- 1State Environmental Protection Key Laboratory of Wetland Ecology and Vegetation Restoration, School of Environment, Northeast Normal University, Changchun, China
- 2Key Laboratory of Vegetation Ecology, Ministry of Education, Northeast Normal University, Changchun, China
- 3Wetland Ecological Resources Research Center, Jiangxi Academy of Forestry, Nanchang, Jiangxi, China
Soil microbial carbon use efficiency (CUE) refers to the efficiency of microorganisms in converting absorbed carbon into their own biomass carbon. Soil microbial CUE is a key parameter to understanding the soil carbon cycle. Biotic and abiotic factors are widely considered to be important factors influencing CUE. However, the related underlying mechanisms remain unclear. This review elaborates on the concept of soil microbial CUE and the various approaches used for its measurement. We reviewed the effects of various abiotic factors, such as temperature, soil moisture, pH, nutrient addition, and substrate type, and biotic factors, such as microbial community structure and diversity, on CUE. Finally, we discussed the focus areas that future studies need to further explore. We hope this review can provide a comprehensive understanding of the factors impacting soil microbial CUE, which is a fundamental step to improving soil carbon storage capacity.
1 Introduction
Carbon cycling is one of the key biogeochemical cycling processes in terrestrial ecosystems (Schimel and Schaeffer, 2012). During this cycle, atmospheric carbon dioxide (CO2) is absorbed and fixed through several processes, including biological, geological, and anthropogenic disturbance processes (Zhang W. et al., 2024). Generally, terrestrial ecosystems are crucial to reducing atmospheric CO2 levels (Davidson and Janssens, 2006). Exploring the mechanisms of carbon cycling is fundamental to countering global climate change (Sun and Chen, 2024). Moreover, the soil carbon pool is the biggest carbon pool in terrestrial ecosystems, with carbon levels ~3- and 2-fold higher than those in the atmosphere and the plants, respectively (Soong et al., 2020). Thus, changes in the soil carbon pool might profoundly impact global carbon cycling (Lou et al., 2024). Soil microorganisms play an indispensable role in terrestrial ecosystem carbon cycling (Frey et al., 2013), participating in nearly all soil transformations, connecting soil, biosphere, atmosphere, hydrosphere, and lithosphere fluxes (Chu et al., 2017).
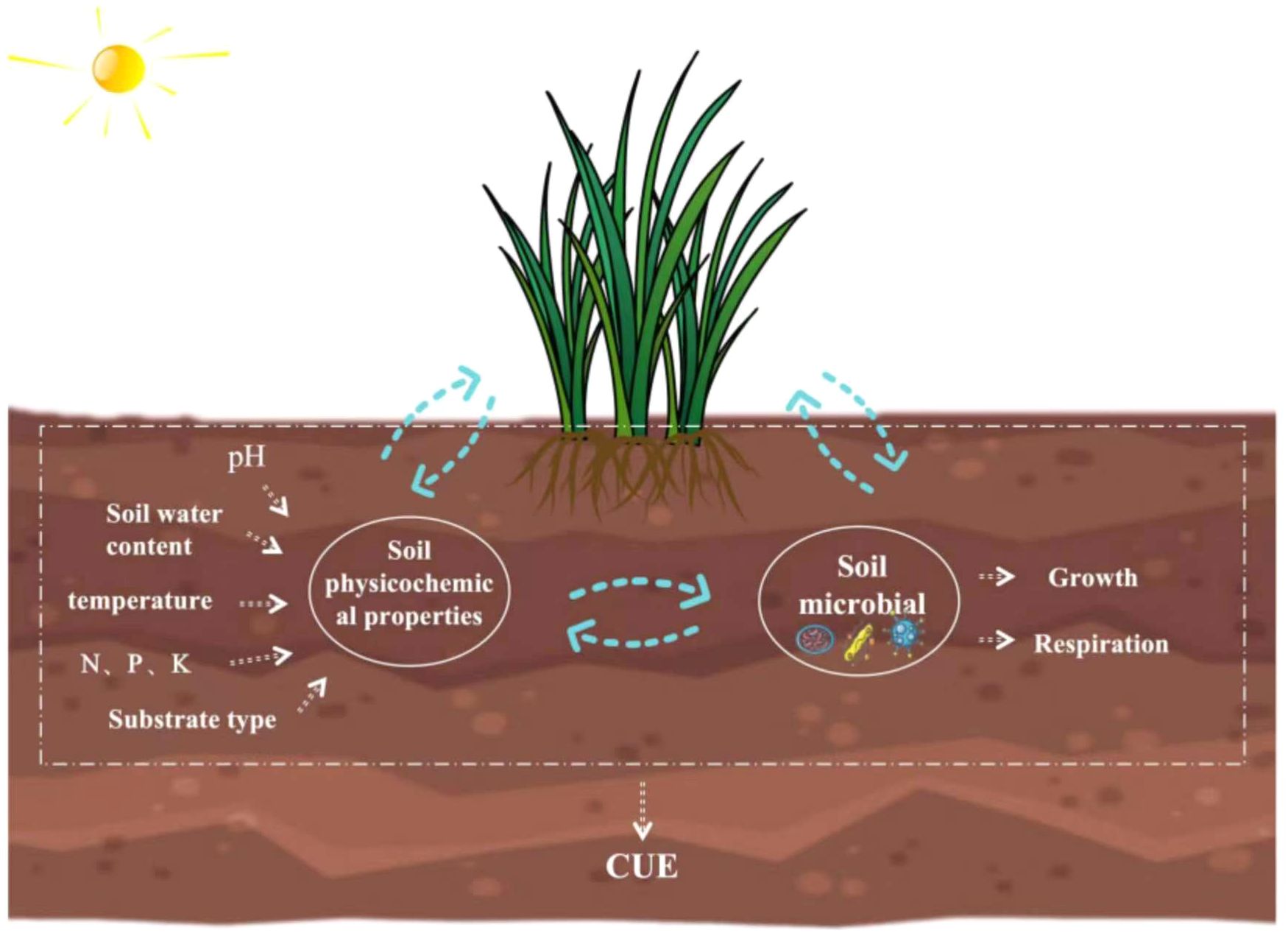
The carbon use efficiency (CUE) of soil microorganisms is defined as their ability to convert the absorbed carbon into biomass (Chen and Yu, 2020), which might directly influence the ecosystem carbon storage rate and storage capacity (Wieder et al., 2013; Miltner et al., 2012). Therefore, exploring soil microbial CUE is important for better understanding ecosystem carbon allocation patterns, carbon flux changes, and carbon storage status and accurately predicting the effects of global climate change on carbon cycling (Xu et al., 2014). Microbial CUE has always been depicted as a fixed variable in many soil carbon cycling models (Parton et al., 1987; Hansen et al., 1991; Kätterer and Andrén, 2001). However, several studies have found that biotic and abiotic factors influence the CUE (Adu and Oades, 1978; Song et al., 2012), such as soil moisture and water effectiveness (Tiemann and Billings, 2011), temperature (Apple et al., 2006; Wetterstedt and Ågren, 2011), pH (Malik et al., 2018; Silva-Sánchez et al., 2019), and nutrients (Ågren et al., 2001; Manzoni et al., 2012). However, the mechanisms underlying the impact of these environmental factors of soil microbial CUE remain unclear (Malik et al., 2020; Manzoni et al., 2012). For instance, some studies have shown that the CUE decreases with increasing temperature (Allison et al., 2010; Devêvre and Horwáth, 2000) because rising temperature leads to elevated respiration and subsequent substrate depletion and nutrient limitation, reducing the CUE (López-Urrutia and Morán, 2007; Allison et al., 2010). In contrast, some studies have shown that increasing temperature only mildly impacts the CUE (Hagerty et al., 2014; Tucker et al., 2013; Dijkstra et al., 2011b; Hagerty et al., 2014). Furthermore, Spohn et al. (2016b) reported a decrease in CUE with increasing nitrogen levels. However, Liu et al. (2018) found that CUE increased with rising nitrogen levels. Thus, the degrees of impact of different environmental factors on soil microbial CUE and the related underlying mechanisms are still not fully understood (Figure 1) (Jones et al., 2018).
