Abstract
Introduction:
Aberrant motor function is an integral part of schizophrenia. In fact, abnormalities are frequently found in patients, in populations at risk, and in unaffected relatives. Motor abnormalities are suspected to be relevant for the clinical outcome and could probably predict the conversion from at-risk individuals to schizophrenia. Furthermore, motor function has been argued as endophenotype of the disorder. Yet, which particular motor domain may classify as a potential endophenotype is unknown. We aimed to compare schizophrenia patients, unaffected first-degree relatives and healthy controls for different motor domains. We expected impairments in all domains in patients and in some domains in relatives.
Method:
We included 43 schizophrenia patients, 34 unaffected first-degree relatives of schizophrenia patients, and 29 healthy control subjects, matched for age, gender, and education level. We compared motor function of four motor domains between the groups. The domains comprise neurological soft signs (NSS), abnormal involuntary movements (dyskinesia), Parkinsonism, and fine motor function including simple [finger tapping (FT)] and complex fine motor function, (i.e., dexterity as measured with the coin rotation test). Furthermore, we tested the association of motor function of the four domains with working memory, frontal lobe function, and nonverbal intelligence for each group separately using within-group bivariate correlations.
Results:
Schizophrenia patients showed poorer motor function in all tested domains compared to healthy controls. First-degree relatives had intermediate ratings with aberrant function in two motor domains. In detail, relatives had significantly more NSS and performed poorer in the FT task than controls. In contrast, complex fine motor function was intact in relatives. Relatives did not differ from controls in dyskinesia or Parkinsonism severity.
Discussion:
Taken together, schizophrenia patients have motor abnormalities in all tested domains. Thus, motor abnormalities are a key element of the disorder. Likewise, first-degree relatives presented motor deficits in two domains. A clear difference between relatives and healthy controls was found for NSS and FT. Thus, NSS and FT may be potential markers of vulnerability for schizophrenia. The lack of association between genetic risk and dyskinesia or Parkinsonism suggests distinct pathobiological mechanisms in the various motor abnormalities in schizophrenia.
Introduction
Motor abnormalities constitute an integral part of schizophrenia. Aberrant motor function was included as one of eight dimensions of psychopathology in schizophrenia spectrum disorders in the current version of the diagnostic and statistical manual (DSM-5) (2014). Aberrant motor function was frequently observed in both medicated and unmedicated patients (1, 2). In addition, aberrant motor function often wax and wane during follow-up (3, 4). At least one motor sign was present in about 66% of patients in first-episodes (5), in 59% of patients on admission (6), and 80% of chronic patients (1, 7). Alterations incorporate various domains of motor function. Domains include for instance neurological soft signs (NSS) (i.e., motor coordination, sequencing, and sensory integration) (8), dyskinesia (abnormal involuntary movements typically manifest as involuntary continuous orofacial movements and dyskinetic movements of the extremities or trunk) (9, 10), parkinsonism (bradykinesia, rigor, tremor) (10), catatonic symptoms (pure motor signs, disturbance of volition, inability to suppress motor reactions and vegetative instability) [for review, see e.g., Ref. (1, 11, 12)], deficits in fine motor function [i.e., the coin rotation task, the Moberg pick-up test (13, 14), finger sequencing and the pegboard test (15)], and psychomotor slowing (16). In sum, schizophrenia patients show generalized aberrant motor function.
Another important line of evidence stems from first-degree relatives of psychosis patients. Aberrant motor function in these subjects may help find markers who are associated with the genetic risk for schizophrenia. However, a comprehensive assessment of a wide range of domains of motor function in first-degree relatives is missing. Thus, it remains unknown, which particular motor domain may serve as a potential genetic risk marker.
In detail, previous reports suggest delayed motor development as a genetic risk marker. For instance, it was hypothesized that NSS arise due to neurodevelopmental delay in motor function in young subjects at risk and first-degree relatives. NSS are subtle neurological deficits (17, 18) which are seen in children and normally vanish during the motor development in late childhood. An association of NSS with the genetic risk for schizophrenia has consistently been reported (19), possibly even predictive for the transition to psychosis (20). Nearly, all studies investigating NSS in unaffected first-degree relatives detected higher prevalence of NSS in relatives compared to healthy controls. This has been substantiated by recent meta-analyses (8, 21).
Whether dyskinesia and Parkinsonism are associated with the genetic risk to develop schizophrenia is less clear than for NSS (22, 23). Even though, most reports show increased incidence of dyskinesia and Parkinsonism in schizophrenia relatives, which has been confirmed in a recent meta-analysis (24). Yet, for instance, Tarbox and Pogue-Geile (25) detected no increased prevalence of dyskinesia in relatives. As for dyskinesia, from the available data, it is difficult to state whether Parkinsonism may serve as a genetic risk marker for psychosis. Some reports showed increased incidence in unaffected relatives (24, 26) with some inconsistency (27).
One further important domain of motor function is fine motor function (i.e., manual skills and dexterity) (28). Schizophrenia patients suffer from defective complex fine motor function, as measured by the Line Copying Task or the coin rotation test (14) as well as simple fine motor function as measured by the finger tapping (FT) task [e.g., Ref. (14, 29, 30)]. Fine motor impairments are already present at the beginning of the disorder in the first episode. These deficits seem to be relatively stable over time (31). Moreover, defective fine motor function may impact activities of daily living and may be associated with poor functional outcome as has been shown in other conditions (i.e., Parkinson’s disease) (32, 33) In general, a loss of complex fine motor skills may not solely be explained by elemental motor deficits such as motor slowing (bradykinesia) but might also reflect disturbances of more complex motor function such as selectively control and coordination of upper limb and specifically finger movements (34, 35). Whether fine motor function is linked to the genetic risk for psychosis is unclear. One single report detected aberrant simple fine motor function (measured with FT) in unaffected first-degree relatives of psychotic patients (36). Likewise another study found reduced complex fine motor function (i.e., tested with inserting long and short pins into holes in a platform) in subjects at risk (37). However, complex fine motor function of the fingers (i.e., finger dexterity measured with the coin rotation task) has not been tested.
In sum, there is considerable and consistent evidence for the association of NSS and the risk to develop schizophrenia. A moderate number of studies suggest an association of dyskinesia and Parkinsonism with schizophrenia risk with some inconsistency. In addition, only few studies addressed the association of complex fine motor function and psychosis risk. Moreover, former investigations focused only on one motor domain. Thus, which domain of motor function is associated with a genetic psychosis risk and how they are interlinked is still unknown.
Therefore, the aim of our study was to compare for the first time a comprehensive motor battery, including motor function in four domains (NSS, dyskinesia, Parkinsonism and fine motor function) in three well-characterized groups (patients, unaffected first-degree relatives and healthy controls). In line with the literature, we expected generalized aberrant motor function in schizophrenia patients. Further, we expected aberrant motor function in some but not all motor domains in unaffected first-degree relatives. In detail, we hypothesized increased incidence of NSS and aberrant fine motor function in unaffected relatives, but no increased incidence of dyskinesia and Parkinsonism.
Materials and Methods
Subjects
This study included 41 patients with schizophrenia, 34 first-degree relatives (parents, siblings, or children) and 29 healthy controls matched for gender, age, and education. All participants were right-handed as determined by the Edinburgh handedness inventory (38) and were native German speakers. This study was carried out in accordance with the recommendations of the local Ethics Committee (Kantonale Ethikkommission: KEK Bern) with written informed consent from all subjects.
Patients were in- and outpatients recruited at the University Hospital of Psychiatry, Bern and diagnosed according to DSM-5. Relatives were contacted via patients with schizophrenia and the Association of Schizophrenia Patients’ Relatives, Bern. Inclusion criterion for relatives was a history or presence of any physician-diagnosed schizophrenia spectrum disorder in at least one first-degree relative. Controls were volunteers recruited from the hospital staff and the community without first-degree relatives with schizophrenia spectrum disorders.
General exclusion criteria for all participants were substance abuse or dependence other than nicotine and past or current medical or neurological condition associated with impaired movement (i.e., dystonia, idiopathic Parkinsonism, or stroke).
All Participants were interviewed by trained psychiatrists using the Mini-International Neuropsychiatric Interview (39) (adapted for DSM-5). In addition, we assessed frontal lobe function using the Frontal Assessment Battery (FAB) (40), verbal working memory with the digit span backwards (DSB) task [subtest from the Wechsler Memory Scale (WMS-III)] (41) as well as nonverbal intelligence using the short Test of nonverbal Intelligence (TONI) (42).
