
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 27 August 2014
Sec. Neuroimaging
Volume 5 - 2014 | https://doi.org/10.3389/fpsyt.2014.00116
 Jie-Yu Chuang1*
Jie-Yu Chuang1* Graham K. Murray1,2,3
Graham K. Murray1,2,3 Antonio Metastasio4
Antonio Metastasio4 Nuria Segarra1
Nuria Segarra1 Roger Tait2
Roger Tait2 Jenny Spencer1,3
Jenny Spencer1,3 Hisham Ziauddeen1,3,5
Hisham Ziauddeen1,3,5 Robert B. Dudas1,2,3,4
Robert B. Dudas1,2,3,4 Paul C. Fletcher1,3
Paul C. Fletcher1,3 John Suckling1,2,3
John Suckling1,2,3
Negative symptoms occur in several major mental health disorders with undetermined mechanisms and unsatisfactory treatments; identification of their neural correlates might unveil the underlying pathophysiological basis and pinpoint the therapeutic targets. In this study, participants with major depressive disorder (n = 24), schizophrenia (n = 22), and healthy controls (n = 20) were assessed with 10 frequently used negative symptom scales followed by principal component analysis (PCA) of the scores. A linear model with the prominent components identified by PCA was then regressed on gray and white-matter volumes estimated from T1-weighted magnetic resonance imaging. In depressed patients, negative symptoms such as blunted affect, alogia, withdrawal, and cognitive impairment, assessed mostly via clinician-rated scales were inversely associated with gray matter volume in the bilateral cerebellum. In patients with schizophrenia, anhedonia, and avolition evaluated via self-rated scales inversely related to white-matter volume in the left anterior limb of internal capsule/anterior thalamic radiation and positively in the left superior longitudinal fasiculus. The pathophysiological mechanisms underlying negative symptoms might differ between depression and schizophrenia. These results also point to future negative symptom scale development primarily focused on detecting and monitoring the corresponding changes to brain structure or function.
Negative symptoms are prevalent, presenting in more than 50% of psychotic patients (1). Based on the analysis of the National Institute of Mental Health, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial, negative symptoms have greater impact on functioning than positive or any other symptoms (2). Moreover, they have undetermined etiology and are resistant to treatment (3) with existing psychotropic drugs. Despite initially being thought to be restricted to schizophrenia, negative symptoms are also present in other major psychiatric disorders, notably major depressive disorder (4) with possibly different clinical presentations. For instance, anhedonia, one of the core domains within negative symptoms (5) appears to covary with clinical state in depressed patients but instead reflects an enduring trait in schizophrenia (6). Clinical differences such as these may imply distinct pathophysiology of negative symptoms in depression and schizophrenia, though as yet this remains uncertain.
Understanding more about the neuroanatomical correlates of negative symptoms in depression and schizophrenia may help elucidate whether negative symptoms in the two conditions represent the same phenomena or not. Although a number of studies have been conducted examining neural correlates of negative symptoms, the results have not been consistent and a variety of regions have been implicated, which prevented us from formulating an a priori hypothesis. In young women at high risk of depression, fractional anisotropy (FA) of bilateral cingulum bundles and left subgenual cingulum are negatively correlated with anhedonia (7). In depressed adolescents, FA was also found to be negatively correlated with anhedonia in anterior limb of internal capsule, posterior cingulum, and inferior-fronto-occipital fasciculus (8). In schizophrenia, negative symptoms inversely correlated with gray matter volume in cerebellum (9), left precuneus (10), right posterior cingulate (10); white-matter volume in regions near bilateral anterior cingulate and right internal capsule (11); FA in internal capsule (12), anterior thalamic radiation (12), parietal portion of the superior longitudinal fasciculus (12), fronto-occipital fasciculus (12), corpus callosum (12–14), medial frontal gyrus (15), and inferior frontal white matter (16). Positive correlation with negative symptoms in schizophrenia was also found in gray matter volume of right thalamus (17) and FA of an area near right insula (18), left cingulum and left superior longitudinal fasciculus II (19). However, some researchers have failed to identify any significant correlation between brain structure and negative symptoms (20, 21). Part of the reason for the heterogeneity of results may result from the heterogeneity in ways in which negative symptoms were measured. Many psychological scales have been devised for the evaluation of negative symptoms. In spite of the appreciable quantity of scales, individually, none covers all aspects of negative symptoms and none has been demonstrated to be superior in terms of severity evaluation or prognostic prediction.
In order to avoid selection bias, we decided to assess negative symptoms by using a large number of negative symptom scales, then employing a data reduction approach with PCA to identify the major dimensions of negative symptoms and specify their magnetic resonance imaging (MRI) anatomical correlates; namely gray and white-matter volumes estimated with voxel-based morphometry (VBM) (22). To our knowledge, this is the first study exploring negative symptoms in schizophrenia and major depressive disorder using integrative information from a large number of negative symptom scales. We hypothesize that distinct patterns of neural correlates will be associated with different domains of negative symptoms in depression and schizophrenia, respectively.
Twenty-two participants with DSM-IV schizophrenia, 24 patients with DSM-IV major depressive disorder, and 20 control participants, were recruited to take part in the study (Table 1). Inclusion criteria were: adequate English proficiency and age between 18 and 65 years. Exclusion criteria were: brain structural abnormality, history of major medical or neurological disorder, substance dependence, and any contraindications to MRI scanning contraindications. First-degree family history of schizophrenia or bipolar disorder was an additional exclusion criterion for depressed and control subjects. The study was approved by the Cambridgeshire 3 National Health Service research ethics committee with written informed consent obtained from every participant.
