- 1Department of Plants and Crops, Ghent University, Faculty of Bioscience Engineering, Ghent, Belgium
- 2Council for Agricultural Research and Economics (CREA), Research Centre for Vegetable and Ornamental Crops, Pontecagnano, Italy
Fusarium wilt, caused by Fusarium oxysporum f. sp. spinaciae, causes a significant challenge on vegetative spinach and seed production. Addressing this issue necessitates continuous research focused on innovative treatments and protocols through comprehensive bioassays. Recent studies have highlighted the potential of plant-based compounds in controlling fungal diseases. The present work aims to conduct a series of experiments, encompassing both in vitro and in planta assessments, to investigate the biocontrol capabilities of different essential oils (EOs) at various application rates, with the ultimate goal of reducing the incidence of Fusarium wilt in spinach. The inhibitory effect of four plant EOs (marjoram, thyme, oregano, and tea tree) was initially assessed on the spore germination of five unknown Fusarium strains. The outcomes revealed diverse sensitivities of Fusarium strains to EOs, with thyme exhibiting the broadest inhibition, followed by oregano at the highest concentration (6.66 μL/mL) in most strains. The tested compounds displayed a diverse range of median effective dose (ED50) values (0.69 to 7.53 µL/mL), with thyme and oregano consistently showing lower ED50 values. The direct and indirect inhibitory impact of these compounds on Fusarium mycelial growth ranged from ~14% to ~100%, wherein thyme and oregano consistently exhibiting the highest effectiveness. Following the results of five distinct inoculation approaches and molecular identification, the highly pathogenic strain F-17536 (F. oxysporum f.sp. spinaciae) was chosen for Fusarium wilt assessment in spinach seedlings, employing two promising EO candidates through seed and soil treatments. Our findings indicate that colonized grain (CG) proved to be a convenient and optimal inoculation method for consistent Fusarium wilt assessment under greenhouse conditions. Seed treatments with thyme and oregano EOs consistently resulted in significantly better disease reduction rates, approximately 54% and 36% respectively, compared to soil treatments (P > 0.05). Notably, thyme, applied at 6.66 µL/mL, exhibited a favorable emergence rate (ERI), exceeding seven, in both treatments, emphasizing its potential for effective disease control in spinach seedlings without inducing phytotoxic effects. This study successfully transitions from in vitro to in planta experiments, highlighting the potential incorporation of EOs into integrated disease management for Fusarium wilt in spinach production.
Introduction
Fungal diseases caused by Fusarium species cause significant economic threats, contributing to a wide range of plant diseases such as damping off, root rot, stem rot, leaf spots, crown rot, and vascular wilts, thereby posing challenges to crops cultivation worldwide. Furthermore, the secondary metabolites produced by the genus Fusarium, known as mycotoxins, exhibit a broad range of toxic effects, leading to both acute and chronic diseases in animals and humans (Antonissen et al., 2014). Over 50 species of Fusarium have been identified, encompassing both plant and animal pathogens, but only a few of these species are known to cause infections in humans (Nucci and Anaissie, 2007). Spinach (Spinacia oleracea L.) is prone to various diseases, leading to reduced production worldwide. Amongst them, Fusarium wilt, caused by the fungus Fusarium oxysporum f. sp. spinaciae, is recognized as the most prevalent and severe disease of spinach. It leads to symptoms ranging from seed decay, wilting, stunted growth, and discoloration of the vascular system to damping-off and plant death, in both young seedlings and fully grown plants (Mowlick et al., 2013; Ekwomadu and Mwanza, 2023). This disease is also a concern in spinach seed production because the pathogen is readily seedborne and can spread through soil, enabling its survival for very long periods even in the absence of host plants (Larena et al., 2003; Sharma et al., 2017). Additionally, the long-lasting chlamydospores, which can persist for over 10 years, pose a significant challenge in controlling Fusarium wilt disease (Egel and Martyn, 2007; Bahadur, 2022). Despite the development of various control strategies, their effectiveness in controlling Fusarium diseases has been insufficient. Specifically, the use of synthetic fungicides to fight Fusarium wilt has been discouraged as they can harm soil health and potentially affect humans and other non-target organisms in the environment (De Cal et al., 2009; Sharma et al., 2017). Hence, exploring potent bioactive compounds for controlling Fusarium diseases directly or in combination with other strategies has become the new emphasis.
Plant-derived bioactive compounds are highlighted for their environmental safety, as they are biodegradable and exhibit minimal to no toxicity towards mammals and non-target organisms (Linde et al., 2010; Kumar et al., 2014; Sharma et al., 2017; Moungthipmalai et al., 2023). Essential oils (EOs), derived from organic sources, offer a range of benefits such as low residue levels, broad-range antimicrobial effectiveness and diverse mechanisms of action, making them promising eco-friendly green preservative agents and alternatives to synthetic fungicides (Han et al., 2019; Najdabbasi et al., 2020; Prakash et al., 2024). They possess remarkable permeability and high volatility, and are composed of a diverse array of secondary metabolites obtained from aromatic plants. Indeed, aromatic plants are abundant sources of bioactive compounds, including tannins, sterols, carvacrol, flavonoids, phenols, alkaloids, quinones, and saponins (Khan et al., 2015; Han et al., 2019). These compounds have demonstrated strong antifungal impacts by affecting cell wall/membrane, inhibiting quorum sensing, altering hyphal morphology, preventing biofilm formation, and suppressing mycotoxin synthesis/production (Chen et al., 2013; Sardi et al., 2014; Haque et al., 2016; Najdabbasi et al., 2020; Abdi-Moghadam et al., 2023). EOs may exert their effects through the active components, either directly influencing the pathogen or inducing systemic resistance in host plants, ultimately leading to a reduction in disease development. Previous studies have reported the antifungal activity of different EOs against various phytopathogens, such as Didymella bryoniae, Colletotrichum lupini, C. lindemuthianum, Penicillium digitatum, Botrytis cinerea, Pleiochaeta setosa, Rhizoctonia solani, Sclerotinia sclerotiorum, Pythium ultimum and Fusarium spp (Fiori et al., 2000; Soylu et al., 2007; Sharma et al., 2017; Dewitte et al., 2018; Bounar et al., 2020; Najdabbasi et al., 2020; Khaleil et al., 2021; Parikh et al., 2021). Many EOs and their major components are classified as “generally recognized as safe” (GRAS) by regulatory authorities such as the Food and Drug Administration (FDA) and the Environmental Protection Agency (EPA), underlining their safety and highlighting their potential effectiveness in combatting fungal pathogens (Tolouee et al., 2010; Lu et al., 2013). Besides extensive research on various target pathogens, the potential of EOs to be used in combination with other EOs or biocontrol agents (Abdel-Kader et al., 2011; Tejeswini et al., 2014; Pane et al., 2017; Zimmermann et al., 2023) makes them promising for controlling Fusarium diseases. In addition, given the modest biological activity of individual components and the synergistic or antagonistic interactions among them, it is important to consider the entire EO for disease management rather than isolating and synthesizing individual components (Bi et al., 2012; Stevic et al., 2014; Nazzaro et al., 2017; Perczak et al., 2019). Despite these, the existing literature on the utilization of EOs for controlling Fusarium wilt in spinach is currently limited. Similarly, there is less information available on the efficiency of artificial inoculation methods for pathogenicity assessment of Fusarium oxysporum f. sp. spinaciae, thereby establishing an optimal inoculation technique is crucial in evaluating the effectiveness of biological control strategies.
Consequently, the objectives of this study were i) to assess the potential of pure EOs as a safe and environmentally-friendly alternative to synthetic chemicals. This encompassed a series of in vitro bioassays that examined the fungitoxicity of EOs on spore germination and mycelial growth of Fusarium strains; ii) to compare various pathogenicity tests and determine the optimal inoculation method for assessing disease severity of Fusarium oxysporum f. sp. spinaciae on spinach; and iii) to investigate the efficacy of chosen EOs in reducing Fusarium wilt of spinach through in planta bioassays.
Materials and methods
Fungal isolates, inoculum preparation and plant EOs
A total of 11 unknown Fusarium strains were isolated from various host plants and diverse geographical locations. Among these, eight were obtained from Enza Zaden (Vegetable-Breeding Company) in the Netherlands, and three from the Research Centre for Vegetable and Ornamental Crops (CREA) in Italy. However, only five strains out of the total of eleven were randomly selected for use in the in vitro experiments, with selection criteria based on their respective host plants and geographical origins. To prepare spore suspensions, each isolate was sub-cultured onto a Potato Dextrose Agar (PDA, 39 gr/L) (Sigma Aldrich, Overijse, Belgium) plate and incubated at 25°C in darkness for 5 days. The mature mycelium was then transferred to fresh PDA plates and subjected to near-ultraviolet (NUV) light and dark cycles, following a 12-hour photoperiod, for 10 days to induce sporulation (International Seed quality Assurance (ISTA) (2023). Afterward, the mycelial surface was gently rubbed with 10 mL of sterile distilled water (SDW), and the resulting mixture was filtered through a sterilized Mira-cloth layer (Merck, Darmstadt, Germany). The spore suspensions were quantified using a hemocytometer and diluted to a concentration of 5 × 105 conidia/mL. Five commercially pure pharmaceutical grade EOs (Omega Pharma, Phytosun aroms, France) including tea tree (Melaleuca alternifoliai), thyme (Thymus vulgaris), oregano (Origanum vulgare) and marjoram (Origanum majorana) were examined for their antifungal activity against Fusarium strains. The EOs were stored at low temperature (4°C) and kept protected from light and humidity for future use. To obtain suitable preparations, the aforementioned EOs were dissolved in SDW at the following concentrations: 0.83, 1.66, 3.33, and 6.66 μL/mL, with 0.1% Tween 80 (Sigma-Aldrich) added.
Inhibitory effects of EOs on spore germination
Agar well diffusion method
For the initial screening, the standard well diffusion assay was employed by incorporating each EO into molten PDA (at approximately 50°C) to assess their antifungal activity against five Fusarium strains. For each strain, spore suspension (5 × 105 conidia/mL) was dispensed onto the surface of solidified PDA plate. Following a drying period of approximately 5 minutes, two wells with a diameter of 6 mm were created on each agar plate (90 mm) using a sterile cork-borer. These wells were then filled with 100 µL of EOs at different concentrations (0.83, 1.66, 3.33, and 6.66 µL/mL). Subsequently, the culture plates were incubated at 25°C for 10 days, and screened for the presence of a zone of inhibition, also known as the zone of clearance. The effectiveness of the tested EOs activity was obtained based on diameter of zone of inhibition (with 6 mm of the well) around the well.
Microtiter plate method
The inhibitory effect of EOs at various concentrations on the spore germination of Fusarium species was assessed using a 96-well microtiter plate assay, based on photometric measurements, following a previously described method (Najdabbasi et al., 2020). In each well of the microtiter plate, a mixture of 20 μL of the respective compound’s concentrations, 180 μL of potato dextrose broth (Potato dextrose broth, 24 g/L) (Sigma Aldrich, Overijse, Belgium), and 20 μL of spore suspension (5 × 105 conidia/mL) was added. The negative control wells in each replicate block contained 20 μL of water instead of the spore suspension. After 8 days of incubation, spore germination in each plate was quantified using a microplate photometer (Asys UVM 330 Plate Reader) to measure the optical density at 620 nm (OD 620). Consequently, the median effective dose (ED50) was derived from the net OD, representing the point at which spore germination was reduced by 50% compared to the control.
Inhibitory effects of EOs on mycelial growth
Agar dilution method
The mycelial growth of Fusarium strains was evaluated through a dose-response assay using petri plates containing PDA media supplemented with different concentrations of the EOs. Mycelial plugs (6 mm) of fungal isolates were taken from the actively growing edges of the cultures and transferred to the center of each pre-amended PDA petri plate. The control comprised PDA without any EO. Following incubation in darkness at 25°C, the average radial growth was determined by measuring the diameters of the colonies in two perpendicular directions using a digital caliper (Solna, Sweden) every two days, until the mycelia fully covered the medium in the control petri plate. Consequently, the inhibitory percentage of different concentrations of EOs was calculated using Abbott’s formula (Abbott, 1925):
wherein, MI represents the percentage of mycelial growth inhibition, and C and T refer to the diameter of the fungus colony (mm) in the control and the relevant treatment, respectively. Furthermore, the minimum inhibitory concentration (MIC) and the minimum fungicidal concentration (MFC) of each EO were determined by sub-culturing the inhibited fungal plugs from the EO-treated plates to newly prepared PDA plates without EO (Najdabbasi et al., 2020). After incubation for an additional seven days at 25°C, the lowest EO concentration that inhibited mycelial growth but allowed growth revival upon transfer was identified as the MIC. Conversely, the lowest EO concentration that prevented mycelial growth after transfer to fresh EO-free PDA plates was recognized as the MFC.
