- 1Guangxi Zhuang and Yao Ethnic Medicine Key Laboratory, Guangxi University of Chinese Medicine, Nanning, China
- 2Nutrition and Bromatology Group, Analytical Chemistry and Food Science, Instituto de Agroecoloxía e Alimentación (IAA) – CITEXVI, Universidade de Vigo, Vigo, Spain
Light provides the necessary energy for plant photosynthesis, which allows plants to produce organic matter and energy conversion, during plant growth and development. Light provides material energy to plants as the basis for cell division and differentiation, chlorophyll synthesis, tissue growth and stomatal movement, and light intensity, photoperiod, and light quality play important roles in these processes. There are several regulatory mechanisms involved in sugar metabolism in plants, and light, as one of the regulatory factors, affects cell wall composition, starch granules, sucrose synthesis, and vascular bundle formation. Similarly, sugar species and genes are affected in the context of light-regulated sugar metabolism. We searched the available databases and found that there are fewer relevant reviews. Therefore, this paper provides a summary of the effects of light on plant growth and development and sugar metabolism, further elaborates on the mechanisms of light effects on plants, and provides some new insights for a better understanding of how plant growth is regulated under different light conditions.
1 Introduction
Light provides a source of energy for plant photosynthesis and acts as an environmental signal that regulates multiple aspects of plant physiological processes. Plants can sense changes in external light conditions through a variety of photoreceptors, such as phytochromes and phototropins, with corresponding signaling pathways to regulate their own growth and development processes. Under low light conditions, the dry matter of the whole plant was reduced, as well as photosynthetic rate, transpiration, stomatal conductance, and stem thickness (Jin et al., 2023; Huang et al., 2024). In addition, light intensity is used as an important variable factor in controlling processes such as plant germination, leaf proliferation and expansion, stomatal development, photosynthesis, and cell division (Bialevich et al., 2022; Bueno and Vendrame, 2024; Xu et al., 2024a). Light quality regulates the whole life cycle of plants through light receptor conduction, and the morphological structure, photosynthesis and organ growth and development of plants will have different effects under different light quality (Wei et al., 2023; Wang et al., 2024b; Wu et al., 2024). Plants can adjust their growth and development by sensing photoperiods for processes such as seed germination, flowering, and fruit ripening, and are also involved in plant responses to adversity to adapt to different seasonal changes (Bao et al., 2024; Chen et al., 2024; Shibaeva et al., 2024).
Sugars as a class of material basis for plant growth and development, its transportation and accumulation process is very complex, subject to the influence and regulation of a variety of factors. Plant sugar metabolism is a series of processes involving the synthesis, catabolism, utilization, and transformation of saccharides in plants, which involve sucrose transport, signaling, starch and cellulose synthesis (Kudo et al., 2023; Li et al., 2023b; Lo Piccolo et al., 2024). In addition, sugar metabolism efficiently utilizes and regulates sugars, participates in plant adaptation to environmental changes, and provides energy for plant growth and development. During plant sugar metabolism, light affects plant sugar metabolism through photosynthesis, sugar signaling, and photoperiodic regulation, and different light conditions cause changes in plant metabolites (Lopes et al., 2024; Zhang et al., 2024). This paper reviews the effects of light on the photosynthetic properties, growth, and sugar metabolism changes in plants, and describes the progress of research on the physiological properties of plants by light, in order to provide theoretical references for the regulation of plant growth by light, and to improve the yield and quality of plants. The relationship between light and them is not clear and could be a direction for research.
2 Importance of light for plant growth and development
There are many properties of light, among which the intensity and quality of light have obvious influence on plants. Light intensity is usually used to measure the brightness of a light source or the intensity of a light beam. According to the different wavelengths, it can be divided into ultraviolet light, visible light and infrared light, of which visible light can be divided into red, orange, yellow, green, blue, indigo and purple colors. For plants, they mainly absorb red and blue light as the basic energy for photosynthesis (Liang et al., 2021).
As an environmental signal, light acts on plants, which is the most important condition among many external environments (light, temperature, gravity, water, minerals, etc.) that affect plant growth and development. On the one hand, light can directly affect plant growth and development by influencing photosynthesis, as far as light intensity is concerned, within a certain range, the rate of photosynthesis is accelerated with the enhancement of light intensity, but after reaching the light saturation point, photosynthesis is no longer affected by light intensity. Gao et al. (2019) screened out the shading conditions that are more favorable for the growth of Aralia elata by studying the law of the influence of light intensity on plant photosynthesis. Qin et al. (2024) studied the effects of different light intensities on the growth, nutritional quality and accumulation of flavonoid components of celery, and the results showed that with the enhancement of light intensity, the celery plant height and aboveground biomass also increased. The effects of different light quality on the rate of photosynthesis and stomatal opening differently (Liu and Van Iersel, 2021; Trivellini et al., 2023; Wang et al., 2024b). On the other hand, light not only serves as the final energy source of green plants metabolism (Wei et al., 2023), but also able to act as a signal to indirectly regulate plant growth and development through the activation of plant photoreceptors, including phytochromes, cryptophylls, and phototropins, which are capable of sensing different wavelengths of light and converting the light signals into biosignals that transmit information through complex pathways (Xu et al., 2021; Spaninks and Offringa, 2023; Jeong et al., 2024). In addition, plant underground root growth is significantly affected by light from plant branches, and long-distance signaling pathways are utilized to modulate root architecture in response to light stimulation (Miotto et al., 2021; Hamon-Josse et al., 2022). Light, as the main source of energy on which plants depend for survival, is the basis for growth, development, and influences plant growth and development by playing a role in seedling differentiation and nutrient growth in plant bodies. As shown in Figure 1, in plants, different light qualities produce different effects on the plant. Red and blue light are most commonly used to regulate the quality of light for plant growth and development, and have similar effects on plants, involving a variety of mechanisms such as the growth of plant roots, stems, leaves, flowers, fruits and seed germination the absorption of water and mineral elements, and the regulation of photosynthetic pigments, stomatal formation, and the process of sugar metabolism (Park and Jeong, 2023; Bueno and Vendrame, 2024; Vatistas et al., 2024; Wang et al., 2024b). Orange light primarily regulates photosynthetic pigments, stomatal formation, and absorption of mineral elements (Leonardos et al., 2019; Samuoliene et al., 2019; Milia et al., 2022). Yellow light affects water absorption and regulates photosynthetic pigments and sugar metabolism processes (Huang et al., 2020; Araújo et al., 2021; Zhuang et al., 2022). Green light regulates photosynthetic pigments, water absorption, stomatal formation and stem and leaf growth (Schenkels et al., 2020; Nakonechnaya et al., 2023; Cossa et al., 2024; Li et al., 2024a). Purple light regulates photosynthetic pigments, water absorption and seed germination (Araújo et al., 2022; Xie et al., 2022; Lee and Nam, 2023).
3 The effect of light on the structure of plant cells and tissues
3.1 Cell division and differentiation
The “cellular theory” suggests that cell division and cell differentiation can determine the growth and development of organismal tissues and play an important role in organ differentiation and morphogenesis, with the mechanism of cell division ensuring the accurate transfer of genetic information between cells and cell differentiation being a key step in the formation of different types of tissues and cells. The process of plant cell division and differentiation occurs throughout the growth and development of plants, and light regulates the growth and development of plants throughout their life cycle, directly regulating the physiological processes of plants through photosynthesis and photomorphogenesis (Gálvez et al., 2020; Deng et al., 2024). In addition, light can act as a signal that affects cell division and differentiation by regulating the activation of plant photoreceptors and signal transduction pathways, causing changes in hormone levels and altering plant developmental, physiological and morphological processes (Deepika et al., 2020; Bao et al., 2024; Hernandez-Castellano et al., 2024).
3.2 Cell wall
Light is necessary for plants to photosynthesize, and the organic matter produced by photosynthesis is an important raw material for cell wall synthesis. Blue light promotes cell wall structural composition and nonstructural carbohydrates by modulating the expression of enzymes and metabolites associated with cell wall structural composition and nonstructural carbohydrates. In the presence of blue light, the cross-sectional area of soybean hypocotyls and xylem increased, the longitudinal length of pith cells decreased, hypocotyl elongation was inhibited, and diameters increased and blue light promotes the establishment of the structural composition of the cell wall through the regulation of enzymes and metabolites related to cell wall structural composition and nonstructural carbohydrates (Wang et al., 2023). In addition, light inhibits wall deposition by affecting the accumulation of cellulose, hemicellulose, and pectin, so that cell wall plasticity and growth are affected, altering the mechanical properties of the cell walls of the tissues in the shoots, with an increase in the thickness of the cell wall as well as the amount of cellulose (Brüggenwirth and Knoche, 2017; Xu et al., 2024b). During inflorescence stem growth, blue light signaling is involved in regulating secondary cell wall biosynthesis in fiber cells. Zhang et al. (2018) found that blue light can promote secondary cell wall thickening in plant stem fiber cells by activating NST-1 transcription factor through MYC2/MYC4 signaling.
3.3 Chloroplast
Light is necessary for plants to photosynthesize and light energy is absorbed by chlorophyll and other pigments in chloroplasts and used to drive the photochemical reactions of photosynthesis. Plants grown under full light conditions suffered from photoinhibition due to overlighting, while plants grown under 25% irradiance experienced light deficiency, and under both over-light and under-light conditions, chloroplasts underwent swelling, irregular seed shape, reduced lamellae, and disintegration of the cyst-like membrane system, which could be attributed to the loss of membrane integrity due to lipid peroxidation and accumulation of reactive oxygen species (ROS), which led to the irregular shape of the chloroplasts, but plant growth was strongest at 75% irradiation due to increased photosynthesis, reduced accumulation of reactive oxygen species, and maintenance of stomata and chloroplast structure (Ma et al., 2015). In addition, red light promotes radial elongation, increases stomatal density, and increases glucose, sucrose, fructose, and starch content in leaves as well as cellulose content in stems in cassava, resulting in shorter fenestrated cells, denser chloroplasts, and starch granules, which may be due to the fact that light quality regulates the accumulation of chlorophyll by altering the expression of the MeLHCA gene, thereby affecting the efficiency of photosynthesis (Zhou et al., 2023). It has been shown that light from the top and side improves the structure and anatomy of leaves, photosynthesis and chlorophyll fluorescence, while side light greatly promotes plant growth, improves chloroplast arrangement, induces a higher density of small stomata, and promotes stomatal opening and photosynthetic efficiency (Yang and Jeong, 2021). Under the condition of insufficient light energy, the chloroplast volume of shade-tolerant peanut increased, the number of chloroplast grana layers increased, and the content of leaf chlorophyll a, chlorophyll b, and chlorophyll a+b increased, which contributed to the acceleration of the transfer of light energy to the vesicles, and the utilization of light energy increased, which indicated that peanut was more adaptive to the shading stress (Wang et al., 2024a).
3.4 Citochondria and vacuole
Mitochondria are the main site of energy metabolism, and miRNA is an important factor in the regulation of gene expression. Under high light conditions, mitochondria enable indigo to have a high level of energy metabolism and promote growth and development by affecting miRNAs (Zhao et al., 2024a). Light affects the function of chloroplasts and mitochondria, the structure of chloroplasts is gradually damaged with the decrease of light intensity, and the structure of mitochondria is degraded or destroyed with the decrease of light intensity, which affects the metabolism of material and energy. The structure of chloroplasts and mitochondria of Viola yedoensis is normal under the light intensity of 6000-8500 lx, and it can obtain more energy to maintain its growth and metabolism (Yang et al., 2020). Light induces morphological changes in the packaging of anthocyanins, the distribution of vacuole compartments and subvesicular compartments, and the diffusion of anthocyanins from inclusions into the vacuole sap, so that vacuoles containing anthocyanins can be fused (Irani and Grotewold, 2005).
3.5 Tissue structure
Investigating the effects of light on plant tissues and organs can help optimize plant growth conditions, thereby improving crop yield and quality in agricultural production and horticultural practices. Studies have shown that controlling light conditions can regulate plant growth and development processes for more efficient production management, that light affects many aspects of plant morphogenesis, growth, development and reproduction, and that light influences the formation of meristematic tissues to lateral organs through the regulation of growth hormones and cytokinins (Yoshida et al., 2011). Blue light increases the production of high quality daughter bulbs in saffron and alters the biomass allocation of bulbs and flowers, whereas under high red light, it stimulates the production of lateral buds, induces vegetative leaf production, and an increase in the blue/red light ratio induces the production of heavier flower bulbs (Moradi et al., 2021). Kalve et al. (2014) found that low light has a specific stimulating effect on petiole formation and expansion, which reduces the rate of increase in leaf area and affects the expansion of leaf thickness. Different light qualities produce different effects on plant tissues; red light accelerates stem elongation, blue light facilitates biomass accumulation as well as leaf and root growth, however a combination of red and blue light increases plant height, stem thickness, total leaf area, stomatal aperture, crown width and total root length (Si et al., 2024; Zhao et al., 2024b).
