
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci., 17 January 2025
Sec. Plant Abiotic Stress
Volume 15 - 2024 | https://doi.org/10.3389/fpls.2024.1504970
In recent decades, climate change has caused a decrease in rainfall, increasing sea levels, temperatures rising, and as a result, an expansion in salt marshes across the globe. An increase in water and soil salinity has led to a decline in the cultivated areas in different areas, and consequently, a substantial decrease in crop production. Therefore, it has forced scientists to find cheap, effective and environmentally friendly methods to minimize salinity’s impact on crops. One of the best strategies is to use beneficial soil microbes, including arbuscular mycorrhizal fungi, in order to increase plant tolerance to salt. The findings of this review showed that salinity can severely impact the morphological, physiological, and biochemical structures of plants, lowering their productivity. Although plants have natural capabilities to deal with salinity, these capacities are limited depending on plant type, and variety, as well as salinity levels, and other environmental factors. Furthermore, result of the present review indicates that arbuscular mycorrhizal fungi have a significant effect on increasing plant resistance in saline soils by improving the soil structure, as well as stimulating various plant factors including photosynthesis, antioxidant defense system, secondary metabolites, absorption of water and nutrients.
Many environmental stresses adversely affect plants’ metabolisms and growth, which ultimately impacts their performance (Hashem et al., 2018). The salt stress is one of the most significant abiotic stresses around the globe, and it has caused severe ecological (including the reduction of biological diversity, destruction of pastures and forests, desertification, and soil erosion) and agricultural problems, particularly in arid and semiarid regions (Himabindu et al., 2016; Jia et al., 2019). Global warming and climate change are contributing to soil salinity by reducing rainfall and increasing transpiration and evaporation, which, along with unsustainable farming practices (including chemical overuse, and watering with saline water), have led to the spread of salt marshes worldwide (Guo et al., 2020; Trenberth et al., 2014). This phenomenon, which is expanding by creating a continuous shortage of atmospheric and pedosphere water, has caused a reduction in the quality and quantity of agricultural products, damage to agricultural land, and a decrease in farmland, and strongly affects human food security and diet (Chaves et al., 2009; Estrada et al., 2013; Ashraf and Harris, 2013; Pan et al., 2020).
According to their salt sensitivity, plants are classified in two major groups: halophytes and glycophytes (Yuan et al., 2019; Pan et al., 2020). Plants called halophytes are salt-tolerant plants that can thrive in soil or water with high saline concentrations (200 mM NaCl or more), whereas glycophytes are salt-sensitive (Wang et al., 2019a; Himabindu et al., 2016). Glycophytes are the predominant crop and forage species used in modern agriculture, which have limited mechanisms of salt tolerance, whereas halophytes have effective mechanisms for protecting themselves from salt damage (Flowers and Muscolo, 2015; Pan et al., 2020). Nevertheless, at the beginning of the growth process, both plants are sensitive to salinity (Himabindu et al., 2016).
Food security for the world’s expanding populace in the face of deteriorating agricultural land is one of humanity’s most important missions, which requires effective strategies to mitigate salinity (Chinnusamy et al., 2005; Duc et al., 2021), this includes the cultivation of salt-resistant crops, adding solutes and growth regulators, implementation of better irrigation systems, plant breeding, and supplying plant growth promoting microorganisms (PGPM) (Khan et al., 2010; Munns, 2002; Diagne et al., 2020). Among the methods mentioned above, the inoculation of plants by beneficial soil microorganisms is an efficient method that, in addition to increasing plant resistance against salt stress, also improves their productivity (Janah et al., 2021b; Ait-El-Mokhtar et al., 2019).
Among the PGPM, arbuscular mycorrhizal fungi are crucial because they establish symbiosis with 80% of plants, including glycophytes, and halophytes (Khan et al., 2010; Gao et al., 2020). Arbuscular mycorrhizal fungi survive in most environments, and provide a variety of ecological services, especially enhancing the rhizosphere properties chemically and physically, strengthening the ecosystem function, and increasing host plant growth and performance (Mathur et al., 2019; Liang et al., 2021; Frosi et al., 2018). Coexistence with mycorrhizal fungi, which is known as the “mother of all plant root symbioses,” is one of the most common strategies used to resist abiotic, and biotic stresses (Romero-Munar et al., 2017). Since mycorrhizal fungi are discovered throughout the planet, including in highly salty conditions, their association with plant roots can be a valuable ecological method to sustain plants in salinity environments (Aroca et al., 2013; Evelin et al., 2009; López-Ráez, 2016).
The mycorrhizal fungi are capable of regulating numerous physiological and biochemical processes within plants, and reduce salinity’s negative effects on them (Augé et al., 2014; Ait-El-Mokhtar et al., 2019). Among the processes that arbuscular mycorrhizal fungi regulate in host plants, and enhance their flexibility in salinity-stressed conditions are the following: facilitating water and nutrient absorption, enhancing photosynthetic ability, modulating antioxidant responses, inhibiting ion absorption, increasing root and shoot biomass, expression of aquaporin genes, gene expression encoding membrane transport proteins, and accumulating compatible solutes (Hashem et al., 2018; Chen et al., 2017; Chang et al., 2018; Ding et al., 2016; Chaichi et al., 2017). Plants that are salt-tolerant under mycorrhizal fungi treatment include soybean, sorghum, wheat, tomato, rice, watermelon, cucumber, safflower, and pistachio (Abbaspour et al., 2021; Diao et al., 2021). The purpose of this review is to describe, existing knowledge about the interaction of crops with arbuscular mycorrhizal fungi and salt stress resistance is explored, to make the findings and unknowns regarding the current topic available to researchers for future studies. In this review, the effect of salt stress on the morphophysiological and biochemical structures of plants has been studied. Furthermore, it has been attempted to investigate the recent findings in the field of the Plants’ natural ability to deal with salinity. This review also discusses the mycorrhizal fungi role in increasing plant morpho-physiological, and biochemical structure, as well as its role in enhancing plant performance in saline environments.
Plants in nature are confronted with various stresses, and one of the main abiotic stresses is salinity that limit the growing, development, and metabolism of plants (Abdel Latef and Chaoxing, 2014; Diagne et al., 2020). Land degradation due to soil salinization has become a deteriorating environmental crisis in farming ecosystems, especially in arid and semiarid areas, and has severely impacted food security worldwide (Porcel et al., 2012; Chen et al., 2017; Van Zelm et al., 2020; Deinlein et al., 2014). It is estimated that one billion hectares worldwide are influenced by saline conditions, and approximately, salinization is affected 10% of arable land (Tufail et al., 2018; Duc et al., 2021; Zaman et al., 2018). Areas affected by salinity are increasing by 15 to 20 million hectares per year, which reduces production of crops by 20% (Evelin et al., 2019; Pan et al., 2020), and by 2050, it is expected that half of arable land could be negatively impacted by salinization due to climate change (Porcel et al., 2012; Janah et al., 2021b; Diao et al., 2021). Soil salinity has impacted agricultural productivity in a number of countries, including Iran, Pakistan, Thailand, Iraq, China, Egypt, India, Australia, Argentina, and the United States more than in other places around the world (Rengasamy, 2010; Abeer et al., 2014; Shrestha, 2006). There are roughly 100 million hectares of salt marshes in China (Huang, 2018), while 1.84 million hectares of saline soil exist in the northeastern regions of Thailand (Arunin and Pongwichian, 2015). 55% of Senegal’s arable land and nearly 37% of Morocco’s soil are impacted by salt (Diagne et al., 2020; Farissi et al., 2011).
Salinity in soil can be caused by different kinds of salts, such as NaCl, Na2CO3, MgCl2, CaSO4, MgSO4, and Na2SO4, among which sodium chloride is most common in arid and semiarid soils (Flowers et al., 1977). Saline soils have an electrical conductivity (EC) of more than 4 decisiemens per meter (dS/m), and an osmotic pressure of -0.2 MPa, which is equal to about 40 mM NaCl (Santander et al., 2019). Due to salinity, the soil’s biological, chemical, and physical properties are destroyed, decreasing fertility and increases desertification (Diagne et al., 2020; Romero-Munar et al., 2019). A high salt concentration in soil solution reduces the osmotic potential of the soil and also suppresses the activities related to nutrients in plants, and leads to unfavourable Na+/Ca2+ and Na+/K+ ratios (Romero-Munar et al., 2019; Kumar and Verma, 2018) (Table 1).
Soil salinity is the result of natural factors such as capillary rise due to evaporation, seawater infiltration, dissolution of rocks, and lack of rainfall, as well as human factors like poor agricultural practices, including improper irrigation and drainage systems (Legros, 2009; Latef and Chaoxing, 2011; Wang et al., 2003). A rise in soil salt levels causes osmotic, and ionic stress in plants, directly reducing crop productivity by disrupting important biochemical, and physiological processes (Abd_Allah et al., 2015). Therefore, in addition to agricultural production, salinity also threatens ecosystem performance (Santos et al., 2016). Thus, developing effective methods for the restoration of saline lands despite the climatic and economic limitations seems to be necessary, Including 1) biological control through the restoration of saline soils with organic materials and cultivation of halophytes, 2) chemical control by applying chemical amendments such as sulfuric acid, sulfur, and gypsum to neutralize alkaline condition, and 3) mechanical control by installation of anti-salt structures such as dams (Fall et al., 2018; Litalien and Zeeb, 2020).
Too much salt in the soil disrupts all the morphological, physiological, and biochemical reactions in plants and drastically reduces crop production (Nawaz et al., 2010; Aroca et al., 2013; Balliu et al., 2015). Salinity hurts plants through three physiological aspects (Zheng et al., 2008; Boorboori, 2023), which are: 1) osmotic tension: increasing the salt concentration in the soil changes the basic texture, and reduce water conductivity, aeration of the soil, and a decrease in osmotic potential, leading to a physiological dryness in plants, as well as the nutritional imbalance (Evelin et al., 2012; Hashem et al., 2016; Chaichi et al., 2017), 2) toxic ions: the overabsorption of toxic ions, including sodium (Na+), and chlorine (Cl−) by plants can result in changes to enzyme structure, damage to cell organelles, decrease the activity of metabolic enzymes, change in macromolecule structure, inhibition of photosynthesis, protein synthesis inhibition, and ion balance disruption (Porcel et al., 2012; Boorboori and Zhang, 2023), and 3) oxidative damage: an increase in soil salinity causes secondary stress in plants called oxidative stress, which leads to disturbances in plant cell structures such as mitochondria, chloroplasts, membranes, etc. (Abdel Latef et al., 2019; Kumar et al., 2015; Porcel et al., 2012) (Figure 1).
Since salts are also plant nutrients, excess salt in the soil imposes competition during the absorption, transfer, or distribution of nutrients, leading to an unbalance in the plant ionic composition, and thus affecting the physiological characteristics of the plant (Rabie and Almadini, 2005; Farissi et al., 2011; Munns and Tester, 2008). Studies in saline environments have indicated that an abundance of Na+ and Cl− ions hinder the solubility, mobility and absorption of nutrients, for instance, nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), copper (Cu), and zinc (Zn) in plants (Zai et al., 2021; Hasegawa et al., 2000; Evelin et al., 2019). Na+ can enter the roots through non-selective cation channels (NSCCs) in the plasma membrane, increasing Na+ content in roots and shoots, and leading to unfavourable Ca2+/Na+ and K+/Na+ ratios (Abdel-Fattah and Asrar, 2012; Van Zelm et al., 2020; Pedranzani et al., 2016). Since Na+ competes with K+ and Ca2+ for membrane transfer sites, maintaining a high ratio of Ca2+/Na+ and K+/Na+ in the cytosol is crucial for increasing plants’ tolerance to salinity and enhancing enzymatic processes (Evelin et al., 2019; Ahanger et al., 2015; Samaddar et al., 2019). A reduction in the ratio of K+/Na+ in the cytosol causes a disturbance in stomatal movement, turgor maintenance, photosynthesis, activity of enzymes, and protein synthesis (Benito et al., 2014; Maathuis and Amtmann, 1999). In contrast, a decrease in the ratio of Ca2+/Na+ causes photosynthetic tissue destruction, disruption of Ca2+ signalling pathways, and a reduction in hydraulic conductivity (Ait-El-Mokhtar et al., 2020) (Figures 1, 2).
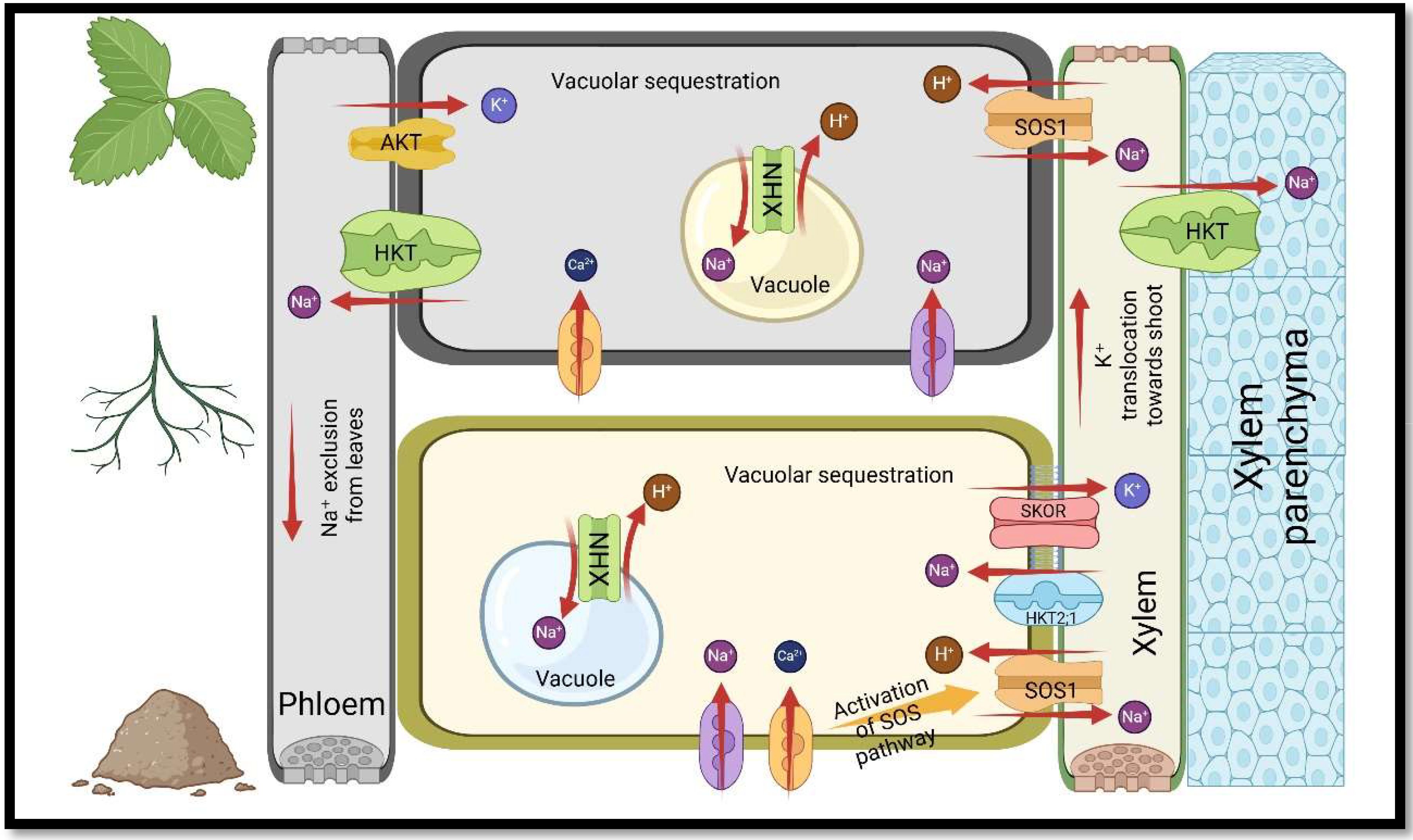
Figure 2. The function of transporter proteins in sustaining acceptable K+/Na+ ratios in plants under salt stress. Nomenclature is as proposed by Evelin et al. (2019), and Boorboori and Zhang (2022).
