- 1College of Agronomy and Biotechnology, Southwest University/Engineering Research Center of South Upland Agriculture, Ministry of Education, Chongqing, China
- 2Institute of Grain Crops, Agricultural Science Extension Research Institute of Dali Bai Autonomous Prefecture, Dali, Yunnan, China
- 3Dali Prefecture Branch of Yunnan Tobacco Company, Dali, Yunnan, China
- 4Yunnan Agricultural University, Kunming, Yunnan, China
With the intensification of global climate change, high-temperature and drought stress have emerged as critical environmental stressors affecting tobacco plants’ growth, development, and yield. This study provides a comprehensive review of tobacco’s physiological and biochemical responses to optimal temperature conditions and limited irrigation across various growth stages. It assesses the effects of these conditions on yield and quality, along with the synergistic interactions and molecular mechanisms associated with these stressors. High-temperature and drought stress induces alterations in both enzymatic and non-enzymatic antioxidant activities, lead to the accumulation of reactive oxygen species (ROS), and promote lipid peroxidation, all of which adversely impact physiological processes such as photosynthetic gas exchange, respiration, and nitrogen metabolism, ultimately resulting in reduced biomass, productivity, and quality. The interaction of these stressors activates novel plant defense mechanisms, contributing to exacerbated synergistic damage. Optimal temperature conditions enhance the activation of heat shock proteins (HSPs) and antioxidant-related genes at the molecular level. At the same time, water stress triggers the expression of genes regulated by both abscisic acid-dependent and independent signaling pathways. This review also discusses contemporary agricultural management strategies, applications of genetic engineering, and biotechnological and molecular breeding methods designed to mitigate adverse agroclimatic responses, focusing on enhancing tobacco production under heat and drought stress conditions.
Introduction
With the intensification of global climate change, the frequency and intensity of extreme weather events are gradually increasing, with heat and drought stress becoming the primary abiotic stress factors affecting plant growth, development, productivity and crop quality (Hossain et al., 2012; Lobell et al., 2015). According to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC), global temperatures have increased by approximately 0.69–1.08°C over the past century, with projections indicating a further rise of 0.3–4.8°C by the end of the 21st century. Concurrently, issues related to water scarcity and their uneven distributions have exacerbated the prevalence of drought. Tobacco is a significant economic cash crop, cultivated worldwide, and has various products, including cigarettes, cigars, and pipe tobacco (Van Liemt, 2001). The tobacco industry plays a vital role in enhancing national revenue and gross domestic product (GDP), fostering local economic development, and increasing tobacco farmers’ income while exerting a substantial influence on the global economy. However, tobacco production is susceptible to adverse agroclimatic conditions, and climate change has significantly affected its production with significant challenges (Fahad et al., 2017).
Heat and drought are the most common abiotic stress, significantly affecting physiological and metabolic processes and downregulating photosynthetic efficiency (Mohammadi et al., 2024). These stresses induce oxidative stress, leading to lipid peroxidation of cell membranes, protein denaturation, and nucleic acid damage, triggering molecular and biochemical responses (Figure 1). In recent years, extensive research has been conducted to investigate the effects of heat and drought stress on tobacco production and the underlying physiological mechanisms. This research encompasses various dimensions, including growth, development, physiological responses, yield, and quality (Farooq et al., 2009; Zhou et al., 2017; Hu et al., 2023). However, heat and drought stress often co-occur in practical agricultural production, exhibiting more complex interactions and synergistic effects, exacerbating plant stress responses and leading to more severe physiological damage and yield loss. The synergistic effects of heat and drought stress have not yet been fully explored (Lamaoui et al., 2018). Consequently, this article examines current research findings regarding the physio-biochemical and molecular mechanisms of action in response to heat and drought stress at various growth stages of tobacco plants and their effects on yield and quality. However, it discusses strategies for mitigating stress to enhance tobacco production’s resilience to heat and drought in the context of global climate change.
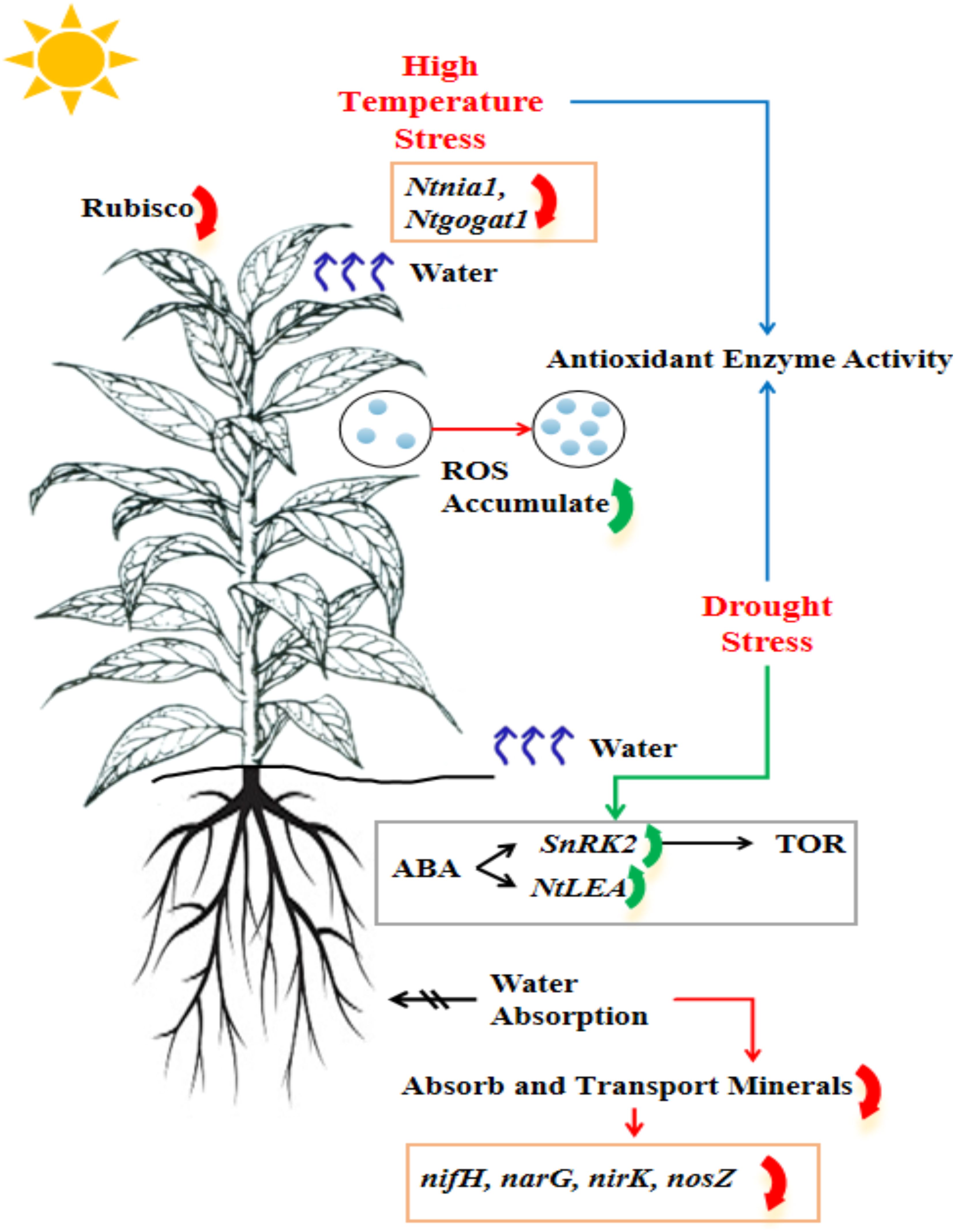
Figure 1. Influence of high temperature and limited water supply on tobacco plant production to tolerate these stress conditions.
