- 1Department of Plant and Soil Sciences, University of Pretoria, Pretoria, South Africa
- 2Forestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria, South Africa
- 3Department of Plant Biotechnology and Bioinformatics, Ghent University, Ghent, Belgium
- 4Centre for Plant Systems Biology, VIB, Ghent, Belgium
- 5Department of Biochemistry, Genetics and Microbiology, University of Pretoria, Pretoria, South Africa
- 6College of Horticulture, Academy for Advanced Interdisciplinary Studies, Nanjing Agricultural University, Nanjing, China
Seagrasses are a paraphyletic group of marine angiosperms and retain certain adaptations from the ancestors of all embryophytes in the transition to terrestrial environments. Among these adaptations is the production of flavonoids, versatile phenylpropanoid secondary metabolites that participate in a variety of stress responses. Certain features, such as catalytic promiscuity and metabolon interactions, allow flavonoid metabolism to expand to produce novel compounds and respond to a variety of stimuli. As marine environments expose seagrasses to a unique set of stresses, these plants display interesting flavonoid profiles, the functions of which are often not completely clear. Flavonoids will likely prove to be effective and versatile agents in combating the new host of stress conditions introduced to marine environments by anthropogenic climate change, which affects marine environments differently from terrestrial ones. These new stresses include increased sulfate levels, changes in salt concentration, changes in herbivore distributions, and ocean acidification, which all involve flavonoids as stress response mechanisms, though the role of flavonoids in combatting these climate change stresses is seldom discussed directly in the literature. Flavonoids can also be used to assess the health of seagrass meadows through an interplay between flavonoid and simple phenolic levels, which may prove to be useful in monitoring the response of seagrasses to climate change. Studies focusing on the genetics of flavonoid metabolism are limited for this group, but the large chalcone synthase gene families in some species may provide an interesting topic of research. Anthocyanins are typically studied separately from other flavonoids. The phenomenon of reddening in certain seagrass species typically focuses on the importance of anthocyanins as a UV-screening mechanism, while the role of anthocyanins in cold stress is discussed less often. Both of these stress response functions would be useful for adaptation to climate change-induced deviations in tidal patterns and emersion. However, ocean warming will likely lead to a decrease in anthocyanin content, which may impact the performance of intertidal seagrasses. This review highlights the importance of flavonoids in angiosperm stress response and adaptation, examines research on flavonoids in seagrasses, and hypothesizes on the importance of flavonoids in these organisms under climate change.
1 Introduction
Seagrasses are a paraphyletic group of marine angiosperms consisting of approximately 72 species (Unsworth et al., 2022), all belonging to the order Alismatales, which includes 11 freshwater and 4 fully marine families. The four seagrass families (the Posidoniaceae, Zosteraceae, Hydrocharitaceae, and Cymodoceaceae) (Effrosynidis et al., 2019; Pfeifer and Classen, 2020) arose from at least three independent lineages transitioning from freshwater to a marine habitat, which has not occurred in any other angiosperm lineage (Wissler et al., 2011; Chen et al., 2022). Despite the limited species diversity, seagrasses boast a large distribution, spanning an area of over 160,000 km2 along most of the world’s temperate and tropical coastlines in large meadows (McKenzie et al., 2020; Short et al., 2007). Seagrasses are of great importance to marine ecosystems and the planet, providing a variety of ecosystem services such as carbon sequestration (Duarte and Krause-Jensen, 2017; Serrano et al., 2021; Marbà et al., 2015), sediment production (East et al., 2023; Keulen and Borowitzka, 2003), food and habitat for vertebrate and invertebrate marine life (Jackson et al., 2015; Gartner et al., 2013; Bloomfield and Gillanders, 2005), and many others, as reviewed by Mtwana Nordlund et al. (2016). Besides human coastal development and activities causing loss of seagrasses (Short et al., 2006), climate change also threatens seagrasses and their ecosystem services, as it is predicted to have a profound impact on seagrass endemism and distribution, with many species becoming more restricted and meadows shifting to currently unprotected areas (Daru and Rock, 2023; Marbà et al., 2015). Understanding the stress response systems of seagrasses will aid us in predicting the extent to which seagrasses will tolerate the coming climatic changes.
The ocean has absorbed the majority of the heat gained over the last 50 years of global warming, mostly in the upper 700 m, leading to ocean warming, with an average increase of 0.9°C in this upper layer during the 20th century (Domingues et al., 2008). Other impacts of anthropogenic climate change on the ocean include ocean acidification due to increased CO2 levels, increased stratification, intensified storms, rising sea levels, and hypoxia (Hoegh-Guldberg and Bruno, 2010; Venegas et al., 2023). Although increased CO2 levels should benefit seagrasses as photosynthetic organisms (Zimmerman et al., 2017; Listiawati and Kurihara, 2021), it is outweighed by the negative impact of high temperatures from ocean warming and marine heatwaves; ocean warming is therefore expected to be the most important factor impacting seagrass health and distribution (Diaz-Almela et al., 2007; Repolho et al., 2017; Moore et al., 2012; Zhang et al., 2022; Short et al., 2016). It is predicted that climate change will cause massive shifts in the distributions of different seagrass species due to shifts in climatically suitable environments, which will negatively impact species that rely on seagrass meadows as a food source or habitat (Daru and Rock, 2023; Duarte et al., 2018). Seagrasses, unlike seaweed which occupies a similar ecological niche, are angiosperms and therefore descended from land plants. This unique ancestry affords them certain traits that may aid them in resisting some of the stress imposed by climate change.
The transition from an aquatic to a terrestrial habitat by the ancestors of all land plants required several key adaptations, as functions previously fulfilled by the surrounding aqueous medium, such as UV screening and mechanical support, now required adaptive mechanisms from the organism, leading to the advent of compounds such as flavonoids and lignins (De Vries and Archibald, 2018). Seagrasses, descended from terrestrial angiosperms, retain certain adaptations from their ancestors’ switch to terrestrial ecosystems, which have greatly expanded in the angiosperms in the millennia on land. Seagrasses therefore possess traits for a variable terrestrial environment in a more stable marine environment, which presents a completely different host of stresses, such as high levels of salinity, a shifting substrate, and low light (Ma et al., 2024). While some adaptations to terrestrial life are lost in seagrasses, like stomata which are not beneficial outside of a gaseous environment (Papenbrock, 2012; Chater et al., 2017; Larkum et al., 2017), other adaptations are retained and may actually be beneficial in a marine environment. Among these retained adaptations are the flavonoids, a group of secondary metabolites restricted to the embryophytes, which function in a variety of stress responses and are hypothesized to have been important in the shift to terrestrial ecosystems by the ancestors of land plants. With these compounds and the genes underlying their biosynthesis, seagrasses maintain a powerful and flexible repertoire of stress-response compounds, which are very useful to plants inhabiting high-stress environments and may play an important role in allowing them to survive the conditions imposed by climate change (Di Ferdinando et al., 2014; Laoué et al., 2022). This review discusses the evolution and function of flavonoids and how they might be beneficial to seagrasses as angiosperms in a marine environment under conditions of climate change.
2 Origin of flavonoids
The phenylpropanoid pathway allows for the production of flavonoids and lignin, which are both argued to be vital elements in allowing the ancestor of land plants to survive in terrestrial environments (Weng and Chapple, 2010; Kenrick and Crane, 1997; De Vries and Archibald, 2018). In this pathway, phenylalanine is converted to trans-cinnamic acid through the action of phenylalanine ammonia-lyase (PAL), which is then converted to p-coumaric acid by cinnamic acid 4-hydrolase (C4H). p-coumaroyl:CoA ligase (4CL) can then produce p-coumaroyl-CoA from p-coumaric acid, which can be used for lignin and flavonoid biosynthesis. This pathway is ubiquitous among land plants, which supports the importance of flavonoids and lignin in the move to terrestrial environments. PAL seems to have been acquired by horizontal gene transfer during symbiotic interactions with soil bacteria or fungi and most likely functioned in the production of antimicrobial or UV-protectant secondary metabolites (Emiliani et al., 2009). Three malonyl-CoA molecules and a p-coumaroyl-CoA produced by 4CL are then used to produce chalcones through the action of chalcone synthase (CHS), a type III polyketide synthase, which marks the beginning of flavonoid biosynthesis (Figure 1). There are several classes of flavonoids comprising thousands of different molecules, namely, aurones, flavones, flavonols, proanthocyanidins, and anthocyanins, with these compounds all being synthesized by branches of flavonoid metabolism which share in enzymes to differing degrees, leading to competition for reaction intermediates. Flavonoids are found in vascular plants, mosses, and liverworts, and although flavonoids have been detected in various algae (Goiris et al., 2014), the quantities are extremely low compared to the typical contents of land plants (Davies et al., 2020), and the diversity is very low, being mostly limited to the flavone apigenin (Goiris et al., 2014). Type III PKS genes have been identified in some algal lineages, including homologs for CHS, as well as homologs for 4CL (De Luca and Lauritano, 2020). Some of these taxa, however, are missing homologs for other key genes involved in cinnamic and coumaric acid biosynthesis, which are important intermediates for the synthesis of the CHS substrate (Jiao et al., 2020). It is not unlikely for flavonoids to have first appeared in photosynthetic marine organisms, where they had limited diversity and functionality and served as an excellent exaptation for the transition to terrestrial habitats, where selection favored their increased production and diversification (De Vries and Archibald, 2018).
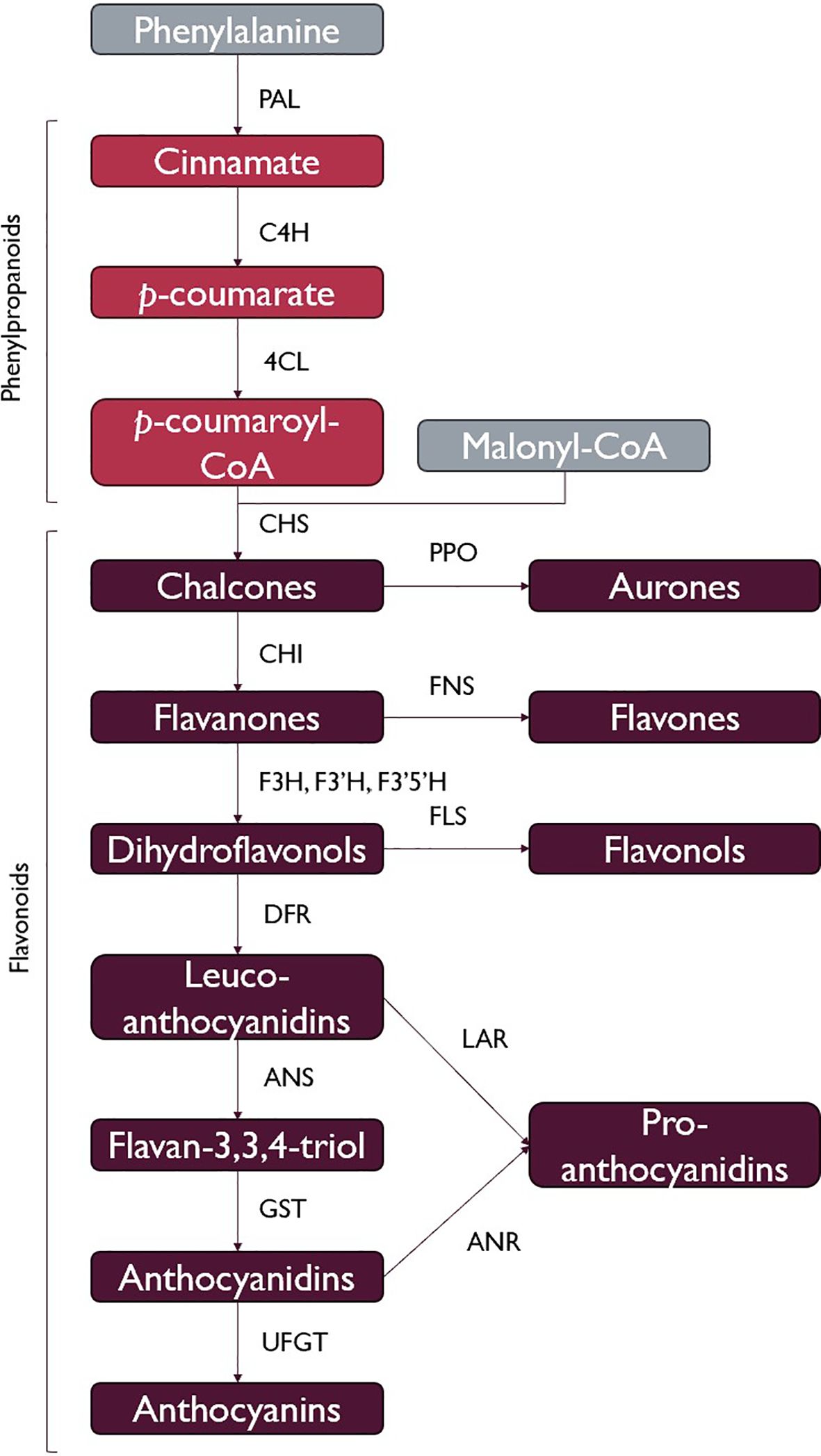
Figure 1. A simplified view of flavonoid biosynthesis. Flavonoids are derived from the phenylpropanoid pathway, as p-coumaroyl-CoA is a product of the phenylpropanoid pathway (phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydrolase (C4H), p-coumaroyl:coA ligase (4CL)). Chalcone synthase (CHS) catalyses the first committed step of flavonoid biosynthesis, and regulation of CHS therefore regulates flavonoid production. Other enzymes of the pathway include polyphenol oxidase (PPO), chalcone isomerase (CHI), flavone synthase (FNS), flavanone 3-hydroxylase (F3H), flavonoid 3’- and flavonoid 3’5’-hydroxylase (F3’H and F3’5’H), flavonol synthase (FLS), dihydroflavonol reductase (DFR), anthocyanidin synthase (ANS), anthocyanin-related glutathione S-transferase (GST), leucoanthocyanidin reductase (LAR), anthocyanidin reductase (ANR), UDP-glucose:flavonoid 3-O-glucosyltransferase (UFGT). Figure simplified from Eichenberger et al. (2023), following the discovery of the catalytic involvement of GST in anthocyanin biosynthesis. This is not a comprehensive list of all flavonoids, and many types of flavonoids are only produced in a handful of lineages.
