
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci. , 20 January 2025
Sec. Plant Abiotic Stress
Volume 15 - 2024 | https://doi.org/10.3389/fpls.2024.1502438
This article is part of the Research Topic Salinity and Drought Stress in Plants: Understanding Physiological, Biochemical and Molecular Responses Volume II View all 26 articles
 Shanxia Huang
Shanxia Huang Songheng Jin*
Songheng Jin*Abiotic stresses are considered as a significant factor restricting horticultural crop productivity and quality. Drought stress is a major environmental constraint among the emerging concerns. Plants have significant susceptibility to drought stress, resulting in a marked decline in production during the last several decades. The development of effective strategies to mitigate drought stress is essential for sustainable agriculture and food security, especially considering the continuous growth of the world population. Several studies suggested that exogenous application of phytohormone to plants can improve drought stress tolerance by activating molecular and physiological defense systems. Phytohormone pretreatment is considered a potential approach for alleviating drought stress in horticultural plants. In addition, melatonin, salicylic acid, jasmonates, strigolactones, brassinosteroids, and gamma-aminobutyric acid are essential phytohormones that function as growth regulators and mitigate the effects of drought stress. These hormones frequently interact with one another to improve the survival of plants in drought-stressed environments. To sum up, this review will predominantly elucidate the role of phytohormones and related mechanisms in drought tolerance across various horticulture crop species.
Plants are affected by abiotic stresses, prompting various internal changes inside them. These abiotic variables affect plant growth and productivity. Abiotic variables refer to the interactions between living organisms and plants that have both beneficial and detrimental consequences. Positive influences may have a favorable impact on plant development. Adverse effects considerably decreased the horticultural plant yield and productivity (Iqbal et al., 2022; Nazir et al., 2024). Plant defense mechanisms using diverse chemical components mitigate adverse effects (Zulfiqar et al., 2022). These climatic changes have intensified drought stress, hence garnering considerable attention lately. Drought is a critical factor that limits worldwide agricultural productivity (Yang et al., 2021). The intensity of the drought is escalating, leading to elevated prices for food. In addition, by 2050, the world population is projected to rise to 9.8 billion (Farooqi et al., 2020). Agricultural output must increase by 70% to satisfy the food needs of an increasing population (Cao et al., 2024). To save future generations from impending crises, it is imperative to advance technology and policies addressing climate change and drought stress, including reforestation, effective water utilization, population management, and the cultivation of drought-resistant crops (Şimşek et al., 2024). Recent years have seen substantial advancements in clarifying the molecular pathways related to drought stress responses in plants (Table 1; Figure 1).
Drought stress induces several morpho-physiological and metabolic alterations that negatively influence plant growth and yield (Sako et al., 2020). Drought stress presents a significant problem to global agriculture, requiring a deep understanding of plants’ adaptation systems. In addition to rapid physiological reactions, new research has shown the intriguing phenomena of epigenetic memory in drought-adapted plants (Kaya et al., 2024). The epidermal wax of plants inhibits non-stomatal water loss and enhances water usage efficiency, facilitating adaptation to arid conditions (Gao et al., 2024). Stomatal regulation and stress signaling are the mechanisms by which plants respond to drought stress. Plants regulate their response mechanisms through the utilization of phytohormones (Haider et al., 2024). Drought stress effectively hindered different physiological function such as leaf area, root length, stem mass, lateral root development, node number, reduced canopy size, and even cause cell death (Feng et al., 2024). Drought stress damage leaf growth, restricted leaf photosynthetic activity, reduced chlorophyll content, stomatal conductance, water potential, and reduced pigments level in leaf (Sári et al., 2024). In another study, Xie et al. (2024) reported that drought stress significantly reduced the seedling growth, pigments concentration, chlorophyll content, and antioxidant enzymes activity and caused oxidative damage in tomatoes. The leaf water potential, antioxidant enzymes activity, secondary metabolites production, proline uptake, and growth were reduced in cucumber under drought stress (Ahmad et al., 2024). Plant growth is significantly affected by water scarcity, mostly owing to the suppression of cell elongation. Water-stressed plants exhibit reduced height and diminished leaf area, resulting in less absorption of photo synthetically active radiation, a lowered rate of photosynthesis, and, ultimately, a reduced yield (Abbas et al., 2023). Water shortage induces stomatal closure, leading to limited CO2 uptake by the leaves and reducing the operational efficiency of Calvin cycle enzymes, particularly Rubisco, due to substrate scarcity (Nguyen et al., 2018). Due to increased photorespiration and decreased stomatal conductance, net photosynthesis is the main physiological measurement that is restricted by drought stress (Chieb and Gachomo, 2023). Excessive reactive oxygen species (ROS) production can also cause damage to the photosynthetic system when the stomata are closed, resulting in a decrease in growth and photosynthesis (Figure 2) (Bouremani et al., 2023).
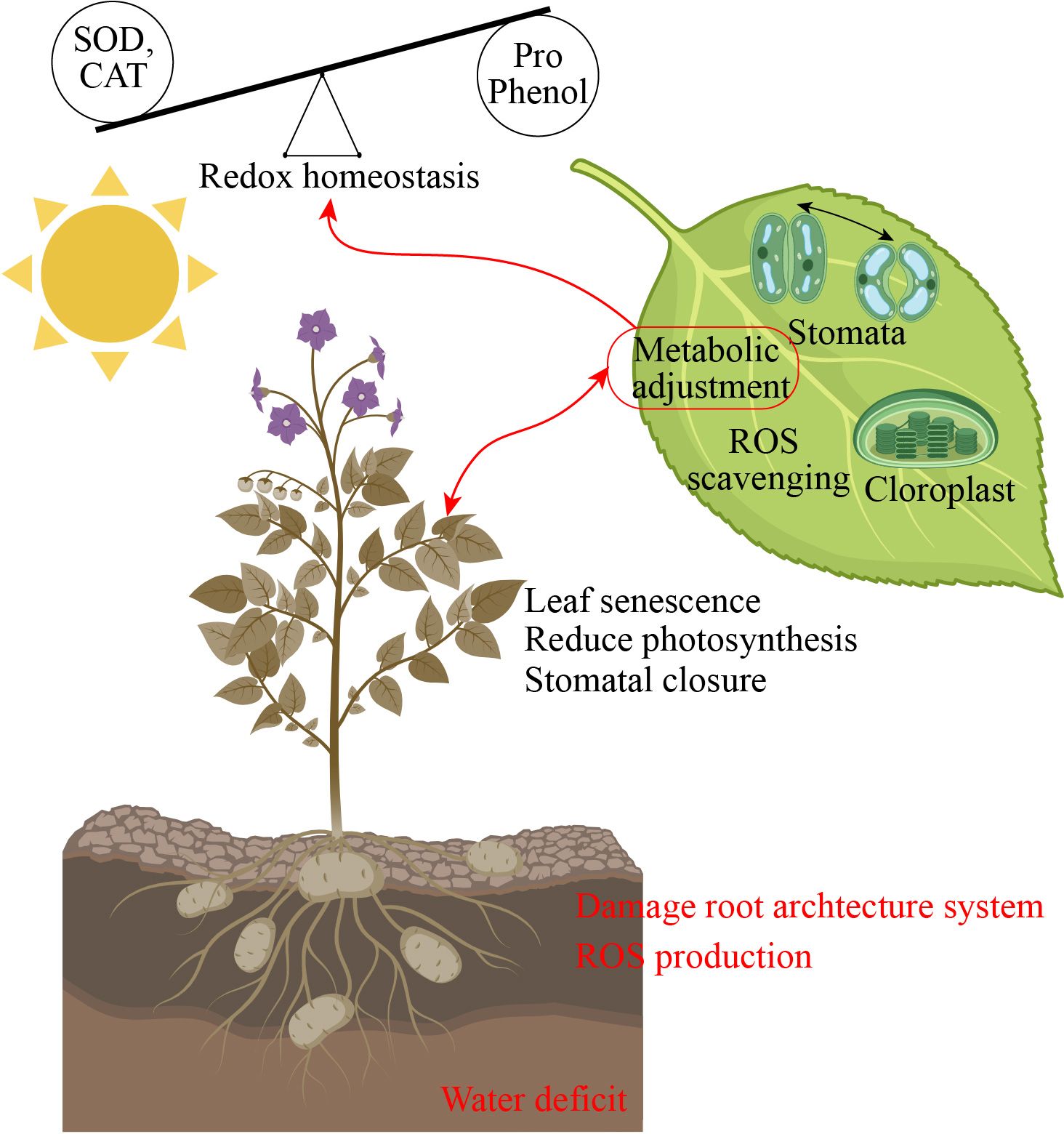
Figure 2. Drought stress altered redox homeostasis and leaf photosynthesis performance. SOD, superoxide dismutase; CAT, catalase; Pro, proline; ROS, reactive oxygen species.
Phytohormone is an important plant growth regulator having low molecular weight (Pandey et al., 2017). The synthesis of several plant hormones occurs in response to drought stress, regulating activities associated with drought tolerance mechanisms (Figure 3). Phytohormones such as melatonin, salicylic acid, jasmonates, strigolactones, brassinosteroids, GABA, auxin, gibberellin, cytokinins, ethylene, abscisic acid, glycine betaine, polyamines proline, and trehalose have a role in osmotic adjustment and enhanced drought stress tolerance mechanism (Ciura and Kruk, 2018). Drought stress also triggers the antioxidant defense system that participates in the elimination of ROS uptake in plants (Fahad et al., 2015). Phytohormones regulate wide range of functions in horticultural plants such as protected photosynthesis, lateral root development, secondary metabolites production, redox balanced, mineral nutrient accumulation, osmotic adjustment, upregulated antioxidant defense, and enhanced drought stress tolerance in horticultural plants (Ullah et al., 2018; Jogawat et al., 2021; Salvi et al., 2021).
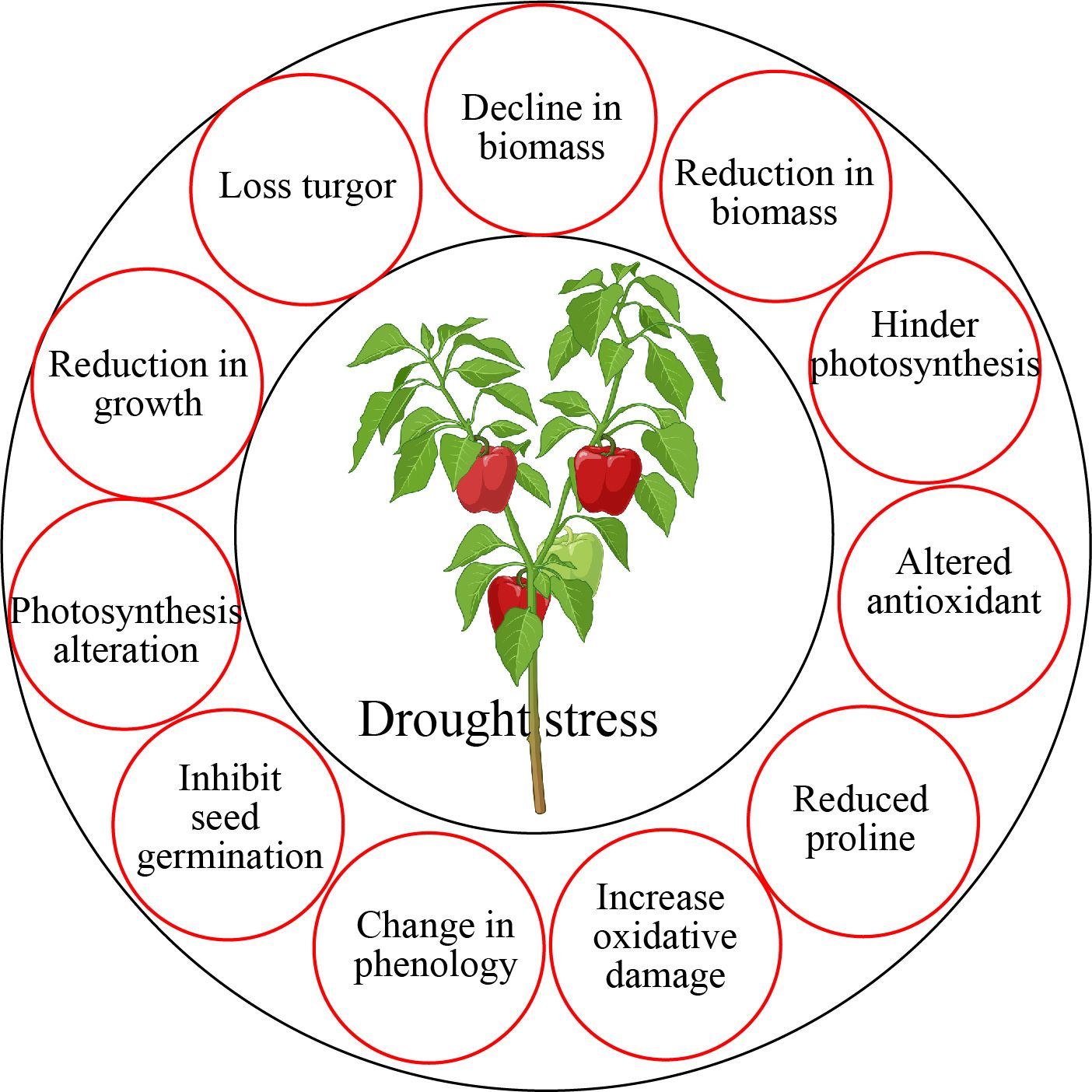
Figure 3. Drought stress altered wide range of physiological and morphological functions in horticultural crops.