Effects of heat and drought stress on the growth and development of tobacco plants
Severe drought stress and prolonged exposure to temperatures exceeding the optimal range can damage plant growth and development (Lipiec et al., 2013). Tobacco plants exhibit differential responses to temperature variations at different developmental stages. Specifically, when temperatures surpass 30°C, tobacco plants are susceptible to leaf scorching during the seedling stage, which manifests as dark yellow and wrinkled foliage (Pearce et al., 2015). During the rosette to vigorous growth stages, continuous high temperatures cause wilting, drooping, and scorching of the upper plant leaves, with the leaf tissue on one side of the midrib showing wilting, deformity, or necrotic spots. In severe conditions, the entire plant can wilt and die (Bittner et al., 2016). In the late growth stage, high temperatures reduce the plant growth rate, reduce the stratification of leaf yellowing, and may even cause premature ripening (Li et al., 2021).
Tobacco plants require sufficient water throughout their entire growth period, with different stages demanding varying water levels (Driedonks et al., 2016). The soil moisture below 50% of the field capacity affects tobacco growth and development, reducing yield and quality (Prasad et al., 2008). Insufficient soil moisture reduces the water absorption capacity of tobacco seeds, thereby downregulating metabolic and enzymatic activities, which extends germination time and frequency (Chen et al., 2016). Research findings showed that water deficiency significantly reduces the seed germination frequency and vigor index (Abrantes et al., 2019). However, agronomic traits such as seedling and root length and fresh and dry biomass are significantly reduced in response to drought stress than control conditions (Su et al., 2017). Research results indicate that if tobacco seedlings experience water stress during transplanting, their photosynthetic CO2 assimilation rate will significantly downregulate (Synková and Valcke, 2001). During the rooting stage, mild drought conditions lead to rapid root volume growth, enhanced fresh and dry mass, root activity and dry matter accumulation (Aharon et al., 2003). However, drought significantly inhibits root development during vigorous growth, resulting in a noticeable decline in root-related indicators and slowdown of dry matter accumulation (Kumar et al., 2021). Severe water shortage at any growth stage leads to poor root development, with dry matter being allocated higher to the roots than aboveground parts, increasing the root-to-shoot ratio (Martiniello and Teixeira da Silva, 2011). Tang et al. (2021) demonstrated that soil moisture content (SMC) positively correlates with the number of tobacco leaves and leaf area expansion. Insufficient moisture negatively impacts leaf development by reducing the total number of leaves, inducing premature ageing of lower leaves, causing upper leaves to shrink and thicken, and roughening leaf tissue. Additionally, it diminishes stem circumference, impedes the growth of stem dry weight, and significantly reduces overall dry matter accumulation.
Role of physiological and biochemical responses during growth and development stage
Photosynthesis is widely recognized as one of the most sensitive physiological processes in response to heat stress (Hasanuzzaman et al., 2013a). Under stress conditions, the main limiting factors for photosynthesis are disruptions in chloroplast function and reduced efficiency of the photosynthetic system (Salvucci and Crafts-Brandner, 2004; Wang et al., 2012). The optimum temperature significantly inhibits photosynthesis in tobacco leaves (Yanhui et al., 2020). Even under the same light conditions, the transpiration rate, stomatal conductance, and photosynthetic intensity of the upper leaves of the Yunyan 87 variety under heat treatment were significantly downregulated. At the same time, the activities of critical enzymes in the xanthophyll cycle, such as violaxanthin de-epoxidase and zeaxanthin epoxidase, were also reduced (Martín et al., 2015). It suggests that the impact of high temperatures on photosynthesis is primarily reflected in the inhibition of enzyme activities related to photosynthesis in the leaves (Song et al., 2014). Under moderate stress, photosynthetic carbon assimilation is the first to be affected, mainly due to the reduced activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). At the same time, the impact on photosystem II (PSII) is relatively minor (Mathur et al., 2014). Heat stress can cause irreversible damage to the photosystem, disrupt the electron transport chain (ETC) and thylakoid membrane structure, and decline photosynthetic efficiency (Ergo et al., 2018). When temperatures reach a certain threshold, PSII suffers irreversible damage, the thylakoid membrane structure is disrupted, and the electron transport process becomes disordered (Yan et al., 2012). Prolonged exposure to high temperatures can lead to the death of cells, leaves, and entire plants (Feller and Vaseva, 2014).
Additionally, heat stress directly affects the activity of respiratory enzymes, reducing respiration intensity (Mathur et al., 2014). The intercellular CO2 concentration in tobacco cultivar Yunyan 87 initially decreases, then increases, and decreases again, indicating that prolonged heat stress weakens tobacco leaf respiration (Hu et al., 2021). During drought condition, stomatal limitations and reduced CO2 assimilation enzyme activities are the main factors causing to decline photosynthetic responses (Fahad et al., 2017; Zandalinas et al., 2018). Tobacco plant leaves typically exhibit noticeable yellowing and chlorosis, and as the severity increases, chlorophyll gradually degrades, leading to red or brown spots on the leaf surface (Raina et al., 2021). Drought stress significantly reduces chlorophyll content in tobacco leaves, thereby adversely impacting photosynthetic efficiency (Qi et al., 2023).The reduction in photosynthetic intensity under drought stress is attributed to stomatal and non-stomatal limitations. During mild drought conditions, the reduction in photosynthetic rate is primarily due to stomatal limitations. Insufficient water causes stomatal closure, hindering CO2 uptake and reducing photosynthesis due to inadequate raw materials. When tobacco plants are exposed to moderate or severe drought for extended periods, the decline in photosynthetic rate is due to the combined effects of both factors (Yan et al., 2024).
Under adverse climatic conditions, antioxidant enzyme activities are affected, leading to excessive accumulation of reactive oxygen species (ROS) in cells, which induces lipid peroxidation of membranes, disrupts membrane lipids and membrane proteins, increases membrane permeability, and damages membrane structural integrity in tobacco plants. This leads to protein and nucleic acid denaturation, causing plant metabolic disorders (Che et al., 2024). Under heat stress, the H2O2 content in tobacco leaves upregulated significantly, along with an increase in malondialdehyde (MDA) content. However, the activities of superoxide dismutase (SOD) and peroxidase (POD) activities are significantly enhanced. Excessive POD activity may enhance the POD-H2O2 decomposition system, promoting chlorophyll degradation, which decreases chlorophyll content (Tan et al., 2020). Different drought frequencies have varying effects on the activities of SOD, POD and catalase (CAT) in tobacco plant leaves. Under drought stress, SOD activity initially increases and then gradually decreases according to time and duration of stress frequency (Zhang and Kirkham, 1994; Li-Ping et al., 2006)). The type of tobacco varieties and degree and duration of stress affect the extent of cell membrane damage (Rizhsky et al., 2002; Li et al., 2011). Therefore, when studying stress resistance mechanisms, it is essential to consider and analyze multiple factors comprehensively.
Impact of heat and drought stress on biomass, yield and quality
Heat and drought stress significantly affect tobacco biomass and yield (Dobra et al., 2010). High-temperature conditions severely inhibit the growth and development of tobacco plants, leading to diminished biomass and production (Yang et al., 2018). These stressors adversely affect physiological processes, including photosynthesis, respiration, and water metabolism, ultimately weakening growth potential and reducing dry matter accumulation (Wahid et al., 2007). Optimal temperature ranges prompt stomata closure in tobacco leaves, diminishing the plant’s capacity to absorb CO2 and inhibiting photosynthetic responses. This reduction in photosynthetic efficiency directly affects the accumulation of photosynthetic products, further decreasing tobacco biomass and disrupting water metabolism (Demirevska et al., 2010). Under elevated temperatures, the transpiration rate and water loss are accelerated, and the roots’ ability to absorb water diminishes, resulting in an insufficient water supply within various plant structures (Ben-Yehoshua and Rodov, 2002). Water deficit adversely affects cell turgor, constraining cell division and expansion, which ultimately leads to a reduction in tobacco biomass. This decline in biomass results in limited leaf area expansion and fewer leaves, affecting the number of harvestable tobacco leaves (Razi and Muneer, 2021; Fang and Xiong, 2015).