Volatile method
The impact of the volatile compounds from the same EOs on the mycelial growth of Fusarium strains was also evaluated at different concentrations, according to a previously reported method (Parikh et al., 2021). Briefly, a mycelial plug (6 mm) obtained from a 10-day-old culture grown on PDA was transferred to the center of a new PDA plate. A volume of 10 µl of the EOs was applied to the inner side of the inverted lid of the fresh PDA plate, and then the plate containing the mycelial plug was inverted and placed onto the lid. The control plates were left untreated without any addition of EO. To prevent any leakage of the active components, the petri plates were tightly sealed with parafilm. The percentage of mycelial growth inhibition was determined for each treatment by measuring the colony diameter of each pathogen 10 days after inoculation, as previously described. Furthermore, the fungal plugs from treatments showing no fungal growth were transferred to untreated PDA culture medium and placed in an incubator at 25°C for an additional week for further evaluation to ascertain fungistatic or fungicidal activity of each EO, as described earlier. The in vitro bioassays were conducted twice, and each treatment was subjected to six replications. Following the evaluation of results obtained from in vitro bioassays, the EO that exhibited the most effective antifungal activity against Fusarium strains at its applied concentrations was chosen for subsequent investigation in in vivo bioassays.
Molecular identification
The subsequent molecular analysis aimed to confirm the identity of Fusarium strains, which was critical for designing the subsequent in planta experiments targeting disease control. The identification process was carried out for all 11 unidentified strains. Total genomic DNA was extracted from mycelia cultured in PDB for seven days at 22°C using a DNeasy Plant Mini Kit (Qiagen, USA) according to the manufacturer’s instructions. The identification of each fungus involved amplification of the internal transcribed spacer (ITS) region (ITS1-5.8SITS4) of the ribosomal RNA (rRNA) using primers ITS1 (5’-TCCGTAGGTGAACCCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) (White et al., 1990), along with amplification of the elongation factor (TEF-1α) using primers EF-1α (5’-ATGGGTAAGGAAGACAAGAC-3’) and EF2 (5’-GGAAGTACCAGTGATCATGTT-3’) (O’Donnell et al., 1998). For PCR, all samples were amplified with a final volume of 25 μl, consisting of 12.5 μl of GoTaq® Colorless Master Mix (Promega Corporation, Madison, WI), 0.25 μl each of 50 μM forward and 50 μM reverse primers, 2 μl of DNA template, and 10 μl of nuclease-free water, and the amplification was performed using the GeneAmp PCR system 97,000 PCR (Applied Biosystem, Foster City, CA, USA). The amplification parameters for ITS1/4 included an initial denaturation step at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 40 s, annealing at 58°C for 40 s, and elongation at 72°C for 40 s, with a final extension step at 72°C for 5 min. The amplification conditions for EF1/2 involved an initial denaturation at 94°C for 5 min, followed by 33 cycles of denaturation at 94°C for 30 s, annealing at 51°C for 30 s, elongation at 72°C for 30 s, and a final extension at 72°C for 5 min. After PCR amplification, the amplicons were separated on a 1% (w/v) agarose gel, stained with ethidium bromide for 30 minutes, and visualized using the Molecular Imager® Gel Doc™ XR+ System with Image Lab™ Software (BIO-RAD, Hercules, CA, USA). Following that, the amplicons were purified using the E.Z.N.A.® Cycle-Pure Kit (VWR International, Leuven, Belgium), and the resulting purified PCR products were sent to LGC Genomics (LGC group, Berlin, Germany) for Sanger sequencing. Identification of Fusarium strains was accomplished by performing a BLAST-search on NCBI (National Center for Biotechnology Information) database.
Assessments of the antifungal activity of EOs - in planta
Inoculation method
Following molecular identification, various inoculation methods were performed on a single strain of Fusarium species isolated from spinach host plant. This aimed to identify the most effective and efficient assessment method suitable for subsequent greenhouse experiments. To set up plant materials, spinach seeds were placed on two layers of blotting paper in petri plates, and subsequently transplanted into standard potting soil after germination. In the first pathogenicity test, the soil drench (SD) method, the fungus spore suspension (5 × 105 conidia/mL) was drenched using a repeater syringe onto the root plugs of two-week-old spinach seedlings (var. Crosstrek F1) grown in 0.5 L plastic pots containing standard potting soil (organic/mineral fertilizers NPK 14-16-18, pH (H2O) 5–6.5, organic and dry matters: 25% and 20%, respectively), with three seedlings per pot. In pathogenicity test 2, the root-dip inoculation (RD) method, the bottom 20% of the root plugs of two-week-old seedlings were cut manually to provide wounding for infection, then swirled in 100 ml of the fresh spore suspension for two minutes. Inoculated seedlings were then transplanted into plastic pots containing standard potting soil. The pathogenicity test 3, seed soaking (SS) method, involved soaking spinach seeds in spore suspension for one hour. Following this soaking period, the seeds were allowed to dry before being planted into the soil. In pathogenicity test 4, the mycelial plug (MP) inoculation method, seedlings roots were inoculated with fungal mycelial plugs. Two weeks after transplantation, the root zones of the seedlings were inoculated with 6 mm fungal mycelial plugs. In pathogenicity test 5, the colonized grain (CG) method, spinach seeds were planted in pots filled with the mixture of standard potting soil and ground wheat grains colonized by the fungus at a ratio of 10 g inoculum/L of substrate. Fusarium strain had been grown on sterilized wheat grains at 25°C for two weeks. Throughout all tests, a non-inoculated treatment was also included in each pathogenicity test as a negative control. Pots were maintained in the growth chamber at 21°C and 12 h photoperiod for four weeks. A rating scale was developed to qualitatively determine the disease severity index (DSI) based on visual foliar symptoms, ranging from 0 to 3: 0 for a healthy plant, 1 for slight wilting, 2 for severe wilting, and 3 for a dead plant. The pathogenicity tests were conducted twice with 10 pots per treatment. The assessment was conducted on a percentage basis using the following formula:
Phytotoxic effect assessment
The phytotoxicity of the tested oils on spinach seeds was also assessed using a germination test following the standard “top of paper method” detailed in the International Seed Testing Association (ISTA), with slight modifications. Two EOs, at concentrations of 3.33 and 6.66 µL/mL, were chosen due to their superior efficacy observed across all strains in vitro, aiming for potential application in future in vivo experiments. To conduct the experiment, a total of 240 previously disinfected spinach seeds (var. Crosstrek F1) were immersed in aqueous EO solutions for 10 minutes, then air-dried on sterile filter paper (Whatman no. 1) under controlled, sterile conditions at room temperature. For each treatment, sets of 15 seeds were placed on top of two layers of filter paper in 140 mm petri plate, ensuring uniform spacing. Petri plates were pre-moistened with 2 ml sterile distilled water (SDW), and covered with lids. Petri plates containing only distilled water served as the control group. The set up was kept in the growth chamber under suitable consistent conditions (85–90% RH, 20°C, and 12 h photoperiod). The experiment followed a completely randomized design (CRD) with four replicates. Seeds were considered germinated once 1 mm of the radicle had protruded through the seed coat. The seed germination percentage was evaluated seven days post-treatment according to the equation stated below.
Fusarium wilt control
Two types of pot experiments were conducted to assess the effectiveness of promising EOs in mitigating Fusarium wilt. These experiments involved treatments applied to both seeds and soil, enabling a comprehensive evaluation of their impact on disease development. As mentioned earlier, fungal inoculum was incorporated into the soil at a rate of 1%, thoroughly mixed, and filled into sterile plastic pots two weeks prior to sowing. For the seed treatment, EOs were prepared in conical flasks at concentrations of 3.33 and 6.66 µL/mL. Spinach seeds were immersed in the prepared oils and shaken for 10 minutes using a shaker to ensure thorough saturation. Following this, the seeds were air-dried and planted with four seeds per pot (0.5 L). In the control treatment, seeds were soaked in SDW. Moreover, the same concentrations of these EOs, as used for seed treatment, were applied for soil treatment. During the planting process, the oils (15 ml) were initially added to each planting hole until saturation. As a control treatment, SDW was employed prior to planting. Pots were subjected to a temperature of 21°C and a photoperiod of 12 h in the growth chamber for a period of four weeks to monitor the development of Fusarium wilt. Disease incidence was evaluated using the aforementioned 0-3 scale for foliar symptoms, and subsequently the disease incidence reduction (DIR) was determined by using the following formula:
The values calculated for DSI on the assessment day were used to compute the DIR in the respective experiment. Additionally, daily assessments were conducted over a period of 14 days post-sowing to determine the seedling emergence rate index (ERI), using the formula outlined by the Association of Official Seed Analysts (AOSA) (2002):
where Gt is the number of the germinated seed on day t and Dt is time corresponding to Gt in days. The irrigation process was performed as necessary, and all experiments were repeated twice.
Statistical analyses
All experiments followed a randomized complete block design (RCBD), and the data are presented as means ± standard deviations (SD) and visually represented using box plots. Due to the lack of normality (Shapiro-Wilk test) and homogeneity of variances (Levene’s test), non-parametric hypothesis testing, specifically the Kruskal-Wallis test, was employed to assess the statistical significance of differences between treatments. In cases where significant differences were observed (p< 0.05), pairwise comparisons of the groups were conducted using Dunn’s test in the “R” software package (version 2.15.3). Probit analysis was utilized to calculate the ED50 values based on the OD values.
Results
Agar well diffusion
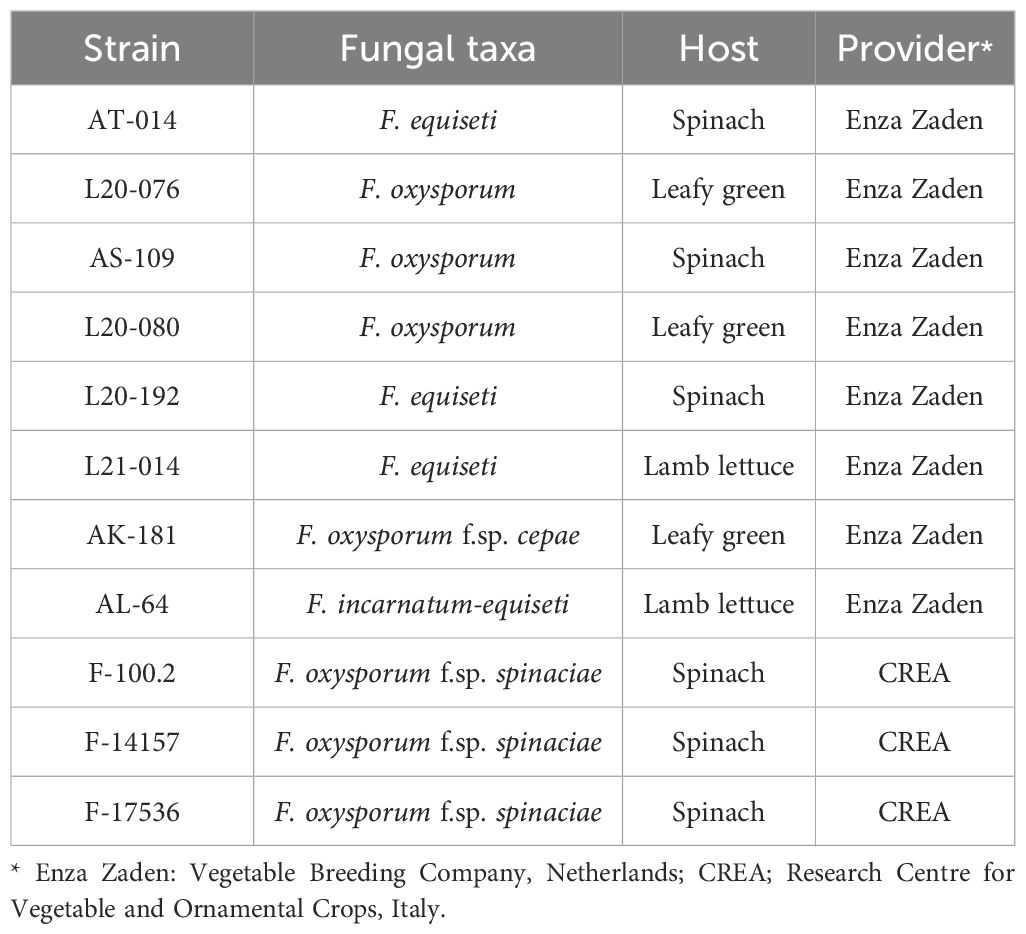
A sensitivity assessment using a well diffusion assay evaluated the response of five Fusarium strains (AL64, AK181, F17536, AT014, and L20-076) to four different EOs at concentrations ranging from 0.83 to 6.66 μL/mL, with clearance zones measured to determine their effects, as shown in Figure 1. The susceptibility of the selected fungal strains varied (P< 0.05) across different concentrations of EOs. Thyme and oregano EOs exhibited the highest level of antifungal activity among the tested oils. Marjoram also exhibited effectiveness, albeit to a lesser extent, by inhibiting only one strain (AK181). For strains AL64, L20-076, and F17536, thyme EO displayed the broadest inhibition zones, with average diameters of 15.46, 14.54, and 14.01 mm, respectively, followed by oregano at the highest concentrations (6.66 µL/mL). Similarly, for strains AT014 and AK181, oregano EO produced the widest inhibition zones of 15.33 and 14.01 mm (at 6.66 µL/mL), respectively, followed by thyme. Overall, among Fusarium strains, thyme and oregano EOs exhibited heightened antifungal activity in most cases, with AK181 emerging as particularly susceptible to marjoram. Conversely, marjoram and tea tree EOs demonstrated minimal variations in sensitivity across the tested strains, with some strains showing little to no response.
