- 1 Kimel Family Translational Imaging Genetics Research Laboratory, Research Imaging Centre, Centre for Addiction and Mental Health, Toronto, ON, Canada
- 2 Department of Psychiatry, University of Toronto, Toronto, ON, Canada
- 3 Neurogenetics Section, Department of Neuroscience, Centre for Addiction and Mental Health, Toronto, ON, Canada
- 4 Program in Neuroscience and Mental Health, The Hospital for Sick Children, Toronto, ON, Canada
- 5 Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
- 6 Geriatric Mental Health Program, Centre for Addiction and Mental Health, Toronto, ON, Canada
Disrupted-in-schizophrenia 1 was originally discovered in a large Scottish family with abnormally high rates of severe mental illness, including schizophrenia, bipolar disorder, and depression. An accumulating body of evidence from genetic, postmortem, and animal data supports a role for DISC1 in different forms of mental illness. DISC1 may play an important role in determining structure and function of several brain regions. One brain region of particular importance for several mental disorders is the striatum, and DISC1 mutant mice have demonstrated an increase in dopamine (D2) receptors in this structure. However, association between DISC1 functional polymorphisms and striatal structure have not been examined in humans. We, therefore hypothesized that there would be a relationship between human striatal volume and DISC1 genotype, specifically in the Leu607Phe (rs6675281) and Ser704Cys (rs821618) single nucleotide polymorphisms. We tested our hypothesis by automatically identifying the striatum in 54 healthy volunteers recruited for this study. We also performed an exploratory analysis of cortical thickness, cortical surface area, and structure volume. Our results demonstrate that Phe allele carriers have larger striatal volume bilaterally (left striatum: p = 0.017; right striatum: p = 0.016). From the exploratory analyses we found that the Phe carriers also had larger left hemisphere volumes (p = 0.0074) and right occipital lobe surface area (p = 0.014) compared to LeuLeu homozygotes. However, these exploratory findings do not survive a conservative correction for multiple comparisons. Our findings demonstrate that a functional DISC1 variant influences striatal volumes. Taken together with animal data that this gene influences D2 receptor levels in striatum, a key risk pathway for mental illnesses such as schizophrenia and bipolar disorder may be conferred via DISC1’s effects on the striatum.
Introduction
Schizophrenia is a highly heritable brain disorder. Twin and family studies have demonstrated that genetic liability for SZ is strong (0.6–0.7; Saha et al., 2005) and several candidate genes have been identified (Brenner et al., 2010). A candidate gene previously identified in this context, namely disrupted-in-schizophrenia 1 (DISC1), was originally discovered at the breakpoint of a chromosomal translocation t(1; 11) (q42.1; q14.3) that segregated with schizophrenia, bipolar disorder, and major depression in a large Scottish family (Millar et al., 2000; Blackwood et al., 2001). After this initial finding, several other genetic linkage studies associated DISC1 with a number of different forms of mental illness; including schizophrenia (Chubb et al., 2007), schizoffective disorder (Hodgkinson et al., 2004), bipolar disorder (Hennah et al., 2008), and autism spectrum disorders (Kilpinen et al., 2007; Williams et al., 2009). There have also been additional associations in individuals with specific cognitive profiles such as long- and short-term memory (Cannon et al., 2005), visual memory in families with high densities of schizophrenia (Hennah et al., 2005), hippocampal structure and function (Callicott et al., 2005), psychosis-related personality traits in healthy control populations (Tomppo et al., 2009), and even subtle differences in healthy cognitive aging (Thomson et al., 2005; Harris et al., 2010).
Mutagenized mice have provided significant information regarding the role of DISC1 in healthy development and maturation. In DISC1 transgenic mice there is evidence of a reduction in neuron number, decreased neurogenesis, altered neuronal morphology, depressive- and schizophrenia-like behavioral phenotypes, and reduced regional brain volumes (Clapcote et al., 2007; Lee et al., 2011). In humans, DISC1 has been shown to have a strong modulatory effect on frontal-temporal cortical development (Carless et al., 2011; Raznahan et al., 2011a) and thinning of the prefrontal cortex (Szeszko et al., 2008) and the left supramarginal gyrus (Brauns et al., 2011). To date, no studies have explicitly focused on the analysis of the relationship between subcortical structures in the human and DISC1. However, evidence from the animal literature suggests that DISC1 may effect the proportion of dopamine receptors located in the striatum, providing an intriguing risk mechanism that may be a final common pathway for several mental disorders. Lipina et al. (2010) demonstrate DISC1 is related to a 2.1-fold increase in the proportion of striatal dopamine receptors without a significant change in dopamine release in the DISC1-L100P mutant (Clapcote et al., 2007).
Two single nucleotide polymorphisms (SNP) are studied here, specifically Leu607Phe (rs6675281) and Ser704Cys (rs821618). These SNPs were chosen as both the Phe and Ser alleles have been previously linked to increased risk for mental illness. Specifically, Raznahan et al. (2011a) demonstrated cortical thinning associated with both of these alleles and Szeszko et al. (2008) showed reduction in the superior frontal gyrus and anterior cingulate gyrus in Phe carriers. Based on these findings and the previous striatum-related finding in the animal model literature we hypothesized that the striatum is one of the neuroanatomical substrates through which DISC1 elevates risk for mental illness. Here, we study this hypothesis using a cohort of healthy control individuals and use automated image processing techniques to accurately identify subcortical structures (Chakravarty et al., 2006, 2008). We also performed an exploratory analysis to better understand the relationship of DISC1 with cortical thickness and cortical surface area.