Motor Behavior
We assessed four different domains of motor function using a comprehensive battery including the neurological evaluation scale (NES) (18) to assess NSS, the Abnormal Involuntary Movement Scale (AIMS) (43) for dyskinesia, and the Unified Parkinson’s Disease Rating Scale (UPDRS) (44) for Parkinsonism. In addition, participants performed a complex and a simple fine-motor tasks. During the complex fine-motor task, the coin rotation task (CR), a coin (Swiss 50-Rappen coin, corresponding exactly in size to a US-Nickel) is rotated between thumb and fingers one and two (index and middle finger) in 10 s as fast as possible. A correction for drops is used [the adjusted score is the number of rotations in 10 s minus (0.1 × rotations × drops)] (34, 45, 46). After a short training period, each participant performed three trials of 10 s with each hand. Performance was videotaped and analyzed in slow motion by a blinded rater. The last half-turn was included when at least half of the movement was completed. Finally, participants performed a simple fine motor task, the FT task. They were instructed to tap their index finger and thumb with the maximum amplitude as fast as possible. This task was also performed for three times during 10 s with each hand. Again, performance was video recorded and analyzed by a blinded rater.
Statistical Analysis
Statistical tests were performed using SPSS 24.0 (SPSS Inc., Chicago, IL, USA). Univariate analysis of variance, chi-square tests (χ2), and general linear models were used to test the continuous and categorical clinical variables between groups (relatives, patients and controls). Our main interest was to assess whether patients and relatives had more severe motor abnormalities than controls. Therefore, we first compared ratings of motor scales between groups using univariate analyses of variance. Post hoc tests included Sidak correction for multiple comparisons. Next, we calculated two separate repeated measures ANOVAs of performance in the two fine motor tasks with side (left and right hand) as within-subject factor. To test the association of aberrant motor function as well as working memory and frontal lobe function, we correlated scores of each scale and task for each group (patients, relatives and controls) separately, applying within-group bivariate correlations. Significance level was set at p < 0.05 two-tailed.
Results
Clinical and demographic data are given in Table 1. Groups did not differ in age, gender, and education. However, groups differed regarding nonverbal intelligence and working memory, for which patients had inferior performance compared to relatives or controls. In addition, patients had poorer frontal lobe function at trend level (p = 0.081) compared to healthy controls (see Table 2).
Table 1
| Controls (C); N 29 | Relatives (R); N 34 | Patients (P); N 43 | df | F/χ2 | p-Value | |
|---|---|---|---|---|---|---|
| Age | 40.86 ± 14.38 | 42.74 ± 15.73 | 37.98 ± 11.37 | 2 | 1.18 | 0.312 |
| Gender | 55.2% (f) | 64.7% (f) | 62.8% (f) | 2 | 0.67 | 0.768 |
| EDU | 13.79 ± 2.32 | 14.18 ± 3.02 | 13.52 ± 3.12 | 2 | 0.49 | 0.617 |
| DOI (years) | – | – | 12.92 ± 12.48 | |||
| PANSS tot | – | – | 71.14 ± 16 | |||
| PANSS pos | – | – | 17.98 ± 6.28 | |||
| PANSS neg | – | – | 18.14 ± 5.15 |
Demographic data and clinical status of the groups.
N, number; f, female; EDU, education (years); DOI, duration of illness; PANSS, Positive and Negative Syndrome Scale; tot, total; pos, positive; neg, negative.
Table 2
| Controls (C); N 29 | Relatives (R); N 34 | Patients (P); N 43 | df | F/χ2 | p-Value | Post hoc Sidak corrected | |
|---|---|---|---|---|---|---|---|
| TONI | 109.24 ± 10.50 | 108.15 ± 8.43 | 97.68 ± 11.40 | 2 | 14.28 | <0.001 | P < C (p < 0.001) P < R (p < 0.001) |
| FAB | 17.17 ± 0.97 | 16.85 ± 1.54 | 16.10 ± 2.72 | 2 | 2.78 | 0.067 | P < C (p < 0.081) |
| DSB | 5.31 ± 0.71 | 5.26 ± 0.79 | 4.42 ± 1.05 | 2 | 9.45 | <0.001 | P < C (p = 0.001) P < R (p = 0.001) |
Nonverbal intelligence, frontal lobe and working memory function of the groups.
N, number; TONI, test of nonverbal intelligence (index scores); FAB, frontal assessment battery; DSB, digit span backwards.
Note: significant group differences are highlighted in bold.
Motor Function
Groups differed in incidence of aberrant motor function in the applied motor scales (see Table 3 and Figure 1). In detail, patients and relatives had higher NSS ratings than healthy controls, indicating more pathological signs. Next, we explored group differences in NSS subdomains (NES subscales). Again, groups differed in all measured motor functions (sensory motor function, motor coordination, motor sequencing, and others). In detail, performance of patients was worse in all subscales compared to healthy controls. Relatives demonstrated inferior performance in “motor sequencing” and “others,” as well as a trend of inferiority in “sensory integration” (p = 0.067) compared to healthy controls. For dyskinesia and Parkinsonism differences became evident between patients and healthy controls, but not between relatives and controls.
Table 3
| Controls (C); N 29 | Relatives (R); N 34 | Patients (P); N 43 | F | p-Value | Post hoc Sidak corrected | |
|---|---|---|---|---|---|---|
| NES total | 3.90 ± 3.59 | 11.65 ± 8.18 | 13.74 ± 11.75 | 10.83 | <0.001 | R > C (p = 0.003) P > C (p < 0.001) |
| NES Sensory integration | 1.14 ± 1.19 | 2.94 ± 2.15 | 3.58 ± 5.12 | 4.23 | 0.017 | P > C (p = 0.015) |
| NES Motor coordination | 0.55 ± 0.78 | 1.79 ± 1.82 | 2.60 ± 2.84 | 8.09 | 0.001 | P > C (p < 0.001) |
| NES Sequencing | 1.03 ± 1.96 | 3.21 ± 2.72 | 2.60 ± 2.96 | 6.39 | 0.002 | R > C (p = 0.005) P > C (p = 0.008) |
| NES Others | 1.14 ± 1.43 | 3.71 ± 3.44 | 4.88 ± 4.73 | 9.12 | <0.001 | R > C (p = 0.020) P > C (p < 0.001) |
| AIMS total | 0.14 ± 0.52 | 1.15 ± 2.36 | 1.91 ± 2.7 | 5.62 | 0.005 | P > C (p = 0.003) |
| AIMS Facial expression | 0.04 ± 0.19 | 0.27 ± 0.75 | 0.26 ± 0.74 | 1.3 | 0.277 | |
| AIMS Lips and perioral area | 0.10 ± 0.41 | 0.32 ± 0.73 | 0.23 ± 0.43 | 1.27 | 0.285 | |
| AIMS Jaw | 0.0 ± 0.0 | 0.09 ± 0.38 | 0.11 ± 0.40 | 1.03 | 0.363 | |
| AIMS Tongue | 0.0 ± 0.0 | 0.15 ± 0.58 | 0. 17 ± 0.51 | 1.31 | 0.276 | |
| AIMS Upper extremity | 0.0 ± 0.0 | 0.15 ± 0.50 | 0.37 ± 0.77 | 3.77 | 0.027 | P > C (p = 0.024) |
| AIMS Lower extremity | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.31 ± 0.68 | 6.79 | 0.002 | P > C (p = 0.008) P > R (p = 0.005) |
| AIMS Neck/shoulders/hips | 0.0 ± 0.0 | 0.12 ± 0.48 | 0.143 ± 0.43 | 1.23 | 0.296 | |
| UPDRS III | 0.10 ± 0.56 | 2.50 ± 3.93 | 7.21 ± 7.46 | 17.19 | <0.001 | P > R (p = 0.001) P > C (p < 0.001) |
Group differences of neurological soft sign, dyskinesia, and Parkinsonism in patients, relatives, and controls.
AIMS, Abnormal Involuntary Movement Scale; NES, Neurological Evaluation Scale; UPDRS III, Unified Parkinson’s Disease Rating Sale motor part III.
Note: significant group differences are highlighted in bold.
