- Plant Production and Technologies Department, Faculty of Applied Sciences, Mus Alparslan University, Mus, Türkiye
As an ornamental plant, Tulipa sintenisii (Muş tulip) has great potential for potting and cut- flowers in floriculture. However, its low number of bulb production per plant is a major constraint to it becoming one of the common cultivated tulip species. This study was conducted to determine the impacts of 10 Bacillus species on bulb number increase as well as other plant parameters of T. sintenisii in the Mus province of Turkey in the 2020/2021 growing season. Selected, equally sized T. sintenisii bulbs were soaked with Bacillus spp. solution (3.4 × 107 CFU/cm3) for 2 s, and the inoculated bulbs were planted in the experimental field in autumn. The experiment was organized in a completely randomized block design with six replications. The investigated bulb parameters were taken at their physiological maturity. The tulip bulbs treated with Bacillus spp. had higher plant height (28.6 cm), bulb number/plant (2.25), total bulb weight (14.7 g), central bulb weight (13.1 g), central bulb length (40.9 mm), and central bulb diameter (26.8 mm) than the control treatment. The Bacillus strain EZF13 had the highest bulb number while EZF104 had the highest total bulb weight, central bulb weight, central bulb length, and central bulb diameter. These findings suggest that Bacillus treatment has great potential to increase bulb number per plant as well as other bulb parameters of native tulip species T. sintenisii. At the same time, an environmentally friendly production model was put forward without fertilizer application with bacteria application in tulips. At the same time, since the application of bacteria increases the usefulness of plant nutrients in the soil, it can be effective in reducing both the costs and the negative effects of fertilizers on the environment with less fertilizer use.
1 Introduction
Tulips are garden and cut-flower favorites from the Liliaceae family that belongs to the genus Tulipa having 150−160 species originating from central Asia (Coskuncelebi et al., 2008). Tulips are known as the third most important segment of cut flowers worldwide (Jhon and Neelofar, 2006; Marasek-Ciolakowska et al., 2012). The tulip bulb production throughout the world continues to increase over the years. Tulips are generally propagated vegetatively since propagation by seeds requires a long time and effort.
There are many tulip (Tulipa spp.) species growing in Turkey’s natural flora. One of these species is Tulipa sintenisii Baker (Muş tulip), an endemic species. It usually blooms in late April and early May, and has a flowering period of approximately 15–20 days. A single flower is formed from each germinated T. sintenisii bulb. The distribution areas of T. sintenisii are generally uncultivated fields and flat meadow areas (Yenikalayci et al., 2019). T. sintenisii has thick, red, and shiny petals with 35–40-cm plant height, which is suitable for growing as cut flowers, for potting, and as border plants.
Tulip bulbs are planted in autumn when the temperature drops and the flower stem begins to elongate with increasing air temperatures (approximately 14°C –20°C) at the beginning of spring. When the leaves dry completely, physiological maturation processes begin in the bulbs. During these development stages of the plant, while the main bulb begins to dry, the development of the baby bulbs reaches the highest level. In late spring, the upper part of the plant dries completely and after this period, the physiological maturation stages of tulip bulbs occur (Van Tuyl and Van Creij, 2007). During the physiological maturity process, leaf blades and male and female organs are formed in the internal structure of tulip bulbs. The completion of the physiological formation stage of the tulip, also known as the ‘G’ developmental stage, is one of the necessary processes for the flowering of tulip bulbs (De Hertogh and Le Nard, 1993). Although there have been many studies carried out to shorten the breeding process (Fortanier, 1973; Kuijpers and Langens-Gerrits, 1997; Ghaffoor et al., 2004), it takes approximately 5–6 years to propagate well-developed tulip bulbs by seeds (Zhang et al., 2023).
