- 1College of Agronomy and Biotechnology, China Agricultural University, Beijing, China
- 2Research Institute of Grain Crops, Xinjiang Academy of Agricultural Sciences, Urumqi, Xinjiang, China
- 3Key Laboratory of Desert-Oasis Crop Physiology, Ecology and Cultivation, Urumqi, Xinjiang, China
Agroforestry intercropping is an effective way to optimize land use and ensure food security. However, the physiological mechanism by which the shading of dominant plants inhibits the yield of non-dominant plants in this mode remains to be investigated. A two-year location experiment of walnut-winter wheat intercrop combined with exogenous 6-benzyladenine (6-BA, the first synthetic cytokinin) treatment was conducted to reveal the mechanism of 6-BA in inhibiting wheat growth and yield formation under shade stress by measuring the photosynthetic characteristics, antioxidant capacity, hormone homeostasis of wheat flag leaves and yield. The results showed that compared with far canopy area (FCA), antioxidant enzyme activity [e.g. superoxide dismutase (SOD), peroxidase (POD)], zeatin (ZT) and abscisic acid (ABA) content and photosynthesis of wheat flag leaves were significantly reduced in below canopy area (BCA) treatment during flowering and grain filling stages, thereby inhibiting wheat dry matter accumulation and yield formation. Exogenous 6-BA significantly increased hormone [i.e. indoleacetic acid (IAA), ZT and gibberellin (GA)] levels, antioxidant enzyme activities and photosynthesis in flag leaves, thereby increasing dry matter and yield, especially in the FCA condition. Furthermore, net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), activities of ribulose bisphosphate carboxylase (RuBPCase) and phosphoenolpyruvate carboxylase (PEPCase), ABA and ZT concentrations of flag leaves at flowering and filling stages had a significant contribution to yield formation under 6-BA and shade treatments. Overall, cytokinin regulates the inhibitory effects of shade stress on wheat photosynthesis, antioxidant capacity and hormone homeostasis to reduce wheat yield loss.
Introduction
Agroforestry systems serve as a buffer measure to optimize land use and mitigate the effects of climate change on forest and cereal yields, which plays an important role in addressing the dual pressures of food security challenges from population growth and climate change (Liu et al., 2020; Ivezić et al., 2021; Yang et al., 2023). However, the inhibitory effect of canopy shading on cereal crop yields under this mode is the main concern for farmers (Ai et al., 2021). It is vital to investigate the potential mechanisms by which shading reduces cereal crop yields, and to evaluate appropriate mitigation measures.
Light is the main source of energy for plants to produce assimilates through photosynthesis and also provides a fundamental signal to regulate plant growth and development (Wu et al., 2019). In agroforestry, canopy shading by dominant trees in the canopy causes disadvantaged crops to grow in poor light conditions for long periods of time, significantly reducing the intensity of blue and red light intercepted by disadvantaged crops, as well as photosynthetically active radiation (400-700 nm) (Wolz and DeLucia, 2018; Ullah et al., 2019). Under shade conditions, plants often exhibit shade avoidance responses, including increased cell elongation in various organs (e.g., hypocotyls, stems) and accelerated flowering (Franklin, 2008; Casal, 2013; Ma and Li, 2019). When plants are stressed by prolonged shading, a series of morphological and physiological changes are observed, such as reduction in leaf thickness, damage to chloroplast ultrastructure, decrease in chlorophyll a:b ratio and inhibition of photosystem II activity, which significantly affect photosynthesis and energy homeostasis in plants (Millenaar et al., 2009; Ren et al., 2016). However, the effect of shading on the physiological activity and regulation of growth and development of wheat under intercropping of walnut and winter wheat is still unclear.
Plant hormones are critical in regulating wheat growth under stress (Han et al., 2024), and the role of hormones in the plant response to shade has been reported previously (Stamm and Kumar, 2010). Pons et al. (2001) reported the role of cytokinins in shade avoidance in the response of plants to vertical light intensity gradients in the canopy. Shading significantly reduces cytokinin levels in young, middle and old leaves of soybean (Wu et al., 2017). In leaves, accumulated auxin stimulates the expression of cytokinin oxidase to degrade cytokinins and inhibit leaf growth (Yang and Li, 2017). In the panicles of shade-tolerant rice varieties, most cytokinin pathway genes are up-regulated (Panigrahy et al., 2019). 6-BA is the first artificially synthesised cytokinin (Greene et al., 2016). Spraying with 6-BA increased the number of basal grains and basal lamina, reduced malondialdehyde levels and improves membrane chloroplast structure in maize leaves (Ren et al., 2017). Application of exogenous 6-BA application affected root morphology by altering auxin levels and the expression of key genes involved in auxin synthesis in lateral roots of Malus hupehensis (Mao et al., 2020). However, the physiological effects of 6-BA under shade conditions are still unclear.
In the present study, a two-year study based on the walnut-wheat intercropping model was conducted to explore the potential mechanisms by which shading and cytokinin affect leaf photosynthesis, antioxidant capacity and hormone homeostasis to regulate wheat yield. We investigated two hypotheses: (1) shading inhibits the photosynthetic characteristics of wheat, disrupts endogenous hormone homeostasis and reduces assimilate accumulation; (2) exogenous 6-BA enhances photosynthesis and alleviates yield loss by regulating the physiological activity of wheat flag leaves.
Materials and methods
Site description
The experiment was conducted during 2020-2022 in the 5 villages in Ayikule Township, Zepu County, Xinjiang, China (38°18′N, 77°17′E). The altitude ranges from 1215 to 1490 m. The climate is warm temperate continental arid, and the daily average temperature and rainfall during the growth period of winter wheat are shown in Figure 1A. The daily solar radiation and total solar radiation for two growing seasons are shown in Figure 1B. The former crop was summer soybean, and the soil of the experimental site was sandy loam. The initial soil nutrients of the upper soil layer (0-20 cm) contained 1.5 g kg-1 soil organic matter, 0.7 g kg-1 total nitrogen, 38.4 mg kg-1 alkaline nitrogen, 17.9 mg kg-1 available phosphorus, and 102.6 mg kg-1 available potassium.

Figure 1. The precipitation, air temperature (A) and total/daily solar radiation in each growing season (B) of the experimental site in the 2020-2022 growing season. Tmean, Tmax and Tmin represent daily average temperature, daily maximum temperature and daily minimum temperature, respectively.