4 Effect of light on plant physiological function
4.1 Photosynthesis
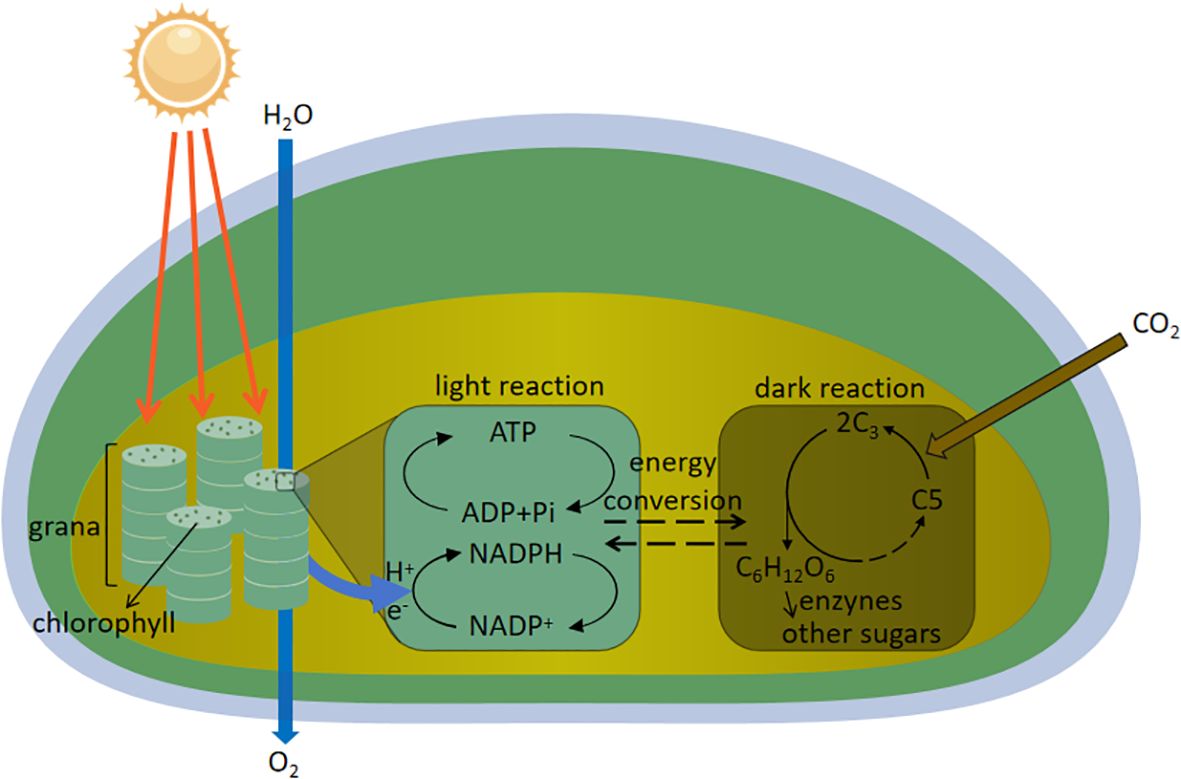
Photosynthesis is the process by which plants use light energy to convert carbon dioxide and water into organic matter and oxygen, and is divided into two main stages: the light reaction and the dark reaction. The light reaction takes place in the chloroplast’s cyst-like membrane, where photosynthetic pigments absorb light energy, excite electrons, and produce ATP and NADPH, while water molecules are broken down to release oxygen. The dark reaction takes place in the stroma of the chloroplasts and utilizes ATP and NADPH produced by the light reaction to convert carbon dioxide to organic matter and produce glucose through energy conversion. And then, under the action of enzymes, other sugars are formed to provide material and energy for plant growth and development, the specific mechanism is shown in Figure 2. Light enhances the photosynthetic activity of plants, maximizes light energy absorption, and affects their response to photosynthetic regulation and environmental stresses (Malekzadeh et al., 2024). Supplemental light improves photosynthetic efficiency and reduces stomatal closure, and increased blue light content better stimulates stomatal opening and promotes photosynthetic electron transfer activity, leading to better photosynthetic rates, and stomatal morphology is highly correlated with leaf photosynthesis and plant development, and is an important determinant of plant photosynthesis and growth (Song et al., 2016; Wang et al., 2024b). Proper combination of red and blue LED lighting improves plant growth and photosynthetic capacity of Mesembryanthemum crystallinum (He et al., 2017). Deeper penetration of light into the canopy improves crop photosynthesis at high light intensities (summer) but not at low light intensities (winter), and internode length and leaf shape effect the vertical distribution of light in the canopy, and in optimizing light absorption and photosynthesis, internode length and leaf shape influence the vertical distribution of light in the canopy, and internodes and narrower leaf length increase crop photosynthesis by 10% (Sarlikioti et al., 2011). Blue light has a positive effect on photosynthesis and carbohydrate production, as well as providing sufficient energy for flowering and growth processes (Li et al., 2024b; Yang et al., 2024).
4.2 Nutrition absorption
4.2.1 Effect on water absorption of plants
Water is one of the key factors for plant growth and survival, helping plants to absorb essential minerals and nutrients from the soil, and the proper use of water can increase the plant’s resistance to disease. Du et al. (2011) investigated the effects of three light environments on plant growth and leaf physiological characteristics, and the relative utilization of water was highest under 30% shade, and the net photosynthetic rate, transpiration rate, stomatal conductance, and chlorophyll content of leaves decreased as shading increased. Nutrient solution replacement based on conductivity and the use of different light emitting diode (LED) spectra, especially red and blue light combinations, can reduce lettuce water and nutrient consumption (Soufi et al., 2023). Interestingly, the growth and development of larch seedlings were significantly affected under light-water coupling, with plant height growth, net photosynthetic rate, stomatal conductance, transpiration rate, chlorophyll A and phenolic compounds content reaching their highest levels under the 60% soil saturated water and 50% shading coupling treatment, whereas more than 75% shading produced inhibitory effects (Jin et al., 2024). Under certain moisture conditions, the biomass of branches, leaves and roots of a plant decreases as light intensity decreases (Zhou et al., 2022).
4.2.2 Effect on the absorption of mineral elements in plants
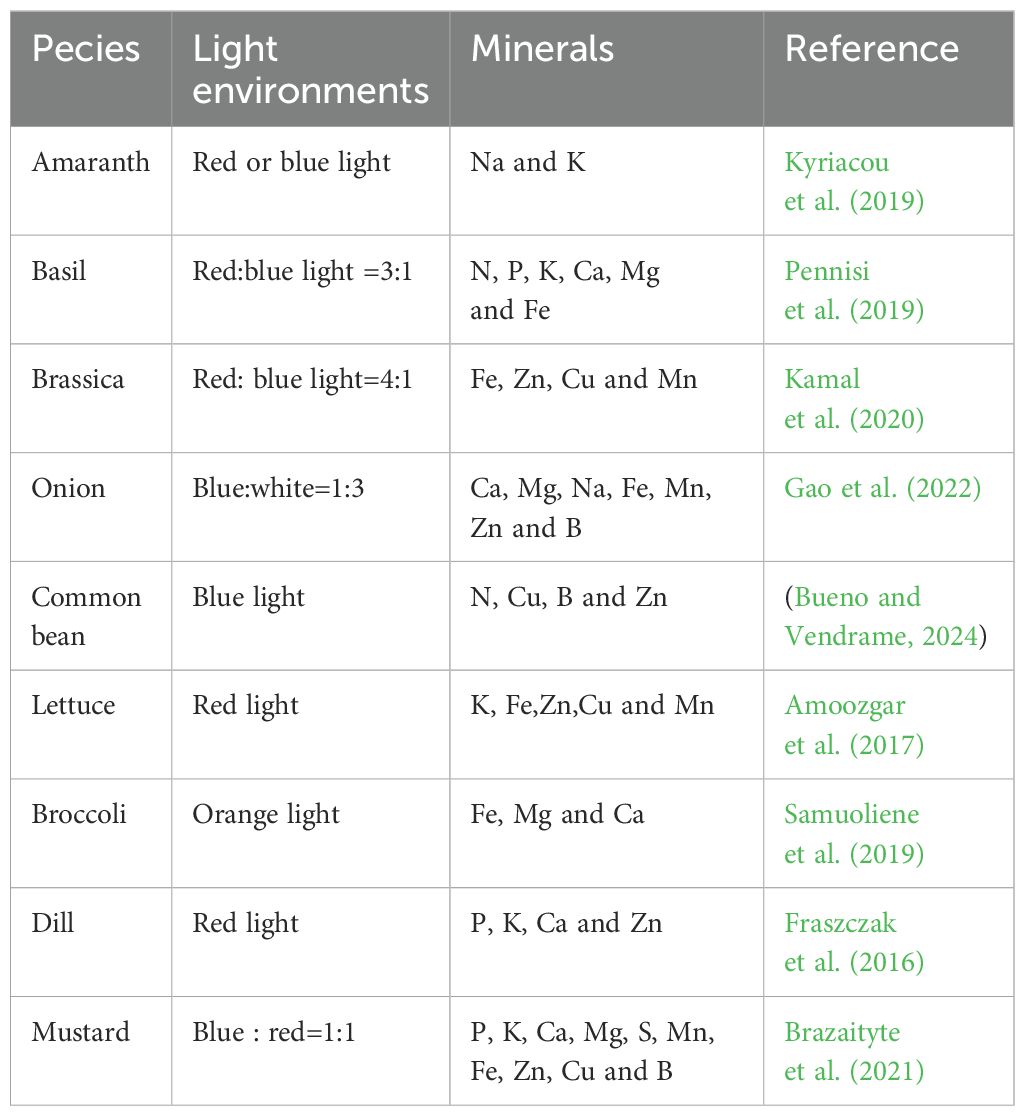
The effects of the light environment on plant mineral composition are multifaceted and include modulation of plant mineral nutrient uptake and growth and development. For example, blue light causes the opening of ion channels located in the cell membrane by controlling the blue light receptor phototropin, which promotes the positive effects of ion transport channels on the uptake and accumulation of mineral nutrients in various plants; whereas red light can promote the uptake of water and minerals by the plant root system, which helps the transportation and utilization of minerals in plants (Kopsell et al., 2015; Suh et al., 2020; An et al., 2023). The effect of light on plant mineral content is shown in Table 1.
4.3 Effect of light on plant reproductive modes
Plant reproduction refers to the methods by which a plant produces offspring, which can be either sexual or asexual. The effect of light on plant reproduction is multifaceted and consists mainly of the regulation of reproductive processes such as flowering and fruiting. Studies have shown that low light adversely affects flowering, fruiting and seed germination of Medicago sativa, as evidenced by delayed flowering, shortened flowering period, decreased flower color, and significant reductions in pollen viability, stigma receptivity, number of flowers, and number and quality of seeds, suggesting that M.sativa reproductive growth is extremely dependent on light intensity, and that M.sativa is a light-demanding species, and that shading can be an important strategy for its reproductive growth (Qin et al., 2022). Cao et al. (2017) evaluated the effects of localized light effects on pollinator flower visitation, pollen and female reproductive resource limitation of yucca populations in two different growing environments (under-forest environment growth and open environment growth), and the results showed that flower visitation increased by 8~11 time in the open environment, and the production of fruits and seeds per flower increased significantly, indicating that localized variations in light conditions affect pollinator activity and affect female reproduction through resource availability. For plants that require sufficient sunlight to maintain their growth and development, plants grown in low light conditions had significantly wider petals than those grown in high light conditions, and plants in low light conditions produced fewer flowers overall and fewer flowers at a time, with light availability affecting a number of floral traits such as number of flowers and amount of nectar, which in turn affects nectar robbing (Fitch and Vandermeer, 2020; Ulum et al., 2020; Ren et al., 2024; Yang et al., 2024).
