
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Plant Sci., 12 February 2025
Sec. Crop and Product Physiology
Volume 16 - 2025 | https://doi.org/10.3389/fpls.2025.1547174
This article is part of the Research TopicOlive ScienceView all 21 articles
Local olive germplasm of the southern Levant includes wild populations of var. sylvestris and local traditional cultivars that are thought to be well-adapted to the region’s arid conditions. By controlling water availability, we tested the response of the Barnea cultivar, two local traditional cultivars (MLL1 and MLL7) and var. sylvestris to low (100%), moderate (33%), and severe (10%) evapotranspiration (ETa) conditions. Measurements of stomatal conductance, relative water content, stem water potential, and the net photosynthesis showed a stronger response of the Barnea cultivar to reduced ETa conditions in comparison to the other three investigated groups. Additionally, when exposed to 100% ETa, the net photosynthesis capacity of MLL1 was significantly higher than that measured in MLL7. Therefore, net photosynthesis, as an indicator of tree productivity, can explain the dominance of MLL1 (Souri cultivar) in local traditional orchards and the negligible abundance of MLL7 (unknown cultivar) as a fruit-bearing tree. Considering that climate change is already influencing olive cultivation, the results of this study stress the potential of the southern Levant local olive germplasm in maintaining sustainable olive horticulture.
Since antiquity, olive oil and table olives have been major components of the daily diet and culture of people in the Mediterranean, specifically in the eastern Mediterranean (Galili et al., 2021; Barazani et al., 2023). Thus, for thousands of years, olive orchards have been a major feature of the rural Mediterranean landscape. Data from the Food and Agriculture Organization of the United Nations (FAO) (https://www.fao.org/faostat/en/) indicates that there are currently 9.4 million hectares of olive orchards worldwide, with 90% concentrated in the Mediterranean Basin. In the southern Levant (modern Israel and the Palestinian Authority), most of the olive cultivation area is still based on traditional agriculture practices (92.5% of a total of 120,000 ha) (Dag et al., 2024). Traditional olive horticulture in this region, based on local cultivars growing under rain-fed conditions, is mostly limited to temperate semi-arid zones (≥350 mm annual rainfall) (Figure 1). Nevertheless, rain-fed orchards are expanding into more arid zones, and individual olive trees, along with relicts of ancient abandoned orchards, are scattered throughout the region and even in the Negev desert (Ashkenazi et al., 2018; Tepper et al., 2022). Moreover, new irrigation and fertilization agro-techniques have expanded olive cultivation zone into more arid regions (Dag et al., 2004).