Experimental design and setup
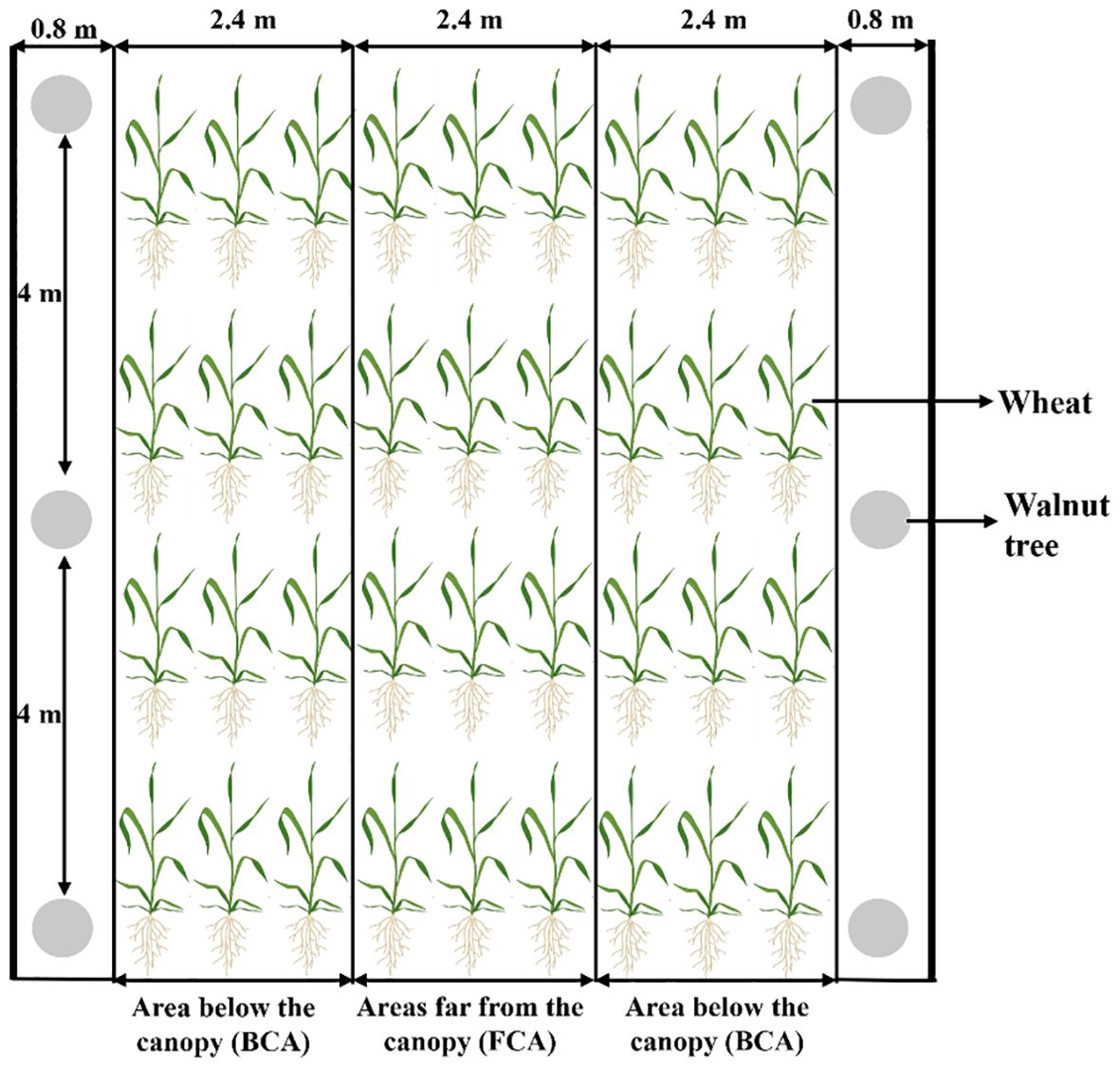
Field experiment was conducted based on the mode of walnut intercropping with wheat, and a two-factor split-plot experimental design was used in the experiment. The degree of shade corresponding to different canopy areas of the walnut trees were the main factor, including below canopy area (BCA) and far canopy area (FCA) (Figure 2); exogenous spraying as secondary factors, including exogenous 6-benzylaminopurine (6-BA) spraying (BCA+6-BA and FCA+6-BA, respectively) and water spraying as control. 6-BA was sprayed once at the booting stage and once at the jointing stage of wheat at a concentration of 60 mg L-1, with a spray rate of 750 L ha-1, and each treatment was repeated three times. The wheat variety Xindong 60 was sown on October 6 and 8 in 2020, 2021, and harvested on June 10 and 12 in 2021, 2022.The sowing rate was 300 kg ha-1 and row spacing of row planting was 15 cm. The walnut tree was 8 years old and evenly spaced north-south with 8 m row spacing and 4 m plant spacing. The width of the walnut-wheat belt was 7.2 m and the minimum distance between the walnut trees and the wheat rows was 1.0 m. The FCA was the area between two corresponding tree trunks located 0.4-2.8 m from the trunk; the BCA referred to the area between two corresponding tree trunks located 2 m from the trunks, as shown in Figure 2. The data in the BCA was the average data from two BCA. Before sowing, 150 kg ha-1 of urea (containing 46% N) and 300 kg ha-1 of diammonium phosphate (containing 46% P2O5, 18% N) were applied as basic fertilizers. Flood irrigation was applied six times during the whole growing season (overwintering, regreening, jointing, booting, flowering and filling) with a total irrigation volume of 4350-4950 m3 ha-1. Other cultural practices remained constant in all treatments.
Plant sampling and measurements
Yield and its components
At maturity, select three representative 1 m2 to record the number of spikes. Select 15 representative samples of complete wheat spikes to record the number of grains per spike. All wheat spikes are cut and dried naturally, then threshed and 1000 whole grains randomly selected to calculate the weight of 1000 grains. The grains are then weighed and the yield recorded. Three replicates were performed for each plot.
Accumulation of assimilates and leaf area index
At the jointing, booting, flowering, filling and maturity stages, two 1-m inner rows of plants were harvested from each plot. The plants were dried at 105 °C for half an hour, and then dried at 75 °C to a constant weight. At the flowering and grain filling stages, leaf area of ten plants was measured manually and LAI was calculated according to Jha (2018).
Relative chlorophyll content (SPAD value), photosynthetic and chlorophyll fluorescence parameters
The SPAD values of flag leaves were measured using a SPAD 502 Plus system (Konica Minolta, Japan) at the flowering and filling stages of winter wheat between 9:00 and 11:00 on sunny days. Ten leaves were randomly selected for each treatment. The net photosynthesis rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and intercellular carbon dioxide concentration (Ci) of flag leaves were determined by a Li-6400XT system (Li-COR, USA) at the flowering and filling stages of winter wheat during 10:30-12:00 am on sunny days, and six leaves were selected at random to measure for each plot. The light intensity was maintained at 1700 μmol·m-2·s-1. The ambient CO2 concentration was measured to be 380 μmol mol-1. At the flowering and filling stages, the maximum fluorescence (Fm’) and steady-state fluorescence (Fs) under natural light conditions, as well as the initial fluorescence and maximum fluorescence of the leaves after 60 min of dark adaptation of six flag leaves randomly selected from each treatment, were measured using the Pocket PEA plant efficiency analyzer. Then, the maximum light energy conversion efficiency (Fv/Fm) and quantum yield (Φ psII) of photosystem II were calculated according to Wang et al. (2015).
Activities of ribulose bisphosphate carboxylase (RuBPCase) and phosphoenolpyruvate carboxylase (PEPCase)
The activities of RuBPCase (EC 4.1.1.39) and PEPCase (EC 4.1.1.31) of flag leaves at flowering and filling stages were measured by detecting the rate of decrease of NADH at 340nm using enzyme-linked immunosorbent assay kits from Geruisi Biotechnology Co., Ltd. (Suzhou, China) according to the manufacturer’s instructions.
Activities of antioxidative enzymes
Superoxide dismutase (SOD; EC 1.15.1.1) activity was assayed by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium (NBT) at 550 nm, and catalase (CAT; EC 1.11.1.6) activity was assayed by measuring the disappearance of H2O2 at 240 nm (Jiang and Zhang, 2002). Peroxidase (POD) activity was determined by the method of Maehly and Chance (1954), in which guaiacol was converted to tetraguaiacol and monitored at 470 nm (Jiang et al., 2020). Ascorbate peroxidase (APX; EC 1.11.1.11) activity was determined by the decrease in absorbance at 290 nm according to Sharma and Dubey (2004). Glutathione reductase (GR; EC 1.6.4.2) activity was calculated by measuring the rate of decrease in absorbance at 340nm to determine the NADPH dehydrogenation rate (Wang et al., 2014).
Endogenous hormone content
The extraction, purification and determination of zeatin (ZT), gibberellin (GA), indole-3-acetic acid (IAA) and abscisic acid (ABA) were performed according to the methods of Lv et al. (2017) and Yang et al. (2001). Specifically, fresh samples (0.5 g) were homogenised with 5 mL of 80% (v/v) methanol containing 1 mmol L-1 butylated hydroxytoluene (BHT). The extraction solution was passed through Chromosep C18 columns, the fractions were vacuole-dried at 40 °C and dissolved in 1 mL phosphate-buffered saline (PBS) containing 0.1% (v/v) Tween 20 and 0.1% (w/v) gelatin (pH 7.5). Quantification of ZR, GA, IAA and ABA were performed by enzyme-linked immunosorbent assay.
Statistical analysis and graphing
The data were subjected to analysis of variance (ANOVA) using SPSS Statistics 26.0 (Chicago, USA). The differences between means were compared using the least significant difference (LSD) test with a P value of < 0.05. The random forest model analysis to rank the contribution of physiological indices at flowering and filling stage to yield was performed using the “ randomForest “ and “ rfPermute “ packages in R. Graphs were generated using Origin 2024b and Microsoft PowerPoint 2016 (Microsoft, Redmond, USA).
