
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry , 18 May 2016
Sec. Neuroimaging
Volume 7 - 2016 | https://doi.org/10.3389/fpsyt.2016.00082
This article is part of the Research Topic Third-generation neuroimaging: translating research into clinical utility View all 22 articles
Executive function deficits, such as working memory, decision-making, and attention problems, are a common feature of several psychiatric disorders for which no satisfactory treatment exists. Here, we transdiagnostically investigate the effects of pharmacological interventions (other than methylphenidate) on the fronto-cingulo-parietal cognitive control network, in order to identify functional brain markers for future procognitive pharmacological interventions. Twenty-nine manuscripts investigated the effect of pharmacological treatment on executive function-related brain correlates in psychotic disorders (n = 11), depression (n = 4), bipolar disorder (n = 4), ADHD (n = 4), OCD (n = 2), smoking dependence (n = 2), alcohol dependence (n = 1), and pathological gambling (n = 1). In terms of impact on the fronto-cingulo-parietal network, the preliminary evidence for catechol-O-methyl-transferase inhibitors, nicotinic receptor agonists, and atomoxetine was relatively consistent, the data for atypical antipsychotics and anticonvulsants moderate, and interpretation of the data for antidepressants was hampered by the employed study designs. Increased activity in task-relevant areas and decreased activity in task-irrelevant areas were the most common transdiagnostic effects of pharmacological treatment. These markers showed good positive and moderate negative predictive value. It is concluded that fronto-cingulo-parietal activity changes can serve as a marker for future procognitive interventions. Future recommendations include the use of randomized double-blind designs and selective cholinergic and glutamatergic compounds.
How are pharmacological interventions for psychiatric disorders related to changes in functional brain correlates of executive functions? Impairments in executive functions, such as working memory, decision-making, planning, and attention, are a common feature of several psychiatric disorders. Extensive evidence in depression (1, 2), schizophrenia (SCZ) and psychosis (3, 4), bipolar disorder (5, 6), obsessive–compulsive disorder (OCD) (7, 8), substance dependence (9, 10), anxiety (11), autism spectrum disorders (12, 13), and attention-deficit/hyperactivity disorder (ADHD) (14, 15) suggests consistent impairments on a broad range of neuropsychological tests. For many of these disorders, there is compelling evidence that executive function deficits may be present before illness onset (16–18) and often persist beyond the acute phase of the disease (9, 19–21). With the exception of ADHD, there currently exists no successful pharmacological treatment for executive function deficits in psychiatric disorders.
The fact that assessments of executive functions in psychiatric disorders predict relapse/remission (9, 22, 23), functional outcome (24, 25), and in some cases, treatment response (26, 27) suggests that it is a core transdiagnostic symptom domain that requires adequate treatment. Recent reviews have suggested that increasing patient functioning and outcome may be achieved by boosting executive functions (28, 29), further increasing attractiveness of this symptom domain as a treatment target.
Then, do the available pharmacological interventions for the treatment of psychiatric disorders modulate executive function deficits? Based on the available literature, especially the dopamine- and noradrenaline-increasing effects of methylphenidate (MPH) have been consistently associated with normalizing effects in ADHD (30, 31) and procognitive effects in healthy volunteers (32). And, many other pharmacological agents with glutamatergic [e.g., memantine (33)], cholinergic [e.g., rivastigmine (33)], dopaminergic [e.g., modafinil (34) and atomoxetine (35)], and noradrenergic [e.g., atomoxetine (35)] properties have also been shown to modulate executive functions. Moreover, while not the primary aim of these agents, there is evidence for a modest improvement in some executive function domains for atypical antipsychotics (36), likely related to their dopamine- and serotonin-modulating properties.
It may be the case that pharmacological agents for psychiatric disorders, which may or may not have the primary aim to modulate executive functions, at least partly impact common substrates. Although executive functions are underlain by widely distributed networks, one brain network consistently associated with executive functions is the fronto-cingulo-parietal cognitive control network (37). Essential hubs in the fronto-cingulo-parietal network have consistently been associated with executive function domains such as the anterior cingulate cortex with cognitive control (38), dorsolateral prefrontal cortex (DLPFC) with working memory (37, 39), inferior frontal gyrus (IFG) and (pre-)supplementary motor area (SMA) with response inhibition (40–42), and the parietal lobules with visual attention and attention control (43, 44). As such, the fronto-cingulo-parietal network is ideally suited as a reference network, which can be used to evaluate the effect of pharmacological agents on executive function-related brain networks.
While especially the effect of MPH on cognitive control networks has been critically evaluated in relation to its pharmacological mechanism (30, 31), the aim of the current review was to systematically review the evidence for other available pharmacological interventions in a transdiagnostic fashion. This includes not only pharmacological agents with demonstrated procognitive effects but also agents that are not primarily used to ameliorate executive function deficits, such as antidepressants or antipsychotics. Relating plausible mechanisms of action to the effect of pharmacological interventions on functional brain correlates of executive functions could assist in further (I) validating the therapeutical effects of these agents and (II) elucidating brain mechanisms that could be targeted by future procognitive agents.
The use of functional magnetic resonance imaging (fMRI) to investigate brain correlates of executive functions is well established (45). More recently, this method has increasingly been used to evaluate the effects of pharmacological agents on brain function (46, 47). The strength of pharmacological fMRI is its ability to quantify activity changes in functional brain networks related to direct or downstream consequences of the pharmacological intervention. This enables the investigation of common and distinct drug effects on functional brain networks.
In this transdiagnostic systematic review, we aim to provide an overview of the effects of pharmacological interventions (other than MPH) on the fronto-cingulo-parietal cognitive control network in psychiatric disorders and to relate these to plausible neuropharmacological mechanisms. Using recent meta-analyses, we start off with a brief definition of the fronto-cingulo-parietal network. Next, we specifically evaluate original studies employing strictly executive functioning fMRI paradigms before treatment and after stable therapeutically efficacious dosing (monotherapy or adjunctive) had been implemented. We conclude with common transdiagnostic effects of pharmacological agents on the fronto-cingulo-parietal network, which could serve as markers for future procognitive interventions.
PubMed was searched for studies published before October 23, 2015 using the initial Boolean phrase: (“fMRI” OR “functional magnetic resonance imaging”) AND (“cognition” OR “working memory” OR “attention” OR “decision-making” or “verbal learning” or “vigilance” or “processing speed” or “reasoning” or “problem solving” or “social cognition” or “verbal memory” or “visual learning” or “visual memory”) AND (“treatment”) AND (“pharmacology”). We followed up this initial search with a number of targeted searches in psychiatric disorders. To these aims, we replaced (“treatment”) AND (“pharmacology”) with (“pharmacology” OR “treatment”) AND (i) (“depression” OR “MDD”), (ii) (“schizophrenia” OR “psychosis”), (iii) (“bipolar” OR “mania” OR “cyclothymia” OR “rapid cycling”), (iv) (“substance dependence” OR “addiction” OR “substance abuse” OR “alcoholism”), (v) (“Tourette syndrome” OR “Tourette” OR “tic”), (vi) (“borderline” OR “personality disorder”), (vii) (“autism” OR “pervasive developmental disorder” OR “Asperger”), (viii) (“obsessive compulsive disorder” OR “OCD” OR “impulse control”), (ix) (“PTSD” or “post traumatic stress disorder”), and (x) (“anxiety” OR “fear” OR “phobia”). Titles and abstracts of all results were screened. Cross-referencing was performed on all included manuscripts and relevant reviews. Given the high number of recent meta-analyses and systematic reviews on the subject (30, 31, 48), we did not systematically review results of MPH treatment in psychiatric disorders. Treatments other than MPH in ADHD were included in the review if they met criteria.
Manuscripts were only considered if
(i) they were published in a peer-reviewed journal.
(ii) they were written in the English language.
(iii) they used the same fMRI paradigm at baseline and follow-up.
(iv) they reported group-level statistics; case studies were not included.
(v) pharmacological agents were specified, and results of the medicated group were reported (e.g., manuscripts combining samples of non-pharmacologically and pharmacologically treated patients were excluded).
(vi) the entire sample of patients was drug-free (in the case of monotherapy) or on stable monotherapy (in the case of adjunctive therapy) at baseline (washout allowed if necessary) and were stably and actively on (adjunctive) medication (no washout) during follow-up session(s). Concretely, “stably on medication” refers to repeated administration (>1; single dosing studies excluded) of the same efficacious drug dose.
(vii) they used fMRI paradigms that only measured aspects of executive functions. Tasks with stressful, painful, emotional, and/or rewarded components were excluded.
(viii) participants had a diagnosis of a psychiatric disorder according to DSM-IV criteria. Neurological disorders, such as stroke, dementia, and Parkinson’s disease, were excluded.
The functional brain networks underlying higher cognitive and attention functions are widespread and complex with among others demonstrated cerebellar (49), occipital cortex (50, 51), striatal (52), and frontal cortical (39) involvement. In order to provide a clear delineation of the topic and facilitate the use of a reference network, we decided to review the effect of pharmacological agents on the fronto-cingulo-parietal cognitive control network. The cognitive control network has been hypothesized to play an essential role in orchestrating higher order behavior such as decision-making, action selection, and working memory (53). A comprehensive meta-analysis by Niendam et al. (37) demonstrated recurrent activity of a fronto-cingulo-parital cognitive control network during paradigms that assess working memory, response inhibition, behavioral flexibility, and other higher order skills (37). These results are in line with previous meta-analyses on functional brain networks underlying working memory, response inhibition, and selective attention, specifically and consistently revealing ACC, DLPFC, and superior and inferior parietal lobule (IPL) activity (41, 54–56). Therefore, the reported results in this manuscript (Table 1) include observed changes in parietal cortex (superior and IPL), insula, ACC, and frontal cortical areas, including DLPFC, ventrolateral PFC (VLPFC), and orbitofrontal cortex (OFC). In the discussion, these results will be linked to other notable findings of the available studies.
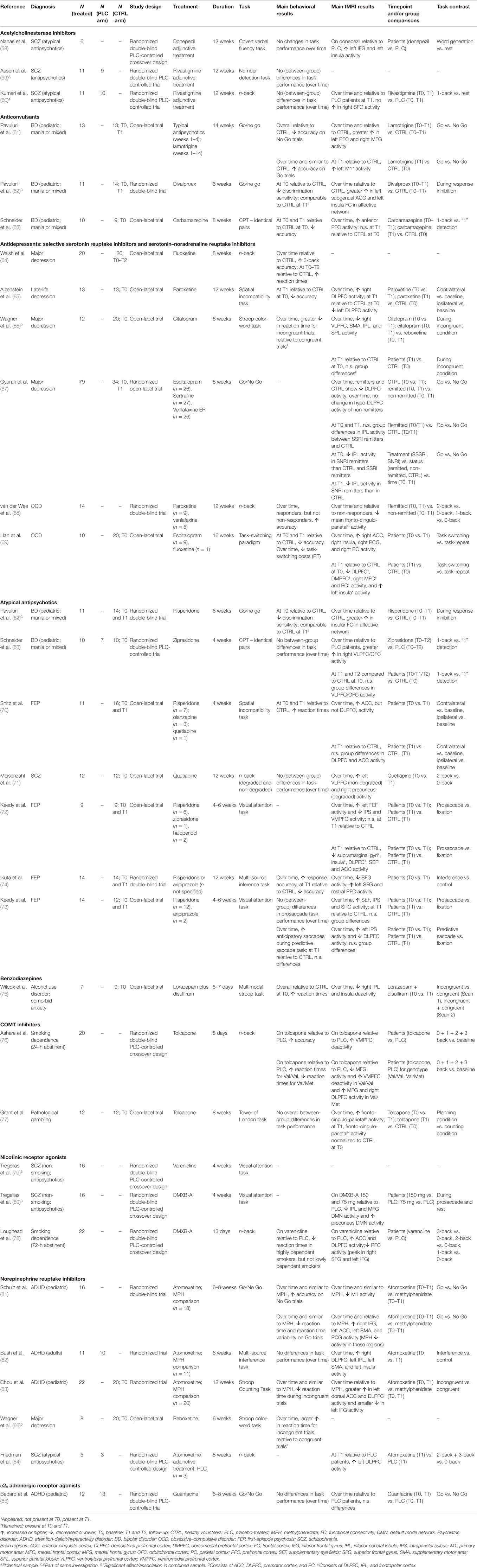
Table 1. Overview of the effect of pharmacological agents on the fronto-cingulo-parietal cognitive control network.
