
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 11 July 2014
Sec. Mood Disorders
Volume 5 - 2014 | https://doi.org/10.3389/fpsyt.2014.00080
This article is part of the Research TopicPsychobiological Research in Psychosomatic MedicineView all 7 articles
A commentary has been posted on this article:
Anxiety as a Risk Factor for Cardiovascular Diseases
Background: Anxiety disorders increase risk of future cardiovascular disease (CVD) and mortality, even after controlling for confounds including smoking, lifestyle, and socioeconomic status, and irrespective of a history of medical disorders. While impaired vagal function, indicated by reductions in heart rate variability (HRV), may be one mechanism linking anxiety disorders to CVD, prior studies have reported inconsistent findings highlighting the need for meta-analysis.
Method: Studies comparing resting-state HRV recordings in patients with an anxiety disorder as a primary diagnosis and healthy controls were considered for meta-analysis.
Results: Meta-analyses were based on 36 articles, including 2086 patients with an anxiety disorder and 2294 controls. Overall, anxiety disorders were characterized by lower HRV [high frequency (HF): Hedges’ g = −0.29. 95% CI: −0.41 to −0.17, p < 0.001; time domain: Hedges’ g = −0.45, 95% CI: −0.57 to −0.33, p < 0.001] than controls. Panic disorder (n = 447), post-traumatic stress disorder (n = 192), generalized anxiety disorder (n = 68), and social anxiety disorder (n = 90), but not obsessive–compulsive disorder (n = 40), displayed reductions in HF HRV relative to controls (all ps < 0.001).
Conclusion: Anxiety disorders are associated with reduced HRV, findings associated with a small-to-moderate effect size. Findings have important implications for future physical health and well-being of patients, highlighting a need for comprehensive cardiovascular risk reduction.
Anxiety disorders are the most prevalent psychiatric disorders (1), and one of the most costly (2). Anxiety disorders also increase risk of cardiovascular disease (CVD) three- to fourfold, after accounting for sex, substance use, and depression (3, 4) while risk of cardiac mortality is increased twofold (5–7). Here, we examine the impact of the anxiety disorders on a psychophysiological marker of health and well-being, heart rate variability (HRV). Previous studies are characterized by contradictory reports, highlighting the need for a meta-analysis of previously published findings.
Heart rate variability is the fluctuation of heart period over time, commonly measured by electrocardiogram (ECG), and is an important marker of psychological well-being, general cardiovascular health, and is a major predictor of mortality (8–10). The mechanism underpinning the relationship between mental and physical health may, in part, relate to impairment in vagal nerve activity leading to a dysregulation of inflammatory processes (11, 12). Reductions in resting-state HRV reflect decreases in vagal output. We have now published several meta-analyses reporting HRV reductions in depression (13) and alcohol dependence (14). To date, no meta-analysis has been published on HRV and the anxiety disorders; this is a surprising observation considering that anxiety is an early marker of cardiovascular risk and a robust predictor of CVD and sudden cardiac death, independent of demographic risk factors, biological risk factors, and health behaviors (5, 6, 15).
There are a variety of reasons to expect that HRV will be reduced in the anxiety disorders. According to the neurovisceral integration model (16), efferent nerve fibers from the pre-frontal cortex moderate parasympathetic activity and vagal nerve inhibition of cardiac activity, where proper vagus nerve regulation mediates inflammatory processes that lead to a variety of pathologies, including type-II diabetes (17), clinical depression (18), coronary heart disease (19), and neurodegenerative diseases (20). The neurovisceral integration model also characterizes a detailed network of specific neural structures that enable humans to adaptively respond to environmental, physiological, behavioral, cognitive, and emotional influences. In this model, a healthy autonomic nervous system is characterized by high levels of adaptive variability, or rheostasis (21, 22). An illustration of this point can be seen in the observation that a healthy cardiorespiratory system is characterized by complex oscillation patterns in heart period (or high HRV) whereas a diseased system displays little to no variability (9). A key feature of the neurovisceral integration model is the central autonomic network (CAN), a network of brain regions that coordinates autonomic, endocrine, and behavioral responses in goal directed action and in adaptation to environmental challenges. The integrity of this network is compromised in anxiety; sympathoexcitatory responses are unable to be effectively inhibited, leading to behavioral inflexibility. The neurovisceral integration model also links hypervigilance and worry – features observed in all the anxiety disorders (23) – to reductions in HRV. Chronic reductions in resting-state HRV appear to be associated with worry (24–27) and pathological worry has been implicated in the development of CVD and CVD risk factors, where HRV is one mechanism of cardiopathogenesis (28). Further, HRV reductions may also be observed during phasic forms of anxiety, such as panic symptomatology (29–31). Given that many treatments aim to address these features of anxiety, it may be that successful treatments may positively impact on autonomic functioning. Interventions that improve HRV (e.g., exercise) may also help to ameliorate hypervigilance and worry (32–34). These bidirectional associations are supported by reciprocal connections between brain and body (16, 35).
Many studies have reported reduced HRV in people with anxiety disorders. The majority of studies in the literature have focused on panic disorder (PD); over 20 studies have been conducted to date on HRV in PD patients. Researchers have also reported on the impact of post-traumatic stress disorder (PTSD), generalized anxiety disorder (GAD), obsessive–compulsive disorder (OCD), social anxiety disorder (SAD), specific phobias, and mixed/grouped anxiety disorders on HRV, with equivocal results (see Table 1 below for a summary). The reason for the inconsistency in results across studies is unclear, although two possible explanations present themselves: small sample size and common confounds including psychiatric and medical co-morbidity, as well as medication use.
