- 1Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, China
- 2College of Life Sciences and Technology, Mianyang Tearchers’ College, Mianyang, Sichuan, China
- 3College of Ecology and Evironment, Chengdu University of Technology, Chengdu, China
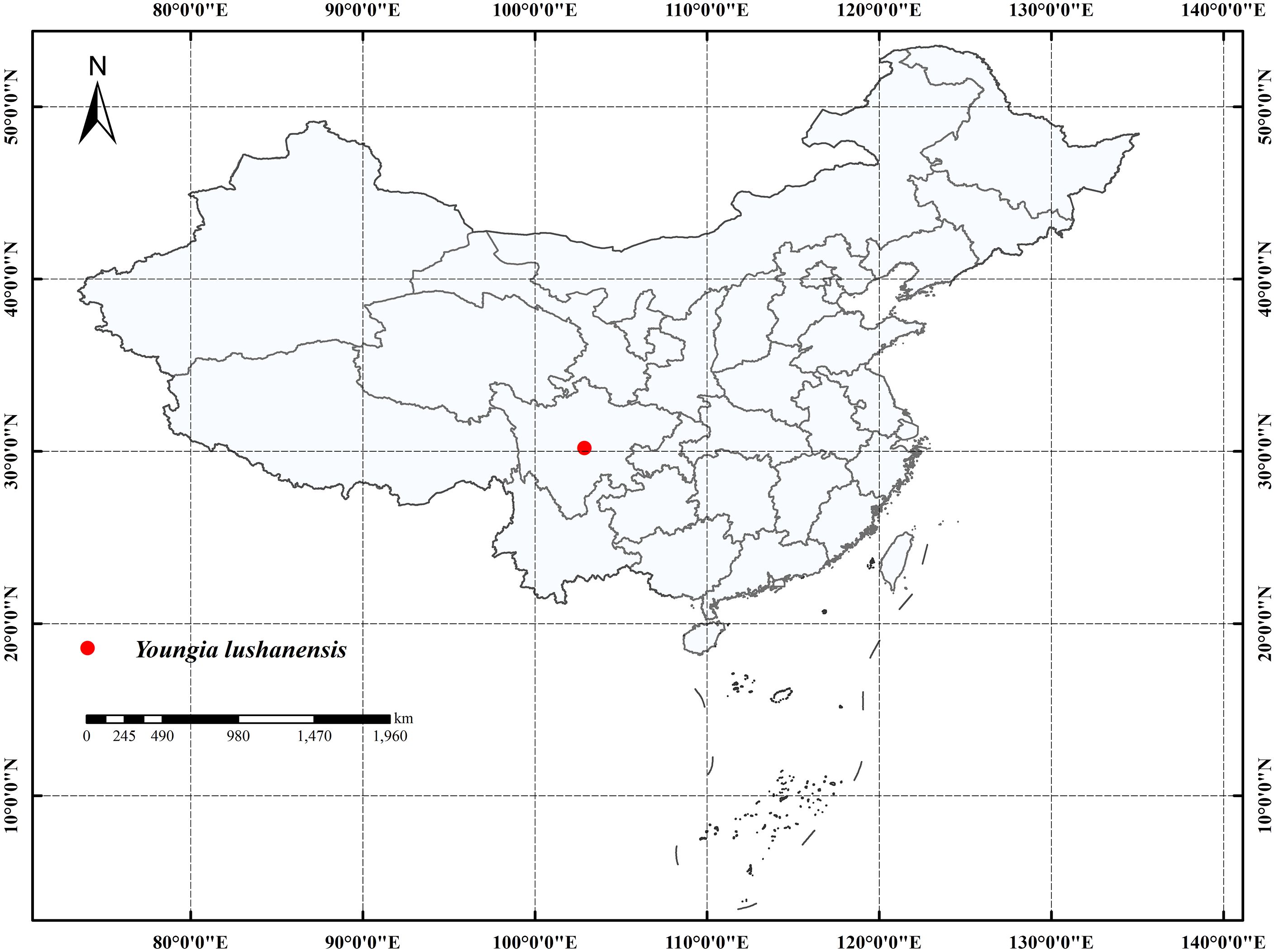
Youngia lushanensis (Asteraceae), a new species of Youngia, is described and illustrated from Lushan in Sichuan, Southwest China. Its systematic position is evaluated based on a molecular phylogenetic analysis of the nuclear ribosomal ITS and on morphological comparison with related species. It is morphologically similar to Y. purpimea and Y. szechuanica, with purplish red abaxially leaves. But it differs from the latter two in that its caudex are glabrous, leaf margins are sparsely dentate, and being glabrous on both surfaces, 9-11 florets in each capitulum, and inner phyllaries are crested. Phylogenetic analysis based on ITS also showed this new species is closely related with Y. purpimea and Y. szechuanica. The three species Y. lushanensis Y. purpimea and Y. szechuanica, formed a basal group of Youngia, which is distributed in moist slopes in Southwest China.
1 Introduction
Youngia Cassini belongs to the Asteraceae family, Cichorioideae subfamily and Tribe Cichoreae, and a member of euasterids II clade of Asterids (APGIV, 2016). Babcock and Stebbins (1937) subdivided this genus into six sections, i.e. “ Sect. Crepidiopsis”, “Sect. Desiphylum”. “Sect. Stenophytum”, Sect. “Hieraciella” and Sect. “Mesomeris” and “sect. Euyoungia”. There is considerable controversy among different scholars regarding the circumscription of Youngia (Babcock and Stebbins, 1937; Hu, 1969; Sennikov and Illarionova, 2008; Maity and Maiti, 2010; Shi and Kilian, 2011). Our former study found that the genus Youngia of Babcock & Stebbins systematics is not a monophyletic group, Youngia diversifolia and Y. nansiensis (“Sect. Crepidiopsis Babcock & Stebbins”), should be a member of the genus Crepidiastrum. Paraixeris humifusa (Dunn) C. Shih should be transferred to the genus Youngia (Peng et al., 2014). There is confusion in the boundary between Youngia and its related genera Crepidiastrum Nakai. Crepidiastrum, is a genus established by Nakai (1920), with about 16 species: C. and E. Asia, including N. Pacific Bonin (Ogasawara) Islands, nine species (two endemic) in China, including Crepidifolium Sennikov, Geblera Kitagawa (1937), and Paraixeris Nakai [=Youngia Cass. sect. Paraixeris (Nakai)] Kitam. (1942) (Shi and Kilian, 2011; Peng et al., 2014). Phylogenic studies showed that Crepidiastrum is the sister group of Youngia (Kilian et al., 2009; Zhang et al., 2011; Peng et al., 2014).