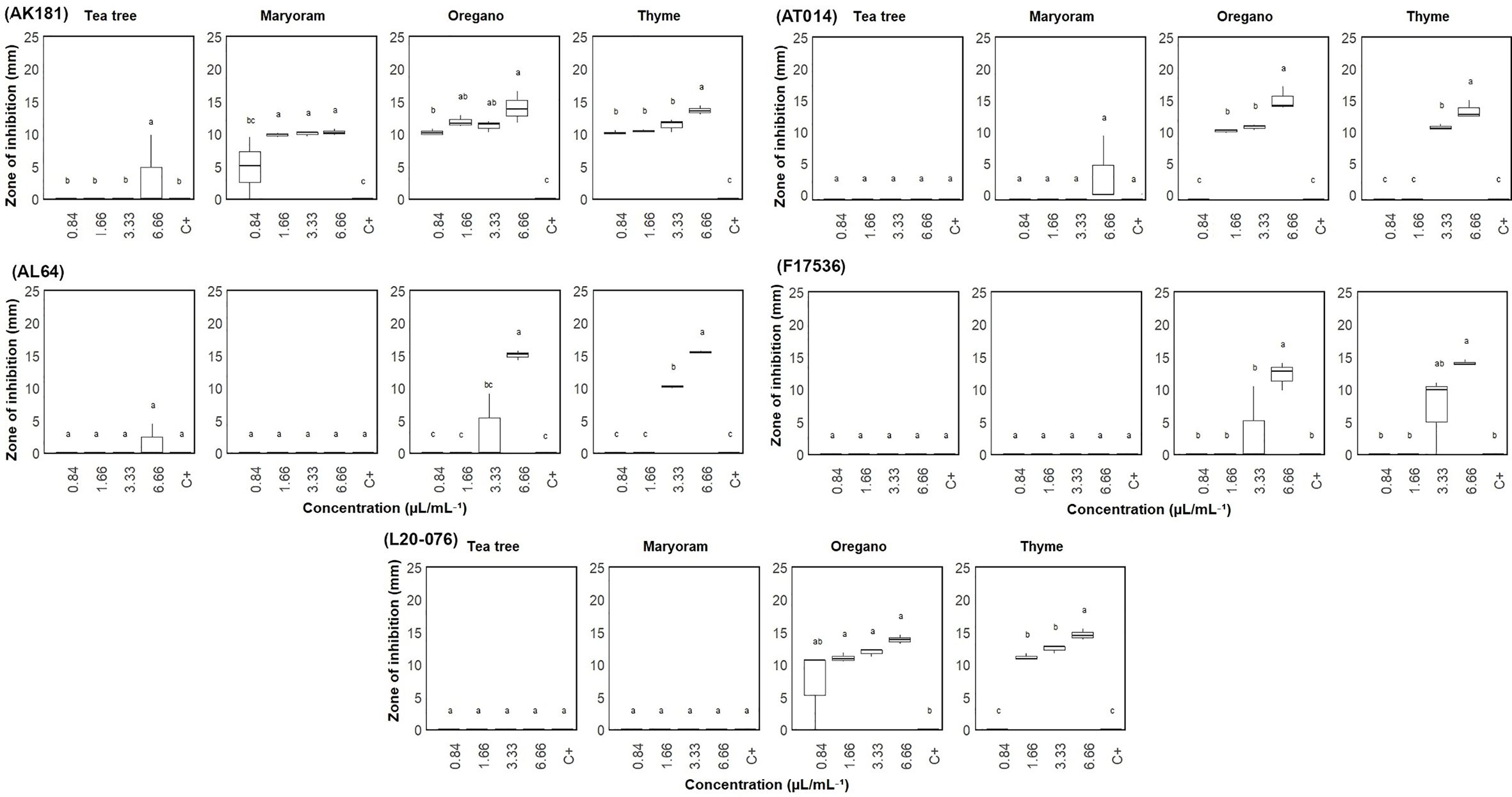
Figure 1 Zone of inhibition (mm) of Fusarium sp. measured via the agar well diffusion test on plates treated with varied concentrations (0.84-6.66 µL/mL) of essential oils. C+: Sterile distilled water served as positive control. Vertical bars represent the standard error (SE) of mean values of six replicates each including 2 wells. Boxes sharing the identical letters indicate values that do not differ significantly based on Dunn’s test (P > 0.05).
Microtiter plate
The baseline assay investigating the impact of four EOs on spore germination of Fusarium strains unveiled a spectrum of ED50 values ranging from 0.69 to 7.53 µL/mL. The statistical analysis revealed significant variations (p< 0.05) in the ED50 values among the tested compounds, indicating highly significant effects of different EOs on the response of various strains (Figure 2). Thyme and oregano EOs consistently exhibited lower ED50 values, indicating robust antifungal properties, while other EOs demonstrated varying levels of efficacy. Tea tree displayed moderate activity, with ED50 values ranging from 1.62 to 6.71 µL/mL across different Fusarium strains. In contrast, marjoram EO generally yielded higher ED50 values, averaging 3.78 µL/mL, suggesting comparatively lower effectiveness against spore germination. Notably, it exhibited an ED50 value of 7.53 µL/mL against AT014, indicating its weaker inhibitory effect in this particular instance.
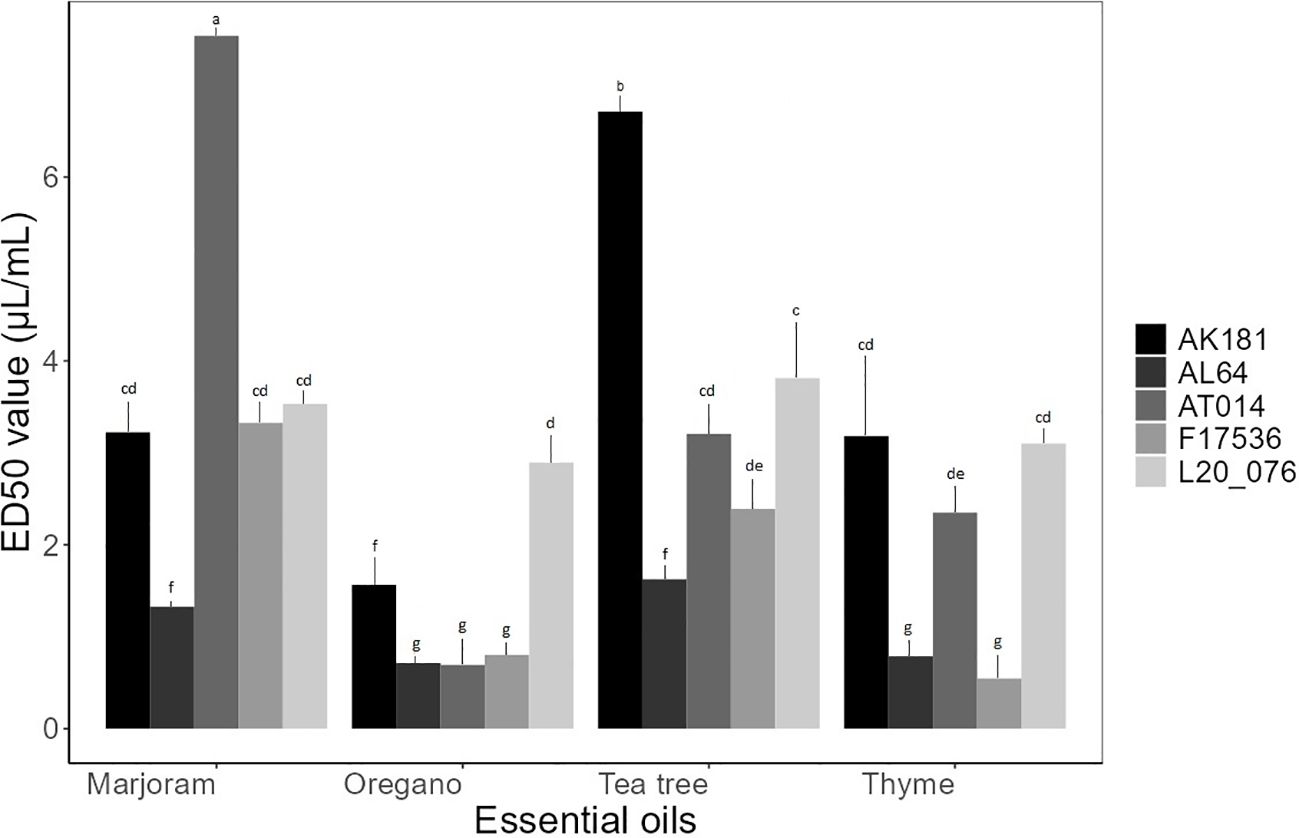
Figure 2 Mean effective dose (μL/mL) of essential oils for spore germination of Fusarium strains. Bars sharing the identical letters indicate values that do not differ significantly based on Dunn’s test (P > 0.05).
Agar dilution
The inhibitory effect of EOs on the mycelial growth of various Fusarium strains was also investigated, revealing a dose-dependent response. As the concentration of EOs increased, the antifungal activity notably improved, resulting in inhibition of mycelial growth ranging from approximately 14% to 100% after seven days of incubation (Figure 3). All tested Fusarium strains exhibited similar sensitivity to the EOs. Notably, thyme and oregano EOs demonstrated complete inhibition (100% mycelial inhibition) against all Fusarium strains, even at the lowest concentration of 0.84 µL/mL. Marjoram EO also exhibited significant (p< 0.05) antifungal activity, with complete growth inhibition observed at concentrations of 3.33 and 6.66 µL/mL, while even at a lower concentration of 1.66 µL/mL, it considerably reduced mycelial growth of most tested strains. In contrast, tea tree EO displayed the weakest inhibitory effect, failing to entirely suppress mycelial growth in some strains, particularly AL64 and F17536, even at its maximum concentration of 6.66 µL/mL.
Figure 3 Mycelial growth inhibition (%) of Fusarium sp. on media amended with varied concentrations (0.84-6.66 µL/mL) of essential oils. Vertical bars represent the standard error (SE) of mean values of six replicates for each compound concentration. Boxes sharing the identical letters indicate values that do not differ significantly based on Dunn’s test (P > 0.05).
Furthermore, the assessment of fungistatic activity revealed that tee tree and marjoram EOs were effective at inhibiting the fungal growth at a concentration of 3.33 µL/mL. Thyme and oregano oils, however, displayed this activity even at 0.84 µL/mL, unlike the other EOs (Table 1). Indeed, tee tree and marjoram exhibited the highest MICs, indicating a lack of fungicidal impact on the fungal strains. Thyme EO displayed fungicidal activity ranging between 1.66 and 6.66 μL/mL, while Oregano ranged from 0.84 to 6.66 μL/mL across all strains tested. However, the importance of the surfactant Tween 80 added to the growth medium should not be overlooked, as it facilitated the even dispersion of the EOs, resulting in reducing the risk of inaccurately elevated MICs and MFCs. Overall, thyme and oregano consistently demonstrated the highest effectiveness among the four EOs, with a MIC of 0.84 µL/mL against nearly all tested strains in this study.
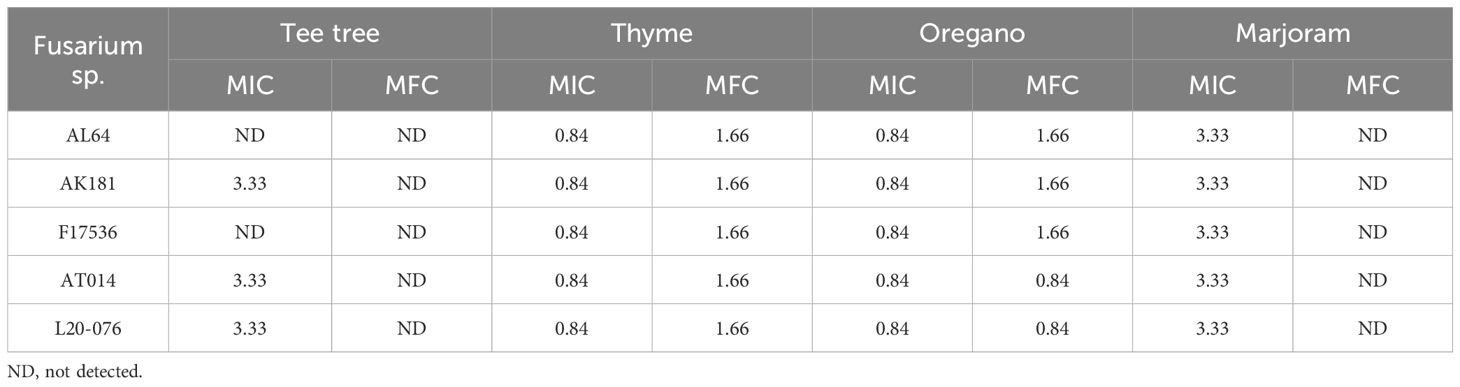
Table 1 Minimum inhibitory concentrations (MIC) and minimum fungicidal concentrations (MFC) of tee tree, thyme, oregano, and marjoram EOs (µL/mL) against the tested Fusarium strains.
