- 1Department of Pharmacy, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, China
- 2Department of Pediatrics, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, China
Background: Our study is an analysis of multiple publications involving assessing the comparable efficacy and tolerability of six interventions, which are lisdexamfetamine dimesylate (LDX), atomoxetine (ATX), methylphenidate (MPH), clonidine hydrochloride (CLON), guanfacine extended release (GXR), and bupropion, for young patients (6–18 years old) suffering from attention deficit hyperactivity disorder (ADHD).
Methods: A conventional meta-analysis (MA) was performed to give direct comparisons and a network meta-analysis (NMA) was used to show the combination of direct and indirect evidence. Ranking preference for all the interventions under a certain outcome was given by the surface of cumulative ranking curve area (SUCRA).
Results: Overall, 15,025 participants from 73 studies were involved in our analysis. In the pairwise MA, LDX was associated with less withdrawal than ATX for lack of efficacy. MPH showed less effectiveness than LDX according to ADHD Rating Scale score. Based on the analysis of our NMA, significant results of efficacy that LDX is a competitive drug were observed when evaluating LDX in comparison with other drugs except for CLON. ATX and GXR presented higher rates of abdominal pain morbidity versus inactive treatment.
Conclusion: The stimulants LDX and MPH are still highly recommended because they are highly effective and are tolerated well by patients. Among the non-stimulants, CLON can be taken into consideration for its appreciable effectiveness and tolerability. ATX and GXR can be seen as moderate choices.
Introduction
Attention deficit hyperactivity disorder (ADHD) is a common kind of psychological behavior disorder that occurs in approximately 5% of children or adolescents (6–18 years of age) worldwide (1, 2). It is a relatively long-lasting disorder, the effects of which can be present for several years and in some cases even last an entire lifetime (2). Children or adolescents with ADHD are characterized by behaviors such as inattention, hyperactivity, and impulsivity (3). Such symptoms are usually detected during youth, between the ages of three and six (2). ADHD has increasingly attracted the attention of parents, doctors, and scientists because of its severely impact on daily activities in patients’ academic and social life (1). The exact causes of ADHD are unclear, but it is thought that it is the result of an imbalance of catecholamine metabolism in the cerebral cortex, or inhibitory dopaminergic and decrease of noradrenergic activities, or a mixture of the two (1, 4).
Drugs therapies treating ADHD can be classified into dopaminergic and noradrenergic pathways (4). Several drugs have been employed for patients suffering from ADHD, including stimulants and non-stimulants. For example, atomoxetine (ATX) is widely used and is considered as a non-psychostimulant (5). It can reduce the symptoms of ADHD and has other clinical advantages such as drowsiness, comorbidities with tics, and anxiety (6). Bupropion (BUP) is a monocyclic phenylaminoketone structurally related to the phenylisopropylamines, with significant antidepressant effects (7). Clonidine hydrochloride (CLON) is α2-adrenergic agonist developed to reduce and delay the release and has recently been used in combination with psychostimulants to treat ADHD (8, 9). Guanfacine is the most widely prescribed psychostimulants as the selective α2A-adrenoceptor agonist for the treatment of ADHD. The efficacy of guanfacine extended release (GXR) is considered to be among first-line treatments for ADHD (10). Lisdexamfetamine dimesylate (LDX) is a stimulant, normally used as a monotherapy or supplement for psychostimulants in ADHD treatment (10, 11). In general, LDX is employed as prodrug due to its fast absorption and hydroxylation, which leads to a gradual and long lasting release (12–14). Methylphenidate (MPH) is a psych stimulant and is considered as the first-line therapy (6). It can increase the concentration of serotonin, dopamine, and norepinephrine to control the extent of inattention and impulsivity (5). MPH is also recognized for its significant antidepressant effects (7). All of the treatments described above have been effective in the treatment of ADHD, but there are aspects of their relative efficacy and safety that still remain unclear. Therefore, it is necessary to establish a system of evaluation in order to make comparisons among these various therapies.
A variety of studies have been conducted in the attempt to create a system of comparison for these diverse treatments, however, a large proportion of these studies have only focused on the pairwise comparison with placebo-controlled treatment (7, 15–19), or were restricted to three or four medications (20). Besides, the majority of these current literatures for systematic analysis have only considered the efficacy of these therapies without incorporating an evaluation of their safety (21). Moreover, the characteristics of the samples chosen in some studies are limited to small subgroups and this has led to limitations in comparing the differences between treatments (4). More importantly, previous studies have used different indicators to measure the efficacy of treatment options. This has led to inconsistencies and contradictions that further increase the need to perform a network meta-analysis (NMA) to estimate the effectiveness and reliability of medications used to cure ADHD.
Our study tries to combine studies involving seven interventions (non-stimulants: ATX, BUP, CLON, and GXR; stimulants: LDX and MPH). Seven outcomes have been considered, including ADHD Rating Scale (ADHD-RS), all cause withdrawal, withdrawal due to adverse event, withdrawal due to lack of efficacy, nausea, abdominal pain, and fatigue.
Materials and Methods
Publication Search
A systematic search was conducted in PubMed, Embase, Cochrane library, and CNKI (up to March 29, 2017), aiming to retrieve randomized controlled trials (RCTs) related to drug therapy in children and juveniles with ADHD. We used the following key words: “randomized controlled trial,” “attention deficit hyperactivity disorder” (including synonyms), and “drug therapy.” The cited articles of references including RCTs, systematic reviews or meta-analyses were also searched manually as supplementary material. Ethical approval was not needed for this study.
Inclusion Criteria
Type of study: mostly RCTs of a minimum of 3-week duration will be included in this review.
Type of participants: children and adolescents aged between 6 and 18 who meet Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria for ADHD.
Type of interventions: studies involving direct comparison with one drug therapy against another or against placebo will be included. All target interventions are ATX, CLON, GXR, BUP, LDX, and MPH.
Type of outcome measures: the efficacy is evaluated by ADHD-RS as a continuous score. And the adverse effects (as a dichotomous outcome) for tolerability are all cause withdrawals, withdraw due to adverse event, withdrawal due to lack of efficacy, nausea, abdominal pain, or fatigue for tolerability. Weight loss is not included because there is limited outcome on it.
Data Extraction
We extracted baseline data and evaluated the risk of bias, including selection bias, performance bias, detection bias and other bias, by the means of random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting. The assessment tool came from Cochrane Handbook (version 5.1.0) for RCTs. Data of interest were blinding, durations, diagnostic criteria, treatment, age of patients, number of patients, and assessment criteria for patients’ conditions.
Statistical Analysis
Conventional meta-analysis (MA) was performed by STATA 12.0 software, which gave us direct comparisons among these drugs, in the forms of the mean deviation (MD) with the corresponding 95% confidence interval (CI) for the primary outcomes of ADHD-RS, and the pooled odds ratios (ORs) with 95% CI for the secondary outcomes of tolerability. Cochran’s Q test (22) and the I2 test (23) were utilized to assess the degree of heterogeneity among studies. A fixed-effects model (Mantel-Haenszel method) was utilized if significant heterogeneity did not exist (P > 0.05 or I2 < 50%). Otherwise, a random-effects model was used.
This NMA was conducted using STATA 12.0 software and WinBUGS software, which showed us the combination of direct and indirect evidence. A Markov chain Monte Carlo method was applied to build Bayesian networks. The data presented in our NMA were similar to that in a pairwise MA. We illustrated the comparison of efficacy and tolerability by computing the MD and OR with the corresponding 95% credible interval (CrI) separately. Ranking preference for all the interventions under a certain outcome was given by the surface of the SUCRA, the value of which was 1 for the best and 0 for the worst.
There are five domains are bias due to (1) the randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported results (24). We used a comparison adjusted funnel plot to illustrate publication bias. Symmetry of the plots indicated no publication bias. P-value was a significant parameter to assess whether there was consistency in comparing direct and indirect evidence, and P < 0.05 showed a statistical inconsistency. The degree of consistency was illustrated by color in the heat plot.
