- 1Temerty Centre for Therapeutic Brain Intervention, Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, ON, Canada
- 2Faculty of Medicine, Department of Psychiatry, University of Toronto, Toronto, ON, Canada
- 3Mayo Clinic Depression Center, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, MN, United States
Adolescent depression is a prevalent disorder with substantial morbidity and mortality. Current treatment interventions do not target relevant pathophysiology and are frequently ineffective, thereby leading to a substantial burden for individuals, families, and society. During adolescence, the prefrontal cortex undergoes extensive structural and functional changes. Recent work suggests that frontolimbic development in depressed adolescents is delayed or aberrant. The judicious application of non-invasive brain stimulation techniques to the prefrontal cortex may present a promising opportunity for durable interventions in adolescent depression. Transcranial direct current stimulation (tDCS) applies a low-intensity, continuous current that alters cortical excitability. While this modality does not elicit action potentials, it is thought to manipulate neuronal activity and neuroplasticity. Specifically, tDCS may modulate N-methyl-d-aspartate receptors and L-type voltage-gated calcium channels and effect changes through long-term potentiation or long-term depression-like mechanisms. This mini-review considers the neurobiological rationale for developing tDCS protocols in adolescent depression, reviews existing work in adult mood disorders, surveys the existing tDCS literature in adolescent populations, reviews safety studies, and discusses distinct ethical considerations in work with adolescents.
Introduction
Transcranial direct current stimulation (tDCS) is a form of non-invasive brain stimulation (NIBS) that has demonstrated efficacy in treating depression (1). Although several authors have reviewed tDCS applications in pediatric patients with neuropsychiatric disorders (2–6), herein we review evidence supporting tDCS as a putative alternative to existing treatments for depressed adolescents.
Adolescent Depression and Unmet Needs
Up to one fifth of adolescents may experience major depressive disorder (MDD) before adulthood (7–9). Depressed adolescents experience comorbid psychiatric disorders (10) and recurrences (11), which compound health-care costs and contribute to considerable psychosocial impairment. Adolescent MDD can impact psychosocial development, education, and employability (12), costing society billions of dollars each year (13). Effective treatment options, however, remain limited.
Existing treatments for adolescent MDD, including selective serotonin reuptake inhibitors (SSRIs) and psychotherapy (14, 15), are only marginally effective (16, 17). Treatment adherence in this population is typically poor (18, 19) and limited by side effects (20). Moreover, ongoing controversies regarding the safety and efficacy of antidepressants in adolescents raise concern in clinicians and parents alike (21, 22).
Neurobiology of Adolescent Depression
Prior work has sought to characterize the neurobiology of depression across the lifespan (23, 24) with the goal of identifying biological treatment targets (25). Studies thus far have revealed differences in the neuronal structure and chemical pathways among depressed adolescents compared to healthy controls. Observed differences include heightened activity in the amygdala during facial-emotion recognition tasks (26) and greater connectivity between the amygdala and subgenual anterior cingulate cortex (ACC) (27). Functional imaging studies have revealed altered medial prefrontal cortical connectivity with brain regions involved in executive functioning, emotion regulation, attention, and reward-based decision making (28). These differences from healthy controls may resolve with psychopharmacological intervention (29).
One proton magnetic resonance spectroscopy (1H-MRS) study revealed decreased gamma-aminobutyric acid (GABA) levels in the ACC of depressed adolescents compared with healthy controls (30). GABA is an inhibitory neurotransmitter that plays a key role in the neurocircuitry of reward which, when dysregulated, may be linked to anhedonia. Another 1H-MRS study showed dysregulation in ratios of N-acetyl aspartate to creatine and choline to creatine in the dorsolateral prefrontal white matter (31). Other 1H-MRS studies of adolescents with treatment-resistant depression have shown elevated choline levels in the dorsolateral prefrontal gray matter (32, 33). Collectively, these disruptions may reflect decreased neuronal density and myelination, which may correlate with cognitive impairments commonly observed in depressed adolescents (34, 35). Despite these advances, however, the treatment of adolescent depression is most often a trial-and-error endeavor (25), and the feasibility of monitoring therapeutic response with biomarkers in clinical practice remains low (36).
Anodal tDCS is thought to be neuromodulatory, raising tonic excitation toward threshold levels, increasing the possibility that neurons will fire. Conversely, cathodal stimulation is thought to decrease cortical excitability (37, 38).
Efficacy in Adult MDD
The history of tDCS in psychiatry dates back to the late nineteenth century, when Tigges and Arndt first documented its antidepressant and antipsychotic properties (39, 40). With the reemergence of tDCS in the modern era (41), researchers have sought to develop this portable, low-cost treatment as a frontline intervention for depression (42). Table 1 provides an overview of sham-controlled trials that suggest tDCS is efficacious in mitigating symptoms of adult depression (1, 43–45). However, some experts have argued that these individual trials lack statistical power to detect therapeutic benefit (37), and others have questioned its clinical utility altogether (46, 47). For example, Bennabi et al. (46) found no difference between sham and active tDCS, but noted a subgroup of individuals in the active tDCS arm who experienced significant clinical improvement. The authors suggest that as yet unknown factors may contribute to, or detract from, tDCS response. Variations in stimulation parameters and duration of treatment, along with different degrees of treatment resistance and the presence or absence of concomitant medication therapy, complicate interpretation of the data. Some meta-analyses have found no beneficial effect (48), while others have shown that tDCS is efficacious in adult depression (49–51).
