
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Protistol. , 07 November 2023
Sec. Evolution and Physiology of Protists
Volume 1 - 2023 | https://doi.org/10.3389/frpro.2023.1293531
 Hideki Ishida1,2
Hideki Ishida1,2 Kousei Yamamoto2
Kousei Yamamoto2 Yuki Yano1
Yuki Yano1 Kenichi Ikeda3
Kenichi Ikeda3 Liudmyla Gaponova4
Liudmyla Gaponova4 Rina Higuchi5
Rina Higuchi5 Andrii Kolosiuk6
Andrii Kolosiuk6 Toshinobu Suzaki7*
Toshinobu Suzaki7*Water freeze-drying of biological samples has long been considered unfeasible as a sample preparation method for scanning electron microscopy (SEM) owing to the formation of damaging ice crystals. Contrary to this assumption, however, when live ciliates in water were frozen in contact with pre-cooled copper blocks in this study, they could be freeze-dried without artifact formation, although the success rate was only about 10%. This method offers several advantages over the traditional approach of chemical fixation followed by dehydration and drying. First, the degree of sample shrinkage associated with sample preparation was much lower than with chemical fixation. Second, contractile ciliates such as Spirostomum and Lacrymaria could be sampled in their elongated state, and the metachronal waves of cilia on the cell surface were well preserved. Moreover, this method requires no special equipment and only a few hours at most for sample preparation before SEM observation. Thus, although this method needs to be improved to increase the success rate in the future, it can be used to prepare samples for SEM observation that could not be prepared by other methods.
It has long been held that organisms with soft cells, such as protists, must be chemically fixed and dehydrated in order to be observed by scanning electron microscopy (SEM). Ciliates are protists with delicate cell surface structures and numerous cilia, thus making it difficult to prepare samples and preserve their fine surface structures. Various sample processing techniques have been recommended to preserve the surface structure of living protist cells, but none have been completely satisfactory (Foissner, 2014). He recommended Parducz’ fixative (mixture of HgCl2 and OsO4) as a good fixative for SEM observations of ciliates in general, but it does not always yield good results for all ciliate species. For example, he stated that a mixture of 2% OsO4 and 3% glutaraldehyde is a much better fixative for hypotrichous ciliates (Foissner, 2014). According to Barlow and Sleigh (1979), cryopreservation techniques (quick freezing and freeze-substitution) are recommended as more reliable alternatives to chemical fixation, both in terms of obtaining a much higher proportion of good results and in the improved preservation of ciliated surfaces. Recently, Seah et al. (2022) described that “strong” fixatives such as Parducz’ solution, Stieve’s solution (HgCl2), and Bouin’s solution (picric acid) are good at preserving ciliate morphology but have potential problems in terms of their hazards and additional costs associated with proper handling and disposal. Thus, there is a strong need for improved sample preparation methods for SEM observation of protists.
The water freeze-drying method for SEM preparation of ciliates was first introduced by Suzaki et al. (1978) as a drying method for chemically fixed specimens, taking advantage of the fact that rapid freezing of water in a supercooled state reduces the formation of large ice crystals. Freezing in supercooled conditions results in smaller, more homogeneous ice crystals, preventing recrystallization into larger ice crystals (Kobayashi et al., 2018). The size of the ice crystals is known to depend on the degree of supercooling (LeBail et al., 2002). Water freeze-drying after fixation with glutaraldehyde and osmium tetroxide has been successfully performed on the ciliate Paramecium caudatum (Suzaki et al., 1978) and the heliozoan Echinosphaerium nucleofilum (Suzaki et al., 1980). Recently, we reported that water freeze-drying of unfixed, live Spirostomum cells allows SEM observation of the cells in their elongated state (Ishida et al., 2022a; Ishida et al., 2022b).
Ciliates such as Spirostomum and Stentor strongly contract their cell bodies in a calcium-dependent manner upon simple mechanical stimulation. These ciliates also readily contract their cell bodies when chemical fixatives are added. Therefore, to observe cells in an elongated state, they must be treated with very high concentrations of divalent cation chelating agents prior to fixation. However, the effect of such pretreatment on the ultrastructure of ciliates cannot be ignored (Huang and Pitelka, 1973; Ishida et al., 1996). Thus, a better sample preparation method that allows to preserve the form, size and ultrastructure of protist cells for electron microscopic observation has been sought. In the water freeze-drying method we developed, the sample suspension is rapidly frozen by bringing the cell suspension into contact with a block of copper that has been previously cooled at -80°C. Although the success rate of this method was only about 10%, we succeeded in preparing some samples with no deformation of the cells and no significant artifacts.
This article presents the results of using the water freeze-drying method for various species of freshwater ciliates (Spirostomum ambiguum, Paramecium caudatum, P. bursaria, Euplotes aediculatus, and Lacrymaria olor), which confirm the success of using this method for the study of protists with soft cells. The water freeze-drying method is compared with the traditional dehydration method and its main advantages over the latter are highlighted.
S. ambiguum (strain GJ01) was originally collected from a pond in Hiroshima Prefecture, Japan (34°23’31.0”N 132°18’52.5”E), and cultured in 0.01% Knop medium (0.24 mM Ca(NO3)2, 0.14 mM KNO3, 0.06 mM MgSO4, and 0.1 mM KH2PO4) with boiled wheat grains in a 9 cm Petri dish at 22°C. Cultures were grown in the dark, and a small amount of wheat infusion solution (adjusted to pH 7.0) was added to the culture medium once a week. Subculturing was conducted at intervals of approximately 2–4 weeks. P. bursaria (strain PbKb1, originally collected in Kobe, Japan), P. caudatum (strain H1, originally collected in Hiroshima, Japan), and E. aediculatus (strain Md1, originally collected in Okinawa, Japan) were cultured in 0.01% Knop medium, and the food Chlorogonium capillatum (strain CE, collected in Osaka, Japan) was supplied to the ciliate cultures twice weekly. C. capillatum was cultured axenically in 0.1% Na-acetate and 0.5% yeast extract. L. olor was obtained through the courtesy of Dr. Seiji Sonobe of the University of Hyogo, Japan.