Results
Characteristics of Trials and Patients
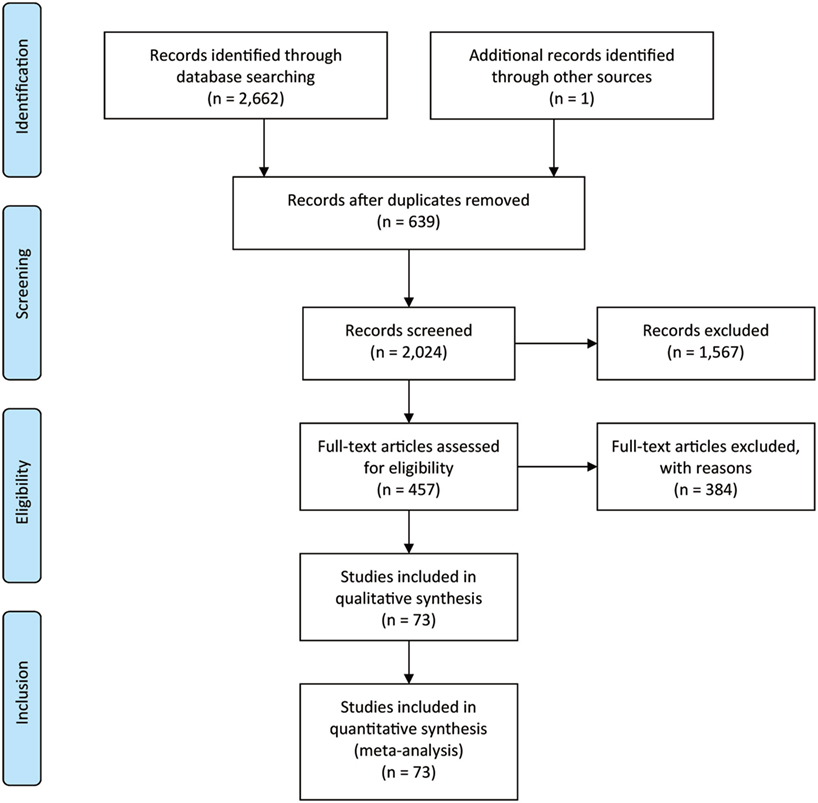
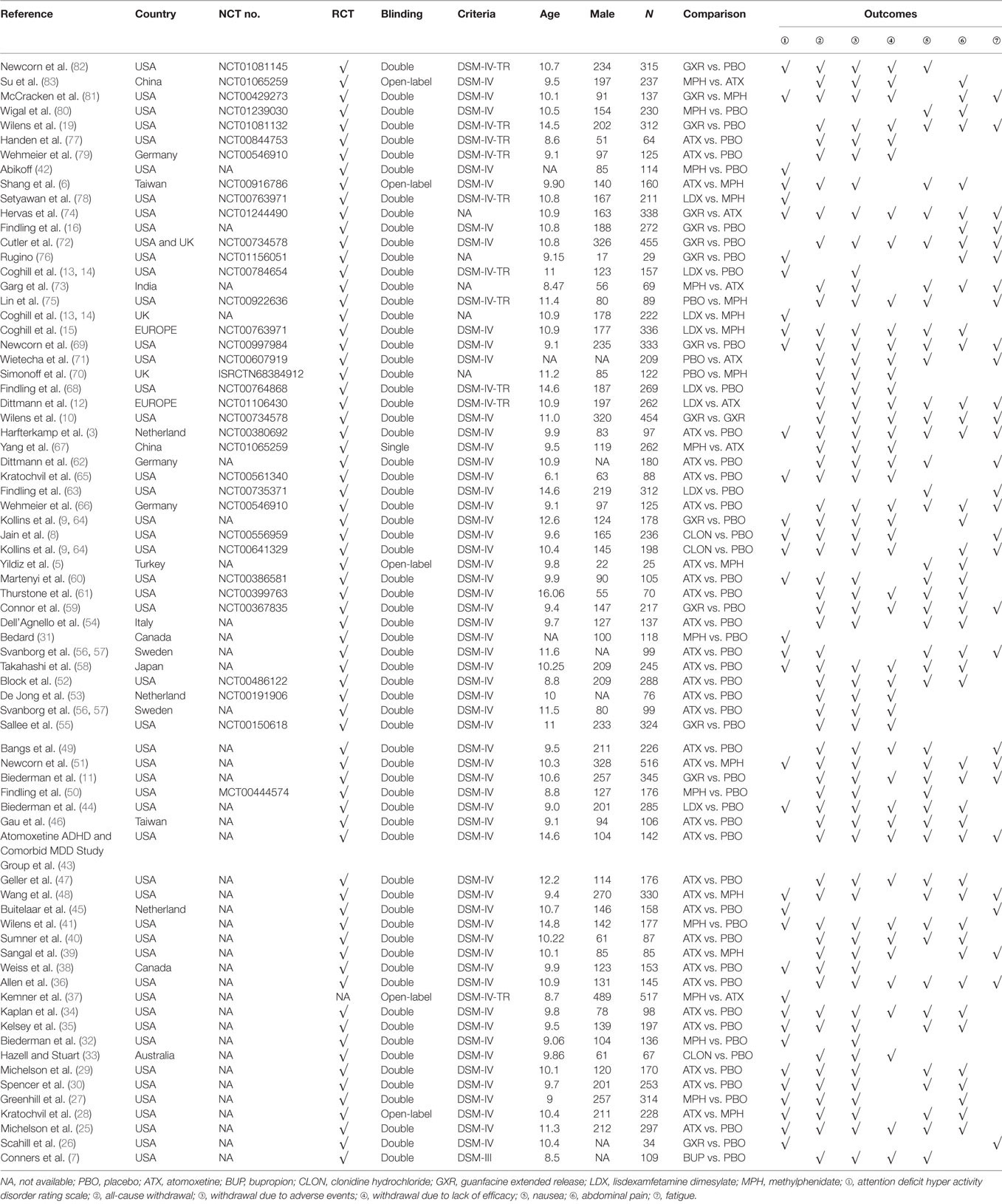
Figure 1 showed the process of literature retrieval and screening. A total of 2,024 studies were obtained after removing duplicates in the primary searches in databases and other sources. Among these studies, 1,567 trials were excluded by title and abstract, according to the inclusion criteria. Full-text reading finally enabled us to identify 73 qualitative trials as sources for data extraction (3, 5–16, 19, 25–83). Overall, 15,025 participants were involved in our analysis. Figures 2 and 3 showed the geometric distribution of RCTs for the included outcomes, which were related to different aspects in efficacy and tolerability. Placebo was taken as the control group in most RCTs. There were considerable quantities of patients in the research results for ATX, MPH, LDX, and GXR. However, the number of patients with BUP and CLON as interventions was limited. Baseline characteristics were listed in Table 1. Double blinding was adopted in most trials, with one single blinding and seven open-label trials.
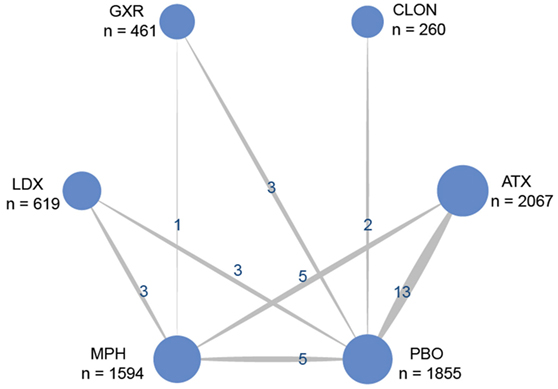
Figure 2. Network of randomized controlled trials (RCTs) comparing attention deficit hyperactivity disorder-rating scale (ADHD-RS) for attention deficit hyper activity disorder. The width of the lines is proportional to the number of trials comparing each pair of treatments and the numbers on the lines illustrate the exact number of trials included in the comparison; the area of the circles represents the cumulative number of patients for each intervention. PBO, placebo; ATX, atomoxetine; BUP, bupropion; CLON, clonidine hydrochloride; GXR, guanfacine extended release; LDX, lisdexamfetamine dimesylate; MPH, methylphenidate.