Thirteen depressed participants were taking antidepressants with the following daily dose ranges: citalopram 30–60 mg, mirtazapine 30–45 mg, venlafaxine 75–225 mg. Nearly, all patients with schizophrenia were on atypical antipsychotic medication and the mean chlorpromazine equivalent dose was 401.243 (standard deviation 91.43) mg/day. Three depressed patients were taking antipsychotics: risperidone 2 mg, quetiapine 100 mg, and quetiapine 100 mg. Eight schizophrenia patients were also taking antidepressant medication: citalopram 20–40 mg, fluoxetine 20 mg, mirtazapine 45 mg, and venlafaxine 150–225 mg.
Ten commonly used negative symptom scales were administered: the negative subscale of the Positive and Negative Syndrome Scale (PANSS) (23); the Snaith–Hamilton Pleasure Scale (SHAPS) (24); Chapman Physical Anhedonia Scale (25); Chapman Social Anhedonia Scale (25); anhedonia subscale of Beck Depression Inventory (BDI) (26); negative symptom subscale of Brief Psychiatric Rating Scale (BPRS) (27); Motivation and Energy Inventory (MEI) social subscale (28); MEI physical subscale (28); Temporal Experience of Pleasure Scale (TEPS) anticipatory subscale (29); and TEPS consummatory subscale (29). Though the PANSS is generally applied to psychotic patients, the PANSS negative subscale has been shown to be a valid instrument in patients with major depressive disorder (30). The BPRS negative symptom scale includes items such as blunted affect, emotional withdrawal, and motor retardation. All scales were self-reported, except the PANSS and BPRS. Higher scores denote more severe negative symptoms, except on the MEI and TEPS. In order to avoid selection bias, we tried to cover all dimensions of negative symptoms: PANSS and BPRS negative symptom subscales are related to impaired cognition, withdrawal, blunted affect, and alogia while the remainder is associated to a greater extent with anhedonia and avolition. It has been suggested that patients with certain mental disorders only suffer a specific type instead of global anhedonia, for instance, anticipatory anhedonia in schizophrenia (29). Many scales have been created for subdivisions of anhedonia, such as the Chapman and TEPS scales used in this study.
Brain MRI T1-weighted images were acquired by a Siemens Tim Trio operating at 3 T (Wolfson Brain Imaging Centre, University of Cambridge) with the following parameters: slice thickness = 1 mm, repetition time = 2000 ms; echo time = 30 ms; flip angle = 78°; in-plane resolution 2 mm × 2mm; matrix size 240 × 256; bandwidth 2232 Hz/pixel.
Magnetic resonance imaging datasets were pre-processed with VBM8 in Statistical Parametric Mapping (SPM8)1 in MATLAB (Math-Works, Natick, MA, USA) following the instructions in the online manual2. To begin with, images were segmented into gray and white matter then Diffeomorphic Anatomical Registration through Exponentiated Lie Algebra (DARTEL) was used to increase the accuracy of inter-subject alignment by modeling the shape of each brain using three parameters for each voxel (31). Following DARTEL, templates were generated and registered to the Montreal Neurological Institute (MNI) space by affine transformation. Afterward, all images were non-linearly warped onto the templates and smoothed with 10 mm full width at half maximum Gaussian kernel as suggested in the manual. Possible spatial expansion or contraction during the normalization process was modulated by the Jacobian determinant to preserve the original tissue volumes.
Principal component analysis simplifies, summarizes, and integrates data along with a reduction in statistical comparisons, thus avoiding inflation of type1 errors. PCA with direct oblimin rotation (32) was performed on all negative symptom scales in all participants via Statistical Package for Social Sciences software (SPSS version 21). Rotation was applied to improve the ease of interpretation, and oblique instead of orthogonal rotation was used due to the presumed association between components. Components with eigenvalues >1 were identified. Participants’ loading scores of these major components were then used for subsequent MRI image analyses in FMRIB Software Library (FSL) version 5.03. A general linear model (GLM) regressed loading scores of the major components onto gray (or white) matter volumes at each intra-cerebral voxel with nuisance covariates of age, gender, and total gray (or white) matter volumes. Four models were regressed for every loading score: gray matter in depressed group; gray matter in schizophrenia group; white matter in depressed group; and white matter in schizophrenia group. In each analysis, a significance level of P ≤ 0.05 family-wise error corrected for multiple comparisons was applied using threshold-free cluster enhancement (TFCE) (33). Regions significantly correlated with loading scores were localized by the Harvard–Oxford Cortical Structural Atlas, Johns Hopkins University White-Matter Tractography Atlas, and Cerebellar Atlas in MNI152 space4. If two or more regions were significantly related to the component scale in the same patient group and the same tissue type, then inter-regional correlation between the extracted volumes of these areas would also be calculated in the patient and control groups.
After controlling for age, gender, and total gray (or white) matter volumes, regions displaying a significant relationship with principal components in patient groups were used as masks to extract tissue volumes from both the patient and control groups. Using SPSS, between-group differences were assessed via independent, two-sample T-tests on these extracted volumes to evaluate the extent of structural group differences related to negative symptoms.
With a Kaiser–Meyer–Olkin test-statistic value of 0.793 and Bartlett’s test of sphericity with p < 0.000001, PCA was adequately completed. Two major components were yielded with eigenvalues >1 (Supplementary Material) collectively explaining 69.93% of the total variance. PANSS negative and BPRS negative subscales loaded more than 0.9 in the pattern matrix onto one component, which was primarily related to impaired cognition, withdrawal, blunted affect, and alogia. The other eight scales loaded onto a second component associated primarily with anhedonia and avolition. We thus identified two principal components, one coding for “cognition/expression” and another coding for “pleasure/motivation” (Tables 1 and 2). Both illness groups expressed each component significantly more than controls (Figure 1). The depressed group scored highest (most impaired) in the “pleasure/motivation” component (t = 2.382, p = 0.026) whereas the schizophrenia scored highest in the “cognition/expression” component (t = 2.627, p = 0.016).