Living cells were first suspended in distilled water. All ciliates used in this study were found in freshwater ponds with very low solute concentrations; thus, placing them in distilled water for up to 24 h had no effect on their cell morphology or motility (unpublished observations). A 12 mm diameter disk-shaped glass coverslip was glued to the top surface of an aluminum sample stub (15 mm diameter, 10 mm height), and a ring-shaped spacer (8–9 mm outer diameter, 3–6 mm inner diameter, 0.5 mm thickness) made of silicone rubber or copper was placed on top (Figure 1). A small amount of the sample suspension (4-15 μL) was placed in the spacer ring such that the sample surface was slightly raised above the top level of the spacer. The sample was then frozen by bringing it into contact with a copper metal block (20 mm diameter, 30 mm height) pre-cooled to approximately -80°C in a deep freezer or on dry ice (YouTube video at https://youtube.com/shorts/2AD-kTd9pG8). The frozen sample on the sample stub was allowed to stand for at least 1 min to ensure temperature reduction before the Cu block was removed. The sample stub was immediately transferred to a freeze-dryer (VFD-21S; Vacuum Device, Mito, Japan) and the sample was freeze-dried at a temperature setting of 0°C. The dried samples were sputter-coated with osmium (Neoc-Pro; Meiwafosis Co. Ltd., Tokyo, Japan) and observed using a field emission-scanning electron microscope (Hitachi S-4800; Tokyo, Japan). The total time required from cell freezing to SEM observation was only 2–3 h.

Figure 1 Schematic diagram of the sample stub. A suspension of cells is added to a spacer ring made of silicone rubber or copper placed on top of a disk-shaped coverslip and the sample stub. The sample suspension is frozen by placing the prepared sample stub in contact with a pre-cooled metal block from above.
For comparison, samples of P. caudatum were prepared by the conventional method of chemical fixation (a slight modification of Song et al., 2017) and freeze-drying with t-butyl alcohol (t-buOH) using the following method. First, cells were suspended in 0.01% Knop medium and collected by centrifugation at 500 × g. This procedure was repeated twice to wash the cells. The cells were then suspended in 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer adjusted to pH 7.0, concentrated by centrifugation, and fixed in 3% glutaraldehyde in 50 mM HEPES buffer (pH 7.0) for 10 min at 20–25°C. Subsequently, the cells were collected by free sedimentation to prevent cell-surface damage. Collected cells were washed three times in 50 mM HEPES buffer (pH 7.0) for 5 min each, followed by post-fixation in 1% OsO4 in 50 mM HEPES buffer (pH 7.0) for 10 min. The cells were then washed three times with 50 mM HEPES buffer (pH 7.0) and dehydrated in a graded ethanol series (50, 60, 70, 80, 90, 95, 99, 100% × 2 and 5 min each). They were then placed in a 1:1 (v/v) solution of t-buOH:100% ethanol for 15 min, and finally in 100% t-buOH for 15 min. Cells suspended in t-buOH were then lowered in temperature, frozen, and freeze-dried in a t-butyl alcohol dryer (VFD-21S; Vacuum Devices). Subsequent manipulations were the same as for the water freeze-drying method. The total time required from fixation to SEM observation was >10 h.
Changes in cell size due to water freeze-drying were examined in comparison with the conventional sample preparation method (chemical fixation and t-buOH freeze-drying). P. caudatum was used as the model organism for this experiment because of its shape, which is suitable for estimating cell volume based on length along the long axis and width at the cell center. Cell size measurements were performed on cells 1) that were alive, 2) immediately after chemical fixation, 3) after ethanol dehydration, 4) after t-buOH freeze-drying, and 5) after direct water freeze-drying of live cells. Measurements of cells described in 1–3 above were made using micrographs taken with an optical microscope, and those of cells described in 4 and 5 were made using SEM images. The length and width of the cells were measured using Image-J software. Welch’s t-test was used for statistical comparisons between the mean values of estimated cell volumes at different stages of sample preparation.
By applying the water freeze-drying method, Paramecium caudatum was successfully dried for SEM observation (Figures 2, 3). Comparing the water freeze-drying method with the conventional method, it was found that the metachronal waves of the cell surface cilia were well preserved using the water freeze-drying method (Figure 2). On the other hand, in samples freeze-dried using t-buOH, cilia were observed to rise in a disordered direction from the cell surface, which may be an artifact of chemical fixation. Stereo-pair photographs (cross-eyed method) of P. caudatum prepared using the water freeze-drying method are shown in Figure 3. The images reveal that the cells were not crushed and the three-dimensional arrangement of cilia on the cell surface could be well observed. For Paramecium bursaria and Euplotes aediculatus, similar results were obtained as for P. caudatum. Figures 4A, B show that cell size and overall shape are normal, and also metachronal waves of cilia on the cell surface were also well preserved for Paramecium bursaria. By using the water freeze-drying method, we succeeded for the first time in observing the proboscis of Lacrymaria olor in the elongated state without special pretreatment (Figure 4C). L. olor is a ciliate with a contractile proboscis; like Spirostomum and Stentor, the proboscis and cell body are easily contracted by chemical fixation. Living Lacrymaria cells frequently extend and retract their proboscis as a food-seeking behavior. An image of cells in the contracted state is shown in Figure 4D.
