
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 06 March 2025
Sec. Plant Abiotic Stress
Volume 16 - 2025 | https://doi.org/10.3389/fpls.2025.1541736
This article is part of the Research Topic Impact of Soil Contaminants on Plant Physiology and Crop Productivity View all 7 articles
 Yicheng Zhang†
Yicheng Zhang† Haider Sultan†
Haider Sultan† Asad Shah†
Asad Shah† Yixue Mu
Yixue Mu Yusheng Li
Yusheng Li Lin Li
Lin Li Zheng Huang
Zheng Huang Shaokun Song
Shaokun Song Ye Tao
Ye Tao Zhenxiang Zhou
Zhenxiang Zhou Lixiao Nie*
Lixiao Nie*Direct seeding of rice (DSR) is a widely used method for its labor- and cost-saving advantages. However, the global intensification of soil salinization presents a significant challenge to food security. Increasing sowing rates is a common practice to enhance germination under salt stress, although it leads to higher seed costs. Recently, seed priming has emerged as an effective technique to improve seedling emergence under abiotic stress, but the regulation of seed priming treatment on the sowing rate of DSR under saline soil conditions has rarely been reported. Therefore, field experiments were conducted at two salinity levels of 1.5‰ (1.5 g kg−1) (T2) and 3.0‰ (3 g kg−1) (T3) and under one non-saline condition (0‰) (T1). The control (P1) consisted of non-primed seeds, while priming treatments included 160 mg L−¹ ascorbic acid (P2), γ-aminobutyric acid (P3), and 200 mg L−¹ zinc oxide nanoparticles (P4); three sowing rates were applied: 90 (S1), 150 (S2), and 240 seeds m−2 (S3). Our results demonstrated that under T1–T3, the germination rate, α-amylase activity, and soluble sugar and protein contents were significantly increased after priming treatments. The contents of reactive oxygen species (i.e., O2− and H2O2) and malondialdehyde (MDA) were decreased, while the activities of enzymatic antioxidants (i.e., superoxide dismutase, peroxidase, and catalase) and the K+/Na+ ratio of rice were significantly increased after the above seed priming treatments. Under T1–T3, the grain yield increased by 13.39%–36.94% after priming treatments, primarily due to enhanced seed germination, which boosted panicle number per unit area. Among P2–P4 treatments, P4 treatment consistently resulted in the highest yield increase (26.96%–36.94%) compared to P1, outperforming P2 and P3 under T1–T3. Furthermore, under T1–T3, the grain yield with priming treatment at 90 seeds m−2 was equivalent to that obtained without priming treatment at 240 seeds m−2. The potential mechanisms by which priming treatments enhance rice salt tolerance include increased levels of osmoregulatory substances and elevated activities of antioxidant enzymes, which collectively support improved seed germination. Therefore, to optimize the economic benefits of DSR when the salt concentration is below 3‰, the sowing rate could be reduced to 90 seeds m−2 using ZnO-nanoparticle priming treatment.
Rice (Oryza sativa L.) is a globally vital crop, serving as a staple food for nearly 50% of the world’s population (Jiang et al., 2023; Yu et al., 2016). With the growing global population, coupled with climate change and environmental stressors such as salinity and drought, food security is facing escalating threats worldwide (Cheeseman, 2016; Wen et al., 2020). Recently, environmental pollution, increased marginal land use, and improper irrigation and land management practices have aggravated soil salinization globally, resulting in substantial declines in crop yields and presenting a significant challenge to agricultural productivity and food security (Wang et al., 2022; Eswar et al., 2021). Therefore, the development and utilization of saline soils for rice cultivation is critical for food security in China and worldwide, especially given the challenges of increasing crop yields and the limited arable land (Mukhopadhyay et al., 2021).
Conventional transplanted rice remains the primary method of rice cultivation in China. As rice cultivation expands, direct seeding of rice (DSR) has become an important cultivation method to address resource and labor shortages (Sandhu et al., 2021). Compared to conventional transplanting, DSR is more susceptible to temperature fluctuations, flooding, and ionic stresses such as sodium (Na+) and chloride (Cl−), resulting in lower seedling emergence rates and, consequently, reduced yields (Su et al., 2022). Elevated soil salinity inhibits rice seed germination, thereby reducing the germination rate of seeds using DSR (Xu et al., 2017; Shi et al., 2017). A decrease in germination rate reduces the number of rice seedlings, and uneven seedling emergence directly lowers the final rice yield (Chen et al., 2021).
Increasing sowing rates is a conventional practice to ensure a sufficient number of seedlings in the field (Zhang et al., 2023). Excessive sowing rates can lead to an overly dense rice seedling population, which reduces tiller number and significantly lowers grain yield, despite reduced weed growth (Hui, 2018). Moreover, hybrid rice seeds are more expensive than conventionally cultivated rice seeds. Higher sowing rates are often recommended to ensure proper crop establishment under salt stress. As a result, the use of hybrid rice seeds increases costs for farmers, raising the economic burden and reducing profitability in rice production (Sun et al., 2015). However, seed priming has gradually become a sustainable measure to improve seedling emergence in DSR. Seed priming provides resistance and stability to rice seeds (Paul et al., 2022).
It is well known that reactive oxygen species (ROS), including hydrogen peroxide (H2O2) and superoxide anions (O2·−), are metabolic by-products generated during normal cellular processes. When crops are exposed to salt stress, ROS accumulate rapidly in plant cells, disrupting the balance between ROS production and their scavenging mechanisms (Madkour, 2019; Zavariyan et al., 2015). ROS accumulation leads to lipid peroxidation, causing malondialdehyde (MDA) accumulation, which is a marker of membrane damage under salt stress (Mushtaq, 2020). To mitigate the harmful effects of ROS, crops have developed antioxidant defense mechanisms, which encompass enzymes such as catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) (Rajput et al., 2021). There were some studies that demonstrated that seed priming technology could improve the activities of antioxidant enzymes and decrease ROS (H2O2 and O2·−) levels, optimizing defense mechanisms during seed germination (Guo et al., 2022).
Previous studies have shown that seed priming technology enhanced the emergence rate of rice seedlings, which accelerated the establishment of the rice population, thereby enhancing root fixation and nutrient absorption (Thakur et al., 2022). Seed priming regulates a range of physiological, biochemical, and molecular processes, enhancing crops’ tolerance to abiotic stresses, promoting faster seedling emergence, and improving overall growth and development (Farooq et al., 2006; Rhaman et al., 2020). Seed priming technology has been demonstrated to enhance crop growth and development, with associated improvements in agronomic traits such as increased plant height, leaf area, tiller number, and biomass (Ma et al., 2018). Additionally, research has indicated that seed priming could regulate the absorption of Na+ and K+, maintaining ion homeostasis under salt stress and thereby mitigating the adverse effects of salt stress on rice (Iqbal and Ashraf, 2010).
Key factors influencing rice yield include seedling establishment and quality (Gupta et al., 2022). After priming, rice seeds exhibit strong seedling quality, rapid emergence, and high seedling rates, all of which contribute to increased yield at harvest (Ali et al., 2020). Both increased sowing rates and seed priming increase germination rates and seedling quality under salt conditions, benefiting early seedling establishment and providing a strong physiological foundation (Farooq et al., 2019). Moreover, seed priming techniques have also been proven to reduce the need for high seed sowing rates (Farooq, 2011). Therefore, seed priming technology is a practical and sustainable measure to improve rice emergence and reduce seed costs for DSR under saline conditions. While most seed priming studies have been conducted in pot experiments, few have examined the effects of seed priming on rice growth and the regulation effect on the sowing rate of DSR under salt stress in field settings. Therefore, the objectives of this study were 1) to investigate the influences of seed priming treatments and sowing rates on the growth and yield of DSR under different salt stresses and 2) to specifically examine the role of priming treatments in optimizing sowing rates for DSR cultivated under saline soil conditions.
The experiments were carried out in Dadan Village, Yacheng Township, Yazhou District, Sanya City, Hainan Province, China (18°36′N, 109°15′E) and Leyi Village, Jiusuo Township, Ledong Lizu Autonomous County, China (18°44′N, 108°89′E), in 2022 and 2023, respectively. The experimental sites were situated in proximity to the sea and equipped with a water diversion irrigation system suitable for large-scale saline-tolerant rice cultivation. The system pumps seawater and underground freshwater through pipelines and mixes them proportionally in a distribution tank to meet the saline water demand for irrigation of experimental fields. The average temperature, precipitation, and total solar radiation in 2022 were lower than those in 2023 (Figure 1). Specifically, the maximum, minimum, and average temperatures recorded in 2022 were 29.11°C, 24.32°C, and 26.43°C, respectively, while in 2023, these values were 30.19°C, 26.04°C°, and 27.88°C, respectively. The average precipitation in 2022 and 2023 were 741.41 mm and 761.93 mm, respectively. The total solar radiation in 2022 was 2,749.10 MJ m−2, representing a 14.74% decrease compared to 2023, which recorded 3,154.29 MJ m−2. The chemical properties of the soil in the two-season experimental field are detailed in Table 1. In 2022, the soil pH, organic matter content, total nitrogen, available phosphorus, and available potassium were 7.61, 0.31%, 0.25 g kg−1, 13.62 mg kg−1, and 280.51 mg kg−1, respectively. In contrast, in 2023, these values were 6.02, 3.68%, 2.26 g kg−1, 80.79 mg kg−1, and 213.25 mg kg−1, respectively.
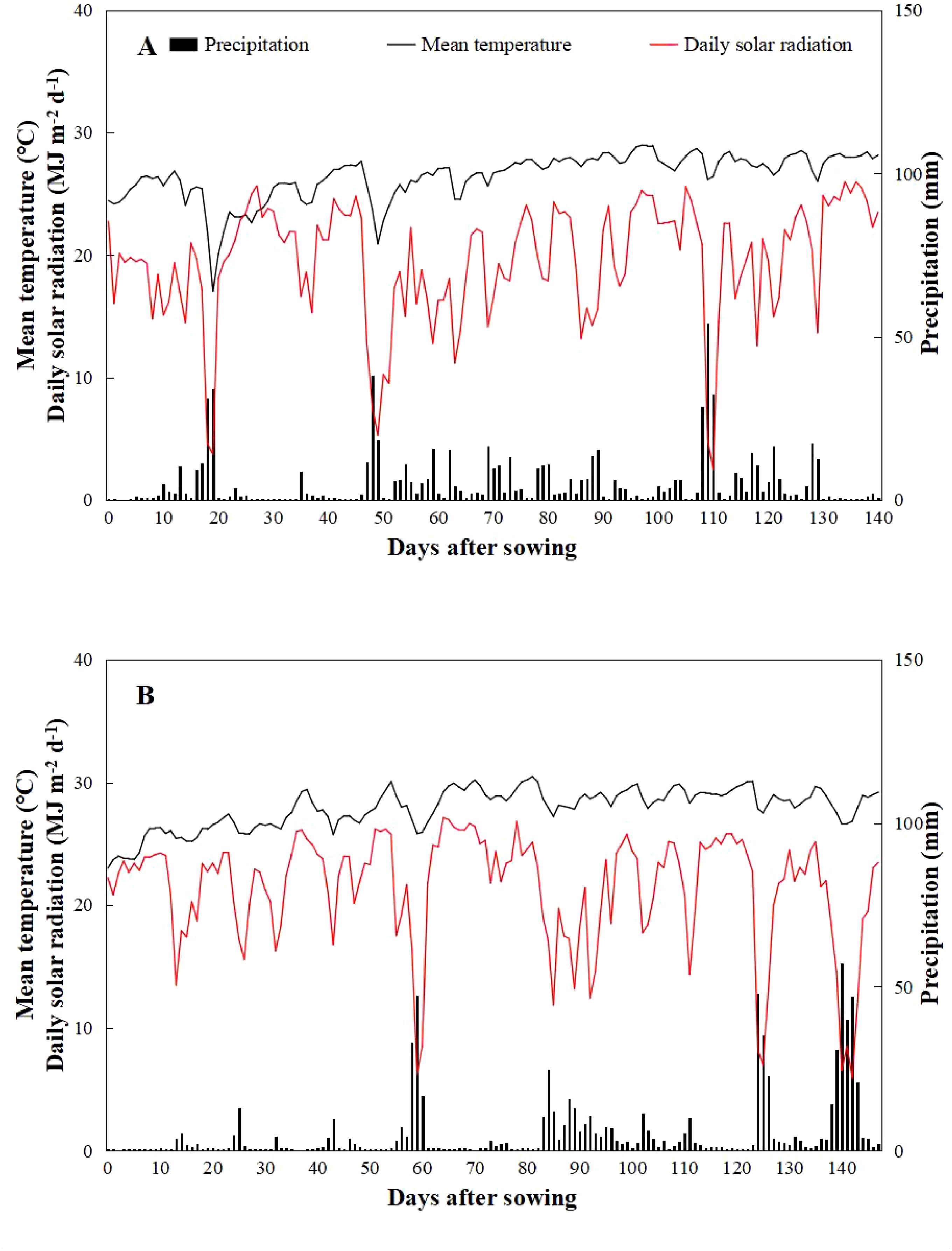
Figure 1. Daily mean temperature, precipitation, and total solar radiation from sowing to maturity of rice in experimental fields of Yazhou District (A), Sanya City, Hainan Province, China, in 2022 and Ledong Li Autonomous County in 2023 (B).