The review was carried out according to PRISM (57) guidelines. The literature search in PubMed yielded 586 unique hits, of which 557 were excluded because they did not (i) utilize a pharmacological intervention or used MPH (38.2%), (ii) recruit patient populations (28.5%), (iii) employ a longitudinal fMRI design (17.8%), (iv) utilize a repeated dosing scheme (8.3%), (v) employ strictly cognitive tasks (4.4%), or (vi) were not written in the English language (2.8%). Thus, 29 manuscripts were included in this review, of which 26 used unique samples. In total, 431 individuals were scanned while medication free or on stable monotherapy at baseline and on monotherapy or adjunctive treatment at follow-up, in addition to placebo-treated patients and healthy volunteers.
Study designs, tasks, sample size, medication distribution, and main findings of the included studies are reported in Table 1. If available, results regarding (i) changes over time in patients vs. controls and (ii) patients vs. controls at follow-up were reported. Else, results regarding (i) change over time in patients and (ii) patients at follow-up vs. controls at follow-up, or (iii) patients at follow-up vs. controls at baseline were reported.
Three studies (two unique samples) investigated the effect of adjunctive actylcholinesterase inhibitors on executive function-related brain correlates before and after treatment. All studies were conducted in SCZ, and a total of 17 patients who were on stable antipsychotic medication were scanned before and after adjunctive treatment with actylcholinesterase inhibitors. Six patients were administered donepezil; the remaining 11 patients received rivastigmine.
None of the reported studies observed significant changes in task performance between placebo and medication sessions or between groups.
In a crossover design, Nahas et al. (58) investigated the effect of 12-week adjunctive donepezil treatment on verbal fluency-related brain activity. On donepezil relative to placebo, participants displayed increased left IFG and insula activity.
Aasen et al. (59) investigated brain activity during a number detection task in a 12-week rivastigmine trial. At follow-up and relative to placebo-treated patients, rivastigmine-treated patients did not show changes in fronto-cingulo-parietal activity. On the n-back task, which assesses selective attention and working memory, Kumari et al. (60) found a smaller increase in right superior frontal gyrus (SFG) activity from baseline to follow-up in the same sample of rivastigmine-treated patients, relative to placebo-treated patients.
We identified three manuscripts investigating the effect of anticonvulsants on the functional brain correlates of executive functions. All studies were conducted in pediatric bipolar disorder (age <18 years). A total of 34 pediatric patients were scanned before and after treatment. Thirteen patients were treated with lamotrigine, 11 with divalproex, and 10 with carbamazepine.
Pavuluri et al. (61) reported that, on a response inhibition task, pediatric bipolar patients displayed poorer overall (i.e., average of baseline and follow-up) accuracy on No-Go trials than healthy volunteers. However, similar to healthy volunteers, performance accuracy on Go trials increased from baseline to follow-up. From baseline to follow-up and compared to healthy volunteers, bipolar patients showed greater increases in left PFC and right medial frontal gyrus activity. At follow-up relative to healthy volunteers, increased left motor cortex activity appeared.
Another study by Pavuluri and colleagues (62) investigated the effect of divalproex on response inhibition and related functional connectivity. When divalproex-treated patients were combined with a parallel arm of risperidone-treated patients (see Atypical Antipsychotic treatment in Bipolar Disorder), discrimination sensitivity during a Go/No-Go task at baseline was poorer in patients than in healthy volunteers, but seemed to have normalized at follow-up. From baseline to follow-up and compared to healthy volunteers, divalproex-treated patients showed a greater increase in left subgenual ACC and left insula functional connectivity in an affective network. Directly comparing the divalproex- and risperidone-treated patient group, the latter group showed a trend-significant greater increase in left insula functional connectivity in the affective network.
Finally, carbamazepine-treated patients displayed poorer accuracy on a sustained attention task at baseline and follow-up compared to healthy volunteers at baseline (63). An increase in sustained attention-related anterior PFC from baseline to follow-up was observed. At follow-up, no significant differences were observed in anterior PFC activity compared to healthy volunteers at baseline.
For treatment with selective serotonin reuptake inhibitors (SSRIs) and serotonin–noradrenaline reuptake inhibitors (SNRIs), six manuscripts were identified. Four studies were conducted in mood disorders, with a total of 124 patients scanned before and after treatment. Two studies were conducted in OCD, with a total of 24 patients scanned before and after treatment. Medication type distribution was as follows: 35 escitalopram (26 mood disorders), 31 venlafaxine (26 mood disorders), 27 sertraline (all mood disorders), 22 paroxetine (13 mood disorders), 21 fluoxetine (20 mood disorders), and 12 citalopram (all mood disorders).
On an n-back task, major depression patients compared to healthy volunteers had slower reaction times at baseline, which did not change after 2 and 8 weeks of SSRI treatment (64). However, compared to healthy volunteers, performance accuracy on the 3-back condition increased over time. Compared to healthy volunteers and over time, no activity changes in the fronto-cingulo-parietal cognitive control network were observed.
Aizenstein and colleagues (65) investigated the effect of SSRI treatment on task performance and brain activity during the preparing to overcome prepotency (spatial incompatibility) task, which assesses cognitive control. Compared to healthy volunteers at baseline, poorer performance accuracy at follow-up was observed in the sample of late-life depression patients treated with paroxetine. From baseline to follow-up, rule applying-related DLPFC, but not response overriding-related ACC, activity increased in patients. At follow-up, DLPFC activity was still lower than healthy volunteers at baseline.
Wagner et al. (66) investigated the effects of SSRI treatment on Stroop task performance and attention and interference-related brain activity. The decrease in response time between baseline and follow-up was greater for incongruent trials than for congruent trials when the SSRI-treated sample was combined with a sample receiving noradrenaline reuptake inhibitors (NRIs; see Noradrenaline Reuptake Inhibitors treatment in Major Depression). From baseline to follow-up, SSRI treatment was associated with widespread decreases in the fronto-cingulo-parietal network during the incongruent condition of the Stroop task (Table 1). When SSRI- and NRI-treated patients were combined, no differences in brain activity during the incongruent condition were observed at follow-up, relative to healthy volunteers.
In a large multicenter endeavor, healthy volunteers and remitted depression patients (treated with SSRIs or SNRIs) showed a decrease in response inhibition-related DLPFC activity from baseline to follow-up (67). Relative to healthy volunteers and remitters, non-remitters displayed DLPFC hypoactivity at baseline, which did not increase at follow-up. SSRI remitters and healthy volunteers showed a similar decrease in IPL activity from baseline to follow-up. At baseline, SNRI remitters compared to SSRI remitters and healthy volunteers displayed IPL hypoactivity, which did not increase to the level of healthy volunteers at follow-up.
Relative to non-responders, SSRI or SNRI treatment responders displayed increased performance accuracy on the n-back over time (68). From baseline to follow-up, responders relative to non-responders correspondingly displayed decreased activity in the fronto-cingulo-parietal network during the 1-back and 2-back condition.
Using a task-switching paradigm, Han et al. (69) showed that task-switching costs (task-switching reaction time − task-repeat reaction time) decreased in SSRI-treated patients from baseline to follow-up. However, patients displayed poorer accuracy at baseline and follow-up than healthy volunteers at baseline. From baseline to follow-up, task-switching activity increased in the fronto-cingulo-parietal network (Table 1). Compared to healthy volunteers at baseline, decreased frontal and parietal activity remained and increased insular activity appeared at follow-up (Table 1).
For treatment with typical and atypical antipsychotics, seven manuscripts were identified. Two studies were conducted in bipolar disorder, with a total of 21 patients scanned before and after treatment. The remaining five studies were conducted in psychotic disorders, with a total of 60 patients scanned before and after treatment. The 36 patients received risperidone (11 bipolar), 11 ziprasidone (10 bipolar), 13 quetiapine, 3 olanzapine, 2 haloperidol, and 2 aripiprazole. Another 14 patients with psychotic disorder received either risperidone or aripiprazole, but numbers for each treatment were not reported.
In addition to divalproex (see Anticonvulsant treatment in Bipolar Disorder), Pavuluri and colleagues (62) also investigated the effect of risperidone on response inhibition-related functional connectivity in pediatric mania. From baseline to follow-up and compared to healthy volunteers, a greater increase in insular functional connectivity during response inhibition was observed in an affective functional connectivity network.
Schneider et al. (63) investigated the effect of ziprasidone on sustained attention in pediatric mixed/mania patients. Performance parameters on the continuous performance task (identical pairs version, similar to 1-back task) did not differ among treatment and control groups (over time). From baseline to follow-up and compared to a placebo-treated patient group, the zipradisone-treated group showed a larger increase in right sustained attention-related VLPFC and OFC activity. At days 7 and 28, no significant differences in sustained attention-related brain activity were observed between the entire patient sample (ziprasidone plus placebo) and healthy volunteers at baseline.
In another study using the preparing to overcome prepotency task, SCZ patients compared to healthy volunteers displayed increased reaction times at baseline and follow-up (70). From baseline to follow-up, response overriding-related ACC activity increased in SCZ patients, whereas rule applying-related DLPFC activity did not. At follow-up, task-induced ACC and DLPFC activity was comparable between SCZ patients and healthy volunteers.
In an open-label quetiapine trial, performance on the n-back task did not improve over time (71). However, task-induced left VLPFC and right precuneus activity increased in SCZ patients from baseline to follow-up.
Following treatment with atypical antipsychotics, Keedy et al. (72) reported activity normalization in anterior frontal and parietal areas during a visual attention task (Table 1). At follow-up, not all activity had normalized: compared to healthy volunteers, widespread reductions in fronto-cingulo-parietal activity had appeared or remained in SCZ patients (Table 1).
In a second study by Keedy et al. (73), patients and healthy volunteers did not differ in performance on the same visual attention task at baseline or follow-up. Activity in supplementary eye fields and parietal cortex normalized from baseline to follow-up (Table 1). During a presaccadic task, which probes frontostriatal motor and attention functions, increased left parietal and decreased DLPFC activity was observed from baseline to follow-up.
A final study using atypical antipsychotics in first-episode psychosis reported increased accuracy over time on an attention control task, which was still poorer than healthy volunteers at follow-up (74). In PFC, left SFG and anterior PFC activity increased from baseline to follow-up, although decreases in SFG activity were also reported.
We identified one manuscript using lorazepam plus disulfiram in substance dependence with comorbid anxiety disorder. A total of seven patients were scanned before and after treatment.