Materials and Methods
Study Participants
Fifty-four healthy Caucasian volunteers were recruited for this study (33 male and 21 female; mean age: 33.5; standard deviation of age: 10.0; age range: 18–55 years old). All subjects were right handed and had no history of a mental disorder. None of the participants were currently abusing substance nor had any previous history of substance abuse, a positive toxicological screening result, a history of head trauma with loss of consciousness or seizure (or other neurological disorder), and had no first-degree relative with a history of a psychotic mental disorder. All subjects were assessed with the Edinburgh handedness inventory (Oldfield, 1971), Wechsler Test for Adult Reading for IQ, and Hollingshead index for socio-economic status. They were also interviewed by a psychiatrist, completed the structured interview for DSM-IV disorders (First et al., 1995) and the Mini-Mental State Examination (Folstein et al., 1975), and completed a urine toxicology exam. The study was approved by the Research Ethics Board of the Centre for Addiction and Mental Health (Toronto, ON, Canada), and all participants provided informed, written consent.
Genetics
Subjects were genotyped for the DISC1 SNPs, Leu607Phe (rs6675281) and Ser704Cys (rs821616) in this study. Genotyping of this polymorphism was performed using a standard 5″ nuclease TaqMan assay-on-demand (Applied Biosystems Inc, Foster City) protocol in a total volume of 10 μL. Postamplification products were analyzed on the ABI 7500 Sequence Detection System (Applied Biosystems), and genotype calls were performed manually. Results were verified independently by two laboratory personnel masked to demographic and phenotypic information. Quality control analysis was performed on 10.0% of the sample.
Magnetic Resonance Imaging
High-resolution magnetic resonance images were acquired as part of a multimodal imaging protocol using an 8-channel head coil on a 1.5-T GE Echospeed system (General Electric Medical Systems, Milwaukee, WI, USA), which permits maximum gradient amplitudes of 40 mT/m. Axial inversion recovery – prepared spoiled gradient recall images were acquired: echo time = 5.3 ms; repetition time = 12.3 ms; time to inversion = 300.0 ms; flip angle = 20°; and number of excitations = 1 (for a total of 124 contiguous images, 1.5-mm thickness).
Segmentation of Subcortical Structures
Subcortical structures (striatum, globus, pallidus, and thalamus) were automatically identified using a atlas of the basal ganglia and thalamus derived from serial histological data (Chakravarty et al., 2006) and warped to a high-contrast and -resolution neuroanatomical template derived from the average of 27 MRI volumes from the same individual (Holmes et al., 1998). The atlas was then customized to the unique neuroanatomy of the subjects being studied using a region-of-interest non-linear registration estimation approach (Chakravarty et al., 2008, 2009b) that has been validated against manually defined gold-standards, intra-operative recordings, and brain activations recorded using functional magnetic resonance imaging techniques (Chakravarty et al., 2008, 2009a,b). All linear and non-linear transformations were estimated using the ANIMAL algorithm (Collins et al., 1994, 1995; Collins and Evans, 1997), which is part of the MINC suite of medical image processing tools1.
Cortical Thickness Analysis
Cortical thickness was analyzed using the CIVET processing pipeline (version 1.1.10; Montreal Neurological Institute at McGill University, Montreal, QC, Canada). T1-weighted images were registered to the ICBM152 non-linear sixth-generation template with a 9-parameter linear transformation (Collins et al., 1994), inhomogeneity corrected (Sled et al., 1998) and tissue classified (Zijdenbos et al., 2002). Deformable models were then used to create white and gray matter surfaces for each hemisphere separately, resulting in four surfaces of 40,962 vertices each (MacDonald, 1998; Kim et al., 2005). From these surfaces, the t-link metric was derived for determining the distance between the white and gray surfaces (Lerch and Evans, 2005). The thickness data were subsequently blurred using a 20-mm surface-based diffusion-blurring kernel in preparation for statistical analyses (Lerch and Evans, 2005). Unnormalized, native-space thickness values were used in all analyses owing to the poor correlation between cortical thickness and brain volume. Normalizing for global brain size when it has little pertinence to cortical thickness risks introducing noise and reducing power. A mid-surface (between pial and white matter surfaces) were non-linearly normalized (Robbins et al., 2004a,b) using a novel depth potential function (Boucher et al., 2009) to minimally biased surface template (Lyttelton et al., 2007). Where pertinent, all vertex-wise analyses were performed using the RMINC2 package and corrected for multiple comparisons using the false-discovery rate (FDR; Genovese et al., 2002).
Results
Genotyping yielded 45 LeuLeu homozygotes, 8 PheLeu heterozygotes, and a single PhePhe homozygote for the Leu607Phe genotype. For subsequent neuroimaging anaylses, all Phe carriers were pooled together in the same group yielding 45 LeuLeu homozygotes (18 female and 27 male; mean age: 28.25) and 9 Phe carriers (3 female and 6 male; mean age: 33). For Ser704Cys, genotyping yielded one Ser homozygote, 20 SerCys heterzygotes, and 33 Cys homozygotes. As in the Leu607Phe genotype, we pooled minor allele carrier (i.e., Ser carriers). In subsequent analyses we compared the 21 Ser carriers (9 female and 12 male) to the 33 Cys homozygotes (12 female, 21 male). Neither SNP violated Hardy–Weinberg equilibrium (Leu607Phe: χ2 = 0.76, p = 0.38; Ser704Cys: χ2 = 1.08, p = 0.29).