N is an essential macronutrient that plants absorb it as nitrate (NO3-), and ammonium (NH4+) ions, whereas salinity interferes with their absorption by immobilizing them (Frechilla et al., 2001; Munns and Tester, 2008). In the saline environment, the NH4+ uptake is challenged by Na+, whereas the NO3- uptake is inhibited by Cl− in the membrane, which causes the low flux of NH4+ and NO3- from soil to roots, leading to a reduction in nitrate reductase activity (NR) in plants (Hoff et al., 1992; Evelin et al., 2019). P is another nutrient whose absorption by roots is interfered with salinity because of its precipitation with other cations, for example, Zn2+, Mg2+, and Ca2+ (Bano and Fatima, 2009; Evelin et al., 2019). The decrease in P content as an essential macronutrient in plants causes the stoppage of growth and premature death of leaves (Giri et al., 2003; Taiz et al., 2015). Furthermore, salt in the soil renders water unavailable to plant roots, resulting in a decline in turgor pressure in plant cells, cell dehydration, and water stress (Füzy et al., 2008; Zou et al., 2013; Ait-El-Mokhtar et al., 2020) (Figure 2).
A plant’s root system absorbs water and nutrients, and it also comes in direct contact with a salty environment, so it contributes greatly in protecting the plant from salinity stress (Rana et al., 2019; Evelin et al., 2019). There is a decrease in growth of roots with increasing salinity of soil, and this is the first response of plants to excessive soil salts (Amanifar et al., 2019; Greenway and Munns, 1980). When salt is present in the rhizosphere, primary roots grow slower because salt inhibits cell division and root epidermal cells elongation, while lateral roots grow (Bernstein et al., 2013; Jung and McCouch, 2013). Furthermore, there is evidence that the toxic effects of salinity on root growth and development are related to the inhibition of endogenous phytohormones levels, such as Indole-3-acetic acid (IAA), and indole-3-butyric acid (IBA) (Khan et al., 2015; Egamberdieva, 2009; Egamberdieva et al., 2016).
Photosynthesis, as a primary metabolism’s key process, is highly sensitive to salinity (Jia et al., 2019; Porcel et al., 2012), and salinity of the soil inhibits plant photosynthetic ability through its effect on leaf surface area, photosystem II efficiency, stomatal conductance, and content of chlorophyll (Farissi et al., 2011; Miransari et al., 2008). Salinity directly damages the complete ultrastructure of photosynthetic organelles such as chloroplasts and also reduces photosynthetic pigment content (total chlorophyll, carotenoids, Chl a and Chl b) (Liang et al., 2021; Akram and Ashraf, 2011; Ait-El-Mokhtar et al., 2020). Chloroplasts are the most susceptible cellular organelles to salt, and in high salinity conditions, grana and thylakoids begin to disintegrate, and vanish as a result of concentration of cations changes in chloroplasts, membrane damage and swelling of thylakoids, which consequently affects the efficiency of light energy utilization (Kaya et al., 2009; Peng et al., 2019). According to studies, salinity suppressed enzymes involved in the synthesis of photosynthetic pigments, including chlorophyll synthetase, and increased chlorophyll degradation enzyme activity, reducing chlorophyll and content of photosynthetic pigments (Wang et al., 2019a; Zai et al., 2021; Tsunekawa et al., 2009). In addition, under salinity stress, chlorophyll levels can decrease due to low mineral absorption, particularly magnesium (Giri and Mukerji, 2004). However, photosynthetic pigment synthesis, and efficiency of photosynthesis in saline environments strongly depends on the plant species and even the genotypes (Bistgani et al., 2019; Khan et al., 2015) (Figure 1).
In addition to directly damaging photosynthetic machinery, salt stress can also damage the photosystem II (PSII) reaction center, interfere with electron transfer from PSII to photosystem I (PSI), and ultimately decrease photosynthesis (Evelin et al., 2013; Liu and Shi, 2010; Jia et al., 2019). Furthermore, the researchers found that under salinity stress potential photochemical efficiency (Fv/Fo), maximum quantum efficiency of PSII (Fv/Fm), and photochemical quenching coefficient (qP) decreased while non-photochemical quenching (NPQ) increased (Liang et al., 2021; Sheng et al., 2008). Osmotic stress caused by salinity reduces the water content in shoots and leaves, thus closing the stomata and reducing CO2 availability (Gao et al., 2020; Duarte et al., 2013; Zu et al., 2005). In the absence of CO2 absorption, excess electrons are accumulated in the thylakoid membranes (disruption of electron transfer between PSII and PSI), resulting in PSII degradation and damage to other photosynthetic apparatus components (Gao et al., 2020; Chang et al., 2018).
Salt-induced stress leads plants to produce an excessive amount of reactive oxygen species (ROS), which causes oxidative degradation to components of cells (Amanifar et al., 2019; Parihar et al., 2020; Boorboori and Zhang, 2022). Oxidative stress process in plants occurs when multiple metabolic pathways are disrupted, and high-speed and energy electron transfers to molecular oxygen (Gill and Tuteja, 2010; Miller et al., 2010). Oxidative damage caused by ROS to plants includes hydroxyl radicals (·OH), superoxide radicals (O2•−), hydrogen peroxide (H2O2), and singlet oxygen (1O2) (Abd_Allah et al., 2015; Gao et al., 2020). ROS buildup disrupts important cellular structures, including organelles, membrane lipids, nucleic acids, proteins, macromolecules (especially enzymes), leading to a reduction in nutrient absorption and transfer, disruption of respiratory and photosynthesis systems, and ultimately limiting growth and development of plants (Porcel et al., 2016; Janah et al., 2021b; Abeer et al., 2014) (Figures 1, 3).
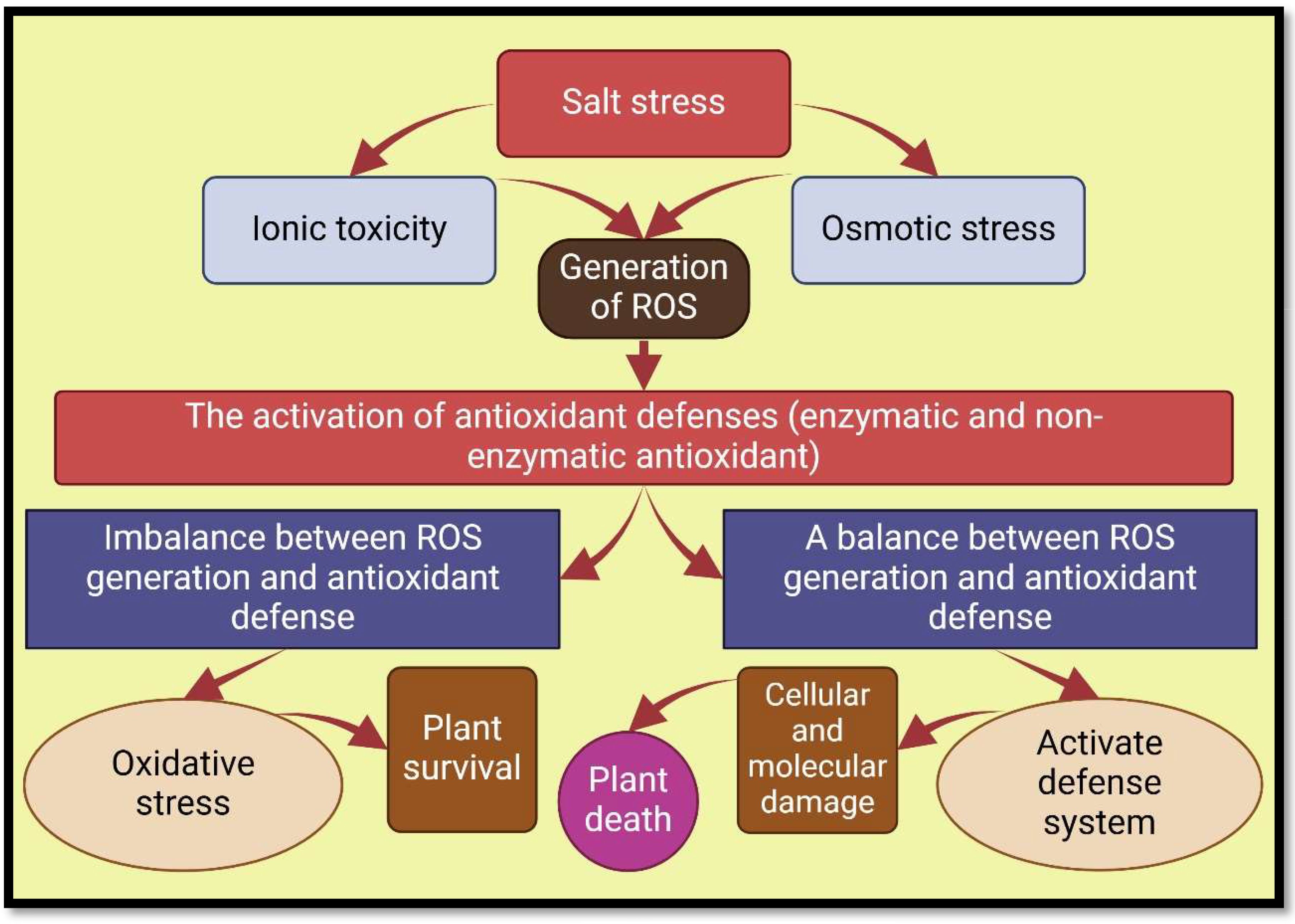
Figure 3. Oxidative stress, and antioxidant defenses in salt-exposed plants. Nomenclature is as proposed by Hasanuzzaman et al. (2021).
Lipid peroxidation changes cell membrane’s selective permeability, and eventually causes membrane leakage, and membrane integrity loss (Abeer et al., 2014; Huang et al., 2010). Maintaining the integrity of the cell membrane is essential for plants to cope with salinity stress, since the damaged cell membrane loses its biological function and affects plant’s natural metabolism (Stevens et al., 2006; Jabeen et al., 2014). According to Nat et al., cell membranes of halophytes can be damaged by peroxidation of lipids when salt concentrations are above 300 mM for a week or more (Nath et al., 2016). Malondialdehyde content (MDA) as lipid peroxidation’s final product, in combination with relative electrolyte leakage (REL) is used as an oxidative damage index, and to identify cell membrane damage extent (Adolfsson et al., 2017; Pedranzani et al., 2016).
Plants contain a very flexible system for adjusting their morpho-physiological, molecular, biochemical and metabolic mechanisms for survival in salinity environments (Porcel et al., 2012; Evelin et al., 2019). Plants enhance their resistance against stress salinity by raising the accumulation of compatible osmolytes, regulating water absorption, maintaining the endogenous levels of growth regulators, increasing chlorophyll synthesis, regulating antioxidant molecules, and compartmentalizing toxic ions in vacuoles (Bistgani et al., 2019; Abbaspour et al., 2021; Munns and Tester, 2008). Additionally, plants regulate the rate of leaf transpiration, stomatal conductance, and photosynthesis rate by adjusting the stomata aperture, leading to greater photosynthesis efficiency (Kaya et al., 2009; Elhindi et al., 2017; Liang et al., 2021).
Osmotic regulation is another defense mechanism that helps plants control osmotic and ionic toxic impacts through the expression of genes involved in nutrient transport and partitioning, accumulation of solutes, and aquaporins (Li et al., 2020; Apse and Blumwald, 2007; Van Zelm et al., 2020). Salt Overly Sensitive 1 (SOS1) is a Na+/H+ antiporter in plasma membrane, which has an essential function in maintaining ions homeostasis and managing Na+ and K+ transport in plasma membrane and tonoplast (Li et al., 2020; Gao et al., 2020). The study conducted on Suaeda salsa showed that at 400mM NaCl concentration, Na+ was actively removed from the cytoplasm into the rhizosphere, while the expression of SOS1 was the highest in the roots (Gao et al., 2020). Furthermore, vacuolar Na+/H+ antiporters (NHXs) are responsible for the sequestration of Na+ into vacuoles, which is driven through the proton motive force generated by H+-PPase and H+-ATPase (VHA) (Diao et al., 2021; Gao et al., 2020). Moreover, The Stelar K+ outward rectifier channel (SKOR) is a K+ channel in plants that transports K+ from the roots to aerial parts (Li et al., 2023). SKOR identify as a transport protein with a role in loading K+ to the xylem, and if it was disrupted K+ content in shoots was significantly reduced, while K+ content in roots was not affected (Abbaspour et al., 2021; Sharma et al., 2013). Moreover, plasma membrane intrinsic proteins (PIPs), a plant’s aquaporin subfamily, was found to be the primary water absorption and transport channels in plant cells and can mediate the plant cell water loss under the stress of salt (Abbaspour et al., 2021).
Increasing the production of ROS can be harmful to cells, but they also regulate numerous fundamental plant processes, including salt stress response (Chen et al., 2020). Nevertheless, plants have evolved detoxification mechanisms to combat oxidative damage, including the induction of secondary metabolite production and a wide range of enzymatic and non-enzymatic antioxidants (Behdad et al., 2020; Toscano et al., 2019; Sharma et al., 2012). The antioxidant enzymes that reduce salinity-induced ROS include catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), and polyphenol oxidases (PPO) (Janah et al., 2021a; Waszczak et al., 2018; Apel and Hirt, 2004). In contrast, non-enzymatic antioxidants that destroy ROS include glutathione (GSH), ascorbic acid (AsA), carotenoids, flavonoids, and α-tocopherol (Foyer and Noctor, 2011; Chaparzadeh et al., 2004).
Salt stress stimulates phenolic acid, phenolic compounds, and flavonoids accumulation in plants as stress-resistance mechanism (Boughalleb et al., 2020; Duc et al., 2021; Liang et al., 2021). A change in the accumulation of phenolic compounds may be related to changes in other metabolic processes, such as phenolic acids, fatty acids, organic acids, amino acids (Saleh et al., 2020), and sugars, which leads to an improvement in the defense system of the plant, ROS reduction, and osmotic regulation (Abeer et al., 2014; Arzani and Ashraf, 2016; Saxena et al., 2015). Further, the increase in total phenolic acids is mainly due to the slope of quinic, protocatechuic, and gallic acids, followed by epicatechin, catechin, and quercetin-3-O-galactoside (Boughalleb et al., 2020). Additionally, plants are able tolerating salt by osmolytes accumulation in their cell cytoplasm for osmotic regulation, including polyamines (spermine, spermidine, and putrescine), proline, glycine, organic acids, total suspended solids (maltose, dextrin, sucrose, and glucose), α-amino nitrogen, and betaine (Evelin et al., 2013, 2019; Dodd and Pérez-Alfocea, 2012; Chen and Murata, 2011; Yang and Guo, 2018).
The AMF are a common soil microbe that colonizes the roots of most terrestrial plants (Duc et al., 2021). According to studies, these symbiotic fungi provide significant benefits to their host plants, including growing their resistance to stress, especially salt stress (Duc et al., 2021; Evelin et al., 2019). AMF increases plant resistance to salt by various biochemical and physiological mechanisms, which can be categorized into three groups: 1) increasing nutrient absorption and maintaining ionic homeostasis in plants, and improving water absorption and maintaining osmotic balance, 2) enhancing photosynthesis efficiency and protecting the photosynthetic apparatus, and 3) plant hormone profile modulation and antioxidant system induction to prevent ROS damage (Evelin et al., 2019; Tavarini et al., 2018; Smith et al., 2010) (Figure 4).