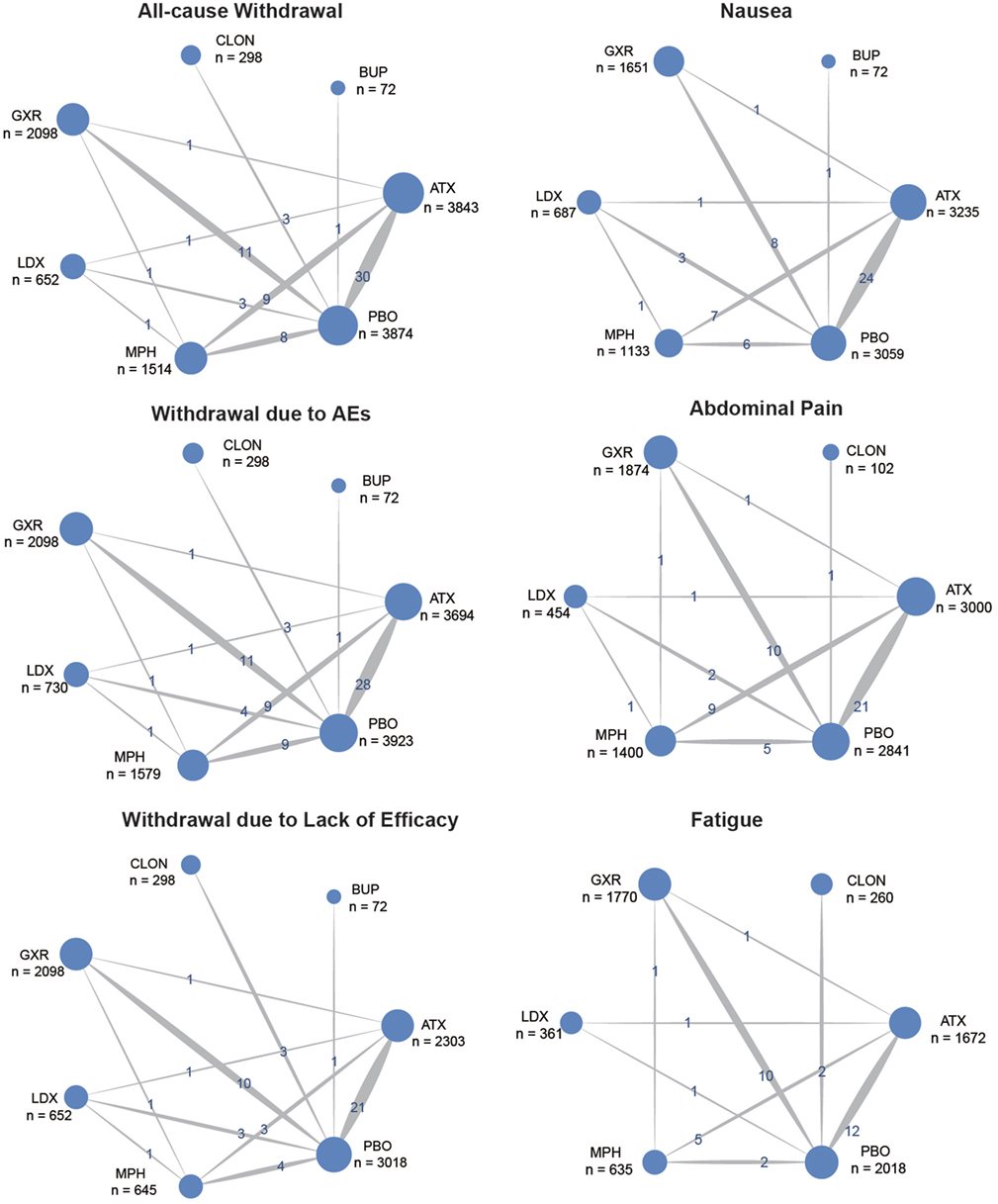
Figure 3. Network of randomized controlled trials (RCTs) comparing secondary outcomes of different treatments for attention deficit hyperactivity disorder. The width of the lines is proportional to the number of trials comparing each pair of treatments and the numbers on the lines illustrate the exact number; the area of the circles represents the cumulative number of patients for each intervention. PBO, placebo; ATX, atomoxetine; BUP, bupropion; CLON, clonidine hydrochloride; GXR, guanfacine extended release; LDX, lisdexamfetamine dimesylate; MPH, methylphenidate.
Result from Pairwise MA
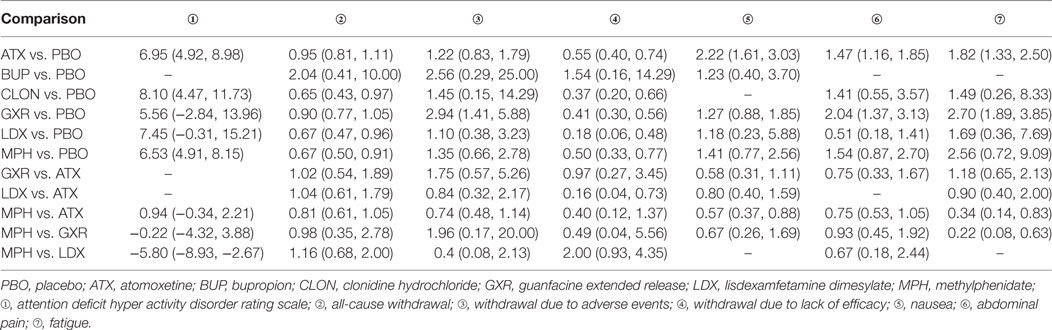
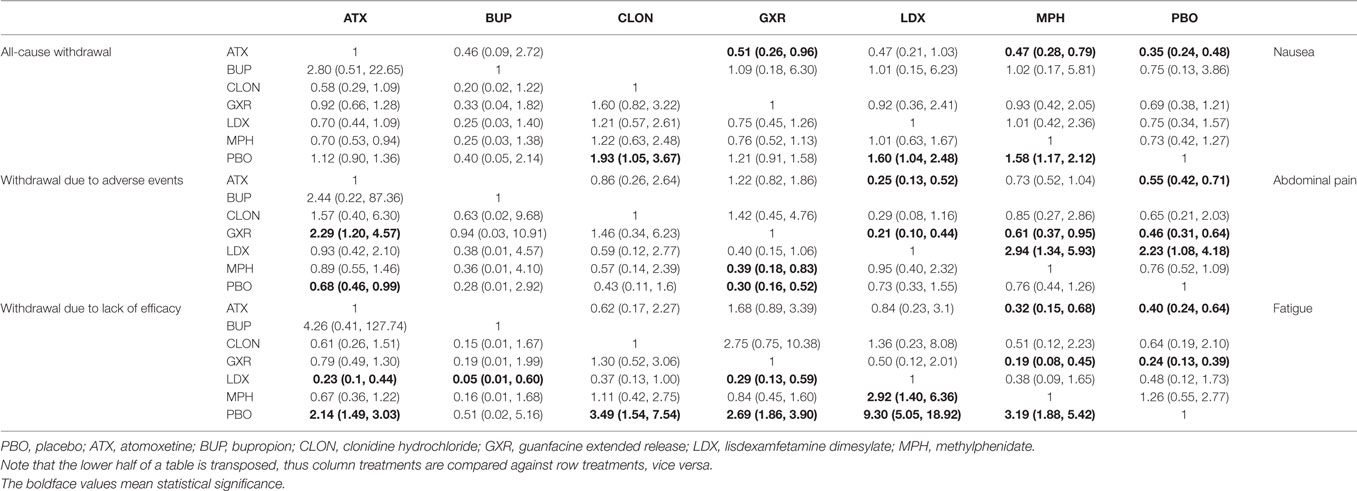
The results of the direct MA were shown in Table 2. All the included therapies had comparisons with placebo. In comparison to placebo, ATX had significantly better ADHD-RS. (MD = 6.95, 95% CI: 4.92–8.98) and decreasing withdrawal due to lack of efficacy (OR = 0.55, 95% CI: 0.40–0.74), but an increase the chance of adverse events (nausea: OR = 2.22, 95% CI: 1.61–3.03; abdominal pain: OR = 1.47, 95% CI: 1.16–1.85; fatigue: OR = 1.82, 95% CI: 1.33–2.50). There were no statistically significant results in the comparison of BUP versus placebo. CLON showed great improvement in the primary outcome of ADHD-RS (MD = 8.10, 95% CI: 4.47–11.73) and less withdrawal was observed (all-cause withdrawal: OR = 0.65, 95% CI: 0.43–0.97; withdrawal due to lack of efficacy: OR = 0.37, 95% CI: 0.20–0.66). Comparing GXR versus placebo, significant results were obtained concerning its ability to cause adverse effects (abdominal pain: OR = 2.04, 95% CI: 1.37–3.13; fatigue: OR = 2.70, 95% CI: 1.89–3.85) and related withdrawal due to adverse events (OR = 2.94, 95% CI: 1.41–5.88), but the chance of withdrawal due to lack of efficacy was reduced (OR = 0.41, 95% CI: 0.30–0.56). LDX significantly resulted in less withdrawal (all-cause withdrawal: OR = 0.67, 95% CI: 0.47–0.96; withdrawal due to lack of efficacy: OR = 0.18, 95% CI: 0.06–0.48) versus placebo. MPH showed a similar decrease in withdrawal (all-cause withdrawal: OR = 0.67, 95% CI: 0.50–0.91; withdrawal due to lack of efficacy: OR = 0.50, 95% CI: 0.33–0.77), plus an improvement in ADHD-RS (MD = 6.53, 95% CI: 4.91–8.15). In the direct comparisons among the seven interventions, LDX was associated with less withdrawal due to lack of efficacy (OR = 0.16, 95% CI: 0.04–0.73) than ATX and MPH showed less effectiveness than LDX according to ADHD-RS (MD = −5.80, 95% CI: −8.93 to −2.67).