There were no differences in age, sex, IQ, socio-economic status, and years of education observed based on genotype (see Table 1).
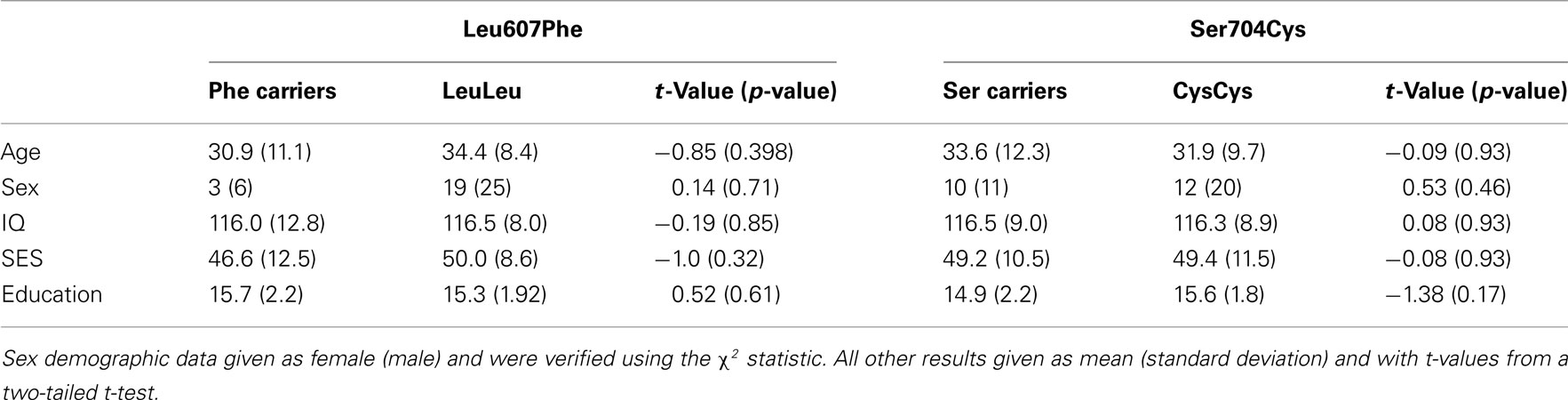
Table 1. Summary of differences with respect to age, sex, IQ, socio-economic status (SES), and education based on Leu607Phe and Ser704Cys genotypes.
All volumes were analyzed using a linear model that included age and sex. These analyses were performed with and without total brain volume was included as an additional covariate (Table 2 reports values with brain volume included in the statistical model; see also Figure 1). For the Leu607Phe genotype, significant increased left and right striatal volume were observed without including brain volumes in the model (left striatum; mean volume, Phe carriers: 10,240 mm3, LeuLeu homozygotes: 9043 mm3, t = 3.06, p = 0.0036; right striatum mean volume, Phe carriers: 10,167 mm3, LeuLeu homozygotes: 8995 mm3, t = 3.06, p = 0.0036). The effect of genotype remains significant for both hemispheres when including brain volume in the model (left striatum: t = 2.48, p = 0.017; right: t = 2.49, p = 0.016). No other significant differences were demonstrated for the other subcortical structures (i.e., the globus pallidus or the thalamus). No significant differences were found for any of the subcortical volumes identified for the Ser704Cys genotype.
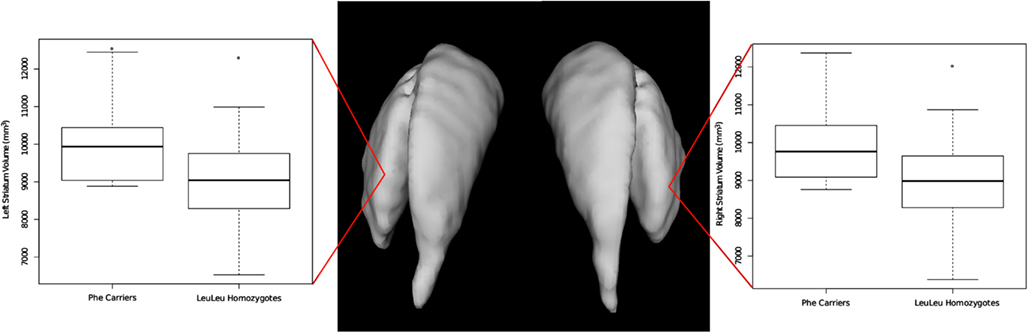
Figure 1. Differences in left and right striatal volume based on the Leu607Phe genotype. Phe carriers exhibit larger striatal volume bilaterally.