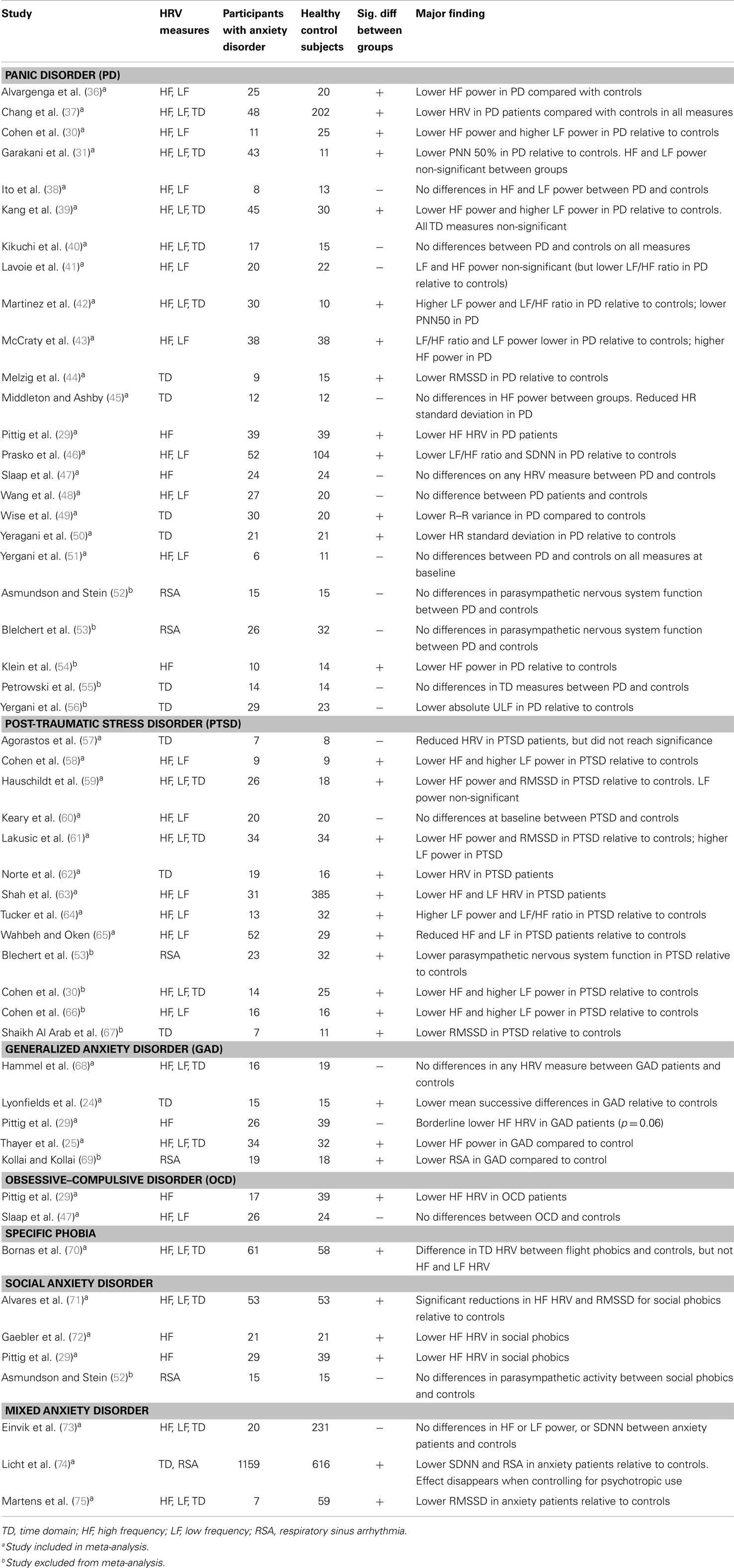
Table 1. Summary of studies reporting comparisons in HRV between patients with anxiety disorders and controls.
Overall, the impact of anxiety and their treatments on HRV is mixed. While many studies report significant HRV reductions in anxiety disorder patients, some report no significant findings. This pattern of results could be considered to reflect an underlying negative “true” relationship, a hypothesis we set to test meta-analytically. A narrative review published 7 years ago (21) comprehensively reviewed studies on the impact of the anxiety disorders on HRV, concluding that “reports of aberrant HRV features of panic outnumber negative findings by a ratio of greater than 6:1” [(21) p.191]. A limitation of this approach is that it does not take into account the magnitude of the effects, whether effect sizes are consistent across studies, or the possibility that non-significant outcomes may have been afflicted by insufficient power. Conversely, meta-analysis provides a robust statistical method of synthesizing effect sizes across studies, and is a valuable tool for clarifying contradictory findings. In summary, there is a need for an up-to-date systematic review of the literature given the important implications that reductions in HRV may have over the long term.
In the present study, we sought to determine the impact of anxiety on resting-state HRV, a psychophysiological marker of health and well-being, minimizing variation across studies. To our knowledge, the present report is the first to address this question using meta-analytic methodology. Specifically, we examine whether patients with any anxiety disorder exhibit reductions in HRV relative to healthy controls, and determine the size of this effect across specific anxiety disorders. We hypothesized that anxiety patients overall would display reductions in HRV relative to healthy participants.
The search strategy followed guidelines outlined in the preferred reporting items for systematic reviews and meta-analyses (PRISMA) (76) statement. Peer-reviewed studies were located in Embase, MEDLINE, and PsychINFO, with all relevant combinations of the following words and phrases: “heart rate variability,” “HRV,” “vagal,” “parasympathetic,” “autonomic nervous system,” “generalized anxiety disorder,” “generalized anxiety disorder,” “GAD,” “obsessive-compulsive disorder,” “obsessi*,” “compulsi*,” “OCD,” “panic disorder” “panic disorder with* agoraphobia,” “PD,” “post-traumatic stress disorder,” “PTSD,” “acute stress disorder,” “social anxiety disorder,” “SAD,” “social phobi*,” “specific phobia,” and “anxi*.” In addition to these electronic searches, each report’s citation list was examined for additional studies. The search was performed during October 2013, with no limitation on time-period. The inclusion criteria were: (1) the comparison of HRV in patients with a diagnosis of an anxiety disorder as defined by DSM-III (77), DSM-III-R (78), DSM-IV (79), DSM-IV-TR (80), or ICD-10 (81), and a control group free from psychiatric diagnosis; (2) satisfactory reporting of statistics (i.e., mean, SD, p, t, r, or F value); and (3) the study was written in English. No limitations were made concerning medication status, physical illness including CVD, or disorder co-morbidity; however these factors were included in follow-up moderator analyses. All potentially relevant manuscripts were independently reviewed by two investigators (JAC and DSQ) and areas of disagreement or uncertainty were adjudicated by a third investigator (AHK).
Meta-analyses were conducted to answer the primary research question of whether patients with anxiety disorders exhibit reductions in resting-state HRV relative to controls, and to determine the size of this effect across specific anxiety disorders. HRV is recorded under short-term (2 min to 1 h) or long term (24 h) conditions, both of which provide reliable indicators of autonomic functioning and are robust predictors of mortality (8) and future cardiac events (82). A variety of HRV measures are reported in the literature, which generally fall under time domain (TD) and frequency domain (83–85). A third domain of HRV is respiratory sinus arrhythmia (RSA), which reflects parasympathetic nervous system activity by indexing the coupling of heart period and respiration. The TD measures extracted for analysis included RMSSD, SDNN, pNN50, and SDANN. If more than one TD measure was reported RMSSD was given preference, followed by SDNN, pNN50 then SDANN. RMSSD was given preference as it closely represents parasympathetic activity and is highly correlated with the high frequency (HF) HRV component. Of the frequency domain HRV measures, HF HRV reflects parasympathetic (vagal) nervous system output (86) and is the most commonly reported measure of HRV in the anxiety literature. The frequency domain measures extracted were high and low frequency (LF) HRV. There is an ongoing debate as to the interpretation of LF HRV (85, 87), and consequently there are sound arguments against the utility and meaningfulness of the oft reported measure LF/HF ratio (88, 89). As such, this measure was not extracted. There are also a number of non-linear methods used to evaluate HRV (85); however, anxiety studies using non-linear methods [e.g., Ref. (71, 90)] have not accumulated to the point that meta-analysis is feasible. Using moderator analyses, we examined possible impacts of known confounds, including medical and psychiatric co-morbidity as well as medication status. Moderator analyses also examined the potential impact of study characteristics (i.e., diagnostic criteria) on HRV parameters. Finally, while short and long-term assessments of HRV have been found to be significantly related to one another, the reported correlation coefficients are rather weak (91). Thus, moderator analyses also compared studies reporting short-term versus long-term recordings to examine if recording method impacted the results.