Results from NMA
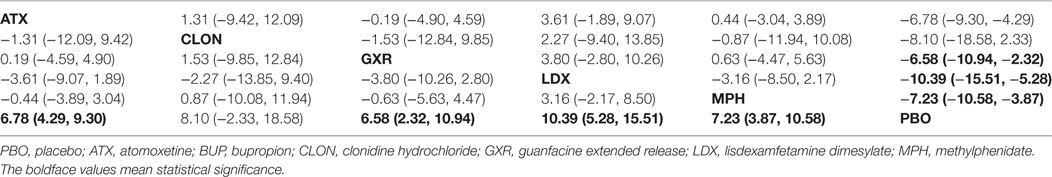
Bayesian models allowed for more refined estimates. Comparisons without direct connection were compared indirectly through Bayesian NMA. Available data from NMA was recorded in Tables 3 and 4 and the results were graphically presented in the forest plots in Figures 4–6. For ADHD-RS, statistically significant improvement was obtained in comparisons with placebo (ATX: MD = 6.78, 95% CrI: 4.29–9.30; GXR: MD = 6.58, 95% CrI: 2.32–10.94; LDX: MD = 10.39, 95% CrI: 5.28–15.51; MPH: MD = 7.23, 95% CrI: 3.87–10.58). In terms of all-cause withdrawal, a significant decrease was observed in CLON, LDX and MPH versus placebo (CLON: OR = 0.52, 95% CrI: 0.27–0.96; LDX: OR = 0.63, 95% CrI: 0.40–0.96; MPH: OR = 0.63, 95% CrI: 0.47–0.85). For withdrawal due to adverse events, ATX and GXR showed a higher possibility versus placebo (ATX: OR = 1.48, 95% CrI: 1.01–2.18; GXR: OR = 3.39, 95% CrI: 1.93–6.30). GXR showed more association with adverse response which had led to withdrawal than ATX (OR = 2.29, 95% CrI: 1.20–4.57), and MPH presented reduction versus GXR (OR = 0.39, 95% CrI: 0.18–0.83). When it came to withdrawal caused by lack of efficacy, all interventions except for BUP presented greater effectiveness than placebo (ATX: OR = 0.47, 95% CrI: 0.33–0.67; CLON: OR = 0.29, 95% CrI: 0.13–0.65; GXR: OR = 0.37, 95% CrI: 0.26–0.54; LDX: OR = 0.11, 95% CrI: 0.05–0.20; MPH: OR = 0.31, 95% CrI: 0.18–0.53). Significant results were acquired when evaluating LDX with other drugs except for CLON (ATX: OR = 0.23, 95% CrI: 0.10–0.44; BUP: OR = 0.05, 95% CrI: 0.01–0.60; GXR: OR = 0.29, 95% CrI: 0.13–0.59; MPH: OR = 0.34, 95% CrI: 0.16–0.72), which indicated the considerable clinical performance of LDX as the participants expected.
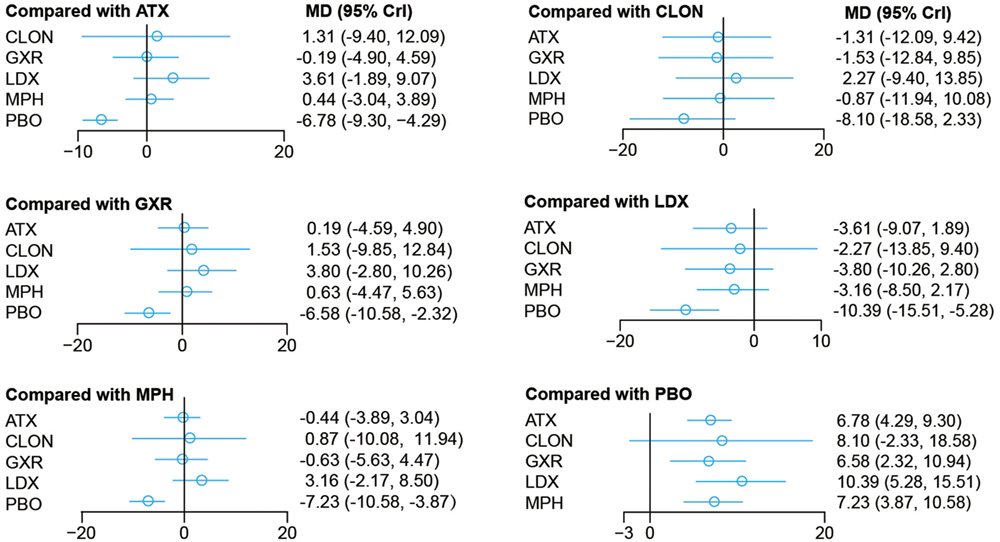
Figure 4. Odds ratios (95% credential intervals) for network comparison of ADHD-RS for attention deficit hyperactivity disorder. PBO, placebo; ATX, atomoxetine; BUP, bupropion; CLON, clonidine hydrochloride; GXR, guanfacine extended release; LDX, lisdexamfetamine dimesylate; MPH, methylphenidate.
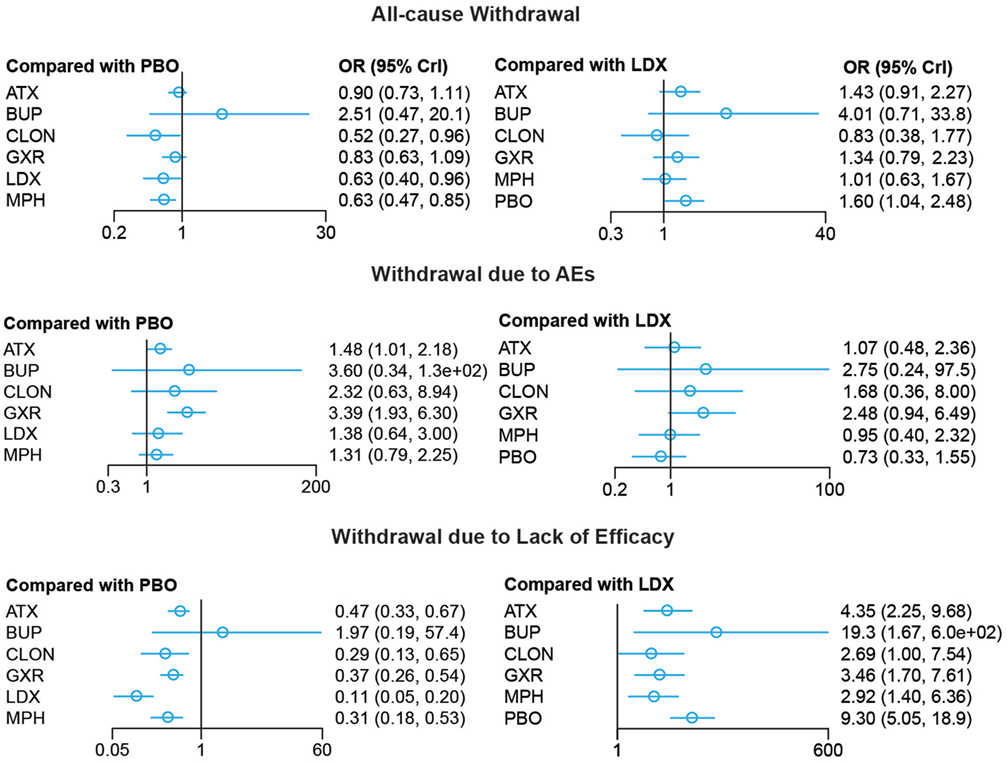
Figure 5. Odds ratios (95% credential intervals) for network comparison of withdrawal outcomes of different treatments for attention deficit hyperactivity disorder. PBO, placebo; ATX, atomoxetine; BUP, bupropion; CLON, clonidine hydrochloride; GXR, guanfacine extended release; LDX, lisdexamfetamine dimesylate; MPH, methylphenidate.
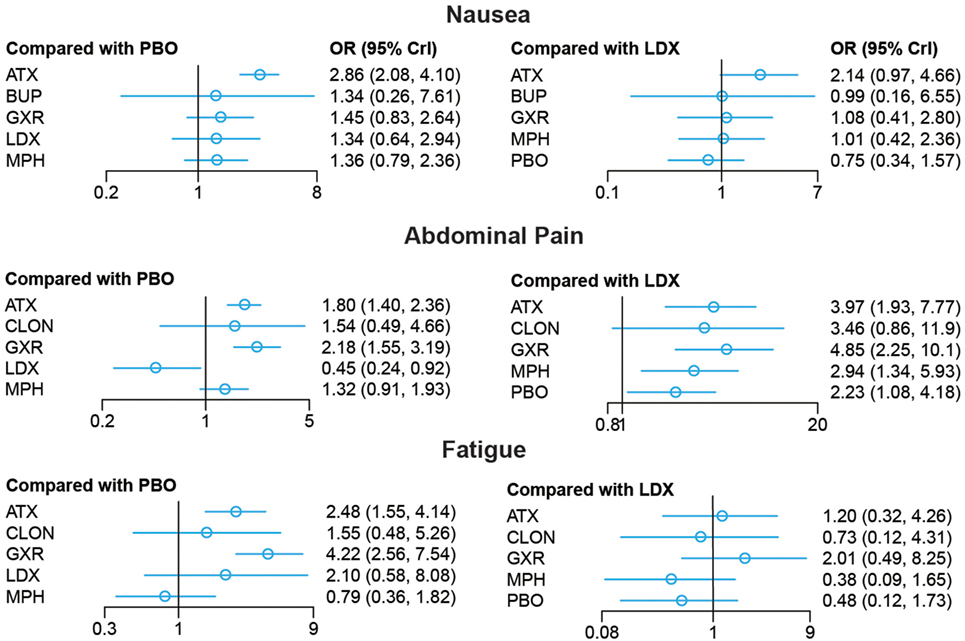
Figure 6. Odds ratios (95% credential intervals) for network comparison of adverse events of different treatments for attention deficit hyperactivity disorder. PBO, placebo; ATX, atomoxetine; BUP, bupropion; CLON, clonidine hydrochloride; GXR, guanfacine extended release; LDX, lisdexamfetamine dimesylate; MPH, methylphenidate.
