
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry , 20 February 2018
Sec. Psychopathology
Volume 9 - 2018 | https://doi.org/10.3389/fpsyt.2018.00029
 Igor Elman1*
Igor Elman1* David Borsook2
David Borsook2
Pain is essential for avoidance of tissue damage and for promotion of healing. Notwithstanding the survival value, pain brings about emotional suffering reflected in fear and anxiety, which in turn augment pain thus giving rise to a self-sustaining feedforward loop. Given such reciprocal relationships, the present article uses neuroscientific conceptualizations of fear and anxiety as a theoretical framework for hitherto insufficiently understood pathophysiological mechanisms underlying chronic pain. To that end, searches of PubMed-indexed journals were performed using the following Medical Subject Headings’ terms: pain and nociception plus amygdala, anxiety, cognitive, fear, sensory, and unconscious. Recursive sets of scientific and clinical evidence extracted from this literature review were summarized within the following key areas: (1) parallelism between acute pain and fear and between chronic pain and anxiety; (2) all are related to the evasion of sensory-perceived threats and are subserved by subcortical circuits mediating automatic threat-induced physiologic responses and defensive actions in conjunction with higher order corticolimbic networks (e.g., thalamocortical, thalamo-striato-cortical and amygdalo-cortical) generating conscious representations and valuation-based adaptive behaviors; (3) some instances of chronic pain and anxiety conditions are driven by the failure to diminish or block respective nociceptive information or unconscious treats from reaching conscious awareness; and (4) the neural correlates of pain-related conscious states and cognitions may become autonomous (i.e., dissociated) from the subcortical activity/function leading to the eventual chronicity. Identifying relative contributions of the diverse neuroanatomical sources, thus, offers prospects for the development of novel preventive, diagnostic, and therapeutic strategies in chronic pain patients.
Acute pain is essential for survival by avoidance of tissue damage and by promotion of healing. This is not so for chronic (i.e., lasting more than 3 months) pain (1) that has no beneficial value (2). By afflicting over 100 million Americans and costing about $635 billion, chronic pain continues to be a challenge of pandemic enormity for patients, for their families, for medical establishment, and for society as a whole (3). What causes otherwise healthy people to develop pain-related disability, to withdraw from their regular activities, and to cultivate attitudes filled with gloomy perspectives, complaining, misery, and suffering (4)?
An autobiographic book by Ruben Gallego entitled “White on Black” (5) describing the life of a patient afflicted with severe cerebral palsy may provide some insights into these conundrums. Notwithstanding the excruciating pain intensity (6) and the perception of blatant indifference from the nursing staff, the author only mentions “pain” twice throughout the entire text. In one instance, he routinely rolls out of bed to fall onto the floor in order to crawl to the bathroom. In the second instance, pain is likewise devoid of any fear or anxiety and is rather perceived as “senseless.” The author eventually graduates from a technical college, marries thrice and fathers three children. He figures out ways of refusal to accept fear and anxiety. Not so for chronic pain patients. They tend to catastrophize in conjunction with experiencing overwhelming anxiety about the ongoing and future pain episodes (7–9).
Chronic pain is indeed a complex phenomenon engaging, in addition to sensory systems, extensive threat response neurocircuitry with emotional and cognitive constituents merging on the brain networks comprising the nucleus accumbens (NAc), the amygdala, the extended amygdala, and the medial prefrontal cortex (mPFC). Assuming neurobiological overlap between the processing of pain and of other threatening signals the present article focuses on the components of pain that are related to fear and to anxiety and are germane for comorbidity of these conditions and for transition from acute to chronic pain (10).
At the outset of this review, we compare epidemiological and clinical data on pain and fear/anxiety comorbidity and this contrast serves as the foundation for the premise of a neurobiological similitude between these commonly comorbid conditions. We then discuss possible pain mechanisms as they relate to the mediation of fear and anxiety. Next, we depict specific evidence for testable hypotheses on mechanistically informed psycho-therapeutic and -pharmacological interventions. Finally, summary and conclusions are presented.
Preclinical and clinical English language peer reviewed literature search on anxiety and fear in pain along with the mechanisms of normative threat processing and their potential impairments in patients with chronic pain disorders was undertaken using PubMed (http://www.ncbi.nlm.nih.gov/pubmed) from inception until December 2017. Medical Subject Headings’ terms used included pain and nociception plus amygdala, anxiety, cognitive, fear, sensory, and unconscious. Information on the mechanisms and neurobiology of pain, fear, anxiety, cognition, analgesia, and salience were also drawn from recent seminal reviews of these topics (11–15). The scopes of the review were adjusted based on consultations with scientists and clinicians, manual searches for relevant articles from the selected papers’ reference lists along with the utilization of PubMed’s “similar articles” function.
Noxious (mechanical, thermal, or chemical) stimuli activate C and A delta fibers. Nociception involves neurophysiologic mechanisms, including afferent activation in neural pathways responsible for detection or reflexive response to noxious stimuli. Like fear (see below), the response to an acute nociceptive stimulus includes pallor, freezing, tremulousness, diaphoresis, tachycardia, hypertension, and preponderance of the adrenomedullary, as compared to the noradrenergic stimulation (16, 17). The latter may be an adaptive reaction as epinephrine promotes memory consolidation (18) and improves coping with extreme situations by enhancing “gating” (i.e., activating descending modulatory systems) of noxious stimuli from reaching conscious awareness (1). Consequently, to assert prompt and automatic responses to hazardous situations, substantial nociceptive components remain sub- or unconscious (12).
Pain is experienced when the modulatory gating threshold is surpassed; so in acute situations, the attention is drawn to the bodily effects of the noxious stimuli. Chronic pain, by contrast, may occur in the absence of obvious tissue injury. Whatever the cause may be, pain is an unpleasant (1) or distressing (19) state associated with actual or potential tissue damage and comprised of sensory, emotional, cognitive, and social components (19, 20). Hence is the complex interplay among nociceptive perceptions and accompanying cognitive, behavioral, and emotional phenomena (13) so that pain experiences, derived from biological factors (e.g., genetic make-up, concentrations of endorphins and catecholamines, age, sex, underlying medical conditions and neuropsychopathology) are modulated by psychosocial variables (e.g., cultural, societal, and familial milieu in conjunction with upbringing; individual expectations; educational and professional backgrounds; and memories of prior pain episodes). In fact, the division between sensory and psychosocial pain expressions is not that perceptible (21) so co-occurring fear and anxiety not only contribute to the cognitive/behavioral pain aspects (22) but also worsen sensory phenomena (23).
Fear is defined as a fundamental emotion promptly arising in the context of tangible and actual threats. It may be appropriate when reality-based and amenable to cognitive control, but is deemed to be a phobia (e.g., pain phobia or agliophobia) if becomes irrational (24). The emotional disturbance evoked by various threats is only part of the homeostatic regulation, with epinephrine secretion predominating that of norepinephrine in conjunction with the pallor, freezing, diaphoresis, tachycardia, hypertension, and shaking (17). Anxiety refers to a related yet distinct concept encompassing an uncertain source of threat (such as in chronic pain) or future-oriented cognitions linking fear and similar emotions to personal meaning of events and of actions (25, 26). Short-term anxiety states may be appropriate and adaptive whereas long-term ongoing anxiety periods are usually consistent with anxiety disorders (27). See Table 1 for comparison of acute and chronic pain with fear and anxiety.