Meta-analyses were based on a single effect size of a standardized mean. Values were transformed from available statistics (e.g., means and SDs) to determine a standardized effect size, Hedges’ g, using the computer software package comprehensive meta-analysis (92). Hedges’ g is related to Cohen’s d and can be interpreted using the same conventions: small (0.2), medium (0.5), and large (0.8) (93). An added benefit of Hedges’ g is correction for biases found in small sample sizes. The random-effects model was applied in the present meta-analysis, thereby adopting a conservative approach that assumes true effect size may vary from study to study, allowing results to be generalized to populations beyond the study samples (94). To measure homogeneity of effect sizes across studies, the Q statistic was examined. A significant Q statistic is indicative of dissimilar effect sizes across studies; suggesting that methodological or population sample differences might be introducing variance in findings across studies (95). To complement the Q test, we also calculated the I2 statistic, which provides an index of the degree of heterogeneity across studies, where I2 signifies the percentage of the total variability in effect sizes due to between-studies variability, and not due to sampling error within studies. Percentages of around 25% (I2 = 25), 50% (I2 = 50), and 75% (I2 = 75) may be interpreted as low, medium, and high heterogeneity, respectively (96). Begg’s adjusted rank correlation test (97) and Egger’s regression test (98) were used to assess publication bias. The Duval and Tweedie “Trim and Fill” procedure (99) was used to adjust for any suspected publication bias using a random-effects model. This procedure imputes the “missing” studies and recalculates the overall effect size with the inclusion of hypothetical studies.
The search of electronic databases for HRV research in anxiety revealed 4149 studies as at October 2013; these were reduced to 194 after examining study abstracts and then to 36 after reviewing the study methods (Figure 1).
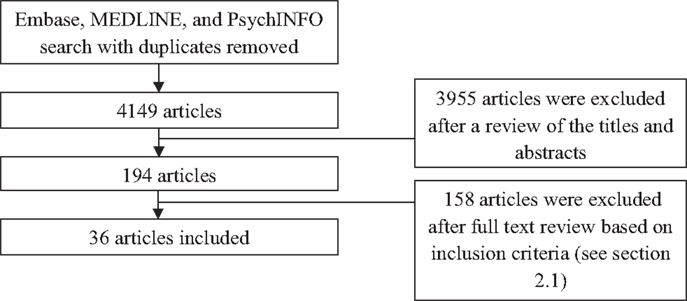
Figure 1. Selection of articles for inclusion. Note: diagnostic grouping of studies do not add to 36 due to some studies reporting on multiple diagnoses.
High frequency HRV was reduced in participants with any anxiety disorder, regardless of specific diagnosis, relative to healthy controls [Hedges’ g = −0.29 (−0.41 to −0.17); SE = 0.06; p < 0.001], a finding associated with a small effect size. Egger’s regression test (p = 0.43) indicated no evidence of publication bias. The Q statistic was significant (Q = 51.39, p = 0.002) indicating study heterogeneity (I2 statistic = 35.8%). To examine findings in further detail, we examined effect sizes in specific anxiety disorders (see Table 2, which displays the summary effect sizes for each disorder; and Supplementary Material, which displays the relevant forest plots for each analysis.). HF HRV was significantly reduced in patients with GAD and SAD, findings associated with a moderate effect size, as well as in those with PD and PTSD, findings associated with a small effect size. Findings for OCD, specific phobia, and those studies in which anxiety disorders were grouped were all non-significant.
Moderator analysis revealed no significant differences across anxiety disorder diagnoses (Q = 6.83; p = 0.34). There was no evidence of heterogeneity between studies using different diagnostic criteria including DSM-III, DSM-IV, or ICD-10 (Q = 0.85, p = 0.65), nor was there heterogeneity for use of medication (Q = 0.1, p = 0.75), co-morbid psychiatric diagnoses (Q = 0.29, p = 0.59), or co-morbid medical disorders (Q = 0.004, p = 0.95). No evidence of heterogeneity between studies using short-term versus long-term recordings was obtained either (Q = 2.92, p = 0.09).
Low frequency HRV did not differ between participants with any anxiety disorder and controls [Hedges’ g = −0.08 (−0.31–0.15); SE = 0.09; p = 0.49]. Egger’s regression test (p = 0.001) indicated evidence of publication bias and the Q statistic was significant (Q = 85.02, p < 0.001) (I2 statistic = 75.3%). However, LF HRV did not differ for any anxiety disorder relative to healthy controls (Table 2). Moderator analyses revealed that LF HRV did not differ across anxiety disorders (Q = 3.73, p = 0.59); nor was there any evidence of heterogeneity from other factors.
Time domain HRV was reduced in participants with any anxiety disorder relative to controls [Hedges’ g = −0.7 (−1.45– 0.05); SE = 0.38; p = 0.07], a finding associated with a moderate effect size. While this finding was at trend levels, the overall Q statistic was significant (Q = 1027.39, p < 0.001) and the I2 statistic was 98.2% indicating high variance between studies, highlighting a need to further inspect these data. Egger’s regression test (p < 0.001) also indicated evidence of publication bias. After the exclusion of a clear outlier (74) (see Supplementary Material), HRV was significantly reduced in those with anxiety disorders relative to healthy controls [Hedges’ g = −0.45 (−0.57 to −0.33); SE = 0.06; p < 0.001], and there was no longer evidence of heterogeneity between studies (Q = 18.46, p = 0.43, I2 statistic = 2.5%). Egger’s regression test still revealed evidence of publication bias (p = 0.01). TD HRV was significantly reduced in patients with PTSD and GAD, findings associated with a moderate effect size, as well as in PD, SAD, and Specific Phobias, findings associated with a small effect size (see Table 2). Follow-up moderator analyses revealed no difference in summary effect sizes between disorders (Q = 2.8, p = 0.73). No evidence of heterogeneity from other factors was observed.
Meta-analysis revealed that anxiety disorders are associated with significant reductions in HRV, effects associated with a small-to-moderate effect size. Importantly, medication use and medical and psychiatric co-morbidity did not impact these findings. The present study revealed that PD, PTSD, GAD, and SAD displayed significant reductions in TD and HF HRV, findings associated with a moderate effect size. Specific phobias also displayed reductions in TD HRV, although these findings were associated with a small effect size. Effects relating to OCD and studies that grouped anxiety disorders were not associated with significant reductions. Further, our results suggest that anxiety disorders do not adversely impact on LF, perhaps highlighting the specificity of effects on the PNS, which is better reflected in HRV measures including RMSSD and HF. Considering that reductions in HRV predicts adverse future outcomes including CVD and sudden cardiac death (9, 10, 15), our findings have important implications for future physical health of patients with a diagnosis of anxiety, and PD, PTSD, GAD, and SAD in particular.
