- 1Flora Conservation Department, Kadoorie Farm and Botanic Garden, Hong Kong, Hong Kong SAR, China
- 2Department of National Parks, Forest Herbarium, Wildlife and Plant Conservation, Bangkok, Thailand
- 3Department of Plant Science, Faculty of Science, Mahidol University, Bangkok, Thailand
- 4Department of Botany, Rhodes University, Grahamstown, Makhanda, South Africa
- 5Mariri Environmental Centre L5 South Concession, Niassa Special Reserve, Mozambique
- 6North American Orchid Conservation Center, Smithsonian Environmental Research Center, Edgewater, MD, United States
- 7Integrated Research Laboratory, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
- 8The Orchid Society of Eastern Himalaya, Daisa Bordoloi Nagar, Tinsukia, Assam, India
- 9Faculty of Agriculture and Marine Science, Kochi University, Monobeotsu, Nankoku, Kochi, Japan
- 10Tochigi Prefectural Museum, Utsunomiya, Japan
- 11Graduate School of Integrative Science and Engineering, Tokyo City University, Tokyo, Japan
- 12Tsukuba Botanical Garden, National Museum of Nature and Science, Tsukuba, Japan
Introduction: The terrestrial orchid genus Nervilia is diagnosed by its hysteranthous pattern of emergence but is nested among leafless myco-heterotrophic lineages in the lower Epidendroideae. Comprising ca. 80 species distributed across Africa, Asia and Oceania, the genus remains poorly known and plagued by vague and overlapping species circumscriptions, especially within each of a series of taxonomically intractable species complexes. Prior small-scale, exploratory molecular phylogenetic analyses have revealed the existence of cryptic species, but little is otherwise understood of origin, the scale and timing of its biogeographic spread, or the palaeoclimatic factors that have shaped its ecology and given rise to contemporary patterns of occurrence.
Methods: Here, we sample widely throughout the generic range, including 45 named taxa and multiple accessions referable to several widespread ‘macrospecies’, as well as material of equivocal identity and probable undescribed status, for the first time enabling an evaluation of taxonomic boundaries at both species and sectional level. Using nuclear (ITS) and plastid (matK, trnL-F) sequence data, we conduct phylogenetic (maximum parsimony and Bayesian inference) and ancestral area analysis to infer relationships and resolve probable origin and colonisation routes.
Results: The genus is strongly supported as monophyletic, as are each of its three sections. However, the number of flowers in the inflorescence and other floral characters are poor indicators of sectional affinity. Dated ancestral area analysis supports an origin in Africa in the Early Oligocene, with spread eastwards to Asia occurring in the Late Miocene, plausibly via the Gomphotherium land bridge at a time when it supported woodland and savanna ecosystems.
Discussion: Taxonomic radiation in Asia within the last 8 million years ties in with dramatic Himalayan-Tibetan Plateau uplift and associated intensification of the Asia monsoon. Multiple long-range migrations appear to have occurred thereafter, as the genus colonised Malesia and Oceania from the Pliocene onwards. The bulk of contemporary species diversity is relatively recent, potentially explaining the ubiquity of cryptic speciation, which leaves numerous species overlooked and unnamed. Widespread disjunct species pairs hint at high mobility across continents, extinction and a history of climate-induced vicariance. Persistent taxonomic challenges are highlighted.
1 Introduction
The Old World terrestrial orchid genus Nervilia Comm. ex Gaudich. is the sole member of subtribe Nerviliinae and the largest genus of tribe Nervilieae (Pridgeon et al., 2005; Chase et al., 2015; POWO, 2024), which is thought to have diverged from its sister tribe Gastrodieae ca. 35 million years ago (Mya; Li et al., 2019). Both tribes are nested within the ‘lower Epidendroid’ clade of the Epidendroideae, which with >21,160 accepted species is the largest orchid subfamily, accounting for more than three-quarters of all orchid diversity (Freudenstein and Chase, 2015). A consensus phylogenetic framework for the lower Epidendroids remains wanting, owing in part to the prevalence of myco-heterotrophy in the basal-most lineages, leading to high substitution rates in, and gene loss from, their plastid genomes (Rothacker, 2007; Górniak et al., 2010; Feng et al., 2016). This has complicated sequence alignment, confounded phylogenetic resolution and undermined stable classification (Chase et al., 2003; Lam et al., 2018). To an extent, whole plastome sequencing has helped clarify basal Epidendroid relationships (Li et al., 2019; Wen et al., 2022), but incongruence between nuclear and plastid trees remains a persistent challenge to the interpretation of evolutionary data sets and the attainment of a reliable taxonomy (Pérez-Escobar et al., 2021). As the only autotrophic member of its tribe and one of relatively few autotrophic lineages at the base of the subfamily, clearer understanding of patterns in speciation and trends in biogeographic occurrence in Nervilia could help shed light on the evolution of the lower Epidendroids as a whole (Chase et al., 2003, 2015; Pérez-Escobar et al., 2021).
Nervilia is diagnosed by its hysteranthous mode of emergence, by which separate generative (flower-bearing) and vegetative (leaf-bearing) shoots sprout in succession, typically with little or no overlap between the two (Pettersson, 1991; Gale et al., 2018). All emergent parts die back at the end of the growing season, with only the subterranean corm perennating through the winter or dry season to the next (Gale et al., 2021). This annual cycle and correspondingly ephemeral above-ground phase – an adaptation thought to have arisen in response either to marked seasonality in rainfall (Pettersson, 1991) or to an interplay of factors including temperature and resource limitation (Howard and Cellinese, 2020) – renders plants easily overlooked in the field and has led to the erroneous claim that some species are leafless myco-heterotrophs (Pettersson, 1991). Combined with their diminutive habit, sporadic occurrence and rarity in many cases, these attributes mean that most species remain poorly known. In fact, because flowers and leaves are rarely present at the same time, herbarium specimens tend to comprise just one or the other, and as two or more species may occur at the same site (Pettersson, 1991; Gale et al., 2015), shoots belonging to different species, or even to different genera, are sometimes mismatched on the same sheet (Pettersson, 1990; Gale et al., 2016, 2021; Ketjarun et al., 2019).
The genus occurs in tropical and subtropical Africa and Madagascar, Asia, Australasia and parts of Micronesia, Melanesia and Polynesia (Pridgeon et al., 2005), and is presently thought to contain in the region of 80 species (POWO, 2024). Tanzania, Thailand and Indonesia appear to be the countries with the greatest diversity, each with 12 or more species, but the majority of species are Asian (Gale et al., 2022; POWO, 2024). Given this geographic bias, Pettersson (1991) hypothesised that the genus originated in Asia. Even so, there are a number of reasons why biogeographic understanding of species diversity might be considered incomplete. Firstly, the very limited herbarium material available means that species circumscriptions and boundaries remain poorly resolved. Many pre-20th century names were published with superficial protologues that did not document morphological details now known to be important for species delimitation (Gale et al., 2007, 2015), but type material of these small, generally membranous plants is delicate and often badly preserved (Pettersson, 1990). This issue is especially problematic for the species of section Linervia, which possess just one flower, restricting options for the observation and analysis of floral traits (Gale et al., 2007). As a result, many names have been misapplied or later proven to be synonyms of incompletely known taxa (Seidenfaden and Smitinand, 1959–1965; Gale et al., 2010; Nusbauer et al., 2011; Gale and Watthana, 2014). Secondly, the lack of any range-wide or continental-scale revision of the genus, other than for the African species (Pettersson, 1991), means that, in the absence of a standard reference, taxonomic confusion has been propagated through the piecemeal misuse and repeated misinterpretation of names in regional or national treatments (Chen and Gale, 2009; Gale et al., 2007). In fact, the only attempt to critically compare all members of the genus known at the time dates back to the early 20th century, when the global tally stood at 37 species plus seven insufficiently known taxa (Schlechter, 1911).
To complicate matters further, Nervilia has been shown to contain a series of species complexes, each characterised by vegetative uniformity and only subtle differences in floral morphology that can nevertheless conceal wide genetic, cytological and biogeographic divergence and thus cryptic diversity (Gale et al., 2010, 2015, 2016, 2018; Ketjarun et al., 2019). Species complexes have been identified in all three presently accepted sections on the genus, but the so-called ‘N. adolphi–punctata alliance’ of section Linervia is the largest, with 30 or more species distributed throughout the generic range (Gale et al., 2015, 2018). Indeed, 21 of the 22 names published in Nervilia as new species since 2010 are referable to this taxonomically challenging complex on account of their one flower with a narrow, predominantly white and usually crimson-spotted, three-lobed lip and glabrous, cordate-polygonal leaf (Figures 1A–F´). This surge in species discovery reinforces that comprehensive taxonomic understanding remains some way off, especially in Asia, where all these new taxa were found. Gale et al. (2016) and Ketjarun et al. (2019) have highlighted that cryptic taxa may also occur in sections Nervilia and Vinerlia, notably within the widespread and polymorphic ‘macrospecies’ N. concolor (Blume) Schltr. and N. plicata (Andrews) Schltr., respectively.

Figure 1. Morphological conservatism and sectional division in Nervilia. (A–H’) Section Linervia. (I–J’) Section Vinerlia. (K–N’) Section Nervilia. (A, A’) Flower and leaf of N. adolphi var. adolphi in Tanzania. (B, B’) Flower and leaf of N. alisanensis in China (Hainan). (C, C’) Flower and leaf of N. juliana in India. (D, D’) Flower and leaf of N. khaoyaica in Thailand. (E, E’) Flower of N. mackinnonii in Thailand and leaf of N. cf. mackinnonii (sample MY73 in Table 1) in Myanmar. (F, F’) Flower and leaf of N. taiwaniana in Taiwan. (G, G’) Flower and leaf of N. simplex in Malawi. (H, H’) Flowers and leaf of N. cumberlegei in Taiwan. (I, I’) Flowers and leaf of N. plicata in China (Hong Kong). (J, J’) Flowers and leaf of N. plicata in Thailand. (K, K’) Flowers and leaf of N. concolor in Thailand. (L, L’) Flowers and leaf of N. kotschyi var. kotschyi in Kenya. (M, M’) Flowers and leaf of N. campestris (=N. holochila) in Indonesia. (N, N’) Flower and leaf of N. maculata in Thailand.
All species produce just one inflorescence and one leaf per annual growth cycle, with the number of flowers borne by the inflorescence, as well as the size, outline and indumentum of the leaf, supposedly varying discretely among the three sections (Schlechter, 1911; Pettersson, 1991; Gale et al., 2021; Figure 1). Thus, as traditionally circumscribed, section Nervilia comprises plants with a four- or more-flowered scape and a comparatively large, glabrous, orbicular leaf; section Vinerlia comprises plants with a two-flowered scape and a pubescent, ovate-reniform leaf; and section Linervia comprises plants with a one-flowered scape and a small, cordate-polygonal or reniform leaf that is usually glabrous but which, in some species, is setose (Schlechter, 1911; Pettersson, 1991). However, there are a number of species that do not conform to this sub-division. For example, Pettersson (1991) reasoned that, despite its normally one-flowered scape and ovate leaf, N. ballii G.Will. is best placed in section Nervilia on account of its lip with a recurved mid-lobe and nectar guides, as is N. shirensis (Rolfe) Schltr., which is two- or three-flowered. On the other hand, Seidenfaden and Smitinand (1959–1965) noted that, despite its two- or three-flowered inflorescence, N. cumberlegei Seidenf. & Smitinand has a fimbriate lip much like the one-flowered N. prainiana (King & Pantl.) Seidenf. and N. crispata (Blume) Schltr. ex K.Schum. & Lauterb., both of which are now generally included in the synonymy of N. simplex (Thouars) Schltr. of section Linervia (Figures 1G–H´). And, acknowledging wide infraspecific variation, Pettersson (1990, 1991) assigned N. kotschyi (Rchb.f.) Schltr. to section Nervilia on account of details of the lip and tepals, even though its usually two-flowered scape and cordate-reniform leaf with fringed keels might justify its placement in section Vinerlia (Figures 1L, L´). Gale et al. (2021) postulated that details of floral anatomy, rather than flower number, will ultimately prove incisive in defining sectional identity.
