
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 03 March 2025
Sec. Plant Systematics and Evolution
Volume 16 - 2025 | https://doi.org/10.3389/fpls.2025.1495796
This article is part of the Research TopicKarst Plants Diversity, Evolution, Taxonomy and ConservationView all 6 articles
 Marjorie D. delos Angeles1,2,3
Marjorie D. delos Angeles1,2,3 Sirilak Radbouchoom1,2
Sirilak Radbouchoom1,2 Boniface K. Ngarega4†
Boniface K. Ngarega4† R. Sedricke Lapuz5
R. Sedricke Lapuz5 Harald Schneider1,2*
Harald Schneider1,2*Karst formations are distinguished by their high levels of species diversity and endemism, including ferns and lycophytes. However, the existing data on plant community composition in karst formations remains deficient. Addressing these knowledge gaps is imperative, given the current accelerated rates of species loss, to enhance efforts to conserve biodiversity in these habitats. This study documents and explains patterns of fern and lycophyte species diversity within karst landscapes (KL) and non-karst landscapes (NKL) in the Philippines. Our comprehensive analysis involved aggregating 19,529 occurrence points encompassing 1,024 fern and lycophyte species sourced from field expeditions, voucher records from local herbaria, and online databases. Indices for species richness, weighted endemism, and corrected weighted endemism were then computed across KL and NKL areas to describe spatial diversity and identify fern and lycophyte hotspot areas. Gap analyses were also performed to determine if established protected areas (PAs) were sufficient to cover the identified fern and lycophyte diversity hotspots. Principal Component Analysis (PCA) was conducted to determine potential ecological drivers of distribution between KL and NKL areas. The findings reveal that most fern and lycophyte species were recorded to occur in NKL areas, with 995 (97.16%) species identified, while 676 (66.02%) species were documented to occur in KLs, including 29 (2.83%) exclusive to karsts. Identified hotspots for NKL are within mountain ecosystems, which are already under existing legal protection. In contrast, KLs have five areas identified as congruent hotspots but considered gap areas due to their exclusion from current PA boundaries. Existing PAs thus provide less protection to karst habitats and their associated floras. PCA did not reveal any significant environmental predictors, suggesting separation of KL and NKL species distributions, possibly due to lack of high-resolution environment data available for karsts. To facilitate the conservation of fern and lycophyte species in karsts, we propose installing and expanding existing PA boundaries, along with conducting more focused surveys in karst regions to better understand their ecological dynamics.
The continuous overexploitation of the world’s natural resources takes global biodiversity towards the sixth mass extinction (Ceballos et al., 2017; Cowie et al., 2022), highlighting the need for prioritizing the conservation of threatened species and ecologically important ecosystems (Clements et al., 2006). Effective conservation of species and habitats, however, cannot be achieved without the necessary data, and it is important to delineate and identify hotspot areas to guide conservation efforts (Myers et al., 2000).
Karst regions, similar to islands, represent an important source of regional biodiversity and endemism (Geekiyanage et al., 2019), providing a wide range of microhabitats due to its highly structured geomorphology resulting from the interaction of water and wind with limestone carbonates (Clements et al., 2006; Tolentino et al., 2020). Karst landscapes are characterized by their fragility, edaphic complexity, and harsh natural ecological environments due to their thin soils that are deficient in nitrogen and phosphorus and have high concentrations of calcium and magnesium (Yang et al., 2022; The Long and Dac Trien, 2019; Geekiyanage et al., 2019). Given these characteristics, karst landscapes provide unique challenges to the plants colonizing them (Green et al., 2019; Liu et al., 2021). Despite hosting several highly specialized species with restricted distribution ranges, karst areas are often overlooked habitats in biodiversity protection efforts. The karst regions of Southeast Asia have particularly been flagged out as a challenge to biodiversity conservation (Clements et al., 2006). While these habitats are rich in species highly dependent on the unique characters of karst landscapes (Ford and Williams, 2007; Fu et al., 2022), they also experience threats from mining activities and the impacts of global climate change (Whitten, 2012; Wong et al., 2016; He et al., 2021). These threats have contributed to increasing species extinctions (Phutthai and Hughes, 2017), highlighting the critical need for comprehensive biological data collection in karst landscapes (Salas et al., 2005, Bystriakova et al., 2019; Fu et al., 2022; Molina-Paniagua et al, 2023).
Surveying plants occurring on tropical karst regions is therefore considered as a priority. This is crucial to support the establishment of protected areas, which target the conservation of species endemic to tropical karst formations. The enhanced availability of digitized historical collections, such as herbarium specimens stored in databases such as the Global Biodiversity Information Facility (GBIF), has recently allowed the assessment of species distribution across different landscapes (Bystriakova et al., 2019; Ramírez-Barahona et al., 2023). Given the recent access to big spatial data, metrics such as species richness, weighted endemism, corrected weighted endemism, and beta diversity have been utilized to determine biodiversity hotspots (Yu et al., 2017; Chen et al., 2022). These have also been used to perform gap analyses in existing conservation measures and protected areas for their possible enhancement and area expansions (Myers, 1988; Scott et al., 1993; Jennings, 2000; Myers et al., 2000; Xu et al., 2017; Brummitt et al., 2021).
Despite challenges brought about by karst landscapes, it can support ferns and lycophytes. These ancient linages with approximately 12,000 recognized species globally (PPG1, 2016) account for 3% of the world’s vascular plant diversity and rank second in diversity after angiosperms (Kress, 1986; Schuettpelz and Pryer, 2009; Nitta et al., 2022). They play pivotal roles in tropical terrestrial ecosystems (Salovaara et al., 2004; Mehltreter, 2010; Watkins and Cardelús, 2012; Bergeron and Pellerin, 2014; Pouteau et al., 2016; Silva et al., 2018), where they exist as terrestrial, epiphytic, and as lithophytic organisms (Page, 2002; Mehltreter, 2010; Watkins et al., 2010). Calcareous ferns were recorded to exhibit high diversity within the families Pteridaceae, Aspleniaceae, and Dyopteridaceae in select Mexican limestone regions (Flores-Galván et al., 2024).
Ferns and lycophytes are sensitive to changes in the environment, making them valuable bioindicators of terrestrial ecosystem health. While their potential as bioindicators have been well explored and have been used in classifying forest types (Salovaara et al., 2004) and determining forest integrity (Bergeron and Pellerin, 2014), their application as bioindicators in tropical karst landscapes remains underutilized. Furthermore, Karst microhabitats, characterized by unique geological formations and microclimates, can act as refugia for site-endemic species. This assertion is supported by the successful recovery of numerous locally endemic fern species within these environments (Takeuchi, 2007; Brownsey and de Lange, 1997; He and Zhang, 2010; Yesilyurt and Schneider, 2010; Tang et al., 2017). The investigation of fern and lycophyte assemblages in limestone forests, particularly on habitats formed over karst formations, remains a relatively understudied aspect of botanical research in the tropics, including the Philippines. Existing studies on ferns and lycophytes of karst landscapes in the country are predominantly centered on enumerations and are primarily taxonomic driven (Price, 1975; Iwatsuki and Price, 1977; Zamora and Co, 1986; Barcelona, 2003; Barcelona et al., 2006; delos Angeles et al., 2022; delos Angeles and Asis, 2022). There is also limited information on drivers of their distribution across both landscapes.
To bridge this knowledge gap, this study therefore undertook a comprehensive analysis of fern and lycophyte diversity, prevalent in both karst and non-karst landscapes within the Philippines and explore the influence of edaphic and bioclimatic factors on their assemblages. To achieve these, we compiled a dataset to illustrate the spatial distributions of ferns and lycophytes across both landscapes. We also performed gap analyses focused on linking the identified biodiversity hotspots in karst and non-karst habitats with existing protected areas. Finally, we propose a list of hotspots on karst areas to be considered as candidates for newly established national parks.
The Philippines, an archipelago of more than 7,600 islands, harbors multiple centers of endemism (Mittermeier et al., 2004). A significant proportion of the country’s land area is characterized by karst formations, which are developed over limestone deposits composed by tertiary and quaternary carbonates (Balázs, 1973). Specifically, approximately 10% of the Philippines I covered by limestone forest formations (Piccini and Rossi, 1994; Balázs, 1973).