There are a number of possible explanations why OCD, in contrast with the other anxiety disorders, was not associated with reductions in HRV. First, the sample size was limited (n = 40). Second, there was inconsistency in reports between the two studies reporting on HRV and OCD. Slaap et al. (47) found that HF HRV parameters did not differ between patients and controls. Pittig et al. (29), however, was the first study to report reduced HF HRV in OCD patients relative to controls. As the authors noted, it is possible that results in the latter study could be accounted for by psychotropic medication use (11 of 17 OCD patients reported current medication use). The inconsistent results cited above mirror the inconsistencies in results on OCD patients and other indices of autonomic activity (100–103). In sum, more research is needed before firm conclusions can be drawn on cardiac autonomic functioning in these patients.
Anxiety, in all its forms, can be seen as a failure of inhibition involving reduced capacity to inhibit cognitive (e.g., apprehension, vigilance, and worry), affective (e.g., panic), behavioral (e.g., avoidance), and physiological (e.g., increased HR) responses, leading to reduced vagal outflow and lowered HRV. The prominent reductions in HRV observed here (with the exception of OCD) are in line with previous theoretical models. The neurovisceral integration model (16, 21) highlights a role for the prefrontal cortex in inhibitory function via a vagally mediated pathway, which can be indexed by HRV. In addition, the polyvagal theory (35) highlights a role for the myelinated vagus in promoting social engagement and communication, and cultivating relaxed behavioral states by inhibiting sympathetic tone on the heart and dampening the hypo-pituitary–adrenal axis. When the environment is perceived as safe, vagal outflow increases, promoting regeneration, homeostatic functions, and social behavior. Critically, impairment of these neural processes leads to a difficulty in detecting whether environments are safe or whether people are trustworthy, which in turn may play a role in the development of the anxiety disorders. Polyvagal theory may therefore help explain the link between anxiety and reduced capacity for inhibition as well as reduced social engagement characteristic of individuals with anxiety.
Our current findings have important implications for the established link between reduced HRV, anxiety, and health. All anxiety disorders exhibit greater threat-related attentional biases relative to controls (23). This perseverance results in chronically high levels of corticotropin releasing factor (a hormone and neurotransmitter released in response to stress) and high basal levels of cortisol, leading to chronically withdrawn parasympathetic activity (i.e., low vagal tone) (104). Additionally, chronic worriers may also display poor cardiac autonomic regulation in response to non-threatening cues (105). It is also possible that impaired vagal function, which usually plays an important role in regulating the hypothalamic–pituitary–adrenal axis (106), leads to heightened activation of stress responses. The inability to disengage from threat detection heightens activation of the sympathetic nervous system underpinned by a chronic withdrawal of parasympathetic activity (and long-term reductions in HRV). This chronicity may in turn contribute to chronic withdrawal of the parasympathetic nervous system, and subsequent impairment in the cholinergic anti-inflammatory reflex (107), leading to an increase in a host of conditions including diabetes, obesity, and CVD (9, 10). A prospective study on worry and future cardiac health has reported that high levels of worry alone increase the risk of future myocardial infarction two to threefold (108). Given the clear impact of chronic worry on future cardiac health, it is especially concerning given that anxiety patients may suffer for as long as 5–10 years before diagnosis and treatment (109). Given the potential health implications of suffering from an anxiety disorder, there are clear implications concerning the importance of discovering whether successful treatments have been shown to increase HRV in anxiety disordered patients.
Previous research concerning the question of the impact of treatment for anxiety on HRV can broadly be grouped into studies that investigated the impact of psychotropic and non-psychotropic therapies. Concerning psychotropic treatments, two studies have investigated the impact of pharmacological intervention in isolation on HRV in anxiety patients. First, Tucker et al. (110) examined the effect of paroxetine (an SSRI) administration on HRV in 17 PD patients. After 4 weeks of 20 mg of paroxetine daily, parasympathetic activity was significantly increased in patients. This finding stands in contrast to reports that SSRI treatments for major depression, including paroxetine, have no impact on HRV (13). Further, the study reports only normalized units of HF HRV, a measure difficult to interpret when not reported alongside raw HF values (88). Thus, the effect of SSRIs in isolation on HRV in anxiety patients has yet to be properly studied, however, we can predict the effect will be similar to the effect of SSRIs in treating major depression, that is, negligible (13). Second, Baker et al. (111) examined the effect of clonazepam (a benzodiazepine) administration on HRV relative to placebo in 27 PD patients. Ten patients received clonazepam while 17 received a placebo. Compared with placebo, patients receiving clonazepam showed a significant decrease in HRV for all time (SDANN, SDNN) and frequency (LF, HF) measures from baseline to 4 weeks (all ps < 0.05). Treatment response was not correlated with HRV. This result is consistent with previous studies observing reductions in HF HRV following benzodiazepine administration (112–114).
The non-psychotropic treatments for anxiety disorders with an evidence base are psychological and behavioral. The impact of treating anxiety with cognitive behavioral therapy (CBT) on HRV has been reported in a number of studies, with varying results. Diveky, Prasko (115) reported that a 6-week CBT program on a sample of 31 panic patients had no significant impact on HRV. Mathewson, Schmidt (116) investigated the impact of 12 weeks of 2 h group CBT sessions in 23 patients with SAD, reporting a decrease in resting RSA levels over the course of the study. The authors note this reduction in RSA may have been due to the anticipation of a stressful task (i.e., anxiety provoking speech) during testing sessions. Two studies have investigated the impact of exposure based treatments on HRV in samples of flight phobics. Bornas et al. (117) investigated the impact of an exposure treatment on HRV in 20 patients, reporting that they exhibited a significant reduction (p < 0.01) in HF HRV after six sessions of computer-assisted or virtual reality exposure over 3 weeks, and curiously that patients with lower pre-treatment HRV responded better to treatment. In contrast to these findings, Busscher et al. (118) reported on the impact of exposure (two 1-h flights) on HRV in 50 flight phobics patients, observing a significant increase in RSA a finding associated with a large effect size, even after a relatively brief treatment. A possible explanation for this discrepancy could be the differential impact between virtual versus in vivo exposure on psychophysiological outcomes. Sack et al. (119) examined the effect of eye movement desensitization and reprocessing (EMDR) treatment on RSA in 11 patients with PTSD. Following an average of 4.7 (ranging from one to eight) EMDR sessions, there was no difference in pre-treatment and post-treatment RSA; however, there was a significant increase in RSA at 6 month follow-up. This reduction at follow-up was preceded by symptom reduction at post-treatment assessment.
Finally, concerning studies that have investigated the effect of concurrent psychotropic and non-psychotropic treatment on HRV, Prasko e al. (46) examined the effect of both CBT and SSRIs on HRV in 19 patients with PD. Following 18 group CBT sessions and pharmacotherapy, all HRV power spectra increased, with HF HRV increasing significantly. Garakani et al. (31) administered either 12 weeks of CBT alone or CBT with sertraline (an SSRI), and in treatment responders, CBT alone was associated with increases in HRV, but not the combined treatment. Clearly, there is considerable heterogeneity in treatment studies with respect to treatment type and the specific disorder being treated. Thus, it is unsurprising that there is considerable variation in reports on HRV outcomes in response to treatment, and it remains to be seen whether successful treatment of anxiety disorders will be paired with increases in HRV. However, given that both anxiety and low HRV predict adverse cardiovascular outcomes, we recommend that anxiety patients consider cardiovascular risk reduction strategies, such as exercise, smoking cessation, dietary change, and meditation, in conjunction with normal treatment.