For adverse effects, including nausea, abdominal pain and fatigue, the results presented further reinforced initial estimates. There was less occurrence of nausea in the groups treated with GXR, MPH and placebo than those with ATX (GXR: OR = 0.51, 95% CrI: 0.26–0.96; MPH: OR = 0.47, 95% CrI: 0.28–0.79; placebo: 0.35, 95% CrI: 0.24–0.48). Statistically significant data was available in comparing morbidity of abdominal pain. A statistical decrease of morbidity was observed in LDX versus other drugs except for CLON (ATX: OR = 0.25, 95% CrI: 0.13–0.52; GXR: OR = 0.21, 95% CrI: 0.10–0.44; MPH: OR = 0.34, 95% CrI: 0.17–0.75; placebo: OR = 0.45, 95% CrI: 0.24–0.92). ATX and GXR presented higher morbidity of abdominal pain versus inactive treatment (ATX: OR = 1.80, 95% CrI: 1.40–2.36; GXR: OR = 2.18, 95% CrI: 1.55–3.19). MPH presented less abdominal pain than GXR (OR = 0.61, 95% CrI: 0.37–0.95). Similarly, ATX and GXR presented more fatigue than placebo (ATX: OR = 2.48, 95% CrI: 1.55–4.14; GXR: OR = 4.22, 95% CrI: 2.56–7.54) and MPH resulted in less fatigue than ATX and GXR (ATX: OR = 0.32, 95% CrI: 0.15–0.68; GXR: OR = 0.19, 95% CrI: 0.08–0.45).
Ranking Scheme Based on SUCRA
The probability of being the best treatment was derived from SUCRA. The result was displayed in Figures 7 and 8. LDX and MPH could be considered as a group with the best comprehensive ranking score, including efficacy and tolerability. LDX had the highest probability of being the best efficacious drug therapy in decreasing ADHD symptoms (0.72) and remitting abdominal pain (0.82). MPH was located in the top three under all the outcomes except nausea. CLON ranked in the secondary group, but there was an absence of data related to nausea and abdominal pain. ATX and GXR had moderate rankings in efficacy, but were associated with the worst evaluation in the morbidity of adverse events. Though BUP was given a high ranking in reducing nausea, the results remained unclear due to the absence of essential information.
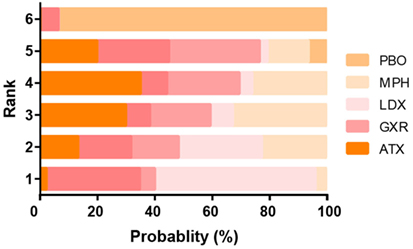
Figure 7. Ranking grams showing probability of each strategy having each specific rank (1–6) for attention deficit hyperactivity disorder-rating scale (ADHD-RS). Ranking indicates the quality of the individual treatment options, with one being the best and six being the worst. PBO, placebo; ATX, atomoxetine; BUP, bupropion; CLON, clonidine hydrochloride; GXR, guanfacine extended release; LDX, lisdexamfetamine dimesylate; MPH, methylphenidate.
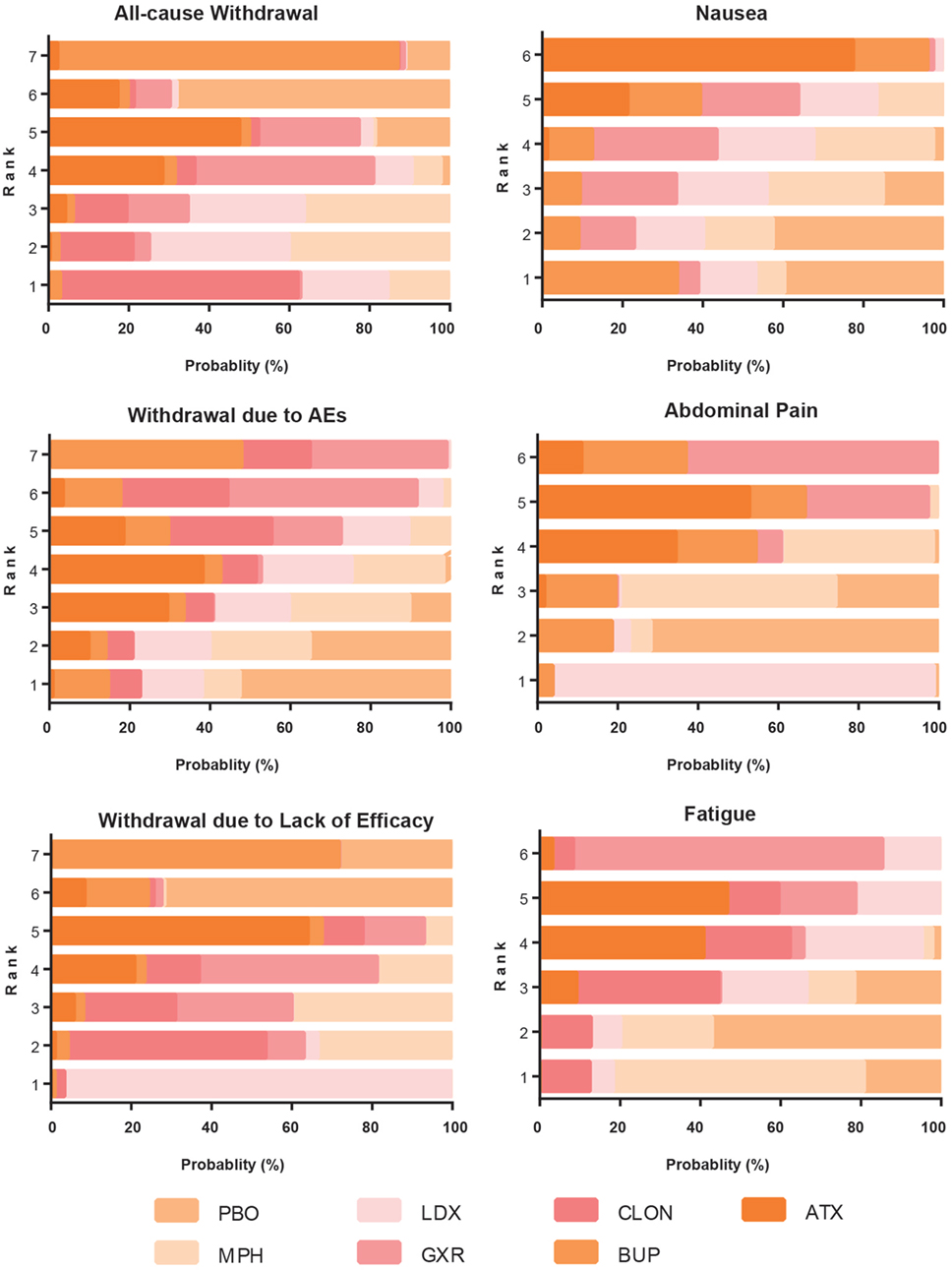
Figure 8. Ranking grams showing the probability of each strategy having each specific rank (1–7) for secondary outcomes. Ranking indicates the quality of the individual treatment options, with one being the best and seven being the worst. PBO, placebo; ATX, atomoxetine; BUP, bupropion; CLON, clonidine hydrochloride; GXR, guanfacine extended release; LDX, lisdexamfetamine dimesylate; MPH, methylphenidate.
Publication Bias and Consistency
The symmetry of the “comparison adjusted” funnel plots in Figure S1 in Supplementary Material suggested no publication bias. The small-study effect was limited in our research. According to the heat plot in Figure S2 in Supplementary Material, there were no statistically inconsistent results between direct and indirect evidence according to the blue color in most area, except for some ambiguity between LDX and MPH.
Discussion
This comprehensive NMA was conducted to compare the efficacy and tolerability of all interventions used to treat young ADHD patients. Our NMA study gave a comprehensive evaluation that included direct and indirect evidence extracted from previous studies. This NMA’s results indicated that the statistical differences between all interventions were compared successfully and their ranking orders under different criteria were determined, respectively. Therefore, it is possible for us to find the optimal treatment option by taking all the outcomes into account.