The experiment was laid out in a split –split plot design with four replications. Each plot area was 4 m2 with a length of 4 m and a width of 1 m. The cultivars Xiangliangyou900 (XLY900) and Longliangyou506 (LLY506) were adopted in the experiment. Both varieties were saline alkali-tolerant hybrid early rice in this experiment. Three salt concentrations were selected, namely, 0‰ (T1), 1.5‰ (1.5 g kg−1) (T2), and 3.0‰ (3 g kg−1) (T3). One non-priming treatment (P1) as a control while three priming treatments were applied in the sub-plot: 160 mg L−1 ascorbic acid (ASA) priming (P2), 160 mg L−1 γ-aminobutyric (GABA) priming (P3), and 200 mg L−1 zinc oxide nanoparticles (ZnO-Nano) priming (P4). We previously conducted screening tests on priming treatments and found that under three salinity levels, the best priming effect on the germination rate of rice seeds was observed on 160 mg L−1 ASA, 160 mg L−1 GABA, and 200 mg L−1 ZnO nanoparticles (Mu et al., 2023). Therefore, these three priming treatments were selected for field tests. The above reagents were purchased from Shanghai Biochemical Technology McLean Reagent Co., Ltd. (Shanghai, China), and the nanoparticle size of ZnO-Nano was 30 ± 10 nm. Three sowing rates were added in the sub-plot, namely, 90 seeds m−2 (S1), 150 seeds m−2 (S2), and 240 seeds m−2 (S3), representing low, medium, and high sowing rates, respectively.
In the 2-year field experiment, nitrogen fertilizer was selected as urea (N ≥ 46.0%) with 150 kg N ha−1, distributed according to the following proportions: basal fertilizer, tiller fertilizer, and booting fertilizer at a 1:1:1 ratio. Phosphorus fertilizer was applied as calcium superphosphate (P2O5 ≥ 14.0%) at a rate of 60 kg P2O5 ha−1, entirely as a basal fertilizer. Potassium fertilizer was applied as potassium chloride (KCl) (K2O ≥ 60.0%) at a rate of 100 kg K ha−1, split between basal and booting fertilizers in a 1:1 ratio. Meticulous management practices were implemented to mitigate potential grain yield loss from pest and disease infestations.
Before the priming treatment, the seeds were placed in a desiccant for dehydration and drying until the moisture content of the seeds was less than 10%. Then, the seeds were stored in an aluminum foil bag under vacuum, with the initial germination rate exceeding 90%. Afterward, the sterilized and dried seeds were placed into a priming solution including the 160 mg L−1 ASA priming, the 160 mg L−1 GABA priming, and the 200 mg L−1 ZnO-Nano priming. The weight of the seed and the volume ratio of the priming solution were 1: 5 (W: V). Among them, the ZnO-Nano priming solution was subjected to ultrasonic dissolution for a duration of 30 minutes. The seeds were incubated at a constant temperature of 25°C under dark conditions for 24 hours, and the priming solution was replaced once after 12 hours of priming. Following 24 hours of priming treatments, the seeds were rinsed three times with distilled water, and the surface water was removed. The seeds were then placed in a blast drying oven at 25°C until their total weight was equivalent to that prior to priming. At the conclusion of the priming treatment, seeds were woven into the sowing belt using a seed weaving machine (SH-BZ-III, Shandong Jiesheng Heavy Machinery Co., Ltd., Jining, China). Strip seeding used wet direct sowing, and the sowing date of both experimental years was March. Before sowing, a rotary tiller was used to rotate the ground to wash salt two to three times until the soil salt content of the field was reduced to below 2‰. The excess water on the soil surface was drained, seeds were sowed when there was no bright water in the field, and row spacing was carried out at 20 cm. Saline irrigation was implemented continuously from sowing to harvest. The salinity concentration was monitored daily using an electrical conductivity meter, and freshwater or seawater was promptly supplemented as needed. This approach ensured the continuity and stability of saline stress throughout the experimental period. In the first week after sowing, the plots were maintained under a moist condition to promote optimal seedling establishment. Subsequently, shallow water irrigation with the appropriate salinity was applied until the five-leaf stage. Following this, fields were flooded with the corresponding saline water, and the water level was maintained at 5–10 cm until 2 weeks before harvest.
Soil samples were collected before sowing from each experimental site at a depth of 0–20 cm with the help of an auger. Each sample comprised a composite of three randomly selected cores from each plot. The fresh soil samples were thoroughly mixed, while a portion of the composite sample was air-dried and subsequently sieved through a 1-mm mesh. After sieving, the soil samples were kept in clean plastic bags for initial soil analysis. The pH of the soil, along with its organic matter content, total nitrogen, available phosphorus, and available potassium, was analyzed. The average temperature, precipitation, and solar radiation were recorded using a micro-meteorological station. The micro-meteorological station was installed 500 m away from the test field. The micro-meteorological station was equipped with various specialized instruments, including an air temperature and humidity sensor, a data logger, a rain gauge, total solar radiation sensors, and wind speed and direction sensors.
In the 2-year field experiment, the seed germination percentage was monitored daily in accordance with the methodology described by AOSA (1990). Seeds were considered germinated when the radicle length exceeded 2 mm. The number of seedlings within 1 m2 in each plot was investigated 5 days after sowing to calculate the emergence rate. The investigation interval was 2 days, and the number of examinations was seven times. The germination rate was measured by the following formula: the germination rate (%) = (number of emerged seeds on a given day/total number of seeds tested) * 100.
Seven days after sowing, seedlings were sampled, washed with double-distilled water, surface-dried using blotting paper, and then immediately stored in liquid nitrogen before being transferred to a −80°C freezer for subsequent analysis. Approximately 0.1– 0.2 g rice seedlings were weighed to quantify α- amylase activity, osmoregulatory substances, ROS levels, and antioxidant enzymes. The kits for testing soluble sugar content, proline content, O2•−, POD, CAT, and H2O2 were obtained from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China), while the kits for analyzing α- amylase activity, SOD, and MDA were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) (Mu et al., 2023).
Throughout the 2-year experiment, one row of 1-m length (0.2 m2) was sampled at the mid-tillering stage (MT), panicle initiation stage (PI), heading stage (HD), and physiological maturity stage (PM) to investigate the tiller or panicle number, and then plant samples were divided into straw and panicles for further plant analysis. After drying the plants at 105°C for 15 minutes, the aboveground biomass of each plot was measured by oven-drying at 85°C for 72 h until a constant weight was achieved.
The grain yield was measured from a 1-m2 sampling area located at the center of each plot and standardized to a moisture content of 0.14 g H2O g−1. Subsequently, a 1-m-long row of plants (0.2 m2) was sampled at each plot to assess additional yield components. Panicle numbers in the sampled rows were counted in order to calculate the panicle number per square meter. The spikelets were obtained through manual threshing, followed by separation into filled and unfilled spikelets by employing the water selection method. After air-drying, unfilled spikelets were distinguished from the half-filled spikelets by winnowing. The total air-dried mass of the filled and unfilled spikelets was weighed, and small samples were taken for analysis: three subsamples of filled spikelets (30 g), three subsamples of unfilled spikelets (2 g), and all the half-filled spikelets were counted to calculate the yield components, including the number of effective panicles per unit area, the number of spikelets per panicle, the filled grain rate, and the 1000-grain weight.
The dried samples of rice straw were ground into powder. Subsequently, 0.1 g of the crushed sample was accurately weighed from each treatment, and the samples were digested using the H2SO4·H2O2 method and concentrated in a microwave oven. The concentration of Na+ and K+ at the PM stage was measured using a flame photometer, and the K+/Na+ ratio was calculated (Li et al., 2023).
In the present study, due to the consistent responses of two rice varieties adopted in all treatments, the mean value of both varieties was present for all data in order to reduce the length of the text (Supplementary Figures S1–S9; Supplementary Tables S1, S2). The data were analyzed using analysis of variance (ANOVA) using the Statistix 9.0 software. The differences between treatments were compared using the least significance difference (LSD) test at the 0.05, 0.01, and 0.001 probability levels. Graphical representations of the data were generated using Origin 2021.
The seed germination of DSR was negatively impacted by increasing salinity levels, whereas all three priming treatments enhanced seed germination across the three salinity conditions (Figure 2). Under the non-saline condition (T1), the average germination rates for rice seeds during 2 years were 50.49%, 61.84%, 62.94%, and 66.82% for treatments P1, P2, P3, and P4, respectively. Under the T2 condition, seed germination for rice was 47.35% under the non-primed (P1) treatment, with germination rates for P2, P3, and P4 showing increases of 9.75%, 8.75%, and 13.56%, respectively, compared to P1 during 2 years. Under the T3 condition, the germination rate was 18.70% in P1, while the rates for P2, P3, and P4 were enhanced by 4.63%, 4.53%, and 6.93%, respectively, compared to P1. The above results suggested that the P4 treatment performed best among the priming treatments under all three salinity conditions. As shown in Figure 2, germination rates under T1 and T2 remained stable over time. However, under T3, the germination rate peaked between days 11 and 13 before declining. This suggests that the higher salt concentration in T3 prolonged exposure to elevated salinity levels, ultimately leading to a reduction in germination over time.
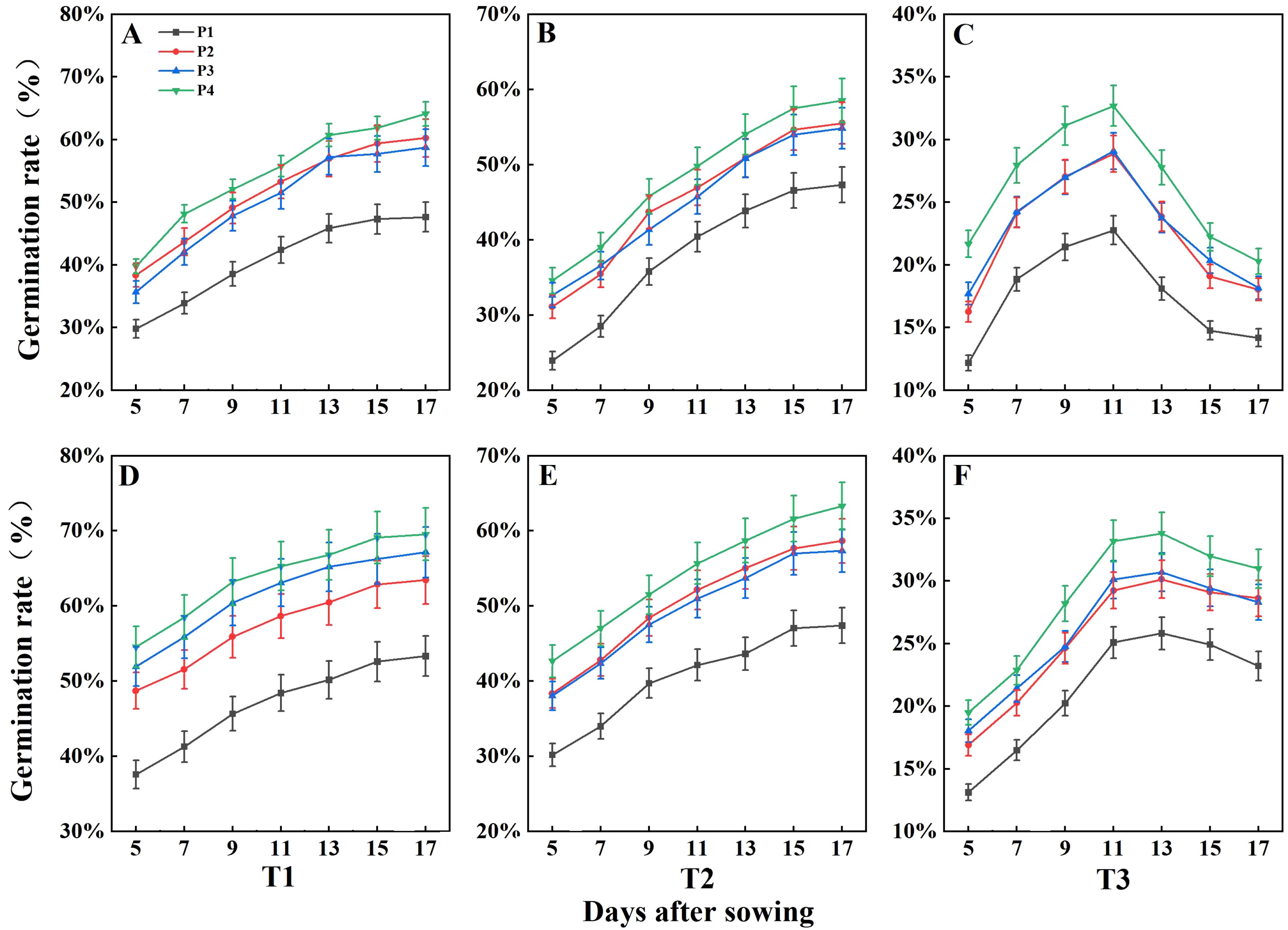
Figure 2. Effects of seed priming treatments on germination rate of direct seeding of rice under salt stress in 2022 (A–C) and 2023 (D–F). Error bars represent the standard error. T1, T2, and T3 represent salinity levels of 0‰, 1.5‰, and 3‰, respectively. P1, P2, P3, and P4 represent no-priming treatment, ASA160mg/L priming treatment, GABA160mg/L priming treatment, and ZnO-Nano200mg/L priming treatment, respectively.