This study has a number of advantages, including the application of a standardized meta-analytic methodology to assess the impact of anxiety disorders on HRV, examination of a range of HRV measures, the inclusion of moderator analyses examining the effect of frequently overlooked confounds including psychiatric and medical co-morbidity, and medication status, assessment of publication bias, and the application of strict inclusion/exclusion criteria for the selection of studies. However, some limitations of the present study are worth noting. There is a paucity of studies reporting on the effects of GAD, OCD, SAD, and SP, placing limits on the conclusions that can be drawn about the effect of these disorders on HRV. This is particularly true for conclusions about HRV and OCD. Only two studies reported on HRV and OCD, with one study report no significant reductions (47), and one study reporting significant reductions with the caveat that the result may have been related to the use of psychotropic medications (29). We also found evidence for study heterogeneity, although this issue was addressed in part, by conducting follow-up analyses to observe the effect of specific anxiety disorders on HRV. Further, patients with an anxiety disorder suffer from co-morbid depression in up to 60% of cases (120), and although the present results indicate that HRV of patients with psychiatric co-morbidity does not differ from those without such co-morbidity, it should be noted that depression and co-morbid anxiety has been associated with greater HRV reductions than depression alone (121), and depression and co-morbid anxiety increases risk of all-cause mortality and CVD two- to threefold (122). We observed a disproportionate number of studies on PD, highlighting the need for future studies on other anxiety disorders. There is also a need for future studies to consider the impact of evidence-based treatments for anxiety disorders on HRV, allowing for the impact of symptom reduction on HRV to be determined and downstream effects on health and well-being to be elucidated. Finally, there is a clear link both between anxiety-disease and HRV-disease, and these have been documented extensively by other authors. However, there are no prospective studies reporting on all three factors, and how HRV mediates the link between anxiety and disease. Future prospective studies examining the link between anxiety and disease while reporting on HRV will be valuable in this regard.
In summary, the present results have important implications for the long-term health and well-being of patients with a variety of anxiety disorders, considering the body of work highlighting a role for impaired vagal regulation as a risk factor for CVD and all-cause mortality [see Ref. (9, 10, 123) for reviews]. In conclusion, we advise clinicians to consider comprehensive cardiovascular risk reduction strategies for anxiety patients.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Andrew H. Kemp acknowledges support from the National Health and Medical Research Council (NHMRC) including Project Grant (464863), and a NHMRC Career Development Award (571101). Andrew H. Kemp is currently supported by an International Research Professorship from the Universidade de São Paulo. Sponsors played no role in analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors John A. Chalmers and Daniel S. Quintana had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The Supplementary Material for this article can be found online at http://www.frontiersin.org/Journal/10.3389/fpsyt.2014.00080/abstract
1. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry (2005) 62(6):593–602. doi: 10.1001/archpsyc.62.6.593
2. Kessler RC, Greenberg PE. The economic burden of anxiety and stress disorders. In Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia: Lippincott Williams & Wilkins (2002). p. 981–92.
3. Härter MC, Conway KP, Merikangas KR. Associations between anxiety disorders and physical illness. Eur Arch Psychiatry Clin Neurosci (2003) 253(6):313–20. doi:10.1007/s00406-003-0449-y
4. Vogelzangs N, Seldenrijk A, Beekman AT, van Hout HP, de Jonge P, Penninx BW. Cardiovascular disease in persons with depressive and anxiety disorders. J Affect Disord (2010) 125(1–3):241–8. doi:10.1016/j.jad.2010.02.112
5. Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol (2010) 56(1):38–46. doi:10.1016/j.jacc.2010.03.034
6. Janszky I, Ahnve S, Lundberg I, Hemmingsson T. Early-onset depression, anxiety, and risk of subsequent coronary heart disease: 37-year follow-up of 49,321 young Swedish men. J Am Coll Cardiol (2010) 56(1):31–7. doi:10.1016/j.jacc.2010.03.033
7. Shibeshi WA, Young-Xu Y, Blatt CM. Anxiety worsens prognosis in patients with coronary artery disease. J Am Coll Cardiol (2007) 49(20):2021–7. doi:10.1016/j.jacc.2007.03.007
8. Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes – the ARIC study. Circulation (2000) 102(11):1239–44. doi:10.1161/01.CIR.102.11.1239
9. Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol (2010) 141(2):122–31. doi:10.1016/j.ijcard.2009.09.543
10. Kemp AH, Quintana DS. The relationship between mental and physical health: insights from the study of heart rate variability. Int J Psychophysiol (2013) 89(3):288–96. doi:10.1016/j.ijpsycho.2013.06.018
11. Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med (2011) 269(1):45–53. doi:10.1111/j.1365-2796.2010.02321.x
12. Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex – linking immunity and metabolism. Nat Rev Endocrinol (2012) 8(12):743–54. doi:10.1038/nrendo.2012.189
13. Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry (2010) 67(11):1067–74. doi:10.1016/j.biopsych.2009.12.012
14. Quintana DS, McGregor IS, Guastella AJ, Malhi GS, Kemp AH. A meta-analysis on the impact of alcohol dependence on short-term resting-state heart rate variability: implications for cardiovascular risk. Alcohol Clin Exp Res (2013) 37(Suppl 1):E23–9. doi:10.1111/j.1530-0277.2012.01913.x
15. La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation (2003) 107(4):565–70. doi:10.1161/01.CIR.0000047275.25795.17
16. Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord (2000) 61(3):201–16. doi:10.1016/S0165-0327(00)00338-4
17. Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes (2003) 52(7):1799–805. doi:10.2337/diabetes.52.7.1799
18. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry (2009) 65(9):732–41. doi:10.1016/j.biopsych.2008.11.029
19. Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ (2000) 321(7255):199–204. doi:10.1136/bmj.321.7255.199
20. Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol (2008) 29(8):357–65. doi:10.1016/j.it.2008.05.002
21. Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol (2007) 74(2):185–99. doi:10.1016/j.biopsycho.2005.08.009
23. Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull (2007) 133(1):1. doi:10.1037/0033-2909.133.1.1
24. Lyonfields JD, Borkovec TD, Thayer JF. Vagal tone in generalized anxiety disorder and the effects of aversive imagery and worrisome thinking. Behav Ther (1995) 26(3):457–66. doi:10.1016/S0005-7894(05)80094-2
25. Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry (1996) 39(4):255–66. doi:10.1016/0006-3223(95)00136-0
26. Brosschot JF, Van Dijk E, Thayer JF. Daily worry is related to low heart rate variability during waking and the subsequent nocturnal sleep period. Int J Psychophysiol (2007) 63(1):39–47. doi:10.1016/j.ijpsycho.2006.07.016
27. Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res (2006) 60(2):113–24. doi:10.1016/j.jpsychores.2005.06.074
28. Tully PJ, Cosh SM, Baune BT. A review of the affects of worry and generalized anxiety disorder upon cardiovascular health and coronary heart disease. Psychol Health Med (2013) 18(6):627–44. doi:10.1080/13548506.2012.749355
29. Pittig A, Arch JJ, Lam CW, Craske MG. Heart rate and heart rate variability in panic, social anxiety, obsessive-compulsive, and generalized anxiety disorders at baseline and in response to relaxation and hyperventilation. Int J Psychophysiol (2013) 87(1):19–27. doi:10.1016/j.ijpsycho.2012.10.012
30. Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Res (2000) 96(1):1–13. doi:10.1016/S0165-1781(00)00195-5
31. Garakani A, Martinez JM, Aaronson CJ, Voustianiouk A, Kaufmann H, Gorman JM. Effect of medication and psychotherapy on heart rate variability in panic disorder. Depress Anxiety (2009) 26(3):251–8. doi:10.1002/da.20533
32. Petruzzello SJ, Landers DM, Hatfield BD, Kubitz KA, Salazar W. A meta-analysis on the anxiety-reducing effects of acute and chronic exercise. Outcomes and mechanisms. Sports Med (1991) 11(3):143–82. doi:10.2165/00007256-199111030-00002
33. McEntee DJ, Halgin RP. Cognitive group therapy and aerobic exercise in the treatment of anxiety. J College Stud Psychother (1999) 13(3):37–55. doi:10.1300/J035v13n03_04
34. Rennie KL, Hemingway H, Kumari M, Brunner E, Malik M, Marmot M. Effects of moderate and vigorous physical activity on heart rate variability in a British study of civil servants. Am J Epidemiol (2003) 158(2):135–43. doi:10.1093/aje/kwg120
35. Porges SW. The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-Regulation. NewYork, NY: W.W. Norton & Company (2011).