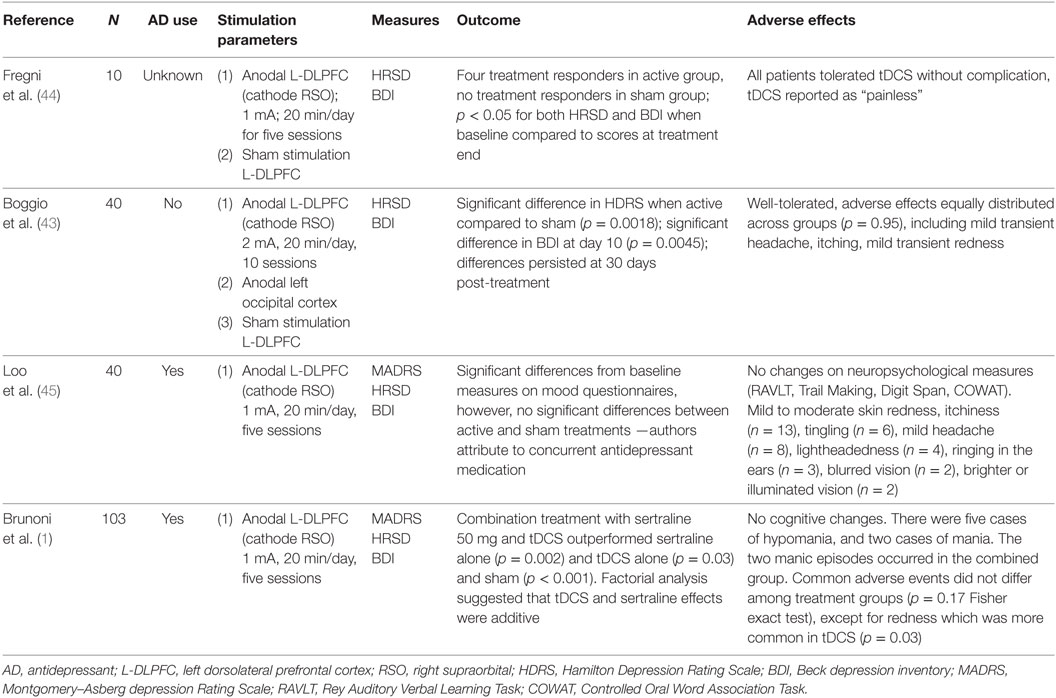
Table 1. Summary of sham-controlled clinical trials in adult major depressive disorder (MDD) reviewed.
Safety in Adults
Bikson and colleagues (52) published an extensive review on the safety literature available to date. Based on their review of current stimulation protocols and computer modeling studies, tDCS was considered safe. No serious adverse events were associated with its administration.
In a systematic review by Brunoni et al. (53), common side effects associated with tDCS—including headache, as well as localized itching, tingling, burning sensations, and discomfort—occurred at similar rates in active and sham stimulation. To date, tDCS has not been shown to induce neuronal damage. Nitsche et al. (54) did not find any elevations in neuronal specific enolase, an indicator of neuronal damage, after a standard course of tDCS. Liebtanz et al. (55) found that a current density of 142.9 A/m2 delivered over more than 10 min was necessary to induce brain damage in rats. This current density is multiple orders of magnitude greater than the 0.096 A/m2 typically delivered to the cortex in human studies (52).
Efficacy in Children and Adolescents
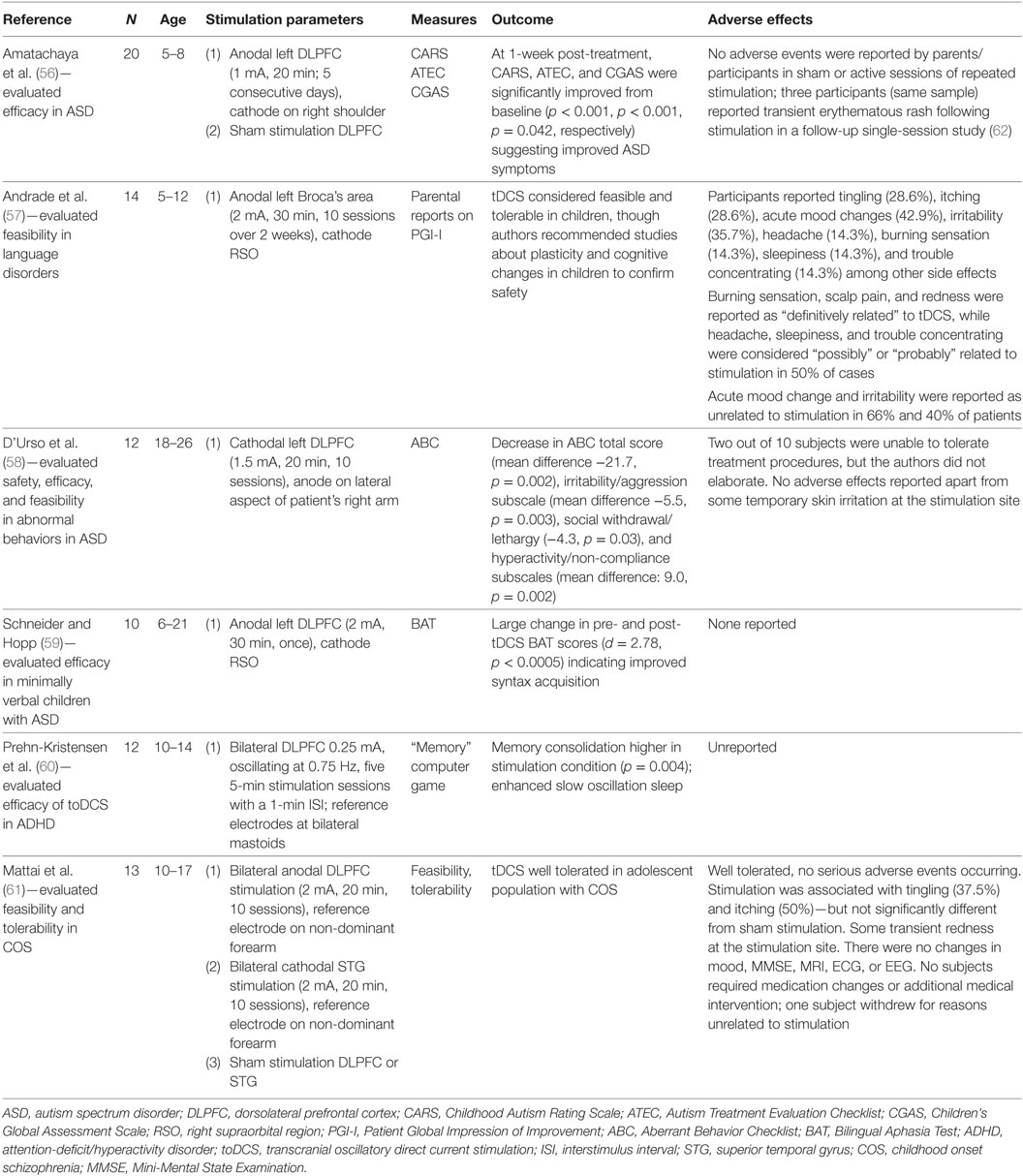
Although studies of adolescent depression are lacking, researchers have applied tDCS to other pediatric psychiatric conditions. The results of these studies are summarized in Table 2. The methodologies of existing studies, including aims and design, as well as tDCS parameters such as current, electrode placement, treatment duration, frequency, and number of sessions, have varied considerably (3).