An attempt to integrate elements from psychological formulations of fear and anxiety symptoms into a neurobiological entity faces a major question: how to define “unconscious” as it relates to brain processes that do not produce conscious percepts. “Unconscious” is a somewhat clichéd entity given multiple definitions ranging Freudian Topographic Model, e.g., dreams, parapraxes, traumatic, and painful memories (28) to universal archetypal images by Jung (29), and the System One fast and intuitive thinking processes in Kahneman and Tversky’s Prospect Theory (30).
“Unconscious” may be defined from the cognitive, emotional, neurological, psychopathological, pharmacological, and legal perspectives (among other things). We address unconscious processing in conditions such as pain, anxiety and fear (31, 32) from the neurobiological standpoint. Specifically, we will consider high-amplitude low-frequency endogenous excitation of the limbic system normatively subordinated to the cortical default mode network containment as a valid version of “unconscious” (12, 15, 33–35). Although only one of many acceptable ways that “unconscious” might be conceptualized, this approach’s advantages include: (a) clearly defined neuroanatomical and electrophysiological criteria (33); (b) a firm foundation of cognitive neuroscience establishing links to the related memory and attention networks research foundation (36), and (c) its relationship to neuropsychopathology has been extensively accepted (33, 37).
Numerous epidemiological surveys suggest that anxiety disorders are particularly prevalent in pain patients (11, 38, 39) and are associated with worsened functional outcomes (7). The most comprehensive of these studies, the US National Comorbidity Survey Part II, found the odds ratio of 4.27 for the association between chronic arthritic pain and anxiety disorders (40). Similar figures were reported for other patients with arthritis and for those with migraine, back pain (41), spinal pain (42), fibromyalgia (43), and the complex regional pain syndrome (44). Such findings are consistent with the international chronic pain and anxiety data from 17 countries (n = 85,088) in various parts of the world (45).
The mechanisms of the above links are likely to be bidirectional (46) and to involve environmental, psychosocial, and neurobiological causes (47). Four potential categories of interaction may (co)exist between pain and fear/anxiety (11), including: (1) causality; (2) mutual influence; (3) common predisposing factor; or (4) independence (i.e., no interaction). Accordingly, anxiety accompanied by depression (48) and by other negative affective and cognitive states (49) may be a direct cause for emotional pain, that is to say psychache (50) and/or to produce pain via excessive muscle contraction as well as via endocrine or other stress-induced pathophysiological end organ alterations (51, 52).
“Mutual maintenance” (i.e., influence) appears to be the most notable interaction. That is why, pain commonly (53, 54) arising in the context of abuse and violence (11) can become a conditioned stimulus eliciting fear and anxiety that in turn enhance subjective pain experience (55, 56) with concurrent avoidance of both pain- and fear-related situations and ensuing deterioration of both conditions (46, 50). In a course of pain and fear/anxiety sensitization, cross-sensitization might likewise occur. If that is the case, pain episodes could increase susceptibility to the development of fear and anxiety syndromes (or even trigger relapse) and vice versa. In view of that, “pre-pain” state may turn into a bona fide pain state as increased fear or anxiety serves as a tipping point disrupting the equilibrium. This is observed in acute exacerbations of pain by anxiety in healthy subjects (57) and by tempering anxiety with drugs (e.g., gabapentin) that have both anxiolytic (55, 58, 59) and analgesic (60) properties. Serotonergic impairments (61) may be a common predisposing factor. Another predisposing factor is a chronic or excessive use of opioid analgesics that amplify pain [viz., opioid-induced hyperalgesia and/or pain chronification (61)] on top of evocation of anxiety, fear, and of other negative affective states (62, 63).
Aside from the clinical impression, including the vignette in the Introduction Section (5), no studies to date have examined outcomes of pain conditions that are devoid (i.e., independent) from fear and anxiety. On the other hand, due to recurring stress accompanied by hopelessness, catastrophizing (57), horror (64), or avoidance of pain-related situations (11, 65), chronic pain may be viewed as a version of post-traumatic stress disorder (PTSD) (65, 66). Since the lack of peritraumatic fear and horror confers resilience to the development of post-traumatic symptomatology (DSM-IV-TR) (67, 68), it may also be plausible to find paucity of chronic pain cases in the absence fear and anxiety responses. Consistent with this assumption, timely and adequate peritraumatic analgesia may prevent the development of fear learning in both laboratory animals (69) and in humans (70). In short, understanding neurobiology of pain as it may relate to that of anxiety/fear could further support the former’s inclusion within the disorders of the threat response system (65, 71).
Pain may indeed be considered within the spectrum of the fear/anxiety disorders, which is encoded in the “persistently high level of anxiety” criterion of the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (27) diagnostic category for Somatic Symptom Disorder with Predominant Pain in consort with “chest pain” and “muscle aches or soreness” respective criteria of panic- and generalized anxiety disorders. While no clinical studies specifically link the neural bases of pain and fear/anxiety, multiple lines of evidence suggest that pain is embedded within extensive threat response circuitry (72) that is critical for the survival of individuals and species via the evasion of real and/or perceived hazards. Thus, both pain and fear may be considered as intervening variables connecting threatening stimuli and consequent pathophysiological alterations with behaviors aimed at regaining the homeostatic equilibrium (73).
In chronic pain, tissue injury and other threats are not directly related to the above changes but are rather modulated by biopsychosocial variables as well as changes in brain plasticity that may alter an individual’s responsivity. Explicitly, while pain and fear/anxiety symptomatology are derived from precipitating factors, their clinical manifestations may be shaped by psychosocial setting, pre-existing neuropsychopathology and prior exposure to the same type of stimuli (see section Key Terms). Moreover, responses to various threats may be interrelated, be they sensory, visual, or interoceptive (72), which is critical for coordination, prioritizing, and selecting the most advantageous choices (74).
Responses to goal-objects comprise diverse informational features of the stimuli, events, or internal states. These features include but not limited to rate, incidence, proximity, timing, and quantity of the stimuli (44, 75). Evaluation of these informational features is critical for higher level cognitive and valuational processing with consequent behavioral choices. Multiple brain regions are involved in the assessment of informational features, their valuation, and probability estimates.
Sensory inputs concerning threats are relayed to the lateral amygdala and are subsequently conveyed from there to the central nucleus (CeA) and to the NAc after passing through the basal nucleus (BA) for generation of fear-related physiological (e.g., autonomic nervous system), emotional, and behavioral (e.g., freezing, escape, fight, and avoidance) responses. The CeA’s laterocapsular division (namely, the nociceptive amygdala) is involved in the descending pain modulation system (i.e., gating) determining pain-related affect, motivations, and behaviors by integrating nociception with interoceptive and environmental information with higher order cognitive percepts of objectives, their valuation, and context (76–78).
