled in the study. Exclusion criteria included having a CVD event in the prior 6 months, a serious medical condition limiting life expectancy, or an active alcohol or substance use disorder, pregnancy, planning on leaving the community in 6 months or the geographic area in 18 months. Serious mental illness diagnosis was assessed by chart review. The study was conducted in partnership with a large community mental health organization that provides outpatient services including psychiatric rehabilitation programs (PRPs). PRPs serve persons with SMI offering vocational and skills training, coordination of behavioral health and social services, and normally provide breakfast and lunch to attendees. We implemented the study at four organization sites with PRPs. As part of our partnership with the community mental health organization and to encourage environments conducive to healthy behaviors, we provided training and resources to promote group physical activity classes open to all program attendees, and a dietician to consult with PRP kitchen staff to provide healthier meals.
We adopted the American Heart Association (AHA) Life's Simple 7 goals and modified some (e.g., 10 lb. weight loss instead of BMI < 25) to provide intermediate targets achievable over the 18-month intervention (11). (Table 1) To work toward these goals, participants in the active intervention met individually with a health coach and a nurse who provided: (1) tailored CVD risk reduction education and counseling; (2) collaboration with physicians to advocate for appropriate management of CVD health risks; and (3) coordination with mental health staff and social supports to encourage and motivate participants to reach individually tailored CV health goals.
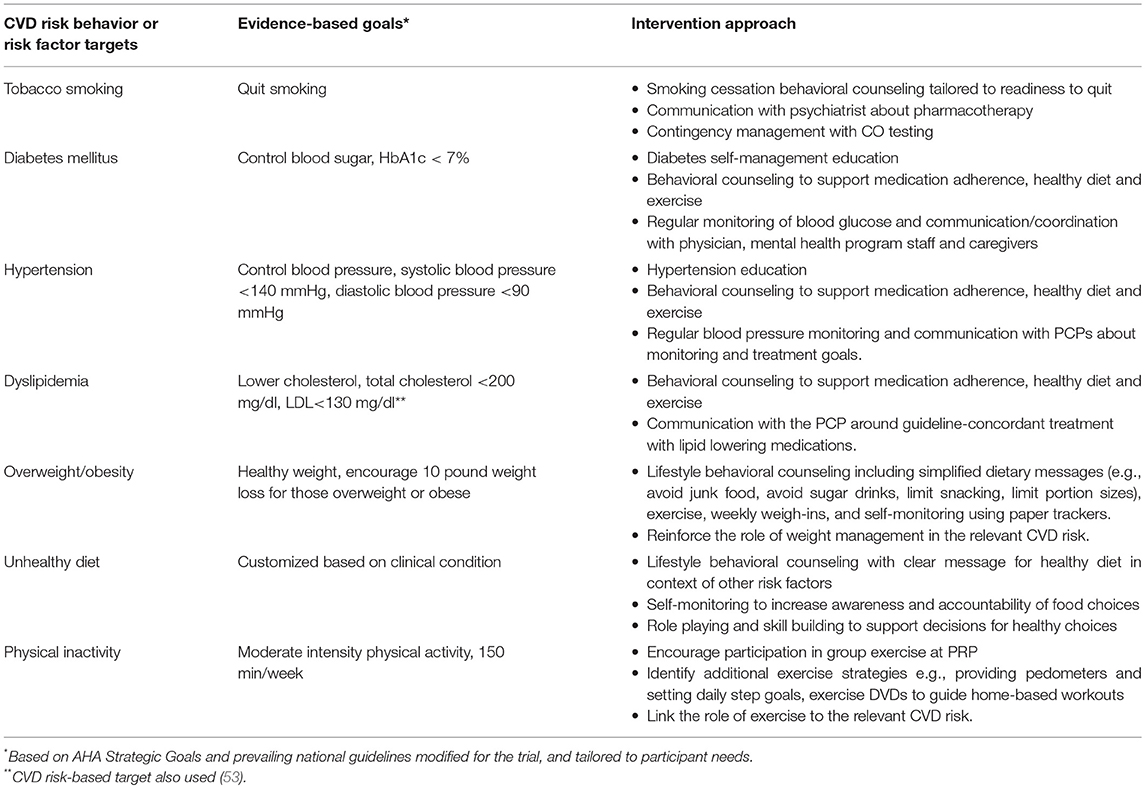
Table 1. Intervention approaches in comprehensive cardiovascular risk reduction intervention for adults with serious mental illness.
Behavioral aspects of the IDEAL intervention incorporate social cognitive theory and behavioral self-management concepts (54, 55). Motivational interviewing, is well suited for adults with SMI in that it is patient-centered, and uses a guiding style to enhance intrinsic motivation to improve CVD risk-related behaviors and promote medication adherence (56–59). The intervention also used solution-focused therapy, a positive-oriented, goal-directed technique emphasizing solutions rather than problems (60, 61). These approaches are useful in helping individuals gain confidence from small successes while staying focused on positive long-term changes.