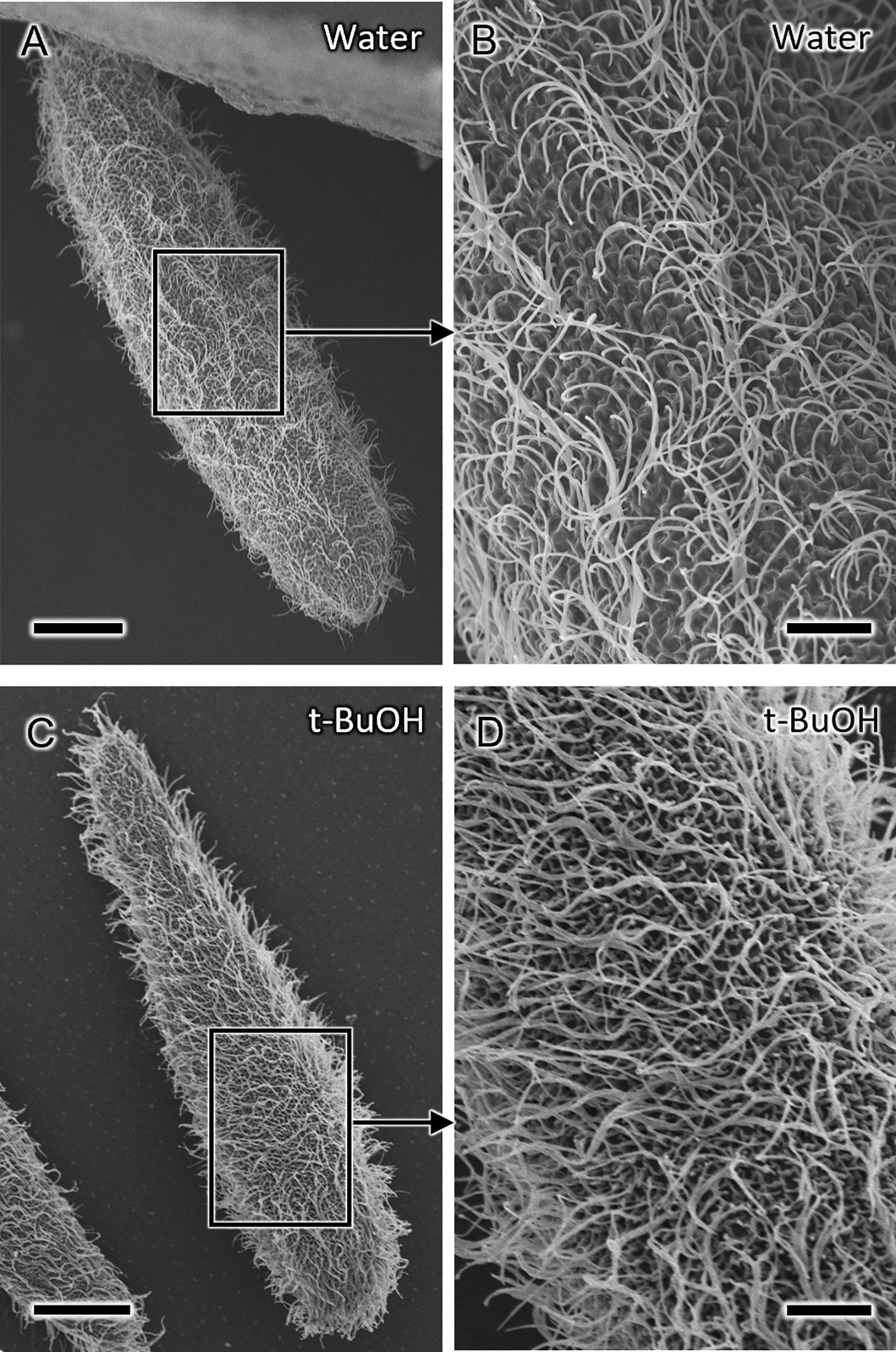
Figure 2 Scanning electron microscopy (SEM) micrographs of Paramecium caudatum prepared by different drying methods. (A, B) Cells prepared by water freeze-drying. (B) Magnified view of the area indicated by the square in (A) showing well-preserved metachronal waves of the cilia. (C, D) Cells chemically fixed, dehydrated with ethanol, and freeze-dried with t-butyl alcohol (t-buOH). Scale bars are 20 μm (A, C) and 5 μm (B, D).
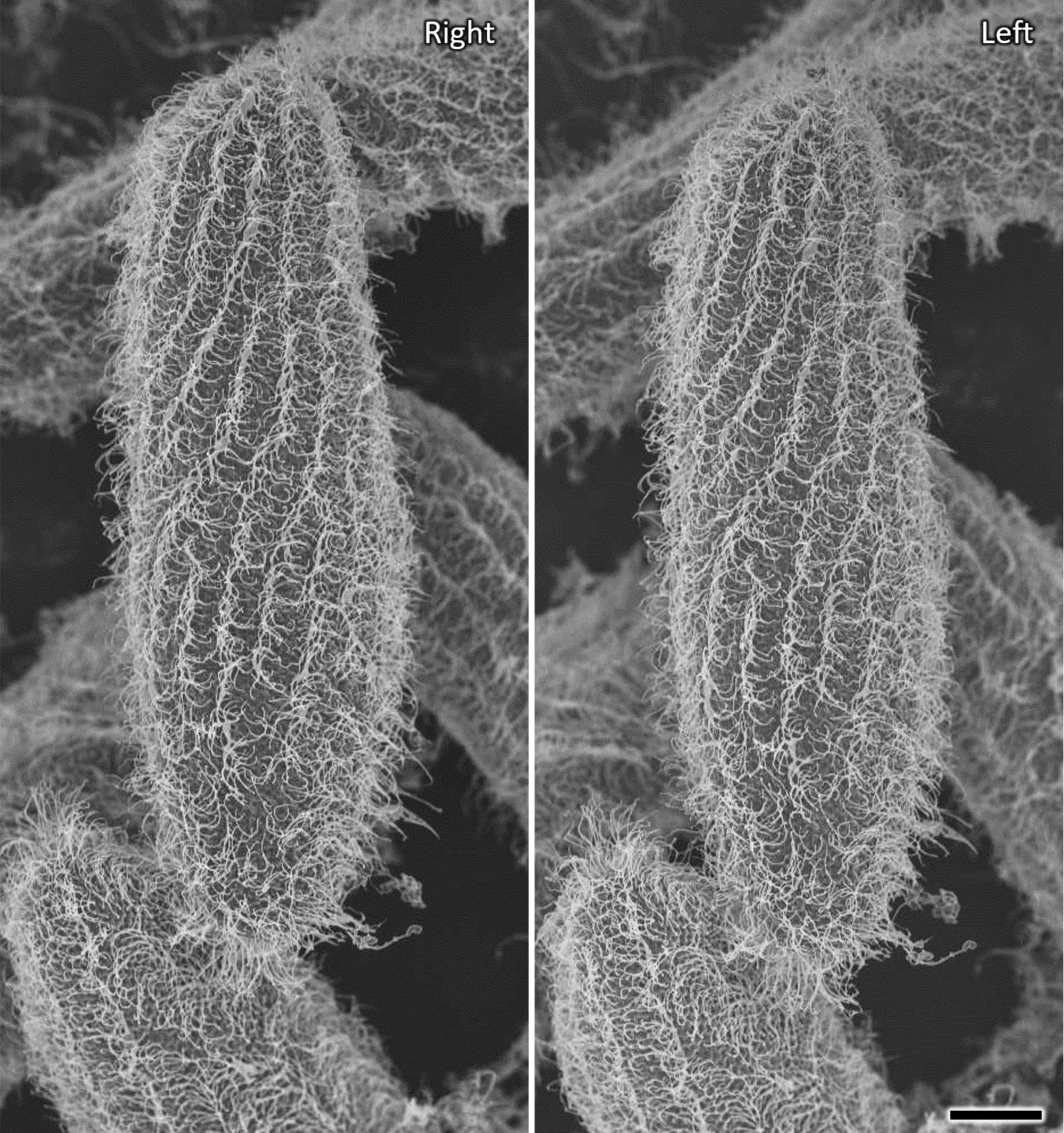
Figure 3 Stereo SEM micrographs of Paramecium caudatum prepared by water freeze-drying showing details of the metachronal waves of the cilia in three dimensions (cross-eyed stereo-pair images). Viewing angles differ by 5 degrees. Scale bar indicates 20 μm.