In addition to the widespread distribution of Y. japonica L., Youngia is a genus consisting of approximately 30 species mainly distributed in East Asia (Shi and Kilian, 2011). China is the diversity center of this genus, with 28 species (22 endemic) in China (Shi and Kilian, 2011). Recently, as botanical research continues, more new species of Youngia have been discovered in China. Youngia zhengyiana T. Deng, D.G. Zhang, J.W. Zhang & H. Sun was found in Lipo County, Guizhou Province (Deng et al., 2014). Later, three new species Y. purpimea Y.L. Peng, W.B. Ju, X.F. Gao & Y.D. Gao (Peng et al., 2015), Y. jiulongensis Y.L. Peng, X.F. Gao & Li Bing Zhang (Peng et al., 2017), and Youngia baoxingensis Y.S. Chen (Chen, 2018) were reported from Sichuan Province. Youngia gongshanensis Y.S. Chen & R. Ke was found from Yunnan Province by Ke and Chen (2016). Youngia jiulongshanensis. Cai, Y.L. Xu & X.F. Jin was found from south-western Zhejiang Province of East China by Cai et al. (2019). Youngia hangii T. Deng, D.G. Zhang, Qun Liu & Z.M. Li from Hubei Province of Central China by Liu et al. (2021). The systematics and diversity of Youngia still need to be studied, especially for the biennial or perennial taxa, due to significant morphological variations in flowers and leaves. In 2023, the author collected a unique member of this genus in Lushan County, Sichuan Province, China. In this paper, new materials were collected, morphology evaluation and molecular analyses were employed to determine the phylogeny of Youngia.
2 Methods and materials
2.1 Plant materials and morphological assessment
The plants were collected in Lushan County. A comparative study was conducted on the morphological characteristics of this species with its closely related species. Five species of Crepidiastrum and 19 specimens of Youngia were sampled. All species of Youngia were used as in-groups, and those of Crepidiastrum were used as outgroups.
Leaf material for DNA analysis was generally obtained (as silica gel-dried material) from wild populations. All vouchers of samples in our experiments and the holotype specimens of Y. lushanensis were deposited in the Herbarium of the Chengdu Institute of Biology (CDBI). In total, 8 out of 33 DNA sequences were newly generated for this study (see Table 1). Taxa sampled, voucher information, and GenBank accession numbers for the datasets were listed in Table 1. The plant material used for morphological studies were listed in Supplementary Material S1. The shape of the leaves, leaf attachment pattern, the morphology of the stems, the number of florets per capitulum, pappus color and whether the inner phyllaries have appendages are important taxonomic features of this genus. Thus, inner phyllaries, basal part of stem, pappus, leaves were chosen for qualitative characters. Florets per capitulum, length of inner phyllaries, pappus and achenes, number of capitula and leaf size were chosen for quantitative characters to study the related species morphology. Indumentum of leaves and petioles, achenes were observed and then were taken photos using a light microscope (Zeiss 2000). T-test method was used to analyzing the significance of differences in quantitative traits between new species and related species.
Table 1. Voucher information and GenBank accession numbers for the 25 species of Crepidiastrum and Youngia genera.
2.2 Study area
All the Youngia species were collected from China. The presumed new species Y. lushanensis were collected from Lushan County, Sichuan, Southwest of China (Figure 1). Lushan County is located at “Huaxi Rain Screen Belt” (Zhuang and Gao, 2002). This area is subtropical monsoon climate with an average annual precipitation of 1950-2000 milliliters, was famous for its high diversity (Sun et al., 2019).
2.3 DNA extraction, PCR amplification and sequencing
Total genomic DNA was isolated and cleaned with TIANGEN plant genomic DNA extraction kit (TIANGEN Biotech., Beijing, China). One nuclear region (ITS) was analyzed. The quality of DNA extraction was detected by gel electrophoresis methods. Electrophoresis was carried out on 0.8% agarose gel, and the gel with clear and bright target bands was recovered. The obtained DNA was stored in 4°Crefrigerators for DNA amplification. All amplifications were performed in 50 µl volumes containing 34 µl deionized sterile water, 3 µl of 25 mmol/L MgCl2 solution, 5 µl Taq reaction buffer, 4 µl of a 2.5 mmol/L dNTP solution, 1 µl of each primer at 10 p mol/mL, 1 µl (2.5 unit) Taq DNA polymerase (Tiangen), and 1 µl genomic DNA (20–100 ng). All amplifications were conducted on a DNA Engine Peltier Thermal Cycler (Bio-Rad Laboratories, USA). All the regions used the same thermal profiles, including a 5 min denaturation at 94°C followed by 33 cycles of 94°C for 45 s, 54°C for 1 min, and extension at 72°C for 1 min, with a final extension of 72°C for 7 min prior to holding at 12°C forever. The PCR products were separated by electrophoresis using 0.8–1.0% agarose gels and visualized with ethidium bromide. Amplified products were purified using E. Z. N. A. gel extraction kit (OMEGA, Biotech., USA). ITS primers its1 (White et al., 1990) and itsb (Blattner, 1999). All ITS fragment sequencing results of the samples were normal with single peak between 600-700 bp. The comparison results using Blast software in GenBank also indicate that the sequenced samples were the ITS gene, and then were used for phylogenic analysis.
2.4 Phylogenetic analysis
The sequences were aligned with Bioedit 7. 1.11 (Hall, 1999), followed by manual adjustment of the misaligned bases by the software. The gaps caused by mononucleotide repeat units were not considered in the phylogenetic analysis, because their homology can be highly uncertain (Kelchner, 2000). Prior to the model-based MP and BI approaches, the best fit model of nucleotide substitutions for the ITS sequence data was explored with the hierarchical likelihood ratio test as implemented in jModelTest2 (Darriba et al., 2012), available at https://groups.google.com/forum/#!forum/jmodeltest, which suggested TIMef+G as the best model. We used the aligned sequences to construct an ML phylogenetic tree was performed using RAxML-HPC2 (8.1.2) (Stamatakis, 2014), available at http://bioinformatics.oxfordjournals.org/content/early/2014/01/21/bioinformatics.btu033.abstract) with 1000 rapid bootstrap analyses followed by a search for the best scoring tree in one single run. BI was run using MrBayes v.3.1.2 (Ronquist et al., 2012). Bayesian tree topology was determined from two independent Markov chain Monte Carlo (MCMC) runs of four incrementally heated chains. Runs were performed for 10 million generations with sampling of trees every 1000 generations. When the log-likelihood scores were found to have stabilized, a consensus tree was calculated after discarding the first 25% of trees as burn-in.