To date, only two small-scale attempts have been made to integrate molecular phylogenetic data into analyses of species relationships in the genus (Gale et al., 2015, 2018). Employing nuclear (ITS) and plastid (matK and trnL-F) sequences, those studies uncovered surprisingly wide vegetative variation within some narrowly distributed species on the one hand, as well as broad uniformity in overall morphology among genetically and biogeographically distinct taxa on the other. But few species were sampled and most were from seasonal tropical Asia. So, although that work flagged cryptic speciation as a feature of the genus and hinted at divergence that reflects the morphology-based sectional classification, numerous ambiguities remain, particularly with regards to interpreting species taxonomy in the light of occurrence and ecology (Gale et al., 2010, 2015, 2018, 2021; Niissalo et al., 2020). For the first time, the present study samples throughout the generic range to enable the validity of the three sections to be tested. In doing so, we assess origin, examine how palaeoecological history has driven diversification and biogeographic spread, and explore the relationship between genetic divergence and morphological differentiation within each of the species complexes.
2 Materials and methods
2.1 Taxon sampling
Owing to the difficulty in positively identifying Nervilia species in the absence of correctly matched flowers and leaves, we included a mix of both named and unnamed accessions to account for as wide a cross section of the genus as possible and so permit an examination of patterns of genetic disparity among morphologically similar plants. Further, we included two or more accessions from different locations for some species in order to test vague or questionable species boundaries, particularly the taxonomically problematic ‘macrospecies’ N. infundibulifolia Blatt. & McCann, N. simplex, N. plicata and N. concolor, which have been subject to unstable and sometimes conflicting interpretation in different parts of their widespread geographic ranges. To help assess the merit of morphology-based assumptions within variable taxa, three names presently treated as synonyms by POWO (2024) were maintained for the purposes of this study: N. campestris (J.J.Sm.) Schltr. [now placed under N. holochila (F.Muell.) Schltr.], N. carinata (Roxb.) Schltr. (now placed under N. concolor) and N. prainiana (now placed under N. simplex).
In total, 96 Nervilia plants were sampled (Table 1). These included 86 samples representing 45 named taxa, four samples that could only be doubtfully referred to a particular named species and were thus qualified with “conferatur” [viz N. cf. mackinnonii (Duthie) Schltr., N. cf. viridis S.W.Gale, Watthana & Suddee and N. cf. concolor], and a further six samples that could not be matched with any published species and were thus suspected to represent undescribed taxa. Our sampling covered all three sections of the genus, including several taxa with a flower number ‘atypical’ of the section in which they are placed (Table 1). Also included was N. stolziana Schltr. which, on account of its spurred lip, was previously assigned (together with N. pectinata P.J.Cribb, not included in this study) to section Kyimbilaea, which has since been subsumed under section Linervia (Pettersson, 1991). In all, we included 11 taxa (13 samples) from Africa and Madagascar, 30 species (60 samples) from the seasonal Asian tropics, seven species (nine samples) from the moist Asian tropics, and seven species (14 samples) from Oceania. Based on the phylograms presented by Chase et al. (2015) and Freudenstein and Chase (2015), we included one sample each of Corymborkis veratrifolia (Reinw.) Blume (Tribe Tropidieae), Monophyllorchis maculata Garay (Tribe Triphoreae) and Gastrodia peichatieniana S.S.Ying (Tribe Gastrodieae) as outgroups from subfamily Epidendroideae for phylogenetic analysis, plus one sample of Habenaria dentata (Sw.) Schltr. (Tribe Orchideae) as an additional outgroup from subfamily Orchidoideae for biogeographic analysis. All samples were collected and transported with permission [CITES permits: ROP-008-2018, ROP-045-2019, PCIP-20-00094, CA-307/2012, 2014-TH006062/CA, 2021-TH010447/BE, JPHTN/PPP/BO-100-24/1(40), 003/16-01, 008/16-01].
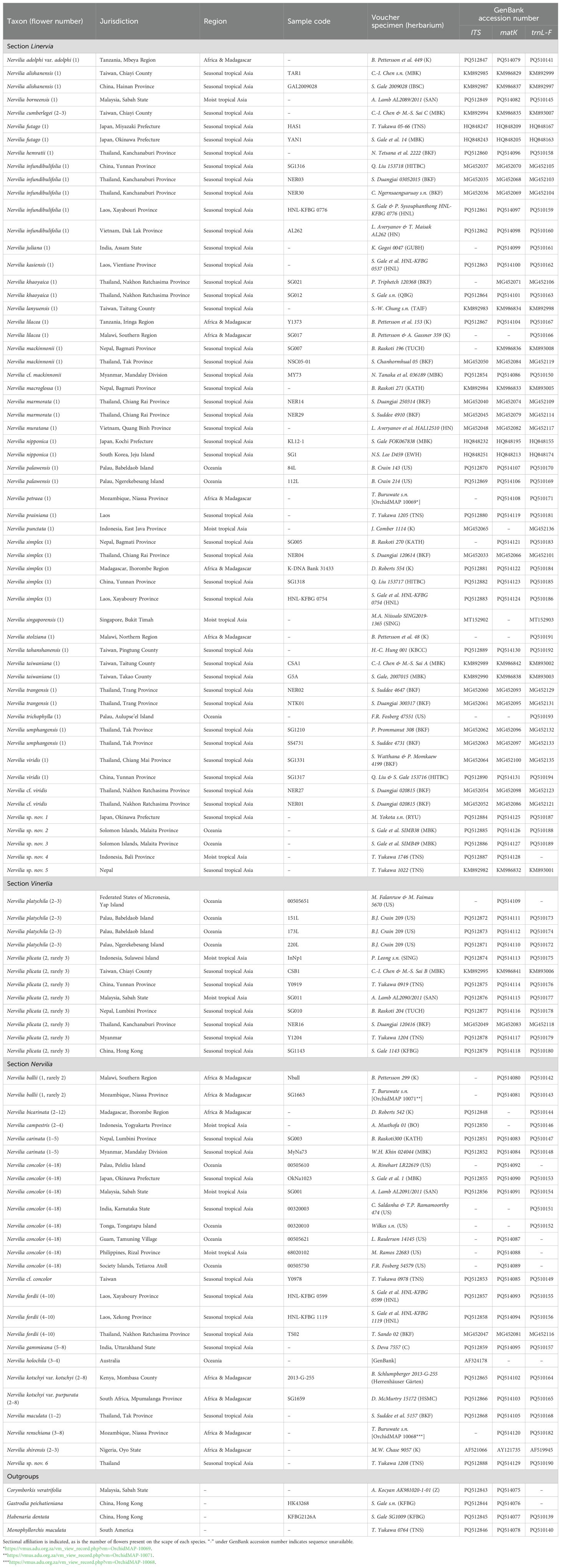
Table 1. Collection localities, jurisdiction, voucher specimens and GenBank accession numbers for the samples used in this study.
2.2 DNA extraction, PCR and sequencing
Of the 100 samples used in this study, 63 were newly sequenced, either from fresh material (48 samples) or from well-preserved herbarium specimens (15 samples). Total DNA was extracted using a QIAGEN DNeasy® plant DNA kit (Hilden, Germany) according to the manufacturer’s instructions. The internal transcribed spacer (ITS) region of nuclear ribosomal DNA was amplified using the primers of White et al. (1990) or Sun et al. (1994), the plastid maturase K gene (matK) region (including part of the flanking trnK introns) was amplified in three sections using the primers of Hidayat et al. (2005), and the entire trnL-F region (comprising the trnL intron and the trnL-F intergenic spacer) was amplified using the c and f primers of Taberlet et al. (1991). PCR was performed in a total reaction mixture of 25 µl containing 1 µl of template DNA (2–10 ng), 5 µl of 5 × Phire® reaction buffer with MgCl2, 0.5 µl 10 mM of dNTP mix, 0.5 µl of Phire® hot start II DNA polymerase (Finnzymes, Finland) and 10 pmol of each primer (Beijing Genomics Institute). The thermal cycler programme consisted of an initial denaturation step at 98°C for 30 s, followed by 35 cycles of 5 s at 98°C, 5 s at 60°C for ITS, 10 s at 55°C for matK and 5 s at 55°C for the trnL-F region, 20 s at 72°C, and a final extension at 72°C for 1 min. Amplification products were purified using a DNA purification Kit (Beijing Genomics Institute). Purified PCR products were sequenced using an ABI 3730 DNA Sequencer (Applied Biosystems, Foster City, California). All sequences have been deposited in GenBank (Table 1).
2.3 Phylogenetic analysis
Alignments were constructed using the MAFFT multiple alignment plugin in Geneious v11.1.4 (Kearse et al., 2012), with subsequent adjustment by eye. We excluded two poly-A regions comprising 41 and 61 positions in the trnL–F and matK genes, respectively (Supplementary File S1). An incongruence length difference (ILD) test (Farris et al., 1995) was performed in PAUP* v4.0b10 (Swofford, 2003) to assess whether the individual matK and trnL–F data sets, and the ITS and combined cpDNA data sets (Supplementary File S1), reflect similar potential phylogenies; 1,000 replicates, each with 1,000 random addition sequence replicates and tree bisection-reconnection (TBR) branch swapping, were performed in each test, and a P value of <0.05 was considered significant (Sullivan, 1996; Darlu and Lecointre, 2002). A “hard” incongruence test was also performed by directly comparing respective topologies, as well as resolution, for each clade generated in the separate analyses, with bootstrap percentages (BP) of ≥85% (Chase et al., 2000) and posterior probabilities (PP) of ≥0.95 (Martínez-Azorín et al., 2011) being taken as evidence of strong support.
Both the homogeneity test for the matK and trnL-F data sets (P = 0.881) and visual node-by-node comparisons of trees generated for either region individually revealed no major topological disparities for nodes of BP ≥85% and PP ≥0.95, and so the two ptDNA regions were combined. Tree topologies generated for the individual ITS and ptDNA data sets using Bayesian inference (BI) were also largely congruent with those using maximum parsimony (MP; Supplementary File S2). However, the ILD test indicated significant incongruence between the ITS and ptDNA data sets (P = 0.001). Even so, visual comparison of the trees generated from the two data sets uncovered no topological disparities with nodes of BP ≥85% and PP ≥0.95, except for the position of a single clade containing four samples representing three species [N. bicarinata (Blume) Schltr., N. kotschyi and N. shirensis; Supplementary File S2]. Since Cunningham (1997) and Yoder et al. (2001) have argued that combined data sets improve phylogenetic accuracy regardless of incongruence, and numerous phylogenetic studies have found that trees generated from combined data sets with or without samples responsible for topological disparities remain highly consistent (e.g. Li et al., 2011; Kumar et al., 2022), we concatenated the ITS and ptDNA data sets and interpreted the resulting combined phylograms.
Phylogenetic analysis of individual and multilocus alignments were carried out using MP in PAUP* v4.0b10 and BI in MrBayes v3.2 (Huelsenbeck and Ronquist, 2003). For MP analyses, heuristic searches were conducted with 1,000 random addition replicates followed by TBR branch swapping. All characters were unordered and equally weighted with gaps (including unavailable sequences) treated as missing data. Topological robustness was assessed using 1,000 bootstrap replicates. For BI analyses, each DNA region was assigned its own model of nucleotide substitution, as determined by the Akaike information criterion (AIC) in Modeltest v3.06 (Posada and Crandall, 1998). Four simultaneous Monte Carlo Markov Chains (MCMC) were run, with sampling one tree every 1,000 generations for 30,000,000 generations, starting with a randomly generated tree. Majority rule (>50%) consensus trees were constructed after removing the first 25% of sampled trees as burn-in.