The most extensively studied pediatric population to date is children and adolescents with autism spectrum disorders (ASDs), a heterogeneous neurodevelopmental condition affecting communication, social functioning, and repetitive/stereotypical behaviors. In an open-label study of minimally verbal individuals with ASD (aged 6–21 years), Schneider and Hopp (59) examined the impact of a single session of anodal tDCS (2 mA) to the left dorsolateral prefrontal cortex (DLPFC) on vocabulary and syntax acquisition. Following the 30-min treatment session, participants demonstrated significant improvement in vocabulary and syntax scores of the Bilingual Aphasia Test, with a substantial effect size for syntax acquisition (Cohen’s d = 2.78). The authors reported no adverse effects from their intervention (59). Amatachaya and colleagues performed two studies with the same sample of 20 male ASD patients aged 5–8 years, both of which followed a double-blinded, sham-controlled crossover design. In the first study (56), participants underwent five sessions of anodal tDCS (1 mA, 20 min) to the left DLPFC or sham stimulation over one week, followed by a 4-week washout period, and then five sessions of the opposite condition. Researchers completed assessments of ASD symptoms at baseline and 7 days after each treatment phase. Active tDCS resulted in significant reductions in overall ASD symptom severity as well as improvements in social, sensory and cognitive awareness, and health and behavioral problem subscales. No such changes occurred with sham stimulation. In a follow-up single-session study (62), the researchers obtained resting state electroencephalography (EEG) at baseline and 24, 48, and 72 h after stimulation. Active tDCS induced increases in peak alpha frequency (PAF) at 0 and 24 h at the treatment site. Conversely, sham stimulation produced no such changes. At one week post-treatment, ASD symptom total scores, and social and health/behavioral problem subscale scores, were significantly lower in those who received active tDCS than in those who received sham. Regression analyses demonstrated significant associations between change in PAF and improvement in measures of ASD symptoms. The authors noted only mild, transient erythematous rash as the sole adverse event.
In a heterogeneous sample of children, Andrade and colleagues (57) conducted a study of the feasibility and tolerability of tDCS. Fourteen participants aged 5–12 years with learning disorders and a variety of comorbidities [which included pervasive developmental disorders/ASD, attention-deficit/hyperactivity disorder (ADHD), and intellectual disability] underwent ten 30-min sessions over two weeks of open-label anodal tDCS (2 mA) applied to Broca’s area during social and speech-related activities. Mood changes (42.9%) and irritability (35.7%) were the most common adverse effects, although participants’ parents did not uniformly attribute these to tDCS. Otherwise, the majority of other side effects (e.g., headache, itching, burning sensation/tingling, and localized erythema) were mild and transient. D’Urso and colleagues (58) also investigated the use of tDCS in an open-label study of adolescents and young adults (aged 18–26) with ASD and intellectual disability; approximately half also had a language disorder. In contrast to other studies, the authors utilized 1.5 mA cathodal stimulation to the left DLPFC for ten 20-min sessions over two weeks, with the aim of restoring inhibitory function and reducing behavioral symptoms. Significant reductions in the Aberrant Behavior Checklist total score and several subscale scores (irritability, social withdrawal, and hyperactivity) were observed between baseline assessment and reassessment one week after tDCS. Minor, transient skin irritation was the only noted adverse effect.
One study to date has examined the effects of tDCS specifically in children and adolescents with ADHD. In a sample of 12 male patients (aged 10–14), Prehn-Kristensen and colleagues (60) investigated whether transcranial oscillatory direct current stimulation (toDCS) improved memory consolidation during slow-wave sleep. Utilizing a double-blind, sham-controlled crossover design, the authors administered a single session of anodal toDCS (0.25 mA, oscillating at 0.75 Hz, five 5-min stimulation sessions with a 1-min interstimulus interval) to the DLPFC bilaterally during slow-wave sleep (determined by EEG). Performance on a declarative memory task was measured before and after stimulation. Active toDCS enhanced slow oscillations on EEG and improved declarative memory performance to a level comparable with a comparator group of 12 healthy control children, while sham toDCS did not result in enhanced slow oscillations or improvement in sleep-dependent memory consolidation.
A single study examined tDCS in youth with schizophrenia. Mattai and colleagues (61) conducted a double-blinded, sham-controlled tolerability study in a sample of 13 patients, aged 10–17, with early-onset schizophrenia. Notably, all patients in the study were receiving concurrent treatment with clozapine. Some participants were also prescribed other psychotropic medications. Participants received either active or sham anodal tDCS (2 mA) to the DLPFC bilaterally, or active or sham cathodal tDCS (2 mA) to the superior temporal gyrus bilaterally. Patients received ten 20-min sessions over two weeks, and those undergoing sham treatment were eligible for additional ten sessions of active tDCS. Tingling sensations, itching, and fatigue were the most common side effects reported, with no difference in rates between active tDCS and sham groups. No serious adverse effects occurred during the 4- or 6-week participation period. Additionally, neither sham nor active tDCS induced significant changes on structural head MRI, EEG, or electrocardiogram (ECG). This study was not powered to evaluate tDCS efficacy.