Figure 1
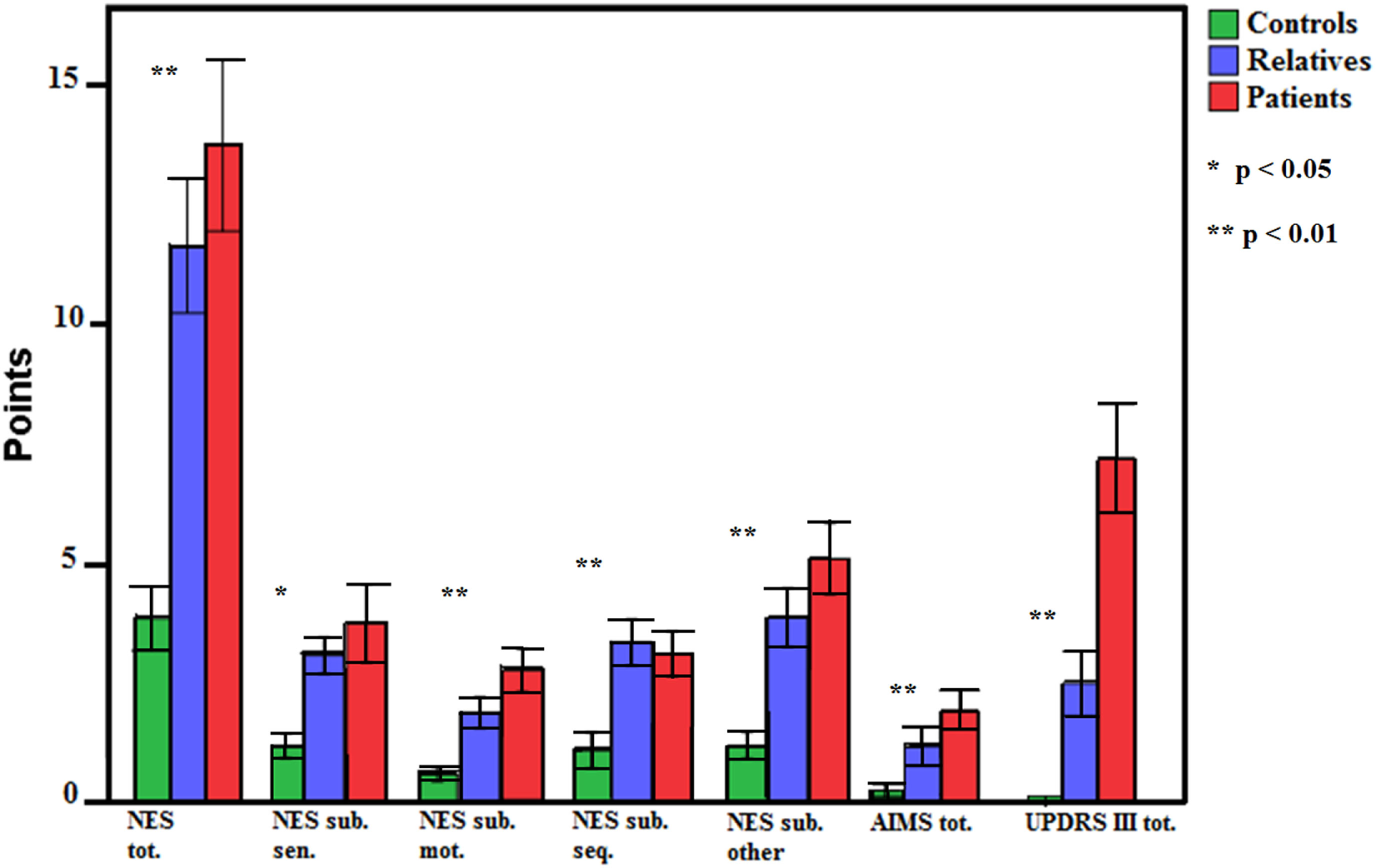
Group differences of neurological soft sign, dyskinesia, and Parkinsonism in patients, relatives, and controls. Error bars indicate SE. NES, Neurological Evaluation Scale; sub., subscale; sen., sensory motor function; mot., motor coordination; seq., motor sequencing; AIMS, Abnormal Involuntary Movement Scale; UPDRS III, Unified Parkinson’s Disease Rating Sale motor part III.
In addition, we tested complex (CR) and simple fine motor function (FT) (Table 4; Figure 2) exploring effects of side (left or right hand), group and group*side interactions. For CR, we found an effect of side in favor of the right hand, an effect of group but no group*side interaction. Post hoc Sidak tests indicated CR was impaired in patients compared to controls. Relatives did not differ from controls. Likewise, in FT, we detected an effect of side in favor of the right hand, an effect of group but no group*side interaction. Post hoc Sidak tests indicated that patients and relatives performed inferior to controls.
Table 4
| Controls (C); N 29 | Relatives (R); N 34 | Patients (P); N 43 | |
|---|---|---|---|
| CR right (±SE) | 14.61 ± 2.97 | 14.47 ± 2.87 | 11.96 ± 3.67 |
| CR left (±SE) | 12.82 ± 3.09 | 12.49 ± 2.87 | 10.31 ± 4.05 |
| FT right (±SE) | 39.95 ± 8.40 | 33.77 ± 7.55 | 35.07 ± 9.49 |
| FT left (±SE) | 37.44 ± 9.15 | 32.10 ± 6.75 | 32.82 ± 9.29 |
Performance of fine motor function in patients, relatives and controls.
CR, coin rotation; FT, finger tapping.
Figure 2
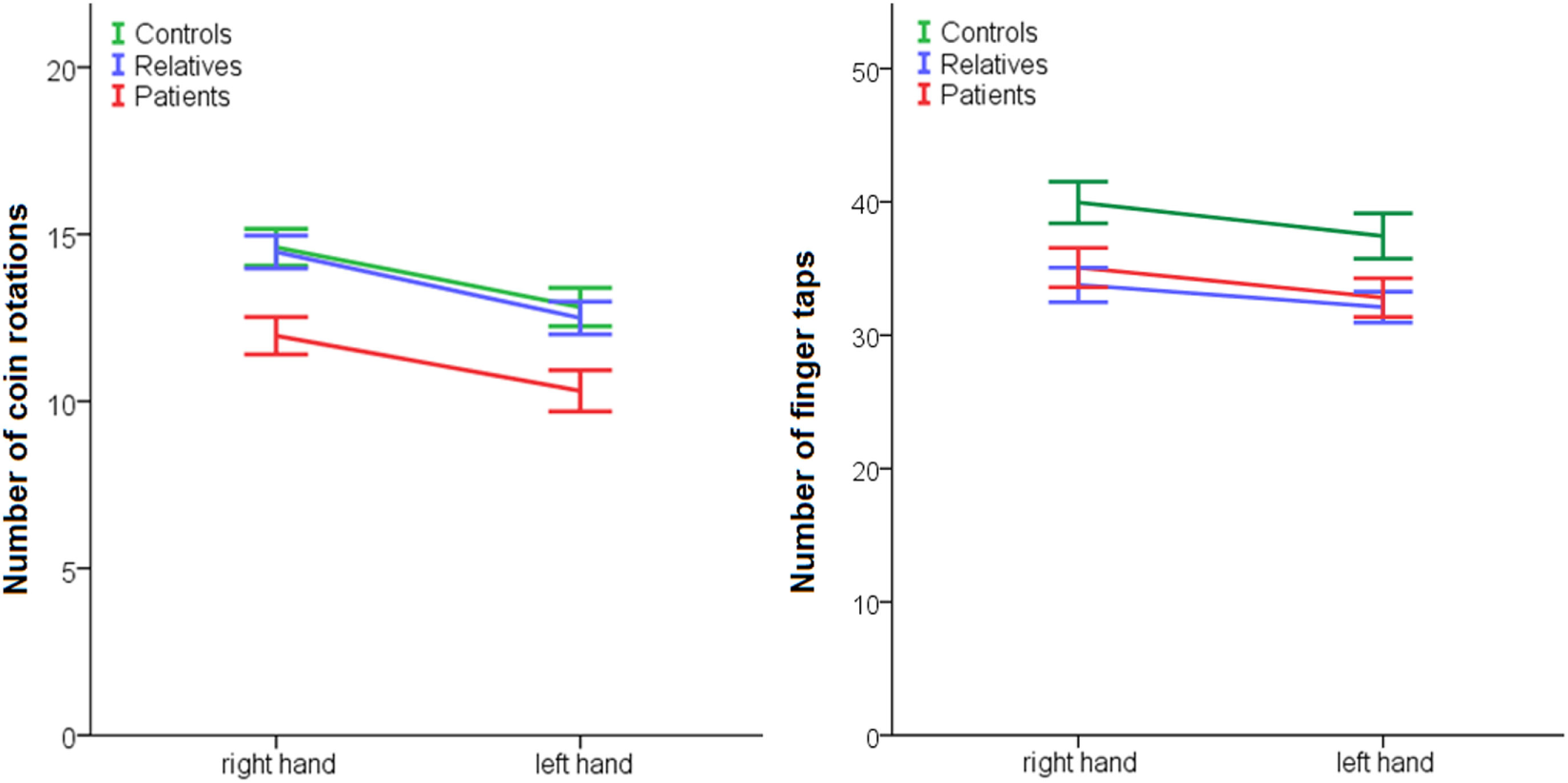
Performance of fine motor function in patients, relatives, and controls. Coin rotation: effect of group p = 0.001, effect of side p < 0.001, no effect side × group: p = 0.822; post hoc: patients < controls: p = 0.003; and relatives < controls p = 0.005. Finger tapping: effect of group: p = 0.018, effect of side: p < 0.001, no effect side × group: p = 0.646; post hoc: patients < controls: p = 0.061 and relatives < controls p = 0.022.
