- 1College of Life Science and Technology, Central South University of Forestry and Technology, Changsha, China
- 2School of Forestry and Biotechnology, Zhejiang Agriculture and Forestry University, Hangzhou, China
Introduction: The Dicranopteris dichotoma fern community plays vital roles in nutrient sequestration, succession regulation, and ecological threshold control. However, the mechanisms underlying the formation of the D. dichotoma–dominant community remain unclear.
Methods: This study established four different community types to investigate the effects of environmental factors on the formation of a D. dichotoma–dominant community.
Results: We found that climate was the primary factor affecting the formation of patches dominated by D. dichotoma at the regional scale. Specifically, higher annual mean temperature and annual mean precipitation were associated with larger single-dominant-species patches of D. dichotoma. Understory light intensity was the major factor affecting the formation of the D. dichotoma community at the community scale. Light intensity ranging from 200 to 500 µmol·m⁻²·s⁻¹ was most conducive to the development of a large D. dichotoma community. Additionally, understory light intensity enhanced the importance value of D. dichotoma in the herb community by decreasing its biomass proportion of support modules and increasing its biomass proportion of photosynthetic and reproductive modules. Soil properties and D. dichotoma characteristics showed interactions with each other. Acidic red-yellow soil was most suitable for the formation of single-dominant-species patches of D. dichotoma, and the growth of D. dichotoma further decreased the soil pH. Soil total phosphorus content was identified as a limiting factor for formation of the D. dichotoma community.
Discussion: In summary, the formation of single-dominant-species patches of D. dichotoma is mainly influenced by a combination of climate, community, and soil.
1 Introduction
As important components of tropical and subtropical ecosystems, ferns play crucial roles in microclimate maintenance, nutrient cycling, and energy circulation (Mehltreter et al., 2010; Tůma et al., 2012; Dalling et al., 2024). Dicranopteris dichotoma is a pioneer fern in subtropical low mountainous and hilly terrains. D. dichotoma thrives with a high light intensity and exhibits characteristics of high-temperature tolerance, acidity resistance, and adaptability to poor soils and drought conditions (Chen et al., 2016a; Wang et al., 2022). In degraded forests, D. dichotoma reproduces via sexual reproduction (spores) during the early colonization stage and then rapidly forms a single-dominant-species community via vegetative reproduction (clones) (Zhu et al., 2016). Therefore, the D. dichotoma single-dominant-species community is commonly found under pioneer tree species such as Pinus massoniana in the subtropical region of China (Qian and Chen, 1959).
D. dichotoma is primarily a clonal plant, and rhizomes are its main clonal reproductive organs. The rhizomes of D. dichotoma grow horizontally in the 0–4 cm soil depth, bearing leaf buds, adventitious roots, and new rhizome branches. These rhizome branches interweave to form a network that, together with the adventitious roots, envelops the detritus and soil, creating a “root sieve” with a thickness up to 10 cm (Zhang et al., 2010a). The leaf buds develop into fronds, forming a dense aerial layer. Due to the dense aerial layer and strong filtering effect of the root sieve, a significant number of seeds are prevented from entering the soil. Additionally, D. dichotoma inhibits the germination and growth of the seeds of other plant species through allelopathy (Juma et al., 2019). Various biological and chemical properties have been proposed as crucial factors in the formation and persistence of single-dominant-species patches of D. dichotoma in the last few decades (Yang et al., 2021).
Previously, the D. dichotoma community was considered a negative factor in the regeneration of the evergreen broadleaf forest posing potential risks such as forest fires, reduced nutrient cycling rates, and diminished plant diversity (Zhang et al., 2010a). However, recent studies have demonstrated the contribution of herbaceous layers to ecosystem nutrient cycling and accumulation, leading to reevaluation of the potential beneficial roles of D. dichotoma in ecosystem restoration and community succession. Indeed, recent studies have found that D. dichotoma slows the regeneration rate of the subtropical forest through “ecological filtering” but does not change the direction of succession (Liu et al., 2021). These findings indicate that D. dichotoma might slow down the succession process and prolong the soil recovery time. Therefore, D. dichotoma appears to play vital roles in nutrient sequestration, succession regulation, and ecological threshold control (Yang et al., 2021).
Nevertheless, the detailed formation mechanism of the single-dominant community of D. dichotoma in subtropical forests remains unclear. It is generally agreed that a lack of light in the understory is a crucial factor restricting the growth of D. dichotoma. This observation is aligned with the principle of heliotic plant withdrawal from the community. To address this question, we conducted controlled greenhouse experiments to evaluate the physiological characteristics of potted D. dichotoma under various light intensities. Additionally, a semi-field experiment was conducted by placing potted D. dichotoma under shrubs, P. massoniana, and evergreen broadleaved forest species. The results were not consistent with the previously established hypothesis. Instead, the experiments revealed that D. dichotoma exhibits a preference for medium-intensity light (300–800 µmol·m−2·s−1) and is intolerant to strong (>1800 µmol·m−2·s−1) and weak (<100 µmol·m−2·s−1) light intensities (Wang et al., 2021; Liao et al., 2023).
The asexual reproduction mode of D. dichotoma is closely linked to rhizome growth and leaf bud development. The rhizomes can branch indefinitely, and the fronds grow in a lush manner in moist and fertile soil, reaching heights of over 1 m (Wang et al., 2022). In contrast, in poor and arid soil conditions, the growth of the rhizomes ceases and the fronds are sparse and stunted, with heights reaching only 10–15 cm (Liu, 2017). Therefore, soil physicochemical properties, particularly the soil nutrient content, greatly impact D. dichotoma clonal reproduction (Lin, 2020; Bai et al., 2020; Wang et al., 2021). In turn, the D. dichotoma community can also impact the soil’s physical and chemical properties (Wan et al., 2014; Chen et al., 2016b; Lyu et al., 2019). However, previous studies have primarily involved small-scale field or pot planting experiments, with only a few large-scale field studies on D. dichotoma conducted to date. Consequently, our understanding of the interaction mechanisms between D. dichotoma community formation and environmental factors remain limited.