On a multisensory Stroop task, alcohol use disorder patients displayed overall slower reaction times than healthy volunteers at baseline (75). While at baseline, alcohol use disorder patients displayed attention and interference-related deactivity in parietal areas, there was slight activity at follow-up (i.e., decreased deactivity) (Table 1).
For treatment with catechol-O-methyl transferase (COMT) inhibitors, two studies were identified. One study was carried out in smoking dependence; the other was carried out in pathological gambling. The 32 patients were scanned before and after tolcapone treatment.
In a randomized double-blind placebo-controlled crossover design, Ashare et al. (76) observed that performance accuracy on the n-back task, relative to placebo, increased after 8 days of treatment with tolcapone. Relative to placebo, patients displayed increased ventromedial PFC (VMPFC) deactivity. On tolcapone relative to placebo, Val/Val carriers of the COMT gene displayed decreased medial PFC (MPFC) activity and increased (i.e., more) VMPFC deactivity during the task, while Val/Met carriers displayed increased VMPFC and right DLPFC activity.
Pathological gambling patients and healthy volunteers showed similar overall accuracy on a Tower of London planning task (77). From baseline to follow-up, planning-related fronto-cingulo-parietal activity increased and seemingly normalized to that of healthy volunteers at baseline (Table 1).
We identified three papers (two unique samples) using nicotinic receptor agonists, with a total of 38 patients scanned before and after treatment. One study used α4β2 nicotinic receptor agonist varenicline in 22 smoking-dependent individuals. Another study used 3-(2,4-dimethoxybenzylidene)-anabaseine (DMXB-A), a partial α7 nicotinic agonist, as an adjunctive treatment in 16 non-smoking SCZ patients on stable antipsychotic treatment.
Response times for correct trials on the n-back decreased on varenicline relative to placebo in highly dependent abstinent smokers, but not in their lowly dependent counterparts (78). Moreover, on varenicline relative to placebo, MPFC and DLPFC activity increased, specifically on the 2-back and 3-back level. In addition, whole brain analyses revealed frontal cortex activity decreases on varenicline relative to placebo, with peak deactivations detected in the right SFG and left IFG.
Relative to placebo, no changes in the fronto-cingulo-parietal network were observed during a visual attention task after a 1-month treatment with 75 and 150 mg of DMXB-A (79). However, differences in frontal and parietal default mode network activity were observed between the placebo and DMXB-A session (Table 1) (80).
For treatment with NRIs, five studies were identified. Three studies were conducted in ADHD (two pediatric and one adult sample), one in major depression, and one in SCZ (adjunctive treatment). A total of 64 patients were scanned before and after treatment, of which 56 received atomoxetine and 8 received reboxetine (all major depression).
Similar to MPH-treated patients, accuracy increased on No-Go trials from baseline to follow-up in those receiving atomoxetine (81). Moreover, reaction times and reaction time variability decreased. From baseline to follow-up and similar to the MPH-treated group, primary motor cortex activity decreased during the response inhibition task. MPH and atomoxetine seemed to produce differential effects on the fronto-cingulo-parietal cognitive control network (Table 1): atomoxetine increased and MPH decreased activity from baseline to follow-up.
Bush et al. (82) reported that performance on an attention control task did not change over time or relative to placebo-treated patients. From baseline to follow-up, frontal cortical and parietal activity (Table 1) during an interference task increased in an atomoxetine-treated cohort of patients.
Similar to MPH-treated patients, reaction times on a Stroop task decreased over time in atomoxetine-reated patients (83). At brain level, differential effects of MPH and atomoxetine were observed. From baseline to follow-up and relative to MPH, greater increases in left DLPFC and ACC activity and a smaller increase in left IFG activity were reported.
In addition to SSRIs (see Antidepressant treatment in Mood Disorders), Wagner et al. (66) also investigated the effects of NRIs on response interference-related brain activity. Whereas SSRIs decreased fronto-cingulo-parietal network activity (Table 1), reboxetine did not.
Friedman et al. (84) investigated the effect of adjunctive atomoxetine pharmacotherapy on n-back-related brain activity in SCZ patients on stable treatment with atypical antipsychotics. Performance parameter changes were not observed among groups or over time. At follow-up relative to placebo-treated patients, increases in left DLPFC activity were observed.
We identified one paper using α2A noradrenergic receptor agonist guanfacine, investigating 12 pediatric ADHD patients before and after treatment.
From baseline to follow-up and compared to placebo-treated patients, 6- to 8-week treatment with guanfacine was not associated with improved accuracy, reaction times, or response inhibition-related brain activity in the fronto-cingulo-parietal network (85).
The aim of this systematic review was to summarize the impact of pharmacological interventions for psychiatric disorders on the fronto-cingulo-parietal cognitive control network and to relate this to plausible neurochemical mechanisms of action. Here, we will first discuss the evidence per treatment class, followed by a review of transdiagnostic commonalities as potential markers for future procognitive treatments.
Despite the pivotal role of acetylcholine in executive functions (86, 87), few potentially procognitive cholinergic agents are currently available, with actylcholinesterase inhibitors and nicotinic receptor agonists used most frequently.
With a placebo-controlled and placebo-controlled crossover design, the available preliminary evidence for adjunctive acetylcholinesterase inhibitors in SCZ was of good quality. In line with a large number of placebo-controlled clinical trials (88–92), adjunctive rivastigmine or donepezil treatment did not improve performance on attention and working memory tasks in SCZ. Moreover, in contrast to studies in Alzheimer’s disease (93, 94), two out of three manuscripts did not report task-related increases in fronto-cingulo-parietal activity (59, 60). Only Nahas et al. (58) observed increased IFG activity during a verbal fluency task after 12 weeks of treatment, a region also previously associated with verbal fluency (95) and likely associated with the Go/No-Go behavior required for this task. Kumari et al. (60) observed increased left SFG activity in placebo-treated patients over time, but not in the rivastigmine-treated patient group. These between-study differences may be explained by sample size (6 vs. 11 participants) or task demands, with verbal fluency tasks being dependent on lexical access speed and vocabulary size (96), in addition to attention and working memory.
There was, however, evidence for increases in occipital cortex and cerebellum activity (58, 59), previously reported to be related to visual attention, stimulus encoding, or motor speed (97, 98). Indeed, meta-analytic evidence suggests that acetylcholinesterase inhibitors may increase motor speed in SCZ (99), possibly explaining increased cerebellar activity.
The absence of any marked and consistent effects of acetylcholinesterase inhibitors on the fronto-cingulo-parietal cognitive control network in SCZ may be related to the suspected abnormalities in the cholinergic system. In SCZ, abnormalities have been observed at the receptor level, with evidence for a decrease in α7 nicotonic (100), β2 (101), and muscarinic receptors (102). Acetylcholinesterase inhibition, compared to direct targeting of receptors, may therefore not be the most efficient way to improve executive functions in SCZ. Second, tobacco desensitizes α7 and α4β2 nicotonic receptors (103, 104) and many participants in the available studies smoked tobacco. This could further decrease the procognitive potential of acetylcholinesterase inhibitors, although upregulated β2 receptors in smoking SCZ have also been associated with improved executive functions (105), requiring further investigation. Finally, all participants were already on atypical antipsychotics, which have been shown to increase frontal cortex acetylcholine release (106), fronto-cingulo-parietal activity (107, 108), and modestly improve performance on neuropsychological tests (36) (also see Atypical Antipsychotics), perhaps indicating a ceiling effect.
Thus, the current preliminary evidence suggests that adjunctive acetylcholinesterase inhibitors in SCZ do not markedly impact the fronto-cingulo-parietal cognitive control network, but may increase occipital cortex and cerebellum activity. Given the quality of the available evidence, these results likely reflect treatment effects, rather than practice or iatrogenic effects. In order to determine if the cholinergic system can be targeted to increase executive functions in SCZ, selective and direct targeting of nicotinic and muscarinic receptors may be a more fruitful strategy, although lack of specificity for the receptor subtypes has hampered the development of suitable drugs.
The only available study for acetylcholine nicotinic receptor agonists in smoking dependence was a placebo-controlled crossover design, which reported decreased n-back reaction times in highly dependent smokers while on varenicline. Moreover, on varenicline relative to placebo, DLPFC and ACC activity increased in the entire sample of smoking-dependent individuals, while activity in other frontal cortical areas decreased. Modulation of these areas is in line with recent meta-analytic evidence and likely reflects varenicline’s ability to optimize activity of the cognitive control network while suppressing task-irrelevant activity of the default mode network (109). Behaviorally, these observations are in agreement with repeated dosing evidence showing that varenicline improves several aspects of executive functions in smokers (110–112) and non-smokers (113). Varencline’s ability to modulate fronto-cingulo-parietal activity is promising and eagerly awaits replication in other psychiatric disorders.
In non-smoking SCZ patients, partial α7 agonist DMXB-A was not associated with activity changes in the fronto-cingulo-parietal network during a visual attention task. However, task-related changes in hippocampal activity (79) and suppression of default mode network activity in frontal cortical and parietal areas (80) were observed. While no other data for SCZ were available, the trial evidence for DMXB-A (114, 115) and varenicline (116–120) in psychotic disorders has been mixed, perhaps related to underlying cholinergic abnormalities or smoking status. Here, the use of fMRI and neuropsychological tasks that emphasize working memory, response inhibition, and action selection abilities could be useful in assessing DMXB-A’s potential procognitive abilities in SCZ.
Varenicline is a nicotinic receptor partial agonist at α4β2 receptors and full agonist at α7 receptors, and DMXB-A is a partial agonist at α7 receptors. Both β2- and α7-selective compounds increase frontal cortex dopamine release in the rat (121), offering an explanation for varenicline and DMXB-A’s putative activity-modulating abilities in frontal cortical areas. Neuropharmacologically, α7 and α4β2 in frontal cortex (121), α4β2 receptors expressed on ventral tegmental area neurons (122) and α7 receptors expressed on glutamatergic projections to the ventral tegmental area (123) seem to underlie the increase in dopamine release.
While anticonvulsants were originally developed to treat epileptic seizures, the majority of them have been shown to exert mood-stabilizing effects in bipolar (124, 125). Given that modulation of glutamatergic and γ-aminobutyric acid (GABA) activity is a common mechanism of these agents, and that excessive PFC glutamatergic signaling may contribute to cognitive impairments (126), it seems plausible to suggest that they could modulate executive functions.
With two open-label trials and one placebo-controlled study, the evidence for anticonvulsants in bipolar disorder was of moderate quality. In the open-label trials, there was no evidence for improvements in task performance (61, 63). This is in contrast to the only available placebo-controlled study, where divalproex treatment seemingly normalized response inhibition, but only when combined with a parallel group of risperidone-treated bipolar patients (62).
In the open-label trials, widespread normalization (increases) of anterior PFC and MPFC hypoactivity was observed after anticonvulsant treatment (61, 63). However, practice and placebo effects could not be excluded because of the lack of a placebo-controlled group and absence of prospective data for healthy volunteers (Table 1). In line with the findings of increased PFC activity in the open-label studies, subgenual ACC and insular functional connectivity increased during a Go/No-Go task in the placebo-controlled study (62). The regional specificity of these findings is noteworthy, given the consistent involvement of the ACC and insula in conflict monitoring and response inhibition (30). This may point toward treatment effects, but it is important that the findings for carbamazepine and lamotrigine are now replicated in placebo-controlled studies.