2 Ecological concepts of microbial CUE
The term “growth yield” refers to the efficiency of an organism to convert substrate to biomass. This term was coined in the early 20th century as a physiological indicator to assess the efficiency of bacteria to assimilate substrate (Monod, 1949). In the mid-1990s, Gifford introduced the term “growth yield” into the field of Ecology as a way to characterize the potential carbon sequestration capacity of organisms, but termed it Carbon Use Efficiency (CUE) (Gifford, 1995). Since then, CUE has gradually been classified into plant CUE, microbial CUE, and ecosystem CUE, referring to plant carbon assimilation, soil carbon sequestration, and ecosystem carbon use efficiencies, respectively (Manzoni et al., 2012). Of these, microbial CUE is the ratio of the amount of organic carbon substrate used by microorganisms for growth and assimilation to the amount of substrate carbon used during decomposition and alienation (Sinsabaugh et al., 2017), reflecting the partitioning of carbon between the growth and respiration of the microorganisms (Sinsabaugh et al., 2013).
In ecological research, microbial CUE is usually expressed as the ratio of microbial growth (µ) to absorption (U), that is, (Manzoni et al., 2012). Microbial CUE also reflects the several processes affecting carbon metabolism at different temporal and spatial scales, such as physiological processes at individual cell and species levels or kinetic features at the community and ecosystem levels (Geyer et al., 2016). Thus, it directly influences the carbon retention time, turnover rate, and soil carbon storage capacity of the ecosystem (Tucker et al., 2013). In summary, exploring the responses of soil microbial CUE to environmental factors can help assess the potential of carbon storage and accurately predict the impacts of global changes, anthropogenic disturbances, and the related management measures on carbon sequestration in ecosystems, making it a hotspot in current ecological research.
3 Determination of soil microbial CUE
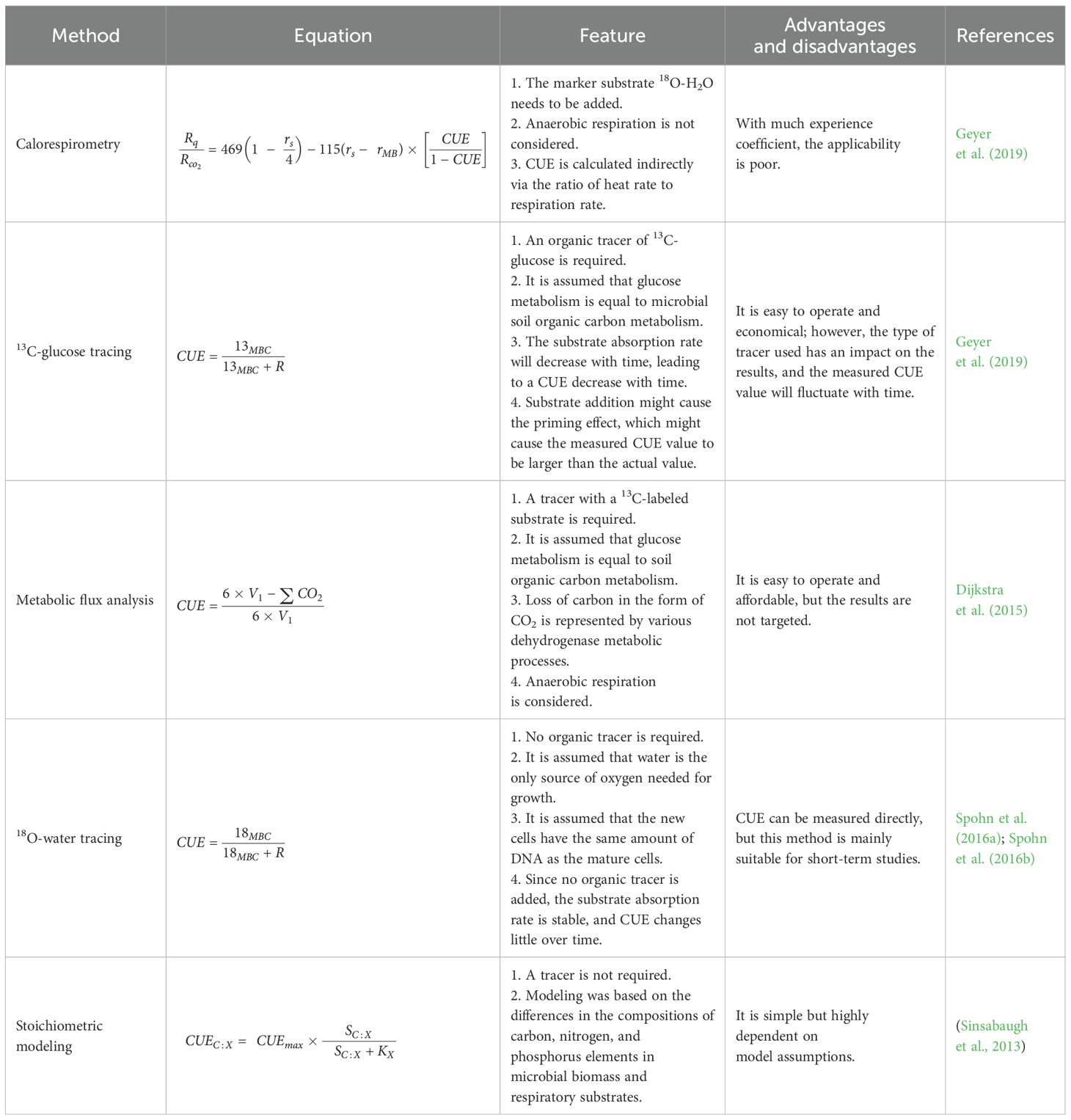
Several methods have been used to estimate soil microbial CUE, such as the calorimetric ratio method, 13C-glucose tracer method, metabolic flux analysis method, 18O-water labeling method, and stoichiometric modeling (Manzoni et al., 2012) (Table 1). In 2004, Hansen et al. found that calorimetric respirometry uses the ratio of heat production to respiration to measure the CUE (Hansen et al., 2004; Spohn et al., 2016a). Calorimetric methods have poor universal applicability (Chakrawal et al., 2020) primarily because they require some empirical coefficients for CUE measurement, such as microbial biomass and oxidation state of substrate carbon. However, the range of such empirical coefficients is often limited, leading to a high CUE (Geyer et al., 2019). In 2006, while examining the differences in the growth yield efficiencies of forest ecosystems, Brant et al. suggested that 13C-glucose tracing might trace the uptake and mineralization of 13C-labeled substrates, where growth is inferred from 13C-incorporation into microbial biomass (Brant et al., 2006). However, this method leads to high CUE values because it uses biologically easily available carbon, inaccurately reflecting the real substrate utilization by microorganisms in the environment (Chen and Yu, 2020). In 2011, Dijkstra et al. proposed that metabolic flux analysis could be used to measure CO2 production for individual C atoms using position-specific 13C-labeled substrates (Dijkstra et al., 2011a; Spohn et al., 2016a). Metabolic flux analysis is a relatively simple method but has a low specificity, making it unsuitable for measuring the impacts of microbial turnover and secretions (Dijkstra et al., 2015). In 2016, Spohn et al. used a novel substrate-independent method of CUE assessment based on the incorporation of 18O from labeled water into the microbial DNA. They measured CUE by combining the conversion factors of microbial DNA and carbon biomass (Spohn et al., 2016a) to study the effects of long-term fertilization on microbial CUE and microbial biomass turnover time. However, they also showed that this method is suitable for assessing CUE in short-term culture treatments but could not be used to estimate the CUE over a long-term period (Spohn et al., 2016a). Furthermore, Sinsabaugh et al. (2016) suggested that CUE could be calculated via the activities of several soil enzymes, such as microbial extracellular enzyme activity and the carbon-to-nitrogen ratio of microbial biomass. They called this method the “stoichiometric modeling method” (Sinsabaugh et al., 2016). This method is easy but cannot reflect the actual microbial metabolic processes, and thus, has always been a surrogate indicator of CUE (Sinsabaugh et al., 2016).