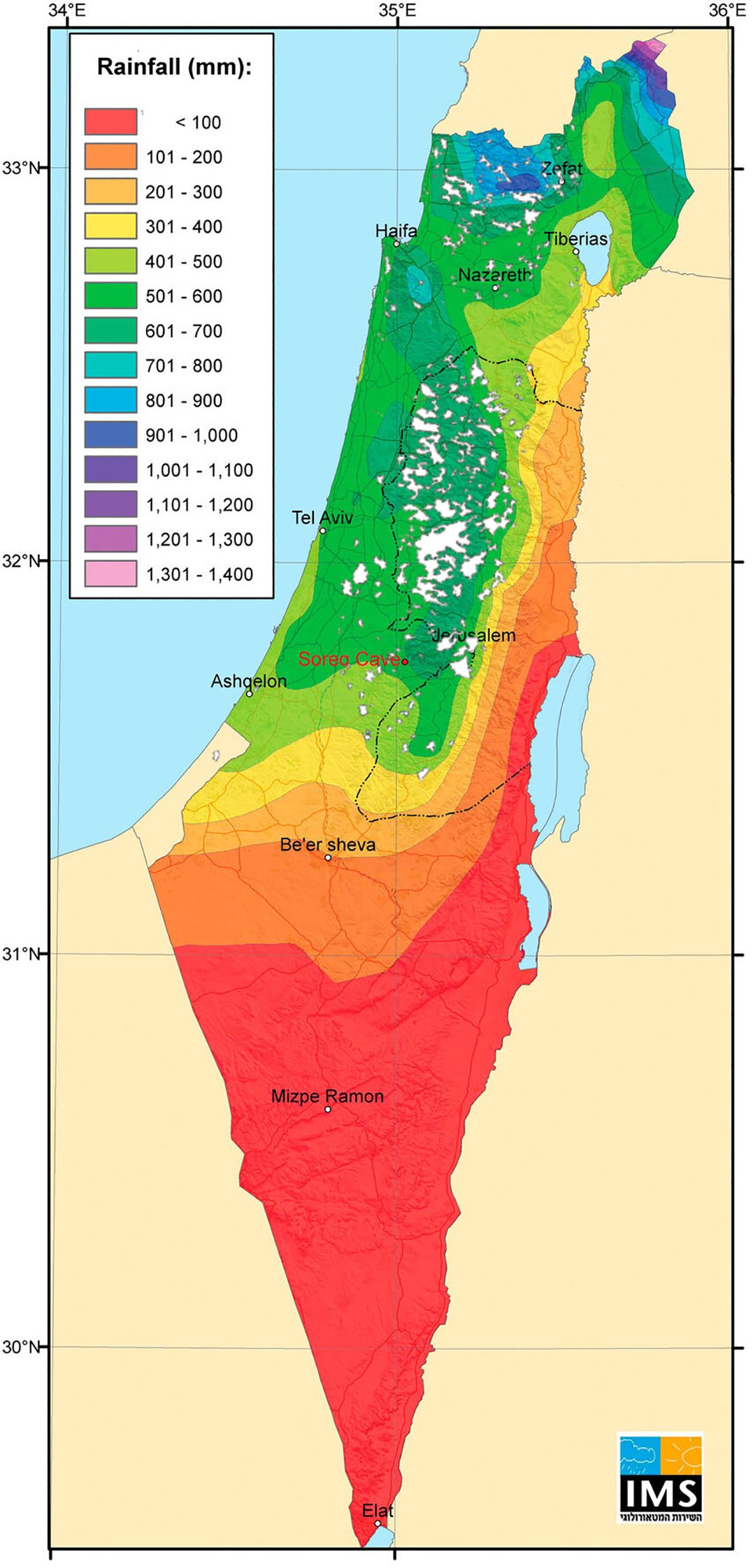
Figure 1. The spread of traditional olive groves in the Southern Levant. The map illustrates the distribution of olive orchards in 1935 (Rafael Frankel et al., 1994), overlaid with the region’s annual participation gradient (data source: the Israel Meteorological Service).
Using multi-locus linage analysis (MLL) of 14 microsatellite loci, Barazani et al. (2014) detected two main local cultivars among more than 300 old living trees: MLL1 and MLL7. The widespread abundance of MLL1 as a fruit-bearing tree confirmed its identification as the common Souri cultivar. In contrast, the nomenclature of MLL7 is still unknown (Barazani et al., 2014). Genetic analysis also indicated that the majority of rootstocks (55%) of old olive trees originated from sexually reproduced saplings (Barazani et al., 2014), most likely collected in the wild from Olea europaea subsp. europaea var. sylvestris (Barazani et al., 2016).
Several populations of var. sylvestris, the wild ancestor of cultivated olives, are still spread around the Mediterranean Basin (Barazani et al., 2016; Tourvas et al., 2023; Ben-Dor et al., 2024; Zunino et al., 2024). Moreover, studies have shown the utilization of var. sylvestris as a rootstock of grafted old olives in the southern Levant (Barazani et al., 2014, 2016), north-western Africa (Ater et al., 2016; Aumeeruddy-Thomas et al., 2017), and the Iberian Penisula (Díez et al., 2011). The genetic variation in wild populations and traditional cultivars is considered a valuable source of adaptive traits (Hannachi et al., 2009; Barazani et al., 2023, 2024). Thus, hypothesizing that local olive germplasm evolved to withstand the southern Levantine semi-arid conditions (Barazani et al., 2008), we aimed to test the response of MLL1 (Souri), MLL7, and local var. sylvestris to drought stress under controlled irrigation conditions.
Stem cuttings, 5 mm in diameter, were taken from trees of MLL1 and MLL7 growing in the Gilat germplasm collection (Barazani et al., 2023), and from three individuals of O. europaea subsp. europaea var. sylvestris growing naturally in Atlit (Ben-Dor et al., 2024). Stem cuttings were also collected from the Barnea cultivar (Gilat germplasm), to represent a local modern variety adapted for intensive irrigation practices (Lavee, 2009).