2.1 Flavonoids in terrestrial plants
It is hypothesized that the success of plants in colonizing terrestrial environments is coupled with the advent of flavonoids, due to their ubiquitous distribution and incredible functional diversity (Kenrick and Crane, 1997; Weng and Chapple, 2010). A few classes of flavonoids and their biosynthesis are summarized in Figure 1. Flavonoids are involved in biotic and abiotic stress responses (Fini et al., 2011; Dong and Lin, 2021), hormonal regulation (Peer et al., 2004), and signaling for microbial and animal interactions (Rahme et al., 2014; Shimada et al., 2003; Dong and Lin, 2021). There are two classic hypotheses for the initial role performed by flavonoids in early land plants. One hypothesis is that the importance of flavonoids in the colonization of terrestrial environments comes from their UV-B screening properties (Li et al., 1993). UV-B radiation decreases photosynthetic efficiency by causing damage to photosystem II (Szilárd et al., 2007) and leads to the production of reactive oxygen species (ROS), which can damage DNA (Li et al., 2002). This hypothesis was based on flavonoid-deficient mutants, showing an increase in sensitivity to UV-B radiation (Li et al., 1993) and the accumulation of flavonoids in the epidermis in response to UV-B exposure (Tevini et al., 1991).
There are several pieces of evidence that oppose this hypothesized initial function of flavonoids. The efficacy of flavonoids as UV-screening compounds requires their accumulation in high quantities, which would not have been possible without certain enzymes to transport them to the cell wall and vacuole, and these enzymes likely would not have existed when flavonoids first arose (Stafford, 1991). This does not discredit the importance of UV-B defense for survival on land, but non-flavonoid compounds may have fulfilled this role, with the UV-B screening role of flavonoids being an exaptation of these molecules when novel enzymatic functions allowed them to accumulate at high levels. Indeed, hydroxycinnamates, which also belong to the phenylpropanoid class of secondary metabolites, can effectively absorb UV-B radiation and play a role in UV-B screening alongside flavonols (Burchard et al., 2000). Lignins may also have played a role in photoprotection in early land plants: lignin epitopes have been identified in the cell walls of bryophytes and are not associated with vascular or mechanical tissues (Ligrone et al., 2008). Non-flavonoid-producing organisms, including fungi, cyanobacteria, algae, lichens, and dinoflagellates, employ mycosporine-like amino acids to protect photosystem II against UV damage (Taira and Taguchi, 2017) and macromolecules against ROS, thereby playing the same role in this stress response as flavonoids in plants. Their biosynthesis also seems to be derived from the shikimate pathway, further tying them to the phenylpropanoids, which are derived from the chorismate produced by this pathway.
The second hypothesis is that flavonoids first arose to function as signaling molecules, specifically in the regulation of auxins (Stafford, 1991). Stafford argued for this role of flavonoids in light of the arguments outlined above, as flavonoids would have been able to perform signaling functions at low cytoplasmic concentrations, and other compounds existed in the ancestors of land plants that functioned in UV-B screening. Many flavonoid-deficient mutant angiosperms show altered auxin-related development, including dwarfing, altered root development, and loss of pollen viability under temperature stress (Dare and Hellens, 2013; Muhlemann et al., 2018; Maloney et al., 2014), though these traits and their severity vary between flavonoid-deficient mutants (Burbulis et al., 1996). Flavonols, specifically, are important in regulating auxin transport during nodulation to promote organogenesis (Zhang et al., 2009; Ng et al., 2015), and flavonoids are also involved in the microbial signaling required with root nodulation (Abdel-Lateif et al., 2012). The formation of microbial symbiotic interactions and improved nutrient acquisition were important innovations for the success of plants in terrestrial environments, with flavonoids being involved in both, as reviewed by Hassan and Mathesius (2012). The relationship between auxin transport and flavonoids has also been observed in gymnosperms (Ramos et al., 2016), but there is no evidence that flavonoids play similar roles in the developmental processes of bryophytes, with flavonoid-deficient liverwort mutants showing normal development (Clayton et al., 2018). It therefore cannot be assumed that the ancestral role of flavonoids was auxin regulation.
The first hypothesis has since been adopted, placing the emphasis on the involvement of flavonoids in UV-B stress on their antioxidant activity, rather than their screening properties (Fini et al., 2011; Agati et al., 2013). The ROS-scavenging function of flavonoids also explains their production in response to a host of biotic and abiotic stresses, with chalcone synthase, which catalyzes the first committed reaction in the synthesis of all flavonoids, being upregulated in response to heat and cold stress (Shvarts et al., 1997), UV-B radiation and blue light (Christie and Jenkins, 1996), wounding (Richard et al., 2000), and biotic stress (Dao et al., 2011), which all lead to the accumulation of reactive oxygen species. Acting as ROS-scavenging compounds would not require the accumulation of flavonoids in the vacuole or cell wall but would still allow flavonoids to reduce the damage sustained as a result of increased UV-B exposure when the ancestors of land plants moved to a terrestrial environment.
2.2 Expansion of flavonoid metabolism
Like many other pathways of secondary metabolism, gene duplication events are important in the expansion of flavonoid metabolism and the origin of new enzymes and branchpoints. Some of the important biosynthetic genes arose from enzymes involved in primary metabolism, such as CHI, which is most likely descended from a non-catalytic fatty acid-binding protein (Ngaki et al., 2012; Kaltenbach et al., 2018). This also allows for neofunctionalization, with stilbene synthases having arisen from CHS multiple times independently, a process that requires only a few missense mutations (Tropf et al., 1994). Legume-specific type II CHI enzymes also originate from the typical type I CHI, which allows for the synthesis of flavonoid compounds required for microbial signaling (Shimada et al., 2003). FNS I likely arose from F3H, allowing for flavone synthesis in the Apiaceae (Martens et al., 2003; Gebhardt et al., 2005).
The enzymes involved in flavonoid biosynthesis, shown in Figure 1, are arranged in a metabolon on the cytoplasmic face of the endoplasmic reticulum. The arrangement of enzymes within the metabolon differs between species but is also determined by spatial–temporal expression differences, with some proteins not being present in the metabolon and increased expression of certain enzymes in certain tissues allowing them to outcompete certain other enzymes for shared binding sites (Crosby et al., 2011). The patterns of these interactions are important for the branchpoints of flavonoid metabolism: for example, DFR and FLS compete for dihydroflavonol substrates at the branchpoint between anthocyanin and flavonol synthesis and additionally compete to interact with CHS within the metabolon (Crosby et al., 2011). These competitive interactions are important in determining the flux of intermediates to different branches of the pathway (Watkinson et al., 2018), especially considering the catalytic promiscuity exhibited by many of the enzymes involved in flavonoid metabolism. For some of the enzymes, like F3H, the catalytic promiscuity is more apparent in vitro, as protein–protein interactions within the metabolon aid in channeling the appropriate substrates from one enzyme to the next in the pathway. It can therefore be hypothesized that the ability to interact with other proteins of the metabolon is what shapes the pathway, rather than the substrate and product specificity of the individual enzymes. These factors have allowed for the expansion of flavonoid metabolism when plants adapt to new environments and the advent of novel useful flavonoid functions, which is likely why flavonoids are so well conserved in the land plants and could well have benefited the ancestors of seagrasses when returning to a marine environment.
2.3 Flavonoids in seagrasses
Seagrasses present an interesting case when discussing the various roles performed by flavonoids, as these aquatic higher plants are descended from terrestrial ancestors, with flavonoid production being an ancestral trait and an adaptation toward life on land. Generally, flavonoids perform similar functions in seagrasses compared to land plants, being involved in oxidative stress responses and UV screening —the versatility of these compounds has allowed them to remain relevant outside of the environment in which they originally arose, with the versatility of the biosynthetic pathway allowing for new compounds to evolve.
There have been several studies characterizing the flavonoid contents of various species of seagrasses, with sulfated flavonoids being of particular prevalence in this group (McMillan et al., 1980). Sulfated flavones have been characterized in the genera Zostera, Thalassia, Halophila, Enhalus, and Phyllospadix, with some other genera producing sulfated non-flavonoid phenolic acids (McMillan et al., 1980). Sulfation greatly increases the solubility of these compounds, even more so than the corresponding glycone, which may be related to the function of these compounds (Grignon-Dubois and Rezzonico, 2018). Sulfated flavonoids may be important for the storage of inorganic sulfates in a more soluble form, facilitating transport through the plant cell and allowing for extrusion (Harborne, 1977; Grignon-Dubois and Rezzonico, 2023), as the toxic sulfide ions present in high concentrations in seawater has little effect on the health of certain seagrass species (Hasler-Sheetal and Holmer, 2015). Posidonia oceanica, for example, is much more sensitive to sulfides than Zostera marina or Thalassia testudinum, which both produce sulfated flavonoids (Grignon-Dubois and Rezzonico, 2018).
Sulfated flavonoids are not limited to seagrasses and are strongly associated with plants growing near water bodies rich in mineral salts, indicating that these compounds might act as an adaptation for dealing with high salt exposure (Teles et al., 2018; Harborne, 1975). Most plant families that produce these compounds are not closely related, indicating that this trait arose multiple times independently through the evolution of different sulfotransferases, though flavonols and flavones are generally the targets for sulfation (Teles et al., 2018). The link between flavonols and salt stress has been noted in the past, with increased levels of the flavonols quercetin and kaempferol being noted in Apocynum venetum seedlings following high salt exposure, as well as the upregulation of flavonol biosynthesis genes (F3H, F3′H, and FLS) coupled with the downregulation of general flavonoid biosynthesis genes (CHS and CHI) (Xu et al., 2020). This is consistent with observations by Walia et al. (2005), who noted that a rice genotype susceptible to salt stress had increased expression of CHS, CHI, F3′H, and DFR (the latter of which represents the branchpoint between flavonol and anthocyanin biosynthesis) in response to salinity stress compared to a tolerant line, correlating to the finding that susceptible varieties produced overall less flavonoids and phenolics when exposed to salt stress (Minh et al., 2016). Overexpression of FLS in Arabidopsis greatly increased the tolerance to salt stress, with the transgenic plants suffering far less membrane damage and displaying an overall improved phenotype under salt stress, with improved seed germination, growth, and chlorophyll content compared to the wild type (Guo et al., 2023). These findings seem to indicate that, although some flavonoids can increase tolerance to salinity stress, managing the competing branches of flavonoid biosynthesis is very important in inducing the desired phenotypic response.
Sulfated flavonoids have also been found to play a role in herbivore deterrence by reducing the attractiveness of sugars in Zostera noltei to the sea urchin Paracentrotus lividus (Casal-Porras et al., 2021), which is likely to be a secondary advantage conferred by these compounds in addition to their general function in aquatic higher plants. Sulfation may be important in modulating flavonoid function (Roubalová et al., 2015; Gidda and Varin, 2006) and has also been implicated in the signaling function of flavonoids —quercetin 3-sulfate reverts the auxin efflux inhibition caused by quercetin in Flaveria bidentis, a terrestrial dicot (Ananvoranich et al., 1994), though research regarding this function is limited (Chan et al., 2019), especially in marine angiosperms.
Overall flavonoid content has been observed to change according to the environmental conditions faced by seagrasses, changing with depth, location in the meadow, and season, with higher flavonoid contents generally being associated with increased environmental stress and competition (Casal-Porras et al., 2023; Sævdal Dybsland et al., 2021; Grignon-Dubois and Rezzonico, 2018). The relationship and ratios between flavonoids and non-flavonoid phenolics, especially rosmarinic acid, are typically assessed in these studies, with an increased flavonoid/rosmarinic acid ratio in Z. marina being attributed to more variable and stressful environments, such as the outskirts of a seagrass meadow when compared to the more sheltered interior (Sævdal Dybsland et al., 2021). Although flavonoids fulfill photoprotective functions in land plants (Agati et al., 2013), the importance of flavonoids in photoprotection differs between species of seagrasses, with some species seeming to rely more on rosmarinic acid for screening harmful radiation: in Z. noltei, there is a significant relationship between UV exposure and rosmarinic acid accumulation, but not flavonoid accumulation (Casal-Porras et al., 2023). Similarly, for Halophila johnsonii, flavonoid content was actually found to be higher in shaded plants, even though flavonoids were localized in epidermal cells where they could function as screening compounds (Gavin and Durako, 2012). The decrease in flavonoid content with increasing depth in Z. marina observed by Sævdal Dybsland et al. (2021) was therefore attributed to reduced general stress exposure with the buffering effect of the water, rather than reduced light exposure.
Spatial variation in flavonoid profiles can actually be used to ascribe certain species of seagrasses to distinct chemical phenotypes (Grignon-Dubois and Rezzonico, 2018), reflecting the importance of flavonoids in the ability of seagrasses to become widely distributed and combat different stresses along that distribution. In Z. noltei, chemotypes vary based on the dominant flavonoid, with 71%–83% of the flavonoid content in the Cadiz Bay population consisting of apigenin 7-sulfate, in contrast to the Arcachon Bay population where diosmetin 7-sulfate constituted 85%–93% of the total flavonoid content (Grignon-Dubois and Rezzonico, 2012). Both populations grow in intertidal meadows but are separated by approximately 1,000 km. The authors hypothesize that this dramatic difference is due to low expression of F3′H in the Cadiz population, which reflects the plasticity of flavonoid metabolism and the dramatic impact of a single gene on the metabolic flux of the pathway. The study was extended to include several other populations around the coast of Europe and North Africa (Grignon-Dubois and Rezzonico, 2018), which allowed them to define a third chemotype with high levels of apigenin 7-sulfate, diosmetin 7-sulfate, and luteolin 7-sulfate, though the ecological significance of these three chemotypes is not quite clear.
Changes in flavonoid and non-flavonoid phenolic content can also potentially act as an indicator of the health of seagrass meadows. As long as the phenolic profile is well understood under stable conditions, sudden deviations from the dynamics of certain indicator compounds can indicate shifts in the stress exposure of the meadow (Grignon-Dubois and Rezzonico, 2023; Astudillo-Pascual et al., 2021). As phenolic content can change quickly and specifically in response to certain stress conditions, such as the dynamics in the ratio of flavonoids to rosmarinic acid under different conditions in Z. marina studied by Sævdal Dybsland et al. (2021), the stresses faced by a certain population can be assessed based on these compounds and their relative abundances. Seagrasses require a degree of phenotypic plasticity to combat the multiple stresses they experience as mostly clonal populations (Pazzaglia et al., 2021), which makes polyphenolics and flavonoids very useful, as alternate branches of the pathway can be exploited to respond to differing stresses (Di Ferdinando et al., 2014). These ratios can also be used to easily asses the health of a seagrass meadow (Sævdal Dybsland et al., 2021), which can prove to be a very valuable tool in monitoring the level of stress experienced by a meadow due to anthropogenic climate change.