Taken together, each of these methods has its own set of benefits, drawbacks, and application range (Geyer et al., 2019), warranting the need for a fast, convenient, low-cost, and more accurate CUE assessment method.
4 Environmental factors
4.1 Abiotic factors
4.1.1 Temperature
Temperature is an important environmental factor affecting soil microbial CUE. Several studies have reported significantly negative effects of temperature on CUE (Frey et al., 2013; Qiao et al., 2019). Within a certain temperature range, microbial respiration intensity increases with the rising temperature, causing elevated substrate consumption, nutrient limitation and reduced soil microbial CUE (Kirschbaum, 2004; Li et al., 2019). However, other studies have shown that a rise in temperature only mildly impacted soil microbial CUE (Dijkstra et al., 2011b; Hagerty et al., 2014). In addition, the effects of temperature on soil microbial CUE might depend on the period of the experiments. For instance, Frey et al. (2013) found that short-term temperature increases might produce high levels of microbially derived carbon, leading to a rise in soil microbial CUE. When the temperature rises from 10°C to 25°C, the CUE increases by 10–40%. However, long-term temperature increases might reduce soil carbon, leading to the negative relationships between temperature increases and soil microbial CUE (Frey et al., 2013). Therefore, the precise impact of temperature change on microbial CUE remains unclear.
4.1.2 Soil moisture
Soil moisture plays a very important role in soil productivity by altering the energy balance between vegetation and the atmosphere (Deng et al., 2020), another key environmental factor influencing soil microbial CUE and driving biogeochemical cycling (Tiemann and Billings, 2011). The effects of soil moisture on soil microbial CUE are complex and variable. For instance, high moisture might increase nutrient availability and promote microbial growth, altering soil microbial CUE (Domeignoz-Horta et al., 2020). Domeignoz-Horta et al. (2020) found that at high soil moisture levels (60%), microbial respiration and growth rates increased by 146% and 169%, respectively. However, the growth rate increased more rapidly than the respiration rate, resulting in an 8% increase in CUE. Whereas Tiemann and Billings (2011) showed that CUE decreased in drought conditions.
The duration of the change in the soil moisture can also significantly affect soil microbial CUE. For instance, long-term water stress reduces the solubility and absorption of soil substrates, inhibiting microbial growth (Or et al., 2007), increasing metabolic consumption, and reducing soil microbial CUE (Tiemann and Billings, 2011). In contrast, short-term water stress might stimulate a microbial response to water stress, reducing the impact of drought by increasing osmotic pressure or short-term dormancy, leading to an increased soil microbial CUE (Herron et al., 2009). Thus, the influence of soil moisture on soil microbial CUE still needs to be elucidated.
4.1.3 pH
Acidic, alkaline, and neutral environments have varied impacts on soil microbial CUE. The soil microbial CUE is higher in alkaline conditions, which might be attributed to two factors (Zhang et al., 2020). Firstly, an increase in pH improves the availability of organic matter and the proportion of resources susceptible to bacterial utilization, resulting in a higher soil microbial CUE in bacteria-dominated conditions (Malik et al., 2018). Secondly, soil pH might regulate the balance between the levels of fungi and bacteria in it (F:B = 0.02–0.7), generating more bacteria and increasing microbial CUE (0.05–0.5) (Silva-Sánchez et al., 2019). In acidic conditions, soil microbes need more energy, reducing aluminum stress, and transfer more carbon to physiological processes such as respiration, leading to lower soil microbial CUE (Jones et al., 2019). However, the ability of microbial communities to store carbon might be enhanced when the soil pH is close to neutral, which is the most advantageous condition for soil organic carbon sequestration (Jones et al., 2019).
4.1.4 Nutrient addition
Nutrients can alter soil microbial CUE by impacting microbial growth and biomass (Liu et al., 2018). Different types of nutrients and rate of change in their levels might affect the metabolic decomposition of soil microorganisms (Yang et al., 2024), altering microbial respiration and growth rates and soil microbial CUE (Adingo et al., 2021).