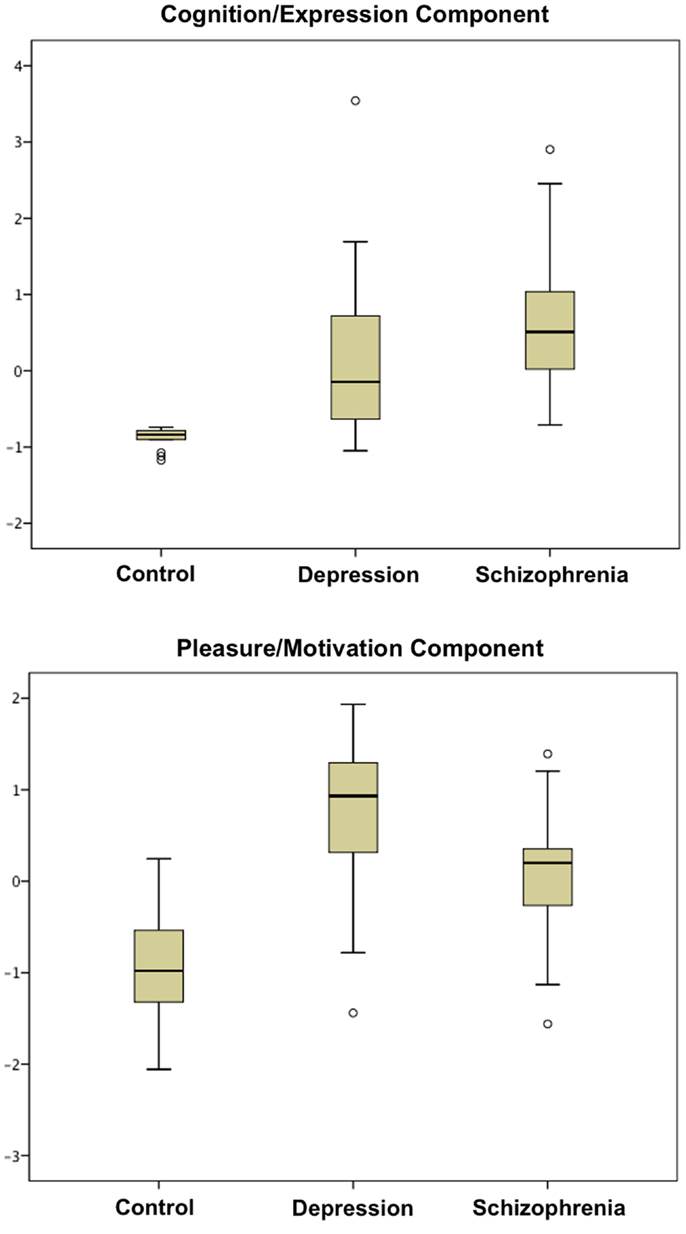
Figure 1. Boxplots of scores in cognition/expression component and pleasure/motivation component are shown. Both patient groups are significantly different from the control group in these two components (Table 1).
In the schizophrenia patient group, the principal component coding for “pleasure/motivation” was found to be inversely correlated with white-matter volume in the left anterior limb of internal capsule/anterior thalamic radiation and positively correlated with left superior longitudinal fasiculus (Figure 2). Since the principal component coding for “pleasure/motivation” in the schizophrenia group was not significantly correlated with the HAMD-17 score with Pearson correlation coefficient r = −0.08 and p = 0.741, the assumption of secondary negative symptoms due to depression is not favored. The principal component coding for “cognition/expression” was significantly inversely correlated with bilateral cerebellar gray matter in the depression patient group (Figure 2). These associations were significantly different between groups (Table 3).