Concurrent and State-Dependent Interventions
While much prior research has examined the effects of tDCS on cognitive, neurophysiologic, or symptomatic measures in neuropsychiatric disorders, some studies have investigated the use of tDCS in conjunction with motor and cognitive tasks as well as therapeutic activities. The interaction between tDCS and concurrent tasks is complex and incompletely understood.
The timing of tDCS delivery in relation to a cognitive or motor task—before, during, or after the task—appears to influence its effects. Stimulation occurring before, but not during or after, motor training increases cortical excitability as measured by transcranial magnetic stimulation (TMS) (63). However, there is also evidence that cognitive (64) and motor (65) tasks administered after tDCS can partially reverse or abolish anodal effects on cortical excitability. Moreover, tDCS-induced excitability changes differ between cognitive and motor tasks performed during anodal and cathodal stimulation (66). The cumulative effects of stimulation paired with tasks may be significant as well; a single session of tDCS with substance-related cues demonstrated opposite effects on event-related potentials compared to repeated sessions of tDCS (67). The specific characteristics of the concurrent task also may determine the effects of the stimulation–task interaction. In a motor learning task, the speed of the task during concurrent tDCS determined whether learning was facilitated or inhibited (68). Active movement in a motor task during stimulation had different effects on excitability than did passive movement (69). Such task-specific effects are not limited to concurrent motor activities; the degree of cognitive demand of an executive functioning task during stimulation affected performance (70).
Researchers have also evaluated more cognitively complex and clinically relevant concurrent tasks. tDCS administered prior to addiction-related cues reduced cravings and substance use (71, 72). By contrast, tDCS administered concurrently with a craving-inducing cue task increased cravings during stimulation but decreased cravings at rest (73). One potential advantage of tDCS is the possibility of enhancing another concurrent therapeutic activity, including psychotherapy, cognitive remediation, skill training, or other modalities. Moreover, its low cost and portability may enable patients to utilize tDCS, with close clinical monitoring, in their homes or other naturalistic settings where such therapeutic activities occur, thereby enhancing the accessibility of tDCS and integrating it into existing intervention plans (38). Although the literature is sparse, there are emerging reports of the use of tDCS concurrently with therapeutic interventions such as integrative speech therapy (74) and aerobic exercise (75) in human patients. However, much remains to be understood regarding the specifics of timing, repetition, and task-specific parameters to maximize the therapeutic effects of concurrent interventions.
Safety in Children and Adolescents
Transcranial direct current stimulation is considered safe in adults (52), and preliminary evidence from studies with child and adolescent patients suggests a similar safety profile (2). Unlike antidepressant medications, tDCS is not associated with sexual side effects, serotonin syndrome, or suicidality. Krishnan and colleagues (2) found tingling, itching, redness, and scalp discomfort to be the most common side effects in their systematic review of adolescent tDCS trials. These effects were self-limited, lasting up to two hours after stimulation. None required additional medical intervention.
Although long-term safety data in children are unavailable, computer models predict that the peak electric field applied to the brain would range from 0.36 V/m for a small adult head to 0.50 V/m for a pediatric head (76), suggesting a range well below of what has been neurotoxic in preclinical studies (52). Other authors have cautioned against the use of tDCS and other brain stimulation modalities in children and adolescents (77, 78), highlighting the vulnerability of the young brain (79) with sensitive periods during which limited intervention can yield unexpected or detrimental results (80). Davis argues that several questions remain unanswered regarding tDCS in pediatric patients, including the potential unknown effects of stimulation, differential side effects in children, limited evidence to guide dosing parameters, and a dearth of translational studies focused on children and adolescents (77).
While few could disagree with Davis’ critique, the same arguments could be levied against electroconvulsive therapy (ECT), which has been used for decades, albeit circumspectly, in child and adolescent psychiatry. ECT is associated with known effects of memory impairment, prolonged and delayed seizures, headaches, confusion, nausea, muscular pain, and anesthetic risks (81–83). The long-term risks of ECT in adolescents are obscure. Moreover, few randomized controlled trials in adolescents exist to guide its use (84). Nonetheless, ECT is an existing clinical option for treatment-resistant depression in adolescents (84).
Unlike ECT, which induces seizures by definition, or TMS, which provokes seizures rarely (85), tDCS is unlikely to induce seizures. In one study, tDCS of 1 mA for 10 min did not induce any detectable epileptiform activity on EEG in pediatric patients (86). Also unlike ECT, tDCS likely does not significantly impair cognition (53, 87, 88). In fact, researchers have evaluated its potential to enhance attention, learning, and memory (89–93). Early applications of tDCS in children and adolescents with ADHD, childhood-onset schizophrenia, and ASD found that tDCS was well tolerated (56, 61, 94). In one study in childhood-onset schizophrenia, tDCS (2 mA, 20 min) did not induce any detectable changes on MRI, EEG, or ECG (61).
Finally, adult treatments are often clinically adapted for pediatric patients without an evidentiary base in this population. Lithium, for example, was approved by the FDA for the treatment of pediatric bipolar disorder entirely on the basis of data gleaned from bipolar adults. Researchers did not evaluate its actual efficacy in pediatric bipolar patients until years after approval (95). In the late 1980s and 1990s, the use of SSRIs for treating depression in youth also was based largely on extrapolation from adult studies. Although the efficacy of SSRIs for adolescent MDD was later supported in some randomized controlled trials in child and adolescent samples, the long-term effects of SSRIs on the adolescent brain remain obscure (96).