Stimulants, including LDX and MPH, have been comprehensively proven superior to non-stimulants in improving children and adolescents’ symptoms of ADHD both in documents and our NMA study. LDX presented the best efficacy. Previous studies have demonstrated that the mechanism of LDX for treating ADHD was related to the sufficient gradual-release of d-amphetamine by hydrolysis from LDX (44). Due to this feature, LDX could provide continuous effects throughout the day. LDX is an effective medication, but its level of toxicity is generally attracted additional attention because it frequently results in adverse events. Nevertheless, it has been confirmed that its toxicity can be controlled via changing the daily dosage (44), which makes it very competitive and consistent with our NMA result.
Besides, our results suggested that MPH is a good candidate, especially with regards to fatigue and the rate of withdrawal, which makes MPHas the routine therapy clinically. LDX is used for patients with ADHD who have an inadequate response to MPH (12, 14). Although MPH releases quickly in vivo as a stimulant, it can be controlled by modification, which is documented as osmotic release oral system (37, 41, 51, 83), leading to moderate side effects (20). It is worth noting that the adverse responses that have been selected in our NMA are related to mild symptoms. There are still arguments concerning the stimulant therapies. For example, some researchers have mentioned that stimulants are not recommended for patients with various cardiovascular problems because there have been reports of sudden death at usual doses and serious cardiovascular adverse events (68).
Non-stimulant therapies can reduce safety concerns to some extent, so they are expected to be substitutes for stimulants. We give an appreciable ranking score to CLON, and its performance in ADHD-RS shows that it tends to be superior to the stimulant MPH. Although its characteristics of releasing in a twinkling induces unwanted effects, for instance, somnolence, restfulness, and sleepiness, and its half-life is relatively short, leading to more side effects, dose optimization and extended-release formulation could be adapted to address the problems (8). However, the probability of overestimation remains due to the relatively limited sample size and direct evidence is absent to provide robust support. ATX and GXR are located at moderate positions under symptom improvement and rate of withdrawal. There are not significant differences in ADHD-RS when compared with another therapy, which indicated comparable efficacy. This result is consistent with existed evidence. However, the unsatisfying SUCRA ranking scores of ATX and GXR in nausea, abdominal pain and fatigue do not mean they are poorly tolerated or unsafe. Statistics about BUP should be updated so that a more comprehensive estimate can be presented.
The overall quality of literatures included can be considered relatively high in terms of publication time, the authority of the journals and the characteristics of the studies. Besides, this NMA study compared all available medications for ADHD and assessed their efficacy and tolerability under various outcomes. However, there are some limitations existing for our study. (1) It is difficult for us to exclude publication bias completely, especially for ADHD-RS and all-cause withdrawal. This may be a result of small sample size and lack of direct comparison between some therapies, such as GXR versus CLON, or CLON versus BUP. (2) Most of the studies compared active treatment to placebo and only a handful of comparative effectiveness studies. (3) Differences in samples, such as mean age, gender ratio, and growth background also affect the accuracy of our NMA. Thus, in order to take all the limitations mentioned above into consideration, better-designed RCT studies should be performed.
In summary, according to the results obtained from our NMA, the stimulants LDX and MPH are still highly recommended because they are highly efficacious and well tolerated by patients. Among the non-stimulants, CLON should be taken into consideration for its appreciable effectiveness and tolerability. ATX and GXR can be seen as moderate choices. We failed to evaluate BUP because of the lack of enhanced evidence. Even though this study provides some guidance for the effectiveness and safety of various medications, clinicians still need to use their professional judgment in assessing the benefits with the efficacy and reliability profile of the therapy, as well as patients’ features and preferences in order to make final treatment decisions.
Author Contributions
Conception or design of the work: RL and ZM. Data acquisition, analysis, and interpretation: FY. Drafting the work: RL and ZM. Revising article critically for important intellectual content: SH. Final approval of the version to be published: all authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/article/10.3389/fpsyt.2017.00229/full#supplementary-material.
Figure S1. Publication bias of all clinical outcomes. The “comparison adjusted” funnel plot evaluates the publication bias of all clinical outcomes with the standard error on the vertical axis.
Figure S2. Heat plot. Consistency can be checked by contrasting effect estimates from direct comparisons with indirect evidence of all clinical outcomes. In combination, we show heat colors corresponding to the change in agreement between direct and indirect estimate. The blue color means the two data possess good consistency while red color indicates inconsistency.
References
1. Punja S, Shamseer L, Hartling L, Urichuk L, Vandermeer B, Nikles J, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev (2016) 2:CD009996. doi:10.1002/14651858.CD009996.pub2
2. Tamminga HG, Reneman L, Huizenga HM, Geurts HM. Effects of methylphenidate on executive functioning in attention-deficit/hyperactivity disorder across the lifespan: a meta-regression analysis. Psychol Med (2016) 46:1791–807. doi:10.1017/S0033291716000350
3. Harfterkamp M, Van De Loo-Neus G, Minderaa RB, Van Der Gaag RJ, Escobar R, Schacht A, et al. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry (2012) 51:733–41. doi:10.1016/j.jaac.2012.04.011
4. Rezaei G, Hosseini SA, Akbari Sari A, Olyaeemanesh A, Lotfi MH, Yassini M, et al. Comparative efficacy of methylphenidate and atomoxetine in the treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review and meta-analysis. Med J Islam Repub Iran (2016) 30:325.
5. Yildiz O, Sismanlar SG, Memik NC, Karakaya I, Agaoglu B. Atomoxetine and methylphenidate treatment in children with ADHD: the efficacy, tolerability and effects on executive functions. Child Psychiatry Hum Dev (2011) 42:257–69. doi:10.1007/s10578-010-0212-3
6. Shang CY, Pan YL, Lin HY, Huang LW, Gau SS. An open-label, randomized trial of methylphenidate and atomoxetine treatment in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol (2015) 25:566–73. doi:10.1089/cap.2015.0035
7. Conners CK, Casat CD, Gualtieri CT, Weller E, Reader M, Reiss A, et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry (1996) 35:1314–21. doi:10.1097/00004583-199610000-00018
8. Jain R, Segal S, Kollins SH, Khayrallah M. Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry (2011) 50:171–9. doi:10.1016/j.jaac.2010.11.005
9. Kollins SH, Jain R, Brams M, Segal S, Findling RL, Wigal SB, et al. Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with ADHD. Pediatrics (2011) 127:e1406–13. doi:10.1542/peds.2010-1260
10. Wilens TE, Bukstein O, Brams M, Cutler AJ, Childress A, Rugino T, et al. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry (2012) 51:74–85.e2. doi:10.1016/j.jaac.2011.10.012
11. Biederman J, Melmed RD, Patel A, Mcburnett K, Konow J, Lyne A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics (2008) 121:e73–84. doi:10.1542/peds.2006-3695
12. Dittmann RW, Cardo E, Nagy P, Anderson CS, Bloomfield R, Caballero B, et al. Efficacy and safety of lisdexamfetamine dimesylate and atomoxetine in the treatment of attention-deficit/hyperactivity disorder: a head-to-head, randomized, double-blind, phase IIIb study. CNS Drugs (2013) 27:1081–92. doi:10.1007/s40263-013-0104-8
13. Coghill DR, Banaschewski T, Lecendreux M, Johnson M, Zuddas A, Anderson CS, et al. Maintenance of efficacy of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder: randomized-withdrawal study design. J Am Acad Child Adolesc Psychiatry (2014) 53:647–57.e1. doi:10.1016/j.jaac.2014.01.017
14. Coghill DR, Banaschewski T, Lecendreux M, Soutullo C, Zuddas A, Adeyi B, et al. Post hoc analyses of the impact of previous medication on the efficacy of lisdexamfetamine dimesylate in the treatment of attention-deficit/hyperactivity disorder in a randomized, controlled trial. Neuropsychiatr Dis Treat (2014) 10:2039–47. doi:10.2147/NDT.S68273