2.4 Flavonoids in response to climate change impacts on the marine environment
Anthropogenic climate change will affect marine ecosystems through ocean warming, acidification, marine heatwaves, and changes in storm patterns. Flavonoids, through their diverse stress response actions, can help mitigate the impact of some of these stress factors. Anthropogenic climate change is likely to increase seagrass exposure to toxic sulfides indirectly through enhancing the effects of eutrophication. Phytoplankton blooms not only limit the light availability for seagrass meadows and create hypoxic conditions but also increase organic matter mineralization in the sediment through enhanced sulfate reduction (Holmer and Bondgaard, 2001; Seidel et al., 2021). Ocean warming leads to further reduced oxygen levels (Schmidtko et al., 2017), further favoring anaerobic metabolic pathways and thereby increasing sulfite levels (Broman et al., 2017). Seagrass species that produce sulfated flavonoids may therefore be more resilient than other species in areas afflicted by eutrophication, leading to changes in distribution and meadow composition. Increased levels of sulfate stress resulting from eutrophication might have an impact on flavonoid signaling if this exists in seagrasses (Roubalová et al., 2015). Upregulation of sulfated flavonoid production could divert more flavonoid pathway intermediates which could interfere with other flavonoid functions, which may require more investment in flavonoid metabolism.
An interesting and troubling implication of climate change on seagrass survival is the reduction of phenolic compounds in response to ocean acidification (Arnold et al., 2012). As has been discussed thus far, seagrasses employ flavonoids and other phenolic compounds as versatile stress response compounds and increase flavonoid levels in more stressful, less stable environments. While increased atmospheric CO2 increases the phenolic content of terrestrial plants, seagrasses growing near volcanic CO2 vents experiencing low pH coupled with high CO2 levels display a loss of phenolic compounds, which makes them more vulnerable to herbivory (Arnold et al., 2012). This vulnerability to herbivory due to alterations in secondary metabolism is made more concerning in light of the shifting range of herbivores in response to climate change, with tropical herbivores shifting poleward and establishing in temperate seagrass meadows (Campbell et al., 2024). Herbivores can greatly affect the health of seagrass meadows and alter the food web by feeding directly on seagrasses instead of seagrass detritus (Lal et al., 2010; Longo, 2024; Hyndes et al., 2016; Campbell et al., 2024). The degree of this loss of phenols does seem to vary between populations, however, as found by a study comparing the metabolic response to elevated CO2 of two eelgrass populations (Zayas-Santiago et al., 2020). This study found that, while the primary metabolism of both populations benefited from increased CO2 concentrations, the population from a colder climate displayed less reduction in defensive compounds derived from the shikimate pathway, including phenolics. The spatial variation in phenolic content in seagrass species may therefore be an important factor in the perseverance of some seagrass meadows and populations over others in the acidifying and warming oceans.
The impact of the shift in herbivore distributions in response to ocean warming on seagrass species will also be affected by the accumulation of sulfated flavonoids. Sulfated flavonoids act as herbivore deterrents in some seagrass species (Casal-Porras et al., 2021), while other species lack these compounds (Grignon-Dubois and Rezzonico, 2018; Astudillo-Pascual et al., 2021), which could leave them vulnerable to herbivory. Seagrasses that do not produce sulfated flavonoids would therefore be confronted with a combination of increased sulfate toxicity and increased herbivory due to ocean warming, which would place them at a competitive disadvantage against other species. Additional monitoring would therefore be necessary for these species, which may have already been threatened due to anthropogenic activity. It is possible for new flavonoid sulfotransferase enzymes to arise within these species convergently, especially as ocean warming is expected to increase the rate of flowering and sexual reproduction in some seagrass species (Marín-Guirao et al., 2019; García-Escudero et al., 2024), which provides more opportunity for recombination from which improved genetic variants may arise. Alternatively, seagrass species which lack sulfated flavonoids may utilize alternative flavonoids or phenolpropanoids for herbivore deterrence and managing the damage caused by sulfates, which can lead to more distinct chemotypes. Shifts in flavonoid metabolism in species lacking sulfated flavonoids can be used to monitor how well different populations are responding to these stress factors and prioritize conservation efforts accordingly.
Seagrasses need to be able to adjust to fluctuations in salinity, especially those growing in estuarine environments where these fluctuations can be significant and rapid (Touchette, 2007). Climate change exacerbates these salinity changes beyond normal levels, with heavy rainfall and flooding leading to the loss of seagrass meadows due to increases in turbidity and decreases in salinity (Campbell and McKenzie, 2004; Chollett et al., 2007), while accelerated evaporations and influx of saltwater into lagoons from rising sea levels will likely lead to increased salinity (Jakimavičius et al., 2018; Shen et al., 2022). Flavonols may therefore be a flexible tool for seagrasses to tolerate the stress associated with increased salinity fluctuations caused by anthropogenic climate change. Flavonol metabolism is often in competition with anthocyanin metabolism, with the two pathways competing for pathway intermediates, metabolon interactions, and transcription factors (Owens et al., 2008; Luo et al., 2016; Yuan et al., 2016; Crosby et al., 2011). Anthocyanin production is likely to decrease under conditions of ocean warming, as this pathway is upregulated in response to cold stress (Sullivan and Koski, 2021), which would allow for increased levels of flavonols under these conditions. Increased levels of evaporation in estuarine habitats, which would lead to increased salt concentrations, would also increase the level of UV exposure faced by these seagrasses. Both flavonols and anthocyanins are antioxidants involved in combatting UV damage, but under conditions of ocean warming and increased salinity in estuaries, flavonols may become more important than anthocyanins in fulfilling a UV-protective function.
3 Phenylpropanoid and flavonoid biosynthesis genes in seagrasses
Figure 2 shows the relative number of gene family members represented in various seagrass and non-seagrass lineages for genes involved in phenylpropanoid metabolism, from a meta-analysis of the annotation data published by Ma et al. (2024). In terms of general phenylpropanoid genes, seagrasses generally possess genes in copy numbers similar to those of other aquatic and non-aquatic plants, showing that there is no significant change in this pathway in the shift to marine ecosystems. The only exception is the enzyme LAC, which is present in fewer copies in both the examined seagrass and freshwater-floating lineages compared to land angiosperms. This enzyme is involved in polymerizing monolignols to produce lignin (Dong and Lin, 2021), with lignin being attributed as another important trait in the evolution of land plants, providing mechanical support and allowing for the advent of vascular tissue (Lei, 2017). Aquatic plants have been found to have lower levels of lignin compared to land plants (Rabemanolontsoa and Saka, 2013), which relates to their increased flexibility (Asaeda et al., 2005). This allows these plants to move with the substrate and suffer reduced mechanical damage from currents and tides, making it an important adaptation to submerged life retained by seagrasses (Ma et al., 2023).
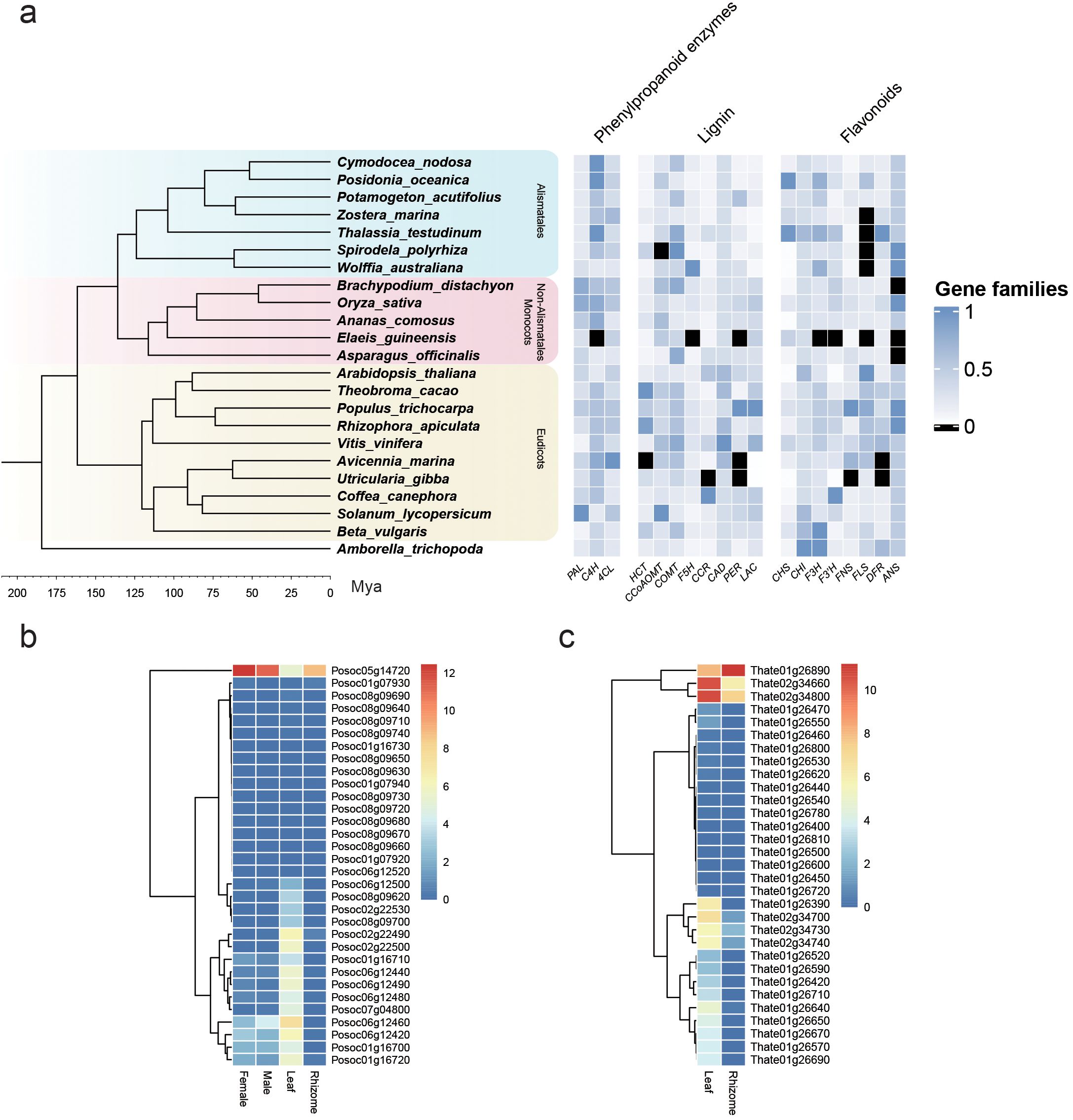
Figure 2. Phenylpropanoid and flavonoid biosynthesis genes in seagrasses. Transcriptome data from Ma et al. (2024) for the annotation of four seagrass genomes (Supplementary Data Sheet 1). (A) Changes in phenylpropanoid gene families in four seagrass species compared to various other terrestrial and aquatic lineages. Seagrasses in this figure are represented by C. nodosa, P. oceanica, Z. marina, and T. testudinum, with other members of the Alismatales being freshwater species. Gene family numbers are normalized by dividing the gene count number of each species by the highest copy number for that family. (B) Gene expression levels of CHS genes in various organs of P. oceanica and the expression values are scaled by log2(TPM + 1). (C) Gene expression levels of CHS genes in various organs of T. testudinum and the expression values are scaled by log2(TPM + 1).
As can be seen in Figure 2, P. oceanica and T. testudinum have an unusually high number of CHS gene representatives. The copy number of this gene varies between species, with Arabidopsis thaliana possessing a single copy, Ipomoea purpurea three (Durbin et al., 1995), and Glycine max eight (Tuteja et al., 2004). The different members of the CHS gene family for a species are often subject to differential spatial–temporal regulation (Deng et al., 2014), allowing for fine control over flavonoid production. Duplication and differentiation of the CHS gene allows for neofunctionalization and subfunctionalization, which Durbin et al. (2000) suggest to be an important strategy in plant adaptation and evolution. Stilbene synthases, for example, have evolved from CHS several times independently, as only a few missense mutations are required to confer stilbene synthase activity to CHS (Tropf et al., 1994). CHS can also produce other polyketide products which cannot be used for further flavonoid biosynthesis —this catalytic promiscuity is likely what allows for the advent of stilbene synthases and other plant-specific polyketide synthases from CHS (Weng and Noel, 2012). The high copy number of CHS in these two seagrass species can therefore represent the need for very fine control over flavonoid production under different environmental conditions or could represent functional diversification of this gene family in these lineages. In Z. marina, 11 CHS homologs were identified in a 2021 paper (Ma et al., 2021a), which could be divided into three clades. These homologs were all found to be functional in producing naringenin chalcone with differing tissue-specific expression patterns and light responses. Studies focusing on chalcone synthases in seagrasses are limited beyond this one and would definitely prove interesting in light of the unusually high copy number found in P. oceanica and T. testudinum. Based on expression data collected for the annotation of the P. oceanica and T. testudinum genomes (Ma et al., 2024), the different CHS copies do display tissue-specific expression differences, as shown in Figures 2B, C, but more data are necessary to capture the full expression profiles of these genes under different conditions.
It is possible that seagrass species with a wider set of CHS copies could respond better to stressful conditions imposed by climate change, due to more specific control over flavonoid upregulation. As discussed earlier, ocean acidification can lead to reduced phenolic content as primary metabolism is favored (Arnold et al., 2012). This is less of a concern for plants which are able to quickly upregulate flavonoid biosynthesis in the appropriate tissue type when exposed to certain stress conditions, such as increased sulfides or sudden increases in temperature.
Another interesting feature is the absence of FLS orthologs in Z. marina and T. testudinum, especially regarding the prevalence of sulfated flavonoids in seagrasses. Flavonol synthase is required for the production of flavonols, which along with flavones are generally the favored substrates for sulfotransferases (Teles et al., 2018). The lack of this gene could therefore point to lineage-specific differences in sulfated flavonoid composition relating to flavone and flavonol levels. As discussed previously, flavonols are associated with salt stress, as well as functioning in various stress responses, inhibiting auxin transport and modulating plant growth under stress conditions (Daryanavard et al., 2023). Flavonols are also very effective as antioxidants and UV-B screening compounds (Pollastri and Tattini, 2011), which makes them useful for plants occupying stressful and variable habitats and potentially very important for combatting the stresses imposed by climate change (Laoué et al., 2022). The absence of FLS orthologs could therefore have interesting implications for the function of flavonols in these lineages and their ability to deal with changing environmental conditions, which warrants further investigation.
The high number of DFR orthologs in T. testudinum has interesting implications for anthocyanin biosynthesis in this lineage compared to the other examined seagrasses. DFR occupies the branchpoint toward anthocyanin biosynthesis, in opposition to FLS directing dihydroflavonols toward flavonol biosynthesis, as can be seen in Figure 1. Different DFR copies are associated with spatial–temporal control of anthocyanin accumulation (Li et al., 2017), with anthocyanin accumulation often being a response to high light and low-temperature cues (Ahmed et al., 2015; Christie et al., 1994; Ma et al., 2021b; Jiang et al., 2016). The high copy number of DFR and the loss of FLS might therefore imply that anthocyanins play a more important role in these stress responses in T. testudinum compared to other seagrass lineages.