To fill this gap, in the present study, we investigated the associations of climate, community, soil, and topography characteristics in four common subtropical communities with the clonal dispersal traits of D. dichotoma in these communities. We established the following hypotheses: (H1) at the regional scale, the formation of large single-dominant-species patches of D. dichotoma is primarily influenced by climate; (H2) moderate levels of understory light (300–800 µmol·m⁻²·s⁻¹) are most favorable for the formation of the D. dichotoma community; and (H3) higher soil nitrogen and phosphorus nutrient contents promote the growth of D. dichotoma.
2 Materials and methods
2.1 Study areas and experimental design
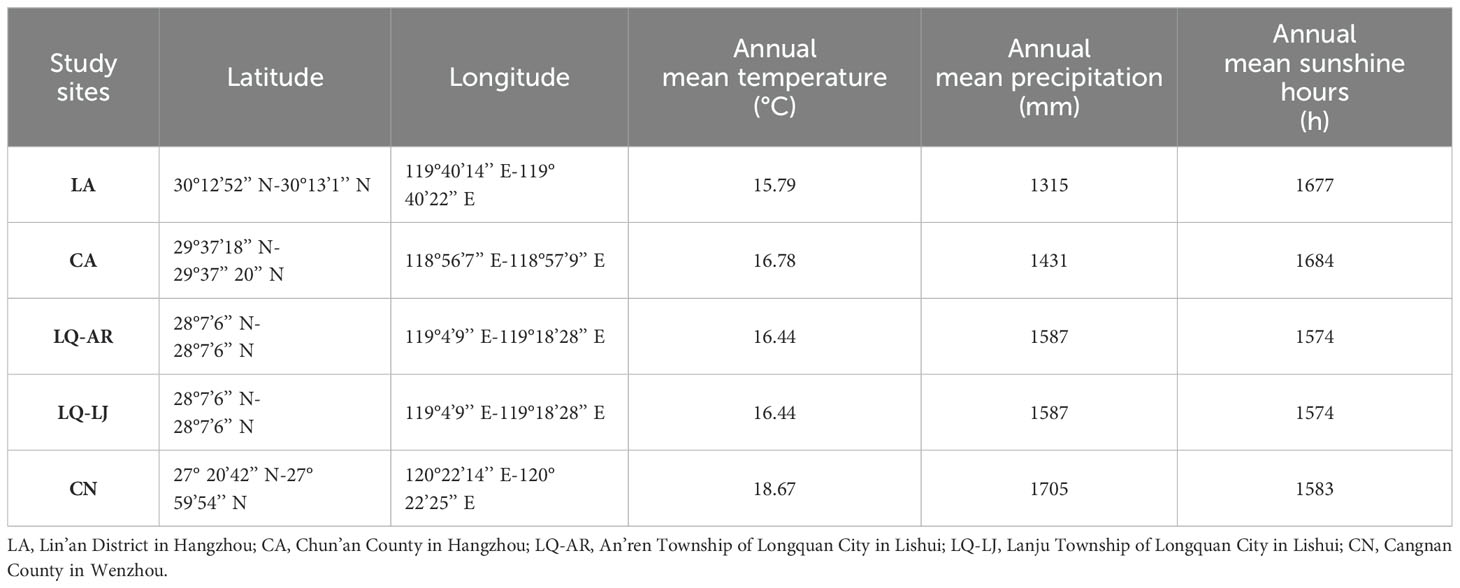
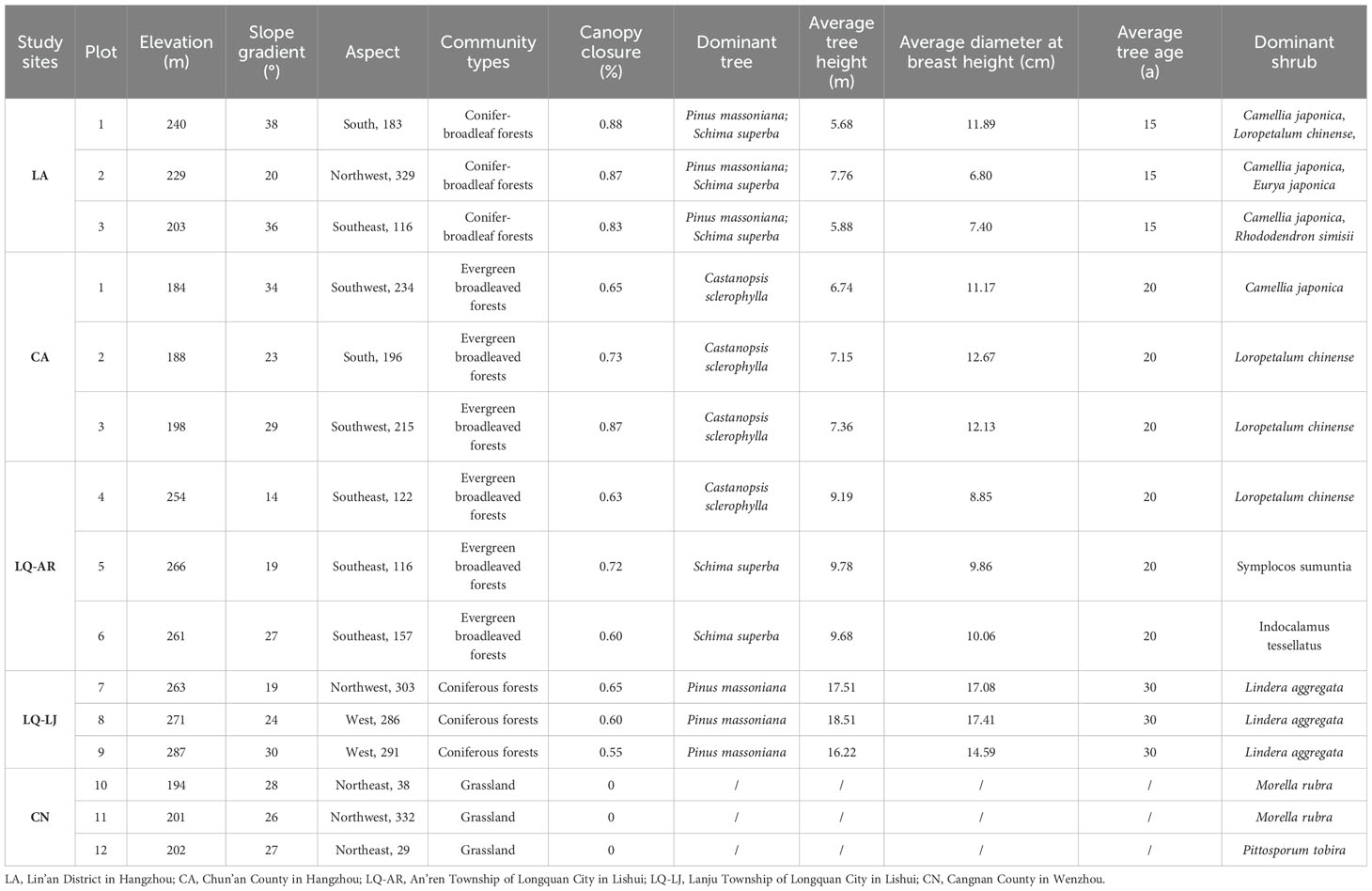
Communities dominated by D. dichotoma in the herb layer were selected as the study stands. The stands were located from north to south across multiple sites in Zhejiang Province, encompassing Lin’an District in Hangzhou (LA), Chun’an County in Hangzhou (CA), Longquan City in Lishui (LQ), and Cangnan County in Wenzhou (CN), as shown in Figure 1. The study region has a mid-subtropical monsoon climate characterized by distinct seasons with prolonged winters and summers and shorter springs and autumns. The predominant soil type is red-yellow loam. The annual mean temperature and annual mean precipitation increased progressively across each study site, while the annual mean sunshine hours decreased accordingly in a north-to-south direction (Table 1). All study sites were located on slopes or gentle slopes at elevations of 200–300 m (Table 2). The community types include grassy slopes, coniferous forests, coniferous and broadleaved mixed forests, and evergreen broadleaved forests, all of which are secondary communities (the community information of the study sites was detailed in Tables 2, 3).
Figure 1. Map showing the location of the study areas (red triangles). LA, Lin’an District in Hangzhou; CA, Chun’an County in Hangzhou; LQ, Longquan City in Lishui; CN, Cangnan County in Wenzhou. This map is based on the standard map No. GS (2024) 0650, downloaded from the Standard Map Service Website of the Ministry of Natural Resources, China. The base map was not modified.
We established 15 tree plots (20 m × 20 m) in the five study areas using a completely randomized design (Figure 1). The vegetation in the three experimental plots in the same study area was relatively consistent. In each plot, three shrub plots (5 m × 5 m) were established diagonally, and three herbaceous plots (1 m × 1 m) were established along the diagonal in each shrub plot, resulting in a total of 15 tree stands, 45 shrub plots, and 135 herbaceous plots across the five study areas. From July to September 2022, we conducted a field survey of plant characteristics (Tables 2, 3). The occurrence probability of each species in all sample plots was recorded to calculate the relative frequencies.
2.2 Plant sampling and measurement
To determine the clonal dispersal traits of D. dichotoma, an herbaceous plot (1 m × 1 m) was harvested from each shrub plot, including both the aboveground parts and root sieve elements. The height of the shoot was measured using a tape measure before harvest. The fronds, stripes, and rhizomes of D. dichotoma were separated (with the adventitious root mass being negligible and therefore not separated from the rhizomes), dried in an oven at 80°C to reach a constant dry weight, and weighed. Before drying, the total length of rhizome, mean length of the rhizomes internodes, and mean length of roots were measured.
2.3 Soil sampling and physicochemical property analysis
Five soil cores, selected by the S-shaped approach, were collected from the 0-cm to 10-cm profiles in each plot using a 5-cm circular soil auger. To ensure consistency, the five soil samples were thoroughly mixed to homogenize each sample. Undisturbed soil samples were collected at the same position in each profile using a cutting ring with a volume of 100 cm3 to measure the soil bulk density and soil water content.
The soil water content was determined using the drying method, the soil bulk density was determined using the cutting ring method (Al-Shammary et al., 2018), and the soil pH was determined using the potentiometric method. The soil samples were air-dried after being sieved (with a 2-mm mesh) for nutrient analysis. The soil total carbon content was determined using a carbon and nitrogen analyzer (Muti-N/C 2100, Analytik Jean AG, Germany). The soil total nitrogen content was determined using the semi-micro-Kjeldahl nitrogen method, as well as with a carbon and nitrogen analyzer. The soil total phosphorus content was determined using acid fusion and the molybdenum-antimony colorimetric method with an ultraviolet spectrophotometer (NanoDrop One, Thermo Fisher Scientific, Waltham, MA, USA). All the analytical methods employed in this study were adapted from the soil agrochemical analysis methods described in Lu (2000).
2.4 Data analysis
The importance value of trees, shrubs, or herbs was calculated using the following formulas (Fang et al., 2009):
where RD, RC, RF, and RH represent the relative density, relative coverage, relative frequency, and relative height, respectively.
To conduct the variance partitioning analysis, parameters such as community type and topographic factor were determined. For community type, the grass slope was set to 1, the coniferous forest was set to 2, the coniferous and broadleaved mixed forest was set to 3, and the evergreen broadleaved forest was set to 4. The slope aspect was transformed into the transformation of the aspect index (TRASP), ranging between 0 and 1, with the following formula:
where TRASP is the aspect index and aspect are the aspect azimuth angle determined by the compass. Through conversion, TRASP falls between 0 and 1, where 0 represents the northeast direction and 1 represents the southwest direction (Wang et al., 2017).
2.5 Statistical analysis
One-way analysis of variance and least significant difference tests were conducted for multiple comparisons, with statistical significance assessed at p < 0.05. Variance partitioning analysis was used to determine the degree to which the environmental factors explain the coverage and important value of D. dichotoma, which was conducted using the R package “vegan.” Regression analysis was used to examine the effects of environmental factors on the growth of D. dichotoma, while Pearson correlation analysis was employed to investigate the interactions between soil characteristics and D. dichotoma. All statistical analyses and mapping were performed in R (version 4.2.0) and Sigmaplot (Systat, version 15.0).
3 Results
3.1 Single-dominant-species patches of D. dichotoma in different study sites
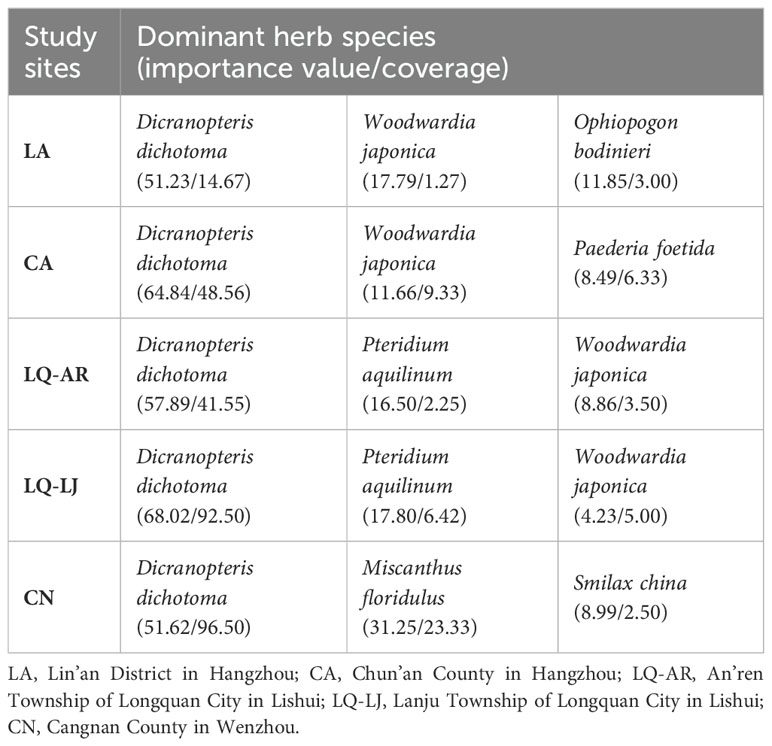
Higher coverage and importance values indicate a stronger capacity for D. dichotoma to form single-dominant-species patches. D. dichotoma exhibited the highest coverage and importance value in LQ-LJ (Figure 2). Although the coverage of D. dichotoma in CN was also high, its importance value was the lowest, suggesting the presence of other herbaceous species within the patches. Table 3 shows that Miscanthus floridulus was the dominant associated species in the herb layer in CN. M. floridulus is a tall herb that readily forms single-species-dominant patches.