Nitrogen addition has both direct and indirect effects on soil microbial CUE (Zhang et al., 2022). The nitrogen concentration and the duration of addition directly impacts soil microbial CUE (Li et al., 2021).
For instance, short-term nitrogen additions might reduce the enzymatic metabolic cost of carbon and nitrogen acquisition by soil microorganisms, inhibit microbial respiration, and increase soil microbial CUE (Riggs et al., 2015). However, Riggs and Hobbie (2016) found that long-term nitrogen additions could lead to the gradual decomposition of active carbon pools, limiting the activities of microbial communities and substantially reducing soil microbial CUE. In addition, low and high nitrogen levels have been shown to markedly increase and decrease the soil microbial CUE by 45.12% and 27.84%, respectively (Li et al., 2021).
Furthermore, nitrogen addition also indirectly affects soil microbial CUE by impacting soil microbial biomass, diversity, and respiration (Lu et al., 2011; Cline and Zak, 2015; Liu et al., 2018). For instance, nitrogen application has been shown to significantly reduce soil microbial biomass and microbial diversity in forest and grassland ecosystems (Lu et al., 2011; Xu et al., 2024). Moreover, nitrogen application can inhibit the secretions of lignocellulosic hydrolases by saprophytic bacteria, inhibiting the ability of saprophytic microbial communities to access carbon sources (such as cellulose and hemicellulose) (Cline and Zak, 2015) and affecting soil microbial CUE. Furthermore, nitrogen addition increases the availability of soil nutrients. Hence, plants need to adjust their resource acquisition strategies, reducing the proportion of carbon allocation to the below-ground part, especially to the inter-root, causing a decrease in microbial activity, reducing excitation effects, inhibiting microbial respiration (Liu et al., 2018), and elevating soil microbial CUE. Finally, nitrogen addition might affect soil pH, leading to soil acidification, increasing the levels of activated aluminum ions, inhibiting microbial biomass and its decomposition (Riggs et al., 2015; Spohn et al., 2016b), and leading to a lower soil microbial CUE.
Several studies have shown that phosphorus addition could increase soil microbial CUE (Cleveland et al., 2002; Elser et al., 2007; Widdig et al., 2020). Phosphorus fertilization can alleviate microbial phosphorus and nitrogen limitation, increasing soil microbial CUE and reducing soil carbon loss. For example, Wang et al. (2022) confirmed that phosphorus addition increasing would alleviate microbial P and N restriction, then increased soil microbial CUE. Phosphorus addition also increases the effective phosphorus levels in the soil, leading to significantly higher microbial respiration rates and biomass, increasing the CUE (Cleveland et al., 2002). Furthermore, the duration of phosphorus addition also affects soil microbial CUE. For instance, Bååth and Anderson (2003) reported an imbalance in the stoichiometric ratio of carbon, nitrogen, and phosphorus due to long-term P addition (Khan et al., 2016), reducing soil microbial CUE. Cui et al. (2020) found that microorganisms need to produce more carbon synthase to increase phosphorus acquisition, leading to a lower CUE and reducing carbon storage in order to maintain the balanced carbon, nitrogen, and phosphorus composition required for microbial biomass homeostasis during soil phosphorus limitations.
Nitrogen and phosphorus co-addition only mildly affects soil microbial CUE (Widdig et al., 2020). This finding might be attributed to the stability of the molecular composition of soil organic carbon and the relatively minor impacts of nitrogen and phosphorus additions on the carbon composition (VandenEnden et al., 2021). Moreover, the soil characteristics, such as soil texture, also influence the soil microbial CUE (Keiblinger et al., 2010; Widdig et al., 2020).
Potassium is the most abundant inorganic cation in plant cells, playing a critical role in various plant functions, which might also affect ecosystem carbon cycling (Chen et al., 2024). However, the impacts of potassium on soil microbial CUE have been studied less than the effects of nitrogen and phosphorus. Several studies found that potassium only mildly impacts the soil microbial CUE. For instance, Spohn et al. (2016b) found that potassium levels did not impact soil microbial CUE because it is not a critical element for microorganisms. Thus, changes in potassium levels do not impact microbial carbon cycling. Onipchenko et al. (2012) also showed that potassium fertilization had insignificant effects on soil microbial CUE. In conclusion, the mechanisms underlying the impact of nutrient addition on soil microbial CUE are complex. The effects of the type and amount of the nutrients added and the duration of nutrient addition on soil microbial CUE still need to be elucidated (VandenEnden et al., 2021).
4.1.5 Substrate type
Soil microbial CUE is also affected by the complexity of the substrate type (Bosatta and Ågren, 1999). Simple substrates or low molecular weight compounds are easily transported inside the cell and have less activation energy (Öquist et al., 2017). In contrast, larger or more complex molecules might undergo multiple oxidation steps to form before they are utilized (Öquist et al., 2017), potentially reducing CUE (Bosatta and Ågren, 1999; Blagodatskaya and Kuzyakov, 2008). For instance, between glucose (Jones et al., 2018) and phenol (Liu et al., 2018), using the former as a substrate leads to a higher soil microbial CUE. However, the effects of substrate type on soil microbial CUE and the underlying mechanisms are still unclear and require further study.
4.2 Biotic factors: microbial community structure and diversity
Both microbial community structure and diversity can affect soil microbial CUE. Interspecific variability in microbial organic matter decomposition and uptake rates (Waldrop and Firestone, 2004; Ziegler and Billings, 2011) might have varied effects on CUE (Maynard et al., 2017b, a). For instance, Adu and Oades (1978) showed that fungi exhibit a higher microbial CUE than bacteria. This finding might be attributed to the indirect impact of the biological interactions among fungi on the function of the community by influencing the community composition or increasing the rates of catabolism (Song et al., 2012; Hiscox et al., 2015). Liu et al. (2018) also found that soil microbial CUE was significantly increased with an increasing proportion of fungi in the microbial community. However, Soares and Rousk (2019) reported that soils with lower fungal-bacterial ratios (F:B) had higher microbial CUE. Therefore, the effects of microbial community structure on soil microbial CUE are still unclear.
Few studies have investigated the effects of microbial diversity on soil microbial CUE. For example, Domeignoz-Horta et al. (2020) used the gradient dilution method and showed positive relationships between microbial diversity and CUE. They also found that microbial diversity more significantly impacted the growth of soil biota than respiration (Domeignoz-Horta et al., 2020). However, the relationships between microbial diversity and CUE and the underlying mechanisms need to be further explored.