4 Anthocyanins
Anthocyanins, as a subclass of flavonoids, may be particularly interesting from a functional point of view in seagrasses, due to them only arising within seed plants (Piatkowski et al., 2020), which makes their function within marine ecosystems interesting. Anthocyanin biosynthesis and its underlying genes have been well characterized for a few decades, with the visual detection of anthocyanin presence and absence allowing for easy detection of mutations in the biosynthetic and regulatory genes. The full anthocyanin pathway is limited to seed plants, with ANS only arising in their common ancestor (Piatkowski et al., 2020), as shown in Figure 3. Seagrasses have retained the ability to synthesize anthocyanins and benefit from them as versatile antioxidants and UV-screening compounds, which non-angiosperm marine organisms do not possess. Like other flavonoids, anthocyanins function in various ROS-producing stress responses, which most likely predates their function in pollinator signaling (Rudall, 2020).
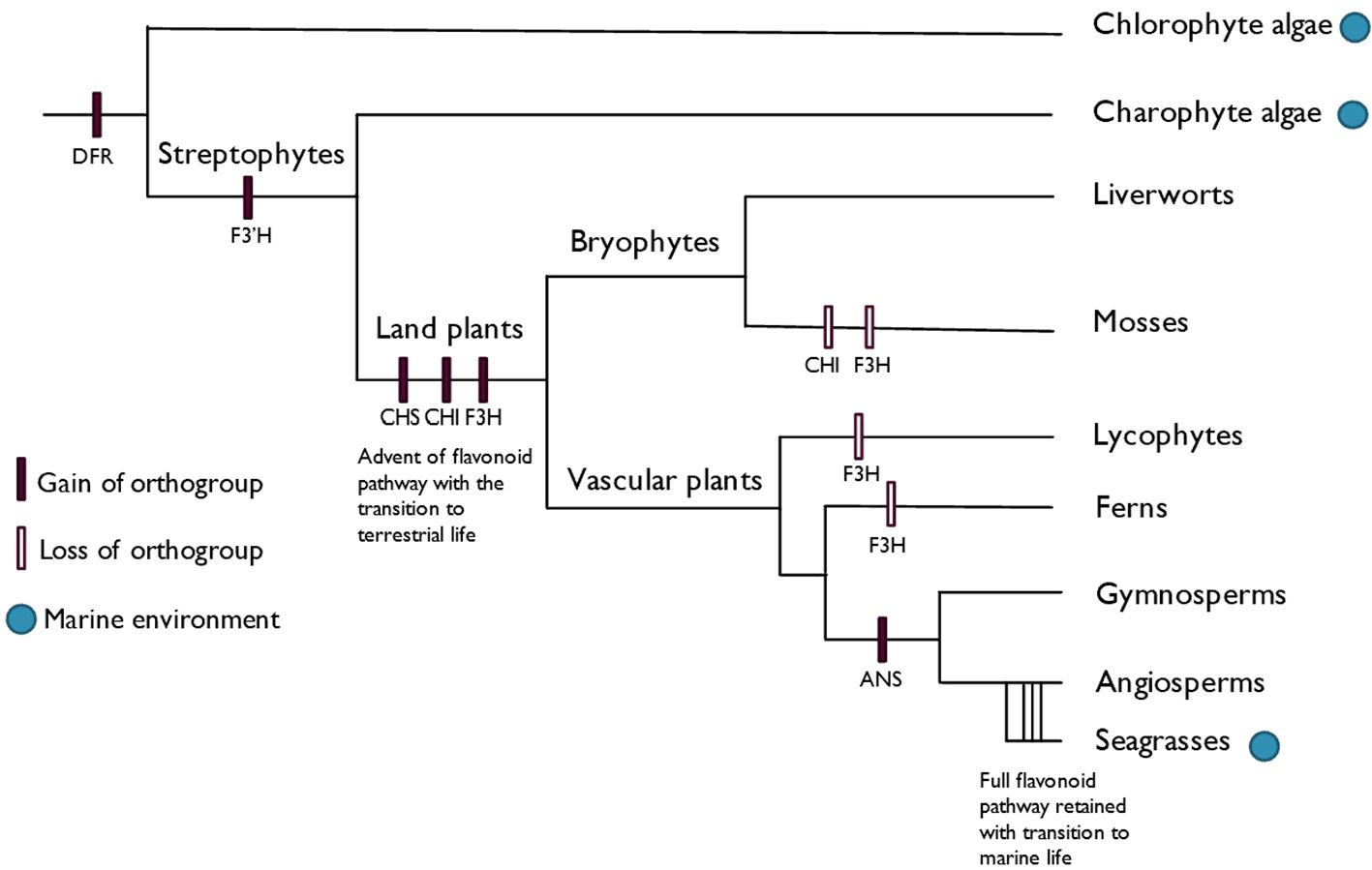
Figure 3. Evolution of the anthocyanin biosynthesis pathway, adapted from Piatkowski et al. (2020). The origin of each orthogroup of the anthocyanin biosynthesis genes is shown, with the complete pathway being limited to the seed plants with the advent of ANS. Seagrasses therefore have a host of genes at their disposal which other photosynthetic marine organisms lack.
Older lineages, such as the bryophytes, produce other red- and purple-pigmented flavonoids, which perform similar protective functions to anthocyanins (Aigner et al., 2013; Ben Saad et al., 2017). Some of these pigments were once thought to be anthocyanidins (Kunz et al., 1993), leading to the hypothesis that anthocyanins arose with the shift of plants to terrestrial habitats along with the first flavonoids, but these pigments have since been found to be a chemically distinct class of flavonoids called auronidins (Berland et al., 2019). This might be a case of convergent evolution, with the compounds having antioxidant activity (Ben Saad et al., 2017) and being produced in response to similar environmental stresses (Snell et al., 2009).
Although the mechanism of anthocyanins in combating oxidative stress is not well understood, the correlation between anthocyanin production and reduced susceptibility to oxidative stress has been observed many times: reduced expression of anthocyanin genes results in increased hydrogen peroxide accumulation after exposure to cold stress (Wang et al., 2013); anthocyanin-deficient mutants show increased chlorophyll and membrane damage and decreased antioxidant capability when exposed to photooxidative stress (Shao et al., 2008); and there is upregulation of anthocyanin biosynthetic genes and their corresponding regulatory genes in response to ROS accumulation (Xu et al., 2017). There is a host of evidence for the importance of anthocyanins in cold stress, with genes specific to anthocyanin production being upregulated under cold stress conditions (He et al., 2020), accumulation of anthocyanin pigments under these conditions (Ahmed et al., 2015; Christie et al., 1994), anthocyanin-deficient mutants being more susceptible to cold stress, and anthocyanin-free tissues accumulating more reactive oxygen species (Meng et al., 2015) and suffering more damage to photosynthetic efficiency than purple-pigmented tissues (Zhang et al., 2019). Reduced expression of anthocyanin biosynthetic genes through RNAi in purple sweet potato (Ipomea batatas) resulted in slower recovery after exposure to cold stress, with increased membrane damage and accumulation of ROS (Wang et al., 2013).
Photoprotection is another important function of anthocyanins, acting both as a sunscreen by absorbing certain wavelengths of light and scavenging free radicals produced by UV radiation (Zeng et al., 2010; Merzlyak et al., 2008; Hughes et al., 2005). Photoinhibition is the result of damage to the reaction center of photosystem II (PSII) caused by strong light, with photosynthetic efficiency suffering when the rate of damage exceeds the rate of de-novo PSII protein synthesis (Adir et al., 2003; Murata et al., 2007). Anthocyanins can be produced in response to high light or constitutively produced in certain vulnerable tissues, such as young leaves, to mitigate the impact of high light on photosynthetic efficiency (Ma et al., 2021b; Karageorgou and Manetas, 2006; Zhang et al., 2016).
Anthocyanin biosynthesis is capable of responding to a variety of stress conditions, with multiple hormonal signals being able to influence anthocyanin production (Lafountain and Yuan, 2021). The late genes of the biosynthetic pathway (DFR, ANS, and 3UGT), which are specific to anthocyanin biosynthesis, are all regulated by an MBW transcriptional activation complex (Quatrocchio et al., 1993), which consists of three proteins. The first is an R2R3 MYB protein, first identified as the maize Myb domain C1 protein that recognizes two consensus sequences in the promoters of the late biosynthesis genes (Sainz and Chandler, 1997). A basic helix-loop-helix (bHLH) transcription factor also plays a role in promoter recognition with a distinct consensus sequence (Ludwig et al., 1990). Plants possess multiple copies of these genes which show tissue-specific and stress-responsive expression (Xu et al., 2014): regulation of the late biosynthesis genes is therefore achieved through the regulation of these proteins. Both the MYB and bHLH families of transcription factors have undergone dramatic expansion in higher plants, and their increase in diversity is associated with an increase in metabolic complexity (Feller et al., 2011). The third protein is a WD40 repeat protein that plays no role in promoter recognition.
Hormonal control of anthocyanin production often relies on the sequestration of the R2R3 MYB and bHLH members of the ternary complex by proteins such as JAZ and DELLA (Qi et al., 2011, Qi et al., 2014). Hormones, such as jasmonate and gibberellic acid, can activate anthocyanin biosynthesis through triggering the ubiquitination of JAZ and DELLA, respectively, resulting in the degradation of these repressor proteins by the 26S proteasome and the formation of the MBW complex. The recent review article by Lafountain and Yuan (2021) gives a good overview of the current knowledge of the negative regulation of anthocyanin biosynthesis, describing over a dozen mechanisms. At the end of the review, they question why so many negative regulators of anthocyanin biosynthesis have evolved: anthocyanins have a range of functions and are produced in response to an array of stimuli, so why evolve repressors instead of activators? The authors point out that many of these repressors function in a similar manner, being degraded in response to a signal to allow for the activation of the pathway genes. This double-negative logic (Davidson and Levine, 2008) removes the need for the constituent proteins of the MBW complex to obtain novel promoter elements in order to respond to a certain stimulus, and the degradation of a repressor allows for rapid upregulation of anthocyanin gene expression upon signal reception. This allows for fine control over anthocyanin biosynthesis under stress conditions and highlights the flexibility of flavonoid metabolism.
4.1 Roles of anthocyanin in abiotic stress responses of seagrasses
While marine environments can be more stable than terrestrial ones with water screening out UV-B radiation and modulating the temperature, intertidal areas can be particularly variable, with strong fluctuations in light intensity requiring photosynthetic organisms to maximize photosynthetic efficiency under low light conditions and protect photosynthetic machinery under extreme levels of irradiance (Kohlmeier et al., 2017; Léger-Daigle et al., 2022). Anthocyanin biosynthesis is subject to extensive negative regulation, as reviewed by Lafountain and Yuan (2021), often involving various plant hormones, which allows for rapid increase in anthocyanin production upon the reception of a signal. This, combined with their UV-screening ability, makes them very useful for seagrasses which can experience variable levels of UV exposure, on account of differences in depth (Dattolo et al., 2014), meadow density (Schubert et al., 2015), and tides (Kaewsrikhaw and Prathep, 2014). Leaf reddening has been observed in a limited number of seagrass species growing in intertidal regions and shallow waters and is associated with stress exposure (Novak and Short, 2010). Anthocyanin production can be upregulated for photoprotection when exposed to high light conditions (Ma et al., 2021b), while energy is not wasted in conditions where UV-screening mechanisms are unnecessary.
The accumulation of anthocyanins to compensate for the shortcomings of other photoprotective mechanisms has also been observed in terrestrial plants (Zhu et al., 2018). In several species of seagrasses, leaves develop red coloration due to anthocyanin accumulation following high light exposure, with the anthocyanins acting as UV-B- screening compounds or compensating for reduced capacity of photoprotective strategies (Buapet et al., 2017; Zhu et al., 2018). The depth and density of the seagrass meadow also influences the degree of light exposure and thereby the degree of anthocyanin accumulation (Kaewsrikhaw et al., 2016; Dattolo et al., 2014). This variation in pigment content between seagrasses can actually discriminate between species by remote sensing and can therefore be a useful tool in monitoring the health of seagrass meadows (Fyfe, 2003). In T. testudinum, foliar anthocyanin accumulation can be a permanent trait or induced in response to light (Novak and Short, 2012), with red leaves displaying increased levels of UV-absorbing compounds besides anthocyanins and maintaining better photosynthetic efficiency than green leaves at midday (Novak and Short, 2011). Interestingly, a study by Kaewsrikhaw and Prathep (2014) found that anthocyanin content in Halophila ovalis was significantly higher during the rainy season, when light intensity was much lower. This contradicts the hypothesis of the involvement of anthocyanins in UV screening in seagrasses, which seems to hold for some other species, like Zostera capricorni and T. testudinum (Fyfe, 2003; Novak and Short, 2011). While anthocyanins are useful in photoprotection in seagrasses, green leaves do not necessarily suffer greater photoinhibition than red leaves in all seagrass species, as other photoprotective mechanisms can still be effective in high light environments (Buapet et al., 2017; Dattolo et al., 2014). A later study by the same authors (Kaewsrikhaw et al., 2016) found that H. ovalis growing in intertidal areas had higher anthocyanin content compared to individuals growing in subtidal areas. The latter population also showed increased chlorophyll content to mitigate the effects of lower light at greater depths. This observation once again supports the role of anthocyanins in photoprotection and UV screening and somewhat contradicts their earlier thoughts regarding anthocyanin accumulation during the rainy season (Kaewsrikhaw and Prathep, 2014). This might in fact be important for managing cold stress during the rainy season, which is a known function of anthocyanins in some plants (Ahmed et al., 2015; Christie et al., 1994; Schulz et al., 2016).
In the shift to aquatic environments, some seagrasses like Z. marina have lost genes involved in UV sensing and resistance, due to the screening effect of seawater rendering these genes redundant (Olsen et al., 2016). Among these lost genes are photoreceptors, specifically CRY2, which play an important role in upregulating anthocyanin biosynthesis genes and anthocyanin accumulation under high light conditions (Jiang et al., 2016), which results in some species of seagrasses being especially vulnerable to photoinactivation of the oxygen-evolving complex (OEC) under harsh light conditions (Wang et al., 2022; Zhao et al., 2021).