Priming treatments significantly influenced α-amylase activity, soluble sugar content, soluble protein content, and proline levels in rice seedlings subjected to salt stress (Figure 3). Under T1, the α-amylase activity, soluble sugar content, and soluble protein content had average increases of 28.66%, 36.70%, and 36.69%, respectively, while the proline content decreased by 29.57% across all priming treatments (P2, P3, and P4) compared to P1. Under T2, the above-mentioned parameters had average decreases of 16.22%, 39.42%, and 18.47%, respectively, and the proline content increased by 27.71% compared to T1. Following priming treatments, the above-mentioned parameters showed increases of 41.29%, 53.51%, and 60.68%, respectively, and the proline content decreased by 16.50% compared with P1. Under T3, the above-mentioned parameters decreased by 40.68%, 58.90%, and 44.38%, respectively, and proline content increased by 48.63% compared to T1. However, with all priming treatments, the above-mentioned parameters increased by 55.68%, 67.16%, and 77.47%, respectively, and proline content decreased by 16.52% compared to P1. Additionally, across all salinity levels, the P4 priming treatment consistently resulted in significantly higher α-amylase activity, soluble sugar content, and protein content and lower proline content compared to P2 and P3 (Figure 3).
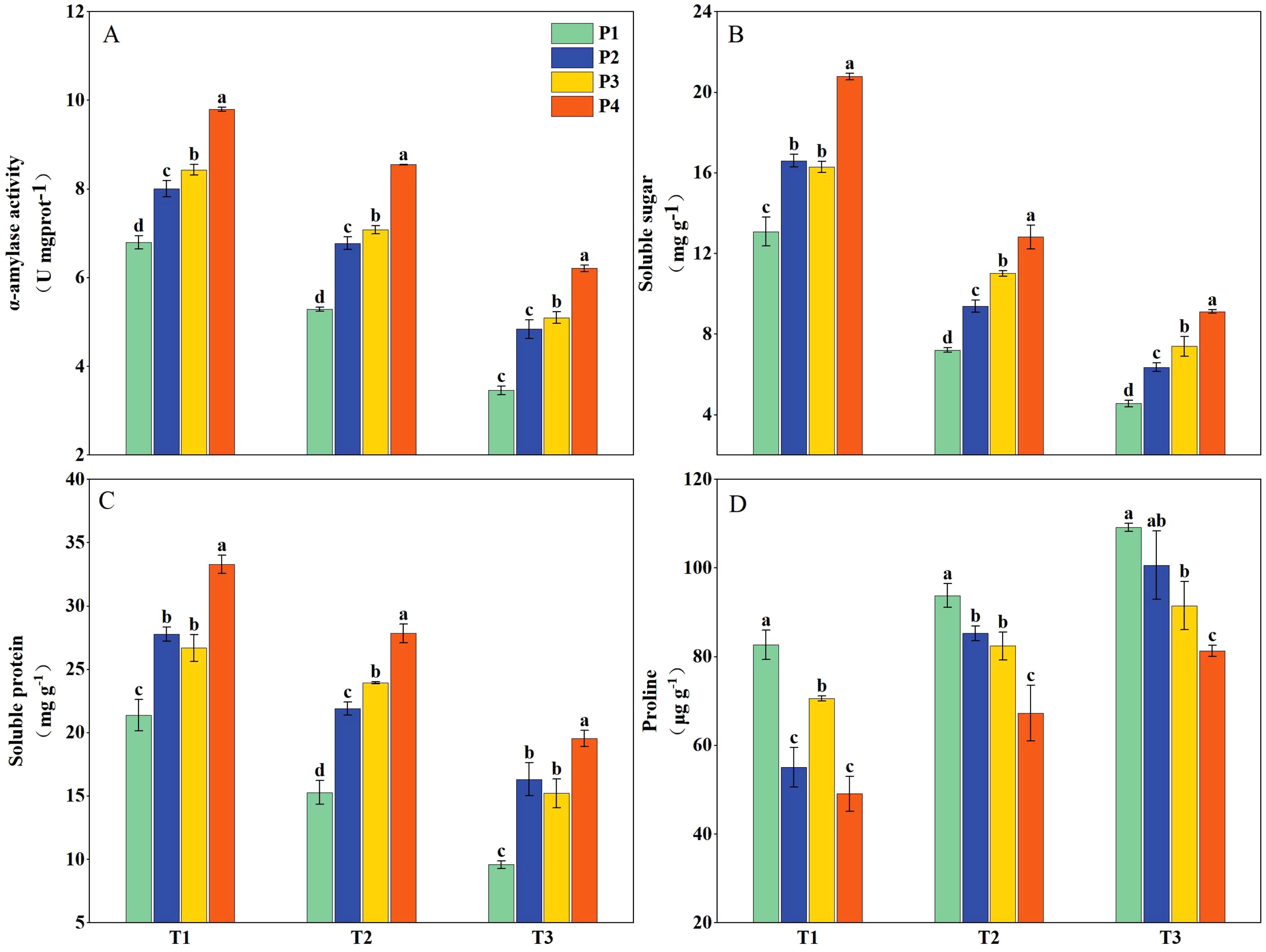
Figure 3. The α-amylase activity (A) and concentration of soluble sugar (B), soluble protein (C), and proline (D) of primed and non-primed seeds under different salt stress at 7 days after sowing (average of two varieties). Within a column, means followed by the same letter are not significantly different at the 0.05 probability level according to the least significant difference test (LSD 0.05). T1, T2, and T3 represent salinity levels of 0‰, 1.5‰, and 3‰, respectively. P1, P2, P3, and P4 represent no-priming treatment, ASA160mg/L priming treatment, GABA160mg/L priming treatment, and ZnO-Nano200mg/L priming treatment, respectively.
The O2•−, H2O2, and MDA contents were significantly increased under salt stress (T2 and T3) (Figure 4), while the ROS content in rice plants was markedly reduced following priming treatments. Under T1, the O2•−, H2O2, and MDA contents had average decreases of 35.26%, 58.10%, and 22.40%, respectively, across all priming treatments compared to P1. Under T2, the O2•−, H2O2, and MDA contents had average decreases of 13.69%, 28.80%, and 15.81%, respectively, under priming treatments compared to P1. Similarly, under T3, the reductions were 11.19%, 25.60%, and 14.43%, respectively, across priming treatments compared to P1. Under all three salinity levels, the P4 treatment resulted in the most substantial reduction of ROS accumulation compared to P2 and P3 (Figure 4).
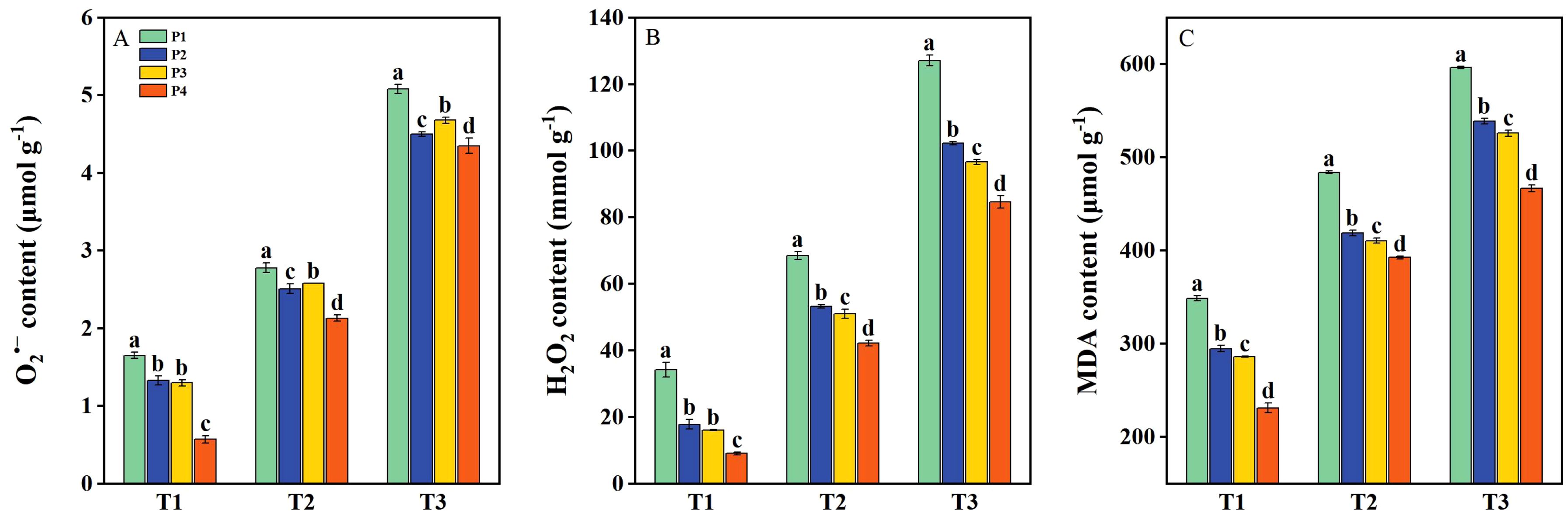
Figure 4. The concentration of O2•− (A), H2O2 (B), and malondialdehyde (MDA) (C) of primed and non-primed seeds under different salt stress at 7 days after sowing (average of two varieties). Note: Within a column, means followed by the same letter are not significantly different at the 0.05 probability level according to the least significant difference test (LSD 0.05). T1, T2, and T3 represent salinity levels of 0‰, 1.5‰, and 3‰, respectively. P1, P2, P3, and P4 represent no-priming treatment, ASA160mg/L priming treatment, GABA160mg/L priming treatment, and ZnO-Nano200mg/L priming treatment, respectively.
The activities of SOD, POD, and CAT in rice seedlings were significantly enhanced under salt stress and further improved by priming treatments (Figure 5). Under T1, the activities of POD, SOD, and CAT had average increases of 31.62%, 67.14%, and 23.69%, respectively, with priming treatments compared to P1. Under T2, the above-mentioned antioxidant enzymes had average increases of 17.75%, 15.98%, and 26.56%, respectively, following priming treatments compared with P1. Under T3, the above-mentioned antioxidant enzymes had average increases of 15.05%, 16.68%, and 19.05%, respectively, across priming treatments compared to P1. Overall, under all three salinity levels, the P4 treatment consistently resulted in the highest increases in the activities of SOD, POD, and CAT compared to P2 and P3 (Figure 5).