The cuttings were rooted under mist conditions as previously described (Dag et al., 2012) and then grown in 3 L pots with commercial potting media composed of coconut fibers, peat, and tuff (Deshanit, Israel). Pots were organized in a block design including 12 pots of each of the four investigated varieties. Water content was decreased to 33% and 10% ETa (actual evapotranspiration), which represents the sum of evaporation from the soil and plant surface along with transpiration. Daily measurement of pot weight were used to calculate daily ETa and subsequently control 33% and 10% ETa in each pot by irrigation. Each treatment group included four plants of each variety (MLL1, MLL7, Barnea, and var. sylvestris). The ETa treatments were maintained for three weeks during which physiological parameters were measured: Stem water potential (SWP) was measured at midday using a Scholander-type pressure chamber (MRC, Israel), and the leaf relative water content (RWC) was determined following Yamasaki and Dillenburg (1999). Measurements of net photosynthesis (µmol CO2 m−2 s−1) and stomatal conductance (mmole H2O m−2 s−1) were performed between 9:00 and 11:00 a.m. using a Licor 6400XT device (LI-COR Inc., Lincoln, NE, USA), keeping light intensity at 1000 µmol photon m−2 s−1 and ambient CO2 concentration at 400 µmole CO2 mol−1 air. Stomatal conductance was measured four times every 7 days during the course of the experiment, while net photosynthesis, SWP and RWC were determined 21 days after exposure to the different irrigation treatments.
Results in the figures are presented as mean ± standard errors (SE), and post-hoc statistical tests were conducted with JMP Pro 16.0.0 (SAS Institute Inc.).
In general, stomatal conductance measured in one-year old olive trees growing under 100% ETa was about two times significantly lower in MLL7 (0.18 ± 0.01 mmole H2O m−2 s−1) than in the other three investigated varieties (Figure 2A). The two low ETa treatments had a significant influence on MLL1 stomatal conductance, as was the case for Barnea and O. europaea subsp. europaea var. sylvestris, but not in MLL7 (ANOVA with repeated measures) (Figure 3). In MLL7, a significant 3.7-fold reduction in stomatal conductance, compared to 100% ETa, was only evident 21 days after exposure to severe drought (10% ETa) (Figure 3). In contrast, in the Barnea cultivar and MLL1, a significant reduction in stomatal conductance was measured already seven days after exposure to 33% ETa (Student’s t-test, P<0.05), and in O. europaea subsp. europaea var. sylvestris, a significant reduction in stomatal conductance was measured 14 days after exposure to both of the drought treatments (Figure 3). Additionally, MLL1, MLL7 and O. europaea subsp. europaea var. sylvestris maintained similar RWC (Figure 2B) and SWP (Figure 2C) in the three ETa treatments. However, in the Barnea cultivar, when exposed to 10% ETa, a significant 1.3- and 2.0-fold reduction was measured in RWC and SWP at the end of the experiment, respectively (ANOVA Tukey HSD, P<0.05) (Figures 2B, C). These results support previous findings showing the sensitivity of the Barnea cultivar to drought (Tugendhaft et al., 2016; Barzilai et al., 2021).