Association of Motor Function With Frontal Lobe Function and Working Memory
The association of motor domains including fine motor function with frontal lobe function and working memory is given in Table 5. Table 4 shows that ratings of some motor domains correlated with performance in motor tests. In fact, fine motor tests (CR and FT) are significantly associated with Parkinsonism in patients and unaffected relatives. Likewise, CR is significantly associated with NSS in patients. In contrast, dyskinesia (AIMS) seems to be independent of fine motor function (CR and FT). In addition, CR is unrelated to frontal lobe function in all groups and to working memory in relatives and controls. Furthermore, frontal lobe function is associated with NSS and Parkinsonism in all groups. Likewise, WM was significantly correlated with motor function in patients. This association is not seen in any other group.
Table 5
| AIMS | UPDRS III | CR | FT | FAB | WM | |
|---|---|---|---|---|---|---|
| Patients | ||||||
| NES | 0.357* | 0.760** | -0.541** | −0.300 | −0.628** | −0.479** |
| AIMS | 0.303* | −0.266 | −0.046 | −0.122 | −0.101 | |
| UPDRS III | −0.479** | −0.412** | −0.522** | −0.264 | ||
| CR | 0.195 | 0.349* | ||||
| FT | 0.077 | 0.116 | ||||
| FAB | 0.492** | |||||
| WM | ||||||
| Relatives | ||||||
| NES | 0.283 | 0.356* | −0.403* | −0.438** | −0.399* | −0.299 |
| AIMS | −0.060 | −0.318 | 0.073 | 0.031 | −0.378* | |
| UPDRS III | −0.341* | −0.496** | −0.419* | −0.230 | ||
| CR | 0.144 | 0.072 | ||||
| FT | 0.464** | 0.017 | ||||
| FAB | 0.307 | |||||
| WM | ||||||
| Controls | ||||||
| NES | 0.471** | 0.488** | −0.337 | −0.271 | −0.613** | −0.197 |
| AIMS | 0.694** | −0.041 | −0.169 | −0.623** | −0.121 | |
| UPDRS III | −0.104 | −0.007 | −0.432* | −0.084 | ||
| CR | 0.335 | −0.155 | ||||
| FT | 0.312 | −0.027 | ||||
| FAB | 0.127 | |||||
| WM | ||||||
Association of motor function with frontal lobe function and working memory function in patients, relatives and controls.
NES, Neurological Evaluation Scale; AIMS, Abnormal Involuntary Movement Scale; UPDRS III, Unified Parkinson’s Disease Rating Scale motor part III; CR, coin rotation; FT, finger tapping; FAB, frontal assessment battery; DSB, digit span backwards.
*p < 0.05; **p < 0.01.
Note: significant associations are highlighted in bold.
Discussion
Increasing evidence has established the clinical relevance of aberrant motor function in schizophrenia (1, 47–49). Here, we investigated a comprehensive motor battery with four domains of motor function in three well-characterized groups (patients, unaffected first-degree relatives and healthy controls). The study aimed to explore, which motor domains were particularly associated with the genetic risk for psychosis. We were able to confirm our main hypotheses, and demonstrated (1) generalized aberrant motor function in schizophrenia patients, and (2) aberrant motor function in some but not all motor domains in schizophrenia relatives. The distinct pattern of associations between genetic psychosis risk and motor domains argues for different pathophysiological mechanisms behind each motor domain.
As hypothesized, aberrant motor function was shown for NSS in schizophrenia relatives compared to controls with intermediate severity. Moreover, NSS are thought to indicate aberrant motor development that is even evident in adults. Aberrant motor development has been associated with a genetic psychosis risk (9). Thus, NSS have been discussed as endophenotype markers in psychosis (50). In contrast, we were not able to confirm our hypothesis of an association between genetic psychosis risk and defective coin rotation, which is a reliable and simple measure of complex fine motor function. In fact, in unaffected first-degree relatives fine motor function was preserved. However, in schizophrenia we detected aberrant complex fine motor function with the coin rotation task. In addition, patients and relatives performed worse than healthy controls in the simple fine motor task. Finally, relatives had similar severity of Parkinsonism and dyskinesia compared to healthy controls, which is different from the findings regarding NSS. Thus, our results confirm the specific importance of NSS for the genetic psychosis risk. Therefore, NSS may in the future be particularly useful to monitor vulnerable subjects.
Generalized Aberrant Motor Function in Schizophrenia
Patients had an increased severity of NSS, dyskinesia and Parkinsonism. In addition, we detected aberrant fine motor function (FT and CR) in patients. Thus, in line with the literature we found generalized aberrant motor function in schizophrenia patients and extended the knowledge by demonstrating further alterations in complex fine motor function, which has been understudied. Complex fine motor function (i.e., manual dexterity as measured with the CR task) adopts an intermediate position between higher-order (i.e., apraxic) and elemental motor function. Aberrant complex fine motor function is characterized by impaired control of selective and coordinated hand and finger movements, not explained by weakness or sensory deficits (51). Aberrant function in this domain may impact performance of skilled movements in schizophrenia, such as hand gestures (14, 52–55). Similar deficits have previously been shown in Parkinson’s disease (35). Taken together, exploring the pathophysiology underlying complex fine motor function, incorporating instrumental approaches, may add to the innovative research in the field of motor function in schizophrenia.
Aberrant Motor Function in Specific Motor Domains in Unaffected First-Degree Relatives
Unaffected first-degree relatives of schizophrenia patients showed aberrant motor function in some but not all investigated motor domains. In line with the literature, we found more NSS in relatives compared to healthy controls. Nearly all studies (56–60) and recent meta-analyses (8, 21) confirmed an increased severity of NSS in relatives of schizophrenia patients. Thus, NSS are very likely to be associated with the genetic risk for psychosis. However, some reports were negative, probably owing to inclusion of a considerable proportion of second-degree relatives (61) or the lack of specific NSS rating scales (62). Besides the total NSS severity, we detected differences between relatives and controls in two of the NES subscales, i.e., “sequencing of motor acts” (i.e., fist-ring test, the fist-edge-palm test, the Ozeretski test) and “others” (i.e., adventitious overflow, Romberg test, tremor, memory, mirror movements). Interestingly, a previous study reported increased heritability in the same subscales, with a correlation between NSS severity of patients and NSS severity of their siblings (56).
In contrast, relatives had no increased severity of dyskinesia and Parkinsonism in our sample. In line with this, some previous reports failed to detect increased dyskinesia in relatives (24, 25). Likewise, one study reported a relatively low incidence of parkinsonism (incidence 3% of the sample in 181 subjects) in unaffected relatives of schizophrenia patients (27). Yet, no control group was included in this latter study. In contrast, other reports (63) demonstrate an increased severity of dyskinesia (63) and Parkinsonism (26, 63) in schizophrenia relatives compared to healthy controls. Thus, whether dyskinesia or Parkinsonism is truly associated with the genetic risk for psychosis requires further clarification in larger samples. Alternatively, dyskinesia and Parkinsonism may exclusively occur in subjects at increased risk for psychosis, who have prodromal signs beyond a genetic risk (23, 64, 65).
Finally, while patients showed impaired complex fine motor function, in relatives, complex fine motor function (CR) was intact. No previous report investigated complex fine motor function (CR) in unaffected first-degree relatives. Currently, our findings argue for an association of impaired complex fine motor function with psychosis but not with genetic psychosis risk. In contrast to complex fine motor function and in line with one previous report, simple fine motor function was impaired in both patients and relatives and may, therefore, be associated with psychosis and the genetic risk (36).
To conclude, we demonstrated increased incidence of NSS and impaired simple fine motor function in schizophrenia relatives arguing for a particular relevance as a genetic risk marker. In contrast, dyskinesia, Parkinsonism and complex fine motor function were not impaired and are, therefore, unlikely to become relevant indicators of the genetic risk for psychosis.
Aberrant Motor Function of Different Domains Linked to Distinct Alterations in the Motor System
In general, motor abnormalities in schizophrenia have been consistently associated with alterations in the cerebral motor system (48, 66–68). In addition, psychosis was associated with altered functional and structural connectivity in the motor system (68–77). Likewise, during motor tasks functional alterations in the motor system were detected in schizophrenia (78). Importantly, while there is some overlap, aberrant motor behavior of distinct motor domains is associated with specific structural and functional alterations (72, 79), i.e., not one mechanism associated with all domains of aberrant motor behavior in psychosis.