2.4 Ancestral area reconstruction
In constructing a dated phylogenetic tree, a single accession was selectively retained for each taxon represented by more than one sample (Supplementary File S3). Divergence times were estimated using a Bayesian uncorrelated relaxed-clock model implemented in BEAST 2.7.6 (Bouckaert et al., 2019) with priors placed on the node for tribes Nervilieae and Gastrodieae (offset 34.93 Mya, mean:1, sigma:1) and the node for subfamilies Epidendroideae and Orchidoideae (offset 64 Mya, mean:1, sigma:1), based on results presented by Givnish et al. (2015); Li et al. (2019) and Li et al. (2022). MCMC searches were run for 50,000,000 generations and sampled every 5,000 generations, with convergence being monitored using Tracer 2.7.6 (Bouckaert et al., 2019). The effective sample sizes (ESSs) of all parameters were assessed as more than 200 and the maximum clade credibility tree was computed using treeAnnotator 2.7.6 (Bouckaert et al., 2019).
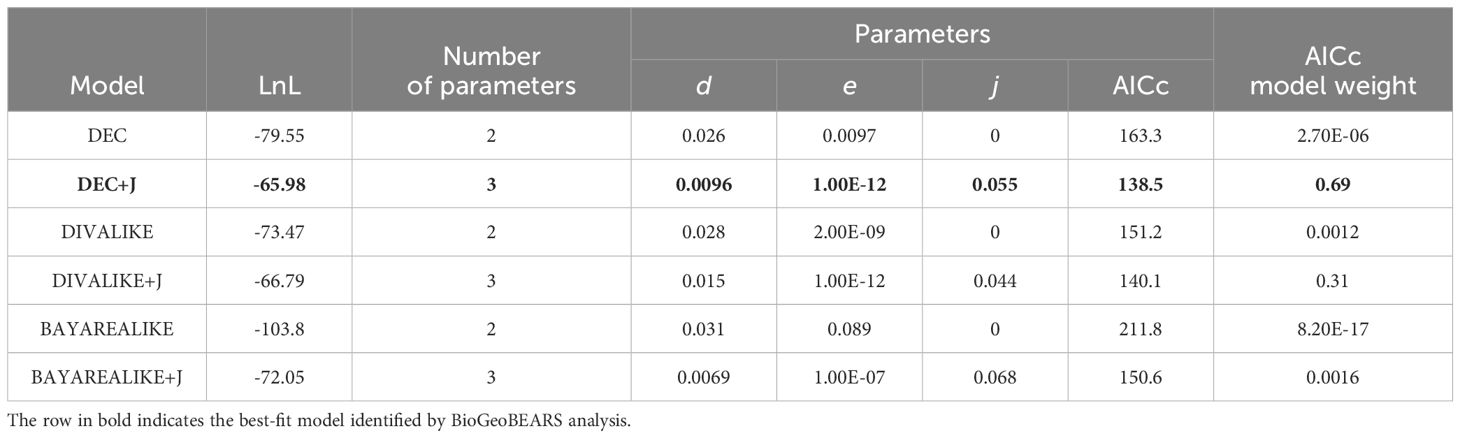
Four areas of endemism were defined for biogeographic analysis, reflecting the extant distribution of Nervilia demarcated by Pridgeon et al. (2005) as well as the climatic zones discernible within this range based primarily on seasonality, which is presumed to be of importance for the hysteranthous habit (Gale et al., 2021): (area 1) tropical Africa & Madagascar, (area 2) seasonal (monsoonal) tropical Asia, (area 3) aseasonal, moist tropical Asia, and (area 4) Oceania (encompassing Australasia, Micronesia, Melanesia and Polynesia). Ancestral area reconstruction was then performed using the package BioGeoBEARS (Matzke, 2016) in R 4.3.2 (R Core Team, 2023), applying the dispersal–extinction–cladogenesis (DEC) model (Ree and Smith, 2008), ML version of Dispersal Vicariance Analysis (DIVALIKE; Ronquist, 1997) and Bayesian biogeographical inference model (BAYAREALIKE; Landis et al., 2013) with the maximum range-size parameter set to three. We tested each of these models with and without founder-event speciation, which was incorporated with J-parameter modelling jump dispersal (Matzke, 2016). All six permutations were compared using likelihood values, and Akaike information criterion (AIC) was performed in BioGeoBEARS using the maximum clade credibility tree from the BEAST analyses described above. The best-fit model was selected based on lower corrected Akaike information criterion (AICc) values with larger weight (wAICc), representing relative support for each model (Burnham and Anderson, 2002). All underlying raw data used in the phylogenetic analyses and ancestral area reconstruction are available in the Dryad Digital Repository, DOI: 10.5061/dryad.tb2rbp0bn.
3 Results
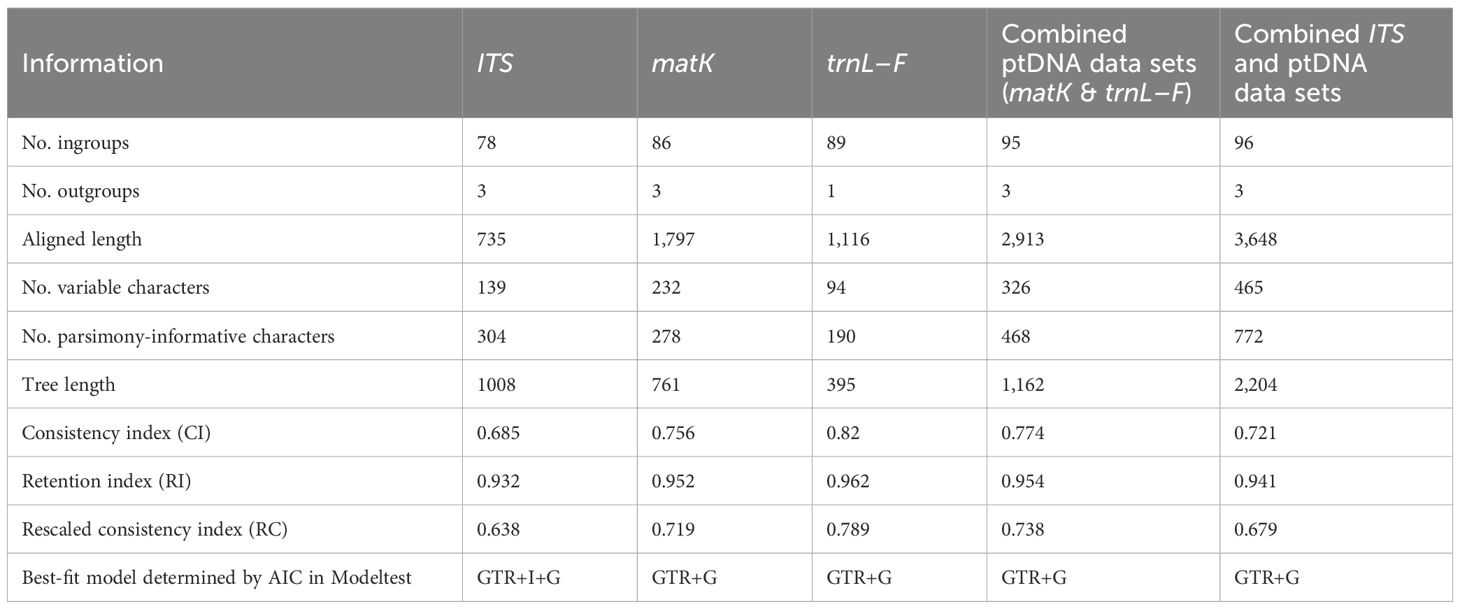
Sequence data for all three regions (ITS, matK and trnL-F) were newly generated for 41 samples, chloroplast data (matK and trnL-F) were generated for a further seven samples, ITS and matK data were generated for three samples, ITS and trnL-F data were generated for two samples, and matK or trnL-F data were individually generated for a further five samples (Table 1). Statistics relating to the aligned matrix for each region and for the combined data sets are shown in Table 2.
3.1 Phylogenetic analysis
The genus Nervilia in its entirety was strongly supported as monophyletic, whether assessed using combined (BP 97%, PP 1.00; Figure 2) or individual ITS and ptDNA data sets (Supplementary File S2). In the combined tree (Figure 2), all ingroup taxa fell into two strongly supported clades, one comprising section Linervia (BP 98%, PP 1.00) and the other composed of sections Vinerlia and Nervilia together (BP 100%, PP 1.00). Represented here by N. plicata and N. platychila Schltr., section Vinerlia was also supported as monophyletic (BP 97%, PP 1.00), as was section Nervilia (BP 99%, PP 1.00). Section Nervilia itself comprised two well resolved sub-clades, one containing five African species [N. bicarinata, N. renschiana (Rchb.f) Schltr., N. shirensis, N. kotschyi and N. ballii; BP 100%, PP 1.00] and the other containing seven named species [N. campestris, N. holochila, N. concolor, N. gammieana (Hook.f.) Pfitzer, N. carinata, N. maculata (C.S.P.Parish & Rchb.f.) Schtlr. and N. fordii (Hance) Schtlr.] plus N. cf. concolor and N. sp. nov. 6 from Asia and Oceania (BP 100%, PP 1.00). Within the latter, N. campestris and N. holochila together formed a single, strongly supported lineage (BP 99%, PP 1.00) that was sister to all remaining members of this sub-clade (BP 75%, PP 1.00), which in turn partitioned the eight positively identified N. concolor accessions (BP 77%, PP 1.00) as separate from an unresolved grade of nine samples representing N. gammieana, N. carinata, N. maculata, N. fordii, N. cf. concolor and N. sp. nov. 6.