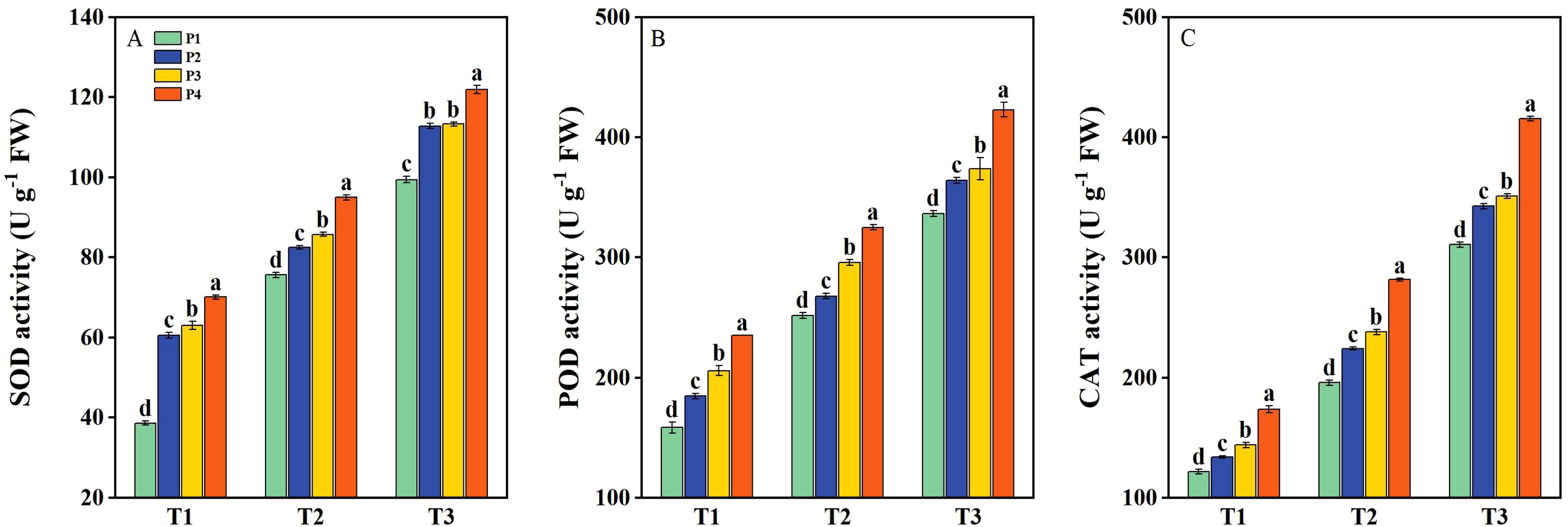
Figure 5. The activity of superoxide dismutase (SOD) (A), peroxidase (POD) (B), and catalase (CAT) (C) of primed and non-primed seeds under different salt stress at 7 days after sowing (average of two varieties). Within a column, means followed by the same letter are not significantly different at the 0.05 probability level according to the least significant difference test (LSD 0.05). T1, T2, and T3 represent salinity levels of 0‰, 1.5‰, and 3‰, respectively. P1, P2, P3, and P4 represent no-priming treatment, ASA160mg/L priming treatment, GABA160mg/L priming treatment, and ZnO-Nano200mg/L priming treatment, respectively.
The tiller number and aboveground biomass of DSR were reduced at various growth stages under salt stress (T2 and T3), with the reduction severity increasing alongside higher salt concentrations (Figures 6, 7). However, both parameters were significantly enhanced under different priming treatments and sowing rate levels. Under T1, the tiller number and aboveground biomass of DSR increased by 13.56%– 18.69%, 13.39%– 19.30%, and 26.37%– 28.67% and by 13.47%– 29.13%, 13.20%– 23.95%, and 19.71%– 40.45% under P2, P3, and P4, respectively, compared to P1 during 2 years. Under T2, the above-mentioned parameters decreased by 13.84%– 29.45% and 12.37%– 29.78%, respectively, compared to T1. However, following P2, P3, and P4 treatments, the above-mentioned parameters increased by 16.80%– 25.11%, 13.33%– 31.36%, and 27.20%– 42.57%, and 17.66%– 33.33%, 16.31%– 39.11%, and 28.70%– 46.67%, respectively, compared to P1. Under T3, the above-mentioned parameters were reduced 39.20%– 60.73% and 36.02%– 53.78%, respectively, compared to T1, However, after P2, P3, and P4 treatments, the above-mentioned parameters increased by 23.60%– 38.40%, 21.60%– 42.98%, and 36.00%– 67.05%, and 17.53%– 65.06%, 23.59%– 26.03%, and 30.63%– 76.51%, respectively, compared to P1. Across all salinity levels and priming treatments, both the tiller number and aboveground biomass increased with higher sowing rates. However, the tiller number and aboveground biomass in the P2S1, P3S1, and P4S1 treatments were not significantly different from those in the P1S3 treatment during 2 years.
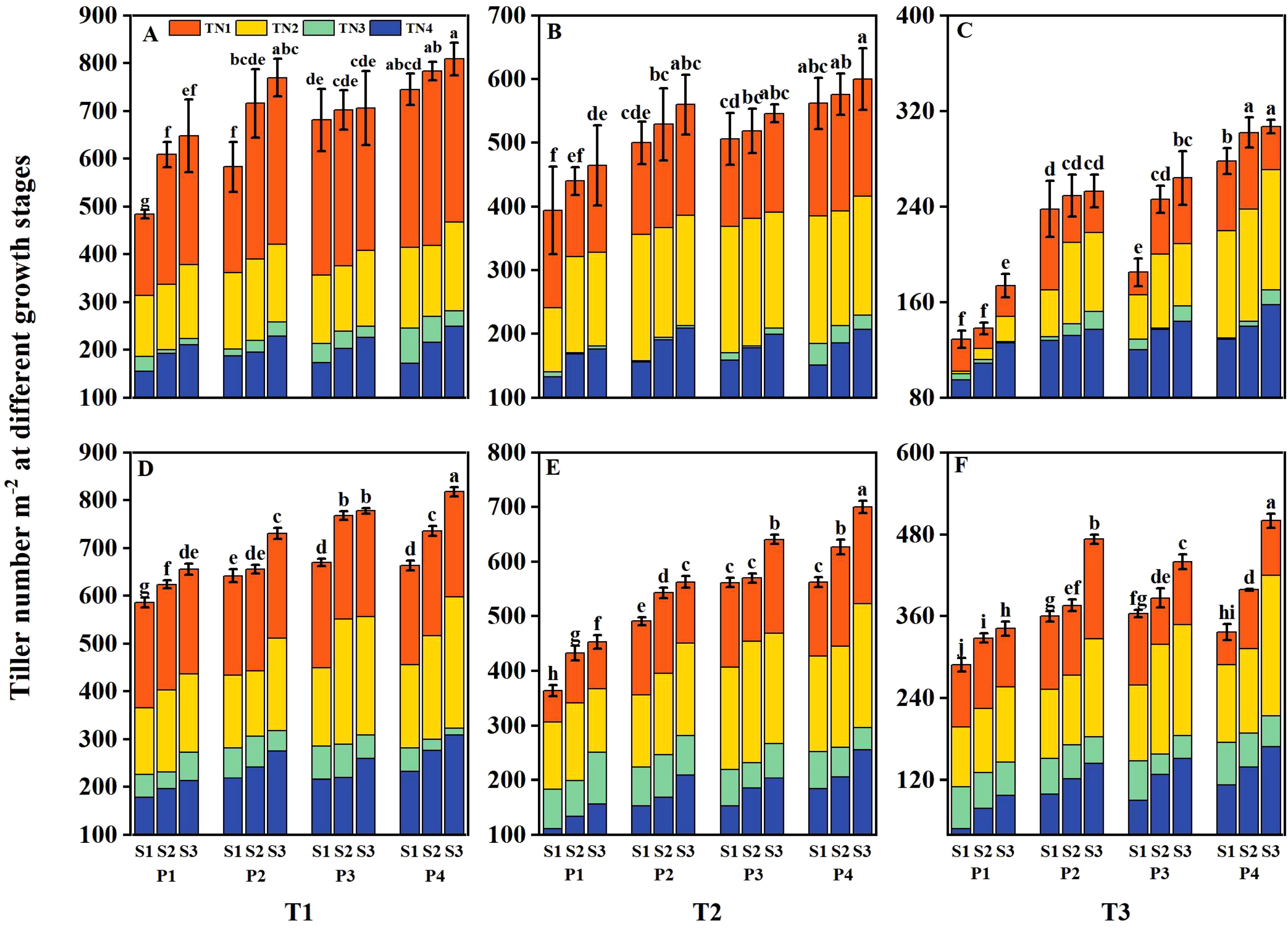
Figure 6. Effects of priming treatments and sowing rates on tiller number at different growth stages in 2022 (A–C) and 2023 (D–F) (average of two varieties). Within a column, means followed by the same letter are not significantly different at the 0.05 probability level according to the least significant difference test (LSD 0.05). TN1 represents tiller number at the mid-tillering stage, TN2 represents tiller number at the panicle initiation stage, TN3 represents tiller number at the heading stage, and TN4 represents tiller number at the physiological maturity stage. T1, T2, and T3 represent salinity levels of 0‰, 1.5‰, and 3‰, respectively. P1, P2, P3, and P4 represent no-priming treatment, ASA160mg/L priming treatment, GABA160mg/L priming treatment, and ZnO-Nano200mg/L priming treatment, respectively. S1, S2, and S3 represent three sowing rates (90, 150, and 240 seeds m−2, respectively).
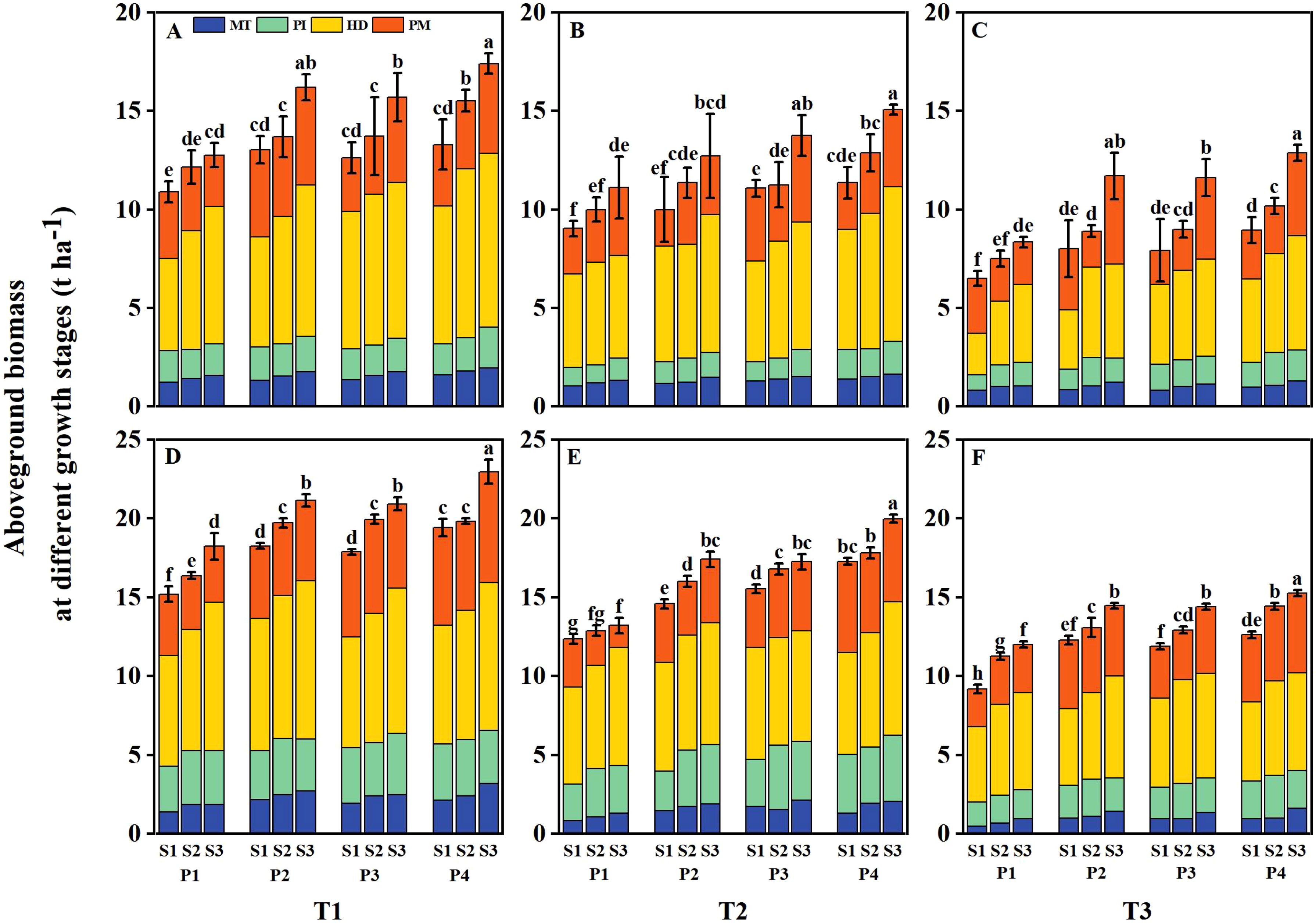
Figure 7. Effects of priming treatments and sowing rates on aboveground biomass at different growth stages in 2022 (A–C) and 2023 (D–F) (average of two varieties). Different lowercase letters represent a significant difference at the maturity stage at 0.05 levels according to the least significance difference (LSD) test. MT represents the mid-tillering stage, PI represents the panicle initiation stage, HD represents the heading stage, and PM represents the physiological maturity stage. T1, T2, and T3 represent salinity levels of 0‰, 1.5‰, and 3‰, respectively. P1, P2, P3, and P4 represent no-priming treatment, ASA160mg/L priming treatment, GABA160mg/L priming treatment, and ZnO-Nano200mg/L priming treatment, respectively. S1, S2, and S3 represent three sowing rates (90, 150, and 240 seeds m−2, respectively).
