
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci., 24 February 2025
Sec. Plant Cell Biology
Volume 16 - 2025 | https://doi.org/10.3389/fpls.2025.1506617
Peptides, currently being considered as a novel class of plant hormones, play diverse roles in plant growth and development. Peptides trigger signaling by binding to receptors and co-receptors, thus activating cellular signaling pathways. Recently, peptides have been extensively investigated in plant reproduction-related processes, ranging from gametes development to gametes fusion. In this review, we summarize recent advancements related to the role of peptides in plant reproduction and discuss challenges that need to be addressed urgently.
Peptides are short proteins that usually containing no more than 100 amino acid residues (Tavormina et al., 2015). In recent decades, numerous studies have revealed the profound involvement of plant peptides in a wide array of growth and developmental processes, thereby establishing their role as plant hormones (De Coninck and De Smet, 2016; Kim et al., 2021; Olsson et al., 2019). Tomato Systemin was the first peptide hormone identified in plants, based on its role in the immune response (McGurl et al., 1992). Since then, extensive research has led scientists to gain an in-depth understanding of the maturation, classification, evolution, and function of plant peptides (De Coninck and De Smet, 2016; Olsson et al., 2019).
Peptides can be classified into different groups, depending on their synthesis pattern: (1) precursor protein-origin peptides and (2) non-precursor protein-origin peptides. The majority of identified peptides belong to the first group, since they are synthesized from specific prepropeptide precursors. Notably, precursor protein-origin peptides are further classified as Cysteine-Rich Peptides (CRPs) and post-translational modification peptides (Olsson et al., 2019). Many plant species have numerous peptide genes. For example, Arabidopsis contains more than 1,000 peptide-coding genes, of which 759 are CRP genes and 431 are Non-CRP genes (Huang et al., 2015). Compared with other genes, CRP genes are more often found in clusters on chromosomes. In most species, 22–39% of the CRP genes are clustered. Considerable collinearities are found between or within species in several syntenic regions containing the CRP genes. Whole-genome duplication is the major force responsible for the expansion of the CRP gene family, with different subfamilies displaying discrepant evolutionary rates, which indicates that these subfamilies are under different selective pressures (Cao and Shi, 2012; Liu et al., 2017).
Sexual plant reproduction necessitates intricate and continuous communication among diverse multicellular organisms (Zhong et al., 2024; Xue et al., 2024). In angiosperms, male and female gametes undergo initiation and development within multicellular structures. Pollen, carrying two sperms, adheres to the stigma, germinates, and transports the sperms to the female gametes through pollen tube polar growth. Subsequently, sperms are released in micropyle, then they fused with the egg cell and central cell separately to develop into embryo and endosperm, respectively. This intricate process involves numerous indispensable peptides-mediated signaling mechanisms (Johnson et al., 2019). In this review, we primarily focus on recent advancements elucidating how peptides regulate this complicated process from gamete maturation to gamete fusion and discuss the current challenges and future prospects of peptide studies related to plant reproduction (view Table 1 for brief information).
In flowering plants, gametes initiate from sporophytic cells while being surrounded by multicellular tissues. Importantly, peptides facilitate communication between different cells during the gamete development phase.
Male gamete development in flowering plants comprises two steps: 1) microsporogenesis, in which somatic cells differentiate into microsporocytes, and 2) male gametogenesis, during which the microsporocyte produces pollen. The microsporocyte is surrounded by a tapetum layer, and frequent material and information exchange between the pollen and the surrounding tapetum layer is essential for successful male gametogenesis (Wilson and Zhang, 2009; Yao et al., 2022). Peptides act as potent signaling molecules during male gametogenesis (Figure 1A). In Arabidopsis, TAPETUM DETERMINANT 1 (TPD1), encoding a small secreted CRP, is expressed in the microsporocyte and tapetum, and loss-of-function mutations in TPD1 lead to excess sporocyte production and absence of tapetum within anthers, causing male sterility. Additionally, EXCESS MICROSPOROCYTES 1 (EMS1), encoding a Leucine-Rich-Repeat (LRR) domain receptor kinase, is expressed in tapetum, and the ems1 mutant is phenotypically similar to the tpd1 mutant. TPD1 interacts with the extracellular LRR region of EMS1, inducing EMS1 phosphorylation (Grelon et al., 2016; Huang et al., 2016b; Jia et al., 2008; Yang et al., 2003). The SOMATIC EMBRYO RECEPTOR KINASE1 (SERK1) and SERK2 display the same expression pattern as EMS1, and serk1/2 mutants exhibit a similar phenotype as the ems1 and tpd1 mutants. Additionally, SERK1 and SERK2 interact with EMS1 in vivo and function as the co-receptors of EMS1, and TPD1 binds to the EMS1-SERK1 heterodimer to phosphorylate EMS1 (Li et al., 2017). Subsequently, it was found that Brassinosteroid (BR) is involved in TPD1-EMS1-SERK1 pathway. BRI1 EMS SUPPRESSOR 1 (BES1) is a key transcription factor specifically regulating BR-mediated gene expression. The null mutants of BES1 family lack the tapetal layer in anthers, similar to the defect of tpd1, ems1 and serk1/2, and gain-of function mutation of BES1 (bes1-D) can significantly suppress the male sterility of tpd1, ems1, and serk1/2. Additionally, EMS1 is necessary for the nuclear localization of BES1. In summary, BES1 mediated BR signaling act downstream of TPD1-EMS1/SERK1 (Chen et al., 2019).
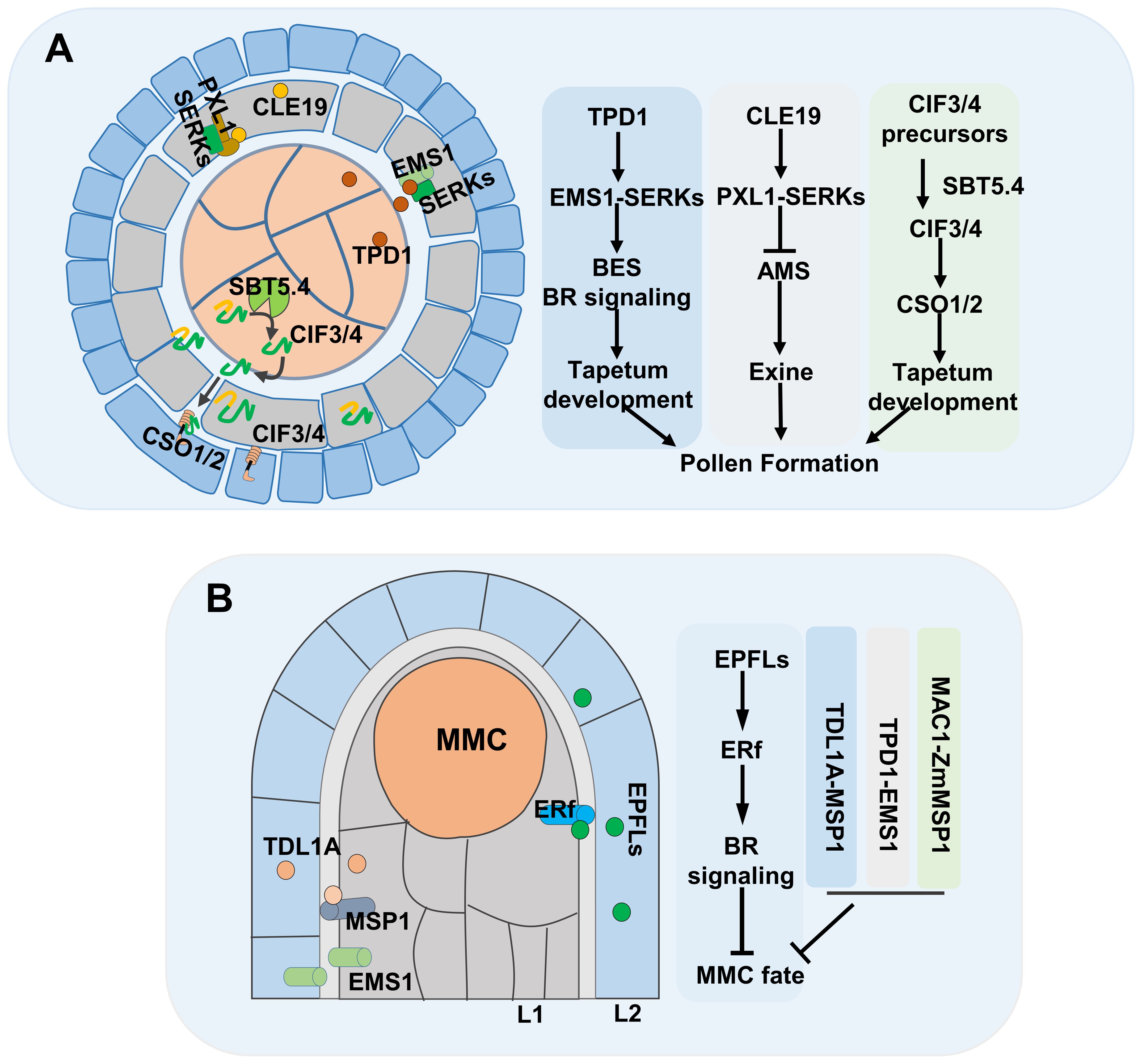
Figure 1. The role of peptides in gametes development. (A) Signaling pathway of peptides during the pollen development. (B) Signaling pathway of peptides in MMC formation, MMC, megaspore mother cell.
In rice (Oryza sativa L.), TPD1-like 1A (TDL1A), an ortholog of TPD1, interacts with MULTIPLE SPOROCYTE1 (MSP1), an ortholog of EMS1. The ostdl1a and msp1 mutants display similar defects in anther phenotype as the tpd1 and ems1 mutants. TDL1A acts as a ligand for MSP1 and regulates pollen development (Nonomura et al., 2003; Zhao T. et al., 2021; Zhao X. et al., 2008). In maize (Zea mays), MULTIPLE ARCHESPORIAL CELLS 1 (MAC1), which is orthologous to TDL1A, interacts with the ZmMSP1 protein to regulate male gametophyte development (Wang et al., 2012). The results of those studies have demonstrated that the function of the TPD1-EMS1 signaling pathway is conserved in different plant species.