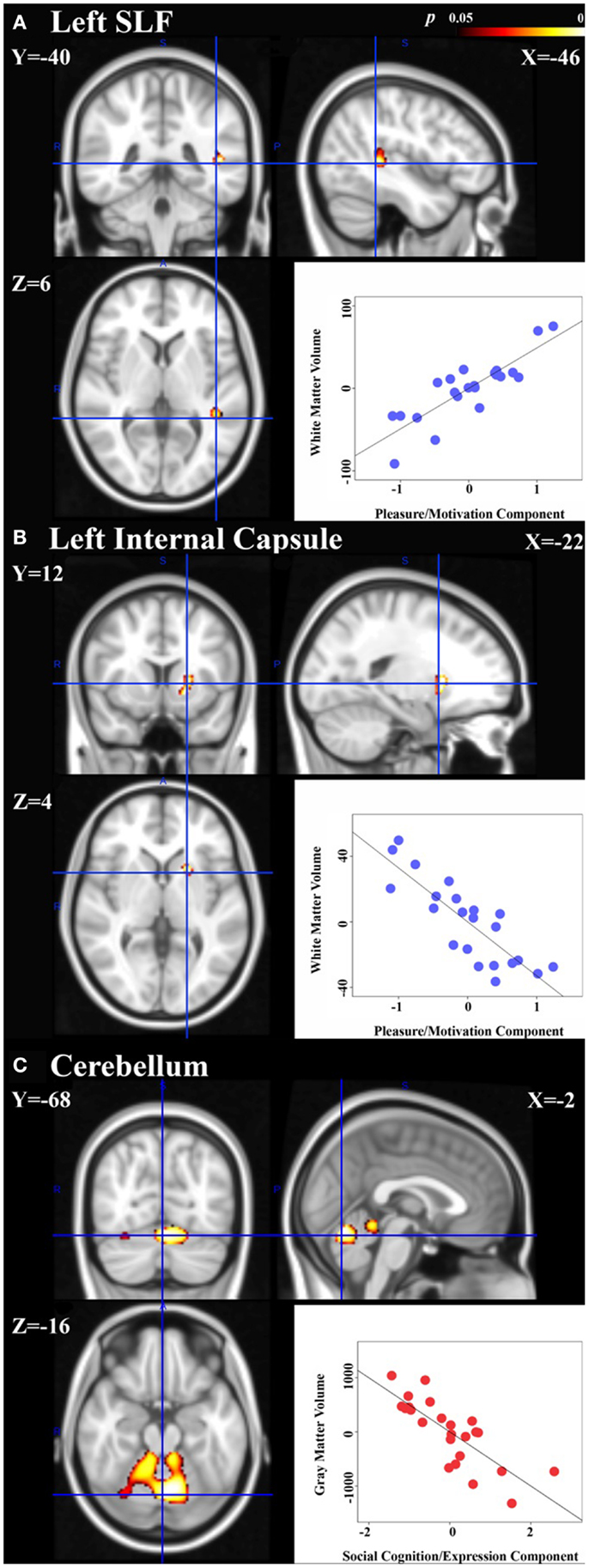
Figure 2. Neural signatures of negative symptoms in schizophrenia and depression groups are shown. Significant association of white-matter volumes with the principal component coding for “pleasure/motivation” was found in the schizophrenia group: (A) left superior longitudinal fasiculus (SLF) with cluster size = 105 voxels and peak p = 0.017. (B) Left anterior limb of internal capsule/anterior thalamic radiation with cluster size = 114 voxels and peak p = 0.014. (C) Significant association of gray matter volumes with the principal component coding for “cognition/expression” was found in the depressed group: cerebellum with a cluster of size = 2212 voxels and peak p = 0.002. Peak voxels shown with corresponding Montreal Neurological Institute (MNI) coordinates. Scatter plots: controlling for age, gender, total gray or white-matter volume, partial correlation coefficients (r) between the extracted volumes and principal component scores are calculated within group and are shown here [(A): r = 0.85, (B) r = −0.84, (C) r = −0.79]. Please note that the partial correlation coefficients are expected to be high as the regions in which we quantify that these correlations were defined as being regions with significant associations between volume and principal component expression.
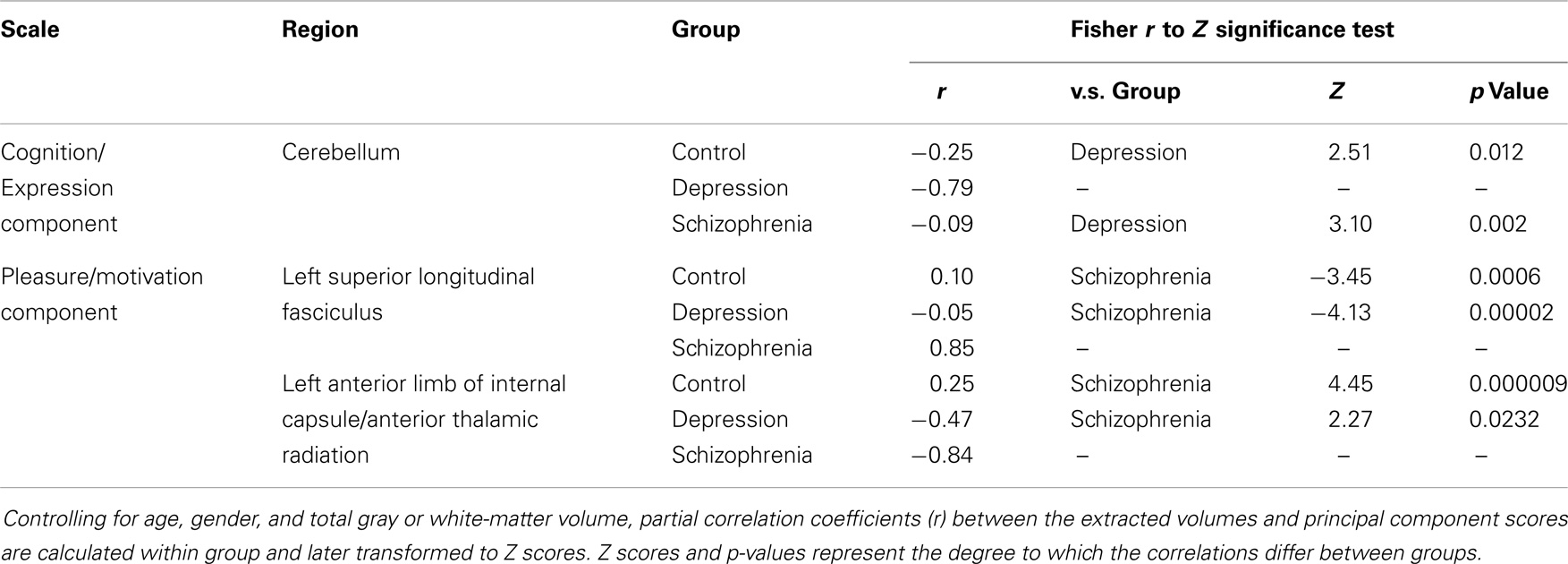
Table 3. Group differences in the correlation of the brain regions shown in Figure 2 with the negative symptoms of first two principal components.