Furthermore, elevated temperatures are often encountered during the maturity stage of tobacco cultivation. Under such conditions, tobacco leaves may experience “forced ripening” due to thermal stress, resulting in the premature yellowing of three or more leaves on a single plant. In severe cases, the entire plant may exhibit abnormal yellowing. Under optimal soil moisture conditions, the yield per plant and acre of tobacco is significantly higher than drought-stress conditions (Moore and Tyson, 1999). Drought stress affects the development of tobacco plant roots. Water is a crucial factor for root growth, and under water-deficient conditions, root growth is hindered, leading to reduced water and nutrient absorption capacity (Bodner et al., 2015). The weakening of root function affects water regulation within the plant and the absorption and transport of minerals, thereby limiting plant growth and biomass accumulation. Dry soil and air conditions result in nutrient deficiency and slow development in plants, with the most obvious manifestation being a slowdown in leaf growth (Lawlor et al., 1981). The impact of drought stress on different parts of the tobacco leaves also varies, with the middle leaves being more affected than the upper leaves (Cakir and Cebi, 2010). Drought-induced cell dehydration inhibits leaf cell growth and leaf area expansion (Alves and Setter, 2004). Current research found that the quality of upper and lower tobacco leaves is significantly reduced during drought stress compared to control conditions (Dobra et al., 2010). The impact of drought stress on tobacco yield also varies with the growth stage. Moderate stress during the rooting is beneficial for root development, while the vigorous growth stage is a critical period for water, and water deficiency during this stage significantly reduces yield and quality. Slightly lower water levels during maturity can improve leaf quality, but severe water deficiency or excess water can reduce quality.
Effects on tobacco leaf quality
Heat and/or drought or combined stress severely affects the quality of tobacco leaves (Hasanuzzaman et al., 2013b; Su et al., 2017). High temperatures can disrupt the balance of carbon and nitrogen metabolism in tobacco plants (Glaubitz et al., 2015). Due to the inhibition of photosynthesis, the synthesis of carbohydrates reduces while respiration increases, consuming more carbohydrates and leading to the reduction in starch content in tobacco leaves. High temperatures stimulate an increase in nicotine content in tobacco leaves. However, the potassium content in tobacco leaves positively correlated with field temperature (Chen et al., 2016). High-temperature treatment significantly accelerates the degradation of soluble proteins in tobacco leaves and increases the MDA content, thereby accelerating tobacco plants’ ageing. Different tobacco varieties respond differently to heat stress, resulting in variations in the chemical composition of tobacco leaves (Leffingwell, 1999; Yang et al., 2018). For example, the yield and quality of the K326 and Yunyan 87 tobacco varieties are mainly influenced by water factors. In contrast, temperature factors primarily affect the yield and quality of the Hongda variety of tobacco.
Under drought stress, tobacco quality typically declines, manifested by the reduction in sugar content and increase in nicotine content. Researchers found that under non-irrigated conditions, the average sugar content dropped about 5% over three years, resulting in poor leaf quality and reduced economic value. The average nicotine content over three years ranged from 1.7 to 2.72%, with no significant impact on nicotine content except under non-irrigated conditions (Biglouei et al., 2010). Water scarcity limits photosynthesis and carbohydrate synthesis. At the same time, drought stress alters metabolic pathways in plants, diverting more nitrogen into alkaloid synthesis (Singh et al., 2018). It also affects the content of minerals like potassium, calcium, and magnesium in tobacco leaves. Typically, these elements’ absorption and transport capacity decreases under drought conditions, reducing mineral content in tobacco leaves. It directly affects the combustibility and flavor of the tobacco leaves. Even when plants grow in nutrient-rich soils, drought can cause nutrient deficiencies by directly affecting the physical and chemical properties of the soil, reducing nutrient mobility in the soil and plant nutrient uptake (Ahanger et al., 2016). During tobacco growth and development, drought at different stages can affect tobacco leaf quality to varying degrees, including chemical composition and aromatic substances. For example, drought during vigorous growth significantly impacts tobacco leaf yield and quality, downregulating biomass, reducing sugar content and increasing total nitrogen and nicotine content (Su et al., 2017).
Synergistic effects of heat and drought stress
The combined effects of heat and drought stress on plants cannot be attributed to the sum of the individual effects of stress; instead, they trigger new defense mechanisms in plants (Rizhsky et al., 2004). Under the combined stress, the physiological responses of tobacco plants exhibit high complexity (Wang et al., 2024). Water deficit is significantly exacerbated when plants experience combined heat and drought stress. High temperatures increase transpiration rates, while drought reduces soil water availability, preventing plants from obtaining sufficient water through their roots (Bodner et al., 2015; da Silva et al., 2010). A significant imbalance between water supply and demand disrupts water metabolism within the plant, resulting in decreased cell turgor, leaf wilting, and potentially irreversible damage. This condition ultimately constrains crop growth and productivity (Wollenweber et al., 2010; Gourdji et al., 2013).
Optimum temperature causes the stomatal functioning of tobacco plants to close, reducing water evaporation and limiting CO2 absorption (Driesen et al., 2020). Under drought conditions, the reduction in soil moisture further exacerbates stomatal closure, decreasing CO2 availability, inhibiting photosynthetic electron transport, lowering leaf photochemical efficiency of PSII, and ultimately reducing photosynthesis. This limitation on photosynthetic productivity downregulates the accumulation of assimilates (Martin-StPaul et al., 2017). Additionally, combined heat and drought stress enhances transpiration rates, accelerating water loss within the plant, which affects the utilization of limited soil moisture and exacerbates the challenges in crop growth and yield formation (Xu et al., 2013).
Key enzymes in nitrogen metabolism, such as nitrate reductase (NR) and glutamine synthetase (GS), played a critical role in assimilating ammonium into organic compounds (Xiong et al., 2018; Tao et al., 2018). Heat and drought stress severely inhibit nitrogen metabolism in plants, reducing NR activity, impairing nitrogen metabolism and absorption, and leading to reduction in nitrogen content in tobacco leaves. This, in turn, affects the synthesis of nitrogen-containing compounds like proteins and chlorophyll, potentially hindering tobacco leaf growth and development, resulting in decreased quality and negatively influencing crop yield (Prasad et al., 2008; Ahanger et al., 2016).
Under combined heat and drought stress, chlorophyll content and the activities of SOD and POD enzymes in tobacco leaves were significantly lower than under heat or drought stress alone, while relative conductivity and MDA content were significantly higher. It indicates that the synergistic stress of heat and drought causes more significant harm to tobacco compared to individual stressors (Wang et al., 2024). Severe drought at different stages of tobacco growth and development affects leaf quality, including changes in the chemical composition and aroma substances (Bahrami-Rad and Hajiboland, 2017). Studies showed that mild drought stress during the maturity stage is detrimental to forming and transforming phenols, higher fatty hydrocarbons, and flavonoids, leading to decreased aroma and taste quality of cured tobacco leaves (Begum et al., 2021). In response to the synergistic stress of heat and drought, tobacco’s ecological adaptation mechanisms are primarily reflected in adjusting the plant’s physiological structure to adapt to adverse environments, such as increasing leaf cuticle thickness and reducing leaf surface area to minimize water loss and altering biochemical pathways to enhance stress tolerance efficiency, such as increasing the activity of antioxidant enzymes, such as CAT and SOD to reduce oxidative stress caused by heat and drought (Carmo-Silva et al., 2012).
Influence of genes expression and signal transduction
Heat and drought stress significantly disrupt protein homeostasis within plant cells, leading to a cascade of physiological and biochemical responses (Feller and Vaseva, 2014). Under heat stress conditions, the structural integrity and properties of tobacco plant cell walls are compromised. This increases membrane fluidity and permeability, facilitating the efflux of extracellular calcium ions (Ca2+), a crucial signaling molecule in various cellular processes (Niu and Xiang, 2018).The elevation of cytosolic Ca2+ levels activates a series of downstream signaling pathways. Concurrently, reactive oxygen species (ROS) and nitric oxide (NO) emerge as critical secondary messengers in response to heat stress. These molecules mediate stress responses by rapidly activating regulatory networks that influence gene expression and metabolic adjustments. The accumulation of ROS can trigger oxidative stress, damaging cellular components, while NO promotes stress tolerance and signaling pathways (Goraya et al., 2017).Heat stress results in the overproduction of cytosolic Ca2+, ROS, and NO, disrupting the endoplasmic reticulum’s protein-folding capacity (ER). This leads to the accumulation of misfolded or unfolded proteins, a condition known as ER stress. Such proteins can exert toxic effects on cellular functions and ultimately compromise cell viability. The accumulation of misfolded proteins triggers the unfolded protein response (UPR), a protective mechanism to restore protein homeostasis and mitigate cellular damage. In response to these imbalances, plants have evolved various adaptive mechanisms to cope with heat stress and minimize cellular damage. These include the upregulation of heat shock proteins (HSPs), which assist in protein folding and stabilization, and the activation of antioxidant systems to counteract ROS-induced damage. However, plants may alter metabolic pathways to enhance osmoregulation and improve stress resilience (Chaudhry and Sidhu, 2022). Plants strive to maintain cellular integrity and functionality through these complex responses in adverse environmental conditions.