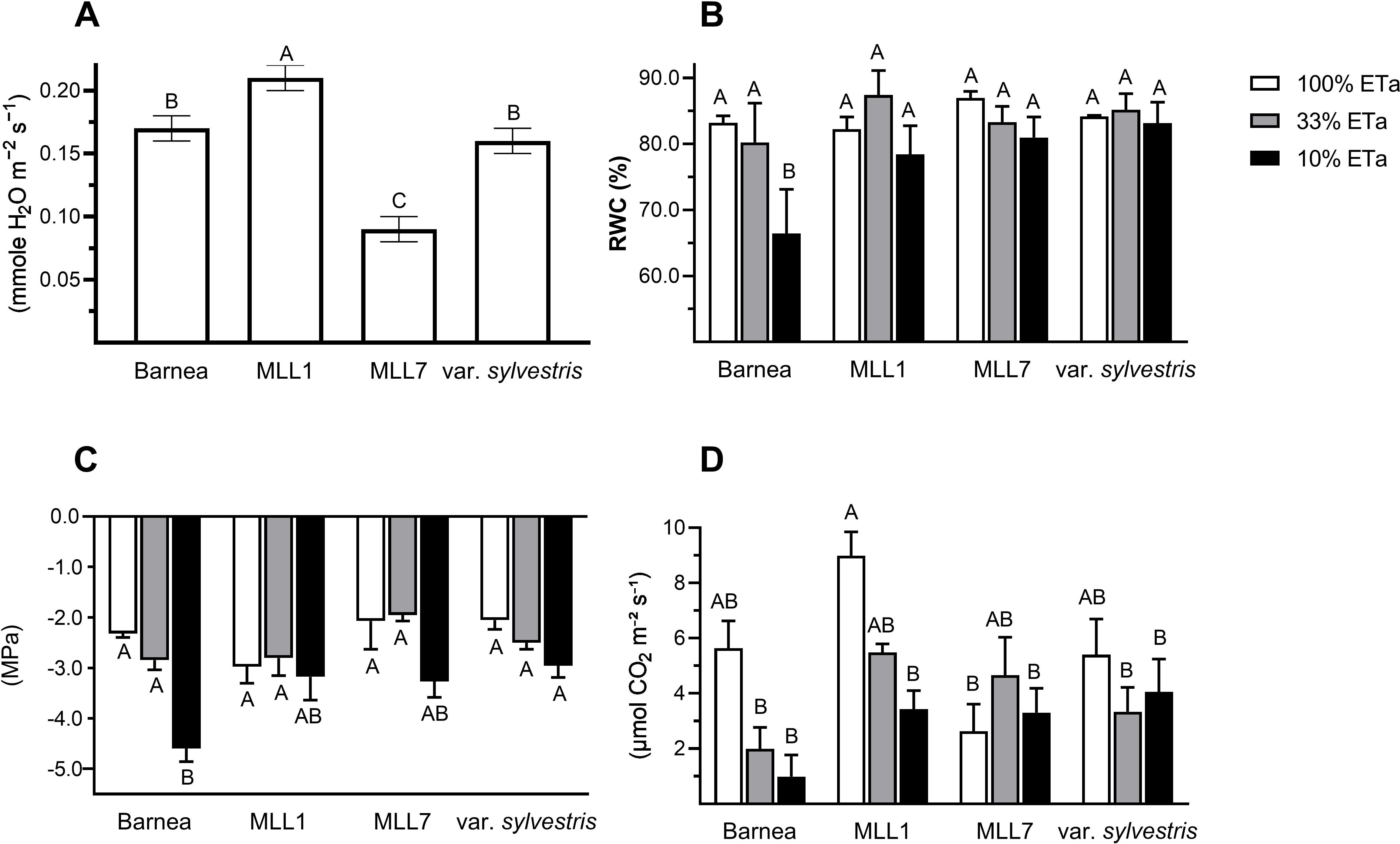
Figure 2. The influence of moderate (33% ETa) and severe drought stress (10% ETa) on physiological parameters, compared to 100% ETa. Results of the stomatal conductance summarizing the measurements that were taken in olives growing in 100% ETa conditions at various time points throughout the experiment (A). Measurements of relative water content (B), stem water potential (C), and net photosynthesis (D) were taken three weeks after the commencement of the drought treatment. Different letters above bars indicate statistically significant differences between cultivars and treatments (ANOVA Tukey’s HSD).