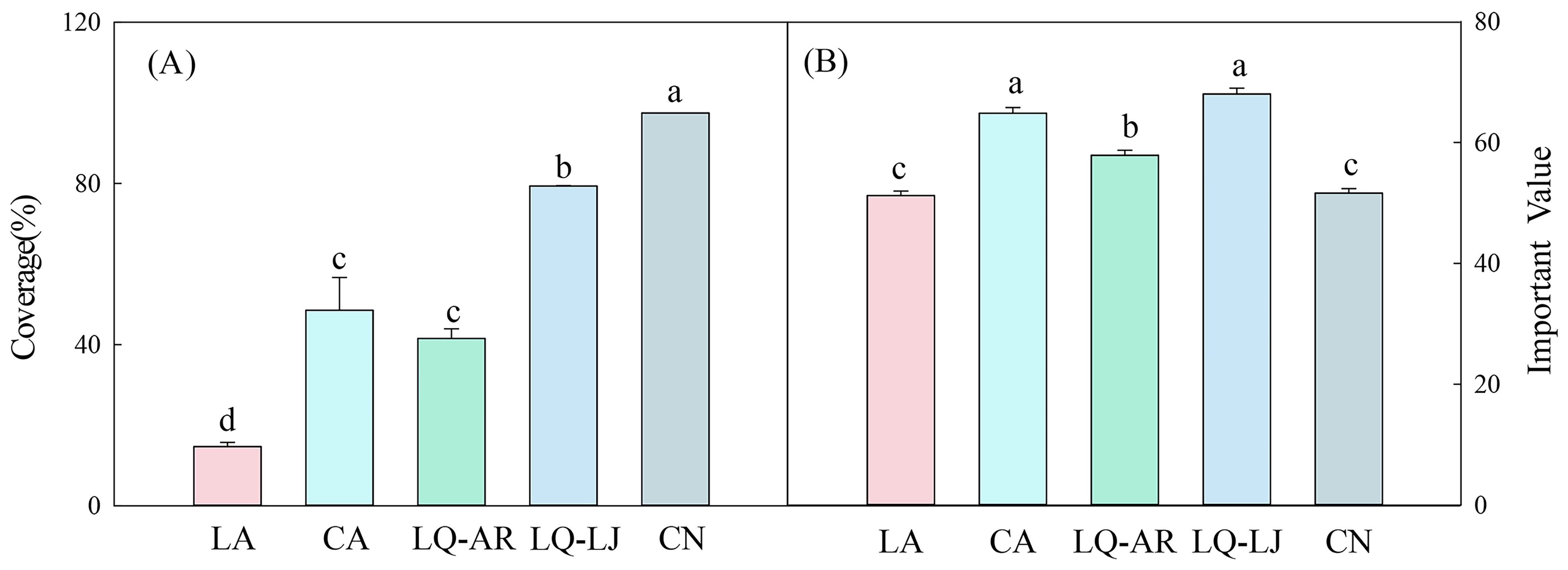
Figure 2. The coverage (A) and important value (B) of D. dichotoma in different study sites. LA, Lin’an District in Hangzhou; CA, Chun’an County in Hangzhou; LQ-AR, An’ren Township of Longquan City in Lishui; LQ-LJ, Lanju Township of Longquan City in Lishui; CN, Cangnan County in Wenzhou. Different lowercase letters represent significant differences among the study sites (P < 0.05). Data represent mean ± SD (n=9).
In CA and LQ-AR, the coverage of D. dichotoma was approximately 50%, roughly half that observed in LQ-LJ; however, the importance values were relatively higher. The coverage of D. dichotoma in LA was the lowest at 14%, which also had the lowest importance value among the sites. Consequently, the formation of single-dominant-species patches of D. dichotoma appears to be the most conducive in LQ-LJ, followed by CN, LQ-AR, CA, and is the least conducive in LA.
3.2 Clonal dispersal traits of D. dichotoma in different study sites
The highest biomass of D. dichotoma was observed in LQ-LJ (454.27 g·m−2), followed by LQ-AR and CN, with the lowest biomass recorded in LA and CA (Figure 3A), representing a significant difference (p < 0.05). The biomass of fronds in LQ-LJ, LQ-AR, and CN was significantly higher than that in CA and LA (Figure 3B, p < 0.05), while the biomass of stipes in CA was significantly lower than that in the other four study sites (Figure 3C, p < 0.05). The biomass of the rhizomes was the greatest in LQ-LJ, indicating that the reproductive ability of D. dichotoma is the strongest in that area (Figure 3D, p < 0.05). The total length of rhizomes and the mean length of rhizome internodes, which reflect the plant’s clonal dispersal ability, were the highest in LQ-LJ and LQ-AR (Figures 4A, B, p < 0.05). The highest mean root length was observed in LA and LQ-AR, followed by LQ-LJ, while the lowest was in CN and CN (Figure 4C, p<0.05). Among the study sites, CN had the lowest mean height of shoots, with the remaining four sites exhibiting no significant differences, averaging approximately 60 cm (Figure 4D).
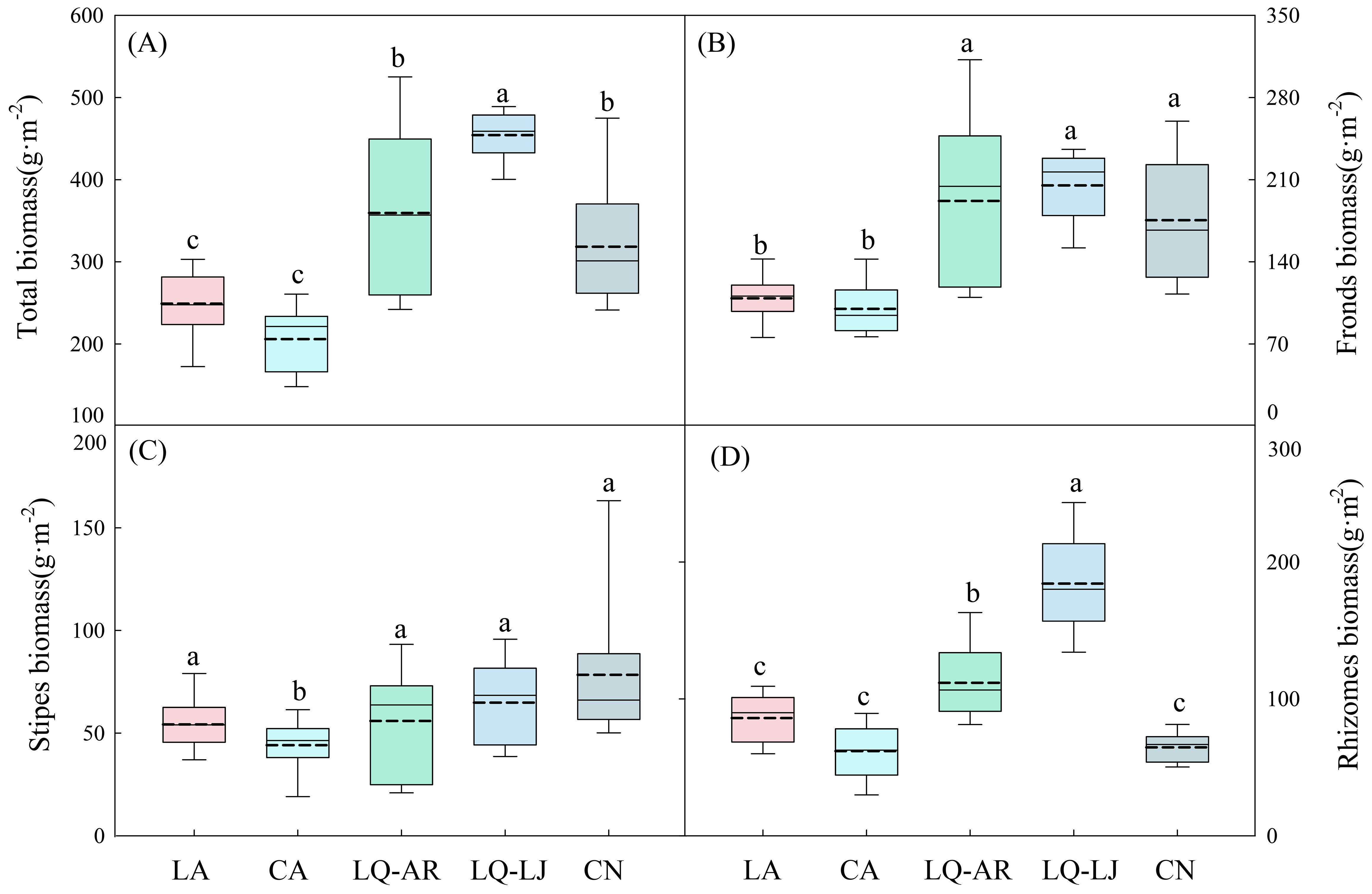
Figure 3. Total biomass (A), fronds biomass (B), stipes biomass (C), and rhizomes biomass (D) of D. dichotoma in different study sites (n = 9). LA, Lin’an District in Hangzhou; CA, Chun’an County in Hangzhou; LQ-AR, An’ren Township of Longquan City in Lishui; LQ-LJ, Lanju Township of Longquan City in Lishui; CN, Cangnan County in Wenzhou. Different lowercase letters represent significant differences among the study sites (p < 0.05). The upper and lower boundaries of the box represent the upper quartile and the lower quartile, respectively. The middle solid line and dashed line indicate the median and mean value, respectively. The whisker lines extend to the maximum and minimum values.