Fear dulls acute pain, which may be advantageous from a phylogenetic standpoint by promoting fight-or-flight operations; anxiety conversely worsens pain experience (79). In terms of neuroanatomy, fear vs. anxiety are differentiated by the engagement of the of the extended amygdala structure, the bed nucleus of the stria terminalis (BNST) (15) in the latter (but not in the former) perhaps by reason of inadequate extinction (80–82) and/or due to top-down suppression by the hippocampus (56) and by the mPFC (80, 83). In the bottom-up fashion fear- and anxiety-related subcortical limbic structures indirectly (15) affect lateral, medial, and insular cortices to contribute to respective conscious experiences (14).
Acute pain (84, 85) and fear (86, 87) are associated with phasic homeostatic responses to proximal and tangible threats. Chronic pain (88) and anxiety (15), in contrast, are of tonic and allostatic (89) nature with similar to each other autonomic changes (e.g., low heart rate variability) (90, 91); they are derived from uncertain threats and/or from contextual learning of the remote stimuli (Table 1). In psychological terms anxiety represents a failure to defend against unconscious libidinal drives (66). Acute pain with ensuing chronicity may be likewise conceptualized to be the limbic system’s respective failures to gate the nociceptive information from reaching conscious awareness and to properly process such information soon thereafter (12). Furthermore, recurrent dopaminergic trafficking consequent to ongoing pain episodes gives rise to between system “anti–reward adaptations” (88, 92–94) recruiting CeA, NAc, BA, and BNST that in concert contribute to allostatic load (Figure 1) in the form of massive outpouring of stress hormones viz., corticotropin-releasing factor, epinephrine, norepinephrine, glutamate, glucocorticoids, and pituitary adenylate cyclase activating polypeptide (95) manifested in fear, anxiety, and other negative affective states like those arising in the context of opioid overuse, that is to say, “hyperkatifeia” (63, 88, 92).
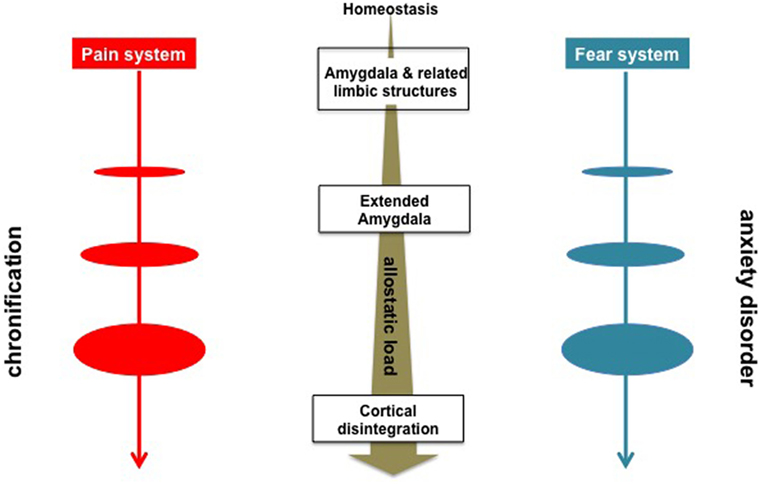
Figure 1. Schematic overview of the neurobiological processes underlying evolution of chronic pain and anxiety disorders. Sensory inputs concerning threats are relayed to the amygdala and other limbic structures, e.g., the central nucleus, the nucleus accumbens, the basal nucleus for generation of fear- or acute pain-related physiological (e.g., autonomic nervous system) behavioral (e.g., freezing, escape, fight, and avoidance), and cognitive responses (77, 78). Anxiety- and chronic pain symptomatology are characterized by the engagement of the of the extended amygdala structures, e.g., the bed nucleus of the stria terminalis along with allostatic load in the form of massive outpouring of stressogenic neurotransmitters and hormones manifested in negative affective states (10, 92).
Amygdala and related corticolimbic regions are conventionally considered to be the key component of the threat processing system involved in the experience of fear and it is commonly hypothesized that they simultaneously control conscious awareness of fear in conjunction with automatic defense responses (96). However, recent findings from human clinical (97, 98) and neuroimaging (99, 100) studies, as well as preclinical work (14), suggests that anxiety symptomatology may be attributed to a two-system construct (Figure 2) comprised of subcortical circuits mediating unconscious and automatic threat-related physiologic and behavioral responses in conjunction with closely linked, yet potentially independent higher corticolimbic networks producing conscious anxiety experiences with corresponding sets of drives and behaviors (14, 15). Their distinctiveness is supported by different temporal features of anxiety cognitions vs. automatic events (15, 101) c.f., preponderance of cognitive experiences lacking recognizable nociceptive input in chronic pain patients (102). The fact that conscious experiences of chronic pain (17) or of anxiety (103) are substantially more bothersome for patients than sensory percepts or defensive (re)actions potentially explains the relative inefficiency of analgesics that mostly target subcortical regions (104).
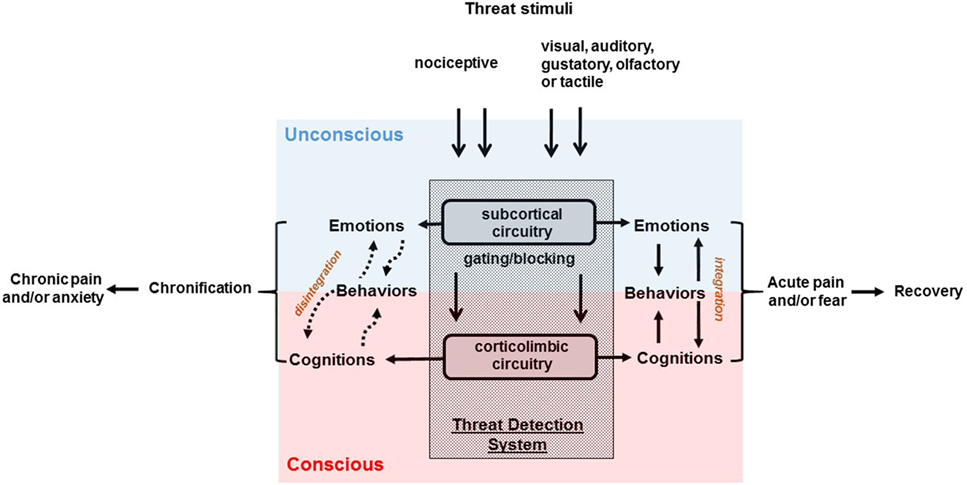
Figure 2. Schematic overview of the two-system construct underlying chronic pain and anxiety disorders. Sensory inputs of nociceptive nature may give rise to pain; while threatening visual, auditory, gustatory, olfactory, or tactile stimuli may bring out fear. Pain is conceptualized to be a failure of the limbic system to gate components of nociceptive information from reaching conscious awareness (12). In psychological terms, anxiety represents a failure to defend against unconscious libidinal threats (66). Chronic pain and anxiety symptoms may be attributed to a disintegration of two-system construct (15) comprised of subcortical circuits mediating unconscious threat-related physiologic, emotional, and behavioral responses in conjunction with linked, yet potentially independent higher corticolimbic network producing cognitive experiences.