4.4 Effect of different light conditions on sugar metabolism
As the main source of energy for plants to carry out various life activities, sugars provide energy for plant growth, metabolism and reproduction, and are also involved in regulating plant adaptation to environmental changes. Changes in light conditions affect the process of sugar metabolism in plants, which in turn affects the accumulation of plant sugars. Under high light, the stems of Dendrobium officinale changed from green to red, with a large amount of red pigment accumulated in the epidermal cells, and the anthocyanin level and total polysaccharide content in the stems increased significantly, which was related to the role of DoHY5 in synergistically regulating the biosynthesis of anthocyanin and polysaccharide under high expression (Li et al., 2023a). Similarly, the content of polysaccharides in Changium smyrnioides increased from 6.53% to 10.80% when the relative light intensity was reduced from 100% to 44.84%, and then decreased to 8.79% as the relative light intensity was reduced to 10.56% (Wang et al., 2017). Under medium light intensity (200 μmol m-2s-1) treatment, Bletilla striata plants increased in height and the level of total polysaccharide content, which effectively improved photosynthetic performance and light energy utilization, and promoted carbon metabolism and carbohydrate accumulation, indicating that appropriate light conditions, carbon metabolism-mediated polysaccharide biosynthesis regulation influences B.striata leaves growth (Zhu et al., 2024). By comparing different light qualities and analyzing the dynamic change patterns of fresh weight, dry weight, chlorophyll a and sugar content of Anoectochilus roxburghii, blue light significantly increased the fresh weight, dry weight, and chlorophyll a of A.roxburghiig, while yellow light significantly increased the content of soluble sugars and polysaccharides of A. roxburghii (Wang et al., 2018). Under different light quality treatments, blue light contributed to the accumulation of Ganoderma lucidum polysaccharide content, and G.lucidum polysaccharide content was significantly higher in the bud stage and the opening umbrella stage than in the white light control group (Hao et al., 2010). In addition, under the effect of blue light, it was beneficial to the morphogenesis of Cordyceps militaris, promoting the differentiation and growth and development of the substrate, and promoting the increase of cordycepin and cordyceps polysaccharide content in C.militaris (Wang et al., 2014). Promotion of plant growth as well as sugar accumulation in Phalaenopsis under the effect of red light (Anuchai and Hsieh, 2017). Yang et al. (2017) found that the effects of different light qualities on sugars in tobacco varied, with blue light increasing total soluble sugar and reducing sugar content compared to white light, while red and green light decreased reducing sugar content. Fructose, glucose and sucrose are the main sugars in grape skins, and by comparing the two light qualities acting on the grapes, the blue light treatment reached the highest total sugar content in the skins, followed by the red light treatment of the grape skins (Kondo et al., 2014). The activities of Sucrose Synthase (SUS), Sucrose Phosphate Synthase(SPS) and invertase(INV) were decreased under reduced light intensity, and the changes in light intensity showed a significant positive correlation with the activities of SUS, SPS and INV, and the amount of sugar accumulation, suggesting that the reduced activity of key enzymes for sugar synthesis and conversion in melon fruits caused by reduced light had extremely unfavorable physiological effects on the amount of sugar accumulation (Yang et al., 2019), a result that has been similarly reported in sweet cherry (Chen et al., 2022), bayberry (Shi et al., 2016) and banana (Choudhury et al., 2008) have been similarly reported.
4.5 Regulation of sugar metabolism genes by light
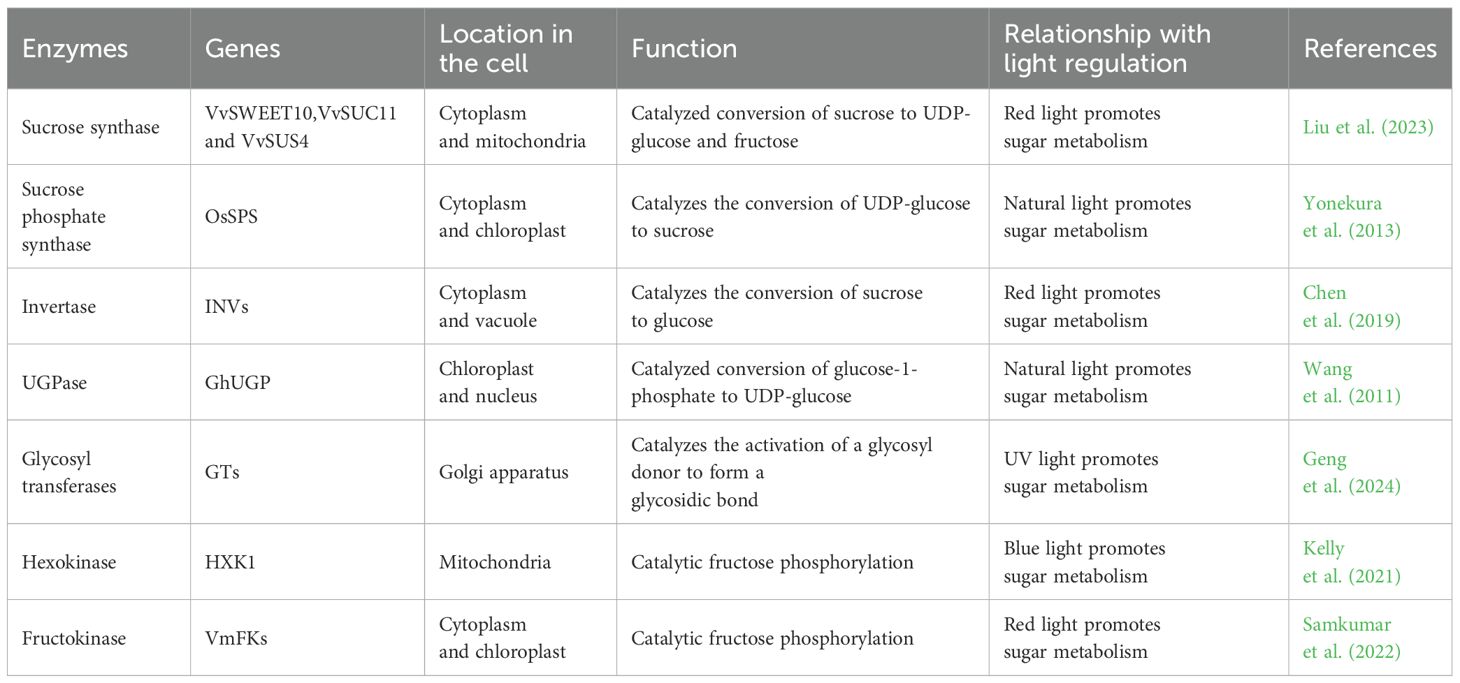
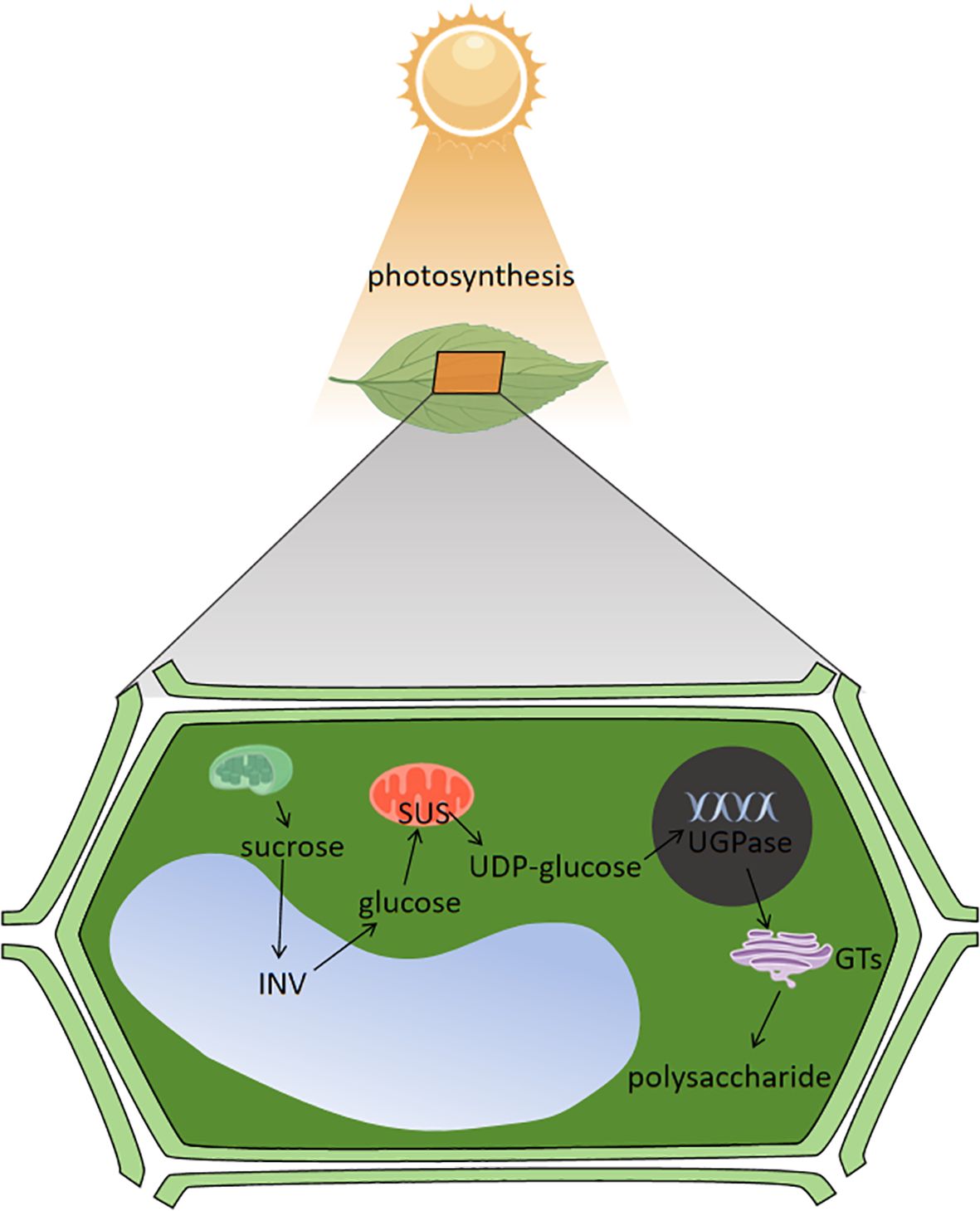
Enzymes play a crucial role in sugar metabolism, and the key enzymes involved in this synthesis process are mainly SUS, SPS, INV, UDP-glucose pyrophosphorylase (UGPase), Glycosyl transferases (GTs), and so on (Davis, 2012; Zabotina et al., 2021). Light conditions also affect sugar synthesis and transformation in plants. SUS, SPS and INV play extremely important roles in the process of sugar synthesis, conversion and accumulation, in which SUS catalyzes the metabolism of sucrose, SPS catalyzes the synthesis of sucrose and INV is the hydrolysis of sucrose into glucose and fructose (Chen et al., 2004).The Cellulose Synthase-Like A subfamily of cellulose-like synthase genes are involved in mannan biosynthesis; Cellulose Synthase-Like D is involved in polysaccharide synthesis, of which is able to promote plant growth and regulate sugar metabolism processes;UDP-arabinopyranose mutase can regulate polysaccharide biosynthesis in plant cell walls; and β-1,3 glucan synthase, UGPase, phosphoenolpyruvate carboxykinase, and deoxyribonuclease I affect polysaccharide synthesis in plants, and the polysaccharide content increases with increased expression of UGPase, β-1,3 glucan synthase, and deoxyribonuclease I, and decreases with increased expression of phosphoenolpyruvate carboxykinase (Ciereszko et al., 2001; Gao et al., 2016; Saqib et al., 2019; Lou et al., 2022). UDP-glucose is the basis for the synthesis of other NDP monosaccharides during sugar metabolism and plays a crucial role in the overall synthesis. At the critical moment of natural product biosynthesis, after the formation of UDP-monosaccharide and GDP-monosaccharide in plant cytoplasm, they are transported to the golgi apparatus by nucleotide sugar transport proteins, and under the action of GTs, these monosaccharides residues are transferred from the active nucleotide sugars to the extended chains to form polysaccharides by dehydration, condensation, and transported to different parts of the body through the form of secretory vacuoles for accumulation (Thibodeaux et al., 2008; Gutmann et al., 2017). Light can act on the enzymes involved in the process of sugar metabolism, by affecting the sugar metabolism produced by photosynthesis in the plant, which are the basic substances for sugar synthesis, and thus affecting the changes in sugar content, the enzymes acted on are shown in Table 2. Under photosynthesis in plants, chloroplasts absorb light energy and generate sucrose through sugar metabolism, sucrose is catalyzed by INV in the vacuole to generate glucose, and glucose is catalyzed by SUS in the mitochondria to generate UDP-glucose, which is then acted upon by UGPase in the cell nucleus, and finally converted into polysaccharides by GTs, and these processes are carried out with the participation of light to ensure the plant’s energy in sugar metabolism supply (Ciereszko et al., 2005; Weiszmann et al., 2018; Stein and Granot, 2019) and the specific mechanism of action is shown in Figure 3.