Beneficial soil microorganisms, a major part of the natural ecosystems, can stimulate plant growth and development, consequently, increasing the yield and quality of crops, and contributing a considerable amount of mineral solubility that can be easily adsorbed by the crop plant (Weller, 1988; Joshi et al., 2006; Balla et al., 2022). Due to the adverse impact of artificial fertilizers on human health and the environment, beneficial soil microorganism usage has been increasing globally in sustainable crop production systems (Cakmakci et al., 2007) since they promote plant growth and development, maintain or improve soil function and structure, enhance bioaccumulation and biogeochemical cycling of inorganic compounds, and control or inhibit plant pathogen growth and development (Doran and Zeiss, 2000; Ehrlich, 1990). In the rhizosphere of nutrient-deficient soils, nutrient mobilization and transformation highly depend on plant and microorganism interactions. At present, the use of beneficial soil microorganisms has become popular as a supplement to chemical fertilizers to have a satisfactory yield increase in sustainable crop production systems (Sturz et al., 2000). To enhance crop productivity, several symbiotic (Rhizobium sp.) and non-symbiotic bacteria have been used worldwide (Burd et al., 2000; Cocking, 2003). Among the huge number of microorganisms, Acinetobacter, Alcaligenes, Azospirillum, Azotobacter, Bacillus, Burkholderia, Derxia, Enterobacter, Gluconacetobacter, Herbaspirillum, Klebsiella, Lysobacter, Paenibacillus, Pseudomonas, and Rahnella have great potential as growth promoters or biofertilizers (Rodriguez et al., 2006).
The beneficial effects of Bacillus spp. inoculations on plant growth and development have been attributed to the production of plant growth regulators, enzymes, and natural antibiotics as well as biological control properties and antagonism effects against phytopathogenicity (Subiramani et al., 2020; Chandran et al., 2021; Etesami et al., 2021; Mahapatra et al., 2022; Ortiz and Sansinenea, 2022; Shen et al., 2023; Khoso et al., 2024). In addition to these, the growth and development of the root system of crop plants are improved by Bacillus spp., consequently, they enhance water and nutrient absorptions (Hungria et al., 2013; Ji et al., 2014; Galindo et al., 2018). Therefore, bulb number and bulb weight could be increased with the application of Bacillus spp.
Unlike other tulip species, the ability of T. sintenisii to produce daughter bulbs is very low. Bulb size and weight are characteristics that directly affect tulip flower quality and size. This situation is a factor that limits the cultivation and commercial production of T. sintenisii. Beneficial soil microorganisms can improve the growth and development of T. sintenisii by supplying nutrients, producing growth hormones, improving soil structure, and inhibiting pathogens. Therefore, the aim of this study was to investigate the effects of different Bacillus spp. strains on bulb number, size, and weight of T. sintenisii.
2 Materials and methods
The tested Bacillus species were obtained from the Department of Agricultural Biotechnology, Erciyes University (Table 1). Bacillus species were grown in Tryptic Soy Broth (pH 7.0) (Merck, Germany) under aerobic conditions at 30°C with shaking at 250 rpm for 48 h. Each bacterial solution was prepared and the number of bacteria in the solution was adjusted to 1 × 109 colony-forming unit CFU mL-1.
The T. sintenisii bulbs were obtained from Muş Alparslan University, Tulip Research Center. Several T. sintenisii plant selection programs were completed at Muş Alparslan University, Tulip Research Center. However, none of the selected clones of T. sintenisii has not been registered yet and there has not been any registered variety present in the market. Therefore, T. sintenisii bulbs used in the current study were a clone of selected T. sintenisii.
This experiment was conducted in the Experimental Field of Muş Alparslan University, (38°77′38”N latitude and 41°42′77”E longitude at an elevation of approximately 1,243 m above sea level). The soil of the experimental plots was a deep well-drained clay silt loam with a pH of 6.61, 2.21% organic matter, 22.1 kg ha-1 available phosphorus, and 780 kg ha-1 available potassium. Based on soil analysis, fertilizer was applied and incorporated into the soil prior to planting at a rate of 40, and 40 kg ha-1 N and P, respectively. Monthly maximum and minimum temperature, monthly average temperature (°C), monthly average relative humidity (%), and monthly total precipitation (mm) values of the experimental year are provided (Table 2). Total precipitation during the growing season was 385 mm and no irrigation was applied.
The T. sintenisii bulbs were obtained from Muş Alparslan University, Tulip Research Center. The tulip bulbs were washed under tap water and dried on filter paper 1 day before bacterial treatment. The bulbs were soaked with bacteria solution (3.4 × 107 CFU/cm3) for 2 s, and then the bulbs were immediately planted at a rate of 4 bulbs/m per row and 15-cm deep on 3 November 2020 and on 7 November 2021. The design of the experiment was a completely randomized block with four replications. The tulip bulbs were planted in a four-row 6-m long plots. The inter-row and intra-row spacing were 0.35 m and 0.25 m, respectively. The height of each plant in the two middle rows was measured at the flowering stage on 28 April 2020. At physiological maturity, all plants were dug up in the two middle rows on 10 June 2021. After digging, the bulbs on each plant were dissected into daughter bulb and central bulb.