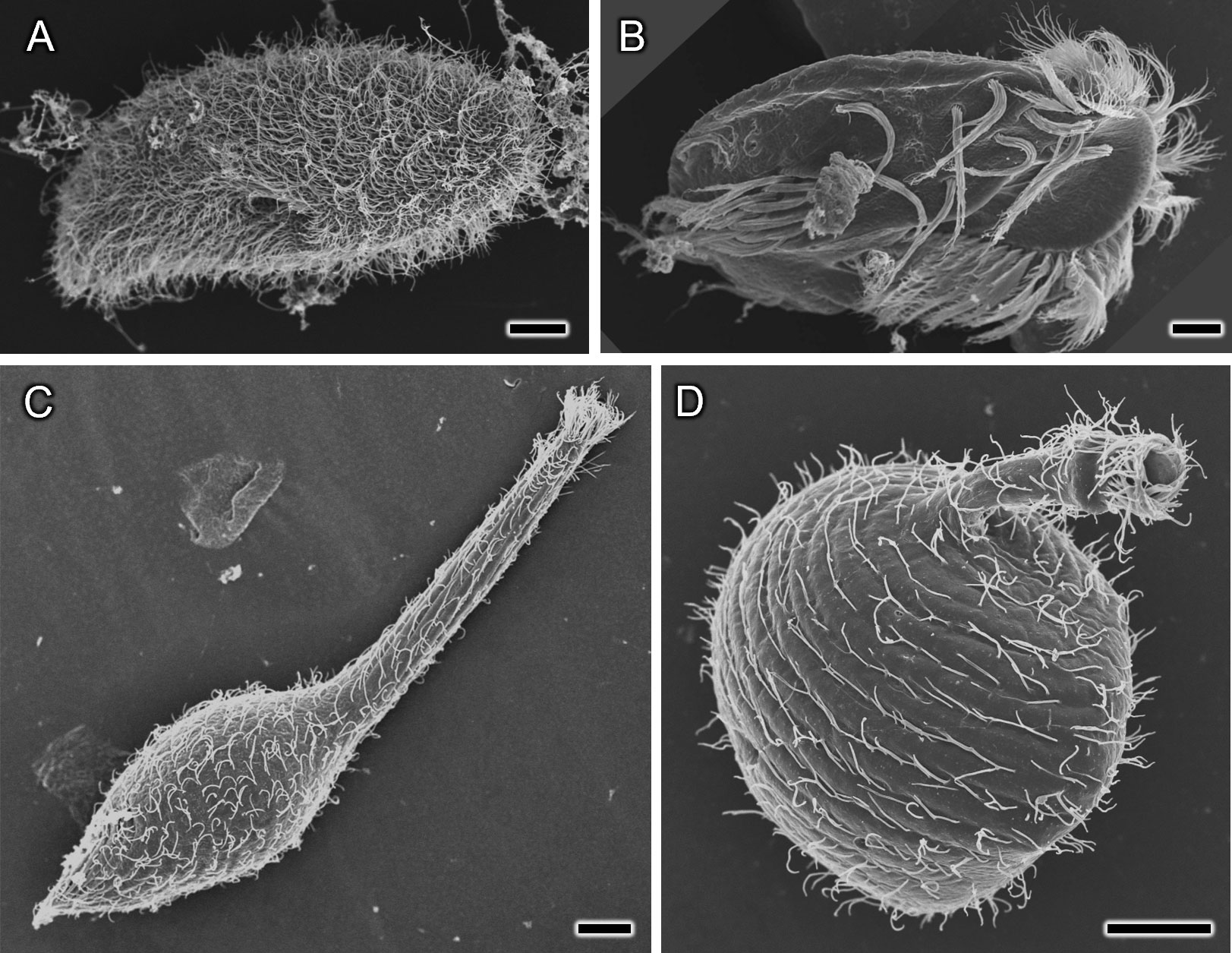
Figure 4 SEM photographs of various ciliates prepared using the water freeze-drying method. (A) Paramecium bursaria, (B) Euplotes aediculatus, (C, D): Lacrymaria olor in which the proboscis is in the elongated state (C) and the shortened state (D). Scale bars indicate 10 μm.
Also, water freeze-drying method allows observing cell organelles. For this purpose, after freezing, the sample was lightly pressed down from above by a copper block to create cracks. An SEM image of S. ambiguum in a contracted state with a fractured surface is shown in Figure 5. No noticeable signs of ice crystal formation can be seen inside the cell, whereas structures that appear to be a macronucleus and a contractile vacuole are observed (Figure 5A). In addition, magnified images of the cytoplasm show many scattered granular structures ~1 µm in diameter (arrows in Figure 5B), which are probably mitochondria. However, the identification of these cellular structures and the extent of ice crystal formation require verification by transmission electron microscopy of ultrathin sections made using the freeze-substitution technique.