5 Effect of light on plant resistance
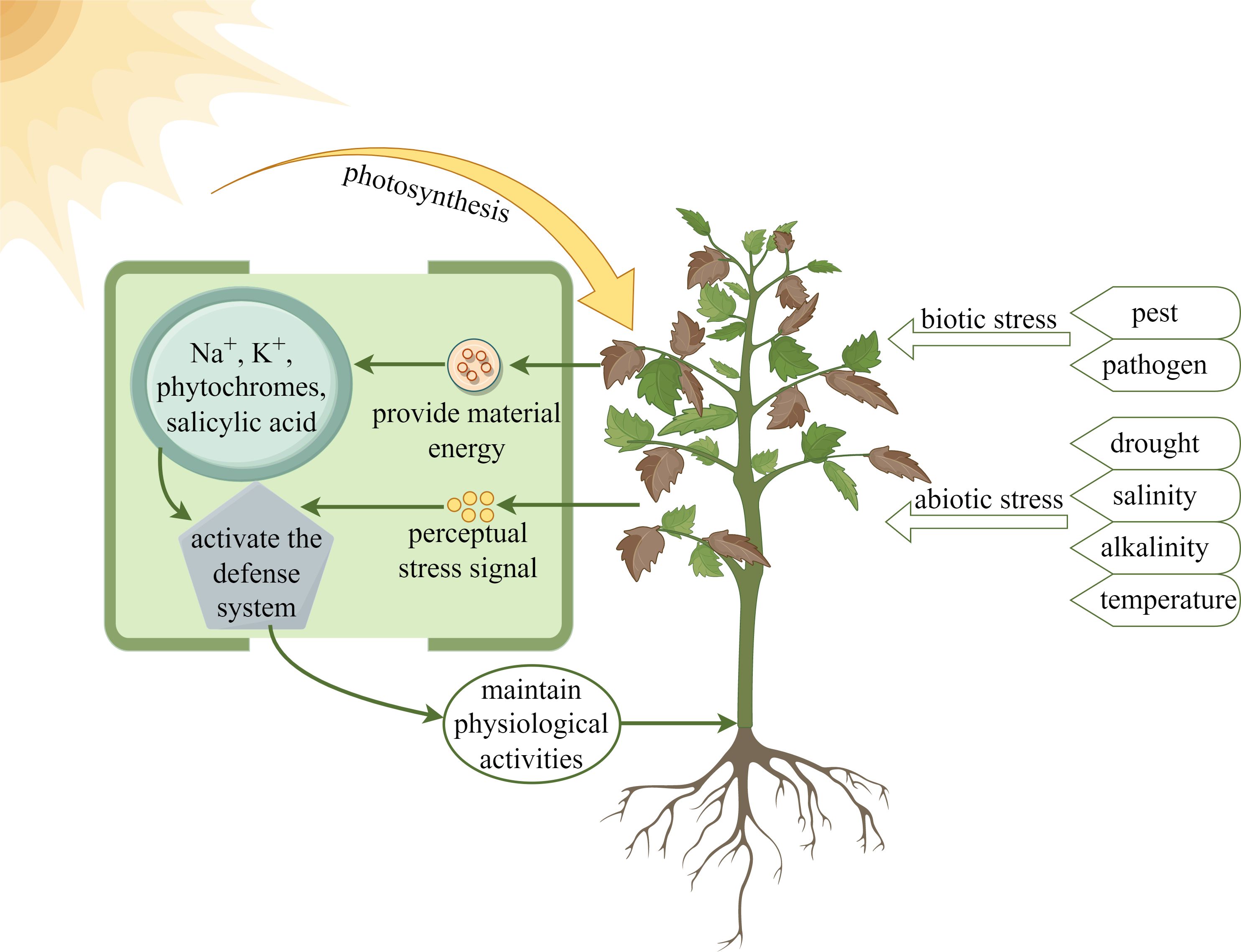
Light, as one of the important environmental factors affecting plant growth and development, can influence and regulate plant growth and development together with other environmental factors, such as water, salinity and temperature (Didaran et al., 2024). In addition, phytochromes in plants can regulate the growth and development of plants from germination to flowering as a way to adapt to the harsh environment (Carvalho et al., 2011; Qiu et al., 2023; Spaninks et al., 2023). As shown in Figure 4, under biotic and abiotic stresses, plants detect stress signals, and through the action of light, absorb light energy to provide them with material energy, such as Na+, K+, phytochromes and salicylic acid, to activate the defense system and maintain physiological activities.
5.1 Drought stress
Any life activities are inseparable from water, green plant water consumption is mainly produced by leaf transpiration, light intensity can directly affect the opening and closing of stomata on the leaves, regulating plant water consumption. It has been shown that high light intensity increases the stomatal index on plant leaves (Zheng and Van Labeke, 2017; Liu et al., 2022; Zhou et al., 2022). In addition, stomata are also related to photoreceptors in plants (Lee, 2021). Kang et al. (2009) found that in Arabidopsis thaliana, cryptochromes and phytochromes jointly mediate the regulation of stomata by light signals, and stomatal development is inhibited when mutant plants are irradiated by light of the corresponding wavelengths, which then affects water transpiration in the plant. PIFs are a class of transcription factors that interact with phytochromes proteins. Gao et al. (2018) investigated the effects of maize ZmPIF1 on drought tolerance in rice and Arabidopsis thaliana, and found that overexpression of ZmPIF1 reduced the stomatal opening and closing and transpiration rate of rice and Arabidopsis thaliana, and reduced the consumption of water to improve drought tolerance. Different light quality treatments were used to explore the drought tolerance mechanism of Cucumis melo. The results showed that green light could regulate stomata, improve water utilization, increase plant height and fresh weight, and reduce leaf stomatal conductance and reactive oxygen species content, suggesting that green light could improve the drought tolerance of C. melo seedlings (Li et al., 2024a).
5.2 Salinity and alkalinity stress
Salinity and alkalinity is a major environmental factor that hinders plant growth and development. Plants have evolved a complex and precise set of mechanisms to adapt to salinity and alkalinity environments, in which the maintenance of Na+, K+ balance is essential for plant growth and development in salinity and alkalinity environments (Sun et al., 2024). Osmoregulation in plants, on the one hand, is through the synthesis of some large molecular weight organic matter, such as sugars, alcohols, etc. to improve the osmotic pressure of the cell, enhance the ability to absorb water in the hypertonic environment, to achieve osmotic balance in the plant cell body, and on the other hand, through the activation of photoreceptors by light to play the role of regulator (Indorf et al., 2007). Yang et al. (2018) found that phytochrome A and phytochrome B negatively regulate salinity tolerance in tobacco. Gavassi et al. (2017) found that tomato phytochrome negatively regulate plant pigment maintenance, growth and development, and osmoprotectant accumulation by studying them. Therefore, screening of phytochrome-deficient mutants is beneficial for plant survival in salinity and alkalinity environments. Under salinity and alkalinity stress, plant photosynthetic efficiency was significantly reduced, whereas supplemental light, especially blue, red and blue/red light, mitigated the unfavorable effects on photosynthetic efficiency and enhanced plant resilience in the face of salinity and alkalinity stress (Malekzadeh et al., 2024).
5.3 Temperature stress
Temperature is a key factor in plant growth and development, both low and high temperatures. Low-temperature stress includes cold damage (0 ~ 15°C) and freezing damage (below 0°C), and prolonged low-temperature environments can lead to slow plant growth and possibly even frostbite and death. Light signaling factors are able to modulate the effect of temperature on plants and improve the cold tolerance of plants (Jiang et al., 2020). Yang et al. (2021) studied the effect of CaPIF8, a phytochrome protein-interacting transcription factor of pepper, on their cold tolerance, and found that CaPIF8 was able to improve cold tolerance of pepper by promoting CBF1 gene expression. Wang et al. (2020) revealed that plants are able to integrate light and temperature signals as a means of adapting to cold environments. In a certain range, raising the temperature increases the activity of enzymes in the plant, while too high a temperature causes damage to the plant such as sunburn and scorching, leading to the denaturation and inactivation of proteins in the plant, which ultimately leads to the death of the plant. Plants can adapt to high temperatures by changing their morphology, including elongation of stems and hypocotyls and thinning of leaves to better adapt to high temperatures (Kerbler and Wigge, 2023). Phytochrome B1 mutant stems grow longer than wild-type stems in high-temperature environments (Gavassi et al., 2017). Song et al. (2017) found that phytochrome B mutant vegetative beads exhibited greater heat tolerance and higher survival rates relative to the wild type.
5.4 Pest resistance
Plants are susceptible to fungi, bacteria, viruses, and phytophagous insects in nature and it surface can serve as a first line of defense to prevent the invasion of pathogens, while some pathogens are able to ignore plants surface and invade the interior of plants, affecting the physiological functions of plants (Clin et al., 2022). After being invaded by pathogenic bacteria, plants activate autoimmune responses through two triggering modes, PAMP-triggered immunity and effector-triggered immunity, which initiate defense signaling pathways such as jasmonic acid, salicylic acid, and ethylene, and activate the expression of related defense genes as a way to achieve the effect of resistance to pests and diseases (Howe and Jander, 2008; Dodds and Rathjen, 2010; Campos et al., 2016; Cortés et al., 2016). In addition, light affects plant resistance to Pseudomonas syringae infection and is able to induce the production of salicylic acid, which promotes stomatal opening and inhibits stomatal closure and water-soaked lesions caused by Pseudomonas syringae infection (Lajeunesse et al., 2023). Gangappa and Kumar (2018) studied the effect of light on the immune response of Arabidopsis thaliana and found that the phytochrome interacting factor PIF4 plays a key role in the expression of Arabidopsis thaliana defense genes and disease resistance. Liu et al. (2019) found that under far-red light, phytochrome can regulate the accumulation of jasmonic acid and participate in the regulation of defense gene expression.
6 Application research
With the continuous development of modern artificial cultivation technology, artificial light cultivation technology has also moved to new heights. Different light conditions, such as intermittent light supply patterns, have a significant impact on plant growth, and have great potential to increase plant yields and promote sustainable development. By studying and understanding the effects of different complementary spectra on plants under different growth conditions, it is possible to modulate the spectra received by plants to improve their resistance. The application of supplemental lighting techniques may increase plant growth during the winter months when daylight hours are short (Bisi et al., 2024). Different wavelengths of light are not only a source of energy for photosynthesis, but also an effective plant growth regulator (Cope and Bugbee, 2013). Multi-color LED lamps are suitable for indoor cultivation of a wide range of experimental plants used for general research, such as Arabidopsis thaliana, Nicotiana Benthamiana, Glycine Max, Solanum tuberosum and Brasilia napus (Janda et al., 2015). Studies have shown that red light increases internode length and decreases leaf length, leaf area and carotenoids in Mentha pulegium; blue light increases leaf area and root length of M.pulegium, and the hydroponic greenhouse cultivation system produces the highest content of chlorophyll, carotenoids and phenolic compounds, and both greenhouse- and field-cultivated M.pulegium outperform the plant factories under different spectral conditions (Roosta et al., 2023). Dramatic differences in Arabidopsis thaliana stem and root elongation, organ formation, and development were observed under red and blue LED conditions, with the red light treatment leading to accelerated stem growth and development and delayed flowering, whereas the blue light treatment led to slower stem growth and development and earlier flowering (Spaninks et al., 2020). Under suitable low light environment (50% ~ 75% of normal light conditions), Betula platyphylla effectively utilizes low light resources and promotes its own growth and development by regulating its morphology, material distribution, photosynthetic rate and antioxidant enzyme system (Zhou et al., 2022). When carrying out planting of Aralia elata seedlings, it is preferable to select understory space with shade intensity less than 75% and apply fertilizers at half the intensity of normal fertilization levels, which can effectively promote seedling growth (Zhang et al., 2022). Extended light/dark cycle improves light use efficiency in tomatoes and sweet peppers compared to conventional photoperiods, resulting in lower production costs (Shibaeva et al., 2024). Optical seed stimulation technology is commercially viable in improving seed germination, crop yield and plant growth, and studies have shown that seed stimulation with different light qualities improves seed germination, with blue light resulting in seed germination rates of up to 180% (Atta et al., 2023). The effects of different light conditions on tissue culture vary, light has an important effect on the proliferation of cultured cells and the differentiation of organs, light intensity affects ectoplasm, cell division, light quality affects the proliferation of healing and cultured tissues as well as the differentiation of organs, while photoperiod affects the differentiation of organ tissues. In order to inhibit the spinning growth of cucumber seedlings under low irradiance and to improve the quality of cucumber seedlings, Zhang et al. (2020) investigated the effects of supplemental LEDs on the morphology and physiological characteristics of cucumber seedlings at different fertility stages under very low irradiance, and the results showed that a red:blue of 1:2 might be the optimal condition to inhibit the spinning growth of cucumber seedlings.