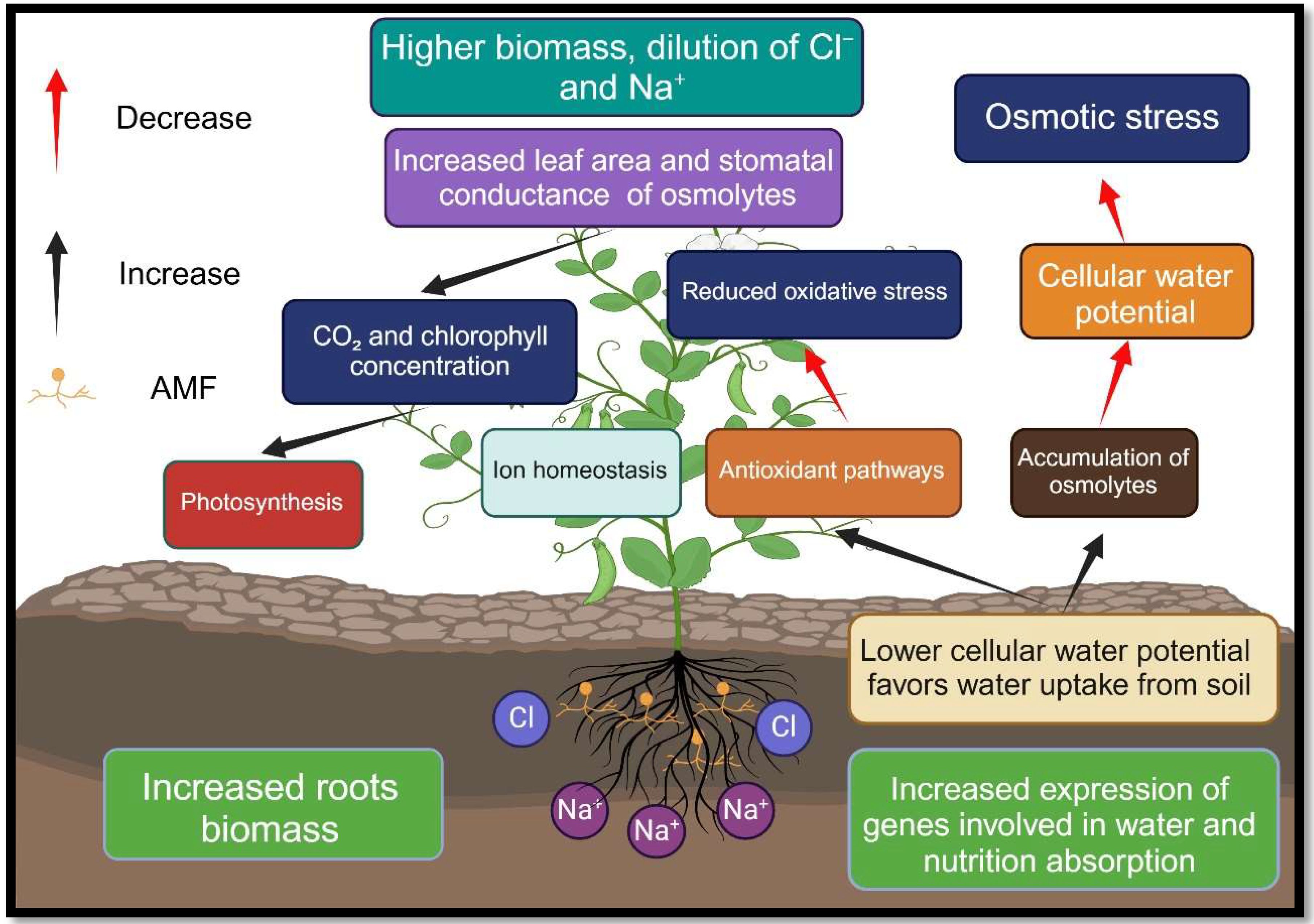
Figure 4. Advantages of colonizing plant roots with arbuscular mycorrhizal fungi in saline soils. Nomenclature is as proposed by Gupta et al. (2021).
AMF can adapt and survive in a salty habitat, however, high salt stress may inhibit spore germination, reduce spore viability, prevent hyphae growth, decrease spore density, and generally diminish AMF biomass (Ahanger et al., 2014; Juniper and Abbott, 2006; Hammer et al., 2011; Estrada et al., 2013). Moreover, Na+ exerts a direct toxic affects AMF, and reduces colonization rate, indicating suppression of the AMF symbiotic effect by salinity (Sheng et al., 2008; Chaichi et al., 2017). According to research on licorice, it was discovered that salinity greatly reduced the ability of Funneliformis mosseae to infect the roots (Amanifar et al., 2019). High level of salt reduces AMF colonization percentage by reducing mycelium growth, vesicles and arbuscules in plants (Bistgani et al., 2019; Abeer et al., 2014; Rivero et al., 2018). Nevertheless, the adverse effect of excessive salt exposure on capacity of AMF colonization is greater in plant growth in its early stages, however, AMF eventually adapts to such a salt level over time (Duc et al., 2021). A study conducted on Eclipta prostrata L. was shown that in high-salinity environments (200 mM NaCl), the colonization of different AMF species (Acaulospora lacunose, Septoglomus deserticola, and Funneliformis mosseae) was significantly less in the first four weeks of growing a plant, however, colonization rates increased in the later stages of plant growth (Duc et al., 2021).
There are a number of factors that determine whether AMF symbiosis with plants increases in saline soil, including the type of plant, AMF genotype, and the external agro-environment (Johnson et al., 2015; Estrada et al., 2013). In general, AMF inoculation efficiency increases among plant species with salinity tolerance capabilities (Fan et al., 2012; Aliasgharzadeh et al., 2001). Additionally, monocotyledonous plant species with fibrous root systems can better coexist with AMF than dicotyledonous species with tap root systems (Abd Rahim et al., 2016; Jia et al., 2019). The researchers also found that AMF symbiosis effect is diverse in different organs of a plant [above organs (e.g., leaves) and underground organs (e.g., roots)] exposed to salt (Gao et al., 2020). Meanwhile, native AMF ecotypes isolated from saline environments maintain higher colonization characteristics compare to the salt-sensitive genotypes, and show a greater level of salinity resistance in plants (Bistgani et al., 2019; Samaddar et al., 2019; Campagnac and Khasa, 2014; Yamato et al., 2008).
Several studies have shown that combining AMF with other beneficial soil microorganisms increases plant resistance to salinity, however, species closely related to AMFs with similar phenotypic characteristics compete fiercely for limited space and resources with them (Ait-El-Mokhtar et al., 2019; Maherali and Klironomos, 2007). Additionally, according to previous research, some compounds affect AMF symbiosis, including dopamine, hydroxyl fatty acids, phenols, sesquiterpenoids, and flavonoids (Gao et al., 2020; Lanfranco et al., 2018). Numerous reports indicate AMF inoculation reduces salt stress in different plants, including lettuce, alfalfa, tomato, wheat, maize, oleaster, black locust, rice, castor, peanut, swamp she-oak, mandarin, basil, cucumber, lychee, Panicum turgidum, Senegalia senegal, Acacia mangium, Acacia auriculiformis, and etc (Diagne et al., 2020; Hashem et al., 2018; Guo et al., 2020; Tisarum et al., 2020; Jia et al., 2019; Santander et al., 2019; Pan et al., 2020; Elboutahiri et al., 2010).
The mutual interaction between AMF, and salt-stressed plants enhances the selective absorption of some elements (Ca, K), limits the absorption of some other elements (Na+), increases the efficiency of water consumption, and promotes host plant growth (Hammer et al., 2011; de Varennes and Goss, 2007; Santander et al., 2017). In saline environments, plant root and soil colonization by AMF can improve the rhizosphere condition of the soil through strengthening the absorption of organic carbon in the soil, increasing N, P and K pools, improving the content of organic matter, adjusting pH of the soil, and preventing soil erosion (Zai et al., 2021; Chang et al., 2014; El Kinany et al., 2019; Aliasgharzadeh et al., 2001). Moreover, AMF improves water and nutrient absorption for host plants by forming a wide hyphal network and spreading myciniums outside the rhizosphere (Ortiz et al., 2015; Lin et al., 2016; Yan et al., 2016) (Figure 4; Table 2).
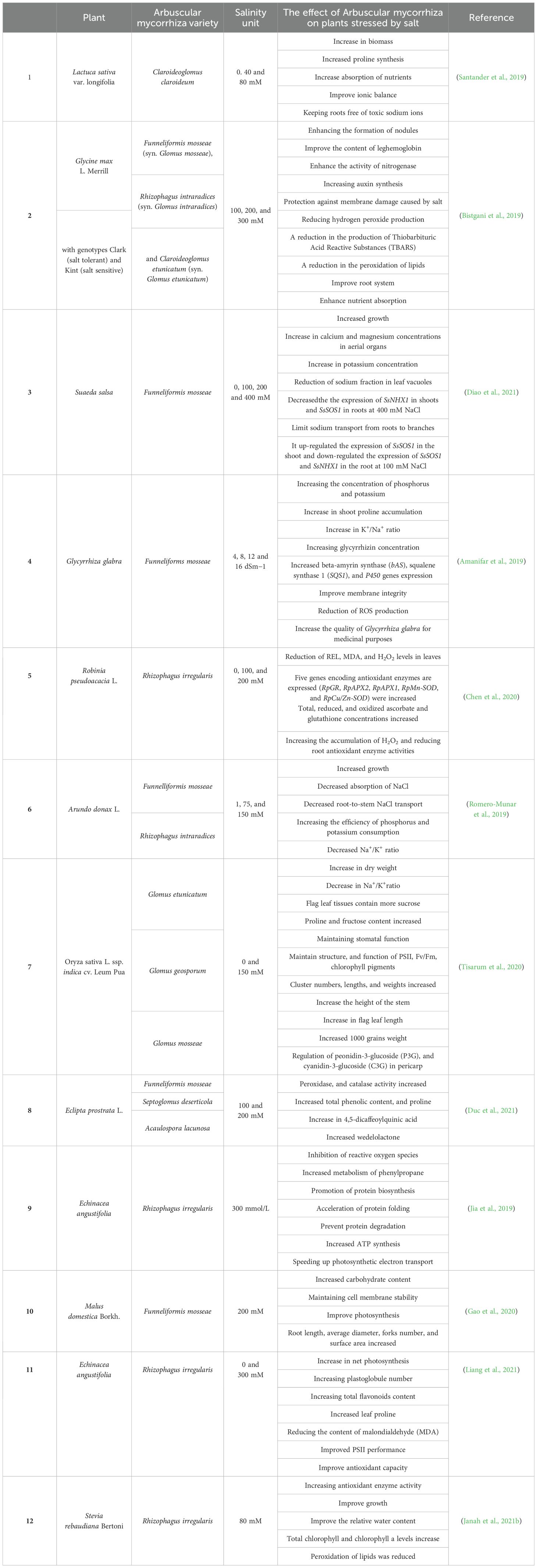
Table 2. The effect of different arbuscular microbial varieties on the decrease of salinity impact in different plants.
Colonization of AMF also affects the concentrations and characteristics of polyamines and organic acids in plants (Sheng et al., 2011). Polyamines by increasing nutrient and water absorption, while organic acids by reducing the electrical conductivity of the soil and increasing the availability of N, P, and K in the soil, helping maintain ionic homeostasis in plant cells (Sheng et al., 2011; Evelin et al., 2019). However, different AMF species have varying abilities to obtain and provide nutrients to salt-stressed plants (Taylor et al., 2015) (Table 2).
Adding AMF to plants stressed by salt enhances their growth through allowing them to increase water and nutrient absorption (Pedranzani et al., 2016; Parihar et al., 2020). Researchers have found that AMF in saline environments increases biomass production, shoot and root dry weight, number of branches, plant height, seedling diameter, leaf area and plant yield (Qiu et al., 2007; Zhao et al., 2015; Tao et al., 2012; Parihar et al., 2020). There are several plants whose biomass has increased under saline conditions due to AMF, including Chrysanthemum morifolium, Elaeagnus angustifolia, Gossypium hirsutum, Medicago sativa, Phoenix dactylifera, Zelkova serrata, Oryza sativa, Verbena officinalis, and Trigonella foenum-graecum (Jia et al., 2019; Duc et al., 2021; Evelin et al., 2019). Furthermore, AMF improves plant growth in saline environments by increasing endogenous production of growth regulators like IBA and IAA (Waqas et al., 2012; Abd_Allah et al., 2015). Research conducted by Guo et al. on Malus domestica Borkh exposed to salt, Funneliformis mosseae inoculation resulted in a positive regulation of genes involved in IAA-responsive proteins in aerial parts (TRINITY_DN10259_c0_g1), and roots (TRINITY_ DN17372_c0_g1) (Gao et al., 2020). Furthermore, AMF colonization can enhance the plant’s ability to explore water and food through rising the length and conductivity of the roots, and ultimately improve the plant’s adaptation to salinity (Chandrasekaran et al., 2019; Kumar et al., 2010).
Plant roots inoculated with AMF when stressed by salt helps to enhance nitrogen absorption, and studies have shown that up to 25% of plant nitrogen is supplied by AMF hyphae (Evelin et al., 2019; Younesi et al., 2013). The improvement of nitrate uptake by symbiotic plants with AMF could be due to maintaining membrane stability and increasing Nitrate reductase (NR) (Evelin et al., 2019). In plants stressed by salt, coexistence of AMF increases the expression of ammonium transporters (AMT1.1, and AMT1.2) and nitrate transporters (NRT1.1، and NAR2.2) (Fileccia et al., 2017; Saia et al., 2015). Meanwhile, under salinity conditions, phosphorus absorption by plants is greatly reduced, while AMF symbiosis is considered as an effective biological method to increase P absorption by plants and stimulate their growth (Romero-Munar et al., 2019; Shokri and Maadi, 2009; Ghorchiani et al., 2018). The findings of a study on Zelkova serrata seedlings grown in saline environments indicated that root inoculation with Funneliformis mosseae resulted in a noticeable enhance in P levels in root and leaf (Jia et al., 2019). The rise in P uptake in AMF-inoculated plants depends on several factors, including 1) increasing the P availability in soil due to alkaline and acid phosphatases secreted by hyphae, 2) P’s continuous movement into the root, because AMF is able to accumulate large amounts of absorbed P to the root, 3) maintaining the intrinsic concentration of phosphate (Pi) through forming polyphosphates within the hyphae, and 4) AMF is able to absorb P at a lower threshold because of high-affinity phosphate transporter genes expression (GmosPT, GiPT, and GvPT) (Vassilev et al., 2012; Abdel-Fattah and Asrar, 2012; Jia et al., 2019). Furthermore, P’s effective absorption by mycorrhizal plants under salinity stress conditions leads to the following benefits: 1) the selective absorption of ions and thus reducing the adverse effects of salinity, 2) partitioning of toxic ions in vacuoles, 3) reduction of ion leakage, and 4) maintaining cell membrane integrity (Bothe, 2012; Cantrell and Linderman, 2001; Evelin et al., 2019) (Table 2).
The researchers observed that the coexistence of AMF rises the absorption of K+ in salt-exposed plants, and plant use K+ uptake as a coping mechanism to salt stress (Giri et al., 2007; Amanifar et al., 2019). Experiments have demonstrated that inoculating salt-exposed roots with AMF upregulates SKOR, resulting in passive secretion of K+ in the xylem flow, ultimately increasing K+ accumulation in the aerial organs and enhancing K+/Na+ ratios (Wang et al., 2021; Evelin et al., 2019). A significant increase in K+ concentration in the roots and leaves of mycorrhizal seedlings, in addition to preventing disturbances in cellular enzymatic processes and inhibiting protein synthesis, enhances stomatal conductance, enhancing water requirement for transpiration (Garg and Bhandari, 2016; Sheng et al., 2008; Van Zelm et al., 2020). Additionally, the results of several studies indicate AMF helps to overcome salinity-induced deficiencies of Mg2+, and Ca2+ by an observable rise in the content of these ions in roots and leaves (Navarro et al., 2014; Evelin et al., 2012; Van Zelm et al., 2020) (Table 2).