3 Results
3.1 Morphological assessment
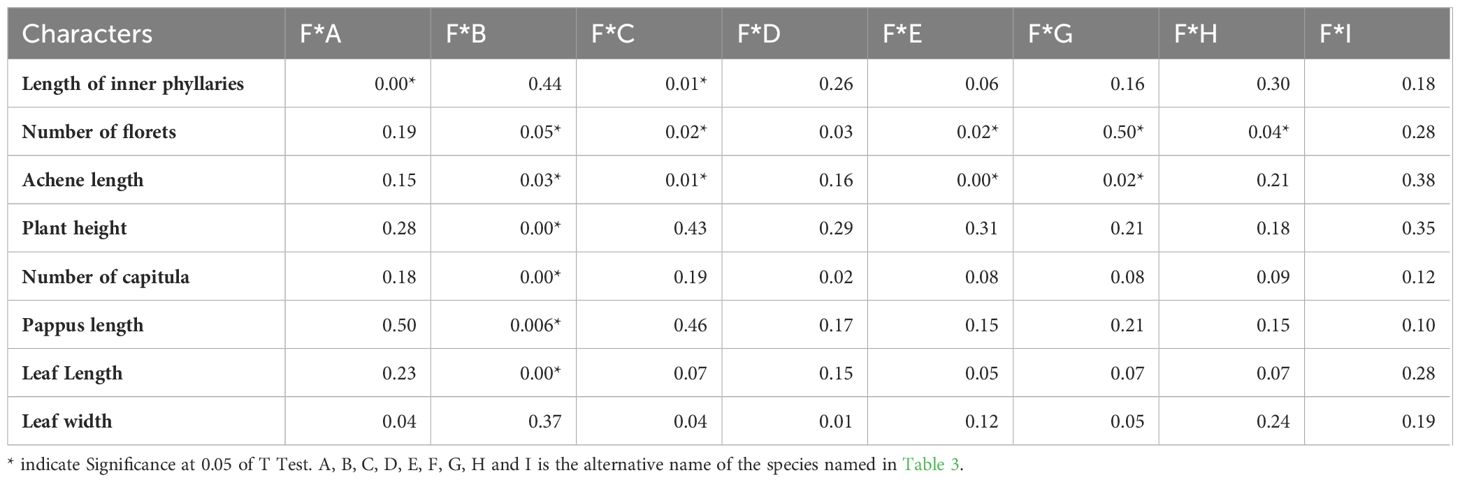
Qualitative characters comparison between Youngia lushanensis, Y. szechuanica, Y. purpimea, Y. zhenyiana, Y. baoxingensis, Y. heterophylla, Y. gracilipes, Y. cineripappa, and Y. paleacea were listed in Table 2. The inner phyllaries of three species including Y. lushanensis, Y. paleacea and Y. baoxingensis are crested with a narrow curved claw near tips. While the inner phyllaries of the other species are smooth and without coronal appendages. The leaf color of Y. lushanensis, Y. szechuanica, Y. purpimea and Y. zhenyiana is similar, with green surface green, purplish red abaxial surface, that of the rest is green on both surface. Indumentum on the surface of leaves and petioles varies among different species (Figure 2, Table 2). The leaves and petioles of Y. szechuanica have brown multicellular pubic hairs on both surfaces, those of Y. paleacea are glabrous or very sparsely white tomentose, those of Y. heterophylla & Y. gracilipes, are sparsely pubescent. And those of Y. lushanensis and the other surface are glabrous. The three species including Y. lushanensis, Y. szechuanica and Y. purpimea have yellowish pappus. The pappus of Y. cineripappa are grayish, that of Y. baoxingensis are brown, and the rest are white (Figure 3). The achenes of Y. lushanensis are yellowish brown, and those of the other compared species are dark brown or blackish brown. Quantitative characters comparison between the related species were listed in Table 3. T-test showed that the flores number per capitulum of the new species Y. lushanensis are significantly different from Y. szechuanica, Y. purpimea, Y. zhenyiana, Y. baoxingensis, Y. gracilipes and Y. cineripappa (Table 4). Number of florets per capitulum, number of capitula in the whole inflorescences, leaf length and plant height are significantly different between Y. lushanensis and Y. baoxingensis.

Table 2. Qualitative character comparison between Youngia lushanensis, Youngia szechuanica, Youngia purpimea, Youngia zhenyiana, Youngia baoxingensis, Youngia heterophylla, Youngia gracilipes, Youngia cineripappa and Youngia paleacea.

Figure 2. Indumentum of leaves, margin and petioles of Youngia. (A–C) Y. Paleacea, (A) Abaxial surface, (B) Ventral surface, (C) Petiole; (D–G) Y. purpimea, (D) Abaxial surface, (E) Ventral surface, (F) Petioles, (G) Margin; (H–K) Y. szechuanica, (H) Abaxial surface, (I) Ventral surface, (J) Petiole, (K) Margin, (L–O) Y. heterophylla, (L) Abaxial surface, (M) Ventral surface, (N) Petiole, (O) Margin; (P–S) Y. cineripappa, (P) Ventral surface, (Q) Abaxial surface, (R) Petiole, (S) Margin; (T, U) Y. gracilipes, (T) Abaxial surface and margin, (U) Ventral surface and margin; (V–X) Y. lushanensis, (V) Ventral surface & margin, (W) Abaxial surface, (X) Petiole.