The plant height (cm), number of bulbs per plant, total bulb weight (g), central bulb weight (g), and central bulb length and diameter (cm) were measured.
Field data were subjected to one-way analysis of variance using the GLM procedure of the SAS statistical package version 9.0. Means were compared using Fisher’s protected least significance difference (LSD) at type-I error of 0.05.
3 Results
Data from 2020 and 2021 trials were combined and analyzed together because there were no significant differences between the two cropping seasons for all of the investigated plant parameters.
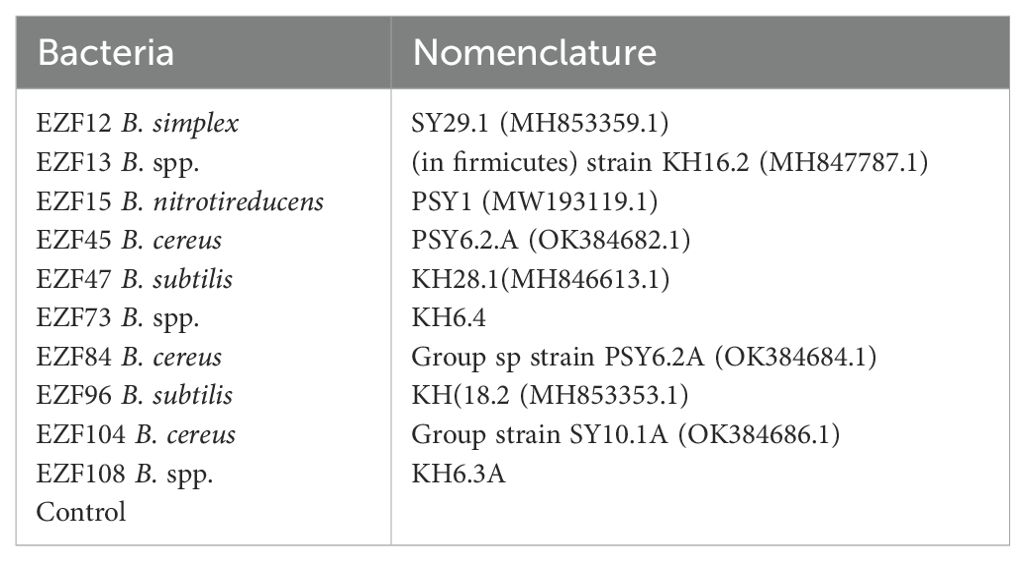
Among the mature bulbs treated with different Bacillus species, significant differences (P > 0.05) were observed for plant height and central bulb diameter (Figure 1).
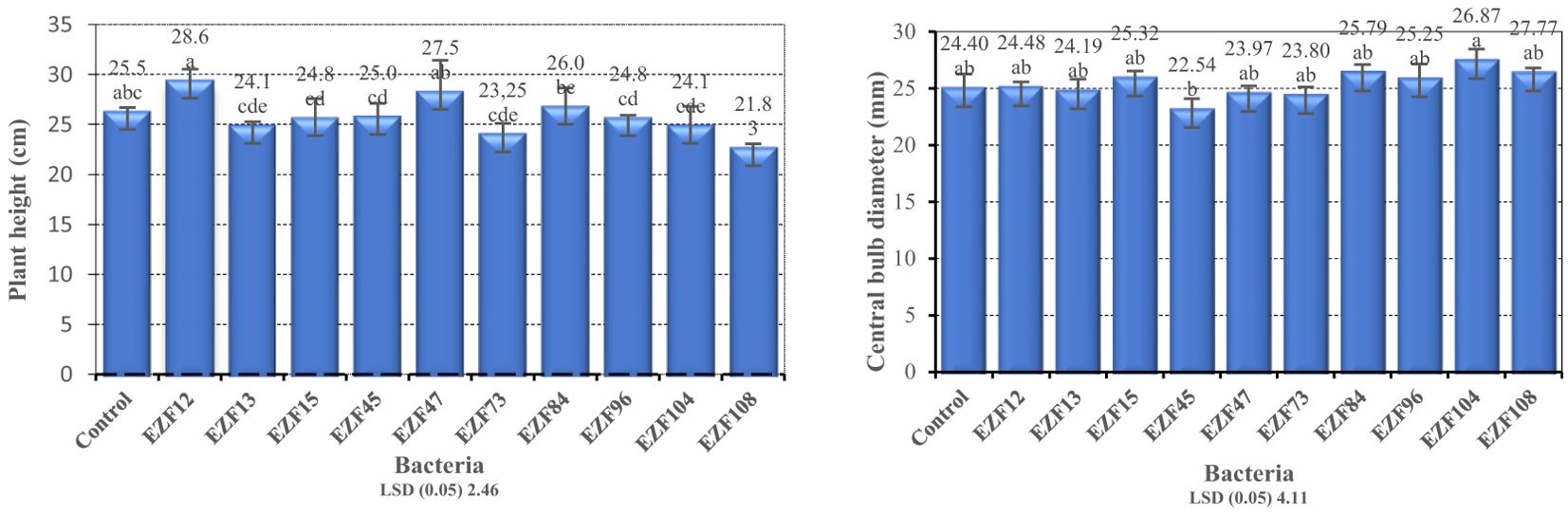
Figure 1. The plant height and central bulb diameter of Muş tulip treated with different Bacillus species. Different letters indicate significant differences at p < 0.05.
The highest plant height was obtained from EZF12-treated plants with 28.6 cm followed by EZF47 and the lowest was obtained from EZF108-treated plants with 21.8 cm (Figure 1). Seven of the plants treated with Bacillus spp. had the lowest height than the control treatment.
The effects of Bacillus on the central bulb diameter of Muş tulip were significant (Figure 1). Central bulb diameter varied between 22.5 and 26.8 mm. Plants treated with EZF45 B. spp. had the lowest central bulb diameter while plants treated with EZF104 B. spp. had the highest central bulb diameter.
