
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 29 January 2025
Sec. Plant Systematics and Evolution
Volume 16 - 2025 | https://doi.org/10.3389/fpls.2025.1525022
Introduction: Pollen is usually presented by the anthers after maturity. However, in some plants, pollen is presented to pollinators on other floral structures (other than the anthers), or via particular expulsion mechanisms, resulting in secondary pollen presentation. The unusual petal morphology in Berchemia mediates pollen presentation, characterised by a combination of secondary pollen presentation and primary pollen presentation. However, the function, the role in reproduction, and the evolutionary significance of the unusual petals remain unclear.
Methods: In this study, we took Berchemia flavescens and Berchemia polyphylla var. leioclada as examples, and used field observations, semi-thin sections technology, scanning electron microscopy, and pollination ecology detection methods to explore the unique pollen presentation strategies, petal functions, and reproductive strategies in Berchemia.
Results: This is a unique pollen presentation process mediated by petals. In the advanced bud stage, petals curl inward, enclosing the stamens. Following anther dehiscence, pollen is released into the petal tube, where the filament and cone-shaped anthers act as pistons, extruding pollen or pollen clumps through gaps at the petal tube apex (secondary pollen presentation). Subsequently, as the anthers emerge from the petal tube, residual pollen is directly presented to pollinators (primary pollen presentation).
Discussion: In Berchemia, the petals enclosing the stamens, effectively shield the pollen from extreme environmental conditions. The petal-stamen complex slow movement (first centrifugal, later centripetal) and pollen presentation in Berchemia suggest a unique reproductive strategy. This mechanism promotes outcrossing, minimizing interference between the pistil and stamens, and offers reproductive assurance by delayed self-pollination.
The diversity of angiosperm is largely reflected in the diversity of flora structures. Flowers are unique reproductive organs of angiosperms and show higher variability than those of all other groups (Zhang, 2004), making them ideal structures for pollination, fertilisation, and seed setting. Flowers achieve reproductive success through pollination (the pistil receives pollen to produce seeds) and pollen dispersal (the anther of the stamen disperses pollen). Under selection pressure to increase pollination and pollen distribution efficiency, the morphology and structure of stamens and pistils adapt to different pollination patterns, improving both pollination and reproductive success (Barrett, 2002; Ren, 2008), increasing the reproductive capacity of angiosperms (Bynum and Smith, 2001; He et al., 2005; Clark and Husband, 2007; Wang et al., 2010; Ren and Tang, 2012; Wiemer et al., 2012; Song et al., 2013; Abdusalam and Tan, 2014; Li et al., 2022).
Angiosperms can control the timing of pollen presentation and limit the amount of pollen carried by pollinators mainly through packaging and dispensing mechanisms (Harder and Wilson, 1994; Castellanos et al., 2006). Secondary pollen presentation (2PP) is one of the dispensing mechanism. While most plants present pollen directly by their anthers, plants with 2PP present pollen on other floral structures (other than the anthers) or via particular expulsion mechanisms (Howell et al., 1993; Yeo, 1993; Ladd, 1994). This phenomenon has been confirmed in at least 29 families of angiosperms (El Ottra et al., 2024). Pollen transfer typically occurs during the advanced bud stage, after pollen has been released at the advanced bud stage but before stigma reception (i.e., protandry) (El Ottra et al., 2024). The gradual release of pollen via 2PP allows pollinators to remove pollen in batches and deposit it on the stigma prolonging the duration of the male stage and improving the efficiency and accuracy of pollen transfer or deposition onto the stigma. Therefore, 2PP in angiosperms is biologically important for improving the fitness of males and females avoiding interference between male and female functions and promoting heterogametic fertilisation (El Ottra et al., 2024).
Petals are the most characteristic and eye-catching organs in eudicot flowers. The colour, morphology, size, structure, and function of petals have shown a wide variety throughout evolution (Endress and Matthews, 2006; Thien et al., 2009). Although petal diversity has been thought to be related to the different visual attractiveness functions of pollinators (Endress, 1994; Irish, 2009; Whitney et al., 2009a; Irish, 2010; Sulborska et al., 2012; De Craene, 2018; Konarska and Masierowska, 2020), this alone cannot fully explain all floral diversity (Endress, 1994). Beyond attraction, petals protect developing stamens and pistils (Delph, 1996), store nectar (Kramer and Hodges, 2010), apply pollen to pollinators (Yeo, 1993; De Almeida et al., 2013), emit olfactory attractants (Balao et al., 2011), and act as a landing foothold or platform for pollinators (Whitney et al., 2009b; Alcorn et al., 2012; Giovanetti and Aronne, 2013; Katsuhara et al., 2017). Additionally, “corolla dragging,” where withered corolla drag anthers across the stigma, can facilitate delayed selfing (Sun et al., 2005; Qu et al., 2007; Duan et al., 2010).
In most angiosperms, petals and stamens alternate (Gu and Hultgård, 2001). However, in families, such as Vitaceae (Chen et al., 2007; Ma et al., 2021; Parmar et al., 2021; Rabarijaona et al., 2023), Loasaceae (Weigend et al., 2010; Henning and Weigend, 2013; Leite et al., 2016; Acuna et al., 2018; Henning et al., 2018), Berberidaceae (Ying et al., 2011; Li et al., 2022; Zhang et al., 2023), and Rhamnaceae (Medan and D’ambrogio, 1998; Medan, 2003; Calvillo-Canadell and Cevallos-Ferriz, 2007; Chen and Schirarend, 2007; Wang G. et al., 2021; Patel et al., 2024), petals are mostly opposite to the stamens. Moreover, in Rhamnaceae the presence, absence, and morphology of petals vary among the different genera (Chen and Schirarend, 2007). The petals of Rhamnus are vestigial. In Ziziphus, the petals are obovate or spatulate but do not enclose the stamens. In contrast, in other genera, petals are typically obovate, hooded, or spatulate, and often enclose the stamens to varying degrees (Chen and Schirarend, 2007; Figure 1).
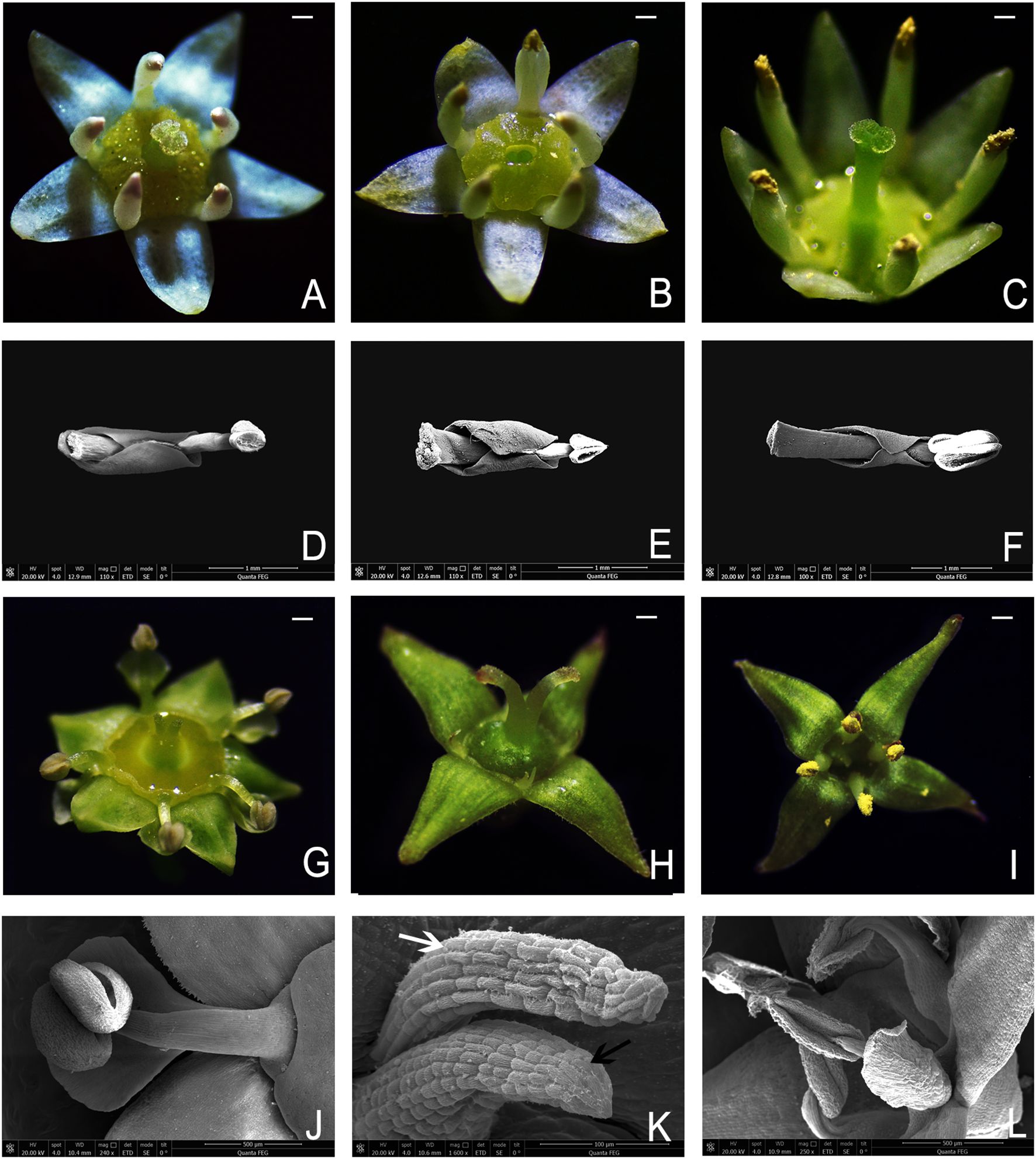
Figure 1. Flowers and petal–stamen complex of several plants in Rhamnaceae. (A) Hermaphroditic flower of B. flavescens. (B) Male flower of B. flavescens. (C) Hermaphroditic flower of B. polyphylla var. leioclada. (D) Petal–stamen complex of B. flavescens (hermaphroditic flower). (E) Petal–stamen complex of B. flavescens (male flower). (F) Petal–stamen complex of B. polyphylla var. leioclada. (hermaphroditic flower). (G) Hermaphroditic flower of Z. jujuba var. spinosa. (H) Female flower of R. tangutica. (I) Male flower of R. tangutica. (J) Petal and stamen of Z. jujuba var. spinosa. (K) Vestigial petal (white arrow) and stamen (black arrow) in the female flower of R. tangutica. (L) Stamens and petals in the male flower of R. tangutica. Scale bars = 1 mm.