Anthocyanins provide a mechanism of resilience to cold stress and photooxidative damage, but as ocean water provides a buffering effect on both of these stresses, seagrasses may have a smaller set of stress response mechanisms (Olsen et al., 2016; Zhang et al., 2022). In a study by Zhang et al., 2022 it was found that low temperatures combined with high light damaged the photosynthetic machinery of three tropical seagrass species (Enhalus acoroides, Thalassia hemperichii, and Cymodocea rotundata), limiting their distribution to the Indo-Pacific convergence regions. Enhalus acoroides and T. hemperichii do experience leaf reddening (Apichanangkool and Prathep, 2014), and anthocyanins are probably an important mechanism in mitigating cold stress during emersion combined with high light in the absence of other mechanisms. As anthocyanins are cold-induced, they have been found to decrease under temperature increases in terrestrial plants (Sullivan and Koski, 2021), though most research pertaining to the subject is focused on anthocyanin content of grapes for the wine industry. Ocean warming and marine heatwaves may therefore have adverse effects on anthocyanin accumulation in seagrasses, which may be especially detrimental to species that rely heavily on anthocyanins as a photoprotective and cold stress resistance mechanism.
4.2 Anthocyanins in seagrass reproduction
Seagrasses generally reproduce asexually through stolons, though sexual reproduction does occasionally occur, which is important in maintaining standing genetic variation in populations; however, the success rate is poor. Seagrasses generally rely on hydrophilous pollination and engage in various strategies to avoid self-pollination, including dioecy, reduced perianths, and separation of male and floral structures in monoecious species (Van Tussenbroek et al., 2016). Ocean warming and marine heatwaves affect the flowering behavior of different seagrass species differently, with the flowering of cold-adapted P. oceanica seemingly being triggered by marine heat waves (Marín-Guirao et al., 2019; García-Escudero et al., 2024), while Z. marina has displayed a decrease in flowering frequency and intensity as oceans warmed (Qin et al., 2020). Although the flowers of seagrasses are generally green or pale yellow, in accordance with the abiotic pollination strategy, a transcriptomics study in P. oceanica actually found anthocyanin biosynthesis genes to be upregulated in floral tissues, which results in a slight red pigmentation of the male reproductive structures appearing prior to pollen release (Entrambasaguas et al., 2017). The purpose of this anthocyanin accumulation is currently unclear, but anthocyanin production is generally downregulated in response to increased temperatures (Sullivan and Koski, 2021), which will likely lead to a loss of red pigmentation in these flowers as oceans continue to warm.
Anthocyanins are generally produced in the flowers of angiosperms to act as an attractive cue for pollinators, allowing them to be distinguished from the surrounding foliage visually and thermally (Harrap et al., 2017; Takács et al., 2009; Seymour and Matthews, 2006). The discovery of invertebrate-mediated pollination in T. testudinum (Van Tussenbroek et al., 2012, Van Tussenbroek et al., 2016), in combination with the discovery of floral anthocyanin accumulation in P. oceanica, indicates that the possibility of anthocyanins functioning in pollinator attraction in seagrasses cannot be ruled out. Alternatively, the floral anthocyanin accumulation may act as a strategy to deter herbivores, as anthocyanin accumulation can signal metabolic investment in a tissue or decrease its palatability (Gould, 2004). Though biotic pollination was until recently believed to not occur in aquatic ecosystems at all, this discovery in this one species of seagrass spurred additional research regarding pollination benefits arising from other observed biotic interactions involving red algae (Lavaut et al., 2022), which indicates that invertebrate pollination in aquatic ecosystems may be very ancient. Ocean warming is predicted to shift the ranges of herbivores poleward, which may negatively impact seagrass meadows losing invertebrate pollinators and positively affect the sexual reproduction of seagrass meadows that are introduced to this new layer of interaction. The accompanying loss of anthocyanins in the reproductive tissues of some seagrass species may make them more difficult to seek out by potential pollinators or make them more palatable to herbivores, with both scenarios negatively impacting sexual reproduction.
5 Priorities for future research
The availability of genomic data from representatives of the four seagrass families (the Posidoniaceae, Zosteraceae, Hydrocharitaceae, and Cymodoceaceae) provides a valuable framework for future research into the role of flavonoids and anthocyanins in these marine organisms. A priority would be to establish a genomics database for seagrasses, where gene family classifications and protein functions can be updated in real time with the sequencing of additional seagrass species. Gene catalogs from RNA sequencing projects can be especially useful in this regard. Genomic technologies also provide a powerful framework for studying the diversity of individual seagrass species at the population level by linking chemotyping with gene expression profiling and proteomics. Documenting these molecular differences between seagrass populations can also help in understanding and predicting differing responses to stress imposed by climate change.
This review highlights some areas for immediate inquiry. The expansion of the CHS gene family in T. testudinum and P. oceanica compared to other seagrass species can be investigated by determining whether this expansion is present in other species of these families and evaluating neo- or subfunctionalization of flavonoids by metabolite and RNA profiling of different tissues and populations of each species. It would also be interesting to examine whether species with large CHS gene families are able to use flavonoids more effectively in dealing with stress. The absence of FLS orthologs in Z. marina and T. testudinum points to an absence of flavonols in these species. These compounds have been implicated in the detoxification of sulfur in seawater and play important roles in the management of salt stress in many species. Metabolite profiling would confirm the absence of these compounds, which could have interesting implications for how these species will respond to changes in salt concentration caused by more frequent storms. A further area of investigation is the high number of DFR orthologs in T. testudinum, which implies an important role of anthocyanins in this species, as DFR represents the first committed step in anthocyanin biosynthesis. Ocean warming will probably lead to a decrease in anthocyanin content, which will likely affect species that rely on anthocyanins more heavily more so than species that employ additional stress response mechanisms. Experiments testing the effects of UV radiation under warmer conditions on seagrasses that undergo leaf reddening will help to elucidate the potential impact of ocean warming on photoprotection efficiency. The vulnerability of seagrass reproductive structures to herbivory under warming conditions could also help to understand the function of anthocyanin accumulation in these organs.
6 Conclusions
There are several take-home messages from this review on flavonoids in seagrasses and the impacts of climate change.
The ability to produce flavonoids may have aided seagrasses in adapting to aquatic environments, even though these compounds were important for the shift to terrestrial ecosystems in the ancestors of land plants.
● Flavonoids are useful compounds in variable habitats, due to the interplay between different pathways allowing for reactions to different types of stress.
● There is an interplay between flavonoids and other simple phenolic compounds in seagrasses to manage different types of stress, with flavonoid content generally being higher in more variable or stressful environments.
● Seagrasses that produce sulfated flavonoids may be more resilient to increased eutrophication resulting from anthropogenic climate change.
● The role of flavonols in seagrasses is not well understood but may have important implications for dealing with changes in salinity.
● Ocean acidification may impact secondary metabolism, including flavonoid production, negatively.
● Flavonoid content can be used as a measure for assessing the health of seagrass meadows, with changes in flavonoid content reflecting the degree of stress faced by the plants, which may prove to be especially important in understanding how different populations are affected by climate change.
● Seagrasses display several interesting genetic features relating to flavonoid metabolism, with a very high number of CHS orthologs in T. testudinum and P. oceanica and the absence of FLS orthologs in the former and Z. marina, which warrants further research.
● Anthocyanin accumulation may allow for flexible protection against UV radiation in seagrasses, with the loss of other protective screening mechanisms. However, the precise function of anthocyanin accumulation in certain species of seagrasses remains unclear.
● Ocean warming will likely have a more severe impact on seagrass species that heavily rely on anthocyanins as a method of photoprotection.
● Understudied marine biotic interactions may have important implications for flavonoid and anthocyanin accumulation and chemistry in seagrasses, and ocean warming will likely change the patterns of these interactions.
Author contributions
JB: Investigation, Visualization, Writing – original draft. XM: Visualization, Writing – review & editing, Data curation, Formal analysis. JC: Writing – review & editing, Data curation, Formal analysis. YP: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. DB: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding to DB is acknowledged: i) a sabbatical grant from the Oppenheimer Memorial Trust (OMT), South Africa; and ii) grant # FBIS2204041924 of the Foundational Biodiversity Information Programme (FBIP) administered by the National Research Foundation (NRF), South Africa. YP acknowledges the funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant No. 833522).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1520474/full#supplementary-material
References
Abdel-Lateif, K., Bogusz, D., Hocher, V. (2012). The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signaling Behav. 7, 636–641. doi: 10.4161/psb.20039
Adir, N., Zer, H., Shochat, S., Ohad, I. (2003). Photoinhibition - a historical perspective. Photosynthesis Res. 76, 343–370. doi: 10.1023/a:1024969518145
Agati, G., Brunetti, C., Di Ferdinando, M., Ferrini, F., Pollastri, S., Tattini, M. (2013). Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 72, 35–45. doi: 10.1016/j.plaphy.2013.03.014
Ahmed, N. U., Park, J.-I., Jung, H.-J., Hur, Y., Nou, I.-S. (2015). Anthocyanin biosynthesis for cold and freezing stress tolerance and desirable color in Brassica rapa. Funct. Integr. Genomics 15, 383–394. doi: 10.1007/s10142-014-0427-7
Aigner, S., Remias, D., Karsten, U., Holzinger, A. (2013). Unusual phenolic compounds contribute to ecophysiological performance in the purple-colored green alga Zygogonium ericetorum (Zygnematophyceae, Streptophyta) from a high-alpine habitat. J. Phycology 49, 648–660. doi: 10.1111/jpy.12075
Ananvoranich, S., Varin, L., Gulick, P., Ibrahim, R. (1994). Cloning and regulation of flavonol 3-sulfotransferase in cell-suspension cultures of Flaveria bidentis. Plant Physiol. 106, 485–491. doi: 10.1104/pp.106.2.485
Apichanangkool, P., Prathep, A. (2014). Changes in seagrass leaf reddening and morphology in response to emersion. Botanica Marina 57, 433–440. doi: 10.1515/bot-2014-0004
Arnold, T., Mealey, C., Leahey, H., Miller, A. W., Hall-Spencer, J. M., Milazzo, M., et al. (2012). Ocean acidification and the loss of phenolic substances in marine plants. PloS One 7, e35107. doi: 10.1371/journal.pone.0035107
Asaeda, T., Fujino, T., Manatunge, J. (2005). Morphological adaptations of emergent plants to water flow: a case study with Typha angustifolia, Zizania latifolia and Phragmites australis. Freshw. Biol. 50, 1991–2001. doi: 10.1111/j.1365-2427.2005.01445.x
Astudillo-Pascual, M., Domínguez, I., Aguilera, P. A., Garrido Frenich, A. (2021). New phenolic compounds in Posidonia oceanica seagrass: A comprehensive array using high resolution mass spectrometry. Plants 10, 864. doi: 10.3390/plants10050864
Ben Saad, H., Gargouri, M., Kallel, F., Chaabene, R., Boudawara, T., Jamoussi, K., et al. (2017). Flavonoid compounds from the red marine alga Alsidium corallinum protect against potassium bromate-induced nephrotoxicity in adult mice. Environ. Toxicol. 32, 1475–1486. doi: 10.1002/tox.22368
Berland, H., Albert, N. W., Stavland, A., Jordheim, M., McGhie, T. K., Zhou, Y., et al. (2019). Auronidins are a previously unreported class of flavonoid pigments that challenges when anthocyanin biosynthesis evolved in plants. Proc. Natl. Acad. Sci. 116, 20232–20239. doi: 10.1073/pnas.1912741116
Bloomfield, A. L., Gillanders, B. M. (2005). Fish and invertebrate assemblages in seagrass, mangrove, saltmarsh, and nonvegetated habitats. Estuaries 28, 63–77. doi: 10.1007/bf02732754