The correlation between white-matter volume in the left anterior limb of internal capsule/anterior thalamic radiation and left superior longitudinal fasciculus in the schizophrenia group was high with a Pearson correlation coefficient r = −0.66 and p < 0.001 (Figure 3) but non-significant in the control group with r = 0.093 and p = 0.696. After Fisher’s r to z transformation, the group difference of the correlations was significant (Z = 2.65, p < 0.01). No regions in the gray matter of schizophrenia group or white matter of depression group were significantly correlated with these components.
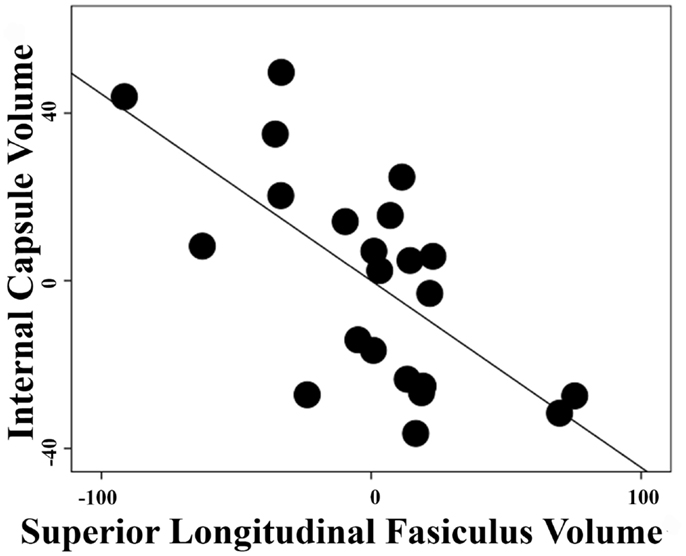
Figure 3. Inter-regional correlation in schizophrenia group. Linear regression was performed with a dependent variable of extracted volumes in the left superior longitudinal fasiculus or left anterior limb of internal capsule/anterior thalamic radiation and covariates of age, gender, and total white-matter volumes. Pearson correlation coefficient between the residuals of extracted volumes from these two regions is r = −0.66 with p < 0.001 in the schizophrenia group.
At a significance level of P ≤ 0.05, group differences between controls and patients were revealed in extracted volumes from only one region identified as having a significant relationship to negative symptom scores: the anterior limb of the internal capsule/anterior thalamic radiation (Table 4).
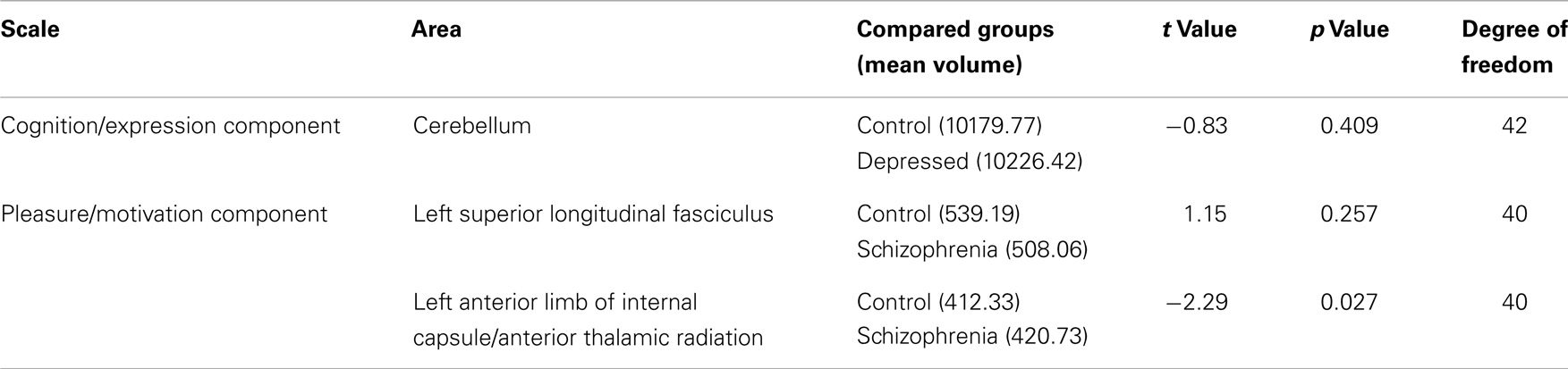
Table 4. Differences between patients and controls in the extracted volumes of areas with significant relationships to the negative symptoms principal components, controlling with age, gender, and total gray or white-matter volume.
Neuroanalysis following PCA identified neural signatures of two distinct dimensions of negative symptoms in schizophrenia and depression, respectively; that is, a principal component coding for “cognition/expression” in gray matter in depression and a principal component coding for “pleasure/motivation” in white matter in schizophrenia. Therefore, as opposed to a common underlying process proposed by Foussias et al. (34), fundamental discrepancies in the brain structural basis of negative symptoms may exist between different diagnoses and distinct domains. Recruiting nearly 500 participants, a recent study has attempted to develop a new negative symptom assessment for schizophrenia (CAINS: Clinical Assessment Interview for Negative Symptoms). Interestingly, structural analysis of all the items in the CAINS revealed two major elements of negative symptoms similar to our findings, termed by the CAINS researchers as “expression,” a measure of behavioral, social, and emotional expression, and “motivation and pleasure” (3).