We briefly examined the significance of phytohormones in plant growth regulation. We want to address these research questions: (i) how does phytohormone govern plant growth and development physiologically? How does phytohormone influence plant growth? (iii) Are there any ignored physiological features important for understanding phytohormone stress regulation? We addressed new scientific advances to differentiate our review from others. This work helps researchers and policymakers create efficient abiotic stress mitigation measures for horticulture crops, improving global food security.
Endogenous plant hormones are essential for both developmental processes and the plant’s reaction to abiotic variables. The primary mediators of plant responses to drought stress are phytohormones (Asghar et al., 2022). Phytohormones are further classified according to the chemical structures of some groups (Mubarik et al., 2021). The primary stress-responsive hormone generated upon drought signal detection is abscisic acid (ABA). It is primarily synthesized in the root and then transported to the leaves to regulate stomatal opening, channel activity, and the expression pattern of ABA-responsive genes (Wani et al., 2016). Furthermore, phytohormones exist at very low amounts inside plants, complicating their measurement analysis (Checker et al., 2018). Phytohormones enhanced abiotic stress tolerance in many plant species such as tomato, radish, strawberry, eggplant, and carrot (Ciura and Kruk, 2018). Phytohormones application regulated mineral nutrient accumulation, maintained osmotic adjustment, balanced leaf water potential, reduced oxidative damage, and upregulated antioxidant enzymes activity in horticultural plants (Han et al., 2018). Several studies suggested that phytohormone application showed considerable improvement in the field of horticulture (Parmar et al., 2017).
Salicylic acid is a versatile plant growth regulator known to participate in plant responses to stressors (Khan et al., 2015). Foliar treatments of salicylic acid may mitigate the deleterious effects of oxidative stress induced by drought via several mechanisms (Kang et al., 2014). Salicylic acid application promotes lateral root development, increases secondary metabolites production, and boosts osmolytes accumulation, thus sustaining the water potential of horticultural plants during drought stress conditions (Table 2) (Ullah et al., 2018). In addition, salicylic acid may function by sustaining the overall chlorophyll concentration in plants, therefore safeguarding their photosynthetic machinery (Horváth et al., 2007). Salicylic acid alleviates the adverse effects of drought stress and functions as a signaling molecule to stimulate the gene expression pattern of stress-related genes and protein (Song et al., 2023). It was extensively documented and studied that salicylic acid directly engages in the activation of plant defense systems (Chen et al., 2023). In addition, salicylic acid has the ability to increase the production and activity of antioxidant enzymes while also activating plant defense mechanisms (Rasheed et al., 2022). Salicylic acid enhances chlorophyll content and improves photosynthetic efficiency, consequently considerably increasing crop production and other yield-related physiological indices under drought stress environment (Iqbal et al., 2022).
Foliar treatments of salicylic acid have been shown to enhance growth in major horticultural (tomato, potato, strawberry, and cucumber) crops under drought stress (Damalas and Koutroubas, 2021). Salicylic acid application regulates normal plant growth, such as increasing flowering, promoting bud differentiation, regulating seed dormancy, and enhancing the number of flowering (Joseph et al., 2010). Several studies suggested that salicylic acid is a multifaceted biomolecule in the response to drought stress. It can regulate the cell wall expansion, regulate hormonal production, and reduce oxidative damage in horticultural plants (Saleem et al., 2021). Salicylic acid application enhanced ion homeostasis, balanced cellular membrane integrity, and regulated antioxidant defense system in response to drought stress. In addition, salicylic acid promoting lateral root growth development, osmotic adjustment, and antioxidant enzymes activity protected photosynthesis in plants (Sharma et al., 2020). Drought stress treatment showed a considerable reduction in chlorophyll content, photosynthesis, relative water content, membrane damage, antioxidant enzymes activity, and pigments concentration in tomato seedlings. A subsequent treatment with salicylic acid mitigated water-induced stress and markedly enhanced the aforementioned metrics. Secondary metabolites production, proline content, and antioxidant enzyme concentration increased in tomato seedlings in response to both salicylic acid and drought treatments (Aires et al., 2022). Salicylic acid treatment significantly affected the growth status, net photosynthetic rate, leaf water potential, and antioxidant enzyme concentration of strawberry plants exposed to drought stresses (Dakheel et al., 2022). The antioxidant enzymes activity, osmolytes production, and growth of sugar beet increased in sugar beet plant by the application of salicylic acid under drought stress environment (Khodadadi et al., 2020). The supplementation of salicylic acid to drought-stressed seedling decreased cellular membrane damage by stimulating the antioxidant enzyme activity and maintained osmotic adjustments in two sweet potato genotypes (Huang et al., 2022a). When watermelon seedlings exposed to drought stress environment because of water deficit grow faster, as salicylic acid promotes the secondary metabolites production, increased osmolytes accumulation, and reduced electrolyte leakage level in watermelon seedling (Silva et al., 2023). Furthermore, recent molecular research has shown that salicylic acid may modulate many gene-level processes in plants, hence enhancing their tolerance to abiotic stress (Zulfiqar et al., 2022). The foliar application of salicylic acid increased growth attributes while reducing stomatal conductance under severe water shortage stress. However, significant water deficiency stress markedly increased the value of SPAD (relative chlorophyll content) index. Exogenous supplementation of salicylic acid may enhance the characteristics of cucumber seedlings and increase their resistance to water stress (Mardani et al., 2012). Salicylic acid treatment promoted yield traits, leaf water content, proline uptake flavonoids, soluble solid concentration, and balanced membrane stability in cantaloupe during drought stress environment (Alam et al., 2022). Spray treatment of salicylic acid enhanced relative chlorophyll content, prolonged fruit ripening period, and elevated secondary metabolites production and antioxidant enzymes activity in melon under drought environment (Nasrabadi et al., 2015).
Foliar application of salicylic acid significantly enhanced growth of grape tomato seedling (Chakma et al., 2021), increased chlorophyll content in strawberry (Mozafari et al., 2018), enhanced the non-photochemical efficiency in sugar beet (Li et al., 2022), increased tuber yield and quality of sugar beet (Youssef and Abdelaal, 2023), protected photosynthetic apparatus in cucumber (Baninasab, 2010), and promoted yield and quality of watermelon (Nastari Nasrabadi et al., 2023). Continuing study into the molecular processes of salicylic acid will enhance our comprehension of plant stress tolerances. This review emphasizes the importance of salicylic acid in improving plant resistance to drought stress environment, therefore facilitating their survival and production under adverse environmental circumstances. Subsequent research in this domain may enhance the formulation of efficacious techniques for crop enhancement and stress mitigation (Figure 4).
Melatonin is a universal biomolecule (Zhang et al., 2015). With its function in horticultural plant growth, melatonin significantly contributes to plant stress defense (Dzinyela et al., 2024) (Table 3). Plants often face challenges under abiotic stress conditions (Zhao et al., 2022). Numerous plant species that are abundant in melatonin have demonstrated a greater ability for maintaining stress tolerance (Debnath et al., 2019). Melatonin may assume markedly distinct functions in the regulation of plant growth and development at low and high concentrations within the same species (Huang et al., 2022b). In cherry plants, melatonin facilitates roots at low doses but suppresses growth at elevated levels in cherry tissue culture. Excessive concentrations may induce hazardous consequences (Zhang et al., 2022). This indicates that melatonin may function differently at low and high doses. Elevated levels of melatonin may significantly diminish ROS in cells, thereby influencing ROS-dependent signaling pathways and impeding cellular proliferation (Ayyaz et al., 2022). Melatonin regulates the concentrations of ROS and enhances molecular defenses that increase plant resilience to drought stress (Moustafa-Farag et al., 2020). Melatonin is a potent antioxidant compound and master growth regulator that protected plants from oxidative damage and modulate numerous responses to environmental disruptions, particularly water stress (Colombage et al., 2023). Melatonin serves as a signaling molecule at the cellular level and enhances the expression of many antioxidant enzymes, hence increasing its efficiency as an antioxidant (Raza et al., 2022).
Melatonin is a stress relief molecule (Tiwari et al., 2021; Ahmad et al., 2023). Melatonin application efficiently enhanced drought stress tolerance in carrot, radish, pepper, sweet potato, and cucumber (Shi et al., 2015; Raza et al., 2022). The supplementation of melatonin to tomato seedlings improves root vigor, alleviates stress-induced damage to PSII response centers, diminishes the adverse effects of dehydration by modulating the antioxidant system, and decreases the cellular concentration of harmful chemicals in the plants (Liu et al., 2015). In another study, Altaf et al. (2022) reported that drought stress considerably decreased growth, hindered photosynthetic activity, restricted pigments concentration, and caused oxidative damage in tomato seedling. In contrast, melatonin application potently increased the tomato seedling growth by recovering the above traits under drought environment. Melatonin treatment promoted lateral root development, increased seed germination, regulated antioxidant enzymes activity, and reduced oxidative damage in cucumber seedling under water stress environment (Zhang et al., 2013). Furthermore, Khan et al. (2023) reported that drought restricted strawberry seedling growth by decreasing the pigments concentration level, hindering enzymes level, and damaging oxidative stress biomarkers. In contrast, melatonin application resorted the strawberry seedling growth by decreasing oxidative damage and increasing antioxidant enzymes activity under drought environment. Foliar application of melatonin enhances the growth capacity of sugar beet plants under drought stress mostly by diminishing cellular membrane integrity level, elevating antioxidant enzyme activities, protecting photosynthetic capacity, and facilitating chlorophyll production (He et al., 2023). Ardıç et al. (2023) revealed that melatonin considerable improved chlorophyll content, antioxidant enzymes activity, and mineral nutrient content, and reduced oxidative damage by reducing the MDA accumulation in pepper under drought condition. In another study, the authors described the essential function of melatonin generation in response to osmotic stress via plant hormone signal transduction. It is shown that ABA signaling is pivotal in melatonin production under osmotic stress (Yan et al., 2023). Seed pretreatment with melatonin showed considerable improvement in the growth of carrot, seed germination, and osmotic adjustment under drought stress environment (Rosińska et al., 2023). Furthermore, Xia et al. (2020) reported that drought treatment dramatically reduced the kiwifruit growth by reducing the enzymatic activity, chlorophyll content, and pigments concentration, and increasing the oxidative damage by enhancing the MDA concentration, EL level, and H2O2 content in kiwifruit leaves. In contrast, melatonin treatment significantly recovered these traits such as protecting photosynthetic apparatus, upregulating antioxidant enzymes activity, and reducing oxidative damage in kiwifruit under drought conditions. Under drought stress environment, melatonin significantly regulated the tuber yield of potato plants by impeding ABA transfer from the root to the shoot system while simultaneously enhancing the levels of non-reducing sugars (El-Yazied et al., 2022). Melatonin remarkably promoted drought stress tolerance via regulating the leaf photosynthesis and maintained membrane stability and lateral root development in tomato (Mushtaq et al., 2022).
Melatonin application potential improved secondary metabolites production in tomato (Huang et al., 2023), regulated antioxidant defense system in cucumber (Lee and Back, 2019), maintained glyoxalase enzymes system in pepper (Kaya and Shabala, 2023), maintained osmotic adjustment in pepper (Li et al., 2019), upregulated mineral metabolism and nutritional status in strawberry (Safa Eynaladin et al., 2025), and decreased MDA and H2O2 accumulation in kiwifruit (Liang et al., 2019). Melatonin is a multifaceted biomolecule that promotes growth and yield improvement during drought conditions, making it an appropriate choice for sustainable agricultural practices aimed at ensuring food security (Zeng et al., 2022).
Jasmonates, including jasmonic acid and methyl jasmonates, are recognized for their involvement in several physiological processes (Ahmad et al., 2016). The exogenous supplementation of jasmonates evaluated on several plants under stress circumstances has shown efficiency in enhancing plant stress resistance (Table 4) (Ali and Baek, 2020). Jasmonates are universally present throughout the plant kingdom (Iqbal et al., 2022). Jasmonate and methyl jasmonates actively contribute to leaf senescence (Siddiqi and Husen, 2019). Jasmonates prominently increased drought stress tolerance by upregulating the antioxidant defense system and maintaining redox homeostasis, flowering, fruit ripening, and hormonal production (Per et al., 2018). In addition, physiological functions associated with jasmonic acid included seed germination, protein accumulation, leaf chlorosis, flowering development, and secondary metabolites production in horticultural plants (Santino et al., 2013; Wasternack, 2014).