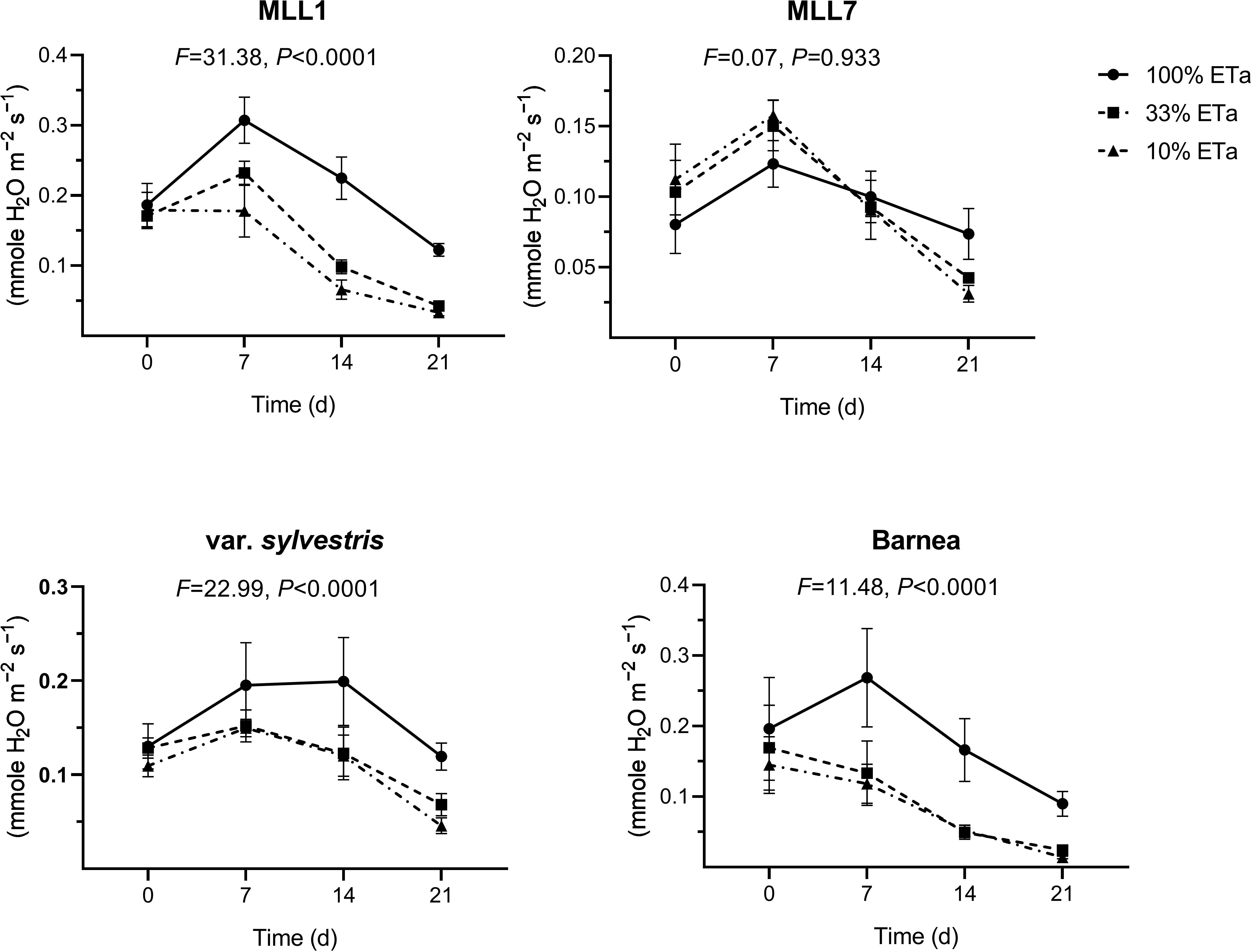
Figure 3. The influence of moderate (33% ETa) and severe drought stress (10% ETa) on stomatal conductance in the four investigated olive varieties. Measurements were conducted at the beginning of the experiments and at three subsequent time points. Results of the ANOVA with repeated measures, assessing the interaction between treatment and time, are presented above each graph.
Altogether, the ability of MLL7 to maintain similar low stomatal conductance, WRC, SWP, and net photosynthesis across all ETa treatments, and throughout the experiment (Figures 2, 3), suggests that its physiological response to water stress is less pronounced than in MLL1, Barnea and O. europaea subsp. europaea var. sylvestris. Thus, our results suggest that past selection processes favored cultivars well adapted to local conditions. The drought resilience of MLL7 may also explain the prominence of MLL7rootstock/MLL1scion combination among ancient living olive trees in the Southern Levant (Barazani et al., 2014, 2017). Supporting its resilience to harsh arid environments, MLL7 survived as a fruit-bearing tree in an agricultural plot in the Negev desert (<50 mm annual rainfall year-1) for at least 500 years without any irrigation (Tepper et al., 2022). However, the low photosynthesis capacity in MLL7 growing in 100% ETa was significantly 3.4 times lower than that measured in MLL1 (Figure 2D). Low photosynthetic capacity is associated with reduced productivity and lower yields (Zhu et al., 2010), which might explain the negligible abundance of MLL7 as a fruit-bearing tree compared to MLL1 (Barazani et al., 2014).