The grain yield of DSR was decreased under salt stress (T2 and T3), with greater reductions observed at higher salinity levels (Tables 2, 3), while grain yield was increased under priming and sowing rate treatments primarily due to the increased panicle number. Under T1, the grain yield increased by 14.40%, 13.39%, and 26.96% under P2, P3, and P4 treatments, respectively, compared with P1 during 2 years. Under T2, the grain yield decreased by 28.76% compared to T1, while under P2, P3, and P4 treatments, the grain yield increased by 15.33%, 14.41%, and 29.35%, respectively, compared with P1. Under T3, the grain yield decreased by 70.75% compared with T1, while under P2, P3, and P4 treatments, it increased by 25.76%, 23.38%, and 36.94%, respectively, compared with P1. The above results suggested that under all three salinity levels, the grain yield under the ZnO-nanoparticle priming (P4) treatment was the highest in the three priming treatments. Furthermore, under all three salinity levels, the grain yield was increased with higher sowing rates, peaking at S3. No significant difference was observed in grain yield among three sowing rates within the same priming treatment in 2022, while significant differences were observed in 2023. Under T1, the grain yield had average increases of 9.18% and 20.05% under S2 and S3, respectively, compared with S1 during 2 years. Under T2, the grain yield had average increases of 5.91% and 17.36% under S2 and S3, respectively, compared with S1. Under T3, the grain yield had average increases of 16.61% and 33.87% under S2 and S3, respectively, compared with S1. Additionally, across all salinity levels, grain yield under the P2S1, P3S1, and P4S1 treatments was not significantly different from the P1S3 treatment in both 2022 and 2023.
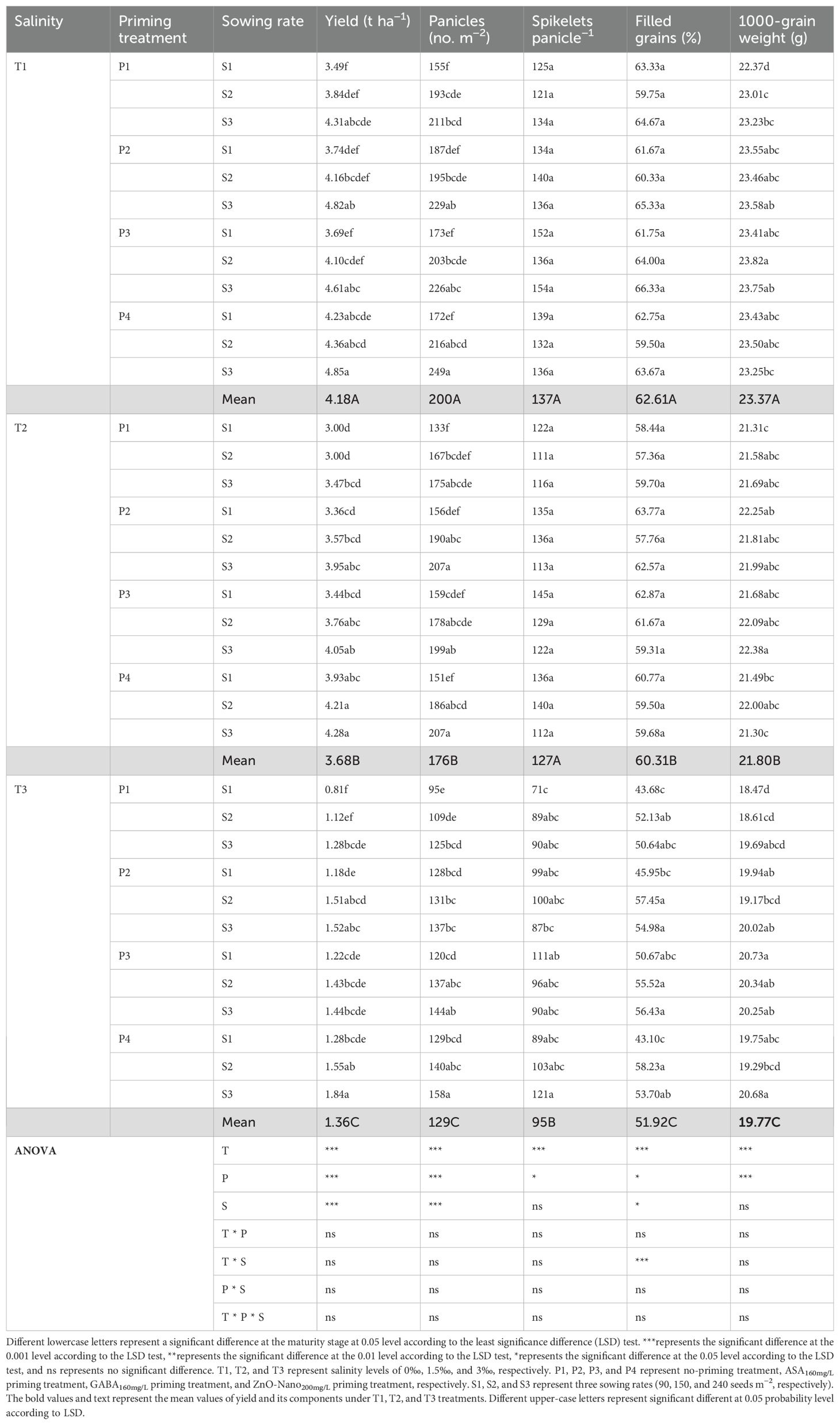
Table 2. Effects of priming treatments and sowing rates on grain yield and its components in rice under salt stress in 2022 (average of two varieties).
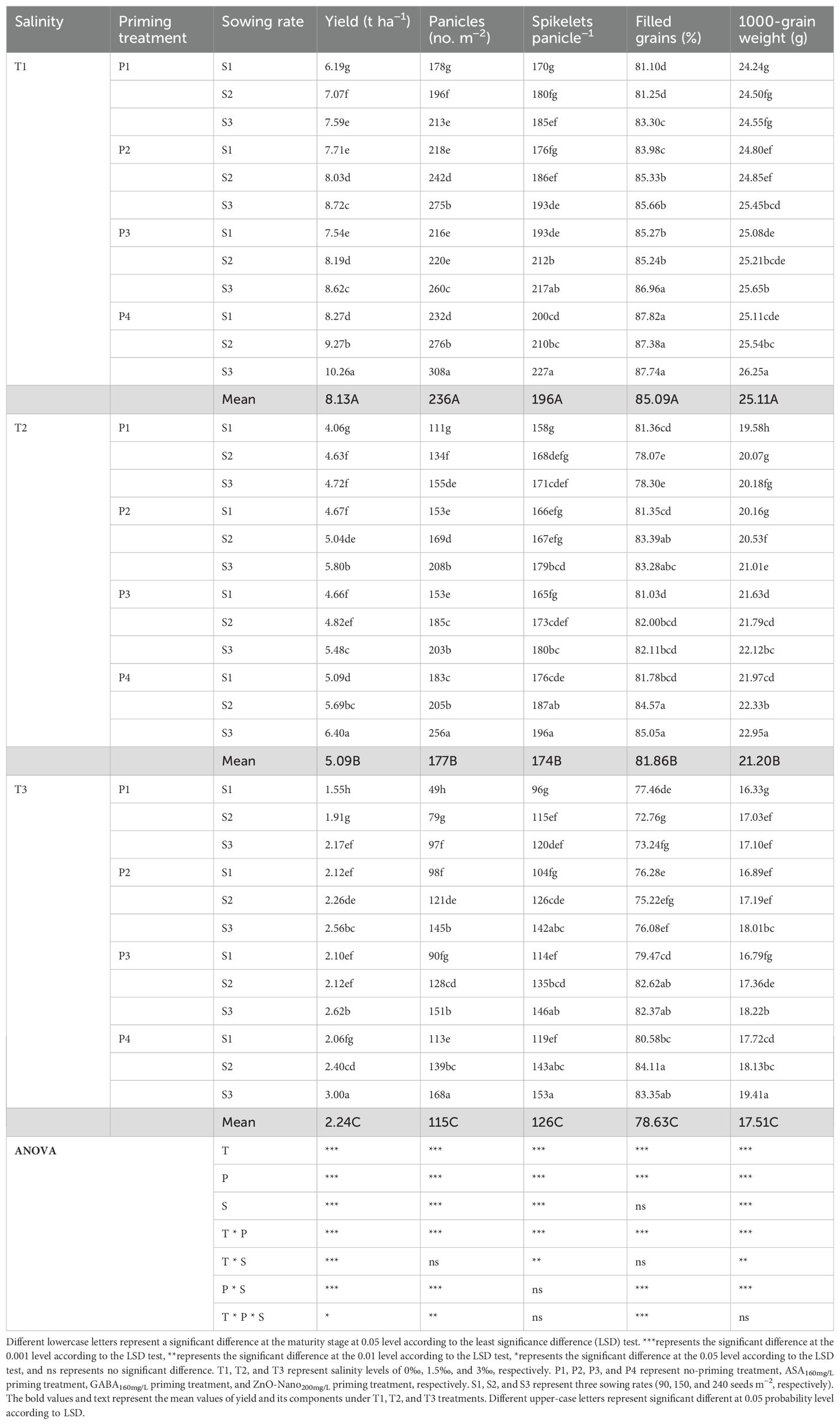
Table 3. Effects of priming treatments and sowing rates on grain yield and its components in rice under salt stress in 2023 (average of two varieties).
The aboveground Na+ and K+ contents of rice were influenced by salt stress and priming treatments. The K+/Na+ ratio in rice was decreased with increasing salt concentration (Figure 8). However, K+/Na+ was increased under priming and sowing rate treatments. Under T1, the K+/Na+ had average increases of 37.13%, 37.77%, and 75.14% under P2, P3, and P4 treatments, respectively, compared to P1 during 2 years. Under T2, the K+/Na+ had an average decrease of 86.76% compared to T1. Following P2, P3, and P4 treatments, the K+/Na+ had average increases of 34.52%, 40.48%, and 61.31%, respectively, compared to P1. Similarly, under T3, the K+/Na+ had an average decrease of 94.33% compared to T1, while after P2, P3, and P4 treatments, it had average increases of 43.48%, 42.03%, and 75.36%, respectively, compared to P1. Among all treatments, P4 consistently outperformed the other priming treatments under all salinity levels. Additionally, the K+/Na+ ratio increased with higher sowing rates under all salinity levels, with the sequence of changes at each priming treatment being S3 > S2 > S1. Moreover, across all salinity levels, the K+/Na+ ratio under the P2S1, P3S1, and P4S1 treatments was significantly higher than that under P1S3 during 2 years.