The supplementation of methyl jasmonates enhances growth, promotes the accumulation of secondary metabolites, and influences endogenous hormone levels, along with other metabolic processes in stressed horticultural plants (Yu et al., 2018; Delgado et al., 2021; Rehman et al., 2023). Jasmonic acid application increased drought stress tolerance by regulating growth traits and promoting polyamines accumulation and antioxidant enzymes activity in tomato seedling under drought environment (Zhang and Huang, 2013). Methyl jasmonates application increased biomass and secondary metabolites production and stimulated antioxidant defense system in cucumber under water stress environment (Wang et al., 2022). Water stress treatment considerably decreased the pigments concentration, leaf water potential, and protein concentration, while it enhanced antioxidant enzymes activity, MDA, H2O2, and proline accumulation in strawberry. In contrast, jasmonic acid application along with drought treatment showed significant improvement in the growth of strawberry seedling (Yosefi et al., 2020). Jasmonic acid application to Brassica rapa significantly mitigated drought-induced damage by altering secondary metabolites production, promoting antioxidant defense system, and protecting photosynthetic machinery (Ahmad Lone et al., 2022). Methyl jasmonates stimulated the production of anthocyanin, flavonoids, and osmolytes production, regulating antioxidant enzymes activity and maintaining photosystem functions while also reducing EL level and maintaining leaf water potential, protecting leaf photosynthesis, and seedling growth. Methyl jasmonates significantly alleviated drought stress in purple basil by increasing its secondary metabolism, photosynthetic apparatus, secondary metabolism, and quality- and yield-related attributes (Lopes et al., 2024). Methyl jasmonates application positively influences the growth of radish by increasing the osmolytes production and reducing the oxidative damage under drought environment (Chen et al., 2019). Methyl jasmonates application along with water stress treatments substantially enhanced flavonoid content, total phenolic levels, and antioxidant capability in peppermint (Gholamreza et al., 2019). In another study, jasmonic acid application regulated antioxidant defense system and reduced H2O2 accumulation in Cucumis melon under drought environment (Nafie et al., 2011). Supplementation with jasmonic acid significantly enhanced growth status and boosted antioxidant defense system and the resilience of sugar beet under drought stress environment (Ghaffari et al., 2019). Methyl jasmonates application potentially improved leaf photosynthetic apparatus, increased antioxidant enzymes activity, and reduced MDA accumulation in cauliflower leaves (Wu et al., 2012).
Jasmonates application promoted seedling growth of tomato (Muñoz-Espinoza et al., 2015), enhanced pigments concentration and altered leaf ultrastructure in cucumber (Wen et al., 2023), and decreased oxidative damage in strawberry leaves (Wang, 1999). Furthermore, methyl jasmonates may enhance drought stress tolerance by elevating photosynthetic assimilation rate, maintaining stomatal conductance, improving proline uptake and osmotic adjustment compounds and antioxidant activity, and reducing H2O2 and MDA accumulation in Citrus (Xiong et al., 2020). Fugate et al. (2018) reported that drought stress treatment dramatically decreased fresh weights and leaf gas exchange traits, damaged PSII system, declined leaf water potential, and increased oxidative damage. In contrast, methyl jasmonates supplementation significantly recovered growth status and restored the above parameters by enhancing the drought stress tolerance in sugar beet. The author described the efficiency of MeJA in boosting Impatiens walleriana capacity to endure water stress in vitro. Impatiens walleriana enhanced water stress resistance by stimulating defense-related metabolic processes, mostly triggered by pretreatment with the minimal methyl jasmonates concentration used (Đurić et al., 2023). Foliar application of methyl jasmonates considerably increased growth, chlorophyll content, and antioxidant enzymes activity, and decreased membrane damage in cowpea under drought environment (Sadeghipour, 2018). Seed or foliar supplementation of methyl jasmonates reinstates normal growth and morphological functions via activating the antioxidant enzymes activity under drought stress environment (Mohi-Ud-Din et al., 2021). Therefore, it may be said that jasmonates has a beneficial regulatory role in horticultural plants during adaptation to drought stress (Figure 5).
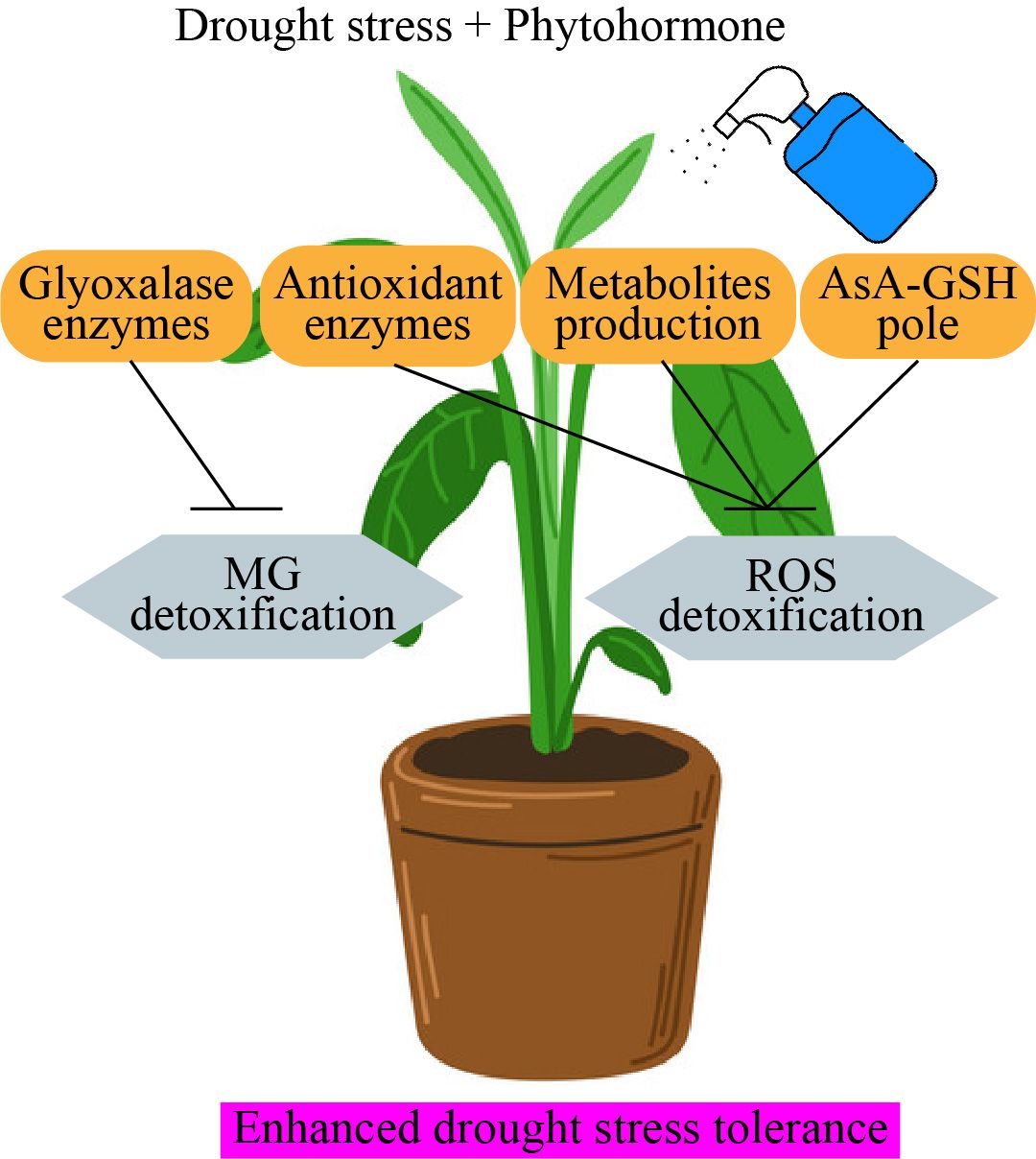
Figure 5. Phytohormone regulates antioxidant enzymes activity under drought stress environment. AsA-GSH, glutathione-ascorbate; ROS, reactive oxygen species; MG, methyl glycolase.
Brassinosteroids are a group of plant hormones that regulate diverse range of functions, including photosynthesis, cell elongation, flowering, root system architecture, and responses to stresses (Ahammed et al., 2015; Ali et al., 2019). BRs have demonstrated the ability to alleviate the adverse impacts of drought by regulating various metabolic functions, such as seed germination, stomatal control, leaf senescence, lateral root development, antioxidant enzymes system, redox homeostasis, osmotic adjustment, maintained leaf water potential, and nutrient absorption in horticultural plants (Table 5) (Bhandari and Nailwal, 2020; Zhang et al., 2023). The function of brassinosteroids in protecting plants from environmental challenges is crucial for sustained production. Furthermore, the application of brassinosteroids has dramatically decreased fruit cracking in litchi (Sharma, 2021). Brassinosteroids increased both the quantity and quality of fruit produce in several horticultural fruit crops (Ali, 2017). They also influence cotyledon development, root extension, leaf creation and growth, and plant biomass accumulation. Another key physiological reaction in plants associated with brassinosteroids activity is ethylene synthesis (Ali, 2019).
Drought treatment considerably decreased pepper plant growth status and caused oxidative damage. In contrast, 24-epibrassinolide application significantly mitigated the oxidative damage caused by drought stress. In addition, the 24-epibrassinolide treatment elevated endogenous nitric oxide levels and regulated antioxidant defense mechanisms in pepper plants (Kaya et al., 2019). The total soluble solid content, proline uptake, pigments concentration, and leaf gas exchange elements were considerably improved, while the excessive production of H2O2, EL, and MDA content were decreased after brassinosteroids treatment (Khamsuk et al., 2018; Siddiqi and Husen, 2021). Foliar application of brassinosteroids effectively increased pigments concentration, chlorophyll content, growth attributes, seedling growth, chlorophyll fluorescence elements, and proline concentration, whereas there was decreased MDA and H2O2 concentration in Leymus chinensis under drought treatment (Lv et al., 2020). The exogenous use of brassinosteroids alleviated the adverse impacts of drought and enhanced drought tolerance by stimulating the antioxidant enzymes activity, fruit production, and pigments concentration in tomato leaf under drought stress (Jangid and Dwivedi, 2017). Kumari et al. (2020) described that brassinosteroids application significantly sustained essential growth and physiological–biochemical activities under drought stress environment. In addition, foliar application of brassinosteroid prior to the onset of stress may mitigate the adverse effects of drought stress on apple plants. Brassinolide enhances the physiological and biochemical characteristics by boosting the antioxidant system and photosynthetic efficiency in Brassica juncea. The increased synthesis of proline, enhancement of the antioxidant system, and decreased stress markers provide resilience to plants in coping with stress environment (Naveen et al., 2021). Under drought stress environment, foliar application of brassinosteroids on pepper seedlings effectively preserves vegetative characteristics, mitigates the adverse effects of stress, and diminishes stress indicators (Khosravi and Haghighi, 2021). In a recent study, Zhou et al. (2024) suggested that exogenous brassinosteroids regulates drought tolerance and elucidates the unique roles of CqBIN2 in the regulation of drought resistance in plants. The author described that ethylene was implicated in brassinosteroid-induced alternative oxidase enzymes activity, which is crucial for tolerance to abiotic stressors in cucumber seedlings (Wei et al., 2015).
Brassinosteroids application potential enhanced the secondary metabolites accumulation in pepper leaf (Samancıoğlu et al., 2014), protected photosynthetic capacity in apple leaf (Kumari and Thakur, 2019), regulated nitrogen and antioxidant defense mechanism in potato (Guo et al., 2024), enhanced carbohydrates and total soluble sugar concentration in radish (Balaraju et al., 2015), and decreased excessive ROS accumulation in cucumber (Xia et al., 2009). The author clearly indicates that local brassinosteroids application may stimulate the sustained generation of H2O2, and the self-propagating characteristic of the ROS signal subsequently facilitates EBR-induced systemic tolerance in cucumber (Xia et al., 2011). Zheng et al. (2022) described that leaf water content, chlorophyll content, pigments concentration declined, while brassinosteroids application signifcantly increased the leaf water status and photosynthetic assimilation rate and reduced oxidative damage in potato plants under drought stress. Exogenous 24-EBL application significantly increased the growth and petioles elongation in carrot (Que et al., 2017). The radish seedling growth and seed germination were increased with Brassinosteroids supplementation under water stress environment. In addition, the antioxidant enzymes activity, chlorophyll content, and proline uptake were increased, while MDA accumulation, H2O2 production, and EL level were considerably decreased in radish seedling under water stress conditions (Mahesh et al., 2013). Brassinosteroids are essential phytohormones that modulate signals to improve resilience to stress in plants. The results suggest that brassinosteroids are potentially beneficial, eco-friendly, naturally occurring compounds that may be extensively used to mitigate the impacts of abiotic stress (Figure 6).