Results
Dry matter weight, leaf area index (LAI) and yield
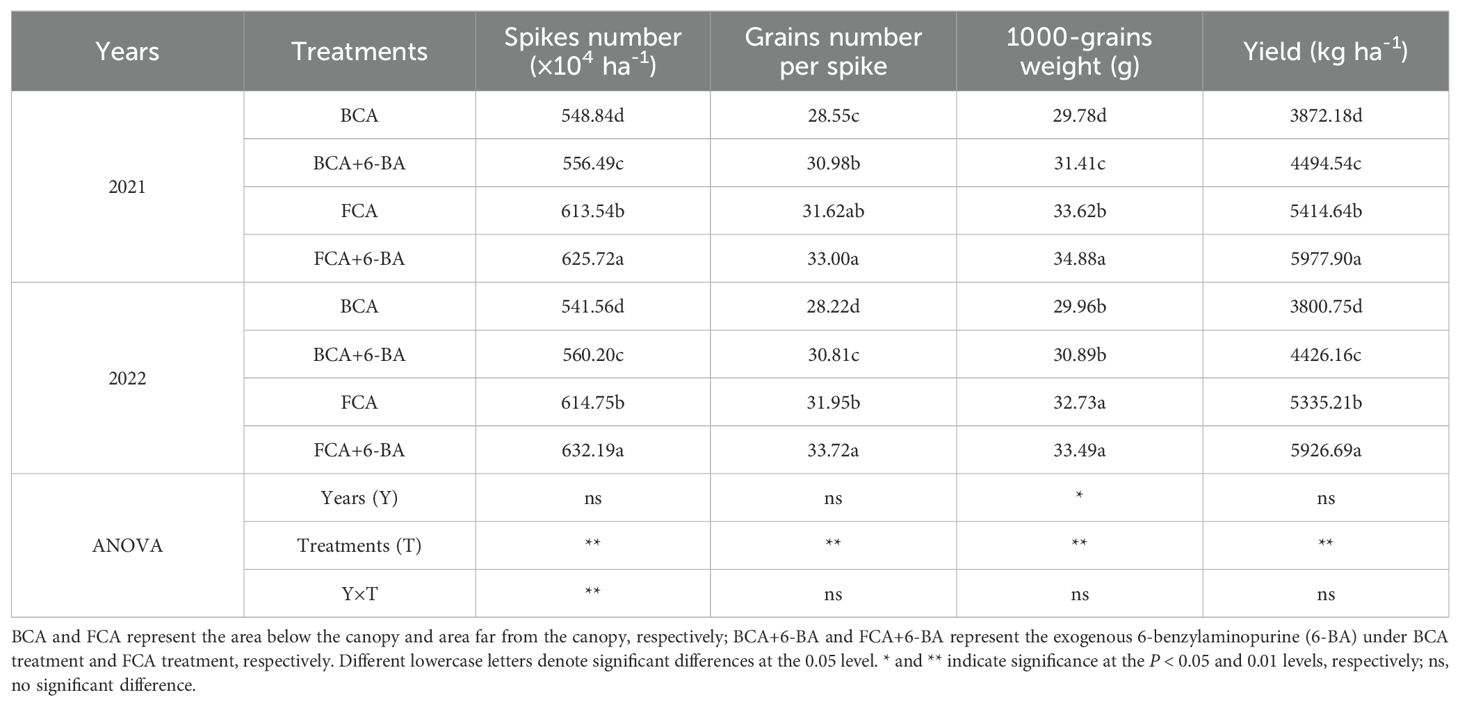
In the walnut-wheat intercrop, shade stress significantly inhibited wheat growth (Figure 3). Specifically, the dry matter weight of single wheat stems was significantly lower in the BCA treatment compared to FCA, especially at maturity (Figure 3A). Meanwhile, compared to the FCA treatment, the LAI of wheat in the BCA treatment was reduced by 10.4%-23.9% at the flowering and filling stages (Figure 3B). Interestingly, wheat dry matter accumulation and LAI (except for 2022) from flowering to filling were increased by exogenous 6-BA, both under BCA and FCA conditions. Correspondingly, affected by canopy shading of walnut trees, the number of effective spikes decreased by 10.81%-11.64%, number of grains per spikes decreased by 7.9%-10.1%, thousand grain weight decreased by 8.1%-10.7%, and grain yield of wheat decreased by 26.6%-27.0% in 2 years in BCA treatment compared with FCA. In the BCA and FCA treatments, the number of effective spikes increased by 1.39% to 3.44%, the number of grains per spike by 4.4% to 9.18%, the thousand grain weight by 2.3% to 5.5% and the yield by 10.4% to 16.5% when exogenous 6-BA was applied compared to spray water (Table 1). There was a significant positive correlation between wheat dry matter weight at flowering and grain filling and yield at maturity, with R2 values of 0.78 and 0.81, respectively (Figure 3C).
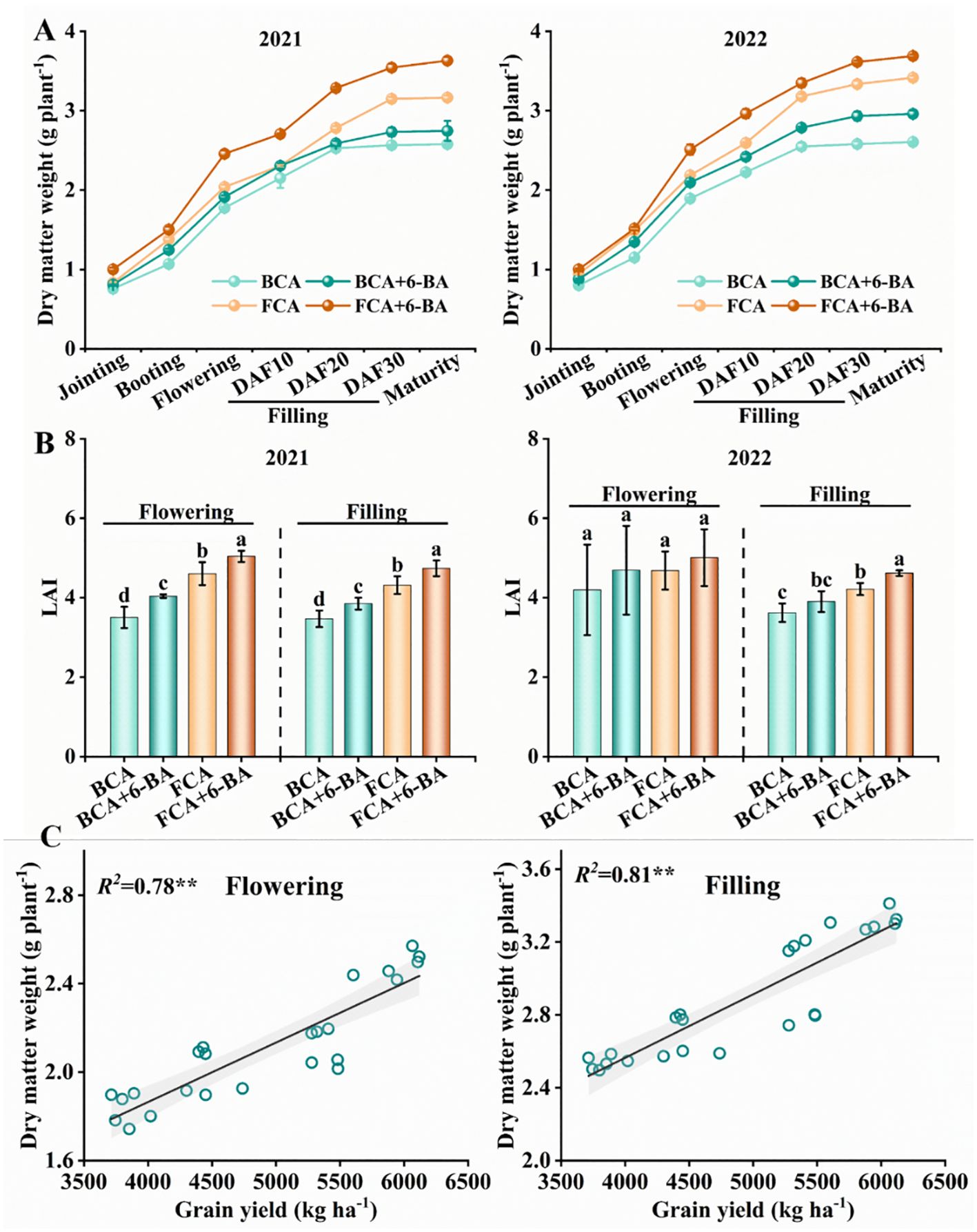
Figure 3. Dry matter accumulation (A), LAI (B) and linear fit between dry matter and yield (C) in wheat under shade and exogenous 6-BA treatments. LAI, leaf area index; BCA, below canopy area; FCA, far canopy area; BCA+6-BA, exogenous spraying of 6-BA under BCA; FCA+6-BA, exogenous spraying of 6-BA under FCA. Values in (B) are means ± SD (n=3). Different lowercase letters denote significant differences (ANOVA, Fisher’s least significant difference test, P<0.05). ** indicate significance at the P < 0.01 level.