Intact pollen wall, consisting of exine (outer wall), intine (inner wall), and pollen coat, plays a crucial role in protecting pollen from various stresses and is essential for pollen germination. The tapetum layer is necessary for pollen wall formation. Tapetum cells express CLAVATA3/ESR-RELATED 19 (CLE19), and loss-of-function and dominant-negative mutants as well as overexpression transgenic lines of CLE19 exhibit significant male fertility defects. Additionally, cle19 mutants show abnormal accumulation of pollen exine, indicating that CLE19 signaling is vital for pollen exine formation (Wang et al., 2017b). PXY-LIKE1 (PXL1) is present in both tapetum cells and pollen grains. Anthers of pxl1-1 and pxl1-2 mutants display a slight reduction in size with abnormally filled pollen exine. Dominant-negative mutation of PXL1 can suppress the developmental defects caused by CLE19 overexpression. Furthermore, physical interaction between CLE19 and PXL1 promotes the phosphorylation of PXL1, confirming its role as a receptor in the CLE19 signal transduction pathway (Yu et al., 2023). SERKs act as co-receptors in multiple Receptor-Like-Kinases (RLKs)-mediated signaling pathways, including the PXL-CLE cascade where they serve as co-receptors alongside PXL to form complexes dependent on CLE19 activity (Yu et al., 2023). These findings contribute to our understanding of a novel mechanism underlying the pollen wall formation process. Tapetum produced sulfo-peptide precursors CASPARIAN STRIP INTEGRITY FACTOR 3 (CIF3) and CIF4 are processed by the pollen localized subtilase AtSBT5.4, a pollen-specific subtilisin serine protease. Then, these mature CIF3 and CIF4 peptides diffuse between tapetal cells to bind to GASSHO (GSO) receptors located in the middle layer which is a tissue surrounding the tapetum and developing pollen, thereby triggering GSO-dependent tapetum activation and consequently leading to polarized sporopollenin secretion (Truskina et al., 2022; Tsuwamoto et al., 2008).
The initiation of the female germline in most flowering plants occurs in the L2 (subepidermal) layer of ovule primordia, resulting in the formation of a single Megaspore Mother Cell (MMC), which then undergoes a series of developmental processes to generate the female gametophyte (Yang et al., 2010). The L1 (epidermal) layer is capable of secreting signals that restrict MMC formation to a single cell (Figure 1B) (Cai et al., 2022). EPIDERMAL PATTERNING FACTOR-like (EPFLs), encoding CRP ligands, are expressed in the L1 layer, while ERECTA family (ERf) receptor kinases are enriched in the plasma membrane of both L1 and L2 layer cells of ovule primordia. Genetic evidence shows that epfl1/2/4/6 and er/erl1/2 mutants exhibit supernumerary enlarged MMC-like cells. Furthermore, EPFLs have been shown to interact with ERf receptor kinases to regulate female germline specification through BR signaling (Cai et al., 2023; Franco, 2023; Li et al., 2023b). The TPD1-EMS1 cascade also plays a role in female gametophyte development. In rice, TDL1A and MSP1 are highly co-expressed in the nucellus epidermis that surrounds the MMC, disruption of TPD1–EMS1-like signaling leads to the formation of extra MMCs in ovules (Zhao et al., 2008). However, in Arabidopsis ovule primordia, TPD1 is weakly expressed at the distal end of ovule integuments; EMS1 is expressed in the nucellus epidermis; and ectopic expression of TPD1 causes ovule developmental defects (Huang et al., 2016a).
While the function of peptides in gametes development is under investigation, progress is slow, primarily because of the presence of multiple layers of tissue surrounding the plant gametes. This greatly challenges the study of cellular-level functions of small peptides. Recently, the utilization of single-cell RNA sequencing (scRNA-seq) technology facilitated the construction of comprehensive single-cell transcriptomes during ovule or anther development (Rodriguez-Villalon and Brady, 2019; Song et al., 2020). This advancement will enable us to explore a wider range of peptides involved in gamete developmental processes.
In angiosperms, compatible pollen grains that land on the stigma of the pistil undergo a series of processes, including capture, adhesion, hydration, and germination (Dresselhaus and Franklin-Tong, 2013). Frequent information exchange takes place between the pollen and stigma, forming a precise “lock-and-key” mechanism that determines pollen acceptable germination (Figures 2A, B) (Cheng and Li, 2023). Pollen Coat Proteins (PCPs), produced in the pollen coat, play crucial roles in this “lock-and-key” system (Wang et al., 2017a). In tomato (Solanum lycopersicum) plants, the pollen autocrine peptide LATE ANTHER TOMATO52 (LAT52) interacts with the pollen-specific protein POLLEN-SPECIFIC RECEPTOR KINASE2 (LePRK2), promoting pollen hydration and germination (Tang et al., 2002). In the self-compatible (SC) species Arabidopsis thaliana, papilla cell produced peptides RAPID ALKALINAZATION FACTOR23/33 (RALF23/33) bind to the FERONIA/ANJEA/LORELEI-like-GPI-anchored protein (FER/ANJ–LLG1) complex, activating the RopGEF–ROP–RBOHD signaling pathway and promoting ROS generation, forming a “lock” to inhibit pollen hydration. Upon compatible pollen landing, pollen-secreted “key” peptides, including PCP-Bs (AtPCP-Bα, AtPCP-Bβ, AtPCP-Bγ, AtPCP-Bδ), compete with RALF23/33 to bind to FER/ANJ, thereby repressing ROS production and initiating pollen hydration (Liu et al., 2021a). Recently, a similar mechanism was reported in which FERONIA/CURVY1/ANJEA/HERCULES RECEPTOR KINASE 1 (FER/CVY1/ANJ/HERK1) and cell wall proteins LEUCINE-RICH REPEAT EXTENSIN3/4/5 (LRX3/4/5) on papilla cell surfaces interact with autocrine stigmatic RALF1/22/23/33 peptide ligands (sRALFs), establishing a “lock” that prevents undesired pollen tube penetration. Compatible pollen-derived RALF10/11/12/13/25/26/30 peptides (pRALFs) act as a “key” by outcompeting sRALFs and enabling successful pollen tube penetration. Furthermore, researchers have utilized pRALFs as mentors to breakdown the lock system and facilitate interspecific pollen tube penetration and fertilization (Lan et al., 2023a). In the self–incompatible (SI) plant species Brassica napus, S–LOCUS CYSTEINE RICH PROTEIN/S-LOCUS PROTEIN11 (SCR/SP11), secreted from the incompatible pollen and recognized by the papilla cell-localized protein S-LOCUS RECEPTOR KINASE (SRK), determines the self-incompatibility response (Nasrallah, 2019; Shiba et al., 2001; Takayama and Isogai, 2005; Takayama et al., 2001). M-LOCUS PROTEIN KINASE (MLPKs), acting as co-receptors of SRK, play positive roles in self-incompatibility mechanisms (Kakita et al., 2007). Additionally, THIOREDOXIN H-LIKE proteins (THL1 and THL2) interact with SRK to inhibit its kinase activity, and SCR/SP11 competitively interact with SRK to release THL1/2, resulting in the activation of the downstream gene ARMADILLO-REPEAT-CONTAINING1(ARC1), encoding an E3 ubiquitin ligase in stigma cells. This leads to the rejection of SI pollen through the degradation of germination-promoting proteins such as GLYOXALASE 1 (GLO1) and EXO70A1 (Goring et al., 2014; Indriolo et al., 2012; Yamamoto and Nasrallah, 2013). More recently, the classical SCR-SRK cascade has been further expanded. SCR-SRK can recruit the receptor protein FER, which activates the production of ROS in stigma cells, and high ROS levels inhibit pollen germination, thereby aiding in the rejection of self-pollen or interspecific pollen (Huang et al., 2023; Zhang et al., 2021). When intraspecific pollen landing on the stigma of Brassica napus, PCP-Bs from pollen coat could trigger Nitric Oxide (NO), which nitrosates FER and RBOHD/F to suppress ROS production in stigmas to facilitate intraspecific pollen germination and growth (Huang et al., 2023; Zhang et al., 2021).
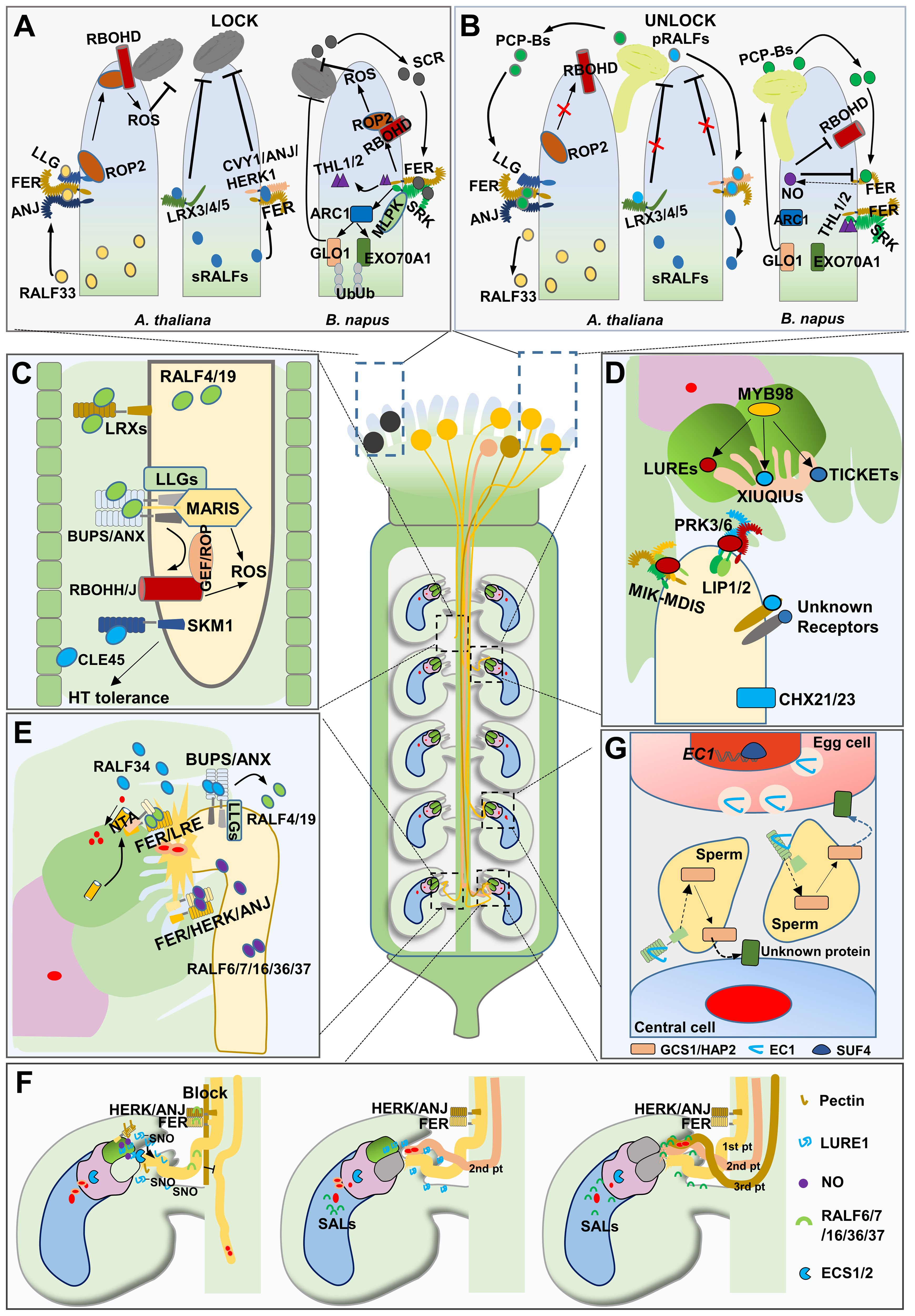
Figure 2. Peptides in pollen/pollen tube-pistil interaction. (A) the schematic diagram of “Lock” state on stigma. Incompatible pollen triggers an incompatibility response in the stigma of Brassicaceae. (B) the schematic diagram of “Unlock” state on stigma. “Key” peptides from compatible pollen activate the “Lock” system to promote pollen germination. The schematic diagram shows the growth of pollen tube in transmitting tract (C) and attractted by the ovule (D). (E) the schematic diagram of pollen tube reception. (F) Polytubey block and fertilization recovery mechanisms in Arabidopsis. (G) the schematic diagram of gamete fusion and activation.