The inhibitory actions of lamotrigine, carbamazepine, and sodium valproate (127–130) at ion channels and N-methyl-d-aspartate (NMDA) receptors (131) are essential in modulating extracellular glutamate and GABA concentrations. Magnetic resonance spectroscopy studies in bipolar disorder have revealed alterations in frontal cortex, particularly ACC, glutamate, and glutamine concentrations (132, 133). Moreover, treatment with anticonvulsants (132), notably divalproex (134), alters VLPFC and ACC glutamate and glutamine levels, reflecting changes in glutamate concentrations, GABA concentrations, or both.
Summarizing, there is preliminary evidence that anticonvulsants impact, specifically increase, fronto-cingulo-parietal, especially ACC, activity in bipolar disorder, possibly reflecting changes in glutamate and/or GABA release at the neuronal level, and increased response inhibition at the behavioral level.
Although impressive in total sample size, the quality of evidence for antidepressants in depression and OCD was suboptimal, with five out of six studies employing an open-label design. Moreover, four out of six studies only had access to healthy control data at baseline or did not utilize a control group (Table 1). This complicates interpretation of the findings and increases susceptibility to practice, placebo and, more generally, iatrogenic effects.
With the exception of one study reporting a greater increase in 3-back performance over time in patients vs. healthy volunteers (64), there was no evidence for improved task performance following antidepressant treatment in depression. These results are generally in line with clinical trials showing no marked effects of antidepressants on cognitive control in depression (135), a domain that all available studies in depression assessed. Nonetheless, they are puzzling, given the presence of response inhibition deficits in depression (136), the proposed role of serotonin in inhibitory control (40), and the observation that serotonergic manipulations modulate the cognitive control network in healthy volunteers (137–139).
Two studies in depression reported activity decreases over time, observing normalized DLPFC activity during a response inhibition task (67) and normalized frontal cortical and parietal activity during an interference/conflict resolution task (66) following antidepressant treatment. Moreover, there was an overall greater engagement of frontoparietal regions with increasing n-back difficulty in patients vs. healthy volunteers (64), consistent with the observation of DLPFC hyperactivity during the n-back task in untreated depression (140). Taken together, these data suggest that SSRIs decrease frontal cortical and parietal hyperactivity in treatment responders, in spite of any marked performance changes.
A modest increase in DLPFC activity was observed during a cognitive control task, which at follow-up was still lower than healthy volunteers at baseline (65). The reported increase in DLPFC activity could have been attenuated by practice effects, given that steady declines in task-related activity have been reported over time in healthy volunteers (64, 67) and the fact that this study only had access to healthy volunteer data at baseline. Moreover, the seemingly contrasting observations of increased and decreased PFC activity may also be related to diagnosis: DLPFC activity increases were observed in late-life depression, while studies reporting anterior PFC decreases were conducted in major depression (Table 1). A final explanation could be the significant variation in treatment duration, ranging from 6 to 12 weeks, which is especially noteworthy in light of the delayed actions of SSRIs (141, 142).
Serotonin–noradrenaline reuptake inhibitor treatment in depression did not seem to impact fronto-cingulo-parietal activity (67). This is in line with the only available study in depression directly comparing serotonergic and noradrenergic agents (66), where SSRIs were associated with decreased parahippocampal and amygdalar activity, and reboxetine with slightly increased activity in these regions (NRIs in other disorders discussed in Section “Noradrenaline Reuptake Inhibitors”). The differential effect of SSRIs, SNRIs, and NRIs on attention and cognition-related brain function may be related to regional variation in serotonin transporter (143), noradrenaline transporter (144), and serotonin receptor subtype 1A (5HT1A) (145) expression. Still, they are surprising in light of the well-established modulating effects of NRIs on fronto-cingulo-parietal, specifically IFG, activity in ADHD (81, 83), and healthy volunteers (146).
All in all, the available results in depression suggest an effect of serotonergic treatment on the fronto-cingulo-parietal cognitive control network, but interpretation is severely hampered by a lack of randomized double-blind trials. In the absence of clear performance changes, we speculate that these activity changes could reflect improvement in the affective domain. Indeed, fronto-cingulo-parietal activity (changes) correlated with Hamilton Rating Scale for Depression scores (64, 66), and ACC metabolism predicts treatment response in depression (147).
The preliminary results in OCD were mixed on the level of performance and activity (changes), which could be related to study design (open label vs. double blind) or reference group (treatment responder vs. healthy volunteers at baseline) (68, 69). There were some indications of performance improvements, such as increased accuracy, in SSRI/SNRI treatment responders (68) and decreased reaction times from baseline to follow-up (69). However, comparison with a control group over multiple time points is essential to disentangle practice effects from treatment effects.
The quality of the evidence for atypical antipsychotics was moderate: three out of seven studies employed a randomized double-blind design, and six out of seven studies had baseline and follow-up data of a control group (Table 1).
Especially in psychotic disorders, atypical antipsychotics have been associated with modest improvements in executive function domains such as response inhibition, planning, and immediate recall (36, 148, 149). One out of two included studies in bipolar disorder reported increased task performance, with risperidone-treated patients showing comparable response inhibition to healthy volunteers but only when combined with a divalproex-treated group (also see Anticonvulsants) (62). In psychotic disorder, only performance on a visual attention task normalised (73) and two studies reported no performance differences at baseline and follow-up, relative to healthy volunteers (71, 72). Thus, there seems to be some overlap with clinical trials in terms of modestly improved executive functions on atypical antipsychotics.
Regardless of psychiatric disorder, the most consistent observation was increased, often normalized, anterior PFC (63, 70, 71) ACC (70), and parietal (73) activity following atypical antipsychotics treatment, the great majority being risperidone. Increased fronto-cingulo-parietal activity has also been observed when SCZ patients were switched from typical to atypical antipsychotics (107, 108) and from 4 to 8 weeks of treatment with olanzapine (150), all in all showing a consistent picture of increased fronto-cingulo-parietal activity on atypical antipsychotics. Outside of the fronto-cingulo-parietal network, decreases in ventral (74) and dorsal (72) striatal activity were observed in psychotic disorder. A correlation between caudate activity and risperidone dose (73) was also reported, consistent with the notion that antipsychotics decrease striatal dopaminergic hyperactivity.
Atypical antipsychotics are thought to increase frontal cortex DA release via 5HT1A agonism (106, 151–153) and 5HT2A antagonism (154, 155). In addition, increased frontal cortex acetylcholine and serotonin release has also been observed following administration of atypical antipsychotics (106, 151, 154). The observed increases in fronto-cingulo-parietal activity following atypical antipsychotics treatment may therefore be underlain by increased dopaminergic, serotonergic, and/or cholinergic activity in frontal cortex and their downstream effects. While the behavioral, neuroimaging, and neuropharmacological evidence seems to point toward modest procognitive effects of atypical antipsychotics, especially psychotic disorders are in need of replication with double-blind randomized designs to understand the exact extent of normalization within the fronto-cingulo-parietal network.
With only one open-label study identified, the results for benzodiazepines were preliminary. There was no evidence for improved performance, and some hints at decreased parietal hyperactivity and decreased temporal hypoactivity at follow-up, although these activity changes might partly reflect practice effects.
A placebo-controlled crossover and open-label study were identified for COMT inhibitors, suggesting moderate quality of the available preliminary evidence.
One out of two studies reported performance changes; in smoking dependence, n-back performance accuracy increased on tolcapone, and there was an effect of COMT genotype on reaction times (76) (Table 1). Task-related activity changes were observed in both smoking dependence and pathological gambling, with the former showing increased VMPFC deactivity on tolcapone (76) and the latter normalized fronto-cingulo-parietal hypoactivity, albeit to activity of healthy volunteers at baseline and in the absence of a placebo-controlled group (77). These results are in line with tolcapone’s effect in healthy volunteers, where it has been reported to increase n-back performance (156) and optimize task-related brain activity (156, 157).
Although preliminary and modest in nature, the results for COMT are favorable and could suggest potential procognitive effects that remain to be replicated in large placebo-controlled clinical trials. Interestingly, tolcapone does not affect extracellular catecholamine concentrations when administered alone, but increases striatal and frontal cortex dopamine release after l-DOPA (158, 159) and clozapine (160) administration. This may suggest that the catecholamine-increasing effect of tolcapone on the fronto-cingulo-parietal network could be even greater in medicated individuals with Parkinson’s disease or psychotic disorder, opening up new avenues for adjunctive treatment.
With three randomized studies (one reported to be double-blind), all having included a MPH-treated patient group and one additional placebo-treated patient group (Table 1), the evidence for atomoxetine in ADHD was of moderate to good quality. In line with clinical trial evidence (35), increased performance on response inhibition tasks was observed following atomoxetine treatment (81, 83).
Meta-analytical evidence in ADHD suggests that MPH among others consistently impacts IFG, dorsal ACC, and SMA activity (30), which were all areas consistently modulated by atomoxetine treatment (Table 1) and involved in conflict monitoring, response inhibition, and action selection (40). There were, however, differences between the two agents, such that atomoxetine and MPH exerted opposing effects on the same regions (81, 82) or that atomoxetine modulated task-relevant activity in one part of the fronto-cingulo-parietal network (e.g., ACC and DLPFC activity), while MPHs actions affected another part of the network (e.g., IFG) (83).
These results suggest that atomoxetine and MPH impact common substrates of the fronto-cingulo-parietal network, but also have a certain local and global task-dependent uniqueness. One tentative explanation could be that atomoxetine and MPH have almost comparable procognitive effects via differing underlying neurochemical mechanisms. MPH and atomoxetine’s dopamine- and noradrenaline-increasing properties (161) are for a major part directed to D1 and α2A adrenergic receptors, the former involved in suppressing noise (162) and the latter in enhancing signal (163) of neuronal networks. Moreover, the atomoxetine-induced increase in noradrenaline levels in frontal cortex decreases after prolonged exposure, while this is not the case for MPH (164). The neuropharmacological properties of MPH and atomoxetine may differentially affect the reorganization of functional brain networks.
In contrast to amphetamine (165) and in line with previous work (166), adjunctive atomoxetine treatment in antipsychotics-treated SCZ patients did not improve task performance. Despite any performance changes, task-related activity increases in DLPFC were observed, as well as an increase in posterior cingulate gyrus activity. These are regions typically modulated by atomoxetine in ADHD (81). While preliminary, the combination of atypical antipsychotics and atomoxetine may have led to overstimulation of frontal cortex dopamine and noradrenaline release, thereby missing the narrow window in which frontal cortex dopamine and noradrenaline levels optimally mediate cognitive performance (163, 167). Replication with SCZ patients on typical antipsychotics or MPH as an adjunctive treatment may shed further light on these findings.
While there were no main effects of α2A noradrenergic agonist guanfacine on response inhibition or associated brain function, this agent is clinically efficacious in treating symptoms of ADHD (168). Moreover, in smoking dependence, guanfacine modulates response inhibition and conflict resolution-related activity in among others SMA, DLPFC, VMPFC, and insula (169). In comparison to MPH and atomoxetine, guanfacine’s actions are confined to α2A noradrenergic receptors, thereby specifically enhancing signal in working memory networks (163). Direct comparison of guanfacine with atomoxetine and MPH could be valuable in elucidating the degree to which the latter two compounds modulate fronto-cingulo-parietal activity via a signal-increasing mechanism.
While executive function deficits are a universal symptom of psychiatric disorders, the underlying causes are not known. What is clear is that executive functions are underlain by an intricate and widely distributed network of brain regions. Theoretically, alterations in any of these regions can negatively affect network integrity, thereby giving rise to executive function deficits. As a result, pharmacological treatment of executive function deficits remains complex, reflected in the unsatisfactory treatment across psychiatric disorders. We identified common transdiagnostic changes in fronto-cingulo-parietal activity, which could serve as treatment markers.