Photosynthetic characteristics
The photosynthetic characteristics of wheat flag leaves were significant differences during the flowering and grain filling stages in different canopy regions. In particular, the SPAD value of wheat flag leaf was significantly higher in the BCA than in the FCA (Figure 4). The SPAD value of flag leaves was significantly increased by exogenous 6-BA under BCA and FCA conditions. Meanwhile, Pn, Gs and Tr of winter wheat flag leaves were significantly reduced in BCA compared with FCA, while Ci was significantly increased (Figure 4). The Pn, Tr and Gs of wheat flag leaves during the flowering and grain filling stages were significantly increased under exogenous 6-BA, while the Ci of flag leaves was reduced. Further analysis of photosynthetic physiological parameters revealed that the activity of RuBPCase and PEPCase in wheat flag leaves gradually decreased from flowering to grain filling stage (Figure 4). The RuBPCase and PEPCase activities of wheat flag leaves in BCA were higher than those in FCA, and the change pattern of 2-year experimental results between treatments was consistent; the RuBPCase and PEPCase activities of wheat flag leaves in BCA and FCA under the intercropping mode of walnut wheat were increased to different degrees by spraying exogenous 6-BA. During the two years, the Fv/Fm and φ II of wheat flag leaves in the flowering period showed similar trends (Figure 4).
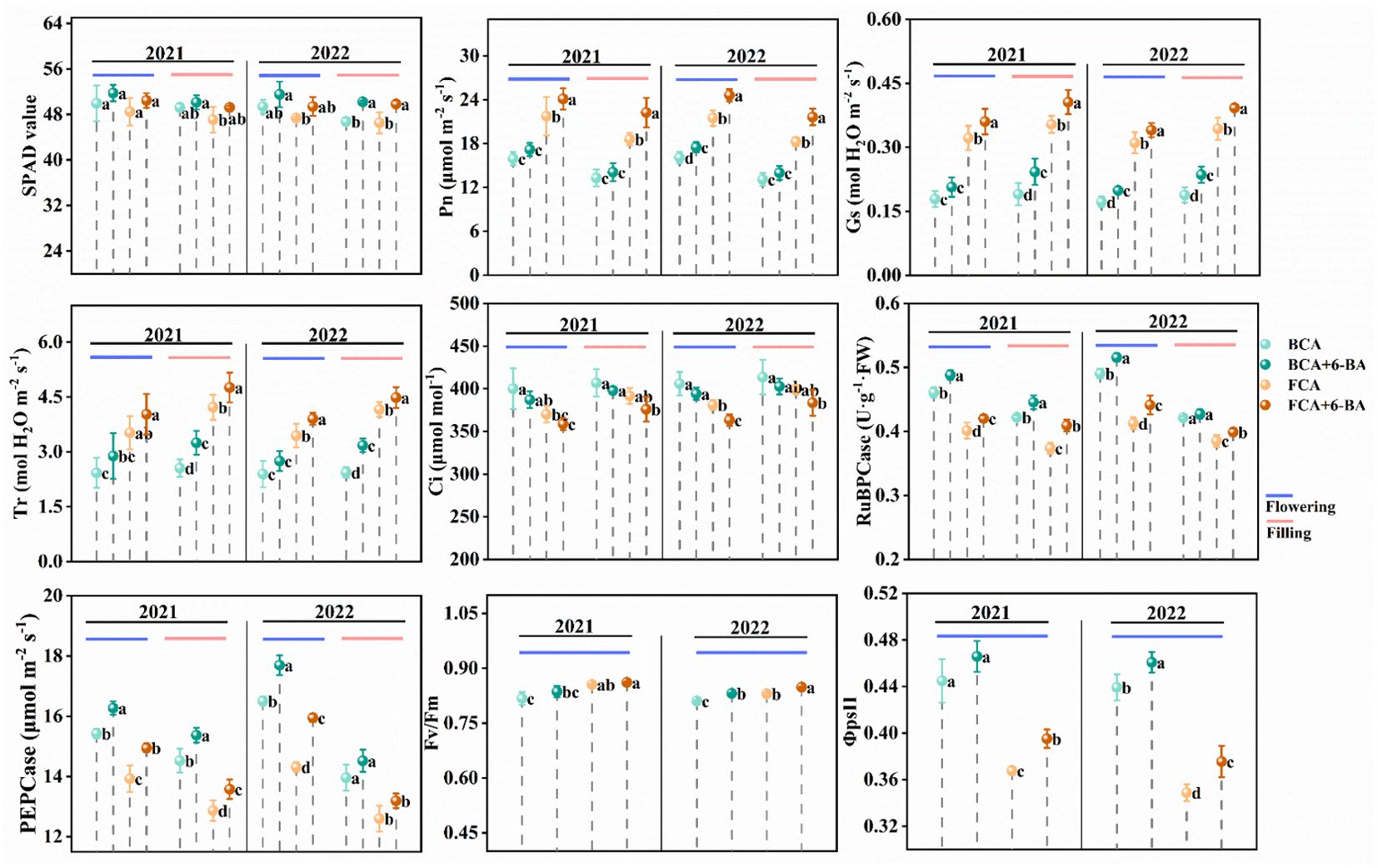
Figure 4. Effect of shading and exogenous 6-BA on SPAD, photosynthetic parameters and fluorescence parameters of wheat flag leaves. SPAD, relative chlorophyll content; Pn, net photosynthetic rate; Tr, Transpiration rate; Gs, Stomatal conductance; Ci, intercellular CO2 concentration; RuBPCase, activity of ribulose bisphosphate carboxylase; PEPCase, activity of phosphoenolpyruvate carboxylase; Fv/Fm, maximum light energy conversion efficiency; Φ psII, quantum yield. BCA, below canopy area; FCA, far canopy area; BCA+6-BA, exogenous spraying of 6-BA under BCA; FCA+6-BA, exogenous spraying of 6-BA under FCA. Values are means ± SD (n=3). Different lowercase letters denote significant differences (ANOVA, Fisher’s least significant difference test, P<0.05).
Enzymatic antioxidant system
During the flowering and filling stages, the activities of SOD, POD, CAT and APX in wheat flag leaves were significantly lower in BCA than in FCA (Figure 5). The activity of GR in the flowering period was higher in BCA than in FCA, but during the grain filling period was opposite. Compare with spraying water, the activities of SOD, POD, CAT and APX in wheat leaves were significantly increased after spraying 6-BA under intercropping mode, especially under FCA. Moreover, the activity of GR was significantly increased by exogenous 6-BA under FCA. Therefore, exogenous 6-BA enhanced the antioxidant capacity of wheat flag leaf under shade stress caused by walnut-wheat intercrop. There was also a highly significant positive correlation between APX and GR activity and Pn, Tr and Gs (Figure 6).