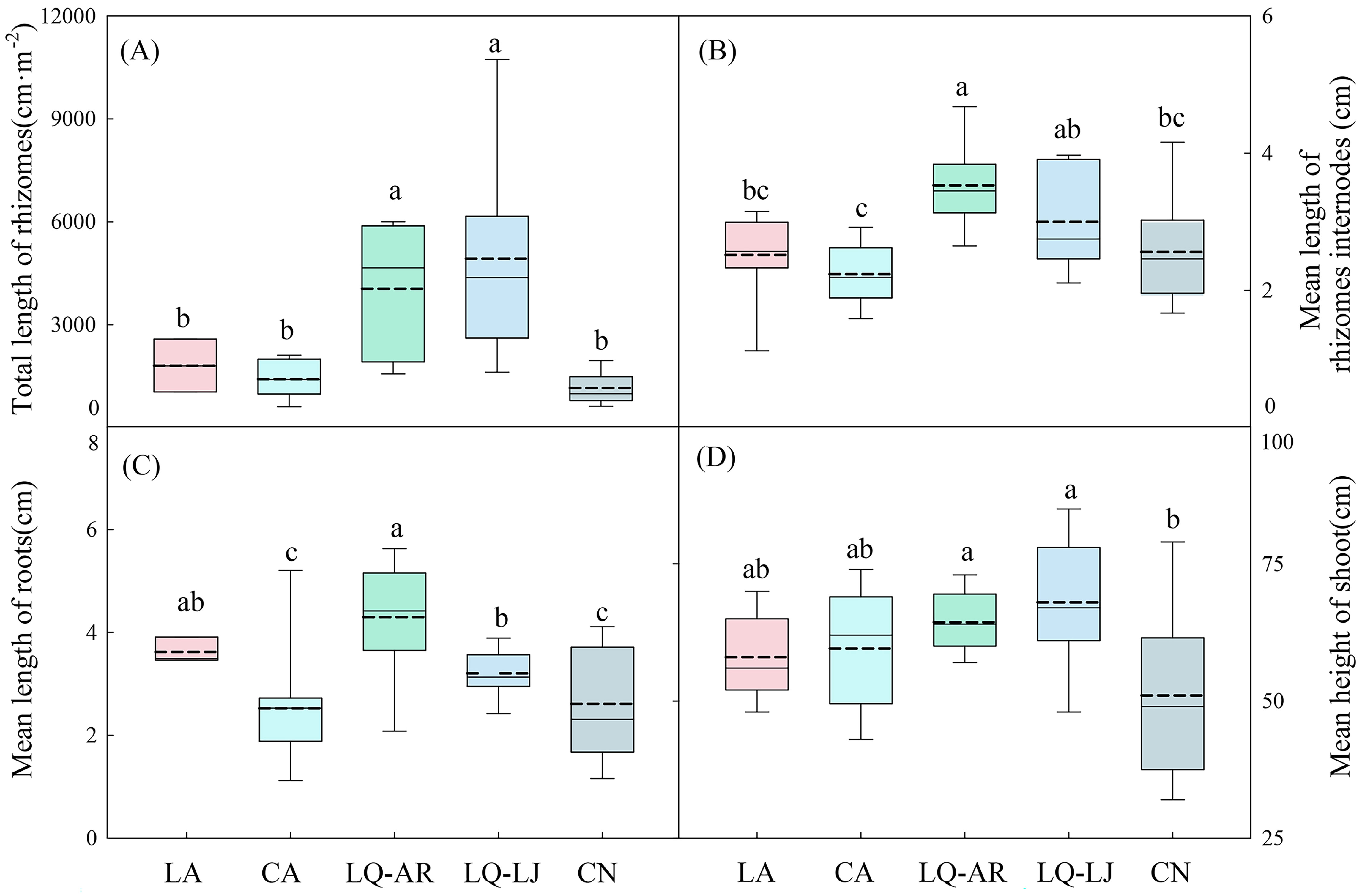
Figure 4. Total length of rhizomes (A), Mean length of rhizomes internodes (B), Mean length of roots (C), and Mean length of shoot (D) of D. dichotoma in different study sites (n = 9). LA, Lin’an District in Hangzhou; CA, Chun’an County in Hangzhou; LQ-AR, An’ren Township of Longquan City in Lishui; LQ-LJ, Lanju Township of Longquan City in Lishui; CN, Cangnan County in Wenzhou. Different lowercase letters represent significant differences among the study sites (p < 0.05). The upper and lower boundaries of the box represent the upper quartile and the lower quartile, respectively. The middle solid line and dashed line indicate the median and mean value, respectively. The whisker lines extend to the maximum and minimum values.
D. dichotoma was found to allocate the majority of its biomass to the fronds and rhizomes, with a smaller portion allocated to the stipes (Figure 5). However, there were significant differences in biomass allocation ratios among different study sites. In LQ-AR and LQ-LJ, the biomass allocation ratio of D. dichotoma to the stipes was only 14%–16%, while those to fronds and rhizomes reached up to 84%. In other sites, the biomass allocation ratio to the stipes exceeded 21%, with those to the fronds and rhizomes ranging from 76% to 79%.
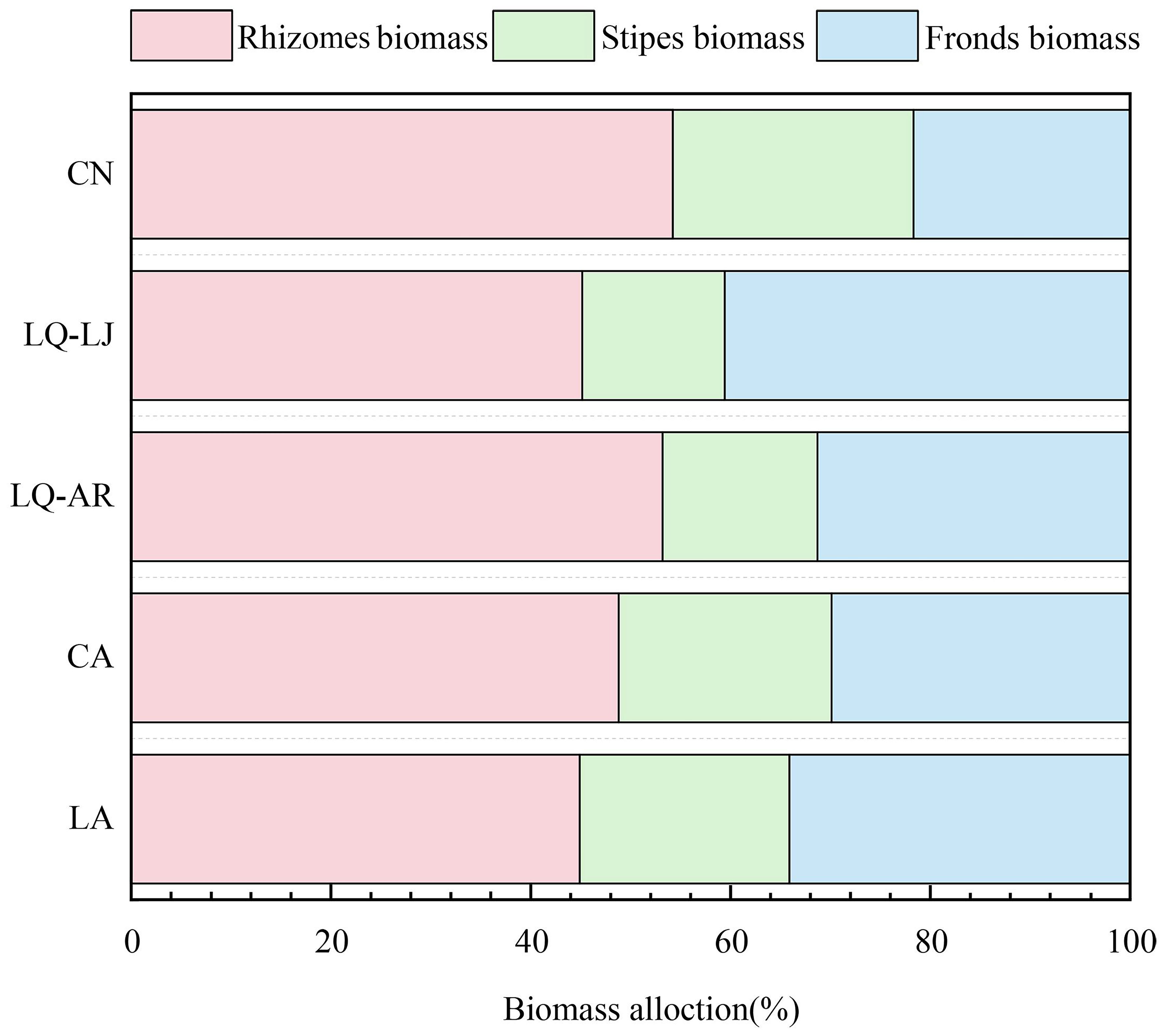
Figure 5. Biomass allocation of D. dichotoma in different study sites (n = 9). LA, Lin’an District in Hangzhou; CA, Chun’an County in Hangzhou; LQ-AR, An’ren Township of Longquan City in Lishui; LQ-LJ, Lanju Township of Longquan City in Lishui; CN, Cangnan County in Wenzhou.
3.3 Effects of climate and topography on single-dominant-species patches of D. dichotoma
Climate and topography had a minimal impact on the importance value of D. dichotoma but significantly influenced its coverage (Figure 6). Topography affected the coverage and importance value of D. dichotoma by 22.1% and 0.6%, respectively. Table 4 shows that the clonal dispersal traits of D. dichotoma, which determined the internal factors affecting its coverage, were significantly positively correlated with elevation and significantly negatively correlated with slope. It explains why D. dichotoma prefers to grow on gentle slopes below an elevation of 300 m (Table 2).