A large body of evidence associates NSS in schizophrenia with structural and functional alterations within cortical premotor and motor areas, the cerebellum and thalamus (48). In detail, NSS are linked to gray matter (GM) abnormalities in cortical areas comprising the pre- and postcentral gyri, premotor areas, inferior and middle frontal gyri (80, 81). Likewise, studies using cortical thickness or sulcation found NSS to be associated with aberrant morphology of frontal, temporal, and parietal areas (82, 83). Subcortical structures related to NSS in schizophrenia included the thalamus, caudate nucleus, putamen, globus pallidus (84, 85) as well as the cerebellum (80, 86, 87). Importantly, even medication-naive first-episode patients show an association of aberrant motor sequencing and GM alterations of cortical and subcortical structures (88). Interestingly, a longitudinal study further confirmed that persisting compared to decreasing NSS in first-episode schizophrenia is related to a progressive decrease of GM volume (89). White matter density has shown to be related to NSS in the inferior frontal gyrus, cerebellum, and corpus callosum in schizophrenia (80, 81). Furthermore, abnormal white matter cerebellar–thalamic tract development predicted NSS after 12 months in ultra-high risk individuals (90). Besides these structural abnormalities, some studies explored functional neural underpinnings of NSS (91). In a paradigm that compared the fist-edge-palm task with a simple motor task, patients with schizophrenia failed to activate the left middle frontal gyrus which was the case in healthy controls (92). In addition, with increasing task difficulty, functional connectivity changed between the sensorimotor cortex and the right frontal gyrus in patients compared to healthy controls (92). Thus, altered brain morphology and function may account for NSS in patients.
Brain imaging studies in schizophrenia and Parkinson’s disease reported associations of dyskinesia and Parkinsonism with basal ganglia dysfunction. For instance, patients with dyskinesia had reduced GM volume in the caudate nucleus, putamen, globus pallidus, and the thalamus (93). Likewise, altered volume of the striatum was shown in medication naïve chronic schizophrenia patients with dyskinesia (94) and volume alterations in the putamen were evident in subjects at risk with dyskinesia (95). Furthermore, white matter alterations within cortico-basal ganglia circuits correlated with involuntary movements in schizophrenia (96). Accordingly, early functional imaging studies reported that activity in the basal ganglia (higher relative metabolic rates in the putamen) was associated with dyskinesia severity in schizophrenia (97). In addition, D2/D3 receptor availability in the striatum was linked to Parkinsonism in schizophrenia (98). In addition, there is some conceptual overlap between Parkinsonism and reduced spontaneous motor activity, which is also paralleled by alterations of the cerebral motor system (72, 76, 77). The relatives in the current study had no increased severity of Parkinsonism. We may speculate that alterations in the suspected underlying brain areas are lacking in relatives. In contrast, basal ganglia shape abnormalities were also reported in unaffected siblings of schizophrenia patients (99). However, a recent study detected no alterations of caudate and putamen laterality indices in schizophrenia siblings, who did not significantly differ from controls (100) and abnormal striatal and ventricle volumes showed no sign of heritability (101). Thus, whether alterations in the basal ganglia are critical for dyskinesia and Parkinsonism in relatives of schizophrenia patients remains to be explored.
Finally, patients had significantly impaired complex fine motor function. Until now, no study investigated neural correlates of impaired coin rotation (complex fine motor function) in schizophrenia. In general, for complex fine motor function, temporal, and spatial information of the movement has to be retrieved and translated into motor output. According to modern models (102) brain areas of the so-called praxis network (103) are involved. Inferior parietal lobule (IPL) and intact connections between IPL and left premotor cortex and supplementary motor area are expected to critically contribute to complex fine motor function (51, 104–107). In line with these proposed models we recently detected differentially altered activation and altered functional coupling in key areas of the left praxis network to be linked to altered complex fine motor function (CR) in Parkinson’s disease (35). Likewise we detected associations of functional and structural alterations in the praxis network with gesture performance in schizophrenia patients (108–110). However, future studies are needed to address the neural correlates of impaired complex fine motor function in schizophrenia.
Association of Motor Function With Frontal Lobe Function and Working Memory
Critical contributors to aberrant motor skills in schizophrenia are working memory and frontal lobe functioning. We, therefore, tested, whether frontal lobe function and working memory correlated with the motor domains in each group. In fact, in our study some motor domains (NSS and Parkinsonism) were associated with frontal lobe function across groups. However, there is also considerable conceptual overlap between motor skills and frontal lobe function. For instance the FAB and the NES share some items such as the fist-edge-palm test.
The pattern of correlations was different for working memory. Only in patients working memory was correlated with motor domains. This may be due to the fact that in our study working memory performance was relatively intact in relatives and healthy controls. Thus, given that motor abilities rely on frontal lobe and working memory function, working memory deficits may have impaired motor function in patients, while altered frontal lobe function compromised motor skills in all three groups. In contrast, working memory deficits were unlikely to account for motor abnormalities in relatives.
Limitations
Some limitations of this study require discussion. The sample was predominantly female as women more frequently agreed to participation. As groups were matched for age, gender and education, the proportion of women was equal across groups. Still, former studies showed no gender effect on NSS severity in patients (111). In addition, most of our patients were medicated. Antipsychotic medication may impact motor behavior. For instance dyskinesia and Parkinsonism were typically attributed to medication (i.e., tardive dyskinesia), even though evidence suggests that these motor abnormalities may also occur spontaneously in drug naïve patients (1, 5, 10, 112–114). We used the NES to assess NSS, but whether our results are comparable to other instruments remains unclear due to inconsistent terminology across instruments (59, 115) [e.g., overlap of motor coordination (116) and sequencing of motor acts in the NES]. Finally, although we assessed a comprehensive battery of various motor domains, other important domains have not been studied. For example, we did not test postural sway (117), which is an interesting measure of cerebellar function.
Conclusion
Modern theories for the development of psychosis suggest that environmental stressors, such as early childhood adversities, together with neurodevelopmental abnormalities may lead to psychosis. One particular marker of neurodevelopmental abnormalities is aberrant motor function. We were able to confirm generalized aberrant motor function in schizophrenia patients and aberrant motor function in some but not all tested motor domains in first-degree relatives of schizophrenia patients. The pattern of alterations in relatives suggests distinct mechanisms in each of the motor domains typically affected in psychosis. Particularly, relatives had increased NSS severity, which may reflect abnormal motor skill development. Thus, NSS could become a specific marker of the genetic risk for psychosis. Importantly such objective markers may not only inform on the etiology of psychosis but may be relevant for screening and staging of psychosis. Finally, markers of aberrant motor function may guide individualized treatment regimen in the future. Yet, future studies with sensitive technical instruments to measure and quantify different motor abnormalities are warranted to confirm our results in longitudinal studies.
Statements
Ethics statement
This study was carried out in accordance with the recommendations of the local Ethics Committee (Kantonale Ethikkommission: KEK Bern) with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Kantonale Ethikkommission: KEK Bern.
Author contributions
LS contributed data acquisition, analysis and interpretation, drafting and critical revision of the manuscript. KS and SW contributed to study concept and design, study supervision, data acquisition, analysis and interpretation, drafting, and critical revision of the manuscript. SW wrote the protocol and acquired funding for the study. PV contributed to data analysis and interpretation, drafting, and critical revision of the manuscript.
Funding
This study was supported by Bangerter-Rhyner Foundation and the Swiss National Science Foundation (grant 152619 to SW).
Acknowledgments
We are grateful for the help of Jeanne Moor who partially recruited relatives of schizophrenia patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer CD and handling Editor declared their shared affiliation.