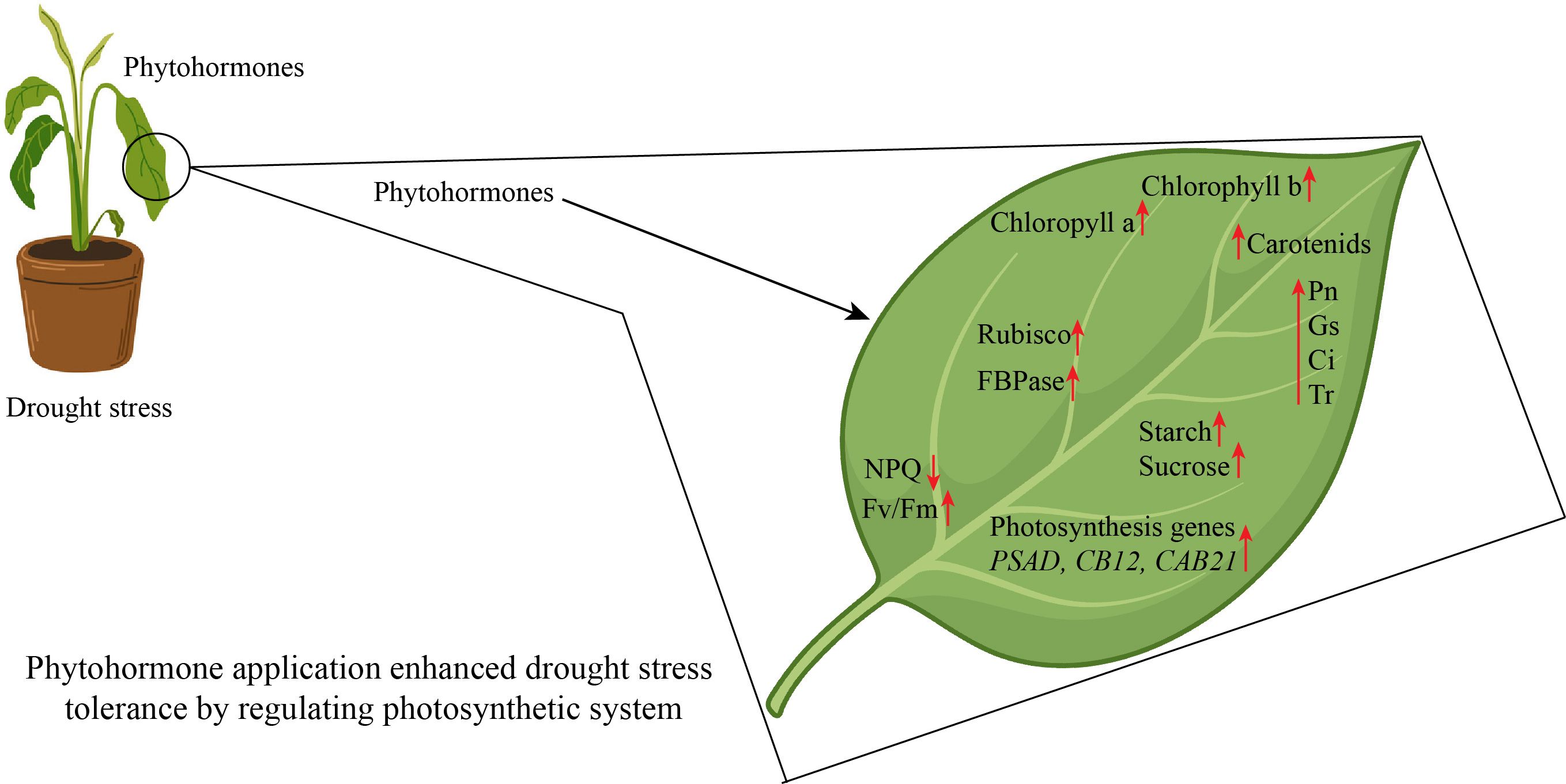
Figure 6. Phytohormone application protected photosynthetic apparatus in horticultural plants. NPQ, non-photochemical quenching; Pn, Net.
Strigolactones are derivatives of carotenoid that respond to different environmental stimuli by acting as both endogenous and external signaling molecules (Pandey et al., 2016). The strigolactones have a positive influence on horticultural plants (Siddiqi and Husen, 2017). Strigolactones regulate different functions in horticultural plants such as seed germination, flowering, seedling growth, cell elongation, photosynthesis, hormonal production, fruit ripening, redox balanced, antioxidant enzymes activity, secondary metabolites production, and leaf water potential in horticultural plants (Table 6) (Kaniganti et al., 2022; Sharma et al., 2024). Additionally, strigolactones cause the vascular cambium to become more active meristematically, which promotes secondary growth (Naseer et al., 2024). Strigolactones are associated with chlorophyll synthesis (Jiang et al., 2024). Under low light, plants produce more auxin, which enhances the synthesis of strigolactones. Strigolactones enhance root hair elongation but prevent the formation of interfascicular cambium in buds. Numerous functions of strigolactones has been described such as root system architecture, root morphology, plant defense mechanisms, and nutrient absorption (Soliman et al., 2022). Strigolactones serve as multifaceted signaling molecules, regulating numerous plant metabolic functions, including tolerance to drought stress (Makhzoum et al., 2017). Strigolactones were first recognized for their function in stimulating germination in root-parasitic plants (Khalid et al., 2024). Strigolactones provide the potential to improve our capacity to safeguard plants against the effects of drought stress.
Foliar application of strigolactones signifcantly protected the photosynthetic apparatus, regulated antioxidant defense mechanism, maintained redox homeostasis, and enhanced drought stress tolerance in pepper (Shu et al., 2023). Strigolactones (GR24) pretreatment mitigates the detrimental effects of drought stress on grapevine seedlings. Under drought stress, strigolactones (GR24) might more effectively promote stomatal closure. Strigolactones (GR24) may regulate chlorophyll constituents and mitigate the reduction of photosynthesis caused by dryness. The interaction of strigolactones with other hormones, particularly abscisic acid, may represent a significant factor in drought response (Min et al., 2019). The foliar application of strigolactones may positively influence the responses of Brassica rapa plants under drought environment (Ali et al., 2023). Furthermore, strigolactones treatment enhances the photosynthetic efficiency of Pennisetum purpureum leaves under drought conditions and elevates the antioxidant capacity of the leaves, thereby mitigating the detrimental effects of drought, fostering the growth of Pennisetum purpureum, and significantly enhancing its drought resistance (Li Y. et al., 2022). Strigolactones (GR24) substantially alleviates the drought-induced damage caused in apple. In addition, strigolactones (GR24) mitigate the drought-induced reduction in photosynthesis via modulating pigment molecules concentration and stomatal aperture. Strigolactones (GR24) mitigate oxidative damage by increasing antioxidant defense system. Strigolactones (GR24) improve drought resistance in apple via activating the expression of Ca2+ signaling-associated genes (Xu et al., 2023).
Strigolactones application positively regulates the growth of cucumber (Zhou et al., 2022), modulates the photosystem II efficiency in tomato and lettuce (Ruiz-Lozano et al., 2016), upregulates antioxidant enzymes activity in tomato (Baltacıer et al., 2023), and maintains redox homeostasis and reduces oxidative damage in pepper (Shu et al., 2024). Omoarelojie et al. (2020) reported that strigolactone-pretreated lupine seeds exhibited enhanced germination and seedling development, along with elevated proline levels and reduced MDA concentration. Foliar application of strigolactones markedly enhanced stomatal sensitivity in tomato plants under drought stress environment (Visentin et al., 2016). Furthermore, the supplementation of strigolactones enhanced the activity of the glyoxalase system and antioxidant enzymes in lupine seedlings. Furthermore, strigolactones modulate many hormone-responsive pathways, enabling plants to overcome environmental stressors and mitigate adverse effects on horticultural crop productivity (Bhoi et al., 2021). In response to various environmental stresses, strigolactones appear to be slightly important in the stress physiology of horticultural plants.
Gamma-aminobutyric acid (GABA) is a newly discovered plant growth regulator (Hasan et al., 2021). GABA regulated secondary metabolites accumulation, maintained redox homeostasis, upregulated antioxidant enzymes activity, balanced mineral accumulation, protected photosynthetic apparatus, and enhanced seedling growth in horticultural plants (Sita and Kumar, 2020). GABA protects plants from drought stress by boosting secondary metabolites production and leaf turgor while lowering oxidative damage via regulation of antioxidant defense system (Kinnersley and Turano, 2000). The application of GABA may enhance the growth and production of pepper under drought stress condition. Furthermore, foliar application of GABA enhanced secondary metabolites accumulation and the activity of antioxidant enzymes associated with pepper plant defense mechanisms (Iqbal et al., 2023). The supplementation of GABA under water stress in snap bean plants enhanced field performance, shown by upregulation of antioxidant enzymes activity, and maintained cellular membrane integrity level, higher pod production, and quality traits. In conclusion, exogenous GABA serves as an efficient priming agent to mitigate drought-induced oxidative damage in snap bean plants under drought stress condition (Abd El-Gawad et al., 2021). GABA application enhanced seed germination, osmolytes production, and antioxidant enzymes activity in white clover under drought stress (Zhou et al., 2021). The foliar treatment of GABA significantly enhanced the drought stress tolerance of cucumber seedlings by elevating antioxidant enzymes activity, free proline concentrations, protected photosynthetic capacity, and leaf relative water content. In addition, GABA treatment may serve as an effective approach to mitigate the detrimental impacts of drought stress on cucumber cultivation (Ghahremani et al., 2023). GABA application enhanced pigments concentration, soluble sugar content, protein concentration, and secondary metabolites production in pea leaves under drought stress environment (Al-Quraan et al., 2021). Cheng et al. (2023) reported that foliar application of GABA improved photosynthetic assimilation rate, increased leaf water content, and reduced EL level in apple leaves (Table 7).
The phytohormones participate in the interaction with other growth regulators, resulting in notable alterations in the phenology of horticultural plants (Figure 7). Abscisic acid has a crucial function in regulating situations of drought stress (Kleman and Matusova, 2023). Stomatal closure is a significant morphological characteristic that is rigorously maintained and mostly influenced by drought stress (Ciura and Kruk, 2018). Under drought stress, abscisic acid application along with jasmonic acid and nitric oxide promote stomatal closure (Altaf et al., 2023). The interaction between the salicylic acid pathway and the abscisic acid, jasmonic acid, and ethylene pathways is essential for the regulation of plant growth and stress responses (Jogawat et al., 2021). Furthermore, the combination of salicylic acid and melatonin significantly enhances the drought tolerance of tomato plants. The combination hormone treatment considerably increased antioxidant enzymes activity, promoted hormone production, and maintained methylglyoxal enzymes pool, which increased the tolerance of tomato plants to drought stress (Kaya et al., 2023). Drought stress has the greatest detrimental impact on lettuce productivity. The combined salicylic acid and melatonin improved lettuce resilience to drought stress. The molecular mechanisms and biochemical interactions of melatonin and melatonin-mediated phytohormonal crosstalk in horticultural plants play a multifaceted role in enhancing drought stress tolerance (Dzinyela et al., 2024). Phytohormones are essential molecules that facilitate drought tolerance, thus offering new opportunities for the preservation of sustainable crop yields for addressing global food demand in the face of changing the environment (Tiwari et al., 2017). Phytohormones coordinate essential developmental signals and transmit environmental information via synergistic or antagonistic interactions known as signaling crosstalk (Seif El-Yazal et al., 2015). Gibberellic acid signaling and its interaction with other hormonal pathways elucidate the multifaceted function of DELLA proteins in conjunction with components of many hormonals signaling pathways (Niharika et al., 2021). Auxin, gibberellins, and cytokinins are pivotal in controlling development under stress situations, whereas abscisic acid and ethylene inhibit growth by modifying the actions of GA, auxin, and CK under adverse conditions (Ullah et al., 2018). The interplay between brassinosteroids (BRs) and gibberellins (GAs) is well established. Mutant BR signaling in Arabidopsis thaliana showed many changes in the expression of GA biosynthetic genes, which might be because bioactive GA synthesis was disrupted (Bano et al., 2023). Auxin, an additional hormone, stimulates hypocotyl development. The auxin signaling pathway entails the modulation of transcription factor auxin response factors via the degradation of AUX/IAA family members (Emenecker and Strader, 2020). The connectivity between GA and ABA facilitates the balance between seed germination and dormancy, which is crucial for stress tolerance. These hormones have an antagonistic connection, resulting in elevated GA and diminished ABA levels under favorable circumstances and reduced GA and increased ABA levels under adverse environmental conditions in seeds (Altaf et al., 2023). The combined application of salicylic acid and melatonin more efficiently mitigates stress in lettuce. In addition, salicylic acid application along with melatonin enhanced the nutritional status vitamin C and antioxidant potential and reduced nitrate concentration in lettuce (Kiremit et al., 2024). Melatonin application crosstalk with other phytohormone significantly enhanced stomatal regulation and photosynthetic capacity in horticultural plants (Sun et al., 2023). The advancement of mechanistic methodologies is essential to mitigate the detrimental impacts of drought on horticultural crops.
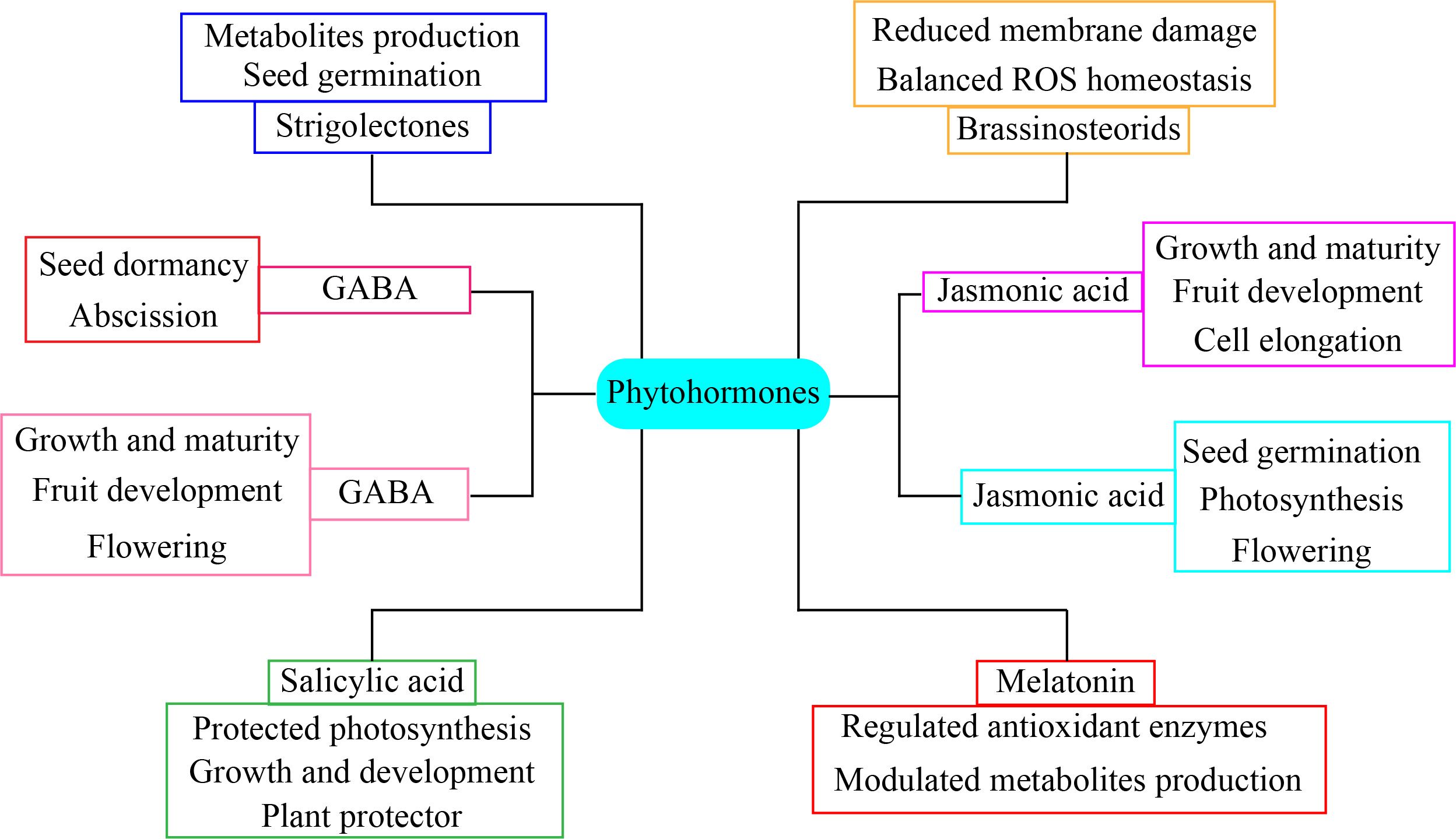
Figure 7. Phytohormone crosstalk application enhanced drought stress tolerance in horticultural plants.