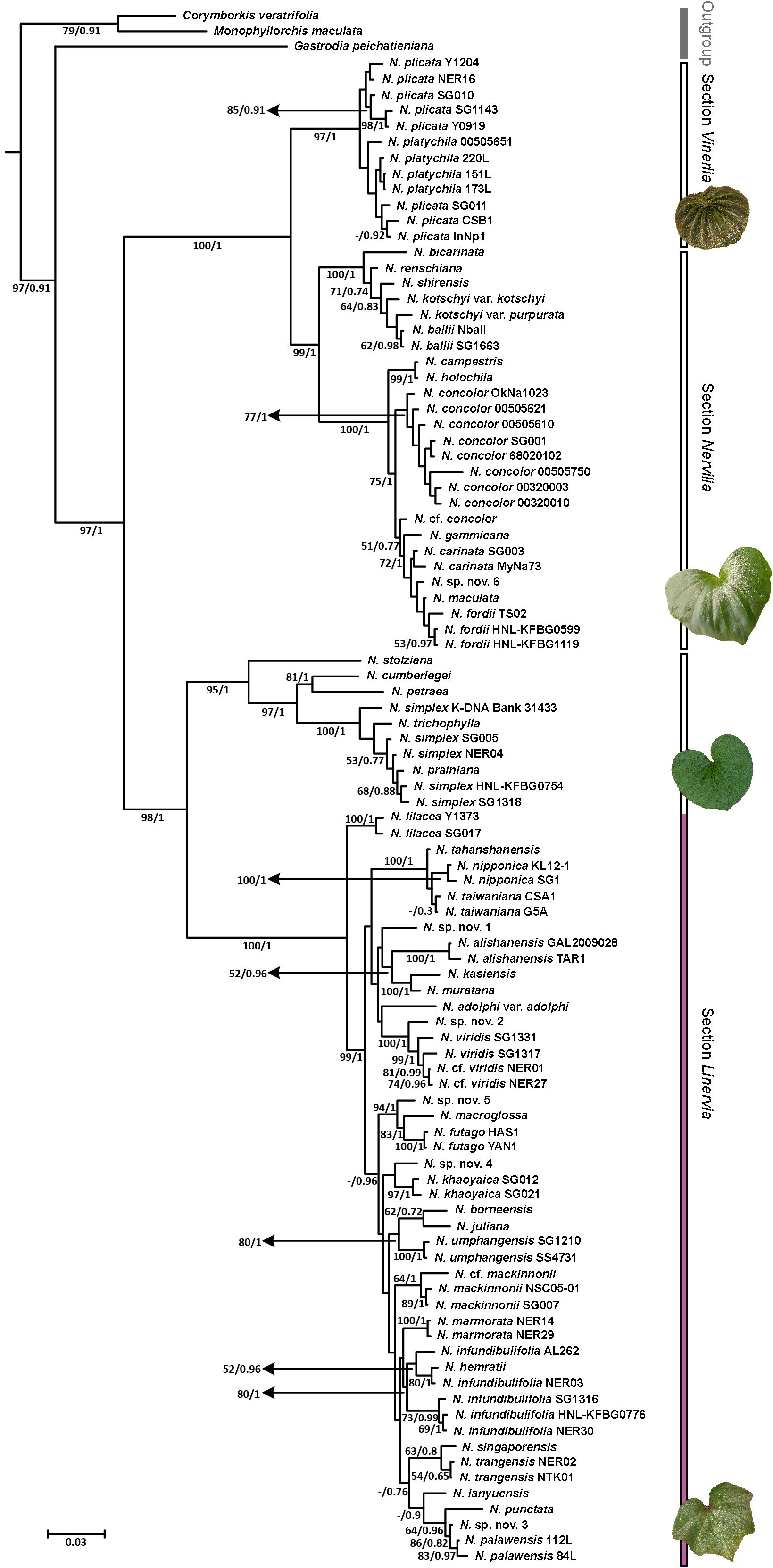
Figure 2. Phylogram obtained from Bayesian inference (BI) analysis of the combined ITS and ptDNA data sets. Numbers at the nodes indicate bootstrap percentages and Bayesian posterior probabilities, respectively. “-” indicates that the node collapsed in MP analysis. Sectional affiliation is indicated at right, as is the extent of the species-rich Nervilia adolphi-punctata alliance (shown in purple).
Section Linervia was also composed of two strongly supported sub-clades, one comprising N. stolziana, N. cumberlegei, N. petraea (Afzel. ex Sw.) Summerh. and the various samples belonging to the N. simplex complex, including N. prainiana and N. trichophylla Fukuy. (BP 95%, PP 1.00), and the other containing 24 other named species plus two unverified accessions (N. cf. viridis and N. cf. mackinnonii) and five putatively undescribed species (BP 100%, PP 1.00), all of which are referable to the ‘N. adolphi–punctata alliance’ on the basis of leaf and floral characters. Within this latter sub-clade, the two N. lilacea Jum. & H.Perrier accessions from Africa (BP 100%, PP 1.00) were strongly supported as sister to all other accessions from Asia and Oceania (BP 99%, PP 1.00). A derived internal sub-clade of the latter containing N. tahanshanensis T.P.Lin & W.M.Lin, N. nipponica Makino and N. taiwaniana S.S.Ying also received strong support (BP 100%, PP 1.00), as did a sister relationship between N. kasiensis S.W.Gale & Phaxays. and N. muratana S.W.Gale & S.K.Wu (BP 100%, PP 1.00). Similarly, the undescribed N. sp. nov. 2 from the Solomon Islands was strongly supported as sister to a clade containing the two Thai and Chinese N. viridis samples plus the two Thai N. cf. viridis accessions (BP 100%, PP 1.00), with the monophyly of the latter four also being strongly supported (BP 99%, PP 1.00). Further, the pair of samples included for each of N. nipponica, N. alishanensis T.C.Hsu, S.W.Chung & C.M.Kuo, N. futago S.W.Gale & T.Yukawa, N. khaoyaica Suddee, Watthana & S.W.Gale, N. umphangensis Suddee, Rueangr. & S.W.Gale, N. mackinnonii, N. marmorata S.W.Gale, Suddee & Duangjai and N. palawensis Schtlr. all formed strongly supported clades; but this was not the case for the pair of N. trangensis S.W.Gale, Suddee & Duangjai and N. taiwaniana samples. Meanwhile, the five N. infundibulifolia samples formed a clade inclusive of N. hemratii S.W.Gale, Tetsana & Suddee (BP 80%, PP 1.00), among which posterior probabilities hinted at a degree of internal structure.
3.2 Biogeographic analysis
Estimated divergence times for the genus encompassing 52 taxa are presented in Figure 3. The results support a close relationship between the tribes Nervilieae (represented by Nervilia) and Gastrodieae (represented by Gastrodia R.Br.), with divergence between the two genera placed at around 31.01 Mya in the Early Oligocene, with a 95% highest posterior density (HPD) interval ranging from 18.5 to 38.45 Mya. The common ancestral age of Nervilia was inferred to be 24.4 Mya (95% HPD 14.77–34.93 Mya). Ancestral lineage Clade A diverged at 12.53 Mya (95% HPD 6.24–25.14 Mya), giving rise to two derived lineages, one representing section Vinerlia (Clade B) and the other representing section Nervilia (Clade C). Clade C subsequently split at 8.94 Mya (95% HPD 3.98–20.88 Mya), generating clades D and E, each of which underwent further differentiation at 4.01 Mya (95% HPD 1.52–11.08 Mya) and 3.66 Mya (95% HPD 1.16–11.31 Mya), respectively. Ancestral lineage Clade F, representing section Linervia, split at 19.11 Mya (95% HPD 10.31–30.76 Mya) into two derived clades, G and H, each of which underwent further divergence at 11.85 Mya (95% HPD 3.81–24.07 Mya) and 9.68 MYA (95% HPD 5.47–21.63 Mya), respectively. Diversification of all derived lineages within the genus occurred from the Late Miocene onwards but this process was concentrated in the Pliocene and appears to have persisted into the Pleistocene (Figure 3).
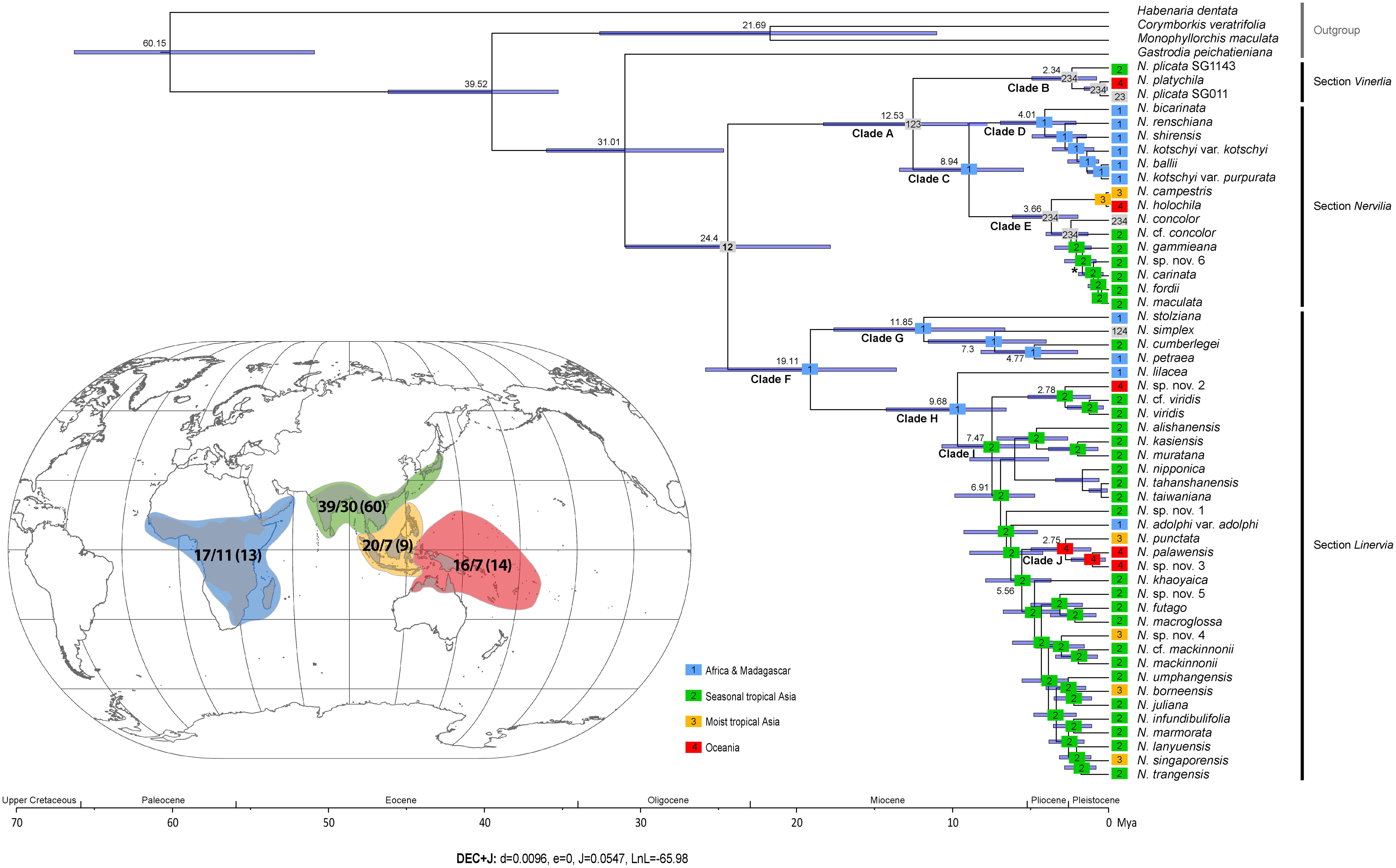
Figure 3. Spatio-temporal reconstruction of Nervilia according to the best-fit model (DEC+J) inferred by BioGeoBEARS and the maximum clade credibility tree obtained by BEAST analysis. The blue bar at each node indicates the 95% credibility intervals with mean node ages (Mya) shown above. Coloured rectangles correspond to the four biogeographic regions defined for the purposes of this study: blue (1) indicates Africa & Madagascar, green (2) seasonal tropical Asia, orange (3) moist tropical Asia and red (4) Oceania. Grey boxes indicate taxa that span two or more biogeographic regions. The worldwide occurrence of Nervilia is shown inset (grey shaded area), with the coloured polygons corresponding to the four biogeographic regions. Numbers shown in each polygon indicate the total number of Nervilia species known in that region according to POWO (2024) on the left of the forward-slash, followed by the number of species sampled in this study and the total number of samples in brackets.
BioGeoBEARS analysis identified the DEC (dispersal–extinction–cladogenesis) +J model as the best-fit model, recovering the lowest AICc value (138.5) and highest wAICc value (0.69) of the six models considered (Table 3). This model suggests that the ancestral Nervilia population had a distribution encompassing either Africa & Madagascar (area 1) or seasonal tropical Asia (area 2), and that it diverged into two distinct lineages, Clades A and F, in the Late Oligocene (Figure 3). Species derived from the earliest settlers of Clade A eventually gave rise to the common ancestor of Clades B and C in the Middle Miocene. Clade C appears to have originated in Africa & Madagascar, with subsequent divergence in the Late Miocene leading to diversification in that region during the Pliocene (Clade D), as well as contemporaneous dispersal to seasonal and moist tropical Asia (areas 2 and 3) initially, followed by Oceania (area 4) in the Pliocene (Clade E). Most diversification in the latter appears to have occurred in seasonal tropical Asia, but the rapid colonisation of seasonal and moist tropical Asia as well as Oceania by N. concolor over this timeframe is noteworthy. In contrast, diversification of Clade B occurred entirely outside of Africa & Madagascar, with its spread firstly across seasonal tropical Asia being followed by more recent colonisation of moist tropical Asia and Oceania during the Pleistocene.