The intervention fits well within a psychiatric rehabilitation framework because rehabilitation models emphasize skill building and goal setting and incorporate reinforcements as part of a behavioral change strategy (62–65). Evidence demonstrates the success of points/reward systems for persons with SMI in improving social interactions, work-related task performance, and other adaptive behaviors (66–74). The IDEAL intervention uses a point system to reward recommended behaviors (e.g., low carbon monoxide (CO) values consistent with no recent smoking, attending group exercise classes). Points can be traded for inexpensive items (e.g., pen, key chain) or saved for larger rewards (e.g., CD player).
The intervention is tailored to meet the cognitive needs of adults with SMI, characterized by memory and executive function deficits (75, 76). Participant materials have high readability and simplicity of messages, and streamlined paper tracking logs correspond to the intervention's lifestyle change approach. In addition, in-person sessions include techniques such as emphasizing learning through repetition, modeling and demonstrating a few specific skills repeatedly; breaking material into small units; and rehearsing behavioral skills (12, 77, 78).
The intervention utilizes concepts drawn from the overlapping constructs of care management and care coordination. Care management is a patient-centered, team-based approach to increase delivery of evidenced-based care for chronic medical conditions (79). Care coordination emphasizes the deliberate organization of patient activities and the sharing of relevant information among all concerned parties to achieve better patient care (80). The IDEAL intervention team includes a nurse and a health coach, who work together to facilitate communication and coordination between participants, providers, and mental health program staff for all aspects of CVD risk factor management.
The health coach is a facilitator with training in health behavior change and individual-level counseling with a skill level typical for a community health educator. When possible, the coach is an employee of the mental health organization; the position is modeled after one that would be feasible and sustainable in a community mental health setting. The coach, embedded in the PRP, is well-positioned to work with (other) community mental health program staff and take advantage of everyday interactions with staff and participants to encourage and support health behavior change and facilitate coordination of care for CVD risk factors. The registered nurse is an intervention team member who serves as a clinical resource addressing all aspects of CVD risk factor status. The coach and nurse report to an intervention director with motivational interviewing and health behavior change expertise. The intervention director implements quality assurance (Table 2), and may also provide direct services to participants.
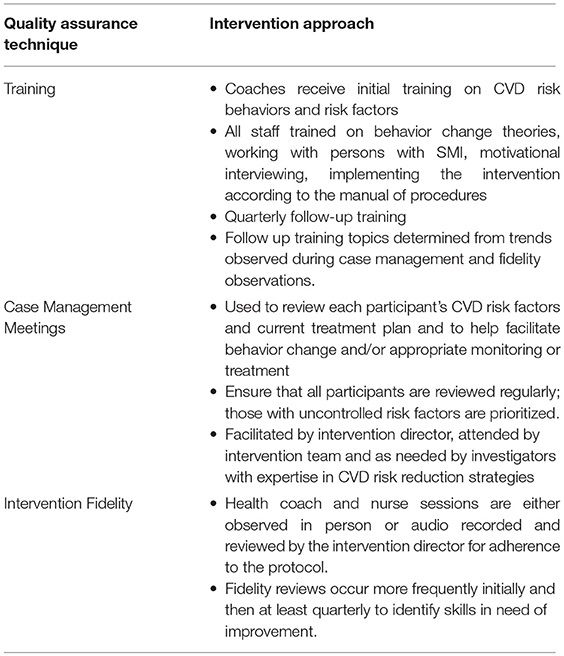
Table 2. Quality assurance techniques used in comprehensive cardiovascular risk reduction intervention for adults with serious mental illness.
The core elements of the intervention include health behavior coaching, coordination of care and care management.
Participants received 20–30 min individual coaching sessions, with weekly sessions for the first six months and at least biweekly thereafter, based on participant need. Guided by the participant's abilities and interests, the health coach addressed CVD risk and targeted behaviors either simultaneously or sequentially. The sessions used motivational interviewing strategies with an initial focus on building rapport and establishing a working alliance. Sessions included a review of the participant's risk behaviors to identify knowledge deficits and to set short-term behavioral goals. The coach used solution-focused therapy to support attainment of individual goals. Participants were encouraged to self-monitor with simple tracking tools as appropriate for their cognitive abilities. The coach reviewed tracking data with participants to reinforce progress, identified triggers and high-risk situations, and formulated new behavioral plans. The nurse provided educational and counseling sessions related to specific CVD risk factors (e.g., diabetes). The intervention sessions may include family members or peers to support the participant for better control of risk factors (e.g. tobacco smoking).
The nurse mets with participants for medication-related counseling to address issues with adherence and plan for physician appointments, prioritizing those with uncontrolled risk factors. He/she shared information on participant's CVD risk factor profile with the primary care physician (PCP) and accompanied the participant on selected office visits. The nurse provided information on guidelines for diabetes, hypertension, dyslipidemia and smoking cessation as needed to participants' physicians, and advocated for evidenced-based monitoring and treatment (19, 53, 81–91). If the participant smoked and was interested in pharmacotherapy, the nurse communicated with the psychiatrist or PCP about prescribing smoking cessation medication and supported the participant through the process. The intervention staff coordinated with mental health staff, therapists, caregivers, physicians and/or office staff to support and reinforce participant health goals. This included facilitating the scheduling of and participant attendance at medical appointments, and removing barriers to obtaining medications and supplies as needed.