7 Conclusion and future perspectives
Light is the basic energy source for photosynthesis and affects plant energy production and material synthesis. As an important environmental factor for plant growth, rational use of the light environment can improve plant quality and regulate sugar metabolism. Different combinations of light condition treatments will have different photosynthetic and physiological characteristics of plants. During plant growth and development, light affects its stem elongation, leaf size, flower opening time, seed germination, and biomass distribution and accumulation (Sahoo et al., 2021; Atta et al., 2023). Under abiotic stress, plants can respond to changes in the environment by sensing light and preventing cell or tissue damage caused by the stress and light regulates plant hormones, such as jasmonic acid, salicylic acid, and ethylene, which increase the plant’s resistance to pests and microbes (Li et al., 2024a). Therefore, plants can adapt to different lighting environments to promote their own growth, such as photoperiod, light quality, light intensity (Zhang et al., 2023; Shibaeva et al., 2024). There are indeed many technical bottlenecks in the study of light in the natural environment, and we cannot control the state of light in an artificial way. Influenced by the natural environment, light will change at any time, in order to study the change of light, we can artificially create some light energy by artificial the simulated ecological way, and study the light under the simulated ecological environment. However, in the process of artificial simulation, there is a certain difference with natural light, so only some specific light quality, light intensity and photoperiod can be studied to examine individually, so as to verify in the wild, which may be an effective method, and further in-depth research is needed.
However, the application of different light qualities in the field, the control of the photoperiod, and the effects of changes in light intensity on plants are challenges that need to be addressed. Therefore, we believe that by rationally utilizing light conditions, can effectively promote the plant’s mimicry of ecological development, graded utilization of matter and energy, and thus improve the plant’s growth efficiency and ecological benefits. Adaptation of plant photosynthesis to medium-long term light is incorporated into an earth system model using ecological optimization theory to reduce uncertainty in photosynthesis simulations and improve model accuracy (Luo and Keenan, 2020). Plants are universally adapted to medium-long term light and can be induced to increase their photosynthetic capacity and light-energy use efficiency in the medium and long term, leading to more efficient the simulated ecological cultivation under field conditions. In addition, when applying planting in the field, the light intensity is adjusted in combination with plant species and growth characteristics to promote healthy plant growth, and at the same time, by adjusting the ratio of light quality and simulating the natural photoperiod, it promotes or delays the plant’s blossoming and fruition, so as to realize a more accurate the simulated ecological control in the field.
Light regulates the process of sugar metabolism in plants, which helps to improve growth and development and adaptability, and enhances the resistance of plants to environmental stresses. Sugars act as signaling molecules by interacting with other signaling molecules, such as phytohormones, to regulate plant growth and development, which in turn affects plant physiological processes (Mukarram et al., 2023). We believe that by exploring the regulatory mechanisms between the light environment and plant growth and sugar metabolism, it will be an effective economic strategy to guide plant breeding and production practices. With the development of science and technology, future research can be conducted in the direction of light source selection, such as artificial light replenishment technology and the application of light emitting diode, in order to improve the efficiency of light replenishment and plant yield, which will provide more regulatory means for the study of plant growth and development (Ngcobo and Bertling, 2024). Nevertheless, in most of the current studies on the effects of light qualities on plants, red and blue light are the most utilized wavelength regions, so we can develop the mechanism of action on plants under multiple light sources by exploring the effects of more light qualities on the growth and development of plants, such as orange, yellow, and green light, in order for the different light qualities to be well utilized in practice, which needs to be further explored and improved. Many processes of plant growth and development are regulated by phytochromes and phytohormones. Therefore, the effects of interactions between spectral energy and endogenous substances on plant growth will be the focus of subsequent research. Meanwhile, in-depth studies on the molecular mechanisms of different light environments on plant growth and development and changes in sugar metabolism, as well as the effects of synergistic effects of multiple environmental factors on plants, will allow the potential of various plants to be further developed and utilized for applications in agriculture and biomedicine.
Author contributions
WW: Writing – original draft, Conceptualization, Validation, Resources, Methodology, Investigation, Data curation. LC: Writing – original draft, Supervision, Methodology, Investigation, Data curation, Conceptualization. RL: Writing – original draft, Methodology, Investigation, Data curation. SH: Writing – original draft, Methodology, Investigation, Data curation. XL: Writing – original draft, Investigation, Data curation. BH: Writing – original draft, Software, Data curation. HL: Writing – original draft, Methodology, Investigation, Data curation. MZ: Writing – original draft, Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Funding acquisition, Conceptualization. XW: Writing – original draft, Writing – review & editing, Visualization, Validation, Project administration, Investigation, Funding acquisition, Conceptualization. HZ: Writing – original draft, Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financed by the National Natural Science Foundation of China (No. 82060695), Guang Xi Key Laboratory of Zhuang and Yao Ethnic Medicine (Gui Ke Ji Zi (2014) No. 32), Collaborative Innovation Center of Zhuang and Yao Ethnic Medicine (Gui Jiao Ke Yan (2013) No. 20), Guangxi Zhuang Autonomous Region Ethnic Medicine Resources and Application Research Center of Engineering (Gui Fa Gai Gao Ji Han (2020) No. 2605), Guangxi Science and Technology Base and Talent Special Project (Gui Ke AD21238031), Guangxi Key Research and Development Program Project (Gui Ke AB21196016), Guangxi First-class Discipline of Traditional Chinese Medicine (Ethnopharmacology) (Gui Jiao Ke Yan (2018) No. 12), Doctoral Starting up Foundation of Guangxi University of Chinese Medicine in 2018 (2018BS019).
Acknowledgments
Some cartoon elements were obtained from https://www.figdraw.com for mechanism drawing.
Conflict of interest
The authors declare the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amoozgar, A., Mohammadi, A., Sabzalian, M. R. (2017). Impact of light-emitting diode irradiation on photosynthesis, phytochemical composition and mineral element content of lettuce cv. Grizzly. Photosynthetica 55, 85–95. doi: 10.1007/s11099-016-0216-8
An, H. S., Zhang, J. Y., Zhang, L. Q., Li, S. G., Zhou, B. Q., Zhang, X. Y. (2023). Effects of nutrition and light quality on the growth of southern highbush blueberry (Vaccinium corymbosum L.) in an advanced plant factory with artificial lighting (PFAL). Horticulturae 9, 16. doi: 10.3390/horticulturae9020287
Anuchai, J., Hsieh, C. H. (2017). Effect of Change in Light Quality on Physiological Transformation of in vitro Phalaenopsis ‘Fortune Saltzman’ Seedlings during the Growth Period. Hortic. J. 86, 395–402. doi: 10.2503/hortj.MI-151
Araújo, D. X., Rocha, T. T., De Carvalho, A. A., Bertolucci, S. K. V., Medeiros, A. P. R., Ribeiro, F. N. S., et al. (2021). Photon flux density and wavelength influence on growth, photosynthetic pigments and volatile organic compound accumulation in Aeollanthus suaveolens (Catinga-de-mulata) under in vitro conditions. Ind. Crops Prod. 168, 13. doi: 10.1016/j.indcrop.2021.113597
Araújo, R. C., Rodrigues, F. A., Dória, J., Pasqual, M. (2022). In vitro germination of Adenium obesum under the effects of culture medium and light emitting diodes of different colors. Plant Cell Tissue Organ Culture 149, 523–533. doi: 10.1007/s11240-021-02184-2
Atta, B. M., Saleem, M., Abro, S., Rizwan, M., Sarwar, G., Farooq, A. (2023). Enhancement of germination and yield of cotton through optical seed priming: Lab. and diverse environment studies. PloS One 18, 25. doi: 10.1371/journal.pone.0288255
Bao, Y., Liu, X., Feng, C. H., Niu, M. X., Liu, C., Wang, H. L., et al. (2024). Light and light signals regulate growth and development in woody plants. Forests 15, 18. doi: 10.3390/f15030523
Bialevich, V., Zachleder, V., Bisová, K. (2022). The effect of variable light source and light intensity on the growth of three algal species. Cells 11, 16. doi: 10.3390/cells11081293
Bisi, R. B., Bowman, K. D., Albrecht, U. (2024). Increasing sweet orange growth in the winter nursery with supplemental light and heating. Horticulturae 10, 16. doi: 10.3390/horticulturae10090897
Brazaityte, A., Miliauskiene, J., Vastakaite-Kairiene, V., Sutuliene, R., Lauzike, K., Duchovskis, P., et al. (2021). Effect of different ratios of blue and red LED light on Brassicaceae microgreens under a controlled environment. Plants-Basel 10, 20. doi: 10.3390/plants10040801
Brüggenwirth, M., Knoche, M. (2017). Cell wall swelling, fracture mode, and the mechanical properties of cherry fruit skins are closely related. Planta 245, 765–777. doi: 10.1007/s00425-016-2639-7
Bueno, P. M. C., Vendrame, W. A. (2024). Wavelength and light intensity affect macro- and micronutrient uptake, stomata number, and plant morphology of common bean (Phaseolus vulgaris L.). Plants-Basel 13, 14. doi: 10.3390/plants13030441
Campos, M. L., Yoshida, Y., Major, I. T., Ferreira, D. D., Weraduwage, S. M., Froehlich, J. E., et al. (2016). Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat. Commun. 7, 10. doi: 10.1038/ncomms12570
Cao, G. X., Wu, B. X., Xu, X. J., Wang, X., Yang, C. P. (2017). The effects of local variation in light availability on pollinator visitation, pollen and resource limitation of female reproduction in Hosta ventricosa. Botanical Stud. 58, 7. doi: 10.1186/s40529-017-0180-z
Carvalho, R. F., Campos, M. L., Azevedo, R. A. (2011). The role of phytochrome in stress tolerance. J. Integr. Plant Biol. 53, 920–929. doi: 10.1111/j.1744-7909.2011.01081.x
Chen, C. Q., Chen, H. X., Yang, W. L., Li, J., Tang, W. J., Gong, R. G. (2022). Transcriptomic and metabolomic analysis of quality changes during sweet cherry fruit development and mining of related genes. Int. J. Mol. Sci. 23, 25. doi: 10.3390/ijms23137402
Chen, J. W., Patloková, K., Pokluda, R. (2024). The effect of the daily light integral and spectrum on Mesembryanthemum crystallinum L. in an indoor plant production environment. Horticulturae 10, 13. doi: 10.3390/horticulturae10030266
Chen, X. L., Wang, L. C., Li, T., Yang, Q. C., Guo, W. Z. (2019). Sugar accumulation and growth of lettuce exposed to different lighting modes of red and blue LED light. Sci. Rep. 9, 10. doi: 10.1038/s41598-019-43498-8
Chen, J., Zhang, S., Zhang, L. (2004). Sugar transport, metabolism, accumulation and their regulation in fruits. Zhi wu sheng li yu fen zi sheng wu xue xue bao = J. Plant Physiol. Mol. Biol. 30, 1–10.