Plants require sophisticated signaling pathways to regulate and develop in a variety of environmental conditions. Horticultural crops have increased vulnerability to variable environmental conditions. Phytohormones are compounds that affect the vegetative and reproductive development of plants while alleviating different abiotic stressors. These dynamic molecules substantially modify the metabolic fluxes inside the plant cell under stress circumstances to promote a more resistant phenotype. Phytohormones are essential stress mitigator throughout all phases of crop growth. Information accumulates about the advantageous impacts of phytohormones like salicylic acid, melatonin, brassinosteroids, jasmonate, and strigolactones in horticultural crops. Substantial data indicate that phytohormone responses vary across different phases of organ development, perhaps owing to distinct cellular and tissue contexts. A complex network of phytohormones affects root shape, emphasizing the need of understanding transcriptional and post-transcriptional processes and their genes. These investigations will enhance understanding of how roots detect internal and external signals and convert them into cellular responses while also allowing breeders to develop predictive models to identify crucial regulators and integrators of root system architecture under different environmental circumstances. Future investigations into phytohormone interactions with other signaling molecules for drought resilience in horticulture crops should concentrate on many critical domains as follows:
● Exploring the precise molecular processes by which phytohormones interact with other signaling molecules to help various horticulture crops tolerate drought.
● Finding and analyzing novel phytohormones and signaling molecules linked to drought tolerance and comprehending how they interact with another pathway.
● Establishing new instruments and technologies, as genome editing and sophisticated imaging methods, to investigate complicated signaling pathways connected to drought tolerance.
● Investigating how temperature and light affect the way that phytohormones interact with other signaling molecules to help plants withstand drought.
Future study in these domains might substantially enhance our comprehension of the molecular processes underlying drought tolerance in horticulture crops and facilitate the identification of novel ways for boosting agricultural yields and securing global food security amid climate change.
SH: Writing – original draft. SJ: Conceptualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbas, K., Li, J., Gong, B., Lu, Y., Wu, X., Lü, G., et al. (2023). Drought stress tolerance in vegetables: the functional role of structural features, key gene pathways, and exogenous hormones. Int. J. Mol. Sci. 24, 13876. doi: 10.3390/ijms241813876
Abd El-Gawad, H. G., Mukherjee, S., Farag, R., Abd Elbar, O. H., Hikal, M., Abou El-Yazied, A., et al. (2021). Exogenous γ-aminobutyric acid (GABA)-induced signaling events and field performance associated with mitigation of drought stress in Phaseolus vulgaris L. Plant Signal. Behav. 16, 1853384. doi: 10.1080/15592324.2020.1853384
Ahammed, G. J., Xia, X. J., Li, X., Shi, K., Yu, J. Q., Zhou, Y. H. (2015). Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr. Protein Pept. Sci. 16, 462–473. doi: 10.2174/1389203716666150330141427
Ahmad, S., Munawar, M., Ullah, M., Khalid, S., Waris, I., Sher, N., et al. (2024). ). Crop responses and strategies for mitigating cold, salt, and drought stress in vegetables: A review. Asian J. Res. Crop Sci. 9, 168–180. doi: 10.9734/ajrcs/2024/v9i2277
Ahmad, P., Rasool, S., Gul, A., Sheikh, S. A., Akram, N. A., Ashraf, M., et al. (2016). Jasmonates: multifunctional roles in stress tolerance. Front. Plant Sci. 7, 813. doi: 10.3389/fpls.2016.00813
Ahmad, I., Zhu, G., Zhou, G., Liu, J., Younas, M. U., Zhu, Y. (2023). Melatonin role in plant growth and physiology under abiotic stress. Int. J. Mol. Sci. 24, 8759. doi: 10.3390/ijms24108759
Ahmad Lone, W., Majeed, N., Yaqoob, U., John, R. (2022). Exogenous brassinosteroid and jasmonic acid improve drought tolerance in Brassica rapa L. genotypes by modulating osmolytes, antioxidants and photosynthetic system. Plant Cell Rep. 41, 603–617. doi: 10.1007/s00299-021-02763-9
Aires, E. S., Ferraz, A. K. L., Carvalho, B. L., Teixeira, F. P., Putti, F. F., de Souza, E. P., et al. (2022). Foliar application of salicylic acid to mitigate water stress in tomato. Plants 11, 1775. doi: 10.3390/plants11131775
Alam, A., Ullah, H., Thuenprom, N., Tisarum, R., Cha-Um, S., Datta, A. (2022). Seed priming with salicylic acid enhances growth, physiological traits, fruit yield, and quality parameters of cantaloupe under water-deficit stress. South Afr. J. Bot. 150, 1–12. doi: 10.1016/j.sajb.2022.06.056
Ali, B. (2017). Practical applications of brassinosteroids in horticulture—some field perspectives. Sci. Hortic. 225, 15–21. doi: 10.1016/j.scienta.2017.06.051
Ali, B. (2019). “Brassinosteroids: the promising plant growth regulators in horticulture,” in Brassinosteroids: plant growth and development eds, Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A. (Singapore: Springer). 349–365.
Ali, S., Anjum, M. A., Nawaz, A., Naz, S., Hussain, S., Ejaz, S. (2019). Effects of brassinosteroids on postharvest physiology of horticultural crops: a concise review. J. Hortic. Sci. Technol. 2, 62–68. doi: 10.46653/jhst
Ali, M. S., Baek, K. H. (2020). Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int. J. Mol. Sci. 21, 621. doi: 10.3390/ijms21020621
Ali, A., Shah, T., Haider, G., Awan, M. I., Gohar, M., Munsif, F., et al. (2023). Strigolactone-mediated oxidative stress alleviation in Brassica rapa through upregulating antioxidant system under water deficit conditions. J. Plant Growth Regul. 42, 4675–4687. doi: 10.1007/s00344-023-10925-0
Al-Quraan, N. A., Al-Ajlouni, Z. I., Qawasma, N. F. (2021). Physiological and biochemical characterization of the GABA shunt pathway in pea (Pisum sativum L.) seedlings under drought stress. Horticulturae 7, 125. doi: 10.3390/horticulturae7060125
Altaf, M. A., Shahid, R., Kumar, R., Altaf, M. M., Kumar, A., Khan, L. U., et al. (2023). Phytohormones mediated modulation of abiotic stress tolerance and potential crosstalk in horticultural crops. J. Plant Growth Regul. 42, 4724–4750. doi: 10.1007/s00344-022-10812-0
Altaf, M. A., Shahid, R., Ren, M. X., Naz, S., Altaf, M. M., Khan, L. U., et al. (2022). Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 11, 309. doi: 10.3390/antiox11020309
Ardıç, Ş. K., Szafrańska, K., Havan, A., Karaca, A., Aslan, M. Ö., Sözeri, E., et al. (2023). Endogenous melatonin content confers drought stress tolerance in pepper. Environ. Exp. Bot. 216, 105536. doi: 10.1016/j.envexpbot.2023.105536
Asghar, M. A., Ahmad, B., Raza, A., Adil, B., Hassan Javed, H., Farooq, M. U., et al. (2022). Shade and microbes enhance drought stress tolerance in plants by inducing phytohormones at molecular levels: a review. J. Plant Eco. 15, 1107–1117. doi: 10.1093/jpe/rtac038
Ayyaz, A., Shahzadi, A. K., Fatima, S., Yasin, G., Zafar, Z. U., Athar, H. U. R., et al. (2022). Uncovering the role of melatonin in plant stress tolerance. Theor. Exp. Plant Physiol. 34, 335–346. doi: 10.1007/s40626-022-00255-z
Balaraju, P., Ayodhya-Ramulu, C., Venkateshwarlu, M., Ugandhar, T. (2015). Influence of PEG imposed water stress and exogenous application of brassinosteroids on metabolites in radish. Asian J. Sci. Tech. 6, 951–955.
Baltacıer, G., Donat, S., Acar, O. (2023). The effects of exogenous salicylic acid and strigolactone applications on seedling growth and antioxidant activity in tomato seedlings under short-term drought stress. J. Inst. Sci. Technol. 13, 89–101.
Baninasab, B. (2010). Induction of drought tolerance by salicylic acid in seedlings of cucumber (Cucumis sativus L.). J. Hortic. Sci. Biotechnol. 85, 191–196. doi: 10.1080/14620316.2010.11512653
Bano, A., Singh, K., Singh, S. P., Sharma, P. (2023). Abscisic acid: metabolism, signaling, and crosstalk with other phytohormones under heavy metal stress. Stresses 3, 665–686. doi: 10.3390/stresses3040046
Bhandari, S., Nailwal, T. K. (2020). Role of brassinosteroids in mitigating abiotic stresses in plants. Biologia 75, 2203–2230. doi: 10.2478/s11756-020-00587-8
Bhoi, A., Yadu, B., Chandra, J., Keshavkant, S. (2021). Contribution of strigolactone in plant physiology, hormonal interaction and abiotic stresses. Planta 254, 28. doi: 10.1007/s00425-021-03678-1
Bouremani, N., Cherif-Silini, H., Silini, A., Bouket, A. C., Luptakova, L., Alenezi, F. N., et al. (2023). Plant growth-promoting rhizobacteria (PGPR): A rampart against the adverse effects of drought stress. Water 15, 418. doi: 10.3390/w15030418
Cao, Y., Yang, W., Ma, J., Cheng, Z., Zhang, X., Liu, X., et al. (2024). An integrated framework for drought stress in plants. Int. J. Mol. Sci. 25, 9347.
Chakma, R., Biswas, A., Saekong, P., Ullah, H., Datta, A. (2021). Foliar application and seed priming of salicylic acid affect growth, fruit yield, and quality of grape tomato under drought stress. Sci. Hortic. 280, 109904. doi: 10.1016/j.scienta.2021.109904
Checker, V. G., Kushwaha, H. R., Kumari, P., Yadav, S. (2018). Role of phytohormones in plant defense: signaling and cross talk. Mol. aspects plant-pathogen interact. (Singapore: Springer), 159–184. doi: 10.1007/978-981-10-7371-7_7
Chen, W., Wang, Y., Xu, L., Dong, J., Zhu, X., Ying, J., et al. (2019). Methyl jasmonate, salicylic acid and abscisic acid enhance the accumulation of glucosinolates and sulforaphane in radish (Raphanus sativus L.) taproot. Sci. Hortic. 250, 159–167. doi: 10.1016/j.scienta.2019.02.024
Chen, S., Zhao, C. B., Ren, R. M., Jiang, J. H. (2023). Salicylic acid had the potential to enhance tolerance in horticultural crops against abiotic stress. Front. Plant Sci. 14, 1141918. doi: 10.3389/fpls.2023.1141918
Cheng, P., Yue, Q., Zhang, Y., Zhao, S., Khan, A., Yang, X., et al. (2023). Application of γ-aminobutyric acid (GABA) improves fruit quality and rootstock drought tolerance in apple. J. Plant Physiol. 280, 153890. doi: 10.1016/j.jplph.2022.153890
Chieb, M., Gachomo, E. W. (2023). The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Bio. 23, 407. doi: 10.1186/s12870-023-04403-8
Ciura, J., Kruk, J. (2018). Phytohormones as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 229, 32–40. doi: 10.1016/j.jplph.2018.06.013
Colombage, R., Singh, M. B., Bhalla, P. L. (2023). Melatonin and abiotic stress tolerance in crop plants. Int. J. Mol. Sci. 24, 7447. doi: 10.3390/ijms24087447
Dakheel, M. G., Ullah, I., Doğan, D. E., Demirsoy, L. (2022). “Potential role of salicylic acid on drought stress tolerance of strawberry plants,” in International Symposium on Soil Science and Plant Nutrition. 269–275.