It seems that, in addition to preventing Na+ absorption, AMF can reduce salinity’s effects on plants through limiting its transfer to plant aerial parts and also diluting Na+ (Janah et al., 2021b; Borde et al., 2011; Talaat and Shawky, 2011). Vesicles in the AMF can store ions such as Na+ and Cl− under salt stress, and increase plant adaptation to saline conditions by inhibiting their uptake through roots (Ait-El-Mokhtar et al., 2020; Miransari, 2010). When salt is present, AMF participates in the selective ion absorption such as Mg, N, P, Ca, and K, and reduces the absorption of Na+ (Paul and Sinha, 2017; Goussi et al., 2018). Furthermore, mycorrhizal plants can control Na+ transport to the upper parts of plant, as well as regulate the internal concentration of Na+ (Evelin et al., 2019; Santander et al., 2019). Mycorrhizal plants also reduce the accumulation of Na+ in leaves and aerial parts by sequestering Na+ in root vacuoles or taking it out of the cytosol (Evelin et al., 2019; Chen et al., 2017). Moreover, AMF assist the host plant in collecting Na+ from the xylem and diverting it from the tissues responsible for photosynthesis to the roots (Evelin et al., 2012). Further, there is a possibility that the reduction of ionic toxicity in salinity conditions is due to the dilution effect, since AMF boosts growth and biomass by improving the nutritional status of plants, leading to Na+ and Cl− dilution (Talaat and Shawky, 2011; Amanifar et al., 2019).
According to previous studies, plants inoculated with AMF up-regulate aquaporin genes (such as PIP), H+-ATPase genes (such as VHA-B), and Na+/H+ antiporters genes (such as NHX1 and SOS1), and the function of these genes mediates sodium flow and water potential in tissues of plants (Diao et al., 2021; Pedranzani et al., 2016; Evelin et al., 2019; Munns, 2005). Various plant species express these genes differently, in such a way that the expression of NHX is regulated by AMF symbiosis in rice under salt conditions, while there is no significant difference for Robinia pseudoacacia (Diao et al., 2021). Meanwhile, AMF coexistence increased the expression of SOS1 in rice shoots and roots of Robinia pseudoacacia, but decreased this gene’s expression in root of rice (Chen et al., 2017). In addition, Several studies have shown AMF coexistence in saline environments increases the expression of PIP1;1, PIP2;1, and PIP1;3 in Robinia pseudoacacia, while it decreases the expression of PIP1 in tomato roots (Diao et al., 2021; Ouziad et al., 2006).
AMF’s extensive hyphae allow higher hydraulic conductivity, and water absorption capacity even in low water potential and improve soil water availability (Medina and Azcón, 2010; Sheng et al., 2008). In addition, plant root inoculation by AMF in saline environments enhances relative water content (RWC), shoot water content and water use efficiency (WUE) (Al-Khaliel, 2010; Kapoor et al., 2008; Sheng et al., 2008). AMF also rises leaf relative water content (LRWC), and leaf water potential (LWP) in plants grown in saline environments, possibly because it improves water absorption capacity and hydraulic conductivity (Kapoor et al., 2008; Chandrasekaran et al., 2019). The improvement of WUE resulting from root inoculation by AMF resulting in a boost in gas exchange capacity, stomatal conductance and subsequent transpiration in plants stressed by salinity (Elhindi et al., 2017; Sheng et al., 2008; Chandrasekaran et al., 2019).
Studies in the past have stated AMF-inoculated plants have shown a higher photosynthetic capacity stressed by salinity (Liang et al., 2021; Abdel Latef and Chaoxing, 2014). AMF can increase photosynthesis of plants suffering from salinity stress by improving transpiration rate, stomatal conductance, water status of leaves, strengthen of photosynthesis machinery, pigment content, photochemistry and non-photochemistry of PSII, carbon uptake and transfer to mycorrhizae (Zai et al., 2021; Cho et al., 2006; Ait-El-Mokhtar et al., 2020). The coexistence of AMF in salty environments affects age-related changes (ARCs) in metabolome of leaves and partially prevents leaf aging and leads to the better metabolites accumulation (Shtark et al., 2019). In addition, AMF treatment protects leaf cells of saline-grown plants by preventing cell wall detachment, reducing plasma membrane damage, inhibiting chloroplasts vanish, stopping thylakoid destruction, and maintaining chloroplast structure (Liang et al., 2021; Evelin et al., 2013). Less damage to chloroplast structure caused by AMF inoculation might be as a result of a higher osmolyte concentration (sugars, betaine, proline, glycine) and polyamines, and larger and more plastoglobules (higher concentration of alpha-tocopherol) in plants (Evelin et al., 2013; Talaat and Shawky, 2014). Osmolyte accumulation in mycorrhizal plants protects CO2 fixing enzymes [rubisco activase (Rca), and PSII pigment protein complexes ribulose-1,5-bisphosphate (RuBisCO)] (Talaat and Shawky, 2014). Plastoglobules, as tocopherol synthesis sites, also can play a protective role in membrane of thylakoids and proteins (Austin et al., 2006). However, tocopherol probably protects PSII by preventing photooxidation of membrane lipids (Liang et al., 2021). AMF leads to the upregulation of the expression of chloroplast-related genes (RppsbA, and RppsbD), which gives the plant a higher PSII efficiency and then boosts the photosynthetic capacity under salinity stress (Chen et al., 2017). Furthermore, AMF treatment improves photosynthetic capacity by increasing N absorption, because N is considered to be a major component of Rubisco enzymes (Ahanger et al., 2014) (Figure 4; Table 2).
AMF inoculation of plant roots reduces the adverse effects of salinity on chlorophyll content and photosynthetic pigments by removing toxic Na+ and inhibiting its transfer to aerial organs (Abeer et al., 2014; Chandrasekaran et al., 2019; Khalil et al., 2011). AMF can also promote chlorophyll production and accelerate photosynthetic activity by improving the chlorophyll synthetase enzyme activity (Wright et al., 1998; Liang et al., 2021). The study conducted on Echinacea angustifolia has shown that the inoculation of plant roots by Rhizophagus irregularis under salinity stress increased the chlorophyll content of leaves 2 to 3 times more than the treatments without AMF (Liang et al., 2021). Liang et al. also demonstrated that AMF treatment gave a much greater increase in Chl a content compared to Chl b and carotenoids, such that the AMF-induced improvement in Chl a was 25%, however, it was less than 20% for Chl b (Liang et al., 2021). The results of the studies conducted on Sesbania sesban, Solanum lycopersicum, Zelkova serrata, Panicum turgidum, Arundo donax, and Ocimum basilicum grown in saline environments also indicated that the inoculation of roots by AMF species increases the photosynthetic pigments and chlorophyll content (Elhindi et al., 2017; Liang et al., 2021; Pollastri et al., 2018; Wang et al., 2019b). Additionally, research has shown that the mediating AMF’s effect in saline environments on leaf surface, photosynthesis rate, total chlorophyll, Chl a and Chl b in C3 plants is more than C4 plants (Chandrasekaran et al., 2019). Since there is a reciprocal relationship between Soil-Plant Analysis Development (SPAD), leaf nitrogen content, and total chlorophyll, research has shown that colonization of AMF in saline conditions increases SPAD (Campanelli et al., 2013; Porcel et al., 2015; Wu et al., 2007). Moreover, AMF application enhances the absorption of Mg2+ by plants in saline environments, and since the Mg2+ ion is the chlorophyll molecule’s central ion, it boosts the content of chlorophyll in the inoculated plants (Rao and Chaitanya, 2016; Jia et al., 2019). Mg2+ is also necessary for the appropriate function of several enzymes such as glutathione synthase, protein kinases, ATPases, carboxylases, phosphatases, and RNA polymerases (Shaul, 2002).
It is well known that fixation of photosynthetic CO2 is essential for rapid plant growth process, and is extremely sensitive to changes in the environment, including salt stress (Liang and Shi, 2021). Research has indicated that a higher content of photosynthetic pigments is the basis of better photosynthetic gas exchange, and since plant-AMF symbiosis increases chlorophyll content and photosynthetic pigments, thus also improving gas exchange and carbon fixation (Takai et al., 2010; Elhindi et al., 2017). The response of mycorrhizal plants under salinity stress can be complicated in relation to gas exchange, so that the results of studies have shown that although CO2 exchange increases, however, plants face a decrease in intercellular CO2 concentration by increasing photosynthesis, which is due to the effective use of CO2 with the coexistence of AMF (Sheng et al., 2008; Chandrasekaran et al., 2019). Further, the researchers’ results have shown that the coexistence of plants with AMF in saline environments reduces stomatal conductance and transpiration rate (Liang and Shi, 2021). Research also has shown that although C3 plants show higher photosynthesis rate and transpiration rate than C4 plants, however, C4 plants have a higher amplitude in stomatal conductance, WUE, and RWC (Pinto et al., 2014; Chandrasekaran et al., 2019). Additionally, there is a significant relationship among stomatal conductance and photosynthetic capacity (Amax) in C4 plants inoculated with AMF in salty conditions, so that increasing stomatal conductance indicates the enhancement of Amax (Chandrasekaran et al., 2019).
Using AMF in saline environments can improve the ability of plants to dissipate excessive energy through increasing the regulation of energy splitting among photochemical and non-photochemical events and protect the photosynthetic apparatus from excessive light (Sheng et al., 2008; Jia et al., 2019). Increasing photochemical efficiency in plants improves CO2 fixation, photosynthetic activities, Rubisco activities, water status of plants, and stomatal conductance (Gao et al., 2020; Porcel et al., 2015; Navarro et al., 2014). Additionally, AMF balances the absorption and use of light energy to obtain photoprotection and reduces salt stress damage to PSII by decreasing the values of ФPSII (actual PSII efficiency), and qP (photochemical quenching) (Zribi et al., 2009; Chen et al., 2017; Hanachi et al., 2014; Ait-El-Mokhtar et al., 2019). When the salinity is high, AMF inoculation’s positive effects on the level of PSII photoinhibition decreases, which can be due to the host plant ionic imbalance and ultimately disrupting the normal cellular function (Jia et al., 2019). Moreover, several studies have found increasing in non-photochemical quenching (NPQ) in leaves of AMF-inoculated plants in salt-stressed conditions, which is an energy dissipation mechanism that provides protection for the photosynthetic apparatus from excess light under salinity (Jia et al., 2019; Zribi et al., 2009). However, AMF’s effects in saline environments on NPQ was different, so that it increased in Zea mays leaves, while it decreased in Cucumis melo leaves and did not show any change in Robinia pseudoacacia leaves (Jia et al., 2019). AMF also up-regulates the synthesizing genes expression of abscisic acid 8′-hydroxylase, capsanthin/capsorubicin synthase and NAD(P)H-ubiquinone oxidoreductase to enhance the photoprotection mechanisms of chloroplasts (Begum et al., 2019) (Table 2).
AMF reduces ROS, oxidative damage and cell leakage in salt-exposed plants by enhancing the enzymatic, and non-enzymatic antioxidant defense system, Phytohormone synthesis, solute accumulation, stimulation of synthesis of osmolytes, and protection of membrane lipids (Parihar et al., 2020; Bistgani et al., 2019; Begum et al., 2019). In addition, the inoculation of plants grown in saline environments by AMF reduces oxidative stress markers (MDA, and H2O2), and lipid peroxidation (Ait-El-Mokhtar et al., 2020; Gao et al., 2020). Some studies have shown that the content of H2O2 and MDA with AMF inoculation is variable in different plant organs, such that the leaves have a lower content of H2O2 and MDA while the roots have a higher level of them, which is due to more Na+ accumulation in the roots (Chen et al., 2017, 2020; Sewelam et al., 2016) (Table 2).
During saline conditions, symbiosis with AMF reduces the threshold concentration of ROS required to cause oxidative degradation by increasing antioxidants activities such as APX, CAT, SOD, POD, GSH, monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), POX, glutathione reductase (GR), and AsA (Bose et al., 2014; Shahvali et al., 2020; Ait-El-Mokhtar et al., 2020; Apel and Hirt, 2004; Samaddar et al., 2019). In their study on Robinia pseudoacacia stressed by salt, Chen et al. showed that root inoculation by Rhizophagus irregularis caused antioxidant enzyme gene expression (RpMn-SOD, RpCu/Zn-SOD, RpAPX2, RpAPX1, RpGR), which resulted in the reduction of H2O2, MDA, and REL in the leaves (Chen et al., 2020). Coexistence with AMF through increasing the secondary metabolites accumulation in tissues of plants causes morphophysiological and hormonal changes in host plants, including removal of ROS, and production of antioxidant (Pan et al., 2020; de Lazzari Almeida et al., 2018; Evelin et al., 2013). Furthermore, according to studies, AMF not only increases secondary metabolite production and accumulation in medicinal plants under salt stress, but also improves their medicinal value (Zeng et al., 2013; Amanifar et al., 2019) (Figure 5; Table 2).
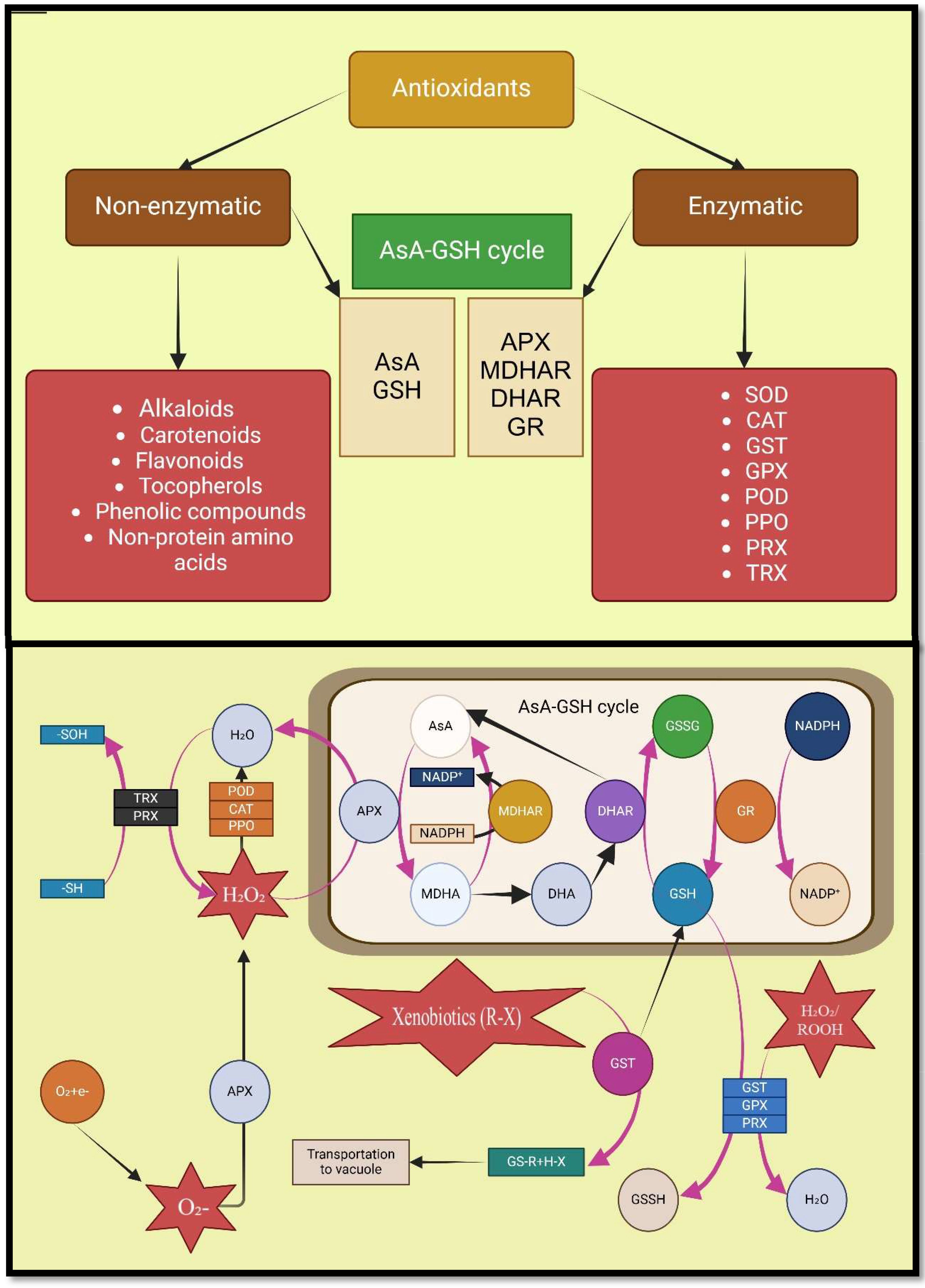
Figure 5. Mechanisms involved in action of various antioxidants. Nomenclature is as proposed by Hasanuzzaman et al. (2021).