In response to heat stress, tobacco plants activate multiple heat stress-responsive genes, typically regulated by heat shock transcription factors (HSFs) (Dos Santos et al., 2022). HSFs are key regulators in plants’ rapid response to heat stress. It binds to the heat shock proteins (HSPs) gene promoter regions, initiating their expression. HSPs function as molecular chaperones, assisting in proper protein folding, preventing protein aggregation and denaturation caused by heat, and thus protecting cellular protein function (Becker and Craig, 1994). In tobacco, genes like HSP70 and HSP90 have been extensively studied, and their upregulated expression under heat stress plays a significant role in maintaining cellular homeostasis and enhancing heat tolerance efficiency (Li et al., 2015).
However, genes associated with antioxidative responses, such as SOD and CAT, are also induced under heat-stress conditions. These genes protect cells from oxidative damage by scavenging ROS, enhancing tobacco’s stress tolerance (Devireddy et al., 2021). Studies have shown that the overexpression of specific heat shock protein genes, likeZmHSP16.9, RcHSP17.8, BcHSP70, and LeHSP21, can maintain photosynthetic responses by increasing seed germination rates, chlorophyll content, and antioxidant capacity while reducing MDA content and conductivity, thereby significantly enhancing plant heat tolerance apparatus (Sun et al., 2012). Furthermore, overexpression of transcription activator genes like CAP2, seed dormancy delay family gene NtDOG1L-T, and heat shock transcription factor gene BcHsfA1 in tobacco seedlings significantly enhances promoter activity under heat stress conditions, with notable increases in the expression levels of HSPs and heat shock factor genes (Zhu et al., 2018). Research has also noticed that N-acetyl glutamate kinase (NAGK) enhances plant heat tolerance by activating antioxidant defense signals such as ascorbate peroxidase 2 (APX2) and superoxide dismutase C (SODC), as well as the heat shock network genes.
Drought stress triggers the activation of abscisic acid (ABA)-dependent and independent signaling pathways in tobacco plants, which regulate the expression of related genes (Soma et al., 2021). ABA is a key hormone in plant response to drought stress. Under drought conditions, the synthesis and accumulation of ABA significantly increase, activating the ABA-dependent signaling pathway (Muhammad Aslam et al., 2022). ABA binds to its receptors, such as the PYR/PYL/RCAR family proteins, inhibiting the activity of PP2C phosphatases and releasing the inhibition of SnRK2 protein kinases. Once activated, SnRK2 kinases can phosphorylate and activate a series of downstream target proteins, including transcription factors, ion channels, and metabolic enzymes, thereby initiating the expression of drought-resistant genes (Kobayashi et al., 2005; Zhu, 2016). ABA can activate ABA-responsive element (ABRE)-binding proteins, including transcription factors like ABF/AREB, which regulate the expression of drought-resistant genes. These genes often encode proteins related to drought resistance, such as LEA proteins, aquaporins, and osmotic regulatory proteins, helping plants maintain cellular homeostasis and water balance (Yoshida et al., 2010; Soma et al., 2021).
Reactive oxygen species (ROS) act as signaling molecules and damaging agents in plant stress responses (Mittler et al., 2022). The production of ROS typically increases under temperature and drought stress, triggering a series of stress responses (Czarnocka and Karpiński, 2018). ROS can initiate signal transduction by oxidatively modifying specific proteins, altering their function or activity. For example, ROS can activate the MAPK (mitogen-activated protein kinase) cascade, amplifying stress signals and promoting downstream responses (Jalmi and Sinha, 2015). Under the regulation of ROS signaling, plants activate the expression of antioxidant genes, such as those encoding SOD, CAT and APX. These enzymes mitigate oxidative stress by scavenging excess ROS, thereby protecting cellular structure and function (Azarabadi et al., 2017).
Studies have shown that Late Embryogenesis Abundant (LEA) protein genes in tobacco are significantly upregulated under drought stress. The proteins encoded by these genes stabilize cellular membranes and proteins, reducing cell damage caused by drought (Bao et al., 2017). Dehydration-responsive Element Binding Protein (DREB) transcription factors play an essential role in the drought stress response in tobacco. DREB transcription factors can recognize and bind to the drought-responsive element (DRE) sequence, activating the expression of drought resistance-related genes, such as aquaporin and osmotic regulation-related genes. The products of these genes help tobacco cells maintain water balance and enhance drought tolerance capacity (Joshi et al., 2016; Siefritz et al., 2002).
Mitigation strategies and prospects for heat and drought stress
Agricultural management and cultivation techniques
Effective management of water and fertilizers plays a crucial role in influencing tobacco leaf yield and structure (Li et al., 2009). Implementing appropriate irrigation practices can mitigate soil temperature under elevated thermal conditions, simultaneously enhancing the uptake of essential nutrients such as nitrogen, phosphorus, and potassium. This, in turn, increases the activity of key enzymes involved in carbon and nitrogen metabolism, thereby alleviating the detrimental effects of heat stress on tobacco plants (Ferrante and Mariani, 2018; Kurt and Kinay, 2021).Elements such as silicon and potassium act as regulatory elements that can enhance plant growth and increase resistance to stress. Research shows that the increasing potassium fertilizer application can stabilize the plasma membrane, reduce membrane permeability, and reduce leaf wilting efficiency, thereby effectively enhancing tobacco resistance (Kumari et al., 2022).
Moreover, applying appropriate amounts of calcium and magnesium fertilizers under heat-stress conditions can significantly improve tobacco stress conditions (Waraich et al., 2012). Calcium maintains the structural and functional integrity of cell membranes and acts as a secondary messenger in plant responses to the adaptation of environmental variables (Thor, 2019). Under heat stress, exogenous calcium and calcium chloride can effectively regulate photosynthesis in tobacco, improve stomatal conductance in plant leaves, enhance the thermal stability of the oxygen-evolving complex, and reduce ROS accumulation, thus alleviating heat stress (Tan et al., 2011).
Genetic engineering strategies
Genetic engineering provides essential avenues for mitigating heat and drought stress in tobacco plants. Researchers can precisely regulate stress-responsive genes through gene editing technologies such as CRISPR/Cas9 (Doudna and Charpentier, 2014). For example, overexpressing antioxidant enzyme genes or osmotic regulatory substance synthesis genes can enhance the ability of tobacco to scavenge ROS, thereby improving cellular osmotic protection. However, regulating essential genes such as transcription factors like DREB and HSF can further activate downstream stress response genes, enhancing tobacco’s stress resistance capacity (Manna et al., 2021). However, genetic engineering approaches still face challenges regarding public acceptance, ecological safety and regulatory policies.
Application of biotechnology
Biotechnology shows great promise in improving tobacco’s tolerance to heat and drought stress conditions. For example, treating tobacco with endophytes or microbial inoculants can enhance its stress resistance. Studies have found that certain endophytic fungi or bacteria can improve tobacco’s tolerance to environmental stress by regulating the plant’s hormonal balance, increasing antioxidant capacity, or enhancing osmotic regulation (Begum et al., 2022). The use of plant growth regulators, such as abscisic acid analogues (ABA analogues), to induce stress responses in plants is an effective strategy (Wang et al., 2024).