Both component scores of depression and schizophrenia patients differ significantly from the controls (Table 1, Figure 1). However, patients showed significant neural correlations with the component, which they did not clinically express predominantly. For instance, the schizophrenia group scored higher in the “cognition/expression” component, with which they failed to demonstrate neural correlates. Further, despite prominent differences in clinical negative symptom severity (Table 1), controls and patients did not differ significantly in all of the extracted volumes within the neural signatures (Table 4). Thus, brain regions in which there are inter-individual differences that relate to symptom severity do not necessarily show differences between groups in average brain volume. This suggests that there may be some neural deficits that contribute to the category of developing either disorder or some separate deficits that influence how severely the illness is expressed.
Although initially considered as a motor coordinator, the cerebellum is now known to play a role in emotional regulation and cognition, supported by its widespread connections to the limbic system, the frontal, parietal, prefrontal, occipital, and temporal cortex. The cerebellum is activated in a variety of mental activities, including facial recognition, emotion attribution, theory of mind attributions, directed attention, memory, and empathy (35, 36) and has been referred to as the “emotional pacemaker” (37). Functional mapping of the cerebellum has been established based on functional MRI studies and clinical lesion reports. The posterior lobules VI, VII, and Crus were found to be linked to cognition, the posterior vermis to be associated with emotion and lobules I–V related to sensorimotor function (37). Indeed, the cerebellar cognitive affective syndrome following posterior cerebellar lobe lesion includes negative symptoms such as passivity, blunted affect, and withdrawal (37). Also, early in 1992, Dolan et al. reported increased regional cerebral blood flow of the cerebellar vermis in depressed patients with cognitive disturbance (38). Furthermore, in an fMRI meta-analysis, the cerebellum has been identified as an important area of dysfunction in depression (39). In our depressed group, the bilateral lobules I–IV, V, VI, Crus, and vermis were found to be negatively correlated with the principal component coding for “cognition/expression” corresponding to the functional topography.
Compared to control participants and based on post-mortem and positron emission tomography studies, patients with schizophrenia are suspected to have higher metabolic rates in the superior longitudinal fasciculus and anterior limb of the internal capsule (40, 41) suggesting roles in the pathophysiology of the disorder. The anterior limb of the internal capsule serves as a bridge between the thalamus, cingulate, and prefrontal cortices and thus plays an important role in motivation, reward, and emotion (8). We identified neural correlates of negative symptoms in the superior longitudinal fasciculus III, which connects the supramarginal gyrus with ventral premotor and prefrontal areas (42). The supramarginal gyrus has been reported to participate in execution and mental simulation of action (43). Furthermore, associations between negative symptoms and these two areas have been reported in terms of FA (12) and now white-matter volume in this study. Taken together, this evidence justifies the association of anterior limb of the internal capsule and superior longitudinal fasciculus with a pleasure/motivation domain of negative symptoms. In the schizophrenia group, we demonstrated a significant correlation between extracted volumes in these two regions (r = −0.66) (Figure 3), which was non-significant in the controls. Certainly, schizophrenia has been regarded as a disease of disconnectivity (44) and this evidence strengthens that assertion.
Encouraged by the National Institute of Mental Health Consensus Development Conference on Negative Symptoms in Rockville, MD, USA, 2005 (45), several attempts have been made in recent years by developing a new negative symptom scale. However, the absence of scales emphasizing the correspondence between scores and structural, or indeed functional, brain changes associated with mental disorders might be accounted for by the diverse findings in the neural correlates of negative symptoms provided here. Despite being difficult to develop, scales reflecting alterations in brain structure and function could be clinically valuable enabling prognosis prediction, disease progress monitoring, and the probing of etiological mechanisms.
Current scales tend to mix several domains of negative symptoms. Nevertheless as shown in our study, it is likely that instead of the whole spectrum, only specific components of negative symptoms correspond to alterations of brain structures. According to our findings, anhedonia and avolition were linked to brain alteration in schizophrenia whereas blunted affect, alogia, withdrawal, and cognitive dysfunction were associated with depression. However, we were unable to distinguish neural correlates of different anhedonia types. Future studies may delve deeper into this topic.
Several limitations should be considered when interpreting our result. We included more participants than some published studies (9, 11, 16, 17), but sample size was still small-to-moderate. Besides, the information regarding disease duration was absent. Furthermore, although we tried to cover all dimensions of negative symptoms by including a large number of frequently used scales, our list of assessments was by no means exhaustive. Other negative symptom scales could be added in future studies. Additionally, the positive correlation in the left superior longitudinal fasciculus with negative symptoms in the schizophrenia group might indicate a compensatory mechanism in response to the deficit. However, we were prevented from providing any cellular explanation of results due to the nature of MRI image analysis. Atypical antipsychotics and cannabis have been associated with brain structural change (46, 47) and were widely used in our patients. Based on two systematic reviews, antipsychotics may alter gray matter volume (48, 49). However, there has been no consensus regarding the directionality of the effect (i.e., increasing or decreasing). Furthermore, we did not find any significant structural difference between patients and controls in the regions in which inter-individual variability of symptoms within patients was associated with inter-individual variability in brain structure. Consequently, we are prevented from determining the exact medication effects. Drug-naïve patients should be recruited in future studies.
In conclusion, in correspondence with the large CAINS study, we identified two major domains of negative symptoms. Neural signatures of these domains were found in depression and schizophrenia. Future research may investigate the association of these regions with negative symptoms. New scales linking negative symptoms with structural brain change are anticipated.