Phenols are one of the bioactive compounds that increases in medicinal plants organs by AMF inoculating (Kapoor et al., 2017). Among the polyphenols that rise under the influence of AMF are quercetin-3-arabinoside, luteolin, 4-O-caffeoylquinic acid, 4,5-dicaffeoylquinic acid, and protocatechuic (Aseel et al., 2019). However, in a salt-salt stressed mycorrhizal plant, conflicting observations have been published about phenolic compounds accumulation (Duc et al., 2021). Although research conducted on Echinacea angustifolia showed that mycorrhization increased flavonoids’ total content, the research performed on lettuce leaves identified that the content of phenol decreased significantly (Liang et al., 2021; Santander et al., 2019). Nevertheless, according to Duke et al., these differences are due to the fact that the majority of prior research investigated polyphenol profiles only at a harvest, while polyphenol content varies with plant age (Duc et al., 2021). Additionally, researchers have found that stress intensity affects both increasing and decreasing trends in phenolic compounds in plants colonized by AMF, and the best performance of AMF is in moderate salt stress (Duc et al., 2021; Amanifar and Toghranegar, 2020). Moreover, changes in the metabolism of carbohydrates and primary metabolites, which can be caused by the improvement of nutrients, water absorption and photosynthesis in colonized plants, enhance Phenolic compounds (Arzani and Ashraf, 2016; Pedone-Bonfim et al., 2018).
The synthesis mechanisms of proline, as a proteinogenic amino acid, can be improved by AMF colonization and enhance tolerance of plants to salt (Abd_Allah et al., 2015; Ahanger et al., 2013). Proline reduces the risk of salt stress by preventing free radical damage, maintaining osmotic balance, protein degradation induced by stress, redox enzyme stabilization, protecting membrane integrity, improving cell water retention, and increasing K concentration in the cell (Duc et al., 2021; Lin et al., 2016; Reddy et al., 2015; Ait-El-Mokhtar et al., 2020; Parihar et al., 2020). Plants’ increased proline content can be attributed to factors, such as 1) inactivation of proline dehydrogenase (catalyzes the breakdown of proline), 2) a greater glutamate dehydrogenase enzyme activity (involved in glutamate synthesis, a precursor of proline), 3) a higher level of Pyrroline-5-carboxylate synthase (P5CS), and 4) increasing in P5CS gene expression (Abo-Doma et al., 2011; Garg and Baher, 2013). Nevertheless, studies on the effects of AMF on the concentration of proline in plants under salinity have been contradictory, although several researches have informed higher content of proline in mycorrhizal plants, while others have shown lower proline in plants (Tuo et al., 2015; Elhindi et al., 2017; Shekoofeh et al., 2012; Yooyongwech et al., 2016). Since the proline molecule is regarded as a stress marker, the reduction of proline synthesis in mycorrhizal plants possibly as a result of AMF-mediated stress reduction (Duc et al., 2021; Evelin et al., 2019).
Organic acids are essential osmolytes in the vacuoles of plants, and AMF colonization plays an important involvement in regulating their concentrations and metabolism, increasing tolerance to salinity in plants (Guo et al., 2010; Evelin et al., 2019). A number of organic acids are increased in mycorrhizal plants stressed by salt, including oxalic, malic, fumaric, citric, acetic acids (Sheng et al., 2011). Moreover, plants with AMF symbiosis accumulate more soluble carbohydrates that reduce the harm resulting from excessive salt exposure, through stabilizing the structure and activities of protein complexes, membrane integrity, maintaining the activity of mature leaves, and balancing energy transfer (Gao et al., 2020; Dodd and Pérez-Alfocea, 2012; Diao et al., 2021). Trehalose (α-D-glucopyranosyl-1,1-α-D-glucopyranoside) as a non-reducing disaccharide controls carbohydrate metabolism, and AMF symbiosis can increase this osmolyte accumulation in plants in saline environments (Lunn et al., 2014; Chang et al., 2014). The higher concentration of trehalose in mycorrhizal plants can be assigned to the increased Trehalose-6-phosphate phosphatase (TPP), and Trehalose-6-phosphate synthase (TPS) activities (enzymes responsible for trehalose biosynthesis) by AMF and the decreased TRE activity (trehalose-degrading enzymes, including Trehalase, and Trehalose phosphorylase) (Garg and Bhandari, 2016).
On the other hand, studies have shown that in roots inoculated with AMF, glycyrrhizin biosynthesis is rised in salinity-stressed conditions, that reduces oxidative stress (Orujei et al., 2013; Amanifar et al., 2019). Further, the observed increase in the concentration of glycyrrhizin in mycorrhizal plants might be due to the rise in enzyme molecule number, as well as the boost in the production of terpenoid biosynthesis precursors (Amanifar et al., 2019). A rise in P uptake by mycorrhizal plants results in a rise in the biosynthesis of terpenoids through enhancing the production of pyrophosphate compounds such as dimethylallyl diphosphate (DMAPP), and isopentenyl pyrophosphate (IPP), which are key precursor molecules for the biosynthesis of terpenoids (Xie et al., 2018). In addition, P contribution in the formation of other precursors of terpenoids through MVA (mevalonic acid) [NADPH (Nicotinamide adenine dinucleotide phosphate), ATP (Adenosine triphosphate), and acetyl-CoA (Acetyl coenzyme A)], and MEP (phosphoglyceraldehyde and pyruvate) pathways is crucial (Kapoor et al., 2017). As the major component of glycyrrhizin biosynthesis, farnesyl pyrophosphate (FPP) is synthesized with IPP and DMAPP condensation sequentially (Kapoor et al., 2017) (Table 2).
The review showed that salinity stress can be reduced in plants by using AMF. AMF strengthens plants’ resistance to salinity by enhancing the absorption of nutrients, and water, selective absorption of elements, the photosynthetic apparatus and the antioxidant defense mechanisms. However, the present review indicates that more studies are needed in various fields, including 1) the role of AMF in reducing salinity stress in field experiments, 2) the contribution of different AMF species in improving plant biochemical and molecular structures under salt stress, 3) interaction of different AMF species in saline environments, 4) the effect of AMF on soil physical and chemical structures, as well as plant root architecture, and 5) AMF’s role in simultaneously reducing salinity stress and other abiotic, and biotic stresses.
MB: Conceptualization, Funding acquisition, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. LL: Funding acquisition, Software, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research is the result of the projects: VEGA 1/0186/23 Windbreaks in the agricultural landscape - ecological, environmental and economic value of multifunctional structures acting as soil degradation measures.
The sincere gratitude goes out to our colleagues at the College of Environment and Surveying and Mapping Engineering, Suzhou University, Anhui, who supported us during this review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbaspour, H., Pour, F. S., Abdel-Wahhab, M. A. (2021). Arbuscular mycorrhizal symbiosis regulates the physiological responses, ion distribution and relevant gene expression to trigger salt stress tolerance in pistachio. Physiol. Mol. Biol. Plants 27, 1765–1778. doi: 10.1007/s12298-021-01043-w
Abd_Allah, E. F., Hashem, A., Alqarawi, A. A., Bahkali, A. H., Alwhibi, M. S. (2015). Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 22, 274–283. doi: 10.1016/j.sjbs.2015.03.004
Abdel-Fattah, G. M., Asrar, A.-W. A. (2012). Arbuscular mycorrhizal fungal application to improve growth and tolerance of wheat (Triticum aestivum L.) plants grown in saline soil. Acta Physiologiae Plantarum 34, 267–277. doi: 10.1007/s11738-011-0825-6
Abdel Latef, A. A. H., Chaoxing, H. (2014). Does inoculation with Glomus mosseae improve salt tolerance in pepper plants? J. Plant Growth Regul. 33, 644–653. doi: 10.1007/s00344-014-9414-4
Abdel Latef, A. A. H., Kordrostami, M., Zakir, A., Zaki, H., Saleh, O. M. (2019). Eustress with H2O2 facilitates plant growth by improving tolerance to salt stress in two wheat cultivars. Plants 8, 303. doi: 10.3390/plants8090303
Abd Rahim, N., Jais, H. M., Hassan, H. M. (2016). Environment and host affects arbuscular mycorrhiza fungi (AMF) population. Trop. Life Sci. Res. 27, 9. doi: 10.21315/tlsr2016.27.3.2
Abeer, H., Abd_Allah, E., Alqarawi, A., El-Didamony, G., Alwhibi, M., Egamberdieva, D., et al. (2014). Alleviation of adverse impact of salinity on faba bean (Vicia faba L.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 46, 2003–2013.
Abo-Doma, A., Edrees, S., Abdel-Aziz, S. (2011). The effect of mycorrhiza growth and expression of some genes in barley. Egyptian J. Genet. Cytology 40, 301–313. doi: 10.21608/ejgc.2011.10794
Adolfsson, L., Nziengui, H., Abreu, I. N., Šimura, J., Beebo, A., Herdean, A., et al. (2017). Enhanced secondary-and hormone metabolism in leaves of arbuscular mycorrhizal Medicago truncatula. Plant Physiol. 175, 392–411. doi: 10.1104/pp.16.01509
Ahanger, M. A., Agarwal, R., Tomar, N. S., Shrivastava, M. (2015). Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L cultivar Kent). J. Plant Interact. 10, 211–223. doi: 10.1080/17429145.2015.1056260
Ahanger, M. A., Hashem, A., Abd-Allah, E. F., Ahmad, P. (2014). “Arbuscular mycorrhiza in crop improvement under environmental stress,” in Emerging technologies and management of crop stress tolerance (Cambridge, MA: Academic Press), 69–95.
Ahanger, M. A., Tyagi, S. R., Wani, M. R., Ahmad, P. (2013). “Drought tolerance: role of organic osmolytes, growth regulators, and mineral nutrients,” in Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment: Volume 1 (New York, NY: Springer).
Ait-El-Mokhtar, M., Baslam, M., Ben-Laouane, R., Anli, M., Boutasknit, A., Mitsui, T., et al. (2020). Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front. Sustain. Food Syst. 4, 131. doi: 10.3390/plants8090303
Ait-El-Mokhtar, M., Laouane, R. B., Anli, M., Boutasknit, A., Wahbi, S., Meddich, A. (2019). Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Scientia Hortic. 253, 429–438. doi: 10.1016/j.scienta.2019.04.066
Akram, M. S., Ashraf, M. (2011). Exogenous application of potassium dihydrogen phosphate can alleviate the adverse effects of salt stress on sunflower. J. Plant Nutr. 34, 1041–1057. doi: 10.1080/01904167.2011.555585
Aliasgharzadeh, N., Rastin, S. N., Towfighi, H., Alizadeh, A. (2001). Occurrence of arbuscular mycorrhizal fungi in saline soils of the Tabriz Plain of Iran in relation to some physical and chemical properties of soil. Mycorrhiza 11, 119–122. doi: 10.1007/s005720100113
Al-Khaliel, A. (2010). Effect of salinity stress on mycorrhizal association and growth response of peanut infected by Glomus mosseae. Plant Soil Environ. 56, 318–324. doi: 10.17221/204/2009-PSE
Amanifar, S., Khodabandeloo, M., Fard, E. M., Askari, M. S., Ashrafi, M. (2019). Alleviation of salt stress and changes in glycyrrhizin accumulation by arbuscular mycorrhiza in liquorice (Glycyrrhiza glabra) grown under salinity stress. Environ. Exp. Bot. 160, 25–34. doi: 10.1016/j.envexpbot.2019.01.001
Amanifar, S., Toghranegar, Z. (2020). The efficiency of arbuscular mycorrhiza for improving tolerance of Valeriana officinalis L. and enhancing valerenic acid accumulation under salinity stress. Ind. Crops Products 147, 112234. doi: 10.1016/j.indcrop.2020.112234
Apel, K., Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Apse, M. P., Blumwald, E. (2007). Na+ transport in plants. FEBS Lett. 581, 2247–2254. doi: 10.1016/j.febslet.2007.04.014
Aroca, R., Ruiz-Lozano, J. M., Zamarreño, Á.M., Paz, J. A., García-Mina, J. M., Pozo, M. J., et al. (2013). Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 170, 47–55. doi: 10.1016/j.jplph.2012.08.020
Arunin, S., Pongwichian, P. (2015). Salt-affected soils and management in Thailand. Bull. Soc. Sea Water Science Japan 69, 319–325. doi: 10.11457/swsj.69.319
Arzani, A., Ashraf, M. (2016). Smart engineering of genetic resources for enhanced salinity tolerance in crop plants. Crit. Rev. Plant Sci. 35, 146–189. doi: 10.1080/07352689.2016.1245056
Aseel, D. G., Rashad, Y. M., Hammad, S. M. (2019). Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against Tomato Mosaic Virus. Sci. Rep. 9, 9692. doi: 10.1038/s41598-019-46281-x
Ashraf, M., Harris, P. J. (2013). Photosynthesis under stressful environments: An overview. Photosynthetica 51, 163–190. doi: 10.1007/s11099-013-0021-6
Augé, R. M., Toler, H. D., Saxton, A. M. (2014). Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: a meta-analysis. Front. Plant Sci. 5, 111035. doi: 10.3389/fpls.2014.00562
Austin, J. R., Frost, E., Vidi, P.-A., Kessler, F., Staehelin, L. A. (2006). Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 18, 1693–1703. doi: 10.1105/tpc.105.039859
Balliu, A., Sallaku, G., Rewald, B. (2015). AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 7, 15967–15981. doi: 10.3390/su71215799
Bano, A., Fatima, M. (2009). Salt tolerance in Zea mays (L). following inoculation with Rhizobium and Pseudomonas. Biol. fertility soils 45, 405–413. doi: 10.1007/s00374-008-0344-9
Begum, N., Qin, C., Ahanger, M. A., Raza, S., Khan, M. I., Ashraf, M., et al. (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front. Plant Sci. 10, 1068. doi: 10.3389/fpls.2019.01068
Behdad, A., Mohsenzadeh, S., Azizi, M., Moshtaghi, N. (2020). Salinity effects on physiological and phytochemical characteristics and gene expression of two Glycyrrhiza glabra L. populations. Phytochemistry 171, 112236. doi: 10.1016/j.phytochem.2019.112236
Benito, B., Haro, R., Amtmann, A., Cuin, T. A., Dreyer, I. (2014). The twins K+ and Na+ in plants. J. Plant Physiol. 171, 723–731. doi: 10.1016/j.jplph.2013.10.014
Bernstein, N., Eshel, A., Beeckman, T. (2013). Effects of salinity on root growth. Plant roots: hidden half 10.