Damalas, C. A., Koutroubas, S. D. (2021). Foliar applications of salicylic acid for improving crop tolerance to drought stress: A review. Salicylic Acid-A Versatile Plant Growth Regul., 65–76. doi: 10.1007/978–3-030–79229-9_5
Debnath, B., Islam, W., Li, M., Sun, Y., Lu, X., Mitra, S., et al. (2019). Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 20, 1040. doi: 10.3390/ijms20051040
Delgado, C., Mora-Poblete, F., Ahmar, S., Chen, J. T., Figueroa, C. R. (2021). Jasmonates and plant salt stress: Molecular players, physiological effects, and improving tolerance by using genome-associated tools. Int. J. Mol. Sci. 22, 3082. doi: 10.3390/ijms22063082
Đurić, M., Subotić, A., Trifunović-Momčilov, M., Milošević, S. (2023). Improvement of water deficit stress tolerance of Impatiens walleriana shoots grown in vitro by methyl jasmonate. Plant Cell Tissue Organ Cult. 154, 351–365. doi: 10.1007/s11240-022-02432-z
Dzinyela, R., Hwarari, D., Opoku, K. N., Yang, L., Movahedi, A. (2024). Enhancing drought stress tolerance in horticultural plants through melatonin-mediated phytohormonal crosstalk. Plant Cell Rep. 43, 272. doi: 10.1007/s00299-024-03362-0
El-Yazied, A. A., Ibrahim, M. F., Ibrahim, M. A., Nasef, I. N., Al-Qahtani, S. M., Al-Harbi, N. A., et al. (2022). Melatonin mitigates drought induced oxidative stress in potato plants through modulation of osmolytes, sugar metabolism, ABA homeostasis and antioxidant enzymes. Plants 11, 1151. doi: 10.3390/plants11091151
Emenecker, R. J., Strader, L. C. (2020). Auxin-abscisic acid interactions in plant growth and development. Biomolecules 10, 281. doi: 10.3390/biom10020281
Fahad, S., Nie, L., Chen, Y., Wu, C., Xiong, D., Saud, S., et al. (2015). Crop plant hormones and environmental stress. Sustain. Agri. Rev. 15, 371-400.
Farooqi, Z. U. R., Ayub, M. A., ur Rehman, M. Z., Sohail, M. I., Usman, M., Khalid, H., et al. (2020). “Regulation of drought stress in plants,” in Plant life under changing environment, Eds. Tripathi, D. K., Pratap Singh, V., Chauhan, D. K., Sharma, S., Prasad, S. M., Dubey, N. K., et al. (Cambridge, Massachusetts, USA: Academic Press), 77–104.
Feng, D., Liu, W., Chen, K., Ning, S., Gao, Q., Chen, J., et al. (2024). Exogenous substances used to relieve plants from drought stress and their associated underlying mechanisms. Int. J. Mol. Sci. 25, 9249. doi: 10.3390/ijms25179249
Fugate, K. K., Lafta, A. M., Eide, J. D., Li, G., Lulai, E. C., Olson, L. L., et al. (2018). Methyl jasmonate alleviates drought stress in young sugar beet (Beta vulgaris L.) plants. J. Agron. Crop Sci. 204, 566–576. doi: 10.1111/jac.2018.204.issue-6
Gao, Y., Ma, X., Zhang, Z., Wang, Y. (2024). Transcription factors and plant hormones mediate wax metabolism in response to drought stress. Physiol. Plantar. 176, e14478. doi: 10.1111/ppl.v176.4
Ghaffari, H., Tadayon, M. R., Nadeem, M., Razmjoo, J., Cheema, M. (2019). Foliage applications of jasmonic acid modulate the antioxidant defense under water deficit growth in sugar beet. Spanish J. Agric. Res. 17, e0805. doi: 10.5424/sjar/2019174-15380
Ghahremani, Z., Fathollahi, A., Barzegar, T., Nikbakht, J., Ranjbar, M. E., Nezamdoost, D. (2023). Role of plant genetic resources and γ-aminobutyric acid (GABA) to enhance drought tolerance of cucumber (Cucumis sativus). Agric. Res. 12, 257–265. doi: 10.1007/s40003-023-00650-1
Gholamreza, A. B. D. I., Shokrpour, M., Karami, L., Salami, S. A. (2019). Prolonged water deficit stress and methyl jasmonate-mediated changes in metabolite profile, flavonoid concentrations and antioxidant activity in peppermint (Mentha× piperita L.). Notulae Bot. Horti Agrobot. Cluj-Napoca 47, 70–80. doi: 10.15835/nbha47110952
Guo, X., Zhang, S., Gong, L., He, Y., Qu, R., Teng, Y., et al. (2024). Brassinosteroid improves resistance to phosphorus deficiency stress through regulating nutrient balance and reactive oxygen species scavenging in potato. Environ. Exp. Bot. 227, 105954. doi: 10.1016/j.envexpbot.2024.105954
Haider, S., Bibi, K., Munyaneza, V., Zhang, H., Zhang, W., Ali, A., et al. (2024). Drought-induced adaptive and ameliorative strategies in plants. Chemosphere 364, 143134. doi: 10.1016/j.chemosphere.2024.143134
Han, X., Zeng, H., Bartocci, P., Fantozzi, F., Yan, Y. (2018). Phytohormones and effects on growth and metabolites of microalgae: a review. Fermentation 4, 25. doi: 10.3390/fermentation4020025
Hasan, M. M., Alabdallah, N. M., Alharbi, B. M., Waseem, M., Yao, G., Liu, X. D., et al. (2021). GABA: A key player in drought stress resistance in plants. Int. J. Mol. Sci. 22, 10136. doi: 10.3390/ijms221810136
He, M., Mei, S., Zhai, Y., Geng, G., Yu, L., Wang, Y. (2023). Effects of melatonin on the growth of sugar beet (Beta vulgaris L.) seedlings under drought stress. J. Plant Growth Regul. 42, 5116–5130. doi: 10.1007/s00344-022-10860-6
Horváth, E., Szalai, G., Janda, T. (2007). Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 26, 290–300. doi: 10.1007/s00344-007-9017-4
Huang, C., Liao, J., Huang, W., Qin, N. (2022a). Salicylic acid protects sweet potato seedlings from drought stress by mediating abscisic acid-related gene expression and enhancing the antioxidant defense system. Int. J. Mol. Sci. 23, 14819. doi: 10.3390/ijms232314819
Huang, X., Tanveer, M., Min, Y., Shabala, S. (2022b). Melatonin as a regulator of plant ionic homeostasis: implications for abiotic stress tolerance. J. Exp. Bot. 73, 5886–5902. doi: 10.1093/jxb/erac224
Huang, Q., Yan, H., You, M., Duan, J., Chen, M., Xing, Y., et al. (2023). Enhancing drought tolerance and fruit characteristics in tomato through exogenous melatonin application. Horticulturae 9, 1083. doi: 10.3390/horticulturae9101083
Iqbal, B., Hussain, F., Khan, M. S., Iqbal, T., Shah, W., Ali, B., et al. (2023). Physiology of gamma-aminobutyric acid treated Capsicum annuum L.(Sweet pepper) under induced drought stress. PloS One 18, e0289900. doi: 10.1371/journal.pone.0289900
Iqbal, S., Wang, X., Mubeen, I., Kamran, M., Kanwal, I., Díaz, G. A., et al. (2022). Phytohormones trigger drought tolerance in crop plants: outlook and future perspectives. Front. Plant Sci. 12, 799318. doi: 10.3389/fpls.2021.799318
Jangid, K. K., Dwivedi, P. (2017). Physiological and biochemical changes by nitric oxide and brassinosteroid in tomato (Lycopersicon esculentum Mill.) under drought stress. Acta Physiol. Plantar. 39, 1–10. doi: 10.1007/s11738-017-2373-1
Jiang, W., Fei Lu, C., Xu, X., Riaz, M. W., Aimin, L. V., Shao, Q. (2024). Strigolactones: Biosynthetic regulation, hormonal interaction, and their involvement in abiotic stress adaption. Sci. Hortic. 325, 112689. doi: 10.1016/j.scienta.2023.112689
Jogawat, A., Yadav, B., Chhaya, Lakra, N., Singh, A. K., Narayan, O. P. (2021). Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: a review. Physiol. Plantar. 172, 1106–1132. doi: 10.1111/ppl.v172.2
Joseph, B., Jini, D., Sujatha, S. (2010). Insight into the role of exogenous salicylic acid on. Asian J. Crop Sci. 2, 226–235. doi: 10.3923/ajcs.2010.226.235
Kang, G., Li, G., Guo, T. (2014). Molecular mechanism of salicylic acid-induced abiotic stress tolerance in higher plants. Acta Physiol. Plantar. 36, 2287–2297. doi: 10.1007/s11738-014-1603-z
Kaniganti, S., Bhattacharya, J., Petla, B. P., Reddy, P. S. (2022). Strigolactone, a neglected plant hormone, with a great potential for crop improvement: Crosstalk with other plant hormones. Environ. Exp. Bot. 204, 105072. doi: 10.1016/j.envexpbot.2022.105072
Kaya, C., Ashraf, M., Wijaya, L., Ahmad, P. (2019). The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol. Biochem. 143, 119-128.
Kaya, C., Shabala, S. (2023). Melatonin improves drought stress tolerance of pepper (Capsicum annuum) plants via upregulating nitrogen metabolism. Funct. Plant Bio. 51, 1–17. doi: 10.1071/FP23060
Kaya, C., Uğurlar, F., Adamakis, I. D. S. (2024). Epigenetic modifications of hormonal signaling pathways in plant drought response and tolerance for sustainable food security. Int. J. Mol. Sci. 25, 8229. doi: 10.3390/ijms25158229
Kaya, C., Ugurlar, F., Ashraf, M., AlYemeni, M. N., Ahmad, P. (2023). Exploring the synergistic effects of melatonin and salicylic acid in enhancing drought stress tolerance in tomato plants through fine-tuning oxidative-nitrosative processes and methylglyoxal metabolism. Sci. Hortic. 321, 112368. doi: 10.1016/j.scienta.2023.112368
Khalid, M. F., Shafqat, W., Khan, R. I., Jawaid, M. Z., Hussain, S., Saqib, M., et al. (2024). Unveiling the resilience mechanism: Strigolactones as master regulators of plant responses to abiotic stresses. Plant Stress. 12, 100490. doi: 10.1016/j.stress.2024.100490
Khamsuk, O., Sonjaroon, W., Suwanwong, S., Jutamanee, K., Suksamrarn, A. (2018). Effects of 24-epibrassinolide and the synthetic brassinosteroid mimic on chili pepper under drought. Acta Physiol. Plantar. 40, 1–12. doi: 10.1007/s11738-018-2682-z
Khan, M. I. R., Fatma, M., Per, T. S., Anjum, N. A., Khan, N. A. (2015). Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 6, 462. doi: 10.3389/fpls.2015.00462
Khan, M. Q. N., Sevgin, N., Rizwana, H., Arif, N. (2023). Exogenous melatonin mitigates the adverse effects of drought stress in strawberry by upregulating the antioxidant defense system. South African J. Bot. 162, 658-666.