15. Coghill D, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol (2013) 23:1208–18. doi:10.1016/j.euroneuro.2012.11.012
16. Findling RL, Mcburnett K, White C, Youcha S. Guanfacine extended release adjunctive to a psychostimulant in the treatment of comorbid oppositional symptoms in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol (2014) 24:245–52. doi:10.1089/cap.2013.0103
17. Schwartz S, Correll CU. Efficacy and safety of atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: results from a comprehensive meta-analysis and metaregression. J Am Acad Child Adolesc Psychiatry (2014) 53:174–87. doi:10.1016/j.jaac.2013.11.005
18. Storebo OJ, Krogh HB, Ramstad E, Moreira-Maia CR, Holmskov M, Skoog M, et al. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ (2015) 351:h5203. doi:10.1136/bmj.h5203
19. Wilens TE, Robertson B, Sikirica V, Harper L, Young JL, Bloomfield R, et al. A randomized, placebo-controlled trial of guanfacine extended release in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry (2015) 54:916–25.e2. doi:10.1016/j.jaac.2015.08.016
20. Stuhec M, Munda B, Svab V, Locatelli I. Comparative efficacy and acceptability of atomoxetine, lisdexamfetamine, bupropion and methylphenidate in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis with focus on bupropion. J Affect Disord (2015) 178:149–59. doi:10.1016/j.jad.2015.03.006
21. Asherson P, Bushe C, Saylor K, Tanaka Y, Deberdt W, Upadhyaya H. Efficacy of atomoxetine in adults with attention deficit hyperactivity disorder: an integrated analysis of the complete database of multicenter placebo-controlled trials. J Psychopharmacol (2014) 28:837–46. doi:10.1177/0269881114542453
22. Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med (2012) 31:3805–20. doi:10.1002/sim.5453
23. Chang TW, Weinstein L. Prevention of herpes keratoconjunctivitis in rabbits by silver sulfadiazine. Antimicrob Agents Chemother (1975) 8:677–8. doi:10.1128/AAC.8.6.677
24. Cheng HY, Elbers RG, Higgins JP, Taylor A, Macarthur GJ, Mcguinness L, et al. Therapeutic interventions for alcohol dependence in non-inpatient settings: a systematic review and network meta-analysis (protocol). Syst Rev (2017) 6:77. doi:10.1186/s13643-017-0462-2
25. Michelson D, Faries D, Wernicke J, Kelsey D, Kendrick K, Sallee FR, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics (2001) 108:E83. doi:10.1542/peds.108.5.e83
26. Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry (2001) 158:1067–74. doi:10.1176/appi.ajp.158.7.1067
27. Greenhill LL, Findling RL, Swanson JM, ADHD Study Group. A double-blind, placebo-controlled study of modified-release methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics (2002) 109:E39. doi:10.1542/peds.109.3.e39
28. Kratochvil CJ, Heiligenstein JH, Dittmann R, Spencer TJ, Biederman J, Wernicke J, et al. Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry (2002) 41:776–84. doi:10.1097/00004583-200207000-00008
29. Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry (2002) 159:1896–901. doi:10.1176/appi.ajp.159.11.1896
30. Spencer T, Heiligenstein JH, Biederman J, Faries DE, Kratochvil CJ, Conners CK, et al. Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry (2002) 63:1140–7. doi:10.4088/JCP.v63n1209
31. Bedard AC, Ickowicz A, Logan GD, Hogg-Johnson S, Schachar R, Tannock R. Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. J Abnorm Child Psychol (2003) 31:315–27. doi:10.1023/A:1023285614844
32. Biederman J, Quinn D, Weiss M, Markabi S, Weidenman M, Edson K, et al. Efficacy and safety of Ritalin LA, a new, once daily, extended-release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Paediatr Drugs (2003) 5:833–41. doi:10.2165/00148581-200305120-00006
33. Hazell PL, Stuart JE. A randomized controlled trial of clonidine added to psychostimulant medication for hyperactive and aggressive children. J Am Acad Child Adolesc Psychiatry (2003) 42:886–94. doi:10.1097/01.CHI.0000046908.27264.00
34. Kaplan S, Heiligenstein J, West S, Busner J, Harder D, Dittmann R, et al. Efficacy and safety of atomoxetine in childhood attention-deficit/hyperactivity disorder with comorbid oppositional defiant disorder. J Atten Disord (2004) 8:45–52. doi:10.1177/108705470400800202
35. Kelsey DK, Sumner CR, Casat CD, Coury DL, Quintana H, Saylor KE, et al. Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics (2004) 114:e1–8. doi:10.1542/peds.114.1.e1
36. Allen AJ, Kurlan RM, Gilbert DL, Coffey BJ, Linder SL, Lewis DW, et al. Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology (2005) 65:1941–9. doi:10.1212/01.wnl.0000188869.58300.a7
37. Kemner JE, Starr HL, Ciccone PE, Hooper-Wood CG, Crockett RS. Outcomes of OROS methylphenidate compared with atomoxetine in children with ADHD: a multicenter, randomized prospective study. Adv Ther (2005) 22:498–512. doi:10.1007/BF02849870
38. Weiss M, Tannock R, Kratochvil C, Dunn D, Velez-Borras J, Thomason C, et al. A randomized, placebo-controlled study of once-daily atomoxetine in the school setting in children with ADHD. J Am Acad Child Adolesc Psychiatry (2005) 44:647–55. doi:10.1097/01.chi.0000163280.47221.c9
39. Sangal RB, Owens J, Allen AJ, Sutton V, Schuh K, Kelsey D. Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep (2006) 29:1573–85. doi:10.1093/sleep/29.12.1573
40. Sumner CR, Schuh KJ, Sutton VK, Lipetz R, Kelsey DK. Placebo-controlled study of the effects of atomoxetine on bladder control in children with nocturnal enuresis. J Child Adolesc Psychopharmacol (2006) 16:699–711. doi:10.1089/cap.2006.16.699
41. Wilens TE, Mcburnett K, Bukstein O, Mcgough J, Greenhill L, Lerner M, et al. Multisite controlled study of OROS methylphenidate in the treatment of adolescents with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med (2006) 160:82–90. doi:10.1001/archpedi.160.1.82
42. Abikoff HB, Vitiello B, Riddle MA, Cunningham C, Greenhill LL, Swanson JM, et al. Methylphenidate effects on functional outcomes in the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS). J Child Adolesc Psychopharmacol (2007) 17:581–92. doi:10.1089/cap.2007.0068
43. Atomoxetine ADHD and Comorbid MDD Study Group; Bangs ME, Emslie GJ, Spencer TJ, Ramsey JL, Carlson C, et al. Efficacy and safety of atomoxetine in adolescents with attention-deficit/hyperactivity disorder and major depression. J Child Adolesc Psychopharmacol (2007) 17:407–20. doi:10.1089/cap.2007.0066
44. Biederman J, Krishnan S, Zhang Y, Mcgough JJ, Findling RL. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther (2007) 29:450–63. doi:10.1016/S0149-2918(07)80083-X
45. Buitelaar JK, Michelson D, Danckaerts M, Gillberg C, Spencer TJ, Zuddas A, et al. A randomized, double-blind study of continuation treatment for attention-deficit/hyperactivity disorder after 1 year. Biol Psychiatry (2007) 61:694–9. doi:10.1016/j.biopsych.2006.03.066
46. Gau SS, Huang YS, Soong WT, Chou MC, Chou WJ, Shang CY, et al. A randomized, double-blind, placebo-controlled clinical trial on once-daily atomoxetine in Taiwanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol (2007) 17:447–60. doi:10.1089/cap.2006.0091
47. Geller D, Donnelly C, Lopez F, Rubin R, Newcorn J, Sutton V, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry (2007) 46:1119–27. doi:10.1097/chi.0b013e3180ca8385
48. Wang Y, Zheng Y, Du Y, Song D, Shin YJ, Cho S, et al. Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Aust N Z J Psychiatry (2007) 41:222–30. doi:10.1080/00048670601057767
49. Bangs ME, Hazell P, Danckaerts M, Hoare P, Coghill DR, Wehmeier PM, et al. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics (2008) 121:e314–20. doi:10.1542/peds.2006-1880
50. Findling RL, Bukstein OG, Melmed RD, Lopez FA, Sallee FR, Arnold LE, et al. A randomized, double-blind, placebo-controlled, parallel-group study of methylphenidate transdermal system in pediatric patients with attention-deficit/hyperactivity disorder. J Clin Psychiatry (2008) 69:149–59. doi:10.4088/JCP.v69n0120
51. Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, et al. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry (2008) 165:721–30. doi:10.1176/appi.ajp.2007.05091676
52. Block SL, Kelsey D, Coury D, Lewis D, Quintana H, Sutton V, et al. Once-daily atomoxetine for treating pediatric attention-deficit/hyperactivity disorder: comparison of morning and evening dosing. Clin Pediatr (Phila) (2009) 48:723–33. doi:10.1177/0009922809335321
53. De Jong CGW, Van De Voorde S, Roeyers H, Raymaekers R, Allen AJ, Knijff S, et al. Differential effects of atomoxetine on executive functioning and lexical decision in attention-deficit/hyperactivity disorder and reading disorder. J Child Adolesc Psychopharmacol (2009) 19:699–707. doi:10.1089/cap.2009.0029
54. Dell’Agnello G, Maschietto D, Bravaccio C, Calamoneri F, Masi G, Curatolo P, et al. Atomoxetine hydrochloride in the treatment of children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a placebo-controlled Italian study. Eur Neuropsychopharmacol (2009) 19:822–34. doi:10.1016/j.euroneuro.2009.07.008