Bistgani, Z. E., Hashemi, M., Dacosta, M., Craker, L., Maggi, F., Morshedloo, M. R. (2019). Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Products 135, 311–320. doi: 10.1016/j.indcrop.2019.04.055
Boorboori, M. R. (2023). Investigating the role of silicon in reducing the risk of arsenic, cadmium, drought and salinity stresses in wheat (Triticum aestivum L.). J. Crop Sci. Biotechnol. 26, 387–404. doi: 10.1007/s12892-022-00191-z
Boorboori, M. R., Zhang, H.-Y. (2022). The role of Serendipita indica (Piriformospora indica) in improving plant resistance to drought and salinity stresses. Biology 11, 952. doi: 10.3390/biology11070952
Boorboori, M. R., Zhang, H. (2023). The mechanisms of trichoderma species to reduce drought and salinity stress in plants. Phyton (0031-9457) 92. doi: 10.32604/phyton.2023.029486
Borde, M., Dudhane, M., Jite, P. (2011). Growth photosynthetic activity and antioxidant responses of mycorrhizal and non-mycorrhizal bajra (Pennisetum glaucum) crop under salinity stress condition. Crop Prot. 30, 265–271. doi: 10.1016/j.cropro.2010.12.010
Bose, J., Rodrigo-Moreno, A., Shabala, S. (2014). ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 65, 1241–1257. doi: 10.1093/jxb/ert430
Bothe, H. (2012). Arbuscular mycorrhiza and salt tolerance of plants. Symbiosis 58, 7–16. doi: 10.1007/s13199-012-0196-9
Boughalleb, F., Abdellaoui, R., Mahmoudi, M., Bakhshandeh, E. (2020). Changes in phenolic profile, soluble sugar, proline, and antioxidant enzyme activities of Polygonum equisetiforme in response to salinity. Turkish J. Bot. 44, 25–35. doi: 10.3906/bot-1908-2
Campagnac, E., Khasa, D. (2014). Relationship between genetic variability in Rhizophagus irregularis and tolerance to saline conditions. Mycorrhiza 24, 121–129. doi: 10.1007/s00572-013-0517-8
Campanelli, A., Ruta, C., De Mastro, G., Morone-Fortunato, I. (2013). The role of arbuscular mycorrhizal fungi in alleviating salt stress in Medicago sativa L. var. icon. Symbiosis 59, 65–76. doi: 10.1007/s13199-012-0191-1
Cantrell, I. C., Linderman, R. G. (2001). Preinoculation of lettuce and onion with VA mycorrhizal fungi reduces deleterious effects of soil salinity. Plant Soil 233, 269–281. doi: 10.1023/A:1010564013601
Chaichi, M., Keshavarz-Afshar, R., Lu, B., Rostamza, M. (2017). Growth and nutrient uptake of tomato in response to application of saline water, biological fertilizer, and surfactant. J. Plant Nutr. 40, 457–466. doi: 10.1080/01904167.2016.1246567
Chandrasekaran, M., Chanratana, M., Kim, K., Seshadri, S., SA, T. (2019). Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress–a meta-analysis. Front. Plant Sci. 10, 457. doi: 10.3389/fpls.2019.00457
Chang, W., Sui, X., Fan, X.-X., Jia, T.-T., Song, F.-Q. (2018). Arbuscular mycorrhizal symbiosis modulates antioxidant response and ion distribution in salt-stressed Elaeagnus angustifolia seedlings. Front. Microbiol. 9, 338240. doi: 10.3389/fmicb.2018.00652
Chang, B., Yang, L., Cong, W., Zu, Y., Tang, Z. (2014). The improved resistance to high salinity induced by trehalose is associated with ionic regulation and osmotic adjustment in Catharanthus roseus. Plant Physiol. Biochem. 77, 140–148. doi: 10.1016/j.plaphy.2014.02.001
Chaparzadeh, N., D'amico, M. L., Khavari-Nejad, R.-A., Izzo, R., Navari-Izzo, F. (2004). Antioxidative responses of Calendula officinalis under salinity conditions. Plant Physiol. Biochem. 42, 695–701. doi: 10.1016/j.plaphy.2004.07.001
Chaves, M. M., Flexas, J., Pinheiro, C. (2009). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 103, 551–560. doi: 10.1093/aob/mcn125
Chen, T. H., Murata, N. (2011). Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ. 34, 1–20. doi: 10.1111/j.1365-3040.2010.02232.x
Chen, J., Zhang, H., Zhang, X., Tang, M. (2017). Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 8, 1739. doi: 10.3389/fpls.2017.01739
Chen, J., Zhang, H., Zhang, X., Tang, M. (2020). Arbuscular mycorrhizal symbiosis mitigates oxidative injury in black locust under salt stress through modulating antioxidant defence of the plant. Environ. Exp. Bot. 175, 104034. doi: 10.1016/j.envexpbot.2020.104034
Chinnusamy, V., Jagendorf, A., Zhu, J. K. (2005). Understanding and improving salt tolerance in plants. Crop Sci. 45, 437–448. doi: 10.2135/cropsci2005.0437
Cho, K., Toler, H., Lee, J., Ownley, B., Stutz, J. C., Moore, J. L., et al. (2006). Mycorrhizal symbiosis and response of sorghum plants to combined drought and salinity stresses. J. Plant Physiol. 163, 517–528. doi: 10.1016/j.jplph.2005.05.003
Deinlein, U., Stephan, A. B., Horie, T., Luo, W., Xu, G., Schroeder, J. I. (2014). Plant salt-tolerance mechanisms. Trends Plant Sci. 19, 371–379. doi: 10.1016/j.tplants.2014.02.001
de Lazzari Almeida, C., Sawaya, A. C. H. F., De Andrade, S. A. L. (2018). Mycorrhizal influence on the growth and bioactive compounds composition of two medicinal plants: Mikania glomerata Spreng. and Mikania laevigata Sch. Bip. ex Baker (Asteraceae). Braz. J. Bot. 41, 233–240. doi: 10.1007/s40415-017-0436-6
de Varennes, A., Goss, M. J. (2007). The tripartite symbiosis between legumes, rhizobia and indigenous mycorrhizal fungi is more efficient in undisturbed soil. Soil Biol. Biochem. 39, 2603–2607. doi: 10.1016/j.soilbio.2007.05.007
Diagne, N., Ndour, M., Djighaly, P. I., Ngom, D., Ngom, M. C. N., Ndong, G., et al. (2020). Effect of plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) on salt stress tolerance of Casuarina obesa (Miq.). Front. Sustain. Food Syst. 4, 601004. doi: 10.3389/fsufs.2020.601004
Diao, F., Dang, Z., Cui, X., Xu, J., Jia, B., Ding, S., et al. (2021). Transcriptomic analysis revealed distinctive modulations of arbuscular mycorrhizal fungi inoculation in halophyte Suaeda salsa under moderate salt conditions. Environ. Exp. Bot. 183, 104337. doi: 10.1016/j.envexpbot.2020.104337
Ding, X., Jiang, Y., Hao, T., Jin, H., Zhang, H., He, L., et al. (2016). Effects of heat shock on photosynthetic properties, antioxidant enzyme activity, and downy mildew of cucumber (Cucumis sativus L.). PloS One 11, e0152429. doi: 10.1371/journal.pone.0152429
Dodd, I. C., Pérez-Alfocea, F. (2012). Microbial amelioration of crop salinity stress. J. Exp. Bot. 63, 3415–3428. doi: 10.1093/jxb/ers033
Duarte, B., Santos, D., Marques, J., Caçador, I. (2013). Ecophysiological adaptations of two halophytes to salt stress: photosynthesis, PS II photochemistry and anti-oxidant feedback–implications for resilience in climate change. Plant Physiol. Biochem. 67, 178–188. doi: 10.1016/j.plaphy.2013.03.004
Duc, N. H., Vo, A. T., Haddidi, I., Daood, H., Posta, K. (2021). Arbuscular mycorrhizal fungi improve tolerance of the medicinal plant Eclipta prostrata (L.) and induce major changes in polyphenol profiles under salt stresses. Front. Plant Sci. 11, 612299. doi: 10.3389/fpls.2020.612299
Egamberdieva, D. (2009). Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiologiae Plantarum 31, 861–864. doi: 10.1007/s11738-009-0297-0
Egamberdieva, D., Jabborova, D., Berg, G. (2016). Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, and nodulation of soybean under salt stress. Plant Soil 405, 35–45. doi: 10.1007/s11104-015-2661-8
Elboutahiri, N., Thami-Alami, I., Udupa, S. M. (2010). Phenotypic and genetic diversity in Sinorhizobium meliloti and S. medicae from drought and salt affected regions of Morocco. BMC Microbiol. 10, 1–13. doi: 10.1186/1471-2180-10-15
Elhindi, K. M., El-Din, A. S., Elgorban, A. M. (2017). The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi J. Biol. Sci. 24, 170–179. doi: 10.1016/j.sjbs.2016.02.010
El Kinany, S., Achbani, E., Faggroud, M., Ouahmane, L., EL Hilali, R., Haggoud, A., et al. (2019). Effect of organic fertilizer and commercial arbuscular mycorrhizal fungi on the growth of micropropagated date palm cv. Feggouss. J. Saudi Soc. Agric. Sci. 18, 411–417. doi: 10.1016/j.jssas.2018.01.004
Estrada, B., Aroca, R., Maathuis, F. J., Barea, J. M., Ruiz-Lozano, J. M. (2013). Arbuscular mycorrhizal fungi native from a M editerranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ. 36, 1771–1782. doi: 10.1111/pce.2013.36.issue-10
Evelin, H., Devi, T. S., Gupta, S., Kapoor, R. (2019). Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: current understanding and new challenges. Front. Plant Sci. 10, 450967. doi: 10.3389/fpls.2019.00470
Evelin, H., Giri, B., Kapoor, R. (2012). Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 22, 203–217. doi: 10.1007/s00572-011-0392-0
Evelin, H., Giri, B., Kapoor, R. (2013). Ultrastructural evidence for AMF mediated salt stress mitigation in Trigonella foenum-graecum. Mycorrhiza 23, 71–86. doi: 10.1007/s00572-012-0449-8
Evelin, H., Kapoor, R., Giri, B. (2009). Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann. Bot. 104, 1263–1280. doi: 10.1093/aob/mcp251
Fall, D., Bakhoum, N., Fall, F., Diouf, F., Ndiaye, C., Faye, M. N., et al. (2018). Effect of peanut shells amendment on soil properties and growth of seedlings of Senegalia Senegal (L.) Britton, Vachellia seyal (Delile) P. Hurter, and Prosopis juliflora (Swartz) DC in salt-affected soils. Ann. For. Sci. 75, 1–11. doi: 10.1007/s13595-018-0714-x
Fan, L., Fang, C., Dubé, C., Deschênes, M., Dalpé, Y., Tao, S., et al. (2012). Arbuscular mycorrhiza alleviates salinity stress of strawberry cultivars under salinity condition. Acta Hortic. 926, 491–496. doi: 10.17660/ActaHortic.2012.926.69
Farissi, M., Bouizgaren, A., Faghire, M., Bargaz, A., Ghoulam, C. (2011). Agro-physiological responses of Moroccan alfalfa (Medicago sativa L.) populations to salt stress during germination and early seedling stages. Seed Sci. Technol. 39, 389–401. doi: 10.15258/sst.2011.39.2.11
Fileccia, V., Ruisi, P., Ingraffia, R., Giambalvo, D., Frenda, A. S., Martinelli, F. (2017). Arbuscular mycorrhizal symbiosis mitigates the negative effects of salinity on durum wheat. PloS One 12, e0184158. doi: 10.1371/journal.pone.0184158
Flowers, T. J., Muscolo, A. (2015). Introduction to the special issue: halophytes in a changing world. AoB Plants 7, plv020. doi: 10.1093/aobpla/plv020
Flowers, T., Troke, P., Yeo, A. (1977). The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Physiol. 28, 89–121. doi: 10.1146/annurev.pp.28.060177.000513
Foyer, C. H., Noctor, G. (2011). Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 155, 2–18. doi: 10.1104/pp.110.167569
Frechilla, S., Lasa, B., Ibarretxe, L., Lamsfus, C., Aparicio-Tejo, P. (2001). Pea responses to saline stress is affected by the source of nitrogen nutrition (ammonium or nitrate). Plant Growth Regul. 35, 171–179. doi: 10.1023/A:1014487908495
Frosi, G., Barros, V. A., Oliveira, M. T., Santos, M., Ramos, D. G., Maia, L. C., et al. (2018). Arbuscular mycorrhizal fungi and foliar phosphorus inorganic supply alleviate salt stress effects in physiological attributes, but only arbuscular mycorrhizal fungi increase biomass in woody species of a semiarid environment. Tree Physiol. 38, 25–36. doi: 10.1093/treephys/tpx105
Füzy, A., Biró, B., Tóth, T., Hildebrandt, U., Bothe, H. (2008). Drought, but not salinity, determines the apparent effectiveness of halophytes colonized by arbuscular mycorrhizal fungi. J. Plant Physiol. 165, 1181–1192. doi: 10.1016/j.jplph.2007.08.010
Gao, T., Liu, X., Shan, L., Wu, Q., Liu, Y., Zhang, Z., et al. (2020). Dopamine and arbuscular mycorrhizal fungi act synergistically to promote apple growth under salt stress. Environ. Exp. Bot. 178, 104159. doi: 10.1016/j.envexpbot.2020.104159
Garg, N., Baher, N. (2013). Role of arbuscular mycorrhizal symbiosis in proline biosynthesis and metabolism of Cicer arietinum L.(chickpea) genotypes under salt stress. J. Plant Growth Regul. 32, 767–778. doi: 10.1007/s00344-013-9346-4
Garg, N., Bhandari, P. (2016). Silicon nutrition and mycorrhizal inoculations improve growth, nutrient status, K+/Na+ ratio and yield of Cicer arietinum L. genotypes under salinity stress. Plant Growth Regul. 78, 371–387. doi: 10.1007/s10725-015-0099-x
Ghorchiani, M., Etesami, H., Alikhani, H. A. (2018). Improvement of growth and yield of maize under water stress by co-inoculating an arbuscular mycorrhizal fungus and a plant growth promoting rhizobacterium together with phosphate fertilizers. Agriculture Ecosyst. Environ. 258, 59–70. doi: 10.1016/j.agee.2018.02.016
Gill, S. S., Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Giri, B., Kapoor, R., Mukerji, K. (2003). Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biol. Fertility Soils 38, 170–175. doi: 10.1007/s00374-003-0636-z
Giri, B., Kapoor, R., Mukerji, K. (2007). Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microbial Ecol. 54, 753–760. doi: 10.1007/s00248-007-9239-9
Giri, B., Mukerji, K. G. (2004). Mycorrhizal inoculant alleviates salt stress in Sesbania aEgyptiaca and Sesbania grandiflora under field conditions: evidence for reduced sodium and improved magnesium uptake. Mycorrhiza 14, 307–312. doi: 10.1007/s00572-003-0274-1
Goussi, R., Manaa, A., Derbali, W., Cantamessa, S., Abdelly, C., Barbato, R. (2018). Comparative analysis of salt stress, duration and intensity, on the chloroplast ultrastructure and photosynthetic apparatus in Thellungiella salsuginea. J. Photochem. Photobiol. B: Biol. 183, 275–287. doi: 10.1016/j.jphotobiol.2018.04.047
Greenway, H., Munns, R. (1980). Mechanisms of salt tolerance in nonhalophytes. Annu. Rev. Plant Physiol. 31, 149–190. doi: 10.1146/annurev.pp.31.060180.001053
Guo, J., Du, M., Lu, C., Wang, B. (2020). NaCl improves reproduction by enhancing starch accumulation in the ovules of the euhalophyte Suaeda salsa. BMC Plant Biol. 20, 1–16. doi: 10.1186/s12870-020-02468-3
Guo, L., Shi, D., Wang, D. (2010). The key physiological response to alkali stress by the alkali-resistant halophyte Puccinellia tenuiflora is the accumulation of large quantities of organic acids and into the rhyzosphere. J. Agron. Crop Sci. 196, 123–135. doi: 10.1111/j.1439-037X.2009.00397.x
Gupta, S., Schillaci, M., Walker, R., Smith, P. M., Watt, M., Roessner, U. (2021). Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil 461, 219–244. doi: 10.1007/s11104-020-04618-w