The progenitor of Clade F was unequivocally of African & Madagascan origin, giving rise to two Clades, G and H, during the Early Miocene. Clade G underwent further differentiation primarily in the same region in the Middle to Late Miocene, but with the N. simplex lineage exhibiting enormous dispersal to seasonal tropical Asia and Oceania in the Late Miocene, mirroring the rapid spread of N. concolor. Clade H is also evidently of African & Madagascan origin and has the Afro-Malagasy N. lilacea at its base, but is otherwise characterised by dispersal to, and subsequent explosive diversification within, seasonal tropical Asia from the Late Miocene onwards, with several more recent, independent onward dispersals to both moist tropical Asia and Oceania. Clade J presents an interesting case of wide dispersal to and differentiation across the Pacific Islands, with subsequent colonisation of moist tropical Asia, from the late Pliocene onwards. Nervilia adolphi, in contrast, appears to represent an unusual dispersal back to Africa in the Late Miocene.
Overall, several long-distance dispersal events are revealed in the evolution of the genus. These include at least three independent migrations from Africa & Madagascar to seasonal tropical Asia in Clades C, G and H, and multiple colonisations of moist tropical Asia from seasonal tropical Asia in Clade I and possibly Clade E. Dispersal to Australasia and the Pacific appears to represent the most recent biogeographic step, having occurred both from seasonal tropical Asia in Clade I (and possibly Clade B) and from moist tropical Asia in Clade E. Moist tropical Asia was also colonised from Oceania in Clade J and, as already noted, Africa was ‘re-colonised’ from seasonal tropical Asia in Clade I.
4 Discussion
As has previously been surmised (Dressler, 1993; Pettersson, 1991; Chase et al., 2003, 2015; Górniak et al., 2010; Freudenstein and Chase, 2015), Nervilia is here resolved as monophyletic and phylogenetically isolated, with our results adding to mounting evidence of a close affiliation with Gastrodia at tribal level (Li et al., 2019; Pérez-Escobar et al., 2021). Although broader relationships among the basal-most Epidendroids remain contentious, the diminutive stature and ephemeral, often leafless habit of many of the constituent taxa renders them difficult to sample and challenging to analyse as compared with the generally showier and more robust higher Epidendroids (Li et al., 2018). The insights that our results provide are thus an important step in unraveling biogeographic trends and patterns in speciation across the grade. Though not yet exhaustive, the phylogenetic framework presented here sheds light on probable geographic origin and modes of dispersal and divergence, with ramifications for the evolution, biogeography, taxonomy and classification of Nervilia, a key lower Epidendroid genus.
4.1 First phylogenetic insights into the origin of Nervilia
Nervilia has been represented by up to just three samples in prior phylogenetic analyses of tribal- or genus-level relationships in the Orchidaceae (Rothacker, 2007; Li et al., 2019; Pérez-Escobar et al., 2021). Our findings corroborate a nested placement among the basal Epidendroids but, by virtue of much broader taxon sampling, we elaborate farther reaching hypotheses relating to the temporal and spatial scale of its evolution. Firstly, our analyses imply an origin in the Early Oligocene in either Africa & Madagascar or the seasonal Asian tropics, contrasting the one previous assertion by Pettersson (1991) that the genus arose in Asia, and most likely the wet Asian tropics. In fact, our results suggest that the genus was not present in that region until much more recently. Whilst further outgroup optimisation could yet alter this perspective, its affiliation with Gastrodia in the Gastrodieae on the one hand, and with Epipogium Borkh. and Stereosandra Blume in tribe Nervilieae itself on the other (Chase et al., 2015), might be expected to recover the same equivocal position, since all three genera are similarly widespread across the Old World tropics (Pridgeon et al., 2005). However, because Africa and Asia have never been geographically connected, it is necessary to discern which is the more likely ancestral area. In this regard, the prevalence of African branches at the more basal stem nodes (i.e. Clades C, F, G and H in Figure 3) lends weight to an African origin, all the more so for the generally eastward trajectory witnessed in the evolution of the genus as a whole: by and large, from Africa to seasonal tropical Asia, and from there onto the wet Asian tropics and Oceania. Movement from Africa to seasonal tropical Asia is also apparent in the transition from Clade H to Clade I in the Late Miocene (ca. 10 Mya onwards; Figure 3), a pattern probably repeated from Clade C to Clade E more or less contemporaneously and, given the absence of section Vinerlia in Africa (Pettersson, 1991), from Clade A to B over the same period, too.
The inferred timing of the origin of the genus is especially illuminating, since the Early Oligocene (from around 33.5 Mya onwards) was marked by the onset of an icehouse climate (Coxall and Pearson, 2007; Liu et al., 2009). This global transition is associated with significant sea-level drop, major aridification and a shift to more pronounced seasonality in rainfall as compared with the preceding warmer and more humid later Eocene (Berggren and Prothero, 1992; Miller et al., 2005; Guo et al., 2008). Although a more northerly inter-tropical convergence zone is thought to have delivered generally higher precipitation and thus wetter conditions to a band stretching across northern Africa and the Tethys oceans (Couvreur et al., 2021), the resulting expansion of savanna-like grassland in Africa and southern Eurasia, as well as subtropical woody savanna in central and southern China, is postulated to have led to fragmentation of closed tropical forest across these land masses (Morley, 2007; Guo et al., 2008). The advent of hysteranthy in the Nervilia lineage could thus be intrinsically linked to this period of increased seasonality in rainfall and lower mean temperatures, with the development of more open habitats at low to middle latitudes potentially offering distinct advantages for a terrestrial, seasonally dormant habit. Though the Early Oligocene is generally viewed as a time of widespread extinction of terrestrial biodiversity (Berggren and Prothero, 1992), compelling evidence for the first appearance of, and diversification within, numerous plant lineages at this time is accumulating (e.g. Zhou et al., 2012; Couvreur et al., 2021; Xue et al., 2024).
4.2 Both incremental inter-continental spread and long-range migration underpin the occurrence of Nervilia today
Progressive northward drift of the African plate through the Oligocene resulted in reconnection with Eurasia in the Middle Miocene (ca. 19–15 Mya) via formation of the Gomphotherium land bridge and eventual closure of the east Tethys Seaway ca. 14 Mya (Hamon et al., 2013; Couvreur et al., 2021). The Arabian plate, which had been contiguous with Africa throughout the Cenozoic and remained so at this juncture, is thought to have supported woodland and savanna ecosystems comprising warm and wet-adapted elements prior to undergoing aridification once in its modern position from the Late Miocene onwards (Steinthorsdottir et al., 2021). In light of this tectonic-cum-palaeoclimatic sequence and the phylogenetic chronology presented here, it seems reasonable to deduce that the very limited occurrence of Nervilia in the Arabian Peninsula today, with only the widespread Afro-Malagasy N. bicarinata being found in isolated parts of Yemen and Oman (Pettersson, 1991), is relictual and plausibly the result of climate-induced vicariance, as has been inferred in the biogeography of numerous sub-Saharan African lineages (Couvreur et al., 2021). The presence of Nervilia in seasonal tropical Asia within the last 10 Mya – apparently in the form of all three sections of the genus – might therefore be congruent with incremental spread through open woodland across the Gomphotherium land bridge to the Indian subcontinent, and from there to continental Southeast Asia, a pattern of incremental inter-continental migration that has been invoked in the dispersal of many ‘out-of-Africa’ palaeotropical taxa, including members of the disparate families Annonaceae, Asparagaceae and Hyacinthaceae (Zhou et al., 2012; Ali et al., 2013; Howard et al., 2022).
The prevailing occurrence of the African Nervilia species in deciduous and semi-deciduous forest, woodland savanna and grassland today (Pettersson, 1991) further hints towards an ancestral association with seasonally arid landscapes. In contrast, the species of topical Asia occur in a wider range of habitats, encompassing grassland and sparse forest types (Roxburgh, 1832; Su, 2000; Gale et al., 2014) but favouring closed-canopy communities, including semi-evergreen, mixed deciduous (or monsoon) and montane forest (e.g. Gale et al., 2015, 2018; Gale and Phaxaysombath, 2017), as well as true lowland rainforest (e.g. Smith, 1909, 1918). Expansion through continental and insular tropical Asia therefore appears to have gone hand-in-hand with extensive niche differentiation, including colonisation of the dark, moist, evergreen forest understorey. The recent discovery of partial mycoheterotrophy in N. nipponica, an Asian, forest-dwelling member of section Linervia in which reliance on fungal partners is most pronounced at lower light intensities (Nomura et al., 2013; Gale et al., 2021), invites closer scrutiny of the eco-physiological factors that could have facilitated this radiation, whether underpinned by vicariance or geodispersal. By combining carbon gain measurements with phylogenetic analysis of a cross-section of the genus representative of different habitat types in both Africa and Asia, it would be possible to address climate-linked landscape-scale patterns of divergence in light of the evolution of variable mixotrophy. But perhaps even more tellingly in this respect is the apparent loss of hysteranthy in a few derived Asiatic species – N. borneensis J.J.Sm., N. muratana and N. kasiensis – which produce successive, temporally overlapping flowering and leafing shoots along a persistent stolon (Smith, 1909; Gale and Wu, 2007; Gale and Phaxaysombath, 2017), implicating exceptional adaptive convergence that warrants finer phylogenetic reconstruction using next generation sequencing.
Whilst a comparatively ‘short hop’ overland from Africa to Arabia and onto seasonal tropical Asia via India therefore seems plausible and parsimonious in the palaeoclimatic contexts of the Middle to Late Miocene, our ancestral area analysis points to further, more complex patterns of migration thereafter. All three sections of the genus bear the same signature of recent arrival in moist tropical Asia and Oceania, as evidenced by the appearance within the past ca. 2.8 million years of section Vinerlia (represented here by N. plicata and N. platychila) in Malesia, Micronesia, New Caledonia and Fiji, section Nervilia (represented here by N. concolor and N. campestris/N. holochila) in Malesia, New Guinea, tropical Australia and the Southwest Pacific, and various species of section Linervia at various locations throughout this vast region. This timing broadly coincides with the Pliocene-Pleistocene boundary, a period of further global cooling, decreasing availability of growing season moisture and forest fragmentation (Couvreur et al., 2021; Steinthorsdottir et al., 2021). The geographic (and taxonomic) expansion of Nervilia across these land masses can probably be attributed at least in part to emergence of the Sunda shelf, since a terrestrial Sundaland was a consistent feature of the Cenozoic at least until the early Pliocene (5 Mya; Hall, 2009) with subsequent exposure occurring episodically through the Pleistocene (Voris, 2000; Sarr et al., 2019). However, permanent separation of Sundaland from both Wallacea and Oceania (Hall, 2009) implicates longer range onward dispersal in at least those lineages that gave rise to N. platychila, N. holochila, N. palawensis and, independently, two undescribed species (sp. nov. 2 and 3) both found on Malaita in the Solomon Islands, as well as in the lineage that gave rise to N. punctata, apparently through migration from Oceania ‘back’ to Malesia. Moreover, the surprising placement of the African N. adolphi within the overwhelmingly Asian ‘N. adolphi–punctata alliance’ of section Linervia is indicative of a somewhat deeper, long-range dispersal back to Africa, meriting further investigation of the origin and spread of the few other Afro-Malagasy members of this complex not included in this study (in particular, N. fuerstenbergiana Schltr. and N. subintegra Summerh.). Evidence of similar long-range dispersal from tropical Asia to Africa during the late Miocene has been uncovered in other plant groups with a marked Africa-Asia-Australasia disjunction (e.g. Li et al., 2009), though not yet, to our knowledge, in the Orchidaceae. We contend that the minute, mobile Orchidaceous dust seed could have been instrumental in facilitating both the stepwise spread and longer distance migrations uncovered here (McCormick and Jacquemyn, 2014; Givnish et al., 2016).