Volatile
The antifungal activity of tea tree, thyme, oregano, and marjoram EOs was investigated to detect their indirect effects in the vapor phase against Fusarium strains (Figure 4), contrasting with direct contact in the agar dilution method. Our results indicate that tea tree and marjoram oils had minimal to negligible effects on all strains, with mycelial growth inhibition consistently below 20% across all concentrations. In contrast, thyme and oregano notably EOs triggered complete growth inhibition in AL64 and L20-076 when applied at the highest concentration tested (6.66 µL/mL). Furthermore, volatiles from these oils consistently (P< 0.05) inhibited growth by over 80% across all strains at this concentration. Notably, the volatile compound derived from thyme exhibited the highest inhibitory potency, effectively halting the mycelial growth of at least one strain (AL64) at a concentration of 3.33 µL/mL.
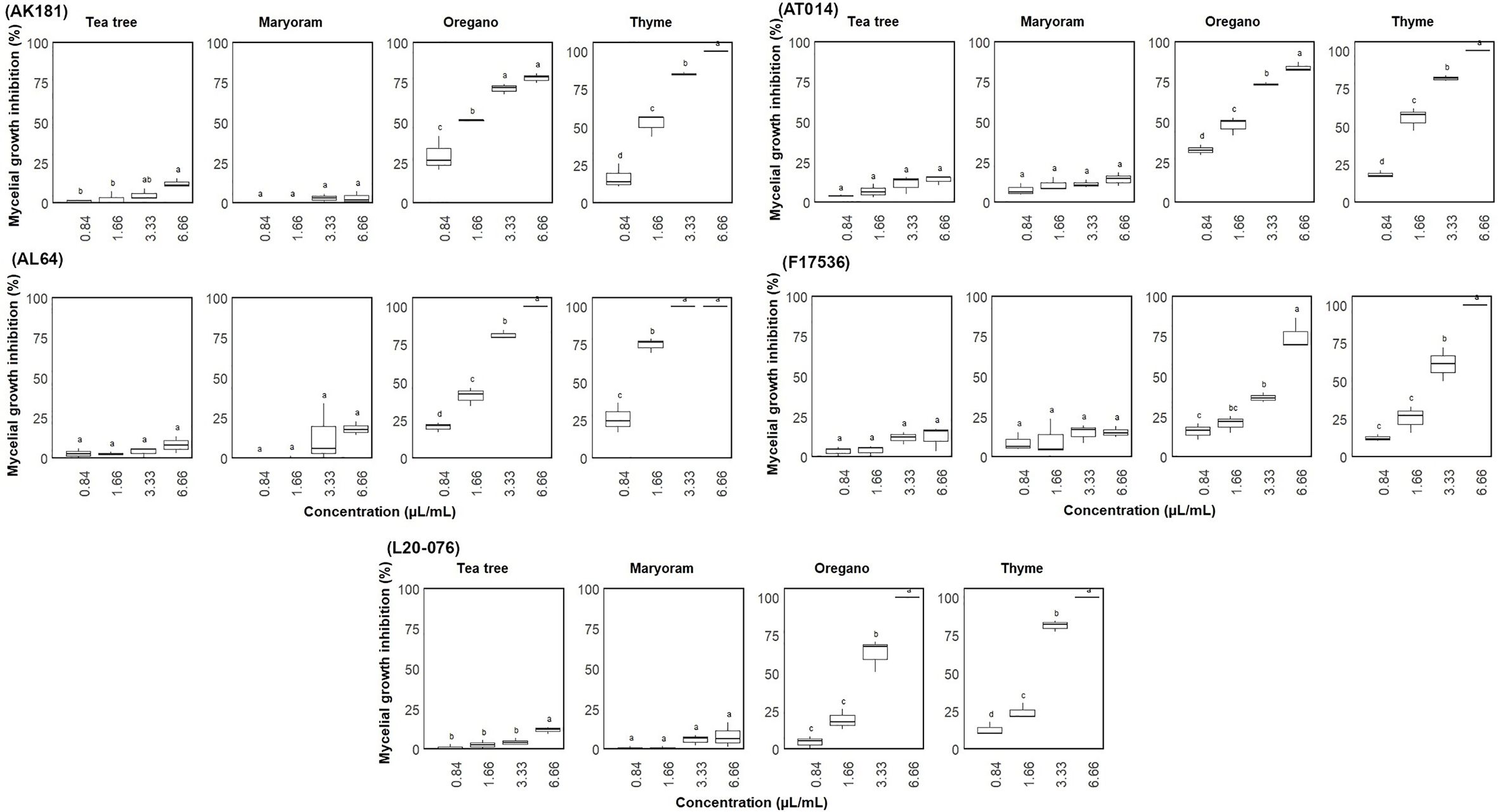
Figure 4 Mycelial growth inhibition (%) of Fusarium sp. on media through exposure to different concentrations (0.84-6.66 µL/mL) of essential oils. Vertical bars represent the standard error (SE) of mean values of six replicates for each compound concentration. Boxes sharing the identical letters indicate values that do not differ significantly based on Dunn’s test (P > 0.05).
Determining the lowest EO concentration resulting in 100% mycelial growth inhibition classified whether the effect was fungicidal or fungistatic. Fungistatic characterization involved observing growth upon transfer from the amended PDA plates to fresh PDA, while the absence of regrowth on fresh PDA indicated fungicidal activity. In this assay, thyme and oregano EOs, particularly at their highest concentration (6.66 µL/mL), exhibited varying degrees of antifungal activity. They demonstrated fungistatic effects against the tested Fusarium strains, except for AL64 and L20-076, where they showed a fungicidal effect (Table 2). Notably, thyme at a concentration of 3.33 µL/mL also displayed fungicidal activity against AL64. Additionally, while these oils effectively inhibited mycelial growth by 100% at the higher concentration, they did not demonstrate fungicidal or fungistatic effects on the Fusarium strains at lower concentrations. After comprehensive assessment of the outcomes derived from the in vitro bioassays, it was concluded that thyme and oregano EOs exhibited superior antifungal efficacy against Fusarium strains, particularly at concentrations of 3.33 and 6.66 µL/mL, prompting their selection for subsequent investigation in in vivo bioassays.
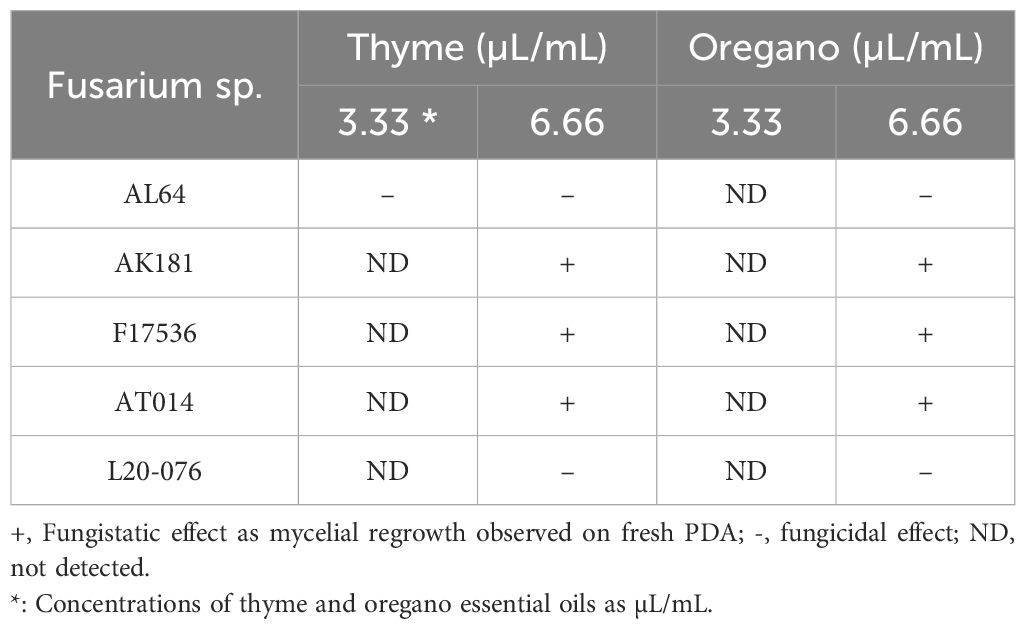
Table 2 Fungicidal or fungistatic effects of volatiles from thyme and oregano essential oils against the tested Fusarium strains.
Molecular identification of Fusarium strains
To strengthen confidence in strains identification and minimize the risk of result misinterpretation, we amplified the ITS rDNA and TEF-1α regions of all isolates. The BLAST search showed the PCR products from three major Fusarium species including F. equiseti, F. oxysporum, and F. incarnatum-equiseti exhibited a 99-100% homology with the associated sequences in the GenBank database (Table 3). Our analysis identified three strains belonging to the F. oxysporum species complex (L20-076, AS-109, and L20-080), three strains belonging to F. equiseti (AT-014, L20-92 and L21-014), one strain belonging to F. oxysporum f.sp. cepae (AK-181), one strain belonging to the F. incarnatum-equiseti species complex (AL-64), and three strains belonging to F. oxysporum f.sp. spinaciae (F-100.2, F14157 and F-17536). Consequently, we selected a single strain of F. oxysporum f.sp. spinaciae (F-17536) for in planta experiments involving spinach, considering its relevance to the target plant and its prior assessment in in vitro experiments.
Assessments of the antifungal activity of EOs - in planta
Inoculation method
To ascertain the most efficient approach for greenhouse-based inoculation, an assessment was conducted on five distinct inoculation methods, namely SD, RD, SS, MP, and CG. This evaluation was performed on a specific strain of F. oxysporum f.sp. spinaciae (F-17536) following preliminary in vitro bioassays and considering its original host plant. All five inoculation techniques induced symptoms of Fusarium wilt in the spinach plants, resulting in significantly varied levels of disease severity (P< 0.05) (Figure 5). Reddening attributed to the presence of fungal mycelium was observed alongside the root vascular tissue in infected seedlings, which exhibited a water-soaked appearance or brown to black discoloration. All inoculation methods displayed initial disease symptoms approximately 14 days after inoculation (DAI), except for the CG method, which manifested symptoms at 9 DAI, characterized by poor germination, stunted growth, and post-emergence damping off. However, the RD, SD, and MP methods displayed comparable levels of disease severity which were significantly (P< 0.05) different from the control. Though the SS method did induce mild foliar symptoms, it was inconsistent throughout the experiments. All of the non-inoculated control plants remained asymptomatic over the duration of each pathogenicity test. As a general observation, the effectiveness of inoculating methods could be arranged in the following order: CG method exhibited the highest efficacy, succeeded by methods MP, RD, SD, and SS, respectively. Consequently, the GC method was selected for conducting further in vivo experiments.