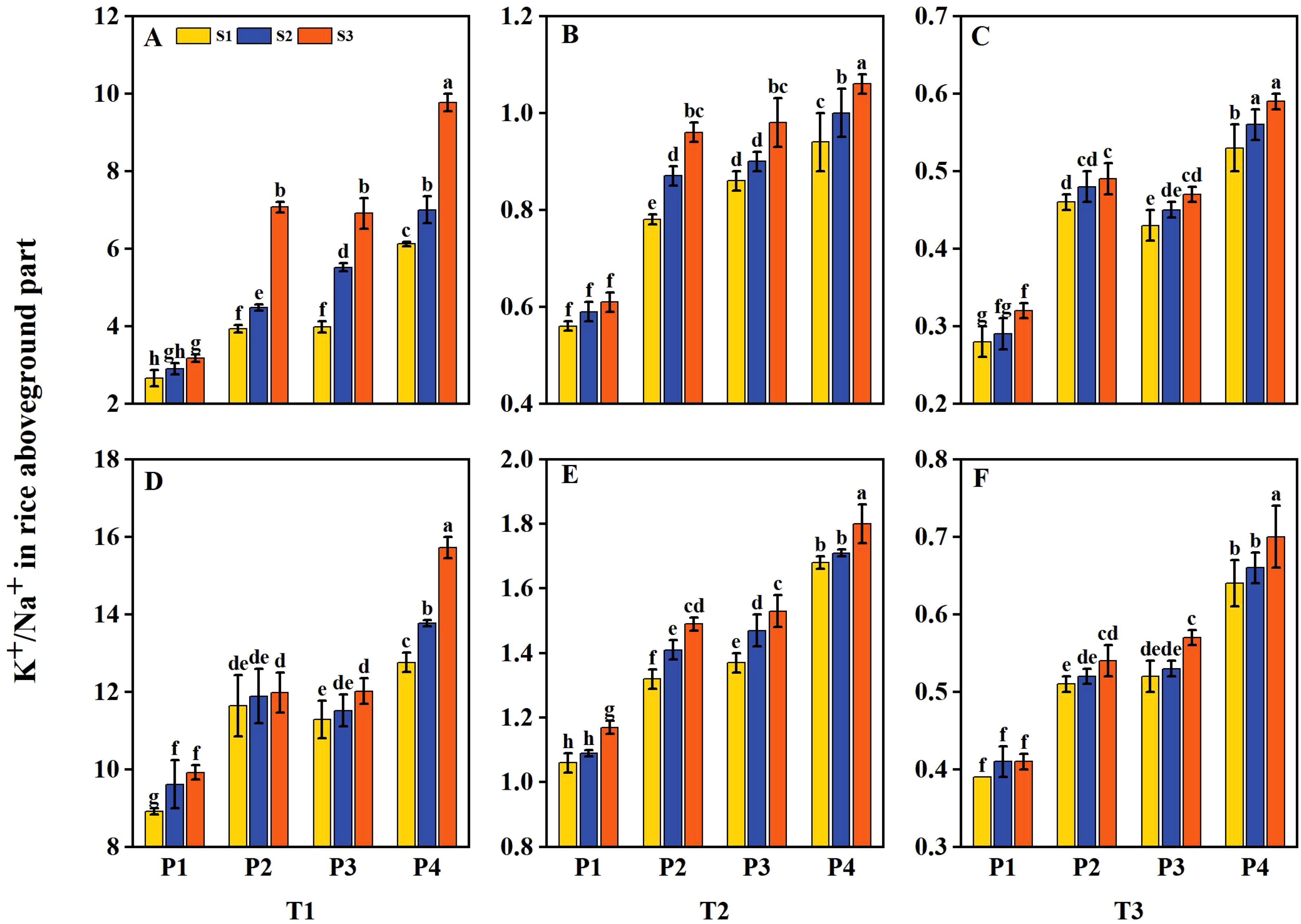
Figure 8. Effects of priming treatments and sowing rates on the K+/Na+ in rice aboveground part under salt stress in 2022 (A–C) and 2023 (D–F) (average of two varieties). Within a column, means followed by the same letter are not significantly different at the 0.05 probability level according to the least significant difference test (LSD 0.05). T1, T2, and T3 represent salinity levels of 0‰, 1.5‰, and 3‰, respectively. P1, P2, P3, and P4 represent no-priming treatment, ASA160mg/L priming treatment, GABA160mg/L priming treatment, and ZnO-Nano200mg/L priming treatment, respectively. S1, S2, and S3 represent three sowing rates (90, 150, and 240 seeds m−2, respectively).
The seed germination rate of DSR under all priming treatments was significantly decreased under salt stress (T2 and T3) during 2 years (Figure 2). Liu et al. (2023) found that rice seed germination rate was closely linked to the structural integrity of cell membranes. The inhibition of germination under salt stress was primarily caused by plasma membrane damage and reduced amylase activity, leading to decreased or lost seed viability. ROS in seeds regulate cellular growth, protect against pathogens, and control redox status, playing a vital role in seed germination and seedling growth (Singh et al., 2016). However, under salt stress, the rapid accumulation of ROS induces oxidative damage to biomolecules, leading to necrosis and cell death (Abeed et al., 2023). According to our study, the decreased seed germination of DSR was caused by the decrease of α- amylase activity and soluble sugar and protein contents and the increase of ROS (O2•− and H2O2) and MDA contents.
However, seed priming treatments could enhance seedlings’ emergence and stand establishment under salt stress. Seed priming initiates various germination-related activities, including early reserve mobilization and improved energy metabolisms (Adetunji, 2020). Furthermore, elevated levels of organic solutes, such as sugars and proteins, support plant metabolic adaptation, reduce cellular osmotic potential, and facilitate osmotic regulation under stress conditions (Matias et al., 2015). Seed priming treatments significantly increase sugars, proteins, α-amylase activity, and antioxidant system activity in seeds under abiotic stress (Sheteiwy et al., 2019; Sharma et al., 2021; Ibrahim, 2016). The process involves pre-exposure to salt stress, which augments the seeds’ resilience to subsequent stress conditions. Seed priming activates pre-germination metabolic pathways, enhancing physiological readiness for germination (Ibrahim, 2016). Our results demonstrated that under all three salinity levels, the seed germination rate of DSR was significantly improved after priming treatments. This enhancement was associated with elevated α-amylase activity, increased levels of soluble sugars and proteins, and reduced proline content, collectively improving seed vigor and salt stress tolerance. Under salt stress, ROS disrupts normal cellular metabolism by elevating MDA levels (Hasanuzzaman et al., 2021). Seed priming treatments activate ROS scavenging mechanisms (SOD, CAT, and POD), facilitating biochemical changes that enhance stress tolerance (Bussotti et al., 2014). The antioxidant system, comprising both enzymatic and non-enzymatic components, safeguards seeds by neutralizing ROS. A significant inverse correlation has been observed between antioxidant enzyme activity and MDA levels (Younesi and Moradi, 2014; Das et al., 2015). According to our study, the decreased ROS (O2•− and H2O2) and MDA contents alongside increased activities of POD, SOD, and CAT ultimately improved the seed germination rate of DSR after priming treatments. These results align with the previous studies that confirmed ROS scavenging through activation of the antioxidant defense system (Ben Rejeb et al., 2014; Sedghi et al., 2010). Furthermore, our study also showed that under all three salinity levels, the seed germination rate was significantly higher under P4 compared to P2 and P3. This improvement was linked to elevated α-amylase activity, increased soluble sugar and protein contents, and enhanced antioxidant enzyme activities in rice seedlings. In recent years, the application of nanomaterials as catalysts to enhance seed stress resistance has gained significant attention (Khan et al., 2023). Studies have shown that seed nano-priming using nanoceria (CeO2 NPs) and silver nanoparticles (Ag NPs) elevates and sustains higher soluble protein levels in crops, thereby boosting metabolic processes. Other studies have indicated that nano-priming treatments facilitate greater water uptake during seed imbibition, further augmenting α-amylase activity and soluble sugar content, which collectively enhance seed germination under stress conditions (Mahakham et al., 2017). Moreover, Rai-Kalal and Jajoo (2021) demonstrated that faster water uptake, upregulation of aquaporin and hydrolytic transcript factors, and increased α-amylase activity to facilitate starch conversion into soluble sugars are key mechanisms of nano-priming. Our findings showed that under all three salinity levels, P4 treatment (ZnO-nanoparticle priming) achieved the highest germination rate among priming treatments. This was associated with higher α-amylase activity, increased soluble sugar and protein contents, and enhanced antioxidant enzyme activities in rice seedlings, aligning with findings from prior studies (Mu et al., 2023; Sultan et al., 2025). Therefore, seed priming technology especially the application of nano-priming, represents an effective strategy to enhance seedling emergence under salt stress conditions. Although nano-priming can promote crop growth, development, and yield, certain studies have reported that nanoparticles may inhibit crop growth, depending on their concentration and size (Mathur et al., 2023). Our research showed that a concentration of 200 mg L−¹ of ZnO nanoparticles did not inhibit crop development, consistent with the results of Liu et al. (2016), who reported that low concentrations (< 500 mg L−¹) of micronutrient metal oxide nanoparticles, such as ZnO, MnOx, and FeOx, improve seed germination and plant growth. Moreover, the complex interaction between nanoparticles, soil properties, and environmental factors appears to influence their impact on plants, warranting further investigation. Therefore, to mitigate nanoparticle phytotoxicity, future research should focus on stabilizing nanomaterials to maximize their potential (Phan and Haes, 2019).
Grain yield was significantly reduced under salt stress, primarily attributed to declines in panicle number, spikelets per panicle, filled grain rate, and 1000-grain weight (Mumtaz et al., 2017; Gerona et al., 2019). In the present study, grain yield under elevated salinity levels (T2 and T3) was markedly reduced, primarily due to a decrease in panicle number (Table 3). Itroutwar et al. (2020) found that seed priming treatments can strengthen rice plants throughout the entire growth period. They also reported a strong correlation between rice yield and growth stage agronomic traits such as tiller number and aboveground biomass. Seed priming treatments could increase seedling density per unit area of crops under stress conditions, helping establish a strong seedling structure during the early stages of rice growth (Mondal and Bose, 2021). In this study, under all salinity levels, the seed germination rate was increased after priming treatments. This established a strong seedling structure, accelerated the tillering, and significantly increased the tillers (Figure 6) and panicle number per m2, thereby boosting rice yield. Moreover, the aboveground biomass of rice was increased (Figure 7) because seed priming promoted vegetative growth and enhanced stem length through cell division (Das et al., 2022).
An optimal seeding density can modulate rice population structure, mitigating the conflict between individual plant development and population growth (Cheng et al., 2012). Appropriately increasing the sowing rate can promote crop growth, enhance photosynthetic potential, and improve photosynthetically active radiation interception, which are crucial for the development of a dominant population (Jiang et al., 2021; Anjorin and Adebayo, 2024). Our results showed that under all three salinity levels, both aboveground biomass and tiller number increased with higher sowing rates under each priming treatment. Grain yield increased with higher sowing rates, primarily driven by a rise in panicle number. These findings align with those of Li et al. (2020), who reported that an increased sowing rate could boost tiller and panicle numbers and aboveground biomass, thereby enhancing the grain yield. In our study, under all three salinity conditions, the grain yield with priming treatment at 90 seeds m−2 was equivalent to that of rice without priming at 240 seeds m−2. Although increasing the sowing rate can improve rice emergence rate and grain yield, our study showed that reducing the sowing rate to 90 seeds m−2 with ZnO-nanoparticle priming treatment did not result in yield loss, significantly reducing cultivation costs in saline-alkali land. These results are consistent with the findings of Wang et al. (2014), who identified the optimal planting density for hybrid rice to be within the range of 70–118 seeds m−2. Therefore, the seed priming treatment could reduce the sowing rate under salt stress, thus lowering the cost of rice cultivation in saline-alkali soil. This cultivation measure provides practical feasibility for rice farming in saline areas.
Additionally, there was a substantial difference in average yields between the two years. The grain yield in 2023 (5.15 t ha−1) was 67.75% higher than that in 2022, mainly due to the increase in total solar radiation in 2023 (Figure 1), which significantly enhanced the light interception in the rice canopy, enhanced photosynthetic efficiency, and increased aboveground biomass, ultimately boosting yield (Lu et al., 2022). Furthermore, soil nutrient content significantly influenced yield. Studies have shown that soil nutrient levels affect nitrogen, phosphorus, and potassium absorption by plants, with a positive linear relationship observed between yield and the accumulation of these nutrients. Thus, differences in yield were primarily due to variations in soil fertility (Wang et al., 2015). Xiong et al. (2004) found that under the same fertilization and management conditions, soil with high organic matter content and elevated levels of one or two nutrients (N, P, or K) could increase yield, mainly due to the enhanced nutrient supply capacity of nutrient-rich soil. Other studies have shown that soil potassium has less effect on yield than nitrogen and phosphorus, and excessively high potassium levels can restrict the absorption of nitrogen and phosphorus in rice, thereby reducing yield (Yin et al., 2001). In the current study, notable variations in soil chemical properties were observed between the two seasons of the experimental field (Table 1). Therefore, the yield in 2023 was higher than that in 2022, mainly due to the higher nutrient content and stronger nutrient supply capacity of the 2023 experimental field. Additionally, the coordinated contents of N, P, and K in soil promoted nutrient absorption by rice, ultimately increasing yield.