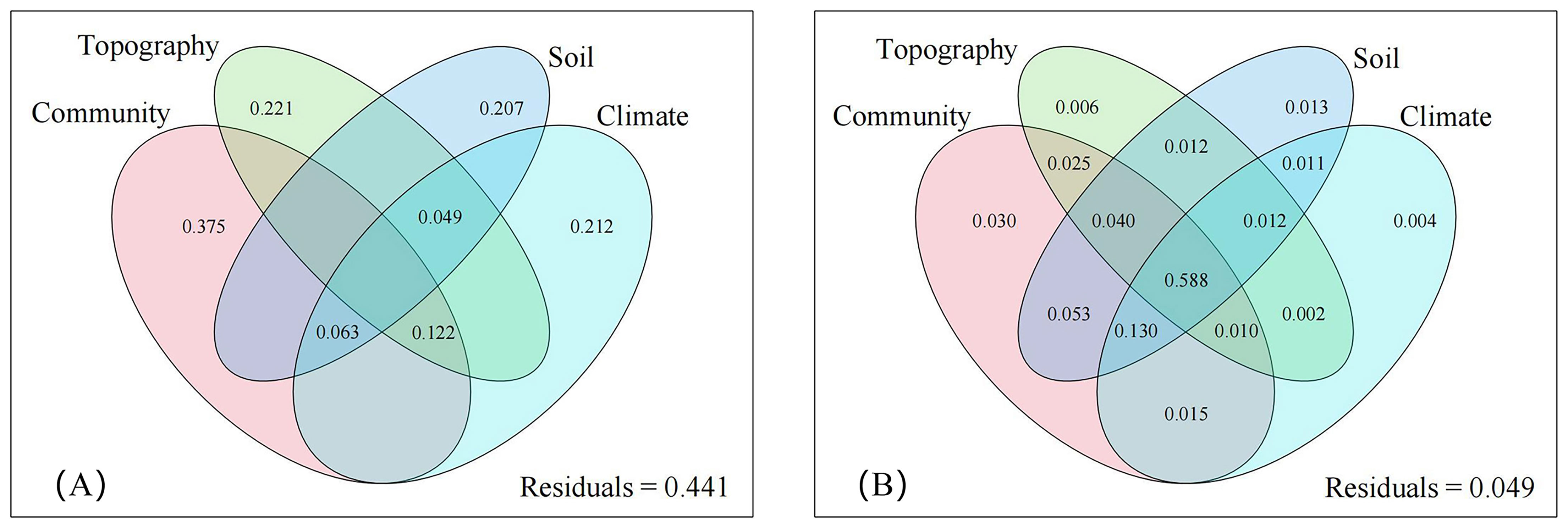
Figure 6. Explanatory of different environmental factors for coverage (A) and important value (B) of D. dichotoma. The climate was annual mean temperature, annual mean precipitation, and annual mean sunshine hours. The topography was elevation, slope, and aspect. The community were canopy closure, community type, and understory light intensity. The soil was pH, soil water content, soil bulk density, total carbon content, total nitrogen content, and total phosphorus content.
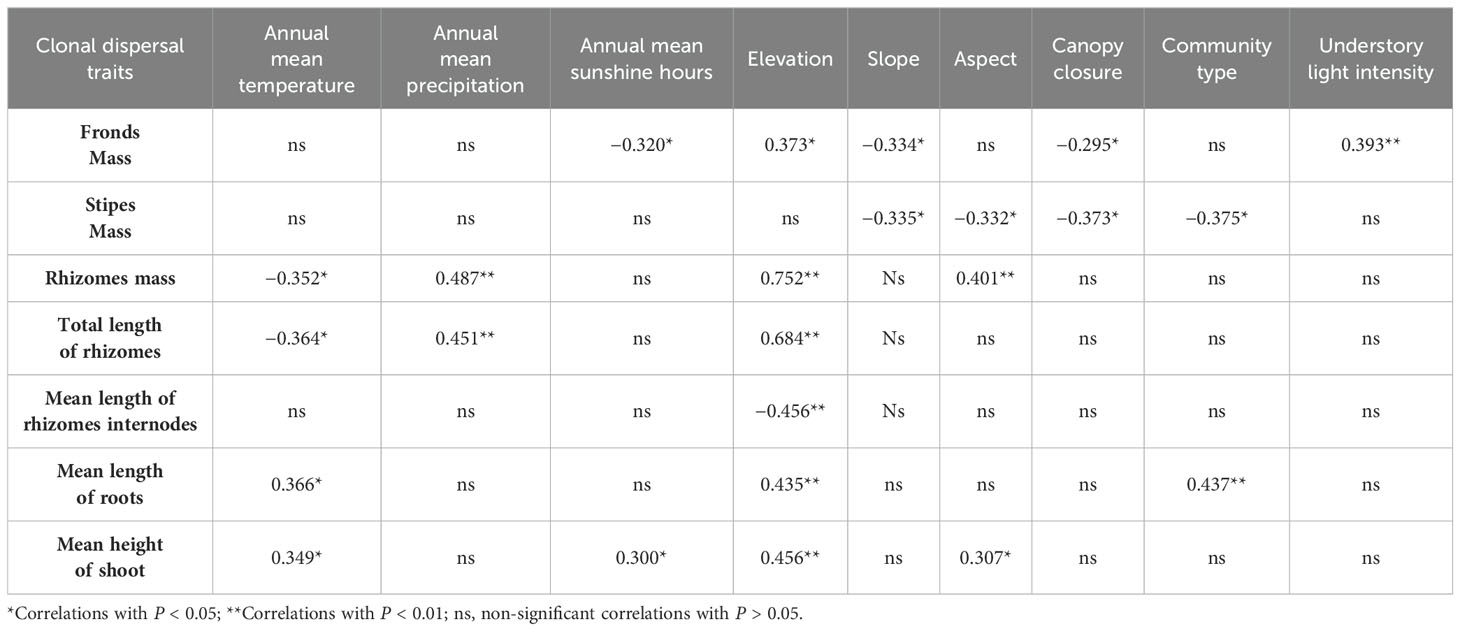
Table 4. Correlation of clonal dispersal traits of D. dichotoma with climate, community, and topography characteristics.
Climate affected both the coverage and importance value of D. dichotoma by 21.2% and 0.4%, respectively. The coverage of D. dichotoma showed a significant positive correlation with annual mean temperature (r = 0.697, Figure 7A), whereas its importance value did not correlate significantly with this temperature variable (Figure 7B). The coverage of D. dichotoma did not show a significant correlation with annual mean precipitation (Figure 7C). However, its importance value had a significant positive correlation with annual mean precipitation, although the correlation coefficient was low at 0.360 (Figure 7D). Therefore, climate and topography mainly affected the coverage of D. dichotoma, while its impact on the importance value was not significant. In addition, the rhizomes biomass and total length of rhizomes were significantly negatively correlated with the annual mean temperature and significantly positively correlated with the annual mean precipitation (Table 4).
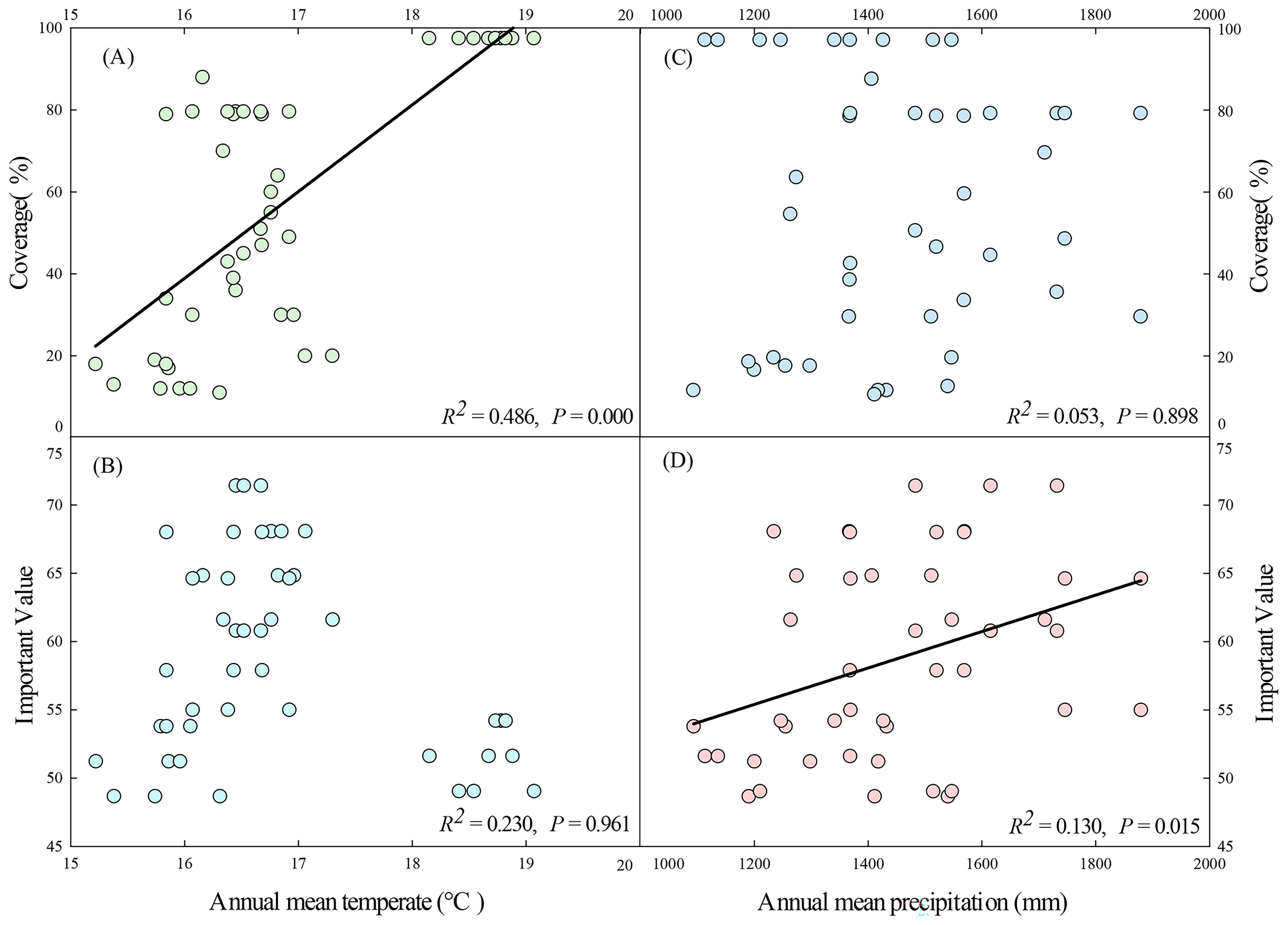
Figure 7. Annual mean temperature in relation to coverage (A) and important value (B) of D. dichotoma. Annual mean precipitation in relation to coverage (C) and important value (D) of D. dichotoma (n=45). Solid lines represent the fitted linear regression model and indicate significant relationship (P < 0.05).
3.4 Effects of community factors on patches of D. dichotoma
The influence of community factors on the coverage of D. dichotoma was the greatest among the four environmental factors, reaching up to 37.5% (Figure 6A). Community types, canopy closure, and understory light intensity exhibit strong autocorrelation; thus, only the effects of understory light intensity on the formation of single-dominant-species patches of D. dichotoma were analyzed.