Participants received intervention sessions tailored to their individual CVD risk profiles and readiness to address each risk factor. When participants had multiple risk factors, in keeping with a patient-centered approach, intervention staff worked collaboratively with the participant to identify the focus of the sessions. They used options tools, cards with pictures, for possible conversation topics based on the participant's risk factors (e.g., for someone who smokes and has diabetes the options listed would read: Smoking, exercise, medication, sugar drinks). These tools gave the participant the autonomy to choose specific behaviors to discuss, while also allowing the coach to provide direction to the session.
Intervention staff assessed the motivation of participants who smoke with questions based on the transtheoretical stages of change (92) and placed them in one of three tracks: interested in quitting in 30 days; interested in quitting/reducing smoking but not ready to set a quit date; and expressing no interest in quitting. Those ready to quit within the next month received smoking cessation sessions and those interested but not ready to set a quit date received a motivational enhancement program (19, 93–95). The programs identified personalized reasons to stop smoking, provided training in strategies to respond to urges to smoke (The 4Ds: delay, do something else, deep breathe, drink water), and educated patients about the benefits of quitting and of using smoking-cessation pharmacotherapy. Those in the motivational enhancement program transitioned to quit smoking sessions when ready. These interventions incorporated a breathalyzer that measures expired CO as a marker of recent smoking, with incentives for low CO values. For those willing to consider recommended pharmacotherapy, the nurse worked with participants' physicians to facilitate prescription of varenicline or bupropion, and/or provided dual form nicotine replacement therapy as appropriate. Participants who expressed no interest in quitting smoking focused on other behavior change topics, however, the coach recommended change in smoking behavior monthly and re-assessed willingness to quit. If participants expressed interest in quitting smoking or were ready to set a quit date, they transitioned to another track.
Intervention staff helped participants optimally manage their diabetes through self-management education sessions, supporting medication adherence, and encouraging self-monitoring of blood sugar as appropriate. They facilitated hemoglobin A1c monitoring and other guideline concordant care for diabetes with the PCP. For those with uncontrolled diabetes, the nurse and coach coordinated care as needed to decrease barriers to blood glucose control (e.g., obtaining and using glucometer) with mental health staff, caregivers, PCPs and/or specialists. They encouraged high impact dietary behaviors (e.g., no sugar drinks), participation in regular exercise, and weight loss when appropriate.
The nurse met with participants for hypertension education sessions including discussion of medication adherence. Intervention staff monitored blood pressure regularly and communicated with participants and PCPs about monitoring and treatment goals. The nurse also reinforced the dietary (e.g., avoid salty/processed foods) weight loss and exercise goals set with the coach.
The nurse met with participants who met recommended guidelines for lipid lowering therapy for medication education and counseling before communicating with the PCP for guideline-concordant treatment. Both the coach and nurse encouraged high impact dietary behaviors (e.g., no greasy foods), participation in regular exercise, and weight loss if needed.
The coach used lifestyle strategies that have proven effective in previous clinical trials including individual counseling sessions, weekly weigh-ins, simplified dietary messages (e.g., avoid junk food, avoid sugar drinks, limit snacking, limit portion sizes) and paper trackers to help monitor these behaviors (18). The nurse also linked the roles of weight management to the participant's other CVD risks.
Intervention staff provided guidance on ways to eliminate unhealthy dietary choices and incorporate healthier options in the context of other CVD risk factors and the participant's health goals. The conversations used simplified messages and incorporate paper trackers to facilitate awareness and goal setting. Dietary topics included: Avoiding salty/greasy foods, sugar drinks, sweets and processed foods, and eating smart portions and more fruits and vegetables. The coach used role playing and hands-on activities (e.g., visiting the soda machine) to model decision making for healthy diet choices.
The intervention staff encouraged participation in regular moderate-intensity physical activity. We trained PRP staff to deliver site-wide group exercise classes using either a study-made DVD or commercially available walking DVD and recommend that classes are offered three times per week. The health coach encouraged exercise class attendance for intervention participants, participated in some of the classes, and substituted for PRP staff if requested. The coach worked with intervention participants to identify additional strategies to meet physical activity recommendations, for example, providing pedometers and setting daily step goals, and providing exercise DVDs to guide home-based workouts. The nurse reinforced exercise goals set with the coach and linked the importance of exercise as an independent health behavior and to the relevant CVD risks.