References
1
WaltherSStrikW. Motor symptoms and schizophrenia. Neuropsychobiology (2012) 66(2):77–92.10.1159/000339456
2
MorrensMDocxLWaltherS. Beyond boundaries: in search of an integrative view on motor symptoms in schizophrenia. Front Psychiatry (2014) 5:145.10.3389/fpsyt.2014.00145
3
WaltherSStegmayerKHornHRampaLRazaviNMullerTJet alThe longitudinal course of gross motor activity in schizophrenia – within and between episodes. Front Psychiatry (2015) 6:10.10.3389/Fpsyt.2015.00010
4
WaltherSStegmayerKHornHRazaviNMullerTJStrikW. Physical activity in schizophrenia is higher in the first episode than in subsequent ones. Front Psychiatry (2015) 5:191.10.3389/Fpsyt.2014.00191
5
PeraltaVCamposMSDe JalonEGCuestaMJ. Motor behavior abnormalities in drug-naive patients with schizophrenia spectrum disorders. Mov Disord (2010) 25(8):1068–76.10.1002/mds.23050
6
PeraltaVCuestaMJ. Motor features in psychotic disorders. I. Factor structure and clinical correlates. Schizophr Res (2001) 47(2–3):107–16.10.1016/S0920-9964(00)00035-9
7
UngvariGSGogginsWLeungSKGerevichJ. Schizophrenia with prominent catatonic features (‘catatonic schizophrenia’). II. Factor analysis of the catatonic syndrome. Prog Neuropsychopharmacol Biol Psychiatry (2007) 31(2):462–8.10.1016/j.pnpbp.2006.11.012
8
ChanRCXuTHeinrichsRWYuYWangY. Neurological soft signs in schizophrenia: a meta-analysis. Schizophr Bull (2010) 36(6):1089–104.10.1093/schbul/sbp011
9
WhittyPFOwoeyeOWaddingtonJL. Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull (2009) 35(2):415–24.10.1093/schbul/sbn126
10
PappaSDazzanP. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: a systematic review. Psychol Med (2008) 39(07):1065.10.1017/s0033291708004716
11
StuivengaMMorrensM. Prevalence of the catatonic syndrome in an acute inpatient sample. Front Psychiatry (2014) 5:174.10.3389/fpsyt.2014.00174
12
UngvariGSCaroffSNGerevichJ. The catatonia conundrum: evidence of psychomotor phenomena as a symptom dimension in psychotic disorders. Schizophr Bull (2010) 36(2):231–8.10.1093/schbul/sbp105
13
TeremetzMCarmentLBrenugat-HerneLCrocaMBletonJPKrebsMOet alManual dexterity in schizophrenia – a neglected clinical marker?Front Psychiatry (2017) 8:120.10.3389/fpsyt.2017.00120
14
WaltherSVanbellingenTMuriRStrikWBohlhalterS. Impaired pantomime in schizophrenia: association with frontal lobe function. Cortex (2013) 49(2):520–7.10.1016/j.cortex.2011.12.008
15
MidorikawaAHashimotoRNoguchiHSaitohOKunugiHNakamuraK. Impairment of motor dexterity in schizophrenia assessed by a novel finger movement test. Psychiatry Res (2008) 159(3):281–9.10.1016/j.psychres.2007.04.004
16
MorrensMHulstijnWSabbeB. Psychomotor slowing in schizophrenia. Schizophr Bull (2007) 33(4):1038–53.10.1093/schbul/sbl051
17
HeinrichsDWBuchananRW. Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry (1988) 145(1):11–8.10.1176/ajp.145.1.11
18
BuchananRWHeinrichsDW. The neurological evaluation scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res (1989) 27(3):335–50.10.1016/0165-1781(89)90148-0
19
ChanRCKCuiHRChuMYZhangTHWangYWangYet alNeurological soft signs precede the onset of schizophrenia: a study of individuals with schizotypy, ultra-high-risk individuals, and first-onset schizophrenia. Eur Arch Psychiatry Clin Neurosci (2018) 268(1):49–56.10.1007/s00406-017-0828-4
20
CallawayDPerkinsDWoodsSLiuLAddingtonJ. Movement abnormalities predict transitioning to psychosis in individuals at clinical high risk for psychosis. Schizophr Bull (2015) 41:S13.10.1016/j.schres.2014.09.031
21
NeelamKGargDMarshallM. A systematic review and meta-analysis of neurological soft signs in relatives of people with schizophrenia. BMC Psychiatry (2011) 11:139.10.1186/1471-244x-11-139
22
KindlerJSchultze-LutterFMichelCMartz-IrngartingerALinderCSchmidtSJet alAbnormal involuntary movements are linked to psychosis-risk in children and adolescents: results of a population-based study. Schizophr Res (2016) 174(1–3):58–64.10.1016/j.schres.2016.04.032
23
MittalVANeumannCSaczawaMWalkerEF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch Gen Psychiatry (2008) 65(2):165–71.10.1001/archgenpsychiatry.2007.23
24
KoningJPTenbackDEvan OsJAlemanAKahnRSvan HartenPN. Dyskinesia and parkinsonism in antipsychotic-naive patients with schizophrenia, first-degree relatives and healthy controls: a meta-analysis. Schizophr Bull (2010) 36(4):723–31.10.1093/schbul/sbn146
25
TarboxSIPogue-GeileMF. Spontaneous dyskinesia and familial liability to schizophrenia. Schizophr Res (2006) 81(2–3):125–37.10.1016/j.schres.2005.09.013
26
MolinaJLGonzalez AlemanGFlorenzanoNPadillaECalvoMGuerreroGet alPrediction of neurocognitive deficits by parkinsonian motor impairment in schizophrenia: a study in neuroleptic-naive subjects, unaffected first-degree relatives and healthy controls from an indigenous population. Schizophr Bull (2016) 42(6):1486–95.10.1093/schbul/sbw023
27
McCreadieRGTharaRSrinivasanTNPadmavathiR. Spontaneous dyskinesia in first-degree relatives of chronically ill, never-treated people with schizophrenia. Br J Psychiatry (2003) 183:45–9.10.1192/bjp.183.1.45
28
MakofskeB. Manual dexterity. In: KreutzerJDeLucaJCaplanB, editors. Encyclopedia of Clinical Neuropsychology. New York: Springer (2011). p. 1522–3.
29
BervoetsCDocxLSabbeBVermeylenSVan Den BosscheMJMorselAet alThe nature of the relationship of psychomotor slowing with negative symptomatology in schizophrenia. Cogn Neuropsychiatry (2014) 19(1):36–46.10.1080/13546805.2013.779578
30
DocxLMorrensMBervoetsCHulstijnWFransenEDe HertMet alParsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatr Scand (2012) 126(4):256–65.10.1111/j.1600-0447.2012.01846.x
31
DocxLSabbeB. Longitudinal Evaluation of the Psychomotor Syndrome in Schizophrenia. (2014).
32
PoharSLAllyson JonesC. The burden of Parkinson disease (PD) and concomitant comorbidities. Arch Gerontol Geriatr (2009) 49(2):317–21.10.1016/j.archger.2008.11.006
33
FokiTVanbellingenTLunguCPirkerWBohlhalterSNyffelerTet alLimb-kinetic apraxia affects activities of daily living in Parkinson’s disease: a multi-center study. Eur J Neurol (2016) 23(8):1301–7.10.1111/ene.13021
34
GebhardtAVanbellingenTBarontiFKerstenBBohlhalterS. Poor dopaminergic response of impaired dexterity in Parkinson’s disease: bradykinesia or limb kinetic apraxia?Mov Disord (2008) 23(12):1701–6.10.1002/Mds.22199
35
KubelSStegmayerKVanbellingenTPastore-WappMBertschiMBurgunderJMet alAltered praxis network underlying limb kinetic apraxia in Parkinson’s disease – an fMRI study. Neuroimage Clin (2017) 16:88–97.10.1016/j.nicl.2017.07.007
36
FlycktLSydowOBjerkenstedtLEdmanGRydinEWieselFA. Neurological signs and psychomotor performance in patients with schizophrenia, their relatives and healthy controls. Psychiatry Res (1999) 86(2):113–29.10.1016/S0165-1781(99)00027-X
37
GschwandtnerUPflugerMAstonJBorgwardtSDreweMStieglitzRDet alFine motor function and neuropsychological deficits in individuals at risk for schizophrenia. Eur Arch Psychiatry Clin Neurosci (2006) 256(4):201–6.10.1007/s00406-005-0626-2
38
OldfieldRC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia (1971) 9(1):97–113.10.1016/0028-3932(71)90067-4
39
SheehanDVLecrubierYSheehanKHAmorimPJanavsJWeillerEet alThe Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry (1998) 59(Suppl 20):22–33; quiz 4–57.
40
DuboisBSlachevskyALitvanIPillonB. The FAB: a frontal assessment battery at bedside. Neurology (2000) 55(11):1621–6.10.1212/WNL.55.11.1621
41
WechslerD. Wechsler Memory Scale (WMS-III). San Antonio, TX: Psychological Corporation (1997).
42
RitterNKilincENavruzBBaeY. Test Review: L. Brown, R. J. Sherbenou, & S. K. Johnsen Test of Nonverbal Intelligence-4 (TONI-4). Austin, TX: PRO-ED, 2010. J Psychoeduc Assess (2011) 29(5):484–8.10.1177/0734282911400400
43
GuyW. ECDEU Assessment Manual for Psychopharmacology, Revised ed. Washington, DC: US Department of Health, Education, and Welfare (1976). A nice practical discussion can be found in Munetz MR, Benjamin S. How to examine patients using the Abnormal Involuntary Movement Scale. Hospital and Community Psychiatry Nov 1988, 39 (11):1172-1177 (1976).
44
FahnSEltonRL. Unified Parkinson’s disease rating scale. In: FahnSMarsdenCDCalneDGoldsteinM, editors. Recent Developments in Parkinson′s Disease. Florham Park, NJ: Macmillan Healthcare Information (1987) p. 153–63.