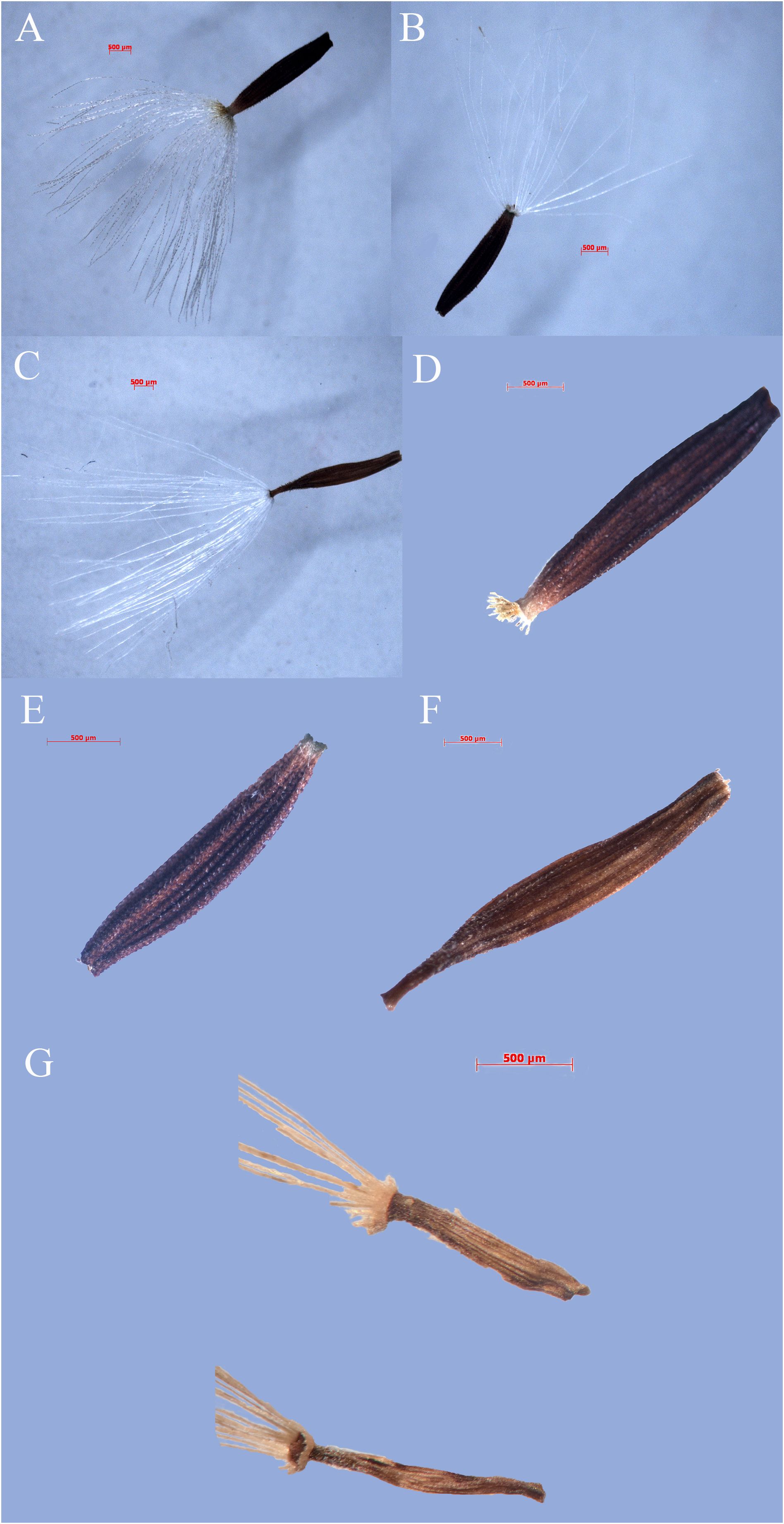
Figure 3. Fruits of Youngia lushanensis and related species. (A, F) Y. paleacea; (B, E) Y. heterophylla; (C, D) Y. cineripappa; (G) Y. lushanensis.
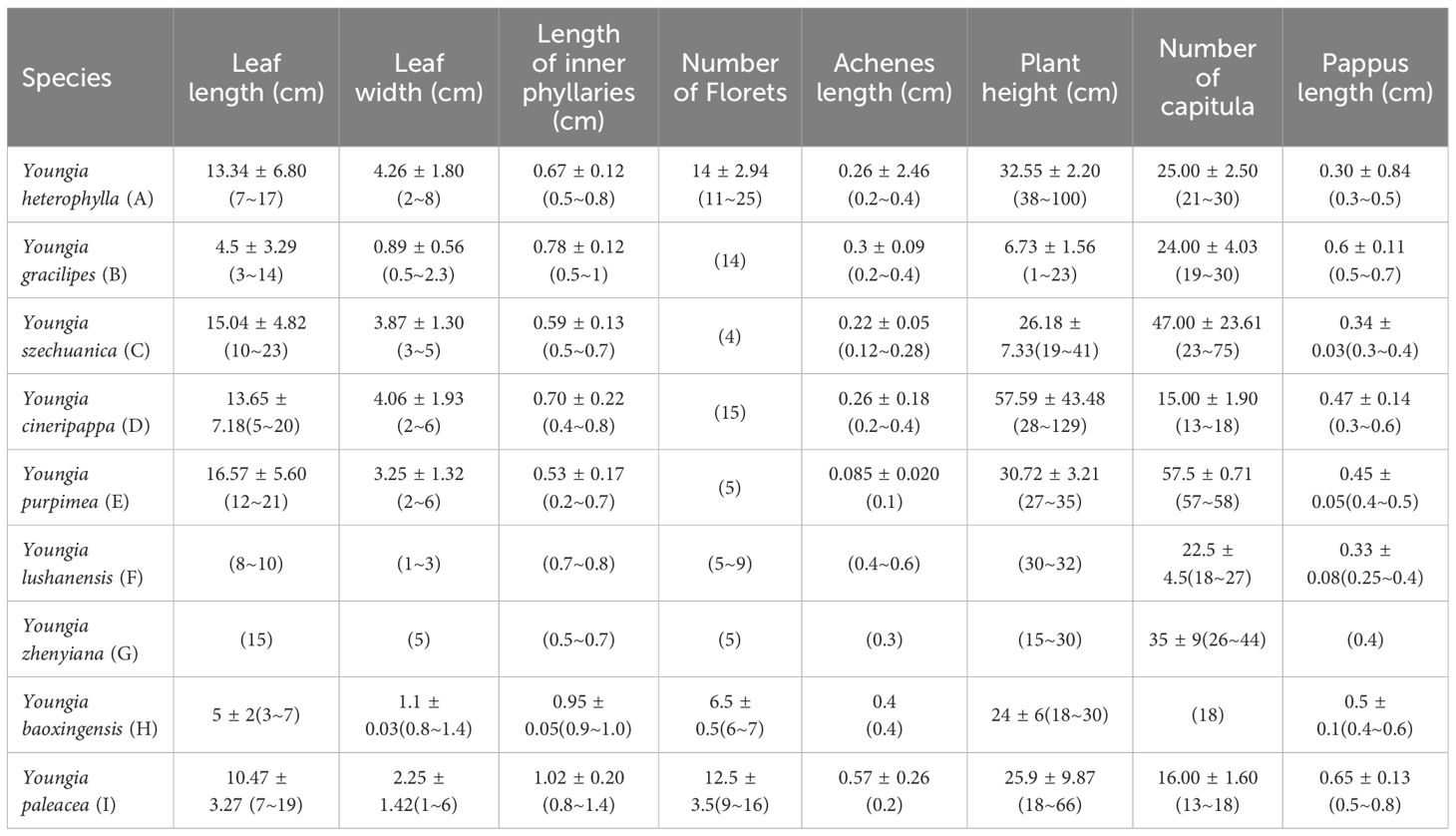
Table 3. Quantitative character comparison between Youngia lushanensis, Y. szechuanica, Y. purpimea, Y. zhenyiana, Y. baoxingensis, Y. heterophylla, Y. gracilipes, Y. cineripappa and Y. paleacea.