Given the extraordinarily high and persistent burden of CVD risk factors and resultant CVD in persons with SMI, there is an urgent need for tailored, effective interventions to promote CV health. We describe a comprehensive health behavior coaching and care coordination/care management intervention designed to address each CVD risk behavior and risk factor for adults with SMI in community mental health settings. The IDEAL intervention design has important strengths. First, the intervention uniquely focuses on addressing all of the seven CVD risk factors and behaviors with strategies directed at reaching clinically significant targets consistent with treatment guidelines. Second, the health coach is embedded in the PRP, facilitating coordination and communication with mental health staff in the everyday community mental health outpatient environment. This provides a practical real-world design to optimize future implementation feasibility and sustainability.
The IDEAL intervention faces some challenges to success. The intervention is ambitious in its attempt to address multiple risk factors in persons with SMI given that accomplishing reduction for a single risk factor in those without SMI often takes intensive efforts (96). Fortunately, the intervention's lifestyle-based recommendations to help address risk factors such as high blood pressure or high blood glucose involve similar approaches. Having both a coach and nurse use a coordinated approach for each participant should help to address this challenge. Another potential barrier to success is that the intervention team does not prescribe medications, and PCPs in the community have no specific incentive to participate in the intervention's care coordination efforts.
If shown to be effective in the RCT, the IDEAL intervention could be implemented through programs, like the BHH, which provide a financing mechanism for staff (e.g., nurse) and infrastructure to integrate care coordination and management into specialty mental health settings. BHHs are currently operating in 15 U.S. states and D.C. through the Affordable Care Act's Medicaid health home waiver program, and have been implemented through the Substance Abuse and Mental Health Services Administration's Primary Behavioral Health Care Integration initiative and other local programs (35, 36, 38).
However, BHHs generally do not require use of a specific care coordination/management protocol or intervention, thus the strategies currently employed in BHH models vary considerably across sites; they also typically do not emphasize evidenced-based behavioral counseling for CVD risk behaviors (35–44). These features likely contribute to the mixed results to-date for BHHs. The two US RCTs evaluating BHHs found the programs improved quality of primary care delivery; while one showed small reductions in cardiovascular risk among a subset of participants, the second showed no effects on CVD health outcomes (37, 48). These findings suggest that care coordination alone, while potentially capable of improving processes of care, may not be enough to achieve improved clinical outcomes. In Denmark, the CHANGE trial tested care coordination vs. care coordination plus lifestyle coaching vs. control and found no change in CVD risk or individual risk factors over 2 years (97). These null results may be due to the high quality of the health system in Denmark such that many participants at baseline had controlled risk factors; also, the lifestyle coaching may not have been as intense as needed to affect additional CVD risk reduction.
The IDEAL intervention may be well suited for integration into and could improve the effectiveness of models like the BHH. When embedded into a BHH-like structure with its financing, the intervention with its standards of procedure for all aspects of care coordination and tailored, evidenced-based behavioral counseling, could make considerable progress in improving the CVD health for a population that is not routinely receiving quality CV care.
Unless effective interventions that effect CV health outcomes are implemented, populations with SMI will continue to lag far behind the nation's CVD goals. Interventions and models incorporating CVD risk factor treatment in mental health settings to-date have not shown meaningful changes in CV risk factors. The IDEAL intervention incorporates care coordination and care management concepts with intensive health behavior change coaching to address all CVD risks in persons with SMI in community mental health outpatient program settings. If successful, the intervention could be adopted within current integrated care models and significantly improve the physical health of persons with SMI.
This study was carried out in accordance with the recommendations of the Johns Hopkins Medicine IRB with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by The Johns Hopkins Medicine IRB.
AD, GD, and GJ wrote the manuscript. GD, LA, GJ, FD, N-YW, DY, JC, RC, AD, SG, JG, CC, EE, and AH contributed to the conception, design and implementation of the study. EM wrote a section of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
This study has been supported by National Heart Lung and Blood Institute (grant number R01112299) and National Institute of Mental Health (grant number P50115842).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. National Center for Health Statistics. Health, United States 2009: With Special Features on Medical Technology. Hyattsville, MD: National Center for Health Statistics (2010).
2. Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry (1997) 171:502–8. doi: 10.1192/bjp.171.6.502
3. Daumit GL, Anthony CB, Ford DE, Fahey M, Skinner EA, Lehman AF, et al. Pattern of mortality in a sample of Maryland residents with severe mental illness. Psychiatry Res. (2010) 176:242–5. doi: 10.1016/j.psychres.2009.01.006
4. Osby U, Correia N, Brandt L, Ekbom A, Sparen P. Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr Res. (2000) 45:21–8. doi: 10.1016/S0920-9964(99)00191-7
5. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry (2007) 64:1123–31. doi: 10.1001/archpsyc.64.10.1123
6. Allison DB, Newcomer JW, Dunn AL, Blumenthal JA, Fabricatore AN, Daumit GL. Obesity among those with mental disorders: a National Institute of Mental Health Meeting Report. Am J Prev Med. (2009) 36:341–50. doi: 10.1016/j.amepre.2008.11.020
7. Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. (2005) 80:45–53. doi: 10.1016/j.schres.2005.08.010
8. Nasrallah HA, Meyer JM, Goff DC, McEvoy JP, Davis SM, Stroup TS, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: Data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. (2006) 86:15–22. doi: 10.1016/j.schres.2006.06.026
9. Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J., et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. (2008) 10:1691–715. doi: 10.1080/14622200802443569
10. NIMH. Prevalence of Serious Mental Illness Among U.S. Adults by Age, Sex, and Race. (2008). Available online at https://www.nimh.nih.gov/health/statistics/shtml (Accessed February 27, 2011).
11. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation (2010) 121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703
12. Velligan DI, Bow-Thomas CC, Huntzinger C, Ritch J, Ledbetter N, Prihoda TJ, et al. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry (2000) 157:1317–23. doi: 10.1176/appi.ajp.157.8.1317
13. Mueser KT, McGurk SR. Schizophrenia. Lancet (2004) 363:2063–72. doi: 10.1016/S0140-6736(04)16458-1
14. Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA (1990) 264:2511–8. doi: 10.1001/jama.1990.03450190043026
15. Perkins R, Rinaldi M. Unemployment rates among patients with long-term mental health problems: A decade of rising unemployment. Psychiatric Bull. (2002) 26:295–8. doi: 10.1192/pb.26.8.295
16. Olfson M, Mechanic D, Hansell S, Boyer CA, Walkup J. Prediction of homelessness within three months of discharge among inpatients with schizophrenia. Psychiatric Serv. (1999) 50:667–73. doi: 10.1176/ps.50.5.667
17. Frank RG, Glied SA. Better But Not Well: Mental Health Policy in the United States Since 1950. Baltimore: Johns Hopkins University Press (2006).
18. Daumit GL, Dickerson FB, Wang NY, Dalcin A, Jerome GJ, Anderson CA, et al. A behavioral weight loss intervention in persons with serious mental illness. N Engl J Med. (2013) 368:1594–602. doi: 10.1056/NEJMoa1214530
19. Evins AE, Cather C, Pratt SA, Pachas GN, Hoeppner SS, Goff DC, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA (2014) 311:145–54. doi: 10.1001/jama.2013.285113
20. Green CA, Yarborough BJ, Leo MC, Yarborough MT, Stumbo SP, Janoff SL, et al. The STRIDE weight loss and lifestyle intervention for individuals taking antipsychotic medications: a randomized trial. Am J Psychiatry (2015) 172:71–81. doi: 10.1176/appi.ajp.2014.14020173
21. McGinty EE, Baller J, Azrin ST, Juliano-Bult D, Daumit GL. Interventions to address medical conditions and health-risk behaviors among persons with serious mental illness: a comprehensive review. Schizophr Bull (2015) 42:96–124. doi: 10.1093/schbul/sbv101.
22. Renders C, Valk G, Griffin S, Wagner E, Thm van Eijk J, Assendelft W. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochr Datab Syst Rev. (2000) 2000:CD001481. doi: 10.1002/14651858.CD001481
23. Glynn L, Murphy A, Smith S, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochr Datab Syst Rev. (2010) 3:CD005182. doi: 10.1002/14651858.CD005182.pub4
24. Schedlbauer A, Davies P, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochr Datab Syst Rev. (2010) 2010:CD004371. doi: 10.1002/14651858.CD004371.pub3
25. Haskell WL, Berra K, Arias E, Christopherson D, Clark A, George J, et al. Multifactor cardiovascular disease risk reduction in medically underserved, high-risk patients. Am J Cardiol. (2006) 98:1472–9. doi: 10.1016/j.amjcard.2006.06.049
26. Haskell WL, Alderman EL, Fair JM, Maron DJ, Mackey SF, Superko HR, et al. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP). Circulation (1994) 89:975–90. doi: 10.1161/01.CIR.89.3.975
27. Becker DM, Yanek LR, Johnson WR Jr, Garrett D, Moy TF, Reynolds SS, et al. Impact of a community-based multiple risk factor intervention on cardiovascular risk in black families with a history of premature coronary disease. Circulation (2005) 111:1298–304. doi: 10.1161/01.CIR.0000157734.97351.B2
28. Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B., et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. (2010) 363:2611–20. doi: 10.1056/NEJMoa1003955
29. Parks J, Svendsen D, Singer P, Foti ME. Morbidity and Mortality in People With Serious Mental Illness. Alexandria: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council (2006).
30. Lawrence D, Kisely S. Inequalities in healthcare provision for people with severe mental illness. J Psychopharmacol. (2010) 24:61–8. doi: 10.1177/1359786810382058
31. Sajatovic M, Dawson NV. The emerging problem of diabetes in the seriously mentally ill. Psychiatr Danub. (2010) 22(Suppl 1):S4–5.
32. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA (2007) 298:1794–6. doi: 10.1001/jama.298.15.1794
33. Horvitz-Lennon M, Kilbourne AM, Pincus HA. From silos to bridges: meeting the general health care needs of adults with severe mental illnesses. Health Aff (2006) 25:659–69. doi: 10.1377/hlthaff.25.3.659
34. McGinty EE, Baller J, Azrin ST, Juliano-Bult D, Daumit GL. Quality of medical care for persons with serious mental illness: a comprehensive review. Schizophr Res. (2015) 165:227–35. doi: 10.1016/j.schres.2015.04.010
35. Centers for Medicare and Medicaid Services. Health Homes. (2017). Available online at: https://www.medicaid.gov/medicaid/ltss/health-homes/index.html, (Accessed May 14, 2017).
36. Scharf DM, Eberhart, NK, Hackbarth, NS, Horvitz-Lennon, M, Beckman, R, Han, B, et al. Substance Abuse Mental Health Administration (SAMHSA). SAMHSA Primary and Behavioral Health Care Integration Program. (2013). Available online at: http://www.integration.samhsa.gov/integrated-care-models.
37. Druss BG, von Esenwein SA, Glick GE, Deubler E, Lally C, Ward MC, et al. Randomized trial of an integrated behavioral health home: the health outcomes management and evaluation (HOME) study. Am J Psychiatry (2017) 174:246–55. doi: 10.1176/appi.ajp.2016.16050507
38. Scharf DM, Eberhart NK, Hackbarth NS, Horvitz-Lennon M, Beckman R, Han B, et al. Evaluation of the SAMHSA Primary and Behavioral Health Care Integration (PBHCI) Grant Program (2013). Available online at: http://www.integration.samhsa.gov/integrated-care-models/RAND_Evaluation_SAMHSA_PBHCI_GRANT_Program.pdf.
39. Missouri Department of Health. Missouri CMHC Healthcare Homes: Progress Report 2012–2015. (2016). Available online at: https://dmh.mo.gov/mentalillness/provider/docs/cmhchch2016report.pdf, (Accessed October 10, 2017).
40. McGinty EE, Kennedy-Hendricks A, Linden S, Choksy S, Stone E, Daumit GL. An innovative model to coordinate healthcare and social services for people with serious mental illness: a mixed-methods case study of Maryland's Medicaid health home program. Gen Hos Psychiatry (2018) 51:54–62. doi: 10.1016/j.genhosppsych.2017.12.003
41. Bao Y, Casalino LP, Pincus HA. Behavioral health and health care reform models: patient-centered medical home, health home, and accountable care organization. J Behav Health Serv Res. (2013) 40:121–32. doi: 10.1007/s11414-012-9306-y
42. Mechanic D. Seizing opportunities under the affordable care act for transforming the mental and behavioral health system. Health Affairs (2012) 31:376–82. doi: 10.1377/hlthaff.2011.0623
43. Druss BG, Goldman HH. Integrating health and mental health services: a past and future history. Am J Psychiatry (2018) 2018:appiajp201818020169. doi: 10.1176/appi.ajp.2018.18020169
44. Druss BG, Newcomer JW. Challenges and solutions to integrating mental and physical health care. J Clin Psychiatry (2007) 68:e09. doi: 10.4088/JCP.0407e09
45. Kilbourne AM, Barbaresso MM, Lai Z, Nord KM, Bramlet M, Goodrich DE., et al. Improving physical health in patients with chronic mental disorders: twelve-month results from a randomized controlled collaborative care trial. J Clin Psychiatry (2017) 78:129–37. doi: 10.4088/JCP.15m10301
46. Breslau J, Leckman-Westin E, Han B, Pritam R, Guarasi D, Horvitz-Lennon M, et al. Impact of a mental health based primary care program on emergency department visits and inpatient stays. Gen Hosp Psychiatry (2018) 52:8–13. doi: 10.1016/j.genhosppsych.2018.02.008
47. Breslau J, Leckman-Westin E, Yu H, Han B, Pritam R, Guarasi D, et al. Impact of a mental health based primary care program on quality of physical health care. Adm Policy Ment Health. (2018) 45:276–85. doi: 10.1007/s10488-017-0822-1
48. Druss BG, von Esenwein SA, Compton MT, Rask KJ, Zhao L, Parker RM. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry (2010) 167:151–9. doi: 10.1176/appi.ajp.2009.09050691
49. Gilmer TP, Henwood BF, Goode M, Sarkin AJ, Innes-Gomberg D. Implementation of integrated health homes and health outcomes for persons with serious mental illness in Los Angeles County. Psychiatr Serv. (2016) 67:1062–7. doi: 10.1176/appi.ps.201500092
50. Krupski A, West II, Scharf DM, Hopfenbeck J, Andrus G, Joesch JM, et al. Integrating primary care into community mental health centers: impact on utilization and costs of health care. Psychiatr Serv. (2016) 67:1233–9. doi: 10.1176/appi.ps.201500424
51. Tepper MC, Cohen AM, Progovac AM, Ault-Brutus A, Leff HS, Mullin B, et al. Mind the gap: developing an integrated behavioral health home to address health disparities in serious mental illness. Psychiatr Serv. (2017) 68:1217–24. doi: 10.1176/appi.ps.201700063
52. D'Agostino RB, Ramachandran SV, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation (2008) 17:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579
53. Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension (2006) 47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6
54. Bandura A, editor. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs (1986).
55. Watson DL, Tharp RG. Self-directed Behavior: Self-modification for Personal Adjustment. 5th ed. Monterey: Brooks/Cole Publishing Company (1989).