Choudhury, S. R., Roy, S., Das, R., Sengupta, D. N. (2008). Differential transcriptional regulation of banana sucrose phosphate synthase gene in response to ethylene, auxin, wounding, low temperature and different photoperiods during fruit ripening and functional analysis of banana SPS gene promoter. Planta 229, 207–223. doi: 10.1007/s00425-008-0821-2
Ciereszko, I., Johansson, H., Kleczkowski, L. A. (2001). Sucrose and light regulation of a cold-inducible UDP-glucose pyrophosphorylase gene via a hexokinase-independent and abscisic acid-insensitive pathway in Arabidopsis. Biochem. J. 354, 67–72. doi: 10.1042/0264-6021:3540067
Ciereszko, I., Johansson, H., Kleczkowski, L. A. (2005). Interactive effects of phosphate deficiency, sucrose and light/dark conditions on gene expression of UDP-glucose pyrophosphory lase in Arabidopsis. J. Plant Physiol. 162, 343–353. doi: 10.1016/j.jplph.2004.08.003
Clin, P., Grognard, F., Andrivon, D., Mailleret, L., Hamelin, F. M. (2022). Host mixtures for plant disease control: Benefits from pathogen selection and immune priming. Evol. Appl. 15, 967–975. doi: 10.1111/eva.13386
Cope, K. R., Bugbee, B. (2013). Spectral effects of three types of white light-emitting diodes on plant growth and development: absolute versus relative amounts of blue light. Hortscience 48, 504–509. doi: 10.21273/hortsci.48.4.504
Cortés, L. E., Weldegergis, B. T., Boccalandro, H. E., Dicke, M., Ballaré, C. L. (2016). Trading direct for indirect defense? Phytochrome B inactivation in tomato attenuates direct anti-herbivore defenses whilst enhancing volatile-ediated attraction of predators. New Phytol. 212, 1057–1071. doi: 10.1111/nph.14210
Cossa, M. C. V., Rocha, J. P. M., De Assis, R. M. A., Leite, J. J. F., Texeira, L. F., Bertolucci, S. K. V., et al. (2024). Impact of photon flux density and light spectral quality on biomass production and arbutin compound accumulation in Origanum majorana L. plantlets. Plant Cell Tissue Organ Culture 156, 16. doi: 10.1007/s11240-023-02658-5
Davis, J. K. (2012). Combining polysaccharide biosynthesis and transport in a single enzyme: dual-function cell wall glycan synthases. Front. Plant Sci. 3. doi: 10.3389/fpls.2012.00138
Deepika, Ankit, Sagar, S., Singh, A. (2020). Dark-induced hormonal regulation of plant growth and development. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.581666
Deng, Q. Q., Du, P. X., Gangurde, S. S., Hong, Y. B., Xiao, Y., Hu, D. X., et al. (2024). ScRNA-seq reveals dark- and light-induced differentially expressed gene atlases of seedling leaves in Arachis hypogaea L. Plant Biotechnol. J. 22, 1848–1866. doi: 10.1111/pbi.14306
Didaran, F., Kordrostami, M., Ghasemi-Soloklui, A. A., Pashkovskiy, P., Kreslavski, V., Kuznetsov, V., et al. (2024). The mechanisms of photoinhibition and repair in plants under high light conditions and interplay with abiotic stressors. J. Photochem. Photobiol. B Biol. 259, 15. doi: 10.1016/j.jphotobiol.2024.113004
Dodds, P. N., Rathjen, J. P. (2010). Plant immunity: towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 11, 539–548. doi: 10.1038/nrg2812
Du, S. N., Bai, G. S., Liang, Y. L. (2011). Effects of soil moisture content and light intensity on the plant growth and leaf physiological characteristics of squash. Ying Yong Sheng Tai Xue Bao 22, 1101–1106. doi: 10.13287/j.1001-9332.2011.0113
Fitch, G., Vandermeer, J. H. (2020). Light availability influences the intensity of nectar robbery and its effects on reproduction in a tropical shrub via multiple pathways. Am. J. Bot. 107, 1635–1644. doi: 10.1002/ajb2.1559
Fraszczak, B., Gasecka, M., Golcz, A., Zawirska-Wojtasiak, R. (2016). The effect of radiation of LED modules on the growth of dill (Anethum graveolens L.). Open Life Sci. 11, 61–70. doi: 10.1515/biol-2016-0008
Gálvez, A., Albacete, A., Del Amor, F. M., López-Marín, J. (2020). The use of red shade nets improves growth in salinized pepper (Capsicum annuum L.) plants by regulating their ion homeostasis and hormone balance. Agronomy-Basel 10, 19. doi: 10.3390/agronomy10111766
Gangappa, S. N., Kumar, S. V. (2018). DET1 and COP1 modulate the coordination of growth and immunity in response to key seasonal signals in Arabidopsis. Cell Rep. 25, 29–2+. doi: 10.1016/j.celrep.2018.08.096
Gao, F., Cao, X. F., Si, J. P., Chen, Z. Y., Duan, C. L. (2016). Characterization of the alkaline/neutral invertase gene in Dendrobium officinale and its relationship with polysaccharide accumulation. Genet. Mol. Res. 15, 8. doi: 10.4238/gmr.15027647
Gao, Z., Khalid, M., Jan, F., Saeed Ur, R., Jiang, X., Yu, X. (2019). Effects of light-regulation and intensity on the growth, physiological and biochemical properties of Aralia elata (miq.) seedlings. South Afr. J. Bot. 121, 456–462. doi: 10.1016/j.sajb.2018.12.008
Gao, S., Kong, Y. W., Lv, Y., Cao, B. L., Chen, Z. J., Xu, K. (2022). Effect of different LED light quality combination on the content of vitamin C, soluble sugar, organic acids, amino acids, antioxidant capacity and mineral elements in green onion (Allium fistulosum L.). Food Res. Int. 156, 8. doi: 10.1016/j.foodres.2022.111329
Gao, Y., Wu, M. Q., Zhang, M. J., Jiang, W., Ren, X. Y., Liang, E. X., et al. (2018). A maize phytochrome-interacting factors protein ZmPIF1 enhances drought tolerance by inducing stomatal closure and improves grain yield in Oryza sativa. Plant Biotechnol. J. 16, 1375–1387. doi: 10.1111/pbi.12878
Gavassi, M. A., Monteiro, C. C., Campos, M. L., Melo, H. C., Carvalho, R. F. (2017). Phytochromes are key regulators of abiotic stress responses in tomato. Scientia Hortic. 222, 126–135. doi: 10.1016/j.scienta.2017.04.035
Geng, D. J., Sun, Y. Q., Liu, S. Z., Chen, W., Gao, F., Bai, Y., et al. (2024). Study on synthesis and regulation of PPVI and PPVII in Paris polyphylla with UV. Metabolites 14, 14. doi: 10.3390/metabo14080427
Gutmann, A., Lepak, A., Diricks, M., Desmet, T., Nidetzky, B. (2017). Glycosyltransferase cascades for natural product glycosylation: Use of plant instead of bacterial sucrose synthases improves the UDP-glucose recycling from sucrose and UDP. Biotechnol. J. 12, 10. doi: 10.1002/biot.201600557
Hamon-Josse, M., Villaécija-Aguilar, J. A., Ljung, K., Leyser, O., Gutjahr, C., Bennett, T. (2022). KAI2 regulates seedling development by mediating light-induced remodelling of auxin transport. New Phytol. 235, 126–140. doi: 10.1111/nph.18110
Hao, J., Chen, X., Lan, J. (2010). Effect of light quality on growth and polysaccharides content of Ganoderma lucidum. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J. Chin. materia Med. 35, 2242–2245.
He, J., Qin, L., Chong, E. L. C., Choong, T. W., Lee, S. K. (2017). Plant Growth and Photosynthetic Characteristics of Mesembryanthemum crystallinum Grown Aeroponically under Different Blue-and Red-LEDs. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00361
Hernandez-Castellano, S., Galaz-Avalos, R. M., Loyola-Vargas, V. M., De-La-Pena, C. (2024). Protocol for generating Arabidopsis thaliana cell suspension cultures under different light conditions. Methods Mol. Biol. (Clifton N.J.) 2827, 145–153. doi: 10.1007/978-1-0716-3954-2_9
Howe, G. A., Jander, G. (2008). Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59, 41–66. doi: 10.1146/annurev.arplant.59.032607.092825
Huang, L. P., Liao, M. C., Liao, H. X., Liu, Z. F., Cai, H. Y., Zhou, W. M., et al. (2024). High phosphorus availability and low light intensity reduce the competitive ability of the invasive plant Chromolaena odorata in tropical coral islands. Biol. Invasions 26, 471–487. doi: 10.1007/s10530-023-03186-1
Huang, L., Xiao, Y., Ran, J., Wei, L., Li, Z., Li, Y., et al. (2020). Drought tolerance of faba bean (Vicia faba L.) can be improved by specific LED light wavelengths. Photosynthetica 58, 1040–1052. doi: 10.32615/ps.2020.052
Indorf, M., Cordero, J., Neuhaus, G., Rodríguez-Franco, M. (2007). Salt tolerance (STO), a stress-related protein, has a major role in light signalling. Plant J. 51, 563–574. doi: 10.1111/j.1365-313X.2007.03162.x
Irani, N. G., Grotewold, E. (2005). Light-induced morphological alteration in anthocyanin-accumulating vacuoles of maize cells. BMC Plant Biol. 5, 15. doi: 10.1186/1471-2229-5-7
Janda, M., Navrátil, O., Haisel, D., Jindrichová, B., Fousek, J., Burketová, L., et al. (2015). Growth and stress response in Arabidopsis thaliana, Nicotiana benthamiana, Glycine max, Solanum tuberosum and Brassica napus cultivated under polychromatic LEDs. Plant Methods 11, 14. doi: 10.1186/s13007-015-0076-4
Jeong, S. J., Niu, G. H., Zhen, S. Y. (2024). Far-red light and temperature interactively regulate plant growth and morphology of lettuce and basil. Environ. Exp. Bot. 218, 11. doi: 10.1016/j.envexpbot.2023.105589
Jiang, B. C., Shi, Y. T., Peng, Y., Jia, Y. X., Yan, Y., Dong, X. J., et al. (2020). Cold-induced CBF-PIF3 interaction enhances freezing tolerance by stabilizing the phyB thermosensor in Arabidopsis. Mol. Plant 13, 894–906. doi: 10.1016/j.molp.2020.04.006
Jin, L., Song, X. Q., Shi, Y., Guan, X., Tang, H. M., Huang, H. Y., et al. (2024). Photosynthetic acclimation of larch to the coupled effects of light intensity and water deficit in regions with changing water availability. Plants-Basel 13, 16. doi: 10.3390/plants13141891
Jin, Z. Q., Wang, Y. D., Si, C. C., Kumar, S., Nie, L. X., Khan, M. N. (2023). Effects of shading intensities on the yield and contents of anthocyanin and soluble sugar in tubers of purple sweet potato. Crop Sci. 63, 3013–3024. doi: 10.1002/csc2.21076
Kalve, S., Fotschki, J., Beeckman, T., Vissenberg, K., Beemster, G. T. S. (2014). Three-dimensional patterns of cell division and expansion throughout the development of Arabidopsis thaliana leaves. J. Exp. Bot. 65, 6385–6397. doi: 10.1093/jxb/eru358
Kamal, K. Y., Khodaeiaminjan, M., El-Tantawy, A. A., Moneim, D. A., Salam, A. A., Ash-Shormillesy, S., et al. (2020). Evaluation of growth and nutritional value of Brassica microgreens grown under red, blue and green LEDs combinations. Physiol. Plant. 169, 625–638. doi: 10.1111/ppl.13083
Kang, C. Y., Lian, H. L., Wang, F. F., Huang, J. R., Yang, H. Q. (2009). Cryptochromes, phytochromes, and COP1 regulate light-controlled stomatal development in Arabidopsis. Plant Cell 21, 2624–2641. doi: 10.1105/tpc.109.069765
Kelly, G., Brandsma, D., Egbaria, A., Stein, O., Doron-Faigenboim, A., Lugassi, N., et al. (2021). Guard cells control hypocotyl elongation through HXK1, HY5, and PIF4. Commun. Biol. 4, 14. doi: 10.1038/s42003-021-02283-y
Kerbler, S. M., Wigge, P. A. (2023). Temperature sensing in plants. Annu. Rev. Plant Biol. 74, 341–366. doi: 10.1146/annurev-arplant-102820-102235
Kondo, S., Tomiyama, H., Rodyoung, A., Okawa, K., Ohara, H., Sugaya, S., et al. (2014). Abscisic acid metabolism and anthocyanin synthesis in grape skin are affected by light emitting diode (LED) irradiation at night. J. Plant Physiol. 171, 823–829. doi: 10.1016/j.jplph.2014.01.001
Kopsell, D. A., Sams, C. E., Morrow, R. C. (2015). Blue wavelengths from LED lighting increase nutritionally important metabolites in specialty crops. Hortscience 50, 1285–1288. doi: 10.21273/hortsci.50.9.1285
Kudo, S. N., Bello, C. C. M., Artins, A., Caldana, C., Satake, A. (2023). Assessing the impacts of genetic defects on starch metabolism in Arabidopsis plants using the carbon homeostasis model. J. R. Soc. Interface 20, 12. doi: 10.1098/rsif.2023.0426
Kyriacou, M. C., El-Nakhel, C., Pannico, A., Graziani, G., Soteriou, G. A., Giordano, M., et al. (2019). Genotype-specific modulatory effects of select spectral bandwidths on the nutritive and phytochemical composition of microgreens. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01501