In Berchemia, the petals opposite the stamens are typically spoon or pocket shaped and curl inwards to enclose the stamens forming a petal–stamen complex. These inward-curled petals lose their ability to attract pollinators. Medan and D’ambrogio (1998) suggested that in andromonoecious Trevoa quinquenervia, cucullate petals protect the anthers during the male phase, contributing to pollen delivered in different doses and reducing pollen theft. However, the function of the petal–stamen complex in Berchemia, which exhibits both androdioecious and hermaphroditic traits, remains unclear.
In this study, we investigated B. flavescens (at least morphologically androdioecious) and B. polyphylla var. leioclada (hermaphroditic), analysing their floral characteristics, and conducting experiments such as manual petal removal and separation. Our aim was to understand how the components of these plants coordinate to ensure successful pollination process and improve male reproductive fitness. We propose the following hypotheses: (1) B. flavescens and B. polyphylla var. leioclada exhibit 2PP, with petals playing a key role; (2) the slow movement of the petal–stamen complex may be related to the function of “petal dragging”; and (3) inward-curled petals protect the stamens from environmental stress, enhance pollen viability, increase pollen output, and reduce the likelihood of pollen theft.
This study primarily utilised live specimens collected from natural and artificial populations. Specimens of B. flavescens and Rhamnus tangutica were collected from natural populations in the Qinling Mountains, Shaanxi, China (E108°47′32.84″, N33°49′47.53″; 1,803-m elevation), while Berchemia sinica and Hovenia acerba were collected from another natural population in the Qinling Mountains, Shaanxi, China (E107°45′33.37″, N34°03′38.76″; 1,219-m elevation). Specimens of B. polyphylla var. leioclada were obtained from artificially cultivated populations at Northwest University, Xi’an, Shaanxi, China (E108°55′39.72″, N34°14′58.61″). Additionally, Ziziphus jujuba var. spinosa was collected from Qingliangshan Forest Park, Xi’an, Shaanxi, China (E108°55′29.54″, N34°10′29.90″).
The populations of B. flavescens and B. sinica consist of both hermaphroditic (Figure 1A) and male (Figure 1B) adult individuals. However, only hermaphroditic adult individuals were found in the populations of B. polyphylla var. leioclada (Figure 1C), Z. jujuba var. spinosa (Figure 1G), and H. acerba. In contrast, the population of R. tangutica consisted of female (Figure 1H) and male (Figure 1I) adult individuals.
Voucher specimens of all plants collected for this study were deposited in the Northwest University Herbarium (Xi’an, China) under accession numbers NWU19349 to NWU19353.
Flowers of B. flavescens and B. polyphylla var. leioclada are yellow-green and actinomorphic, and their five petals are spatulate, obovate, or hood shaped. The five stamens, which are antepetalous, are often enclosed by the petals forming a petal–stamen complex (Figures 1A–F). Since the petals of hermaphroditic flowers resemble those of male flowers, we focused on hermaphroditic flowers of B. flavescens and B. polyphylla var. leioclada to observe their floral characteristics. Flowering process, petal morphology features, and stamen changes were observed on 30 tagged well-developed flower buds. In addition, we also observed the morphological characteristics of petals and the changes of stamens in B. sinica, H. acerba, R. tangutica, and Z. jujuba var. spinosa. Dissected floral organs were photographed with a stereomicroscope (Leica EZ4 W, Germany).
Thirty flower buds of B. flavescens and 30 flower buds of B. polyphylla var. leioclada were randomly selected and tagged in each population. The state of the flowers and anthers was observed and photographed hourly from 7 a.m. on the day before flowering until the flowers withered.
Pollen viability was determined using a modified Alexander staining method (Peterson et al., 2010). Briefly, pollen was placed in Alexander dye for 15–30 min at 37°C. The number of non-aborted (magenta-red) and aborted pollen grains (blue-green) in each field was counted under a microscope (Nikon Eclipse 50i, equipped with a DS-Fil camera, Japan), and pollen viability was calculated.
Stigmatic receptivity was determined using the benzidine–hydrogen peroxide method (Dafni, 1992). Briefly, flowers in different flowering stages were collected during peak flowering, and the stigmas were immersed in a benzidine–hydrogen peroxide solution [4:11:22, benzidine: 1% hydrogen peroxide: 3% water (v/v/v)] for 3 min. Receptivity was indicated by the presence of bubbles and blue stain. Degrees of receptivity were assigned based on the intensity of the reaction: no reaction (−), weak positive (+), strong positive (++), or very strong positive (+++) (Dafni and Maués, 1998).
To investigate pollen presentation strategies, pollination efficiency, and whether the petals act as key organs for 2PP, we empirically studied pollen removal as an indicator of reproductive success in B. flavescens and B. polyphylla var. leioclada. Twenty flower buds (undehisced anthers) were selected and fixed directly in FAA (50%) solution for statistical analysis of pollen grain numbers within the anthers. In addition, 20 flowers of each species were randomly selected to calculate the amount of pollen removed between the pollen was extruded through the cleft or gap in the petal tube and before the anther protruded from the petal tube. After the anthers had fully protruded from the petal tube, the anthers from 20 flowers of each species were removed, and the remaining pollen was counted. When the anthers moved to the top of the stigma, the anthers from 20 flowers of each species were removed, and the remaining pollen was counted.
To count pollen, stamens from each flower were hydrolysed in 8 M NaOH for 10 min and then crushed using a dissecting needle. The volume was brought up to 1 ml using 10% KCl solution. Pollen grains were counted using a microscope (Nikon Eclipse 50i equipped with a DS-Fil camera, Japan). This process was repeated five times.
During the peak flowering phase, the species and behaviour of visiting insects were observed and recorded continuously from 7:00 to 18:00 every day over 2–3 days of fine weather. Pollinators were identified by observing their effective contact with the anthers and/or stigmas and confirming the presence of pollen on their bodies. In addition, the flower-visiting behaviour was photographed, and specimens were collected for identification.
To investigate how petals, as key organs of 2PP, coordinate with stamens to disperse pollen during pollen presentation, we studied the morphology and structure of petals and examined their development.
Flowers and petals of B. flavescens and B. polyphylla var. leioclada were collected in EP tubes and fixed in 50% FAA. The specimens were then dehydrated through an ethanol series (70%, 85%, 95%, and 100%) and subsequently through a series of anhydrous ethanol–isoamyl acetate mixtures [2:1, 1:1, and 1:2 (all v/v)], finishing with 100% isoamyl acetate. After dehydration, the flowers and petals were then dried in a CO2 critical point dryer, sputter-coated with gold, examined and photographed using a Quanta FEG 450 scanning electron microscope.
Flowers at different developmental stages were collected and fixed in 2.5% glutaraldehyde in 0.1 mol L−1 phosphate buffer (pH 7.0) at 4°C. Semi-thin sections were prepared using routine methods (Liu et al., 2022; Ma et al., 2024), dehydrated using a gradient series of ethanol, and transitioned through ethanol and 1,2-epoxypropane to 1,2-epoxypropane before being embedded in Epon 812 resin. Subsequently, 2-μm sections were cut using a Leica RM2155 microtome with glass knives. The sections were then stained with toluidine blue O and observed under a microscope (Nikon Eclipse 50i equipped with a DS-Fil camera, Japan).
To verify that the petals mediate pollen removal and the function of “petal dragging” of B. flavescens and B. polyphylla var. leioclada, we conducted empirical research to determine whether the removal or separation of petals affected reproductive success.
One hundred well-developed and open flower buds were randomly selected and labelled, then divided into five treatment groups as follows: (1) petals removed, (2) petals and stamens separated, (3) stigmas destroyed, (4) artificial cross-pollination, and (5) control (no treatment). The position and morphology of the stamens were recorded every hour until the flowers wilted. In addition, the time at which the stamens moved slowly above the stigma after treatment was recorded.
All statistical analyses were performed using SPSS software (version 17.0). Analysis of variance was used to determine whether there was a significant difference in the amount of pollen dispersed between 2PP and PPP, with a significance level set at p < 0.05. Results are presented as mean ± SE. All experiments were repeated three times. Additional statistical analyses were performed using GraphPad Prism 8.0.2.263 software.
The petals of B. flavescens and B. polyphylla var. leioclada enclosed the stamens forming a petal–stamen complex (Figures 1A–F). This complex moved slowly during flowering (Figure 2), and the shape of the petals changed constantly (Figure 3). During the small flower bud stage, the petals covered or pressed against the anthers and surrounded the pistils (Figures 3A, B, N, O). Before blooming, the middle sections of the petals partially overlapped giving them a “cucullate” appearance as they curl inwards (Figures 3C, P). At the advanced bud stage, the anthers had already dehisced longitudinally (Figures 3D, Q, 4E, F), releasing pollen grains into the tube formed by the wrapped petals (Figures 3C, D, P, Q). As the petals, and filaments continued to elongate, the tops of the cucullate petals appeared as a cleft or gap (Figures 3F, S). The filament and cone-shaped anther acted as pistons pushing pollen grains in the petal tube to be extruded out in clumps or clusters through a cleft or gap at the top of the petal tube (2PP; Figures 4A–D). As the filaments and petals grew, the anthers gradually protruded from the petal tube, with some pollen remaining in the anthers. The mode of pollen presentation changed from the secondary pollen presentation (2PP) to the primary pollen presentation (PPP). Additionally, the petals gradually transitioned from a “cucullate” to a “shawl-shaped” form (Figures 3H–M, U–Z). Initially, the petal–stamen complex moved slowly in a centrifugal direction, which obviously increases the distance between the anther and stigma (Figures 2A–C, E–G, I–K). Subsequently, the complex moved slowly in a centripetal direction. Finally, the anthers of hermaphroditic flowers gathered above the stigma (Figure 2D, L), and the anthers of male flowers gathered at the centre of the flower (Figure 2H).