4.3 Recent diversification and the prevalence of cryptic species boundaries
One the most striking features in the evolution of the genus, however, is the enormous taxonomic diversification that appears to have occurred from around 8 Mya, notably in section Linervia and particularly in seasonal tropical Asia. This timing and regionalisation coincide with the most active phase in the uplift of the Himalaya-Tibetan Plateau, which precipitated significant intensification of the Indian and East Asian monsoons (Zhisheng et al., 2001). Replacing Oligocene subtropical aridity (Guo et al., 2008), the evolution of this atmospheric system is tightly correlated with phased Himalayan orogeny through the Miocene, transforming the geography and biology of the continent through alternating circulations of moist, oceanic air during the summer and dry, inland air during the winter (Li et al., 2015; Nguyen et al., 2024). This process is believed to have reached its zenith by around 3.6–2.6 Mya, although the East Asia winter monsoon continued to strengthen thereafter (Zhisheng et al., 2001). Given that our results reveal both ongoing speciation and independent but broadly synchronous dispersal events between subtropical and tropical Asia and Oceania in all three sections of the genus from the upper Pliocene well into the Pleistocene, it is probable that monsoonal oscillations over tropical East Asia and Oceania played an important role in this dynamism, providing further evidence of the role of seasonality in the evolution of the genus as a whole. A similar explanation was proposed by Ji et al. (2024) in interpreting patterns of diversification within certain lineages of the orchid tribe Collabieae, and especially in the genus Calanthe R.Br.
The apparent link between rapid, recent diversification and the ubiquity of cryptic taxa across the genus warrants deeper examination. All three sections contain species complexes (Gale et al., 2016, 2018; Ketjarun et al., 2019) but the present study reveals the enormous geographic scale of their spread, mostly from the Pliocene onwards. Cursory appraisal of the multi-flowered N. concolor and allies in tropical Asia and Oceania has led to considerable taxonomic discord, with the name N. aragoana having been widely applied across Asia and the Pacific (e.g., Lewis and Cribb, 1989; Pearce and Cribb, 2002; Gale and Watthana, 2014) before being subsumed under the synonymy of the former without detailed analysis (POWO, 2024). Whilst our results support the recognition of a single though variable species ranging from southern Japan to Borneo and from the Western Ghats to the Society Islands, the inclusion also of N. carinata in its synonymy is unfounded, demanding critical review of its circumscription with respect to certain names not sampled in our study [e.g. N. scottii (Rchb.f.) Schltr. and N. tibetensis Rolfe; POWO, 2024]. Indeed, it is clear that this alliance harbours unrecognised diversity, given the presence of both morphologically distinct (N. sp. nov. 6) and anomalous (N. cf. concolor) entities here. Moreover, the placement of the strikingly different – and one-flowered – N. maculata (Figures 1N, N´) in this clade underscores the need for caution before lumping grossly similar ‘floral types’ together without fully evaluating finer characters and phylogenetic distance in the light of ecological differentiation.
The same may apply to the macrospecies N. plicata, which is here found to comprise a grade of morphologically diverse, continental Southeast Asian and insular tropical Asian and Pacific elements, the latter including N. platychila, with a degree of structure suggested among some samples within each of these two vast regions hinting at a possible link between biogeographic history and taxonomic divergence (Ketjarun et al., 2019). But on an even more remarkable scale, species diversity within the N. adolphi–punctata alliance appears to have been generated across the entire generic range predominantly within the last ca. 8 million years, and much of it far more recently than that. Despite vegetative uniformity, this complex patently still conceals cryptic taxa, including the five undescribed species sampled here. In contrast, N. simplex exhibits little genetic discontinuity across its enormous range, supporting the incorporation of both the continental Southeast Asian N. prainiana and Micronesian N. trichophylla despite the morphological disparities that have been used to define them (e.g. Seidenfaden, 1978). Next generation sequencing, as well as analyses of polyploidy, reticulate evolution and possible hybridisation (Chennaveeraiah and Jorapur, 1966; Gale et al., 2015) and introgression, are recommended to further disentangle the evolutionary history of these taxonomically intractable lineages. In addition, since knowledge of pollination biology in Nervilia remains fragmentary (Pettersson, 1989; Gale, 2007), clarification of taxonomic, ecological and geographic biases in rewarding, deceptive and autogamous systems, for example, could further shed light on how floral divergence and pollinator shifts have shaped speciation and biogeographic spread (Ackerman et al., 2023).
4.4 Taxonomic implications
That said, sufficient clarity is already achieved to draw several taxonomic conclusions. Firstly, though flower number is confirmed as an unreliable basis for defining a sectional classification of the genus, the three presently recognised sections are nevertheless clearly natural. Section Vinerlia occurs only in Asia and Oceania, not Africa and Madagascar, and is typified by the widespread N. plicata. Higher resolution, integrated phylogenetic and morphological research is needed to ascertain whether N. platychila can be maintained as distinct from that species, possibly reflecting a biogeographic split between insular tropical Asia plus Oceania on the one hand, versus inland, continental Asia on the other. Though both are two-flowered, the pubescent, reniform leaf and longitudinally folded labellum are diagnostic. Examination of other species that probably belong here, including N. ignobilis Tuyama and N. umenoi Fukuyama, is needed to better define the section. Pridgeon et al. (2005) list N. maculata as the type of section Vinerlia, but that species is here unequivocally placed in section Nervilia.
Section Nervilia occurs throughout the range of the genus and is highly variable not only in flower number, but also in terms of leaf shape and indumentum, as well as floral morphology. Pettersson (1991) used two labellum characters to define the section in Africa – the presence of nectar guides and a recurved mid-lobe – but these do not apply outside that continent. He also referred to a possible distinction in pollination ecology, with Eumenid wasps known to pollinate two African species (N. bicarinata and N. shirensis), but no pollination studies have yet been conducted on Asian or Australasian members of the section to either confirm or refute this as a reliable sectional trait. Therefore, though the African species appear to be monophyletic and sister to all remaining members, section Nervilia lacks a clear synapomorphy at present. As concluded elsewhere (POWO, 2024), we confirm that N. campestris is most likely conspecific with N. holochila, presenting an intriguing case of vicariance across Wallace’s Line, albeit highly localised to Java on the western side. Despite wide morphological variation throughout its enormous range, N. concolor is monophyletic and there is little evidence of internal genetic structure. However, N. carinata is not conspecific.
Section Linervia comprises two natural sub-groups: the fimbriate-lipped species typified by the extremely widespread N. simplex plus the spurred African species (represented here by N. stolziana) previously placed in section Kyimbilaea, and those species with an entire labellum mid-lobe that constitute the N. adolphi–punctata alliance. Within the latter, all four N. viridis samples included here were found to be monophyletic and almost certainly conspecific, even though Gale et al. (2018) refrained from combining the two “N. cf. viridis” samples from eastern Thailand on the grounds that Bayesian coalescence analysis resolved them as distinct. Intriguingly, this continental Asiatic species falls sister to an unnamed species from the Solomon Islands. The Himalayan N. macroglossa and southern Japanese N. futago present another interesting disjunction, potentially alluding to historic extinction of other closely related, geographically contiguous taxa, as suggested by the selected BioGeoBEARS model. The widespread continental Asian N. infundibulfolia exhibits considerable internal genetic structure worthy of further examination but is monophyletic only if N. hemratii is considered synonymous. Nervilia punctata is here placed in a clade with the Micronesian N. palawensis and an unnamed species from the Solomon Islands. Our analyses corroborate Gale et al. (2018) in determining N. punctata to be Malesian, with prior records of this entity from continental Southeast Asia (e.g. Seidenfaden, 1978; Gale and Watthana, 2014) probably amounting to misidentifications of N. mackinnonii or other members of this problematic complex. Though the section is overwhelmingly one-flowered, the two- or rarely three-flowered N. cumberlegei also belongs here, and thus the only synapomorphy for the section appears to be the elongating fruiting scape (Pettersson, 1991; Gale et al., 2006).
5 Conclusions
The cumulative effects of multiple dispersal events coupled with isolation through extinction or vicariance here emerge as predominant drivers shaping the current geographical distribution of species within Nervilia. Africa is singled out as the probable ancestral centre (or ‘cradle’) of the genus as well as that of sections Nervilia and Linervia, whilst seasonal tropical Asia is identified as a radiative reservoir (or ‘museum’) of species diversity, especially for section Linervia, and probably gave rise to section Vinerlia. Despite the relatively ancient origin of the genus as a whole, speciation appears to have accelerated from the Late Miocene onwards, correlating to Himalayan uplift and intensification of the Asian monsoon. Other than the widespread macrospecies N. concolor, N. plicata and N. simplex, most species probably arose through speciation within areas, with high levels of regional endemism.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
SG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JL: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SS: Investigation, Resources, Writing – review & editing. PT: Investigation, Methodology, Writing – review & editing. CP: Investigation, Methodology, Project administration, Resources, Writing – review & editing. TB: Investigation, Methodology, Resources, Writing – review & editing. BC: Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Project administration, Writing – review & editing. MM: Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing. DW: Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing. AM: Formal analysis, Investigation, Methodology, Resources, Writing – review & editing. KG: Investigation, Methodology, Resources, Writing – review & editing. KI: Investigation, Methodology, Writing – review & editing. YM: Investigation, Methodology, Writing – review & editing. TF: Investigation, Methodology, Resources, Supervision, Writing – review & editing. SL: Investigation, Methodology, Writing – review & editing. TY: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the authors’ institutions. The USDA Institute of Pacific Islands Forestry provided funding for collections in Palau by BC and MKM via a collaboration between the Smithsonian’s North American Orchid Conservation Center, the Scholarly Studies Program, the American Orchid Society and the U.S. Forest Service.
Acknowledgments
We are extremely grateful to Philip Cribb, Boris Schlumpberger, Leonid Averyanov, Larry Zettler, staff at the Orchid Recovery Program at Illinois College and the late Jeffrey Wood for assistance with sampling. Numerous government and traditional leaders in Palau, as well as staff at the Palauan Protected Areas Network, are gratefully acknowledged for organising research permits and facilitating field work. Relevant authorities are acknowledged for providing permission to collect samples and for assistance with obtaining CITES paperwork where necessary. The following people are thanked for allowing us to reproduce their images in Figure 1: Phillip Cribb (A, A'), Narong Jirawatkavi (D, D'), Santi Watthana (E, K), Isobyl la Croix (G, G'), Shih-Wen Chung (H), Obchang Thaithong (J, J'), Boris Schlumpberger (L, L')and Indri Arina Khasanati (M, M'). Mang Lung Cheuk is thanked for helping to prepare the map used in Figure 3. This paper is dedicated to the memory of Anthony Lamb and Tetsuo Koyama, whose steadfast and heartfelt support of this project from the outset laid the foundation for its eventual completion.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1495487/full#supplementary-material
References
Ackerman, J. D., Phillips, R. D., Tremblay, R. L., Karremans, A., Reiter, N., Peter, C. I., et al. (2023). Beyond the various contrivances by which orchids are pollinated: global patterns in orchid pollination biology. Bot. J. Linn. Soc 202, 295–324. doi: 10.1093/botlinnean/boac082
Ali, S. S., Pfosser, M., Wetschnig, W., Martínez-Azorín, M., Crespo, M. B., Yu, Y. (2013). Out of Africa: Miocene dispersal, vicariance, and extinction within Hyacinthaceae subfamily Urgineoideae. J. Integr. Plant Biol. 55, 950–964. doi: 10.1111/jipb.v55.10
Berggren, W. A., Prothero, D. R. (1992). “Eocene-Oligocene climatic and biotic evolution: An overview,” in Eocene-Oligocene Climatic and Biotic Evolution. Eds. Berggren, W. A., Prothero, D. R. (Princeton University Press, Princeton), 1–28.