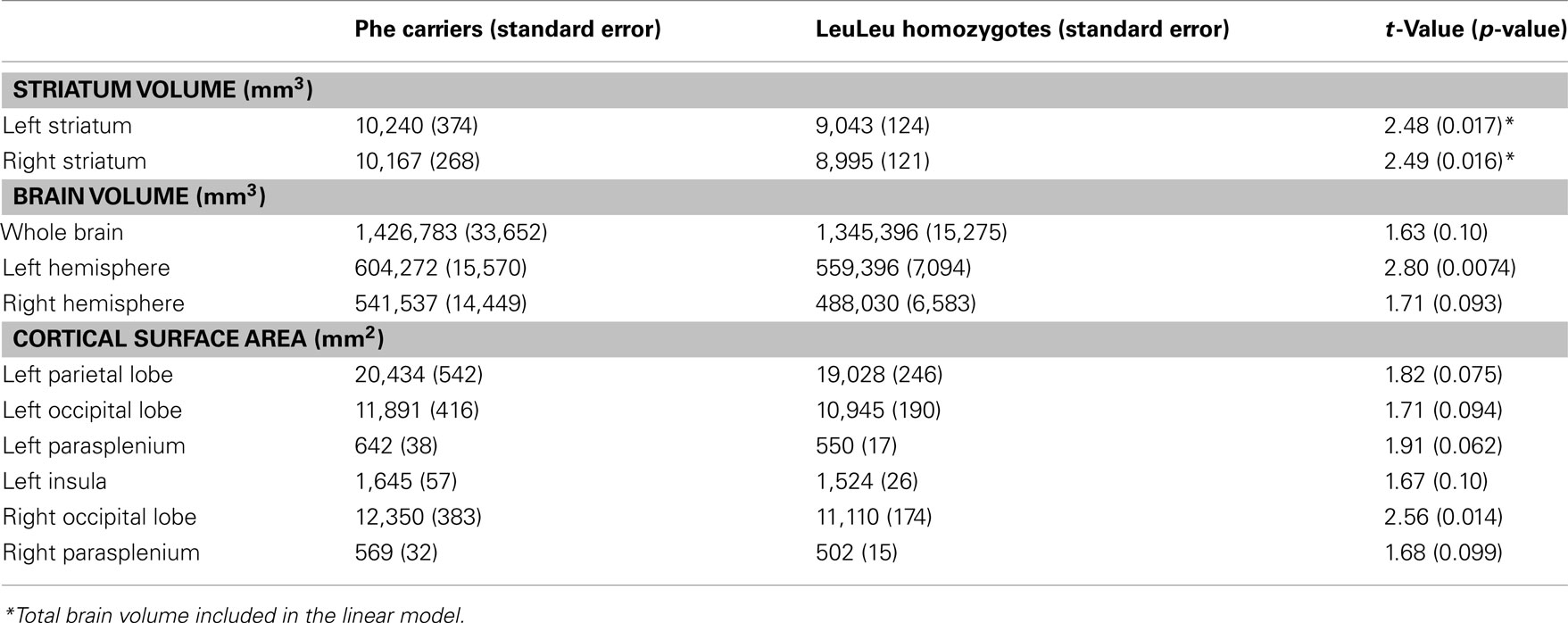
Table 2. Results from the volumetric analysis, cortical thickness, and surface area for Leu607Phe genotypes.
In our exploratory analyses we found other volume differences based on Leu607Phe genotype. Phe carriers had significantly increased right hemisphere volume in comparison to LeuLeu homozygotes (mean volume, Phe carriers: 1,426,683 mm3, LeuLeu homozygotes: 1,345,396mm3, t = 2.80, p = 0.0074). Trend-level differences in total brain volume (t = 1.63, p = 0.10) and left hemisphere volume (t = 1.71, p = 0.093) between these groups were also found. Phe carriers had significantly larger surface area in the right occipital lobe (mean surface area, Phe carriers: 12,350 mm2, LeuLeu homozygotes: 11,110 mm2, t = 2.56, p = 0.014). In addition, trend-level increases in surface area in Phe carriers were observed in the left parietal and right occipital lobes, and bilaterally in the parasplenium (see Table 2). No vertex-wise differences in cortical thickness or surface area survived comparison for mulitple comparisons at a 5% FDR (Genovese et al., 2002) and no structure-wise differences in cortical thickness were observed.
No neuroanatomical differences in volume, cortical thickness, or surface area were observed based on the Ser704Cys genotype.
Discussion
In this manuscript we present an analysis of the relationship between neuroanatomy and two DISC1 SNPs, namely and Leu607Phe andSer704Cys, that have been previously associated with increased risk for mental illness such as schizophrenia (Chubb et al., 2007), schizoaffective disorder (Hodgkinson et al., 2004), bipolar disorder (Hennah et al., 2008), and autism spectrum disorders (Kilpinen et al., 2007; Williams et al., 2009). We hypothesized that these genotypes would be related to striatal volume as DISC1 has previously been associated with the number of striatal dopamine receptors (Lipina et al., 2010). After an analysis of subcortical structures, we found that Phe carriers had larger striatal volume. Further, Phe carrers also exhibited trend-level increases in cortical surface area in comparison to LeuLeu homozygotes (none of which survives a conservative Bonfferroni correction). No volumetric or morphological differences were observed based on Ser704Cys genotype.
To the best of our knowledge, the relationship between striatal volume and DISC1 genotype has not been previously studied. Our finding is in keeping with a 2.1-fold increase in striatal dopamine receptors previously reported in DISC1 mutant mice (Lipina et al., 2010), that is consistent with the dopamine hypothesis of schizophrenia. The striatum is one of the brain regions with the highest concentration of D2 receptors. There is a substantial body of evidence supporting dopaminergic dysfunction in schizophrenia, including from in vivo positron emission tomography studies of the D2 receptor (Seeman, 2009; Heinz and Schlagenhauf, 2010). A more refined version of this hypothesis states that the synthesis and availability of the releasable striatal dopamine are increased in patients suffering from schizophrenia (Grunder et al., 2003; Guillin et al., 2007; Hietala et al., 1995), and that dopaminergic activity may be altered in the context of stress (Mizrahi et al., 2012).