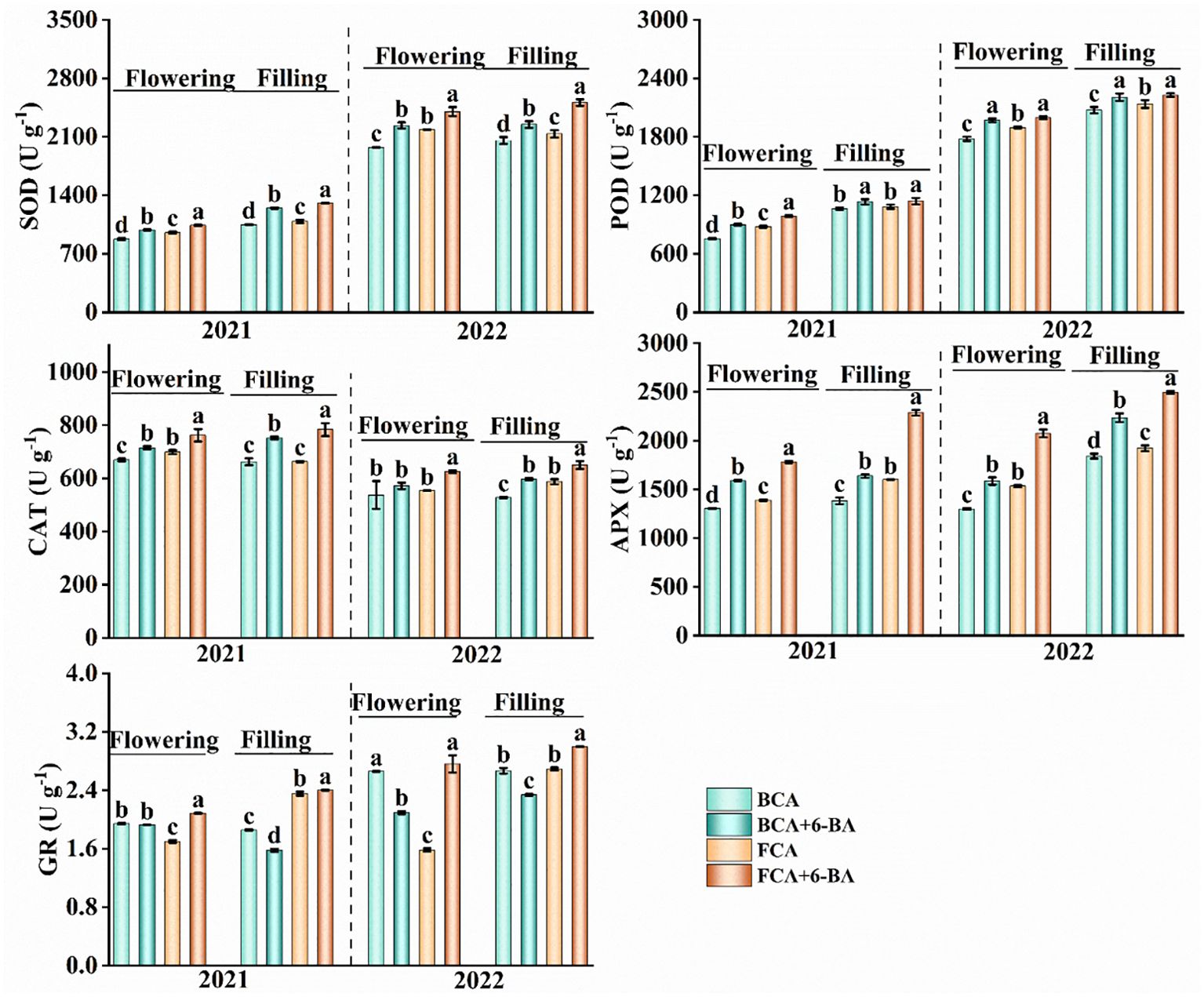
Figure 5. Effect of shade and exogenous 6-BA on antioxidant enzyme activities of wheat flag leaves. SOD, superoxide dismutase activity; POD, peroxidase activity; CAT, catalase activity; APX, ascorbate peroxidase activity; GR, glutathione reductase. BCA, below canopy area; FCA, far canopy area; BCA+6-BA, exogenous spraying of 6-BA under BCA; FCA+6-BA, exogenous spraying of 6-BA under FCA. Values are means ± SD (n=3). Different lowercase letters denote significant differences (ANOVA, Fisher’s least significant difference test, P<0.05).
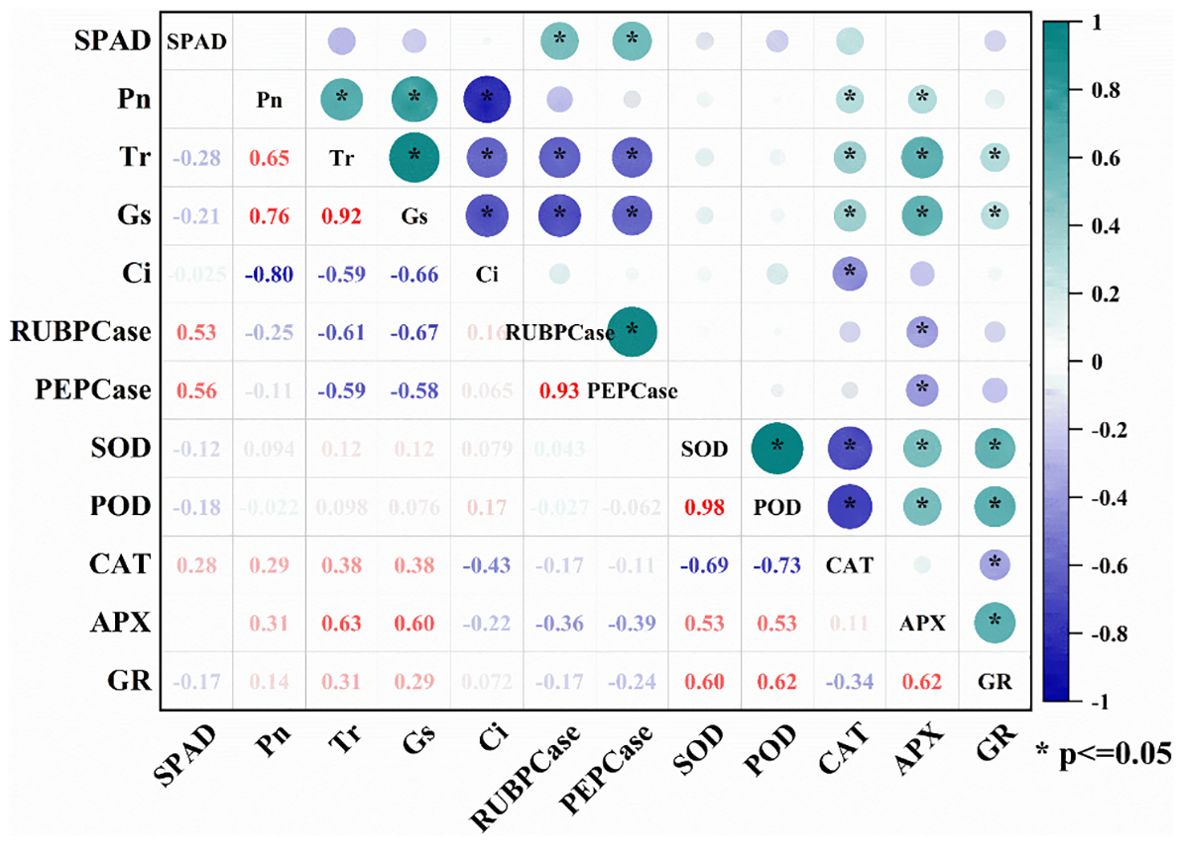
Figure 6. Correlation analysis between antioxidant enzyme activities and photosynthetic parameters in wheat flag leaves at flowering and filling stages. SPAD, relative chlorophyll content; Pn, net photosynthetic rate; Tr, Transpiration rate; Gs, Stomatal conductance; Ci, intercellular CO2 concentration; RuBPCase, activity of ribulose bisphosphate carboxylase; PEPCase, activity of phosphoenolpyruvate carboxylase; SOD, superoxide dismutase activity; POD, peroxidase activity; CAT, catalase activity; APX, ascorbate peroxidase activity; GR, glutathione reductase. * indicate significance at the P<0.05.
Endogenous hormone homeostasis
Shade stress and exogenous 6-BA treatments regulated endogenous hormone homeostasis in wheat flag leaves during both the flowering and grain filling stages. Specifically, IAA and GA contents in wheat flag leaves under BCA were significantly higher than those under FCA, while the ABA and ZT contents were much lower than those under FCA. Further analysis shows that the endogenous IAA, GA and ZT contents in wheat leaves were significantly increased by spraying 6-BA under BCA and FCA compared to not spraying 6-BA, with the most pronounced increase at maturity, while the ABA content of wheat flag leaves was significantly reduced (Figure 7).