A logarithmic correlation was found between understory light intensity and coverage of D. dichotoma, with a correlation coefficient of 0.827 (Figure 8A, p < 0.05). The coverage of D. dichotoma initially increased rapidly with increasing understory light intensity and then leveled off. At light intensities of 200–400 µmol·m⁻²·s⁻¹, the coverage ranged from 40% to 80%, whereas at light intensities of 400–500 µmol·m⁻²·s⁻¹ the coverage approached 100%.
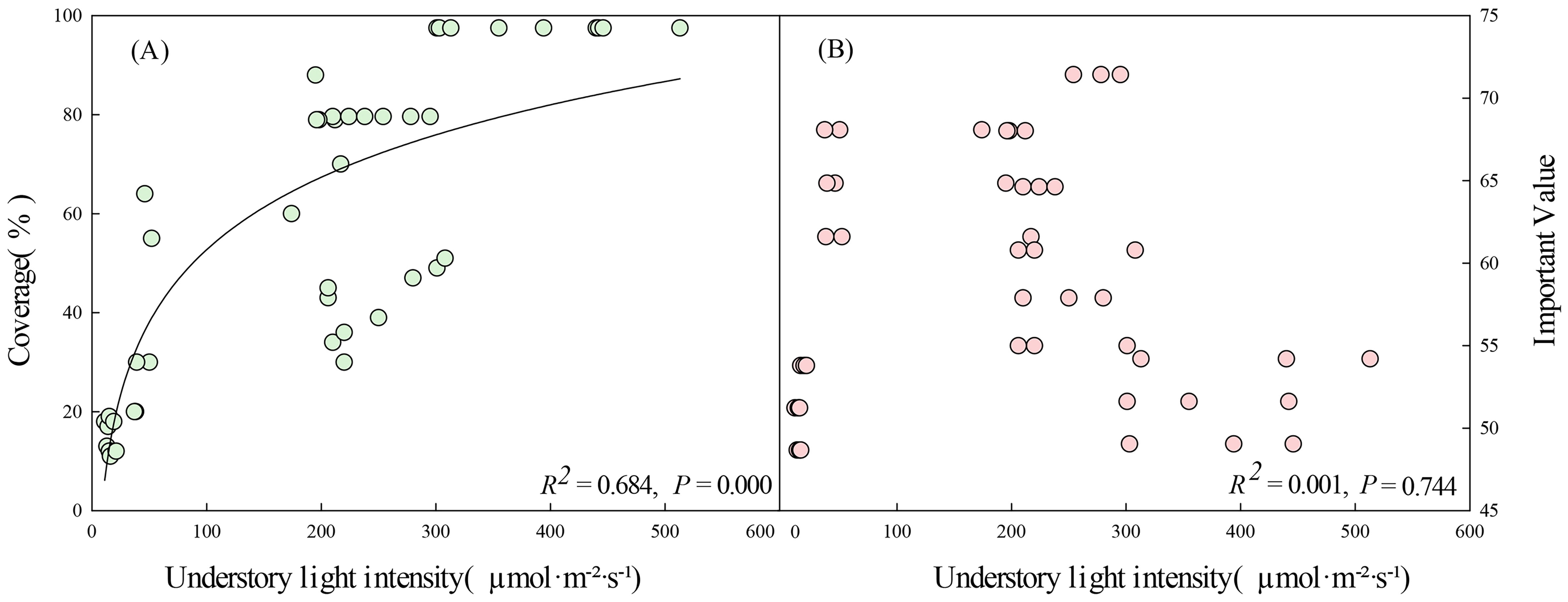
Figure 8. Understory light intensity in relation to coverage (A) and important value (B) of D. dichotoma (n=45). Solid lines represent the fitted linear regression model and indicate a significant relationship (P < 0.05).
By contrast, community factors affected the importance value of D. dichotoma only by 3.0% (Figure 6B), and understory light intensity also did not have a significant impact on the importance value of D. dichotoma (Figure 8B). Furthermore, the fronds and stipes biomass of D. dichotoma was significantly negatively correlated with the community canopy, and significantly positively correlated with the understory light intensity (Table 4).
3.5 Correlations between soil properties and patches of D. dichotoma
D. dichotoma was mainly found in acidic and moist soil. Figure 9 shows that the soil pH was in the range of 4.53–5.11, the soil water content was 15.38%–22.96%, and the soil bulk density was 0.98–1.48 g·cm−3. D. dichotoma exhibits strong adaptability to soil nutrients and was able to grow in soils with very low total carbon, nitrogen, and phosphorus contents (Figures 9D–F).
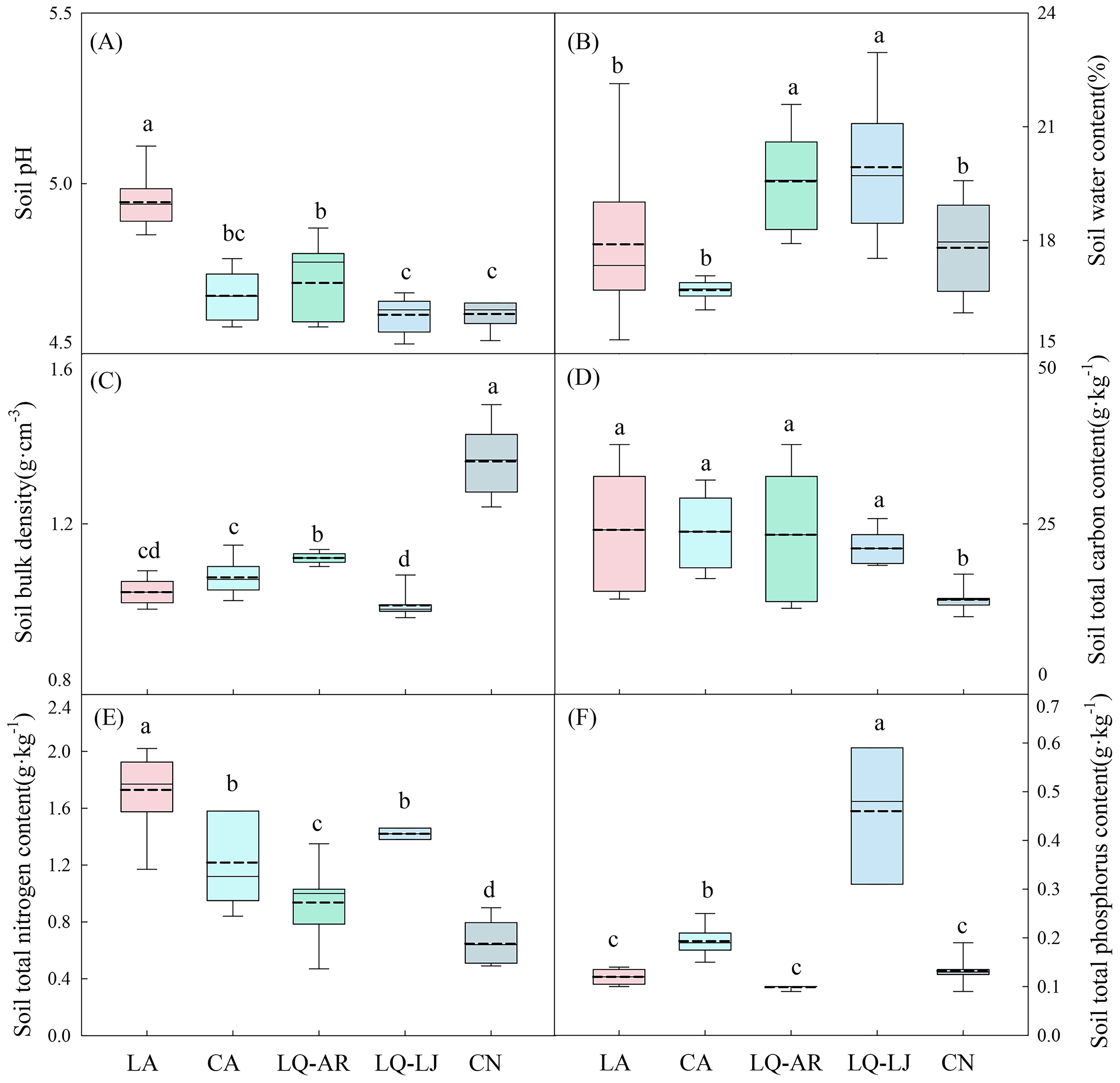
Figure 9. Soil pH (A), soil water content (B), soil bulk density (C), soil total carbon content (D), soil total nitrogen content (E), soil total phosphorus content (F) in different study sites (n=9). LA, Lin’an District in Hangzhou; CA, Chun’an County in Hangzhou; LQ-AR, An’ren Township of Longquan City in Lishui; LQ-LJ, Lanju Township of Longquan City in Lishui; CN, Cangnan County in Wenzhou. Different lowercase letters represent significant differences among the study sites (p < 0.05). The upper and lower boundaries of the box represent the upper quartile and the lower quartile, respectively. The middle solid line and the dashed line indicate the median and mean value, respectively. The whisker lines extend to the maximum and minimum values.