Previous studies examining the relationship between human neuroanatomy have focused on cortical thickness (Brauns et al., 2011; Raznahan et al., 2011a), gray matter density (Cannon et al., 2005), and hippocampal structure (Callicott et al., 2005). Raznahan et al. (2011a)demonstrated decreased thickness in temporal-parietal areas of Phe carriers. In addition, with a longitudinal design, they demonstrated that the rate of cortical thinning in normally developing LeuLeu homozygotes resembled a typical developmental trajectory whereas a more abnormal developmental trajectory was observed in the Phe carriers. Other studies report reduced cortical thickness in the left supra-marginal gyrus (Brauns et al., 2011) and reduced gray matter density (Cannon et al., 2005) in the dorsolateral prefrontal cortex of Phe carriers. Our analyses showed no cortical thickness differences for either SNP. It is possible that such differences may only be detectable at an earlier time point in the human lifespan, since the study by Raznahan et al. (2011a) focuses on a normally developing cohort of adolescents and young adults. The remaining studies on cortical anatomy and DISC1 present conflicting results (Szeszko et al., 2008). While our study may have been underpowered to detect cortical thickness changes we do demonstrate that Phe carriers actually have larger brain volume, specifically in the right hemisphere. This relative increase in Phe carrier brain volume may be a reflection of an abnormal developmental process that may, in turn, increase susceptibility to mental illness (Gogtay et al., 2004; Paus et al., 2008; Raznahan et al., 2011b).
Our study is also the first to report results related to cortical surface area in DISC1. Cortical volume changes through the course of development can be fractionated into cortical thickness, surface area, and convex hull area subcomponents (Raznahan et al., 2011b). Further it has been demonstrated that the elements that comprise total brain volume have specific evolutionary (Rakic, 1995a,b), genetic (Voineskos et al., 2010, 2011), and cellular processes (Chenn and Walsh, 2002). Other than the whole brain and hemispheric volume differences reported, no structure-wise volumetric trends were found in our analyses. It is possible that these differences may be related to abnormal development of the cortical manifold that is influenced by DISC1 (as seen in the results for surface area). However, given the fact that results for our surface area findings were no longer significant following multiple comparison correction, our findings should be considered as exploratory.
One of the limitations of the current study is that we did not examine the effects of DISC1 variation in other populations such as ultra high-risk or disease cohorts. Future work in these populations may enable improved prediction of risk of conversion to psychosis, as well as provide insight into phenotypic heterogeneity in disease populations. Another limitation is that reports of heritability of striatal volumes and of alterations of striatal volumes in ultra high-risk subjects are mixed. (Ettinger et al., 2012) have demonstrated no differences in striatal volumes between UHR subjects that convert and those that do not convert to a form of psychosis (Hannan et al., 2010) or in monozygotic twins discordant for psychosis (Ettinger et al., 2012). Groups that rely on manual tracing either omit portions of the striatum (the tail of the caudate; Hannan et al., 2010) or define volumes using sub-sampled MRI image volumes (to facilitate manual labeling using stereology-based techniques). In both these cases, one cannot expect the accuracy of the manual labeling to be as consistent as an automated segmentation technique that has been shown to have lower variability than manual raters (Chakravarty et al., 2009b). Furthermore, there is evidence in the UHR literature that supports our findings. For instance, larger caudate nucleus volumes were demonstrated in a study of non-psychotic UHR individuals who are members of a multicomplex families where at least one parent suffers from psychosis (Hajek et al., 2009). In addition, studies of patients with schizotypal personality disorders, where medication confounds are not present, also support alterations of striatal volumes in schizophrenia spectrum disorders (Koo et al., 2006; Levitt et al., 2009).
Other groups have also demonstrated neuroanatomical differences in brain morphology based on the Ser704Cys variant. We show no such differences in the striatal volumes or the other cortical measures. One interpretation of these results is that only the Leu607Phe variant influences striatal anatomy. On the other hand, it is also possible that we may simply be underpowered to detect subtle changes related to Ser704Cys genotype that others have found (Cannon et al., 2005; Brauns et al., 2011; Raznahan et al., 2011a). One of the more robust findings of morphological differences that were shown to be associated with the Ser704Cys genotype occurred in a cohort of healthy developing younger individuals (Raznahan et al., 2011a), suggesting that the strength of this relationship may change across the course of brain development.
This is the first paper demonstrating a potential role for DISC1 in human striatal structure. Further, there are now converging lines of evidence from both animal and human studies that confirm this role. We have delineated this new pathway using an imaging-genetics analysis approach and have demonstrated how DISC1 confers risk for severe mental illness through the striatum. Further research is required to better understand the link between DISC1 and the striatal deficits reported in this article and cortical deficits previously described.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to acknowledge support from the Michael and Sonja Koerner Foundation, the Kimel Family, and the Paul E. Garfinkel New Investigator Catalyst Award. Aristotle N. Voineskos is funded by NARSAD, the Canadian Institutes of Health Research, and the Schizophrenia Society of Ontario.