Georeferenced occurrence points of ferns and lycophytes were primarily obtained from the following sources to compile data for analyses. They are as follows: i) occurrences points sampled during field research, detailed below; (ii) voucher records from physical examinations in select herbaria (College of Agriculture Herbarium UP or CAHUP, Los Baños Collection or LBC, & Jose Vera Santos Memorial Herbarium or PUH); and (iii) and historical records accessed via the Global Biodiversity Information Facility database (GBIF, https://doi.org/10.15468/dl.698jtt). Additional occurrence data were harvested from these published works on Philippine ferns and lycophytes: Barcelona and Pelser (2014), Coritico et al. (2019); Amoroso et al. (2020a); Amoroso et al. (2020b); Amoroso et al. (2021); Chao et al. (2022); Chen et al. (2022), and Coritico et al. (2022).
As for the field occurrences, the first author conducted a series of field surveys in select provinces of the Philippines from the year 2020–2023. All surveys were carried out with the necessary permissions provided by the administrations overseeing the protection of biodiversity. Both karst areas and non-karst areas were surveyed to account fern and lycophyte species diversity. In total, fieldwork was conducted across nine municipalities from seven provinces of the Philippines, which were 1) Carranglan, Nueva Ecija; 2) Dinapigue, Northern Sierra Madre; 3) Divilacan, Northern Sierra Madre; 4) Masungi Georeserve, Rizal; 5) Mt. Makiling Forest Reserve, Laguna; 6) Puerto Princesa Subterranean River National Park, Palawan; 7) Narra, Palawan; 8) Paranas, Samar Island Natural Park, Samar Island; and 9) Taft, Eastern Samar, Samar Island (Figure 1). Species occurrences and geographic locations were recorded using a Global Positioning System (GPS) device, and voucher specimens were deposited at CAHUP and LBC.
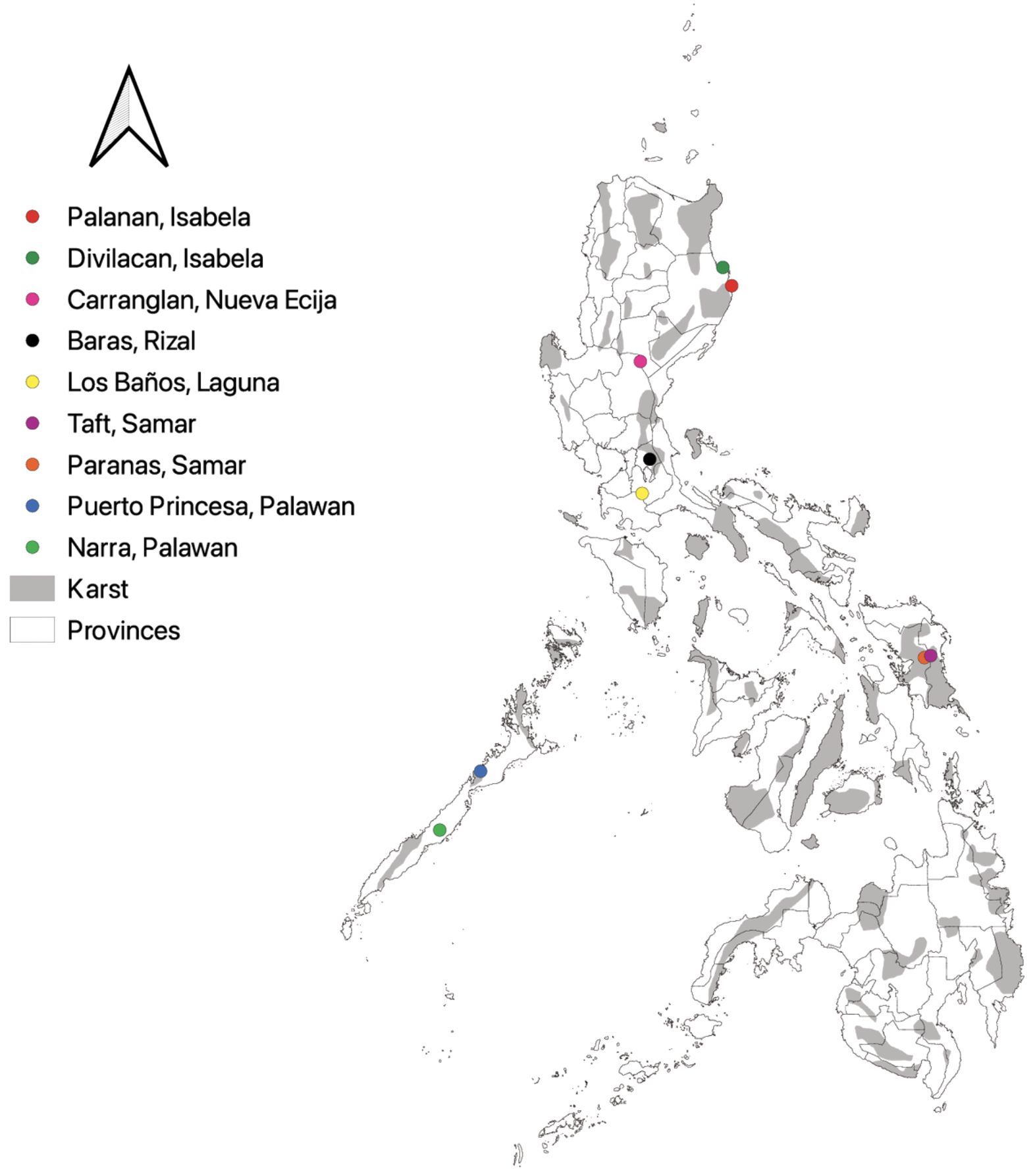
Figure 1. Map showing the survey locations of pteridophytes in Philippine provinces. Gray areas represent karst formations, while lined areas within islands distinguish between and among provinces. Field survey localities include: Palanan, Isabela (red); Divilacan, Isabela (green); Carranglan, Nueva Ecija (pink); Baras, Rizal (black); Los Baños, Laguna (yellow); Taft, Samar (violet); Paranas, Samar (orange); Puerto Princesa, Palawan (blue); and Narra, Palawan (yellow green). Map generated using QGIS 3.32.0 Lima.
Occurrence records on historical Philippine records of ferns and lycophytes were downloaded from the Global Biodiversity Information Facility (GBIF.org, 2023a,b,c,d,e,f,g,h,I,j,k,l,m,n,o,p,q,r,s,t,u,v,w,x,y,z, aa,bb,cc,dd,ee,ff,gg,hh,ii,jj,kk,ll,mm,nn,oo,pp,qq,rr,ss,tt). These data were compiled together with the records obtained from field surveys, herbarium vouchers, and literature records. To ensure the quality of the final dataset, a multi-step occurrence cleaning process was conducted. Those with mismatched described location and GPS coordinates were reassigned based on the description given in the voucher labels. Data points with erratic and incomplete information (e.g., mentioned only country or region names) were excluded. Data points outside the country border were also manually removed.
The species names of the assembled occurrence records were further curated and updated. Family and genus names were standardized in accordance with PPG I (2016), while synonyms were replaced with accepted names based on the nomenclature ascribed in Co’s Digital Flora (Pelser et al., 2011) and World Plants (Hassler, 2004–2023) to reduce transcription errors. In addition, duplicated and misplaced accessions were manually excluded. Finally, spatial autocorrelation between occurrence data was reduced by spatial rarefaction using R package “spThin” v.0.1.0 (Aiello-Lammens et al., 2015) and retained one occurrence point per species within each 1 km2 pixel for analyses.
To examine and analyze spatial indices for the two forest formations, the data set was further divided into occurrence records in Karst Landscapes (KL) only and Non-Karst Landscapes (NKL). This was achieved by overlaying the gathered occurrence points with the map of karst formations in the Philippines, then appropriately tagging each occurrence as “KL” or “NKL” based on their location. The Karst map was obtained from the World Karst Aquifer Map available at WHYMAP (World-wide Hydrologeological Mapping and Assessment Programme, 2023), (https://www.whymap.org, downloaded on 9 May 2023).
To explore the distribution of ferns and lycophytes in the Philippines, the software Biodiverse version 4.0 (Laffan et al., 2010) was employed to carry out spatial analysis on a grid scale of 0.083333°C (5 arcmin). Three biodiversity indices, namely, (1) species richness (SR), (2) weighted endemism (WE), and (3) corrected weighted endemism (CWE), were calculated for each KL and NKL grid cell. The three indices were defined as follows: (1) species richness represents the sum of the number of distinct/unique species occurring in a grid cell (Chen et al., 2022); (2) weighted endemism, or range-size rarity, assigns high weights to species with small ranges and smaller weights to widespread species (Xue et al., 2022)—with a high weighted endemism value suggesting that there are more range-restricted species in a particular region (Linder, 2001; Huang et al., 2016; Baldwin et al., 2017; Yu et al., 2017); and (3) the corrected weighted endemism, on the other hand, separates the existing trend between weighted endemism and species richness by dividing it by the species richness at each location (Crisp et al., 2001).