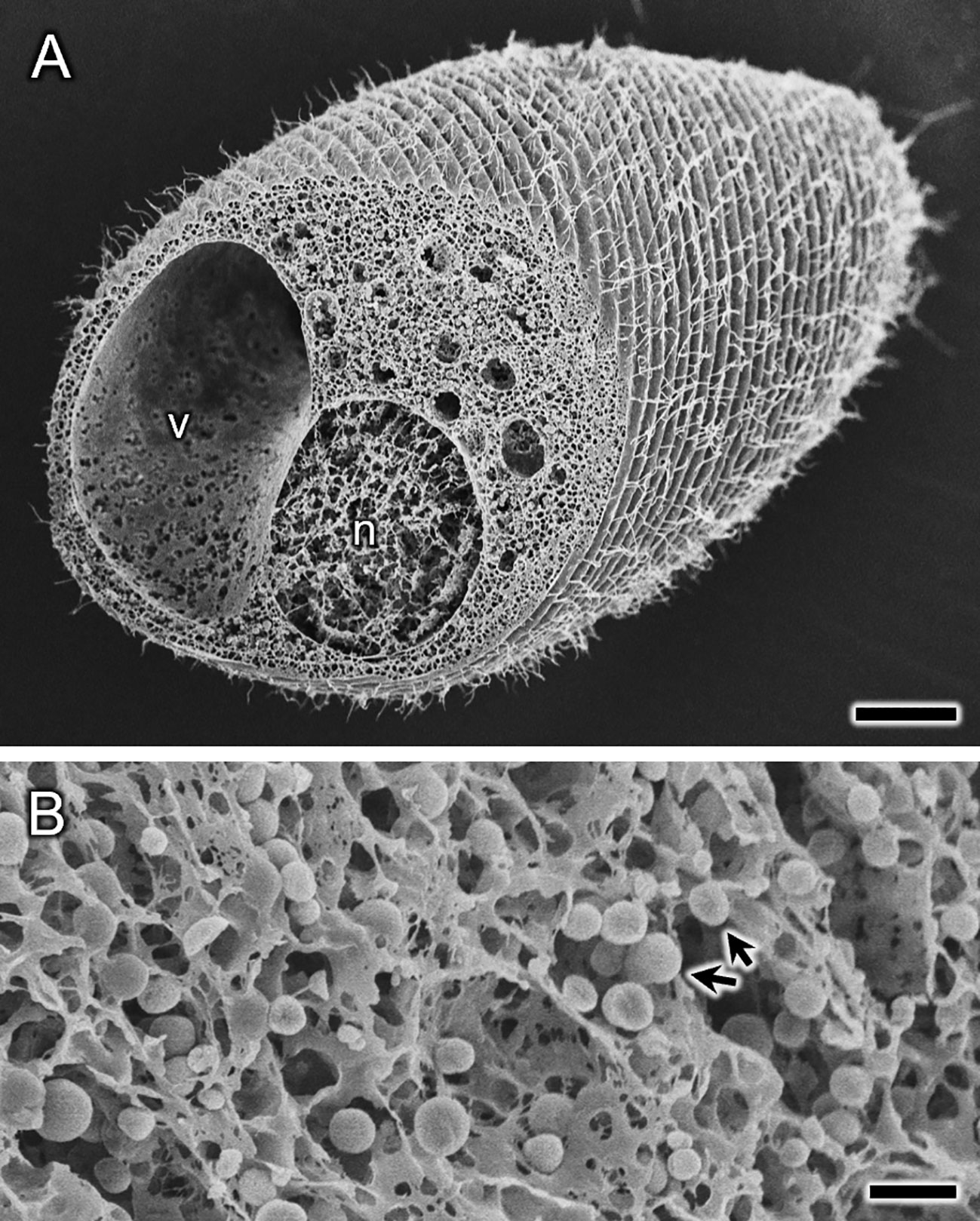
Figure 5 Spirostomum ambiguum in a contracted state prepared using the water freeze-drying method. Cells were contracted before freezing on the sample stub. Not only the cell surface, but also the interior of the cell (A) appears to be frozen with no significant ice crystal formation. The many small spherical structures (arrows) with diameters of ~1 μm seen in the magnified image of the fractured surface (B) are probably mitochondria, but future confirmation is needed. In (A), large structures labeled n and v appear to be the macronucleus and the contractile vacuole, respectively, which also need to be confirmed. Scale bars indicate 20 μm (A) and 2 μm (B).
To compare methods of sample preparation with chemical fixation and without its application, and determining the effectiveness of these techniques, we examined the cell size at different stages of sample preparation (Table 1; Figure 6). First, in living cells, the length along the long axis of the cell and the width at the center of the cell were measured (Figure 6A). Cells belonging to the same cell population were then chemically fixed with glutaraldehyde and OsO4, and subsequent cell size was examined in the same manner (Figure 6B). Samples were then dehydrated with ethanol (Figure 6C) and subsequently freeze-dried with t-buOH (Figure 6D). Samples taken from the same cell population, unfixed, and water freeze-dried, were also measured for cell size (Figure 6E). The data (Table 1; Figure 6F) showed that cell shrinkage began at the chemical fixation stage and was more pronounced during the dehydration process. Assuming that the cell shape of P. caudatum is a prolate spheroid, the volume of the cells after t-buOH drying was reduced to about 25% of that in the living state. In cells subjected to water freeze-drying, on the other hand, the length of the cells was slightly shortened and their width was slightly increased (Table 1), but the estimated volume of the cells was almost equal to that of the live state. No significant differences in mean values were observed (Figure 6F). Thus, the water freeze-drying method was found to be a better method for preparing samples for SEM while preserving cell shape and size. However, a major drawback of the water freeze-drying method was that only about 10% of the cells were observed to not have artifacts. In many cases, the cells preserved their size or overall shape, but some areas of the cell surface appeared to be externally compressed or had detached cilia, as illustrated in Figure 7. Thus, the percentage of cells that appeared to contain even a small number of artifacts accounted for about 90% of the total.