Despite differences in experimental designs, treatment duration, and dosage, there were notable transdiagnostic effects of pharmacological agents on the fronto-cingulo-parietal network. In studies that also showed beneficial behavioral effects, or for which clinical trial data suggested beneficial effects, common activity changes in the fronto-cingulo-parietal network were (I) enhancement of activity in task-relevant areas and (II) suppression of activity in task-irrelevant areas.
In smoking dependence, tolcapone further suppressed activity in task-irrelevant areas such as the VMPFC (76), while in pathological gambling, it increased task-relevant fronto-cingulo-parietal activity (77). In line with the agent’s neuropharmacological action, the exact nature of activity changes was dependent on COMT genotype (Table 1). Similarly, nicotinic receptor agonist varenicline increased DLPFC and ACC activity in smoking dependence (78) and suppressed parietal and frontal cortical default mode network activity in SCZ (80). Atomoxetine, in most cases, increased task-relevant activity in DLPFC, ACC, IFG, and IPL in ADHD (81–83). Lastly, atypical antipsychotics increased DLPFC, ACC, and parietal cortex activity (70, 73), and decreased VMPFC activity (72). Overall, these results suggest positive predictive value of increased task-related fronto-cingulo-parietal activity as a marker of successful pharmacological treatment.
These were also transdiagnostic commonalities that displayed a degree of negative predictive value. Notably, increased task-relevant DLPFC activity was observed after adjunctive atomoxetine treatment in SCZ, together with increased task-irrelevant posterior cingulate activity, a region thought to be associated with the default mode network (80). Moreover, in depression, non-remitters showed task-relevant DLPFC hypoactivity (67). Finally, acetylcholinesterase inhibitors in SCZ did not consistently modulate fronto-cingulo-parietal network activity (58–60).
A final common observation was that the effects of pharmacological agents on the fronto-cingulo-parietal network were often lateralized. Unilateral effects could be related to lateralization of frontoparietal networks at rest (170). Indeed, changes in unilateral frontoparietal networks have also been observed following dopaminergic challenges (171). Lateralized functional networks could be the result of lateralized projections at the cellular level, which has been shown for among others dopamine (172), serotonin (173), and glutamate (174). Assessing laterality of the observed activity changes could be helpful in determining the specificity of treatment effects.
The available evidence suggests that dopamine, noradrenaline, and acetylcholine agonists are most consistently associated with activity changes in the fronto-cingulo-parietal network, as measured with fMRI. Across disorders, increased activity in task-relevant areas or decreased activity in task-irrelevant areas were the most consistent findings, demonstrated by good positive and moderate negative predictive value. However, in order to fully assess the potential of these markers, more randomized double-blind studies are necessary.
The current review highlights the potential of a dimensional, transdiagnostic approach in future (pharmacological) neuroimaging studies, thereby paralleling a shift that is currently taking place in the field of clinical diagnostics and management. The search for procognitive agents has thus far been carried out within the context of categorical classifications, which has not lead to any major breakthroughs in the development of treatments.
A surprising observation was the lack of selective cholinergic, especially muscarinic, and glutamatergic, notably NMDA receptor, agents. Preclinical and proof-of-concept trial evidence have demonstrated promising procognitive potential for M1 allosteric modulators (86, 175) and selective NMDA antagonists (176). The lack of clinical trial and neuroimaging evidence may reflect unavailability of such compounds or potential safety issues of the available compounds (177). A recent initiative that may facilitate testing of novel compounds is the Medicine Chest initiative by the ECNP (www.ecnp.eu), which could aid clinical researchers in gaining access to new pharmacological tools.
DH and TA performed literature search, writing, and interpretation of the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
DH was supported by a Kootstra post-doctoral Fellowship. TA was supported by an NWO VIDI.
1. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull (2013) 139(1):81–132. doi:10.1037/a0028727
2. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med (2014) 44(10):2029–40. doi:10.1017/S0033291713002535
3. Bora E, Yucel M, Pantelis C. Cognitive impairment in affective psychoses: a meta-analysis. Schizophr Bull (2010) 36(1):112–25. doi:10.1093/schbul/sbp093
4. Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L. Meta-analysis of cognitive performance in drug-naive patients with schizophrenia. Schizophr Res (2014) 158(1–3):156–62. doi:10.1016/j.schres.2014.06.034
5. Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med (2008) 38(6):771–85. doi:10.1017/S0033291707001675
6. Lee RS, Hermens DF, Scott J, Redoblado-Hodge MA, Naismith SL, Lagopoulos J, et al. A meta-analysis of neuropsychological functioning in first-episode bipolar disorders. J Psychiatr Res (2014) 57:1–11. doi:10.1016/j.jpsychires.2014.06.019
7. Morein-Zamir S, Craig KJ, Ersche KD, Abbott S, Muller U, Fineberg NA, et al. Impaired visuospatial associative memory and attention in obsessive compulsive disorder but no evidence for differential dopaminergic modulation. Psychopharmacology (2010) 212(3):357–67. doi:10.1007/s00213-010-1963-z
8. Segalas C, Alonso P, Labad J, Jaurrieta N, Real E, Jimenez S, et al. Verbal and nonverbal memory processing in patients with obsessive-compulsive disorder: its relationship to clinical variables. Neuropsychology (2008) 22(2):262–72. doi:10.1037/0894-4105.22.2.262
9. Kopera M, Wojnar M, Brower K, Glass J, Nowosad I, Gmaj B, et al. Cognitive functions in abstinent alcohol-dependent patients. Alcohol (2012) 46(7):665–71. doi:10.1016/j.alcohol.2012.04.005
10. van Holst RJ, Schilt T. Drug-related decrease in neuropsychological functions of abstinent drug users. Curr Drug Abuse Rev (2011) 4(1):42–56. doi:10.2174/1874473711104010042
11. O’Toole MS, Pedersen AD. A systematic review of neuropsychological performance in social anxiety disorder. Nord J Psychiatry (2011) 65(3):147–61. doi:10.3109/08039488.2011.565801
12. Robinson S, Goddard L, Dritschel B, Wisley M, Howlin P. Executive functions in children with autism spectrum disorders. Brain Cogn (2009) 71(3):362–8. doi:10.1016/j.bandc.2009.06.007
13. Verte S, Geurts HM, Roeyers H, Oosterlaan J, Sergeant JA. Executive functioning in children with an autism spectrum disorder: can we differentiate within the spectrum? J Autism Dev Disord (2006) 36(3):351–72. doi:10.1007/s10803-006-0074-5
14. Joseph MF, Frazier TW, Youngstrom EA, Soares JC. A quantitative and qualitative review of neurocognitive performance in pediatric bipolar disorder. J Child Adolesc Psychopharmacol (2008) 18(6):595–605. doi:10.1089/cap.2008.064
15. Schoechlin C, Engel RR. Neuropsychological performance in adult attention-deficit hyperactivity disorder: meta-analysis of empirical data. Arch Clin Neuropsychol (2005) 20(6):727–44. doi:10.1016/j.acn.2005.04.005
16. Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry (2012) 69(6):562–71. doi:10.1001/archgenpsychiatry.2011.1592
17. Christensen MV, Kyvik KO, Kessing LV. Cognitive function in unaffected twins discordant for affective disorder. Psychol Med (2006) 36(8):1119–29. doi:10.1017/S0033291706007896
18. Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry (2003) 160(6):1078–85. doi:10.1176/appi.ajp.160.6.1078
19. Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. J Affect Disord (2011) 134(1–3):20–31. doi:10.1016/j.jad.2010.11.011
20. Daglas R, Yucel M, Cotton S, Allott K, Hetrick S, Berk M. Cognitive impairment in first-episode mania: a systematic review of the evidence in the acute and remission phases of the illness. Int J Bipolar Disord (2015) 3:9. doi:10.1186/s40345-015-0024-2
21. McAuley T, Crosbie J, Charach A, Schachar R. The persistence of cognitive deficits in remitted and unremitted ADHD: a case for the state-independence of response inhibition. J Child Psychol Psychiatry (2014) 55(3):292–300. doi:10.1111/jcpp.12160
22. Majer M, Ising M, Kunzel H, Binder EB, Holsboer F, Modell S, et al. Impaired divided attention predicts delayed response and risk to relapse in subjects with depressive disorders. Psychol Med (2004) 34(8):1453–63. doi:10.1017/S0033291704002697
23. Gruber SA, Rosso IM, Yurgelun-Todd D. Neuropsychological performance predicts clinical recovery in bipolar patients. J Affect Disord (2008) 105(1–3):253–60. doi:10.1016/j.jad.2007.04.014
24. Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry (2006) 163(3):418–25. doi:10.1176/appi.ajp.163.3.418
25. Miller M, Hinshaw SP. Does childhood executive function predict adolescent functional outcomes in girls with ADHD? J Abnorm Child Psychol (2010) 38(3):315–26. doi:10.1007/s10802-009-9369-2
26. Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord (2000) 60(1):13–23. doi:10.1016/S0165-0327(99)00157-3
27. Trampush JW, Lencz T, DeRosse P, John M, Gallego JA, Petrides G, et al. Relationship of cognition to clinical response in first-episode schizophrenia spectrum disorders. Schizophr Bull (2015) 41(6):1237–47. doi:10.1093/schbul/sbv120
28. Insel TR, Voon V, Nye JS, Brown VJ, Altevogt BM, Bullmore ET, et al. Innovative solutions to novel drug development in mental health. Neurosci Biobehav Rev (2013) 37(10 Pt 1):2438–44. doi:10.1016/j.neubiorev.2013.03.022
29. Husain M, Mehta MA. Cognitive enhancement by drugs in health and disease. Trends Cogn Sci (2011) 15(1):28–36. doi:10.1016/j.tics.2010.11.002
30. Rubia K, Alegria AA, Cubillo AI, Smith AB, Brammer MJ, Radua J. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry (2014) 76(8):616–28. doi:10.1016/j.biopsych.2013.10.016
31. Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, et al. Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies. J Clin Psychiatry (2013) 74(9):902–17. doi:10.4088/JCP.12r08287
32. Linssen AM, Sambeth A, Vuurman EF, Riedel WJ. Cognitive effects of methylphenidate in healthy volunteers: a review of single dose studies. Int J Neuropsychopharmacol (2014) 17(6):961–77. doi:10.1017/S1461145713001594