To detect the congruence among the distribution patterns of the three indices, we calculated all pairwise Pearson’s correlation coefficients. Pearson’s correlation coefficients (r) were separated into five classes according to Schober et al. (2018): negligible correlation (0 < |r| < 0.1), weak correlation (0.10 ≤ |r |<0.40), moderate correlation (0.40 ≤ |r| < 0.70), strong correlation (0.70 ≤ |r| < 0.90), and very strong correlation (0.90 ≤ |r| < 1). Data cleaning, wrangling, analyses, and visualization were performed in R (v. 2023.06.0 + 421; R Core Development Team, 2023).
To estimate conservation gaps, the protected areas were overlapped with the generated distribution maps based on the three spatial diversity indices. Protected area boundary shapefiles of the Philippines were downloaded from the World Database on (Protected Planet (2014-2023), available at https://www.protectedplanet.net/en/thematic-areas/wdpa?tab=WDPA, downloaded on 1 June 2023). Grid cells within the top 10%, 20%, 30%, and 50% for each spatial diversity metrics were identified as hotspots (Yu et al., 2017). For the gap analysis, the three metrics were overlapped with Philippine protected areas. By overlapping the hotspots resulting from the different metrics, species hotspots and areas that are outside protected area boundaries were visually identified. Maps were generated using QGIS 3.32.0 (QGIS.org, 2023) with WGS-84 EPSG:4326 projection.
To understand the drivers of distribution among pteridophyte species from KL and NKL, Principal Component Analysis (PCA) (“PCA” function in the R package FactoMineR) was conducted. Due to the imbalance between KL and NKL points, 2,669 species occurrence points were first randomly selected from the NKL species to match the number of available KL species occurrence points. Analyses were then conducted using three set of parameters: i) all abiotic variables, ii) bioclimatic variables only, and iii) physical/chemical variables only. Bioclimatic variables (BIO1–BIO19) data were obtained from WorldClim 2.1 database at 30 arcs resolution (Fick & Hijmans, 2021). Soil physical/chemical variables were obtained from World Soil Information (ISRIC) at 250 m resolution then resample to 30 arcs. Abiotic variable values were extracted for each species occurrence point. Values were then normalized prior to the PCA run.
Predictor variables that had correlations with response variables between 0.1 and −0.1 due to weak explanatory power were removed (Fløjgaard et al., 2011). On account of collinearity, when two or more predictor variables were strongly correlated (r > ± 0.70), we only retained variables that had the stronger correlation with the response variable and makes biological sense with the taxa (Dormann et al., 2007). Data was analyzed using the software R, version 2023.06.0 + 421 (R Development Core Team, 2023).
A total of 62,887 occurrence points were compiled by incorporating 302 physical voucher examinations (PUH, LBC, and CAHUP), 683 field derived coordinate points, and 61,902 GBIF-derived occurrence data. Of the 1,430 species, a total of 406 were excluded from the analysis due to erratic and/or controversial information such as unplaced names, no formal species records, or incorrect distribution occurrence records. A total of 19,259 points remained after spatial thinning and were utilized for analysis (Supplementary Table 1). In total, there are 1,024 fern and lycophyte species, 992 species of which were in non-karst areas. Out of the 679 species recovered to occur in karst areas, 5% were found only in karst and 95% in both karst and non-karst areas (Fick and Hijmans, 2017).
The reconstructed fern and lycophyte species spatial patterns in karst areas reveal 10 hotspot areas. The distributional range of species occurring in karst formations of the Philippines comprised of 2,669 occurrence records in 248 grid cells. SR per grid cell ranged from 1 to 139 species (Figure 2). Most of the karst landscape identified to have fern and lycophyte species rich flora were found at the foot or near mountain ecosystems. Species-rich provinces comprised i) Kabayan, Benguet; ii) Baco, Mindoro Oriental; iii) San Teodoro, Mindoro Oriental; iv) Paranas, Samar; v) Hinabangan, Samar; vi) Dingalan, Aurora; vii) Samarenana, Palawan; viii) Caramoan, Catanduanes; ix) Valencia, Bohol; and x) Guinayangan, Quezon. A consistent pattern was observed by exploring the spatial distribution for WE. The spatial distribution of SR was highly correlated with WE (R = 0.95). In turn, SR and WE had a weak correlation with CWE (R = 0.19; R = 0.25). Notably, records were rare or totally missing for six provinces with karst formations in the Philippines. These areas were identified to be Davao del Norte, Masbate, Quirino, Saranggani, Southern Leyte, and Surigao del Sur. Five from these 10 landscapes have existing natural parks and other protected landscapes.
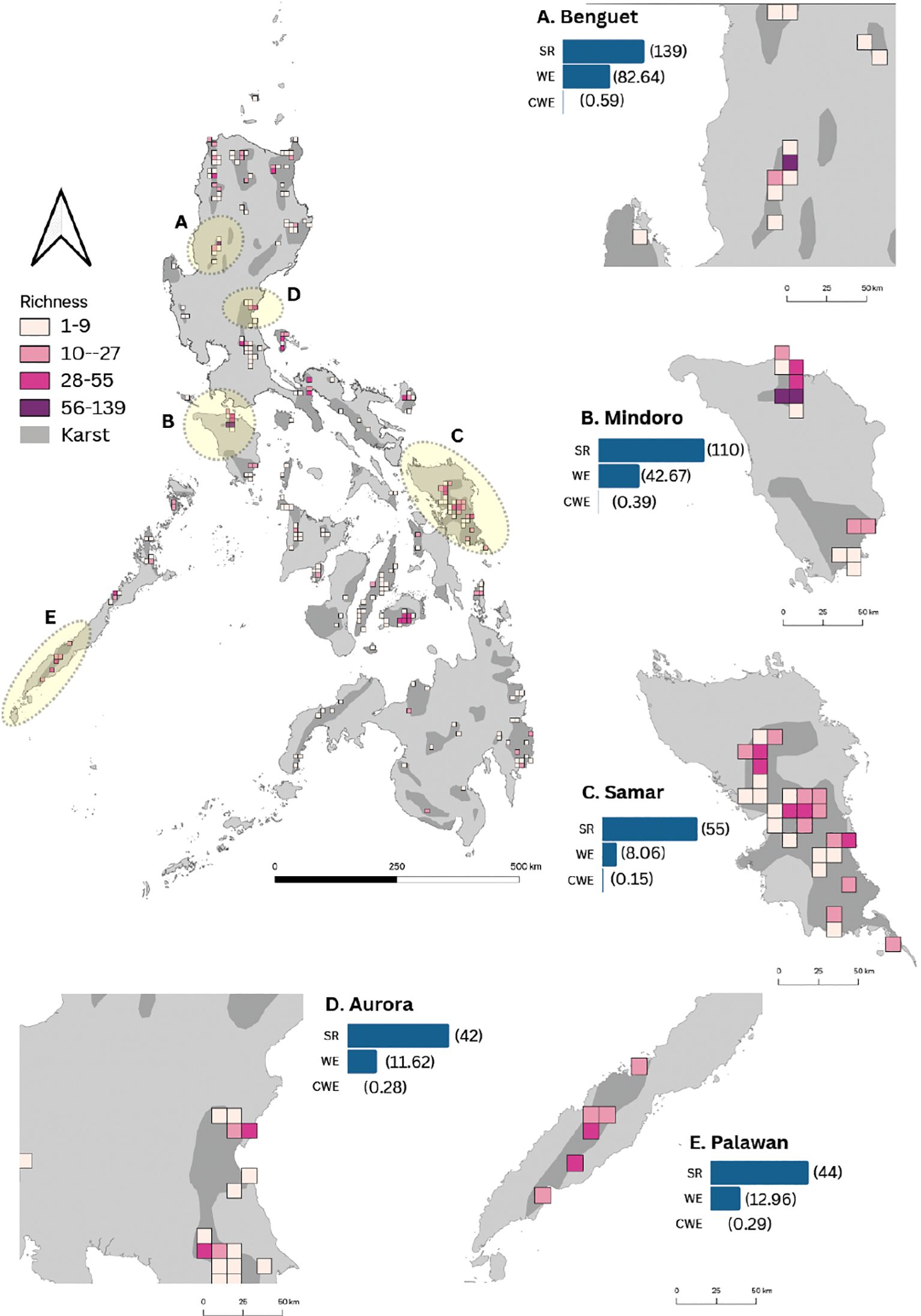
Figure 2. Distribution of fern and lycophyte species richness and endemism in karst habitats across the Philippines. Karst formations are shaded in gray. Provinces with high species richness and endemism are highlighted in yellow and zoomed in. The color code represents the four classes of species richness: Class I (1–9 species) in light pink, Class II (10–27 spp.) in pink, Class III (28–55 spp.) in dark pink, and Class IV (56–139 spp.) in purple. The identified hotspot provinces (A–E) are accompanied by bars indicating species richness (SR), weighted endemism (WE), and corrected weighted endemism (CWE). Numbers in parentheses correspond to the index values for SR, WE, and CWE.