Bouckaert, R., Vaughan, T. G., Barido-Sottani, J., Duchêne, S., Fourment, M., Gavryushkina, A., et al. (2019). BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 15, e1006650. doi: 10.1371/journal.pcbi.1006650
Burnham, K. P., Anderson, D. R. (2002). Model Selection and Multimodel Inference: A Practical Information Theoretic Approach. 2nd ed (New York: Springer Verlag).
Chase, M. W., Cameron, K. M., Barrett, R. L., Freudenstein, J. V. (2003). “DNA data and Orchidaceae systematics: A new phylogenetic classification,” in Orchid Conservation. Eds. Dixon, K. M., Kell, S. P., Barrett, R. L., Cribb, P. J. (Natural History Publications, Borneo, Kota Kinabalu), 69–89.
Chase, M. W., Cameron, K. M., Freudenstein, J. V., Pridgeon, A. M., Salazar, G., van den Berg, C., et al. (2015). An updated classification of Orchidaceae. Bot. J. Linn. Soc 177, 151–174. doi: 10.1111/boj.12234
Chase, M. W., de Bruijn, A. Y., Cox, A. V., Reeves, G., Rudall, P. J., Johnson, M. A. T., et al. (2000). Phylogenetics of Asphodelaceae (Asparagales): An analysis of plastid rbcL and trnL-F DNA sequences. Ann. Bot. 86, 935–951. doi: 10.1006/anbo.2000.1262
Chen, S.-C., Gale, S. W. (2009). Nervilia, in Flora of China Vol. 25. Eds. Wu, Z.-Y., Raven, P. H., Hong, D.-Y. (Beijing: Science Press and St. Louis: Missouri Botanical Garden Press), 197–120.
Chennaveeraiah, M. S., Jorapur, S. M. (1966). Chromosome number and morphology in five species of Nervilia Gaud. Nucleus 9, 39–44.
Couvreur, T. L. P., Dauby, G., Blach-Overgaard, A., Deblauwe, V., Dessein, S., Droisssart, V., et al. (2021). Tectonics, climate and the diversification of the tropical African flora and fauna. Biol. Rev. 96, 16–51. doi: 10.1111/brv.12644
Coxall, H. K., Pearson, P. N. (2007). “The eocene-oligocene transition,” in Deep-Time Perspectives on Climate Change: Marrying the Signal from Computer Models and Biological Proxies. Eds. Williams, M., Haywood, A. M., Gregory, F. J., Schmidt, D. N. (The Geological Society, London), 351–387.
Cunningham, C. W. (1997). Can three incongruence tests predict when data should be combined? Mol. Biol. Evol. 14, 733–740. doi: 10.1093/oxfordjournals.molbev.a025813
Darlu, P., Lecointre, G. (2002). When does the incongruence length difference test fail? Mol. Biol. Evol. 19, 432–437. doi: 10.1093/oxfordjournals.molbev.a004098
Dressler, R. L. (1993). Phylogeny and Classification of the Orchid Family (Cambridge: Cambridge University Press).
Farris, J. S., Källersjö, M., Kluge, A., Bult, G. C. (1995). Constructing a significance test for incongruence. Syst. Biol. 44, 570–572. doi: 10.2307/2413663
Feng, Y.-L., Wicke, S., Li, J.-W., Han, Y., Lin, C.-S., Li, D.-Z., et al. (2016). Lineage-specific reductions of plastid genomes in an orchid tribe with partially and fully mycoheterotrophic species. Genome Biol. Evol. 8, 2164–2175. doi: 10.1093/gbe/evw144
Freudenstein, J. V., Chase, M. W. (2015). Phylogenetic relationships in Epidendroideae (Orchidaceae), one of the great flowering plant radiations: progressive specialization and diversification. Ann. Bot. 115, 665–681. doi: 10.1093/aob/mcu253
Gale, S. (2007). Autogamous seed set in a critically endangered orchid in Japan: pollination studies for the conservation of Nervilia nipponica. Plant Syst. Evol. 268, 59–73. doi: 10.1007/s00606-007-0570-x
Gale, S. W., Duangjai, S., Li, J., Ito, Y., Watthana, S., Termwutthipreecha, P., et al. (2018). Integrative analyses of Nervilia (Orchidaceae) section Linervia reveal further undescribed cryptic diversity in Thailand. Syst. Biodivers. 16, 377–396. doi: 10.1080/14772000.2017.1415233
Gale, S. W., Li, J., Kinoshita, A., Yukawa, T. (2015). Studies in Asian Nervilia (Orchidaceae) V: N. futago, a cryptic new species from southwest Japan confirmed by morphological, cytological and molecular analyses. Syst. Bot. 40, 413–425. doi: 10.1600/036364415X688772
Gale, S. W., Madea, A., Miyashita, A., Sugiura, D., Ogura-Tsujita, Y., Kinoshita, A., et al. (2021). International biological flora: Nervilia nipponica. J. Ecol. 109, 2780–2799. doi: 10.1111/1365-2745.13683
Gale, S. W., Maeda, A., Chen, C. I., Yukawa, T. (2010). Inter-specific relationships and hierarchical spatial genetic structuring in Nervilia nipponica, an endangered orchid in Japan. J. Plant Res. 123, 625–637. doi: 10.1007/s10265-010-0314-9
Gale, S. W., Maeda, A., Kuroiwa, N. (2006). Observations on the phenology and reproductive success of the critically endangered Nervilia nipponica (Orchidaceae) in Kochi Prefecture, Japan. Acta Phytotax. Geobot. 57, 81–93.
Gale, S. W., Phaxaysombath, T. (2017). Studies in Asian Nervilia (Orchidaceae) VII: N. kasiensis, a new Lao endemic. Blumea 62, 1–5. doi: 10.3767/000651917X694732
Gale, S. W., Rueangruea, S., Suddee, S. (2014). Studies in Asian Nervilia (Nervilieae, Epidendroideae, Orchidaceae) IV: N. umphangensis, a new species from the Thai-Myanmar border. Phytotaxa 166, 139–144. doi: 10.11646/phytotaxa.166.2.5
Gale, S. W., Schuiteman, A., Watthana, S., Sando, T., Souvannakhoummane, K., Averyanov, L., et al. (2016). Studies in Asian Nervilia (Nervilieae, Epidendroideae, Orchidaceae) VI: N. mekongensis, a new species from Thailand, Cambodia, Laos and Vietnam. Phytotaxa 247, 267–273. doi: 10.11646/phytotaxa.247.4.4
Gale, S. W., Tetsana, N., Suddee, S. (2022). Studies in Asian Nervilia (Orchidaceae) VIII: N. hemratii, another new member of section Linervia from Thailand. Kew Bull. 77, 569–574. doi: 10.1007/s12225-022-10024-5
Gale, S. W., Watthana, S. (2014). Nervilia, in Flora of Thailand Vol. 12. Eds. Santisuk, T., Balslev, H. (Bangkok: Forest Herbarium, Department of National Parks, Wildlife and Plant Conservation), 553–569.
Gale, S., Wu, S. K. (2007). Studies in Asian Nervilia (Orchidaceae) II: N. muratana, a new species from southern Yunnan, China. Makinoa New Ser. 7, 79–86.
Gale, S., Yukawa, T., Kuroiwa, N. (2007). Studies in Asian Nervilia (Orchidaceae) I: Neotypification and circumscription of N. nipponica in Japan. Kew Bull. 62, 85–94.
Givnish, T. J., Spalink, D., Ames, M., Lyon, S. P., Hunter, S. J., Zuluaga, A., et al. (2016). Orchid historical biogeography, diversification, Antarctica and the paradox of orchid dispersal. J. Biogeogr. 43, 1905–1916. doi: 10.1111/jbi.2016.43.issue-10
Givnish, T. J., Spalink, D., Ames, M., Lyon, S. P., Hunter, S. J., Zuluaga, A., et al. (2015). Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proc. R. Soc Lond. B 282, 20151553. doi: 10.1098/rspb.2015.1553
Górniak, M., Paun, O., Chase, M. W. (2010). Phylogenetic relationships within Orchidaceae based on a low-copy nuclear coding gene, Xdh: Congruence with organellar and nuclear ribosomal DNA results. Mol. Phylogenet. Evol. 56, 784–795. doi: 10.1016/j.ympev.2010.03.003
Guo, Z. T., Sun, B., Zhang, Z. S., Peng, S. Z., Xiao, G. Q., Ge, J. Y., et al. (2008). A major reorganization of Asian climate by the early Miocene. Climate Past 4, 153–174. doi: 10.5194/cp-4-153-2008
Hall, R. (2009). Southeast Asia’s changing palaeogeography. Blumea 54, 148–161. doi: 10.3767/000651909X475941
Hamon, N., Sepulchre, P., Lefebvre, V., Ramstein, G. (2013). The role of eastern Tethys seaway closure in the Middle Miocene Climatic Transition (ca. 14 Ma). Climate Past 9, 2687–2702. doi: 10.5194/cp-9-2687-2013
Hidayat, T., Yukawa, T., Ito, M. (2005). Molecular phylogenetics of subtribe Aeridinae (Orchidaceae): insights from plastid matK and nuclear ribosomal ITS sequences. J. Plant Res. 118, 271–284. doi: 10.1007/s10265-005-0217-3
Howard, C. C., Cellinese, N. (2020). Tunicate bulb size variation in monocots explained by temperature and phenology. Ecol. Evol. 10, 2299–2309. doi: 10.1002/ece3.v10.5
Howard, C. C., Crowl, A. A., Harvey, T. S., Cellinese, N. (2022). Peeling back the layers: First phylogenomic insights into the Ledebouriinae (Scilloideae, Asparagaceae). Mol. Phylogenet. Evol. 169, 107430. doi: 10.1016/j.ympev.2022.107430
Huelsenbeck, J. P., Ronquist, F. (2003). MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Ji, H.-Y., Ye, C., Chen, Y.-Q., Li, J.-W., Hidayat, A., Miao, J.-L., et al. (2024). Phylogenomics and biogeographical diversification of Collabieae (Orchidaceae) and its implications in the reconstruction of the dynamic history of Asian evergreen broadleaved forests. Mol. Phylogenet. Evol. 196, 108084. doi: 10.1016/j.ympev.2024.108084
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649. doi: 10.1093/bioinformatics/bts199
Ketjarun, K., Traiperm, P., Suddee, S., Watthana, S., Gale, S. W. (2019). Labellar anatomy of the Nervilia plicata complex (Orchidaceae: Epidendroideae) in tropical Asia. Kew Bull. 74, 1–13. doi: 10.1007/s12225-018-9788-8
Kumar, P., Li, J., Gale, S. W. (2022). Integrative analyses of Crepidium (Orchidaceae, Epidendroideae, Malaxideae) shed more light on its relationships with Dienia, Liparis and Malaxis and justify reinstatement of narrow endemic C. allanii. Bot. J. Linn. Soc 198, 285–305. doi: 10.1093/botlinnean/boab048
Lam, V. K. Y., Darby, H., Merckx, V. S. F. T., Lim, G., Yukawa, T., Neubig, K. M., et al. (2018). Phylogenomic inference in extremis: A case study with mycoheterotroph plastomes. Amer. J. Bot. 105, 480–494. doi: 10.1002/ajb2.2018.105.issue-3
Landis, M. J., Matzke, N. J., Moore, B. R., Huelsenbeck, J. P. (2013). Bayesian analysis of biogeography when the number of areas is large. Syst. Biol. 62, 789–804. doi: 10.1093/sysbio/syt040
Li, Y., Dressler, S., Zhang, D., Renner, S. S. (2009). More Miocene dispersal between Africa and Asia – The case of Bridelia (Phyllanthaceae). Syst. Bot. 34, 521–529. doi: 10.1600/036364409789271263
Li, J., Gale, S. W., Kumar, P., Zhang, J., Fischer, G. A. (2018). Prioritizing the orchids of a biodiversity hotspot for conservation based on phylogenetic history and extinction risk. Bot. J. Linn. Soc 186, 473–497. doi: 10.1093/botlinnean/box084
Li, Y.-X., Li, Z.-H., Schuiteman, A., Chase, M. W., Li, J.-W., Huang, W.-C., et al. (2019). Phylogenomics of Orchidaceae based on plastid and mitochondrial genomes. Mol. Phylogenet. Evol. 139, 106540. doi: 10.1016/j.ympev.2019.106540
Li, M. H., Liu, K. W., Li, Z., Lu, H. C., Ye, Q. L., Zhang, D., et al. (2022). Genomes of leafy and leafless Platanthera orchids illuminate the evolution of mycoheterotrophy. Nat. Plants 8, 373–388. doi: 10.1038/s41477-022-01127-9
Li, J. H., Liu, Z. J., Salazar, G. A., Bernhardt, P., Perner, H., Yukawa, T., et al. (2011). Molecular phylogeny of Cypripedium (Orchidaceae: Cypripedioideae) inferred from multiple nuclear and chloroplast regions. Mol. Phylogenet. Evol. 61, 308–320. doi: 10.1016/j.ympev.2011.06.006
Li, S. F., Mao, L. M., Spicer, R. A., Lebreton-Anberrée, J., Su, T., Sun, M., et al. (2015). Late Miocene vegetation dynamics under monsoonal climate in southwestern China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 425, 14–40. doi: 10.1016/j.palaeo.2015.02.030
Liu, Z., Pagani, M., Zinniker, D., DeConto, R., Huber, M., Brinkhuis, H., et al. (2009). Global cooling during the Eocene-Oligocene climate transition. Science 323, 1187–1190. doi: 10.1126/science.1166368
Martínez-Azorín, M., Crespo, M. B., Juan, A., Fay, M. F. (2011). Molecular phylogenetics of subfamily Ornithogaloideae (Hyacinthaceae) based on nuclear and plastid DNA regions, including a new taxonomic arrangement. Ann. Bot. 107, 1–37. doi: 10.1093/aob/mcq207
Matzke, N. J. (2016).Stochastic mapping under biogeographical models: PhyloWiki BioGeoBEARS. Available online at: http://phylo.wikidot.com/biogeobearsstochastic_mapping. (accessed April 18, 2024)
McCormick, M. K., Jacquemyn, H. (2014). What constrains the distribution of orchid populations? New Phytol. 202, 392–400. doi: 10.1111/nph.12639
Miller, K. G., Kominz, M. A., Browning, J. V., Wright, J. D., Mountain, G. S., Katz, M. E., et al. (2005). The Phanerozoic record of global sea-level change. Science 310, 1293–1298. doi: 10.1126/science.1116412
Morley, R. J. (2007). “Cretaceous and tertiary climate change and the past distribution of megathermal rainforests,” in Tropical Rainforest Responses to Climatic Changes. Eds. Bush, M. B., Flenley, J. (Praxis Publishing, Chichester), 1–31.