3.2 Phylogenetic tree
From the Bayesian tree (Figure 4), the species of Youngia formed a monophyletic group with strong support (PP= 1.0, LP = 98), which demonstrated that the presumed new species belonged to the genus. It revealed that the samples were clustered into three major clades of Youngia species (Figure 1). Clade I is the base group includes Y. szechuanica of “sect. Hieraciella”, Y. lushanensis, Y. purpimea, which is the sister group of Clade II and Clade III. Clade II includes “sect. Euyoungia”. Clade III include species of “sect. Mesomeris” and species of “sect. Desiphylum”. The presumed new species was nested within the Clade I, was closely related to Y. purpimea and Y. szechuanica (E. S. Soderberg) S. Y. Hu.
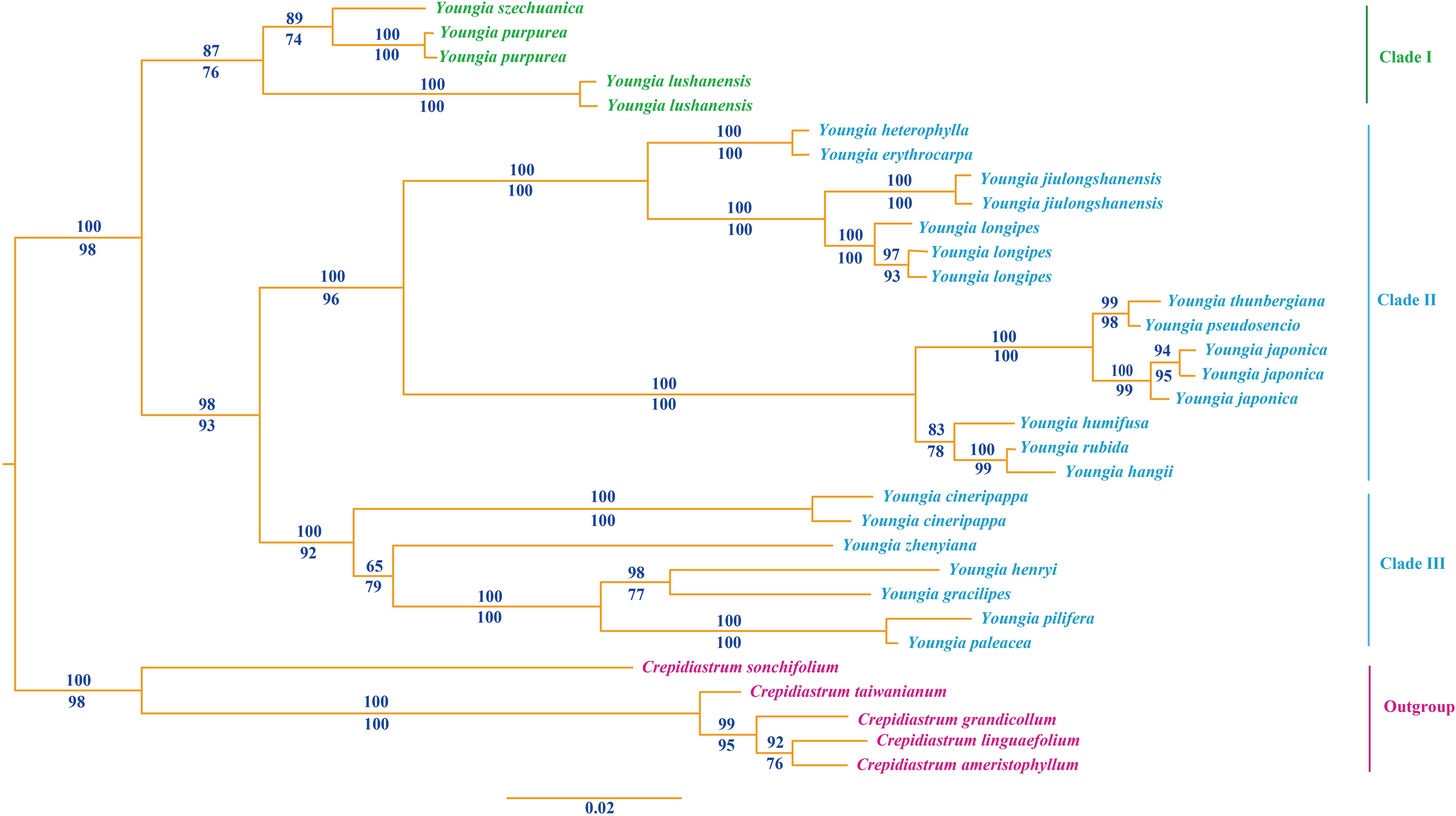
Figure 4. Bayesian consensus tree of the new species and related species of Youngia based on ITS sequences. Numbers above branches indicate Bayesian posterior probability [PP], numbers below branches are ML bootstrap [LP]. The new species and its sister groups are shown in green font.