Broman, E., Sjöstedt, J., Pinhassi, J., Dopson, M. (2017). Shifts in coastal sediment oxygenation cause pronounced changes in microbial community composition and associated metabolism. Microbiome 5, (1). doi: 10.1186/s40168-017-0311-5
Buapet, P., Makkliang, F., Thammakhet-Buranachai, C. (2017). Photosynthetic activity and photoprotection in green and red leaves of the seagrasses, Halophila ovalis and Cymodocea rotundata: implications for the photoprotective role of anthocyanin. Mar. Biol. 164, (9). doi: 10.1007/s00227-017-3215-9
Burbulis, I. E., Iacobucci, M., Shirley, B. W. (1996). A null mutation in the first enzyme of flavonoid biosynthesis does not affect male fertility in Arabidopsis. Plant Cell 8, 1013–1025. doi: 10.1105/tpc.8.6.1013
Burchard, P., Bilger, W., Weissenbock, G. (2000). Contribution of hydroxycinnamates and flavonoids to epidermal shielding of UV-A and UV-B radiation in developing rye primary leaves as assessed by ultraviolet-induced chlorophyll fluorescence measurements. Plant Cell Environ. 23, 1373–1380. doi: 10.1046/j.1365-3040.2000.00633.x
Campbell, J. E., Kennedy Rhoades, O., Munson, C. J., Altieri, A. H., Douglass, J. G., Heck, K. L., et al. (2024). Herbivore effects increase with latitude across the extent of a foundational seagrass. Nat. Ecol. Evol. 8, 663–675. doi: 10.1038/s41559-024-02336-5
Campbell, S. J., McKenzie, L. J. (2004). Flood related loss and recovery of intertidal seagrass meadows in southern Queensland, Australia. Estuarine Coast. Shelf Sci. 60, 477–490. doi: 10.1016/j.ecss.2004.02.007
Casal-Porras, I., Jiménez-Ramos, R., Zubía, E., Brun, F. G. (2021). Importance of the chemical defenses and sugars in the feeding preference of Paracentrotus lividus over two sympatric template seagrass species. Estuarine Coast. Shelf Sci. 259, 107466. doi: 10.1016/j.ecss.2021.107466
Casal-Porras, I., Muñoz, K., Ortega, M. J., Brun, F. G., Zubía, E. (2023). Rosmarinic acid and flavonoids of the seagrass Zostera noltei: new aspects on their quantification and their correlation with sunlight exposure. Plants 12, 4078. doi: 10.3390/plants12244078
Chan, K. X., Phua, S. Y., Van Breusegem, F. (2019). Secondary sulfur metabolism in cellular signalling and oxidative stress responses. J. Exp. Bot. 70, 4237–4250. doi: 10.1093/jxb/erz119
Chater, C. C. C., Caine, R. S., Fleming, A. J., Gray, J. E. (2017). Origins and evolution of stomatal development. Plant Physiol. 174, 624–638. doi: 10.1104/pp.17.00183
Chen, L.-Y., Lu, B., Morales-Briones, D. F., Moody, M. L., Liu, F., Hu, G.-W., et al. (2022). Phylogenomic analyses of Alismatales shed light into adaptations to aquatic environments. Mol. Biol. Evol. 39, (5). doi: 10.1093/molbev/msac079
Chollett, I., Bone, D., Pérez, D. (2007). Effects of heavy rainfall on Thalassia testudinum beds. Aquat. Bot. 87, 189–195. doi: 10.1016/j.aquabot.2007.05.003
Christie, P. J., Alfenito, M. R., Walbot, V. (1994). Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 194, 541–549. doi: 10.1007/BF00714468
Christie, J. M., Jenkins, G. I. (1996). Distinct UV-B and UV-A/blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell 8, 1555–1567. doi: 10.1105/tpc.8.9.1555
Clayton, W. A., Albert, N. W., Thrimawithana, A. H., McGhie, T. K., Deroles, S. C., Schwinn, K. E., et al. (2018). UVR8-mediated induction of flavonoid biosynthesis for UVB tolerance is conserved between the liverwort Marchantia polymorpha and flowering plants. Plant J. 96, 503–517. doi: 10.1111/tpj.14044
Crosby, K. C., Pietraszewska-Bogiel, A., Gadella, T. W. J., Winkel, B. S. J. (2011). Förster resonance energy transfer demonstrates a flavonoid metabolon in living plant cells that displays competitive interactions between enzymes. FEBS Lett. 585, 2193–2198. doi: 10.1016/j.febslet.2011.05.066
Dao, T. T. H., Linthorst, H. J. M., Verpoorte, R. (2011). Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 10, 397–412. doi: 10.1007/s11101-011-9211-7
Dare, A., Hellens, R. (2013). RNA interference silencing of CHS greatly alters the growth pattern of apple (Malus domestica). Plant Signaling Behav. 8, e25033. doi: 10.4161/psb.25033
Daru, B. H., Rock, B. M. (2023). Reorganization of seagrass communities in a changing climate. Nat. Plants 9, 1034–1043. doi: 10.1038/s41477-023-01445-6
Daryanavard, H., Postiglione, A. E., Mühlemann, J. K., Muday, G. K. (2023). Flavonols modulate plant development, signaling, and stress responses. Curr. Opin. Plant Biol. 72, 102350. doi: 10.1016/j.pbi.2023.102350
Dattolo, E., Ruocco, M., Brunet, C., Lorenti, M., Lauritano, C., D’Esposito, D., et al. (2014). Response of the seagrass Posidonia oceanica to different light environments: Insights from a combined molecular and photo-physiological study. Mar. Environ. Res. 101, 225–236. doi: 10.1016/j.marenvres.2014.07.010
Davidson, E. H., Levine, M. S. (2008). Properties of developmental gene regulatory networks. Proc. Natl. Acad. Sci. 105, 20063–20066. doi: 10.1073/pnas.0806007105
Davies, K. M., Jibran, R., Zhou, Y., Albert, N. W., Brummell, D. A., Jordan, B. R., et al. (2020). The evolution of flavonoid biosynthesis: A bryophyte perspective. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00007
De Luca, D., Lauritano, C. (2020). In silico identification of type III PKS chalcone and stilbene synthase homologs in marine photosynthetic organisms. Biology 9, 110. doi: 10.3390/biology9050110
Deng, X., Bashandy, H., Ainasoja, M., Kontturi, J., Pietiäinen, M., Laitinen, R. A. E., et al. (2014). Functional diversification of duplicated chalcone synthase genes in anthocyanin biosynthesis of Gerbera hybrida. New Phytol. 201, 1469–1483. doi: 10.1111/nph.12610
De Vries, J., Archibald, J. M. (2018). Plant evolution: landmarks on the path to terrestrial life. New Phytol. 217, 1428–1434. doi: 10.1111/nph.14975
Diaz-Almela, E., Marbà, N., Duarte, C. M. (2007). Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Global Change Biol. 13, 224–235. doi: 10.1111/j.1365-2486.2006.01260.x
Di Ferdinando, M., Brunetti, C., Agati, G., Tattini, M. (2014). Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ. Exp. Bot. 103, 107–116. doi: 10.1016/j.envexpbot.2013.09.012
Domingues, C. M., Church, J. A., White, N. J., Gleckler, P. J., Wijffels, S. E., Barker, P. M., et al. (2008). Improved estimates of upper-ocean warming and multi-decadal sea-level rise. Nature 453, 1090–1093. doi: 10.1038/nature07080
Dong, N.-Q., Lin, H.-X. (2021). Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 63, 180–209. doi: 10.1111/jipb.13054
Duarte, C. M., Krause-Jensen, D. (2017). Export from seagrass meadows contributes to marine carbon sequestration. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00013
Duarte, B., Martins, I., Rosa, R., Matos, A. R., Roleda, M. Y., Reusch, T. B. H., et al. (2018). Climate change impacts on seagrass meadows and macroalgal forests: an integrative perspective on acclimation and adaptation potential. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00190
Durbin, M. L., Learn, G. H., Huttley, G. A., Clegg, M. T. (1995). Evolution of the chalcone synthase gene family in the genus Ipomoea. Proc. Natl. Acad. Sci. 92, 3338–3342. doi: 10.1073/pnas.92.8.3338
Durbin, M. L., McCaig, B., Clegg, M. T. (2000). Molecular evolution of the chalcone synthase multigene family in the morning glory genome. Plant Mol. Biol. 42, 79–92. doi: 10.1023/a:1006375904820
East, H. K., Johnson, J. A., Perry, C. T., Finlay, G., Musthag, A., Zahir, H., et al. (2023). Seagrass meadows are important sources of reef island-building sediment. Commun. Earth Environ. 4, (1). doi: 10.1038/s43247-023-00675-y
Effrosynidis, D., Arampatzis, A., Sylaios, G. (2019). Seagrass and hydrographic data for the Mediterranean Sea. Data Brief 25, 104286. doi: 10.1016/j.dib.2019.104286
Eichenberger, M., Schwander, T., Hüppi, S., Kreuzer, J., Mittl, P. R. E., Peccati, F., et al. (2023). The catalytic role of glutathione transferases in heterologous anthocyanin biosynthesis. Nat. Catalysis 6, 927–938. doi: 10.1038/s41929-023-01018-y
Emiliani, G., Fondi, M., Fani, R., Gribaldo, S. (2009). A horizontal gene transfer at the origin of phenylpropanoid metabolism: a key adaptation of plants to land. Biol. Direct 4, 7. doi: 10.1186/1745-6150-4-7
Entrambasaguas, L., Jahnke, M., Biffali, E., Borra, M., Sanges, R., Marín-Guirao, L., et al. (2017). Tissue-specific transcriptomic profiling provides new insights into the reproductive ecology and biology of the iconic seagrass species Posidonia oceanica. Mar. Genomics 35, 51–61. doi: 10.1016/j.margen.2017.05.006
Feller, A., Machemer, K., Braun, E. L., Grotewold, E. (2011). Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 66, 94–116. doi: 10.1111/j.1365-313x.2010.04459.x
Fini, A., Brunetti, C., Di Ferdinando, M., Ferrini, F., Tattini, M. (2011). Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signaling Behav. 6, 709–711. doi: 10.4161/psb.6.5.15069
Fyfe, S. K. (2003). Spatial and temporal variation in spectral reflectance: Are seagrass species spectrally distinct. Limnology Oceanography 48, 464–479. doi: 10.4319/lo.2003.48.1_part_2.0464
García-Escudero, C. A., Litsi-Mizan, V., Efthymiadis, P. T., Gerakaris, V., Serrano, O., Apostolaki, E. T. (2024). Strong marine heatwaves trigger flowering in seagrass. Limnology Oceanography 69, 1494–1507. doi: 10.1002/lno.12589
Gartner, A., Tuya, F., Lavery, P. S., McMahon, K. (2013). Habitat preferences of macroinvertebrate fauna among seagrasses with varying structural forms. J. Exp. Mar. Biol. Ecol. 439, 143–151. doi: 10.1016/j.jembe.2012.11.009
Gavin, N., Durako, M. (2012). Localization and antioxidant capacity of flavonoids in Halophila johnsonii in response to experimental light and salinity variation. J. Exp. Mar. Biol. Ecol. 416–417, 32–40. doi: 10.1016/j.jembe.2012.02.006
Gebhardt, Y., Witte, S., Forkmann, G., Lukačin, R., Matern, U., Martens, S. (2005). Molecular evolution of flavonoid dioxygenases in the family Apiaceae. Phytochemistry 66, 1273–1284. doi: 10.1016/j.phytochem.2005.03.030
Gidda, S. K., Varin, L. (2006). Biochemical and molecular characterization of flavonoid 7-sulfotransferase from Arabidopsis thaliana. Plant Physiol. Biochem. 44, 628–636. doi: 10.1016/j.plaphy.2006.10.004
Goiris, K., Muylaert, K., Voorspoels, S., Noten, B., De Paepe, D., E Baart, G. J., et al. (2014). Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycology 50, 483–492. doi: 10.1111/jpy.12180
Gould, K. S. (2004). Nature′s swiss army knife: the diverse protective roles of anthocyanins in leaves. BioMed. Res. Int. 5), 314–320. doi: 10.1155/s1110724304406147
Grignon-Dubois, M., Rezzonico, B. (2012). First phytochemical evidence of chemotypes for the seagrass Zostera noltei. Plants 1, 27–38. doi: 10.3390/plants1010027
Grignon-Dubois, M., Rezzonico, B. (2018). Phenolic chemistry of the seagrass Zostera noltei Hornem. Part 1: First evidence of three infraspecific flavonoid chemotypes in three distinctive geographical regions. Phytochemistry 146, 91–101. doi: 10.1016/j.phytochem.2017.12.006
Grignon-Dubois, M., Rezzonico, B. (2023). Seasonal and interannual variability in the phenolic content of the seagrass nanozostera noltei: characterization of suitable candidates for the monitoring of seagrass health. Diversity 15, (12). doi: 10.3390/d15121210
Guo, X., Li, J., Cai, D. (2023). Overexpression of a Flavonol Synthase Gene from Apocynum venetum Improves the Salinity Stress Tolerance of Transgenic Arabidopsis thaliana. J. Soil Sci. Plant Nutr. 24, 2317–2333. doi: 10.1007/s42729-023-01590-z
Harborne, J. B. (1975). Flavonoid sulphates: A new class of sulphur compounds in higher plants. Phytochemistry 14, 1147–1155. doi: 10.1016/S0031-9422(00)98585-6
Harborne, J. (1977). The plant and its biochemical adaptation to the environment. Introduction to ecological biochemistry (London, UK: Academic Press), 1–27.
Harrap, M. J., Rands, S. A., Hempel De Ibarra, N., Whitney, H. M. (2017). The diversity of floral temperature patterns, and their use by pollinators. eLife 6, e31262. doi: 10.7554/elife.31262
Hasler-Sheetal, H., Holmer, M. (2015). Sulfide intrusion and detoxification in the seagrass Zostera marina. PloS One 10, e0129136. doi: 10.1371/journal.pone.0129136
Hassan, S., Mathesius, U. (2012). The role of flavonoids in root-rhizosphere signalling: opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 63, 3429–3444. doi: 10.1093/jxb/err430
He, Q., Ren, Y., Zhao, W., Li, R., Zhang, L. (2020). Low temperature promotes anthocyanin biosynthesis and related gene expression in the seedlings of purple head chinese cabbage (Brassica rapa L.). Genes 11, 81. doi: 10.3390/genes11010081
Hoegh-Guldberg, O., Bruno, J. F. (2010). The impact of climate change on the world’s marine ecosystems. Science 328, 1523–1528. doi: 10.1126/science.1189930
Holmer, M., Bondgaard, E. J. (2001). Photosynthetic and growth response of eelgrass to low oxygen and high sulfide concentrations during hypoxic events. Aquat. Bot. 70, 29–38. doi: 10.1016/S0304-3770(00)00142-X
Hughes, N. M., Neufeld, H. S., Burkey, K. O. (2005). Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytol. 168, 575–587. doi: 10.1111/j.1469-8137.2005.01546.x
Hyndes, G. A., Heck, K. L., Vergés, A., Harvey, E. S., Kendrick, G. A., Lavery, P. S., et al. (2016). Accelerating tropicalization and the transformation of temperate seagrass meadows. BioScience 66, 938–948. doi: 10.1093/biosci/biw111