Interspecies hybridization is a powerful tool for hybrid breeding; however, one of the major challenges lies in the reproductive barriers present on stigma. Leveraging the elucidated knowledge of the “lock-and-key” system, researchers have developed specific mutants, such as fer-4, srk, and anj, and chemical compounds, including ROS scavenger Na-SA, NO donor and SRK disruptor AS-ODN, to interfere with this system at the stigma level (Huang et al., 2023). The mentoring effect of pRALFs has been employed by researchers to overcome compatibility barriers by allowing both incompatible and compatible pollen to deceive the “lock” mechanism, thereby facilitating the germination and penetration of otherwise incompatible pollen grains (Lan et al., 2023a). Additionally, certain abiotic factors play significant roles in determining pollen tube penetration; for instance, high temperatures disrupt the targeting of SRK to plasma membranes, leading to weakened self-incompatibility response in plants (Yamamoto et al., 2019). Similarly, application of the NaCl solution and other saline water to field-cultivated Brassica napus plants facilitates self-pollination in this SI species, although the underlying molecular mechanisms remain unknown (Li et al., 2024).
After pollen germination and penetration, the pollen tube grows through the style into the transmitting tract and then emerges from the transmitting tract, targeting the ovule micropyle (Li et al., 2018). Peptide signals play a key regulatory role in keeping growth and guidance of the pollen tube in different plant species (Figures 2C, D). In solid and enclosed pistil tissues, integrity of the pollen tube is a prerequisite for its polar growth. In Arabidopsis, RALFs, together with CrRLK1L homologs, and LLGs form signaling modules that are essential for preserving pollen tube integrity until it is accepted by female gametes (Zhou et al., 2024). RALF4/19 are pollen-expressed proteins, ralf4/19 pollen tubes germinated normally in vitro, but their pollen tubes burst prematurely in vivo, producing abnormally short siliques and were male sterile. The CrRLK1L homologs ANXUR1/2 (ANX1/2) and BUDDHA’S PAPER SEAL 1/2 (BUPS1/2) are highly expressed paralogs of FER specifically in pollen grains. In anx1/2 and bups1/2 mutants, pollen tubes ruptured prematurely during growth within pistils, leading to pollen tube growth arrest, the loss of either pair of CrRLK1L homologs leads to male sterility, similar to ralf4/19. Biochemical assays revealed that ANXs and BUPSs form a heteromer, all four receptor kinases (i.e., ANX1/2 and BUPS1/2) interact with LLG2/3 to form a large complex, while RALF4/19 act as ligands of the ANXs–BUPSs–LLGs complex (Feng et al., 2019; Ge et al., 2017, 2019a, 2019b, 2019c). COBRA-like protein 11 (COBL11) interacts with RALF4/19, ANX1/2, and BUPS1/2, and the functional loss of COBL11 disrupts the proper distribution of RALF4/19 and the membrane localization of ANXs (Li et al., 2023a). MARIS and RBOH oxidases (RBOHH/J) act downstream of the tripartite complexes, indicating the involvement of ROS in maintaining pollen tube integrity (Boisson-Dernier et al., 2015; Kaya et al., 2014; Liao et al., 2016). The application of RALF4/19 on pollen could inhibits pollen germination and growth, pollen-expressed LRX8/9/10/11 interact directly with RALF4/19 to control their growth-inhibitory function, suggesting that the RALF-LRX module works as a brake to regulate pollen tube growth (Mecchia et al., 2017; Moussu et al., 2020; Sede et al., 2018). Interestingly, RALF4/19 orchestrate both pollen tube growth and integrity, and slower growth appears to be better for integrity. However, the connection between ANX–BUPS–RALFs and LRXs–RALFs mechanisms remains unclear. In maize, ZmRALFs mediate both pollen tube integrity and growth through the FER–like receptor kinases, ZmLLGs and extension–like proteins (Zhou et al., 2024). Additionally, RALFs in regulating pollen tube integrity is also observed in rice (Kim et al., 2023). Together, these studies suggest that the RALFs–controlled mechanism of pollen tube integrity maintenance during growth is considerably conserved in different plant species. Besides RALFs, other peptides are also involved in regulating pollen tube growth. In tomato plants, LeSTIG, a small cysteine-rich protein from pistil, replaces LAT52 to bind to LePRK1/2 receptors, thus activating RAC/ROP GTPases for pollen tube growth regulation (Huang et al., 2014; Kaothien et al., 2005; Liu et al., 2020). Under heat stress, pistils secrete peptides, such as CLE45, which bind to STERILITY-REGULATING KINASE MEMBER1 (SKM1) and SKM2 receptors, promoting the growth of pollen tube through the pistil while protecting sperm delivery from high temperatures (Endo et al., 2013).
The final destination of pollen tubes is the ovule micropyle; however, before reaching the micropyle, pollen tubes must leave the transmitting tract, travel through the septum and grow along the funiculus. This process can be divided into two specific steps (Wang et al., 2023). In the first step, the pollen tube needs to emerge from the transmitting tract and elongate to the funiculus, also known as funicular guidance. The pollen tube puncture points are uniformly distributed across the septum, suggesting that pollen tube emergence is not random and that specific mechanisms must regulate the funicular guidance. Recent imaging analysis shows that this emergence from transmitting tract depends on sporophytic signals from the ovule (Mizuta et al., 2024).
K+ transporters CATION/PROTON EXCHANGERS 21/23 (CHX21/23) are proteins specific to pollen tubes. Loss of these proteins in chx21/23 results in pollen tubes being unable to emerge out of the transmitting tract. In vivo experiments show that chx21/23 pollen tubes fail to target the ovule correctly (Lu et al., 2011). These results indicate that CHX21/23 potentially respond to unknown signals involved in pollen tube emergence from transmitting tract. In Arabidopsis, Mitogen-Activated Protein Kinase (MPK3) and MPK6, which are involved in various biotic and abiotic stress responses, are enriched in pollen tube nuclei. Pollen tubes of the mpk3/6 mutant were observed to be defective in targeting funiculus (Guan et al., 2014). Phytosulfokine (PSK), a disulfated pentapeptide that is processed from a 90–100 amino acid precursor, further catalyzed by a tyrosylprotein sulfotransferase (TPST) in trans-Golgi, and PSK is perceived by LRR receptor PSKR (Matsubayashi and Sakagami, 1996; Yang et al., 1999; Komori et al., 2009; Matsubayashi et al., 2002). In Arabidopsis, PSKs, TPST, and PSKRs are expressed in both pollen tubes and pistil tissues. Phenotypic analyses of tpst-1 and pskr1-3 pskr2-1 mutants revealed that the PSK signaling pathway in pollen tubes and maternal tissues plays a crucial role in funicular guidance (Stührwohldt et al., 2015). While CHXs, MPKs, and PSK signaling pathways have been implicated in funicular guidance, several questions remain unresolved. Specifically, it is unclear whether CHXs and MPKs are involved in the PSK pathway and whether PSK serves as a direct signal for controlling funicular guidance. The current priority is to identify a direct signal that regulates funicular guidance. Previous research has indicated that pollen tube attraction signals produced by embryo sac are short-range and may not reach the surface of the guiding tissue (Mizuta and Higashiyama, 2018; Li et al., 2018). In contrast, funiculus or ovule integument, which are closer to the point where pollen tube penetrates the septum, may generate signals that directly influence pollen tube’s exit from transmitting tract and its subsequent guidance towards funiculus.
The second step is ovular guidance, with the pollen tube elongating from funicular to micropyle opening. A series of peptides secreted from synergid cells guide the pollen tube to the micropyle. TfLUREs is the first group of peptides identified as pollen tube attractants in Torenia fournieri. TfLUREs are defensin-like CRPs secreted by ovule synergid cells that diffuse towards the micropylar and funicular region to attract pollen tubes (Okuda et al., 2009). Arabidopsis contains seven homologs of TfLUREs (Okuda and Higashiyama, 2010; Takeuchi and Higashiyama, 2012). Pollen tube tip-localized Receptor-like Kinase 6 (PRK6) interacts with LURE1 through its extracellular LRR domain, in vitro, prk6 pollen tubes fail to target LURE peptides. Additionally, two receptor-like cytoplasmic kinases (RLCKs), LOST IN POLLEN TUBE GUIDANCE1 (LIP1) and LIP2, are also required for LURE-mediated pollen tube guidance. Furthermore, LIP1/2 could directly interact with PRK6, suggesting that PRK6-LIP1/2 act as a receptor complex for LUREs (Liu et al., 2013; Takeuchi and Higashiyama, 2016). Recent reports have indicated that PRKs can interact with ROPGEFs, which activate RAC/ROPs, playing a crucial role in polar cell growth, further expanding PRK6 signaling (Gu et al., 2006; Takeuchi and Higashiyama, 2016; Luo et al., 2017).