Lajeunesse, G., Roussin-Léveillée, C., Boutin, S., Fortin, E., Laforest-Lapointe, I., Moffett, P. (2023). Light prevents pathogen-induced aqueous microenvironments via potentiation of salicylic acid signaling. Nat. Commun. 14, 13. doi: 10.1038/s41467-023-36382-7
Lee, J. S. (2021). The stomatal openings occurred from blue light photoreceptors mediated signal transduction pathway may be enhanced by a blue light stimulated photosynthesis. Russian J. Plant Physiol. 68, 818–827. doi: 10.1134/s1021443721050095
Lee, J. H., Nam, S. Y. (2023). Vegetative propagation of six pachyphytum species as influenced by different LED light qualities. Hortic. Sci. Technol. 41, 237–249. doi: 10.7235/hort.20230022
Leonardos, E. D., Ma, X., Lanoue, J., Grodzinski, B. (2019). Leaf and whole-plant gas exchange and water-use efficiency of chrysanthemums under HPS and LEDs during the vegetative and flower-induction stages. Can. J. Plant Sci. 99, 639–653. doi: 10.1139/cjps-2018-0245
Li, H. B., Feng, B. H., Li, J. C., Fu, W. M., Wang, W. T., Chen, T. T., et al. (2023b). RGA1 alleviates low-light-repressed pollen tube elongation by improving the metabolism and allocation of sugars and energy. Plant Cell Environ. 46, 1363–1383. doi: 10.1111/pce.14547
Li, Y. T., Qi, Z. B., Ren, X. Y., Li, Y. C., Zhang, N. B., Liu, Q. (2024b). Effects of red and blue light on red clover (Trifolium pratense L.) growth and secondary metabolism. Plant Growth Regul. 20, 1087–1106. doi: 10.1007/s10725-024-01161-x
Li, D. X., Ye, G. Y., Li, J., Lai, Z. Q., Ruan, S. Y., Qi, Q., et al. (2023a). High light triggers flavonoid and polysaccharide synthesis through DoHY5-dependent signaling in Dendrobium officinale. Plant J. 115, 1114–1133. doi: 10.1111/tpj.16284
Li, X., Zhao, S. W., Cao, Q. Q., Qiu, C., Yang, Y. Y., Zhang, G. Z., et al. (2024a). Effect of Green Light Replacing Some Red and Blue Light on Cucumis melo under Drought Stress. Int. J. Mol. Sci. 25, 19. doi: 10.3390/ijms25147561
Liang, Y., Kang, C. Q., Kaiser, E., Kuang, Y., Yang, Q. C., Li, T. (2021). Red/blue light ratios induce morphology and physiology alterations differently in cucumber and tomato. Scientia Hortic. 281, 9. doi: 10.1016/j.scienta.2021.109995
Liu, B. S., Meng, C., Wang, X. R., Luo, J., Zhao, Y. (2022). Effects of light intensity on morphological structure and physiological characteristics of Gleditsia sinensis seedlings. Russian J. Plant Physiol. 69, 11. doi: 10.1134/s1021443722602506
Liu, J., Van Iersel, M. W. (2021). Photosynthetic physiology of blue, green, and red light: light intensity effects and underlying mechanisms. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.619987
Liu, Y., Wei, H. B., Ma, M. D., Li, Q. Q., Kong, D. X., Sun, J., et al. (2019). Arabidopsis FHY3 and FAR1 regulate the balance between growth and defense responses under shade conditions. Plant Cell 31, 2089–2106. doi: 10.1105/tpc.18.00991
Liu, M. L., Zhao, Y., Fan, P. G., Kong, J. H., Wang, Y. J., Xu, X. B., et al. (2023). Grapevine plantlets respond to different monochromatic lights by tuning photosynthesis and carbon allocation. Hortic. Res. 10, 13. doi: 10.1093/hr/uhad160
Lopes, F. L., Formosa-Jordan, P., Malivert, A., Margalha, L., Confraria, A., Feil, R., et al. (2024). Sugar signaling modulates SHOOT MERISTEMLESS expression and meristem function in Arabidopsis. Proc. Natl. Acad. Sci. United States America 121, e2408699121. doi: 10.1073/pnas.2408699121
Lo Piccolo, E., Torre, S., Lauria, G., De Quattro, C., Sebastiani, F., Guidi, L., et al. (2024). LED streetlamps alter tree architecture, downregulate the photosynthetic process and alter the sugar metabolism of Populus alba L. Environ. Exp. Bot. 226, 11. doi: 10.1016/j.envexpbot.2024.105861
Lou, H. Y., Tucker, M. R., Shirley, N. J., Lahnstein, J., Yang, X. J., Ma, C., et al. (2022). The cellulose synthase-like F3 (CslF3) gene mediates cell wall polysaccharide synthesis and affects root growth and differentiation in barley. Plant J. 110, 1681–1699. doi: 10.1111/tpj.15764
Luo, X. Z., Keenan, T. F. (2020). Global evidence for the acclimation of ecosystem photosynthesis to light. Nat. Ecol. Evol. 4, 1351–135+. doi: 10.1038/s41559-020-1258-7
Ma, X. H., Song, L. L., Yu, W. W., Hu, Y. Y., Liu, Y., Wu, J. S., et al. (2015). Growth, physiological, and biochemical responses of Camptotheca acuminata seedlings to different light environments. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00321
Malekzadeh, M. R., Roosta, H. R., Esmaeilizadeh, M., Dabrowski, P., Kalaji, H. M. (2024). Improving strawberry plant resilience to salinity and alkalinity through the use of diverse spectra of supplemental lighting. BMC Plant Biol. 24, 19. doi: 10.1186/s12870-024-04984-y
Milia, M., Corrias, F., Addis, P., Zitelli, G. C., Cicchi, B., Torzillo, G., et al. (2022). Influence of different light sources on the biochemical composition of Arthrospira spp. Grown in model systems. Foods 11, 13. doi: 10.3390/foods11030399
Miotto, Y. E., Da Costa, C. T., Offringa, R., Kleine-Vehn, J., Maraschin, F. D. (2021). Effects of light intensity on root development in a D-root growth system. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.778382
Moradi, S., Kafi, M., Aliniaeifard, S., Salami, S. A., Shokrpour, M., Pedersen, C., et al. (2021). Blue Light Improves Photosynthetic Performance and Biomass Partitioning toward Harvestable Organs in Saffron (Crocus sativus L.). Cells 10, 23. doi: 10.3390/cells10081994
Mukarram, M., Ali, J., Dadkhah-Aghdash, H., Kurjak, D., Kacik, F., Durkovic, J. (2023). Chitosan-induced biotic stress tolerance and crosstalk with phytohormones, antioxidants, and other signalling molecules. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1217822
Nakonechnaya, O. V., Kholin, A. S., Subbotin, E. P., Burkovskaya, E. V., Khrolenko, Y. A., Gafitskaya, I. V., et al. (2023). The influence of LED lights of different spectra on the development of Lactuca sativa. Biol. Bull. 50, 371–378. doi: 10.1134/s1062359023700176
Ngcobo, B. L., Bertling, I. (2024). An overview of the recent developments and current status on the preharvest application of LED technology in controlled environment agriculture. J. Hortic. Sci. Biotechnol. 99, 531–538. doi: 10.1080/14620316.2024.2341906
Park, Y. G., Jeong, B. R. (2023). Shift in the light quality of night interruption affects flowering and morphogenesis of Petunia hybrida. Plants-Basel 12, 12. doi: 10.3390/plants12102049
Pennisi, G., Blasioli, S., Cellini, A., Maia, L., Crepaldi, A., Braschi, I., et al. (2019). Unraveling the role of red : blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00305
Qin, Y. M., Liu, X. X., Li, C. Y., Chu, Q. W., Cheng, S. B., Su, L. H., et al. (2024). Effect of light intensity on celery growth and flavonoid synthesis. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1326218
Qin, F. F., Shen, Y. X., Li, Z. H., Qu, H., Feng, J. X., Kong, L. N., et al. (2022). Shade delayed flowering phenology and decreased reproductive growth of Medicago sativa L. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.835380
Qiu, X., Sun, G. H., Liu, F., Hu, W. M. (2023). Functions of plant phytochrome signaling pathways in adaptation to diverse stresses. Int. J. Mol. Sci. 24, 21. doi: 10.3390/ijms241713201
Ren, C., Jiang, L. M., Chen, W. Z., Wang, Z. Y. (2024). Effect of Different Post-Flowering Photoperiods on Main Agronomic Traits of Strawberry (Fragaria x ananassa Duch. cv. Akihime). Agronomy-Basel 14, 10. doi: 10.3390/agronomy14092039
Roosta, H. R., Samadi, A., Bikdeloo, M. (2023). Different cultivation systems and foliar application of calcium nanoparticles affect the growth and physiological characteristics of pennyroyal (Mentha pulegium L.). Sci. Rep. 13, 13. doi: 10.1038/s41598-023-47855-6
Sahoo, G. R., Swamy, S. L., Mishra, A., Thakur, T. K. (2021). Effect of seed source, light, and nitrogen levels on biomass and nutrient allocation pattern in seedlings of Pongamia pinnata. Environ. Sci. pollut. Res. 28, 15005–15020. doi: 10.1007/s11356-020-11734-8
Samkumar, A., Karppinen, K., Dhakal, B., Martinussen, I., Jaakola, L. (2022). Insights into sugar metabolism during bilberry (Vaccinium myrtillus L.) fruit development. Physiol. Plant. 174, 14. doi: 10.1111/ppl.13657
Samuoliene, G., Brazaityte, A., Virsile, A., Miliauskiene, J., Vastakaite-Kairiene, V., Duchovskis, P. (2019). Nutrient levels in Brassicaceae microgreens increase under tailored light-emitting diode spectra. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01475
Saqib, A., Scheller, H. V., Fredslund, F., Welner, D. H. (2019). Molecular characteristics of plant UDP-arabinopyranose mutases. Glycobiology 29, 839–846. doi: 10.1093/glycob/cwz067
Sarlikioti, V., De Visser, P. H. B., Buck-Sorlin, G. H., Marcelis, L. F. M. (2011). How plant architecture affects light absorption and photosynthesis in tomato: towards an ideotype for plant architecture using a functional-structural plant model. Ann. Bot. 108, 1065–1073. doi: 10.1093/aob/mcr221
Schenkels, L., Saeys, W., Lauwers, A., De Proft, M. P. (2020). Green light induces shade avoidance to alter plant morphology and increases biomass production in Ocimum basilicum L. Scientia Hortic. 261, 6. doi: 10.1016/j.scienta.2019.109002
Shi, L. Y., Cao, S. F., Shao, J. R., Chen, W., Yang, Z. F., Zheng, Y. H. (2016). Chinese bayberry fruit treated with blue light after harvest exhibit enhanced sugar production and expression of cryptochrome genes. Postharvest Biol. Technol. 111, 197–204. doi: 10.1016/j.postharvbio.2015.08.013
Shibaeva, T. G., Sherudilo, E. G., Ikkonen, E., Rubaeva, A. A., Levkin, I. A., Titov, A. F. (2024). Effects of extended light/dark cycles on solanaceae plants. Plants-Basel 13, 11. doi: 10.3390/plants13020244
Si, C. C., Lin, Y., Luo, S. M., Yu, Y. H., Liu, R. Q., Naz, M., et al. (2024). Effects of LED light quality combinations on growth and leaf colour of tissue cultureGenerated plantlets in Sedum rubrotinctum. Hortic. Sci. Technol. 42, 53–67. doi: 10.7235/hort.20240005
Song, Y., Jiang, C. Y., Gao, L. H. (2016). Polychromatic supplemental lighting from underneath canopy is more effective to enhance tomato plant development by improving leaf photosynthesis and stomatal regulation. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01832
Song, J. Y., Liu, Q. J., Hu, B. R., Wu, W. J. (2017). Photoreceptor PhyB involved in Arabidopsis temperature perception and heat-tolerance formation. Int. J. Mol. Sci. 18, 13. doi: 10.3390/ijms18061194
Soufi, H. R., Roosta, H. R., Hamidpour, M. (2023). The plant growth, water and electricity consumption, and nutrients uptake are influenced by different light spectra and nutrition of lettuce. Sci. Rep. 13, 15. doi: 10.1038/s41598-023-48284-1
Spaninks, K., Lamers, G., Van Lieshout, J., Offringa, R. (2023). Light quality regulates apical and primary radial growth of Arabidopsis thaliana and Solanum lycopersicum. Scientia Hortic. 317, 9. doi: 10.1016/j.scienta.2023.112082
Spaninks, K., Offringa, R. (2023). Local phytochrome signalling limits root growth in light by repressing auxin biosynthesis. J. Exp. Bot. 74, 4642–4653. doi: 10.1093/jxb/erad163
Spaninks, K., Van Lieshout, J., Van Ieperen, W., Offringa, R. (2020). Regulation of Early Plant Development by Red and Blue Light: A Comparative Analysis Between Arabidopsis thaliana and Solanum lycopersicum. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.599982
Stein, O., Granot, D. (2019). An overview of sucrose synthases in plants. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00095