Differences in bulb number per plant were significant among Bacillus spp. treatments (P < 0.05; Figure 2). Bulb number per plant varied between 1.25 and 2.25, the maximum bulb number per plant was obtained from EZF13 and the minimum was obtained from EZF45. Except for EZF45, all treated bacteria species had significantly higher bulb number per plant compared with the control treatment. Under natural growth conditions, T. sintenisii generally produces one bulb per year. Seven of the Bacillus species increased the bulb number per plant. When bulb production was the main target, EZF13 treatment could be the best application to increase bulb number per plant.

Figure 2. Bulb number/plant and bulb weight of Muş tulip treated with different Bacillus species. Different letters indicate significant differences at p < 0.05
A similar trend was observed for total bulb weight. Analysis of variance showed a significant total bulb weight difference among Bacillus spp. treatments (P < 0.05; Figure 2). Total bulb weight varied between 9.2 and 14.7 g. The heaviest total bulb weight was obtained in EZF104-treated bulbs and the lightest bulb was recorded in EZF45-treated bulbs. Eight of the Bacillus species-treated bulbs had greater total bulb weight than the control treatment.
When central bulb weight was in consideration, EZF73 had the highest central bulb weight with 13.14 g followed by EZF104 and EZF84. The lowest central bulb weight was noted in treatment EZF45 with 8.74 g followed by EZF47 (Figure 3).