Footnotes
References
Blackwood, D. H., Fordyce, A., Walker, M. T., St Clair, D. M., Porteous, D. J., and Muir, W. J. (2001). Schizophrenia and affective disorders – cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am. J. Hum. Genet. 69, 428–433.
Boucher, M., Whitesides, S., and Evans, A. (2009). Depth potential function for folding pattern representation, registration and analysis. Med. Image Anal. 13, 203–214.
Brauns, S., Gollub, R. L., Roffman, J. L., Yendiki, A., Ho, B. C., Wassink, T. H., Heinz, A., and Ehrlich, S. (2011). DISC1 is associated with cortical thickness and neural efficiency. Neuroimage 57, 1591–1600.
Brenner, R., Madhusoodanan, S., Puttichanda, S., and Chandra, P. (2010). Primary prevention in psychiatry – adult populations. Ann. Clin. Psychiatry 22, 239–248.
Callicott, J. H., Straub, R. E., Pezawas, L., Egan, M. F., Mattay, V. S., Hariri, A. R., Verchinski, B. A., Meyer-Lindenberg, A., Balkissoon, R., Kolachana, B., Goldberg, T. E., and Weinberger, D. R.. (2005). Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 102, 8627–8632.
Cannon, T. D., Hennah, W., van Erp, T. G., Thompson, P. M., Lonnqvist, J., Huttunen, M., Gasperoni, T., Tuulio-Henriksson, A., Pirkola, T., Toga, A. W., Kaprio, J., Mazziotta, J., and Peltonen, L. (2005). Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch. Gen. Psychiatry 62, 1205–1213.
Carless, M. A., Glahn, D. C., Johnson, M. P., Curran, J. E., Bozaoglu, K., Dyer, T. D., Winkler, A. M., Cole, S. A., Almasy, L., MacCluer, J. W., Duggirala, R., Moses, E. K., Göring, H. H., and Blangero, J. (2011). Impact of DISC1 variation on neuroanatomical and neurocognitive phenotypes. Mol. Psychiatry 16, 1096–104, 1063.
Chakravarty, M. M., Bertrand, G., Hodge, C. P., Sadikot, A. F., and Collins, D. L. (2006). The creation of a brain atlas for image guided neurosurgery using serial histological data. Neuroimage 30, 359–376.
Chakravarty, M. M., Broadbent, S., Rosa-Neto, P., Lambert, C. M., and Collins, D. L. (2009a). Design, construction, and validation of an MRI-compatible vibrotactile stimulator intended for clinical use. J. Neurosci. Methods 184, 129–135.
Chakravarty, M. M., Sadikot, A. F., Germann, J., Hellier, P., Bertrand, G., and Collins, D. L. (2009b). Comparison of piece-wise linear, linear, and nonlinear atlas-to-patient warping techniques: analysis of the labeling of subcortical nuclei for functional neurosurgical applications. Hum. Brain Mapp. 30, 3574–3595.
Chakravarty, M. M., Sadikot, A. F., Germann, J., Bertrand, G., and Collins, D. L. (2008). Towards a validation of atlas warping techniques. Med. Image Anal. 12, 713–726.
Chenn, A., and Walsh, C. A. (2002). Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365–369.
Chubb, J. E., Bradshaw, N. J., Soares, D. C., Porteous, D. J., and Millar, J. K. (2007). The DISC locus in psychiatric illness. Mol. Psychiatry 13, 36–64.
Clapcote, S. J., Lipina, T. V., Millar, J. K., Mackie, S., Christie, S., Ogawa, F., Lerch, J. P., Trimble, K., Uchiyama, M., Sakuraba, Y., Kaneda, H., Shiroishi, T., Houslay, M. D., Henkelman, R. D., Sled, J. G., Gondo, Y., Porteous, D. J., and Roder, J. C. (2007). Behavioral phenotypes of Disc1 missense mutations in mice. Neuron 54, 387–402.
Collins, D. L., and Evans, A. C. (1997). ANIMAL: validation and applications of non-linear registration-based segmentation. Int. J. Pattern Recogn. Artif. Intell. 11, 1271–1294.
Collins, D. L., Holmes, C. J., Peters, T. M., and Evans, A. C. (1995). Automatic 3-D model-based neuroanatomical segmentation. Hum. Brain Mapp. 3, 190–208.
Collins, D. L., Neelin, P., Peters, T. M., and Evans, A. C. (1994). Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 18, 192–205.
Ettinger, U., Schmechtig, A., Toulopoulou, T., Borg, C., Orrells, C., Owens, S., Matsumoto, K., van Haren, N. E., Hall, M.-H., Kumari, V., McGuire, P. K., Murray, R. M., and Picchioni, M. (2012). Prefrontal and striatal volumes in monozygotic twins concordant and discordant for schizophrenia. Schizophr. Bull. 38, 192–203.
First, M. B. S., Gibbon, M., and Williams, J. B. W. (1995). “Structured clinical interview for DSM-IV axis I disorders, patient addition (SCID-P),” in Biometric Research. New York, NY: New York Psychiatric Institute.
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). ”Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198.
Genovese, C. R., Lazar, N. A., and Nichols, T. (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15, 870–878.
Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., Nugent, T. F. III, Herman, D. H., Clasen, L. S., Toga, A. W., Rapoport, J. L., and Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 101, 8174–8179.
Grunder, G., Siessmeier, T., Piel, M., Vernaleken, I., Buchholz, H. G., Zhou, Y., Hiernke, C., Wong, D. F., Rösch, F., and Bartenstein, P. (2003). Quantification of D2-like dopamine receptors in the human brain with 18F-desmethoxyfallypride. J. Nucl. Med. 44, 109–116.