45
VanbellingenTKerstenBBellionMTemperliPBarontiFMuriRet alImpaired finger dexterity in Parkinson’s disease is associated with praxis function. Brain Cogn (2011) 77(1):48–52.10.1016/j.bandc.2011.06.003
46
BarkemeyerCASanta MariaMPBrowndykeJNCallonEBDunnAM. The coin rotation task: a convenient and sensitive measure of fine motor control. Arch Clin Neuropsychol (1998) 13(1):18.10.1016/S0887-6177(98)90379-1
47
van HartenPNWaltherSKentJSSponheimSRMittalVA. The clinical and prognostic value of motor abnormalities in psychosis, and the importance of instrumental assessment. Neurosci Biobehav Rev (2017) 80:476–87.10.1016/j.neubiorev.2017.06.007
48
WaltherS. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res (2015) 233(3):293–8.10.1016/j.pscychresns.2015.06.010
49
WaltherSMittalVA. Motor system pathology in psychosis. Curr Psychiatry Rep (2017) 19(12):97.10.1007/s11920-017-0856-9
50
GottesmanIIGouldTD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry (2003) 160(4):636–45.10.1176/appi.ajp.160.4.636
51
HeilmanKM. Apraxia. Continuum (Minneap Minn) (2010) 16:86–98.10.1212/01.CON.0000368262.53662.08
52
DutschkeLLStegmayerKRamseyerFBohlhalterSVanbellingenTStrikWet alGesture impairments in schizophrenia are linked to increased movement and prolonged motor planning and execution. Schizophr Res (2017) 19(12):97.10.1016/j.schres.2017.07.012
53
StegmayerKMoorJVanbellingenTBohlhalterSMuriRMStrikWet alGesture performance in first- and multiple-episode patients with schizophrenia spectrum disorders. Neuropsychobiology (2016) 73(4):201–8.10.1159/000446116
54
WaltherSEisenhardtSBohlhalterSVanbellingenTMuriRStrikWet alGesture performance in schizophrenia predicts functional outcome after 6 months. Schizophr Bull (2016) 42(6):1326–33.10.1093/schbul/sbw124
55
WaltherSVanbellingenTMuriRStrikWBohlhalterS. Impaired gesture performance in schizophrenia: particular vulnerability of meaningless pantomimes. Neuropsychologia (2013) 51(13):2674–8.10.1016/j.neuropsychologia.2013.08.017
56
YaziciAHDemirBYaziciKMGogusA. Neurological soft signs in schizophrenic patients and their nonpsychotic siblings. Schizophr Res (2002) 58(2–3):241–6.10.1016/S0920-9964(01)00338-3
57
ChenYLChenYHMakFL. Soft neurological signs in schizophrenic patients and their nonpsychotic siblings. J Nerv Ment Dis (2000) 188(2):84–9.10.1097/00005053-200002000-00004
58
RossiADe CataldoSDi MicheleVMannaVCeccoliSStrattaPet alNeurological soft signs in schizophrenia. Br J Psychiatry (1990) 157:735–9.10.1192/bjp.157.5.735
59
IsmailBCantor-GraaeEMcNeilTF. Neurological abnormalities in schizophrenic patients and their siblings. Am J Psychiatry (1998) 155(1):84–9.10.1176/ajp.155.1.84
60
KinneyDKWoodsBTYurgeluntoddD. Neurologic abnormalities in schizophrenic-patients and their families 0.2. Neurologic and Psychiatric Findings in Relatives. Arch Gen Psychiatry (1986) 43(7):665–8.10.1001/archpsyc.1986.01800070051007
61
ComptonMTBolliniAMMcKenzie MackLKrydaADRutlandJWeissPSet alNeurological soft signs and minor physical anomalies in patients with schizophrenia and related disorders, their first-degree biological relatives, and non-psychiatric controls. Schizophr Res (2007) 94(1–3):64–73.10.1016/j.schres.2007.04.002
62
AppelsMCSitskoornMMde BooMKlumpersUMKempsAEldersonAet alNeurological signs in parents of schizophrenic patients. Neuroreport (2002) 13(5):575–9.10.1097/00001756-200204160-00008
63
KoningJPTenbackDEKahnRSVollemaMGCahnWvan HartenPN. Movement disorders are associated with schizotypy in unaffected siblings of patients with non-affective psychosis. Psychol Med (2011) 41(10):2141–7.10.1017/S0033291711000389
64
MittalVADaleyMShiodeMFBeardenCEO’NeillJCannonTD. Striatal volumes and dyskinetic movements in youth at high-risk for psychosis. Schizophr Res (2010) 123(1):68–70.10.1016/j.schres.2010.08.002
65
DeanDJMittalVA. Spontaneous parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. NPJ Schizophr (2015) 1.10.1038/npjschz.2014.6
66
HirjakDThomannPAKuberaKMWolfNDSambataroFWolfRC. Motor dysfunction within the schizophrenia-spectrum: a dimensional step towards an underappreciated domain. Schizophr Res (2015) 169(1–3):217–33.10.1016/j.schres.2015.10.022
67
BernardJARussellCENewberryREGoenJRMittalVA. Patients with schizophrenia show aberrant patterns of basal ganglia activation: evidence from ALE meta-analysis. Neuroimage Clin (2017) 14:450–63.10.1016/j.nicl.2017.01.034
68
WaltherSSchappiLFederspielABohlhalterSWiestRStrikWet alResting-state hyperperfusion of the supplementary motor area in catatonia. Schizophr Bull (2017) 43(5):972–81.10.1093/schbul/sbw140
69
WoodwardNDKarbasforoushanHHeckersS. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry (2012) 169(10):1092–9.10.1176/appi.ajp.2012.12010056
70
AnticevicAColeMWRepovsGMurrayJDBrumbaughMSWinklerAMet alCharacterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex (2014) 24(12):3116–30.10.1093/cercor/bht165
71
KaufmannTSkatunKCAlnaesDDoanNTDuffEPTonnesenSet alDisintegration of sensorimotor brain networks in schizophrenia. Schizophr Bull (2015) 41(6):1326–35.10.1093/schbul/sbv060
72
WaltherSStegmayerKFederspielABohlhalterSWiestRViherPV. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull (2017) 43(5):982–92.10.1093/schbul/sbx091
73
ViherPVStegmayerKGiezendannerSFederspielABohlhalterSVanbellingenTet alCerebral white matter structure is associated with DSM-5 schizophrenia symptom dimensions. Neuroimage Clin (2016) 12:93–9.10.1016/j.nicl.2016.06.013
74
StegmayerKHornHFederspielARazaviNBrachtTLaimbockKet alSupplementary motor area (SMA) volume is associated with psychotic aberrant motor behaviour of patients with schizophrenia. Psychiatry Res (2014) 223(1):49–51.10.1016/j.pscychresns.2014.05.002
75
BrachtTSchnellSFederspielARazaviNHornHStrikWet alAltered cortico-basal ganglia motor pathways reflect reduced volitional motor activity in schizophrenia. Schizophr Res (2013) 143(2–3):269–76.10.1016/j.schres.2012.12.004
76
WaltherSFederspielAHornHRazaviNWiestRDierksTet alAlterations of white matter integrity related to motor activity in schizophrenia. Neurobiol Dis (2011) 42(3):276–83.10.1016/j.nbd.2011.01.017
77
WaltherSFederspielAHornHRazaviNWiestRDierksTet alResting state cerebral blood flow and objective motor activity reveal basal ganglia dysfunction in schizophrenia. Psychiatry Res (2011) 192(2):117–24.10.1016/j.pscychresns.2010.12.002
78
MartinelliCRigoliFShergillSS. Aberrant force processing in schizophrenia. Schizophr Bull (2017) 43(2):417–24.10.1093/schbul/sbw092
79
HirjakDKuberaKMThomannPAWolfRC. Motor dysfunction as an intermediate phenotype across schizophrenia and other psychotic disorders: progress and perspectives. Schizophr Res (2017) pii:S0920-9964(17)30616-3.10.1016/j.schres.2017.10.007
80
ThomannPAWustenbergTSantosVDBachmannSEssigMSchroderJ. Neurological soft signs and brain morphology in first-episode schizophrenia. Psychol Med (2009) 39(3):371–9.10.1017/S0033291708003656
81
Mouchet-MagesSRodrigoSCachiaAMouaffakFOlieJPMederJFet alCorrelations of cerebello-thalamo-prefrontal structure and neurological soft signs in patients with first-episode psychosis. Acta Psychiatr Scand (2011) 123(6):451–8.10.1111/j.1600-0447.2010.01667.x
82
GayOPlazeMOppenheimCMouchet-MagesSGaillardROlieJPet alCortex morphology in first-episode psychosis patients with neurological soft signs. Schizophr Bull (2013) 39(4):820–9.10.1093/schbul/sbs083
83
HirjakDWolfRCStieltjesBHauserTSeidlUSchroderJet alCortical signature of neurological soft signs in recent onset schizophrenia. Brain Topogr (2014) 27(2):296–306.10.1007/s10548-013-0292-z
84
DazzanPMorganKDOrrKGHutchinsonGChitnisXSucklingJet alThe structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain (2004) 127(Pt 1):143–53.10.1093/brain/awh015
85