56. Swanson AJ, Pantalon MV, Cohen KR. Motivational interviewing and treatment adherence among psychiatric and dually diagnosed patients. J Nervous Mental Dis. (1999) 187:630–5. doi: 10.1097/00005053-199910000-00007
57. McCracken S, Corrigan P. Motivational interviewing for medication adherence in individuals with schizophrenia. In: Arkowitz H, Westra H, Miller W, Rollnick S, editors. Motivational Interviewing in the Treatment of Psychological Problems. New York, NY: Guilford Press (2007).
58. Bellack AS, Mueser KT, Gingerich S, Agresta J. Social Skills Training for Schizophrenia: A Step by Step Guide, 2nd ed. New York, NY: The Guilford Press (2004).
59. Corrigan PW, McCracken SG, Holmes EP. Motivational interviews as goal assessment for persons with psychiatric disability. Community Ment Health J. (2001) 37:113–22. doi: 10.1023/A:1002757631131
60. Knekt P, Laaksonen MA, Raitasalo R, Haaramo P, Lindfors O. Changes in lifestyle for psychiatric patients three years after the start of short- and long-term psychodynamic psychotherapy and solution-focused therapy. Eur Psychiatry (2010) 25:1–7. doi: 10.1016/j.eurpsy.2009.03.006
61. Gingerich WJ, Eisengart S. Solution-focused brief therapy: a review of the outcome research. Fam Process (2000) 39:477–98. doi: 10.1111/j.1545-5300.2000.39408.x
62. Anthony WA. Psychiatric rehabilitation: key issues and future policy. Health Aff. (1992) 11:164–71. doi: 10.1377/hlthaff.11.3.164
63. Mueser KT, Drake RE, Bond GR. Recent advances in psychiatric rehabilitation for patients with severe mental illness. Harv Rev Psychiatry (1997) 5:123–37. doi: 10.3109/10673229709000298
64. Heinssen RK. The cognitive exoskeleton: environmental interventions in cognitive rehabilitation. In: Corrigan PW, Yudofsky SC, editor. Coginitive Rehabilitation for Neuropsychiatric Disorders. Washington, DC: American Psychiatric Press (1996). p. 395–423.
65. Mueser KT, Corrigan PW, Hilton DW, Tanzman B, Schaub A, Gingerich S, et al. Illness management and recovery: a review of the research. Psychiatr Serv. (2002) 53:1272–84. doi: 10.1176/appi.ps.53.10.1272
66. Dickerson FB, Tenhula WN, Green-Paden LD. The token economy for schizophrenia: review of the literature and recommendations for future research. Schizophr Res. (2005) 75:405–16. doi: 10.1016/j.schres.2004.08.026
67. Glynn SM. Token economy approaches for psychiatric patients. Progress and pitfalls over 25 years. Behav Modif. (1990) 14:383–407. doi: 10.1177/01454455900144002
68. Lippman MR, Motta RW. Effects of positive and negative reinforcement on daily living skills in chronic psychiatric patients in community residences. J Clin Psychol. (1993) 49:654–62. doi: 10.1002/1097-4679(199309)49:5<654::AID-JCLP2270490507>3.0.CO;2-B
69. Menditto AA, Baldwin LJ, O'Neal LG, Beck NC. Social-learning procedures for increasing attention and improving basic skills in severely regressed institutionalized patients. J Behav Ther Exp Psychiatry (1991) 22:265–9. doi: 10.1016/0005-7916(91)90043-5
70. Paul GL, Menditto AA. Effectiveness of inpatient treatment programs for mentally ill adults in public psychiatric facilities. Appl Prevent Psychol. (1992) 1992:41–63. doi: 10.1016/S0962-1849(05)80133-7
71. Rimmerman A, Finn H, Schnee J, Klein I. Token reinforcement in the psychosocial rehabilitation of individuals with chronic mental illness: is it effective over time? Int J Rehabil Res. (1991) 14:123–30. doi: 10.1097/00004356-199106000-00004
72. Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Reid N. Effects of contingency management and bupropion on cigarette smoking in smokers with schizophrenia. Psychopharmacology (2011) 217:279–87. doi: 10.1007/s00213-011-2282-8
73. Lubman DI, King JA, Castle DJ. Treating comorbid substance use disorders in schizophrenia. Int Rev Psychiatry (2010) 22:191–201. doi: 10.3109/09540261003689958
74. Sigmon SC, Higgins ST. Voucher-based contingent reinforcement of marijuana abstinence among individuals with serious mental illness. J Subst Abuse Treat. (2006) 30:291–5. doi: 10.1016/j.jsat.2006.02.001
75. Anthony WA, Rogers ES, Cohen M, Davies RR. Relationships between psychiatric symptomatology, work skills, and future vocational performance. Psychiatr Serv. (1995) 46:353–8. doi: 10.1176/ps.46.4.353
76. Green MF, Nuechterlein KH. Should schizophrenia be treated as a neurocognitive disorder? Schizophr Bull. (1999) 25:309–19. doi: 10.1093/oxfordjournals.schbul.a033380
77. Bellack AS, Bennett ME, Gearson JS. Behavioral Treatment for Substance Abuse Treatment in Schizophrenia (BTSAS). Baltimore: University of Maryland School of Medicine (2001).