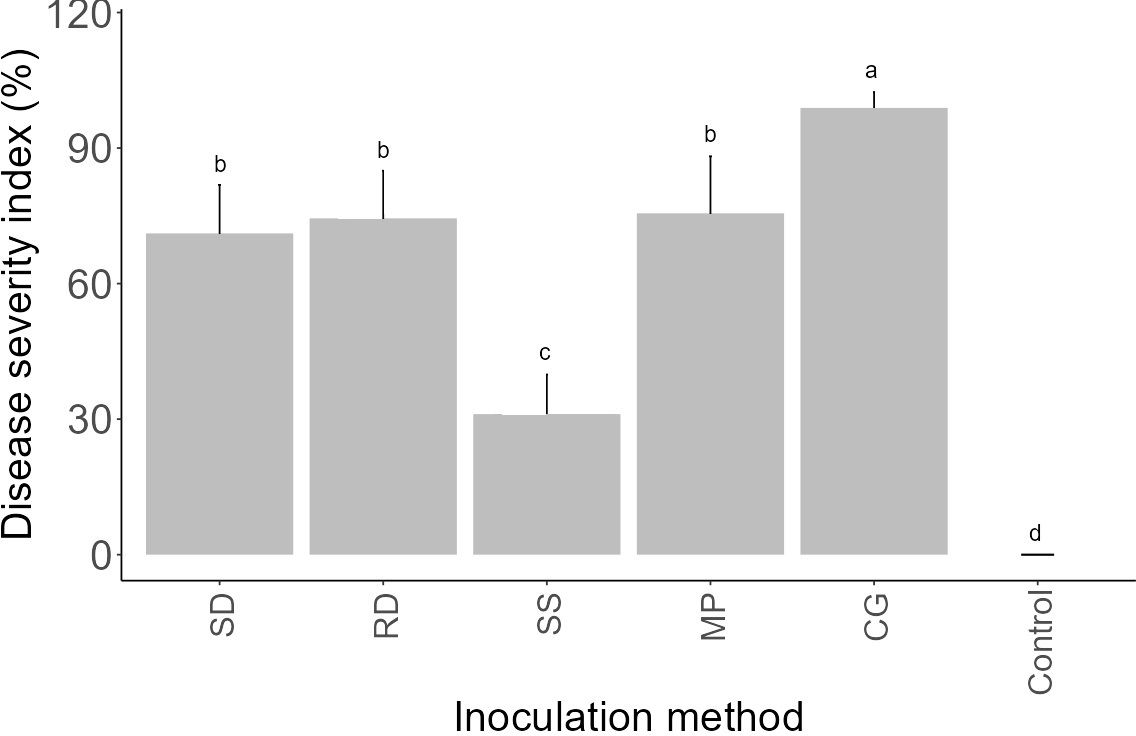
Figure 5 Mean Fusarium wilt severity index (%) on spinach (var. Crosstrek F1) induced by F. oxysporum f.sp. spinaciae 22 days post-inoculation using five distinct inoculation methods. SD: soil drenching; RD: root dipping; SS: seed soaking; MP: mycelial plug; CG: colonized grain. Vertical bars represent the standard error (SE) of mean values of 10 pots each including 3 plants. Barplots sharing the identical letters indicate values that do not differ significantly based on Dunn’s test (P > 0.05).
Phytotoxic effect
The impact of thyme and oregano EOs on the germination rate of spinach seeds is presented in Table 4. By comparing the average germination rates of different treatments, including various concentrations of EOs, with those of the control group, we could determine if there were any harmful effects on seed germination. At 6.66 µL/mL, oregano oil showed a marginal 2% decrease in germination percentage compared to the control, which is practically negligible and might be compensated for by increasing the seed amount. Thyme oil at 3.33 and 6.66 µL/mL and oregano oil at 3.33 µL/mL did not affect germination rates, indicating no phytotoxic impact of treatments on seed germination across all concentrations used when compared to the untreated control.
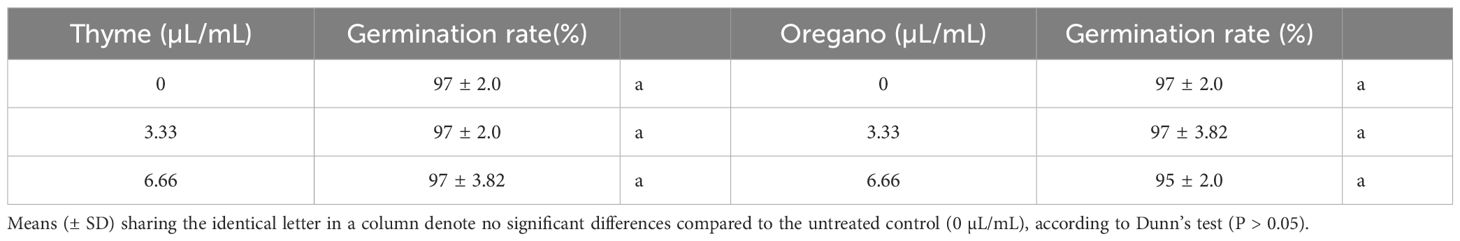
Table 4 Percentage of germination rate on day seven after treatment of spinach seeds with thyme and oregano essential oils.
Fusarium wilt control
In this phase of our study, the primary aim was to mitigate Fusarium wilt in spinach seedlings through the application of two efficient EOs candidates in both seed and soil treatments. Focusing on oregano and thyme at concentrations of 3.33 and 6.66 µL/mL, we sought to evaluate the impact of these treatments on disease incidence and the emergence rate of seedlings. There was no significant difference (p > 0.05). in the mean ERI values 14 days after sowing spinach seeds treated with EOs compared to the untreated control (Table 5). Soil treatment with varying concentrations of EOs also resulted in no significant difference in the ERI values, indicating that the pathogen was not able to interfere the development of spinach seeds. Additionally, a clear relationship found between the application of these EOs and their concentrations in terms of DIR. Indeed, thyme and oregano EOs consistently reduced disease occurrence across various concentration levels (P< 0.05), with no discernible impact on seeds emergence. For instance, thyme, in seed treatment at 6.66 µL/mL, exhibited an ERI of ≈ 7 and the highest DIR of ≈ 54%. The remarkable performance of thyme emphasizes its potential for effective disease control in spinach seedlings, making it a promising candidate for further exploration in Fusarium wilt management strategies. The data from the experiment also revealed that seed treatments with thyme and oregano EOs at various concentrations consistently led to considerable DIR compared to soil treatments, highlighting the superior efficacy of seed-based interventions in controlling disease occurrence. In essence, using EOs as seed dressing proved more effective than soil treatment, with thyme oil showing the most promising outcomes among the oils tested.
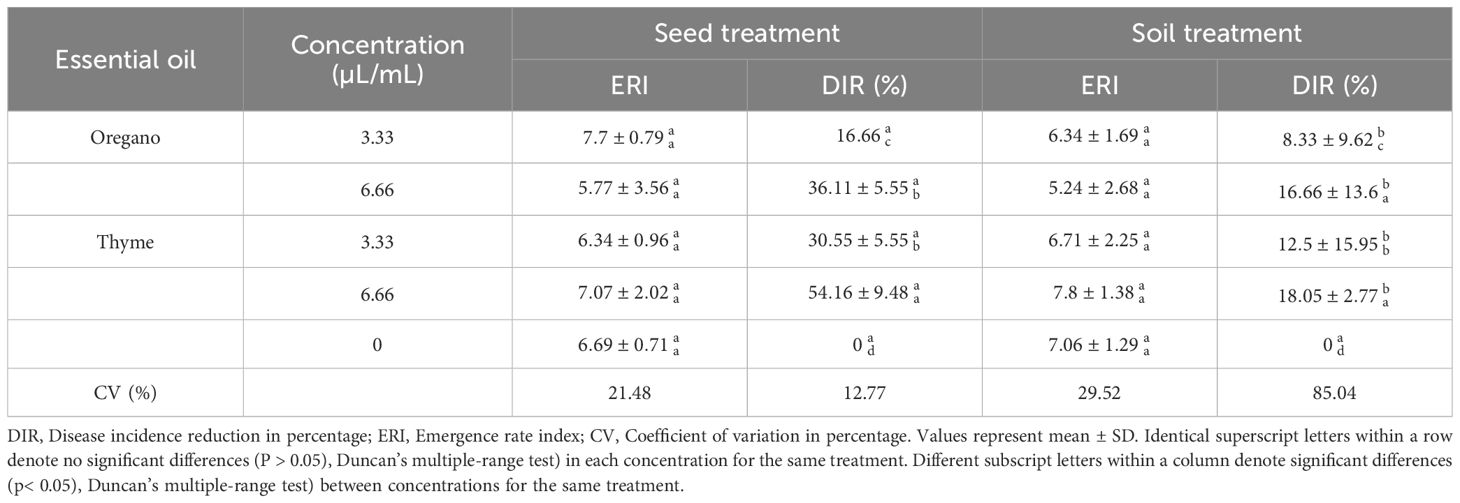
Table 5 Emergence rate index and reduction of Fusarium wilt incidence in spinach seedlings through the application of oregano and thyme EOs in seed and soil treatments.
Discussion
In response to the growing need to reduce reliance on chemical substances for plant protection and combat resistance to existing chemicals, there has been a notable increase in the investigation of natural-based compounds. This aims to alleviate the adverse impacts of synthetic pesticides on human health and the environment. Additionally, spinach, an important leafy green crop, is predominantly threatened by soilborne diseases that target its vascular tissue, posing a considerable challenge to its cultivation worldwide. Therefore, the current study aimed to assess antifungal activity of certain plant-derived EOs through both in vitro and in planta experiments, with the overarching objective of managing Fusarium wilt disease in spinach. Fungal growth was influenced by all the tested EOs, although the degree of inhibition differed depending on the specific oil and the strains investigated. The EOs utilized in our study, commercially produced, are known for their effectiveness as biocontrol agents against various phytopathogens. Also, their extraction through standardized procedures enhances the reliability and repeatability of outcomes. Nevertheless, aside from environmental factors, inconsistencies in the efficiency of EOs may occur as a result of their extraction from different plant parts and under varying laboratory conditions, as indicated in previous studies (Dan et al., 2010; Amini et al., 2016; Sarkhosh et al., 2018; Najdabbasi et al., 2020).
We found notable efficacy in inhibiting spore germination and mycelial growth across strains for both thyme and oregano EOs. Oregano EO revealed the most extensive inhibition zone and consistently lower ED50 values, highlighting its potent antifungal attribute. Following oregano, thyme EO also showed considerable effectiveness, while other EOs demonstrated varying levels of efficacy against the tested strains. Such inhibitory effects could be linked to the enzymatic activity of the EOs by disrupting cell wall structure and impeding membrane synthesis (Rasooli et al., 2006; Li et al., 2014). In a study by Parikh et al. (2021), the inhibitory effects of 38 EOs on 10 important pathogenic fungi and oomycetes were examined, revealing that thyme, clove, lemongrass, cinnamon, and oregano EOs exhibited the most potent inhibitory effects on spore germination, sporangia production, and mycelial growth of the pathogens. However, the levels of inhibitory effect varied among the different plant pathogens. In our investigation, thyme and oregano EOs could also effectively suppress Fusarium growth through fungicidal activity, at concentrations exceeding 0.84 μL/mL, as opposed to the fungistatic effects observed with tea tree and marjoram at 3.33 µL/mL. Diverse volatile bioactive compounds such as tannins, carvacrol, flavonoids, borneol, sterols, quinones, saponins, alkaloids, and phenols present in EOs cause a range of biological activities, including antibacterial, insecticidal, antiviral, and antifungal properties (Daferera et al., 2003; Kim et al., 2003; Burt, 2004; Schnitzler et al., 2007; Halmer, 2008; Kerekes et al., 2013; Nazzaro et al., 2017). For instance, the antifungal capability of oregano oil may be linked to the presence of its primary components, carvacrol and thymol, which are phenolic compounds known for their antioxidant effects (Mechergui et al., 2010). Utilizing GC-MS analysis, Soylu et al. (2006) also identified 16 bioactive compounds in thyme oil, with carvacrol constituting the major component at 37.9%. Considering the significant presence and activity of phenols and monoterpenic alcohols, it was not surprising that oregano and thyme oils were among the most effective in inhibiting all tested Fusarium strains in our study, consistent with previous research (Kishore et al., 2007; Regnier et al., 2014; Dewitte et al., 2018; Najdabbasi et al., 2020). In our study, a uniform level of MFC values exhibiting fungicidal activity was observed for most strains, with the exception of oregano, which showed reduced activity for two Fusarium strains (AT014 and L20-076). EOs with the lower MFCs are not only more effective and preferable for disease management but are also desirable for reducing both the volume and cost of applications. On the contrary, their efficacy without fungicidal activity might be weakened upon initial contact with the pathogen, especially through evaporation and washing away (Parikh et al., 2021).
To assess the possible phytotoxic impact of the chosen EOs on germination, spinach seeds were subjected to seed treatments with the EOs. Indeed, treating seeds to prevent soil-borne pathogens when planting leafy green vegetables is a widespread disease management practice. In our study, a standard germination rate exceeding 90% was observed across all treated seeds, affirming that even at a concentration of 6.66 µL/mL, these oils do not exhibit phytotoxic effects on spinach seeds germination. Similar findings were observed by Parikh et al. (2021), where EOs derived from oregano, clove, cinnamon, palmarosa, and thyme did not affect the germination of chickpea seeds, with a germination rate of 90 to 95%, comparable to that of untreated control seeds. Orzali et al. (2020) also reported no influence of Origanum vulgare EO on the germination rate of tomato seeds, consistent with the findings of Flores et al. (2018), who investigated the antibacterial efficacy of oregano, thyme, and cinnamon EOs without observing any phytotoxic effects on tomato seedlings. However, the phytotoxic impacts of EOs on various crop seeds have been previously documented (Mahdavikia and Saharkhiz, 2015; Boukaew et al., 2017; Ibáñez and Blázquez, 2018; Perczak et al., 2019; Jouini et al., 2020), which are mainly associated with allelopathy, a non-selective phenomenon that becomes serious only if seeds or plants possess enzymatic mechanisms of eliminating toxicogenic potential (Sumalan et al., 2019; Benarab et al., 2020; Bota et al., 2022). Overall, the type and concentration of the EO, along with the plant species exposed to it, determine the phytotoxic level (Somda et al., 2007; Orzali et al., 2014),. Hence, establishing the ideal concentration of EOs possessing potent antifungal properties yet are free of phytotoxicity is always crucial.