The respective contributions from the amygdala and from the extended amygdala to acute pain/fear and to chronic pain/anxiety are only modulatory and indirect (15) so that the circuits that are directly responsible for the subjective cognitions, valuations, and experiences may function independently from the subcortical limbic input. Such disintegration between cognition, perception, and emotions may occur due to (1) substantial dopaminergic surges in reward, motivation, and learning centers leading to “hardwired” neuroplasticity in the striato-thalamic-frontal cortical loop, with insuring top-down dissociation from the subcortical activity (105, 106); and/or (2) hypofunctionality of the excitatory glutamatergic afferents from the amygdala–hippocampus complex failing to produce bottom-up restrain of the striato-thalamic-frontal cortical loops (105, 107, 108).
Impartments of the bottom-up striato-thalamic-frontal cortical modulations may be observed in a number of neuropsychiatric conditions associated, such as pain (109), with heightened dopaminergic bursts in reward, motivation, and learning centers (88, 110, 111). For instance, positive symptoms of schizophrenia may become dissociated from the mesolimbic subcortical activity and persist notwithstanding presumably complete dopamine blockade by antipsychotic agents in about a quarter of psychotic patients (112, 113). Moreover, craving in patients addicted to opioids persists even in the face of fully occupied opioid receptors (114). This is also the case for cocaine craving in cocaine dependent subjects receiving agonist substitution therapy (115). Akin to drug addiction (116, 117), pain and anxiety chronicity lacking normal sensory input may be attributable to neuroplastic changes that become ingrained in the corticolimbic (e.g., thalamocortical, thalamo-striato-cortical, and amygdalo-cortical) synapses driving compulsive thoughts and repetitious actions (118).
Psycho-diagnostic and -metric assessments may define and monitor cognitive neuroadaptational states, while neuroimaging combined with cognitive and biochemical challenges could be instrumental for demonstration of subcortical emotional and physiological aberrations. Thus, rather than targeting pain along the entire biopsychosocial continuum it may be useful to segregate this multidimensional system into cognitive, emotional, and sensory domains based on the distinct underlying circuitry. Addressing cognitive/subjective domain separately may provide a sound footing for understanding its role in the therapeutic armamentarium for chronic pain.
While none of the professionally delivered therapies for chronic pain appears to be superior, generic types of cognitive-behavioral techniques is the commonplace practice supported by clinical trials (118); other methodologies include motivational intervention, self-help and peer support. Suboptimal outcomes of these intervention call for a more personalized approach accounting for unique biological susceptibilities along with secondary gains (e.g., pain as proxy for gaining pity, appreciation, or exemption from routine chores and responsibilities), catastrophizing and other cognitive distortions and problematic decision-making processes.
Opioid analgesic agents improve sensory components of acute pain and their short term use in chronic pain (119) can ameliorate autonomic responses by aborting stress-induced catecholamines releases in part via blockade of the locus ceruleus activity (120, 121). These beneficial properties are, however, outweighed by severe side effects, including those resulting from opioidergic and dopaminergic stimulation with secondary worsening of reward and motivational deficits (109), as well as opioid induced endocrinopathies (e.g., hypogonadism) (122). Moreover, opioid analgesics providing instant pain relief (i.e., negative reinforcement) can become a conditioned stimulus eliciting future painful episodes. Opioid-induced changes in the mesolimbic dopaminergic pathway may underlie heightened incentive salience attributed to opioids or to related cues (i.e., drug craving) as well as the amplification of hyperkatifeia and of sensory pain components (63, 109). Overall, opioid analgesics possess advantageous therapeutic properties for the treatment of acute pain and for mitigation of traumatic memories. These qualities explain to some extent the rise of opioids as the drug of choice in the pharmacopeia of chronic pain. However, opioids’ efficacy has been questioned by modern (123) research suggesting relative inefficacy of these agents in up to 70% of patients in pooled analyses of rigorously designed clinical trials (124, 125).
There are other potential psychopharmacological strategies for the management of the reentrant (126) autonomous thalamocortical circuits (105). Anti-glutamatergic agents may be helpful (127) and have already been successfully used in pain patients (128–130). As an example, ketamine administration produces long-term analgesia lasting at least 3 months (131). The findings of neocortical/cortical glutamatergic desynchronization support this sort of strategies, but more research is needed for understanding cortical mechanisms of chronic pain (132). Glutamate inhibition may be also instrumental because of excitatory glutamatergic neurotransmission (133) sensitization arising in the context of stress-like anti-reward phenomena (92) resulting from pain-induced activation of dopaminergic pathways. Similar to chronic pain, PTSD’s cognitive symptoms of flashbacks and intrusive recollections may be temporarily disconnected from the anti-reward stress (27, 134) and respond to anti-glutamatergic agents (135). These findings add further support for the proposed and therapeutic strategies for chronic pain patients.
Striato-thalamic-frontal cortical pathways coordinate motor, cognitive, and emotional functions within the brain, including regulation of fear- and anxiety-related amygdala activity (136) while the limbic system represents a set of subcortical and cortical structures engaged (among other tasks) in the processing of emotions, motivation, stress, and fear (137). This review compares the roles played by the systems above in acute/chronic pain and in fear/anxiety conditions to indicate that some features are shared. For example, there are parallels in the acute harm prevention motivation typical of both acute pain and fear. Conspicuous similarities between chronic pain and anxiety include lack of survival value, involvement of the adrenomedullary system, autonomic responses, the key role of the extended amygdala, and related limbic structures in the emotional/physiological components and disintegration of the cognitive and behavioral/physiological phenomena. On the other hand, nociceptive, neuropathic, immune, degenerative, traumatic, and malignant pain sources may be associated with diffused tissue damage (88), which is not typical of patients with fear or anxiety.
Although limbic system is commonly implicated in the pathophysiology of chronic pain syndromes (12), here chronic pain is also postulated to result from dissociation of limbic structures from physiologically linked striato-thalamic-frontal cortical pathways. Prefrontal cortex, amygdala, NAc, and thalamic nuclei are the key information hubs (138) and their firing/communication impairments underlie fundamental psychiatric symptoms involving perception, arousal, cognition, and emotions (139). Accordingly, the symptoms may be derived from top-down or bottom-up dysfunction or combination. Abnormal bottom-up activity results in excessive responses to painful (hyperalgesia) or even normally non-painful (allodynia) stimuli with corresponding deficiency in reward function and an overwhelming urge to eradicate pain (88, 92). These may become disintegrated from the top–down changes manifested in unrealistic and even catastrophic (140) expectation of continued pain and/or of unsuccessful analgesia, subjective pain overvaluation with regard to a subjectively acceptable amount of pain, i.e., framing (88).