Conclusion
Existing therapies are inadequate to meet the needs of adolescents with depression. tDCS may offer hope, as available evidence suggests it is safe, tolerable, and acceptable. Furthermore, available data suggest that tDCS is efficacious for depression in adults. Although caution is warranted in the context of neurodevelopment, measured research efforts could develop tDCS as a novel and effective intervention for adolescent depression.
Author Contributions
All authors (JL, CL, ZJD, and PC) contributed to the conception, structure, and literature review for the manuscript. JL and CL prepared the initial draft, and ZJD and PC critically revised the draft. All authors (JL, CL, ZJD, and PC) prepared the final draft. All authors (JL, CL, ZJD, and PC) approved the final draft for publication and agreed to assume accountability for the accuracy and composition of the manuscript.
Disclaimer
The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Statement
JL has no disclosures. CL receives research support from the Mayo Clinic Foundation Departmental Small Grant Program and is a site investigator for a multicenter study funded by Neuronetics, Inc. ZJD has received research and equipment in-kind support for an investigator-initiated study through Brainsway Ltd.; he also has served on the advisory board for F. Hoffmann-La Roche Ltd. and Merck & Co., Inc. and has received speaker support from Eli Lilly and Co. PC has received research grant support from Pfizer, Inc., NIMH (K23 MH100266), the Brain and Behavior Research Foundation, and the Mayo Clinic Foundation. He has served as a site subprincipal or principal investigator (without additional compensation) for Eli Lilly and Co., Forest Laboratories, Inc., Merck & Co., Inc., and Pfizer, Inc.; has received equipment support from Neuronetics, Inc.; and receives supplies and genotyping services from Assurex Health, Inc. for an investigator-initiated study. He is a site primary investigator for a multicenter study funded by Neuronetics, Inc.
Acknowledgments
We would like to thank Hedva Chiu for her contributions to the literature review and manuscript preparation.
Funding
PC is supported by the Brain and Behavior Research Foundation, the Mayo Clinic Foundation, the Paul and Betty Woolls Foundation, and the National Institute of Mental Health (K23 MH100266). ZJD is supported by the Ontario Mental Health Foundation, the Canadian Institutes of Health Research, the Brain and Behavior Research Foundation, the Temerty Family, the Grant Family, the Centre for Addiction and Mental Health Foundation, and the Campbell Family Mental Health Research Institute.
References
1. Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, et al. The sertraline vs electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry (2013) 70:383–91. doi: 10.1001/2013.jamapsychiatry.32
2. Krishnan C, Santos L, Peterson MD, Ehinger M. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul (2015) 8:76–87. doi:10.1016/j.brs.2014.10.012
3. Muszkat D, Polanczyk GV, Dias TGC, Brunoni AR. Transcranial direct current stimulation in child and adolescent psychiatry. J Child Adolesc Psychopharmacol (2016) 26:590–7. doi:10.1089/cap.2015.0172
4. Palm U, Segmiller FM, Epple AN, Freisleder F-J, Koutsouleris N, Schulte-Körne G, et al. Transcranial direct current stimulation in children and adolescents: a comprehensive review. J Neural Transm (2016) 123(10):1219–34. doi:10.1007/s00702-016-1572-z
5. Rajapakse T, Kirton A. Non-invasive brain stimulation in children: applications and future directions. Transl Neurosci (2013) 4:217–33. doi:10.2478/s13380-013-0116-3
6. Vicario CM, Nitsche MA. Non-invasive brain stimulation for the treatment of brain diseases in childhood and adolescence: state of the art, current limits and future challenges. Front Syst Neurosci (2013) 7:94. doi:10.3389/fnsys.2013.00094
7. Costello EJ, Foley DL, Angold A. 10 year research update review: the epidemiology of child and adolescent psychiatric disorders: II. Developmental epidemiology. J Am Acad Child Adolesc Psychiatry (2006) 45:8–25. doi:10.1097/01.chi.0000184929.41423.c0
8. Merikangas KR, He J-P, Brody D, Fisher PW, Bourdon K, Koretz DS. Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES. Pediatrics (2010) 125:75–81. doi:10.1542/peds.2008-2598
9. Ryan ND. Treatment of depression in children and adolescents. Lancet (2005) 366:933–40. doi:10.1016/S0140-6736(05)67321-7
10. Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry (1999) 40:57–87. doi:10.1111/1469-7610.00424
11. Fergusson DM, Boden JM, Horwood LJ. Recurrence of major depression in adolescence and early adulthood, and later mental health, educational and economic outcomes. Br J Psychiatry (2007) 191:335–42. doi:10.1192/bjp.bp.107.036079
12. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry (2002) 59:225–31. doi:10.1001/archpsyc.59.3.225
13. Mrazek DA, Hornberger JC, Altar CA, Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr Serv (2014) 65:977–87. doi:10.1176/appi.ps.201300059