Figure 2. Flower dynamics of B. flavescens and B. polyphylla var. leioclada. (A–D) Hermaphroditic flower of B. flavescens. (E–H) Male flower of B. flavescens. (I–L) Hermaphroditic flower of B. polyphylla var. leioclada. Scale bars = 1 mm.
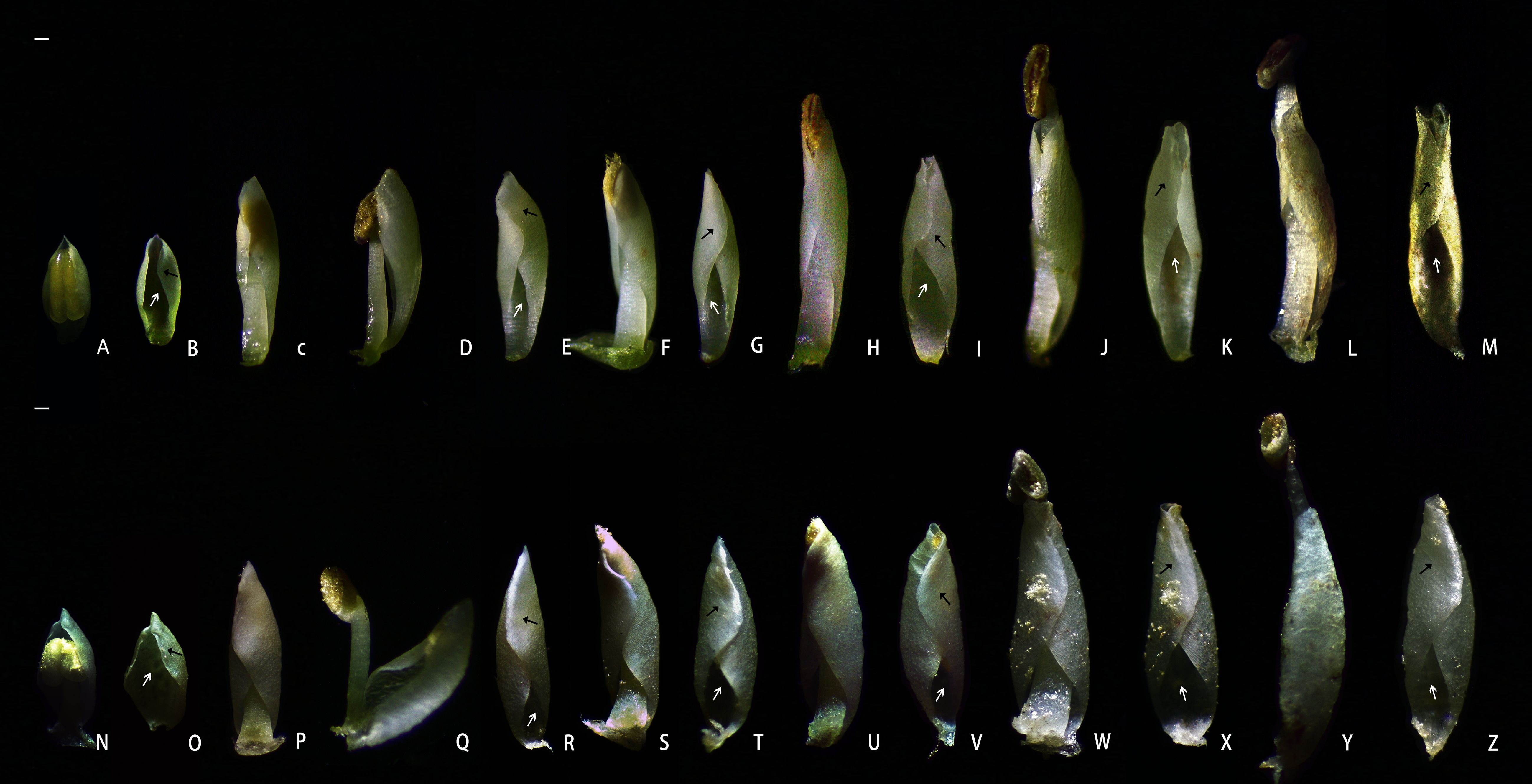
Figure 3. Morphological changes in the petal–stamen complex during pollen presentation of hermaphroditic flowers in B. polyphylla var. leioclada and B. flavescens. (A–M) Petal–stamen complex of B. polyphylla var. leioclada (hermaphroditic flower). (N–Z) Petal–stamen complex of B. flavescens (hermaphroditic flower). Adaxial surface of the flower petal indicated by a white arrow; abaxial surface of the flower petal indicated by a black arrow. Scale bars = 0.5 mm.
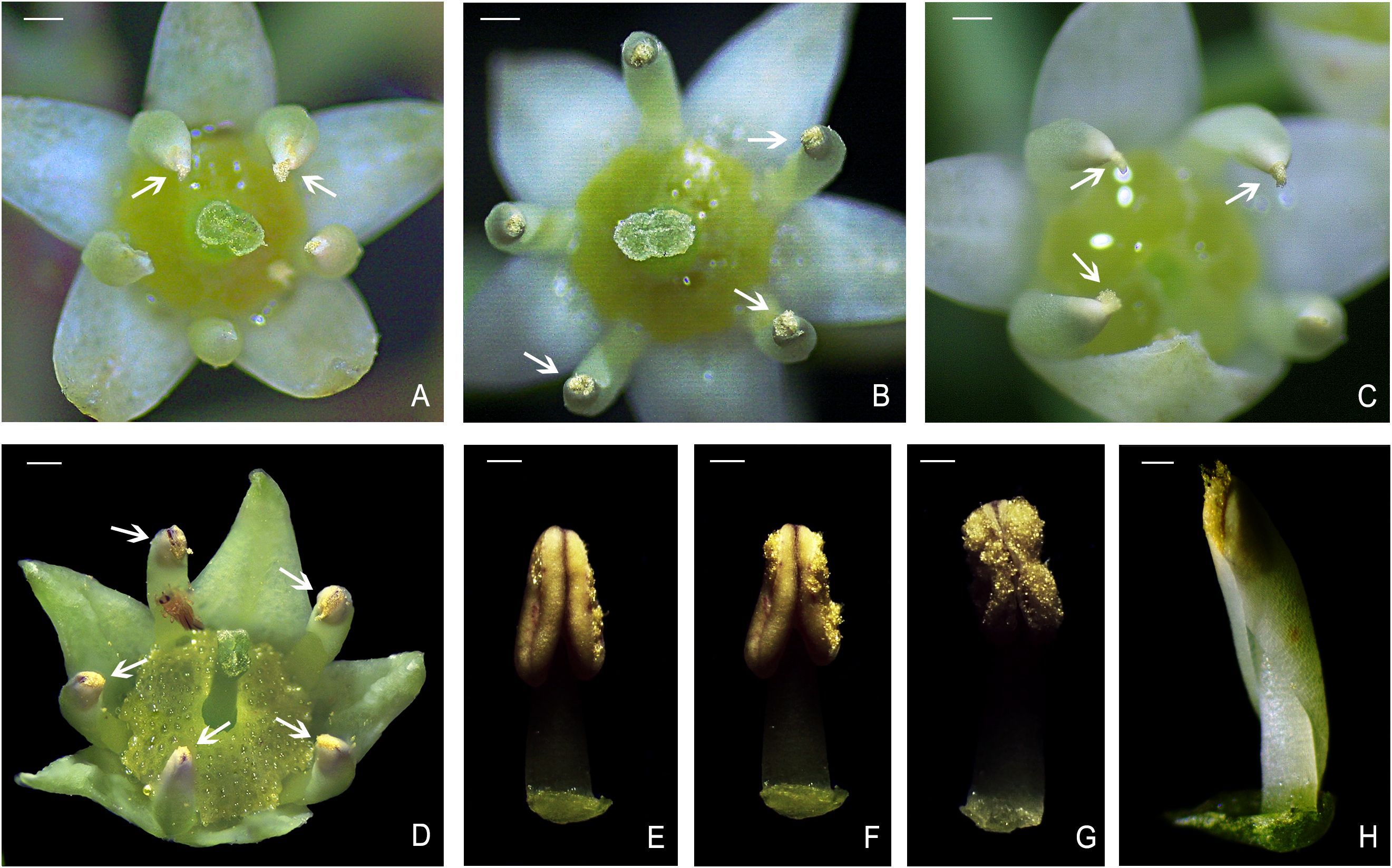
Figure 4. Secondary pollen presentation (2PP) in B. flavescens and B. polyphylla var. leioclada. (A, B) Pollens pushed out of the gap in the petal tube of the hermaphroditic flower in B. flavescens (arrow). (C) Pollens pushed out of the gap in the petal tube of the male flower in B. flavescens (arrow). (D) Pollens pushed out of the gap in the petal tube of the hermaphroditic flower in B. polyphylla var. leioclada flower (arrow). (E–G) Process of anther dehiscence after petal removal in B. polyphylla var. leioclada. (H) Petal–stamen complex of B. polyphylla var. leioclada. Scale bars (A–D) = 1 mm. Scale bars (E–H) = 0.5 mm.
The flowers of B. flavescens and B. polyphylla var. leioclada exhibited typical protandrous characteristics. Before blooming (the advanced bud stage), the anthers were enclosed within the petals (anther dehiscence occurred), and pollen grains were first released into the petal tube (Figures 3C, D, P, Q). At this point, pollen viability was high, while stigmatic receptivity was almost absent (Table 1).