4.3 Combined effects
Furthermore, the combined effects of multiple environmental factors on soil microbial CUE can help in better comprehension of carbon sequestration in natural ecosystems than the effects of individual factors. It is still unclear whether biotic or abiotic factors impact soil microbial CUE more significantly. Some studies have found that abiotic factors, such as temperature (Hagerty et al., 2014), pH (Silva-Sánchez et al., 2019; Zhang et al., 2020), and soil moisture (Tiemann and Billings, 2011), might impact soil microbial CUE more prominently, whereas biotic factors only play a secondary role. For instance, Jones et al. (2019) reported pH to be a fundamental factor affecting soil microbial CUE in acidic conditions, altering CUE by regulating the proportion of fungi and bacteria. In contrast, Soares and Rousk (2019) suggested that the microbial community structure more prominently affects soil microbial CUE, whereas abiotic factors might indirectly affect soil microbial CUE by regulating microbial diversity (Fengling et al., 2018; Zhang L. et al., 2024). Thus, biotic (such as fungal-bacterial ratios) and abiotic factors (such as water and temperature) might simultaneously regulate the diversity and structure of soil microorganisms. Hence, exploring their combined effects might help better assess soil microbial CUE. The results of previous studies showed that the combined effects of environmental factors on soil microbial CUE are still unclear. Hence, the factors that prominently impact soil microbial CUE still need to be elucidated.
5 Future prospects
5.1 Addition of multiple nutrients
The growth of soil microorganisms is influenced by the combined effects of multiple nutrients, indicating that the addition of only a single nutrient might not effectively elucidate how the nutrients affect microbial growth. Moreover, the effects of the type and amount of the added nutrients and the duration of nutrient addition on soil microbial CUE are still unclear. Thus, future studies should focus on exploring the combined effects of multiple nutrients on soil microbial CUE, especially over the long term.
5.2 Microbial diversity
The soil microbial diversity contributes to maintaining the stability and sustainability of soil ecosystems and preventing the deterioration of the soil environment (Wertz et al., 2007). Different microbial populations exhibit varying growth and respiration rates, indicating that soil microbial diversity exhibits varying effects on soil microbial CUE. However, there is a lack of knowledge about the effects of soil microbial diversity on soil microbial CUE. Thus, the relationship between soil microbial diversity, including microbial structure and quantity, and soil microbial CUE needs to be further explored.
5.3 Biotic and abiotic factors
The activities of soil microorganisms are simultaneously affected by biotic and abiotic factors (McIntire and Fajardo, 2014). However, most studies only focus on the effects of individual biotic or abiotic factors on soil microbial CUE. The combined effects of biotic and abiotic factors on soil microbial CUE are still poorly understood. Exploring the combined effects of biotic and abiotic factors on soil microbial CUE might help in a more accurate prediction of the changes in soil microbial CUE with changing climate. Thus, future studies should focus on the combined effects of biotic and abiotic factors on soil microbial CUE.
Author contributions
XT: Writing – original draft, Writing – review & editing. ZL: Funding acquisition, Project administration, Writing – review & editing. JY: Writing – review & editing, Conceptualization, Investigation. WY: Conceptualization, Investigation, Writing – review & editing. WL: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Key R&D Program of China, grant numbers 2023YFF1304600; and Jiangxi Province Forestry Innovation special project (Grant No. 2023 (25)).
Conflict of interest
The authors declare the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adingo, S., Yu, J. R., Xuelu, L., Li, X., Jing, S., Xiaong, Z. (2021). Variation of soil microbial carbon use efficiency (CUE) and its Influence mechanism in the context of global environmental change: a review. PeerJ 9, e12131. doi: 10.7717/peerj.12131
Adu, J. K., Oades, J. M. (1978). Utilization of organic materials in soil aggregates by bacteria and fungi. Soil Biol. Biochem. 10, 117–122. doi: 10.1016/0038-0717(78)90081-0
Ågren, G. I., Bosatta, E., Magill, A. H. (2001). Combining theory and experiment to understand effects of inorganic nitrogen on litter decomposition. Oecologia 128, 94–98. doi: 10.1007/s004420100646
Allison, S. D., Wallenstein, M. D., Bradford, M. A. (2010). Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 3, 336–340. doi: 10.1038/ngeo846
Apple, J., Giorgio, P. A., Kemp, W. (2006). Temperature regulation of bacterial production, respiration, and growth efficiency in a temperate salt-marsh estuary. Aquat. Microbial Ecol. 43, 243–254. doi: 10.3354/ame043243
Bååth, E., Anderson, T. H. (2003). Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 35, 955–963. doi: 10.1016/S0038-0717(03)00154-8
Blagodatskaya, Е., Kuzyakov, Y. (2008). Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol. Fertility Soils 45, 115–131. doi: 10.1007/s00374-008-0334-y
Bosatta, E., Ågren, G. I. (1999). Soil organic matter quality interpreted thermodynamically. Soil Biol. Biochem. 31, 1889–1891. doi: 10.1016/S0038-0717(99)00105-4
Brant, J. B., Sulzman, E. W., Myrold, D. D. (2006). Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol. Biochem. 38, 2219–2232. doi: 10.1016/j.soilbio.2006.01.022
Chakrawal, A., Herrmann, A. M., Šantrůčková, H., Manzoni, S. (2020). Quantifying microbial metabolism in soils using calorespirometry — A bioenergetics perspective. Soil Biol. Biochem. 148, 107945. doi: 10.1016/j.soilbio.2020.107945
Chen, B., Fang, J., Piao, S., Ciais, P., Black, T. A., Wang, F., et al. (2024). A meta-analysis highlights globally widespread potassium limitation in terrestrial ecosystems. New Phytol. 241, 154–165. doi: 10.1111/nph.19294