4 Discussion
Both phylogeny analysis based on ITS and morphology study showed that the presumed new species Youngia lushanensis was closely related with Y. purpimea and Y. szechuanica. However, it can be easily recognized by its crested inner phyllaries and 9–11 florets in each capitulum (Figure 5A, C, D). Youngia lushanensis is similar to Y. purpimea (Peng et al., 2015) for its leaves are undivided, glabrous and purplish red on the back. While in Y. lushanensis, the middle and lateral veins are concave in the leaf surface and margins with inconspicuous short teeth, caudex is glabrous, each capitulum contains 9–11 florets (vs 5 florets in Y. purpimea), and inner phyllaries are crested (Table 2; Figure 5B). Youngia lushanensis is similar to Y. szechuanica (Hu, 1969), with fewer florets in the capitula, comparing with the other species in the Youngia genus. But the leaves of the latter are pinnately deeply divided, both surfaces covered with brown ruffled multicellular stub hairs, and the petiole base is covered with dense brown villus (Table 2). Youngia lushanensis is also similar to Y. baoxingensis (Chen, 2018) with crested inner phyllaries. But is distinctive from the later for ovate leaves and 9–11 florets (vs 6–7 florets in Y. baoxingensis) (Figure 2, Figure 3). The habitat of Y. lushanensis is similar to Y. purpimea, growing in the rocky slopes with flowing water in lower altitude 780–795 m. Youngia purpimea were found in the rocky slopes with flowing water in Xuyong and Gulin County, Southwest of Sichuan, with the altitude from 650–1200 m, according to the investigation of Peng et al. (2014), and specimen records [S.N. 890 (SM); S.N. 0221 (SM), S.N. 77-503 (SM)]. Youngia szechuanica was also found in moist stone slopes in Hejiang County of Sichuan Province, Qijiang and Nanchuan County of Chongqing City, with the altitude from 650–1700 m, according to in our field survey and specimen records [Zhang 1030 (KUN), Z.Y. Liu 183148(PE), W.P. Fang (PE); J.H. Xiong & Z.L. Zhou 92210 (PE), J.H. Xiong & Z.L. Zhou 91831(PE); Gu-0821 (SM); J.H. Xiong & B.Q. Li 95266 (SM); J.H. Xiong & B.Q. Li 95060 (SM); Z.C. Zhong 01430 (SM)]. The three species formed a basal clade of the genus Youngia (“sect. Hieraciella”). While Y. baoxingensis grows on grassy slopes at the margins of mixed forests at elevations of 2950–3200 m (Chen, 2018). Changes in environmental moisture conditions may lead to the rapid diversification of the genus Youngia, as observed in the critical genera linked to peculiar environment condition (Cheng et al., 2022; Perrino et al., 2022; Calvo et al., 2020; Perrino et al., 2023). Due to the lack of molecular materials, this could not be included in the phylogenetic tree. However, based on morphology and habitat, it could belong to the species of “sect. Mesomeris”, which is consistent with viewpoint of Chen (2018). Strangely, although only five florets per capitulum in Y. zhenyiana, which is similar to the Y. szechuanica and Y. purpimea of the “sect. Hieraciella”. But the pappus of Y. zhenyiana is white and leaf texture is papery, which make it is significantly different from the “sect. Hieraciella”. Phylogeny tree based on ITS suggests that it belongs to another group (“sect. Mesomeris”) (Figure 4). The presumed species, Y. purpimea and Y. szechuanica could be easily distinguished from the other species of Youngia by their special leaf texture, fewer florets and yellowish brown pappus. We will provide classification and retrieval keys for relevant species in the flowing paraphrases.

Figure 5. Flowers & Fruits of Youngia lushanensis. (A) Inflorescence. (B). Capitulum & phyllaries. (C–F). Florets. (G) Pappus, immature achenes and ligulate flowers.
4.1 Key to Youngia lushanensis and related species
1a Perennial, florets 5–11 per capitulum, pappus yellowish, leaves slightly leathery or thicker, growing in wet slopes with flowing water…………………………………………2
2a Florets 5 per capitulum, inner phyllaries glabrous, the main stem base covered with dense brown or brown long soft hairs…………………………………………………………3
3a Leaves, lyrately pinnatilobate, pinnatipartite, or pinnatisect, both faces pubescent with brown multicellular crinkled hairs………………………………...…Youngia szechuanica
3b Leaves undivided, margin sparsely and shallowly sinuate-dentate with long-acuminate teeth…Youngia purpimea
2b Florets 9–11 per capitulum, inner phyllaries crested, stem base glabrous…………………………..Youngia lushanensis
1b Perennial or annual, florets5–16 per capitulum, grayish or white, usually growing in drier habitat……………………4
4a Annual or biennial…………………………………………5
5a Biennial, achenes dark brown……….Youngia heterophylla
5b Annual, achene reddish brown………….Youngia japonica
4b Perennial……………………………………………………6
6a Pappus grayish, leaves undivided to pinnatifid or lyrately pinnatifid……………………………...Youngia cineripappa
6b Pappus white or brown, leaves undivided to pinnatifid or lyrately pinnatifid…………………………………………..7
7a Florets 5, Papppus white………………Youngia zhenyiana
7b Florets more than six, pappus white or brown……………8
8a 1–23 cm tall, rosulate, subacaulescent or dwarf, caudex short …………………………………………....Youngia gracilipes
8b 18–66 cm tall, rosulate, caudex long, stem erect …….………………………………………Youngia paleacea
5 Taxonomy
Youngia lushanensis X.H. Xiong, Y.L. Peng, X.M. Pu, L.J. Cheng & W.B. Ju sp. nov. (Figures 5, 6).

Figure 6. Youngia lushanensis X.H. Xiong, Y.L. Peng, X.M. Pu, L.J. Cheng & W.B. Ju sp. nov. (A, B) Habitat. (C, D, G) Whole living plants. (E). Leaf ventral surface. (F). Leaf dorsal surface. (H). Inflorescence.
Diagnosis: —The new species most closely resembles Y. szechuanica and Y. purpimea, but differs in having thick and leathery leaves, with concave midrib and lateral veins on the ventral surface, margins sparsely dentate, and being glabrous on both surfaces (Figures 6C–H), and 9–11 florets per capitulum, inner phyllaries crested with a narrow curved claw near tip.
Type: —CHINA. Sichuan Province: Luyang Street, Lushan County, 788.7 m, 2 June 2023. X.H. Xiong 20230602-1 (holotype, CDBI)! (Paratype, X.H. Xiong 20230602-2 CDBI).
Herbs, 30–32 cm tall, perennial, often rosulate, with rhizomatic rootstock. Fibrous roots vertically extended or skewed. Caudex glabrous. Stem upright, solitary, thick and robust, glabrous. Caudical leaves 4–5, thick and leathery, narrowly ovate, 8–10 cm long and 1–3 cm wide, glabrous, green on the ventral surface and purple red on abaxial surface, the midrib of the leaf blade concave on the surface, with 3–4 pairs lateral veins that arise from the midrib of the blade, margin entire or sparsely dentate, apex acuminate, base attenuate, petiole 2–2.5 cm long, petiole base smooth and glabrous. Cauline leaves 1–2, linear, bract-like. Involucre cylindrical, green. Synflorescence corymbiform; capitula 18–27; peduncles slender, 3–4 cm, glabrous. Outer phyllaries 5, 0.9 mm long, ovate, inner phyllaries 9–10, 8–9 mm long, lanceolate, crested with a narrow curved claw near tip. Florets 9–11, ligule, yellow, 10–15 mm long, 5-toothed. Anther tube yellow to brown, ca. 3 mm long; Style branches yellow, 1.0 mm long. Immature achenes light brown, fusiform, 2–3 mm, mature achenes 4-6 mm, with six uneven ribs, apex slightly attenuate, with small prickly hairs. Pappus, yellowish-brown, 2.5–4 mm long (Figures 5E–G).