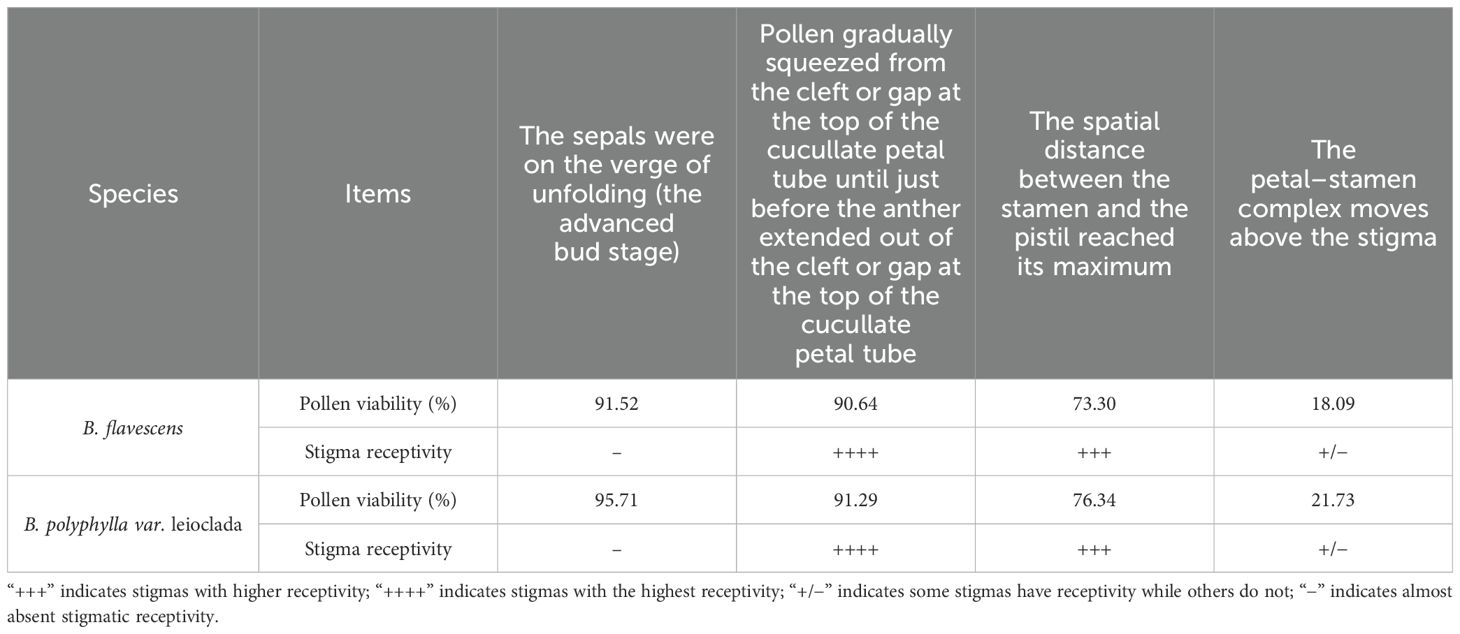
Table 1. Pollen viability and stigmatic receptivity of B. flavescens and B. polyphylla var. leioclada.
Stigmatic receptivity reached its peak when pollen was extruded from the cleft or gap of the petal tube during the secondary pollen presentation (2PP) stage. As the flowering process advanced, pollen viability gradually declined, and stigmatic receptivity weakened after the anthers emerged from the petal tube cleft. Notably, when the anthers approached the stigma later in the flowering process, a small number of pollen grains remained in the anthers. These remaining pollen grains retained viability, and the stigma maintained some receptivity (Table 1).
When the anthers of B. flavescens and B. polyphylla var. leioclada dehisced, the released pollen was enveloped by the petal tube (Figures 3C, D, P, Q). The pollen presentation process can be divided into two stages. In the first stage (2PP), a cleft or gap created at the top of the petal tube due to the elongation of filaments and petals (Figures 3F, S), allowing clusters of pollen grains to be extruded from this opening and gradually presented to pollinators (Figure 4). Petals are key organs for 2PP. The time taken for pollen to be pushed out of the petal tube by the anthers until the anther begins to extend the petal tube of B. flavescens was approximately 7.643 ± 0.508 h (indicating that 2PP lasts about this duration). During this time, approximately 14,081 ± 253.3 pollen grains were released constituting approximately 73.4% of the total pollen. For B. polyphylla var. leioclada, the time from when pollen was extruded from the petal tube gap to the point when the anthers began to protrude out of the petal tube was approximately 2.686 ± 0.108 h (indicating that 2PP lasts about this duration). Approximately 7,637 ± 163.6 pollen grains were released accounting for roughly 29.8% of the total pollen.
With the elongation of the filaments and petals, the anthers gradually protruded from the petal tubes (Figures 3H, J, L, U, W, Y). Pollens were still present in the anthers at this time, and the mode of pollen presentation changed from the secondary pollen presentation (2PP) to the primary pollen presentation (PPP). The number of pollen grains in B. flavescens remaining in the anthers after they protruded from the top of the petal tube was approximately 5,094 ± 141.5. It took approximately 63.5 ± 1.080 h from the beginning of the anther protruding from the petal tube to slow the movement above the stigma. In B. polyphylla var. leioclada, approximately 17,947 ± 467.4 pollen grains remained in the anthers when the anther protruded from the petal tube; the duration is approximately 58.514 ± 4.022 h until they slowly move above the stigma.
The floral nectaries around the base of the ovary in B. flavescens and B. polyphylla var. leioclada belong to disc nectary (Figure 5). The nectary is usually composed of a secretory epidermis, nectariferous tissue, and vascular bundles. The epidermal cells are nearly square or rectangular and arranged neatly. The nectariferous tissue is composed of three to six layers of small, polygonal cells with large nuclei and dense cytoplasm (Figures 5A, B). After flowering, colourless and transparent nectar is visible on the surface of the flower disc, attracting floral insects (Figures 4A–D). In the population of B. flavescens, approximately 19 insect species from seven orders were recorded (Figures 6A–D). The largest order was Diptera, which accounted for 36.9% of the total insect visitors, followed by Hymenoptera (26.3%), Coleoptera (15.8%), and Lepidoptera, Hemiptera, Araneae, and Acarina accounted for 5.25% each. Approximately 31 insect species were observed in B. polyphylla var. leioclada belonging to five orders (Figures 6E–O). The largest order was Hymenoptera accounting for 35.5% of the total insect visitors, followed by Diptera (32.3%), Lepidoptera (22.6%), Hemiptera (6.4%), and Neuroptera (3.2%).

Figure 5. Disc and disc nectary of B. flavescens and B. polyphylla var. leioclada. (A) Longitudinal section of the disc of hermaphroditic flower in B. flavescens. (B) Longitudinal section of the disc of hermaphroditic flower in B. polyphylla var. leioclada. (C) SEM of the disc in B. flavescens (hermaphroditic flower). (D) SEM of the disc in B. polyphylla var. leioclada (hermaphroditic flower). Scale bars = 50 μm.

Figure 6. Flower-visiting insects of B. flavescens and B. polyphylla var. leioclada. (A) Apidae. (B) Syrphidae. (C) Muscidae. (D) Syrphidae. (E) Apidae. (F) Syrphidae. (G, H) Apidae. (I) Vespidae. (J) Crabronidae. (K) Syrphidae. (L) Calliphoridae. (M) Scoliidae. (N) Papilionidae. (O) Gasteruptiidae.
The mature petal epidermal cells of B. flavescens (both male and hermaphroditic) and B. polyphylla var. leioclada (hermaphroditic) predominantly displayed an arrangement of flat rectangles, spindles, or fusiform shapes. The adaxial epidermal cells exhibited numerous longitudinal, straight, and fine stripes, with a few displaying wavy stripes (Figures 7A–C, G–I). However, the abaxial epidermal cells primarily featured transverse, oblique, or twisted stripe arrangements (Figures 7D–F, J–L).
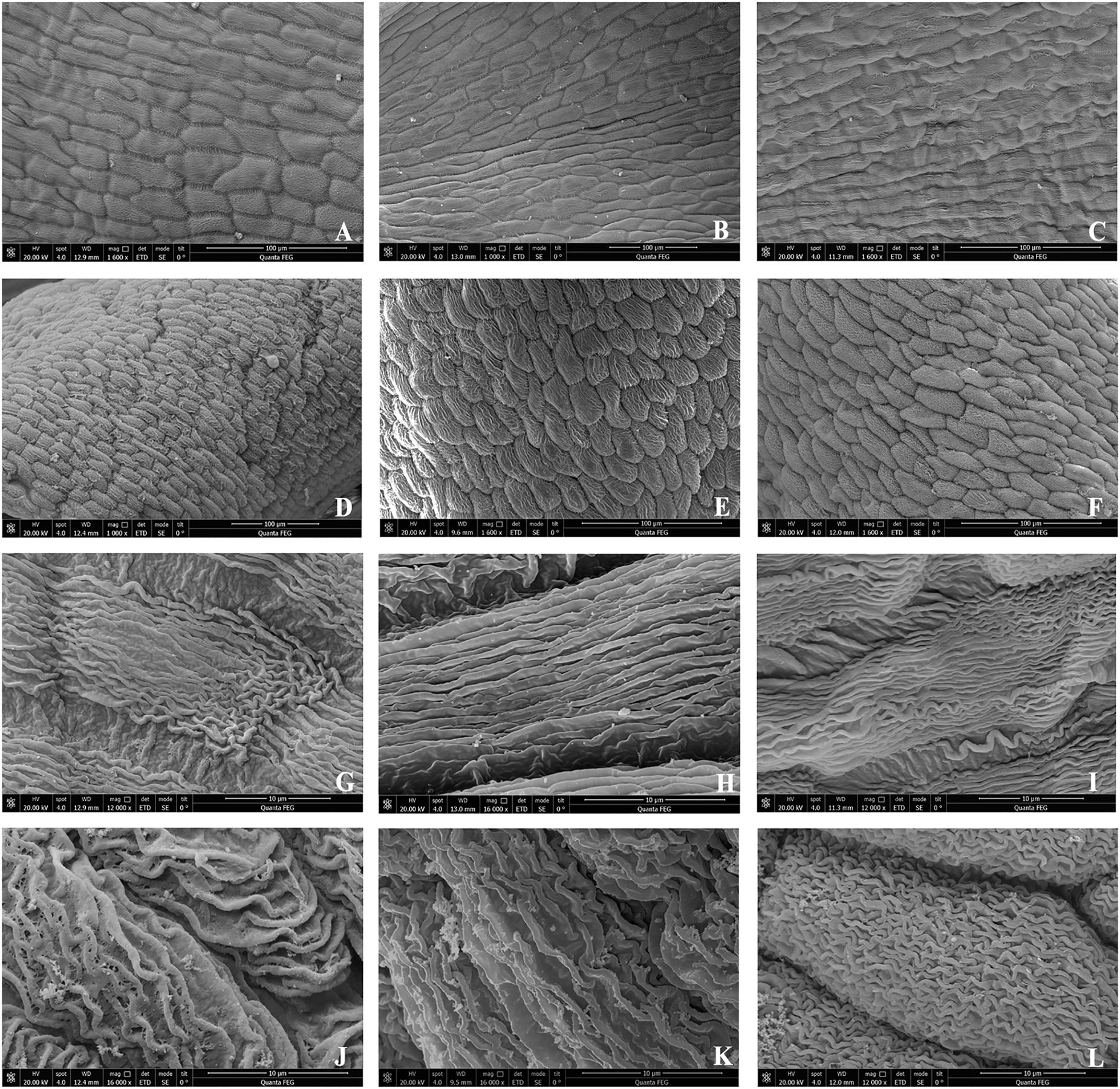
Figure 7. SEM showing the surface of the petals in B. flavescens and B. polyphylla var. leioclada. (A, G) Adaxial surface of the male flower petal of B. flavescens. (D, J) Abaxial surface of the male flower petal of B. flavescens. (B, H) Adaxial surface of the hermaphroditic flower petal of B. flavescens. (E, K) Abaxial surface of the hermaphroditic flower petal of B. flavescens. (C, I) Adaxial surface of the hermaphroditic flower petal of B. polyphylla var. leioclada. (F, L) Abaxial surface of the hermaphroditic flower petal of B. polyphylla var. leioclada.