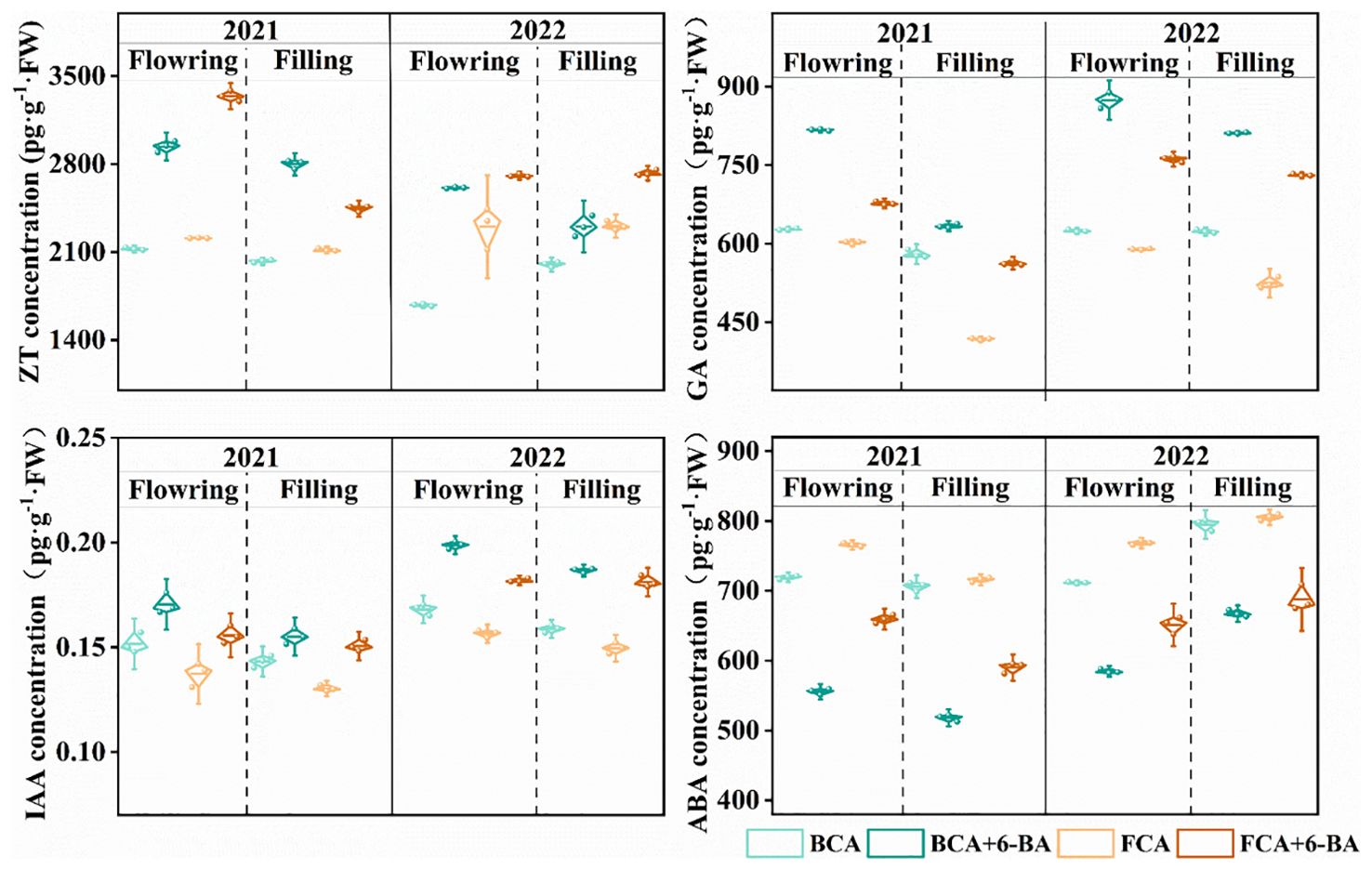
Figure 7. Effect of shade and exogenous 6-BA on endogenous hormone levels in wheat. ZT, zeatin; ABA, abscisic acid; GA, gibberellin; IAA, indoleacetic acid. BCA, below canopy area; FCA, far canopy area; BCA+6-BA, exogenous spraying of 6-BA under BCA; FCA+6-BA, exogenous spraying of 6-BA under FCA.
Principal component analysis and random forest model
The results of the principal component analysis between the photosynthetic parameters, antioxidant enzymes, hormone content and dry matter accumulation in wheat during the flowering and grain filling stages showed that PC1 and PC2 together explained 57% of the changes in dry matter weight (Figure 8A). The main factors influencing dry matter accumulation in wheat were Pn, Tr, Gs, ABA content and APX activity in flag leaves. Furthermore, the random forest model quantified the contributions of photosynthetic and physiological indicators of wheat to mature yield (Figure 8B) and the results showed that the activities of RuBPCase and PEPCase, the value of Gs, Tr, Pn and Ci, the content of ABA and ZT of flag leaves during flowering significantly contributed to yield, when the activity of RuBPCase, PEPCase and APX, the value of Gs, Pn, Ci and Tr, the content of GA, ZT, ABA of flag leaves during grain filling stage significantly contributed to yield of maturity.

Figure 8. Relationships between physiological indicators and dry matter weight and yield revealed by principal component analysis (A) and random forest modelling (B). SOD, superoxide dismutase activity; POD, peroxidase activity; CAT, catalase activity; APX, ascorbate peroxidase activity; GR, glutathione reductase; SPAD, relative chlorophyll content; Pn, net photosynthetic rate; Tr, Transpiration rate; Gs, Stomatal conductance; Ci, intercellular CO2 concentration; RuBP, activity of ribulose bisphosphate carboxylase; PEPC, activity of phosphoenolpyruvate carboxylase; ZT, zeatin; ABA, abscisic acid; GA, gibberellin; IAA, indoleacetic acid; DW, dry matter weight. BCA, below canopy area; FCA, far canopy area; BCA+6-BA, exogenous spraying of 6-BA under BCA; FCA+6-BA, exogenous spraying of 6-BA under FCA. * and ** indicate significance at the P < 0.05 and 0.01 levels, respectively; ns indicate no significance (P > 0.05).
Discussion
Shading inhibits the physiological activity of the flag leaves and thus reduces the wheat yield in the intercropping mode of walnut with wheat
In the fruit-grain intercropping mode of land use optimization, grain crops are disadvantaged in growth, especially for cereal crops such as wheat (Charbonnier et al., 2017; Liu et al., 2020). Similar to the results of Nasielski et al. (2015), who found that soybean growth was significantly inhibited in agroforestry intercropping areas, the present study found a significant decrease in dry matter accumulation and yield of wheat in BCA compared to FCA in a walnut-wheat intercropping pattern in Xinjiang, China (Figure 3A; Table 1). A highly significant positive correlation was found between dry matter weight and yield at flowering and filling stages (Figure 2C). Photosynthesis provides the material basis for yield formation, with more than 90% of wheat yield derived from photosynthesis (Parry et al., 2011), and flag leaf photosynthesis contributing about 40% of yield (Carmo-Silva et al., 2017). The efficiency and regulation of Rubisco and PEPCase are reasonable strategies to increase photosynthesis and yield (Carmo-Silva et al., 2015). However, despite the increase in RuBPCase and PEPCase activities induced by shade stress, Pn, Tr and Gs were significantly suppressed in wheat flag leaves under BCA (Figure 4; Figure 6), which may be related to the insufficient CO2 supply. Interestingly, this study also found a significant positive correlation between APX and CAT activities and photosynthetic parameters (Figure 6). Hussain et al. (2020) showed that shade increased antioxidant enzyme activities in soybean and that the magnitude of the effect was greater in the early than in the late stages of shading. However, in the present study, enzymatic antioxidant systems such as SOD, POD, APX were significantly weakened in wheat flag leaves in the near-canopy area (Figure 5), which is connected with the plant adaptation mechanism to shade. Hormones are key physiological indicators that balance plant growth and stress tolerance (Stamm and Kumar, 2010; Xu et al., 2021). Previous studies have shown that post-anthesis shade reduces IAA levels but increases ABA levels in maize kernels (Wang et al., 2020). The study on Rumex palustris showed that flooding and shading not only induced GA production but also enhanced sensitivity to GA (Benschop, 2004). In the present study, shading significantly increased IAA and GA contents and decreased ABA and ZT contents in wheat flag leaves (Figure 7). The contribution of ZT and ABA contents to yield was significant in wheat flag leaves at the flowering and filling stages (Figure 8B). Thus, walnut tree shading significantly altered flag leaf photosynthesis, antioxidant enzyme and hormone contents, which in turn reduced dry matter accumulation and yield of winter wheat.