Treatment with opioid analgesics does not adequately address such cognitive distortions and may even worsen them (109, 141). If corroborated by human studies, the abovementioned insights will have implications for the primary and secondary prevention of pain chronification. Identification of neurobiologic risk factors for chronic pain could lead to screening of vulnerable individuals. Those with heightened vulnerability owing to baseline anxiety symptomatology might be counseled to avoid prolonged pain exposure (primary prevention), or selected for early intervention (secondary prevention) even in the presence of mild warning signs (e.g., anxiety, drug seeking, and catastrophizing) early [<3 months (142)] in the course of the pain-related illness. The proposed model of chronic pain could also have treatment implications, as it supports the use of both psychosocial and pharmacological interventions for amelioration of chronic pain problems. Clinical experience suggests that utilization of such combined interventions is lower (143) than what could be projected from positive outcomes of clinical trials (144). And so, this review provides clinical researches and practitioners alike with the important knowledge base for understanding the rationale for anxiolytic therapy and raises their awareness of the unmet psychosocial needs of chronic pain patients. Lastly, this model may also provide important leads for recognition and treatment of pain problems in patients with other neuropsychiatric disorders, including schizophrenia, addictions, and major depression (109).
It is conceivable that future therapeutic interventions targeting pain will address somewhat independent emotional/sensory and cognitive/behavioral components. Patients will be then characterized according to this dichotomy and clear cut cases will be speared side effects by only receiving interventions aimed at the specific system. The time is probably ripe to commence clinical trials to pursue the presented ideas.
IE and DB conceived the idea, performed literature searches, and wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research was supported by the grant 1 I01 CX001118-01A2 from the Veterans Health Administration. IE reported no potential conflicts of interest. DB disclosed consulting fees from Biogen.
1. Mendell LM. Constructing and deconstructing the gate theory of pain. Pain (2014) 155(2):210–6. doi:10.1016/j.pain.2013.12.010
2. Katz J, Rosenbloom BN, Fashler S. Chronic pain, psychopathology, and DSM-5 somatic symptom disorder. Can J Psychiatry (2015) 60(4):160–7. doi:10.1177/070674371506000402
3. Butler AS, Xi J, Cox TL, Pope AM, Randall D, Bowman V. Relieving Pain in America. A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: Institute of Medicine of the National Academies (2011).
6. Pain in Adults with Cerebral Palsy. American Academy for Cerebral Palsy and Developmental Medicine (2016). Available from: https://www.aacpdm.org/UserFiles/file/fact-sheet-pain-011516.pdf
7. Bean DJ, Johnson MH, Heiss-Dunlop W, Lee AC, Kydd RR. Do psychological factors influence recovery from complex regional pain syndrome type 1? A prospective study. Pain (2015) 156(11):2310–8. doi:10.1097/j.pain.0000000000000282
8. Hooten WM. Chronic pain and mental health disorders: shared neural mechanisms, epidemiology, and treatment. Mayo Clin Proc (2016) 91(7):955–70. doi:10.1016/j.mayocp.2016.04.029
9. Sullivan MJL, Scott BR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess (1995) 7(4):524–32. doi:10.1037/1040-3590.7.4.524
10. Hasenbring MI, Chehadi O, Titze C, Kreddig N. Fear and anxiety in the transition from acute to chronic pain: there is evidence for endurance besides avoidance. Pain Manag (2014) 4(5):363–74. doi:10.2217/pmt.14.36
11. Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety (2009) 26(10):888–901. doi:10.1002/da.20600
12. Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron (2015) 87(3):474–91. doi:10.1016/j.neuron.2015.06.005
13. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci (2013) 14:502–11. doi:10.1038/nrn3516
14. LeDoux JE, Moscarello J, Sears R, Campese V. The birth, death and resurrection of avoidance: a reconceptualization of a troubled paradigm. Mol Psychiatry (2017) 22(1):24–36. doi:10.1038/mp.2016.166
15. LeDoux JE, Pine DS. Using neuroscience to help understand fear and anxiety: a two-system framework. Am J Psychiatry (2016) 173(11):1083–93. doi:10.1176/appi.ajp.2016.16030353
16. Feinstein B, Langton JN, Jameson RM, Schiller F. Experiments on pain referred from deep somatic tissues. J Bone Joint Surg Am (1954) 36A(5):981–97. doi:10.2106/00004623-195436050-00007
17. Goldstein DS. Stress, Catecholamines, and Cardiovascular Disease. New York, NY: Marcel Dekker, Inc (1995).
18. McIntyre CK, McGaugh JL, Williams CL. Interacting brain systems modulate memory consolidation. Neurosci Biobehav Rev (2012) 36(7):1750–62. doi:10.1016/j.neubiorev.2011.11.001
19. Williams AC, Craig KD. Updating the definition of pain. Pain (2016) 157:2420–3. doi:10.1097/j.pain.0000000000000613
20. Merskey H, Bogduk N. International Association for the Study of Pain. Task Force on Taxonomy. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Seattle: IASP Press (1994).
21. Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain (1999) 79:105–11. doi:10.1016/S0304-3959(98)00184-5
22. Zale EL, Lange KL, Fields SA, Ditre JW. The relation between pain-related fear and disability: a meta-analysis. J Pain (2013) 14(10):1019–30. doi:10.1016/j.jpain.2013.05.005
23. Zhuo M. Neural mechanisms underlying anxiety-chronic pain interactions. Trends Neurosci (2016) 39(3):136–45. doi:10.1016/j.tins.2016.01.006
24. Hammer AI, Hammer M. Alcohol consumption and pain phobia: toward a unifying theory of alcoholism. Adv Alcohol Subst Abuse (1990) 8(3–4):43–55. doi:10.1300/J251v08n03_04
25. Hofmann SG, Ellard KK, Siegle GJ. Neurobiological correlates of cognitions in fear and anxiety: a cognitive-neurobiological information-processing model. Cogn Emot (2012) 26(2):282–99. doi:10.1080/02699931.2011.579414
26. Izard CE. Basic emotions, natural kinds, emotion schemas, and a new paradigm. Perspect Psychol Sci (2007) 2(3):260–80. doi:10.1111/j.1745-6916.2007.00044.x
27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing (2013).