36. Alvarenga ME, Richards JC, Lambert G, Esler MD. Psychophysiological mechanisms in panic disorder: a correlative analysis of noradrenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability, and psychological variables. Psychosom Med (2006) 68(1):8–16. doi:10.1097/01.psy.0000195872.00987.db
37. Chang HA, Chang CC, Tzeng NS, Kuo TB, Lu RB, Huang SY. Decreased cardiac vagal control in drug-naive patients with panic disorder: a case-control study in Taiwan. Asia Pac Psychiatry (2013) 5(2):80–9. doi:10.1111/appy.12032
38. Ito T, Inoue Y, Sugihara T, Yamada H, Katayama S, Kawahara R. Autonomic function in the early stage of panic disorder: power spectral analysis of heart rate variability. Psychiatry Clin Neurosci (1999) 53(6):667–72. doi:10.1046/j.1440-1819.1999.00623.x
39. Kang EH, Lee IS, Park JE, Kim KJ, Yu BH. Platelet serotonin transporter function and heart rate variability in patients with panic disorder. J Korean Med Sci (2010) 25(4):613–8. doi:10.3346/jkms.2010.25.4.613
40. Kikuchi M, Hanaoka A, Kidani T, Remijn GB, Minabe Y, Munesue T, et al. Heart rate variability in drug-naive patients with panic disorder and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry (2009) 33(8):1474–8. doi:10.1016/j.pnpbp.2009.08.002
41. Lavoie KL, Fleet RP, Laurin C, Arsenault A, Miller SB, Bacon SL. Heart rate variability in coronary artery disease patients with and without panic disorder. Psychiatry Res (2004) 128(3):289–99. doi:10.1016/j.psychres.2004.06.005
42. Martinez JM, Garakani A, Kaufmann H, Aaronson CJ, Gorman JM. Heart rate and blood pressure changes during autonomic nervous system challenge in panic disorder patients. Psychosom Med (2010) 72(5):442–9. doi:10.1097/PSY.0b013e3181d972c2
43. McCraty R, Atkinson M, Tomasino D, Stuppy WP. Analysis of twenty-four hour heart rate variability in patients with panic disorder. Biol Psychol (2001) 56(2):131–50. doi:10.1016/S0301-0511(01)00074-6
44. Melzig CA, Weike AI, Hamm AO, Thayer JF. Individual differences in fear-potentiated startle as a function of resting heart rate variability: implications for panic disorder. Int J Psychophysiol (2009) 71(2):109–17. doi:10.1016/j.ijpsycho.2008.07.013
45. Middleton HC, Ashby M. Clinical recovery from panic disorder is associated with evidence of changes in cardiovascular regulation. Acta Psychiatr Scand (1995) 91(2):108–13. doi:10.1111/j.1600-0447.1995.tb09749.x
46. Prasko J, Latalova K, Diveky T, Grambal A, Kamaradova D, Velartova H, et al. Panic disorder, autonomic nervous system and dissociation-changes during therapy. Neuro Endocrinol Lett (2010) 32(5):641–51.
47. Slaap BR, Nielen MM, Boshuisen ML, van Roon AM, den Boer JA. Five-minute recordings of heart rate variability in obsessive-compulsive disorder, panic disorder and healthy volunteers. J Affect Disord (2004) 78(2):141–8. doi:10.1016/S0165-0327(02)00240-9
48. Wang SM, Yeon B, Hwang S, Lee HK, Kweon YS, Lee CT, et al. Threat-induced autonomic dysregulation in panic disorder evidenced by heart rate variability measures. Gen Hosp Psychiatry (2013) 35(5):497–501. doi:10.1016/j.genhosppsych.2013.06.001
49. Wise V, McFarlane AC, Clark CR, Battersby M. An integrative assessment of brain and body function ‘at rest’ in panic disorder: a combined quantitative EEG/autonomic function study. Int J Psychophysiol (2011) 79(2):155–65. doi:10.1016/j.ijpsycho.2010.10.002
50. Yeragani VK, Balon R, Pohl R, Ramesh C, Glitz D, Weinberg P, et al. Decreased R-R variance in panic disorder patients. Acta Psychiatr Scand (1990) 81(6):554–9. doi:10.1111/j.1600-0447.1990.tb05498.x
51. Yeragani VK, Pohl R, Berger R, Balon R, Ramesh C, Glitz D, et al. Decreased heart rate variability in panic disorder patients: a study of power-spectral analysis of heart rate. Psychiatry Res (1993) 46(1):89–103. doi:10.1016/0165-1781(93)90011-5
52. Asmundson GJ, Stein MB. Vagal attenuation in panic disorder: an assessment of parasympathetic nervous system function and subjective reactivity to respiratory manipulations. Psychosom Med (1994) 56(3):187–93. doi:10.1097/00006842-199405000-00002
53. Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med (2007) 69(9):935–43. doi:10.1097/PSY.0b013e31815a8f6b
54. Klein E, Cnaani E, Harel T, Braun S, Ben-Haim SA. Altered heart rate variability in panic disorder patients. Biol Psychiatry (1995) 37(1):18–24. doi:10.1016/0006-3223(94)00130-U
55. Petrowski K, Herold U, Joraschky P, Mück-Weymann M, Siepmann M. The effects of psychosocial stress on heart rate variability in panic disorder. German J Psychiatry (2010) 13:66–73.