Transverse sections of mature petals revealed that the petals of B. flavescens (male and hermaphroditic flowers), B. sinica (male and hermaphroditic flowers), B. polyphylla var. leioclada (hermaphroditic flowers), H. acerba (hermaphroditic flowers), and Z. jujuba var. spinosa (hermaphroditic flowers) were composed of two layers of epidermal cells (upper and lower), parenchyma cells, and vascular tissues. The epidermal cells were larger in volume and had thicker cytoplasm, whereas the parenchyma cells of the mesophyll were smaller and exhibited an irregular shape. The volume of epidermal cells in the middle area of the petal was notably larger than that at the edge of petal (Figures 8A–G). In the middle sections of petals, approximately three to five layers of parenchyma cells were observed between the upper and lower epidermis, whereas the edge regions contained either no parenchyma cells or a single layer (Figures 8A–G). In contrast, the petals of R. tangutica were vestigial (Figures 8H, I). Specifically, the transverse sections of female petals were oval in shape (Figures 8I).
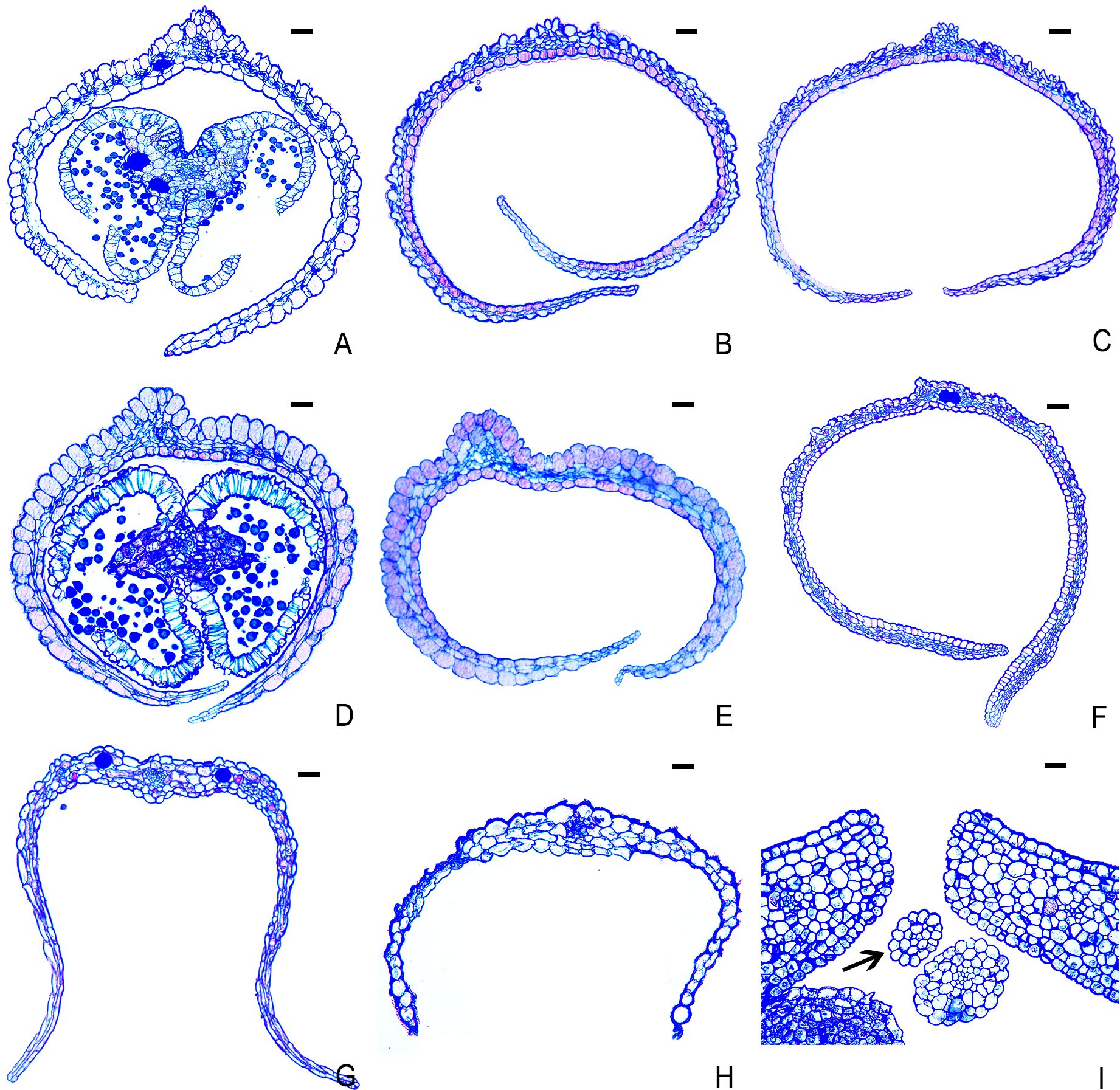
Figure 8. Transverse sections of petals of several plants in Rhamnaceae. (A) B. polyphylla var. leioclada (hermaphroditic flower). (B) B. flavescens (hermaphroditic flower). (C) B. flavescens (male flower). (D) B. sinica (male flower). (E) B. sinica (hermaphroditic flower). (F) H. acerba (hermaphroditic flower). (G) Z. jujuba var. spinosa (hermaphroditic flower). (H) R. tangutica (male flower). (I) R. tangutica (female flower, arrow indicates petal). Scale bars = 10 μm.
Additionally, the adaxial surface of the petals appeared relatively smooth and flat, whereas the abaxial surface was characterised by numerous bumps (Figures 7, 8).
After conducting treatments—petal removal, separation of petals and stamens, destruction of stigmas, artificial cross-pollination, and a control group (no treatment), we observed significant changes in the movement of the petal–stamen complex relative to the stigma (Figure 9). Compared with the control and artificial cross-pollination treatments, the time for stamens to return to the stigma was the shortest in the stigma treatment. In addition, when the petals were removed or the petals and stamens were separated, the stamens of some B. flavescens and B. polyphylla var. leioclada failed to gather near the stigma. In these cases, the stamens curl outwards and lie flat or recline on the flower disc (Figure 10).
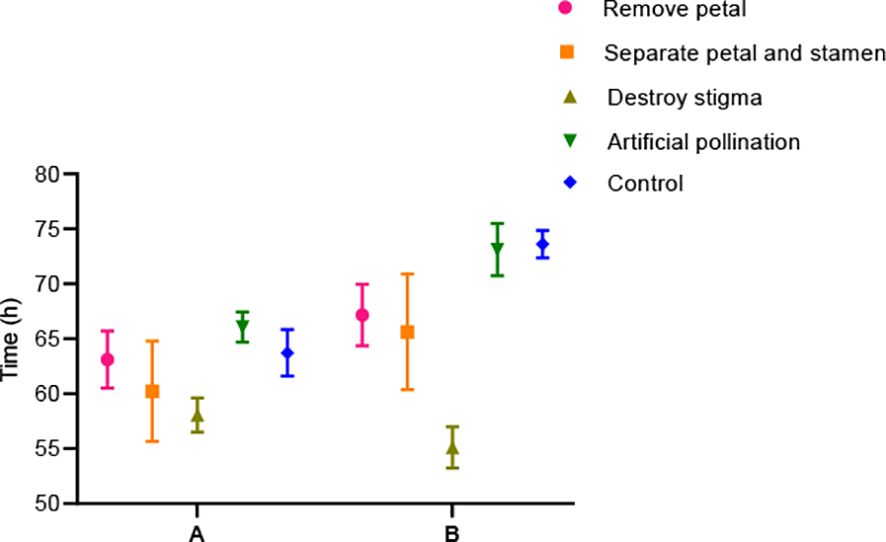
Figure 9. Time for stamen movement to reach above/near the stigma in the hermaphroditic flowers of B. flavescens and B. polyphylla var. leioclada after artificial treatments. (A) B. polyphylla var. leioclada. (B) B. flavescens.

Figure 10. Effects of artificial petal removal and separation of the petal–stamen complex on stamen movement in hermaphroditic flowers of B. flavescens and B. polyphylla var. leioclada. (A–C) B. flavescens. (D–F) B. polyphylla var. leioclada. Arrows indicate those stamens that curl outwards and lie flat or recline on the flower disc. Scale bars = 1 mm.
The male fitness of angiosperms largely depends on the “fate” of their pollen, with successful plant reproduction relying on the efficient transfer of a significant amount of pollen between plants. However, in species with granular pollen, less than 1% of the pollen removed from the anther successfully reaches the stigma (Stanton et al., 1992; Harder, 2000). This low male fitness has driven plants to evolve strategies that enhance pollination accuracy leading to the development of diverse pollen presentation mechanisms.