30. Morewedge CK, Kahneman D. Associative processes in intuitive judgment. Trends Cogn Sci (2010) 14(10):435–40. doi:10.1016/j.tics.2010.07.004
31. Nava E, Romano D, Grassi M, Turati C. Skin conductance reveals the early development of the unconscious processing of emotions. Cortex (2016) 84:124–31. doi:10.1016/j.cortex.2016.07.011
32. Rabellino D, Densmore M, Frewen PA, Theberge J, Lanius RA. The innate alarm circuit in post-traumatic stress disorder: conscious and subconscious processing of fear- and trauma-related cues. Psychiatry Res (2016) 248:142–50. doi:10.1016/j.pscychresns.2015.12.005
33. Carhart-Harris RL, Friston KJ. The default-mode, ego-functions and free-energy: a neurobiological account of Freudian ideas. Brain (2010) 133(Pt 4):1265–83. doi:10.1093/brain/awq010
34. Mantini D, Vanduffel W. Emerging roles of the brain’s default network. Neuroscientist (2013) 19(1):76–87. doi:10.1177/1073858412446202
35. Marini S, Di Tizio L, Dezi S, Armuzzi S, Pelaccia S, Valchera A, et al. The bridge between two worlds: psychoanalysis and fMRI. Rev Neurosci (2016) 27(2):219–29. doi:10.1515/revneuro-2015-0031
36. Raniga P, Bryan P, Egan G. Resting State Functional Coupling between the Ascending Synchronising System, Limbic System and the Default Mode Network via Theta Oscillations. bioRxiv (2016). Available from: https://www.biorxiv.org/content/early/2016/11/08/086058
37. Balthazar ML, Pereira FR, Lopes TM, da Silva EL, Coan AC, Campos BM, et al. Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Hum Brain Mapp (2014) 35(4):1237–46. doi:10.1002/hbm.22248
38. Castro M, Kraychete D, Daltro C, Lopes J, Menezes R, Oliveira I. Comorbid anxiety and depression disorders in patients with chronic pain. Arq Neuropsiquiatr (2009) 67(4):982–5. doi:10.1590/S0004-282X2009000100007
39. Gureje O. Comorbidity of pain and anxiety disorders. Curr Psychiatry Rep (2008) 10(4):318–22. doi:10.1007/s11920-008-0051-0
40. McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain (2003) 106(1–2):127–33. doi:10.1016/S0304-3959(03)00301-4
41. McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain (2004) 111(1–2):77–83. doi:10.1016/j.pain.2004.06.002
42. Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, et al. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain (2005) 113(3):331–9. doi:10.1016/j.pain.2004.11.010
43. Raphael KG, Janal MN, Nayak S, Schwartz JE, Gallagher RM. Psychiatric comorbidities in a community sample of women with fibromyalgia. Pain (2006) 124(1–2):117–25. doi:10.1016/j.pain.2006.04.004
44. Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron (2001) 32(5):927–46. doi:10.1016/S0896-6273(01)00533-5
45. Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, et al. Mental disorders among persons with chronic back or neck pain: results from the world mental health surveys. Pain (2007) 129(3):332–42. doi:10.1016/j.pain.2007.01.022
46. Vlaeyen JW, Crombez G, Linton SJ. The fear-avoidance model of pain. Pain (2016) 157(8):1588–9. doi:10.1097/j.pain.0000000000000574
47. Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: a neural systems approach. Neurosci Biobehav Rev (2014) 39:61–78. doi:10.1016/j.neubiorev.2013.12.006
48. Smitherman TA, Maizels M, Penzien DB. Headache chronification: screening and behavioral management of comorbid depressive and anxiety disorders. Headache (2008) 48:45–50. doi:10.1111/j.1526-4610.2007.00974.x
49. Koleck M, Mazaux JM, Rascle N, Bruchon-Schweitzer M. Psycho-social factors and coping strategies as predictors of chronic evolution and quality of life in patients with low back pain: a prospective study. Eur J Pain (2006) 10:1–11. doi:10.1016/j.ejpain.2005.01.003
50. Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med (2007) 30(1):77–94. doi:10.1007/s10865-006-9085-0
51. Tang J, Gibson SJ. A psychophysical evaluation of the relationship between trait anxiety, pain perception, and induced state anxiety. J Pain (2005) 6(9):612–9. doi:10.1016/j.jpain.2005.03.009
52. Weisenberg M, Aviram O, Wolf Y, Raphaeli N. Relevant and irrelevant anxiety in the reaction to pain. Pain (1984) 20(4):371–83. doi:10.1016/0304-3959(84)90114-3
53. Ellsberg M, Jansen HA, Heise L, Watts CH, Garcia-Moreno C, WHO Multi-Country Study on Women’s Health and Domestic Violence against Women Study Team. Intimate partner violence and women’s physical and mental health in the WHO multi-country study on women’s health and domestic violence: an observational study. Lancet (2008) 371(9619):1165–72. doi:10.1016/S0140-6736(08)60522-X
54. Sachs-Ericsson N, Kendall-Tackett K, Hernandez A. Childhood abuse, chronic pain, and depression in the National Comorbidity Survey. Child Abuse Negl (2007) 31(5):531–47. doi:10.1016/j.chiabu.2006.12.007
55. de-Paris F, Sant’Anna MK, Vianna MR, Barichello T, Busnello JV, Kapczinski F, et al. Effects of gabapentin on anxiety induced by simulated public speaking. J Psychopharmacol (2003) 17(2):184–8. doi:10.1177/0269881103017002006
56. Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci (2001) 21(24):9896–903.
57. Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother (2009) 9(5):745–58. doi:10.1586/ern.09.34
58. Hamner MB, Brodrick PS, Labbate LA. Gabapentin in PTSD: a retrospective, clinical series of adjunctive therapy. Ann Clin Psychiatry (2001) 13(3):141–6. doi:10.3109/10401230109148960
59. Pande AC, Pollack MH, Crockatt J, Greiner M, Chouinard G, Lydiard RB, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol (2000) 20(4):467–71. doi:10.1097/00004714-200008000-00011
60. Wiffen PJ, McQuay HJ, Edwards JE, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database Syst Rev (2005) 3:CD005452. doi:10.1002/14651858.CD005452
61. Smitherman TA, Penzien DB, Maizels M. Anxiety disorders and migraine intractability and progression. Curr Pain Headache Rep (2008) 12(3):224–9. doi:10.1007/s11916-008-0039-9
62. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology (2006) 104(3):570–87. doi:10.1097/00000542-200603000-00025
63. Shurman J, Koob GF, Gutstein HB. Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Med (2010) 11(7):1092–8. doi:10.1111/j.1526-4637.2010.00881.x
64. Haugh R. Hospitals and clinicians confront a new imperative: pain management. Hosp Health Netw (2005) 79(4):51–2.
65. Liedl A, O’Donnell M, Creamer M, Silove D, McFarlane A, Knaevelsrud C, et al. Support for the mutual maintenance of pain and post-traumatic stress disorder symptoms. Psychol Med (2010) 40(7):1215–23. doi:10.1017/S0033291709991310
66. Gabbard GO. Psychodynamic Psychiatry in Clinical Practice. 5th ed. Washington, DC: American Psychiatric Publishing (2014).
67. Green CL, Nahhas RW, Scoglio AA, Elman I. Post-traumatic stress symptoms in pathological gambling: potential evidence of anti-reward processes. J Behav Addict (2017) 6:98–101. doi:10.1556/2006.6.2017.006
68. Horn SR, Charney DS, Feder A. Understanding resilience: new approaches for preventing and treating PTSD. Exp Neurol (2016) 284(Pt B):119–32. doi:10.1016/j.expneurol.2016.07.002
69. Szczytkowski-Thomson JL, Lebonville CL, Lysle DT. Morphine prevents the development of stress-enhanced fear learning. Pharmacol Biochem Behav (2013) 103(3):672–7. doi:10.1016/j.pbb.2012.10.013
70. Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med (2010) 362(2):110–7. doi:10.1056/NEJMoa0903326
71. Liedl A, Knaevelsrud C. Chronic pain and PTSD: the perpetual avoidance model and its treatment implications. Torture (2008) 18(2):69–76.