33. Di Santo SG, Prinelli F, Adorni F, Caltagirone C, Musicco M. A meta-analysis of the efficacy of donepezil, rivastigmine, galantamine, and memantine in relation to severity of Alzheimer’s disease. J Alzheimers Dis (2013) 35(2):349–61. doi:10.3233/JAD-122140
34. Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology (2008) 33(7):1477–502. doi:10.1038/sj.npp.1301534
35. Asherson P, Bushe C, Saylor K, Tanaka Y, Deberdt W, Upadhyaya H. Efficacy of atomoxetine in adults with attention deficit hyperactivity disorder: an integrated analysis of the complete database of multicenter placebo-controlled trials. J Psychopharmacol (2014) 28(9):837–46. doi:10.1177/0269881114542453
36. Desamericq G, Schurhoff F, Meary A, Szoke A, Macquin-Mavier I, Bachoud-Levi AC, et al. Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis. Eur J Clin Pharmacol (2014) 70(2):127–34. doi:10.1007/s00228-013-1600-y
37. Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci (2012) 12(2):241–68. doi:10.3758/s13415-011-0083-5
38. Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci (2005) 8(12):1784–90. doi:10.1038/nn1594
39. Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A (1996) 93(24):13473–80. doi:10.1073/pnas.93.24.13473
40. Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol (2013) 108:44–79. doi:10.1016/j.pneurobio.2013.06.005
41. Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Hum Brain Mapp (2005) 25(1):22–34. doi:10.1002/hbm.20127
42. Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia (2008) 46(1):224–32. doi:10.1016/j.neuropsychologia.2007.07.015
43. Olson IR, Berryhill M. Some surprising findings on the involvement of the parietal lobe in human memory. Neurobiol Learn Mem (2009) 91(2):155–65. doi:10.1016/j.nlm.2008.09.006
44. Nickel J, Seitz RJ. Functional clusters in the human parietal cortex as revealed by an observer-independent meta-analysis of functional activation studies. Anat Embryol (2005) 210(5–6):463–72. doi:10.1007/s00429-005-0037-1
45. Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci (2000) 12(1):1–47. doi:10.1162/08989290051137585
46. Nathan PJ, Phan KL, Harmer CJ, Mehta MA, Bullmore ET. Increasing pharmacological knowledge about human neurological and psychiatric disorders through functional neuroimaging and its application in drug discovery. Curr Opin Pharmacol (2014) 14:54–61. doi:10.1016/j.coph.2013.11.009
47. Mehta MA, O’Daly OG. Pharmacological application of fMRI. Methods Mol Biol (2011) 711:551–65. doi:10.1007/978-1-61737-992-5_28
48. Czerniak SM, Sikoglu EM, King JA, Kennedy DN, Mick E, Frazier J, et al. Areas of the brain modulated by single-dose methylphenidate treatment in youth with ADHD during task-based fMRI: a systematic review. Harv Rev Psychiatry (2013) 21(3):151–62. doi:10.1097/HRP.0b013e318293749e
49. Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum (2012) 11(2):352–65. doi:10.1007/s12311-011-0260-7
50. Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage (2000) 11(5 Pt 1):380–91. doi:10.1006/nimg.2000.0592
51. Soto D, Hodsoll J, Rotshtein P, Humphreys GW. Automatic guidance of attention from working memory. Trends Cogn Sci (2008) 12(9):342–8. doi:10.1016/j.tics.2008.05.007
52. Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol (2015) 66:25–52. doi:10.1146/annurev-psych-010213-115159
53. Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci (2000) 1(1):59–65. doi:10.1038/35036228
54. Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp (2005) 25(1):46–59. doi:10.1002/hbm.20131
55. Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci (2003) 3(4):255–74. doi:10.3758/CABN.3.4.255
56. Rae CL, Hughes LE, Weaver C, Anderson MC, Rowe JB. Selection and stopping in voluntary action: a meta-analysis and combined fMRI study. Neuroimage (2014) 86:381–91. doi:10.1016/j.neuroimage.2013.10.012
57. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med (2009) 6(7):e1000097. doi:10.1371/journal.pmed.1000097
58. Nahas Z, George MS, Horner MD, Markowitz JS, Li X, Lorberbaum JP, et al. Augmenting atypical antipsychotics with a cognitive enhancer (donepezil) improves regional brain activity in schizophrenia patients: a pilot double-blind placebo controlled BOLD fMRI study. Neurocase (2003) 9(3):274–82. doi:10.1076/neur.9.3.274.15563
59. Aasen I, Kumari V, Sharma T. Effects of rivastigmine on sustained attention in schizophrenia: an fMRI study. J Clin Psychopharmacol (2005) 25(4):311–7. doi:10.1097/01.jcp.0000169267.36797.76
60. Kumari V, Aasen I, ffytche D, Williams SC, Sharma T. Neural correlates of adjunctive rivastigmine treatment to antipsychotics in schizophrenia: a randomized, placebo-controlled, double-blind fMRI study. NeuroImage (2006) 29(2):545–56. doi:10.1016/j.neuroimage.2005.08.013
61. Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J Clin Psychiatry (2010) 71(11):1526–34. doi:10.4088/JCP.09m05504yel
62. Pavuluri MN, Ellis JA, Wegbreit E, Passarotti AM, Stevens MC. Pharmacotherapy impacts functional connectivity among affective circuits during response inhibition in pediatric mania. Behav Brain Res (2012) 226(2):493–503. doi:10.1016/j.bbr.2011.10.003
63. Schneider MR, Klein CC, Weber W, Bitter SM, Elliott KB, Strakowski SM, et al. The effects of carbamazepine on prefrontal activation in manic youth with bipolar disorder. Psychiatry Res (2014) 223(3):268–70. doi:10.1016/j.pscychresns.2014.04.011
64. Walsh ND, Williams SC, Brammer MJ, Bullmore ET, Kim J, Suckling J, et al. A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol Psychiatry (2007) 62(11):1236–43. doi:10.1016/j.biopsych.2006.12.022
65. Aizenstein HJ, Butters MA, Wu M, Mazurkewicz LM, Stenger VA, Gianaros PJ, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry (2009) 17(1):30–42. doi:10.1097/JGP.0b013e31817b60af
66. Wagner G, Koch K, Schachtzabel C, Sobanski T, Reichenbach JR, Sauer H, et al. Differential effects of serotonergic and noradrenergic antidepressants on brain activity during a cognitive control task and neurofunctional prediction of treatment outcome in patients with depression. J Psychiatry Neurosci (2010) 35(4):247–57. doi:10.1503/jpn.090081
67. Gyurak A, Patenaude B, Korgaonkar MS, Grieve SM, Williams LM, Etkin A. Frontoparietal activation during response inhibition predicts remission to antidepressants in patients with major depression. Biol Psychiatry (2016) 79(4):274–81. doi:10.1016/j.biopsych.2015.02.037
68. van der Wee NJ, Ramsey NF, van Megen HJ, Denys D, Westenberg HG, Kahn RS. Spatial working memory in obsessive-compulsive disorder improves with clinical response: a functional MRI study. Eur Neuropsychopharmacol (2007) 17(1):16–23. doi:10.1016/j.euroneuro.2006.04.012
69. Han JY, Kang DH, Gu BM, Jung WH, Choi JS, Choi CH, et al. Altered brain activation in ventral frontal-striatal regions following a 16-week pharmacotherapy in unmedicated obsessive-compulsive disorder. J Korean Med Sci (2011) 26(5):665–74. doi:10.3346/jkms.2011.26.5.665
70. Snitz BE, MacDonald A III, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry (2005) 162(12):2322–9. doi:10.1176/appi.ajp.162.12.2322
71. Meisenzahl EM, Scheuerecker J, Zipse M, Ufer S, Wiesmann M, Frodl T, et al. Effects of treatment with the atypical neuroleptic quetiapine on working memory function: a functional MRI follow-up investigation. Eur Arch Psychiatry Clin Neurosci (2006) 256(8):522–31. doi:10.1007/s00406-006-0687-x
72. Keedy SK, Rosen C, Khine T, Rajarethinam R, Janicak PG, Sweeney JA. An fMRI study of visual attention and sensorimotor function before and after antipsychotic treatment in first-episode schizophrenia. Psychiatry Res (2009) 172(1):16–23. doi:10.1016/j.pscychresns.2008.06.003
73. Keedy SK, Reilly JL, Bishop JR, Weiden PJ, Sweeney JA. Impact of antipsychotic treatment on attention and motor learning systems in first-episode schizophrenia. Schizophr Bull (2015) 41(2):355–65. doi:10.1093/schbul/sbu071
74. Ikuta T, Robinson DG, Gallego JA, Peters BD, Gruner P, Kane J, et al. Subcortical modulation of attentional control by second-generation antipsychotics in first-episode psychosis. Psychiatry Res (2014) 221(2):127–34. doi:10.1016/j.pscychresns.2013.09.010
75. Wilcox CE, Mayer AR, Bogenschutz MP, Ling J, Dekonenko C, Cumbo H. Cognitive control network function in alcohol use disorder before and during treatment with lorazepam. Subst Use Misuse (2015) 50(1):40–52. doi:10.3109/10826084.2014.957771
76. Ashare RL, Wileyto EP, Ruparel K, Goelz PM, Hopson RD, Valdez JN, et al. Effects of tolcapone on working memory and brain activity in abstinent smokers: a proof-of-concept study. Drug Alcohol Depend (2013) 133(3):852–6. doi:10.1016/j.drugalcdep.2013.09.003
77. Grant JE, Odlaug BL, Chamberlain SR, Hampshire A, Schreiber LR, Kim SW. A proof of concept study of tolcapone for pathological gambling: relationships with COMT genotype and brain activation. Eur Neuropsychopharmacol (2013) 23(11):1587–96. doi:10.1016/j.euroneuro.2013.07.008
78. Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, et al. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry (2010) 67(8):715–21. doi:10.1016/j.biopsych.2010.01.016
79. Tregellas JR, Olincy A, Johnson L, Tanabe J, Shatti S, Martin LF, et al. Functional magnetic resonance imaging of effects of a nicotinic agonist in schizophrenia. Neuropsychopharmacology (2010) 35(4):938–42. doi:10.1038/npp.2009.196
80. Tregellas JR, Tanabe J, Rojas DC, Shatti S, Olincy A, Johnson L, et al. Effects of an alpha 7-nicotinic agonist on default network activity in schizophrenia. Biol Psychiatry (2011) 69(1):7–11. doi:10.1016/j.biopsych.2010.07.004
81. Schulz KP, Fan J, Bedard AC, Clerkin SM, Ivanov I, Tang CY, et al. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry (2012) 69(9):952–61. doi:10.1001/archgenpsychiatry.2011.2053
82. Bush G, Holmes J, Shin LM, Surman C, Makris N, Mick E, et al. Atomoxetine increases fronto-parietal functional MRI activation in attention-deficit/hyperactivity disorder: a pilot study. Psychiatry Res (2013) 211(1):88–91. doi:10.1016/j.pscychresns.2012.09.004
83. Chou TL, Chia S, Shang CY, Gau SS. Differential therapeutic effects of 12-week treatment of atomoxetine and methylphenidate on drug-naive children with attention deficit/hyperactivity disorder: a counting Stroop functional MRI study. Eur Neuropsychopharmacol (2015) 25(12):2300–10. doi:10.1016/j.euroneuro.2015.08.024
84. Friedman JI, Carpenter D, Lu J, Fan J, Tang CY, White L, et al. A pilot study of adjunctive atomoxetine treatment to second-generation antipsychotics for cognitive impairment in schizophrenia. J Clin Psychopharmacol (2008) 28(1):59–63. doi:10.1097/jcp.0b013e318161318f