The final spatial dataset for fern and lycophyte occurrences in non-karst areas contained 16,590 occurrence points in 854 grid cells. SR per grid cell ranged from 1 to 359 species (Figure 3). Species-rich provinces consisted of i) Santa Cruz, Davao del Sur; ii) Los Baños, Laguna; iii) Candelaria, Quezon; iv) Bagac, Bataan; v) San Jose, Negros Oriental; vi) Lucban, Quezon; vii) La Trinidad, Benguet; viii) Tayabas, Quezon; ix) Bauko, Mountain Province; and x) Casiguran, Sorsogon. Existing natural parks and other protected landscapes were in all these regions.
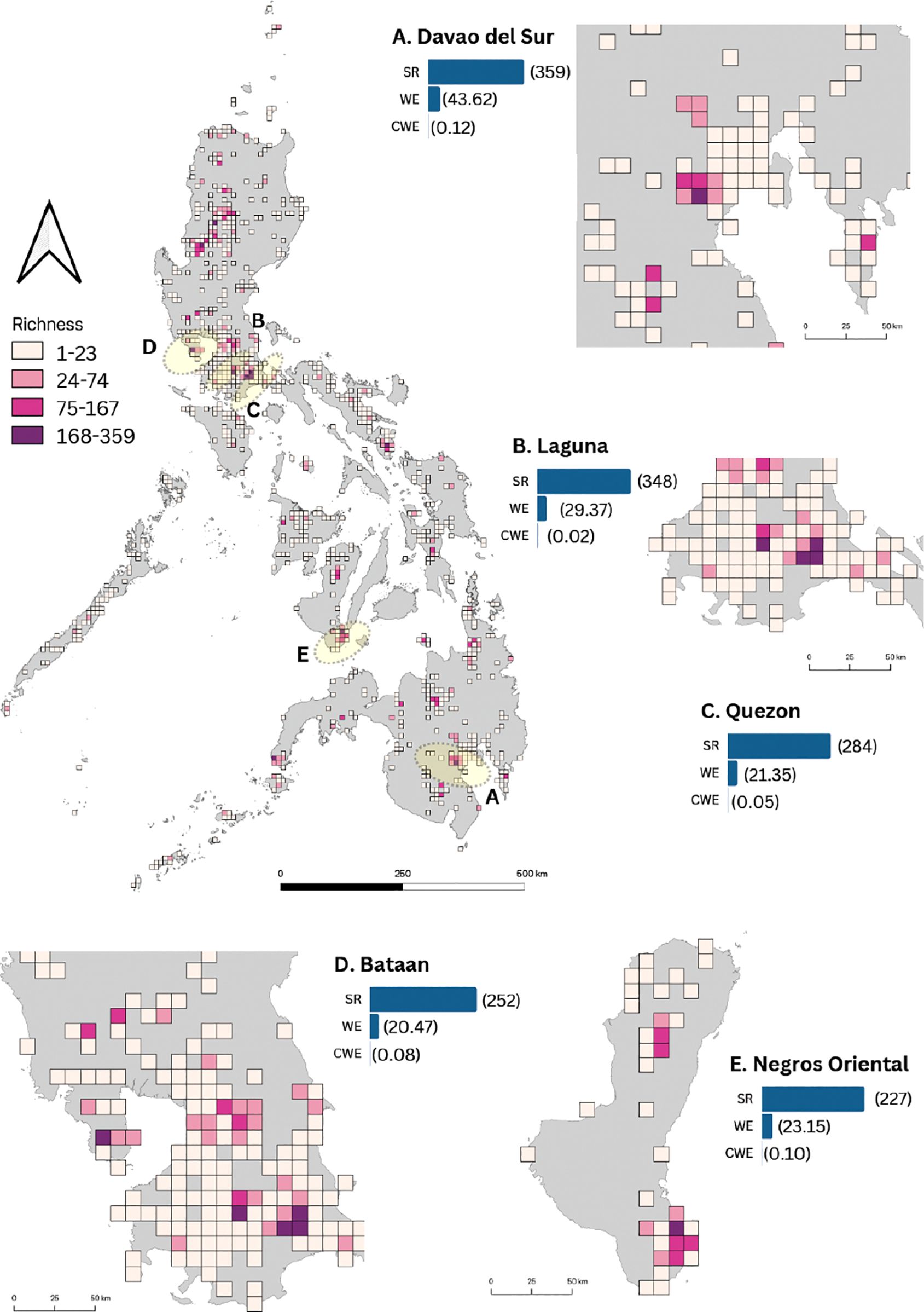
Figure 3. Distribution of fern and lycophyte species richness and endemism in non-karst areas across the Philippines. Provinces with high species richness and endemism are highlighted in yellow and zoomed in. The color code represents four classes of species richness: Class I (1–23 spp.) in light pink, Class II (24–74 spp.) in pink, Class III (75–167 spp.) in dark pink, and Class IV (168–359 spp.) in purple. The maps of these five provinces (A–E) are accompanied by bars indicating species richness (SR), weighted endemism (WE), and corrected weighted endemism (CWE) for the respective province. Numbers in parentheses represent values for SR, WE, and CWE.
These provinces were recognized for the presence of ecologically and culturally significant mountain ecosystems including some dormant volcanoes. Mountain ecosystems representing putative cradles or sanctuaries of diversity included protected landscapes such as i) Mt. Apo National Park (MANP) in Davao del Sur, ii) Mt. Makiling Forest Reserve (MMFR) in Laguna, iii) Mt. Banahaw-San Cristobal Protected Landscape (MBSCPL) in Quezon, iv) Mt. Mariveles in Bagac, Bataan, v) Balinsasayao Twin Lakes Natural Park, Negros Oriental, vi) Mt. Santo Tomas, Benguet, vii) Mt. Polis, Mountain Province, and viii) Bulusan Volcano National Park, Sorsogon. Like the results of KL, the spatial distributions of NKL for SR was highly correlated with WE (R = 0.80). However, SR and WE had a weak correlation with CWE (R = 0.07; R = 0.29).
Based on the diversity metrics SR and WE indices, the top 10% hotspots were concentrated in Batan, Benguet located in Northern Luzon. Extending the selected hotspots to the top 20% revealed by SR and WE expanded the list towards Mangan II and Caagutayan of Mindoro Oriental. Further extending to the top 30% included (1) Paranas and Bagacay, Samar; (2) Valencia, Bohol; (3) Samerana, Palawan; (4) Dikapanikian, Aurora; (5) Caramoan, Catanduanes; (6) Guinayangan, Quezon; (7) Bilar, Bohol; (8) Gandara, Samar; and (9) Tagkawayan, Quezon. Finally, when expanded further to 50%, grid cells with high values for SR and WE now included also (1) Carmen, Bohol; (2) San Teodoro, Mindoro Oriental; (3) Rizal, Palawan; (4) Verde Island Passage; (5) Cabayugan, Puerto Princesa, Palawan; (6) Sierra Bullones, Bohol; and (7) Nueva Era, Ilocos Norte. Most of the hotspot areas for SR and WE were concentrated in the Luzon and Visayas islands.
Most of the differences in overlap among these diversity indices were found at 50%. Once 50% was considered as the extent of congruence, results revealed more unique hotspots and was thus chosen as the ideal threshold for defining pteridophyte hotspots. A total of 11 areas were identified as hotspot areas. These congruent hotspots cover approximately 93.5 km2. Six of the 11 congruent hotspot areas are found within protected areas: 1) Batan, Benguet; 2) Samarenana, Palawan; 3) Caramoan, Catanduanes; 4) Gandara, Samar; 5) Rizal, Palawan; and 6) Matalud, Samar. However, the five other areas that were identified to be congruent hotspots, now considered as gap areas, that are not within protected areas include the following: 1) Nueva Era, Ilocos Norte; 2) Dikapanikian, Aurora; 3) Bagong Silang, Tagkawayan, Quezon; 4) Mangangan, Baco, Mindoro Oriental; and 5) San Teodoro, Caagutayan, Mindoro Oriental (Figure 4).