LRR-RLKs MDIS1-INTERACTING RECEPTOR LIKE KINASE 1/2 (MIK1/2) and MALE DISCOVERER 1/2 (MDIS1/2) are membrane proteins located at the tip of pollen tube. Pollen tubes lacking mik1/2 and mdis1 show reduced sensitivity to LURE1, indicating that the MIKs-MDISs complex serves as another receptor for LURE1 (Wang et al., 2016). The PRK6-LURE complex, which mediates pollen tube guidance, also functions as a prezygotic isolation barrier. Wild-type Arabidopsis ovules preferentially attract conspecific pollen tubes, but septuple atlure mutant ovules can attract a significant amount of Arabidopsis lyrata pollen tubes. This suggests that AtLURE1/PRK6-mediated signaling promotes conspecific micropylar pollen tube attraction (Liu et al., 2021b). Interestingly, the atlure mutant displays normal fertility, suggesting the existence of other attractants. Currently, the LURE-mediated pollen tube guidance mechanism appears to be relatively well-established; however, several unresolved issues remain. First, structural studies have shown that a C-terminal loop of the LRR domain is responsible for the recognition of AtLURE1.2. This recognition is mediated by a set of residues that are largely conserved among PRK6 homologs from Arabidopsis lyrata and Capsella rubella but not in Torenia fournieri, where the first LURE was identified, which introduces some confusion (Zhang et al., 2017).
The defensin-like peptides XIUQIUs and TICKETs have been found to possess pollen tube attractant activity (Meng et al., 2019; Zhong et al., 2019). Specifically, the nonspecies-specific attractant activity of XIUQIUs is independent of PRK6, although the XIUQIU receptor remains unclear (Zhong et al., 2019). Recently, species-specific pollen tube attraction activity was identified among sister species of the Brassicaceae family through the discovery of NPA1 (Wang et al., 2024), a synergid-secreted non-defensin-like peptide that contributes to species barriers. Interestingly, MYB98 transcription factor regulates the expression of all aforementioned attractants in synergid cells (Kasahara et al., 2005; Meng et al., 2019; Punwani et al., 2007; Takeuchi and Higashiyama, 2012; Zhong et al., 2019); however, offspring production still occurs in myb98 mutants, indicating the presence of non-synergid cell-mediated or non-MYB98-regulated attractant signaling. In maize, an egg apparatus is developed to mediate pollen tube guidance. The egg-apparatus-secreted polymorphic peptide ZmEA1 exhibits pollen tube attractant activity, indicating that the egg cell, or potentially other types of cells, is also capable of secreting attractants (Marton et al., 2005). Intriguingly, when ZmEA1 is expressed in Arabidopsis synergid cells, the secreted ZmEA1 enables Arabidopsis ovules to attract maize pollen tubes in vitro towards the micropylar opening (Marton et al., 2012).
After entering the micropyle, the pollen tube ruptures by interacting with synergid cells (Figure 2E). During this interaction, one of the two synergid cells undergoes elimination and the synergid cell that persists is called persistent synergid cell (Maruyama et al., 2015).
RALF4/19 is responsible for maintaining the integrity of pollen tubes. However, when the pollen tube arrives at the micropyle, ovule-derived RALF34 competes with RALF4/19 to bind to BUPS1 and ANX1. The replacement of RALF4/19 by RALF34 disrupts pollen tube integrity, leading to pollen tube rupture and sperm release (Ge et al., 2017). Five pollen-expressed RALFs (RALF6/7/16/36/37) are involved in the bursting of pollen tubes, as evident from the failure of pollen tubes to burst in the ralf6/7/16/36/37 quintuple mutant. FER, HERK, and ANJ are expressed in ovules and redundantly participate in pollen tube reception; the pollen tubes of fer and anj herk1 mutants fail to burst and show overgrowth in the micropylar region. Furthermore, FER, HERK, and ANJ physically interact with each other, and RALF6/7/16/36/37 proteins function as ligands for the FER-HERK-ANJ complex (Zhong et al., 2022). RALF4/19 also act as ligands for the FER-LRE complex and enhance the Ca2+ channel activity of NORTIA (NTA) in synergid cells, which is essential for pollen tube reception; however, the mechanisms underlying Ca2+-induced rupture of pollen tube and elimination of one synergid cell remain unclear (Gao et al., 2023, 2022). In maize, pollen tube plasma membrane-localized potassium channel KZM1 is activated by the synergid cell-secreted peptide EMBRYO SAC4 (ZmES4). The interaction between ZmES4 and KZM1 induces K+ influx, resulting in altered osmotic pressure that promotes water uptake and potentially leads to pollen tube rupture (Amien et al., 2010).
Polyspermy is a phenomenon where multiple sperm cells enter a single egg cell. This leads to aberrant chromosome numbers and consequently failed embryo development. In angiosperms, polyspermy always occurs when multiple pollen tubes enter the same ovule. Plants have evolved various strategies to prevent polyspermy (Figure 2F).
Once the pollen tube penetrates the transmitting tract, a local block is established. In Arabidopsis, pollen tube-derived peptides RALF6/7/16/36/37 interact with septum-localized receptors FER/ANJ/HERK to establish the early polyspermy block, inhibiting the local emergence of other pollen tubes from the transmitting tract, however, what happens downstream the RALFs-FER/ANJ/HERK remains unknown (Zhong et al., 2022). After the pollen tube ruptures in the female gametocyte, RALFs cease to exist and the block is temporarily lost, creating an opportunity for another pollen tube to emerge and target the same ovule. To avert polyspermy at this stage, the pollen tube, after reaching the micropyle, induces the production and accumulation of NO at the filiform apparatus in a process that is dependent on FER and mediated by de-esterified pectin. NO inhibits the activity of the already secreted LUREs and prevents further secretion of these attractants from synergid cells by nitrosating both precursor and mature forms of LUREs (Duan et al., 2020). After fusing with the sperm, the egg cell secretes two aspartic proteases, ECS1/2, that specifically cleave the pollen tube attractants LUREs (Yu et al., 2021). However, if the fertilization conducted by the first pollen tube fails, ovules must attract another pollen tube to achieve successful fertilization, this process is known as fertilization recovery. Plants need to coordinate fertilization recovery with the polyspermy block. RALFs-induced polyspermy block expired after the rupture or fertilization defect of the first pollen tube, allowing another pollen tube to emerge and target the ovule (Zhong et al., 2022). The persistent synergid cell continues to secrete attractants towards the micropylar and funicular region in order to guide the pollen tube (Kasahara et al., 2012, 2013; Maruyama and Higashiyama, 2016; Maruyama et al., 2015). However, it is still unknown how the NO-mediated polyspermy block expired. Recently, it was discovered that central cells play a crucial role in fertilization recovery. When both synergid cells are eliminated or defected, the central cell secretes CRPs SALVAGER1 (SAL1) and SAL2, which function to attract pollen tubes. This backup mechanism may explain why myb98 and atlure xiuqiu mutants are fertile (Dresselhaus and van der Linde, 2023; Lan et al., 2023b; Meng et al., 2023, 2024).
After two sperm cells are released, they migrate separately towards the central cell and egg cell. Subsequently, one sperm fuses with egg cell to initiate embryo formation, while the second sperm fuses with central cell to facilitate endosperm development. Despite limited knowledge regarding gamete fusion and activation, EGG CELL1 (EC1) peptides have been identified as crucial players in this process. In Arabidopsis, five EC1-like genes (EC1.1–EC1.5) are specifically expressed in egg cell, and EC1 peptides accumulate in storage vesicles prior to sperm arrival. Upon contact with sperm cells, exocytosis occurs within the EC1 vesicles. When EC1 peptides were applied to sperm cells, the protein HAPLESS2/GENERATIVE CELL SPECIFIC1 (HAP2/GCS1), essential for gamete fusion, redistributes from the endomembrane system to the cell surface in order to facilitate gamete fusion. Furthermore, ovules lacking EC1 exhibit a blockage in gamete fusion (Sprunck et al., 2012). These findings suggest that EC1 promotes the ability of sperm to fuse with egg cell (Figure 2G); however, the receptor of EC1 remains a significant unresolved mystery. The C2H2 transcription factor SUPPRESSOR OF FRIGIDA4 (SUF4) directly regulates the expression of EC1 genes, and suf4-1 mutant exhibits a moderate ec1 phenotype, indicating that SUF4 controls sperm fusion by regulating EC1 expression (Resentini et al., 2017).
Despite the increasing attention given to small peptides, the practical application of this research remains a challenging issue. However, there is considerable potential for certain aspects of this knowledge to be applied in agricultural breeding in the future (Figure 3). Many peptides play critical roles in gametic development, aiding in the construction of sterile lines in various crops to accelerate breeding processes (Erbasol Serbes et al., 2019; Liu et al., 2023; Xiong et al., 2023). The ecs1/2 double mutant can induce maternal haploids in Arabidopsis, and ECS mutations are also capable of producing haploids in rice. Given that ECS homologs exist in other species such as Brassica napus and Raphanus sativus, it suggests that ECSs-HI (ECSs-haploid induction) technology can be widely utilized in agricultural breeding (Mao et al., 2023; Zhang et al., 2023).The identification of “key” peptides on the stigma and the investigation of pollen tube attractants can help overcome reproductive barriers and facilitate the creation of distant hybrid species (Marton et al., 2012; Uebler et al., 2013; Wang et al., 2016; Zhong et al., 2019, 2022; Liu et al., 2021a; Liu et al., 2021b; Huang et al., 2023). Several investigations have indicated that specific peptides participate in seed development and consequently adjust the size and morphology of seeds (Jin et al., 2016; Sui et al., 2016). Recent studies have revealed that the peptide microRPG1 regulates the dehydration process of corn grains during seed maturation (Yu et al., 2024). Manipulating microRPG1 to alter the dehydration rate of corn grains holds significant potential for developing varieties that are more suitable for easy harvesting.