Grafting is considered to increase the survival and growth of propagated olives (Foxhall, 2007), especially of cultivars that do not root easily (Barazani et al., 2014). The utilization of specific clones as rootstocks was reported by Zohary et al. (2012) as a common propagation technique used by traditional olive growers in Turkey. Additionally, it has been shown that grafting the Picual cultivar (scion) on rootstocks of O. europaea subsp. europaea var. sylvestris improved tree vigor (Díaz-Rueda et al., 2020). Although our experiment did not include grafted olives, the findings support the resilience of O. europaea subsp. europaea var. sylvestris to Mediterranean abiotic stress conditions (e.g., Ozturk et al., 2010; Kassout et al., 2021, 2024; Tadić et al., 2024). The role of saplings (segregating populations) as rootstocks in promoting fruit tree vigor is well-known (Warschefsky et al., 2016). In olives, grafting of the cultivar Coratina on various rootstocks emphasized the importance of grafting in ensuring the sustainability of olives under drought conditions (El Said et al., 2024). Accordingly, further comparative common garden experiments with grafted olives, utilizing both southern Levant local cultivars and var. sylvestris as rootstocks and scions (e.g., var. sylvestrisrootstock/MLL1scion, MLL7rootstock/Barneascion, var. sylvestrisrootstock/Barneascion), are needed to examine the potential advantage of this agronomic technique and local germplasm in controlling tree vigor under abiotic stress conditions.
Although the olive tree is considered well-adapted to Mediterranean environments, a decrease in its resilience to changing environmental conditions has already been reported (Fraga et al., 2020; Kaniewski et al., 2023). Assuming that the rootstocks of ancient grafted olive trees are, and have been, essential for survival and resilience over centuries in arid and semi-arid conditions in the region, the overall results of this study underscore the agronomic potential of local germplasm in agriculture and breeding programs, ensuring olive horticulture better suited for the changing environments.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
BA: Formal analysis, Investigation, Writing – review & editing. AD: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. EB-D: Formal analysis, Investigation, Writing – review & editing. GG: Conceptualization, Methodology, Supervision, Writing – review & editing. OB: Conceptualization, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Israel Science Foundation (ISF), grant no. 332/21.
We thank the Israel Science Foundation (ISF) for the support of our study on the history of olive cultivation in the southern Levant.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ashkenazi, E., Chen, Y., Avni, Y. (2018). Olive tree survival and adaptation to the harsh growing conditions in the arid desert environment of the Negev Highlands, Southern Israel. Israel J. Plant Sci. 65, 147–152. doi: 10.1163/22238980-00001040
Ater, M., Barbara, H., Kassout, J. (2016). “Importance of local varieties, oleaster and traditional practices of olive cultivation in the region of Chefchaouen (Northern Morocco),” in In L’oléiculture au Maroc de la préhistoire à nos jours: pratiques, diversité, adaptation, usages, commerce et politiques. Eds. Ater, M., Essalouh, L., Ilbert, H., Moukhli, A., Khadari, B. (CIHEAM, Montpellier), 109–121.
Aumeeruddy-Thomas, Y., Moukhli, A., Haouane, H., Khadari, B. (2017). Ongoing domestication and diversification in grafted olive–oleaster agroecosystems in Northern Morocco. Region. Environ. Change 17, 1315–1328. doi: 10.1007/s10113-017-1143-3
Barazani, O., Dag, A., Dunseth, Z. (2023). The history of olive cultivation in the southern Levant. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1131557
Barazani, O., Dag, A., Kerem, Z., Lavee, S., Kadereit, J. W. (2008). Local old olive landrace varieties in Israel—Valuable plant genetic resources in olive cultivation. Israel J. Plant Sci. 56, 265–271. doi: 10.1560/IJPS.56.3.265
Barazani, O., Keren-Keiserman, A., Westberg, E., Hanin, N., Dag, A., Ben-Ari, G., et al. (2016). Genetic variation of naturally growing olive trees in Israel: from abandoned groves to feral and wild? BMC Plant Biol. 16, 1–11. doi: 10.1186/s12870-016-0947-5
Barazani, O., Lifshitz, D., Mayzlish-Gati, E. (2024). Conservation of plant genetic resources in the southern Levant. Sci. Hortic. 331, 113124. doi: 10.1016/j.scienta.2024.113124