14. Birmaher B, Brent D; AACAP Work Group on Quality Issues, Bernet W, Bukstein O, Walter H, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry (2007) 46:1503–26. doi:10.1097/chi.0b013e318145ae1c
15. Hopkins K, Crosland P, Elliott N, Bewley S. Diagnosis and management of depression in children and young people: summary of updated NICE guidance. Br J Sports Med (2016) 50:184–6. doi:10.1136/bjsports-2015-h824rep
16. Cheung AH, Emslie GJ, Mayes TL. Review of the efficacy and safety of antidepressants in youth depression. J Child Psychol Psychiatry (2005) 46:735–54. doi:10.1111/j.1469-7610.2005.01467.x
17. Weisz JR, McCarty CA, Valeri SM. Effects of psychotherapy for depression in children and adolescents: a meta-analysis. Psychol Bull (2006) 132:132–49. doi:10.1037/0033-2909.132.1.132
18. Bulloch AGM, Patten SB. Non-adherence with psychotropic medications in the general population. Soc Psychiatry Psychiatr Epidemiol (2009) 45:47–56. doi:10.1007/s00127-009-0041-5
19. Krivoy A, Balicer RD, Feldman B, Hoshen M, Zalsman G, Weizman A, et al. The impact of age and gender on adherence to antidepressants: a 4 year population-based cohort study. Psychopharmacology (Berl) (2015) 232:3385–90. doi:10.1007/s00213-015-3988-9
20. Brunoni AR, Fraguas R, Fregni F. Pharmacological and combined interventions for the acute depressive episode: focus on efficacy and tolerability. Ther Clin Risk Manag (2009) 5:897–910. doi:10.214/TCRM.S5751
21. Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA (2007) 297:1683–96. doi:10.1001/jama.297.15.1683
22. Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet (2016) 388:881–90. doi:10.1016/S01406736(16)30385-3
23. Henje Blom E, Ho TC, Connolly CG, LeWinn KZ, Sacchet MD, Tymofiyeva O, et al. The neuroscience and context of adolescent depression. Acta Paediatr (2016) 105:358–65. doi:10.1111/apa.13299
24. Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology (2009) 35:192–216. doi:10.1038/npp.2009.104
25. Leuchter AF, Cook IA, Hamilton SP, Narr KL, Toga A, Hunter AM, et al. Biomarkers to predict antidepressant response. Curr Psychiatry Rep (2010) 12:553–62. doi:10.1007/s11920-010-0160-4
26. Yang DTT, Simmons DAN, Matthews DSC, Tapert DSF, Frank DGK, Max DJE, et al. Adolescents with major depression demonstrate increased amygdala activation. J Am Acad Child Adolesc Psychiatry (2010) 49:42–51. doi:10.1097/00004583-201001000-00008
27. Ho TC, Yang G, Wu J, Cassey P, Brown SD, Hoang N, et al. Functional connectivity of negative emotional processing in adolescent depression. J Affect Disord (2014) 155:65–74. doi:10.1016/j.jad.2013.10.025
28. Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ. Functional brain imaging studies of youth depression: a systematic review. Neuroimage Clin (2014) 4:209–31. doi:10.1016/j.nicl.2013.11.009
29. Tao R, Calley CS, Hart J, Mayes TL, Nakonezny PA, Lu H, et al. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. Am J Psychiatry (2012) 169:381–8. doi:10.1176/appi.ajp.2011.11040615
30. Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. Anterior cingulate cortex γ-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry (2012) 69:139–49. doi:10.1001/archgenpsychiatry.2011.131
31. Mao N, Fang J, Xie H, Liu X, Jiang X, Wang G, et al. Correlation between neurochemical metabolism and memory function in adolescent patients with depression: a multi-voxel 1H magnetic resonance spectroscopy study: neurometabolite changes in depression. Psychiatry Clin Neurosci (2016) 70:167–74. doi:10.1111/pcn.12372
33. Yang X-R, Langevin LM, Jaworska N, Kirton A, Lebel RM, Harris AD, et al. Proton spectroscopy study of the dorsolateral prefrontal cortex in youth with familial depression. Psychiatry Clin Neurosci (2016) 70:269–77. doi:10.1111/pcn.12392
34. Han G, Klimes-Dougan B, Jepsen S, Ballard K, Nelson M, Houri A, et al. Selective neurocognitive impairments in adolescents with major depressive disorder. J Adolesc (2012) 35:11–20. doi:10.1016/j.adolescence.2011.06.009
35. Sommerfeldt SL, Cullen KR, Han G, Fryza BJ, Houri AK, Klimes-Dougan B. Executive attention impairment in adolescents with major depressive disorder. J Clin Child Adolesc Psychol (2016) 45:69–83. doi:10.1080/15374416.2015.1072823
36. Thase ME. Using biomarkers to predict treatment response in major depressive disorder: evidence from past and present studies. Dialogues Clin Neurosci (2014) 16:539–44.
37. Meron D, Hedger N, Garner M, Baldwin DS. Transcranial direct current stimulation (tDCS) in the treatment of depression: systematic review and meta-analysis of efficacy and tolerability. Neurosci Biobehav Rev (2015) 57:46–62. doi:10.1016/j.neubiorev.2015.07.012
38. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul (2009) 2:241–5. doi:10.1016/j.brs.2009.02.004
39. Arndt R. Die Electricität in der Psychiatrie [Electricity in psychiatry]. Arch Psychiatr Nervenkrankh (1870) 2:259–337. doi:10.1007/BF02046767
40. Tigges W. Behandlung der Psychosen mit Elektricität. I. Allgemeines [Treatment of psychosis with electricity. I. General]. Allg Z Psychiatr (1883) 39:697–738.
41. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol (2000) 527:633–9. doi:10.1111/j.1469-7793.2000.t01-1-00633.x
42. Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul (2012) 5:175. doi:10.1016/j.brs.2011.03.002
43. Boggio PS, Rigonatti SP, Ribeiro RB, Myczkowski ML, Nitsche MA, Pascual-Leone A, et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol (2008) 11:249–54. doi:10.1017/S1461145707007833
44. Fregni F, Boggio PS, Nitsche MA, Marcolin MA, Rigonatti SP, Pascual-Leone A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disord (2006) 8:203–4. doi:10.1111/j.1399-5618.2006.00291.x
45. Loo CK, Sachdev P, Martin D, Pigot M, Alonzo A, Malhi GS, et al. A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. Int J Neuropsychopharmacol (2010) 13:61–9. doi:10.1017/S1461145709990411
46. Bennabi D, Nicolier M, Monnin J, Tio G, Pazart L, Vandel P, et al. Pilot study of feasibility of the effect of treatment with tDCS in patients suffering from treatment-resistant depression treated with escitalopram. Clin Neurophysiol (2015) 126:1185–9. doi:10.1016/j.clinph.2014.09.026
47. Blumberger DM, Tran LC, Fitzgerald PB, Hoy KE, Daskalakis ZJ. A randomized double-blind sham-controlled study of transcranial direct current stimulation for treatment-resistant major depression. Front Psychiatry (2012) 3:74. doi:10.3389/fpsyt.2012.00074
48. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res (2013) 47:1–7. doi:10.1016/j.jpsychires.2012.09.025
49. Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM, et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry (2016) 208:522–31. doi:10.1192/bjp.bp.115.164715
50. Kalu UG, Sexton CE, Loo CK, Ebmeier KP. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychol Med (2012) 42:1791–800. doi:10.1017/S0033291711003059
51. Shiozawa P, Fregni F, Benseñor IM, Lotufo PA, Berlim MT, Daskalakis JZ, et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int J Neuropsychopharmacol (2014) 17:1443–52. doi:10.1017/S1461145714000418
52. Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimulat (2016) 9(5):641–61. doi:10.1016/j.brs.2016.06.004
53. Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol (2011) 14:1133–45. doi:10.1017/S1461145710001690
54. Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol (2003) 114:600–4. doi:10.1016/S1388-2457(02)00412-1
55. Liebetanz D, Koch R, Mayenfels S, König F, Paulus W, Nitsche MA. Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol (2009) 120:1161–7. doi:10.1016/j.clinph.2009.01.022
56. Amatachaya A, Auvichayapat N, Patjanasoontorn N, Suphakunpinyo C, Ngernyam N, Aree-Uea B, et al. Effect of anodal transcranial direct current stimulation on autism: a randomized double-blind crossover trial. Behav Neurol (2014) 2014:173073. doi:10.1155/2014/173073
57. Andrade AC, Magnavita GM, Allegro JVBN, Neto CEBP, Lucena Rde CS, Fregni F. Feasibility of transcranial direct current stimulation use in children aged 5 to 12 years. J Child Neurol (2014) 29:1360–5. doi:10.1177/0883073813503710
58. D’Urso G, Bruzzese D, Ferrucci R, Priori A, Pascotto A, Galderisi S, et al. Transcranial direct current stimulation for hyperactivity and noncompliance in autistic disorder. World J Biol Psychiatry (2015) 16:361–6. doi:10.3109/15622975.2015.1014411
59. Schneider HD, Hopp JP. The use of the Bilingual Aphasia Test for assessment and transcranial direct current stimulation to modulate language acquisition in minimally verbal children with autism. Clin Linguist Phon (2011) 25:640–54. doi:10.3109/02699206.2011.570852
60. Prehn-Kristensen A, Munz M, Göder R, Wilhelm I, Korr K, Vahl W, et al. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimul (2014) 7:793–9. doi:10.1016/j.brs.2014.07.036
61. Mattai A, Miller R, Weisinger B, Greenstein D, Bakalar J, Tossell J, et al. Tolerability of transcranial direct current stimulation in childhood-onset schizophrenia. Brain Stimul (2011) 4:275–80. doi:10.1016/j.brs.2011.01.001
62. Amatachaya A, Jensen MP, Patjanasoontorn N, Auvichayapat N, Suphakunpinyo C, Janjarasjitt S, et al. The short-term effects of transcranial direct current stimulation on electroencephalography in children with autism: a randomized crossover controlled trial. Behav Neurol (2015) 2015:928631. doi:10.1155/2015/928631
63. Cabral ME, Baltar A, Borba R, Galvão S, Santos L, Fregni F, et al. Transcranial direct current stimulation: before, during, or after motor training? Neuroreport (2015) 26:618–22. doi:10.1097/WNR.0000000000000397
64. Quartarone A, Morgante F, Bagnato S, Rizzo V, Sant’Angelo A, Aiello E, et al. Long lasting effects of transcranial direct current stimulation on motor imagery. Neuroreport (2004) 15:1287–91. doi:10.1097/01.wnr.0000127637.22805.7c
65. Thirugnanasambandam N, Sparing R, Dafotakis M, Meister IG, Paulus W, Nitsche MA, et al. Isometric contraction interferes with transcranial direct current stimulation (tDCS) induced plasticity: evidence of state-dependent neuromodulation in human motor cortex. Restor Neurol Neurosci (2011) 29:311–20. doi:10.3233/RNN-2011-0601
66. Antal A, Terney D, Poreisz C, Paulus W. Towards unravelling task-related modulations of neuroplastic changes induced in the human motor cortex. Eur J Neurosci (2007) 26:2687–91. doi:10.1111/j.1460-9568.2007.05896.x
67. Conti CL, Moscon JA, Fregni F, Nitsche MA, Nakamura-Palacios EM. Cognitive related electrophysiological changes induced by non-invasive cortical electrical stimulation in crack-cocaine addiction. Int J Neuropsychopharmacol (2014) 17:1465–75. doi:10.1017/S1461145714000522
68. Bortoletto M, Pellicciari MC, Rodella C, Miniussi C. The interaction with task-induced activity is more important than polarization: a tDCS study. Brain Stimulat (2015) 8:269–76. doi:10.1016/j.brs.2014.11.006
69. Miyaguchi S, Onishi H, Kojima S, Sugawara K, Tsubaki A, Kirimoto H, et al. Corticomotor excitability induced by anodal transcranial direct current stimulation with and without non-exhaustive movement. Brain Res (2013) 1529:83–91. doi:10.1016/j.brainres.2013.07.026
70. Gill J, Shah-Basak PP, Hamilton R. It’s the thought that counts: examining the task-dependent effects of transcranial direct current stimulation on executive function. Brain Stimul (2015) 8:253–9. doi:10.1016/j.brs.2014.10.018
71. Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett (2009) 463:82–6. doi:10.1016/j.neulet.2009.07.041
72. Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry (2008) 69:32–40. doi:10.4088/JCP.v69n0105
73. Shahbabaie A, Golesorkhi M, Zamanian B, Ebrahimpoor M, Keshvari F, Nejati V, et al. State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int J Neuropsychopharmacol (2014) 17:1591–8. doi:10.1017/S1461145714000686
74. Carvalho Lima VLC, Collange Grecco LA, Marques VC, Fregni F, Brandão de Ávila CR. Transcranial direct current stimulation combined with integrative speech therapy in a child with cerebral palsy: a case report. J Bodyw Mov Ther (2016) 20:252–7. doi:10.1016/j.jbmt.2015.03.007
75. Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front Hum Neurosci (2016) 10:68. doi:10.3389/fnhum.2016.00068
76. Kessler SK, Minhas P, Woods AJ, Rosen A, Gorman C, Bikson M. Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PLoS One (2013) 8:e76112. doi:10.1371/journal.pone.0076112
77. Davis NJ. Transcranial stimulation of the developing brain: a plea for extreme caution. Front Hum Neurosci (2014) 8:600. doi:10.3389/fnhum.2014.00600
78. Reiner PB. Comment on “can transcranial electrical stimulation improve learning difficulties in atypical brain development? A future possibility for cognitive training” by Krause and Cohen Kadosh. Dev Cogn Neurosci (2013) 6:195–6. doi:10.1016/j.dcn.2013.05.002
79. Kolb B, Teskey GC. Age, experience, injury, and the changing brain. Dev Psychobiol (2012) 54:311–25. doi:10.1002/dev.20515
80. Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci (2004) 16:1412–25. doi:10.1162/0898929042304796
81. Cohen D, Paillère-Martinot ML, Basquin M. Use of electroconvulsive therapy in adolescents. Convuls Ther (1997) 13:25–31.