Dr. Jie-Yu Chuang performed the analysis and wrote the original draft of this paper. Dr. John Suckling directed the analysis and revised the paper with Dr. Graham K. Murray and Dr. Jie-Yu Chuang. Drs Graham K. Murray, Hisham Ziauddeen, Jenny Spencer, Robert B. Dudas, and Paul C. Fletcher designed the data collection. Dr. Graham K. Murray directed the data collection. Drs Antonio Metastasio, Nuria Segarra, Jenny Spencer, Robert B. Dudas collected the data. Drs Antonio Metastasio, Nuria Segarra, Jenny Spencer, and Tait assisted with preliminary analyses. All authors took part in revising the manuscript and approved the final version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by Brain and Behavior Research Foundation (NARSAD) and Medical Research Council (MRC) G0701911 awards to Graham K. Murray, and by the University of Cambridge Behavioural and Clinical Neuroscience Institute, funded by a joint award from the Medical Research Council and Wellcome Trust (G1000183 and 093875/Z/10Z). We thank Dr. Zheng Ye for her help with image analysis and technical support, Niels Reinders, and staff at the Wolfson Brain Imaging Centre for help with data collection, and staff at IAPT, CAMEO, and the Rehabilitation and Recovery Service in the Cambridgeshire and Peterborough NHS Foundation Trust for help with recruitment. The study was supported by infrastructure provided by the Wellcome Trust/MRC Behavioural and Clinical Neuroscience Institute at the University of Cambridge. Paul C. Fletcher is supported by the Bernard Wolfe Health Neuroscience Fund and the Wellcome Trust.
The Supplementary Material for this article can be found online at http://www.frontiersin.org/Journal/10.3389/fpsyt.2014.00116/abstract
1. Bobes J, Arango C, Garcia-Garcia M, Rejas J. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS study. J Clin Psychiatry (2010) 71:280–6. doi: 10.4088/JCP.08m04250yel
2. Rabinowitz J, Levine SZ, Garibaldi G, Bugarski-Kirola D, Berardo CG, Kapur S. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr Res (2012) 137:147–50. doi:10.1016/j.schres.2012.01.015
3. Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry (2013) 170:165–72. doi:10.1176/appi.ajp.2012.12010109
4. Kitamura T, Suga R. Depressive and negative symptoms in major psychiatric disorders. Compr Psychiatry (1991) 32:88–94. doi:10.1016/0010-440X(91)90074-M
5. Strauss GP, Keller WR, Buchanan RW, Gold JM, Fischer BA, McMahon RP, et al. Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophr Res (2012) 142:88–92. doi:10.1016/j.schres.2012.10.012
6. Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull (2006) 32:259–73. doi:10.1093/schbul/sbj009
7. Keedwell PA, Chapman R, Christiansen K, Richardson H, Evans J, Jones DK. Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia. Biol Psychiatry (2012) 72(4):296–302. doi:10.1016/j.biopsych.2012.01.022
8. Henderson SE, Johnson AR, Vallejo AI, Katz L, Wong E, Gabbay V. A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Front Psychiatry (2013) 4:152. doi:10.3389/fpsyt.2013.00152
9. Bergé D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O. Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand (2011) 123:431–9. doi:10.1111/j.1600-0447.2010.01635.x
10. Lee JS, Park H-J, Chun JW, Seok J-H, Park I-H, Park B, et al. Neuroanatomical correlates of trait anhedonia in patients with schizophrenia: a voxel-based morphometric study. Neurosci Lett (2011) 489:110–4. doi:10.1016/j.neulet.2010.11.076
11. Paillère-Martinot M, Caclin A, Artiges E, Poline JB, Joliot M, Mallet L, et al. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res (2001) 50:19–26. doi:10.1016/S0920-9964(00)00137-7
12. Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu K-W, Brickman AM, et al. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophr Res (2007) 92:211–24. doi:10.1016/j.schres.2006.12.029
13. Nakamura K, Kawasaki Y, Takahashi T, Furuichi A, Noguchi K, Seto H, et al. Reduced white matter fractional anisotropy and clinical symptoms in schizophrenia: a voxel-based diffusion tensor imaging study. Psychiatry Res (2012) 202:233–8. doi:10.1016/j.pscychresns.2011.09.006
14. Michael AM, Calhoun VD, Pearlson GD, Baum SA, Caprihan A. Correlations of diffusion tensor imaging values and symptom scores in patients with schizophrenia. Conf Proc IEEE Eng Med Biol Soc (2008) 2008:5494–7. doi:10.1109/IEMBS.2008.4650458
15. Bai YM, Chou K-HH, Lin C-PP, Chen I-YY, Li C-TT, Yang KC, et al. White matter abnormalities in schizophrenia patients with tardive dyskinesia: a diffusion tensor image study. Schizophr Res (2009) 109:167–81. doi:10.1016/j.schres.2009.02.003
16. Wolkin A, Choi SJ, Szilagyi S, Sanfilipo M, Rotrosen JP, Lim KO. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. Am J Psychiatry (2003) 160:572–4. doi:10.1176/appi.ajp.160.3.572
17. Yoshihara Y, Sugihara G, Matsumoto H, Suckling J, Nishimura K, Toyoda T, et al. Voxel-based structural magnetic resonance imaging (MRI) study of patients with early onset schizophrenia. Ann Gen Psychiatry (2008) 7:25. doi:10.1186/1744-859X-7-25
18. Skelly LR, Calhoun V, Meda SA, Kim J, Mathalon DH, Pearlson GD. Diffusion tensor imaging in schizophrenia: relationship to symptoms. Schizophr Res (2008) 98:157–62. doi:10.1016/j.schres.2007.10.009
19. Lee JS, Han K, Lee S, Seok J, Kim J. Altered structural connectivity and trait anhedonia in patients with schizophrenia. Neurosci Lett (2014) 579:7–11. doi:10.1016/j.neulet.2014.07.001
20. Lui S, Deng W, Huang X, Jiang L, Ma X, Chen H, et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry (2009) 166:196–205. doi:10.1176/appi.ajp.2008.08020183
21. Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, et al. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry (2001) 158:234–43. doi:10.1176/appi.ajp.158.2.234
22. Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry applications of the human brain. Methods Imaging (2005) 1:1–9. doi:10.2174/1573405054038726
23. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull (1987) 13:261–76. doi:10.1093/schbul/13.2.261
24. Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry (1995) 167:99–103. doi:10.1192/bjp.167.1.99
25. Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol (1976) 85:374–82. doi:10.1037/0021-843X.85.4.374
26. Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry (2005) 57:319–27. doi:10.1016/j.biopsych.2004.11.026
27. Welham J, Stedman T, Clair A. Choosing negative symptom instruments: issues of representation and redundancy. Psychiatry Res (1999) 87:47–56. doi:10.1016/S0165-1781(99)00042-6
28. Fehnel SE, Bann CM, Hogue SL, Kwong WJ, Mahajan SS. The development and psychometric evaluation of the Motivation and Energy Inventory (MEI). Qual Life Res (2004) 13(7):1321–36. doi:10.1023/B:QURE.0000037502.64077.4d
29. Gard D, Gard M, Kring A, John O. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Pers (2006) 40:1086–102. doi:10.1016/j.jrp.2005.11.001
30. Eisenberg DP, Aniskin DB, White L, Stein JA, Harvey PD, Galynker II. Structural differences within negative and depressive syndrome dimensions in schizophrenia, organic brain disease, and major depression: a confirmatory factor analysis of the positive and negative syndrome scale. Psychopathology (2009) 42:242–8. doi:10.1159/000218522
31. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage (2007) 38:95–113. doi:10.1016/j.neuroimage.2007.07.007
32. Mounce C, Keogh E, Eccleston C. A principal components analysis of negative affect-related constructs relevant to pain: evidence for a three component structure. J Pain (2010) 11:710–7. doi:10.1016/j.jpain.2009.10.014
33. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage (2009) 44:83–98. doi:10.1016/j.neuroimage.2008.03.061
34. Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull (2010) 36:359–69. doi:10.1093/schbul/sbn094
35. Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry (2008) 64:81–8. doi:10.1016/j.biopsych.2008.01.003
36. Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science (2004) 303:1157–62. doi:10.1126/science.1093535
37. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex (2010) 46:831–44. doi:10.1016/j.cortex.2009.11.008
38. Dolan RJ, Bench CJ, Brown RG, Scott LC, Friston KJ, Frackowiak RS. Regional cerebral blood flow abnormalities in depressed patients with cognitive impairment. J Neurol Neurosurg Psychiatry (1992) 55:768–73. doi:10.1136/jnnp.55.9.768
39. Graham J, Salimi-Khorshidi G, Hagan C, Walsh N, Goodyer I, Lennox B, et al. Meta-analytic evidence for neuroimaging models of depression: state or trait? J Affect Disord (2013) 151(2):423–31. doi:10.1016/j.jad.2013.07.002
40. Beasley CL, Dwork AJ, Rosoklija G, Mann JJ, Mancevski B, Jakovski Z, et al. Metabolic abnormalities in fronto-striatal-thalamic white matter tracts in schizophrenia. Schizophr Res (2009) 109:159–66. doi:10.1016/j.schres.2009.01.017
41. Buchsbaum MS, Buchsbaum BR, Hazlett EA, Haznedar MM, Newmark R, Tang CY, et al. Relative glucose metabolic rate higher in white matter in patients with schizophrenia. Am J Psychiatry (2007) 164:1072–81. doi:10.1176/appi.ajp.164.7.1072
42. Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex (2005) 15:854–69. doi:10.1093/cercor/bhh186
43. Grèzes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum Brain Mapp (2000) 12:1–19. doi:10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V
44. Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci (2013) 14(5):322–36. doi:10.1038/nrn3465
45. Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull (2006) 32(2):214–9. doi:10.1093/schbul/sbj053
46. Molina V, Reig S, Sanz J, Palomo T, Benito C, Sánchez J, et al. Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr Res (2005) 80:61–71. doi:10.1016/j.schres.2005.07.031
47. Cookey J, Bernier D, Tibbo PG. White matter changes in early phase schizophrenia and cannabis use: an update and systematic review of diffusion tensor imaging studies. Schizophr Res (2014) 156(2–3):137–42. doi:10.1016/j.schres.2014.04.026
48. Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med (2009) 39:1763–77. doi:10.1017/S0033291709005315
49. Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, et al. The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia? A systematic review. Curr Pharm Des (2009) 15:2535–49. doi:10.2174/138161209788957456
Keywords: negative symptoms, depression, schizophrenia, cerebellum, white matter
Citation: Chuang J-Y, Murray GK, Metastasio A, Segarra N, Tait R, Spencer J, Ziauddeen H, Dudas RB, Fletcher PC and Suckling J (2014) Brain structural signatures of negative symptoms in depression and schizophrenia. Front. Psychiatry 5:116. doi: 10.3389/fpsyt.2014.00116
Received: 18 June 2014; Accepted: 12 August 2014;
Published online: 27 August 2014.
Edited by:
Christoph Mulert, Universitätsklinikum Hamburg–Eppendorf, GermanyReviewed by:
Uner Tan, Cukurova University, TurkeyCopyright: © 2014 Chuang, Murray, Metastasio, Segarra, Tait, Spencer, Ziauddeen, Dudas, Fletcher and Suckling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie-Yu Chuang, Herchel Smith Building for Brain and Mind Sciences, Forvie Site, Addenbrooke’s Hospital, Hills Road, Cambridge, CB2 0QQ, UK e-mail:amM3NTZAY2FtLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.