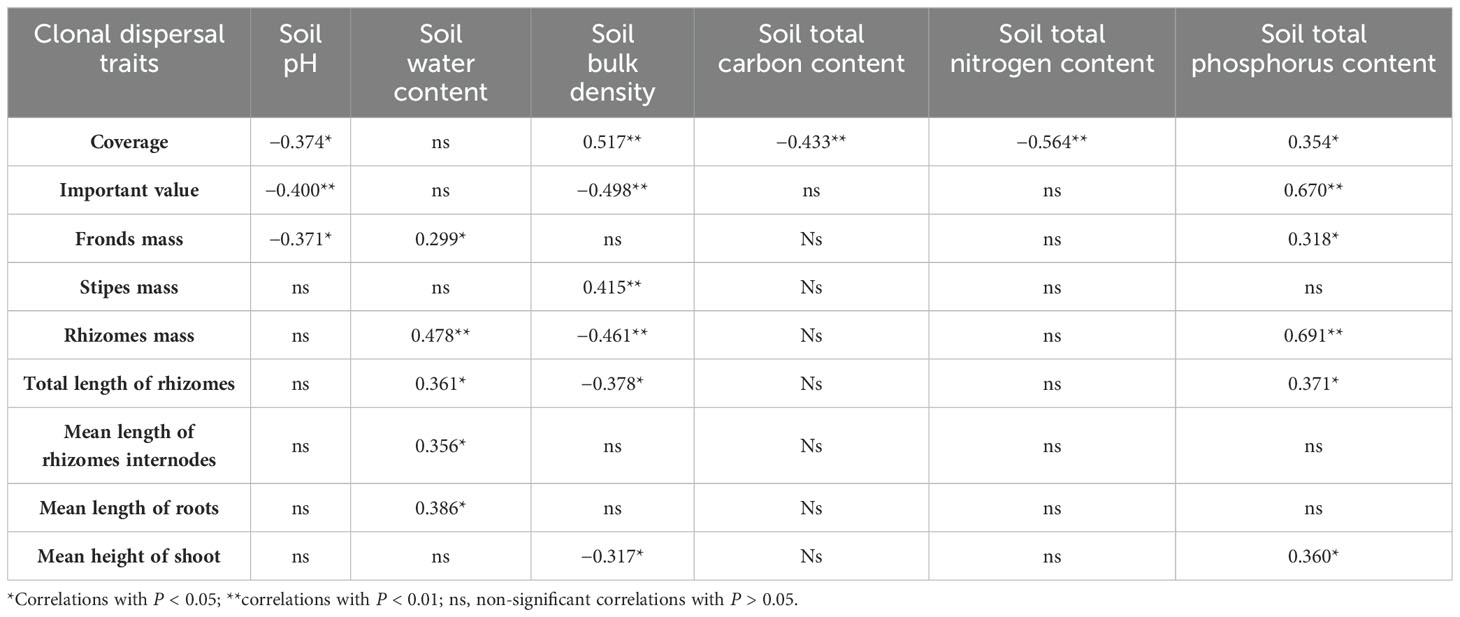
Soil characteristics contributed 20.7% to the cover of D. dichotoma and contributed 1.3% to its importance value (Figure 6). The clonal dispersal traits of D. dichotoma were significantly positively correlated with soil moisture and total phosphorus content and were significantly negatively correlated with soil bulk density (Table 5). The coverage of D. dichotoma was significantly negatively correlated with soil pH, total carbon, and total nitrogen content and was significantly positively correlated with soil bulk density and total phosphorus content. The importance value of D. dichotoma was significantly negatively correlated with soil pH and bulk density and was significantly positively correlated with total nitrogen and total phosphorus contents (Table 5). These findings indicated that soil and D. dichotoma characteristics can indeed influence each other.
4 Discussion
4.1 Effect of climate on single-dominant-species patches of D. dichotoma
In this study, D. dichotoma was typically found on slopes or gentle slopes below an altitude of 300 m. This is consistent with a previous study showing that D. dichotoma mainly grew in subtropical low mountains and hills (Zhang et al., 2010a). We found that D. dichotoma could form large single-dominant-species patches under coniferous forests, evergreen broadleaved forests, conifer-broadleaf forests, and even in unforested lands. Yang et al. (2021) found that the distribution of D. dichotoma primarily stretched from the south of the Yangtze River in China to the Pacific Islands. In summary, at the regional scale, the primary environmental factor influencing the formation of the dominant D. dichotoma community appears more likely to be related to climate than community characteristics.
Although D. dichotoma is widely distributed south of the Yangtze River Delta, field surveys have found that it more readily forms extensive stands in the southern Zhejiang Province, while it is more sporadically dispersed in the northern Zhejiang Province. Our study provides further evidence that higher annual mean temperature and annual mean precipitation are factors that are more conducive to the formation of single-dominant-species patches of D. dichotoma. The mechanism is considered to be due to the fact that high temperature and high humidity promote the clonal growth and clonal dispersal of D. dichotoma. Therefore, at a regional scale, climate is the primary driver for the formation of single-dominant-species patches of D. dichotoma.
Although higher annual mean precipitation was associated with a larger importance value of the D. dichotoma community, the individual contribution of climate to the importance value was not substantial. This can explain the finding that the CN study site had the highest annual mean temperature and annual mean precipitation, but a relatively low importance value of D. dichotoma. This is largely because such a hot and humid climate also benefits the growth of other herbaceous plants, including competing species such as M. floridulus.
In addition, the individual contributions of climate, topography, community, and soil factor to the importance value of D. dichotoma were each very low, their combined effect on the importance value reached 58.8%, indicating that the formation of single-dominant-species patches is determined by the comprehensive interaction of these four environmental factors.
4.2 Effects of community factors on single-dominant-species patches of D. dichotoma
As a light-demanding fern, understory light intensity is considered to be the dominant factor for the growth of D. dichotoma (Zhang et al., 2010b), which was confirmed by our results. Specifically, we found that an understory light intensity of 200–500 µmol·m⁻²·s⁻¹ was the most favorable condition for the formation of the D. dichotoma community. This was consistent with the results of our previous controlled pot and semi-controlled field experiments, which showed that D. dichotoma does not tolerate strong light (Jin, 2019). This is because strong light and high temperatures will damage the donor side, acceptor side, and reaction center of photosystem II. Additionally, these conditions can lead to the degradation of pigment proteins in the leaves, resulting in a reduction in the photosynthetic capacity (Liao et al., 2023).
Community characteristics have minimal and non-significant effects on the rhizomes biomass and total length of rhizomes, but significantly influenced the biomass allocation of D. dichotoma. In communities with an understory light intensity of 200–300 µmol·m⁻²·s⁻¹, D. dichotoma exhibited the best development of dominant layers, with the lowest biomass investment in the supporting structures. This enables D. dichotoma to allocate more resources to the fronds or rhizomes, thereby enhancing its productivity and reproductive capacity (Liu et al., 2008). Therefore, at the community scale, variations in understory light intensity alter biomass investment in different clonal modulars, thereby influencing the formation of single dominant populations of D. dichotoma.
Our previous studies revealed that D. dichotoma had a greater light saturation point and a lower light compensation point than those of many other species (Liao et al., 2023). Yu et al. (2023) demonstrated that D. dichotoma adapted to low-light environments in evergreen broadleaved forests by increasing its specific leaf area, soil total nitrogen, soil total phosphorus, and chlorophyll content in the fronds. The disappearance of D. dichotoma in evergreen broadleaved forests could potentially be linked to a shift in resource allocation. Specifically, more resources tended to be allocated to the fronds (e.g., nitrogen and phosphorus), with a compensatory reduction in the allocation of resources to the reproductive organs. The growth and sprouting of rhizomes is a vital pathway for the rapid expansion of D. dichotoma (Liu, 2017). Furthermore, some studies have reported that mycorrhization in the ferns was bound to light intensity, with fungal hyphae extending across the root surface area of ferns and improving nutrient absorption (Lehnert et al., 2017; Deng et al., 2023; Li et al., 2023). A substantial decrease in the arbuscular mycorrhizal fungi richness and abundance in the roots of Struthiopteris spicant (L.) Weiss, a terrestrial fern distributed in northwestern North America and across Europe, has been reported when they are grown under low light conditions (Molino et al., 2019; Guillen-Otero et al., 2024). Simultaneously, high temperatures and strong light stress are less frequent in evergreen broadleaved forests than coniferous forests. More favorable growth conditions for plant species and increased interspecies competition for D. dichotoma are therefore common in evergreen broadleaved forests (Pang et al., 2018). Consequently, restricted rhizome growth and increased interspecific competition might significantly inhibit the growth of D. dichotoma in forests with low understory light.
4.3 Interaction between soil characteristics and D. dichotoma
Soil is a crucial ecological factor influencing the growth of D. dichotoma, which favors acidic red-yellow soil with low pH. However, the growth of D. dichotoma also significantly impacts soil characteristics. Previous studies showed that D. dichotoma can further lower the soil pH by secreting organic acids and other substances (Liu et al., 2012). The present study confirmed a significant negative correlation between soil pH and coverage of D. dichotoma, validating the reciprocal influence between soil properties and D. dichotoma. Additionally, we discovered that lower soil total carbon and total nitrogen contents were associated with higher D. dichotoma coverage. This was attributed to the ability of D. dichotoma to tolerate nutrient-poor soil where high-nutrient-demand herbaceous plants cannot thrive.
Previous studies proposed that although D. dichotoma can tolerate nutrient-poor soil, it exhibits better growth in loose and fertile soil. However, the specific soil nutrients that limit the formation of a D. dichotoma community have not been determined. Our correlation analysis between clonal dispersal characteristics of D. dichotoma and soil properties indicated a preference for moist and loose soil with a high phosphorus content. This finding further confirms that soil total phosphorus is a limiting factor for the formation of single-dominant-species patches of D. dichotoma. The subtropical region of China is a phosphorus-deprived area, with a soil total phosphorus content ranging between 0.1 and 0.4 g·kg−1, which is lower than that of the red soil in southern China at 0.56 g·kg−1 (Fang et al., 2018). Moreover, the predominant acidic red soil in this region, which is rich in iron and aluminum oxides, strongly adsorbs and fixes phosphorus, resulting in an extremely low effective phosphorus content. However, the specific mechanisms underlying these effects require further in-depth research.
5 Conclusion
Climate was identified as the primary factor influencing the formation of single-dominant-species patches of D. dichotoma at the regional scale. Higher annual mean temperature and annual mean precipitation were associated with an increased coverage and increased importance value of D. dichotoma. At the community scale, community characteristics affected the formation of D. dichotoma-dominant patches. Understory light intensities in the range of 200–500 µmol·m⁻²·s⁻¹ were found to be most suitable for the development of large D. dichotoma patches. Soil total phosphorus content emerged as a limiting factor for the development of the D. dichotoma community, and the growth of D. dichotoma further decreased the soil pH.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
DD: Writing – original draft, Data curation. JT: Writing – original draft, Investigation, Data curation. LL: Writing – original draft, Investigation. LY: Writing – review & editing, Project administration. WY: Writing – review & editing. FY: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the National Natural Science Foundation of China (42001046) and the “Pioneer” and “Leading Goose” R & D Program of Zhejiang (2022C02019).