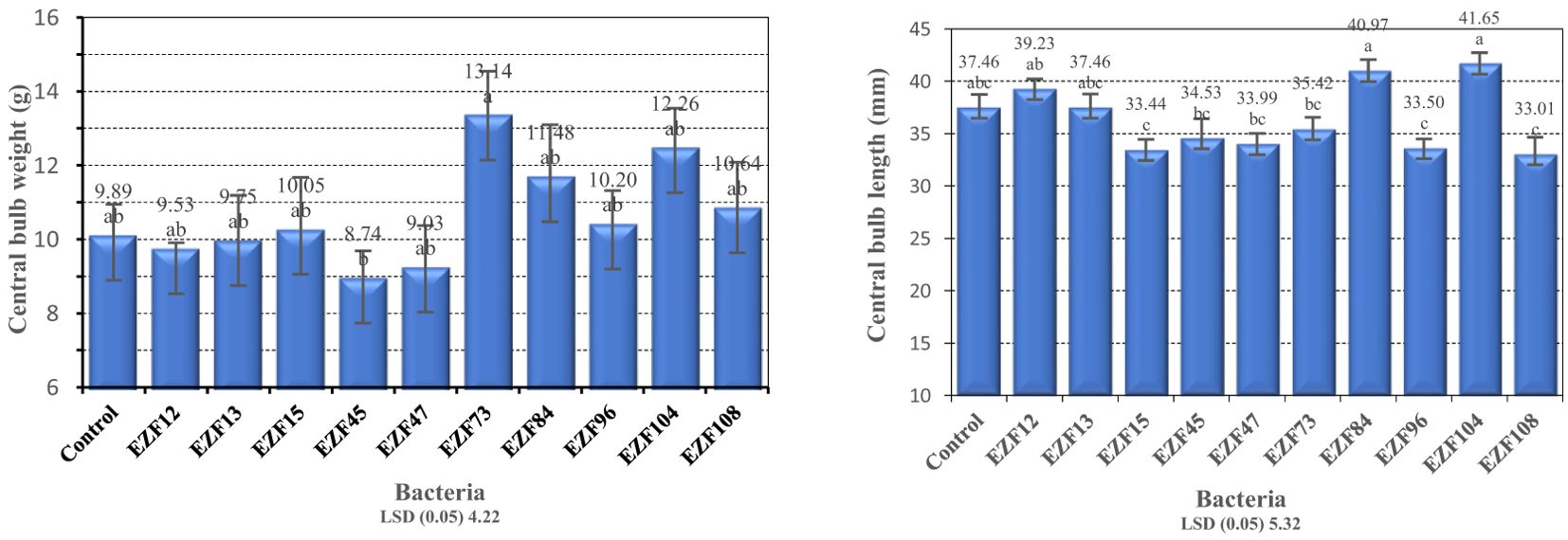
Figure 3. The central bulb weight and central bulb length of Muş tulip treated with different Bacillus species. Different letters indicate significant differences at p < 0.05
The tested Bacillus species differed significantly (P ≤ 0.05) from each other in their response in terms of central bulb lengths (Figure 3). The central bulb length varied between 41.6 and 33.0 mm. The highest and the lowest central bulb lengths were obtained from EZF104 and EZF108, respectively. The results showed that Bacillus treatment increased the bulb diameter. Three of the bacterial treatments (EZF104, EZF84, and EZF12) had higher bulb lengths than the control treatment.
The results of the present study showed that Bacillus spp. treatment had a positive impact on central bulb diameter. Compared with the control, six of the bacterial treatments increased bulb diameter (Figure 3). However, only the inoculation of EZF104 significantly increased bulb diameter. The treatment EZF45 had significantly lower bulb diameter than the control treatment.
4 Discussion
The improvement in the measured bulb parameters of T. sintenisii cannot be attributable to a single factor alone. The inoculation of the strains of B. spp. BEZF13 and B. cereus EZF104 showed the best effect on the evaluated T. sintenisii bulbs. It is well known that plant growth-promoting rhizobacteria are capable of promoting plant growth and development by synthesizing phytohormones, and enhancing plant nutrient acquisition and utilization (Corrales et al., 2014; Chandran et al., 2021; Etesami et al., 2021; Mahapatra et al., 2022; Ortiz and Sansinenea, 2022; Khoso et al., 2024). They are widely used as growth enhancers for many crop plants (Blake et al., 2021; Dobrzyński et al., 2022; Youssfi et al., 2024; Vincze et al., 2024).