55. Sallee FR, Mcgough J, Wigal T, Donahue J, Lyne A, Biederman J, et al. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry (2009) 48:155–65. doi:10.1097/CHI.0b013e318191769e
56. Svanborg P, Thernlund G, Gustafsson PA, Hägglöf B, Poole L, Kadesjö B. Efficacy and safety of atomoxetine as add-on to psychoeducation in the treatment of attention deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled study in stimulant-naïve Swedish children and adolescents. Eur Child Adolesc Psychiatry (2009) 18:240–9. doi:10.1007/s00787-008-0725-5
57. Svanborg P, Thernlund G, Gustafsson PA, Hägglöf B, Schacht A, Kadesjö B. Atomoxetine improves patient and family coping in attention deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled study in Swedish children and adolescents. Eur Child Adolesc Psychiatry (2009) 18:725–35. doi:10.1007/s00787-009-0031-x
58. Takahashi M, Takita Y, Yamazaki K, Hayashi T, Ichikawa H, Kambayashi Y, et al. A randomized, double-blind, placebo-controlled study of atomoxetine in Japanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol (2009) 19:341–50. doi:10.1089/cap.2008.0154
59. Connor DF, Findling RL, Kollins SH, Sallee F, Lopez FA, Lyne A, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs (2010) 24:755–68. doi:10.2165/11537790-000000000-00000
60. Martenyi F, Zavadenko NN, Jarkova NB, Yarosh AA, Soldatenkova VO, Bardenstein LM, et al. Atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: a 6-week, randomized, placebo-controlled, double-blind trial in Russia. Eur Child Adolesc Psychiatry (2010) 19:57–66. doi:10.1007/s00787-009-0042-7
61. Thurstone C, Riggs PD, Salomonsen-Sautel S, Mikulich-Gilbertson SK. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J Am Acad Child Adolesc Psychiatry (2010) 49:573–82. doi:10.1016/j.jaac.2010.02.013
62. Dittmann RW, Schacht A, Helsberg K, Schneider-Fresenius C, Lehmann M, Lehmkuhl G, et al. Atomoxetine versus placebo in children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a double-blind, randomized, multicenter trial in Germany. J Child Adolesc Psychopharmacol (2011) 21:97–110. doi:10.1089/cap.2009.0111
63. Findling RL, Childress AC, Cutler AJ, Gasior M, Hamdani M, Ferreira-Cornwell MC, et al. Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry (2011) 50:395–405. doi:10.1016/j.jaac.2011.01.007
64. Kollins SH, Lopez FA, Vince BD, Turnbow JM, Farrand K, Lyne A, et al. Psychomotor functioning and alertness with guanfacine extended release in subjects with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol (2011) 21:111–20. doi:10.1089/cap.2010.0064
65. Kratochvil CJ, Vaughan BS, Stoner JA, Daughton JM, Lubberstedt BD, Murray DW, et al. A double-blind, placebo-controlled study of atomoxetine in young children with ADHD. Pediatrics (2011) 127:e862–8. doi:10.1542/peds.2010-0825
66. Wehmeier PM, Schacht A, Wolff C, Otto WR, Dittmann RW, Banaschewski T. Neuropsychological outcomes across the day in children with attention-deficit/hyperactivity disorder treated with atomoxetine: results from a placebo-controlled study using a computer-based continuous performance test combined with an infra-red motion-tracking device. J Child Adolesc Psychopharmacol (2011) 21:433–44. doi:10.1089/cap.2010.0142
67. Yang L, Cao Q, Shuai L, Li H, Chan RC, Wang Y. Comparative study of OROS-MPH and atomoxetine on executive function improvement in ADHD: a randomized controlled trial. Int J Neuropsychopharmacol (2012) 15:15–26. doi:10.1017/S1461145711001490
68. Findling RL, Cutler AJ, Saylor K, Gasior M, Hamdani M, Ferreira-Cornwell MC, et al. A long-term open-label safety and effectiveness trial of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol (2013) 23:11–21. doi:10.1089/cap.2011.0088
69. Newcorn JH, Stein MA, Childress AC, Youcha S, White C, Enright G, et al. Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: morning or evening administration. J Am Acad Child Adolesc Psychiatry (2013) 52:921–30. doi:10.1016/j.jaac.2013.06.006
70. Simonoff E, Taylor E, Baird G, Bernard S, Chadwick O, Liang H, et al. Randomized controlled double-blind trial of optimal dose methylphenidate in children and adolescents with severe attention deficit hyperactivity disorder and intellectual disability. J Child Psychol Psychiatry (2013) 54:527–35. doi:10.1111/j.1469-7610.2012.02569.x
71. Wietecha L, Williams D, Shaywitz S, Shaywitz B, Hooper SR, Wigal SB, et al. Atomoxetine improved attention in children and adolescents with attention-deficit/hyperactivity disorder and dyslexia in a 16 week, acute, randomized, double-blind trial. J Child Adolesc Psychopharmacol (2013) 23:605–13. doi:10.1089/cap.2013.0054
72. Cutler AJ, Brams M, Bukstein O, Mattingly G, Mcburnett K, White C, et al. Response/remission with guanfacine extended-release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry (2014) 53:1092–101. doi:10.1016/j.jaac.2014.08.001
73. Garg J, Arun P, Chavan BS. Comparative short term efficacy and tolerability of methylphenidate and atomoxetine in attention deficit hyperactivity disorder. Indian Pediatr (2014) 51:550–4. doi:10.1007/s13312-014-0445-5
74. Hervas A, Huss M, Johnson M, Mcnicholas F, Van Stralen J, Sreckovic S, et al. Efficacy and safety of extended-release guanfacine hydrochloride in children and adolescents with attention-deficit/hyperactivity disorder: a randomized, controlled, phase III trial. Eur Neuropsychopharmacol (2014) 24:1861–72. doi:10.1016/j.euroneuro.2014.09.014
75. Lin DY, Kratochvil CJ, Xu W, Jin L, D’Souza DN, Kielbasa W, et al. A randomized trial of edivoxetine in pediatric patients with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol (2014) 24:190–200. doi:10.1089/cap.2013.0043
76. Rugino TA. Effect on primary sleep disorders when children with ADHD are administered guanfacine extended release. J Atten Disord (2014). doi:10.1177/1087054714554932
77. Handen BL, Aman MG, Arnold LE, Hyman SL, Tumuluru RV, Lecavalier L, et al. Atomoxetine, parent training, and their combination in children with autism spectrum disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry (2015) 54:905–15. doi:10.1016/j.jaac.2015.08.013
78. Setyawan J, Yang H, Cheng D, Cai X, Signorovitch J, Xie J, et al. Developing a risk score to guide individualized treatment selection in attention deficit/hyperactivity disorder. Value Health (2015) 18:824–31. doi:10.1016/j.jval.2015.06.005
79. Wehmeier PM, Kipp L, Banaschewski T, Dittmann RW, Schacht A. Does comorbid disruptive behavior modify the effects of atomoxetine on ADHD symptoms as measured by a continuous performance test and a motion tracking device? J Atten Disord (2015) 19:591–602. doi:10.1177/1087054712456739
80. Wigal SB, Nordbrock E, Adjei AL, Childress A, Kupper RJ, Greenhill L. Efficacy of methylphenidate hydrochloride extended-release capsules (aptensio XR) in children and adolescents with attention-deficit/hyperactivity disorder: a phase iii, randomized, double-blind study. CNS Drugs (2015) 29:331–40. doi:10.1007/s40263-015-0241-3
81. McCracken JT, Mcgough JJ, Loo SK, Levitt J, Del’Homme M, Cowen J, et al. Combined stimulant and guanfacine administration in attention-deficit/hyperactivity disorder: a controlled, comparative study. J Am Acad Child Adolesc Psychiatry (2016) 55:657–66.e1. doi:10.1016/j.jaac.2016.05.015
82. Newcorn JH, Harpin V, Huss M, Lyne A, Sikirica V, Johnson M, et al. Extended-release guanfacine hydrochloride in 6-17-year olds with ADHD: a randomised-withdrawal maintenance of efficacy study. J Child Psychol Psychiatry (2016) 57:717–28. doi:10.1111/jcpp.12492
83. Su Y, Yang L, Stein MA, Cao Q, Wang Y. Osmotic release oral system methylphenidate versus atomoxetine for the treatment of attention-deficit/hyperactivity disorder in chinese youth: 8-week comparative efficacy and 1-year follow-up. J Child Adolesc Psychopharmacol (2016) 26:362–71. doi:10.1089/cap.2015.0031
Keywords: attention deficit hyperactivity disorder, efficacy, tolerability, interventions, network meta-analysis
Citation: Luan R, Mu Z, Yue F and He S (2017) Efficacy and Tolerability of Different Interventions in Children and Adolescents with Attention Deficit Hyperactivity Disorder. Front. Psychiatry 8:229. doi: 10.3389/fpsyt.2017.00229
Received: 19 March 2017; Accepted: 25 October 2017;
Published: 13 November 2017
Edited by:
Fernando Rodriguez De Fonseca, Instituto de Investigación Biomédica de Málaga, SpainReviewed by:
Jorge Manzanares, Universidad Miguel Hernandez-CSIC, SpainScott Edwards, LSU Health Sciences Center New Orleans, United States
Copyright: © 2017 Luan, Mu, Yue and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoying He, c214XzFzeUAxMjYuY29t
†These authors have contributed equally to this work.
 Ruiling Luan1†
Ruiling Luan1† Shaoying He
Shaoying He