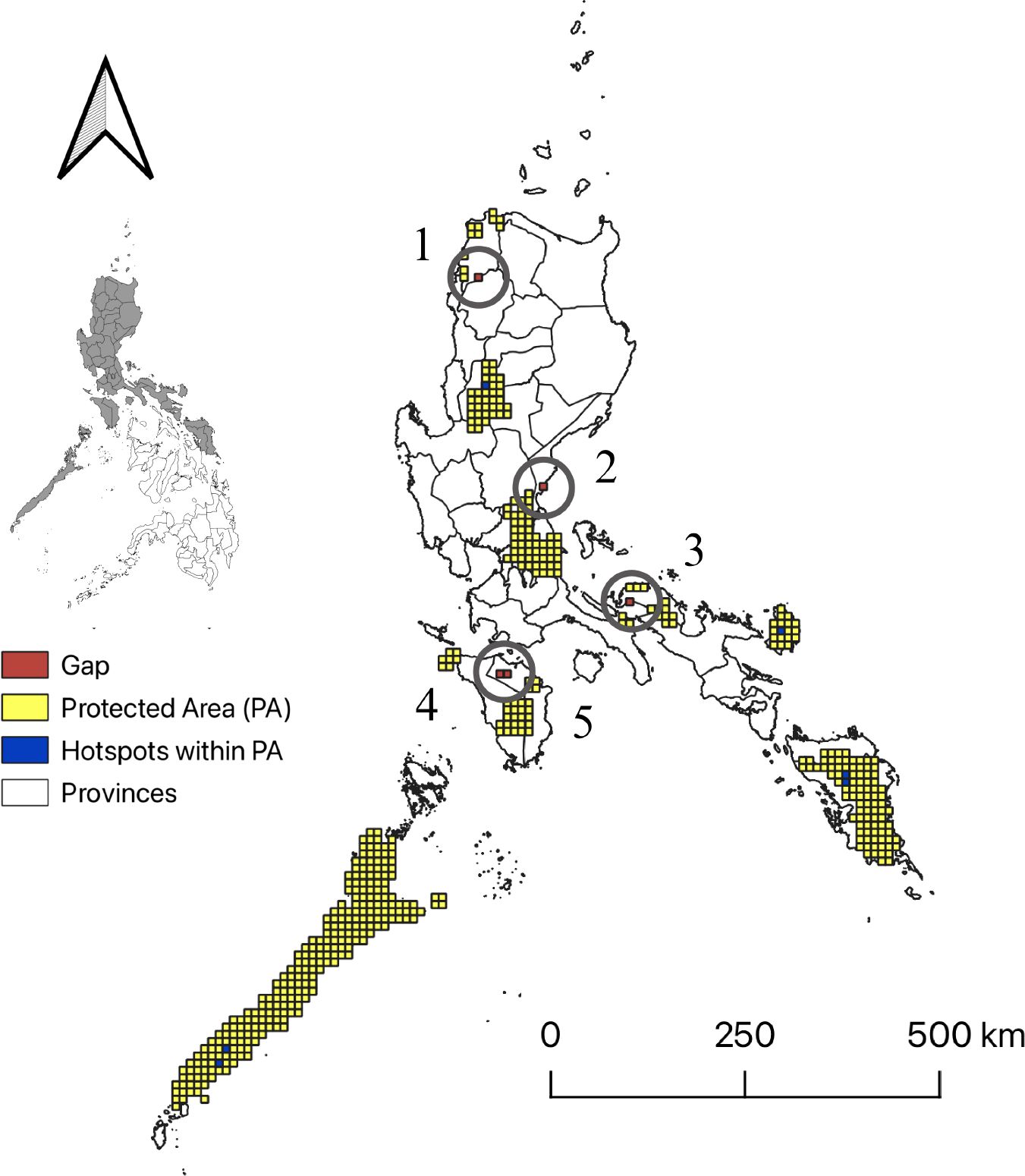
Figure 4. Hotspot areas and gap areas of pteridophyte species occurring in forests formed over karst formations. Hotspots were defined as the overlap of the richest 50% of grid cells for species richness (SR), weighted endemism (WE), and corrected weighted endemism (CWE). Hotspots inside protected areas are shown in blue, while hotspots outside protected areas are shown in red. Identified gap areas (red) include 1) Nueva Era, Ilocos Norte; 2) Dikapanikian, Aurora; 3) Bagong Silang, Tagkawayan, Quezon; 4) Mangangan, Baco, Mindoro Oriental; and 5) San Teodoro, Caagutayan, Mindoro Oriental. Map generated using QGIS 3.32.0.
Principal component analyses did not reveal influential factors driving species distribution between karst and non-karst areas, as there was no discernible clustering between karst and non-karst species occurrence points (Figure 5 and Supplementary File 3). The PCA, which considered bioclimatic variables alone, explained the highest proportion of variance (77.2%) (Supplementary File 10B and Supplementary File 6), followed by the PCA that used soil and elevation variables (71.4%) (Supplementary File 10C and Supplementary File 8). Despite the identification of these explanatory variables, these were insufficient to distinctly separate ferns and lycophytes into habitat groups of karst and non-karst landscapes, with ferns and lycophytes recorded from karst landscapes nested within non-karst landscapes.
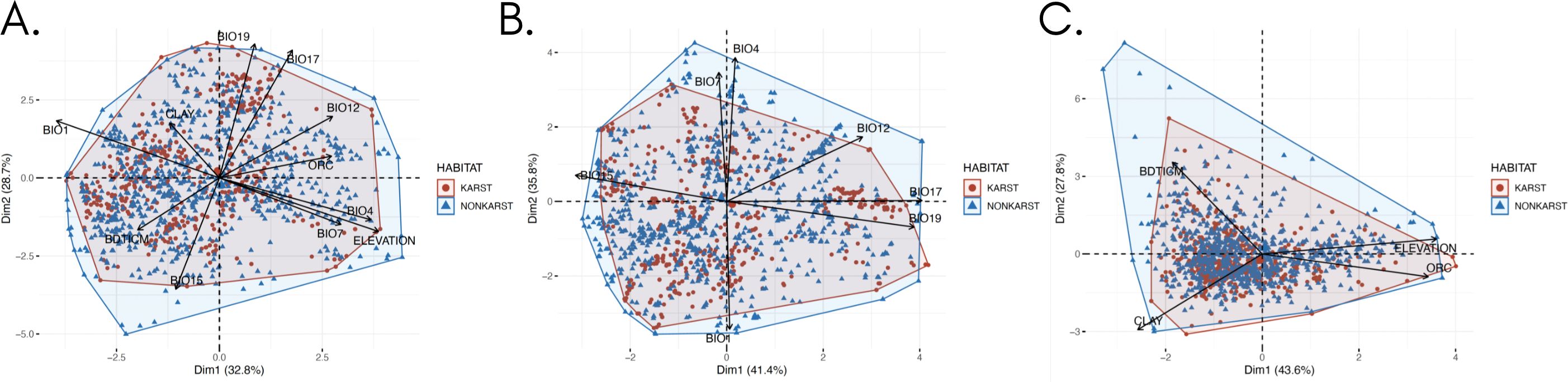
Figure 5. Principal components analysis (PCA) plots illustrating the influence of (A) all variables, (B) bioclimatic variables, and (C) soil variables on the distribution of ferns and lycophytes within Philippine ecospace. The scatterplot represents a PCA focused on dimensions 1 and 2, with data points color-coded by habitat: blue for karst and red for non-karst. Vectors represent the variables: BIO1, BIO4, BIO7, BIO12, BIO15, BIO17, BIO19, absolute depth to bedrock (BDTICM), elevation, organic carbon content (ORC), and clay content.
Limited studies have been conducted to explore the composition of ferns and lycophytes in karst landscapes of the Philippines. The challenging terrain and limited accessibility to these landscapes have significantly hindered botanical exploration. This has resulted in a sampling bias that is evident in the disproportionately higher number of species occurrence records documented from non-karst landscapes. To avoid underestimating ferns and lycophytes species associated with karst environments, it is crucial to conduct more fieldwork. This involves direct documentation of plant specimens in relation to their substrate complemented with the integration of other data sources.
To address data gaps due to the lack of targeted surveys in karst areas, this study used an approach that considers available voucher specimens available and historical data from GBIF. However, unlike that of field-derived data and publications with substrate information, only a limited number of vouchers and historical data contains information regarding the substrate. Thus, the association between plant and substrate are not directly accessible by the collector information. To overcome this limitation, we employed a mapping approach that overlays plant and karst distribution maps to infer associations.