The ability of Bacillus spp. strains to colonize the bulb or root system depends on plant species (Yanti et al., 2017). Inoculated Bacillus spp. strains must be able to establish and interact with the root system (Blake et al., 2021; Rajer et al., 2022; Shen et al., 2023). Compared to control, the inclusion and colonization of Bacillus spp. strains EZF12, EZF47, and EZF84 on bulbs increased plant height by 12.2%, 7.8%, and 1.9%, respectively. The central bulb weight, length, and diameter are important bulb parameters to assess the potential of tulip bulb blooming in the next spring. In the current study, central bulb weight significantly increased with Bacillus spp. strain treatments. The size of bulbs and bulblets depends on tulip species and tulip cultivars (Kleynhans, 2006). The ideal bulb size for most of the tulip species is approximately 12 cm. However, for T. sintenisii, bulb size is approximately 4 cm. For all tulip species, larger bulbs are highly preferred since smaller bulbs have low crop quality with smaller flowers and shorter plant heights. In the current study, the bulb size of plants treated with EZF12, EZF84, and EZF104 Bacillus spp. strains were greater than the control plants. Central bulb weight was one of the most important bulb parameters significantly influenced by inoculation of the Bacillus spp. The size of tulip bulbs in order to flower varies by species; the minimum size is generally from 6 to 8 g. Carl et al. (2015) stated that this range corresponds to approximately 6 to 9 cm in circumference. Since tulip bulbs must reach a critical weight in order to have a flower bud (Sestras et al., 2007). In the present study, central bulb weight was increased 45.5% by inoculation of the Bacillus spp. strain EZF73. Total bulb weight was another parameter positively influenced by the Bacillus spp. Total bulb weight was increased 35.6% by inoculation of the Bacillus spp. strain EZF104. These results can be attributed to the success of these Bacillus spp. strains (EZF73 and EZF104) in associating with tulip roots. After planting tulip bulbs, bacteria inoculation or soil fertility will not affect blooming of the bulbs in the next spring, but it will influence the growth and development of new bulbs. Therefore, inoculation of tulip bulbs with proper strains of Bacillus spp. can increase the size and weight of tulip bulbs.
5 Conclusion
Tulip bulbs treated with Bacillus spp. showed improvement in plant height, total bulb number, and central bulb weight, length, and diameter. Bacillus spp. strain EZF13 has great potential to increase bulb number per plant. Tulip bulb treatment with this strain could be recommended to increase the bulb number of T. sintenisii under field conditions in commercial tulip bulb production. Determining the appropriate bacterial strains for each crop may be beneficial in terms of increasing soil fertility, reducing fertilizer rate, and decreasing the negative impact of fertilizers on the environment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
I would like to thank the staff of the Muş Tulip Application and Research Center Directorate for their contribution to the conduct of this research.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Balla, A., Silini, A., Cherif-Silini, H., Chenari Bouket, A., Alenezi, F. N., Belbahri, L. (2022). Recent advances in encapsulation techniques of plant growth-promoting microorganisms and their prospects in the sustainable agriculture. Appl. Sci. 12, 9020. doi: 10.3390/app12189020
Blake, C., Nordgaard, M., Maróti, N., Maróti, G., Kovács, A. T. (2021). Diversification of Bacillus subtilis during experimental evolution on Arabidopsis thaliana and the complementarity in root colonization of evolved subpopulations. Environ. Microbiol. 23, 6122–6136. doi: 10.1111/1462-2920.15680
Burd, G. I., Dixon, D. G., Glick, B. R. (2000). Plant growth promoting bacteria that decrease heavy metal toxicity in plants. Can. J. Microbiol. 46, 237–245. doi: 10.1139/w99-143
Cakmakci, R., Erat, M., Erdogan, U. G., Donmez, M. (2007). The influence of PGPR on growth parameters, antioxidant andpentose phosphate oxidative cycle enzymes in wheat and spinachplants. J. Plant Nutr. Soil Sci. 170, 288–295. doi: 10.1002/jpln.200625105
Carl, E. N. J., Paul, V. N., Dickey, D. A. (2015). Growth, development, and mineral nutrient accumulation and distribution in tulip from planting through post anthesis shoot senescence. Int. J. Agron. 2015, 341287. doi: 10.1155/2015/341287
Chandran, H., Meena, M., Swapnil, P. (2021). Plant growth-promoting Rhizobacteria as a green alternative for sustainable agriculture. Sustainability 13, 10986. doi: 10.3390/su131910986
Cocking, E. C. (2003). Endophytic colonization of plant roots by nitrogenfixing bacteria. Plant Soil. 252, 169–175. doi: 10.1023/A:1024106605806
Corrales, R. L. C., Sánchez, L. L. C., Arévalo, G. Z. Y., Moreno, B. V. E. (2014). Bacillus: género bacteriano que demuestra ser un importante solubilizador de fosfato. Nova 12, 165–177. Available at: http://www.scielo.org.co/pdf/nova/v12n22/v12n22a06.pdf.