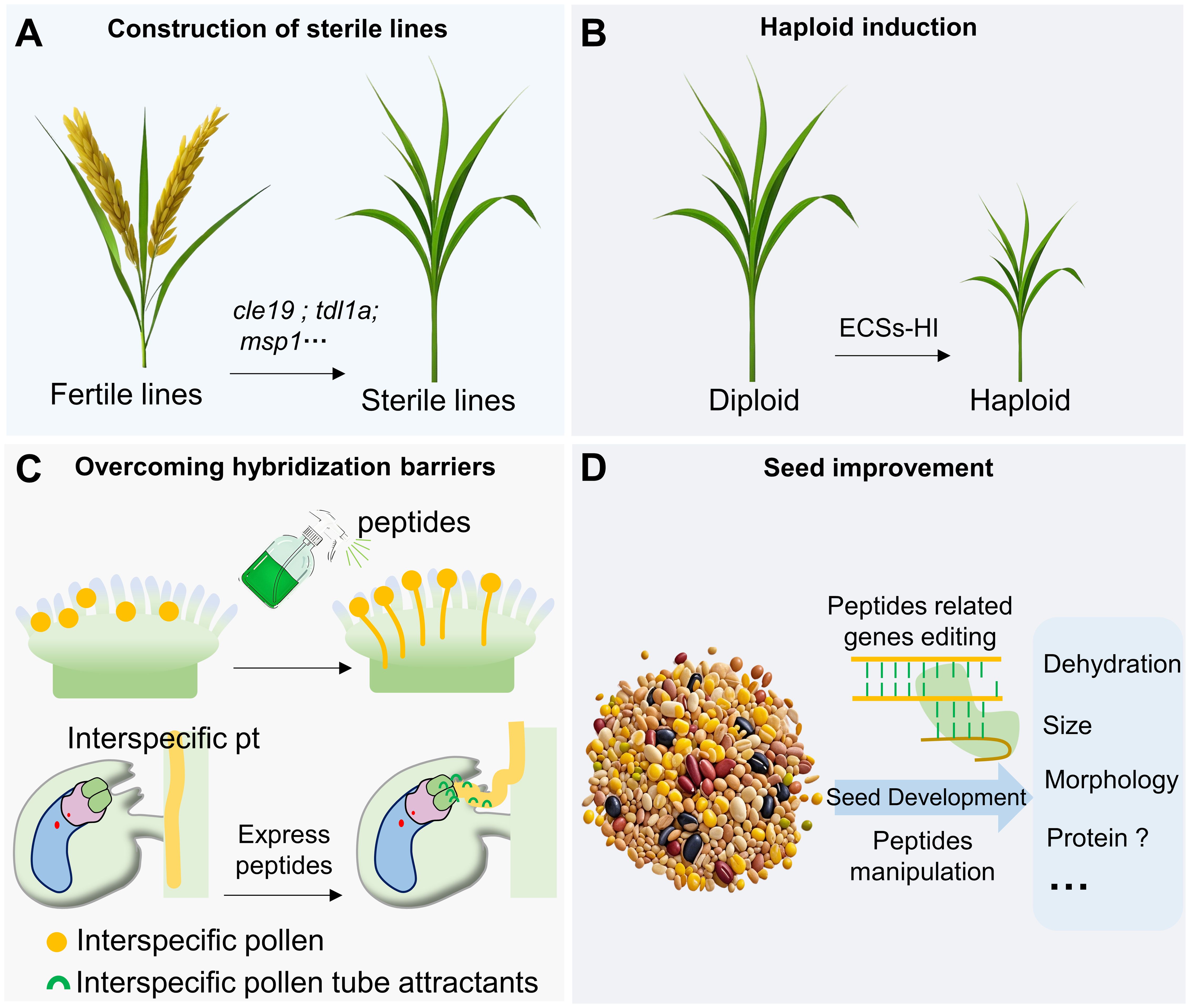
Figure 3. Potential application of peptides research. Peptide studies in plant reproduction are potentially applicable to the construction of sterile lines (A), haploid induction (B), overcoming hybridization barriers (C) and seed improvement (D).
Since the initial investigation of plant sexual reproduction, a plethora of findings have been uncovered, with the discovery of peptide functions standing out as one of the most significant breakthroughs (Kim et al., 2021; Qu et al., 2015). However, several aspects of peptides and their role in plant reproduction remain unaddressed and require dedicated pursuit by researchers. First, it will be important to identify new functional peptides involved in plant reproduction. In Arabidopsis, 139 and 390 CRPs have been identified in male and female gametophytes, respectively (Huang et al., 2015); however, only a limited number of CRPs have been proven to play crucial roles, leaving significant gaps. Furthermore, no non-precursor protein-derived peptides associated with plant reproduction have yet been identified, posing a major challenge for researchers. Second, the identification of receptors for these peptide ligands remains challenging. The receptors for XIUQIUs, TICKETs, and SALs are currently unknown, more than 600 receptor-like kinases were identified in Arabidopsis, and it is difficult to find the specific ligand-receptor combinations (Baudino et al., 2001; Shiu and Bleecker, 2003). Artificial intelligence-based protein interaction prediction tools may facilitate receptor identification. Third, the regulatory mechanisms governing peptide movement remain unclear, including the redistribution of LUREs from the cytoplasm to the filiform apparatus during ovule maturation and the secretion of SALs from the central cell to the micropyle after the elimination of both synergid cells (Meng et al., 2023; Susaki et al., 2022). Finally, the RALFs-FER-ANJ-HERK1 cascade constructs the polytubey block system after pollen tube penetrates through the septum (Zhong et al., 2022). However, what are the precise factors downstream of this system that most directly prevent other pollen tubes from perforating? Are they still peptides? For a long time, it has been postulated that there exists a pollen tube repelling signal, and NO is regarded as a pollen tube repellent (Prado et al., 2004; 2008; Wong et al., 2020). Emerging results have revealed that the production of NO is dependent on FER, is NO a downstream effector of the polytubey mechanism? Additionally, pollen tube itself also generates NO, does the pollen tube directly repel other pollen tubes by releasing NO and participate in the establishment of polytubey block (Duan et al., 2020; Huang et al., 2023; Šírová et al., 2011)? Although these questions present tremendous challenges for us, current discoveries and continuously updated technologies encourage us to carry out further exploration in order to create a comprehensive framework of the regulatory network for plant reproduction.
CY: Writing – original draft. KG: Writing – review & editing. HL: Writing – review & editing. TF: Writing – review & editing. JM: Writing – review & editing, Funding acquisition, Project administration, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project is supported by starting research grant for High-level Talents from Southwest University, Young Elite Scientists Sponsorship Program by CAST (2023QNRC001), National Natural Science Foundation of China (32400289).
The authors thank the reviewers for their helpful and constructive suggestions and comments to improve the quality of the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amien, S., Kliwer, I., Marton, M. L., Debener, T., Geiger, D., Becker, D., et al. (2010). Defensin-like ZmES4 mediates pollen tube burst in maize via opening of the potassium channel KZM1. PloS Biol. 8, e1000388. doi: 10.1371/journal.pbio.1000388
Baudino, S., Hansen, S., Brettschneider, R., Hecht, V. R. G., Dresselhaus, T., Lorz, H., et al. (2001). Molecular characterisation of two novel maize LRR receptor-like kinases, which belong to the SERK gene family. Planta. 213, 1–10. doi: 10.1007/s004250000471
Boisson-Dernier, A., Franck, C. M., Lituiev, D. S., Grossniklaus, U. (2015). Receptor-like cytoplasmic kinase MARIS functions downstream of CrRLK1L-dependent signaling during tip growth. Proc. Natl. Acad. Sci. U.S.A. 112, 12211–12216. doi: 10.1073/pnas.1512375112
Cai, H., Huang, Y., Liu, L., Zhang, M., Chai, M., Xi, X., et al. (2023). Signaling by the EPFL-ERECTA family coordinates female germline specification through the BZR1 family in Arabidopsis. Plant Cell 35, 1455–1473. doi: 10.1093/plcell/koad032
Cai, H., Ma, S., Su, H., Liu, K., Aslam, M., Qin, Y. (2022). Positional signals establishment in the regulation of female germline specification. Seed Biol. 1, 6. doi: 10.48130/SeedBio-2022-0006
Cao, J., Shi, F. (2012). Evolution of the RALF gene family in plants: Gene duplication and selection patterns. Evol. Bioinform. 8, 271–292. doi: 10.4137/EBO.S9652
Chen, W., Lv, M., Wang, Y., Wang, P. A., Cui, Y., Li, M., et al. (2019). BES1 is activated by EMS1-TPD1-SERK1/2-mediated signaling to control tapetum development in Arabidopsis thaliana. Nat. Commun. 10, 4164. doi: 10.1038/s41467-019-12118-4
Cheng, L., Li, C. (2023). Stigma receptors function as barriers between intraspecies and interspecies in Brassicaceae. Seed Biol. 2, 3. doi: 10.48130/SeedBio-2023-0003
De Coninck, B., De Smet, I. (2016). Plant peptides – taking them to the next level. J. Exp. Bot. 67, 4791–4795. doi: 10.1093/jxb/erw309
Dresselhaus, T., Franklin-Tong, N. (2013). Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol. Plant 6, 1018–1036. doi: 10.1093/mp/sst061
Dresselhaus, T., van der Linde, K. (2023). Plant reproduction: Fertilization SALvaged by the central cell. Curr. Biol. 33, R1013–R1015. doi: 10.1016/j.cub.2023.08.078
Duan, Q., Liu, M. J., Kita, D., Jordan, S. S., Yeh, F. J., Yvon, R., et al. (2020). FERONIA controls pectin- and nitric oxide-mediated male-female interaction. Nature 579, 561–566. doi: 10.1038/s41586-020-2106-2
Endo, S., Shinohara, H., Matsubayashi, Y., Fukuda, H. (2013). A novel pollen-pistil interaction conferring high-temperature tolerance during reproduction via CLE45 signaling. Curr. Biol. 23, 1670–1676. doi: 10.1016/j.cub.2013.06.060
Erbasol Serbes, I., Palovaara, J., Groß-Hardt, R. (2019). Development and function of the flowering plant female gametophyte. Curr. Top. Dev. Biol. 131, 401–434. doi: 10.1016/bs.ctdb.2018.11.016
Feng, H., Liu, C., Fu, R., Zhang, M., Li, H., Shen, L., et al. (2019). LORELEI-LIKE GPI-ANCHORED PROTEINS 2/3 regulate pollen tube growth as chaperones and coreceptors for ANXUR/BUPS receptor kinases in Arabidopsis. Mo. Plant 12, 1612–1623. doi: 10.1016/j.molp.2019.09.004
Franco, J. (2023). BZR1, you have an invite: EPFL-ERECTA wants to join your female germline specification network. Plant Cell 35, 1298–1299. doi: 10.1093/plcell/koad045
Gao, Q., Wang, C., Xi, Y., Shao, Q., Hou, C., Li, L., et al. (2023). RALF signaling pathway activates MLO calcium channels to maintain pollen tube integrity. Cell Res. 33, 71–79. doi: 10.1038/s41422-022-00754-3
Gao, Q., Wang, C., Xi, Y., Shao, Q., Li, L., Luan, S. (2022). A receptor-channel trio conducts Ca(2+) signalling for pollen tube reception. Nature 607, 534–539. doi: 10.1038/s41586-022-04923-7
Ge, Z., Cheung, A. Y., Qu, L. J. (2019a). Pollen tube integrity regulation in flowering plants: Insights from molecular assemblies on the pollen tube surface. New Phytol. 222, 687–693. doi: 10.1111/nph.15645
Ge, Z., Dresselhaus, T., Qu, L. J. (2019b). How CrRLK1L receptor complexes perceive RALF signals. Trends Plant Sci. 24, 978–981. doi: 10.1016/j.tplants.2019.09.002
Ge, Z., Zhao, Y., Liu, M. C., Zhou, L. Z., Wang, L., Zhong, S., et al. (2019c). LLG2/3 are co-receptors in BUPS/ANX-RALF signaling to regulate Arabidopsis pollen tube integrity. Cur. Biol. 29, 3256–3265. doi: 10.1016/j.cub.2019.08.032