Molecular breeding and germplasm innovation
Molecular breeding techniques, such as selecting and cultivating tobacco varieties with high resistance to heat and drought, represent a long-term strategy for coping with the era of climate change. Technologies like genome-wide association studies (GWAS) and genomic selection (GS) can be applied to identify quantitative trait loci (QTLs) associated with stress resistance and use them in marker-assisted breeding (Devate et al., 2022). Moreover, modern molecular tools, including transgenic technologies, gene editing and RNA interference, can accelerate the development of new stress-resistant germplasm. However, given the complexity and unpredictability of climate change, continuous exploration of recent stress-resistant traits and breeding strategies will be necessary in the future.
Conclusion and future perspectives
High temperatures and drought represent significant environmental stressors that profoundly influence the growth and development of tobacco plants. These stressors adversely affect both the yield and quality of tobacco post-curing. A comprehensive understanding of the mechanisms by which high temperature and drought exert their effects is essential for implementing effective preventive measures in field demonstrations. Future research should prioritize the exploration of strategies to mitigate these impacts. Moreover, attention should also be directed toward other stressors. For instance, concurrent high temperature and soil flooding stress during the late stages of tobacco growth in Yunnan, China, necessitates further investigation. In the context of climate change, developing strategies to regulate crop growth and development under optimal temperature conditions with limited water irrigation is becoming increasingly pertinent and holds significant implications for global agricultural security and sustainability. Despite the critical nature of these challenges, there is a scarcity of studies examining the development of root ultrastructure, rhizosphere microorganisms, and root exudates in response to the combined stress of optimal temperature and water scarcity. Therefore, intensified research efforts are essential to provide deeper insights into plant responses to high temperatures and drought by linking aboveground and belowground components. Understanding these interactions will be crucial as climate conditions are expected to become hotter and drier. Overall, this study systematically explores the development, trends, and prospects of plant responses to water stress and optimal temperature research, thereby enhancing our understanding of how plants adapt to the challenges posed by climate change.
Author contributions
ML: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft. XL: Investigation, Methodology, Project administration, Resources, Writing – original draft. YS: Software, Supervision, Validation, Visualization, Writing – original draft. YH: Data curation, Methodology, Writing – original draft. CY: Data curation, Methodology, Writing – original draft. JL: Formal analysis, Project administration, Writing – original draft. SJ: Investigation, Writing – original draft. KG: Formal analysis, Methodology, Writing – original draft. ZY: Project administration, Resources, Writing – original draft. WH: Investigation, Methodology, Writing – original draft. JS: Funding acquisition, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LW: Funding acquisition, Resources, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Science and Technology Plan Project of Dali Prefecture Branch of Yunnan Tobacco Company (2022530000241035, DLYC2023001, 2015YN20, 2024530000241012).
Acknowledgments
The authors would like to thank the College of Agronomy and Biotechnology, Southwest University and Engineering Research Center of South Upland Agriculture, Ministry of Education, Chongqing, China for providing the necessary facilities for this study.
Conflict of interest
Authors YS, YH, CY, JL, SJ, and JS were employed by the company Dali Prefecture Branch of Yunnan Tobacco Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Science and Technology Plan Project of Dali Prefecture Branch of Yunnan Tobacco Company (2022530000241035, DLYC2023001, 2015YN20, 2024530000241012). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrantes, F. D. L., Ribas, A. F., Vieira, L. G. E., MaChado-Neto, N. B., Custodio, C. C. (2019). Seed germination and seedling vigor of transgenic tobacco (Nicotiana tabacum L.) with increased proline accumulation under osmotic stress. J. Hortic. Sci. Biotechnol. 94, 220–228. doi: 10.1080/14620316.2018.1499423
Ahanger, M. A., Morad-Talab, N., Abd-Allah, E. F., Ahmad, P., Hajiboland, R. (2016). “Plant growth under drought stress: Significance of mineral nutrients,” in Water Stress and Crop Plants: A Sustainable Approach. 2, 649–668. doi: 10.1002/9781119054450.ch37
Aharon, R., Shahak, Y., Wininger, S., Bendov, R., Kapulnik, Y., Galili, G. (2003). Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15, 439–447. doi: 10.1105/tpc.009225
Alves, A. A., Setter, T. L. (2004). Response of cassava leaf area expansion to water deficit: cell proliferation, cell expansion and delayed development. Ann. Bot. 94, 605–613. doi: 10.1093/aob/mch179
Azarabadi, S., Abdollahi, H., Torabi, M., Salehi, Z., Nasiri, J. (2017). ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L). Eur. J. Plant Pathol. 147, 279–294. doi: 10.1007/s10658-016-1000-0
Bahrami-Rad, S., Hajiboland, R. (2017). Effect of potassium application in drought-stressed tobacco (Nicotiana rustica L.) plants: Comparison of root with foliar application. Ann. Agric. Sci. 62, 121–130. doi: 10.1016/j.aoas.2017.08.001
Bao, F., Du, D., An, Y., Yang, W., Wang, J., Cheng, T., et al. (2017). Overexpression of Prunus mume dehydrin genes in tobacco enhances tolerance to cold and drought. Front. Plant Sci. 8, 151. doi: 10.3389/fpls.2017.00151
Becker, J., Craig, E. A. (1994). Heat-shock proteins as molecular chaperones. Eur. J. Biochem. 219, 11–23. doi: 10.1111/j.1432-1033.1994.tb19910.x
Begum, N., Akhtar, K., Ahanger, M. A., Iqbal, M., Wang, P., Mustafa, N. S., et al. (2021). Arbuscular mycorrhizal fungi improve growth, essential oil, secondary metabolism, and yield of tobacco (Nicotiana tabacum L.) under drought stress conditions. Environ. Sci. pollut. Res. 28, 45276–45295. doi: 10.1007/s11356-021-13755-3
Begum, N., Wang, L., Ahmad, H., Akhtar, K., Roy, R., Khan, M. I., et al. (2022). Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by upregulating antioxidant and mineral nutrition metabolism. Microbial Ecol. 83, 971–988. doi: 10.1007/s00248-021-01815-7
Ben-Yehoshua, S., Rodov, V. (2002). “Transpiration and Water Stress,” in Postharvest Physiology and Pathology of Vegetables (CRC Press), 143–197. Available online at: https://www.taylorfrancis.com/chapters/edit/10.1201/9780203910092-11/transpiration-water-stress-shimshon-ben-yehoshua-victor-rodov.
Biglouei, M. H., Assimi, M. H., Akbarzadeh, A. (2010). Effect of water stress at different growth stages on quantity and quality traits of Virginia (flue-cured) tobacco type. Plant Soil Environ. 56, 67–75. doi: 10.17221/163/2009-PSE
Bittner, R. J., Arellano, C., Mila, A. L. (2016). Effect of temperature and resistance of tobacco cultivars to the progression of bacterial wilt, caused by Ralstonia solanacearum. Plant Soil 408, 299–310. doi: 10.1007/s11104-016-2938-6
Bodner, G., Nakhforoosh, A., Kaul, H. P. (2015). Management of crop water under drought: a review. Agron. Sustain. Dev. 35, 401–442. doi: 10.1007/s13593-015-0283-4
Cakir, R., Cebi, U. (2010). The effect of irrigation scheduling and water stress on the maturity and chemical composition of Virginia tobacco leaf. Field Crops Res. 119, 269–276. doi: 10.1016/j.fcr.2010.07.017
Carmo-Silva, A. E., Gore, M. A., Andrade-Sanchez, P., French, A. N., Hunsaker, D. J., Salvucci, M. E. (2012). Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ. Exp. Bot. 83, 1–11. doi: 10.1016/j.envexpbot.2012.03.001
Chaudhry, S., Sidhu, G. P. S. (2022). Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 41, 1–31. doi: 10.1007/s00299-021-02759-5
Che, Y., Wang, H., Yao, T., Wang, Z., Bo, L., Zhang, H. (2024). Activation of the antioxidant system and transduction of the mediated by exogenous calcium improve drought resistance in tobacco. Plant Stress 4, 100551. doi: 10.1016/j.stress.2024.100551
Chen, X., Chen, Q., Zhang, X., Li, R., Jia, Y., Ef, A., et al. (2016). Hydrogen sulfide mediates nicotine biosynthesis in tobacco (Nicotiana tabacum) under high temperature conditions. Plant Physiol. Biochem. 104, 174–179. doi: 10.1016/j.plaphy.2016.02.033
Chen, Y., Han, Y., Zhang, M., Zhou, S., Kong, X., Wang, W. (2016). Overexpression of the wheat expansin gene TaEXPA2 improved seed production and drought tolerance in transgenic tobacco plants. PloS One 11, e0153494. doi: 10.1371/journal.pone.0153494
Czarnocka, W., Karpiński, S. (2018). Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radical Biol. Med. 122, 4–20. doi: 10.1016/j.freeradbiomed.2018.01.011
da Silva, E. C., Nogueira, R. J. M. C., da Silva, M. A., de Albuquerque, M. B. (2010). Drought stress and plant nutrition. Plant Stress 5, 32–41.