85. Bedard AC, Schulz KP, Krone B, Pedraza J, Duhoux S, Halperin JM, et al. Neural mechanisms underlying the therapeutic actions of guanfacine treatment in youth with ADHD: a pilot fMRI study. Psychiatry Res (2015) 231(3):353–6. doi:10.1016/j.pscychresns.2015.01.012
86. Melancon BJ, Tarr JC, Panarese JD, Wood MR, Lindsley CW. Allosteric modulation of the M1 muscarinic acetylcholine receptor: improving cognition and a potential treatment for schizophrenia and Alzheimer’s disease. Drug Discov Today (2013) 18(23–24):1185–99. doi:10.1016/j.drudis.2013.09.005
87. Nees F. The nicotinic cholinergic system function in the human brain. Neuropharmacology (2015) 96(Pt B):289–301. doi:10.1016/j.neuropharm.2014.10.021
88. Akhondzadeh S, Gerami M, Noroozian M, Karamghadiri N, Ghoreishi A, Abbasi SH, et al. A 12-week, double-blind, placebo-controlled trial of donepezil adjunctive treatment to risperidone in chronic and stable schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2008) 32(8):1810–5. doi:10.1016/j.pnpbp.2008.08.001
89. Fagerlund B, Soholm B, Fink-Jensen A, Lublin H, Glenthoj BY. Effects of donepezil adjunctive treatment to ziprasidone on cognitive deficits in schizophrenia: a double-blind, placebo-controlled study. Clin Neuropharmacol (2007) 30(1):3–12. doi:10.1097/01.WNF.0000240940.67241.F6
90. Freudenreich O, Herz L, Deckersbach T, Evins AE, Henderson DC, Cather C, et al. Added donepezil for stable schizophrenia: a double-blind, placebo-controlled trial. Psychopharmacology (2005) 181(2):358–63. doi:10.1007/s00213-005-2235-1
91. Friedman JI, Adler DN, Howanitz E, Harvey PD, Brenner G, Temporini H, et al. A double blind placebo controlled trial of donepezil adjunctive treatment to risperidone for the cognitive impairment of schizophrenia. Biol Psychiatry (2002) 51(5):349–57. doi:10.1016/S0006-3223(01)01342-7
92. Tugal O, Yazici KM, Anil Yagcioglu AE, Gogus A. A double-blind, placebo controlled, cross-over trial of adjunctive donepezil for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol (2004) 7(2):117–23. doi:10.1017/S1461145703004024
93. Goekoop R, Scheltens P, Barkhof F, Rombouts SA. Cholinergic challenge in Alzheimer patients and mild cognitive impairment differentially affects hippocampal activation – a pharmacological fMRI study. Brain (2006) 129(Pt 1):141–57. doi:10.1093/brain/awh671
94. Miettinen PS, Jauhiainen AM, Tarkka IM, Pihlajamaki M, Grohn H, Niskanen E, et al. Long-term response to cholinesterase inhibitor treatment is related to functional MRI response in Alzheimer’s disease. Dement Geriatr Cogn Disord (2015) 40(5–6):243–55. doi:10.1159/000435948
95. Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Laakso M, Partanen K, et al. Verbal fluency activates the left medial temporal lobe: a functional magnetic resonance imaging study. Ann Neurol (2000) 47(4):470–6. doi:10.1002/1531-8249(200004)47:4<470::AID-ANA10>3.0.CO;2-M
96. Shao Z, Janse E, Visser K, Meyer AS. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol (2014) 5:772. doi:10.3389/fpsyg.2014.00772
97. Bentley P, Driver J, Dolan RJ. Modulation of fusiform cortex activity by cholinesterase inhibition predicts effects on subsequent memory. Brain (2009) 132(Pt 9):2356–71. doi:10.1093/brain/awp176
98. Pa J, Berry AS, Compagnone M, Boccanfuso J, Greenhouse I, Rubens MT, et al. Cholinergic enhancement of functional networks in older adults with mild cognitive impairment. Ann Neurol (2013) 73(6):762–73. doi:10.1002/ana.23874
99. Ribeiz SR, Bassitt DP, Arrais JA, Avila R, Steffens DC, Bottino CM. Cholinesterase inhibitors as adjunctive therapy in patients with schizophrenia and schizoaffective disorder: a review and meta-analysis of the literature. CNS Drugs (2010) 24(4):303–17. doi:10.2165/11530260-000000000-00000
100. Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry (1995) 38(1):22–33. doi:10.1016/0006-3223(94)00252-X
101. D’Souza DC, Esterlis I, Carbuto M, Krasenics M, Seibyl J, Bois F, et al. Lower ss2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. Am J Psychiatry (2012) 169(3):326–34. doi:10.1176/appi.ajp.2011.11020189
102. Raedler TJ, Knable MB, Jones DW, Urbina RA, Gorey JG, Lee KS, et al. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry (2003) 160(1):118–27. doi:10.1176/appi.ajp.160.1.118
103. Fenster CP, Hicks JH, Beckman ML, Covernton PJ, Quick MW, Lester RA. Desensitization of nicotinic receptors in the central nervous system. Ann N Y Acad Sci (1999) 868:620–3. doi:10.1111/j.1749-6632.1999.tb11335.x
104. Gentry CL, Wilkins LH Jr, Lukas RJ. Effects of prolonged nicotinic ligand exposure on function of heterologously expressed, human alpha4beta2- and alpha4beta4-nicotinic acetylcholine receptors. J Pharmacol Exp Ther (2003) 304(1):206–16. doi:10.1124/jpet.102.041756
105. Esterlis I, Ranganathan M, Bois F, Pittman B, Picciotto MR, Shearer L, et al. In vivo evidence for beta2 nicotinic acetylcholine receptor subunit upregulation in smokers as compared with nonsmokers with schizophrenia. Biol Psychiatry (2014) 76(6):495–502. doi:10.1016/j.biopsych.2013.11.001
106. Ichikawa J, Li Z, Dai J, Meltzer HY. Atypical antipsychotic drugs, quetiapine, iloperidone, and melperone, preferentially increase dopamine and acetylcholine release in rat medial prefrontal cortex: role of 5-HT1A receptor agonism. Brain Res (2002) 956(2):349–57. doi:10.1016/S0006-8993(02)03570-9
107. Honey GD, Bullmore ET, Soni W, Varatheesan M, Williams SC, Sharma T. Differences in frontal cortical activation by a working memory task after substitution of risperidone for typical antipsychotic drugs in patients with schizophrenia. Proc Natl Acad Sci U S A (1999) 96(23):13432–7. doi:10.1073/pnas.96.23.13432
108. Schlagenhauf F, Dinges M, Beck A, Wustenberg T, Friedel E, Dembler T, et al. Switching schizophrenia patients from typical neuroleptics to aripiprazole: effects on working memory dependent functional activation. Schizophr Res (2010) 118(1–3):189–200. doi:10.1016/j.schres.2010.01.022
109. Sutherland MT, Ray KL, Riedel MC, Yanes JA, Stein EA, Laird AR. Neurobiological impact of nicotinic acetylcholine receptor agonists: an activation likelihood estimation meta-analysis of pharmacologic neuroimaging studies. Biol Psychiatry (2015) 78(10):711–20. doi:10.1016/j.biopsych.2014.12.021
110. Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, et al. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry (2009) 65(2):144–9. doi:10.1016/j.biopsych.2008.08.028
111. Rhodes JD, Hawk LW Jr, Ashare RL, Schlienz NJ, Mahoney MC. The effects of varenicline on attention and inhibitory control among treatment-seeking smokers. Psychopharmacology (2012) 223(2):131–8. doi:10.1007/s00213-012-2700-6
112. Ashare RL, McKee SA. Effects of varenicline and bupropion on cognitive processes among nicotine-deprived smokers. Exp Clin Psychopharmacol (2012) 20(1):63–70. doi:10.1037/a0025594
113. Mocking RJ, Patrick Pflanz C, Pringle A, Parsons E, McTavish SF, Cowen PJ, et al. Effects of short-term varenicline administration on emotional and cognitive processing in healthy, non-smoking adults: a randomized, double-blind, study. Neuropsychopharmacology (2013) 38(3):476–84. doi:10.1038/npp.2012.205
114. Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry (2008) 165(8):1040–7. doi:10.1176/appi.ajp.2008.07071135
115. Preskorn SH, Gawryl M, Dgetluck N, Palfreyman M, Bauer LO, Hilt DC. Normalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. J Psychiatr Pract (2014) 20(1):12–24. doi:10.1097/01.pra.0000442935.15833.c5
116. Hong LE, Thaker GK, McMahon RP, Summerfelt A, Rachbeisel J, Fuller RL, et al. Effects of moderate-dose treatment with varenicline on neurobiological and cognitive biomarkers in smokers and nonsmokers with schizophrenia or schizoaffective disorder. Arch Gen Psychiatry (2011) 68(12):1195–206. doi:10.1001/archgenpsychiatry.2011.83
117. Shim JC, Jung DU, Jung SS, Seo YS, Cho DM, Lee JH, et al. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology (2012) 37(3):660–8. doi:10.1038/npp.2011.238
118. Smith RC, Amiaz R, Si TM, Maayan L, Jin H, Boules S, et al. Varenicline effects on smoking, cognition, and psychiatric symptoms in schizophrenia: a double-blind randomized trial. PLoS One (2016) 11(1):e0143490. doi:10.1371/journal.pone.0143490
119. Smith RC, Lindenmayer JP, Davis JM, Cornwell J, Noth K, Gupta S, et al. Cognitive and antismoking effects of varenicline in patients with schizophrenia or schizoaffective disorder. Schizophr Res (2009) 110(1–3):149–55. doi:10.1016/j.schres.2009.02.001
120. Wing VC, Wass CE, Bacher I, Rabin RA, George TP. Varenicline modulates spatial working memory deficits in smokers with schizophrenia. Schizophr Res (2013) 149(1–3):190–1. doi:10.1016/j.schres.2013.06.032
121. Livingstone PD, Srinivasan J, Kew JN, Dawson LA, Gotti C, Moretti M, et al. Alpha7 and non-alpha7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. Eur J Neurosci (2009) 29(3):539–50. doi:10.1111/j.1460-9568.2009.06613.x
122. Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol (2007) 74(8):1235–46. doi:10.1016/j.bcp.2007.07.032
123. Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci (2004) 24(50):11244–52. doi:10.1523/JNEUROSCI.3009-04.2004
124. Liu HY, Potter MP, Woodworth KY, Yorks DM, Petty CR, Wozniak JR, et al. Pharmacologic treatments for pediatric bipolar disorder: a review and meta-analysis. J Am Acad Child Adolesc Psychiatry (2011) 50(8):749–62.e39. doi:10.1016/j.jaac.2011.05.011
125. Smith LA, Cornelius VR, Azorin JM, Perugi G, Vieta E, Young AH, et al. Valproate for the treatment of acute bipolar depression: systematic review and meta-analysis. J Affect Disord (2010) 122(1–2):1–9. doi:10.1016/j.jad.2009.10.033
126. Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology (2012) 37(1):4–15. doi:10.1038/npp.2011.181
127. Ahmad S, Fowler LJ, Whitton PS. Effects of acute and chronic lamotrigine treatment on basal and stimulated extracellular amino acids in the hippocampus of freely moving rats. Brain Res (2004) 1029(1):41–7. doi:10.1016/j.brainres.2004.09.016
128. Ahmad S, Fowler LJ, Whitton PS. Lamotrigine, carbamazepine and phenytoin differentially alter extracellular levels of 5-hydroxytryptamine, dopamine and amino acids. Epilepsy Res (2005) 63(2–3):141–9. doi:10.1016/j.eplepsyres.2005.02.002
129. Biggs CS, Pearce BR, Fowler LJ, Whitton PS. The effect of sodium valproate on extracellular GABA and other amino acids in the rat ventral hippocampus: an in vivo microdialysis study. Brain Res (1992) 594(1):138–42. doi:10.1016/0006-8993(92)91038-G
130. Loscher W. Effects of the antiepileptic drug valproate on metabolism and function of inhibitory and excitatory amino acids in the brain. Neurochem Res (1993) 18(4):485–502. doi:10.1007/BF00967253