Ge, Z. X., Bergonci, T., Zhao, Y. L., Zou, Y. J., Du, S., Liu, M. C., et al. (2017). Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358, 1596–1599. doi: 10.1126/science.aao3642
Goring, D. R., Indriolo, E., Samuel, M. A. (2014). The ARC1 E3 ligase promotes a strong and stable self-incompatibility response in Arabidopsis species: response to the Nasrallah and Nasrallah commentary. Plant Cell 26, 3842–3846. doi: 10.1105/tpc.114.131243
Grelon, M., Huang, J., Zhang, T., Linstroth, L., Tillman, Z., Otegui, M. S., et al. (2016). Control of anther cell differentiation by the small protein ligand TPD1 and its receptor EMS1 in Arabidopsis. PloS Genet. 12, e1006147. doi: 10.1371/journal.pgen.1006147
Gu, Y., Li, S., Lord, E. M., Yang, Z. (2006). Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell. 18, 366–381. doi: 10.1105/tpc.105.036434
Guan, Y., Lu, J., Xu, J., McClure, B., Zhang, S. (2014). Two mitogen-activated protein kinases, MPK3 and MPK6, are required for funicular guidance of pollen tubes in Arabidopsis. Plant Physiol. 165, 528–533. doi: 10.1104/pp.113.231274
Huang, Q. P., Dresselhaus, T., Gu, H. Y., Qu, L. J. (2015). Active role of small peptides in Arabidopsis reproduction: Expression evidence. J. Integr. Plant Biol. 57, 518–521. doi: 10.1111/jipb.12356
Huang, W. J., Liu, H. K., McCormick, S., Tang, W. H. (2014). Tomato pistil factor STIG1 promotes in vivo pollen tube growth by binding to phosphatidylinositol 3-phosphate and the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell 26, 2505–2523. doi: 10.1105/tpc.114.123281
Huang, J., Wijeratne, A. J., Tang, C., Zhang, T., Fenelon, R. E., Owen, H. A., et al. (2016a). Ectopic expression of TAPETUM DETERMINANT1 affects ovule development in Arabidopsis. J. Exp. Bot. 67, 1311–1326. doi: 10.1093/jxb/erv523
Huang, J., Yang, L., Yang, L., Wu, X., Cui, X., Zhang, L., et al. (2023). Stigma receptors control intraspecies and interspecies barriers in Brassicaceae. Nature 614, 303–308. doi: 10.1038/s41586-022-05640-x
Huang, J., Zhang, T., Linstroth, L., Tillman, Z., Otegui, M. S., Owen, H. A., et al. (2016b). Control of anther cell differentiation by the small protein ligand TPD1 and its receptor EMS1 in Arabidopsis. PloS Genet. 12, e1006147. doi: 10.1371/journal.pgen.1006147
Indriolo, E., Tharmapalan, P., Wright, S. I., Goring, D. R. (2012). The ARC1 E3 ligase gene is frequently deleted in self-compatible Brassicaceae species and has a conserved role in Arabidopsis lyrata self-pollen rejection. Plant Cell 24, 4607–4620. doi: 10.1105/tpc.112.104943
Jia, G., Liu, X., Owen, H. A., Zhao, D. (2008). Signaling of cell fate determination by the TPD1 small protein and EMS1 receptor kinase. Proc. Natl. Acad. Sci. U.S.A. 105, 2220–2225. doi: 10.1073/pnas.0708795105
Jin, J., Hua, L., Zhu, Z., Tan, L., Zhao, X., Zhang, W., et al. (2016). GAD1 encodes a secreted peptide that regulates grain number, grain length, and awn development in rice domestication. Plant Cell. 28, 2453–2463. doi: 10.1105/tpc.16.00379
Johnson, M. A., Harper, J. F., Palanivelu, R. (2019). A fruitful journey: Pollen tube navigation from germination to fertilization. Annu. Rev. Plant Biol. 70, 809–837. doi: 10.1146/annurev-arplant-050718-100133
Kakita, M., Murase, K., Iwano, M., Matsumoto, T., Watanabe, M., Shiba, H., et al. (2007). Two distinct forms of M-locus protein kinase localize to the plasma membrane and interact directly with S-locus receptor kinase to transduce self-incompatibility signaling in Brassica rapa. Plant Cell 19, 3961–3973. doi: 10.1105/tpc.106.049999
Kaothien, P., Ok, S. H., Shuai, B., Wengier, D., Cotter, R., Kelley, D., et al. (2005). Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J. 42, 492–503. doi: 10.1111/j.1365-313X.2005.02388.x
Kasahara, R. D., Maruyama, D., Hamamura, Y., Sakakibara, T., Twell, D., Higashiyama, T. (2012). Fertilization recovery after defective sperm cell release in Arabidopsis. Curr. Biol. 22, 1084–1089. doi: 10.1016/j.cub.2012.03.069
Kasahara, R. D., Maruyama, D., Higashiyama, T. (2013). Fertilization recovery system is dependent on the number of pollen grains for efficient reproduction in plants. Plant Signal. Behav. 8, e23690. doi: 10.4161/psb.23690
Kasahara, R. D., Portereiko, M. F., Sandaklie-Nikolova, L., Rabiger, D. S., Drews, G. N. (2005). MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell 17, 2981–2992. doi: 10.1105/tpc.105.034603
Kaya, H., Nakajima, R., Iwano, M., Kanaoka, M. M., Kimura, S., Takeda, S., et al. (2014). Ca2+-Activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26, 1069–1080. doi: 10.1105/tpc.113.120642
Kim, E. J., Kim, J. H., Hong, W. J., Kim, E. Y., Kim, M. H., Lee, S. K., et al. (2023). Rice pollen-specific OsRALF17 and OsRALF19 are essential for pollen tube growth. J. Integr. Plant Biol. 65, 2218–2236. doi: 10.1111/jipb.13508
Kim, M.-J., Jeon, B. W., Oh, E., Seo, P. J., Kim, J. (2021). Peptide signaling during plant reproduction. Trends Plant Sci. 26, 822–835. doi: 10.1016/j.tplants.2021.02.008
Komori, R., Amano, Y., Ogawa-Ohnishi, M., Matsubayashi, Y. (2009). Identification of tyrosylprotein sulfotransferase in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 106, 15067–15072. doi: 10.1073/pnas.0902801106
Lan, Z., Song, Z., Wang, Z., Li, L., Liu, Y., Zhi, S., et al. (2023a). Antagonistic RALF peptides control an intergeneric hybridization barrier on Brassicaceae stigmas. Cell 186, 4773–4787. doi: 10.1016/j.cell.2023.09.003
Lan, Z., Song, Z., Zhong, S., Qu, L.-J. (2023b). The central cell: another opportunity for fertilization recovery in plants. Seed Biol. 2, 22. doi: 10.48130/SeedBio-2023-0022
Li, M., Lv, M., Wang, X., Cai, Z., Yao, H., Zhang, D., et al. (2023b). The EPFL-ERf-SERK signaling controls integument development in Arabidopsis. New Phytol. 238, 186–201. doi: 10.1111/nph.18701
Li, H. J., Meng, J. G., Yang, W. C. (2018). Multilayered signaling pathways for pollen tube growth and guidance. Plant Reprod. 31, 31–41. doi: 10.1007/s00497-018-0324-7
Li, Z., Wang, Y., Huang, J., Ahsan, N., Biener, G., Paprocki, J., et al. (2017). Two SERK receptor-like kinases interact with EMS1 to control anther cell fate determination. Plant Physiol. 173, 326–337. doi: 10.1104/pp.16.01219
Li, H., Yang, Y., Zhang, H., Li, C., Du, P., Bi, M., et al. (2023a). The Arabidopsis GPI-anchored protein COBL11 is necessary for regulating pollen tube integrity. Cell Rep. 42, 113353. doi: 10.1016/j.celrep.2023.113353
Li, Y., Zhang, W., Yang, Y., Liang, X., Lu, S., Ma, C., et al. (2024). BnaPLDα1-BnaMPK6 involved in NaCl-mediated overcoming of self-Incompatibility in Brassica napus L. Plant Sci. 345, 112116. doi: 10.1016/j.plantsci.2024.112116
Liao, H. Z., Zhu, M. M., Cui, H. H., Du, X. Y., Tang, Y., Chen, L. Q., et al. (2016). MARIS plays important roles in Arabidopsis pollen tube and root hair growth. J. Integr. Plant Biol. 58, 927–940. doi: 10.1111/jipb.12484
Liu, H. K., Li, Y. J., Wang, S. J., Yuan, T. L., Huang, W. J., Dong, X., et al. (2020). Kinase partner protein plays a key role in controlling the speed and shape of pollen tube growth in tomato. Plant Physiol. 184, 1853–1869. doi: 10.1104/pp.20.01081
Liu, C., Shen, L., Xiao, Y., Vyshedsky, D., Peng, C., Sun, X., et al. (2021a). Pollen PCP-B peptides unlock a stigma peptide-receptor kinase gating mechanism for pollination. Science 372, 171–175. doi: 10.1126/science.abc6107
Liu, D., Shi, J., Liang, W., Zhang, D. (2023). Molecular mechanisms underlying plant environment-sensitive genic male sterility and fertility restoration. Seed Biol. 2, 13. doi: 10.48130/SeedBio-2023-0013
Liu, M., Wang, Z., Hou, S., Wang, L., Huang, Q., Gu, H., et al. (2021b). AtLURE1/PRK6-mediated signaling promotes conspecific micropylar pollen tube guidance. Plant Physiol. 186, 865–873. doi: 10.1093/plphys/kiab105
Liu, X., Zhang, H., Jiao, H., Li, L., Qiao, X., Fabrice, M. R., et al. (2017). Expansion and evolutionary patterns of cysteine-rich peptides in plants. BMC Genomics 18, 610. doi: 10.1186/s12864-017-3948-3
Liu, J., Zhong, S., Guo, X., Hao, L., Wei, X., Huang, Q., et al. (2013). Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr. Biol. 23, 993–998. doi: 10.1016/j.cub.2013.04.043