Barazani, O., Waitz, Y., Tugendhaft, Y., Dorman, M., Dag, A., Hamidat, M., et al. (2017). Testing the potential significance of different scion/rootstock genotype combinations on the ecology of old cultivated olive trees in the southeast Mediterranean area. BMC Ecol. 17, 1–8. doi: 10.1186/s12898-017-0114-3
Barazani, O., Westberg, E., Hanin, N., Dag, A., Kerem, Z., Tugendhaft, Y., et al. (2014). A comparative analysis of genetic variation in rootstocks and scions of old olive trees–a window into the history of olive cultivation practices and past genetic variation. BMC Plant Biol. 14, 1–8. doi: 10.1186/1471-2229-14-146
Barzilai, O., Avraham, M., Sorek, Y., Zemach, H., Dag, A., Hochberg, U. (2021). Productivity versus drought adaptation in olive leaves: Comparison of water relations in a modern versus a traditional cultivar. Physiol. Plant. 173, 2298–2306. doi: 10.1111/ppl.13580
Ben-Dor, E., Dag, A., Perelberg, A., Chen, T., Ben Dor, Y., Low Ramati, D., et al. (2024). Genetic and phenotypic evidence suggest the existence of indigenous olive population of wild var. sylvestris in the Carmel coast, southern Levant. BMC Plant Biol. 24, 896. doi: 10.1186/s12870-024-05575-7
Dag, A., Erel, R., Ben-Gal, A., Zipori, I., Yermiyahu, U. (2012). The effect of olive tree stock plant nutritional status on propagation rates. HortScience 47, 307–310. doi: 10.21273/HORTSCI.47.2.307
Dag, A., Tugendhaft, Y., Yogev, U., Shatzkin, N., Priel, N. (2004). Commercial cultivation of olive (Olea europaea L.) with saline water under extreme desert conditions”, in. V Int. Symp. Olive Grow. 791, 279–284. doi: 10.17660/ActaHortic.2008.791.40
Dag, A., Zipori, I., Tietel, Z. (2024). Factors that affect the quality of oilive oil produced using olives from traditional orchards in the Middle East. HortTechnology 34, 396–404. doi: 10.21273/HORTTECH05407-24
Díaz-Rueda, P., Franco-Navarro, J. D., Messora, R., Espartero, J., Rivero-Núñez, C. M., Aleza, P., et al. (2020). SILVOLIVE, a germplasm collection of wild subspecies with high genetic variability as a source of rootstocks and resistance genes for olive breeding. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00629
Díez, C. M., Trujillo, I., Barrio, E., Belaj, A., Barranco, D., Rallo, L. (2011). Centennial olive trees as a reservoir of genetic diversity. Ann. Bot. 108, 797–807. doi: 10.1093/aob/mcr194
El Said, S. H., Abd Allatif, A. M., Abdel-Fattah, A. A. (2024). Response of grafted olive (Olea europaea L. Cv. Coratina) to water deficit conditions. Basrah J. Agric. Sci. 37, 134–148. doi: 10.37077/25200860.2024.37.1.11
Fraga, H., Moriondo, M., Leolini, L., Santos, J. A. (2020). Mediterranean olive orchards under climate change: A review of future impacts and adaptation strategies. Agronomy 11, 56. doi: 10.3390/agronomy11010056
Galili, E., Langgut, D., Terral, J., Barazani, O., Dag, A., Kolska Horwitz, L., et al. (2021). Early production of table olives at a mid-7th millennium BP submerged site off the Carmel coast (Israel). Sci. Rep. 11, 2218. doi: 10.1038/s41598-020-80772-6
Hannachi, H., Sommerlatte, H., Breton, C., Msallem, M., El Gazzah, M., Ben El Hadj, S., et al. (2009). Oleaster (var. sylvestris) and subsp. cuspidata are suitable genetic resources for improvement of the olive (Olea europaea subsp. europaea var. europaea). Genet. Resour. Crop Evol. 56, 393–403. doi: 10.1007/s10722-008-9374-2
Kaniewski, D., Marriner, N., Morhange, C., Khater, C., Terral, J.-F., Besnard, G., et al. (2023). Climate change threatens olive oil production in the Levant. Nat. Plants 9, 219–227. doi: 10.1038/s41477-022-01339-z
Kassout, J., Ater, M., Ivorra, S., Barbara, H., Limier, B., Ros, J., et al. (2021). Resisting aridification: adaptation of sap conduction performance in Moroccan wild olive subspecies distributed over an aridity gradient. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.663721
Kassout, J., Terral, J.-F., Souali, H., Ater, M. (2024). Environment-dependent and intraspecific variations in leaf and size traits of a native wild olive (Olea europaea L.) along an aridity gradient in Morocco: A functional perspective. Plant Ecol. 225, 943–959. doi: 10.1007/s11258-024-01445-2
Lavee, S. (2009). The revolutionary impact of introducing irrigation-intensification to the olive oil industry. Acta Hort. 888, 21–30. doi: 10.17660/ActaHortic.2011.888.1
Ozturk, M., Dogan, Y., Sakcali, M. S., Doulis, A., Karam, F. (2010). Ecophysiological responses of some maquis (Ceratonia siliqua L., Olea oleaster Hoffm. & Link, Pistacia lentiscus and Quercus coccifera L.) plant species to drought in the east Mediterranean ecosystem. J. Environ. Biol. 31, 233. Available online at: https://www.jeb.co.in./journal_issues/201001_jan10/paper_01.pdf.