78. Bellack AS. Psychosocial Behavior. In: Tasman A, Kay J, Lieberman JA, editors. Psychiatry 2nd ed. London: Wiley (2003).
79. Care Management: Implications for Medical Practice, Health Policy, and Health Services Research. (2018). Available online at: at https://www.ahrq.gov/professionals/prevention-chronic-care/improve/coordination/caremanagement/index.html - overview (Accessed August 20, 2018).
80. Care Coordination. 2018. Available online at: https://www.ahrq.gov/professionals/prevention-chronic-care/improve/coordination/index.html. (Accessed August 20, 2018).
81. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation (2002) 106:3143–421. doi: 10.1161/circ.106.25.3143
82. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA (2003) 289:2560–72. doi: 10.1001/jama.289.19.2560
83. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care (2004) 27:596–58. doi: 10.2337/diacare.27.2.596
84. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care (2008) 53:1217–22.
85. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation (2014) 129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a
86. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA (2014) 311:507–20. doi: 10.1001/jama.2013.284427
87. Ebbert JO, Hughes JR, West RJ, Rennard SI, Russ C, McRae TD, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA (2015) 313:687–94. doi: 10.1001/jama.2015.280
88. Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. (2010) 36:71–93. doi: 10.1093/schbul/sbp116
89. Evins AE. Reassessing the safety of varenicline. Am J Psychiatry (2013) 170:1385–7. doi: 10.1176/appi.ajp.2013.13091257
90. Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet (2016) 387:2507–20. doi: 10.1016/S0140-6736(16)30272-0
91. American Diabetes Association. Standards of Medical Care in Diabetes - 2013. Diabetes Care (2013) 36(Suppl 1):S11–66. doi: 10.2337/dc13-S011
92. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. (1997) 12:38–48. doi: 10.4278/0890-1171-12.1.38
93. Evins AE, Cather C, Deckersbach T, Freudenreich O, Culhane MA, Olm-Shipman CM, et al. A double-blind placebo-controlled trial of bupropion sustained-release for smoking cessation in schizophrenia. J Clin Psychopharmacol. (2005) 25:218–25. doi: 10.1097/01.jcp.0000162802.54076.18
94. Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, et al. A 12-week double-blind, placebo-controlled study of bupropion sr added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J Clin Psychopharmacol. (2007) 27:380–6. doi: 10.1097/01.jcp.0b013e3180ca86fa
95. Cather C, Freidman-Yakoobian M, Gottlieb J, Park E, Goff D, Henderson D. A randomized controlled trial of motivational interviewing compared to psychoeducation for smoking pre-contemplators with severe mental illness. In: Society for Research on Nicotine and Tobacco 16th Annual Meeting Proceedings Baltimore, Society for Research on Nicotine and Tobacco (Baltimore, MD) (2010).
96. Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. (2011) 34:841–59. doi: 10.1016/j.psc.2011.08.006
97. Speyer H, Christian Brix Nørgaard H, Birk M, Karlsen M, Storch Jakobsen A, Pedersen K, et al. The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry (2016) 15:155–65. doi: 10.1002/wps.20318
Keywords: serious mental disorder, cardiovascular risk, intervention, care coordination, behavioral coaching
Citation: Dalcin AT, Jerome GJ, Appel LJ, Dickerson FB, Wang N-Y, Miller ER, Young DR, Charleston JB, Gennusa JV, Goldsholl S, Heller A, Evins AE, Cather C, McGinty EE, Crum RM and Daumit GL (2019) Need for Cardiovascular Risk Reduction in Persons With Serious Mental Illness: Design of a Comprehensive Intervention. Front. Psychiatry 9:786. doi: 10.3389/fpsyt.2018.00786
Received: 15 September 2018; Accepted: 31 December 2018;
Published: 08 February 2019.
Edited by:
Andrea Fiorillo, Università Degli Studi Della Campania Luigi Vanvitelli Naples, ItalyReviewed by:
Luca Steardo, Università Degli Studi Della Campania Luigi Vanvitelli Caserta, ItalyCopyright © 2019 Dalcin, Jerome, Appel, Dickerson, Wang, Miller, Young, Charleston, Gennusa, Goldsholl, Heller, Evins, Cather, McGinty, Crum and Daumit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gail L. Daumit, Z2RhdW1pdEBqaG1pLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.