Hammer, E. C., Nasr, H., Pallon, J., Olsson, P. A., Wallander, H. (2011). Elemental composition of arbuscular mycorrhizal fungi at high salinity. Mycorrhiza 21, 117–129. doi: 10.1007/s00572-010-0316-4
Hanachi, S., Van Labeke, M.-C., Mehouachi, T. (2014). Application of chlorophyll fluorescence to screen eggplant (Solanum melongena L.) cultivars for salt tolerance. Photosynthetica 52, 57–62. doi: 10.1007/s11099-014-0007-z
Hasanuzzaman, M., Raihan, M. R. H., Masud, A. A. C., Rahman, K., Nowroz, F., Rahman, M., et al. (2021). Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 22, 9326. doi: 10.3390/ijms22179326
Hasegawa, P. M., Bressan, R. A., Zhu, J.-K., Bohnert, H. J. (2000). Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol. 51, 463–499. doi: 10.1146/annurev.arplant.51.1.463
Hashem, A., Abd_Allah, E. F., Alqarawi, A. A., Al-Huqail, A. A., Wirth, S., Egamberdieva, D. (2016). The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 7, 1089. doi: 10.3389/fmicb.2016.01089
Hashem, A., Alqarawi, A. A., Radhakrishnan, R., Al-Arjani, A.-B. F., Aldehaish, H. A., Egamberdieva, D., et al. (2018). Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 25, 1102–1114. doi: 10.1016/j.sjbs.2018.03.009
Himabindu, Y., Chakradhar, T., Reddy, M. C., Kanygin, A., Redding, K. E., Chandrasekhar, T. (2016). Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ. Exp. Bot. 124, 39–63. doi: 10.1016/j.envexpbot.2015.11.010
Hoff, T., Stummann, B. M., Henningsen, K. W. (1992). Structure, function and regulation of nitrate reductase in higher plants. Physiologia Plantarum 84, 616–624. doi: 10.1111/j.1399-3054.1992.tb04712.x
Huang, R.-D. (2018). Research progress on plant tolerance to soil salinity and alkalinity in sorghum. J. Integr. Agric. 17, 739–746. doi: 10.1016/S2095-3119(17)61728-3
Huang, Z., He, C.-X., He, Z.-Q., Zou, Z.-R., Zhang, Z.-B. (2010). The effects of arbuscular mycorrhizal fungi on reactive oxyradical scavenging system of tomato under salt tolerance. Agric. Sci. China 9, 1150–1159. doi: 10.1016/S1671-2927(09)60202-9
Jabeen, Z., Hussain, N., Han, Y., Shah, M. J., Zeng, F., Zeng, J., et al. (2014). The differences in physiological responses, ultrastructure changes, and Na+ subcellular distribution under salt stress among the barley genotypes differing in salt tolerance. Acta physiologiae plantarum 36, 2397–2407. doi: 10.1007/s11738-014-1613-x
Janah, I., Elhasnaoui, A., Issa Ali, O., Lamnai, K., Aissam, S., Loutfi, K. (2021a). Physiochemical responses of Stevia rebaudiana Bertoni subjected to sodium chloride (NaCl) salinity and exogenous salicylic acid application. Gesunde Pflanzen 73, 509–520. doi: 10.1007/s10343-021-00570-6
Janah, I., Meddich, A., Elhasnaoui, A., Khayat, S., Anli, M., Boutasknit, A., et al. (2021b). Arbuscular mycorrhizal fungi mitigates salt stress toxicity in Stevia rebaudiana Bertoni through the modulation of physiological and biochemical responses. J. Soil Sci. Plant Nutr. 23, 152–162. doi: 10.1007/s42729-021-00690-y
Jia, T., Wang, J., Chang, W., Fan, X., Sui, X., Song, F. (2019). Proteomics analysis of E. angustifolia seedlings inoculated with arbuscular mycorrhizal fungi under salt stress. Int. J. Mol. Sci. 20, 788. doi: 10.3390/ijms20030788
Johnson, N. C., Wilson, G. W., Wilson, J. A., Miller, R. M., Bowker, M. A. (2015). Mycorrhizal phenotypes and the l aw of the m inimum. New Phytol. 205, 1473–1484. doi: 10.1111/nph.2015.205.issue-4
Jung, J. K., McCouch, S. (2013). Getting to the roots of it: genetic and hormonal control of root architecture. Front. Plant Sci. 4, 186. doi: 10.3389/fpls.2013.00186
Juniper, S., Abbott, L. (2006). Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 16, 371–379. doi: 10.1007/s00572-006-0046-9
Kapoor, R., Anand, G., Gupta, P., Mandal, S. (2017). Insight into the mechanisms of enhanced production of valuable terpenoids by arbuscular mycorrhiza. Phytochem. Rev. 16, 677–692. doi: 10.1007/s11101-016-9486-9
Kapoor, R., Sharma, D., Bhatnagar, A. (2008). Arbuscular mycorrhizae in micropropagation systems and their potential applications. Scientia Hortic. 116, 227–239. doi: 10.1016/j.scienta.2008.02.002
Kaya, C., Ashraf, M., Sonmez, O., Aydemir, S., Tuna, A. L., Cullu, M. A. (2009). The influence of arbuscular mycorrhizal colonisation on key growth parameters and fruit yield of pepper plants grown at high salinity. Scientia Hortic. 121, 1–6. doi: 10.1016/j.scienta.2009.01.001
Khalil, H. A., Eissa, A. M., El-Shazly, S. M., Nasr, A. M. A. (2011). Improved growth of salinity-stressed citrus after inoculation with mycorrhizal fungi. Scientia Hortic. 130, 624–632. doi: 10.1016/j.scienta.2011.08.019
Khan, A. L., Waqas, M., Lee, I.-J. (2015). Resilience of Penicillium resedanum LK6 and exogenous gibberellin in improving Capsicum annuum growth under abiotic stresses. J. Plant Res. 128, 259–268. doi: 10.1007/s10265-014-0688-1
Kumar, A., Dames, J. F., Gupta, A., Sharma, S., Gilbert, J. A., Ahmad, P. (2015). Current developments in arbuscular mycorrhizal fungi research and its role in salinity stress alleviation: a biotechnological perspective. Crit. Rev. Biotechnol. 35, 461–474. doi: 10.3109/07388551.2014.899964
Kumar, A., Sharma, S., Mishra, S. (2010). Influence of arbuscular mycorrhizal (AM) fungi and salinity on seedling growth, solute accumulation, and mycorrhizal dependency of Jatropha curcas L. J. Plant Growth Regul. 29, 297–306. doi: 10.1007/s00344-009-9136-1
Kumar, A., Verma, J. P. (2018). Does plant—microbe interaction confer stress tolerance in plants: a review? Microbiological Res. 207, 41–52. doi: 10.1016/j.micres.2017.11.004
Lanfranco, L., Fiorilli, V., Venice, F., Bonfante, P. (2018). Strigolactones cross the kingdoms: plants, fungi, and bacteria in the arbuscular mycorrhizal symbiosis. J. Exp. Bot. 69, 2175–2188. doi: 10.1093/jxb/erx432
Latef, A. A. H. A., Chaoxing, H. (2011). Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Scientia Hortic. 127, 228–233. doi: 10.1016/j.scienta.2010.09.020
Legros, J.-P. (2009). La salinisation des terres dans le monde. Proc. Academie Des. Sci. Lettres Montpellier Conf. n, 257–269.
Li, Y., Kong, D., Fu, Y., Sussman, M. R., Wu, H. (2020). The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 148, 80–89. doi: 10.1016/j.plaphy.2020.01.006
Li, S., Wang, Y., Wang, C., Zhang, Y., Sun, D., Zhou, P., et al. (2023). Cryo-EM structure reveals a symmetry reduction of the plant outward-rectifier potassium channel SKOR. Cell Discovery 9, 67. doi: 10.1038/s41421-023-00572-w
Liang, J., Shi, W. (2021). Cotton/halophytes intercropping decreases salt accumulation and improves soil physicochemical properties and crop productivity in saline-alkali soils under mulched drip irrigation: A three-year field experiment. Field Crops Res. 262, 108027. doi: 10.1016/j.fcr.2020.108027
Liang, B., Wang, W., Fan, X., Kurakov, A., Liu, Y., Song, F., et al. (2021). Arbuscular mycorrhizal fungi can ameliorate salt stress in Elaeagnus angustifolia by improving leaf photosynthetic function and ultrastructure. Plant Biol. 23, 232–241. doi: 10.1111/plb.v23.s1
Lin, D., Xiao, M., Zhao, J., Li, Z., Xing, B., Li, X., et al. (2016). An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 21, 1374. doi: 10.3390/molecules21101374
Litalien, A., Zeeb, B. (2020). Curing the earth: A review of anthropogenic soil salinization and plant-based strategies for sustainable mitigation. Sci. Total Environ. 698, 134235. doi: 10.1016/j.scitotenv.2019.134235
Liu, J., Shi, D.-C. (2010). Photosynthesis, chlorophyll fluorescence, inorganic ion and organic acid accumulations of sunflower in responses to salt and salt-alkaline mixed stress. Photosynthetica 48, 127–134. doi: 10.1007/s11099-010-0017-4
López-Ráez, J. A. (2016). How drought and salinity affect arbuscular mycorrhizal symbiosis and strigolactone biosynthesis? Planta 243, 1375–1385. doi: 10.1007/s00425-015-2435-9
Lunn, J. E., Delorge, I., Figueroa, C. M., Van Dijck, P., Stitt, M. (2014). Trehalose metabolism in plants. Plant J. 79, 544–567. doi: 10.1111/tpj.2014.79.issue-4
Maathuis, F. J., Amtmann, A. (1999). K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann. Bot. 84, 123–133. doi: 10.1006/anbo.1999.0912
Maherali, H., Klironomos, J. N. (2007). Influence of phylogeny on fungal community assembly and ecosystem functioning. science 316, 1746–1748. doi: 10.1126/science.1143082
Mathur, S., Tomar, R. S., Jajoo, A. (2019). Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynthesis Res. 139, 227–238. doi: 10.1007/s11120-018-0538-4
Medina, A., Azcón, R. (2010). Effectiveness of the application of arbuscular mycorrhiza fungi and organic amendments to improve soil quality and plant performance under stress conditions. J. Soil Sci. Plant Nutr. 10, 354–372. doi: 10.4067/S0718-95162010000100009
Miller, G., Suzuki, N., Ciftci-Yilmaz, S., Mittler, R. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33, 453–467. doi: 10.1111/j.1365-3040.2009.02041.x
Miransari, M. (2010). Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 12, 563–569. doi: 10.1111/j.1438-8677.2009.00308.x
Miransari, M., Bahrami, H., Rejali, F., Malakouti, M. (2008). Using arbuscular mycorrhiza to alleviate the stress of soil compaction on wheat (Triticum aestivum L.) growth. Soil Biol. Biochem. 40, 1197–1206. doi: 10.1016/j.soilbio.2007.12.014
Munns, R. (2002). Comparative physiology of salt and water stress. Plant Cell Environ. 25, 239–250. doi: 10.1046/j.0016-8025.2001.00808.x
Munns, R. (2005). Genes and salt tolerance: bringing them together. New Phytol. 167, 645–663. doi: 10.1111/j.1469-8137.2005.01487.x
Munns, R., Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Nath, M., Bhatt, D., Prasad, R., Gill, S. S., Anjum, N. A., Tuteja, N. (2016). Reactive oxygen species generation-scavenging and signaling during plant-arbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front. Plant Sci. 7, 219102. doi: 10.3389/fpls.2016.01574
Navarro, J. M., Pérez-Tornero, O., Morte, A. (2014). Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on the rootstock salt tolerance. J. Plant Physiol. 171, 76–85. doi: 10.1016/j.jplph.2013.06.006
Nawaz, K., Hussain, K., Majeed, A., Khan, F., Afghan, S., Ali, K. (2010). Fatality of salt stress to plants: Morphological, physiological and biochemical aspects. Afr. J. Biotechnol. 9, 5475–5480.
Ortiz, N., Armada, E., Duque, E., Roldán, A., Azcón, R. (2015). Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: effectiveness of autochthonous or allochthonous strains. J. Plant Physiol. 174, 87–96. doi: 10.1016/j.jplph.2014.08.019
Orujei, Y., Shabani, L., Sharifi-Tehrani, M. (2013). Induction of glycyrrhizin and total phenolic compound production in licorice by using arbuscular mycorrhizal fungi. Russian J. Plant Physiol. 60, 855–860. doi: 10.1134/S1021443713050129
Ouziad, F., Wilde, P., Schmelzer, E., Hildebrandt, U., Bothe, H. (2006). Analysis of expression of aquaporins and Na+/H+ transporters in tomato colonized by arbuscular mycorrhizal fungi and affected by salt stress. Environ. Exp. Bot. 57, 177–186. doi: 10.1016/j.envexpbot.2005.05.011
Pan, J., Peng, F., Tedeschi, A., Xue, X., Wang, T., Liao, J., et al. (2020). Do halophytes and glycophytes differ in their interactions with arbuscular mycorrhizal fungi under salt stress? A meta-analysis. Botanical Stud. 61, 1–13. doi: 10.1186/s40529-020-00290-6
Parihar, M., Rakshit, A., Rana, K., Tiwari, G., Jatav, S. S. (2020). The effect of arbuscular mycorrhizal fungi inoculation in mitigating salt stress of pea (Pisum Sativum L.). Commun. Soil Sci. Plant Anal. 51, 1545–1559. doi: 10.1080/00103624.2020.1784917
Pastia, M. C., Stătescu, F., Stan, A. N. (2016). Soil characterization from osoi-moreni area, iași, by analyzing certain indicators of salinity. Sci. Papers. Ser. A. Agron. 59.
Paul, D., Sinha, S. N. (2017). Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river Ganga, India. Ann. Agrarian Sci. 15, 130–136. doi: 10.1016/j.aasci.2016.10.001
Pedone-Bonfim, M. V. L., Da Silva, D. K. A., Da Silva-Batista, A. R., De Oliveira, A. P., Da Silva Almeida, J. R. G., Yano-Melo, A. M., et al. (2018). Mycorrhizal inoculation as an alternative for the sustainable production of Mimosa tenuiflora seedlings with improved growth and secondary compounds content. Fungal Biol. 122, 918–927. doi: 10.1016/j.funbio.2018.05.009
Pedranzani, H., Rodríguez-Rivera, M., Gutiérrez, M., Porcel, R., Hause, B., Ruiz-Lozano, J. M. (2016). Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 26, 141–152. doi: 10.1007/s00572-015-0653-4
Peng, C., Chang, L., Yang, Q., Tong, Z., Wang, D., Tan, Y., et al. (2019). Comparative physiological and proteomic analyses of the chloroplasts in halophyte Sesuvium portulacastrum under differential salt conditions. J. Plant Physiol. 232, 141–150. doi: 10.1016/j.jplph.2018.10.028
Pinto, H., Sharwood, R. E., Tissue, D. T., Ghannoum, O. (2014). Photosynthesis of C3, C3–C4, and C4 grasses at glacial CO2. J. Exp. Bot. 65, 3669–3681. doi: 10.1093/jxb/eru155
Pollastri, S., Savvides, A., Pesando, M., Lumini, E., Volpe, M. G., Ozudogru, E. A., et al. (2018). Impact of two arbuscular mycorrhizal fungi on Arundo donax L. response to salt stress. Planta 247, 573–585. doi: 10.1007/s00425-017-2808-3
Porcel, R., Aroca, R., Azcon, R., Ruiz-Lozano, J. M. (2016). Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 26, 673–684. doi: 10.1007/s00572-016-0704-5
Porcel, R., Aroca, R., Ruiz-Lozano, J. M. (2012). Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 32, 181–200. doi: 10.1007/s13593-011-0029-x
Porcel, R., Redondo-Gómez, S., Mateos-Naranjo, E., Aroca, R., Garcia, R., Ruiz-Lozano, J. M. (2015). Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 185, 75–83. doi: 10.1016/j.jplph.2015.07.006
Qiu, N., Chen, M., Guo, J., Bao, H., Ma, X., Wang, B. (2007). Coordinate up-regulation of V-H+-ATPase and vacuolar Na+/H+ antiporter as a response to NaCl treatment in a C3 halophyte Suaeda salsa. Plant Sci. 172, 1218–1225. doi: 10.1016/j.plantsci.2007.02.013
Rabie, G., Almadini, A. (2005). Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr. J. Biotechnol. 4, 210.