In B. flavescens and B. polyphylla var. leioclada, pollen presentation can be divided into two stages: 2PP and PPP. The cone-shaped anthers, with a pointed upper end and wide lower end, provide space for pollen release (Figures 3C, P, 4E–H). Pollen grains were first released into the petal tube after anther dehiscence. The cone-shaped anther and filament acted as a piston and gradually presented the pollen in the petal tube to pollinators by pushing out the pollen in clumps or clusters from below to above, rather than releasing it simultaneously. This mode manifested as 2PP mechanism. Petals play a crucial role in 2PP process. The petals on the adaxial surface are relatively smooth and flat (Figures 7, 8), which reduces the friction between the pollen and petals and is conducive to the extrusion of pollen from the petal tube. As the anthers gradually protrude from the petal tube, the remaining pollen is directly presented to pollinators by the anthers, manifested as PPP mechanism. At the end of flowering, the petal–stamen complex moves centripetally, positioning above the stigma, allowing the unpollinated pistil the opportunity for selfing.
Berchemia employs a complex pollen presentation strategy that combines 2PP and PPP, a phenomenon not yet reported in other plant taxa (Fan et al., 2015; Yang et al., 2019; El Ottra et al., 2024). The evolution of the petals in Berchemia is adapted to this mode of pollination.
Owing to the sessile growth habit of flowering plants, sexual reproduction depends on successful pollen transfer. From this point of view, the fate of the pollen (that is, whether the pollen can be transferred to the stigma of the pistil) determines the fate of the future flower. Plants have evolved elaborate flowers to promote outcrossing and increase the genetic diversity of their offspring. Although plants bloom in large numbers to attract pollinators, this inevitably leads to selfing. Breeding strategies of outcrossing and selfing not only exist in different plants but also in many species with mixed mating systems that delay selfing. The decisive factors influencing mating modes include the distance between anthers and stigmas, spatial changes in stamens or pistils, and temporary flower closures (petal movement) (Dole, 1992; Barrett, 2003). Many plants achieve pollination and secure reproduction by the movement of their floral organs.
In B. flavescens and B. polyphylla var. leioclada, the petal–stamen complex exhibits slow movements during flowering. Anthers have dehisced while the complex surrounds the pistil at the advanced bud stage (Figures 3C, D, P, Q). However, as the petals curl inwards on both sides and enclose the stamens, pollen grains are released into the petal tube to prevent selfing. As the flower gradually opens and the petals and filaments grow, pollen is slowly squeezed out of the cleft at the top of the cucullate petal tube. During this period, the petal-stamen complex moves in the centrifugal direction, and the distance between pistil and stamen increases significantly (Figures 2A–C, E–G, I–K). This movement effectively hinders selfing. Subsequently, the petal–stamen complex of hermaphroditic flowers moves back in the centripetal direction to a position above the stigma (Figures 2D, L) allowing the unpollinated pistil the opportunity for selfing. This mechanism, where selfing through anther–stigma contact occurs after the loss of outcrossing opportunities, exemplifies “delayed selfing” (Ippolito and Armstrong, 1993; Sun et al., 2005; Duan et al., 2007; Qu et al., 2007). In this study, pollen was gradually presented from the beginning of pollen release to the end of the petal–stamen complex movement in B. flavescens and B. polyphylla var. leioclada extending the duration of pollen release and enhancing male reproductive fitness through pollen gradual presentation. According to the reproductive assurance hypothesis, the fitness advantage of self-pollination is that cross-pollination occurs first when outcrossing opportunities arise; However, when cross-pollination is not feasible, self-pollination occurs (Aarssen, 2000). Therefore, B. flavescens and B. polyphylla var. leioclada exhibit mixed mating systems where delayed selfing serves as a reproductive assurance strategy in environments where cross-pollination is limited. This floral design and the behaviour of the petal–stamen complex promote outcrossing, avoiding interference between pistils and stamens, and enable delayed selfing, ensuring reproduction (Ren and Tang, 2012; Armbruster et al., 2014; Goodwillie and Weber, 2018; Liu et al., 2020; Abdusalam et al., 2021; Minnaar and Anderson, 2021; Silva-Batista et al., 2021).
Petal diversity is widely believed to play a role in attracting pollinators (Whitney et al., 2009a; Sulborska et al., 2012; De Craene, 2018; Konarska and Masierowska, 2020). However, this function alone cannot fully explain the extensive floral diversity observed across species (Endress, 1994). In Berchemia, the petals are positioned opposite the stamens, enclosing them to provide protection and enhance pollen output efficiency, albeit at the cost of reduced ability to attract pollinators. The presence of open disc nectaries, which are less selective for nectar-feeding insects, suggests that B. flavescens and B. polyphylla var. leioclada attract a diverse range of flower-visiting insects. Consequently, these species experience high pollinator diversity and abundance (Figures 5, 6).
Pollen is released through the petal tube ensuring that each pollinator collects only a small amount of pollen per visit. This mechanism minimizes pollen waste, prolongs the pollen release period, and enhances pollination accuracy (Thomson et al., 2000). Therefore, the secondary pollen presentation (2PP) mechanism, resulting from the stamens being enclosed by the petals, represents an effective adaptive strategy in Berchemia for optimising reproductive success in the context of diverse and abundant pollinator interactions.
In this study, mature petals of B. flavescens and B. polyphylla var. leioclada exhibited differences in cell growth rates between the middle and edge regions. Cells in the middle region of the petal grew faster than the cells at the edge resulting in a significant expansion force in the middle of the petals, while the edges of the petals are subjected to a tensile force. In addition, the number of adaxial epidermal cells in the petal was lower than that of the abaxial epidermal cells, which is conducive to the sides of the petals curling inwards. The petals gradually curled inwards towards both sides and eventually enclosed the stamens under the involution force caused by this uneven growth. After removing the petals or separating the petals and stamens, some of the stamens curl outwards and lie flat or recline on the flower disc indicating that the anthers did not gather above the stigma (Figure 10). This phenomenon indirectly verified the “dragging effect” of the petals indicating that the dragging of petals enhances selfing accuracy in these two species. The dragging effect of petals promotes the slow movement of the petal–stamen complex, thereby promoting cross-pollination, avoiding the interference between the pistils and stamens, and achieving delayed selfing, thereby ensuring reproductive success for the plants.
Pollen is highly susceptible to damage from UV-B radiation and high temperatures caused by direct sunlight (Feng et al., 2000; Sato et al., 2002; Aylor, 2004). For plants without pollen protection measures, the direct exposure of pollen to sunlight (especially strong UV-B radiation) can result in varying degrees of pollen damage (Zhang et al., 2014). Flat epidermal cells have weaker light absorption abilities than conical epidermal cells (Noda et al., 1994; Gorton and Vogelmann, 1996). In B. flavescens and B. polyphylla var. leioclada, flat petal epidermal cells were observed instead of conical ones (Figure 7), illustrating the protective effect of petals on pollen. The petals opposite the stamens, allowing the petals to enclose the stamens as they curl inwards, effectively shielding them from rainwater erosion and intense UV-B radiation, likely prolonging pollen vitality, and improving the pollen output rate.
In Rhamnaceae, petals are mostly opposite the stamens, but their presence or absence and shapes vary among the different genera (Chen and Schirarend, 2007; Figure 1). Moreover, the ability of petals to attract pollinators is greatly degraded in Rhamnaceae. However, the evolution of petals is closely related to the way the pollen is presented and the type of breeding system. In Berchemia, which exhibits androdioecious (at least morphologically androdioecious) and hermaphroditic breeding system, the 2PP formed by petals enclosing the stamens effectively prolongs the time of pollen presentation, reduces interference between stamens and pistils through petal dragging, and promotes cross-pollination and delayed selfing. In Ziziphus, which has hermaphroditic flowers, petals do not enclose stamens but, instead, droop outwardly in late flowering stages (Figure 1G). This mechanism is not intended to promote delayed selfing; rather, it aims to prevent self-pollination and enhance outcrossing rates (Wang F. et al., 2021). This suggests a typical PPP mechanism in Ziziphus, contrasting with those of plants in Berchemia, showing the significant role of petals in the flowering process. Moreover, in Rhamnus (dioecy), the petals are vestigial forming rod-like shapes or even disappear (Chen and Schirarend, 2007). This may be the result of adaptation to complete outcrossing.
Therefore, the unique pollen presentation strategy caused by the inwards curl of the petals of Berchemia may represent a derived floral trait. In Rhamnaceae, the pollen presentation strategy may have evolved from the simplest form—primary pollen presentation (where petals do not enclose the stamens) to a complex pollen presentation strategy combining primary and secondary pollen presentation (with petals enclosing the stamens) and then to complete outcrossing (petals vestigial). This evolution may lead to outcrossing and delayed selfing, characterised by vestigial petal.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
FM: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. QZ: Data curation, Formal analysis, Methodology, Software, Writing – original draft. XT: Data curation, Formal analysis, Methodology, Software, Writing – original draft. Y-lF: Investigation, Writing – original draft. W-zL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from the National Natural Science Foundation of China (Grant no. 32371693).