Coskuncelebi, K., Terzioglu, S., Türkmen, Z., Makbul, S., Usta, A. (2008). A study comporative study on two closely relative Tulipa L. Taxa from NE Anatolia. Plant Syst. Evol. 276, 191. doi: 10.1007/s00606-008-0094-z
De Hertogh, A. A., Le Nard, M. (1993) (Amsterdam: Elsevier Science Publishers). Wold production and horticultural utilization of flower bulbsCap, 2.
Dobrzyński, J., Jakubowska, Z., Dybek, B. (2022). Potential of Bacillus pumilus to directly promote plant growth. Fronters Microbiol. 13. doi: 10.3389/fmicb.2022.1069053
Doran, J. W., Zeiss, M. R. (2000). Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 15, 3–11. doi: 10.1016/S0929-1393(00)00067-6
Etesami, H., Jeong, B. R., Glick, B. R. (2021). Potential advantage of rhizosheath microbiome, in contrast to rhizosphere microbiome, to improve drought tolerance in crops. Rhizosphere 20, 100439. doi: 10.1016/j.rhisph.2021.100439
Fortanier, E. J. (1973). Reviewing the length of the generation period and its shortening, particularly in tulips. Sci. Hortic. 1, 107–116. doi: 10.1016/0304-4238(73)90010-1
Galindo, F. S., Teixeira Filho, M. C. M., Buzetti, S., Ludkiewicz, M. G. Z., Rosa, P. A. L., Tritapepe, C. A. (2018). Technical and economic viability of co-inoculation with Azospirillum brasilense in soybean cultivars in the Cerrado. Rev. Bras. Eng. Agric. Ambient. 22, 51–56. doi: 10.1590/1807-1929/agriambi.v22n1p51-56
Ghaffoor, A., Maqbool, I., Waseem, K., Quraishi, A. (2004). [amp]]Idot;n vitro response of (Tulipa gesteriana L.) to various growth regulators. Int. J. Agric. Biol. 6, 1168–1169.
Hungria, M., Nogueira, M. A., Araujo, R. S. (2013). Co-inoculation of soybeans and common beans with rhizobia and azospirilla: Strategies to improve sustainability. Biol. Fertil. Soils. 49, 791–801. doi: 10.1007/s00374-012-0771-5
Jhon, A. Q., Neelofar (2006). “Tulip,” in Bulbous Ornamental and Aquatic Plants: Adv. in Ornam. Horticulture, vol. 3 . Ed. Bhattacharjee, S. K. (Pointers Publishers, Jaipur, India), 1–72.
Ji, S. H., Gururani, M. A., Chun, S. C. (2014). Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars? Microbiol. Res. 169, 83–98. doi: 10.1016/j.micres.2013.06.003
Joshi, K. K., Kumar, V., Dubey, R. C., Maheswari, D. K. (2006). Effect of chemical fertilizer adaptive variants, Pseudomonas aeruginosa GRC2 and Azotobacter chroococcum AC1 ON Macrophomina phaseolina causing charcoal rot Brassica juncea. Korean J. Environ. Agric. 25, 228–235. doi: 10.5338/KJEA.2006.25.3.228
Khoso, M. A., Wagan, S., Alam, I., Hussain, A., Ali, Q., Saha, S., et al. (2024). Impact of plant growth-promoting rhizobacteria (PGPR) on plant nutrition and root characteristics: Current perspective. Plant Stress. 11, 100341. doi: 10.1016/j.stress.2023.100341
Kleynhans, R. (2006). “Lachenalia, spp,” in Flower Breeding and Genetics: Issues, Challenges and Opportunities for the 21st Century. Ed. Anderson, N. O. (Springer, Dordrecht), 491–516.
Kuijpers, A. M., Langens-Gerrits, M. (1997). Propagation of tulip in vitro. Acta Hortic. 430, 321–324. doi: 10.17660/ActaHortic.1997.430.49
Mahapatra, S., Yadav, R., Ramakrishna, W. (2022). Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 132, 3543–3562. doi: 10.1111/jam.15480
Marasek-Ciolakowska, A., Ramanna, M. S., Aren, P., Van Tuy, J. M. (2012). Breeding and cytogenetics in the genus Tulipa. Floriculture Ornamental Biotech. 6, 90–97.