82. Walter G, Koster K, Rey JM. Electroconvulsive therapy in adolescents: experience, knowledge, and attitudes of recipients. J Am Acad Child Adolesc Psychiatry (1999) 38:594–9. doi:10.1097/00004583-199905000-00022
83. Walter G, Rey JM. An epidemiological study of the use of ECT in adolescents. J Am Acad Child Adolesc Psychiatry (1997) 36:809–15. doi:10.1097/00004583-199706000-00018
84. Ghaziuddin N, Kutcher SP, Knapp P, Bernet W, Arnold V, Beitchman J, et al. Practice parameter for use of electroconvulsive therapy with adolescents. J Am Acad Child Adolesc Psychiatry (2004) 43:1521–39. doi:10.1097/01.chi.0000142280.87429.68
85. Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol (2009) 120:2008–39. doi:10.1016/j.clinph.2009.08.016
86. Moliadze V, Andreas S, Lyzhko E, Schmanke T, Gurashvili T, Freitag CM, et al. Ten minutes of 1 mA transcranial direct current stimulation was well tolerated by children and adolescents: self-reports and resting state EEG analysis. Brain Res Bull (2015) 119(Pt A):25–33. doi:10.1016/j.brainresbull.2015.09.011
87. Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol (2006) 117:845–50. doi:10.1016/j.clinph.2005.12.003
88. Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology (2005) 64:872–5. doi:10.1212/01.WNL.0000152986.07469.E9
89. Brasil-Neto JPM. Learning, memory, and transcranial direct current stimulation. Neuroimaging Stimul (2012) 3:80. doi:10.3389/fpsyt.2012.00080
90. Coffman BA, Clark VP, Parasuraman R. Battery powered thought: enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. Neuroimage (2014) 85(Pt 3):895–908. doi:10.1016/j.neuroimage.2013.07.083
91. Hoy KE, Emonson MRL, Arnold SL, Thomson RH, Daskalakis ZJ, Fitzgerald PB. Testing the limits: investigating the effect of tDCS dose on working memory enhancement in healthy controls. Neuropsychologia (2013) 51:1777–84. doi:10.1016/j.neuropsychologia.2013.05.018
92. Iuculano T, Kadosh RC. The mental cost of cognitive enhancement. J Neurosci (2013) 33:4482–6. doi:10.1523/JNEUROSCI.4927-12.2013
93. Krause B, Cohen Kadosh R. Can transcranial electrical stimulation improve learning difficulties in atypical brain development? A future possibility for cognitive training. Dev Cogn Neurosci (2013) 6:176–94. doi:10.1016/j.dcn.2013.04.001
94. Bandeira ID, Guimarães RSQ, Jagersbacher JG, Barretto TL, de Jesus-Silva JR, Santos SN, et al. Transcranial direct current stimulation in children and adolescents with attention-deficit/hyperactivity disorder (ADHD) a pilot study. J Child Neurol (2016) 31:918–24. doi:10.1177/0883073816630083
95. Geller B, Cooper TB, Watts HE, Cosby CM, Fox LW. Early findings from a pharmacokinetically designed double-blind and placebo-controlled study of lithium for adolescents comorbid with bipolar and substance dependency disorders. Prog Neuropsychopharmacol Biol Psychiatry (1992) 16:281–99. doi:10.1016/0278-5846(92)90080-X
Keywords: adolescent depression, neurostimulation, non-invasive brain stimulation, transcranial current stimulation, transcranial direct current stimulation
Citation: Lee JC, Lewis CP, Daskalakis ZJ and Croarkin PE (2017) Transcranial Direct Current Stimulation: Considerations for Research in Adolescent Depression. Front. Psychiatry 8:91. doi: 10.3389/fpsyt.2017.00091
Received: 29 January 2017; Accepted: 04 May 2017;
Published: 07 June 2017
Edited by:
Christoph Mulert, University Medical Center Hamburg-Eppendorf, GermanyReviewed by:
Mo Chen, University of Minnesota, United StatesAndre R. Brunoni, University of São Paulo, Brazil
Copyright: © 2017 Lee, Lewis, Daskalakis and Croarkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul E. Croarkin, croarkin.paul@mayo.edu
 Jonathan C. Lee
Jonathan C. Lee Charles P. Lewis
Charles P. Lewis Zafiris J. Daskalakis
Zafiris J. Daskalakis Paul E. Croarkin
Paul E. Croarkin