Chen, Z., Yu, G. R. (2020). Advances in the soil microbial carbon use efficiency. Acta Ecol. Sin. 40, 756–767. doi: 10.5846/stxb201811082427
Chu, H. Y., Wang, Y. F., Shi, Y., Lyu, X. T., Zhu, Y. G., Han, X. G. (2017). Current status and development trend of soil microbial biogeography. Bull. Chin. Acad. Sci. 32, 585–592. doi: 10.16418/j.issn.1000-3045.2017.06.005
Cleveland, C. C., Townsend, A. R., Schmidt, S. K. (2002). Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5, 0680–0691. doi: 10.1007/s10021-002-0202-9
Cline, L. C., Zak, D. R. (2015). Soil microbial communities are shaped by plant-driven changes in resource availability during secondary succession. Ecology 96, 3374–3385. doi: 10.1890/15-0184.1
Cui, Y., Zhang, Y., Duan, C., Wang, X., Zhang, X., Ju, W., et al. (2020). Ecoenzymatic stoichiometry reveals microbial phosphorus limitation decreases the nitrogen cycling potential of soils in semi-arid agricultural ecosystems. Soil Tillage Res. 197, 104463. doi: 10.1016/j.still.2019.104463
Davidson, E. A., Janssens, I. A. (2006). Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173. doi: 10.1038/nature04514
Deng, Y., Wang, S., Bai, X., Luo, G., Wu, L., Cao, Y., et al. (2020). Variation trend of global soil moisture and its cause analysis. Ecol. Indic. 110, 105939. doi: 10.1016/j.ecolind.2019.105939
Devêvre, O. C., Horwáth, W. R. (2000). Decomposition of rice straw and microbial carbon use efficiency under different soil temperatures and moistures. Soil Biol. Biochem. 32, 1773–1785. doi: 10.1016/S0038-0717(00)00096-1
Dijkstra, P., Blankinship, J. C., Selmants, P. C., Hart, S. C., Koch, G. W., Schwartz, E., et al. (2011a). Probing carbon flux patterns through soil microbial metabolic networks using parallel position-specific tracer labeling. Soil Biol. Biochem. 43, 126–132. doi: 10.1016/j.soilbio.2010.09.022
Dijkstra, P., Salpas, E., Fairbanks, D., Miller, E. B., Hagerty, S. B., van Groenigen, K. J., et al. (2015). High carbon use efficiency in soil microbial communities is related to balanced growth, not storage compound synthesis. Soil Biol. Biochem. 89, 35–43. doi: 10.1016/j.soilbio.2015.06.021
Dijkstra, P., Thomas, S. C., Heinrich, P. L., Koch, G. W., Schwartz, E., Hungate, B. A. (2011b). Effect of temperature on metabolic activity of intact microbial communities: Evidence for altered metabolic pathway activity but not for increased maintenance respiration and reduced carbon use efficiency. Soil Biol. Biochem. 43, 2023–2031. doi: 10.1016/j.soilbio.2011.05.018
Domeignoz-Horta, L. A., Pold, G., Liu, X.-J. A., Frey, S. D., Melillo, J. M., DeAngelis, K. M. (2020). Microbial diversity drives carbon use efficiency in a model soil. Nat. Commun. 11, 3684. doi: 10.1038/s41467-020-17502-z
Elser, J. J., Bracken, M. E. S., Cleland, E. E., Gruner, D. S., Harpole, W. S., Hillebrand, H., et al. (2007). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x
Fengling, R., Xubo, Z., Nan, S., Minggang, X., Kailou, L. (2018). A meta-analysis of manure application impact on soil microbial biomass across China’s croplands. Sci. Agric. Sin. 51, 119–128. doi: 10.3864/j.issn.0578-1752.2018.01.011
Frey, S. D., Lee, J., Melillo, J. M., Six, J. (2013). The temperature response of soil microbial efficiency and its feedback to climate. Nat. Climate Change 3, 395–398. doi: 10.1038/nclimate1796
Geyer, K. M., Dijkstra, P., Sinsabaugh, R., Frey, S. D. (2019). Clarifying the interpretation of carbon use efficiency in soil through methods comparison. Soil Biol. Biochem. 128, 79–88. doi: 10.1016/j.soilbio.2018.09.036
Geyer, K. M., Kyker-Snowman, E., Grandy, A. S., Frey, S. D. (2016). Microbial carbon use efficiency: accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry 127, 173–188. doi: 10.1007/s10533-016-0191-y
Gifford, R. M. (1995). Whole plant respiration and photosynthesis of wheat under increased CO2 concentration and temperature: long-term vs. short-term distinctions for modelling. Global Change Biol. 1, 385–396. doi: 10.1111/j.1365-2486.1995.tb00037.x
Hagerty, S. B., van Groenigen, K. J., Allison, S. D., Hungate, B. A., Schwartz, E., Koch, G. W., et al. (2014). Accelerated microbial turnover but constant growth efficiency with warming in soil. Nat. Climate Change 4, 903–906. doi: 10.1038/nclimate2361
Hansen, S., Jensen, H. E., Nielsen, N. E., Svendsen, H. (1991). Simulation of nitrogen dynamics and biomass production in winter wheat using the Danish simulation model DAISY. Fertilizer Res. 27, 245–259. doi: 10.1007/BF01051131
Hansen, L. D., Macfarlane, C., McKinnon, N., Smith, B. N., Criddle, R. S. (2004). Use of calorespirometric ratios, heat per CO2 and heat per O2, to quantify metabolic paths and energetics of growing cells. Thermochimica Acta 422, 55–61. doi: 10.1016/j.tca.2004.05.033
Herron, P. M., Stark, J. M., Holt, C., Hooker, T., Cardon, Z. G. (2009). Microbial growth efficiencies across a soil moisture gradient assessed using 13C-acetic acid vapor and 15N-ammonia gas. Soil Biol. Biochem. 41, 1262–1269. doi: 10.1016/j.soilbio.2009.03.010
Hiscox, J., Savoury, M., Vaughan, I. P., Mueller, C. T., Boddy, L. (2015). Antagonistic fungal interactions influence carbon dioxide evolution from decomposing wood. Fungal Ecol. 14, 24–32. doi: 10.1016/j.funeco.2014.11.001
Jones, D. L., Cooledge, E. C., Hoyle, F. C., Griffiths, R. I., Murphy, D. V. (2019). pH and exchangeable aluminum are major regulators of microbial energy flow and carbon use efficiency in soil microbial communities. Soil Biol. Biochem. 138, 107584. doi: 10.1016/j.soilbio.2019.107584
Jones, D. L., Hill, P. W., Smith, A. R., Farrell, M., Ge, T., Banning, N. C., et al. (2018). Role of substrate supply on microbial carbon use efficiency and its role in interpreting soil microbial community-level physiological profiles (CLPP). Soil Biol. Biochem. 123, 1–6. doi: 10.1016/j.soilbio.2018.04.014
Kätterer, T., Andrén, O. (2001). The ICBM family of analytically solved models of soil carbon, nitrogen and microbial biomass dynamics — descriptions and application examples. Ecol. Model. 136, 191–207. doi: 10.1016/S0304-3800(00)00420-8