Demirevska, K., Simova-Stoilova, L., Fedina, I., Georgieva, K., Kunert, K. (2010). Response of oryzacystatin I transformed tobacco plants to drought, heat and light stress. J. Agron. Crop Sci. 196, 90–99. doi: 10.1111/j.1439-037X.2009.00396.x
Devate, N. B., Krishna, H., Parmeshwarappa, S. K. V., Manjunath, K. K., Chauhan, D., Singh, S., et al. (2022). Genome-wide association mapping for component traits of drought and heat tolerance in wheat. Front. Plant Sci. 13, 943033. doi: 10.3389/fpls.2022.943033
Devireddy, A. R., Tschaplinski, T. J., Tuskan, G. A., Muchero, W., Chen, J. G. (2021). Role of reactive oxygen species and hormones in plant responses to temperature changes. Int. J. Mol. Sci. 22, 8843. doi: 10.3390/ijms22168843
Dobra, J., Motyka, V., Dobrev, P., Malbeck, J., Prasil, I. T., Haisel, D., et al. (2010). Comparison of hormonal responses to heat, drought and combined stress in tobacco plants with elevated proline content. J. Plant Physiol. 167, 1360–1370. doi: 10.1016/j.jplph.2010.05.013
Dos Santos, T. B., Ribas, A. F., de Souza, S. G. H., Budzinski, I. G. F., Domingues, D. S. (2022). Physiological responses to drought, salinity, and heat stress in plants: a review. Stresses 2, 113–135. doi: 10.3390/stresses2010009
Doudna, J. A., Charpentier, E. (2014). The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096. doi: 10.1126/science.1258096
Driedonks, N., Rieu, I., Vriezen, W. H. (2016). Breeding for plant heat tolerance at vegetative and reproductive stages. Plant Reprod. 29, 67–79. doi: 10.1007/s00497-016-0275-9
Driesen, E., Van den Ende, W., De Proft, M., Saeys, W. (2020). Influence of environmental factors light, CO2, temperature, and relative humidity on stomatal opening and development: A review. Agronomy 10, 1975. doi: 10.3390/agronomy10121975
Ergo, V. V., Lascano, R., Vega, C. R., Parola, R., Carrera, C. S. (2018). Heat and water stressed field-grown soybean: A multivariate study on the relationship between physiological-biochemical traits and yield. Environ. Exp. Bot. 148, 1–11. doi: 10.1016/j.envexpbot.2017.12.023
Fahad, S., Bajwa, A., Nazir, U., Anjum, S. A., Farooq, A., Zohaib, A., et al. (2017). Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 8, 1147. doi: 10.3389/fpls.2017.01147
Fang, Y., Xiong, L. (2015). General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life sciences: CMLS 72, 673–689. doi: 10.1007/s00018-014-1767-0
Farooq, M., Wahid, A., Kobayashi, N. S. M. A., Fujita, D. B. S. M. A., Basra, S. M. (2009). Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 29, 153–188. doi: 10.1051/agro:2008021
Feller, U., Vaseva, I. I. (2014). Extreme climatic events: impacts of drought and high temperature on physiological processes in agronomically important plants. Front. Environ. Sci. 2, 39. doi: 10.3389/fenvs.2014.00039
Ferrante, A., Mariani, L. (2018). Agronomic management for enhancing plant tolerance to abiotic stresses: High and low values of temperature, light intensity, and relative humidity. Horticulturae 4, 21. doi: 10.3390/horticulturae4030021
Glaubitz, U., Erban, A., Kopka, J., Hincha, D. K., Zuther, E. (2015). High night temperature strongly impacts TCA cycle, amino acid and polyamine biosynthetic pathways in rice in a sensitivity-dependent manner. J. Exp. Bot. 66, 6385–6397. doi: 10.1093/jxb/erv352
Goraya, G. K., Kaur, B., Asthir, B., Bala, S., Kaur, G., Farooq, M. (2017). Rapid injuries of high temperature in plants. J. Plant Biol. 60, 298–305. doi: 10.1007/s12374-016-0365-0
Gourdji, S. M., Sibley, A. M., Lobell, D. B. (2013). Global crop exposure to critical high temperatures in the reproductive period: Historical trends and future projections. Environ. Res. Lett. 8, 24041. doi: 10.1088/1748-9326/8/2/024041
Hasanuzzaman, M., Nahar, K., Alam, M. M., Roychowdhury, R., Fujita, M. (2013b). Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 14, 9643–9684. doi: 10.3390/ijms14059643
Hasanuzzaman, M., Nahar, K., Fujita, M. (2013a). “Extreme Temperature Responses, Oxidative Stress and Antioxidant Defense in Plants,” in Abiotic Stress-Plant Responses and Applications in Agriculture, vol. 13, 169–205. doi: 10.5772/54833
Hossain, A., Lozovskaya, M. V., Zvolinsky, V. P., Tutuma, N. V. (2012). “Effect of soil resources and climatic factors (temperature) on spring wheat and barley in the northern Bangladesh and southern Russia,” in International scientific and practical conference on problems of environmental management and conservation of ecological balance in the arid zones’’, held in ‘‘Caspian Scientific Research Institute of Arid Agriculture’’, Salt Zaymische, Chorniarsky district (Astrakhan State, Russia), 16–18.
Hu, W., Di, Q., Wei, J., Zhang, J., Liu, J. (2021). Grafting tobacco onto nutrient-efficient rootstocks improves photosynthesis. J. Am. Soc. Hortic. Sci. 146, 286–293. doi: 10.21273/JASHS05041-21
Hu, Z., He, Z., Li, Y., Wang, Q., Yi, P., Yang, J., et al. (2023). Transcriptomic and metabolic regulatory network characterization of drought responses in tobacco. Front. Plant Sci. 13, 1067076. doi: 10.3389/fpls.2022.1067076
Jalmi, S. K., Sinha, A. K. (2015). ROS mediated MAPK signaling in abiotic and biotic stress-striking similarities and differences. Front. Plant Sci. 6, 769. doi: 10.3389/fpls.2015.00769
Joshi, R., Wani, S. H., Singh, B., Bohra, A., Dar, Z. A., Lone, A. A., et al. (2016). Transcription factors and plants response to drought stress: current understanding and future directions. Front. Plant Sci. 7, 1029. doi: 10.3389/fpls.2016.01029
Kobayashi, Y., Murata, M., Minami, H., Yamamoto, S., Kagaya, Y., Hobo, T., et al. (2005). Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 44, 939–949. doi: 10.1111/j.1365-313X.2005.02583.x
Kumar, J., Sen Gupta, D., Djalovic, I., Kumar, S., Siddique, K. H. (2021). Root-omics for drought tolerance in cool-season grain legumes. Physiologia Plantarum 172, 629–644. doi: 10.1111/ppl.v172.2
Kumari, V. V., Banerjee, P., Verma, V. C., Sukumaran, S., Chandran, M. A. S., Gopinath, K. A., et al. (2022). Plant nutrition: An effective way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 23, 8519. doi: 10.3390/ijms23158519
Kurt, D., Kinay, A. (2021). Effects of irrigation, nitrogen forms and topping on sun cured tobacco. Ind. Crops Products 162, 113276. doi: 10.1016/j.indcrop.2021.113276
Lamaoui, M., Jemo, M., Datla, R., Bekkaoui, F. (2018). Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 6, 26. doi: 10.3389/fchem.2018.00026
Lawlor, D. W., Day, W., Johnston, A. E., Legg, B. J., Parkinson, K. J. (1981). Growth of spring barley under drought: crop development, photosynthesis, dry-matter accumulation and nutrient content. J. Agric. Sci. 96, 167–186. doi: 10.1017/S002185960003197X
Leffingwell, J. (1999). “BA Basic Chemical Constituents of Tobacco Leaf and Differences Among Tobacco Types,” in Tobacco: Production, Chemistry, and Technology. Eds. Davis, D.L., Nielson, M. T. (Blackwell Science, Hoboken, N. J).