Conducting thorough assessments in karst landscapes presents challenges, as evidenced by the bias towards collecting data from non-karst landscapes. The higher number of species occurrences recorded in NKL may be a consequence of the rugged terrain, topographic isolation, and limited accessibility of karst areas in the Philippines. Additionally, the available edaphic, topographic, and bioclimatic data online is coarse and could be insufficient in capturing changes in microenvironments of karst landscapes. We suspect that the low-resolution data available and used for the PCA could be the reason why no distinct clusters between the two environmental types were apparent. Future related work should consider using field-derived environmental data for ecological analysis. Spatial heterogeneity in soil nutrients and moisture along karst ecosystems, influenced by the rate of karst formation and carbonate rock substrate, highlights the need to gather detailed abiotic data directly from fieldwork (Geekiyanage et al., 2019). Field data play a crucial role not only in providing precise location information but also in helping to understand ecological processes underlying in karst formations that distinguishes them from their non-karst counterparts and in confirming the relationship between fern and lycophyte species and their substrate.
The foundation of effective conservation decision-making relies on the accuracy and precision of the data used to design present and future management strategies (Hughes et al., 2021a, Hughes et al., 2021b). It is essential to formulate data-driven conservation strategies guided by robust scientific principles. Protected areas, both existing and prospective, represent a key strategic approach for biodiversity preservation (Sodhi et al., 2004; Matten et al., 2023). Our analysis identified 11 karst landscapes as critical hotspots for pteridophytes, with the majority of these areas falling within protected area boundaries. While these areas will remain protected for the foreseeable future, some karst areas, such as limestone forests, remain inadequately safeguarded. Through gap analysis, previously overlooked areas requiring conservation intervention were identified. Notably, five of these 11 hotspots areas lack any legal protection, underscoring the urgency to expand the adjacent protected areas to cover these crucial karst regions, thus safeguarding the pteridophyte species they harbor.
The conservation of these areas not only ensures the preservation of karst landscapes and their associated pteridophytes but also safeguards the numerous ecosystem services these unique forest formation provides. The success of the Kunming Montreal Biodiversity Framework (CBD, 2022) depends largely on our knowledge and understanding of plant diversity and distribution; in its absence, there is an overwhelming risk that some of the global commitments—such as pledge to protect 30% of the world’s terrestrial surface by 2030, potentially escalating the risk of plants species loss (Antonelli, 2023). Existing protected areas and future protected areas remain to be one of the most strategic and effective means of preserving biodiversity (Sodhi et al., 2004; Matten et al., 2023).
To enhance conservation efforts, it is crucial to promote the growth and training of experts capable of species identification and proficient in collating high-resolution biological, ecological, and spatial-temporal species data. Establishing standardized long-term monitoring strategies for ferns and lycophyte species is recommended to ensure data reliability and track secure species populations in the wild. Continuous inventories, spatial occurrences, and comparative studies of fern communities are pivotal in bridging biodiversity information gaps and preventing extinctions (Pimm, 2020). A robust database of species occurrences is instrumental in predicting community patterns and determining conservation priorities for ferns and lycophytes in both karst and non-karst landscapes.
This study utilized available ferns and lycophytes occurrence data from multiple sources to visualize spatial diversity of ferns and lycophytes between karst and non-karst landscapes. We demonstrated that fern and lycophyte hotspots are concentrated in mountain ecosystems within established protected areas. However, we also found that historical records available are unevenly distributed across the Philippines and biased towards non-karst landscapes, resulting in survey gaps concentrated on the karst formation of five areas. This study underlines the need to provide special protection for tropical karst forest ecosystems because of their unique species assemblages, many of which exhibit localized distributions and are highly susceptible to habitat loss from mining and other anthropogenic activities. Furthermore, this study proposes to promote the expansion of protected areas to accommodate identified fern and lycophyte gap areas within karst landscapes. This proactive approach aligns with the imperative to conserve and sustainably manage these ecologically valuable habitats for the benefit of both biodiversity and local communities.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
MA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. SR: Formal analysis, Writing – review & editing. BN: Formal analysis, Writing – review & editing. RL: Formal analysis, Writing – review & editing. HS: Conceptualization, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was financially supported by the Alliance of International Science Organizations (ANSO) by giving a stipend to the first author (MDDLA) to enable her to carry out her PhD studies. The senior author (HS) acknowledges the financial support by the Yunnan Province Science and Technology Department (202101AS070012), Yunnan Revitalization Talent Support Program “Innovation Team” Project (202405AS350019), and 14th Five-Year Plan of the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (E3ZKFF8B01).
We express our gratitude to the support provided by the management of protected areas who granted permission to the first author to carry out crucial field surveys. We are grateful to the efforts to provide access to historical collections provided by the Global Biodiversity Information Facility and especially for all the herbaria and related institutions to take the efforts to digitize vouchers to make them freely available online.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1495796/full#supplementary-material
Aiello-Lammens, M. E., Boria, R. A., Radosavljevic, A., Vilela, B., Anderson, R. P. (2015). spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38, 541–545. doi: 10.1111/ecog.01132
Amoroso, V. B., Cariño, Y. L. L., Nobleza, J. C., Coritico, F. P. (2020a). Ophioderma subsessile (Ophioglossaceae), a new snake tongue fern species from Mindanao, Philippines. Philipp J. Sci. 150, 215–221. doi: 10.56899/150.S1.14
Amoroso, V. B., Cariño, Y. L. L., Nobleza, J. C., Coritico, F. P. (2021). A new species of Actinostachys (Schizaeaceae) from Mindanao, Philippines. Phytotaxa 508, 101–106. doi: 10.11646/phytotaxa.508.1.10
Amoroso, V. B., Coritico, F. P., Fritsch, P. W. (2020b). Actinostachys minuta, a new species of grass fern from Mindanao, Philippines. PhytoKeys 151, 59–66. doi: 10.3897/phytokeys.151.53100
Antonelli, A. (2023). Five essentials for area-based biodiversity protection. Nat. Ecol. Evol. 7, 630–631. doi: 10.1038/s41559-023-02023-x
Balázs, D. (1973). “Karst in the Philippines,” in International speleology II, subsection ba: geomorphology of the karst surface, 19–38.
Baldwin, B. G., Thornhill, A. H., Freyman, W. A., Ackerly, D. D., Kling, M. M., Morueta-Holme, N., et al. (2017). Species richness and endemism in the native flora of California. Am. J. Bot. 104, 487–501. doi: 10.3732/ajb.1600326
Barcelona, J. F. (2003). “The taxonomy and ecology of the pteridophytes of Mt. Iraya and Vicinity Batan Islands, Batanes Province, Northern Philippines,” in Pteridology in the new millennium. Eds. Chandra, S., Sricastava, M. (DORDRECHT, Netherlands: Springer, Dordrecht).
Barcelona, J. F., Dolotina, N. E., Madroñero, G. C., Granert, W. G., Sopot, D. D. (2006). The ferns and fern allies of the karst forests of Bohol Island, Philippines. Am. Fern J. 96, 1–20. doi: 10.1640/0002-8444(2006)96[1:TFAFAO]2.0.CO;2
Barcelona, J. F., Pelser, P. B. (2014). Phanerosorus (Matoniaceae), a new fern genus record for the Philippines. Phytotaxa 170, 133–135. doi: 10.11646/phytotaxa.170.2.6
Bergeron, A., Pellerin, S. (2014). Pteridophytes as indicators of urban forest integrity. Ecol. Indic 2014, 38: 40–38: 49. doi: 10.1016/j.ecolind.2013.10.015
Brownsey, P. J., de Lange, P. J. (1997). Asplenium cimmeriorum, a new fern species from New Zealand. N. Z. J. Bot. 35, 283–292. doi: 10.1080/0028825X.1997.10410154
Brummitt, N., Araújo, A. C., Harris, T. (2021). Areas of plant diversity – What do we know? Plants People Planet 3, 33–44. doi: 10.1002/ppp3.10110
Bystriakova, N., De Melo, P. H. A., Moat, J., Lughada, E. N., Monro, A. K. (2019). A preliminary evaluation of the karst flora of Brazil using collections data. Sci. Rep. 9, 17037. doi: 10.1038/s41598-019-5104-6
CBD (2022). “Decision 15/4, U.N. Doc. CBD/COP/DEC/15/4 (2022),” in The Kunming-Montreal global biodiversity framework. Available at: https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-04-en.pdf (Accessed September 24, 2023).