Jackson, E. L., Rees, S. E., Wilding, C., Attrill, M. J. (2015). Use of a seagrass residency index to apportion commercial fishery landing values and recreation fisheries expenditure to seagrass habitat service. Conserv. Biol. 29, 899–909. doi: 10.1111/cobi.12436
Jakimavičius, D., Kriaučiūnienė, J., Šarauskienė, D. (2018). Impact of climate change on the Curonian Lagoon water balance components, salinity and water temperature in the 21st century. Oceanologia 60, 378–389. doi: 10.1016/j.oceano.2018.02.003
Jiang, M., Ren, L., Lian, H., Liu, Y., Chen, H. (2016). Novel insight into the mechanism underlying light-controlled anthocyanin accumulation in eggplant (Solanum melongena L.). Plant Sci. 249, 46–58. doi: 10.1016/j.plantsci.2016.04.001
Jiao, C., Sørensen, I., Sun, X., Sun, H., Behar, H., Alseekh, S., et al. (2020). The Penium margaritaceum genome: hallmarks of the origins of land plants. Cell 181, 1097–1111.e1012. doi: 10.1016/j.cell.2020.04.019
Kaewsrikhaw, R., Prathep, A. (2014). The effect of habitats, densities and seasons on morphology, anatomy and pigment content of the seagrass Halophila ovalis (R.Br.) Hook.f. at Haad Chao Mai National Park, Southern Thailand. Aquat. Bot. 116, 69–75. doi: 10.1016/j.aquabot.2014.01.009
Kaewsrikhaw, R., Ritchie, R. J., Prathep, A. (2016). Variations of tidal exposures and seasons on growth, morphology, anatomy and physiology of the seagrass Halophila ovalis (R.Br.) Hook. f. in a seagrass bed in Trang Province, Southern Thailand. Aquat. Bot. 130, 11–20. doi: 10.1016/j.aquabot.2015.12.006
Kaltenbach, M., Burke, J. R., Dindo, M., Pabis, A., Munsberg, F. S., Rabin, A., et al. (2018). Evolution of chalcone isomerase from a noncatalytic ancestor. Nat. Chem. Biol. 14, 548–555. doi: 10.1038/s41589-018-0042-3
Karageorgou, P., Manetas, Y. (2006). The importance of being red when young: anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiol. 26, 613–621. doi: 10.1093/treephys/26.5.613
Kenrick, P., Crane, P. R. (1997). The origin and early evolution of plants on land. Nature 389, 33–39. doi: 10.1038/37918
Keulen, M. V., Borowitzka, M. A. (2003). Seasonal variability in sediment distribution along an exposure gradient in a seagrass meadow in Shoalwater Bay, Western Australia. Estuarine Coast. Shelf Sci. 57, 587–592. doi: 10.1016/S0272-7714(02)00394-3
Kohlmeier, D., Pilditch, C. A., Bornman, J. F., Bischof, K. (2017). Adjustment of photoprotection to tidal conditions in intertidal seagrasses. J. Mar. Biol. Assoc. United Kingdom 97, 571–579. doi: 10.1017/S0025315416001090
Kunz, S., Burkhardt, G., Becker, H. (1993). Riccionidins a and b, anthocyanidins from the cell walls of the liverwort Ricciocarpos natans. Phytochemistry 35, 233–235. doi: 10.1016/S0031-9422(00)90540-5
Lafountain, A. M., Yuan, Y. W. (2021). Repressors of anthocyanin biosynthesis. New Phytol. 231, 933–949. doi: 10.1111/nph.17397
Lal, A., Arthur, R., Marbà, N., Lill, A. W. T., Alcoverro, T. (2010). Implications of conserving an ecosystem modifier: Increasing green turtle (Chelonia mydas) densities substantially alters seagrass meadows. Biol. Conserv. 143, 2730–2738. doi: 10.1016/j.biocon.2010.07.020
Laoué, J., Fernandez, C., Ormeño, E. (2022). Plant flavonoids in mediterranean species: A focus on flavonols as protective metabolites under climate stress. Plants 11, 172. doi: 10.3390/plants11020172
Larkum, A. W. D., Davey, P. A., Kuo, J., Ralph, P. J., Raven, J. A. (2017). Carbon-concentrating mechanisms in seagrasses. J. Exp. Bot. 68, 3773–3784. doi: 10.1093/jxb/erx206
Lavaut, E., Guillemin, M. L., Colin, S., Faure, A., Coudret, J., Destombe, C., et al. (2022). Pollinators of the sea: A discovery of animal-mediated fertilization in seaweed. Science 377, 528–530. doi: 10.1126/science.abo6661
Léger-Daigle, R., Noisette, F., Bélanger, S., Cusson, M., Nozais, C. (2022). Photoacclimation and light thresholds for cold temperate seagrasses. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.805065
Lei, L. (2017). Lignin evolution: Invasion of land. Nat. Plants 3, 17042. doi: 10.1038/nplants.2017.42
Li, Y., Liu, X., Cai, X., Shan, X., Gao, R., Yang, S., et al. (2017). Dihydroflavonol 4-reductase genes from Freesia hybrida play important and partially overlapping roles in the biosynthesis of flavonoids. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00428
Li, J., Ou-Lee, T. M., Raba, R., Amundson, R. G., Last, R. L. (1993). Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5, 171–179. doi: 10.1105/tpc.5.2.171
Li, S., Paulsson, M., Björn, L. O. (2002). Temperature-dependent formation and photorepair of DNA damage induced by UV-B radiation in suspension-cultured tobacco cells. J. Photochem. Photobiol. B: Biol. 66, 67–72. doi: 10.1016/S1011-1344(01)00277-9
Ligrone, R., Carafa, A., Duckett, J. G., Renzaglia, K. S., Ruel, K. (2008). Immunocytochemical detection of lignin-related epitopes in cell walls in bryophytes and the charalean alga Nitella. Plant Systematics Evol. 270, 257–272. doi: 10.1007/s00606-007-0617-z
Listiawati, V., Kurihara, H. (2021). Ocean warming and acidification modify top-down and bottom-up control in a tropical seagrass ecosystem. Sci. Rep. 11, (1). doi: 10.1038/s41598-021-92989-0
Longo, G. O. (2024). Seagrass vulnerability to tropicalization-induced herbivory. Nat. Ecol. Evol. 8, 600–601. doi: 10.1038/s41559-024-02345-4
Ludwig, S. R., Bowen, B., Beach, L., Wessler, S. R. (1990). A regulatory gene as a novel visible marker for maize transformation. Science 247, 449–450. doi: 10.1126/science.247.4941.449
Luo, P., Ning, G., Wang, Z., Shen, Y., Jin, H., Li, P., et al. (2016). Disequilibrium of flavonol synthase and dihydroflavonol-4-reductase expression associated tightly to white vs. red color flower formation in plants. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.01257
Ma, Y., Ma, X., Gao, X., Wu, W., Zhou, B. (2021b). Light induced regulation pathway of anthocyanin biosynthesis in plants. Int. J. Mol. Sci. 22, 11116. doi: 10.3390/ijms222011116
Ma, X., Vanneste, S., Chang, J., Ambrosino, L., Barry, K., Bayer, T., et al. (2024). Seagrass genomes reveal ancient polyploidy and adaptations to the marine environment. Nat. Plants. 10, 240–255. doi: 10.1038/s41477-023-01608-5
Ma, L., Yang, C., Xiao, D., Liu, X., Jiang, X., Lin, H., et al. (2023). Chromosome-level assembly of Dictyophora rubrovolvata genome using third-generation DNA sequencing and Hi-C analysis. G3: Genes Genomes Genet. 13, (8). doi: 10.1093/g3journal/jkad102
Ma, M., Zhong, M., Zhang, Q., Zhao, W., Wang, M., Luo, C. (2021a). Phylogenetic Implications and Functional Disparity in the Chalcone synthase Gene Family of Common Seagrass Zostera marina. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.760902
Maloney, G.-O., DiNapoli, K. T., Muday, G. A.-O. (2014). The anthocyanin reduced tomato mutant demonstrates the role of flavonols in tomato lateral root and root hair development. Plant Physiol. 166, 614–631. doi: 10.1104/pp.114.240507
Marbà, N., Arias-Ortiz, A., Masqué, P., Kendrick, G. A., Mazarrasa, I., Bastyan, G. R., et al. (2015). Impact of seagrass loss and subsequent revegetation on carbon sequestration and stocks. J. Ecol. 103, 296–302. doi: 10.1111/1365-2745.12370
Marín-Guirao, L., Entrambasaguas, L., Ruiz, J. M., Procaccini, G. (2019). Heat-stress induced flowering can be a potential adaptive response to ocean warming for the iconic seagrass Posidonia oceanica. Mol. Ecol. 28, 2486–2501. doi: 10.1111/mec.15089
Martens, S., Forkmann, G., Britsch, L., Wellmann, F., Matern, U., Lukačin, R. (2003). Divergent evolution of flavonoid 2-oxoglutarate-dependent dioxygenases in parsley. FEBS Lett. 544, 93–98. doi: 10.1016/s0014-5793(03)00479-4
McKenzie, L. J., Nordlund, L. M., Jones, B. L., Cullen-Unsworth, L. C., Roelfsema, C., Unsworth, R. K. F. (2020). The global distribution of seagrass meadows. Environ. Res. Lett. 15, 074041. doi: 10.1088/1748-9326/ab7d06
McMillan, C., Zapata, O., Escobar, L. (1980). Sulphated phenolic compounds in seagrasses. Aquat. Bot. 8, 267–278. doi: 10.1016/0304-3770(80)90055-8
Meng, C., Zhang, S., Deng, Y. S., Wang, G.-D., Kong, F. Y. (2015). Overexpression of a tomato flavanone 3-hydroxylase-like protein gene improves chilling tolerance in tobacco. Plant Physiol. Biochem. 96, 388–400. doi: 10.1016/j.plaphy.2015.08.019
Merzlyak, M. N., Chivkunova, O. B., Solovchenko, A. E., Naqvi, K. R. (2008). Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J. Exp. Bot. 59, 3903–3911. doi: 10.1093/jxb/ern230
Minh, L. T., Khang, D. T., Thu Ha, P. T., Tuyen, P. T., Minh, T. N., Quan, N. V., et al. (2016). Effects of salinity stress on growth and phenolics of rice (Oryza sativa L.). Int. Lett. Natural Sci. 57, 1–10. doi: 10.18052/www.scipress.com/ilns.57.1
Moore, K., Shields, E., Parrish, D., Orth, R. (2012). Eelgrass survival in two contrasting systems: role of turbidity and summer water temperatures. Mar. Ecol. Prog. Ser. 448, 247–258. doi: 10.3354/meps09578
Mtwana Nordlund, L., Koch, E. W., Barbier, E. B., Creed, J. C. (2016). Seagrass ecosystem services and their variability across genera and geographical regions. PloS One 11, e0163091. doi: 10.1371/journal.pone.0163091
Muhlemann, J. K., Younts, T. L. B., Muday, G. K. (2018). Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc. Natl. Acad. Sci. 115, E11188–E11197. doi: 10.1073/pnas.1811492115
Murata, N., Takahashi, S., Nishiyama, Y., Allakhverdiev, S. I. (2007). Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta (BBA) - Bioenergetics 1767, 414–421. doi: 10.1016/j.bbabio.2006.11.019
Ng, J. L. P., Hassan, S., Truong, T. T., Hocart, C. H., Laffont, C., Frugier, F., et al. (2015). Flavonoids and Auxin Transport Inhibitors Rescue Symbiotic Nodulation in the Medicago truncatula Cytokinin Perception Mutant cre1. Plant Cell 27, 2210–2226. doi: 10.1105/tpc.15.00231
Ngaki, M. N., Louie, G. V., Philippe, R. N., Manning, G., Pojer, F., Bowman, M. E., et al. (2012). Evolution of the chalcone-isomerase fold from fatty-acid binding to stereospecific catalysis. Nature 485, 530–533. doi: 10.1038/nature11009
Novak, A. B., Short, F. T. (2010). Leaf reddening in seagrasses. Botanica Marina 53, 93–97. doi: 10.1515/BOT.2010.011
Novak, A. B., Short, F. T. (2011). Leaf reddening in the seagrass Thalassia testudinum in relation to anthocyanins, seagrass physiology and morphology, and plant protection. Mar. Biol. 158, 1403–1416. doi: 10.1007/s00227-011-1658-y
Novak, A. B., Short, F. T. (2012). Permanent and transient leaf reddening in the seagrass Thalassia testudinum. Bull. Mar. Sci. 88, 305–315. doi: 10.5343/bms.2011.1107
Olsen, J. L., Rouzé, P., Verhelst, B., Lin, Y.-C., Bayer, T., Collen, J., et al. (2016). The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 530, 331–335. doi: 10.1038/nature16548
Owens, D. K., Alerding, A. B., Crosby, K. C., Bandara, A. B., Westwood, J. H., Winkel, B. S. J. (2008). Functional analysis of a predicted flavonol synthase gene family in arabidopsis. Plant Physiol. 147, 1046–1061. doi: 10.1104/pp.108.117457
Papenbrock, J. (2012). Highlights in seagrasses’ phylogeny, physiology, and metabolism: what makes them special? ISRN Bot. 2012, 1–15. doi: 10.5402/2012/103892
Pazzaglia, J., Reusch, T. B. H., Terlizzi, A., Marín-Guirao, L., Procaccini, G. (2021). Phenotypic plasticity under rapid global changes: The intrinsic force for future seagrasses survival. Evolutionary Appl. 14, 1181–1201. doi: 10.1111/eva.13212
Peer, W. A., Bandyopadhyay, A., Blakeslee, J. J., Makam, S. N., Chen, R. J., Masson, P. H., et al. (2004). Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana [W. Plant Cell 16, 1898–1911. doi: 10.1105/tpc.021501
Pfeifer, L., Classen, B. (2020). The cell wall of seagrasses: fascinating, peculiar and a blank canvas for future research. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.588754
Piatkowski, B. T., Imwattana, K., Tripp, E. A., Weston, D. J., Healey, A., Schmutz, J., et al. (2020). Phylogenomics reveals convergent evolution of red-violet coloration in land plants and the origins of the anthocyanin biosynthetic pathway. Mol. Phylogenet. Evol. 151, 106904. doi: 10.1016/j.ympev.2020.106904
Pollastri, S., Tattini, M. (2011). Flavonols: old compounds for old roles. Ann. Bot. 108, 1225–1233. doi: 10.1093/aob/mcr234
Qi, T., Huang, H., Wu, D., Yan, J., Qi, Y., Song, S., et al. (2014). Arabidopsis DELLA and JAZ proteins bind the WD-repeat/bHLH/MYB complex to modulate gibberellin and jasmonate signaling synergy. Plant Cell 26, 1118–1133. doi: 10.1105/tpc.113.121731
Qi, T., Song, S., Ren, Q., Wu, D., Huang, H., Chen, Y., et al. (2011). The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 23, 1795–1814. doi: 10.1105/tpc.111.083261
Qin, L.-Z., Kim, S. H., Song, H.-J., Kim, H. G., Suonan, Z., Kwon, O., et al. (2020). Long-term variability in the flowering phenology and intensity of the temperate seagrass Zostera marina in response to regional sea warming. Ecol. Indic. 119, 106821. doi: 10.1016/j.ecolind.2020.106821
Quatrocchio, F., Wing, J. F., Leppen, H. T. C., Mol, J. N. M., Koes, R. E. (1993). Regulatory genes controlling anthocyanin pigmentation are functionally conserved among plant species and have distinct sets of target genes. Plant Cell 5, 1497–1512. doi: 10.2307/3869734
Rabemanolontsoa, H., Saka, S. (2013). Comparative study on chemical composition of various biomass species. RSC Adv. 3, 3946. doi: 10.1039/c3ra22958k
Rahme, J., Suter, L., Widmer, A., Karrenberg, S. (2014). Inheritance and reproductive consequences of floral anthocyanin deficiency in Silene dioica (Caryophyllaceae). Am. J. Bot. 101, 1388–1392. doi: 10.3732/ajb.1400136