To delve deeper into controlling Fusarium wilt in greenhouse settings, we initially assessed different inoculation methods employing a specific strain of F. oxysporum f.sp. spinaciae, F-17536, to induce the infection in spinach, aiming to establish a reliable and reproducible methodology. An optimal inoculation technique guarantees effective colonization of the host plant, enabling a thorough disease assessment. In the comparison of the five inoculation methods, we suggest seeding spinach in a mixture of Fusarium-colonized ground wheat (GC) and substrate at a ratio of 1%. As the preferred protocol for Fusarium wilt screening, we effectively exposed spinach to a concentrated pathogen load, thereby intensifying disease pressure from the soil. This resulted in symptoms appearing as early as 9 DAI, in contrast to the 14 DAI observed with other inoculation methods. However, the presence of conidia in the mycelia might have interfered with this outcome. This inoculation method also simulates the presence of soil-borne fungal inoculum in natural field conditions, representing a primary source of infections (Hörberg, 2002; Hoheneder et al., 2022). Lai et al. (2020) showed that introducing Fusarium-colonized barley inoculum during sugar beet seed planting led to the development of more severe disease symptoms in a shorter timeframe compared to the root-dipping technique. Although the colonized grain-based inoculum technique has been demonstrated as suitable for studying various soil-borne pathogens and host plants (Holmes and Benson, 1994; Bai and Shaner, 2004; Kirk et al., 2008; Mirmajlessi et al., 2012; Zhang et al., 2014; Noor and Khan, 2015; Koch et al., 2020; Lai et al., 2020; Hoheneder et al., 2022), it was applied for the first time to test the pathogenicity of F. oxysporum f.sp. spinaciae on spinach, leveraging strengths from previous studies. The disease symptom induced by MP, RD, and SD inoculation methods also led to comparable severe Fusarium infections. The observed uniformity in DSI among these three methods is likely because of their shared capacity to infiltrate the root epidermis and colonize tissues intracellularly and intercellularly, as reported by previous studies (Van Peer and Schippers, 1992; Mendgen et al., 1996; Czymmek et al., 2007; Lai et al., 2020). However, despite their effectiveness, these methods are time consuming and resource-intensive, requiring large quantities of inoculant and nursery preparation. In contrast, the SS method was found to be less effective, likely due to factors such as limited survival of the inoculant, inadequate spore quantities on seeds, or the removal of attached spores during watering. Nonetheless, it offers cost savings for large-scale experiments as it needs low amount of inoculant compared to other methods (Rocha et al., 2019).
Continuing with greenhouse experiments, we evaluated the effects of the most potent EOs (thyme and oregano), determined in vitro, on the F. oxysporum f.sp. spinaciae-spinach pathosystem in vivo, laying the groundwork for future field experiments. Since greenhouse experiments can be adjusted to mitigate environmental variables, creating optimal growth conditions and gaining fresh insights into disease management within a shorter time frame, they can yield comparable assessments to field evaluations, as reported by previous studies (La Torre et al., 2016; Borrero et al., 2017; Gonçalves et al., 2021). For instance, Ribeiro et al. (2015) found that while field evaluations for testing genotype resistance to Fusarium oxysporum f. sp. cubense isolates typically span two years, greenhouse experiments could achieve similar assessments in approximately three months. We observed that at the highest concentration, thyme oil exhibited greater efficacy against F. oxysporum f.sp. spinaciae compared to oregano oil. This resulted in approximately 54% and 17% reductions in disease incidence on spinach in both seed and soil treatments, respectively, without causing any phytotoxic effects. Indeed, the antifungal activities of these EOs are primarily due to high levels of carvacrol and thymol, as reported in several studies (Bouchra et al., 2003; Neri et al., 2006; Combrinck et al., 2011; Pérez-Alfonso et al., 2012; Zhang et al., 2019). Carvacrol suppresses Cyclooxygenase (COX-2) expression and activates peroxisome proliferator-activated receptors (PPAR) α and γ (Hotta et al., 2010; Mączka et al., 2023), while thymol induces the production of reactive oxygen species (ROS) and lipid peroxidation (LPO) in fungal cells (Ma et al., 2019; Shcherbakova et al., 2021). The reduced efficacy of soil treatment may result from the uneven distribution of EOs in the soil matrix, leading to varying concentrations around treated seeds and limiting their effectiveness in inhibiting fungal growth. Besides, the interaction between EO and soil components can also affect the bioavailability and stability of active compounds, thereby reducing its antifungal activity. In contrast, immersing seeds in the EO could potentially create a protective obstacle against contaminated soil during the germination process. These could explain why Fusarium wilt was satisfactorily inhibited in seeds treated with relatively high concentrations of these oils, which is consistent with findings from other studies (Mbega et al., 2012; Karaca et al., 2017; Cadena et al., 2018; Terzić et al., 2023). Van Der Wolf et al. (2008) demonstrated the antifungal properties of thyme, oregano, cinnamon, and Clove EOs against various seed-borne pathogens, highlighting thyme as the most effective natural compound for mitigating infections in cabbage seeds. In a study conducted by Ben-Jabeur et al. (2015), thyme EO also revealed effective control of gray mold and Fusarium wilt in tomato seedlings. It resulted in a reduction of Botrytis cinerea colonization by 64% and significantly lowered Fusarium wilt severity by 30.76%, compared to the untreated control, especially seven days post-treatment. In practice, adjusting the treatment duration and dosage is crucial when applying oil to seeds before sowing to maximize antifungal action against soil-borne pathogens without causing phytotoxicity. Furthermore, the susceptibility of EOs to environmental factors like light, temperature, and humidity, along with their high volatility, can lead to instability, potentially affecting their effectiveness over time (Turek and Stintzing, 2013; Najdabbasi et al., 2020). To address these challenges, EOs can be integrated into novel formulations like microemulsions, nanoparticles, and encapsulation techniques, which enhance their physicochemical stability, reinforce biocidal effectiveness, and prolong their activity against plant pathogenic fungi and bacteria (Zhang et al., 2014; Pavoni et al., 2019; Weisany et al., 2019; Basavegowda et al., 2020; Tu et al., 2020; Weisany et al., 2022). For instance, Sivalingam et al. (2024) showed that essential oil-loaded nanostructured lipid carriers displayed antifungal activity against Fusarium oxysporum f.sp. lycopersici, comparable to the positive control carbendazim. Taken together, these aspects suggest that although seed treatment with EOs holds promise as a control strategy, improving application methods and formulations is essential to enhance their effectiveness in managing Fusarium wilt in spinach cultivation.
Conclusion
This study collectively assessed various artificial inoculation methods to induce Fusarium wilt in spinach under greenhouse conditions. It found that the colonized grain-based inoculum method was the most effective approach in eliciting symptoms, which appeared at approximately 9 DAI, thus providing valuable insight for future Fusarium research on spinach. Furthermore, given that previous studies have primarily focused on other Fusarium species when assessing the fungitoxicity of EOs, our research provides additional evidence supporting the potential use of thyme and oregano oils for controlling F. oxysporum f.sp. spinacia. To our knowledge, this study is the first to investigate the application of these EOs for treating spinach seeds and soil in a greenhouse environment, resulting in a reduction in Fusarium wilt incidence without adverse effects on plant health. Supported by in vivo experiments, these findings highlight the potential of thyme EO as a green fungicide for integrated pest management in spinach cultivation. However, successful large-scale application of such compounds requires formulation optimization, suitable farm facilities, and consideration of various biological and environmental factors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MM: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NN: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. LS: Resources, Writing – review & editing. GH: Funding acquisition, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful to Sacha Kofman (Enza Zaden) for providing the spinach seeds for the plant experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbott, W. S. (1925). A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18, 265–267. doi: 10.1093/jee/18.2.265a
Abdel-Kader, M. M., El-Mougy, N. S., Lashin, S. M. (2011). Essential oils and Trichoderma harzianum as an integrated control measure against faba bean root rot pathogens. J. Plant Prot. Res. 51, 306–313. doi: 10.2478/v10045-011-0050-8
Abdi-Moghadam, Z., Mazaheri, Y., Rezagholizade-Shirvan, A., Mahmoudzadeh, M., Sarafraz, M., Mohtashami, M., et al. (2023). The significance of essential oils and their antifungal properties in the food industry: A systematic review. Heliyon. 9, e21386. doi: 10.1016/j.heliyon.2023.e21386
Amini, J., Farhang, V., Javadi, T., Nazemi, J. (2016). Antifungal effect of plant essential oils on controlling Phytophthora species. Plant Pathol. J. 32, 16. doi: 10.5423/PPJ.OA.05.2015.0091
Antonissen, G., Martel, A., Pasmans, F., Ducatelle, R., Verbrugghe, E., Vandenbroucke, V., et al. (2014). The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins (Basel). 6, 430–452. doi: 10.3390/toxins6020430
Association of Official Seed Analysts (AOSA). (2002). Seed vigor testing handbook: contribution no. 32 to the Handbook on seed testing (Las Cruces, NM: Association of Official Seed Analysts).
Bahadur, A. (2022). “urrent status of Fusarium and their management strategies,” in Fusarium - an overview of the genus. Ed. Mirmajlessi, S. M. (IntechOpen, London, United Kingdom), 1–17. doi: 10.5772/intechopen.100608
Bai, G., Shaner, G. (2004). Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 42, 135–161. doi: 10.1146/annurev.phyto.42.040803.140340
Basavegowda, N., Patra, J. K., Baek, K. H. (2020). Essential oils and mono/bi/trimetallic nanocomposites as alternative sources of antimicrobial agents to combat multidrug-resistant pathogenic microorganisms: an overview. Molecules. 25, 1058. doi: 10.3390/molecules25051058
Benarab, H., Fenni, M., Louadj, Y., Boukhabti, H., Ramdani, M. (2020). Allelopathic activity of essential oil extracts from Artemisia Herba-Alba Asso. on seed and seedling germination of weed and wheat crops. Acta Sci. Nat. 7, 86–97. doi: 10.2478/asn-2020-0009
Ben-Jabeur, M., Ghabri, E., Myriam, M., Hamada, W. (2015). Thyme essential oil as a defense inducer of tomato against gray mold and Fusarium wilt. Plant Physiol. Biochem. 94, 35–40. doi: 10.1016/j.plaphy.2015.05.006
Bi, Y., Jiang, H., Hausbeck, M. K., Hao, J. J. (2012). Inhibitory effects of essential oils for controlling Phytophthora capsici. Plant Dis. 96, 797–803. doi: 10.1094/PDIS-11-11-0933
Borrero, C., Bascón, J., Gallardo, M.Á., Orta, M. S., Avilés., M. (2017). New foci of strawberry Fusarium wilt in Huelva (Spain) and susceptibility of the most commonly used cultivars. Sci. Hortic. 226, 85–90. doi: 10.1016/j.scienta.2017.08.034
Bota, V., Sumalan, R. M., Obistioiu, D., Negrea, M., Cocan, I., Popescu, I., et al. (2022). Study on the sustainability potential of thyme, oregano, and coriander essential oils used as vapours for antifungal protection of wheat and wheat Products. Sustainability. 14, 4298. doi: 10.3390/su14074298
Bouchra, C., Achouri, M., Hassani, L. M. I., Hmamouchi, M. (2003). Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers. Fr. J. Ethnopharmacol. 89, 165–169. doi: 10.1016/S0378-8741(03)00275-7
Boukaew, S., Prasertsan, P., Sattayasamitsathit, S. (2017). Evaluation of antifungal activity of essential oils against aflatoxigenic Aspergillus flavus and their allelopathic activity from fumigation to protect maize seeds during storage. Ind. Crop Prod. 97, 558–566. doi: 10.1016/j.indcrop.2017.01.005
Bounar, R., Krimat, S., Boureghda, H., Dob, T. (2020). Chemical analyses, antioxidant and antifungal effects of oregano and thyme essential oils alone or in combination against selected Fusarium species. Int. Food Res. J. 27, 66–77. Available at: https://api.semanticscholar.org/CorpusID:229321802.
Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. 94, 223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022
Cadena, M. B., Preston, G. M., van der Hoorn, R. A., Flanagan, N. A., Townley, H. E., Thompson, I. P. (2018). Enhancing cinnamon essential oil activity by nanoparticle encapsulation to control seed pathogens. Ind. Crops Prod. 124, 755–764. doi: 10.1016/j.indcrop.2018.08.043
Chen, Y., Zeng, H., Tian, J., Ban, X., Ma, B., Wang, Y. (2013). Antifungal mechanism of essential oil from Anethum graveolens seeds against Candida albicans. J. Med. Microbiol. 62, 1175–1183. doi: 10.1099/jmm.0.055467-0
Combrinck, S., Regnier, T., Kamatou, G. P. P. (2011). In vitro activity of eighteen essential oils and some major components against common postharvest fungal pathogens of fruit. Ind. Crop Prod. 33, 344–349. doi: 10.1016/j.indcrop.2010.11.011
Czymmek, K. J., Fogg, M., Powell, D. H., Sweigard, J., Park, S. Y., Kang, S. (2007). In vivo time-lapse documentation using confocal and multi-photon microscopy reveals the mechanisms of invasion into the Arabidopsis root vascular system by Fusarium oxysporum. Fungal Genet. Biol. 44, 1011–1023. doi: 10.1016/j.fgb.2007.01.012
Daferera, D. J., Basil, N., Ziogas, N., Polissiou, M. G. (2003). The effectiveness of plant essential oils on Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Protect. 22, 39–44. doi: 10.1016/S0261-2194(02)00095-9
Dan, Y., Liu, H.-Y., Gao, W.-W., Chen, S.-L. (2010). Activities of essential oils from Asarum heterotropoides var. mandshuricum against five phytopathogens. Crop Protect. 29, 295–299. doi: 10.1016/j.cropro.2009.12.007
De Cal, A., Sztejnberg, A., Sabuquillo, P., Melgarejo, P. (2009). Management Fusarium wilt on melon and watermelon by Penicillium oxalicum. Biol. Control. 51, 480–486. doi: 10.1016/j.biocontrol.2009.08.011
Dewitte, K., Landschoot, S., Carrette, J., Audenaert, K., Haesaert, G. (2018). Exploration of essential oils as alternatives to conventional fungicides in lupin cultivation. Organic Agric. 9, 107–116. doi: 10.1007/s13165-018-0212-3
Egel, D. S., Martyn, R. D. (2007). Fusarium wilt of watermelon and other cucurbits. Plant Health Instr. 10, 1094. doi: 10.1094/PHI-I-2007-0122-01
Ekwomadu, T. I., Mwanza, M. (2023). Fusarium fungi pathogens, identification, adverse effects, disease management, and global food security: a review of the latest research. Agric. 13, 1810. doi: 10.3390/agriculture13091810
Fiori, A., Schwan-Estrada, K., Stangarlin, J., Vida, J., Scapim, C., Cruz, M., et al. (2000). Antifungal activity of leaf extracts and essential oils of some medicinal plants against Didymella bryoniae. J. Phytopathol. 148, 483–487. doi: 10.1046/j.1439-0434.2000.00524.x
Flores, J. B., García, J. O., Becheleni, F. R. C., Espinoza, A. V., Wong-Corral, F. J., Rueda-Puente, E. O. (2018). Effect of essential oils in the control of the Clavibacter michiganensis subespecie michiganensis in tomato (Lycopersicum esculentum L.) plants. Biotecnia. 20, 96–101. doi: 10.18633/biotecnia.v20i3.704
Gonçalves, D. C., Tebaldi de Queiroz, V., Costa, A. V., Lima, W. P., Belan, L. L., Moraes, W. B., et al. (2021). Reduction of fusarium wilt symptoms in tomato seedlings following seed treatment with origanum vulgare L. essential Oil carvacrol. Crop Prot. 141, 105487. doi: 10.1016/j.cropro.2020.105487
Halmer, P. (2008). Seed technology and seed enhancement. Acta Hortic. 771, 17–26. doi: 10.17660/ActaHortic.2008.771.1
Han, X.-B., Zhao, J., Cao, J.-M., Zhang, C.-S. (2019). Essential oil of Chrysanthemum indicum L.: potential biocontrol agent against plant pathogen Phytophthora nicotianae. Environ. Sci. pollut. Control Ser. 26, 7013–7023. doi: 10.1007/s11356-019-04152-y
Haque, E., Irfan, S., Kamil, M., Sheikh, S., Hasan, A., Ahmad, A., et al. (2016). Terpenoids with antifungal activity trigger mitochondrial dysfunction in Saccharomyces cerevisiae. Microbiol. 85, 436–443. doi: 10.1134/S0026261716040093
Hoheneder, F., Biehl, E. M., Hofer, K., Petermeier, J., Groth, J., Herz, M., et al. (2022). Host genotype and weather effects on Fusarium head blight severity and mycotoxin load in spring barley. Toxins. 14, 125. doi: 10.3390/toxins14020125
Holmes, K. A., Benson, D. M. (1994). Evaluation of Phytophthora parasitica var. nicotianae for biocontrol of Phytophthora parasitica on Catharanthus roseus. Plant Dis. 78, 193–199. doi: 10.1094/PD-78-0193
Hörberg, H. M. (2002). Patterns of splash dispersed conidia of Fusarium poae and Fusarium culmorum. Eur. J. Plant Pathol. 108, 73–80. doi: 10.1023/A:1013936624884
Hotta, M., Nakata, R., Katsukawa, M., Hori, K., Takahashi, S., Inoue, H. (2010). Carvacrol, a component of thyme oil, activates PPARalpha and gamma and suppresses COX-2 expression. J. Lipid Res. 51, 132–139. doi: 10.1194/jlr.M900255-JLR200
Ibáñez, M., Blázquez, M. (2018). Phytotoxicity of essential oils on selected weeds: Potential hazard on food crops. Plants. 7, 79. doi: 10.3390/plants7040079
International Seed quality Assurance (ISTA) (2023). International rules for seed testing. Available online at: https://www.seedtest.org/en/international-rules-for-seed-testing-rubric-3.html (Accessed February 15, 2023).
Jouini, A., Verdeguer, M., Pinton, S., Araniti, F., Palazzolo, E., Badalucco, L., et al. (2020). Potential effects of essential oils extracted from mediterranean aromatic plants on target weeds and soil microorganisms. Plants. 9, 1–24. doi: 10.3390/plants9101289
Karaca, G., Bilginturan, M., Olgunsoy, P. (2017). Effects of some plant essential oils against fungi on wheat seeds. Indian J. Pharm. Educ. Res. 51, 385–388. doi: 10.5530/ijper.51.3s.53
Kerekes, E. B., Deák, É., Takó, M., Tserennadmid, R., Petkovits, T., Vágvölgyi, C., et al. (2013). Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 115, 933–942. doi: 10.1111/jam.2013.115.issue-4
Khaleil, M. M., Alnoman, M. M., Elrazik, E. S. A., Zagloul, H., Khalil, A. M. A. (2021). Essential oil of Foeniculum vulgare Mill. as a green fungicide and defense-inducing agent against Fusarium Root Rot Disease in Vicia faba L. Biol. (Basel). 10, 696. doi: 10.3390/biology10080696
Khan, A., Ahmad, A., Khan, L. A., Padoa, C. J., van Vuuren, S., Manzoor, N. (2015). Effect of two monoterpene phenols on antioxidant defense system in Candida albicans. Microb. Pathog. 80, 50–56. doi: 10.1016/j.micpath.2015.02.004
Kim, S.-I., Park, C., Ohh, M.-H., Cho, H.-C., Ahn, Y.-J. (2003). Contact and fumigant activities of aromatic plant extracts and essential oils against Lasioderma serricorne (Coleoptera: anobiidae). J. Stored Prod. Res. 39, 11–19. doi: 10.1016/S0022-474X(02)00013-9
Kirk, W. W., Wharton, P. S., Schafer, R. L., Tumbalam, P., Poindexter, S., Guza, C., et al. (2008). Optimizing fungicide timing for the control of Rhizoctonia crown and root rot of sugar beet using soil temperature and plant growth stages. Plant Dis. 92, 1091–1098. doi: 10.1094/PDIS-92-7-1091
Kishore, G. K., Pande, S., Harish, S. (2007). Evaluation of essential oils and their components for broad-spectrum antifungal activity and control of late leaf spot and crown rot diseases in peanut. Plant Dis. 91, 375–379. doi: 10.1094/PDIS-91-4-0375
Koch, E., Zink, P., Pfeiffer, T., von Galen, A., Linkies, A., Drechsel, J., et al. (2020). Artificial inoculation methods for testing microorganisms as control agents of seed- and soil-borne Fusarium-seedling blight of maize. J. Plant Dis. Prot. 127, 883–893. doi: 10.1007/s41348-020-00350-w
Kumar, V., Mathela, C. S., Tewari, A. K., Bisht, K. S. (2014). In vitro inhibition activity of essential oils from some Lamiaceae species against phytopathogenic fungi. Pestic. Biochem. Phys. 114, 67–71. doi: 10.1016/j.pestbp.2014.07.001
Lai, X., Qi, A., Liu, Y., Mendoza, L. E. D. R., Liu, Z., Lin, Z., et al. (2020). Evaluating inoculation methods to infect sugar beet with Fusarium oxysporum f. betae and F. secorum. Plant Dis. 104, 1312–1317. doi: 10.1094/PDIS-09-19-1895-RE
Larena, I., Sabuquillo, P., Melgarejo, P., De Cal, A. (2003). Biocontrol of Fusarium and Verticillium wilt of tomato by Penicillium oxalicum under greenhouse and field conditions. J. Phytopathol. 151, 507–512. doi: 10.1046/j.1439-0434.2003.00762.x
La Torre, A., Caradonia, F., Matere, A., Battaglia, V. (2016). Using plant essential oils to control Fusarium wilt in tomato plants. Eur. J. Plant Pathol. 144, 487–496. doi: 10.1007/s10658-015-0789-2
Li, Y., Fabiano-Tixier, A.-S., Chemat, F. (2014). “Essential oils: from conventional to green extraction,” in Essential oils as reagents in green chemistry. Eds. Li, Y., Fabiano-Tixier, A. S., Chema, F. (Switzerland: Springer Cham), 9–20.
Linde, J. H., Combrinck, S., Regnier, T. J. C., Virijevic, S. (2010). Chemical composition and antifungal activity of the essential oils of Lippia rehmannii from South Africa. S. Afr. J. Bot. 76, 37–42. doi: 10.1016/j.sajb.2009.06.011
Lu, M., Han, Z., Yao, L. (2013). In vitro and in vivo antimicrobial efficacy of essential oils and individual compounds against Phytophthora parasitica var. nicotianae. J. Appl. Microbiol. 115, 187–198. doi: 10.1111/jam.12208
Ma, D., Cui, X., Zhang, Z., Li, B., Xu, Y., Tian, S., et al. (2019). Honokiol suppresses mycelial growth and reduces virulence of Botrytis cinerea by inducing autophagic activities and apoptosis. Food Microbiol. 88, 103411. doi: 10.1016/j.fm.2019.103411
Mączka, W., Twardawska, M., Grabarczyk, M., Wińska, K. (2023). Carvacrol-a natural phenolic compound with antimicrobial properties. Antibiotics. 12, 824. doi: 10.3390/antibiotics12050824
Mahdavikia, F., Saharkhiz, M. J. (2015). Phytotoxic activity of essential oil and water extract of peppermint (Mentha × piperita L. CV. Mitcham). J. Appl. Res. Med. Aromat. Plants. 2, 146–153. doi: 10.1016/j.jarmap.2015.09.003
Mbega, E. R., Mabagala, R. B., Mortensen, C. N., Wulff, E. G. (2012). Evaluation of essential oils as seed treatment for the control of Xanthomonas spp. associated with the bacterial leaf spot of tomato in Tanzania. J. Plant Pathol. 94, 273–281.