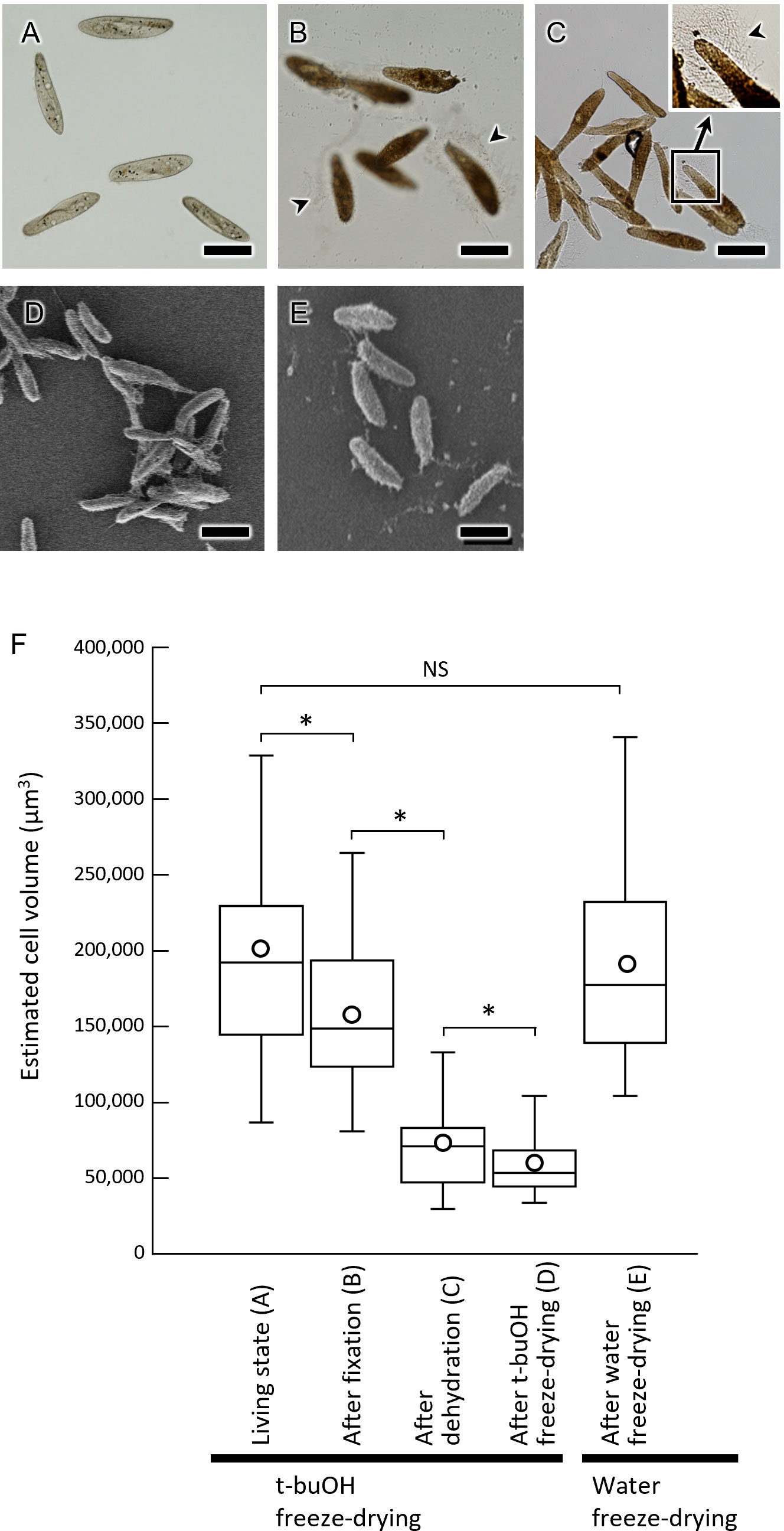
Figure 6 Cell size of Paramecium caudatum at various stages of SEM sample preparation. (A–C) Light micrographs of P. caudatum. (A) live cells, (B) after chemical fixation, and (C) after ethanol dehydration. (D, E) SEM images of P. caudatum. (D) t-buOH freeze-dried cells after chemical fixation and ethanol dehydration. (E) Water freeze-dried cells without fixation. Scale bars indicate 100 μm. (F) Box-and-whisker plots show, from left to right, the distribution of cell volume in live cells, after chemical fixation, after ethanol dehydration, after t-buOH freeze-drying, and after water freeze-drying. Cell volume was estimated by assuming cells to be prolate spheroids and calculated using the cell length and width measurements (see Table 1). Boxes indicate median and interquartile ranges. Vertical lines above and below the boxes indicate maximum and minimum values, respectively. Mean values are shown as circles. NS, no significant difference, *p < 0.01 (Welch’s t-test).