Ramos, P., Guajardo, J., Moya-León, M. A., Herrera, R. (2016). A differential distribution of auxin and flavonols in radiata pine stem seedlings exposed to inclination. Tree Genet. Genomes 12, (3). doi: 10.1007/s11295-016-1003-1
Repolho, T., Duarte, B., Dionísio, G., Paula, J. R., Lopes, A. R., Rosa, I. C., et al. (2017). Seagrass ecophysiological performance under ocean warming and acidification. Sci. Rep. 7, 41443. doi: 10.1038/srep41443
Richard, S., Lapointe, G., Rutledge, R. G. (2000). Induction of chalcone synthase expression in white spruce by wounding and jasmonate. Plant Cell Physiol. 41, 982–987. doi: 10.1093/pcp/pcd017
Roubalová, L., Purchartová, K., Papoušková, B., Vacek, J., Křen, V., Ulrichová, J., et al. (2015). Sulfation modulates the cell uptake, antiradical activity and biological effects of flavonoids in vitro: An examination of quercetin, isoquercitrin and taxifolin. Bioorganic Medicinal Chem. 23, 5402–5409. doi: 10.1016/j.bmc.2015.07.055
Rudall, P. J. (2020). Colourful cones: how did flower colour first evolve? J. Exp. Bot. 71, 759–767. doi: 10.1093/jxb/erz479
Sævdal Dybsland, C., Bekkby, T., Hasle Enerstvedt, K., Kvalheim, O. M., Rinde, E., Jordheim, M. (2021). Variation in Phenolic Chemistry in Zostera marina Seagrass along Environmental Gradients. Plants 10, (2). doi: 10.3390/plants10020334
Sainz, M. B. G. E., Chandler, V. L. (1997). Evidence for direct activation of an anthocyanin promoter by the maize C1 protein and comparison of DNA binding by related Myb domain proteins. Plant Cell 9, 611–625. doi: 10.1105/tpc.9.4.611
Schmidtko, S., Stramma, L., Visbeck, M. (2017). Decline in global oceanic oxygen content during the past five decades. Nature 542, 335–339. doi: 10.1038/nature21399
Schubert, N., Colombo-Pallota, M. F., Enríquez, S. (2015). Leaf and canopy scale characterization of the photoprotective response to high-light stress of the seagrass Thalassia testudinum. Limnology Oceanography 60, 286–302. doi: 10.1002/lno.10024
Schulz, E., Tohge, T., Zuther, E., Fernie, A. R., Hincha, D. K. (2016). Flavonoids are determinants of freezing tolerance and cold acclimation in Arabidopsis thaliana. Sci. Rep. 6, 34027. doi: 10.1038/srep34027
Seidel, L., Broman, E., Turner, S., Ståhle, M., Dopson, M. (2021). Interplay between eutrophication and climate warming on bacterial communities in coastal sediments differs depending on water depth and oxygen history. Sci. Rep. 11, (1). doi: 10.1038/s41598-021-02725-x
Serrano, O., Gómez-López, D. I., Sánchez-Valencia, L., Acosta-Chaparro, A., Navas-Camacho, R., González-Corredor, J., et al. (2021). Seagrass blue carbon stocks and sequestration rates in the Colombian Caribbean. Sci. Rep. 11, (1). doi: 10.1038/s41598-021-90544-5
Seymour, R. S., Matthews, P. G. (2006). The role of thermogenesis in the pollination biology of the Amazon waterlily Victoria amazonica. Ann. Bot. 98, 1129–1135. doi: 10.1093/aob/mcl201
Shao, L., Shu, Z., Peng, C.-L., Lin, Z.-F., Yang, C.-W., Gu, Q. (2008). Enhanced sensitivity of Arabidopsis anthocyanin mutants to photooxidation: a study with fluorescence imaging. Funct. Plant Biol. 35, 714. doi: 10.1071/fp08069
Shen, J., Wu, Z., Yin, L., Chen, S., Cai, Z., Geng, X., et al. (2022). Physiological basis and differentially expressed genes in the salt tolerance mechanism of Thalassia hemprichii. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.975251
Shimada, N., Aoki, T., Sato, S., Nakamura, Y., Ayabe, S. (2003). A cluster of genes encodes the two types of chalcone isomerase involved in the biosynthesis of general flavonoids and legume-specific 5-deoxy(iso)flavonoids in Lotus japonicus. Plant Physiol. 131, 941–951. doi: 10.1104/pp.004820
Short, F., Carruthers, T., Dennison, W., Waycott, M. (2007). Global seagrass distribution and diversity: A bioregional model. J. Exp. Mar. Biol. Ecol. 350, 3–20. doi: 10.1016/j.jembe.2007.06.012
Short, F. T., Koch, E. W., Creed, J. C., Magalhães, K. M., Fernandez, E., Gaeckle, J. L. (2006). SeagrassNet monitoring across the Americas: case studies of seagrass decline. Mar. Ecol. 27, 277–289. doi: 10.1111/j.1439-0485.2006.00095.x
Short, F. T., Kosten, S., Morgan, P. A., Malone, S., Moore, G. E. (2016). Impacts of climate change on submerged and emergent wetland plants. Aquat. Bot. 135, 3–17. doi: 10.1016/j.aquabot.2016.06.006
Shvarts, M., Borochov, A., Weiss, D. (1997). Low temperature enhances petunia flower pigmentation and induces chalcone synthase gene expression. Physiologia Plantarum 99, 67–72. doi: 10.1111/j.1399-3054.1997.tb03432.x
Snell, K. R. S., Kokubun, T., Griffiths, H., Convey, P., Hodgson, D. A., Newsham, K. K. (2009). Quantifying the metabolic cost to an Antarctic liverwort of responding to an abrupt increase in UVB radiation exposure. Global Change Biol. 15, 2563–2573. doi: 10.1111/j.1365-2486.2009.01929.x
Stafford, H. A. (1991). Flavonoid evolution: an enzymic approach. Plant Physiol. 96, 680–685. doi: 10.1104/pp.96.3.680
Sullivan, C. N., Koski, M. H. (2021). The effects of climate change on floral anthocyanin polymorphisms. Proc. R. Soc. B: Biol. Sci. 288, 20202693. doi: 10.1098/rspb.2020.2693
Szilárd, A., Sass, L., Deák, Z., Vass, I. (2007). The sensitivity of Photosystem II to damage by UV-B radiation depends on the oxidation state of the water-splitting complex. Biochim. Biophys. Acta (BBA) - Bioenergetics 1767, 876–882. doi: 10.1016/j.bbabio.2006.11.020
Taira, H., Taguchi, S. (2017). Cellular Mycosporine-like amino acids protect photosystem II of the Dinoflagellate Scrippsiella sweeneyae from ultraviolet radiation damage. J. Photochem. Photobiol. B: Biol. 174, 27–34. doi: 10.1016/j.jphotobiol.2017.07.015
Takács, S., Bottomley, H., Andreller, I., Zaradnik, T., Schwarz, J., Bennett, R., et al. (2009). Infrared radiation from hot cones on cool conifers attracts seed-feeding insects. Proc. R. Soc. B: Biol. Sci. 276, 649–655. doi: 10.1098/rspb.2008.0742
Teles, Y., Souza, M., Souza, M. (2018). Sulphated flavonoids: biosynthesis, structures, and biological activities. Molecules 23, 480. doi: 10.3390/molecules23020480
Tevini, M., Braun, J., Fieser, G. (1991). The protective function of the epidermal layer of rye seedlings against UV-B radiation. Photochem. Photobiol. 53, 329–333. doi: 10.1111/j.1751-1097.1991.tb03636.x
Touchette, B. W. (2007). Seagrass-salinity interactions: Physiological mechanisms used by submersed marine angiosperms for a life at sea. J. Exp. Mar. Biol. Ecol. 350, 194–215. doi: 10.1016/j.jembe.2007.05.037
Tropf, S., Lanz, T., Rensing, S. A., Schroder, J., Schroder, G. (1994). Evidence that stilbene synthases have developed from chalcone synthases several times in the course of evolution. J. Mol. Evol. 38, (6). doi: 10.1007/bf00175881
Tuteja, J. H., Clough, S. J., Chan, W. C., Vodkin, L. O. (2004). Tissue-specific gene silencing mediated by a naturally occurring chalcone synthase gene cluster in Glycine max. Plant Cell 16, 819–835. doi: 10.1105/tpc.021352
Unsworth, R. K. F., Cullen-Unsworth, L. C., Jones, B. L. H., Lilley, R. J. (2022). The planetary role of seagrass conservation. Science 377, 609–613. doi: 10.1126/science.abq6923
Van Tussenbroek, B., Monroy, V., Weis, V. (2012). Meso-fauna foraging on seagrass pollen may serve in marine zoophilous pollination. Mar. Ecol. Prog. Ser. 469, 1–6. doi: 10.3354/meps10072
Van Tussenbroek, B. I., Villamil, N., Márquez-Guzmán, J., Wong, R., Monroy-Velázquez, L. V., Solis-Weiss, V. (2016). Experimental evidence of pollination in marine flowers by invertebrate fauna. Nat. Commun. 7, 12980. doi: 10.1038/ncomms12980
Venegas, R. M., Acevedo, J., Treml, E. A. (2023). Three decades of ocean warming impacts on marine ecosystems: A review and perspective. Deep Sea Res. Part II: Topical Stud. Oceanography 212, 105318. doi: 10.1016/j.dsr2.2023.105318
Walia, H., Wilson, C., Condamine, P., Liu, X., Ismail, A. M., Zeng, L., et al. (2005). Comparative Transcriptional Profiling of Two Contrasting Rice Genotypes under Salinity Stress during the Vegetative Growth Stage. Plant Physiol. 139, 822–835. doi: 10.1104/pp.105.065961
Wang, H., Fan, W., Li, H., Yang, J., Huang, J., Zhang, P. (2013). Functional characterization of dihydroflavonol-4-reductase in anthocyanin biosynthesis of purple sweet potato underlies the direct evidence of anthocyanins function against abiotic stresses. PloS One 8, e78484. doi: 10.1371/journal.pone.0078484
Wang, M., Zhao, W., Ma, M., Zhang, D., Wen, Y., Zhong, M., et al. (2022). Intrinsic photosensitivity of the vulnerable seagrass Phyllospadix iwatensis: photosystem II oxygen-evolving complex is prone to photoinactivation. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.792059
Watkinson, J. I., Bowerman, P. A., Crosby, K. C., Hildreth, S. B., Helm, R. A.-O., Winkel, B. S. J. (2018). Identification of MOS9 as an interaction partner for chalcone synthase in the nucleus. PeerJ 5, 2167–8359. doi: 10.7717/peerj.5598
Weng, J. K., Chapple, C. (2010). The origin and evolution of lignin biosynthesis. New Phytol. 187, 273–285. doi: 10.1111/j.1469-8137.2010.03327.x
Weng, J. K., Noel, J. P. (2012). The remarkable pliability and promiscuity of specialized metabolism. Cold Spring Harbor Symp. Quantitative Biol. 77, 309–320. doi: 10.1101/sqb.2012.77.014787
Wissler, L., Codoñer, F. M., Gu, J., Reusch, T. B., Olsen, J. L., Procaccini, G., et al. (2011). Back to the sea twice: identifying candidate plant genes for molecular evolution to marine life. BMC Evolutionary Biol. 11, 8. doi: 10.1186/1471-2148-11-8
Xu, W., Grain, D., Bobet, S., Le Gourrierec, J., Thévenin, J., Kelemen, Z., et al. (2014). Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB–bHLH–WDR complexes and their targets in Arabidopsis seed. New Phytol. 202, 132–144. doi: 10.1111/nph.12620
Xu, Z., Mahmood, K., Rothstein, S. J. (2017). ROS induces anthocyanin production via late biosynthetic genes and anthocyanin deficiency confers the hypersensitivity to ROS-generating stresses in arabidopsis. Plant Cell Physiol. 58, 1364–1377. doi: 10.1093/pcp/pcx073
Xu, Z., Zhou, J., Ren, T., Du, H., Liu, H., Li, Y., et al. (2020). Salt stress decreases seedling growth and development but increases quercetin and kaempferol content in Apocynum venetum. Plant Biol. 22, 813–821. doi: 10.1111/plb.13128
Yuan, Y.-W., Rebocho, A. B., Sagawa, J. M., Stanley, L. E., Bradshaw, H. D. (2016). Competition between anthocyanin and flavonol biosynthesis produces spatial pattern variation of floral pigments between Mimulus species. Proc. Natl. Acad. Sci. 113, 2448–2453. doi: 10.1073/pnas.1515294113
Zayas-Santiago, C. C., Rivas-Ubach, A., Kuo, L.-J., Ward, N. D., Zimmerman, R. C. (2020). Metabolic profiling reveals biochemical pathways responsible for eelgrass response to elevated CO2 and temperature. Sci. Rep. 10, (1). doi: 10.1038/s41598-020-61684-x
Zeng, X. Q., Chow, W. S., Su, L. J., Peng, X. X., Peng, C. L. (2010). Protective effect of supplemental anthocyanins on Arabidopsis leaves under high light. Physiologia Plantarum 138, 215–225. doi: 10.1111/j.1399-3054.2009.01316.x
Zhang, T.-J., Chow, W. S., Liu, X.-T., Zhang, P., Liu, N., Peng, C.-L. (2016). A magic red coat on the surface of young leaves: anthocyanins distributed in trichome layer protect Castanopsis fissa leaves from photoinhibition. Tree Physiol. 36, 1296–1306. doi: 10.1093/treephys/tpw080
Zhang, J., Subramanian, S., Stacey, G., Yu, O. (2009). Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J. 57, 171–183. doi: 10.1111/j.1365-313x.2008.03676.x
Zhang, Q., Zhai, J., Shao, L., Lin, W., Peng, C. (2019). Accumulation of anthocyanins: an adaptation strategy of Mikania micrantha to low temperature in winter. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01049
Zhang, M., Zhang, L., Li, H., Liu, J. (2022). The lack of low temperature tolerance of tropical seagrasses strongly restricts their geographical distribution. Mar. Environ. Res. 173, 105539. doi: 10.1016/j.marenvres.2021.105539
Zhao, W., Yang, X.-Q., Zhang, Q.-S., Tan, Y., Liu, Z., Ma, M.-Y., et al. (2021). Photoinactivation of the oxygen-evolving complex regulates the photosynthetic strategy of the seagrass Zostera marina. J. Photochem. Photobiol. B: Biol. 222, 112259. doi: 10.1016/j.jphotobiol.2021.112259
Zhu, H., Zhang, T. J., Zheng, J., Huang, X. D., Yu, Z. C., Peng, C. L., et al. (2018). Anthocyanins function as a light attenuator to compensate for insufficient photoprotection mediated by nonphotochemical quenching in young leaves of Acmena acuminatissima in winter. Photosynthetica 56, 445–454. doi: 10.1007/s11099-017-0740-1
Keywords: seagrasses, ocean warming, flavonols, chalcone synthase, phenylpropanoids, phenols, chemical ecology, marine angiosperms
Citation: Botes J, Ma X, Chang J, Van de Peer Y and Berger DK (2025) Flavonoids and anthocyanins in seagrasses: implications for climate change adaptation and resilience. Front. Plant Sci. 15:1520474. doi: 10.3389/fpls.2024.1520474
Received: 31 October 2024; Accepted: 30 December 2024;
Published: 28 January 2025.
Edited by:
Hung Manh Nguyen, Ben-Gurion University of the Negev, IsraelReviewed by:
Deepali Singh, Gautam Buddha University, IndiaBuntora Pasaribu, Padjadjaran University, Indonesia
Copyright © 2025 Botes, Ma, Chang, Van de Peer and Berger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yves Van de Peer, eXZwZWVAcHNiLnZpYi11Z2VudC5iZQ==; Dave Kenneth Berger, ZGF2ZS5iZXJnZXJAZmFiaS51cC5hYy56YQ==
 Jana Botes
Jana Botes Xiao Ma
Xiao Ma Jiyang Chang
Jiyang Chang Yves Van de Peer
Yves Van de Peer Dave Kenneth Berger
Dave Kenneth Berger