Lu, Y., Chanroj, S., Zulkifli, L., Johnson, M. A., Uozumi, N., Cheung, A., et al. (2011). Pollen tubes lacking a pair of K+ transporters fail to target ovules in Arabidopsis. Plant Cell. 23, 81–93. doi: 10.1105/tpc.110.080499
Luo, N., Yan, A., Liu, G., Guo, J., Rong, D., Kanaoka, M. M., et al. (2017). Exocytosis-coordinated mechanisms for tip growth underlie pollen tube growth guidance. Nat. Commun. 8, 1687. doi: 10.1038/s41467-017-01452-0
Mao, Y., Nakel, T., Erbasol Serbes, I., Joshi, S., Tekleyo, D. G., Baum, T., et al. (2023). ECS1 and ECS2 suppress polyspermy and the formation of haploid plants by promoting double fertilization. Elife 12, e85832. doi: 10.7554/eLife.85832
Marton, M. L., Cordts, S., Broadhvest, J., Dresselhaus, T. (2005). Micropylar pollen tube guidance by Egg Apparatus 1 of maize. Science 307, 573–576. doi: 10.1126/science.1104954
Marton, M. L., Fastner, A., Uebler, S., Dresselhaus, T. (2012). Overcoming hybridization barriers by the secretion of the maize pollen tube attractant ZmEA1 from Arabidopsis ovules. Curr. Biol. 22, 1194–1198. doi: 10.1016/j.cub.2012.04.061
Maruyama, D., Higashiyama, T. (2016). The end of temptation: the elimination of persistent synergid cell identity. Curr. Opin. Plant Biol. 34, 122–126. doi: 10.1016/j.pbi.2016.10.011
Maruyama, D., Volz, R., Takeuchi, H., Mori, T., Igawa, T., Kurihara, D., et al. (2015). Rapid elimination of the persistent synergid through a cell fusion mechanism. Cell 161, 907–918. doi: 10.1016/j.pbi.2016.10.011
Matsubayashi, Y., Ogawa, M., Morita, A., Sakagami, Y. (2002). An LRR receptor kinase involved in perception of a peptide plant hormone, phytosulfokine. Science 296, 1470–1472. doi: 10.1126/science.1069607
Matsubayashi, Y., Sakagami, Y. (1996). Phytosulfokine, sulfated peptides that induce the proliferation of single mesophyll cells of Asparagus officinalis L. Proc. Natl. Acad. Sci. U.S.A. 93, 7623–7627. doi: 10.1073/pnas.93.15.7623
McGurl, B., Pearce, G., Orozco-Cardenas, M., Ryan, C. A. (1992). Structure, expression, and antisense inhibition of the Systemin precursor gene. Science 255, 1570–1573. doi: 10.1126/science.1549783
Mecchia, M. A., Santos-Fernandez, G., Duss, N. N., Somoza, S. C., Boisson-Dernier, A., Gagliardini, V., et al. (2017). RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 358, 1600–1603. doi: 10.1126/science.aao5467
Meng, J. G., Li, S. Z., Li, H. J. (2024). Central cell: the key to determine persistent pollen tube attraction or termination. Sci. China Life Sci. 67, 2030–2032. doi: 10.1007/s11427-023-2534-2
Meng, J. G., Xu, Y. J., Wang, W. Q., Yang, F., Chen, S. Y., Jia, P. F., et al. (2023). Central-cell-produced attractants control fertilization recovery. Cell 186, 3593–3605. doi: 10.1016/j.cell.2023.06.024
Meng, J. G., Zhang, M. X., Yang, W. C., Li, H. J. (2019). TICKET attracts pollen tubes and mediates reproductive isolation between relative species in Brassicaceae. Sci. China Life Sci. 62, 1413–1419. doi: 10.1007/s11427-019-9833-3
Mizuta, Y., Higashiyama, T. (2018). Chemical signaling for pollen tube guidance at a glance. J. Cell Sci. 131, jcs208447. doi: 10.1242/jcs.208447
Mizuta, Y., Sakakibara, D., Nagahara, S., Kaneshiro, I., Nagae, T. T., Kurihara, D., et al. (2024). Deep imaging reveals dynamics and signaling in one-to-one pollen tube guidance. EMBO Rep. 25, 2529–2549. doi: 10.1038/s44319-024-00151-4
Moussu, S., Broyart, C., Santos-Fernandez, G., Augustin, S., Wehrle, S., Grossniklaus, U., et al. (2020). Structural basis for recognition of RALF peptides by LRX proteins during pollen tube growth. Proc. Natl. Acad. Sci. U.S.A. 117, 7494–7503. doi: 10.1073/pnas.2000100117
Nasrallah, J. B. (2019). Self-incompatibility in the Brassicaceae: Regulation and mechanism of self-recognition. Curr. Top. Dev. Biol. 131, 435–452. doi: 10.1016/bs.ctdb.2018.10.002
Nonomura, K. I., Miyoshi, K., Eiguchi, M., Suzuki, T., Miyao, A., Hirochika, H., et al. (2003). The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell. 15, 1728–1739. doi: 10.1105/tpc.012401
Okuda, S., Higashiyama, T. (2010). Pollen tube guidance by attractant molecules: LUREs. Cell Struct. Funct. 35, 45–52. doi: 10.1247/csf.10003
Okuda, S., Tsutsui, H., Shiina, K., Sprunck, S., Takeuchi, H., Yui, R., et al. (2009). Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458, 357–361. doi: 10.1038/nature07882
Olsson, V., Joos, L., Zhu, S., Gevaert, K., Butenko, M. A., De Smet, I. (2019). Look closely, the beautiful may be small: Precursor-derived peptides in plants. Annu. Rev. Plant Biol. 70, 153–186. doi: 10.1146/annurev-arplant-042817-040413
Prado, A. M., Colaço, R., Moreno, N., Silva, A. C., Feijó, J. A. (2008). Targeting of pollen tubes to ovules is dependent on nitric oxide (NO) signaling. . Mol. Plant 1, 703–714. doi: 10.1093/mp/ssn034
Prado, A. M., Porterfield, D. M., Feijó, J. A. (2004). Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development 131, 2707–2714. doi: 10.1242/dev.01153
Punwani, J. A., Rabiger, D. S., Drews, G. N. (2007). MYB98 positively regulates a battery of synergid-expressed genes encoding filiform apparatus localized proteins. Plant Cell 19, 2557–2568. doi: 10.1105/tpc.107.052076
Qu, L. J., Li, L., Lan, Z., Dresselhaus, T. (2015). Peptide signalling during the pollen tube journey and double fertilization. J. Exp. Bot. 66, 5139–5150. doi: 10.1093/jxb/erv275
Resentini, F., Cyprys, P., Steffen, J. G., Alter, S., Morandini, P., Mizzotti, C., et al. (2017). SUPPRESSOR OF FRIGIDA (SUF4) supports gamete fusion via regulating Arabidopsis EC1 gene expression. Plant Physiol. 173, 155–166. doi: 10.1104/pp.16.01024
Rodriguez-Villalon, A., Brady, S. M. (2019). Single cell RNA sequencing and its promise in reconstructing plant vascular cell lineages. Curr. Opin. Plant Biol. 48, 47–56. doi: 10.1016/j.pbi.2019.04.002
Sede, A. R., Borassi, C., Wengier, D. L., Mecchia, M. A., Estevez, J. M., Muschietti, J. P. (2018). Arabidopsis pollen extensins LRX are required for cell wall integrity during pollen tube growth. FEBS Lett. 592, 233–243. doi: 10.1002/1873-3468.12947
Shiba, H., Takayama, S., Iwano, M., Shimosato, H., Funato, M., Nakagawa, T., et al. (2001). A pollen coat protein, SP11/SCR, determines the pollen S-specificity in the self-incompatibility of Brassica species. Plant Physiol. 125, 2095–2103. doi: 10.1104/pp.125.4.2095
Shiu, S. H., Bleecker, A. B. (2003). Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 132, 530–543. doi: 10.1104/pp.103.021964
Šírová, J., Sedlářová, M., Piterková, J., Luhová, L., Petřivalský, M. (2011). The role of nitric oxide in the germination of plant seeds and pollen. Plant Sci. 181, 560–572. doi: 10.1016/j.plantsci.2011.03.014
Song, Q., Ando, A., Jiang, N., Ikeda, Y., Chen, Z. J. (2020). Single-cell RNA-seq analysis reveals ploidy-dependent and cell-specific transcriptome changes in Arabidopsis female gametophytes. Genome Biol. 21, 178. doi: 10.1186/s13059-020-02094-0
Sprunck, S., Rademacher, S., Vogler, F., Gheyselinck, J., Grossniklaus, U., Dresselhaus, T. (2012). Egg cell-secreted EC1 triggers sperm cell activation during double fertilization. Science 338, 1093–1097. doi: 10.1126/science.1223944
Stührwohldt, N., Dahlke, R. I., Kutschmar, A., Peng, X., Sun, M. X., Sauter, M. (2015). Phytosulfokine peptide signaling controls pollen tube growth and funicular pollen tube guidance in Arabidopsis thaliana. Physiol. Plant 153, 643–653. doi: 10.1111/ppl.12270
Sui, Z., Wang, T., Li, H., Zhang, M., Li, Y., Xu, R., et al. (2016). Overexpression of peptide-encoding OsCEP6.1 results in pleiotropic effects on growth in rice (O. sativa). Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00228
Susaki, D., Izumi, R., Oi, T., Takeuchi, H., Shin, J. M., Sugi, N., et al. (2022). F-actin regulates the polarized secretion of pollen tube attractants in Arabidopsis synergid cells. Plant Cell. 35, 1222–1240. doi: 10.1093/plcell/koac371
Takayama, S., Isogai, A. (2005). Self-incompatibility in plants. Annu. Rev. Plant Biol. 56, 467–489. doi: 10.1146/annurev.arplant.56.032604.144249