6-BA alleviates inhibition of antioxidant enzymes and hormonal homeostasis in wheat under shade to reduce yield loss
Exogenous hormones alleviate plant stress and promote plant growth under stress conditions by improving plant physiological metabolism (Panigrahy et al., 2019; Mao et al., 2020; Li et al., 2020, 2023). A series of studies have shown that 6-BA plays an important role in plant resistance to drought, waterlogging, high temperature, salinity and other stresses (Wu et al., 2012; Ghaleh et al., 2020; Hu et al., 2022). In maize, 6-BA application improved light energy uptake and dissipation, carbon assimilation and metabolism, and chloroplast ultrastructure, thereby increasing photosynthetic rate in flooded summer maize (Hu et al., 2022). In this study, exogenous 6-BA significantly increased RuBPCase activity in wheat flag leaves (Figure 4), thereby promoting photosystem II efficiency and net photosynthesis in wheat (Figure 3). Similar to the results of Pan et al. (2013) in hybrid rice, exogenous 6-BA significantly increased the activities of SOD, POD, CAT and APX in flag leaves at anthesis and filling stages, especially at FCA (Figure 5), which also indicates that 6-BA application effectively enhanced the scavenging capacity of leaves under shade for superoxide anion, hydrogen peroxide and hydroxyl radical (Li et al., 2020). The most important steps in photosynthesis and the synthesis of hormones for the defensive classes of plants both take place in the chloroplasts (Lu and Yao, 2018). During the flowering and filling stages, exogenous 6-BA increased the content of IAA, ZT, and GA in wheat flag leaves, while reducing the content of ABA (Figure 7). Cytokinin is an essential phytohormone involved in the regulation of photosynthesis (Hudecek et al., 2022). Compared to water spraying, 6-BA application decreased growth hormone content, increased cytokinin content and young spike dry matter, and transported more soluble sugars and sucrose from non-spike organs to young spikes (Li et al., 2019). The main factors influencing dry matter accumulation in wheat were Pn, Tr, Gs, ABA content and APX activity in flag leaves (Figure 8A). Importantly, exogenous 6-BA also significantly increased wheat dry matter accumulation in BCA and FCA (Figure 3). The flowering to grain-filling stage is a critical period for wheat grain development and filling (Djanaguiraman et al., 2020). Increasing photosynthetic capacity by exogenous 6-BA significantly increases wheat thousand kernel weight, which in turn increases yield (Table 1). Overall, exogenous 6-BA facilitated the enhancement of physiological activity and photosynthesis in wheat, which in turn partially mitigated the detrimental effects of shading on wheat yield development. 6-BA is also a potentially valuable chemical control regulator that is expected to be beneficial in intensive agriculture.
Conclusions
When walnut and wheat are intercropped, walnut shading stress significantly reduced antioxidant enzyme activity, ABA and ZT content, and photosynthesis in wheat flag leaves during the flowering and filling stages, thereby reducing dry matter accumulation and mature yield. Under shade stress, exogenous 6-BA significantly increased flag leaf ZT, IAA and GA levels, antioxidant enzymes and photosynthesis, thereby increasing dry matter and yield. The random forest model indicated that under 6-BA and shading treatments, Pn, Gs, Ci, RuBPCase and PEPCase activities, ABA and ZT contents of flag leaves were significant factors influencing wheat yield formation. In conclusion, cytokinins regulate the inhibitory effects of shading stress on wheat photosynthesis, antioxidant capacity and hormone homeostasis, thereby reducing wheat yield loss. Future research will focus on the mechanism by which 6-BA maintains photosynthetic activity in maize leaves and alleviates photoinhibition under shading stress.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YZ: Conceptualization, Data curation, Funding acquisition, Writing – original draft. JL: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft. QX: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. CC: Formal analysis, Investigation, Validation, Visualization, Writing – review & editing. SN: Investigation, Supervision, Validation, Visualization, Writing – review & editing. JJL: Data curation, Funding acquisition, Project administration, Supervision, Visualization, Writing – review & editing. LD: Conceptualization, Investigation, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key Research and Development Projects of Xinjiang Autonomous Region (2022B02001-3, 2021B02002-1), National Natural Science Foundation of China (31960379, 51879267), Xinjiang Uygur Autonomous Region “Tianshan Talents” Training Program (2023TSYCCX0013, 2023TSYCLJ0009), National Wheat Industry Technology System (CARS-03-88), Xinjiang Uyghur Autonomous Region Wheat Industry Technology System Project (XJARS-01-06, XJARS-01-19).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ai, P., Ma, Y., Hai, Y. (2021). Influence of jujube/cotton intercropping on soil temperature and crop evapotranspiration in an arid area. Agr Water Manage 256, 107118. doi: 10.1016/j.agwat.2021.107118
Benschop, J. J. (2004). The role of abscisic acidin ethylene-induced elongation. PhD thesis. Utrecht University, The Netherlands.
Carmo-Silva, E., Andralojc, P. J., Scales, J. C., Driever, S. M., Mead, A., Lawson, T., et al. (2017). Phenotyping of field-grown wheat in the UK highlights contribution of light response of photosynthesis and flag leaf longevity to grain yield. J. Exp. Bot. 68, 3473–3486. doi: 10.1093/jxb/erx169
Carmo-Silva, E., Scales, J. C., Madgwick, P. J., Parry, M. A. (2015). Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ. 8, 1817–1832. doi: 10.1111/pce.12425
Casal, J. J. (2013). Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 64, 403–427. doi: 10.1146/annurev-arplant-050312-120221
Charbonnier, F., Roupsard, O., le Maire, G., Guillemot, J., Casanoves, F., Lacointe, A., et al. (2017). Increased light-use efficiency sustains net primary productivity of shaded coffee plants in agroforestry system. Plant Cell Environ. 40, 1592–1608. doi: 10.1111/pce.12964
Djanaguiraman, M., Narayanan, S., Erdayani, E., Prasad, P. V. V. (2020). Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol. 20, 268. doi: 10.1186/s12870-020-02479-0
Franklin, K. A. (2008). Shade avoidance. New Phytol. 179, 930–944. doi: 10.1111/j.1469-8137.2008.02507.xS
Ghaleh, Z. R., Sarmast, M. K., Atashi, S. (2020). 6-Benzylaminopurine (6-BA) ameliorates drought stress response in tall fescue via the influencing of biochemicals and strigolactone-signaling genes. Plant Physiol. Bioch. 155, 877–887. doi: 10.1016/j.plaphy.2020.08.009
Greene, D. W., Crovetti, A. J., Pienaar, J. (2016). Development of 6-Benzyladenine as an apple thinner. HortScience 51, 1448–1451. doi: 10.21273/HORTSCI10822-16
Han, R., Ma, L., Terzaghi, W., Guo, Y., Li, J. (2024). Molecular mechanisms underlying coordinated responses of plants to shade and environmental stresses. Plant J. 117, 1893–1913. doi: 10.1111/tpj.16653
Hu, J., Ren, B. Z., Dong, S. T., Liu, P., Zhao, B., Zhang, J. W. (2022). Phosphoproteomic and physiological analysis revealed 6-benzyladenine improved the operation of photosynthetic apparatus in waterlogged summer maize. Environ. Exp. Bot. 193, 104679. doi: 10.1016/j.envexpbot.2021.104679
Hudecek, M., Nozkova, V., Plihalova, L., Plihal, O. (2022). Plant hormone cytokinin at the crossroads of stress priming and control of photosynthesis. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1103088
Hussain, S., Pang, T., Iqbal, N., Shafiq, I., Skalicky, M., Brestic, M., et al. (2020). Acclimation strategy and plasticity of different soybean genotypes in intercropping. Funct. Plant Biol. 47, 592–610. doi: 10.1071/FP19161
Ivezić, V., Yu, Y., Werf, W. (2021). Crop yields in European agroforestry systems: A meta-analysis. Front. Sustain Food S 5. doi: 10.3389/fsufs.2021.606631
Jha, S. K. (2018). Effect of irrigation method and scheduling on development and water utilization in winter wheat (Doctoral Dissertation). Chin. Acad. Agric. Sci.