Ortiz, A., Sansinenea, E. (2022). The role of beneficial microorganisms in soil quality and plant health. Sustainability 14, 5358. doi: 10.3390/su14095358
Rajer, F. U., Samma, M. K., Ali, Q., Rajar, W. A., Wu, H., Raza, W., et al. (2022). Bacillus spp.-Mediated Growth Promotion of Rice Seedlings and Suppression of Bacterial Blight Disease under Greenhouse Conditions. Pathogens 11, 1251. doi: 10.3390/pathogens11111251
Rodriguez, H., Fraga, R., Gonzalez, T., Bashan, Y. (2006). Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil. 287, 15–21. doi: 10.1007/s11104-006-9056-9
Sestras, R., Mihalte, L., Sestras, A., Baciu, A., Bondrea, I. (2007). Research regarding the main traits of several genotypes of tulips. J. Agric. Rev. Agricola. 16, 140–145.
Shen, Y., Yang, H., Lin, Z., Chu, L., Pan, X., Wang, Y., et al. (2023). Screening of compound-formulated Bacillus and its effect on plant growth promotion. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1174583
Sturz, A., Christie, B., Nowak, J. (2000). Bacterial endophytes: potential role in developing sustainable systems of crop production. CRC Crit. Rev. Plant Sci. 19, 1–30. doi: 10.1080/07352680091139169
Subiramani, S., Ramalingam, S., Muthu, T., Nile, S. H., Venkidasamy, B. (2020). Development of Abiotic Stress Tolerance in Crops by Plant Growth-Promoting Rhizobacteria (PGPR) (Singapore: Springer), 125–145. doi: 10.1007/978-981-15-2576-6_8
Van Tuyl, J. M. V., Van Creij, M. G. (2007). “Tulip,” in Flower Breeding and Genetics (Springer, Dordrecht), 623–641.
Vincze, É.-B., Becze, A., Laslo, É., Mara, G. (2024). Beneficial soil microbiomes and their potential role in plant growth and soil fertility. Agriculture 14, 152. doi: 10.3390/agriculture14010152
Weller, D. M. (1988). Biological control of soilborne pathogens in the rhizosphere with bacteria. Annu. Rev. Phytopathol. 26, 379–407. doi: 10.1146/annurev.py.26.090188.002115
Yanti, Y., Habazar, T., Reflinaldon, R., Nasution, Chainur, R. N., Felia, S. (2017). Indigenous Bacillus spp. ability to growth promoting activities and control bacterial wilt disease (Ralstonia solanacearum). Biodiversitas 18, 1562–1567. ISSN 1412-033X. doi: 10.13057/biodiv/d180434
Yenikalayci, A., Tufan, Y., Kayaalp, N., Karada, g Y. (2019). “Distribution areas, characteristics and problems of Muş Tulip (Tulipa sistenisii Baker),” in International Agricultural Congress of Mus Plain, Mus/Turkey, September 24-27/2019. 436–446. Available at: https://lalemmaun.alparslan.edu.tr/files/70/TulipasintenisiiMu%C5%9F2019.pdf.
Youssfi, C. E., Soujaa, H., Hammoudani, Y. E., Mohammed, H. Z., Mourabit, N., Aarab, S. (2024). “Overview of insights into the role of Bacillus species in drought stress alleviation and plant disease management,” in E3S Web of Conferences, Vol. 527. 03010. doi: 10.1051/e3sconf/202452703010
Keywords: Bacillus spp., Muş tulip, rhizobacteria, tulip bulb, Tulipa sintenisii
Citation: Yenikalaycı A (2025) Impact of different strains of Bacillus spp. on the bulb production of Tulipa sintenisii Baker. Front. Plant Sci. 15:1456919. doi: 10.3389/fpls.2024.1456919
Received: 29 June 2024; Accepted: 17 December 2024;
Published: 06 February 2025.
Edited by:
Tajamul Hussain, Oregon State University, United StatesReviewed by:
Jiban Shrestha, Nepal Agricultural Research Council, NepalAwad Shala, Horticulre Research Station, Egypt
Copyright © 2025 Yenikalaycı. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmet Yenikalaycı, YS55ZW5pa2FsYXljaUBhbHBhcnNsYW4uZWR1LnRy
 Ahmet Yenikalaycı
Ahmet Yenikalaycı