HirjakDWolfRCStieltjesBSeidlUSchroderJThomannPA. Neurological soft signs and subcortical brain morphology in recent onset schizophrenia. J Psychiatr Res (2012) 46(4):533–9.10.1016/j.jpsychires.2012.01.015
86
HirjakDWolfRCKuberaKMStieltjesBMaier-HeinKHThomannPA. Neurological soft signs in recent-onset schizophrenia: focus on the cerebellum. Prog Neuropsychopharmacol Biol Psychiatry (2015) 60:18–25.10.1016/j.pnpbp.2015.01.011
87
ThomannPARoebelMDos SantosVBachmannSEssigMSchroderJ. Cerebellar substructures and neurological soft signs in first-episode schizophrenia. Psychiatry Res (2009) 173(2):83–7.10.1016/j.pscychresns.2008.07.006
88
VenkatasubramanianGJayakumarPNGangadharBNKeshavanMS. Automated MRI parcellation study of regional volume and thickness of prefrontal cortex (PFC) in antipsychotic-naive schizophrenia. Acta Psychiatr Scand (2008) 117(6):420–31.10.1111/j.1600-0447.2008.01198.x
89
KongLBachmannSThomannPAEssigMSchroderJ. Neurological soft signs and gray matter changes: a longitudinal analysis in first-episode schizophrenia. Schizophr Res (2012) 134(1):27–32.10.1016/j.schres.2011.09.015
90
MittalVADeanDJBernardJAOrrJMPelletier-BaldelliACarolEEet alNeurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophr Bull (2014) 40(6):1204–15.10.1093/schbul/sbt199
91
ZhaoQLiZHuangJYanCDazzanPPantelisCet alNeurological soft signs are not “soft” in brain structure and functional networks: evidence from ALE meta-analysis. Schizophr Bull (2014) 40(3):626–41.10.1093/schbul/sbt063
92
ChanRCHuangJZhaoQWangYLaiYYHongNet alPrefrontal cortex connectivity dysfunction in performing the Fist-Edge-Palm task in patients with first-episode schizophrenia and non-psychotic first-degree relatives. Neuroimage Clin (2015) 9:411–7.10.1016/j.nicl.2015.09.008
93
SarroSPomarol-ClotetECanales-RodriguezEJSalvadorRGomarJJOrtiz-GilJet alStructural brain changes associated with tardive dyskinesia in schizophrenia. Br J Psychiatry (2013) 203(1):51–7.10.1192/bjp.bp.112.114538
94
McCreadieRGTharaRPadmavatiRSrinivasanTNJaipurkarSD. Structural brain differences between never-treated patients with schizophrenia, with and without dyskinesia, and normal control subjects: a magnetic resonance imaging study. Arch Gen Psychiatry (2002) 59(4):332–6.10.1001/archpsyc.59.4.332
95
MittalVAOrrJMTurnerJAPelletierALDeanDJLunsford-AveryJet alStriatal abnormalities and spontaneous dyskinesias in non-clinical psychosis. Schizophr Res (2013) 151(1–3):141–7.10.1016/j.schres.2013.10.003
96
BaiYMChouKHLinCPChenIYLiCTYangKCet alWhite matter abnormalities in schizophrenia patients with tardive dyskinesia: a diffusion tensor image study. Schizophr Res (2009) 109(1–3):167–81.10.1016/j.schres.2009.02.003
97
ShihabuddinLBuchsbaumMSHazlettEAHaznedarMMHarveyPDNewmanAet alDorsal striatal size, shape, and metabolic rate in never-medicated and previously medicated schizophrenics performing a verbal learning task. Arch Gen Psychiatry (1998) 55(3):235–43.10.1001/archpsyc.55.3.235
98
HeinzAKnableMBCoppolaRGoreyJGJonesDWLeeKSet alPsychomotor slowing, negative symptoms and dopamine receptor availability – an IBZM SPECT study in neuroleptic-treated and drug-free schizophrenic patients. Schizophr Res (1998) 31(1):19–26.10.1016/S0920-9964(98)00003-6
99
MamahDHarmsMPWangLBarchDThompsonPKimJet alBasal ganglia shape abnormalities in the unaffected siblings of schizophrenia patients. Biol Psychiatry (2008) 64(2):111–20.10.1016/j.biopsych.2008.01.004
100
CuestaMJLecumberriPCabadaTMoreno-IzcoLRibeiroMLopez-IlundainJMet alBasal ganglia and ventricle volume in first-episode psychosis. A family and clinical study. Psychiatry Res (2017) 269:90–6.10.1016/j.pscychresns.2017.09.010
101
GoldmanALPezawasLMattayVSFischlBVerchinskiBAZoltickBet alHeritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry (2008) 63(5):475–83.10.1016/j.biopsych.2007.06.006
102
GeschwindN. The apraxias: neural mechanisms of disorders of learned movement. Am Sci (1975) 63(2):188–95.
103
NiessenEFinkGRWeissPH. Apraxia, pantomime and the parietal cortex. Neuroimage Clin (2014) 5:42–52.10.1016/j.nicl.2014.05.017
104
KolbBMilnerB. Performance of complex arm and facial movements after focal brain lesions. Neuropsychologia (1981) 19(4):491–503.10.1016/0028-3932(81)90016-6
105
HaalandKYHarringtonDLKnightRT. Neural representations of skilled movement. Brain (2000) 123(Pt 11):2306–13.10.1093/brain/123.11.2306
106
RothiLJHeilmanKMWatsonRT. Pantomime comprehension and ideomotor apraxia. J Neurol Neurosurg Psychiatry (1985) 48(3):207–10.10.1136/jnnp.48.3.207
107
WatsonRTFleetWSGonzalez-RothiLHeilmanKM. Apraxia and the supplementary motor area. Arch Neurol (1986) 43(8):787–92.10.1001/archneur.1986.00520080035016
108
StegmayerKBohlhalterSVanbellingenTFederspielAWiestRMuriRMet alLimbic interference during social action planning in schizophrenia. Schizophr Bull (2018) 44(2):359–68.10.1093/schbul/sbx059
109
StegmayerKBohlhalterSVanbellingenTFederspielAMoorJWiestRet alStructural brain correlates of defective gesture performance in schizophrenia. Cortex (2016) 78:125–37.10.1016/j.cortex.2016.02.014
110
ViherPVStegmayerKKubickiMKarmacharyaSLyallAEFederspielAet alThe cortical signature of impaired gesturing: findings from schizophrenia. Neuroimage Clin (2018) 17:213–21.10.1016/j.nicl.2017.10.017
111
BombinIArangoCBuchananRW. Significance and meaning of neurological signs in schizophrenia: two decades later. Schizophr Bull (2005) 31(4):962–77.10.1093/schbul/sbi028
112
PeraltaVCuestaMJ. Neuromotor abnormalities in neuroleptic-naive psychotic patients: antecedents, clinical correlates, and prediction of treatment response. Compr Psychiatry (2011) 52(2):139–45.10.1016/j.comppsych.2010.05.009
113
PeraltaVCuestaMJ. The effect of antipsychotic medication on neuromotor abnormalities in neuroleptic-naive nonaffective psychotic patients: a naturalistic study with haloperidol, risperidone, or olanzapine. Prim Care Companion J Clin Psychiatry (2010) 12(2).10.4088/PCC.09m00799gry
114
StegmayerKWaltherSvan HartenP. Tardive dyskinesia associated with atypical antipsychotics: prevalence, mechanisms and management strategies. CNS Drugs (2018) 32(2):135–47.10.1007/s40263-018-0494-8
115
MechriASlamaHBourdelMCChebelSMandhoujOKrebsMOet al[Neurological soft signs in schizophrenic patients and their nonaffected siblings]. Encephale (2008) 34(5):483–9.10.1016/j.encep.2007.08.009
116
KrebsMOGut-FayandABourdelMCDischampJOlieJP. Validation and factorial structure of a standardized neurological examination assessing neurological soft signs in schizophrenia. Schizophr Res (2000) 45(3):245–60.10.1016/S0920-9964(99)00206-6
117
DeanDJKentJSBernardJAOrrJMGuptaTPelletier-BaldelliAet alIncreased postural sway predicts negative symptom progression in youth at ultrahigh risk for psychosis. Schizophr Res (2015) 162(1–3):86–9.10.1016/j.schres.2014.12.039
Summary
Keywords
neurological soft signs, fine motor function, relatives of schizophrenia, motor function, dimension, schizophrenia
Citation
Schäppi L, Stegmayer K, Viher PV and Walther S (2018) Distinct Associations of Motor Domains in Relatives of Schizophrenia Patients—Different Pathways to Motor Abnormalities in Schizophrenia?. Front. Psychiatry 9:129. doi: 10.3389/fpsyt.2018.00129
Received
28 November 2017
Accepted
26 March 2018
Published
23 April 2018
Volume
9 - 2018
Edited by
Johannes Schröder, University of Heidelberg, Germany
Reviewed by
Vaibhav A. Diwadkar, Wayne State University School of Medicine, United States; Céline Z. Duval, Universitätsklinikum Heidelberg, Germany
Updates
Copyright
© 2018 Schäppi, Stegmayer, Viher and Walther.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lea Schäppi, lea.schaeppi@upd.unibe.ch
Specialty section: This article was submitted to Schizophrenia, a section of the journal Frontiers in Psychiatry
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.