Jiang, M., Zhang, J. (2002). Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 53, 2401–2410. doi: 10.1093/jxb/erf090
Jiang, N., Yu, P., Fu, W., Li, G., Feng, B., Chen, T., et al. (2020). Acid invertase confers heat tolerance in rice plants by maintaining energy homoeostasis of spikelets. Plant Cell Environ. 43, 1273–1287. doi: 10.1111/pce.13733
Li, Y., Han, X., Ren, H., Zhao, B., Zhang, J., Ren, B., et al. (2023). Exogenous SA or 6-BA maintains photosynthetic activity in maize leaves under high temperature stress. Crop J. 11, 605–617. doi: 10.1016/j.cj.2022.08.006
Li, G., Liang, Z., Li, Y., Liao, Y., Liu, Y. (2020). Exogenous spermidine regulates starch synthesis and the antioxidant system to promote wheat grain filling under drought stress. Acta Physiol. Plant 42, 110. doi: 10.1007/s11738-020-03100-5
Li, S., Song, M., Duan, J., Yang, J., Zhu, Y., Zhou, S. (2019). Regulation of spraying 6-BA in the late jointing stage on the fertile floret development and grain setting in winter wheat. Agronomy 9, 546. doi: 10.3390/agronomy9090546
Liu, Z., Jia, G., Yu, X. (2020). Water uptake and WUE of apple tree-corn agroforestry in the loess hilly region of China. Agr Water Manage 234, 106138. doi: 10.1016/j.agwat.2020.106138
Lu, Y., Yao, J. (2018). Chloroplasts at the crossroad of photosynthesis, pathogen infection and plant defense. Int. J. Mol. Sci. 19, 3900. doi: 10.3390/ijms19123900
Lv, X., Li, T., Wen, X., Liao, Y., Liu, Y. (2017). Effect of potassium foliage application post-anthesis on grain filling of wheat under drought stress. Field Crop Res. 206, 95–105. doi: 10.1016/j.fcr.2017.02.015
Ma, L., Li, G. (2019). Auxin-dependent cell elongation during the shade avoidance response. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00914
Maehly, A. C., Chance, B. (1954). The assay of catalases and peroxidases. Method Enzymol. 1, 357–424.
Mao, J., Niu, C., Li, K., Mobeen Tahir, M., Khan, A., Wang, H., et al. (2020). Exogenous 6-benzyladenine application affects root morphology by altering hormone status and gene expression of developing lateral roots in Malus hupehensis. Plant Biol. 22, 1150–1159. doi: 10.1111/plb.13154
Millenaar, F. F., Van Zanten, M., Cox, M. C. H., Pierik, R., Voesenek, L. A. C. J., Peeters, A. J. M. (2009). Differential petiole growth in Arabidopsis thaliana: photocontrol and hormonal regulation. New Phytol. 184, 141–152. doi: 10.1111/j.1469-8137.2009.02921.x
Nasielski, J., Furze, J. R., Tan, J., Bargaz, A., Thevathasan, N. V., Isaac, M. E. (2015). Agroforestry promotes soybean yield stability and N2-fixation under water stress. Agron. Sustain Dev. 35, 1541–1549. doi: 10.1007/s13593-015-0330-1
Pan, S. G., Rasul, F., Li, W., Tian, H., Mo, Z. W., Duan, M. Y., et al. (2013). Roles of plant growth regulators on yield, grain qualities and antioxidant enzyme activities in super hybrid rice (Oryza sativa L.). Rice 6, 9. doi: 10.1186/1939-8433-6-9
Panigrahy, M., Ranga, A., Das, J., Panigrahi, K. C. S. (2019). Shade tolerance in Swarnaprabha rice is associated with higher rate of panicle emergence and positively regulated by genes of ethylene and cytokinin pathway. Sci. Rep. 9, 6817. doi: 10.1038/s41598-019-43096-8
Parry, M. A., Reynolds, M., Salvucci, M. E., Raines, C., Andralojc, P. J., Zhu, X. G., et al. (2011). Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 62, 453–467. doi: 10.1093/jxb/erq304
Pons, T. L., Jordi, W., Kuiper, D. (2001). Acclimation of plants to light gradients in leaf canopies: evidence for a possible role for cytokinins transported in the transpiration stream. J. Exp. Bot. 52, 1563–1574. doi: 10.1093/jexbot/52.360.1563
Ren, B., Cui, H., Camberato, J. J., Dong, S., Liu, P., Zhao, B., et al. (2016). Effects of shading on the photosynthetic characteristics and mesophyll cell ultrastructure of summer maize. Sci. Nat. 103, 1–12. doi: 10.1007/s00114-016-1392-x
Ren, B., Zhang, J., Dong, S., Liu, P., Zhao, B. (2017). Regulations of 6-benzyladenine (6-BA) on leaf ultrastructure and photosynthetic characteristics of waterlogged summer maize. J. Plant Growth Regul. 36, 743–754. doi: 10.1007/s00344-017-9677-7
Sharma, P., Dubey, R. S. (2004). Ascorbate peroxidase from rice seedlings: properties of enzyme isoforms, effects of stresses and protective roles of osmolytes. Plant Sci. 167, 541–550. doi: 10.1016/j.plantsci.2004.04.028
Stamm, P., Kumar, P. P. (2010). The phytohormone signal network regulating elongation growth during shade avoidance. J. Exp. Bot. 61, 2889–2903. doi: 10.1093/jxb/erq147
Ullah, H., Santiago-Arenas, R., Ferdous, Z., Attia, A., Datta, A. (2019). Improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: A review. Adv. Agron. 156, 109–157. doi: 10.1016/bs.agron.2019.02.002
Wang, J., Shi, K., Lu, W., Lu, D. (2020). Effects of post-silking shading stress on enzymatic activities and phytohormone contents during grain development in spring maize. J. Plant Growth Regul. 40, 1060–1073. doi: 10.1007/s00344-020-10164-7
Wang, X., Vignjevic, M., Liu, F., Jacobsen, S., Jiang, D., Wollenweber, B. (2015). Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul. 75, 677–687. doi: 10.1007/s10725-014-9969-x
Wang, C., Wen, D., Sun, A., Han, X., Zhang, J., Wang, Z., et al. (2014). Differential activity and expression of antioxidant enzymes and alteration in osmolyte accumulation under high temperature stress in wheat seedlings. J. Cereal Sci. 60, 653–659. doi: 10.1016/j.jcs.2014.05.004
Wolz, K. J., DeLucia, E. H. (2018). Alley cropping: Global patterns of species composition and function. Agr Ecosyst. Environ. 252, 61–68. doi: 10.1016/j.agee.2017.10.005
Wu, Y., Gong, W., Yang, W. (2017). Shade Inhibits leaf size by controlling cell proliferation and enlargement in soybean. Sci. Rep. 7, 9259. doi: 10.1038/s41598-017-10026-5
Wu, A., Hammer, G. L., Doherty, A., von Caemmerer, S., Farquhar, G. D. (2019). Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 5, 380–388. doi: 10.1038/s41477-019-0398-8
Wu, X., Zhu, Z., Li, X., Zha, D. (2012). Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters and antioxidative system in seedlings of eggplant (Solanum melongena L.) under salinity stress. Acta Physiol. Plant 34, 2105–2114. doi: 10.1007/s11738-012-1010-2
Xu, H., Chen, P., Tao, Y. (2021). Understanding the shade tolerance responses through hints from phytochrome a-mediated negative feedback regulation in shade avoiding plants. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.813092
Yang, C., Li, L. (2017). Hormonal regulation in shade avoidance. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01527
Yang, T., Ouyang, X., Wang, B., Tian, D., Xu, C., Lin, Z., et al. (2023). Understanding the effects of tree-crop intercropping systems on crop production in China by combining field experiments with a meta-analysis. Agr Syst. 210, 103705. doi: 10.1016/j.agsy.2023.103705
Keywords: walnut-wheat intercropping, 6-benzyladenine, hormone, wheat yield, physiological mechanism
Citation: Zhang Y, Li J, Xu Q, Chen C, Nie S, Lei J and Duan L (2024) Cytokinin modulates the inhibitory effect of shade stress on photosynthesis, antioxidant capacity and hormone homeostasis to regulate the grain yield in wheat. Front. Plant Sci. 15:1498123. doi: 10.3389/fpls.2024.1498123
Received: 18 September 2024; Accepted: 31 October 2024;
Published: 28 November 2024.
Edited by:
Fulai Liu, University of Copenhagen, DenmarkCopyright © 2024 Zhang, Li, Xu, Chen, Nie, Lei and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Lei, bGVpanVuamllQHNvaHUuY29t; Liusheng Duan, RHVhbmxzaEBjYXUuZWR1LmNu
†These authors have contributed equally to this work
 Yongqiang Zhang1,2,3†
Yongqiang Zhang1,2,3† Juan Li
Juan Li Liusheng Duan
Liusheng Duan