Mechergui, K., Coelho, J. A., Serra, M. C., Lamine, S. B., BoukhChina, S., Khouja, M. L. (2010). Essential oils of Origanum vulgare L. subsp. glandulosum (Desf.) Ietswaart from Tunisia: chemical composition and antioxidant activity. J. Sci. Food. Agric. 90, 1745–1749. doi: 10.1002/jsfa.v90:10
Mendgen, K., Hahn, M., Deising, H. (1996). Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 34, 367–386. doi: 10.1146/annurev.phyto.34.1.367
Mirmajlessi, S. M., Safaie, N., Mostafavi, H., Mansouripour, S. M., Mahmoudy, S. B. (2012). Genetic diversity among crown and root rot isolates of Rhizoctonia solani isolated from cucurbits using PCR based techniques. Afr. J. Agric. Res. 7, 583–590. doi: 10.5897/AJAR11.1453
Moungthipmalai, T., Puwanard, C., Aungtikun, J., Sittichok, S., Soonwera, M. (2023). Ovicidal toxicity of plant essential oils and their major constituents against two mosquito vectors and their non-target aquatic predators. Sci. Rep. 13, 2119. doi: 10.1038/s41598-023-29421-2
Mowlick, S., Inoue, T., Takehara, T., Kaku, N., Ueki, K., Ueki, A. (2013). Changes and recovery of soil bacterial communities influenced by biological soil disinfestation as compared with chloropicrin-treatment. AMB Express. 3, 1–12. doi: 10.1186/2191-0855-3-46
Najdabbasi, N., Mirmajlessi, S. M., Dewitte, K., Landschoot, S., Mänd, M., Audenaert, K., et al. (2020). Biocidal activity of plant-derived compounds against Phytophthora infestans: An alternative approach to late blight management. Crop Prot. 138, 105315. doi: 10.1016/j.cropro.2020.105315
Nazzaro, F., Fratianni, F., Coppola, R., Feo, V. D. (2017). Essential oils and antifungal activity. Pharm. (Basel). 10, 86. doi: 10.3390/ph10040086
Neri, F., Mari, M., Brigati, S. (2006). Control of Penicillium expansum by plant volatile compounds. Plant Pathol. 55, 100–105. doi: 10.1111/j.1365-3059.2005.01312.x
Noor, A., Khan, M. F. R. (2015). Efficacy and safety of mixing azoxystrobin and starter fertilizers for controlling Rhizoctonia solani in sugar beet. Phytoparasitica. 43, 51–55. doi: 10.1007/s12600-014-0416-3
Nucci, M., Anaissie, E. (2007). Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 20, 695–704. doi: 10.1128/CMR.00014-07
O’Donnell, K., Kistler, H. C., Cigelnik, E., Ploetz, R. C. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. PNAS. 95, 2044–2049. doi: 10.1073/pnas.95.5.204
Orzali, L., Forni, C., Riccioni, L. (2014). Effect of chitosan seed treatment as elicitor of resistance to Fusarium graminearum in wheat. Seed Sci. Technol. 42, 132–149. doi: 10.15258/sst.2014.42.2.03
Orzali, L., Valente, M. T., Scala, V., Loreti, S., Pucci, N. (2020). Antibacterial activity of essential oils and Trametes versicolor extract against Clavibacter michiganensis subsp. michiganensis and Ralstonia solanacearum for seed treatment and development of a rapid in vivo assay. Antibiotics. 9, 628. doi: 10.3390/antibiotics9090628
Pane, C., Villecco, D., Zaccardelli, M. (2017). Combined use of Brassica carinata seed meal, thyme oil and a Bacillus amyloliquefaciens strain for controlling three soil-borne fungal plant diseases. J. Plant Pathol. 99, 77–84. doi: 10.4454/jpp.v99i1.3829
Parikh, L., Agindotan, B. O., Burrows, M. E. (2021). Antifungal activity of plant-derived essential oils on pathogens of pulse crops. Plant Dis. 105, 1692–1701. doi: 10.1094/PDIS-06-20-1401-RE
Pavoni, L., Maggi, F., Mancianti, F., Nardoni, S., Ebani, V. V., Cespi, M., et al. (2019). Microemulsions: an effective encapsulation tool to enhance the antimicrobial activity of selected EOs. J. Drug Deliv. Sci. Technol. 53, 101101. doi: 10.1016/j.jddst.2019.05.050
Perczak, A., Gwiazdowska, D., Gwiazdowski, R., Juś, K., Marchwińska, K., Waśkiewicz, A. (2019). The inhibitory potential of selected essential oils on Fusarium spp. growth and mycotoxins biosynthesis in maize seeds. Pathogens. 9, 23. doi: 10.3390/pathogens9010023
Pérez-Alfonso, C. O., Martínez-Romero, D., Zapata, P. J., Serrano, M., Valero, D., Castillo, S. (2012). The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and P. italicum involved in lemon decay. Int. J. Food Microbiol. 158, 101–106. doi: 10.1016/j.ijfoodmicro.2012.07.002
Prakash, B., Gupta, V., Raghuvanshi, T. S. (2024). Essential oils as green promising alternatives to chemical preservatives for agri-food products: New insight into molecular mechanism, toxicity assessment, and safety profile. Food Chem. Toxicol. 183, 114241. doi: 10.1016/j.fct.2023.114241
Rasooli, I., Rezaei, M. B., Allameh, A. (2006). Growth inhibition and morphological alterations of Aspergillus Niger by essential oils from Thymus eriocalyx and Thymus xporlock. Food Contr 17, 359–364. doi: 10.1016/j.foodcont.2004.12.002
Regnier, T., Combrinck, S., Veldman, W., Du Plooy, W. (2014). Application of essential oils as multi-target fungicides for the control of Geotrichum citri-aurantii and other postharvest pathogens of citrus. Ind. Crops Prod. 61, 151–159. doi: 10.1016/j.indcrop.2014.05.052
Ribeiro, L. R., da Luz, L. A., Silva, S. O., Bragança, C. A. D., Amorim, E. P., Haddad, F. (2015). “Teste de agressividade de haplótipos de Fusarium oxysporum f. sp. cubense oriundos de regiões produtoras,” in II Simpósio Internacional de Fruticultura - Pragas Quarentenárias e Melhoramento Preventivo. Eds. Haroldo, D., Reinhardt, R. C., Ferraz, F. L. (Salvador-BA: Embrapa Brasília), 51–52.
Rocha, I., Ma, Y., Souza-Alonso, P., Vosátka, M., Freitas, H., Oliveira, R. S. (2019). Seed coating: a tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 6. doi: 10.3389/fpls.2019.01357
Sardi, J. D. C. O., Pitangui, N. D. S., Rodríguez-Arellanes, G., Taylor, M. L., Fusco-Almeida, A. M., Mendes-Giannini, M. J. S. (2014). Highlights in pathogenic fungal biofilms. Rev. Iberoam. De. Micol. 31, 22–29. doi: 10.1016/j.riam.2013.09.014
Sarkhosh, A., Schaffer, B., VargaS, A. I., Palmateer, A. J., Lopez, P., Soleymani, A. (2018). In vitro evaluation of eight plant essential oils for controlling Colletotrichum, Botryosphaeria, Fusarium and Phytophthora fruit rots of avocado, mango and papaya. Plant Protect. Sci. 54, 153–162. doi: 10.17221/49/2017-PPS
Schnitzler, P., Koch, C., Reichling, J. (2007). Susceptibility of drug-resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop, and sandalwood. Antimicrob. Agents Chemother. 51, 1859–1862. doi: 10.1128/AAC.00426-06
Sharma, A., Rajendran, S., Srivastava, A., Sharma, S., Kundu, B. (2017). Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng. 123, 308–313. doi: 10.1016/j.jbiosc.2016.09.011
Shcherbakova, L., Mikityuk , O., Arslanova , L., Stakheev , A., Erokhin , D., Zavriev , S., et al. (2021). Studying the ability of thymol to improve fungicidal effects of tebuconazole and difenoconazole against some plant pathogenic fungi in seed or foliar treatments. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.629429
Sivalingam, S., Sharmila, J. S., Golla, G., Arunachalam, L., Singh, T., Karthikeyan, G., et al. (2024). Encapsulation of essential oil to prepare environment friendly nanobio-fungicide against Fusarium oxysporum f.sp. lycopersici: An experimental and molecular dynamics approach. Colloids and Surfaces A: Physicochemical and Engineering Aspects. Colloid Surface A. 681, 132681. doi: 10.1016/j.colsurfa.2023.132681
Somda, I., Leth, V., Sereme, P. (2007). Antifungal effect of Cymbopogon citratus, Eucalyptus camaldulensis and Azadirachta indica oil extracts on sorghum seed-borne fungi. Asian J. Plant Sci. 6, 1182–1189. doi: 10.3923/ajps.2007.1182.1189
Soylu, E. M., Soylu, S., Kurt, S. (2006). Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathologia. 161, 119–128. doi: 10.1007/s11046-005-0206-z
Soylu, S., Yigitbas, H., Soylu, E. M., Kurt, S. (2007). Antifungal effects of essential oils from oregano and fennel on Sclerotinia sclerotiorum. J. Appl. Microbiol. 103, 1021–1030. doi: 10.1111/jam.2007.103.issue-4
Stevic, T., Berić, T., Šavikin, K., Soković, M., Gođevac, D., Dimkić, I., et al. (2014). Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind. Crops Prod. 55, 116–122. doi: 10.1016/j.indcrop.2014.02.011
Sumalan, R. M., Alexa, E., Popescu, I., Negrea, M., Radulov, I., Obistioiu, D., et al. (2019). Exploring ecological alternatives for crop protection using Coriandrum sativum essential oil. Molecules. 24, 2040. doi: 10.3390/molecules24112040
Tejeswini, M. S., Sowmya, H. V., Swarnalatha, S. P., Negi, P. S. (2014). Antifungal activity of essential oils and their combinations in in vitro and in vivo conditions. Arch. Phytopathol. Plant Prot. 47, 564–570. doi: 10.1080/03235408.2013.814235
Terzić, D., Tabaković, M., Oro, V., Poštić, D., Štrbanović, R., Filipović, V.R. (2023). Impact of essential oils on seed quality and seed-borne pathogens of Althea officinalis seeds of different ages. Chem. Biol. Technol. Agric. 10, 33. doi: 10.1186/s40538-023-00405-8
Tolouee, M., Alinezhad, S., Saberi, R., Eslamifar, A., Zad, S. J., Jaimand, K., et al. (2010). Effect of matricaria chamomilla L. flower essential oil on the growth and ultrastructure of Aspergillus Niger van Tieghem. Int. J. Food Microbiol. 139, 127–133. doi: 10.1016/j.ijfoodmicro.2010.03.032
Tu, Q. B., Wang, P. Y., Sheng, S., Xu, Y., Wang, J.-Z., You, S., et al. (2020). Microencapsulation and antimicrobial activity of plant essential oil against Ralstonia solanacearum. Waste Biomass Valor. 11, 5273–5282. doi: 10.1007/s12649-020-00987-6
Turek, C., Stintzing, F. C. (2013). Stability of essential oils: a review. Comp. Rev. Food Sci. Food Safe. 12, 40–53. doi: 10.1111/1541-4337.12006
Van Der Wolf, J. M., Birnbaum, Y., van der Zouwen, P. S., Groot, S. P. C. (2008). Disinfection of vegetable seed by treatment with essential oils, organic acids and plant extracts. Seed Sci. Technol. 36, 7688. doi: 10.15258/sst.2008.36.1.08
Van Peer, R., Schippers, B. (1992). Lipopolysaccharides of plant-growth promoting Pseudomonas sp. strain WCS417r induce resistance in carnation to Fusarium wilt. Eur. J. Plant Pathol. 98, 129–139. doi: 10.1007/BF01996325
Weisany, W., Amini, J., Samadi, S., Hossaini, S., Yousefi, S., Struik, P. C. (2019). Nano silver-encapsulation of Thymus daenensis and Anethum graveolens essential oils enhances antifungal potential against strawberry anthracnose. Ind. Crop Prod. 141, 111808. doi: 10.1016/j.indcrop.2019.111808
Weisany, W., Yousefi, S., Tahir, N. A. R., Zadeh, N. G., McClements, D. J., Adhikari, B., et al. (2022). Targeted delivery and controlled released of essential oils using nanoencapsulation: A review. Adv. Colloid Interface Sci. 303, 102655. doi: 10.1016/j.cis.2022.102655
White, T. J., Bruns, T. D., Lee, S. B., y Taylor, J. W. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR protocols: A Guide to Methods and Applications. Eds. Innis, M. A., Gelfand, D. H., Sninsky, J. J., White, T. J. (Academic Press, New York, NY), 315–322.
Zhang, J., Ma, S., Du, S., Chen, S., Sun, H. (2019). Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 56, 2611–2620. doi: 10.1007/s13197-019-03747-0
Zhang, Y., Niu, Y., Luo, Y., Ge, M., Yang, T., Yu, L., et al. (2014). Fabrication, characterization and antimicrobial activities of thymol loaded zein nanoparticles stabilized by sodium caseinate chitosan hydrochloride doub layers. Food Chem. 142, 269–275. doi: 10.1016/j.foodchem.2013.07.058
Keywords: anti-fungal activity, disease management, Fusarium oxysporum f.sp. spinaciae, fusarium wilt, plant-based compounds
Citation: Mirmajlessi M, Najdabbasi N, Sigillo L and Haesaert G (2024) An implementation framework for evaluating the biocidal potential of essential oils in controlling Fusarium wilt in spinach: from in vitro to in planta. Front. Plant Sci. 15:1444195. doi: 10.3389/fpls.2024.1444195
Received: 05 June 2024; Accepted: 19 July 2024;
Published: 06 August 2024.
Edited by:
Franco Nigro, University of Bari Aldo Moro, ItalyReviewed by:
Samia Gargouri, Institut National de la Recherche Agronomique de Tunisie (INRAT), TunisiaFilippo De Curtis, University of Molise, Italy
Copyright © 2024 Mirmajlessi, Najdabbasi, Sigillo and Haesaert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahyar Mirmajlessi, bWFoeWFyLm1pcm1hamxlc3NpQHVnZW50LmJl
 Mahyar Mirmajlessi
Mahyar Mirmajlessi Neda Najdabbasi1
Neda Najdabbasi1 Loredana Sigillo
Loredana Sigillo Geert Haesaert
Geert Haesaert