We gratefully acknowledge and thank Professor Jiangli Tan of Northwest University for identifying the insects. Concurrently, we also thank the reviewers for spending their valuable time to provide constructive comments.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aarssen, L. W. (2000). Why are most selfers annuals? A new hypothesis for the fitness benefit of selfing. Oikos 89, 606–612. doi: 10.1034/j.1600-0706.2000.890321.x
Abdusalam, A., Maimaitituerxun, R., Hashan, H., Abdukirim, G. (2021). Pollination adaptations of group-by-group stamen movement in a meadow plant with temporal floral closure. Plant Divers. 43, 308–316. doi: 10.1016/j.pld.2021.04.001
Abdusalam, A., Tan, D. Y. (2014). Contribution of temporal floral closure to reproductive success of the spring-flowering Tulipa iliensis. J. Syst. Evol. 52, 186–194. doi: 10.1111/jse.12036
Acuna, R., Chinchilla, I. F., Weigend, M. (2018). An unusual disjunction in Loasaceae: Central American Chichicaste grandis is nested in Brazilian Aosa. Phytotaxa 365, 273–287. doi: 10.11646/phytotaxa.365.3.5
Alcorn, K., Whitney, H., Glover, B. (2012). Flower movement increases pollinator preference for flowers with better grip. Funct. Ecol. 26, 941–947. doi: 10.1111/j.1365-2435.2012.02009.x
Armbruster, W. S., Corbet, S. A., Vey, A. J. M., Liu, S. J., Huang, S. Q. (2014). In the right place at the right time: Parnassia resolves the herkogamy dilemma by accurate repositioning of stamens and stigmas. Ann. Bot. 113, 97–103. doi: 10.1093/aob/mct261
Aylor, D. E. (2004). Survival of maize (Zea mays) pollen exposed in the atmosphere. Agr. For. Meteorol. 123, 125–133. doi: 10.1016/j.agrformet.2003.12.007
Balao, F., Herrera, J., Talavera, S., Dötterl, S. (2011). Spatial and temporal patterns of floral scent emission in Dianthus inoxianus and electroantennographic responses of its hawkmoth pollinator. Phytochemistry 72, 601–609. doi: 10.1016/j.phytochem.2011.02.001
Barrett, S. C. H. (2002). The evolution of plant sexual diversity. Nat. Rev. Genet. 3, 274–284. doi: 10.1038/nrg776
Barrett, S. C. H. (2003). Mating strategies in flowering plants: the outcrossing-selfing paradigm and beyond. Philos. Trans. R Soc. Lond B Biol. Sci. 358, 991–1004. doi: 10.1098/rstb.2003.1301
Bynum, M. R., Smith, W. K. (2001). Floral movements in response to thunderstorms improve reproductive effort in the alpine species Gentiana algida (Gentianaceae). Am. J. Bot. 88, 1088–1095. doi: 10.2307/2657092
Calvillo-Canadell, L., Cevallos-Ferriz, S. R. S. (2007). Reproductive structures of Rhamnaceae from the Cerro del Pueblo (Late Cretaceous, Coahuila) and Coatzingo (Oligocene, Puebla) Formations, Mexico. Am. J. Bot. 94, 1658–1669. doi: 10.3732/ajb.94.10.1658
Castellanos, M. C., Wilson, P., Keller, S. J., Wolfe, A. D., Thomson, J. D. (2006). Anther evolution: pollen presentation strategies when pollinators differ. Am. Nat. 167, 288–296. doi: 10.1086/498854
Chen, Z. D., Ren, H., Wen, J. (2007). “Vitaceae,” in Flora of China, vol. 12 . Eds. Wu, Z. Y., Raven, P. H., Hong, D. Y. (Beijing/St. Louis: Science Press/Missouri Botanical Garden Press), 173–222.
Chen, Y. L., Schirarend, C. (2007). “Rhamnaceae,” in Flora of China, vol. 12 . Eds. Wu, Z. Y., Raven, P. H., Hong, D. Y. (Beijing/St. Louis: Science Press/Missouri Botanical Garden Press), 115–168.
Clark, M. J., Husband, B. C. (2007). Plasticity and timing of flower closure in response to pollination in Chamerion angustifolium (Onagraceae). Int. J. Plant Sci. 168, 619–625. doi: 10.1086/513486
Dafni, A. (1992). Pollination Ecology: A practical approach (New York: Oxford University Press), 1–57.
Dafni, A., Maués, M. M. (1998). A rapid and simple procedure to determine stigma receptivity. Sex Plant Reprod. 11, 177–180. doi: 10.1007/s004970050138
De Almeida, N. M., De Castro, C. C., De Lima Leite, A. V., Novo, R. R., MaChado, I. C. (2013). Enantiostyly in Chamaecrista ramosa (Fabaceaesta970050138803.12. floral morphology, pollen transfer dynamics and breeding system. Plant Biol. 15, 369–375. doi: 10.1111/j.1438-8677.2012.00651.x
De Craene, L. R. (2018). Understanding the role of floral development in the evolution of angiosperm flowers: clarifications from a historical and physico-dynamic perspective. J. Plant Res. 131, 367–393. doi: 10.1007/s10265-018-1021-1
Delph, L. F. (1996). Flower Size Dimorphism in Plants with Unisexual Flowers (New York: Springer), 217–237.
Dole, J. A. (1992). Reproductive assurance mechanisms in three taxa of the mimulus guttatus complex (scrophulariaceae). Am. J. Bot. 79, 650–659. doi: 10.1002/j.1537-2197.1992.tb14607.x
Duan, Y. W., Dafni, A., Hou, Q. Z., He, Y. P., Liu, J. Q. (2010). Delayed selfing in an alpine biennial gentianopsis paludosa (Gentianaceae) in the Qinghai-Tibetan plateau. J. Integr. Plant Biol. 52, 593–599. doi: 10.1111/j.1744-7909.2010.00951.x
Duan, Y. W., He, Y. P., Zhang, T. F., Liu, J. Q. (2007). Delayed selfing in an alpine species Gentianopsis barbata. J. Plant Ecol. (Chinese Version) 31, 110–117. doi: 10.17521/cjpe.2007.0014
El Ottra, J. H. L., Toni, J. F. G., Thaowetsuwan, P., Dos Santos, P., Jeiter, J., De Craene, L. R., et al. (2024). Pollen transfer within flowers: how pollen is secondarily presented. Int. J. Plant SciI. 185, 15–31. doi: 10.1086/727514
Endress, P. K. (1994). Diversity and Evolutionary Biology of Tropical Flowers (Cambridge, UK: Cambridge University Press).
Endress, P. K., Matthews, M. L. (2006). Elaborate petals and staminodes in eudicots: Diversity, function, and evolution. Org. Divers. Evol. 6, 257–293. doi: 10.1016/j.ode.2005.09.005
Fan, Y. L., Kress, W. J., Li, Q. J. (2015). A new secondary pollen presentation mechanism from a wild ginger (Zingiber densissimum) and its functional roles in pollination process. PloS One 10, e0143812. doi: 10.1371/journal.pone.0143812
Feng, H., An, L., Tan, L., Hou, Z., Wang, X. (2000). Effect of enhanced ultraviolet-B radiation on pollen germination and tube growth of 19 taxa in vitro. Environ. Exp. Bot. 43, 45–53. doi: 10.1016/S0098-8472(99)00042-8
Giovanetti, M., Aronne, G. (2013). Honey bee handling behaviour on the papilionate flower of Robinia pseudoacacia L. Arthropod-Plant Inte 7, 119–124. doi: 10.1007/s11829-012-9227-y
Goodwillie, C., Weber, J. J. (2018). The best of both worlds? A review of delayed selfing in flowering plants. Am. J. Bot. 105, 641–655. doi: 10.1002/ajb2.1045
Gorton, H. L., Vogelmann, T. C. (1996). Effects of epidermal cell shape and pigmentation on optical properties of antirrhinum petals at visible and ultraviolet wavelengths. Plant Physiol. 112, 879–888. doi: 10.1104/pp.112.3.879
Gu, C. Z., Hultgård, U.-M. (2001). “Parnassia,” in Flora of China, vol. 8 . Eds. Wu, Z. Y., Raven, P. H. (Beijing/St. Louis: Science Press/Missouri Botanical Garden Press), 358–379.
Harder, L. D. (2000). “Pollen dispersal and the floral diversity of Monocotyledons,” in Monocots: systematics and evolution. Eds. Wilson, K. L., Morrison, D. (CSIRO Publishing, Melbourne), 243–257.
Harder, L. D., Wilson, W. G. (1994). Floral evolution and male reproductive success: Optimal dispensing schedules for pollen dispersal by animal-pollinated plants. Evol. Ecol. 8, 542–559. doi: 10.1007/BF01238257
He, Y. P., Duan, Y. W., Liu, J. Q., Smith, W. K. (2005). Floral closure in response to temperature and pollination in Gentiana straminea Maxim. (Gentianaceae), an alpine perennial in the Qinghai-Tibetan Plateau. Plant Syst. Evol. 256, 17–33. doi: 10.1007/s00606-005-0345-1
Henning, T., Mittelbach, M., Ismail, S. A., Acuna-Castillo, R. H., Weigend, M. (2018). A case of behavioural diversification in male floral function - the evolution of thigmonastic pollen presentation. Sci. Rep. 8, 14018. doi: 10.1038/s41598-018-32384-4
Henning, T., Weigend, M. (2013). Beautiful, complicated—and intelligent? Novel aspects of the thigmonastic stamen movement in Loasaceae. Plant Signal Behav. 8, e24605. doi: 10.4161/psb.24605
Howell, G. J., Slater, A. T., Knox, R. B. (1993). Secondary pollen presentation in angiosperms and its biological significance. Aust. J. Bot. 41, 417–438. doi: 10.1071/bt9930417
Ippolito, A., Armstrong, J. E. (1993). Floral biology of hornstedtia scottiana (Zingiberaceae) in a lowland rain forest of Australia. Biotropica 25, 281–289. doi: 10.2307/2388786
Irish, V. F. (2009). Evolution of petal identity. J. Exp. Bot. 60, 2517–2527. doi: 10.1093/jxb/erp159
Irish, V. F. (2010). The flowering of Arabidopsis flower development. Plant J. 61, 1014–1028. doi: 10.1111/j.1365-313X.2009.04065.x
Katsuhara, K. R., Kitamura, S., Ushimaru, A. (2017). Functional significance of petals as landing sites in fungus-gnat pollinated flowers of Mitella pauciflora (Saxifragaceae). Funct. Ecol. 31, 1193–1200. doi: 10.1111/1365-2435.12842
Konarska, A., Masierowska, M. (2020). Structure of floral nectaries and female-biased nectar production in protandrous species Geranium macrorrhizum and Geranium phaeum. Protoplasma 257, 501–523. doi: 10.1007/s00709-019-01454-3