Suh, D. H., Kim, Y. X., Jung, E. S., Lee, S., Park, J., Lee, C. H., et al. (2020). Characterization of Metabolic Changes under Low Mineral Supply (N, K, or Mg) and Supplemental LED Lighting (Red, Blue, or Red-Blue Combination) in Perilla frutescens Using a Metabolomics Approach. Molecules 25, 15. doi: 10.3390/molecules25204714
Sun, L. P., Cao, X. Q., Du, J. C., Wang, Y., Zhang, F. H. (2024). Canola (Brassica napus) enhances sodium chloride and sodium ion tolerance by maintaining ion homeostasis, higher antioxidant enzyme activity and photosynthetic capacity fluorescence parameters. Funct. Plant Biol. 51, 14. doi: 10.1071/fp23089
Thibodeaux, C. J., Melancon, C. E., Liu, H. W. (2008). Natural-product sugar biosynthesis and enzymatic glycodiversification. Angewandte Chemie International Edition 47, 9814–9859. doi: 10.1002/anie.200801204
Trivellini, A., Toscano, S., Romano, D., Ferrante, A. (2023). The role of blue and red light in the orchestration of secondary metabolites, nutrient transport and plant quality. Plants-Basel 12, 17. doi: 10.3390/plants12102026
Ulum, F. B., Castro, C. C., Hörandl, E. (2020). Ploidy-dependent effects of light stress on the mode of reproduction in the Ranunculus auricomus complex (Ranunculaceae). Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00104
Vatistas, C., Avgoustaki, D. D., Monedas, G., Bartzanas, T. (2024). The effect of different light wavelengths on the germination of lettuce, cabbage, spinach and arugula seeds in a controlled environment chamber. Scientia Hortic. 331, 11. doi: 10.1016/j.scienta.2024.113118
Wang, C., Chen, Y., Cui, C., Shan, F. X., Zhang, R., Lyu, X., et al. (2023). Blue light regulates cell wall structure and carbohydrate metabolism of soybean hypocotyl. Int. J. Mol. Sci. 24, 15. doi: 10.3390/ijms24021017
Wang, F., Chen, X. X., Dong, S. J., Jiang, X. C., Wang, L. Y., Yu, J. Q., et al. (2020). Crosstalk of PIF4 and DELLA modulates CBF transcript and hormone homeostasis in cold response in tomato. Plant Biotechnol. J. 18, 1041–1055. doi: 10.1111/pbi.13272
Wang, C. L., Guo, Q. S., Zhu, Z. B., Cheng, B. X. (2017). Physiological characteristics, dry matter, and active component accumulation patterns of Changium smyrnioides in response to a light intensity gradient. Pharm. Biol. 55, 581–589. doi: 10.1080/13880209.2016.1263345
Wang, W., Su, M. H., Li, H. H., Zeng, B. Y., Chang, Q., Lai, Z. X. (2018). Effects of supplemental lighting with different light qualities on growth and secondary metabolite content of Anoectochilus roxburghii. Peerj 6, 20. doi: 10.7717/peerj.5274
Wang, T. Q., Sun, Q. Y., Zheng, Y. J., Xu, Y. L., Liu, B. B., Li, Q. M. (2024b). Effects of red and blue light on the growth, photosynthesis, and subsequent growth under fluctuating light of cucumber seedlings. Plants-Basel 13, 19. doi: 10.3390/plants13121668
Wang, J., Yang, D., Li, H., Han, W. (2014). Study on monosaccharide composition of intracellular polysacchride and contents of cordycepin and cordyceps polysacchride produced by Cordyceps militaris induced by blue light. Zhong yao cai = Zhongyaocai = J. Chin. Medicinal Mater 37, 1395–1399. doi: 10.13863/j.issn1001-4454.2014.08.022
Wang, J., Yao, R., Sun, Z. X., Wang, M. W., Jiang, C. J., Zhao, X. H., et al. (2024a). Effects of shading on morphology, photosynthesis characteristics, and yield of different shade-tolerant peanut varieties at the flowering stage. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1429800
Wang, Q. H., Zhang, X., Li, F. G., Hou, Y. X., Liu, X. L., Zhang, X. Y. (2011). Identification of a UDP-glucose pyrophosphorylase from cotton (Gossypium hirsutum L.) involved in cellulose biosynthesis in Arabidopsis thaliana. Plant Cell Rep. 30, 1303–1312. doi: 10.1007/s00299-011-1042-x
Wei, Y., Wang, S., Yu, D. (2023). The role of light quality in regulating early seedling development. Plants-Basel 12, 15. doi: 10.3390/plants12142746
Weiszmann, J., Fürtauer, L., Weckwerth, W., Nägele, T. (2018). Vacuolar sucrose cleavage prevents limitation of cytosolic carbohydrate metabolism and stabilizes photosynthesis under abiotic stress. FEBS J. 285, 4082–4098. doi: 10.1111/febs.14656
Wu, B. S., Mansoori, M., Schwalb, M., Islam, S., Naznin, M. T., Addo, P. W., et al. (2024). Light emitting diode effect of red, blue, and amber light on photosynthesis and plant growth parameters. J. Photochem. Photobiol. B Biol. 256, 11. doi: 10.1016/j.jphotobiol.2024.112939
Xie, C. Y., Tang, J., Xiao, J. X., Geng, X., Guo, L. P. (2022). Purple light-emitting diode (LED) lights controls chlorophyll degradation and enhances nutraceutical quality of postharvest broccoli florets. Scientia Hortic. 294, 9. doi: 10.1016/j.scienta.2021.110768
Xu, P., Chen, H. R., Li, T., Xu, F., Mao, Z. L., Cao, X. L., et al. (2021). Blue light-dependent interactions of CRY1 with GID1 and DELLA proteins regulate gibberellin signaling and photomorphogenesis in Arabidopsis. Plant Cell 33, 2375–2394. doi: 10.1093/plcell/koab124
Xu, Y. F., Chen, S. Y., Zhao, S. Y., Song, J. R., Sun, J., Cui, N., et al. (2024a). Effects of light intensity on the photosynthetic characteristics of Hosta genotypes differing in the glaucousness of leaf surface. Scientia Hortic. 327, 8. doi: 10.1016/j.scienta.2023.112834
Xu, Y. H., Yang, C. N., Zhang, Y. P., Cao, Q., Wan, C. P., Chen, C. Y., et al. (2024b). Blue light treatment delays postharvest ripening of kiwifruit by suppressing ethylene biosynthesis, starch degradation, and cell wall metabolism. Lwt Food Sci. Technol. 199, 10. doi: 10.1016/j.lwt.2024.116105
Yang, L. Y., Chen, J. J., Sun, X. M., Li, J. X., Chen, N. L. (2019). Inhibition of sucrose and galactosyl-sucrose oligosaccharide metabolism in leaves and fruits of melon (Cucumis melo L.) under low light stress. Scientia Hortic. 244, 343–351. doi: 10.1016/j.scienta.2018.09.001
Yang, Y. X., Guang, Y. L., Wang, F., Chen, Y., Yang, W. T., Xiao, X. F., et al. (2021). Characterization of phytochrome-interacting factor genes in pepper and functional analysis of CaPIF8 in cold and salt stress. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.746517
Yang, J., Jeong, B. R. (2021). Side lighting enhances morphophysiology by inducing more branching and flowering in chrysanthemum grown in controlled environment. Int. J. Mol. Sci. 22, 26. doi: 10.3390/ijms222112019
Yang, T., Lv, R., Li, J., Lin, H., Xi, D. (2018). Phytochrome A and B Negatively Regulate Salt Stress Tolerance of Nicotiana tobacum via ABA-Jasmonic Acid Synergistic Cross-Talk. Plant Cell Physiol. 59, 2381–2393. doi: 10.1093/pcp/pcy164
Yang, P., Shi, H., Guo, Q., Wang, Y., Xuan, H., Liu, L., et al. (2020). Effects of light intensity on material and energy metabolism of Viola yedoensis. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J. Chin. materia Med. 45, 5944–5950. doi: 10.19540/j.cnki.cjcmm.20200927.102
Yang, J. L., Song, J. N., Jeong, B. R. (2024). Flowering and runnering of seasonal strawberry under different photoperiods are affected by intensity of supplemental or night-interrupting blue light. Plants-Basel 13, 24. doi: 10.3390/plants13030375
Yang, L. Y., Wang, L. T., Ma, J. H., Ma, E. D., Li, J. Y., Gong, M. (2017). Effects of light quality on growth and development, photosynthetic characteristics and content of carbohydrates in tobacco (Nicotiana tabacum L.) plants. Photosynthetica 55, 467–477. doi: 10.1007/s11099-016-0668-x
Yonekura, M., Aoki, N., Hirose, T., Onai, K., Ishiura, M., Okamura, M., et al. (2013). The promoter activities of sucrose phosphate synthase genes in rice, OsSPS1 and OsSPS11, are controlled by light and circadian clock, but not by sucrose. Front. Plant Sci. 4. doi: 10.3389/fpls.2013.00031
Yoshida, S., Mandel, T., Kuhlemeier, C. (2011). Stem cell activation by light guides plant organogenesis. Genes Dev. 25, 1439–1450. doi: 10.1101/gad.631211
Zabotina, O. A., Zang, N., Weerts, R. (2021). Polysaccharide biosynthesis: glycosyltransferases and their complexes. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.625307
Zhang, X. C., Chen, K. Y., Zhao, Z. Y., Li, S. Y., Li, Y. Y. (2023). A novel LED light radiation approach enhances growth in green and albino tea varieties. Plants-Basel 12, 13. doi: 10.3390/plants12050988
Zhang, Y. T., Dong, H., Song, S. W., Su, W., Liu, H. C. (2020). Morphological and physiological responses of cucumber seedlings to supplemental LED light under extremely low irradiance. Agronomy-Basel 10, 19. doi: 10.3390/agronomy10111698
Zhang, Y. Q., Liu, W., Lu, X., Li, S., Li, Y., Shan, Y. Z., et al. (2024). Effects of different light conditions on morphological, anatomical, photosynthetic and biochemical parameters of Cypripedium macranthos Sw. Photosynth. Res. 160, 97–109. doi: 10.1007/s11120-024-01100-x
Zhang, Q., Xie, Z., Zhang, R., Xu, P., Liu, H. T., Yang, H. Q., et al. (2018). Blue light regulates secondary cell wall thickening via MYC2/MYC4 activation of the NST1-directed transcriptional network in Arabidopsis. Plant Cell 30, 2512–2528. doi: 10.1105/tpc.18.00315
Zhang, T., Yan, Q. L., Yuan, J. F., Zhang, J. X. (2022). Application of fertilization in changing light adaptability and improving growth of Aralia elata (Miq.) Seem. seedlings under various light conditions in temperate forests. J. Plant Physiol. 277, 8. doi: 10.1016/j.jplph.2022.153804
Zhao, S. W., Li, X., Kang, Y. S., Lin, Y. Q., Wu, Y. J., Yang, Z. C. (2024b). Photomorphogenesis and photosynthetic trait changes in melon seedlings responding to red and blue light. Horticulturae 10, 13. doi: 10.3390/horticulturae10090961
Zhao, K., Pu, Y. Y., Shi, H. Z., Guo, Q. S., Su, Y., Yang, F., et al. (2024a). The potential mechanism of response to light intensity in energy metabolism mediated by miRNA in Isatis indigotica. Gene 897, 12. doi: 10.1016/j.gene.2023.148083
Zheng, L., Van Labeke, M. C. (2017). Long-term effects of red- and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00917
Zhou, Q., Li, R. M., Fernie, A. R., Che, Y. N., Ding, Z. P., Yao, Y., et al. (2023). Integrated analysis of morphological, physiological, anatomical and molecular responses of cassava seedlings to different light qualities. Int. J. Mol. Sci. 24, 14. doi: 10.3390/ijms241814224
Zhou, Q., Zhao, F., Zhang, H. H., Zhu, Z. L. (2022). Responses of the growth, photosynthetic characteristics, endogenous hormones and antioxidant activity of Carpinus betulus L. seedlings to different light intensities. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1055984
Zhu, J., Cai, Y. M., Li, X., Yang, L. Y., Zhang, Y. C. (2024). Integrated multi-omic analysis reveals the carbon metabolism-mediated regulation of polysaccharide biosynthesis by suitable light intensity in Bletilla striata leaves. Plant Physiol. Biochem. 214, 12. doi: 10.1016/j.plaphy.2024.108872
Keywords: light, plant, growing development, sugar metabolism, photosynthesis
Citation: Wu W, Chen L, Liang R, Huang S, Li X, Huang B, Luo H, Zhang M, Wang X and Zhu H (2025) The role of light in regulating plant growth, development and sugar metabolism: a review. Front. Plant Sci. 15:1507628. doi: 10.3389/fpls.2024.1507628
Received: 08 October 2024; Accepted: 11 December 2024;
Published: 07 January 2025.
Edited by:
Marina López-Pozo, University of Colorado Boulder, United StatesReviewed by:
Chunhong Yang, Chinese Academy of Sciences (CAS), ChinaMd Ahasanur Rahman, Howard University, United States
Copyright © 2025 Wu, Chen, Liang, Huang, Li, Huang, Luo, Zhang, Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Zhu, emh1aHVhZ3hAMTYzLmNvbQ==; Xiaoxun Wang, d2FuZ3h4MTk3MzAzQDE2My5jb20=; Miao Zhang, MTc1MzU3NjQ4ODhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Wenyuan Wu
Wenyuan Wu Long Chen1,2†
Long Chen1,2† Miao Zhang
Miao Zhang Hua Zhu
Hua Zhu