Khodadadi, S., Chegini, M. A., Soltani, A., Ajam Norouzi, H., Sadeghzadeh Hemayati, S. (2020). Influence of foliar-applied humic acid and some key growth regulators on sugar beet (Beta vulgaris L.) under drought stress: Antioxidant defense system, photosynthetic characteristics and sugar yield. Sugar Tech 22, 765–772. doi: 10.1007/s12355-020-00839-6
Khosravi, S., Haghighi, M. (2021). The Effect of Foliar Spray of Brassinosteroid on Sweet Pepper (Capsicum annuum L.) Seedling under Drought Stress. J. Hortic. Sci. 35, 367–381. doi: 10.22067/jhs.2021.61838.0
Kinnersley, A. M., Turano, F. J. (2000). Gamma aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 19, 479–509. doi: 10.1080/07352680091139277
Kiremit, M. S., Akınoğlu, G., Mitrovica, B., Rakıcıoğlu, S. (2024). Enhancing drought-salinity stress tolerance in lettuce: Synergistic effects of salicylic acid and melatonin. South Afr. J. Bot. 172, 212–226. doi: 10.1016/j.sajb.2024.07.021
Kleman, J., Matusova, R. (2023). Strigolactones: Current research progress in the response of plants to abiotic stress. Biologia 78, 307–318. doi: 10.1007/s11756-022-01230-4
Kumari, S., Thakur, A. (2019). The effects of water stress and brassinosteroid on apple varieties. Int. J. Eco. Plants. 6, 1–6. doi: 10.23910/IJEP/2019.6.1.0278
Kumari, S., Thakur, A., Singh, N., Chandel, J. S., Rana, N. (2020). Influence of drought stress and brassinosteroid on growth and physio-biochemical characteristics of apple plants. Indian J. Hortic. 77, 88–93. doi: 10.5958/0974-0112.2020.00007.9
Lee, H. J., Back, K. (2019). 2-Hydroxymelatonin confers tolerance against combined cold and drought stress in tobacco, tomato, and cucumber as a potent anti-stress compound in the evolution of land plants. Melatonin Res. 2, 35–46. doi: 10.32794/mr11250020
Li, Y., Li, S., Feng, Q., Zhang, J., Han, X., Zhang, L., et al. (2022). Effects of exogenous Strigolactone on the physiological and ecological characteristics of Pennisetum purpureum Schum. Seedlings under drought stress. BMC Plant Bio. 22, 578. doi: 10.1186/s12870-022-03978-y
Li, H., Mo, Y., Cui, Q., Yang, X., Guo, Y., Wei, C., et al. (2019). Transcriptomic and physiological analyses reveal drought adaptation strategies in drought-tolerant and-susceptible watermelon genotypes. Plant Sci. 278, 32–43. doi: 10.1016/j.plantsci.2018.10.016
Li, X., Riaz, M., Song, B., Liang, X., Liu, H. (2022). Exogenous salicylic acid alleviates fomesafen toxicity by improving photosynthetic characteristics and antioxidant defense system in sugar beet. Ecotoxicol. Environ. Saf. 238, 113587. doi: 10.1016/j.ecoenv.2022.113587
Liang, D., Ni, Z., Xia, H., Xie, Y., Lv, X., Wang, J., et al. (2019). Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 246, 34–43. doi: 10.1016/j.scienta.2018.10.058
Liu, X., Liu, D. H., Chen, T., Zhang, J., Wang, C. L. (2022). Watercore pear fruit respiration changed and accumulated γ-aminobutyric acid (GABA) in response to inner hypoxia stress. Genes 13, 977. doi: 10.3390/genes13060977
Liu, J., Wang, W., Wang, L., Sun, Y. (2015). Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul. 77, 317–326. doi: 10.1007/s10725-015-0066-6
Lopes, A. S., Dias, T. J., Henschel, J. M., da Silva, J. H. B., de Oliveira Sousa, V. F., Targino, V. A., et al. (2024). Methyl jasmonate mitigates drought stress in purple basil by enhancing photosynthesis and secondary metabolism. J. Plant Growth Regul. doi: 10.1007/s00344-024-11392-x
Lv, J., Zong, X. F., Shakeel Ahmad, A., Wu, X., Wu, C., Li, Y. P., et al. (2020). Alteration in morpho-physiological attributes of Leymus chinensis (Trin.) Tzvelev by exogenous application of brassinolide under varying levels of drought stress. Chilean J.Agri. Res. 80, 61–71. doi: 10.4067/S0718-58392020000100061
Mahesh, K., Balaraju, P., Ramakrishna, B., Rao, S. S. R. (2013). Effect of brassinosteroids on germination and seedling growth of radish (Raphanus sativus L.) under PEG-6000 induced water stress. Am. J. Plant Sci. 4, 40442. doi: 10.4236/ajps.2013.412285
Makhzoum, A., Yousefzadi, M., Malik, S., Gantet, P., Tremouillaux-Guiller, J. (2017). Strigolactone biology: genes, functional genomics, epigenetics and applications. Crit. Rev. Biotechnol. 37, 151–162. doi: 10.3109/07388551.2015.1121967
Mardani, H., Bayat, H., Saeidnejad, A. H., Rezaie, E. E. (2012). Assessment of salicylic acid impacts on seedling characteristic of cucumber (Cucumis sativus L.) under water stress. Notulae Sci. Biolog. 4, 112–115. doi: 10.15835/nsb417258
Min, Z., Li, R., Chen, L., Zhang, Y., Li, Z., Liu, M., et al. (2019). Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol. Biochem. 135, 99–110. doi: 10.1016/j.plaphy.2018.11.037
Mohi-Ud-Din, M., Talukder, D., Rohman, M., Ahmed, J. U., Jagadish, S. K., Islam, T., et al. (2021). Exogenous application of methyl jasmonate and salicylic acid mitigates drought-induced oxidative damages in french bean (Phaseolus vulgaris L.). Plants 10, 2066. doi: 10.3390/plants10102066
Moustafa-Farag, M., Mahmoud, A., Arnao, M. B., Sheteiwy, M. S., Dafea, M., Soltan, M., et al. (2020). Melatonin-induced water stress tolerance in plants: Recent advances. Antioxidants 9, 809. doi: 10.3390/antiox9090809
Mozafari, A. A., Havas, F., Ghaderi, N. (2018). Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria× ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ Culture 132, 511–523. doi: 10.1007/s11240-017-1347-8
Mubarik, M. S., Khan, S. H., Sajjad, M., Raza, A., Hafeez, M. B., Yasmeen, T., et al. (2021). A manipulative interplay between positive and negative regulators of phytohormones: A way forward for improving drought tolerance in plants. Physiol. Plantar. 172, 1269–1290. doi: 10.1111/ppl.v172.2
Muñoz-Espinoza, V. A., López-Climent, M. F., Casaretto, J. A., Gómez-Cadenas, A. (2015). Water stress responses of tomato mutants impaired in hormone biosynthesis reveal abscisic acid, jasmonic acid and salicylic acid interactions. Front. Plant Sci. 6, 997. doi: 10.3389/fpls.2015.00997
Mushtaq, N., Iqbal, S., Hayat, F., Raziq, A., Ayaz, A., Zaman, W. (2022). Melatonin in micro-tom tomato: Improved drought tolerance via the regulation of the photosynthetic apparatus, membrane stability, osmoprotectants, and root system. Life 12, 1922. doi: 10.3390/life12111922
Nafie, E., Hathout, T., Mokadem, A., Shyma, A. (2011). Jasmonic acid elicits oxidative defense and detoxification systems in Cucumis melo L. cells. Braz. J. Plant Physiol. 23, 161–174. doi: 10.1590/S1677-04202011000200008
Naseer, M. A., Zhang, Z. Q., Mukhtar, A., Asad, M. S., Wu, H. Y., Yang, H., et al. (2024). Strigolactones: A promising tool for nutrient acquisition through arbuscular mycorrhizal fungi symbiosis and abiotic stress tolerance. Plant Physiol. Biochem. 215, 109057. doi: 10.1016/j.plaphy.2024.109057
Nasrabadi, H. N., Nemati, H., Kafi, M., Arouei, H. (2015). Effect of foliar application with salicylic acid on two Iranian melons (Cucumis melo L.) under water deficit. Afr. J. Agric. Res. 10, 3305–3309. doi: 10.5897/AJAR2015.10057
Nastari Nasrabadi, H., Saberali, S. F., ShirmohammadiAliakbarkhani, Z. (2023). Improving growth and fruit yield of watermelon using mycorrhizal fungi and salicylic acid under different irrigation regimes. Isfahan Univ. Technology-J. Crop Product. Process. 13, 109–124. doi: 10.47176/jcpp.13.3.37791
Naveen, N., Kumari, N., Avtar, R., Jattan, M., Ahlawat, S., Rani, B., et al. (2021). Evaluation of effect of brassinolide in Brassica juncea leaves under drought stress in field conditions. Horticulturae 7, 514. doi: 10.3390/horticulturae7110514
Nazir, F., Peter, P., Gupta, R., Kumari, S., Nawaz, K., Khan, M. I. R. (2024). Plant hormone ethylene: A leading edge in conferring drought stress tolerance. Physiol. Plantar. 176, e14151. doi: 10.1111/ppl.v176.1
Nguyen, H. C., Lin, K. H., Ho, S. L., Chiang, C. M., Yang, C. M. (2018). Enhancing the abiotic stress tolerance of plants: from chemical treatment to biotechnological approaches. Physiol. Plantar. 164, 452–466. doi: 10.1111/ppl.2018.164.issue-4
Niharika, Singh, N. B., Singh, A., Khare, S., Yadav, V., Bano, C., et al. (2021). Mitigating strategies of gibberellins in various environmental cues and their crosstalk with other hormonal pathways in plants: a review. Plant Mol. Bio. Rep. 39, 34–49. doi: 10.1007/s11105-020-01231-0
Omoarelojie, L. O., Kulkarni, M. G., Finnie, J. F., Pospíšil, T., Strnad, M., Van Staden, J. (2020). Synthetic strigolactone (rac-GR24) alleviates the adverse effects of heat stress on seed germination and photosystem II function in lupine seedlings. Plant Physiol. Biochem. 155, 965–979. doi: 10.1016/j.plaphy.2020.07.043
Pandey, N., Iqbal, Z., Pandey, B. K., Sawant, S. V. (2017). “Phytohormones and Drought Stress: Plant Responses to Transcriptional Regulation,” in Mechanism of Plant Hormone Signaling under Stress (New Jersey: John Wiley & Sons, Ltd), 477–504. doi: 10.1002/9781118889022.ch34
Pandey, A., Sharma, M., Pandey, G. K. (2016). Emerging roles of strigolactones in plant responses to stress and development. Front. Plant Sci. 7, 434. doi: 10.3389/fpls.2016.00434
Parmar, N., Singh, K. H., Sharma, D., Singh, L., Kumar, P., Nanjundan, J., et al. (2017). Genetic engineering strategies for biotic and abiotic stress tolerance and quality enhancement in horticultural crops: a comprehensive review. 3 Biotech. 7, 1–35. doi: 10.1007/s13205-017-0870-y
Per, T. S., Khan, M. I. R., Anjum, N. A., Masood, A., Hussain, S. J., Khan, N. A. (2018). Jasmonates in plants under abiotic stresses: Crosstalk with other phytohormones matters. Environ. Exp. Bot. 145, 104–120. doi: 10.1016/j.envexpbot.2017.11.004
Que, F., Wang, G. L., Xu, Z. S., Wang, F., Xiong, A. S. (2017). Transcriptional regulation of brassinosteroid accumulation during carrot development and the potential role of brassinosteroids in petiole elongation. Front. Plant Sci. 8, 1356. doi: 10.3389/fpls.2017.01356
Rasheed, F., Anjum, N. A., Masood, A., Sofo, A., Khan, N. A. (2022). The key roles of salicylic acid and sulfur in plant salinity stress tolerance. J. Plant Growth Regul. 41, 1891–1904. doi: 10.1007/s00344-020-10257-3
Raza, A., Charagh, S., García-Caparrós, P., Rahman, M. A., Ogwugwa, V. H., Saeed, F., et al. (2022). Melatonin-mediated temperature stress tolerance in plants. GM Crops. Food. 13, 196–217. doi: 10.1080/21645698.2022.2106111
Rehman, M., Saeed, M. S., Fan, X., Salam, A., Munir, R., Yasin, M. U., et al. (2023). The multifaceted role of jasmonic acid in plant stress mitigation: An overview. Plants 12, 3982. doi: 10.3390/plants12233982
Rosińska, A., Andrzejak, R., Kakkerla, V. (2023). Effect of osmopriming with melatonin on germination, vigor and health of Daucus carota L. seeds. Agriculture 13, 749. doi: 10.3390/agriculture13040749
Ruiz-Lozano, J. M., Aroca, R., Zamarreño, Á. M., Molina, S., Andreo-Jiménez, B., Porcel, R., et al. (2016). Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 39, 441–452. doi: 10.1111/pce.12631
Şimşek, Ö., Isak, M. A., Dönmez, D., Dalda Şekerci, A., İzgü, T., Kaçar, Y. A. (2024). Advanced biotechnological interventions in mitigating drought stress in plants. Plants 13, 717. doi: 10.3390/plants13050717
Sadeghipour, O. (2018). Drought tolerance of cowpea enhanced by exogenous application of methyl jasmonate. Int. J. Mod. Agric. 7, 51–57.
Safa Eynaladin, M., Shokouhian, A. A., Rasoulzadeh, A., Hemati, A. (2025). Effect of melatonin and Fulvic acid on enzymatic activity and physiological properties of strawberry cv Camarosa under drought stress. J. Environ. Sci. Stud. 9, 9660–9645. doi: 10.22034/jess.2024.433794.2202
Sako, K., Nguyen, H. M., Seki, M. (2020). Advances in chemical priming to enhance abiotic stress tolerance in plants. Plant Cell Physiol. 61, 1995–2003. doi: 10.1093/pcp/pcaa119
Saleem, M., Fariduddin, Q., Janda, T. (2021). Multifaceted role of salicylic acid in combating cold stress in plants: a review. J. Plant Growth Regul. 40, 464–485. doi: 10.1007/s00344-020-10152-x
Salvi, P., Manna, M., Kaur, H., Thakur, T., Gandass, N., Bhatt, D., et al. (2021). Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 40, 1305–1329. doi: 10.1007/s00299-021-02683-8
Samancıoğlu, A., Kocaçınar, F., Demirkıran, A. R., Korkmaz, A. (2014). Enhancing water stress tolerance in pepper at seedling stage by 24-epibrassinolid (EBL) applications. Acta Hortic. 1142, 109–416. doi: 10.17660/ActaHortic.2016.1142.62
Santino, A., Taurino, M., De Domenico, S., Bonsegna, S., Poltronieri, P., Pastor, V., et al. (2013). Jasmonate signaling in plant development and defense response to multiple (a) biotic stresses. Plant Cell Rep. 32, 1085–1098. doi: 10.1007/s00299-013-1441-2
Sári, D., Ferroudj, A., Dávid, S., El-Ramady, H., Faizy, S. E. D., Ibrahim, S., et al. (2024). Drought stress under a nano-farming approach: A review. Egypt. J. Soil Sci. 64, 135–151. doi: 10.21608/ejss.2023.239634.1668
Seif El-Yazal, S., Seif El-Yazal, A., M., F., Dwidar, E., Rady, M. (2015). Phytohormone crosstalk research: cytokinin and its crosstalk with other phytohormones. Curr. Protein Pept. Sci. 16, 395–405. doi: 10.2174/1389203716666150330141159
Sharma, A., Sidhu, G. P. S., Araniti, F., Bali, A. S., Shahzad, B., Tripathi, D. K., et al. (2020). The role of salicylic acid in plants exposed to heavy metals. Molecules 25, 540.