Kramer, E. M., Hodges, S. A. (2010). Aquilegia as a model system for the evolution and ecology of petals. Phil. Trans. R. Soc B 365, 477–490. doi: 10.1098/rstb.2009.0230
Ladd, P. G. (1994). Pollen presenters in the flowering plants–form and function. Bot. J. Linn 115, 165–195. doi: 10.1111/j.1095-8339.1994.tb01777.x
Leite, A. V., Nadia, T., MaChado, I. C. (2016). Pollination of Aosa rupestris (Hook.) Weigend (Loasaceae): are stamen movements induced by pollinators? Braz. J. Bot. 39, 559–567. doi: 10.1007/s40415-016-0258-y
Li, D. F., Han, W. L., Renner, S. S., Huang, S. Q. (2022). Touch-sensitive stamens enhance pollen dispersal by scaring away visitors. eLife 11, e81449. doi: 10.7554/eLife.81449
Liu, J., Bi, B. X., Tian, G. H., Li, Z. W., Wang, W. R., Ma, F., et al. (2022). Crystal idioblasts are involved in the anther dehiscence of Nicotiana tabacum. Physiol. Plant. 174, e13753. doi: 10.1111/ppl.13753
Liu, C. C., Gui, M. Y., Sun, Y. C., Wang, X. F., He, H., Wang, T. X., et al. (2020). Doubly guaranteed mechanism for pollination and fertilization in Ipomoea purpurea. Plant Biol. J. 22, 910–916. doi: 10.1111/plb.13121
Ma, F., Fu, Y. L., Wei, W. J., Li, Z. W., Liu, J., Bi, B. X., et al. (2024). Zygotic quiescence prolongs the reproductive cycle in Berchemia sinica (Rhamnaceae). Flora 314, 152493. doi: 10.1016/j.flora.2024.152493
Ma, Z. Y., Nie, Z. L., Ren, C., Liu, X. Q., Zimmer, E. A., Wen, J. (2021). Phylogenomic relationships and character evolution of the grape family (Vitaceae). Mol. Phylogenet. Evol. 154, 106948. doi: 10.1016/j.ympev.2020.106948
Medan, D. (2003). Reproductive biology of the andean shrub discaria nana (Rhamnaceae). Plant Biol. 5, 94–102. doi: 10.1055/s-2003-37980
Medan, D., D’ambrogio, A. C. (1998). Reproductive biology of the andromonoecious shrub Trevoa quinquenervia (Rhamnaceae). Bot. J. Linn Soc. 126, 191–206. doi: 10.1111/j.1095-8339.1998.tb02526.x
Minnaar, C., Anderson, B. (2021). A combination of pollen mosaics on pollinators and floral handedness facilitates the increase of outcross pollen movement. Curr. Biol. 31, 3180–3184.e3. doi: 10.1016/j.cub.2021.04.074
Noda, K., Glover, B. J., Linstead, P., Martin, C. (1994). Flower colour intensity depends on specialized cell shape controlled by a Myb-related transcription factor. Nature 369, 661–664. doi: 10.1038/369661a0
Parmar, G., Dang, V., Rabarijaona, R. N., Chen, Z. D., Jackes, B. R., Barrett, R. L., et al. (2021). Phylogeny, character evolution and taxonomic revision of Causonis, a segregate genus from Cayratia (Vitaceae). Taxon 70, 1188–1218. doi: 10.1002/tax.12562
Patel, R., Rana, R. S., Ali, A., Hazra, T., Khan, A. M. A. (2024). First buckthorn (Rhamnaceae) fossil flowers from India. J. Syst. Evol. 62, 829–841. doi: 10.1111/jse.13024
Peterson, R., Slovin, J. P., Chen, C. (2010). A simplified method for differential staining of aborted and non-aborted pollen grains. Intl J. Plant Biol. 1, e13. doi: 10.4081/pb.2010.e13
Qu, R. M., Li, X. J., Luo, Y. B., Dong, M., Xu, H. L., Chen, X., et al. (2007). Wind-dragged corolla enhances self-pollination: A new mechanism of delayed self-pollination. Ann. Bot. 100, 1155–1164. doi: 10.1093/aob/mcm209
Rabarijaona, R. N., Ranaivoson, R. M., Yu, J. R., You, Y. C., Liu, B., Ye, J. F., et al. (2023). Species delimitation and biogeography of Cyphostemma (Vitaceae), emphasizing diversification and ecological adaptation in Madagascar. Taxon 72, 766–790. doi: 10.1002/tax.12980
Ren, M. X. (2008). Stamen fusion in plants: diversity, adaptive significance, and taxonomic implications. J. Syst. Evol. 46, 452–466. doi: 10.3724/SP.J.1002.2008.06184
Ren, M. X., Tang, J. Y. (2012). Up and down: stamen movements in Ruta graveolens (Rutaceae) enhance both outcrossing and delayed selfing. Ann. Bot. 110, 1017–1025. doi: 10.1093/aob/mcs181
Sato, S., Peet, M. M., Thomas, J. F. (2002). Determining critical pre- and posticaling/m periods and physiological processes in Lycopersicon esculentum Mill. exposed to moderately elevated temperatures. J. Exp. Bot. 53, 1187–1195. doi: 10.1093/jexbot/53.371.1187
Silva-Batista, I. C. D., Da Costa, F. G. C. M., De Assunção, T. S., Koschnitzke, C., Vieira, R. C., Bove, C. P. (2021). First report of osmophores and wet stigma in Podostemaceae with notes on floral biology and pollination of Weddellina squamulosa Tul. Flora 278, 151799. doi: 10.1016/j.flora.2021.151799
Song, B., Zhang, Z. Q., Stöcklin, J., Yang, Y., Niu, Y., Chen, J. G., et al. (2013). Multifunctional bracts enhance plant fitness during flowering and seed development in Rheum nobile (Polygonaceae), a giant herb endemic to the high Himalayas. Oecologia 172, 359–370. doi: 10.1007/s00442-012-2518-2
Stanton, M. L., Ashman, T. L., Galloway, L. F., Young, H. J. (1992). “Estimating mate fitness of plants in natural populations,” in Ecology and evolution of plant reproduction. Ed. Wyatt, R. (Chapman & Hall, London), 62–90.
Sulborska, A., Weryszko-Chmielewska, E., Chwil, M. (2012). Micromorphology of Rosa rugosa Thunb. petal epidermis secreting fragrant substances. Acta Agrobot. 65, 21–28. doi: 10.5586/aa.2012.018
Sun, S. G., Guo, Y. H., Gituru, R. W., Huang, S. Q. (2005). Corolla wilting facilitates delayed autonomous self-pollination in Pedicularis dunniana (Orobanchaceae). Plant Syst. Evol. 251, 229–237. doi: 10.1007/s00606-004-0260-x
Thien, L. B., Bernhardt, P., Devall, M. S., Chen, Z. D., Luo, Y. B., Fan, J. H., et al. (2009). Pollination biology of basal angiosperms (ANITA grade). Am. J. Bot. 96, 166–182. doi: 10.3732/ajb.0800016
Thomson, J. D., Wilson, P., Valenzuela, M., Malzone, M. (2000). Pollen presentation and pollination syndromes, with special reference to Penstemon. Plant Spec. Biol. 15, 11–29. doi: 10.1046/j.1442-1984.2000.00026.x
Wang, Y., Meng, L. L., Yang, Y. P., Duan, Y. W. (2010). Change in floral orientation in Anisodus luridus (Solanaceae) protects pollen grains and facilitates development of fertilized ovules. Am. J. Bot. 97, 1618–1624. doi: 10.3732/ajb.1000010
Wang, G. T., Shu, J. P., Jiang, G. B., Chen, Y. Q., Wang, R. J. (2021). Morphology and molecules support the new monotypic genus Fenghwaia (Rhamnaceae) from south China. PhytoKeys 171, 25–35. doi: 10.3897/phytokeys.171.57277
Wang, F., Sun, X. H., Dong, J. B., Cui, R., Liu, X., Li, X. X., et al. (2021). A primary study of breeding system of Ziziphus jujuba var. spinosa. Sci. Rep. 11, 10318. doi: 10.1038/s41598-021-89696-1
Weigend, M., Ackermann, M., Henning, T. (2010). Reloading the revolver - male fitness as a simple explanation for complex reward partitioning in Nasa macrothyrsa (Loasaceae, Cornales): reloading the revolver. Biol. J. Linn Soc. 100, 124–131. doi: 10.1111/j.1095-8312.2010.01419.x
Whitney, H. M., Chittka, L., Bruce, T. J. A., Glover, B. J. (2009b). Conical epidermal cells allow bees to grip flowers and increase foraging efficiency. Curr. Biol. 19, 948–953. doi: 10.1016/j.cub.2009.04.051
Whitney, H. M., Kolle, M., Andrew, P., Chittka, L., Steiner, U., Glover, B. J. (2009a). Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators. Science 323, 130–133. doi: 10.1126/science.1166256
Wiemer, A. P., Sérsic, A. N., Marino, S., Simões, A. O., Cocucci, A. A. (2012). Functional morphology and wasp pollination of two South American asclepiads (Asclepiadoideae–Apocynaceae). Ann. Bot. 109, 77–93. doi: 10.1093/aob/mcr268
Yang, S. L., Chu, G. M., Shi, X., Wang, S. M. (2019). Elaborated pollen packaging and dispensing mechanism induced by petal architecture from a Papaveraceae species. PeerJ 7, e7066. doi: 10.7717/peerj.7066
Yeo, P. F. (1993). Secondary Pollen Presentation: Form, Function and Evolution (Heidelberg: Springer-Verlag).
Ying, J. S., David, E. B., Anthony, R. B. (2011). “Berberidaceae,” in Flora of China, vol. 19 . Eds. Peter, H. R., Zhang, L. B., Al-Shehbaz, I. A. (Beijing/St. Louis: Science Press/Missouri Botanical Garden Press), 714–800.
Zhang, D. Y. (2004). Plant Life-History Evolution and Reproductive Ecology (Beijing: Science Press).
Zhang, F., Huang, D. H., Jannathan, M. (2023). Sporogenesis and gametogenesis in an early-spring-flowering perennial Leontice incerta (Berberidaceae) in the desert. Acta Bot. Boreal – Occident Sin. 43, 1557–1567. doi: 10.7606/j.issn.1000-4025.2023.09.1557
Keywords: Berchemia, secondary pollen presentation, petal-stamen complex, sexual interference, delayed selfing, reproduction assurance
Citation: Ma F, Zhao Q, Tian X, Fu Y-l and Liu W-z (2025) Stamens enclosed by petals in Berchemia (Rhamnaceae): a unique mechanism for pollen presentation. Front. Plant Sci. 16:1525022. doi: 10.3389/fpls.2025.1525022
Received: 08 November 2024; Accepted: 07 January 2025;
Published: 29 January 2025.
Edited by:
Gerald Matthias Schneeweiss, University of Vienna, AustriaReviewed by:
Lucía Melisa Zini, National Scientific and Technical Research Council (CONICET), ArgentinaCopyright © 2025 Ma, Zhao, Tian, Fu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-zhe Liu, bHdlbnpoZUBud3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.