Etymology: —”Lushan-” refers to the locality of the new species; “ -ensis” is an artificial ending.
Distribution and habitat: —This species was only found in the type locality, i.e., 30.1752 N, 102. 8705 E., Luyang Street, Lushan County, Sichuan Province, at elevations between 789–795 m. A total of 40-55 individual were found in this only population, growing on the slopes with flowing water, at the edge of mixed evergreen and deciduous broad-leaved forest, according to our field survey. This species grows on thin rocky swamp soil (Figure 6A, B).
Phenology: —Flowering and fruiting from May to July.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, PP785561, PP784683, PP784685, PP789654, PP785562, PP785563, PP785560.
Author contributions
YP: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing, Funding acquisition. XP: Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft. XX: Conceptualization, Investigation, Methodology, Writing – original draft. LC: Data curation, Software, Writing – original draft. WJ: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Natural Science Foundation of China (Grant No. 31000096, 32170208), the Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences (Y4ZK111B01) and Sichuan Provincial Department of Science and Technology (2023YFS0378).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1453759/full#supplementary-material
Supplementary Material S1 | Taxon name, voucher information for morphology studies Youngia baoxingensis, Y.S. Chen 7280 (PE); Youngia lushanensis, X.H. Xiong 20230602-2, X.H. Xiong20230602-1 (CDBI); Youngia heterophylla, Y. L. Peng, Y.J. Li & H.L. Zhou SE03311 (CDBI), Y. L. Peng, Y.J. Li & H.L. Zhou SE3341(CDBI), X.F. Gao, Y.L. Peng, B. Xu & X. Zeng 11996 (CDBI), Y.S. Che & Z.H. Wang 9102(PE), PE-Gulin Expedition 023(PE), Li Heng et al. 24217(PE), Z.Y. Liu 17241(PE), K.J. Guan, J.W. Wang &C.L. Li 596(PE), G.F. Li 61926(PE), Wuning Expedition 1029 (KUN), X.F. Gao, Y.D. Gao & W. B. Ju HGX11476 (CDBI), Y.L. Peng 102 (CDBI), X.F. Gao, Y. L. Peng, B. Xu & X. Zheng 11612 (CDBI); C.H. Li 893 (NAS); Youngia cineripappa, ZZ09097 (KUN), ZZ09041 (KUN), Z.Z. Zhu & W.B. Ju714(CDBI), Y. L. Peng 360(CDBI), Z.Q. Fang 836(CDBI), J.J. Zhou & H. Zhoui1406 12008 (CSFI), A. Liu, Y.K. Gong & C.Z. Huang (CSFI), Y.S. Chen 7317(PE), T.T. YU 6167 (KUN), Henry A.11997 (E); Y.S. Chen & Z.H. Wang 9048(PE), Y.S. Chen & Z.H. Wang 9048 (PE); Youngia gracilipes, Boufford & al. 34392 (A, KUN), Y.T. Zhang & K. Y. Lang 2338 (PE), Xiang Expedition 3992 (PE), X.F. Gao, Z.M. Zhu, W.B. Ju & W.T. Jin 14783 (CDBI), J.S. Yang 380 (KUN), Biology Institute Expedition 3992(CDBI), S. Chen & Z.H. Wang 9321 (PE), Y.S. Chen & Z.H. Wang 9358(PE), Y.S. Chen 9499A(PE), J.S. Ying & D.Y. Hong 651229(PE); Youngia paleacea, Y. L. Peng, Y.J. Li & H.L. Zhou SE03326 (CDBI), Y. L. Peng, Y.J. Li & H.L. Zhou SE03341 (CDBI), Boufford & al. 34500 (A, KUN), D.E. Boufford et al. 43368 (PE); Boufford & al.38233 (PE), FLPH Tibet Expedition 12-1502 (PE), FLPH Tibet Expedition 12-0587A (PE), Tibet Expedition 14959 (PE), Y.S. Chen 7541 (PE), K. Iwasuki et al. (KUN), L. Peng pl2011082605-3 (CDBI), Y.L. Peng & L.J. Tong 1309(CDBI); Youngia zhenyiana, Deng 2945 (KUN); Youngia purpimea, 11571 (CDBI), Y. L. Peng, Y.J. Li & H.L. Zhou SE02106 (CDBI), S.N. 890 (SM); S.N. 0221 (SM), S.N. 77-503 (SM); Youngia szechuanica, Y. L. Peng, Y.J. Li & H.L. Zhou SE02557 (CDBI), Zhang 1030 (KUN), Z.Y. Liu 183148(PE), W.P. Fang (PE); J.H. Xiong & Z.L. Zhou 92210 (PE), J.H. Xiong & Z.L. Zhou 91831(PE); Gu-0821 (SM); J.H. Xiong & B.Q. Li 95266 (SM); J.H. Xiong & B.Q. Li 95060 (SM); Z.C. Zhong 01430 (SM).
References
Babcock, E. B., Stebbins, G. L. (1937). The Genus Youngia (Washington DC: Carnegie Institution of Washington), 1–106.
Blattner, F. R. (1999). Direct amplification of the entire ITS region from poorly preserved plant material using recombinant PCR. Bio Techniques 27, 1180–1186. doi: 10.2144/99276st04
Cai, X., Xu, Y. L., Liu, J. L., Zhou, H. L., Jin, X. F. (2019). Youngia jiulongshanensis (Asteraceae: Liguliflorae), a new species from Zhejiang, East China. Phytotaxa 425, 244–252. doi: 10.11646/phytotaxa.425.4.5
Calvo, P., Gagliano, M., Souza, G. M., Trewavas, A. (2020). Plants are intelligent, here’s how. Ann. Bot. 125, 11–28. doi: 10.1093/aob/mcz155
Chen, Y. S. (2018). Youngia baoxingensis, a new species of Asteraceae (tribe Cichorieae) from Sichuan, China. Phytotaxa 382, 239–242. doi: 10.11646/phytotaxa.382.2.10
Cheng, X., Ping, T., Li, Z., Wang, T., Han, H., Epstein, H. E. (2022). Effects of environmental factors on plant functional traits across different plant life forms in a temperate forest ecosystem. New Forests 53, 125–142. doi: 10.1007/s11056-021-09847-0
Darriba, D., Taboada, G. L., Doallo, R., Posada, D. (2012). jModelTest 2: more models, new heuristics and high-performance computing. Nat. Methods 9, 772.