Guillin, O., Abi-Dargham, A., and Laruelle, M. (2007). Neurobiology of dopamine in schizophrenia. Int. Rev. Neurobiol. 78, 1–39.
Hajek, T., Gunde, E., Slaney, C., Propper, L., MacQueen, G., Duffy, A., and Alda, M. (2009). Striatal volumes in affected and unaffected relatives of bipolar patients – high-risk study. J. Psychiatr. Res. 43, 724–729.
Hannan, K. L., Wood, S. J., Yung, A. R., Velakoulis, D., Phillips, L. J., Soulsby, B., Berger, G., McGorry, P. D., and Pantelis, C. (2010). Caudate nucleus volume in individuals at ultra-high risk of psychosis: a cross-sectional magnetic resonance imaging study. Psychiatry Res. 182, 223–230.
Harris, S. E., Hennah, W., Thomson, P. A., Luciano, M., Starr, J. M., Porteous, D. J., and Deary, I. J. (2010). Variation in DISC1 is associated with anxiety, depression and emotional stability in elderly women. Mol. Psychiatry 15, 232–234.
Heinz, A., and Schlagenhauf, F. (2010). Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr. Bull. 36, 472–485.
Hennah, W., Thomson, P., McQuillin, A., Bass, N., Loukola, A., Anjorin, A., Blackwood, D., Curtis, D., Deary, I. J., Harris, S. E., Isometsä, E. T., Lawrence, J., Lönnqvist, J., Muir, W., Palotie, A., Partonen, T., Paunio, T., Pylkkö, E., Robinson, M., Soronen, P., Suominen, K., Suvisaari, J., Thirumalai, S., Clair, D. St., Gurling, H., Peltonen, L., and Porteous, D. (2008). DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder. Mol. Psychiatry 14, 865–873.
Hennah, W., Tuulio-Henriksson, A., Paunio, T., Ekelund, J., Varilo, T., and Partonen, T. (2005). A haplotype within the DISC1 gene is associated with visual memory functions in families with a high density of schizophrenia. Mol. Psychiatry 10, 1097–1103.
Hietala, J., Syvalahti, E., Vuorio, K., Rakkolainen, V., Bergman, J., Haaparanta, M., Kuoppamäki, M., Ruotalainen, U., Vuorio, K., Räkköläinen, V., Bergman, J., Solin, O., Kirvela, O., and Salokangas, R. K. R. (1995). Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet 346, 1130–1131.
Hodgkinson, C. A., Goldman, D., Jaeger, J., Persaud, S., Kane, J. M., Lipsky, R. H., and Malhotra, A. K. (2004). Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am. J. Hum. Genet. 75, 862–872.
Holmes, C. J., Hoge, R., Collins, L., Woods, R., Toga, A. W., and Evans, A. C. (1998). Enhancement of MR images using registration for signal averaging. J. Comput. Assist. Tomogr. 22, 324–333.
Kilpinen, H., Ylisaukko-Oja, T., Hennah, W., Palo, O. M., Varilo, T., Vanhala, R., Nieminen-von Wendt, T., von Wendt, L., Paunio, T., and Peltonen, L. (2007). Association of DISC1 with autism and Asperger syndrome. Mol. Psychiatry 13, 187–196.
Kim, J. S., Singh, V., Lee, J. K., Lerch, J., Ad-Dab’bagh, Y., MacDonald, D., Lee, J. M., Kim, S. I., and Evans, A. C. (2005). Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage 27, 210–221.
Koo, M. S., Levitt, J. J., McCarley, R. W., Seidman, L. J., Dickey, C. C., Niznikiewicz, M. A., Voglmaier, M. M., Zamani, P., Long, K. R., Kim, S. S., and Shenton, M. E. (2006). Reduction of caudate nucleus volumes in neuroleptic-naive female subjects with schizotypal personality disorder. Biol. Psychiatry 60, 40–48.
Lee, F. H., Fadel, M. P., Preston-Maher, K., Cordes, S. P., Clapcote, S. J., Price, D. J., Roder, J. C., and Wong, A. H. (2011). Disc1 point mutations in mice affect development of the cerebral cortex. J. Neurosci. 31, 3197–3206.
Lerch, J. P., and Evans, A. C. (2005). Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage 24, 163–173.
Levitt, J. J., Styner, M., Niethammer, M., Bouix, S., Koo, M. S., Voglmaier, M. M., Dicky, C. C., Niznikiewicz, M. A., Kikinis, R., McCarley, R. W., and Shenton, M. E. (2009). Shape abnormalities of caudate nucleus in schizotypal personality disorder. Schizophr. Res. 110, 127–139.
Lipina, T. V., Niwa, M., Jaaro-Peled, H., Fletcher, P. J., Seeman, P., Sawa, A., and Roder, J. C. (2010). Enhanced dopamine function in DISC1-L100P mutant mice: implications for schizophrenia. Genes Brain Behav. 9, 777–789.
Lyttelton, O., Boucher, M., Robbins, S., and Evans, A. (2007). An unbiased iterative group registration template for cortical surface analysis. Neuroimage 34, 1535–1544.
MacDonald, D. (1998). A Method for Identifying Geometrically Simple Surfaces from Three Dimensional Images. Montreal: McGill University.