Takayama, S., Shimosato, H., Shiba, H., Funato, M., Che, F. S., Watanabe, M., et al. (2001). Direct ligand-receptor complex interaction controls Brassica self-incompatibility. Nature 413, 534–538. doi: 10.1038/35097104
Takeuchi, H., Higashiyama, T. (2012). A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PloS Biol. 10, e1001449. doi: 10.1371/journal.pbio.1001449
Takeuchi, H., Higashiyama, T. (2016). Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531, 245–248. doi: 10.1038/nature17413
Tang, W., Ezcurra, I., Muschietti, J., McCormick, S. (2002). A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell. 14, 2277–2287 doi: 10.1105/tpc.003103
Tavormina, P., De Coninck, B., Nikonorova, N., De Smet, I., Cammue, B. P. (2015). The plant peptidome: An expanding repertoire of structural features and biological functions. Plant Cell. 27, 2095–2118. doi: 10.1105/tpc.15.00440
Truskina, J., Bruck, S., Stintzi, A., Boeuf, S., Doll, N. M., Fujita, S., et al. (2022). A peptide-mediated, multilateral molecular dialogue for the coordination of pollen wall formation. Proc. Natl. Acad. Sci. U.S.A. 119, e2201446119. doi: 10.1073/pnas.2201446119
Tsuwamoto, R., Fukuoka, H., Takahata, Y. (2008). GASSHO1 and GASSHO2 encoding a putative leucine-rich repeat transmembrane-type receptor kinase are essential for the normal development of the epidermal surface in Arabidopsis embryos. Plant J. 54, 30–42. doi: 10.1111/j.1365-313X.2007.03395.x
Uebler, S., Dresselhaus, T., Márton, M. L. (2013). Species-specific interaction of EA1 with the maize pollen tube apex. Plant Signal. Behav. 8, e25682. doi: 10.4161/psb.25682
Wang, L., Clarke, L. A., Eason, R. J., Parker, C. C., Qi, B., Scott, R. J., et al. (2017a). PCP-B class pollen coat proteins are key regulators of the hydration checkpoint in Arabidopsis thaliana pollen-stigma interactions. New Phytol. 213, 764–777. doi: 10.1111/nph.14162
Wang, T., Liang, L., Xue, Y., Jia, P. F., Chen, W., Zhang, M. X., et al. (2016). A receptor heteromer mediates the male perception of female attractants in plants. Nature 531, 241–244. doi: 10.1038/nature16975
Wang, X., Liu, X., Yi, X., Wang, M., Shi, W., Li, R., et al. (2023). The female germ unit is essential for pollen tube funicular guidance in Arabidopsis thaliana. New Phytol. 238, 155–168. doi: 10.1111/nph.18686
Wang, S., Lu, J., Song, X. F., Ren, S. C., You, C., Xu, J., et al. (2017b). Cytological and transcriptomic analyses reveal important roles of CLE19 in pollen exine formation. Plant Physiol. 175, 1186–1202. doi: 10.1104/pp.17.00439
Wang, W. Q., Meng, J. G., Yang, F., Xu, Y. J., Li, S. Z., Li, H. J. (2024). A non-defensin peptide NPA1 attracts pollen tube in Arabidopsis. Seed Biol. 3, e003. doi: 10.48130/seedbio-0024-0001
Wang, C.-J. R., Nan, G.-L., Kelliher, T., Timofejeva, L., Vernoud, V., Golubovskaya, I. N., et al. (2012). Maize multiple archesporial cells 1 (mac1), an ortholog of rice TDL1A, modulates cell proliferation and identity in early anther development. Development 139, 2594–2603. doi: 10.1242/dev.077891
Wilson, Z. A., Zhang, D. B. (2009). From Arabidopsis to rice: pathways in pollen development. J. Exp. Bot. 60, 1479–1492. doi: 10.1093/jxb/erp095
Wong, A., Donaldson, L., Portes, M. T., Eppinger, J., Feijó, J. A., Gehring, C. (2020). Arabidopsis DIACYLGLYCEROL KINASE4 is involved in nitric oxide-dependent pollen tube guidance and fertilization. Development 147, dev183715. doi: 10.1242/dev.183715
Xiong, T., Ye, F., Chen, J., Chen, Y., Zhang, Z. (2023). Peptide signaling in anther development and pollen-stigma interactions. Gene 865, 147328. doi: 10.1016/j.gene.2023.147328
Xue, J., Du, Q., Yang, F., Chen, L. Y. (2024). The emerging role of cysteine-rich peptides in pollen-pistil interactions. J. Exp. Bot. 75, 6228–6243. doi: 10.1093/jxb/erae322
Yamamoto, M., Nasrallah, J. B. (2013). In planta assessment of the role of Thioredoxin h proteins in the regulation of S-locus receptor kinase signaling in transgenic Arabidopsis. Plant Physiol. 163, 1387–1395. doi: 10.1104/pp.113.225672
Yamamoto, M., Nishimura, K., Kitashiba, H., Sakamoto, W., Nishio, T. (2019). High temperature causes breakdown of S haplotype-dependent stigmatic self-incompatibility in self-incompatible Arabidopsis thaliana. J. Exp. Bot. 70, 5745–5751. doi: 10.1093/jxb/erz343
Yang, H., Matsubayashi, Y., Nakamura, K., Sakagami, Y. (1999). Oryza sativa PSK gene encodes a precursor of phytosulfokine-α, a sulfated peptide growth factor found in plants. Proc. Natl. Acad. Sci. U.S.A. 96, 13560–13565. doi: 10.1073/pnas.96.23.13560
Yang, W. C., Shi, D. Q., Chen, Y. H. (2010). Female gametophyte development in flowering plants. Annu. Rev. Plant Biol. 61, 89–108. doi: 10.1146/annurev-arplant-042809-112203
Yang, S. L., Xie, L. F., Mao, H. Z., Puah, C. S., Yang, W. C., Jiang, L., et al. (2003). TAPETUM DETERMINANT1 is required for cell specialization in the Arabidopsis anther. Plant Cell 15, 2792–2804. doi: 10.1105/tpc.016618
Yao, X., Hu, W., Yang, Z. N. (2022). The contributions of sporophytic tapetum to pollen formation. Seed Biol. 1, 5. doi: 10.48130/SeedBio-2022-0005
Yu, Y., Li, W., Liu, Y., Liu, Y., Zhang, Q., Ouyang, Y., et al. (2024). A zea genus-specific micropeptide controls kernel dehydration in maize. Cell. 188, 44–59. doi: 10.1016/j.cell.2024.10.030
Yu, Y., Song, W., Zhai, N., Zhang, S., Wang, J., Wang, S., et al. (2023). PXL1 and SERKs act as receptor–coreceptor complexes for the CLE19 peptide to regulate pollen development. Nat. Commun. 14, 3307. doi: 10.1038/s41467-023-39074-4
Yu, X., Zhang, X., Zhao, P., Peng, X., Chen, H., Bleckmann, A., et al. (2021). Fertilized egg cells secrete endopeptidases to avoid polytubey. Nature 592, 433–437. doi: 10.1038/s41586-021-03387-5
Zhang, L., Huang, J., Su, S., Wei, X., Yang, L., Zhao, H., et al. (2021). FERONIA receptor kinase-regulated reactive oxygen species mediate self-incompatibility in Brassica rapa. Curr. Biol. 31, 3004–3016. doi: 10.1016/j.cub.2021.04.060
Zhang, X., Shi, C., Li, S., Zhang, B., Luo, P., Peng, X., et al. (2023). A female in vivo haploid-induction system via mutagenesis of egg cell-specific peptidases. Mol. Plant 16, 471–480. doi: 10.1016/j.molp.2023.01.001
Zhao, X., de Palma, J., Oane, R., Gamuyao, R., Luo, M., Chaudhury, A., et al. (2008). OsTDL1A binds to the LRR domain of rice receptor kinase MSP1, and is required to limit sporocyte numbers. Plant J. 54, 375–387. doi: 10.1111/tpj.15461
Zhang, X., Liu, W., Nagae, T. T., Takeuchi, H., Zhang, H., Han, Z., et al (2017). Structural basis for receptor recognition of pollen tube attraction peptides. Nat. Commun. 8, 1331. doi: 10.1038/s41467-017-01323-8
Zhao, T., Ren, L., Zhao, Y., You, H., Zhou, Y., Tang, D., et al. (2021). Reproductive cells and peripheral parietal cells collaboratively participate in meiotic fate acquisition in rice anthers. Plant J. 108, 661–671. doi: 10.1111/tpj.15461
Zhong, S., Li, L., Wang, Z., Ge, Z., Li, Q., Bleckmann, A., et al. (2022). RALF peptide signaling controls the polytubey block in Arabidopsis. Science 375, 290–296. doi: 10.1126/science.abl4683
Zhong, S., Liu, M., Wang, Z., Huang, Q., Hou, S., Xu, Y. C., et al. (2019). Cysteine-rich peptides promote interspecific genetic isolation in Arabidopsis. Science 364, eaau9564. doi: 10.1126/science.aau9564
Zhong, S., Zhao, P., Peng, X., Li, H. J., Duan, Q., Cheung, A. Y. (2024). From gametes to zygote: Mechanistic advances and emerging possibilities in plant reproduction. Plant Physiol. 195, 4–35. doi: 10.1093/plphys/kiae125
Keywords: plant reproduction, peptide, receptor, pollen tube, fertilization
Citation: Yan C-L, Guan K-X, Lin H, Feng T and Meng J-G (2025) Peptides in plant reproduction—small yet powerful. Front. Plant Sci. 16:1506617. doi: 10.3389/fpls.2025.1506617
Received: 05 October 2024; Accepted: 03 February 2025;
Published: 24 February 2025.
Edited by:
Giampiero Cai, University of Siena, ItalyReviewed by:
Luigi Parrotta, University of Bologna, ItalyCopyright © 2025 Yan, Guan, Lin, Feng and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang-Guo Meng, bWVuZ2pnMTk5M0Bzd3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.