Ceballos, G., Ehrlich, P. R., Dirzo, R. (2017). Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. PNAS 114, E6089–E6096. doi: 10.1073/pnas.1704949114
Chao, Y. S., Chen, C. W., Chiou, W. L., Coritico, F. P., Chang, Y. H., Aleck Yang, T. Y. (2022). Pteris faba and Pteris rubella, two new species in section Hypsopodium (Pteridaceae). N. Z. J. Bot. 61, 1–13. doi: 10.1080/0028825X.2022.2095918
Chen, K., Khine, P. K., Yang, Z., Schneider, H. (2022). Historical plant records enlighten the conservation efforts of ferns and Lycophytes’ diversity in tropical China. J. Nat. Conserv. 68, 126197. doi: 10.1016/j.jnc.2022.126197
Clements, R., Sodhi, N. S., Schilthuizen, M., Ng, P. K. L. (2006). Limestone karsts of southeast asia: imperiled arks of biodiversity. Biosci. 56, 733. doi: 10.1641/0006-3568(2006)56[733:LKOSAI]2.0.CO;2
Coritico, F. P., Amoroso, V. B., Liy, Y. C. (2019). Athyrium nakanoi Makino (Athyriaceae), a new record from the Philippines and an identification key to the Malesian Athyrium Sect. Polystichoides. Philipp. J. Syst. Biol. 13, 46–50. doi: 10.26757/pjsb2019a13006
Coritico, F. P., Cariño, Y. L. L., Guiang, M. M. M., Amoroso, V. B. (2022). New Record of Ophioderma redactophylla (Ophioglossaceae) in the Philippines and New Insights to its Morphology. Philipp J. Sci. 151, 227–233. doi: 10.56899/151.01.17
Cowie, R. H., Bouchet, P., Fontaine, B. (2022). The Sixth Mass Extinction: fact, fiction or speculation? Biol. Rev. 97, 640–663. doi: 10.1111/brv.12816
Crisp, M. D., Laffan, S., Linder, H. P., Monro, A. (2001). Endemism in the Australian flora. J. Biogeogr. 28, 183–198. doi: 10.1046/j.1365-2699.2001.00524.x
delos Angeles, M. D., Asis, A. A. (2022). “Pteridophyte diversity in the puerto princesa subterranean river national park (PPSRNP), palawan island, Philippines,” in Plant genetic resources, inventory, collection and conservation. Eds. Ramamoorthy, S., Buot, I. E., Jr., Chandrasekaran, R. (Springer Nature Singapore, Singapore).
delos Angeles, M. D., Buot, I. E., Jr., Liu, H. M., Schneider, H. (2022). Pteridophyte diversity in the samar island natural park (SINP), samar island, Philippines. Philipp. J. Sci. 151, 1929–1942. doi: 10.56899/151.05.31
Dormann, C. F., Elith, J., Bacher, S., Buchmann, C., Carl, G., Carré, G., et al. (2007). Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 30, 609–628. doi: 10.1111/j.1600-0587.2012.07348.x
Fick, S. E., Hijmans, R. J. (2017). WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatology 37, 4302–4315. doi: 10.1002/joc.5086
Fløjgaard, C., Normand, S., Skov, F., Svenning, J. C. (2011). Deconstructing the mammal species richness pattern in Europe-towards an understanding of the relative importance of climatic, biogeographic history, habitat heterogeneity and humans. Global Ecol. Biogeography 20, 218–230. doi: 10.1111/j.1466-8238.2010.00604.x
Flores-Galván, C., Márquez-Guzmán, J., Mata-Rosas, M., Watkins, J. E., Jr., Mehltreter, K. (2024). Limestone ferns: a review of the substrate characteristics and species diversity in selected geographic regions and genera. N. Z. J. Bot. 1–18. doi: 10.1080/0028825X.2024.2393294
Ford, D. C., Williams, P. W. (2007). Karst hydrogeology and geomorphology (111 River Street, MS 4-02, Hoboken, New Jersey, 07030-5774, USA: John Wiley & Sons Ltd. England), 576 pp. doi: 10.1002/9781118684986
Fu, L., Monro, A. K., Wei, Y. (2022). Cataloguing vascular plant diversity of karst caves in China. Biodiversity Sci. 30, 21537. doi: 10.17520/biods.2021537
Geekiyanage, N., Goodale, U. M., Cao, K., Kitajima, K. (2019). Plant ecology of tropical and subtropical karst ecosystems. Biotropica 51, 626–640. doi: 10.1111/btp.12696
Green, S. M., Dungait, J. A. J., Tu, C. L., Buss, H. L., Sanderson, N., Hawkes, S. J., et al. (2019). Soil functions and ecosystem services research in the Chinese karst Critical Zone. Chem. Geol. 527, 15. doi: 10.1016/j.chemgeo.2019.03.018
Hassler, M. (2004-2023). World plants. Synonymic checklist and distribution of the world flora. Version 16.1. Available online at: www.worldplants.de (Accessed October 7, 2023).
He, H., Zhang, L. B. (2010). Pteris xiaoyingae sp. nov. (sect. Pteris) from a Karst Cave in China Based on Morphological and Palynological Evidence. Syst. Bot. 35, 695–700. doi: 10.1600/036364410X539781
He, G., Zhao, X., Yu, M. (2021). Exploring the multiple disturbance of karst landscape in Guilin World Heritage Site, China. Catena. 203, 105349. doi: 10.106/j.catena.2021.105349
Huang, J. H., Huang, J. H., Liu, C. R., Zhang, J. L., Lu, X. H., Ma, K. P. (2016). Diversity hotspots and conservation gaps for the Chinese endemic seed flora. Biol. Conserv. 198, 104–112. doi: 10.1016/j.biocon.2016.04.007
Hughes, A. C., Orr, M. C., Yang, Q., Qiao, H. (2021a). Effectively and accurately mapping global biodiversity patterns for different regions and taxa. Glob. Ecol. Biogeogr. 30, 1375–1388. doi: 10.1111/geb.13304
Hughes, A. C., Qiao, H., Orr, M. C. (2021b). Extinction targets are not SMART (Specific, Measurable, Ambitious, Realistic, and Time Bound). Biosci. 71, 115–118. doi: 10.1093/biosci/biaa148
Iwatsuki, K., Price, M. G. (1977). The pteridophytes of mt. Burnay and vicinity, northern luzon. South East Asian Stud. Vol. 14, (4).
Jennings, M. D. (2000). Gap analysis: concepts, methods, and recent results. Landsc. Ecol. 15, 5–20. doi: 10.1023/A:1008184408300
Kress, W. J. (1986). The systematic distribution of vascular epiphytes: an update. Selbyana 9, 2–22.
Laffan, S. W., Lubarsky, E., Rosauer, D. F. (2010). Biodiverse, a tool for the spatial analysis of biological and related diversity. Ecography 33, 643–647. doi: 10.1111/j.1600-0587.2010.06237.x
Linder, H. P. (2001). Plant diversity and endemism in sub-Saharan tropical Africa. J. Biogeogr. 28, 169–182. doi: 10.1046/j.1365-2699.2001.00527.x
Liu, C., Huang, Y., Wu, F., Liu, W., Ning, Y., Huang, Z., et al. (2021). Plant adaptability karst regions. J. Plant Res. 134, 889–906. doi: 10.1007/s10265-021-01330-3
Matten, D. M., Mienna, I. M., Bieker, V. C., Mishler, B. D., Moen, V. S., Nygaard, M., et al. (2023). Spatial patterns of phylogenetic and species diversity of fennoscandian vascular plants in protected areas. Biodiversity Conserv. 32, 4425–4443. doi: 10.1007/s10531-023-02705-9
Mehltreter, K. (2010). “Fern conservation,” in Fern ecology. Eds. Mehltreter, K., Lr, W., Share, J. M. (Cambridge, England: Cambridge University Press), 323–359.
Mittermeier, R. A., Gil, P. R., Hoffman, M., Pilgrim, J., Brooks, T., Mittermeier, C. G., et al. (2004). Hotspots: earth’s biologically richest and most endangered terrestrial ecoregions. Cemex. 392, 11–65.