Millar, J. K., Wilson-Annan, J. C., Anderson, S., Christie, S., Taylor, M. S., Semple, C. A., Devon, R. S., Clair, D. M., Muir, W. J., Blackwood, D. H., and Porteous, D. J.. (2000). Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum. Mol. Genet. 9, 1415–1423.
Mizrahi, R., Addington, J., Rusjan, P. M., Suridjan, I., Ng, A., Boileau, I., Pruessner, J. C., Remington, G., Hooule, S., and Wilson, A. A. (2012). Increased stress-induced dopamine release in psychosis. Biol. Psychiatry 71, 561–567.
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113.
Paus, T., Keshavan, M., and Giedd, J. N. (2008). Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 9, 947–957.
Rakic, P. (1995a). Radial versus tangential migration of neuronal clones in the developing cerebral cortex. Proc. Natl. Acad. Sci. U.S.A. 92, 11323–11327.
Rakic, P. (1995b). A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18, 383–388.
Raznahan, A., Lee, Y., Long, R., Greenstein, D., Clasen, L., Addington, A., Rapoport, J. L., and Giedd, J. N. (2011a). Common functional polymorphisms of DISC1 and cortical maturation in typically developing children and adolescents. Mol. Psychiatry 16, 917–926.
Raznahan, A., Shaw, P., Lalonde, F., Stockman, M., Wallace, G. L., Greenstein, D., Clasen, L., Gogtay, N., and Giedd, J. N. (2011b). How does your cortex grow? J. Neurosci. 31, 7174–7177.
Robbins, S., Evans, A. C., Collins, D. L., and Whitesides, S. (2004a). Tuning and comparing spatial normalization methods. Med. Image Anal. 8, 311–323.
Robbins, S. M., Adviser-Whitesides, S., and Adviser-Evans, A. (2004b). Anatomical Standardization of the Human Brain in Euclidean 3-Space and on the Cortical 2-Manifold. Montreal: McGill University.
Saha, S., Chant, D., Welham, J., and McGrath, J. (2005). A systematic review of the prevalence of schizophrenia. PLoS Med. 2, e141.
Seeman, P. (2009). Glutamate and dopamine components in schizophrenia. J. Psychiatry Neurosci. 34, 143–149.
Sled, J. G., Zijdenbos, A. P., and Evans, A. C. (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging 17, 87–97.
Szeszko, P. R., Hodgkinson, C. A., Robinson, D. G., Derosse, P., Bilder, R. M., Lencz, T., Burdick, K. E., Napolitano, B., Betensky, J. D., Kane, J. M., Goldman, D., and Malhotra, A. K. (2008). DISC1 is associated with prefrontal cortical gray matter and positive symptoms in schizophrenia. Biol. Psychol. 79, 103–110.
Thomson, P. A., Harris, S. E., Starr, J. M., Whalley, L. J., Porteous, D. J., and Deary, I. J. (2005). Association between genotype at an exonic SNP in DISC1 and normal cognitive aging. Neurosci. Lett. 389, 41–45.
Tomppo, L., Hennah, W., Miettunen, J., Jarvelin, M. R., Veijola, J., Ripatti, S., Lahermo, P., Lichtertmann, D., Peltonen, L., and Ekelund, J. (2009). Association of variants in DISC1 with psychosis-related traits in a large population cohort. Arch. Gen. Psychiatry 66, 134.
Voineskos, A. N., Lerch, J. P., Felsky, D., Tiwari, A., Rajji, T. K., Miranda, D., Loubagh, N. J., Pollock, B. G., Mulsant, B. H., and Kennedy, J. L. (2011). The ZNF804A gene: characterization of a novel neural risk mechanism for the major psychoses. Neuropsychopharmacology 36, 1871–1878.
Voineskos, A. N., Rajji, T. K., Lobaugh, N. J., Miranda, D., Shenton, M. E., Kennedy, J. L., Pollock, B. J., and Mulsant, B. H. (2010). Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiol. Aging 33, 21–24.
Williams, J. M., Beck, T. F., Pearson, D. M., Proud, M. B., Cheung, S. W., and Scott, D. A. (2009). A 1q42 deletion involving DISC1, DISC2, and TSNAX in an autism spectrum disorder. Am. J. Med. Genet. A 149, 1758–1762.
Keywords: DISC1, striatum, striatal volume, mental illness, schizophrenia, bipolar disorder, depression
Citation: Chakravarty MM, Felsky D, Tampakeras M, Lerch JP, Mulsant BH, Kennedy JL and Voineskos AN (2012) DISC1 and striatal volume: a potential risk phenotype for mental illness. Front. Psychiatry 3:57. doi: 10.3389/fpsyt.2012.00057
Received: 03 April 2012; Accepted: 24 May 2012;
Published online: 19 June 2012.
Edited by:
Zafiris J. Daskalakis, University of Toronto, CanadaReviewed by:
Paul Croarkin, Mayo Clinic, USALuiz Kobuti Ferreira, Universidade de São Paulo, Brazil
Copyright: © 2012 Chakravarty, Felsky, Tampakeras, Lerch, Mulsant, Kennedy and Voineskos. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: M. Mallar Chakravarty, Centre for Addiction and Mental Health, 250 College Street, Toronto, ON, Canada M5T 1R8. e-mail: mallar_chakravarty@camh.net


 Maria Tampakeras3
Maria Tampakeras3