Li, Z. G., Long, W. B., Yang, S. Z., Wang, Y. C., Tang, J. H., Chen, T. (2015). Involvement of sulfhydryl compounds and antioxidant enzymes in H2S-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension-cultured cells. In Vitro Cell. Dev. Biol. - Plant 51, 428–437. doi: 10.1007/s11627-015-9705-x
Li, Y., Ren, K., Hu, M., He, X., Gu, K., Hu, B., et al. (2021). Cold stress in the harvest period: effects on tobacco leaf quality and curing characteristics. BMC Plant Biol. 21, 1–15. doi: 10.1186/s12870-021-02895-w
Li, S. X., Wang, Z. H., Malhi, S. S., Li, S. Q., Gao, Y. J., Tian, X. H. (2009). Nutrient and water management effects on crop production, and nutrient and water use efficiency in dryland areas of China. Adv. Agron. 102, 223–265. doi: 10.1016/S0065-2113(09)01007-4
Li, F., Xing, S., Guo, Q., Zhao, M., Zhang, J., Gao, Q., et al. (2011). Drought tolerance through over-expression of the expansin gene TaEXPB23 in transgenic tobacco. J. Plant Physiol. 168, 960–966. doi: 10.1016/j.jplph.2010.11.023
Lipiec, J., Doussan, C., Nosalewicz, A., Kondracka, K. (2013). Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophysics 27, 463–477. doi: 10.2478/intag-2013-0017
Li-Ping, B. A. I., Fang-Gong, S. U. I., Ti-Da, G. E., Zhao-Hui, S. U. N., Yin-Yan, L. U., Guang-Sheng, Z. H. O. U. (2006). Effect of soil drought stress on leaf water status, membrane permeability and enzymatic antioxidant system of maize. Pedosphere 16, 326–332. doi: 10.1016/S1002-0160(06)60059-3
Lobell, D. B., Hammer, G. L., Chenu, K., Zheng, B., McLean, G., Chapman, S. C. (2015). The shifting influence of drought and heat stress for crops in northeast Australia. Global Change Biol. 21, 4115–4127. doi: 10.1111/gcb.2015.21.issue-11
Manna, M., Thakur, T., Chirom, O., Mandlik, R., Deshmukh, R., Salvi, P. (2021). Transcription factors as key molecular target to strengthen the drought stress tolerance in plants. Physiologia Plantarum 172, 847–868. doi: 10.1111/ppl.v172.2
Martín, M., Noarbe, D. M., Serrot, P. H., Sabater, B. (2015). The rise of the photosynthetic rate when light intensity increases is delayed in ndh gene-defective tobacco at high but not at low CO2 concentrations. Front. Plant Sci. 6, 34. doi: 10.3389/fpls.2015.00034
Martiniello, P., Teixeira da Silva, J. A. (2011). Physiological and bioagronomical aspects involved in growth and yield components of cultivated forage species in Mediterranean environments: A review. Eur. J. Plant Sci. Biotechnol. 5, 64–98. Available online at: https://www.researchgate.net/publication/283514296_Physiological_and_Bioagronomical_Aspects_Involved_in_Growth_and_Yield_Components_of_Cultivated_Forage_Species_in_Mediterranean_Environments_A_Review.
Martin-StPaul, N., Delzon, S., Cochard, H. (2017). Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 20, 1437–1447. doi: 10.1111/ele.2017.20.issue-11
Mathur, S., Agrawal, D., Jajoo, A. (2014). Photosynthesis: response to high temperature stress. J. Photochem. Photobiol. B: Biol. 137, 116–126. doi: 10.1016/j.jphotobiol.2014.01.010
Mittler, R., Zandalinas, S. I., Fichman, Y., Van Breusegem, F. (2022). Reactive oxygen species signaling in plant stress responses. Nat. Rev. Mol. Cell Biol. 23, 663–679. doi: 10.1038/s41580-022-00499-2
Mohammadi, M., Modarres Sanavy, S. A. M., Heidarzadeh, A., Pirdashti, H., Tahmasebi Sarvestani, Z., Zand, B. (2024). Interactive effects of mycorrhizal, Azospirillum, and nitrogen + phosphorus with limited irrigation on yield and morpho-physiological traits of evening primrose (Oenothera biennis L.) in arid and semi-arid regions. Agric. Water Manage. 301, 108947. doi: 10.1016/j.agwat.2024.108947
Moore, J. M., Tyson, A. W. (1999). Irrigating Tobacco (The University of Georgia College of). Available online at: https://extension.uga.edu/publications/detail.html?number=B892.
Muhammad Aslam, M., Waseem, M., Jakada, B. H., Okal, E. J., Lei, Z., Saqib, H. S. A., et al. (2022). Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 23, 1084. doi: 10.3390/ijms23031084
Niu, Y., Xiang, Y. (2018). An overview of biomembrane functions in plant responses to high-temperature stress. Front. Plant Sci. 9, 915. doi: 10.3389/fpls.2018.00915
Pearce, R. C., Bailey, W. A., Bush, L. P., Green, J. D., Jack, A. M., Miller, R. D., et al. (2015). 2015-2016 Burley and Dark Tobacco Production Guide. Available online at: https://smith.tennessee.edu/wp-content/uploads/sites/209/2020/11/Tobacco-Production-Guide.pdf.
Prasad, P. V. V., Staggenborg, S. A., Ristic, Z. (2008). “Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants,” in Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes. Eds. Ahuja, L. R., Reddy, V. R., Saseendran, S. A., Yu, Q., vol. 1, 301–355. doi: 10.2134/advagricsystmodel1.c11
Qi, M., Zheng, X., Niu, G., Ye, A., Rather, S. A., Ahmed, N., et al. (2023). Supplementation of acetylcholine mediates physiological and biochemical changes in tobacco lead to alleviation of damaging effects of drought stress on growth and photosynthesis. J. Plant Growth Regul. 42, 4616–4628. doi: 10.1007/s00344-022-10642-0
Raina, M., Kumar, A., Yadav, N., Kumari, S., Yusuf, M. A., Mustafiz, A., et al. (2021). StCaM2, a calcium binding protein, alleviates negative effects of salinity and drought stress in tobacco. Plant Mol. Biol. 106, 85–108. doi: 10.1007/s11103-021-01131-1
Razi, K., Muneer, S. (2021). Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 41, 669–691. doi: 10.1080/07388551.2021.1874280
Rizhsky, L., Liang, H., Mittler, R. (2002). The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 130, 1143–1151. doi: 10.1104/pp.006858
Rizhsky, L., Liang, H., Shuman, J., Shulaev, V., Davletova, S., Mittler, R. (2004). When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 134, 1683–1696. doi: 10.1104/pp.103.033431
Salvucci, M. E., Crafts-Brandner, S. J. (2004). Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiologia Plantarum 120, 179–186. doi: 10.1111/j.0031-9317.2004.0173.x
Siefritz, F., Tyree, M. T., Lovisolo, C., Schubert, A., Kaldenhoff, R. (2002). PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. Plant Cell 14, 869–876. doi: 10.1105/tpc.000901
Singh, R., Gupta, P., Khan, F., Singh, S. K., Mishra, T., Kumar, A., et al. (2018). Modulations in primary and secondary metabolic pathways and adjustment in physiological behavior of Withaniasomnifera under drought stress. Plant Sci. 272, 42–54. doi: 10.1016/j.plantsci.2018.03.029
Soma, F., Takahashi, F., Yamaguchi-Shinozaki, K., Shinozaki, K. (2021). Cellular phosphorylation signaling and gene expression in drought stress responses: ABA-dependent and ABA-independent regulatory systems. Plants 10, 756. doi: 10.3390/plants10040756