Keiblinger, K. M., Hall, E. K., Wanek, W., Szukics, U., Hämmerle, I., Ellersdorfer, G., et al. (2010). The effect of resource quantity and resource stoichiometry on microbial carbon-use-efficiency. FEMS Microbiol. Ecol. 73, 430–440. doi: 10.1111/j.1574-6941.2010.00912.x
Khan, K. S., Mack, R., Castillo, X., Kaiser, M., Joergensen, R. G. (2016). Microbial biomass, fungal and bacterial residues, and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 271, 115–123. doi: 10.1016/j.geoderma.2016.02.019
Kirschbaum, M. U. F. (2004). Soil respiration under prolonged soil warming: are rate reductions caused by acclimation or substrate loss? Global Change Biol. 10, 1870–1877. doi: 10.1111/j.1365-2486.2004.00852.x
Li, J., Sang, C., Yang, J., Qu, L., Xia, Z., Sun, H., et al. (2021). Stoichiometric imbalance and microbial community regulate microbial elements use efficiencies under nitrogen addition. Soil Biol. Biochem. 156, 108207. doi: 10.1016/j.soilbio.2021.108207
Li, J., Wang, G., Mayes, M. A., Allison, S. D., Frey, S. D., Shi, Z., et al. (2019). Reduced carbon use efficiency and increased microbial turnover with soil warming. Global Change Biol. 25, 900–910. doi: 10.1111/gcb.14517
Liu, W., Qiao, C., Yang, S., Bai, W., Liu, L. (2018). Microbial carbon use efficiency and priming effect regulate soil carbon storage under nitrogen deposition by slowing soil organic matter decomposition. Geoderma 332, 37–44. doi: 10.1016/j.geoderma.2018.07.008
López-Urrutia, Á., Morán, X. A. G. (2007). Resource limitation of bacterial production distorts the temperature dependence of oceanic carbon cycling. Ecology 88, 817–822. doi: 10.1890/06-1641
Lou, H., Shi, X., Ren, X., Yang, S., Cai, M., Pan, Z., et al. (2024). Limited terrestrial carbon sinks and increasing carbon emissions from the Hu Line spatial pattern perspective in China. Ecol. Indic. 162, 112035. doi: 10.1016/j.ecolind.2024.112035
Lu, M., Zhou, X., Luo, Y., Yang, Y., Fang, C., Chen, J., et al. (2011). Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis. Agriculture Ecosyst. Environ. 140, 234–244. doi: 10.1016/j.agee.2010.12.010
Malik, A. A., Martiny, J. B. H., Brodie, E. L., Martiny, A. C., Treseder, K. K., Allison, S. D. (2020). Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change. ISME J. 14, 1–9. doi: 10.1038/s41396-019-0510-0
Malik, A. A., Puissant, J., Buckeridge, K. M., Goodall, T., Jehmlich, N., Chowdhury, S., et al. (2018). Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 9, 3591. doi: 10.1038/s41467-018-05980-1
Manzoni, S., Taylor, P., Richter, A., Porporato, A., Ågren, G. I. (2012). Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 196, 79–91. doi: 10.1111/j.1469-8137.2012.04225.x
Maynard, D. S., Crowther, T. W., Bradford, M. A. (2017a). Competitive network determines the direction of the diversity-function relationship. Proc. Natl. Acad. Sci. U.S.A. 114, 11464–11469. doi: 10.1073/pnas.1712211114
Maynard, D. S., Crowther, T. W., Bradford, M. A. (2017b). Fungal interactions reduce carbon use efficiency. Ecol. Lett. 20, 1034–1042. doi: 10.1111/ele.12801
McIntire, E. J. B., Fajardo, A. (2014). Facilitation as a ubiquitous driver of biodiversity. New Phytol. 201, 403–416. doi: 10.1111/nph.12478
Miltner, A., Bombach, P., Schmidt-Brücken, B., Kästner, M. (2012). SOM genesis: microbial biomass as a significant source. Biogeochemistry 111, 41–55. doi: 10.1007/s10533-011-9658-z
Monod, J. (1949). The growth of bacterial cultures. Annu. Rev. Microbiol. 3, 371–394. doi: 10.1146/annurev.mi.03.100149.002103
Onipchenko, V. G., Makarov, M. I., Akhmetzhanova, A. A., Soudzilovskaia, N. A., Aibazova, F. U., Elkanova, M. K., et al. (2012). Alpine plant functional group responses to fertiliser addition depend on abiotic regime and community composition. Plant Soil 357, 103–115. doi: 10.1007/s11104-012-1146-2
Öquist, M. G., Erhagen, B., Haei, M., Sparrman, T., Ilstedt, U., Schleucher, J., et al. (2017). The effect of temperature and substrate quality on the carbon use efficiency of saprotrophic decomposition. Plant Soil 414, 113–125. doi: 10.1007/s11104-016-3104-x
Or, D., Smets, B. F., Wraith, J. M., Dechesne, A., Friedman, S. P. (2007). Physical constraints affecting bacterial habitats and activity in unsaturated porous media – a review. Adv. Water Resour. 30, 1505–1527. doi: 10.1016/j.advwatres.2006.05.025
Parton, W. J., Schimel, D. S., Cole, C. V., Ojima, D. S. (1987). Analysis of factors controlling soil organic matter levels in great plains grasslands. Soil Sci. Soc. America J. 51, 1173–1179. doi: 10.2136/sssaj1987.03615995005100050015x
Qiao, Y., Wang, J., Liang, G., Du, Z., Zhou, J., Zhu, C., et al. (2019). Global variation of soil microbial carbon-use efficiency in relation to growth temperature and substrate supply. Sci. Rep. 9, 5621. doi: 10.1038/s41598-019-42145-6
Riggs, C. E., Hobbie, S. E. (2016). Mechanisms driving the soil organic matter decomposition response to nitrogen enrichment in grassland soils. Soil Biol. Biochem. 99, 54–65. doi: 10.1016/j.soilbio.2016.04.023
Riggs, C. E., Hobbie, S. E., Bach, E. M., Hofmockel, K. S., Kazanski, C. E. (2015). Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry 125, 203–219. doi: 10.1007/s10533-015-0123-2
Schimel, J. P., Schaeffer, S. M. (2012). Microbial control over carbon cycling in soil. Front. Microbiol. 3. doi: 10.3389/fmicb.2012.00348
Silva-Sánchez, A., Soares, M., Rousk, J. (2019). Testing the dependence of microbial growth and carbon use efficiency on nitrogen availability, pH, and organic matter quality. Soil Biol. Biochem. 134, 25–35. doi: 10.1016/j.soilbio.2019.03.008
Sinsabaugh, R. L., Manzoni, S., Moorhead, D. L., Richter, A. (2013). Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol. Lett. 16, 930–939. doi: 10.1111/ele.12113
Sinsabaugh, R. L., Moorhead, D. L., Xu, X., Litvak, M. E. (2017). Plant, microbial and ecosystem carbon use efficiencies interact to stabilize microbial growth as a fraction of gross primary production. New Phytol. 214, 1518–1526. doi: 10.1111/nph.14485
Sinsabaugh, R. L., Turner, B. L., Talbot, J. M., Waring, B. G., Powers, J. S., Kuske, C. R., et al. (2016). Stoichiometry of microbial carbon use efficiency in soils. Ecol. Monogr. 86, 172–189. doi: 10.1890/15-2110.1