Rana, M. M., Takamatsu, T., Baslam, M., Kaneko, K., Itoh, K., Harada, N., et al. (2019). Salt tolerance improvement in rice through efficient SNP marker-assisted selection coupled with speed-breeding. Int. J. Mol. Sci. 20, 2585. doi: 10.3390/ijms20102585
Rao, D. E., Chaitanya, K. (2016). Photosynthesis and antioxidative defense mechanisms in deciphering drought stress tolerance of crop plants. Biol. Plantarum 60, 201–218. doi: 10.1007/s10535-016-0584-8
Reddy, P. S., Jogeswar, G., Rasineni, G. K., Maheswari, M., Reddy, A. R., Varshney, R. K., et al. (2015). Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench. Plant Physiol. Biochem. 94, 104–113. doi: 10.1016/j.plaphy.2015.05.014
Rengasamy, P. (2010). Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 37, 613–620. doi: 10.1071/FP09249
Rivero, J., Álvarez, D., Flors, V., Azcón-Aguilar, C., Pozo, M. J. (2018). Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol. 220, 1322–1336. doi: 10.1111/nph.2018.220.issue-4
Romero-Munar, A., Baraza, E., Gulías, J., Cabot, C. (2019). Arbuscular mycorrhizal fungi confer salt tolerance in giant reed (Arundo donax L.) plants grown under low phosphorus by reducing leaf Na+ concentration and improving phosphorus use efficiency. Front. Plant Sci. 10, 452495. doi: 10.3389/fpls.2019.00843
Romero-Munar, A., Del-Saz, N. F., Ribas-Carbó, M., Flexas, J., Baraza, E., Florez-Sarasa, I., et al. (2017). Arbuscular mycorrhizal symbiosis with Arundo donax decreases root respiration and increases both photosynthesis and plant biomass accumulation. Plant Cell Environ. 40, 1115–1126. doi: 10.1111/pce.12902
Saia, S., Rappa, V., Ruisi, P., Abenavoli, M. R., Sunseri, F., Giambalvo, D., et al. (2015). Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 6, 147456. doi: 10.3389/fpls.2015.00815
Saleh, A. M., Abdel-Mawgoud, M., Hassan, A. R., Habeeb, T. H., Yehia, R. S., Abdelgawad, H. (2020). Global metabolic changes induced by arbuscular mycorrhizal fungi in oregano plants grown under ambient and elevated levels of atmospheric CO2. Plant Physiol. Biochem. 151, 255–263. doi: 10.1016/j.plaphy.2020.03.026
Samaddar, S., Chatterjee, P., Choudhury, A. R., Ahmed, S., Sa, T. (2019). Interactions between Pseudomonas spp. and their role in improving the red pepper plant growth under salinity stress. Microbiological Res. 219, 66–73. doi: 10.1016/j.micres.2018.11.005
Santander, C., Aroca, R., Ruiz-Lozano, J. M., Olave, J., Cartes, P., Borie, F., et al. (2017). Arbuscular mycorrhiza effects on plant performance under osmotic stress. Mycorrhiza 27, 639–657. doi: 10.1007/s00572-017-0784-x
Santander, C., Sanhueza, M., Olave, J., Borie, F., Valentine, A., Cornejo, P. (2019). Arbuscular mycorrhizal colonization promotes the tolerance to salt stress in lettuce plants through an efficient modification of ionic balance. J. Soil Sci. Plant Nutr. 19, 321–331. doi: 10.1007/s42729-019-00032-z
Santos, J., Al-Azzawi, M., Aronson, J., Flowers, T. J. (2016). eHALOPH a database of salt-tolerant plants: helping put halophytes to work. Plant Cell Physiol. 57, e10–e10. doi: 10.1093/pcp/pcv155
Saxena, A., Raghuwanshi, R., Singh, H. B. (2015). Trichoderma species mediated differential tolerance against biotic stress of phytopathogens in Cicer arietinum L. J. basic Microbiol. 55, 195–206. doi: 10.1002/jobm.201400317
Sewelam, N., Kazan, K., Schenk, P. M. (2016). Global plant stress signaling: reactive oxygen species at the cross-road. Front. Plant Sci. 7, 170027. doi: 10.3389/fpls.2016.00187
Shahvali, R., Shiran, B., Ravash, R., Fallahi, H., Đeri, B. B. (2020). Effect of symbiosis with arbuscular mycorrhizal fungi on salt stress tolerance in GF677 (peach× almond) rootstock. Scientia Hortic. 272, 109535. doi: 10.1016/j.scienta.2020.109535
Sharma, T., Dreyer, I., Riedelsberger, J. (2013). The role of K+ channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Front. Plant Sci. 4, 224. doi: 10.3389/fpls.2013.00224
Sharma, P., Jha, A. B., Dubey, R. S., Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012. doi: 10.1155/2012/217037
Shaul, O. (2002). Magnesium transport and function in plants: the tip of the iceberg. Biometals 15, 307–321. doi: 10.1023/A:1016091118585
Shekoofeh, E., Sepideh, H., Roya, R. (2012). Role of mycorrhizal fungi and salicylic acid in salinity tolerance of Ocimum basilicum resistance to salinity. Afr. J. Biotechnol. 11, 2223–2235.
Sheng, M., Tang, M., Chen, H., Yang, B., Zhang, F., Huang, Y. (2008). Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18, 287–296. doi: 10.1007/s00572-008-0180-7
Sheng, M., Tang, M., Zhang, F., Huang, Y. (2011). Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza 21, 423–430. doi: 10.1007/s00572-010-0353-z
Shokri, S., Maadi, B. (2009). Effects of arbuscular mycorrhizal fungus on the mineral nutrition and yield of Trifolium alexandrinum plants under salinity stress. J. Agron. 8, 79–83. doi: 10.3923/ja.2009.79.83
Shrestha, R. (2006). Relating soil electrical conductivity to remote sensing and other soil properties for assessing soil salinity in northeast Thailand. Land degradation Dev. 17, 677–689. doi: 10.1002/ldr.v17:6
Shtark, O. Y., Puzanskiy, R. K., Avdeeva, G. S., Yurkov, A. P., Smolikova, G. N., Yemelyanov, V. V., et al. (2019). Metabolic alterations in pea leaves during arbuscular mycorrhiza development. PeerJ 7, e7495. doi: 10.7717/peerj.7495
Smith, S. E., Facelli, E., Pope, S., Andrew Smith, F. (2010). Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 326, 3–20. doi: 10.1007/s11104-009-9981-5
Stevens, J., Senaratna, T., Sivasithamparam, K. (2006). Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regul. 49, 77–83. doi: 10.1007/s10725-006-0019-1
Taiz, L., Zeiger, E., Møller, I. M., Murphy, A. (2015). Plant Physiology and Development. 6th Edition. (Sunderland, CT: Sinauer Associates).
Takai, T., Kondo, M., Yano, M., Yamamoto, T. (2010). A quantitative trait locus for chlorophyll content and its association with leaf photosynthesis in rice. Rice 3, 172–180. doi: 10.1007/s12284-010-9047-6
Talaat, N. B., Shawky, B. T. (2011). Influence of arbuscular mycorrhizae on yield, nutrients, organic solutes, and antioxidant enzymes of two wheat cultivars under salt stress. J. Plant Nutr. Soil Sci. 174, 283–291. doi: 10.1002/jpln.201000051
Talaat, N. B., Shawky, B. T. (2014). Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 98, 20–31. doi: 10.1016/j.envexpbot.2013.10.005
Tao, L., Run-Jin, L., Xin-Hua, H., Bao-Shan, W. (2012). Enhancement of superoxide dismutase and catalase activities and salt tolerance of euhalophyte Suaeda salsa L. by mycorrhizal fungus Glomus mosseae. Pedosphere 22, 217–224. doi: 10.1016/S1002-0160(12)60008-3
Tavarini, S., Passera, B., Martini, A., Avio, L., Sbrana, C., Giovannetti, M., et al. (2018). Plant growth, steviol glycosides and nutrient uptake as affected by arbuscular mycorrhizal fungi and phosphorous fertilization in Stevia rebaudiana Bert. Ind. Crops Products 111, 899–907. doi: 10.1016/j.indcrop.2017.10.055
Taylor, A., Pereira, N., Thomas, B., Pink, D. A., Jones, J. E., Bending, G. D. (2015). Growth and nutritional responses to arbuscular mycorrhizal fungi are dependent on onion genotype and fungal species. Biol. Fertility Soils 51, 801–813. doi: 10.1007/s00374-015-1027-y
Tisarum, R., Theerawitaya, C., Samphumphuang, T., Polispitak, K., Thongpoem, P., Singh, H. P., et al. (2020). Alleviation of salt stress in upland rice (Oryza sativa L. ssp. indica cv. Leum Pua) using arbuscular mycorrhizal fungi inoculation. Front. Plant Sci. 11, 348. doi: 10.3389/fpls.2020.00348
Toscano, S., Trivellini, A., Cocetta, G., Bulgari, R., Francini, A., Romano, D., et al. (2019). Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant Sci. 10, 468818. doi: 10.3389/fpls.2019.01212
Trenberth, K. E., Dai, A., van der Schrier, G., Jones, P. D., Barichivich, J., Briffa, K. R., et al. (2014). Global warming and changes in drought. Nat. Climate Change 4, 17–22. doi: 10.1038/nclimate2067
Tsunekawa, K., Shijuku, T., Hayashimoto, M., Kojima, Y., Onai, K., Morishita, M., et al. (2009). Identification and characterization of the Na+/H+ antiporter Nhas3 from the thylakoid membrane of Synechocystis sp. PCC 6803. J. Biol. Chem. 284, 16513–16521.
Tufail, A., Li, H., Naeem, A., Li, T. (2018). Leaf cell membrane stability-based mechanisms of zinc nutrition in mitigating salinity stress in rice. Plant Biol. 20, 338–345. doi: 10.1111/plb.2018.20.issue-2
Tuo, X.-Q., Li, S., Wu, Q.-S., Zou, Y.-N. (2015). Alleviation of waterlogged stress in peach seedlings inoculated with Funneliformis mosseae: Changes in chlorophyll and proline metabolism. Scientia Hortic. 197, 130–134. doi: 10.1016/j.scienta.2015.09.022
Van Zelm, E., Zhang, Y., Testerink, C. (2020). Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 71, 403–433. doi: 10.1146/annurev-arplant-050718-100005
Vassilev, N., Eichler-Löbermann, B., Vassileva, M. (2012). Stress-tolerant P-solubilizing microorganisms. Appl. Microbiol. Biotechnol. 95, 851–859. doi: 10.1007/s00253-012-4224-8
Wang, H., An, T., Huang, D., Liu, R., Xu, B., Zhang, S., et al. (2021). Arbuscular mycorrhizal symbioses alleviating salt stress in maize is associated with a decline in root-to-leaf gradient of Na+/K+ ratio. BMC Plant Biol. 21, 1–15. doi: 10.1186/s12870-021-03237-6
Wang, J., Fu, Z., Ren, Q., Zhu, L., Lin, J., Zhang, J., et al. (2019b). Effects of arbuscular mycorrhizal fungi on growth, photosynthesis, and nutrient uptake of Zelkova serrata (Thunb.) Makino seedlings under salt stress. Forests 10, 186. doi: 10.3390/f10020186
Wang, F., Sun, Y., Shi, Z. (2019a). Arbuscular mycorrhiza enhances biomass production and salt tolerance of sweet sorghum. Microorganisms 7, 289. doi: 10.3390/microorganisms7090289
Wang, W., Vinocur, B., Altman, A. (2003). Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1–14. doi: 10.1007/s00425-003-1105-5
Waqas, M., Khan, A. L., Kamran, M., Hamayun, M., Kang, S.-M., Kim, Y.-H., et al. (2012). Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17, 10754–10773. doi: 10.3390/molecules170910754
Waszczak, C., Carmody, M., Kangasjärvi, J. (2018). Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 69, 209–236. doi: 10.1146/annurev-arplant-042817-040322
Wright, D., Read, D., Scholes, J. (1998). Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant Cell Environ. 21, 881–891. doi: 10.1046/j.1365-3040.1998.00351.x
Wu, J., Wang, D., Rosen, C. J., Bauer, M. E. (2007). Comparison of petiole nitrate concentrations, SPAD chlorophyll readings, and QuickBird satellite imagery in detecting nitrogen status of potato canopies. Field Crops Res. 101, 96–103. doi: 10.1016/j.fcr.2006.09.014
Xie, W., Hao, Z., Zhou, X., Jiang, X., Xu, L., Wu, S., et al. (2018). Arbuscular mycorrhiza facilitates the accumulation of glycyrrhizin and liquiritin in Glycyrrhiza uralensis under drought stress. Mycorrhiza 28, 285–300. doi: 10.1007/s00572-018-0827-y
Yamato, M., Ikeda, S., Iwase, K. (2008). Community of arbuscular mycorrhizal fungi in a coastal vegetation on Okinawa island and effect of the isolated fungi on growth of sorghum under salt-treated conditions. Mycorrhiza 18, 241–249. doi: 10.1007/s00572-008-0177-2
Yan, W., Zhong, Y., Shangguan, Z. (2016). A meta-analysis of leaf gas exchange and water status responses to drought. Sci. Rep. 6, 20917. doi: 10.1038/srep20917
Yang, Y., Guo, Y. (2018). Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 217, 523–539. doi: 10.1111/nph.2018.217.issue-2
Yooyongwech, S., Samphumphuang, T., Tisarum, R., Theerawitaya, C., Cha-Um, S. (2016). Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Scientia Hortic. 198, 107–117. doi: 10.1016/j.scienta.2015.11.002
Younesi, O., Moradi, A., Namdari, A. (2013). Influence of arbuscular mycorrhiza on osmotic adjustment compounds and antioxidant enzyme activity in nodules of salt-stressed soybean (Glycine max). Acta Agriculturae Slovenica 101, 219–230-219–230. doi: 10.14720/aas.2013.101.2.14913
Yuan, F., Guo, J., Shabala, S., Wang, B. (2019). Reproductive physiology of halophytes: Current standing. Front. Plant Sci. 9, 432034. doi: 10.3389/fpls.2018.01954
Zai, X.-M., Fan, J.-J., Hao, Z.-P., Liu, X.-M., Zhang, W.-X. (2021). Effect of co-inoculation with arbuscular mycorrhizal fungi and phosphate solubilizing fungi on nutrient uptake and photosynthesis of beach palm under salt stress environment. Sci. Rep. 11, 5761. doi: 10.1038/s41598-021-84284-9
Zaman, M., Shahid, S. A., Heng, L. (2018). Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques (Vienna: Springer Nature).
Zeng, Y., Guo, L.-P., Chen, B.-D., Hao, Z.-P., Wang, J.-Y., Huang, L.-Q., et al. (2013). Arbuscular mycorrhizal symbiosis and active ingredients of medicinal plants: current research status and prospectives. Mycorrhiza 23, 253–265. doi: 10.1007/s00572-013-0484-0
Zhao, R., Guo, W., Bi, N., Guo, J., Wang, L., Zhao, J., et al. (2015). Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl. Soil Ecol. 88, 41–49. doi: 10.1016/j.apsoil.2014.11.016
Zheng, Y., Jia, A., Ning, T., Xu, J., Li, Z., Jiang, G. (2008). Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance. J. Plant Physiol. 165, 1455–1465. doi: 10.1016/j.jplph.2008.01.001
Zou, Y.-N., Wu, Q.-S., Huang, Y.-M., Ni, Q.-D., He, X.-H. (2013). Mycorrhizal-mediated lower proline accumulation in Poncirus trifoliata under water deficit derives from the integration of inhibition of proline synthesis with increase of proline degradation. PloS One 8, e80568. doi: 10.1371/journal.pone.0080568
Zribi, L., Fatma, G., Fatma, R., Salwa, R., Hassan, N., Néjib, R. M. (2009). Application of chlorophyll fluorescence for the diagnosis of salt stress in tomato “Solanum lycopersicum (variety Rio Grande). Scientia Hortic. 120, 367–372. doi: 10.1016/j.scienta.2008.11.025
Keywords: mycorrhiza, salinity, plant, resistance, symbiotic relationship
Citation: Boorboori MR and Lackóová L (2025) Arbuscular mycorrhizal fungi and salinity stress mitigation in plants. Front. Plant Sci. 15:1504970. doi: 10.3389/fpls.2024.1504970
Received: 01 October 2024; Accepted: 24 December 2024;
Published: 17 January 2025.
Edited by:
Fernanda Fidalgo, University of Porto, PortugalCopyright © 2025 Boorboori and Lackóová. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Reza Boorboori, Ym9vcmJvb3JpQGFoc3p1LmVkdS5jbg==; Lenka Lackóová, bGVua2EubGFja29vdmFAdW5pYWcuc2s=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.