56. Yeragani VK, Pohl R, Srinivasan K, Balon R, Ramesh C, Berchou R. Effects of isoproterenol infusions on heart rate variability in patients with panic disorder. Psychiatry Res (1995) 56(3):289–93. doi:10.1016/0165-1781(95)02608-Y
57. Agorastos A, Boel JA, Heppner PS, Hager T, Moeller-Bertram T, Haji U, et al. Diminished vagal activity and blunted diurnal variation of heart rate dynamics in posttraumatic stress disorder. Stress (2013) 16(3):300–10. doi:10.3109/10253890.2012.751369
58. Cohen H, Kotler M, Matar MA, Kaplan Z, Miodownik H, Cassuto Y. Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biol Psychiatry (1997) 41(5):627–9. doi:10.1016/S0006-3223(96)00525-2
59. Hauschildt M, Peters MJ, Moritz S, Jelinek L. Heart rate variability in response to affective scenes in posttraumatic stress disorder. Biol Psychol (2011) 88(2–3):215–22. doi:10.1016/j.biopsycho.2011.08.004
60. Keary TA, Hughes JW, Palmieri PA. Women with posttraumatic stress disorder have larger decreases in heart rate variability during stress tasks. Int J Psychophysiol (2009) 73(3):257–64. doi:10.1016/j.ijpsycho.2009.04.003
61. Lakusic N, Fuckar K, Mahovic D, Cerovec D, Majsec M, Stancin N. Characteristics of heart rate variability in war veterans with post-traumatic stress disorder after myocardial infarction. Mil Med (2007) 172(11):1190–3.
62. Norte CE, Souza GGL, Vilete L, Marques-Portella C, Coutinho ESF, Figueira I, et al. They know their trauma by heart: an assessment of psychophysiological failure to recover in PTSD. J Affect Disord (2013) 150(1):136–41. doi:10.1016/j.jad.2012.11.039
63. Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol Psychiatry (2013) 73(11):1103–10. doi:10.1016/j.biopsych.2013.01.019
64. Tucker P, Pfefferbaum B, Jeon-Slaughter H, Khan Q, Garton T. Emotional stress and heart rate variability measures associated with cardiovascular risk in relocated Katrina survivors. Psychosom Med (2012) 74(2):160–8. doi:10.1097/PSY.0b013e318240a801
65. Wahbeh H, Oken BS. Peak high-frequency HRV and peak alpha frequency higher in PTSD. Appl Psychophysiol Biofeedback (2013) 38(1):57–69. doi:10.1007/s10484-012-9208-z
66. Cohen H, Kotler M, Matar M, Kaplan Z. Normalization of heart rate variability in post-traumatic stress disorder patients following fluoxetine treatment: preliminary results. Isr Med Assoc J (2000) 2(4):296–301.
67. Shaikh al arab A, Guedon-Moreau L, Ducrocq F, Molenda S, Duhem S, Salleron J, et al. Temporal analysis of heart rate variability as a predictor of post traumatic stress disorder in road traffic accidents survivors. J Psychiatr Res (2012) 46(6):790–6. doi:10.1016/j.jpsychires.2012.02.006
68. Hammel JC, Smitherman TA, McGlynn FD, Mulfinger AM, Lazarte AA, Gothard KD. Vagal influence during worry and cognitive challenge. Anxiety Stress Coping (2011) 24(2):121–36. doi:10.1080/10615806.2010.490912
69. Kollai M, Kollai B. Cardiac vagal tone in generalised anxiety disorder. Br J Psychiatry (1992) 161(6):831–5. doi:10.1192/bjp.161.6.831
70. Bornas X, Llabrés J, Noguera M, López AM, Gelabert JM, Vila I. Fear induced complexity loss in the electrocardiogram of flight phobics: a multiscale entropy analysis. Biol Psychol (2006) 73(3):272–9. doi:10.1016/j.biopsycho.2006.05.004
71. Alvares GA, Quintana DS, Kemp AH, Van Zwieten A, Balleine BW, Hickie IB, et al. Reduced heart rate variability in social anxiety disorder: associations with gender and symptom severity. PLoS One (2013) 8(7):e70468. doi:10.1371/journal.pone.0070468
72. Gaebler M, Daniels JK, Lamke JP, Fydrich T, Walter H. Heart rate variability and its neural correlates during emotional face processing in social anxiety disorder. Biol Psychol (2013) 94(2):319–30. doi:10.1016/j.biopsycho.2013.06.009
73. Einvik G, Hrubos-Strom H, Randby A, Nordhus IH, Somers VK, Omland T, et al. Major depressive disorder, anxiety disorders, and cardiac biomarkers in subjects at high risk of obstructive sleep apnea. Psychosom Med (2011) 73(5):378–84. doi:10.1097/Psy.0b013e318219e64e
74. Licht CM, de Geus EJ, van Dyck R, Penninx BW. Association between anxiety disorders and heart rate variability in The Netherlands Study of Depression and Anxiety (NESDA). Psychosom Med (2009) 71(5):508–18. doi:10.1097/PSY.0b013e3181a292a6
75. Martens EJ, Nyklicek I, Szabo BM, Kupper N. Depression and anxiety as predictors of heart rate variability after myocardial infarction. Psychol Med (2008) 38(3):375–83. doi:10.1017/S0033291707002097
76. Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med (2009) 151(4):264. doi:10.7326/0003-4819-151-4-200908180-00135
77. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III. Washington, DC: American Psychiatric Association (1980).
78. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. Washington, DC: American Psychiatric Association (1987).
79. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington, DC: American Psychiatric Association (1994).
80. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association (2000).
81. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization (1992).
82. Tsuji H, Larson MG, Venditti FJ Jr, Manders ES, Evans JC, Feldman CL, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation (1996) 94(11):2850–5. doi:10.1161/01.CIR.94.11.2850
83. Cardiology TFotESo. the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation (1996) 93(5):1043–65. doi:10.1161/01.CIR.93.5.1043
84. Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, et al. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur Heart J (1996) 17(3):354–81. doi:10.1093/oxfordjournals.eurheartj.a014868
85. Billman GE. Heart rate variability – a historical perspective. Front Physiol (2011) 2:86. doi:10.3389/fphys.2011.00086
86. Berntson GG, Bigger JT Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology (1997) 34(6):623–48. doi:10.1111/j.1469-8986.1997.tb02140.x
87. Reyes del Paso GA, Langewitz W, Mulder LJ, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology (2013) 50(5):477–87. doi:10.1111/psyp.12027
88. Heathers JA. Everything Hertz: methodological issues in short-term frequency-domain HRV. Card Electrophysiol (2014) 5:177. doi:10.3389/fphys.2014.00177
89. Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol (2013) 4:26. doi:10.3389/fphys.2013.00026
90. Thayer JF, Friedman BH. Assessment of anxiety using heart rate nonlinear dynamics. In: Ditto W, editor. Chaos in Biology and Medicine: SPIE Proceedings (1993) 2036:42–8.