Sharma, S. K. (2021). Brassinosteroids application responses in fruit crops-a review. Int. J. Agric. Environ. Biotechnol. 14, 123–140. doi: 10.30954/0974-1712.02.2021.2
Sharma, P., Jha, A. B., Dubey, R. S. (2024). Strigolactones: coordination with other phytohormones and enhancement of abiotic stress responses. Environ. Exp. Bot. 223, 105782. doi: 10.1016/j.envexpbot.2024.105782
Shi, H., Jiang, C., Ye, T., Tan, D. X., Reiter, R. J., Zhang, H., et al. (2015). Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in Bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 66, 681–694. doi: 10.1093/jxb/eru373
Shu, H., Altaf, M. A., Mushtaq, N., Fu, H., Lu, X., Zhu, G., et al. (2023). Physiological and transcriptome analysis of the effects of exogenous strigolactones on drought responses of pepper seedlings. Antioxidants 12, 2019. doi: 10.3390/antiox12122019
Shu, H., Xu, K., Li, X., Liu, J., Altaf, M. A., Fu, H., et al. (2024). Exogenous strigolactone enhanced the drought tolerance of pepper (Capsicum chinense) by mitigating oxidative damage and altering the antioxidant mechanism. Plant Cell Rep. 43, 106. doi: 10.1007/s00299-024-03196-w
Siddiqi, K. S., Husen, A. (2017). Plant response to strigolactones: current developments and emerging trends. App. Soil Eco. 120, 247–253. doi: 10.1016/j.apsoil.2017.08.020
Siddiqi, K. S., Husen, A. (2019). Plant response to jasmonates: current developments and their role in changing environment. Bull. Nat. Res. Centre. 43, 1–11. doi: 10.1186/s42269-019-0195-6
Siddiqi, K. S., Husen, A. (2021). Significance of brassinosteroids and their derivatives in the development and protection of plants under abiotic stress. Biologia 76, 2837–2857. doi: 10.1007/s11756-021-00853-3
Silva, J. M., da Silva Júnior, G. B., Bonifácio, A., Dutra, A. F., de Mello Prado, R., de Alcântara Neto, F., et al. (2023). Exogenous salicylic acid alleviates water stress in watermelon plants. Ann. App. Bio. 182, 121–130. doi: 10.1111/aab.v182.1
Sita, K., Kumar, V. (2020). Role of Gamma Amino Butyric Acid (GABA) against abiotic stress tolerance in legumes: a review. Plant Physiol. Rep. 25, 654–663. doi: 10.1007/s40502-020-00553-1
Soliman, S., Wang, Y., Han, Z., Pervaiz, T., El-Kereamy, A. (2022). Strigolactones in plants and their interaction with the ecological microbiome in response to abiotic stress. Plants 11, 3499. doi: 10.3390/plants11243499
Song, W., Shao, H., Zheng, A., Zhao, L., Xu, Y. (2023). Advances in roles of salicylic acid in plant tolerance responses to biotic and abiotic stresses. Plants 12, 3475. doi: 10.3390/plants12193475
Sun, H., Jia, M., Wang, Y., Lu, H., Wang, X. (2023). The complexity of melatonin and other phytohormones crosstalk with other signaling molecules for drought tolerance in horticultural crops. Sci. Hortic. 321, 112348. doi: 10.1016/j.scienta.2023.112348
Tilahun, S., Choi, H. R., Baek, M. W., Cheol, L. H., Kwak, K. W., Park, D. S., et al. (2021). Antioxidant properties, γ-aminobutyric acid (GABA) content, and physicochemical characteristics of tomato cultivars. Agronomy 11, 1204. doi: 10.3390/agronomy11061204
Tiwari, R. K., Lal, M. K., Kumar, R., Chourasia, K. N., Naga, K. C., Kumar, D., et al. (2021). Mechanistic insights on melatonin-mediated drought stress mitigation in plants. Physiol. Plantar. 172, 1212–1226. doi: 10.1111/ppl.v172.2
Tiwari, S., Lata, C., Singh Chauhan, P., Prasad, V., Prasad, M. (2017). A functional genomic perspective on drought signalling and its crosstalk with phytohormone-mediated signalling pathways in plants. Curr. Genom. 18, 469–482. doi: 10.2174/1389202918666170605083319
Ullah, A., Manghwar, H., Shaban, M., Khan, A. H., Akbar, A., Ali, U., et al. (2018). Phytohormones enhanced drought tolerance in plants: a coping strategy. Environ. Sci. Poll. Res. 25, 33103–33118. doi: 10.1007/s11356-018-3364-5
Vijayakumari, K., Puthur, J. T. (2016). [amp]]gamma;-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul. 78, 57–67. doi: 10.1007/s10725-015-0074-6
Visentin, I., Vitali, M., Ferrero, M., Zhang, Y., Ruyter-Spira, C., Novák, O., et al. (2016). Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytol. 212, 954–963. doi: 10.1111/nph.2016.212.issue-4
Wang, S. Y. (1999). Methyl jasmonate reduces water stress in strawberry. J. Plant Growth Regul. 18, 127–134. doi: 10.1007/PL00007060
Wang, X., Cui, J., Li, X., Wang, G., Zheng, G., Li, Y., et al. (2022). Comparative study on growth, physiological and biotic stress resistance of cucumber (Cucumis sativus L.) by exogenous application of jasmonic acid and methyl jasmonate under greenhouse conditions. doi: 10.21203/rs.3.rs-1739929/v1
Wani, S. H., Kumar, V., Shriram, V., Sah, S. K. (2016). Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 4, 162–176. doi: 10.1016/j.cj.2016.01.010
Wasternack, C. (2014). Action of jasmonates in plant stress responses and development—applied aspects. Biotechnol. Advanc. 32, 31–39. doi: 10.1016/j.biotechadv.2013.09.009
Wei, L. J., Deng, X. G., Zhu, T., Zheng, T., Li, P. X., Wu, J. Q., et al. (2015). Ethylene is involved in brassinosteroids induced alternative respiratory pathway in cucumber (Cucumis sativus L.) seedlings response to abiotic stress. Front. Plant Sci. 6, 982. doi: 10.3389/fpls.2015.00982
Wen, D., Zheng, Y., Han, Y., Song, J., Sun, S., Yang, N., et al. (2023). Sodium selenite increases drought tolerance by promoting jasmonic acid biosynthesis in cucumber. Hortic. Advanc. 1, 6. doi: 10.1007/s44281-023-00009-0
Wu, H., Wu, X., Li, Z., Duan, L., Zhang, M. (2012). Physiological evaluation of drought stress tolerance and recovery in cauliflower (Brassica oleracea L.) seedlings treated with methyl jasmonate and coronatine. J. Plant Growth Regul. 31, 113–123. doi: 10.1007/s00344-011-9224-x
Xia, H., Ni, Z., Hu, R., Lin, L., Deng, H., Wang, J., et al. (2020). Melatonin alleviates drought stress by a non-enzymatic and enzymatic antioxidative system in kiwifruit seedlings. Int. J. Mol. Sci. 21, 852. doi: 10.3390/ijms21030852
Xia, X. J., Wang, Y. J., Zhou, Y. H., Tao, Y., Mao, W. H., Shi, K., et al. (2009). Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 150, 801–814. doi: 10.1104/pp.109.138230
Xia, X. J., Zhou, Y. H., Ding, J., Shi, K., Asami, T., Chen, Z., et al. (2011). Induction of systemic stress tolerance by brassinosteroid in Cucumis sativus. New Phytol. 191, 706–720. doi: 10.1111/j.1469-8137.2011.03745.x
Xie, G., Xu, R., Chong, L., Zhu, Y. (2024). Understanding drought stress response mechanisms in tomato. Vege. Res. 4. e001. doi: 10.48130/vegres-0023-0033
Xiong, B., Wang, Y., Zhang, Y., Ma, M., Gao, Y., Zhou, Z., et al. (2020). Alleviation of drought stress and the physiological mechanisms in Citrus cultivar (Huangguogan) treated with methyl jasmonate. Bio Sci. Biotechnol. Biochem. 84, 1958–1965. doi: 10.1080/09168451.2020.1771676
Xu, J., Li, L., Liu, Y., Yu, Y., Li, H., Wang, X., et al. (2023). Molecular and physiological mechanisms of strigolactones-mediated drought stress in crab apple (Malus hupehensis Rehd.) seedlings. Sci. Hortic. 311, 111800. doi: 10.1016/j.scienta.2022.111800
Yan, M., Li, M., Ding, Z., Qiao, F., Jiang, X. (2023). Plant hormone signals mediate melatonin synthesis to enhance osmotic stress tolerance in watermelon cells. Hortic 9, 927. doi: 10.3390/horticulturae9080927
Yang, X., Lu, M., Wang, Y., Wang, Y., Liu, Z., Chen, S. (2021). Response mechanism of plants to drought stress. Horticulturae 7, 50. doi: 10.3390/horticulturae7030050
Yosefi, A., Mozafari, A. A., Javadi, T. (2020). Jasmonic acid improved in vitro strawberry (Fragaria× ananassa Duch.) resistance to PEG-induced water stress. Plant Cell Tissue Organ Cult. 142, 549–558. doi: 10.1007/s11240-020-01880-9
Youssef, E., Abdelaal, H. (2023). Influence of water stress and ortho salicylic acid on sweet potato plants (Ipomoea batatas L.) under environment and climate changes. Egyp. J. Chem. 66, 99–106. doi: 10.21608/ejchem.2022.170424.7117
Yu, X., Zhang, W., Zhang, Y., Zhang, X., Lang, D., Zhang, X. (2018). The roles of methyl jasmonate to stress in plants. Func. Plant Bio. 46, 197–212. doi: 10.1071/FP18106
Zahra, G., Marjan, M., Taher, B., Ranjbar, M. E. (2021). Foliar application of ascorbic acid and gamma aminobutyric acid can improve important properties of deficit irrigated cucumber plants (Cucumis sativus cv. Us). Gesunde Pflanzen 73, 77–84. doi: 10.1007/s10343-020-00530-6
Zeng, W., Mostafa, S., Lu, Z., Jin, B. (2022). Melatonin-mediated abiotic stress tolerance in plants. Front. Plant Sci. 13, 847175. doi: 10.3389/fpls.2022.847175
Zhang, Z., Chen, Z., Song, H., Cheng, S. (2023). From plant survival to thriving: exploring the miracle of brassinosteroids for boosting abiotic stress resilience in horticultural crops. Front. Plant Sci. 14, 1218229. doi: 10.3389/fpls.2023.1218229
Zhang, C., Huang, Z. (2013). Effects of endogenous abscisic acid, jasmonic acid, polyamines, and polyamine oxidase activity in tomato seedlings under drought stress. Sci. Hortic. 159, 172–177. doi: 10.1016/j.scienta.2013.05.013
Zhang, N., Sun, Q., Zhang, H., Cao, Y., Weeda, S., Ren, S., et al. (2015). Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 66, 647–656. doi: 10.1093/jxb/eru336
Zhang, T., Wang, J., Sun, Y., Zhang, L., Zheng, S. (2022). Versatile roles of melatonin in growth and stress tolerance in plants. J. Plant Growth Regul. 41, 507–523. doi: 10.1007/s00344-021-10317-2
Zhang, N., Zhao, B., Zhang, H. J., Weeda, S., Yang, C., Yang, Z. C., et al. (2013). Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 54, 15–23. doi: 10.1111/j.1600-079X.2012.01015.x
Zhao, C., Nawaz, G., Cao, Q., Xu, T. (2022). Melatonin is a potential target for improving horticultural crop resistance to abiotic stress. Sci. Hortic. 291, 110560. doi: 10.1016/j.scienta.2021.110560
Zhen, A., Zhang, Z., Jin, X., Liu, T., Ren, W., Hu, X. (2018). Exogenous GABA application improves the NO3—N absorption and assimilation in Ca (NO3) 2-treated muskmelon seedlings. Sci. Hortic. 227, 117–123. doi: 10.1016/j.scienta.2017.09.025
Zheng, H., Ma, J., Huang, W., Di, H., Xia, X., Ma, W., et al. (2022). Physiological and comparative transcriptome analysis reveals the mechanism by which exogenous 24-epibrassinolide application enhances drought resistance in potato (Solanum tuberosum L.). Antioxidants 11, 1701. doi: 10.3390/antiox11091701
Zhou, M., Hassan, M. J., Peng, Y., Liu, L., Liu, W., Zhang, Y., et al. (2021). [amp]]gamma;-aminobutyric acid (GABA) priming improves seed germination and seedling stress tolerance associated with enhanced antioxidant metabolism, DREB expression, and dehydrin accumulation in white clover under water stress. Front. Plant Sci. 12, 776939. doi: 10.3389/fpls.2021.776939
Zhou, X., Tan, Z., Zhou, Y., Guo, S., Sang, T., Wang, Y., et al. (2022). Physiological mechanism of strigolactone enhancing tolerance to low light stress in cucumber seedlings. BMC Plant Bio. 22, 1–17. doi: 10.1186/s12870-021-03414-7
Zhou, Y. L., You, X. Y., Wang, X. Y., Cui, L. H., Jiang, Z. H., Zhang, K. P. (2024). Exogenous 24-epibrassinolide enhanced drought tolerance and promoted BRASSINOSTEROID-INSENSITIVE2 expression of quinoa. Plants 13, 873. doi: 10.3390/plants13060873
Keywords: drought stress, phytohormone, antioxidants, oxidative damage, photosynthesis, pigments content
Citation: Huang S and Jin S (2025) Enhancing drought tolerance in horticultural plants through plant hormones: a strategic coping mechanism. Front. Plant Sci. 15:1502438. doi: 10.3389/fpls.2024.1502438
Received: 26 September 2024; Accepted: 16 December 2024;
Published: 20 January 2025.
Edited by:
Muhammad Waseem, Hainan University, ChinaReviewed by:
Abazar Ghorbani, Guizhou University, ChinaCopyright © 2025 Huang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songheng Jin, aHN4MTFoQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.