Nguyen, H. B., Huang, J., Van Do, T., Nguyen, H. M. T., Li, S. F., Nguyen, M. T., et al. (2024). Monsoon influence on plant diversity in northern IndoChina: Evidence from the late Miocene Yen Bai flora, northern Vietnam. Palaeogeogr. Palaeoclimatol. Palaeoecol. 634, 111925. doi: 10.1016/j.palaeo.2023.111925
Niissalo, M. A., Choo, L. M., Kurzweil, H., Yam, T. W., Khew, G. S. (2020). A new species of Nervilia (Orchidaceae) from Singapore. Gard. Bull. Singapore 72, 1–14. doi: 10.26492/gbs72(1).2020-01
Nomura, N., Ogura-Tsujita, Y., Gale, S. W., Maeda, A., Umata, H., Hosaka, K., et al. (2013). The rare terrestrial orchid Nervilia nipponica consistently associates with a single group of novel mycobionts. J. Plant Res. 126, 613–623. doi: 10.1007/s10265-013-0552-8
Nusbauer, L., Cribb, P., Gautier, L. (2011). Nervilia gassneri Börge Oett. from Africa is conspecific with the Malagasy N. lilacea Jum. & H.Perrier. Candollea 66, 127–139. doi: 10.15553/c2011v661a14
Pérez-Escobar, O. A., Dodsworth, S., Bogarín, D., Bellot, S., Balbuena, J. A., Schley, R. J., et al. (2021). Hundreds of nuclear and plastid loci yield novel insights into orchid relationships. Amer. J. Bot. 108, 1166–1180. doi: 10.1002/ajb2.v108.7
Pearce, N. R., Cribb, P. J. (2002). Orchids of Bhutan (Edinburgh: Royal Botanic Gardens, Edinburgh).
Pettersson, B. (1989). Pollination in the African species of Nervilia (Orchidaceae). Lindleyana 4, 33–41.
Pettersson, B. (1990). Studies in the genus Nervilia (Orchidaceae) in Africa. Nord. J. Bot. 9, 487–497. doi: 10.1111/j.1756-1051.1990.tb00539.x
Pettersson, B. (1991). “The Genus Nervilia (Orchidaceae) in Africa and the Arabian Peninsula,” in Orchid Monographs, vol. 5. (Rijksherbarium/Hortus Botanicus, Leiden).
Posada, D., Crandall, K. A. (1998). MODELTEST: Testing the model of DNA substitution. Bioinformatics 14, 817–818. doi: 10.1093/bioinformatics/14.9.817
POWO (2024). Plants of the World Online (Kew: Facilitated by the Royal Botanic Gardens). Available at: https://powo.science.kew.org.
Pridgeon, A. M., Cribb, P. J., Chase, M. W., Rasmussen, F. N. (2005). “Epidendroideae (Part One),” in Genera orchidacearum, vol. 4. (Oxford University Press, Oxford).
R Core Team (2023). R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna). Available at: https://www.r-project.org/.
Ree, R. H., Smith, S. A. (2008). Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst. Biol. 57, 4–14. doi: 10.1080/10635150701883881
Ronquist, F. (1997). Dispersal – vicariance analysis: a new approach to the quantification of historical biogeography. Syst. Biol. 46, 195–203. doi: 10.1093/sysbio/46.1.195
Rothacker, E. P. (2007). The primitive Epidendroideae (Orchidaceae): Phylogeny, character evolution and the systematics of Psilochilus (Triphoreae). Unpublished doctoral dissertation (Ohio State University, USA).
Sarr, A.-C., Husson, L., Sepulchre, P., Pastier, A.-W., Pedoja, K., Elliot, M., et al. (2019). Subsiding sundaland. Geology 47, 119–122. doi: 10.1130/G45629.1
Schlechter, R. (1911). Die Polychondreae (Neottiinae Pfitz.) und ihre systematische Einteilung. Bot. Jahrb. Syst. 45, 375–410.
Seidenfaden, G. (1978). Orchid genera in Thailand VI. Neottioideae lindl. Dansk Bot. Ark. 32, 1–196.
Seidenfaden, G. H., Smitinand, T. (1959–1965). The Orchids of Thailand: A Preliminary List (Bangkok: The Siam Society).
Smith, J. J. (1909). Neue Orchideen des malaiischen Archipels, III. Bull. Dépt. Agric. Indes Néerl. 22, 1–51.
Steinthorsdottir, M., Coxall, H. K., De Boer, A. M., Huber, M., Barbolini, N., Bradshaw, C. D., et al. (2021). The Miocene: The future of the past. Paleoceanogr. Paleoclimatol. 36, e2020PA004037. doi: 10.1029/2020PA004037
Su, H. J. (2000). “Orchidaceae,” in Flora of Taiwan, vol. 5 . Ed. Huang, T.-C. (National Taiwan University, Taipei), 729–1086.
Sullivan, J. (1996). Combining data with different distributions of among-site rate variation. Syst. Biol. 45, 375–380. doi: 10.1093/sysbio/45.3.375
Sun, Y., Skinner, D. Z., Liang, G. H., Hulbert, S. H. (1994). Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theor. Appl. Genet. 89, 23–32. doi: 10.1007/BF00226978
Swofford, D. L. (2003). PAUP* Phylogenetic Analysis Using Parsimony (and other methods). version 4 (Sunderland: Sinauer Associates).
Taberlet, P. L., Geilly, L., Pautou, G., Bouvet, J. (1991). Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 17, 1105–1109. doi: 10.1007/BF00037152
Voris, H. K. (2000). Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. J. Biogeogr. 27, 1153–1167. doi: 10.1046/j.1365-2699.2000.00489.x
Wen, Y., Qin, Y., Shao, B., Li, J., Ma, C., Liu, Y., et al. (2022). The extremely reduced, diverged and reconfigured plastomes of the largest mycoheterotrophic orchid lineage. BMC Plant Biol. 22, 448. doi: 10.1186/s12870-022-03836-x
White, T. J., Bruns, T., Lee, S., Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR Protocols: A Guide to Methods and Application. Eds. Innis, M., Gelfand, D., Sninsky, J., White, T. J. (Academic Press, San Diego), 315–322.
Xue, B., Huang, E., Zhao, G., Wei, R., Song, Z., Zhang, X., et al. (2024). [amp]]lsquo;Out of Africa’ origin of the pantropical staghorn fern genus Platycerium (Polypodiaceae) supported by plastid phylogenomics and biogeographical analysis. Ann. Bot. 133, 697–710. doi: 10.1093/aob/mcae003
Yoder, A. D., Irwin, J. A., Payseur, B. A. (2001). Failure of the ILD to determine data combinability for slow loris phylogeny. Syst. Biol. 50, 408–424. doi: 10.1080/106351501300318003
Zhisheng, A., Kutzbach, J. E., Prell, W. L., Porter, S. C. (2001). Evolution of Asian monsoon and phased uplift of the Himalaya-Tibetan plateau since Late Miocene times. Nature 411, 62–66. doi: 10.1038/35075035
Keywords: Asia monsoon, diversification, hysteranthy, lower Epidendroideae, out-of-Africa, species complex
Citation: Gale SW, Li J, Suddee S, Traiperm P, Peter CI, Buruwate T, Crain BJ, McCormick MK, Whigham DF, Musthofa A, Gogoi K, Ito K, Minamiya Y, Fukuda T, Landrein S and Yukawa T (2025) Molecular phylogenetic analyses reveal multiple long-distance dispersal events and extensive cryptic speciation in Nervilia (Orchidaceae), an isolated basal Epidendroid genus. Front. Plant Sci. 15:1495487. doi: 10.3389/fpls.2024.1495487
Received: 12 September 2024; Accepted: 11 November 2024;
Published: 20 February 2025.
Edited by:
Xiaohua Jin, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Cássio Van Den Berg, State University of Feira de Santana, BrazilJunwen Zhai, Fujian Agriculture and Forestry University, China
Copyright © 2025 Gale, Li, Suddee, Traiperm, Peter, Buruwate, Crain, McCormick, Whigham, Musthofa, Gogoi, Ito, Minamiya, Fukuda, Landrein and Yukawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephan W. Gale, c3RlcGhhbmdhbGVAa2ZiZy5vcmc=
 Stephan W. Gale
Stephan W. Gale Jihong Li
Jihong Li Somran Suddee
Somran Suddee Paweena Traiperm3
Paweena Traiperm3 Craig I. Peter
Craig I. Peter Tomas Buruwate
Tomas Buruwate Melissa K. McCormick
Melissa K. McCormick Dennis F. Whigham
Dennis F. Whigham Arni Musthofa
Arni Musthofa Katsura Ito
Katsura Ito Sven Landrein
Sven Landrein