Acknowledgments
We thank Dr. Hui Zhang for her assistance with the conception and data analysis, and Changhe Zhou, Peng Ye, and Zaiyang Wen for their help with the investigation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Shammary, A. A. G., Kouzani, A. Z., Kaynak, A., Khoo, S. Y., Norton, M., Gates, W. (2018). Soil bulk density estimation methods: A review. Pedosphere 28, 581–596. doi: 10.1016/S1002-0160(18)60034-7
Bai, K., Wei, Y., Zhang, D., Fu, L., Lv, S., Deng, L. (2020). Contrasting effects of light, soil chemistry and phylogeny on leaf nutrient concentrations in cave-dwelling plants. Plant Soil 448, 105–120. doi: 10.1007/s11104-020-04422-6
Chen, N., Zhang, Q., Chen, T., Yang, Y., Xie, J. (2016a). The N and P stoichiometry characteristics of Dicranopteris dichotoma in the restoration process of degraded red soil. For. Res. 29, 735–742. doi: 10.13275/j.cnki.lykxyj.2016.05.016
Chen, Z., Chen, Z., Yan, X., Bai, L. (2016b). Stoichiometric mechanisms of Dicranopteris dichotoma growth and resistance to nutrient limitation in the Zhuxi watershed in the red soil hilly region of China. Plant Soil 398, 367–379. doi: 10.1007/s11104-015-2670-7
Dalling, J. W., Garcia, E., Espinosa, C., Pizano, C., Ferrer, A., Viana, J. L. (2024). Zombie leaves: Novel repurposing of senescent fronds in the tree fern Cyathea rojasiana in a tropical montane forest. Ecology 105, e4248. doi: 10.1002/ecy.4248
Deng, C., Lyu, M., Xiong, X., Peñuelas, J., Sardans, J., Li, X. J., et al. (2023). Understory ferns removal downregulates microbial carbon use efficiency and carbon accrual in previously degraded lands. Agric. For. Meteorology 340, 109631. doi: 10.1016/j.agrformet.2023.109631
Fang, X., Chen, J. L., Wang, L. F., Li, S. L., Xiang, W. H., Lei, P. F. (2018). Research progress on soil phosphorus availability and its influential factors in subtropical forests. J. Cent. South Univ. Forestry Technol. 38, 1–12. doi: 10.14067/j.cnki.1673-923x.2018.12.001
Fang, J. Y., Wang, X. P., Shen, Z. H., Tang, Z. Y., He, J. S., Yu, D., et al. (2009). Methods and protocols for plant community inventory. Biodiversity Sci. 17, 533. doi: 10.3724/SP.J.1003.2009.09253
Guillen-Otero, T., Lee, S. J., Hertel, D., Kessler, M. (2024). Facultative mycorrhization in a fern (Struthiopteris spicant L. Weiss) is bound to light intensity. BMC Plant Biol. 24, 103. doi: 10.1186/s12870-024-04782-6
Jin, G. H. (2019). The Photosynthetic Physiological Response of Dicranopteris dichotoma under different light conditions. Hangzhou: Zhejiang Agricultural and Forestry University. Available at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201901&filename=1018283139.nh&v.
Juma, G., Zhang, Y., Liu, Q., Yang, Y., Wang, X. P., Yang, S. (2019). Allelopathic effects of native plant species Dicranopteris dichotoma on invasive species Bidens pilosa and Eupatorium catarium. Allelopath. J. 48, 45–58. doi: 10.26651/allelo.j/2019-48-1-1243
Lehnert, M., Krug, M., Kessler, M. (2017). A review of symbiotic fungal endophytes in lycophytes and ferns – a global phylogenetic and ecological perspective. Symbiosis 71, 77–89. doi: 10.1007/s13199-016-0436-5
Li, Q., Zhu, C., Yu, J., Wu, X., Huang, S. (2023). Response of soil bacteria of Dicranopteris dichotoma populations to vegetation restoration in red soil region of China. J. Soil Sci. Plant Nutr. 23, 456–468. doi: 10.1007/s42729-022-01058-6
Liao, L., Yu, F., Yi, L. T., Zhang, M. R., Xu, Y. (2023). Effects of different light environments on photosynthetic physiology of Dicranopteris dichotoma under successional stages in subtropical forests. Acta Ecologica Sin. 43, 1853–1860. doi: 10.5846/stxb202112273661
Lin, Q. (2020). Effect of phosphorus addition on the functional traits of Dicranopteris dichotoma. Sci. Soil Water Conserv. 18, 130–138. doi: 10.16843/j.sswc.2020.04.015
Liu, L. T. (2017). Analysis on population pattern of Dicranopteris pedata during spread process. Beijing: Beijing Forestry University. doi: 10.26949/d.cnki.gblyu.2017.000057
Liu, Y. C., Liu, Q. J., Wang, H. Q., Ma, Z. Q., Xu, W. J. (2008). Characteristics of biomass allocation of Dicranopteris dichotoma. Chin. J. Ecol. 27, 705–711. doi: 10.13292/j.1000-4890.2008.0153
Liu, M. J., Sun, Z. Y., Geng, S. B., Wen, M. L., Dai, J. L., Yang, L. (2021). Ecological filtering effect of Dicranopteris symusia under Eucalyptus plantation in south China. Trop. Geogr. 41, 1338–1346. doi: 10.13284/j.cnki.rddl.003411
Liu, Z., Wu, J., Zhou, L., Lin, Y., Fu, S. (2012). Effect of understory fern (Dicranopteris dichotoma) removal on substrate utilization patterns of culturable soil bacterial communities in subtropical Eucalyptus plantations. Pedobiologia 55, 7–13. doi: 10.1016/j.pedobi.2011.07.014
Lu, R. K. (2000). Soil agrochemical analysis method (Beijing: China Agricultural Science and Technology Press).
Lyu, M. K., Xie, J. S., Giardina, C. P., Vadeboncoeur, M. A., Feng, X. J., Wang, M. H., et al. (2019). Understory ferns alter soil carbon chemistry and increase carbon storage during reforestation with native pine on previously degraded sites. Soil Biol. Biochem. 132, 80–92. doi: 10.1016/j.soilbio.2019.02.004
Mehltreter, K., Walker, L. R., Sharpe, J. M. (2010). Fern ecology (London: Cambridge University Press).
Molino, S., Galán, J. M., Wasowicz, P., Fuente, P., Sessa, E. B. (2019). The Struthiopteris spicant (Blechnaceae, Polypodiopsida) complex in Western Europe, with proposals for taxonomic and nomenclatural changes. Plant Systematics Evol. 305, 255–268. doi: 10.1007/s00606-019-1565-0
Pang, C., Ma, X. K.-K., Hung, T. T., Hau, B. C. (2018). Early ecological succession on landslide trails, Hong Kong, China. Écoscience 25, 153–161. doi: 10.1080/11956860.2018.1431377
Tůma, I., Fiala, K., Záhora, J., Holub, P. (2012). The role of Athyrium distentifolium in reduction of soil acidification and base cation losses due to acid deposition in a deforested mountain area. Plant Soil 354, 107–120. doi: 10.1007/s11104-011-1048-8
Wan, S., Zhang, C., Chen, Y., Zhao, J., Wang, X., Wu, J., et al. (2014). The understory fern Dicranopteris dichotoma facilitates the overstory Eucalyptus trees in subtropical plantations. Ecosphere 5, 1–12. doi: 10.1890/ES14-00017.1
Wang, T., Kang, F. F., Han, H. R., Cheng, X. Q., Bai, Y. C., Ma, J. Y., et al. (2017). Factors influencing spatial heterogeneity of soil moisture content in a small catchment of Mount Taiyue, Shanxi Province. Acta Ecol. Sin. 37, 3902–3911. doi: 10.5846/stxb201604170709
Wang, C., Li, Z., Cai, B., Tan, Q., Li, Y., He, L., et al. (2022). Effect of root system of the Dicranopteris dichotoma on the soil unconfined compressive strength of collapsing walls in hilly granite area of South China. CATENA 216, 106411. doi: 10.1016/j.catena.2022.106411
Wang, J. J., Zhang, M. R., Yi, L. T., Zhang, R. M., He, Y. H. (2021). Effects of light and nitrogen on clonal reproduction characteristics and biomass allocation of Dicranopteris dichotoma. J. Zhejiang A&F Univ. 38, 74–83. doi: 10.11833/j.issn.2095-0756.20200199
Yang, L., Huang, Y., Lima, L. V., Sun, Z., Liu, M., Wang, J., et al. (2021). Rethinking the ecosystem functions of dicranopteris, a widespread genus of ferns. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.581513
Yu, J. B., Li, Q. Y., Wu, X. Y., Zhu, C. L., Huang, S. Q., Yang, F., et al. (2023). Adaptational responses of leaf functional traits of Dicranopteris dichotoma to environmental factors in different vegetational restoration stages. Global Ecol. Conserv. 44, e02484. doi: 10.1016/j.gecco.2023.e02484
Zhang, M. R., He, M., Wen, G. S., Zhang, R. M., Zhang, J. G., Hou, P. (2010a). The characteristics of Dicranopteris Dichotoma population and its effects on the forest regeneration. J. Inner Mongolia Agric. Univ. 31, 303–308.
Zhang, M. R., Wen, G. S., Zhang, R. M., Hou, P. (2010b). A study of the development mechanisms of Dicranopteris dichotoma synusium of the forest communities in thousands lake. J. Inner Mongolia Agric. Univ. 31, 28–34.
Keywords: Dicranopteris dichotoma, subtropics, clonal plant, climate, community characteristics, soil
Citation: Dong D, Tong J, Liao L, Yi L, Yan W and Yu F (2024) Formation of single-dominant-species patches of Dicranopteris dichotoma primarily influenced by understory light intensity. Front. Plant Sci. 15:1444371. doi: 10.3389/fpls.2024.1444371
Received: 27 June 2024; Accepted: 10 October 2024;
Published: 11 November 2024.
Edited by:
Kai Yue, Fujian Normal University, ChinaReviewed by:
Chengming You, Sichuan Agricultural University, ChinaShengqiang Wang, Guangxi University, China
Copyright © 2024 Dong, Tong, Liao, Yi, Yan and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Yu, eXVmZWlAemFmdS5lZHUuY24=; Wende Yan, Y3NmdXl3ZEBob3RtYWlsLmNvbQ==
 Dubin Dong1,2
Dubin Dong1,2 Wende Yan
Wende Yan Fei Yu
Fei Yu