91. Fei L, Copie X, Malik M, Camm AJ. Short- and long-term assessment of heart rate variability for risk stratification after acute myocardial infarction. Am J Cardiol (1996) 77(9):681–4. doi:10.1016/S0002-9149(97)89199-0
92. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. Cornwall: John Wiley & Sons (2011).
93. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Psychology Press (1988).
95. Shadish WR, Haddock CK. Combining estimates of effect size. In: Cooper H, Hedges G, editors. The Handbook of Research Synthesis and Meta-Analysis. New York: Sage (2009). p. 257–77.
96. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi:10.1002/sim.1186
97. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101. doi:10.2307/2533446
98. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi:10.1136/bmj.315.7109.629
99. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics (2000) 56(2):455–63. doi:10.1111/j.0006-341X.2000.00455.x
100. Benkelfat C, Mefford IN, Masters CF, Nordahl TE, King AC, Cohen RM, et al. Plasma catecholamines and their metabolites in obsessive-compulsive disorder. Psychiatry Res (1991) 37(3):321–31. doi:10.1016/0165-1781(91)90067-Y
101. Hoehn-Saric R, McLeod DR, Hipsley P. Is hyperarousal essential to obsessive-compulsive disorder? Diminished physiologic flexibility, but not hyperarousal, characterizes patients with obsessive-compulsive disorder. Arch Gen Psychiatry (1995) 52(8):688. doi:10.1001/archpsyc.1995.03950200078017
102. McCarthy PR, Ray WJ, Foa EB. Cognitive influences on electrocortical and heart rate activity in obsessive-compulsive disorder. Int J Psychophysiol (1995) 19(3):215–22. doi:10.1016/0167-8760(95)00009-H
103. Zahn TP, Leonard HL, Swedo SE, Rapoport JL. Autonomic activity in children and adolescents with obsessive-compulsive disorder. Psychiatry Res (1996) 60(1):67–76. doi:10.1016/0165-1781(95)02846-3
104. Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychol (2000) 55(11):1247. doi:10.1037/0003-066X.55.11.1247
105. Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behav Brain Res (1998) 94(2):225–48. doi:10.1016/S0166-4328(98)00041-2
106. Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci (2006) 1088(1):361–72. doi:10.1196/annals.1366.014
108. Kubzansky LD, Kawachi I, Spiro A, Weiss ST, Vokonas PS, Sparrow D. Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the Normative Aging Study. Circulation (1997) 95(4):818–24. doi:10.1161/01.CIR.95.4.818
109. Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ, Borkovec TD, Rickels K, et al. Consensus statement on generalized anxiety disorder from the International Consensus Group on Depression and Anxiety. J Clin Psychiatry (2001) 62:53–8.
110. Tucker P, Adamson P, Miranda R Jr, Scarborough A, Williams D, Groff J, et al. Paroxetine increases heart rate variability in panic disorder. J Clin Psychopharmacol (1997) 17(5):370–6. doi:10.1097/00004714-199710000-00006
111. Baker B, Khaykin Y, Devins G, Dorian P, Shapiro C, Newman D. Correlates of therapeutic response in panic disorder presenting with palpitations: heart rate variability, sleep, and placebo effect. Can J Psychiatry (2003) 48(6):381–7.
112. Agelink MW, Majewski TB, Andrich J, Mueck-Weymann M. Short-term effects of intravenous benzodiazepines on autonomic neurocardiac regulation in humans: a comparison between midazolam, diazepam, and lorazepam. Crit Care Med (2002) 30(5):997–1006. doi:10.1097/00003246-200205000-00008
113. Vogel LR, Muskin PR, Collins ED, Sloan RP. Lorazepam reduces cardiac vagal modulation in normal subjects. J Clin Psychopharmacol (1996) 16(6):449–53. doi:10.1097/00004714-199612000-00008
114. Michaloudis D, Kochiadakis G, Georgopoulou G, Fraidakis O, Chlouverakis G, Petrou A, et al. The influence of premedication on heart rate variability. Anaesthesia (1998) 53(5):446–53. doi:10.1046/j.1365-2044.1998.00323.x
115. Diveky T, Prasko J, Kamaradova D, Grambal A, Latalova K, Silhan P, et al. Comparison of heart rate variability in patients with panic disorder during cognitive behavioral therapy program. Psychiatr Danub (2013) 25(1):62–7.
116. Mathewson KJ, Schmidt LA, Miskovic V, Santesso DL, Duku E, McCabe RE, et al. Does respiratory sinus arrhythmia (RSA) predict anxiety reduction during cognitive behavioral therapy (CBT) for social anxiety disorder (SAD)? Int J Psychophysiol (2013) 88(2):171–81. doi:10.1016/j.ijpsycho.2013.03.016
117. Bornas X, del Amo AR, Tortella-Feliu M, Llabrés J. Heart rate variability profiles and exposure therapy treatment outcome in flight phobia. Appl Psychophysiol Biofeedback (2012) 37(1):53–62. doi:10.1007/s10484-011-9179-5
118. Busscher B, Spinhoven P, van Gerwen LJ, de Geus EJ. Anxiety sensitivity moderates the relationship of changes in physiological arousal with flight anxiety during in vivo exposure therapy. Behav Res Ther (2013) 51(2):98–105. doi:10.1016/j.brat.2012.10.009
119. Sack M, Lempa W, Lamprecht F. Assessment of psychophysiological stress reactions during a traumatic reminder in patients treated with EMDR. J EMDR Pract Res (2007) 1(1):15–23. doi:10.1891/1933-3196.1.1.15
120. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry (2005) 62(6):617–27. doi:10.1001/archpsyc.62.6.617
121. Kemp AH, Quintana DS, Felmingham KL, Matthews S, Jelinek HF. Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: implications for cardiovascular risk. PLoS One (2012) 7(2):e30777. doi:10.1371/journal.pone.0030777
122. Phillips AC, Batty GD, Gale CR, Deary IJ, Osborn D, MacIntyre K, et al. Generalized anxiety disorder, major depressive disorder, and their comorbidity as predictors of all-cause and cardiovascular mortality: the Vietnam experience study. Psychosom Med (2009) 71(4):395–403. doi:10.1097/Psy.0b013e31819e6706
Keywords: heart rate variability, anxiety, anxiety disorders, meta-analysis, treatment, cardiovascular disease
Citation: Chalmers JA, Quintana DS, Abbott MJ-A and Kemp AH (2014) Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front. Psychiatry 5:80. doi: 10.3389/fpsyt.2014.00080
Received: 13 May 2014; Paper pending published: 02 June 2014;
Accepted: 26 June 2014; Published online: 11 July 2014.
Edited by:
Silvia Raquel Soares Ouakinin, University of Lisbon, PortugalReviewed by:
Nathalie Michels, Ghent University, BelgiumCopyright: © 2014 Chalmers, Quintana, Abbott and Kemp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew H. Kemp, Center for Clinical and Epidemiologic Research, Hospital Universitário, University of São Paulo, Av Lineu Prestes 2565, São Paulo 05508-000, SP, Brazil e-mail:YW5kcmV3LmtlbXBAc3lkbmV5LmVkdS5hdQ==;YW5kcmV3LmtlbXBAaHUudXNwLmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.