Molina-Paniagua, M. E., Alves de Melo, P. H., Ramírez-Barahona, S., Monro, A. K., Burelo-Ramos, C. M., Gómez-Domínguez, H., et al. (2023). How diverse are the mountain karst forests of Mexico? PloS One 18, e0292352. doi: 10.1371/journal.pone.0292352
Myers, N. (1988). Threatened biotas, “Hot spots” in tropical forests. Environmentalist 8, 187–208. doi: 10.1007/BF02240252
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858doi: 10.1038/35002501
Nitta, J. H., Schuettpelz, E., Ramirez-Barahona, S., Iwasaki, W. (2022). An open and continuously updated fern tree of life. Front. Plant Sc. 13. doi: 10.3389/fpls.2022.909768
Page, C. N. (2002). Ecological strategies in fern evolution: a neopteridological review. Rev. Palaeobotany Palynology. 119, 1–33. doi: 10.1016/S0034-6667(01)00127-0
Pelser, P. B., Barcelona, J., Nickrent, D. L. (2011-onwards). Co’s digital flora of the Philippines. Available online at: www.philippineplants.org (Accessed October 10, 2023).
Phutthai, T., Hughes, M. (2017). Four new species of Begonia (Begoniaceae) from Thailand. Edinb. J. Bot. 74, 149–161. doi: 10.1017/S0960428617000051
Piccini, L., Rossi, G. (1994). Italian caving exploration in the island of palawan, Philippines. Speleologia 15, 5–62.
Pimm, S. L. (2020). What we need to know to prevent a mass extinction of plant species. Plants People Planet 3, 7–15. doi: 10.1002/ppp3.10160
Pouteau, R., Meyer, J. Y., Blanchard, P., Nitta, J. H., Terorotua, M., Taputuarai, R. (2016). Fern species richness and abundance are indicators of climate change on high-elevation islands: evidence from an elevational gradient on Tahiti (French Polynesia). Clim. Change 138, 143–156. doi: 10.1007/s10584-016-1734-x
PPGI (Pteridophyte Phylogeny Group) (2016). A community-derived classification for extant lycophytes and ferns. JSE 54, 563–603. doi: 10.1111/jse.12229
Price, M. G. (1975). The pteridophytes of mt. Makiling and vicinity (Institute of Biological Sciences, University of the Philippines Los Baños, College, Laguna, Philippines).
Protected Planet (2014-2023). The world database on protected areas (WDPA). Available online at: https://www.protectedplanet.net/en/thematic-areas/wdpa?tab=WDPA (Accessed June 1, 2023).
QGIS.org (2023). “Geographic information system,” in Open source geospatial foundation project. Available at: http://qgis.org (Accessed May 29, 2023).
Ramírez-Barahona, S., Cuervo-Robayo, A. P., Magallón, S. (2023). Assessing digital accessible botanical knowledge and priorities for exploration and discovery of plant diversity across Mesoamerica. New Phytol. 240, 1659–1672. doi: 10.1111/nph.19190
R Development Core Team (2023). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing, Vienna). Available at: https://www.R-project.org/. (Accessed May 29, 2023)
Salas, L. A., Bedos, A., Deharveng, L., Fryer, S., Hadiaty, R., Heryanto, et al. (2005). Biodiversity, endemism, and the conservation of limestone karsts in the sangkulirang peninsula, borneo. Biodiversity 6 (2), 15–23. doi: 10.1080/14888386.2005.9712762
Salovaara, K. J., Cárdenas, G. G., Tuomisto, H. (2004). Forest classification in an amazonian rainforest landscape using pteridophytes as indicator species. Ecography 27, 689–700.
Schober, P., Boer, C., Schwarte, L. A. (2018). Correlation coefficients: appropriate use and interpretation. Anesth. Analg 126, 1763–1768. doi: 10.1213/ANE.0000000000002864
Schuettpelz, E., Pryer, K. M. (2009). Evidence for a Cenozoic radation of ferns in an angiosperm-dominated canopy. PNAS 106, 1120–11205. doi: 10.1073/pnas.0811136106
Scott, J. M., Davis, F., Csuti, B., Noss, R., Butterfield, B., Wright, R. G. (1993). Gap analysis, a geographical approach to protection of biological diversity. Wildl. Monogr. 123, 3–41.
Silva, V. L., Mehltreter, K., Schmitt, J. L. (2018). Ferns as potential ecological indicators of edge effects in two types of mexican forests. Ecol. Indic. 93, 669–676. doi: 10.1016/j.ecolind.2018.05.029
Sodhi, N. S., Koh, P. L., Brook, B. W., Ng, P. K. L. (2004). Southeast Asian biodiversity: an impending disaster. TREE 19, 654–660. doi: 10.1016/j.tree.2004.09.006
Takeuchi, W. (2007). A new fern and two floristic records from the Karius Limestone of Papua New Guinea. Edinb. J. Bot. 64, 7–15. doi: 10.1017/S0960428606000692
Tang, G. D., Huang, L., Li, J. Y., He, Z. L., Zhang, L. B. (2017). Polystichum hastipinnum (subg. Haplopolystichum; Dryopteridaceae), a new cave fern from Guangdong, China. Phytotaxa 309, 066–072. doi: 10.11646/phytotaxa.309.1.6
The Long, N., Dac Trien, N. (2019). The tropical limestone forest ecosystem: A review of distinctive characteristics. J. Sci. Technol. 17, 44–50.
Tolentino, P. J. S., Navidad, J. R. L., delos Angeles, M. D., Fernandez, D. A. F., Villanueva, E. L. C., Obeña, R. D. R., et al. (2020). Review: Biodiversity of forests over limestone in Southeast Asia with emphasis on the Philippines. Biodiversitas 21, 1597–1613. doi: 10.13057/biodiv/d210441
Watkins, J. E., Jr., Cardelús, C. L. (2012). Ferns in an angiosperm world: Cretaceous radiation into the epiphytic niche and diversification on the forest floor. IJPS 173, 695–710. doi: 10.1086/665974
Watkins, J. E., Jr., Holbrook, N. M., Zwieniecki, M. A. (2010). Hydraulic properties of fern sporophytes: consequences for ecological and evolutionary diversification. Am. J. Bot. 97, 2007–2019. doi: 10.3732/ajb.1000124
Wong, T. C., Luo, T., Zhang, H., Li, S., Chu, W. (2016). The socio-economic transformation of rocky karst areas: case study of qianxinan prefecture, guizhou province, China. Malaysian J. Chin. Stud. 5, 49–65.
World-wide Hydrologeological Mapping and Assessment Programme (WHYMAP) (2023).Available online at: https://www.whymap.org (Accessed May 9, 2023).
Xu, W. H., Xiao, Y., Zhang, J. J., Yang, W., Zhang, L., Hull, V., et al. (2017). Strengthening protected areas for biodiversity and ecosystem services in China. PNAS 114, 1601–1606. doi: 10.1073/pnas.1620503114
Xue, T. T., Yang, X. D., Liu, Q., Qin, F., Zhang, W. D., Janssens, S. B., et al. (2022). Integration of hotspot identification, gap analysis, and niche modeling supports the conservation of Chinese threatened higher plants. JSE 61, 682–697. doi: 10.1111/jse.12901
Yang, L., Zhao, C., Jiao, S., Li, S., Wang, L., Li, Y. (2022). Reconstructing spatial pattern of historical cropland in karst areas of Guizhou, Southwest China. Sci. Rep. 52, 1325–1330. doi: 10.1038/s41598-022-26793-9
Yesilyurt, J. C., Schneider, H. (2010). The new fern genus Calciphilopteris (Pteridaceae). Phytotaxa 7, 52–59. doi: 10.11646/phytotaxa.7.1.7
Yu, F. Y., Skidmore, A. K., Wang, T. J., Huang, J. H., Ma, K. P., Groen, T. A. (2017). Rhododendron diversity patterns and priority conservation areas in China. Divers. Distrib. 23, 1143–1156. doi: 10.1111/ddi.12607
Keywords: diversity hotspots, gap analysis, multisource data, Philippines, pteridophytes
Citation: delos Angeles MD, Radbouchoom S, Ngarega BK, Lapuz RS and Schneider H (2025) Spatial diversity and distribution of fern and lycophyte species in karst and non-karst landscapes towards conservation needs. Front. Plant Sci. 16:1495796. doi: 10.3389/fpls.2025.1495796
Received: 13 September 2024; Accepted: 30 January 2025;
Published: 03 March 2025.
Edited by:
Long-Fei Fu, Guangxi Institute of Botany, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Vladan Djordjević, University of Belgrade, SerbiaCopyright © 2025 delos Angeles, Radbouchoom, Ngarega, Lapuz and Schneider. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harald Schneider, aGFyYWxkQHh0YmcuYWMuY24=
†Present address: Boniface K. Ngarega, Department of Plant Biology, Ecology, and Evolution, Oklahoma State University, Stillwater, OK, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.