Deng, T., Zhang, J. W., Zhu, X. X., Zhang, D. G., Nie, Z. L., Sun, H. (2014). Youngia zhengyiana (Asteraceae, Crepidinae), a new species from south China, with notes on the systematics of Youngia inferred from morphology and nrITS phylogeny. Phytotaxa 170, 259–268. doi: 10.11646/phytotaxa.170.4.3
Hall, T. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT[J]. Nucleic Acids Symposium Series 41 (41), 95–98.
Ke, R., Chen, Y. S. (2016). A new species of Youngia (Asteraceae, tribe Cichorieae) from Yunnan, China. Phytotaxa 275, 140–148. doi: 10.11646/phytotaxa.275.2.5
Kelchner, S. A. (2000). The evolution of non-coding chloroplast DNA and its application in plant systematics. Ann. Missouri Botanical Garden 87, 482–498. doi: 10.2307/2666142
Kilian, N., Gemeinholzer, B., Lack, H. W. (2009). “Tribe Cichorieae,” in Systematics, Evolution, and Biogeography of the Compositae. Eds. Funk, V. A., Susanna, A., Stuessy, T., Bayer, R. (IAPT, Vienna), 343–383.
Kitagawa, M. (1937). Some plants from Inner Mongolia collected by Mr. T. Akagi in the year 1936. J. Japanese Bot. 13, 425–434.
Kitamura, S. (1942). Expositiones plantarum novarum orientali-Asiaticarum VII. Acta. Phytotaxonomica Geobotanica 11, 120–133.
Liu, Q., Huang, G. Y., Zhang, D. G., Zhang, J. W., Deng, T., Li, Z. M. (2021). Youngia hangii (Asteraceae, Crepidinae), a new species from Hubei, China. PhytoKeys 182, 27. doi: 10.3897/phytokeys.182.71063
Maity, D., Maiti, G.G. (2010). Taxonomic delimitation of the genus Tibetoseris Sennikov and the new genus Pseudoyoungia of the Compositae-Cichorieae from eastern Himalaya. Compositae Newsletter 48, 22–42.
Nakai, T. (1920). Notulae ad plantas Japoniae et Koreae, XXIII. Botanical Magazine (Tokyo) 34, 142–159. doi: 10.15281/jplantres1887.34.406_141
Peng, Y. L., Gao, X. F., Zhang, L. B. (2017). Youngia jiulongensis (Asteraceae), a new species from Sichuan, China. Novon 25, 298–301. doi: 10.3417/2015028
Peng, Y. L., Ju, W. B., Gao, X. F., et al. (2015). Youngia purpimea (Asteraceae), a new species from Sichuan, China. Phytotaxa 236, 191–195. doi: 10.11646/phytotaxa.236.2.8
Peng, Y. L., Zhang, Y., Gao, X. F., Tong, L. J., Li, L., Li, R. Y., et al. (2014). A phylogenetic analysis and new delimitation of Crepidiastrum (Asteraceae, tribe Cichorieae). Phytotaxa 159, 241–255. doi: 10.11646/phytotaxa.159.4.1
Perrino, E. V., Mahmoud, Z. N. A., Valerio, F., Tomaselli, V., Wagensommer, R. P., Trani, A. (2023). Synecology of Lagoecia cuminoides L. @ in Italy and evaluation of functional compounds presence in its water or hydroalcoholic extracts. Sci. Rep. 13, 20906. doi: 10.1038/s41598-023-48065-w
Perrino, E. V., Tomaselli, V., Wagensommer, R. P., Silletti, G. N., Esposito, A., Stinca, A. (2022). Ophioglossum lusitanicum L. New records of plant community and 92/43/EEC Habitat. in Italy. Agronomy 12, 3188. doi: 10.3390/agronomy12123188
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Sennikov, A. N., Illarionova, I. D. (2008). Generic delimitation of the subtribe Ixeridinae newly segregated from Crepidiinae (Asteraceae-Lactuceae). Komarovia 5, 57–115.
Shi, C., Kilian, N. (2011). “Youngia,” in Flora of China, vol. 20–21 . Eds. Wu, Z. Y., Raven, P. H., Hong, D. Y. (Science Press, Beijing & Missouri Botanical Garden Press, St. Louis), 252–263.
Stamatakis, A. (2014). RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30 (9), 1312–1313. doi: 10.1093/bioinformatics/btu033
Sun, B., Wang, Q., Zhao, Q., Yang, Y., Sheng, Y. (2019). “Study on the adsorption and desorption characteristics of Phosphorus in Ryegrass and natural grasses in in sloping land of rainy area of west China”, in IOP Conference Series: Earth and Environmental Science, (Gothenburg, Sweden: IOP Publishing), 295, 042126.
The Angiosperm Phylogeny Group. (2016). An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APGIV. Botanical J. Linn. Soc. 181, 1–20. doi: 10.1111/boj.12385
White, T. M., Bruns, T., Lee, S., Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA for phylogenetics,” in PCR protocols: a guide to methods and applications. Eds. Innis, M. A., Gelfand, D. H., Sninsky, J. J., White, T. J. (Academic Press, San Diego, CA), 315–321.
Zhang, J. W., Nie, Z. L., Wen, J., Sun, H. (2011). Molecular phylogeny and biogeography of three closely related genera, Soroseris, Stebbinsia, and Syncalathium (Asteraceae, Cichorieae), endemic to the Tibetan Plateau, SW China. Taxon 60, 15–26. doi: 10.1002/tax.2011.60.issue-1
Keywords: Youngia, Asteraceae, morphology, ITS, new species
Citation: Peng Y, Pu X, Xiong X and Cheng L (2024) Youngia lushanensis, a new species of Asteraceae from Sichuan, China, inferred from morphology and nrITS phylogeny. Front. Plant Sci. 15:1453759. doi: 10.3389/fpls.2024.1453759
Received: 24 June 2024; Accepted: 03 October 2024;
Published: 04 December 2024.
Edited by:
Robert Philipp Wagensommer, Free University of Bozen-Bolzano, ItalyCopyright © 2024 Peng, Pu, Xiong and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulan Peng, cGVuZ3lsQGNpYi5hYy5jbg==; Xianhua Xiong, MTg0OTgzNjMyMkBxcS5jb20=
 Yulan Peng
Yulan Peng Xuemei Pu
Xuemei Pu Xianhua Xiong
Xianhua Xiong Lijie Cheng1,3
Lijie Cheng1,3