72. Zhang S, Mano H, Ganesh G, Robbins T, Seymour B. Dissociable learning processes underlie human pain conditioning. Curr Biol (2016) 26(1):52–8. doi:10.1016/j.cub.2015.10.066
73. Toleman EC. The determiners of behavior at a choice point. Psychol Rev (1938) 45:1–41. doi:10.1037/h0062733
74. Grigson PS. Like drugs for chocolate: separate rewards modulated by common mechanisms? Physiol Behav (2002) 76(3):389–95. doi:10.1016/S0031-9384(02)00779-5
75. McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science (2004) 306(5695):503–7. doi:10.1126/science.1100907
76. Farb N, Daubenmier J, Price CJ, Gard T, Kerr C, Dunn BD, et al. Interoception, contemplative practice, and health. Front Psychol (2015) 6:763. doi:10.3389/fpsyg.2015.00763
77. Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol (2015) 227:261–84. doi:10.1007/978-3-662-46450-2_13
78. Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp (2014) 35(2):527–38. doi:10.1002/hbm.22199
79. Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain (2000) 84:65–75. doi:10.1016/S0304-3959(99)00183-9
80. Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci (2013) 14(6):417–28. doi:10.1038/nrn3492
81. Milad MR, Rosenbaum BL, Simon NM. Neuroscience of fear extinction: implications for assessment and treatment of fear-based and anxiety related disorders. Behav Res Ther (2014) 62:17–23. doi:10.1016/j.brat.2014.08.006
82. Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett (1993) 163(1):109–13. doi:10.1016/0304-3940(93)90241-C
83. Giustino TF, Maren S. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front Behav Neurosci (2015) 9:298. doi:10.3389/fnbeh.2015.00298
84. Burton AR, Fazalbhoy A, Macefield VG. Sympathetic responses to noxious stimulation of muscle and skin. Front Neurol (2016) 7:109. doi:10.3389/fneur.2016.00109
85. Coizet V, Dommett EJ, Klop EM, Redgrave P, Overton PG. The parabrachial nucleus is a critical link in the transmission of short latency nociceptive information to midbrain dopaminergic neurons. Neuroscience (2010) 168(1):263–72. doi:10.1016/j.neuroscience.2010.03.049
86. Craske MG, Rauch SL, Ursano R, Prenoveau J, Pine DS, Zinbarg RE. What is an anxiety disorder? Depress Anxiety (2009) 26(12):1066–85. doi:10.1002/da.20633
87. Stockhorst U, Antov MI. Modulation of fear extinction by stress, stress hormones and estradiol: a review. Front Behav Neurosci (2015) 9:359. doi:10.3389/fnbeh.2015.00359
88. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron (2016) 89(1):11–36. doi:10.1016/j.neuron.2015.11.027
89. McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, et al. Mechanisms of stress in the brain. Nat Neurosci (2015) 18(10):1353–63. doi:10.1038/nn.4086
90. Chalmers JA, Quintana DS, Abbott MJ, Kemp AH. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiatry (2014) 5:80. doi:10.3389/fpsyt.2014.00080
91. Staud R. Heart rate variability as a biomarker of fibromyalgia syndrome. Fut Rheumatol (2008) 3(5):475–83. doi:10.2217/17460816.3.5.475
92. Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev (2016) 68:282–97. doi:10.1016/j.neubiorev.2016.05.033
93. Ehrenreich H, Schuck J, Stender N, Pilz J, Gefeller O, Schilling L, et al. Endocrine and hemodynamic effects of stress versus systemic CRF in alcoholics during early and medium term abstinence. Alcohol Clin Exp Res (1997) 21(7):1285–93. doi:10.1111/j.1530-0277.1997.tb04450.x
94. Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res (2000) 24(12):1836–49. doi:10.1111/j.1530-0277.2000.tb01988.x
95. Missig GM, Linda M, Vizzard M, Braas KM, Wascheck JA, Ressler K, et al. Parabrachial PACAP activation of amygdala endosomal ERK signaling regulates the emotional component of pain. Biol Psychiatry (2016) 81(8):671–82. doi:10.1016/j.biopsych.2016.08.025
96. Harnett NG, Shumen JR, Wagle PA, Wood KH, Wheelock MD, Banos JH, et al. Neural mechanisms of human temporal fear conditioning. Neurobiol Learn Mem (2016) 136:97–104. doi:10.1016/j.nlm.2016.09.019
97. Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol (2008) 18(2):166–72. doi:10.1016/j.conb.2008.06.006
98. Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol (2006) 57:27–53. doi:10.1146/annurev.psych.56.091103.070234
99. Buff C, Brinkmann L, Neumeister P, Feldker K, Heitmann C, Gathmann B, et al. Specifically altered brain responses to threat in generalized anxiety disorder relative to social anxiety disorder and panic disorder. Neuroimage Clin (2016) 12:698–706. doi:10.1016/j.nicl.2016.09.023
100. Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry (2008) 65(5):568–76. doi:10.1001/archpsyc.65.5.568
101. Rachman S, Hodgson RI. Synchrony and desynchrony in fear and avoidance. Behav Res Ther (1974) 12(4):311–8. doi:10.1016/0005-7967(74)90005-9
102. Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron (2010) 66(1):149–60. doi:10.1016/j.neuron.2010.03.002
103. Barry DT, Cutter CJ, Beitel M, Kerns RD, Liong C, Schottenfeld RS. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry (2016) 77(10):1413–9. doi:10.4088/JCP.15m09963
104. Mazei-Robison MS, Nestler EJ. Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harb Perspect Med (2012) 2(7):a012070. doi:10.1101/cshperspect.a012070
105. Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain Res Brain Res Rev (2000) 31(2–3):371–84. doi:10.1016/S0165-0173(99)00054-5
106. O’Donnell P, Grace AA. Dysfunctions in multiple interrelated systems as the neurobiological bases of schizophrenic symptom clusters. Schizophr Bull (1998) 24(2):267–83. doi:10.1093/oxfordjournals.schbul.a033325
107. Grace AA, Moore H, O’Donnell P. The modulation of corticoaccumbens transmission by limbic afferents and dopamine: a model for the pathophysiology of schizophrenia. Adv Pharmacol (1998) 42:721–4. doi:10.1016/S1054-3589(08)60849-2
108. O’Donnell P, Grace AA. Phencyclidine interferes with the hippocampal gating of nucleus accumbens neuronal activity in vivo. Neuroscience (1998) 87(4):823–30. doi:10.1016/S0306-4522(98)00190-0
109. Elman I, Zubieta JK, Borsook D. The missing p in psychiatric training: why it is important to teach pain to psychiatrists. Arch Gen Psychiatry (2011) 68(1):12–20. doi:10.1001/archgenpsychiatry.2010.174
110. Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology (2006) 31(10):2091–120. doi:10.1038/sj.npp.1301051
111. Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol (2013) 109:1–27. doi:10.1016/j.pneurobio.2013.06.003
113. Sommer IE, Slotema CW, Daskalakis ZJ, Derks EM, Blom JD, van der Gaag M. The treatment of hallucinations in schizophrenia spectrum disorders. Schizophr Bull (2012) 38(4):704–14. doi:10.1093/schbul/sbs034
114. Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, et al. Buprenorphine/naloxone collaborative study group. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med (2003) 349(10):949–58. doi:10.1056/NEJMoa022164
115. Mooney ME, Herin DV, Specker S, Babb D, Levin FR, Grabowski J. Pilot study of the effects of lisdexamfetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend (2015) 153:94–103. doi:10.1016/j.drugalcdep.2015.05.042
116. Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories – indications for novel treatments of addiction. Eur J Neurosci (2014) 40(1):2163–82. doi:10.1111/ejn.12644
117. Haass-Koffler CL, Bartlett SE. Stress and addiction: contribution of the corticotropin releasing factor (CRF) system in neuroplasticity. Front Mol Neurosci (2012) 5:91. doi:10.3389/fnmol.2012.00091
118. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology (2010) 35(1):217–38. doi:10.1038/npp.2010.4
119. Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane review. Spine (Phila Pa 1976) (2014) 39(7):556–63. doi:10.1097/BRS.0000000000000249
120. Han MH, Bolanos CA, Green TA, Olson VG, Neve RL, Liu RJ, et al. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci (2006) 26(17):4624–9. doi:10.1523/JNEUROSCI.4701-05.2006
121. Nestler EJ. Reflections on: “a general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function”. Brain Res (2016) 1645:71–4. doi:10.1016/j.brainres.2015.12.039
122. Katz N, Mazer NA. The impact of opioids on the endocrine system. Clin J Pain (2009) 25(2):170–5. doi:10.1097/AJP.0b013e3181850df6
123. Martinez V, Beloeil H, Marret E, Fletcher D, Ravaud P, Trinquart L. Non-opioid analgesics in adults after major surgery: systematic review with network meta-analysis of randomized trials. Br J Anaesth (2017) 118(1):22–31. doi:10.1093/bja/aew391
124. Derry S, Stannard C, Cole P, Wiffen PJ, Knaggs R, Aldington D, et al. Fentanyl for neuropathic pain in adults. Cochrane Database Syst Rev (2016) 10:CD011605. doi:10.1002/14651858.CD011605.pub2
125. McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst Rev (2013) 8:CD006146. doi:10.1002/14651858.CD006146.pub2
126. Edelman GM, Gally JA. Reentry: a key mechanism for integration of brain function. Front Integr Neurosci (2013) 7:63. doi:10.3389/fnint.2013.00063
127. Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci (2016) 19(4):533–41. doi:10.1038/nn.4269
128. Bonicalzi V, Canavero S, Cerutti F, Piazza M, Clemente M, Chio A. Lamotrigine reduces total postoperative analgesic requirement: a randomized double-blind, placebo-controlled pilot study. Surgery (1997) 122(3):567–70. doi:10.1016/S0039-6060(97)90129-X
129. Gegelashvili G, Bjerrum OJ. High-affinity glutamate transporters in chronic pain: an emerging therapeutic target. J Neurochem (2014) 131(6):712–30. doi:10.1111/jnc.12957
130. Vuckovic S, Srebro D, Savic Vujovic K, Prostran M. The antinociceptive effects of magnesium sulfate and MK-801 in visceral inflammatory pain model: the role of NO/cGMP/K(+)ATP pathway. Pharm Biol (2015) 53(11):1621–7. doi:10.3109/13880209.2014.996821
131. Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol (2014) 77(2):357–67. doi:10.1111/bcp.12094
132. Zhuo M. Ionotropic glutamate receptors contribute to pain transmission and chronic pain. Neuropharmacology (2017) 112(Pt A):228–34. doi:10.1016/j.neuropharm.2016.08.014
133. Coderre TJ. The role of excitatory amino acid receptors and intracellular messengers in persistent nociception after tissue injury in rats. Mol Neurobiol (1993) 7(3–4):229–46. doi:10.1007/BF02769177
134. Yehuda R, McFarlane AC, Shalev AY. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry (1998) 44(12):1305–13. doi:10.1016/S0006-3223(98)00276-5
135. McGhee LL, Maani CV, Garza TH, Gaylord KM, Black IH. The correlation between ketamine and posttraumatic stress disorder in burned service members. J Trauma (2008) 64(2 Suppl):S195–8. doi:10.1097/TA.0b013e318160ba1d
136. Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev (2006) 30(1):97–117. doi:10.1016/j.neubiorev.2005.07.002
137. Rolls ET. Limbic systems for emotion and for memory, but no single limbic system. Cortex (2015) 62:119–57. doi:10.1016/j.cortex.2013.12.005
138. Newman J, Grace AA. Binding across time: the selective gating of frontal and hippocampal systems modulating working memory and attentional states. Conscious Cogn (1999) 8(2):196–212. doi:10.1006/ccog.1999.0392
139. Belujon P, Grace AA. Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proc Biol Sci (2015) 282:2516. doi:10.1098/rspb.2014.2516
140. Evers AW, Kraaimaat FW, van Riel PL, Bijlsma JW. Cognitive, behavioral and physiological reactivity to pain as a predictor of long-term pain in rheumatoid arthritis patients. Pain (2001) 93(2):139–46. doi:10.1016/S0304-3959(01)00303-7
141. Dellemijn P. Are opioids effective in relieving neuropathic pain? Pain (1999) 80:453–62. doi:10.1016/S0304-3959(98)00256-5
142. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet (2006) 367:1618–25. doi:10.1016/S0140-6736(06)68700-X
143. Baird E, Williams ACC, Hearn L, Amris K. Interventions for treating persistent pain in survivors of torture. Cochrane Database Syst Rev (2017) 8:CD012051. doi:10.1002/14651858.CD012051.pub2
Keywords: amygdala, cognitive, fear, nociception, sensory, unconscious
Citation: Elman I and Borsook D (2018) Threat Response System: Parallel Brain Processes in Pain vis-à-vis Fear and Anxiety. Front. Psychiatry 9:29. doi: 10.3389/fpsyt.2018.00029
Received: 09 November 2017; Accepted: 25 January 2018;
Published: 20 February 2018
Edited by:
Roumen Kirov, Institute of Neurobiology (BAS), BulgariaReviewed by:
Valentina Nicolardi, Sapienza Università di Roma, ItalyCopyright: © 2018 Elman and Borsook. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Igor Elman, aWdvci5lbG1hbkB3cmlnaHQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.