The Na+ content in rice straw increased significantly, while the K+ content and K+/Na+ ratio decreased under salt conditions. Salt stress inhibited plant growth primarily through ion and osmotic stress (Ran et al., 2023; Farooq et al., 2021). As salt stress increased, Na+ content was increased while K+ content decreased due to the antagonistic interaction between Na+ and K+, resulting in nutrient imbalance in rice plants (García et al., 2010). However, seed priming treatment could improve the physiological resilience of crops under salt stress, reducing ionic toxicity to plants and ultimately promoting growth (Khan et al., 2022; Biswas et al., 2023; Zulfiqar et al., 2022). Under salt stress, elevated activity of the K+ uptake system is a critical adaptive trait. The accumulation of K+ in plant cells plays a vital role in osmotic adjustment, aiding in the maintenance of cellular water balance. In guard cells, K+ is crucial for stomatal closure, thereby minimizing excessive water loss through transpiration. Recent studies have suggested that proteins regulating K+ uptake channels in guard cells may serve as potential targets for enhancing crop abiotic tolerance (Locascio et al., 2019). In the present study, seed priming and sowing rate treatments alleviated salt stress by significantly decreasing Na+ content and increasing K+ content and K+/Na+ ratio. Research has demonstrated that seed priming can reduce Na+ content while enhancing the uptake of K+ and Ca+ in crops, maintaining ion homeostasis of crops and thus enhancing crop salt tolerance (Farhoudi, 2011; Sheteiwy et al., 2021; Tan et al., 2022; Sen and Puthur, 2020). The underlying mechanism may involve priming treatments increasing K+ accumulation in rice, with adequate K+ levels promoting solute deposition, lowering osmotic potential, and enhancing turgor pressure under osmotic stress. Consequently, sufficient K+ concentration supported osmotic adjustment, resulting in increased turgor pressure, improved water uptake, and reduced osmotic potential. These physiological changes strengthened the plant’s capacity to withstand salt stress (Kumar et al., 2020). Moreover, our results showed that under all three salinity levels, both Na+ and K+ contents in rice tended to decrease with increasing sowing rate. The phenomenon may be attributed to increased competition for available resources at higher sowing rates, which reduced Na+ and K+ uptake (Santiago-Arenas et al., 2021). However, after priming treatment, the rice maintained higher α-amylase activity, soluble sugar content, and protein content, reducing K+ absorption loss and ultimately increasing the K+/Na+ ratio. Therefore, priming treatment can enhance the competitive advantage of K+ over other nutrients under high sowing rates through osmotic regulation, ultimately reducing the sowing rate while maintaining the balance of potassium and sodium at salt concentrations below 3‰.
The germination and growth of DSR were inhibited under salt stress, leading to reduced yield. Under salt stress conditions, the α-amylase activity, soluble sugar and protein contents, and the activities of SOD, POD, and CAT were increased by seed priming treatments at each sowing rate, improving seed germination and seedling growth, further contributing to an increase in panicle number and thus improving the grain yield. Under all three salinity conditions, the grain yield of DSR with seed priming at a sowing rate of 90 seeds m−2 was comparable to, or even exceeded, that of non-primed rice at 240 seeds m−2. This can be attributed to seed priming treatments, which promoted a reduction in cell osmotic potential and facilitated osmotic adjustment to the saline stress. Additionally, the increased activity of antioxidant enzymes helped scavenge ROS, thereby improving the germination rate, enhancing the tiller number, and increasing the K+/Na+ ratio. These physiological changes compensated for the challenges posed by the lower sowing rate under saline conditions. In addition, under each salinity level and sowing rate condition, the grain yield under the ZnO-nanoparticle priming treatment was always the highest. In conclusion, to ensure the economic benefits of DSR production when the salt concentration is below 3‰, the sowing rate of DSR could be reduced to 90 seeds m−2 using ZnO-nanoparticle priming treatment. Nevertheless, the molecular mechanisms underlying nano-priming-mediated enhancement of plant salt tolerance remain poorly understood. Therefore, further research is necessary to evaluate the technology on a broader range of rice varieties and assess its applicability under varying environmental conditions.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
YZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HS: Writing – review & editing, Writing – original draft. AS: Writing – original draft, Writing – review & editing. YM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing. YL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing. LL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing. ZH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing. SS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing. YT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. LN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. ZZ: Conceptualization, Data curation, Methodology, Resources, Software, Validation, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Foundation of Major Projects in Hainan Province, China (ZDKJ202001), the Research Initiation Fund of Hainan University (KYQD (ZR) 19104), the earmarked fund for HNARS (HINARS-04-G03), and the National Natural Science Foundation of China (Project No. 32360301).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1541736/full#supplementary-material
Abeed, A. H. A., al-Huqail, A. A., Albalawi, S., Alghamdi, S. A., Ali, B., Alghanem, S. M. S., et al. (2023). Calcium nanoparticles mitigate severe salt stress in Solanum lycopersicon by instigating the antioxidant defense system and renovating the protein profile. S. Afr. J. Bot. 161, 36–52. doi: 10.1016/j.sajb.2023.08.005
Adetunji, A. E. (2020). Effects of inorganic salt solutions on vigour, viability, oxidative metabolism, and germination enzymes in aged cabbage and lettuce seeds. Plants. 9, 1164. doi: 10.3390/plants9091164
Ali, L. G., Nulit, R., Ibrahim, M. H., Yien, C. Y. S. (2020). Enhancement of germination and early seedling growth of rice (Oryza sativa) var. FARO44 by seed priming under normal and drought stressed conditions. J. Plant Nutr. 43, 1579–1593. doi: 10.1080/01904167.2020.1739298
Anjorin, F. B., Adebayo, K. O. (2024). Growth and yield responses of selected open and hybrid maize varieties to plant densities in the rainforest-savanna-transition agroecology of Nigeria. Agrosearch 22, 1–14. doi: 10.4314/agrosh.v22i2.1
Ben Rejeb, K., Abdelly, C., Savouré, A. (2014). How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 80, 278–284. doi: 10.1016/j.plaphy.2014.04.007
Biswas, S., Seal, P., Majumder, B., Biswas, A. K. (2023). Efficacy of seed priming strategies for enhancing salinity tolerance in plants: An overview of the progress and achievements. Plant Stress 9, 100186. doi: 10.1016/j.stress.2023.100186
Bussotti, F., Ferrini, F., Pollastrini, M., Fini, A. (2014). The challenge of Mediterranean sclerophyllous vegetation under climate change: From acclimation to adaptation. Environ. Exp. Bot. 103, 80–98. doi: 10.1016/j.envexpbot.2013.09.013
Cheeseman, J. (2016). “Food security in the face of salinity, drought, climate change, and population growth,” in Halophytes for Food Security in Dry Lands. Eds. Khan, M. Z. R., Shirazi, M. U., Khan, M. A., Subhan, S. M. (Elsevier), Academic Press, 111–123. doi: 10.1016/B978-0-12-801854-5.00007-8
Chen, X., Zhang, R., Xing, Y., Jiang, B., Li, B., Xu, X., et al. (2021). The efficacy of different seed priming agents for promoting sorghum germination under salt stress. PloS One 16, e0245505. doi: 10.1371/journal.pone.0245505
Cheng, J., Zhao, F., Zeng, S., Chen, X., Wang, L., Wu, J., et al. (2012). Effects of mechanical dry direct seeding density on photosynthesis and yield formation of rice population. Hubei Agric. Sci. 51, 5275–5278. doi: 10.14088/j.cnki.issn0439-8114.2012.23.063
Das, D., Basar, N. U., Ullah, H., Attia, A., Salin, K. R., Datta, A. (2022). Growth, yield, and water productivity of rice as influenced by seed priming under alternate wetting and drying irrigation. Arch. Agron. Soil Sci. 68, 1515–1529. doi: 10.1080/03650340.2021.1912320
Das, P., Nutan, K. K., Singla-Pareek, S. L., Pareek, A. (2015). Oxidative environment and redox homeostasis in plants: dissecting out significant contribution of major cellular organelles. Front. Environ. Sci. 2. doi: 10.3389/fenvs.2014.00070
Eswar, D., Karuppusamy, R., Chellamuthu, S. (2021). Drivers of soil salinity and their correlation with climate change. Curr. Opin. Environ. Sustain. 50, 310–318. doi: 10.1016/j.cosust.2020.10.015
Farhoudi, R. (2011). Effect of salt stress on physiological and morphological parameters of rapeseed cultivars. Adv. Environ. Biol. 5, 2501–2509.
Farooq, M. (2011). Rice direct seeding: Experiences, challenges and opportunities. Soil Tillage Res. 111, 87–98. doi: 10.1016/j.still.2010.10.008
Farooq, M., Barsa, S. M. A., Wahid, A. (2006). Priming of field-sown rice seed enhances germination, seedling establishment, allometry, and yield. Plant Growth Regul. 49, 285–294. doi: 10.1007/s10725-006-9138-y
Farooq, M., Park, J. R., Jang, Y. H., Kim, E. G., Kim, K. M. (2021). Rice cultivars under salt stress Show differential expression of genes related to the regulation of Na+/K+ balance. Front. Plant Sci. 12, 680131. doi: 10.3389/fpls.2021.680131
Farooq, M., Usman, M., Nadeem, F., Rehman, H. U., Wahid, A., Basra, S. M. A., et al. (2019). Seed priming in field crops: Potential benefits, adoption, and challenges. Crop Pasture Sci. 70, 731. doi: 10.1071/CP18604
García, M. J., Lucena, C., Romera, F. J., Alcántara, E., Pérez-Vicente, R. (2010). Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. J. Exp. Bot. 61, 3885–3899. doi: 10.1093/jxb/erq203
Gerona, M. E. B., Deocampo, M. P., Egdane, J. A., Ismail, A. M., Dionisio-Sese, M. L. (2019). Physiological responses of contrasting rice genotypes to salt stress at reproductive stage. Rice Sci. 26, 207–219. doi: 10.1016/j.rsci.2019.05.001
Guo, X., Zhi, W., Feng, Y., Zhou, G., Zhu, G. (2022). Seed priming improved salt-stressed sorghum growth by enhancing antioxidative defense. PloS One 17, e0263036. doi: 10.1371/journal.pone.0263036
Gupta, S., Doležal, K., Kulkarni, M. G., Balázs, E., Van Staden, J. (2022). Role of non-microbial biostimulants in regulation of seed germination and seedling establishment. Plant Growth Regul. 97, 271–313. doi: 10.1007/s10725-021-00794-6
Hasanuzzaman, M., Raihan, Md. R.H., Masud, A. A. C., Rahman, K., Nowroz, F., Rahman, M., et al. (2021). Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 22, 9326. doi: 10.3390/ijms22179326
Ibrahim, E. A. (2016). Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 192, 38–46. doi: 10.1016/j.jplph.2015.12.011
Iqbal, M., Ashraf, M. (2010). Changes in hormonal balance: A possible mechanism of pre-sowing chilling-induced salt tolerance in spring wheat. J. Agron. Crop Sci. 196, 440–454. doi: 10.1111/j.1439-037X.2010.00434.x
Itroutwar, P. D., Govindaraju, K., Tamilselvan, S., Kannan, M., Raja, K., Subramanian, K. S. (2020). Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.). J. Plant Growth Regul. 39, 717–728. doi: 10.1007/s00344-019-10012-3
Jiang, H., Thobakgale, T., Li, Y., Liu, L., Su, Q., Cang, B., et al. (2021). Construction of dominant rice population under dry cultivation by seeding rate and nitrogen rate interaction. Sci. Rep. 11, 7189. doi: 10.1038/s41598-021-86707-z
Jiang, H., Xing, X., Meng, X., Chen, J., Yu, K., Xu, X., et al. (2023). Research progress in water-saving cultivation of rice in China. Crop Sci. 63, 2623–2635. doi: 10.1002/csc2.21068
Khan, M. N., Fu, C., Li, J., Tao, Y., Li, Y., Hu, J., et al. (2023). Seed nanopriming: How do nanomaterials improve seed tolerance to salinity and drought? Chemosphere 310, 136911. doi: 10.1016/j.chemosphere.2022.136911
Khan, M. O., Irfan, M., Muhammad, A., Ullah, I., Nawaz, S., Khalil, M. K., et al. (2022). A practical and economical strategy to mitigate salinity stress through seed priming. Front. Environ. Sci. 10, 991977. doi: 10.3389/fenvs.2022.991977
Kumar, P., Kumar, T., Singh, S., Tuteja, N., Prasad, R., Singh, J. (2020). Potassium: A key modulator for cell homeostasis. J. Biotechnol. 324, 198–210. doi: 10.1016/j.jbiotec.2020.10.018
Li, Y., Ai, Z., Mu, Y., Zhao, T., Zhang, Y., Li, L., et al. (2023). Rice yield penalty and quality deterioration is associated with failure of nitrogen uptake from regreening to panicle initiation stage under salinity. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1120755
Li, L., Zhang, Z., Tian, H., Mo, Z., Ashraf, U., Duan, M., et al. (2020). Roles of nitrogen deep placement on grain yield, nitrogen use efficiency, and antioxidant enzyme activities in mechanical pot-seedling transplanting rice. Agronomy 10, 1252. doi: 10.3390/agronomy10091252
Liu, R., Zhang, H., Lal, H. (2016). Effects of stabilized nanoparticles of copper, zinc, manganese, and iron oxides in low concentrations on lettuce (Lactuca sativa) seed germination: nanotoxicants or nanonutrients? Water Air Soil pollut. 227, 42. doi: 10.1007/s11270-015-2738-2
Liu, S., Zhu, X., Wang, H., Yan, M. (2023). Effects of NaCl pretreatment on germination characteristics of rice seeds under salt stress. Northeast Agric. Sci. 48, 12–16. doi: 10.164/j.cnki.1003-8701.2023.05.003
Locascio, A., Marqués, M., García-Martínez, G., Corratgé-Faillie, C., Andres-Col-ás, N., Rubio, L., et al. (2019). BCL2ASSOCIATED ATHANOGENE4 regulates the kat1 potassium channel and controls stomatal movement. Plant Physiol. 181, 1277–1294. doi: 10.1104/pp.19.00224
Lu, J., Liu, K., Deng, J., Feng, X., Xiong, X., Huang, L., et al. (2022). Evaluating the effect of population density and the contribution of early canopy closure to grain yield of hybrid rice. J. Plant Growth Regul. 41, 830–839. doi: 10.1007/s00344-021-10342-1
Ma, H.-Y., Zhao, D.-D., Ning, Q.-R., Wei, J.-P., Li, Y., Wang, M.-M., et al. (2018). A multi-year beneficial effect of seed priming with gibberellic acid-3 (GA3) on plant growth and production in a perennial grass, Leymus chinensis. Sci. Rep. 8, 13214. doi: 10.1038/s41598-018-31471-w
Madkour, L. H. (2019). Function of reactive oxygen species (ROS) inside the living organisms and sources of oxidants. Pharma Sci. Anal. Res. J. 2, 180023.