Song, Y., Chen, Q., Ci, D., Shao, X., Zhang, D. (2014). Effects of high temperature on photosynthesis and related gene expression in poplar. BMC Plant Biol. 14, 1–20. doi: 10.1186/1471-2229-14-111
Su, X., Wei, F., Huo, Y., Xia, Z. (2017). Comparative physiological and molecular analyses of two contrasting flue-cured tobacco genotypes under progressive drought stress. Front. Plant Sci. 8, 827. doi: 10.3389/fpls.2017.00827
Sun, L. P., Liu, Y., Kong, X. P., Zhang, D., Pan, J. W., Zhou, Y., et al. (2012). ZmHSP16.9, a cytosolic class I small heat shock protein in maize (Zea mays), confers heat tolerance in transgenic tobacco. Plant Cell Rep. 31, 1473–1484. doi: 10.1007/s00299-012-1262-8
Synková, H., Valcke, R. (2001). Response to mild water stress in transgenic Pssu-ipt tobacco. Physiologia Plantarum 112, 513–523. doi: 10.1034/j.1399-3054.2001.1120408.x
Tan, W., Meng, Q. W., Brestic, M., Olsovska, K., Yang, X. (2011). Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol. 168, 2063–2071. doi: 10.1016/j.jplph.2011.06.009
Tan, S.-L., Yang, Y.-J., Liu, T., Zhang, S.-B., Huang, W. (2020). Responses of photosystem I compared with photosystem II to combination of heat stress and fluctuating light in tobacco leaves. Plant Sci. 295, 110371. doi: 10.1016/j.plantsci.2019.110371
Tang, Z., Chen, L., Chen, Z., Fu, Y., Sun, X., Wang, B., et al. (2021). Climatic factors determine the yield and quality of Honghe flue-cured tobacco. Sci. Rep. 10, 19868. doi: 10.1038/s41598-020-76919-0
Tao, Z. Q., Wang, D. M., Chang, X. H., Wang, Y. J., Yang, Y. S., Zhao, G. C. (2018). Effects of zinc fertilizer and short-term high temperature stress on wheat grain production and wheat flour proteins. J. Integr. Agric. 17, 1979–1990. doi: 10.1016/S2095-3119(17)61791-0
Thor, K. (2019). Calcium—nutrient and messenger. Front. Plant Sci. 10, 440. doi: 10.3389/fpls.2019.00440
Van Liemt, G. (2001). The World Tobacco Industry: Trends and Prospects (ILO). Available online at: https://www.researchgate.net/publication/228768549_The_World_Tobacco_Industry_Trends_and_Prospects.
Wahid, A., Gelani, S., Ashraf, M., Foolad, M. R. (2007). Heat tolerance in plants: an overview. Environ. Exp. Bot. 61, 199–223. doi: 10.1016/j.envexpbot.2007.05.011
Wang, X., Cai, J., Liu, F., Jin, M., Yu, H., Jiang, D., et al. (2012). Pre-anthesis high-temperature acclimation alleviates the negative effects of post-anthesis heat stress on stem stored carbohydrates remobilization and grain starch accumulation in wheat. J. Cereal Sci. 55, 331–336. doi: 10.1016/j.jcs.2012.01.004
Wang, X., Shi, M., Zhang, R., Wang, Y., Zhang, W., Qin, S., et al. (2024). Dynamics of physiological and biochemical effects of heat, drought, and combined stress on potato seedlings. Chem. Biol. Technol. Agric. 11, 109. doi: 10.1186/s40538-024-00239-7
Waraich, E. A., Ahmad, R., Halim, A., Aziz, T. (2012). Alleviation of temperature stress by nutrient management in crop plants: a review. J. Soil Sci. Plant Nutr. 12, 221–244. doi: 10.4067/S0718-95162012000200003
Wollenweber, B., Porter, J. R., Schellberg, J. (2010). Lack of interaction between extreme high-temperature events at vegetative and reproductive growth stages in wheat. J. Agron. Crop Sci. 189, 142–150. doi: 10.1111/j.1439-037X.2002.tb00231.x
Xiong, X., Chang, L. Y., Muhammad, K., Zhang, J. J., Huang, D. F. (2018). Alleviation of drought stress by nitrogen application in Brassica campestris ssp. Chinensis L. Agronomy 8, 66. doi: 10.3390/agronomy8040066
Xu, Z., Shimizu, H., Yagasaki, Y., Ito, S., Zheng, Y., Zhou, G. (2013). Interactive effects of elevated CO 2, drought, and warming on plants. J. Plant Growth Regul. 32, 692–707. doi: 10.1007/s00344-013-9337-5
Yan, K., Chen, P., Shao, H., Zhao, S., Zhang, L., Zhang, L., et al. (2012). Responses of photosynthesis and photosystem II to higher temperature and salt stress in Sorghum. J. Agron. Crop Sci. 198, 218–225. doi: 10.1111/j.1439-037X.2011.00498.x
Yan, W., Lu, Y., Guo, L., Liu, Y., Li, M., Zhang, B., et al. (2024). Effects of drought stress on photosynthesis and chlorophyll fluorescence in blue honeysuckle. Plants 13, 2115. doi: 10.3390/plants13152115
Yang, L. Y., Yang, S. L., Li, J. Y., Ma, J. H., Pang, T., Zou, C. M., et al. (2018). Effects of different growth temperatures on growth, development, and plastid pigments metabolism of tobacco (Nicotiana tabacum L.) plants. Botanical Stud. 59, 1–13. doi: 10.1186/s40529-018-0221-2
Yanhui, C., Hongrui, W., Beining, Z., Shixing, G., Zihan, W., Yue, W., et al. (2020). Elevated air temperature damage to photosynthetic apparatus alleviated by enhanced cyclic electron flow around photosystem I in tobacco leaves. Ecotoxicology Environ. Saf. 204, 111136. doi: 10.1016/j.ecoenv.2020.111136
Yoshida, T., Fujita, Y., Sayama, H., Kidokoro, S., Maruyama, K., Mizoi, J., et al. (2010). AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 61, 672–685. doi: 10.1111/j.1365-313X.2009.04092.x
Zandalinas, S. I., Mittler, R., Balfagón, D., Arbona, V., Gómez-Cadenas, A. (2018). Plant adaptations to the combination of drought and high temperatures. Physiologia Plantarum 162, 2–12. doi: 10.1111/ppl.2018.162.issue-1
Zhang, J., Kirkham, M. B. (1994). Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol. 35, 785–791. doi: 10.1093/oxfordjournals.pcp.a078658
Zhou, R., Yu, X., Ottosen, C. O., Rosenqvist, E., Zhao, L., Wang, Y., et al. (2017). Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 17, 1–13. doi: 10.1186/s12870-017-0974-x
Zhu, J. K. (2016). Abiotic stress signaling and responses in plants. Cell 167, 313–324. doi: 10.1016/j.cell.2016.08.029
Keywords: high temperature, limited water irrigation, interactive role of high temperature and water stress, plant growth-yield-quality, physiological mechanisms, tobacco
Citation: Liu M, Liu X, Song Y, Hu Y, Yang C, Li J, Jin S, Gu K, Yang Z, Huang W, Su J and Wang L (2024) Tobacco production under global climate change: combined effects of heat and drought stress and coping strategies. Front. Plant Sci. 15:1489993. doi: 10.3389/fpls.2024.1489993
Received: 02 September 2024; Accepted: 08 November 2024;
Published: 26 November 2024.
Edited by:
Krishan K. Verma, Guangxi Academy of Agricultural Sciences, ChinaReviewed by:
Dao-Jun Guo, Hexi University, ChinaAjay Kumar, Amity University, India
Rajan Bhatt, Punjab Agricultural University, India
Copyright © 2024 Liu, Liu, Song, Hu, Yang, Li, Jin, Gu, Yang, Huang, Su and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaen Su, ZGx5Yzg4MTZAMTYzLmNvbQ==; Longchang Wang, d2FuZ2xjQHN3dS5lZHUuY24=
†These authors have contributed equally to this work
 Ming Liu
Ming Liu Xianglu Liu
Xianglu Liu Yuxiao Song2†
Yuxiao Song2† Longchang Wang
Longchang Wang