131. Farber NB, Jiang XP, Heinkel C, Nemmers B. Antiepileptic drugs and agents that inhibit voltage-gated sodium channels prevent NMDA antagonist neurotoxicity. Mol Psychiatry (2002) 7(7):726–33. doi:10.1038/sj.mp.4001087
132. Soeiro-de-Souza MG, Henning A, Machado-Vieira R, Moreno RA, Pastorello BF, da Costa Leite C, et al. Anterior cingulate glutamate-glutamine cycle metabolites are altered in euthymic bipolar I disorder. Eur Neuropsychopharmacol (2015) 25(12):2221–9. doi:10.1016/j.euroneuro.2015.09.020
133. Spencer AE, Uchida M, Kenworthy T, Keary CJ, Biederman J. Glutamatergic dysregulation in pediatric psychiatric disorders: a systematic review of the magnetic resonance spectroscopy literature. J Clin Psychiatry (2014) 75(11):1226–41. doi:10.4088/JCP.13r08767
134. Strawn JR, Patel NC, Chu WJ, Lee JH, Adler CM, Kim MJ, et al. Glutamatergic effects of divalproex in adolescents with mania: a proton magnetic resonance spectroscopy study. J Am Acad Child Adolesc Psychiatry (2012) 51(6):642–51. doi:10.1016/j.jaac.2012.03.009
135. Rosenblat JD, Kakar R, McIntyre RS. The cognitive effects of antidepressants in major depressive disorder: a systematic review and meta-analysis of randomized clinical trials. Int J Neuropsychopharmacol (2015) 19(2):yv082. doi:10.1093/ijnp/pyv082
136. Gohier B, Ferracci L, Surguladze SA, Lawrence E, El Hage W, Kefi MZ, et al. Cognitive inhibition and working memory in unipolar depression. J Affect Disord (2009) 116(1–2):100–5. doi:10.1016/j.jad.2008.10.028
137. Drueke B, Schlaegel SM, Seifert A, Moeller O, Grunder G, Gauggel S, et al. The role of 5-HT in response inhibition and re-engagement. Eur Neuropsychopharmacol (2013) 23(8):830–41. doi:10.1016/j.euroneuro.2013.05.005
138. Klaassens BL, van Gorsel HC, Khalili-Mahani N, van der Grond J, Wyman BT, Whitcher B, et al. Single-dose serotonergic stimulation shows widespread effects on functional brain connectivity. Neuroimage (2015) 122:440–50. doi:10.1016/j.neuroimage.2015.08.012
139. Macoveanu J, Hornboll B, Elliott R, Erritzoe D, Paulson OB, Siebner H, et al. Serotonin 2A receptors, citalopram and tryptophan-depletion: a multimodal imaging study of their interactions during response inhibition. Neuropsychopharmacology (2013) 38(6):996–1005. doi:10.1038/npp.2012.264
140. Matsuo K, Glahn DC, Peluso MA, Hatch JP, Monkul ES, Najt P, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry (2007) 12(2):158–66. doi:10.1038/sj.mp.4001894
141. Blier P, de Montigny C, Chaput Y. Modifications of the serotonin system by antidepressant treatments: implications for the therapeutic response in major depression. J Clin Psychopharmacol (1987) 7(6 Suppl):24S–35S. doi:10.1097/00004714-198712001-00003
142. Hjorth S, Bengtsson HJ, Kullberg A, Carlzon D, Peilot H, Auerbach SB. Serotonin autoreceptor function and antidepressant drug action. J Psychopharmacol (2000) 14(2):177–85. doi:10.1177/026988110001400208
143. Varnas K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp (2004) 22(3):246–60. doi:10.1002/hbm.20035
144. Smith HR, Beveridge TJ, Porrino LJ. Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience (2006) 138(2):703–14. doi:10.1016/j.neuroscience.2005.11.033
145. Marazziti D, Marracci S, Palego L, Rotondo A, Mazzanti C, Nardi I, et al. Localization and gene expression of serotonin 1A (5HT1A) receptors in human brain postmortem. Brain Res (1994) 658(1–2):55–9. doi:10.1016/S0006-8993(09)90010-5
146. Nandam LS, Hester R, Bellgrove MA. Dissociable and common effects of methylphenidate, atomoxetine and citalopram on response inhibition neural networks. Neuropsychologia (2014) 56:263–70. doi:10.1016/j.neuropsychologia.2014.01.023
147. Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport (1997) 8(4):1057–61. doi:10.1097/00001756-199703030-00048
148. Purdon SE, Jones BD, Stip E, Labelle A, Addington D, David SR, et al. Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. The Canadian Collaborative Group for research in schizophrenia. Arch Gen Psychiatry (2000) 57(3):249–58. doi:10.1001/archpsyc.57.3.249
149. Bender S, Dittmann-Balcar A, Schall U, Wolstein J, Klimke A, Riedel M, et al. Influence of atypical neuroleptics on executive functioning in patients with schizophrenia: a randomized, double-blind comparison of olanzapine vs. clozapine. Int J Neuropsychopharmacol (2006) 9(2):135–45. doi:10.1017/S1461145705005924
150. Sambataro F, Blasi G, Fazio L, Caforio G, Taurisano P, Romano R, et al. Treatment with olanzapine is associated with modulation of the default mode network in patients with schizophrenia. Neuropsychopharmacology (2010) 35(4):904–12. doi:10.1038/npp.2009.192
151. Hertel P, Nomikos GG, Iurlo M, Svensson TH. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (1996) 124(1–2):74–86. doi:10.1007/BF02245607
152. Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY. 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem (2001) 76(5):1521–31. doi:10.1046/j.1471-4159.2001.00154.x
153. Rollema H, Lu Y, Schmidt AW, Sprouse JS, Zorn SH. 5-HT(1A) receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biol Psychiatry (2000) 48(3):229–37. doi:10.1016/S0006-3223(00)00850-7
154. Huang M, Panos JJ, Kwon S, Oyamada Y, Rajagopal L, Meltzer HY. Comparative effect of lurasidone and blonanserin on cortical glutamate, dopamine, and acetylcholine efflux: role of relative serotonin (5-HT)2A and DA D2 antagonism and 5-HT1A partial agonism. J Neurochem (2014) 128(6):938–49. doi:10.1111/jnc.12512
155. Zhang W, Bymaster FP. The in vivo effects of olanzapine and other antipsychotic agents on receptor occupancy and antagonism of dopamine D1, D2, D3, 5HT2A and muscarinic receptors. Psychopharmacology (1999) 141(3):267–78. doi:10.1007/s002130050834
156. Magalona SC, Rasetti R, Chen J, Chen Q, Gold I, Decot H, et al. Effect of tolcapone on brain activity during a variable attentional control task: a double-blind, placebo-controlled, counter-balanced trial in healthy volunteers. CNS Drugs (2013) 27(8):663–73. doi:10.1007/s40263-013-0082-x
157. Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology (2007) 32(5):1011–20. doi:10.1038/sj.npp.1301227
158. Huotari M, Gainetdinov R, Mannisto PT. Microdialysis studies on the action of tolcapone on pharmacologically-elevated extracellular dopamine levels in conscious rats. Pharmacol Toxicol (1999) 85(5):233–8. doi:10.1111/j.1600-0773.1999.tb02014.x
159. Li YH, Wirth T, Huotari M, Laitinen K, MacDonald E, Mannisto PT. No change of brain extracellular catecholamine levels after acute catechol-O-methyltransferase inhibition: a microdialysis study in anaesthetized rats. Eur J Pharmacol (1998) 356(2–3):127–37. doi:10.1016/S0014-2999(98)00524-X
160. Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci (2004) 24(23):5331–5. doi:10.1523/JNEUROSCI.1124-04.2004
161. Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology (2002) 27(5):699–711. doi:10.1016/S0893-133X(02)00346-9
162. Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci (2001) 21(10):3628–38.
163. Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry (2010) 49(10):1011–23. doi:10.1016/j.jaac.2010.06.015
164. Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T. Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem (2010) 114(1):259–70. doi:10.1111/j.1471-4159.2010.06750.x
165. Barch DM, Carter CS. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr Res (2005) 77(1):43–58. doi:10.1016/j.schres.2004.12.019
166. Kelly DL, Buchanan RW, Boggs DL, McMahon RP, Dickinson D, Nelson M, et al. A randomized double-blind trial of atomoxetine for cognitive impairments in 32 people with schizophrenia. J Clin Psychiatry (2009) 70(4):518–25. doi:10.4088/JCP.08m04358
167. Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry (2011) 69(12):e89–99. doi:10.1016/j.biopsych.2011.01.027
168. Ruggiero S, Clavenna A, Reale L, Capuano A, Rossi F, Bonati M. Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol (2014) 24(10):1578–90. doi:10.1016/j.euroneuro.2014.08.001
169. McKee SA, Potenza MN, Kober H, Sofuoglu M, Arnsten AF, Picciotto MR, et al. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. J Psychopharmacol (2015) 29(3):300–11. doi:10.1177/0269881114562091
170. Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A (2009) 106(31):13040–5. doi:10.1073/pnas.0905267106
171. Cole DM, Oei NY, Soeter RP, Both S, van Gerven JM, Rombouts SA, et al. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb Cortex (2013) 23(7):1509–16. doi:10.1093/cercor/bhs136
172. Franco R, Casadó-Anguera V, Muñoz A, Petrovic M, Navarro G, Moreno E, et al. Hints on the lateralization of dopamine binding to D1 receptors in rat striatum. Mol Neurobiol (2015). doi:10.1007/s12035-015-9468-8
173. Kranz GS, Hahn A, Baldinger P, Haeusler D, Philippe C, Kaufmann U, et al. Cerebral serotonin transporter asymmetry in females, males and male-to-female transsexuals measured by PET in vivo. Brain Struct Funct (2014) 219(1):171–83. doi:10.1007/s00429-012-0492-4
174. Capper-Loup C, Rebell D, Kaelin-Lang A. Hemispheric lateralization of the corticostriatal glutamatergic system in the rat. J Neural Transm (Vienna) (2009) 116(9):1053–7. doi:10.1007/s00702-009-0265-2
175. Vardigan JD, Cannon CE, Puri V, Dancho M, Koser A, Wittmann M, et al. Improved cognition without adverse effects: novel M1 muscarinic potentiator compares favorably to donepezil and xanomeline in rhesus monkey. Psychopharmacology (2015) 232(11):1859–66. doi:10.1007/s00213-014-3813-x
176. Kumar G, Olley J, Steckler T, Talpos J. Dissociable effects of NR2A and NR2B NMDA receptor antagonism on cognitive flexibility but not pattern separation. Psychopharmacology (2015) 232(21–22):3991–4003. doi:10.1007/s00213-015-4008-9
Keywords: psychopharmacology, fMRI, psychiatric disorders, attention, cognition, prefrontal cortex, executive functioning, treatment
Citation: van Amelsvoort T and Hernaus D (2016) Effect of Pharmacological Interventions on the Fronto-Cingulo-Parietal Cognitive Control Network in Psychiatric Disorders: A Transdiagnostic Systematic Review of fMRI Studies. Front. Psychiatry 7:82. doi: 10.3389/fpsyt.2016.00082
Received: 15 January 2016; Accepted: 26 April 2016;
Published: 18 May 2016
Edited by:
Stefan Borgwardt, University of Basel, SwitzerlandReviewed by:
Felipe Ortuño, Clínica Universidad de Navarra, SpainCopyright: © 2016 van Amelsvoort and Hernaus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dennis Hernaus, ZGVubmlzLmhlcm5hdXNAbWFhc3RyaWNodHVuaXZlcnNpdHkubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.