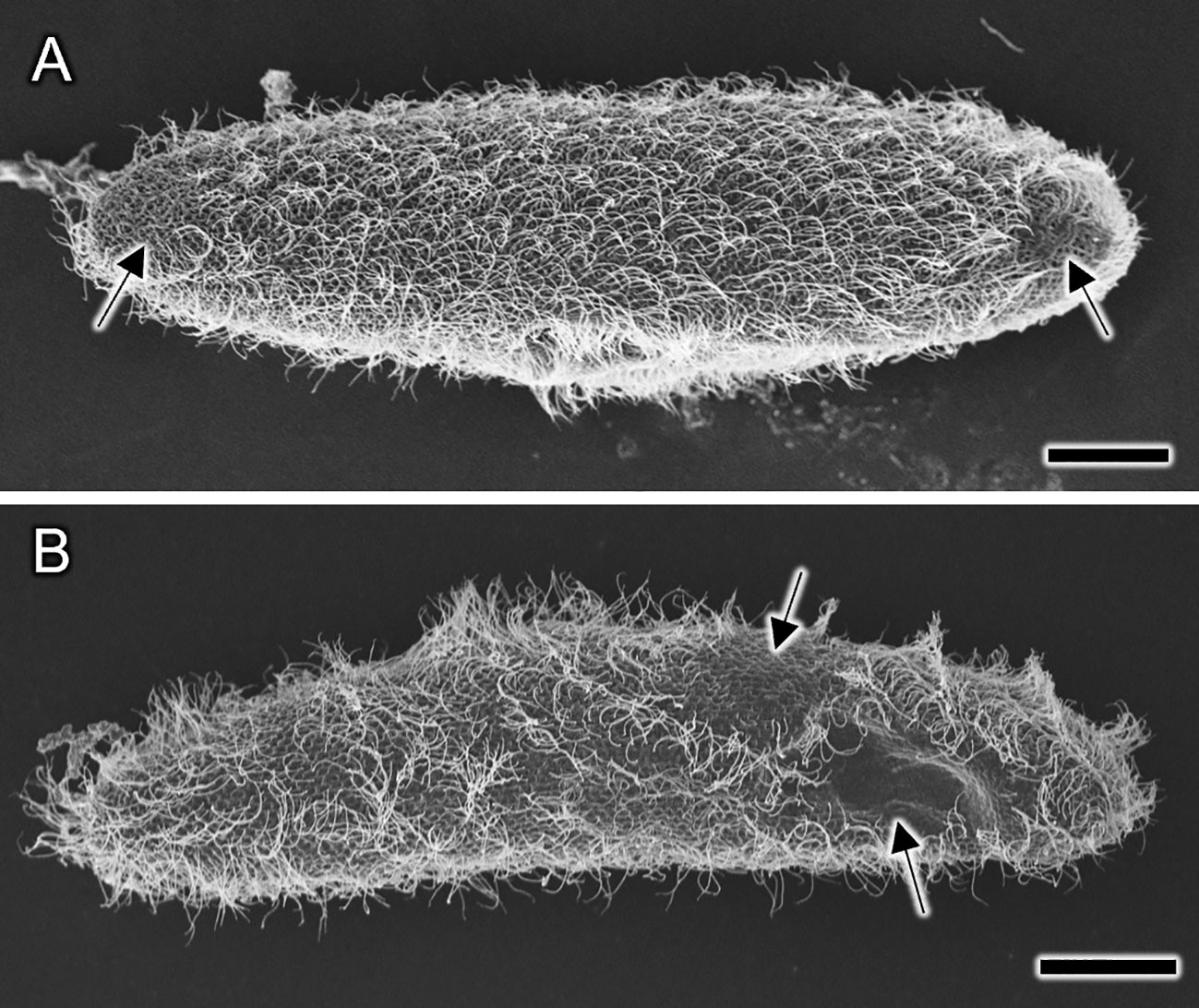
Figure 7 (A, B) Representative examples of SEM images of Paramecium caudatum prepared by water freeze-drying, illustrating artifacts that may have occurred during the freezing process. Cell size and overall shape are normal. However, there are some areas on the cell surface (arrows) that appear to be damaged by external pressure, from which most of the cilia are detached. Scale bars indicate 20 μm.
The main feature of this method is the rapid freezing of water in a supercooled state. It minimizes the formation of ice crystals, thereby allowing the sample to be dried without fixation. Moreover, if a portable cold storage unit with dry ice is available, sample preparation would be possible even in the field or away from the laboratory.
The water freeze-drying method appears to have advantages over the traditional method of dehydration and drying after chemical fixation. First, the degree of sample shrinkage was much lower than with chemical fixation. Second, contractile ciliates could be sampled in their elongated state, and the metachronal waves of cilia on the cell surface are well preserved. Third, only a few hours at most for sample preparation before SEM observation is required. Forth, no special additional equipment or chemicals required. Fifth, accurate elemental analysis of the sample would be possible because SEM observation can be performed without modifying the composition or quantity of elements contained in the sample.
Although this method has many advantages, it also has some disadvantages. The main is only 10% of the samples dried using this method exhibited no artifacts. Nevertheless, it is not critical when we study protist morphology using a monoculture that contains many identical cells.
Various artifacts were observed when we used the water freeze-drying method. The main artifacts that have been found during the examination of the S. ambiguum are described in detail in our previous paper (Ishida et al., 2022b). About 60% of the cells showed traces of pressure stress from one side to various degrees, and about 30% of the cells showed large cell deformations, probably due to the formation of ice crystals. Many artifacts were found on the top surface of the cells, and fewer artifacts were observed on the bottom of the cells near the surface of the specimen stub. A typical example of artifacts is shown in Figure 7, where traces of externally applied pressure on cell P. caudatum are presented. The causes of these artifacts are discussed in association with Figure 8. Stage I is the cell suspension before freezing. When a cooled metal block is pressed against the cell suspension from above, the water inside the container may gradually solidify from the surface that first contacts the metal block (Stages II and III). At this time, in the first freezing area (Space A), freezing progresses gradually due to the generation of solidification heat, and freezing may finally pause. Cells in this region (e.g., Cell 3) would show significant damage from ice crystal formation. Cells located at the border between Space A and Space B (Cell 1) would also be crushed by the gradually forming ice only at its top. The liquid phase (Space B) left behind during the initial freezing would also eventually freeze (Stage III). But this area would become supercooled and might eventually freeze very rapidly. It is known that increasing the water freezing rate reduces ice crystal formation (Kobayashi et al., 2018) and this may explain the absence of artifacts in the area near the surface of the sample stub. The fact that about 10% of the cells showed no artifacts at the end of the drying stage (Stage IV) may indicate that this percentage may reflect the proportion of space B to the total volume.
The above considerations will need to be tested in the future, but assuming this scenario is reasonable, the following modifications may be possible to increase the success rate of water freeze-drying.
1. Increase the volume fraction of Space B. This can be achieved by optimizing the height and size of the sample space, as well as the cooling temperature of the cooling metal block.