Soares, M., Rousk, J. (2019). Microbial growth and carbon use efficiency in soil: Links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol. Biochem. 131, 195–205. doi: 10.1016/j.soilbio.2019.01.010
Song, Z., Vail, A., Sadowsky, M. J., Schilling, J. S. (2012). Competition between two wood-degrading fungi with distinct influences on residues. FEMS Microbiol. Ecol. 79, 109–117. doi: 10.1111/j.1574-6941.2011.01201.x
Soong, J. L., Fuchslueger, L., Marañon-Jimenez, S., Torn, M. S., Janssens, I. A., Penuelas, J., et al. (2020). Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling. Global Change Biol. 26, 1953–1961. doi: 10.1111/gcb.14962
Spohn, M., Klaus, K., Wanek, W., Richter, A. (2016a). Microbial carbon use efficiency and biomass turnover times depending on soil depth – Implications for carbon cycling. Soil Biol. Biochem. 96, 74–81. doi: 10.1016/j.soilbio.2016.01.016
Spohn, M., Pötsch, E. M., Eichorst, S. A., Woebken, D., Wanek, W., Richter, A. (2016b). Soil microbial carbon use efficiency and biomass turnover in a long-term fertilization experiment in a temperate grassland. Soil Biol. Biochem. 97, 168–175. doi: 10.1016/j.soilbio.2016.03.008
Sun, Y., Chen, X. (2024). Phosphorus fertilization enhances terrestrial carbon cycling in phosphorus-deficient ecosystems. J. Environ. Manage. 351, 119941. doi: 10.1016/j.jenvman.2023.119941
Tiemann, L. K., Billings, S. A. (2011). Changes in variability of soil moisture alter microbial community C and N resource use. Soil Biol. Biochem. 43, 1837–1847. doi: 10.1016/j.soilbio.2011.04.020
Tucker, C. L., Bell, J., Pendall, E., Ogle, K. (2013). Does declining carbon-use efficiency explain thermal acclimation of soil respiration with warming? Global Change Biol. 19, 252–263. doi: 10.1111/gcb.12036
VandenEnden, L., Anthony, M. A., Frey, S. D., Simpson, M. J. (2021). Biogeochemical evolution of soil organic matter composition after a decade of warming and nitrogen addition. Biogeochemistry 156, 161–175. doi: 10.1007/s10533-021-00837-0
Waldrop, M. P., Firestone, M. K. (2004). Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities. Oecologia 138, 275–284. doi: 10.1007/s00442-003-1419-9
Wang, X., Cui, Y., Wang, Y., Duan, C., Niu, Y., Sun, R., et al. (2022). Ecoenzymatic stoichiometry reveals phosphorus addition alleviates microbial nutrient limitation and promotes soil carbon sequestration in agricultural ecosystems. J. Soils Sediments 22, 536–546. doi: 10.1007/s11368-021-03094-8
Wertz, S., Degrange, V., Prosser, J. I., Poly, F., Commeaux, C., Guillaumaud, N., et al. (2007). Decline of soil microbial diversity does not influence the resistance and resilience of key soil microbial functional groups following a model disturbance. Environ. Microbiol. 9, 2211–2219. doi: 10.1111/j.1462-2920.2007.01335.x
Wetterstedt, J.Å.M., Ågren, G. I. (2011). Quality or decomposer efficiency – which is most important in the temperature response of litter decomposition? A modelling study using the GLUE methodology. Biogeosciences 8, 477–487. doi: 10.5194/bg-8-477-2011
Widdig, M., Schleuss, P.-M., Biederman, L. A., Borer, E. T., Crawley, M. J., Kirkman, K. P., et al. (2020). Microbial carbon use efficiency in grassland soils subjected to nitrogen and phosphorus additions. Soil Biol. Biochem. 146, 107815. doi: 10.1016/j.soilbio.2020.107815
Wieder, W. R., Bonan, G. B., Allison, S. D. (2013). Global soil carbon projections are improved by modelling microbial processes. Nat. Climate Change 3, 909–912. doi: 10.1038/nclimate1951
Xu, H., Qu, Q., Xue, S., Wang, M. (2024). A global analysis of the effects of forest thinning on soil N stocks and dynamics. Catena 246, 108411. doi: 10.1016/j.catena.2024.108411
Xu, X., Schimel, J. P., Thornton, P. E., Song, X., Yuan, F., Goswami, S. (2014). Substrate and environmental controls on microbial assimilation of soil organic carbon: a framework for Earth system models. Ecol. Lett. 17, 547–555. doi: 10.1111/ele.12254
Yang, Y., Yang, J., Dong, Q., Li, D., Tan, B., Wu, Q., et al. (2024). Heavy nitrogen application rate and long-term duration decrease the soil organic carbon and nitrogen sequestration rates in forest ecosystems. Forests 15, 1585. doi: 10.3390/f15091585
Zhang, K., Chen, L., Li, Y., Brookes, P. C., Xu, J., Luo, Y. (2020). Interactive effects of soil pH and substrate quality on microbial utilization. Eur. J. Soil Biol. 96, 103151. doi: 10.1016/j.ejsobi.2020.103151
Zhang, Y., Qiang, W., Luo, R. Y., Wang, M., Pang, X. Y. (2022). Effects of nitrogen and phosphorus addition on soil microbial growth, turnover, and carbon use efficiency: a review. Chin. J. Appl. Environ. Biol. 28, 526–534. doi: 10.19675/j.cnki.1006-687x.2020.11019
Zhang, W., Wang, L., Chen, J., Zhang, Y. (2024). Preferential flow in soils: review of role in soil carbon dynamics, assessment of characteristics, and performance in ecosystems. Eurasian Soil Sci. 57, 814–825. doi: 10.1134/S1064229323602548
Zhang, L., Xiong, S., Shen, Y., You, C., Li, H., Wang, L., et al. (2024). Changes in soil microbial community and function across stand age of Cryptomeria japonica var. sinensis plantations in subtropical China. Appl. Soil Ecol. 203, 105645. doi: 10.1016/j.apsoil.2024.105645
Keywords: carbon use efficiency, soil microorganisms, biotic factors, abiotic factors, carbon cycle
Citation: Tang X, Li Z, Yuan J, Yu W and Luo W (2024) Biotic and abiotic factors affecting soil microbial carbon use efficiency. Front. Plant Sci. 15:1445230. doi: 10.3389/fpls.2024.1445230
Received: 07 June 2024; Accepted: 29 October 2024;
Published: 15 November 2024.
Edited by:
Shouzheng Tong, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Gang Yang, Southwest University of Science and Technology, ChinaFeng Li, Chinese Academy of Sciences (CAS), China
Xin-Sheng Chen, Anhui University, China
Hongwei Xu, Sichuan Agricultural University, China
Copyright © 2024 Tang, Li, Yuan, Yu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbo Luo, bHVvd2IzMzBAbmVudS5lZHUuY24=; Zhenxin Li, bGl6eDU0MkBuZW51LmVkdS5jbg==
 Xinyu Tang
Xinyu Tang Zhenxin Li
Zhenxin Li Jihong Yuan
Jihong Yuan Weirui Yu
Weirui Yu Wenbo Luo
Wenbo Luo