Rafael Frankel, R., Avitsur, S., Xxxe., A. (1994). History and technology of olive oil in the Holy Land; 0-917526-06-6 hardback $40 (Arlington, VA, Tel Aviv, Israel: Oléarius Editions & Eretz Israel Museum).
Tadić, J., Dumičić, G., Veršić Bratinčević, M., Vitko, S., Liber, Z., Radić Brkanac, S. (2024). Comparative analysis of cultivated and wild olive genotypes to salinity and drought stress. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1423761
Tepper, Y., Porat, N., Langgut, D., Barazani, O., Bajpai, P. K., Dag, A., et al. (2022). Relict olive trees at runoff agriculture remains in Wadi Zetan, Negev Desert, Israel. J. Archaeol. Sci.: Rep. 41, 103302. doi: 10.1016/j.jasrep.2021.103302
Tourvas, N., Ganopoulos, I., Koubouris, G., Kostelenos, G., Manthos, I., Bazakos, C., et al. (2023). Wild and cultivated olive tree genetic diversity in Greece: a diverse resource in danger of erosion. Front. Genet. 14. doi: 10.3389/fgene.2023.1298565
Tugendhaft, Y., Eppel, A., Kerem, Z., Barazani, O., Ben-Gal, A., Kadereit, J. W., et al. (2016). Drought tolerance of three olive cultivars alternatively selected for rain fed or intensive cultivation. Sci. Hortic. 199, 158–162. doi: 10.1016/j.scienta.2015.12.043
Warschefsky, E. J., Klein, L. L., Frank, M. H., Chitwood, D. H., Londo, J. P., Von Wettberg, E. J., et al. (2016). Rootstocks: diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 21, 418–437. doi: 10.1016/j.tplants.2015.11.008
Yamasaki, S., Dillenburg, L. R. (1999). Measurements of leaf relative water content in Araucaria angustifolia. Rev. Brasilleira Fisiol. Vegetal 11, 69–75.
Zhu, X.-G., Long, S. P., Ort, D. R. (2010). Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 61, 235–261. doi: 10.1146/annurev-arplant-042809-112206
Zohary, D., Hopf, M., Weiss, E. (2012). Domestication of Plants in the Old World: The origin and spread of domesticated plants in Southwest Asia, Europe, and the Mediterranean Basin (Oxford, UK: Oxford University Press).
Keywords: adaptation, climate change, drought, olive, traditional cultivars, wild olives
Citation: Adi B, Dag A, Ben-Dor E, Gabay G and Barazani O (2025) Exploring drought tolerance in wild and traditional olive varieties from the Southern Levant. Front. Plant Sci. 16:1547174. doi: 10.3389/fpls.2025.1547174
Received: 17 December 2024; Accepted: 24 January 2025;
Published: 12 February 2025.
Edited by:
Franco Famiani, University of Perugia, ItalyReviewed by:
Annalisa Marchese, University of Palermo, ItalyCopyright © 2025 Adi, Dag, Ben-Dor, Gabay and Barazani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oz Barazani, YmFyYXphbmlAYWdyaS5nb3YuaWw=; Gilad Gabay, Z2dhYmF5QGJndS5hYy5pbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.