2. Accumulate the cells in Space B (i.e., near the surface of the sample stub) as much as possible. For this purpose, it may be possible to guide the cells to the bottom of the water by modifying their swimming in some physiological way (e.g., by exposing the cells to low temperature for a short time or by using some kind of tactic behavior [phototaxis, galvanotaxis, or thermotaxis] of the protist).
As mentioned above, one of the advantages of water freeze-drying is that intracellular components can be preserved without chemical modification or leakage toward the cell exterior. Therefore, the intact elemental composition of the cell surface and interior can be analyzed using an elemental analyzer equipped with a scanning electron microscope. Cellular DNA and RNA would also be preserved in a less damaged state. While observing the external morphology, specific cells of interest could be selected using a focused ion beam scanning electron microscope, cut out and removed from the SEM. As a result, it would be possible to analyze the genetic composition of a particular cell. The water-freezing method may also be used to prepare samples for transmission electron microscopy. Freeze-substitution is a well-known preparation technique for transmission electron microscopy in which rapidly frozen cells are dehydrated and fixed at low temperature. Cells are usually frozen by high-pressure freezing or metal-contact freezing, both of which require expensive equipment and skilled techniques. The water-freezing method introduced here is a simple procedure and requires no special equipment, but it has not proven effective in preserving the ultrastructure within the cells. However, the image of the fractured cell surface shown in Figure 5 suggests some possibility, and future studies on this point are needed.
In this study, water freeze-drying was applied to various fresh-water ciliates (P. caudatum, P. bursaria, E. aediculatus, L. olor, and S. ambiguum) for SEM without fixing the cells. The results showed that this method is superior to the conventional method of preparing specimens using chemical fixation and t-buOH freeze-drying, with much less cell shrinkage and deformation of the specimens. Metachronal waves of cilia on the cell surface as well as form and size of cell were also well preserved. It was also shown that this method may be suitable for observing the cell interior. However, because only 10% of the samples dried using this method exhibited no artifacts, the method needs to be improved to increase the success rate. Nevertheless, this is not critical when studying protist morphology using cultures.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The manuscript presents research on animals that do not require ethical approval for their study.
HI: Conceptualization, Data curation, Investigation, Supervision, Writing – review & editing. KY: Data curation, Investigation, Writing – review & editing. YY: Investigation, Writing – review & editing. KI: Conceptualization, Supervision, Writing – review & editing. LG: Methodology, Writing – review & editing. RH: Writing – review & editing. AK: Methodology, Writing – review & editing. TS: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by JSPS KAKENHI (grant number JP21K19168).The authors thank Dr. Seiji Sonobe of the University of Hyogo for providing living samples of Lacrymaria olor.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors TS, KI, HI, declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Barlow D. I., Sleigh M. A. (1979). Freeze substitution for preservation of ciliated surfaces for scanning electron microscopy. J. Microscopy 115, 81–95. doi: 10.1111/j.1365-2818.1979.tb00154.x
Foissner W. (2014). An update of ‘basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa’. Int. J. Syst. Evol. Microbiol. 64, 271–292. doi: 10.1099/ijs.0.057893-0
Huang B., Pitelka D. R. (1973). The contractile process in the ciliate, Stentor coeruleus. I. The role of microtubules and filaments. J. Cell Biol. 57, 704–728. doi: 10.1083/jcb.57.3.704
Ishida H., Matsumoto C., Shimada M., Suzaki T. (2022a). SEM observation of non-fixed and water freeze-dried Spirostomum ambiguum. Eur. J. Protistol. 85, 125896. doi: 10.1016/j.ejop.2022.125896
Ishida H., Matsumoto C., Shimada M., Suzaki T. (2022b). Use of water freeze-drying to prepare unfixed ciliates for scanning electron microscopy. J. Electr. Microsc. Tech. Med. Biol. 35, 1–7.
Ishida H., Suzaki T., Shigenaka Y. (1996). Cell body contraction of Spirostomum does not involve shortening of inter-kinetosomal distance along ciliary lines. Zool. Sci. 13, 669–672. doi: 10.2108/zsj.13.669
Kobayashi R., Kimizuka N., Watanabe M., Takenaga F., Suzuki T. (2018). Property changes during frozen storage in frozen soybean curds prepared by freezing accompanied with supercooling. Trans. Jpn. Soc Refrig. Air. Cnd. Eng. 35, 269–276. doi: 10.11322/tjsrae.18-25FB
LeBail A., Chevalier D., Mussa D. M., Ghoul M. (2002). High pressure freezing and thawing of foods: A review. Int. J. Refrig. 25, 504–513. doi: 10.1016/S0140-7007(01)00030-5
Seah B. K. B., Emmerich C., Singh A., Swart E. C. (2022). Improved methods for bulk cultivation and fixation of Loxodes ciliates for fluorescence microscopy. Protist 173, 125905. doi: 10.1016/j.protis.2022.125905
Song C., Murata K., Suzaki T. (2017). Intracellular symbiosis of algae with possible involvement of mitochondrial dynamics. Sci. Rep. 7, 1221. doi: 10.1038/s41598-017-01331-0
Suzaki T., Shigenaka Y., Toyohara A., Otsuji H. (1978). A simplified freeze-drying technique for protozoan cells. J. Electron. Microsc. (Tokyo). 27, 153–156. doi: 10.1093/oxfordjournals.jmicro.a050108
Keywords: water freeze-drying, ciliate, dehydration, supercooling, paramecium
Citation: Ishida H, Yamamoto K, Yano Y, Ikeda K, Gaponova L, Higuchi R, Kolosiuk A and Suzaki T (2023) Method of preparing unfixed ciliates for scanning electron microscopy without noticeable artifacts. Front. Protistol. 1:1293531. doi: 10.3389/frpro.2023.1293531
Received: 13 September 2023; Accepted: 24 October 2023;
Published: 07 November 2023.
Edited by:
Federico Buonanno, University of Macerata, ItalyReviewed by:
Santosh Kumar, Zoological Survey of India, IndiaCopyright © 2023 Ishida, Yamamoto, Yano, Ikeda, Gaponova, Higuchi, Kolosiuk and Suzaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshinobu Suzaki, VG9zaGlub2J1LnN1emFraUBwcm90aXN0b2xvZ3kuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.