Mahakham, W., Sarmah, A. K., Maensiri, S., Theerakulpisut, P. (2017). Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 7, 8263. doi: 10.1038/s41598-017-08669-5
Mathur, P., Chakraborty, R., Aftab, T., Roy, S. (2023). Engineered nanoparticles in plant growth: Phytotoxicity concerns and the strategies for their attenuation. Plant Physiol. Biochem. 199, 107721. doi: 10.1016/j.plaphy.2023.107721
Matias, J. R., Ribeiro, R. C., Aragão, C. A., Araújo, G. G. L., Dantas, B. F. (2015). Physiological changes in osmo- and hydro-primed cucumber seeds germinated in biosaline water. J. Seed Sci. 37, 151–160. doi: 10.1590/2317-1545v37n1135472
Mondal, S., Bose, B. (2021). Seed priming: An interlinking technology between seeds, Seed germination and seedling establishment. Plant Reprod. Ecology-Recent Adv. 107-122.
Mu, Y., Li, Y., Zhang, Y., Guo, X., Song, S., Huang, Z., et al. (2023). A comparative study on the role of conventional, chemical, and nanopriming for better salt tolerance during seed germination of direct seeding rice. J. Integr. Agric. 23, 3998–4017. doi: 10.1016/j.jia.2023.12.013
Mukhopadhyay, R., Sarkar, B., Jat, H. S., Sharma, P. C., Bolan, N. S. (2021). Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manage. 280, 111736. doi: 10.1016/j.jenvman.2020.111736
Mumtaz, M. Z., Saqib, M., Abbas, G., Akhtar, J., Qamar, Z. (2017). Genotypic variation in rice for grain yield and quality as affected by salt-affected field conditions. J. Plant Nutr. 40, 1–10. doi: 10.1080/01904167.2017.1385796
Mushtaq, Z. (2020). Salt stress, its impacts on plants and the strategies plants are employing against it: A review. J. Appl. Biol. 8, 81–91. doi: 10.7324/JABB.2020.80315
Paul, S., Dey, S., Kundu, R. (2022). Seed priming: An emerging tool towards sustainable agriculture. Plant Growth Regul. 97, 215–234. doi: 10.1007/s10725-021-00761-1
Phan, H. T., Haes, A. J. (2019). What does nanoparticle stability mean? J. Phys. Chem. 123, 16495–16507. doi: 10.1021/acs.jpcc.9b00913
Rai-Kalal, P., Jajoo, A. (2021). Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 160, 341–351. doi: 10.1016/j.plaphy.2021.01.032
Rajput, V. D., Harish, Singh, R. K., Verma, K. K., Sharma, L., Quiroz-Figueroa, F. R., et al. (2021). Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 10, 267. doi: 10.3390/biology10040267
Ran, C., Gao, D., Bai, T., Geng, Y., Shao, X., Guo, L. (2023). Straw return alleviates the negative effects of saline sodic stress on rice by improving soil chemistry and reducing the accumulation of sodium ions in rice leaves. Agriculture Ecosyst. Environ. 342, 108253. doi: 10.1016/j.agee.2022.108253
Rhaman, M. S., Imran, S., Rauf, F., Khatun, M., Baskin, C. C., Murata, Y., et al. (2020). Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 10, 37. doi: 10.3390/plants10010037
Sandhu, N., Yadav, S., Kumar Singh, V., Kumar, A. (2021). Effective crop management and modern breeding strategies to ensure higher crop productivity under direct seeded rice cultivation system: A review. Agronomy 11, 1264. doi: 10.3390/agronomy11071264
Santiago-Arenas, R., Dhakal, S., Ullah, H., Agarwal, A., Datta, A. (2021). Seeding, nitrogen, and irrigation management optimize rice water and nitrogen use efficiency. Nutr. Cycl. Agroecosyst. 120, 325–341. doi: 10.1007/s10705-021-10153-6
Sedghi, M., Nemati, A., Esmaielpour, B. (2010). Effect of seed priming on germination and seedling growth of two medicinal plants under salinity. Emirates. J. Food Agric. 22, 130. doi: 10.9755/ejfa.v22i2.4900
Sen, A., Puthur, J. T. (2020). “Seed priming-induced physiochemical and molecular events in plants coupled to abiotic stress tolerance: An overview,” in Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants. (Elsevier), Academic Press, 303–316. doi: 10.1016/B978-0-12-817892-8.00018-0
Sharma, D., Afzal, S., Singh, N. K. (2021). Nanopriming with phytosynthesized zinc oxide nanoparticles for promoting germination and starch metabolism in rice seeds. J. Biotechnol. 336, 64–75. doi: 10.1016/j.jbiotec.2021.06.014
Sheteiwy, M. S., Shao, H., Qi, W., Daly, P., Sharma, A., Shaghaleh, H., et al. (2021). Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine max L.) seedlings. J. Sci. Food Agric. 101, 2027–2041. doi: 10.1002/jsfa.10822
Sheteiwy, M. S., Shao, H., Qi, W., Hamoud, Y. A., Shaghaleh, H., Khan, N. U., et al. (2019). GABA-alleviated oxidative injury induced by salinity, osmotic stress, and their combination by regulating cellular and molecular signals in rice. Int. J. Mol. Sci. 20, 5709. doi: 10.3390/ijms20225709
Shi, Y., Gao, L., Wu, Z., Zhang, X., Wang, M., Zhang, C., et al. (2017). Genome-wide association study of salt tolerance at the seed germination stage in rice. BMC Plant Biol. 17, 92. doi: 10.1186/s12870-017-1044-0
Singh, R., Singh, S., Parihar, P., Mishra, R. K., Tripathi, D. K., Singh, V. P., et al. (2016). Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01299
Su, X., Zhan, J., Wang, J., Li, X., Wei, Y., Wu, H., et al. (2022). Development status of direct seeding rice and study on response mechanism of submergence. OALib 9, 1–13. doi: 10.4236/oalib.1108613
Sultan, H., Abbas, H. M. M., Faizan, M., Emamverdian, A., Shah, A., Bahadur, S., et al. (2025). Residual effects of biochar and nano-modified biochar on growth and physiology under saline environment in two different genotype of Oryza sativa L. J. Environ. Manage. 373, 123847. doi: 10.1016/j.jenvman.2024.123847
Sun, L., Hussain, S., Liu, H., Peng, S., Huang, J., Cui, K., et al. (2015). Implications of low sowing rate for hybrid rice varieties under dry direct-seeded rice system in Central China. Field Crops Res. 175, 87–95. doi: 10.1016/j.fcr.2015.02.009
Tan, Z., Zhu, H., He, X., Xi, B., Tian, Y., Sun, X., et al. (2022). Effect of ventilation quantity on electron transfer capacity and spectral characteristics of humic substances during sludge composting. Environ. Sci. pollut. Res. 29, 70269–70284. doi: 10.1007/s11356-022-20808-8
Thakur, M., Tiwari, S., Kataria, S., Anand, A. (2022). Recent advances in seed priming strategies for enhancing planting value of vegetable seeds. Sci. Hortic. 305, 111355. doi: 10.1016/j.scienta.2022.111355
Wang, Q., Chang, B., Sun, B., Gao, P., Liu, F., Gao, Z. (2015). Effects of different soil types on rice yield and nutrient absorption characteristics. Crop J. 23, 116–121. doi: 10.16035/j.issn.1001-7283.2015.03.022
Wang, D., Chen, S., Wang, Z., Ji, C., Xu, C., Zhang, X., et al. (2014). Optimizing hill seeding density for high-yielding hybrid rice in a single rice cropping system in South China. PloS One 9, e109417. doi: 10.1371/journal.pone.0109417
Wang, R., Li, L., Gentine, P., Zhang, Y., Chen, J., Chen, X., et al. (2022). Recent increase in the observation-derived land evapotranspiration due to global warming. Environ. Res. Lett. 17, 024020. doi: 10.1088/1748-9326/ac4291
Wen, W., Timmermans, J., Chen, Q., Bodegom, P. M. (2020). A review of remote sensing challenges for food security with respect to salinity and drought threats. Remote Sens. 13, 6. doi: 10.3390/rs13010006
Xiong, H., Tang, Y., Ren, D., Li, X., Cheng, K., Yao, W., et al. (2004). Relationship between different soil types, different climate conditions and rice yield. Southwest Agric. J. 305–309. doi: 10.16213/j.cnki.scjas.2004.03.008
Xu, E., Chen, M., He, H., Zhan, C., Cheng, Y., Zhang, H., et al. (2017). Proteomic analysis reveals proteins involved in seed imbibition under salt stress in rice. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.02006
Yin, X., Xu, W., Feng, J. (2001). Relationship between soil nutrients and rice yield in paddy fields in Jilin Province. J. Changchun Univ. Sci. Technol. 01, 74–77. doi: 10.13278/j.cnki.jjuese.2001.01.015
Younesi, O., Moradi, A. (2014). Effect of priming of seeds of Medicago sativa ‘Bami’ with gibberellic acid on germination, seedlings growth, and antioxidant enzymes activity under salinity stress. J. Hortic. Res. 22, 167–174. doi: 10.2478/johr-2014-0034
Yu, S., Xiong, Y., Xiao, J., Luo, L., Zhang, Q. (2016). Hybrid rice and green super rice. Chin. Sci. Bull. 61, 3797–3803. doi: 10.1360/N972016-01092
Zavariyan, A. M., Rad, M. Y., Asghari, M. (2015). Effect of seed priming by potassium nitrate on germination and biochemical indices in Silybum marianum L. under salinity stress. Int. J. Life Sci. 9, 23–29. doi: 10.3126/ijls.v9i1.11922
Zhang, H., Liu, C., Mao, L., Li, Y., Shen, Y. (2023). Divergent response of hay and grain yield of oat: Effects of environmental factors and sowing rate. J. Sci. Food Agric. 103, 233–242. doi: 10.1002/jsfa.12135
Keywords: direct seeding rice, salt stress, seed priming, sowing rate, grain yield
Citation: Zhang Y, Sultan H, Shah A, Mu Y, Li Y, Li L, Huang Z, Song S, Tao Y, Zhou Z and Nie L (2025) Regulation effect of seed priming on sowing rate of direct seeding of rice under salt stress. Front. Plant Sci. 16:1541736. doi: 10.3389/fpls.2025.1541736
Received: 08 December 2024; Accepted: 10 February 2025;
Published: 06 March 2025.
Edited by:
Baris Uzilday, Ege University, TürkiyeReviewed by:
Rafaqat Ali Gill, Lushan Botanical Garden (CAS), ChinaCopyright © 2025 Zhang, Sultan, Shah, Mu, Li, Li, Huang, Song, Tao, Zhou and Nie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixiao Nie, bHhuaWVAaGFpbmFudS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.