Abstract
Cold stress is a critical factor affecting rice production worldwide. The application of cold-resistant agents may improve the cold resistance and yield of crops. To screen for suitable cold-resistant agents for machine-transplanted early rice, the effects of uniconazole, abscisic acid, and zinc-amino acids chelate and their spraying times (seed soaking stage, one leaf and one heart stage, two leaves and one heart stage, 7 days before the transplanting stage, and regreening stage) on the yield and cold resistance of machine-transplanted early rice were investigated. Moreover, the application method (spraying amount: 750 and 1125 g ha−1; spraying time: 7 days before the transplanting stage, transplanting stage, regreening stage, and transplanting stage and regreening stage) for the most suitable cold-resistant agent was optimized. The zinc-amino acids chelate was better than the other two cold-resistant agents for promoting rice tillering and increasing the leaf area index, dry matter weight, antioxidant enzyme activities (CAT, SOD, POD) and yield (i.e., 9.22% and 7.14% higher than uniconazole and abscisic acid, respectively), especially when it was applied in the regreening stage. The examination of spraying amounts and times indicated that the zinc-amino acids chelate dosage had no significant effect on the yield and cold resistance of early rice. However, the rice yield and antioxidant enzyme activities were highest when samples were sprayed once in the transplanting stage and the regreening stage. On the basis of the study results, 750 g ha−1 zinc-amino acids chelate applications in the transplanting and regreening stages of machine-transplanted early rice plants may be ideal for increasing cold stress resistance and yield.
1 Introduction
Rice is an important staple food crop consumed by a large proportion of the global population. Unfortunately, because of climate change, extreme weather conditions occur relatively frequently, with cold stress seriously affecting rice yield and quality (Huang et al., 2013). As one of the main grain-producing areas in China, the southern rice-growing region is susceptible to late spring cold conditions, which are mainly caused by long periods of rainfall, frequent intrusion of cold air, or radiative cooling due to cold anticyclones on clear nights (Qian and Zhang, 2012). Cold stress seriously affects the production of early rice (e.g., decreased seedling quality as well as delayed transplanting, regreening of tillers, and panicle differentiation) and may even affect the planting time of late rice (Ai et al., 2022; Crofts et al., 2022; Kwak et al., 2018; Zhou et al., 2018). Extreme low-temperature stress in Hunan province from 1981 to 2010 reportedly decreased early rice and late rice yields by 6.66% and 1.82%, respectively (Liu et al., 2019).
Mechanized rice planting is currently the simplest and most effective cultivation method for rice at least partly because it can decrease the required labor force and optimize the economic benefits of rice production (Li et al., 2022). Machine transplanting has significantly increased rice production efficiency by decreasing costs, time, and labor, thereby increasing productivity and profits. However, the problems associated with this approach include low seedling quality and limited seedling age elasticity, which seriously restricts the utility of machine-transplanted rice seedlings (Gao et al., 2022). In addition, early rice seedlings are susceptible to low-temperature stress in southern China, further limiting the development and application of machine-transplanting technology.
Cold-induced damages significantly affect rice plant growth, development, and physiological metabolism. Earlier research showed that rice plants are most susceptible to low-temperature stress at the seedling and panicle differentiation stages. An exposure to low temperatures will decrease the seedling establishment rate and lead to panicle degeneration, resulting in a significant decrease in rice yield (Zhang et al., 2022). Low temperatures can also directly affect antioxidant enzyme activities, chlorophyll contents, and photosynthetic rates in early rice leaves. Specifically, low-temperature stress during the rice seedling stage decreases superoxide dismutase (SOD) and peroxidase (POD) activities in leaves, while increasing the malondialdehyde content (Theocharis et al., 2012) and decreasing the photosynthetic capacity (Zsiros et al., 2019). Cold-induced damages to rice plants may be restricted by certain defensive measures, including the selection of cold-resistant varieties (Lee and Kwon, 2015), warm water irrigation (Zhang et al., 2020), or soil warming (Ge et al., 2015). However, there are potential issues and problems associated with these measures (e.g., varietal differences among rice-growing regions, high management costs, and detrimental effects on ecological systems). Foliar fertilizers are widely used to increase crop cold resistance and yield because of their low cost and the fact they are not particularly harmful to the environment. Gurav et al. (2019) determined that potassium dihydrogen phosphate can protect plant seedlings from low-temperature stress because it stabilizes the plant cell membrane structure to maintain its protective function and permeability. Li et al. (2015) observed that potassium and zinc in foliar fertilizers can decrease oxidative damage due to reactive oxygen species in plants under low temperature stress, and improve the growth and development of plants. Pretreatment of rice seedlings with SA (salicylic acid) induced enhanced cold tolerance, mainly manifested in increased antioxidant system activity, thereby improving the relationship between yield components and increasing grain yield (Huang et al., 2016; Wang et al., 2021a).
The effects of low temperatures on rice yield formation and physiological characteristics have been extensively studied (Crofts et al., 2022; Jia et al., 2022; Xu et al., 2020). Research on technical measures that affect low-temperature defense responses has mostly focused on cold-induced damages and the expression of cold tolerance-related genes during the seedling stage (Wang et al., 2021b; Yang et al., 2012). Relatively few studies have screened different types of cold-resistant agents useful for treating machine-transplanted early rice in subtropical regions. Furthermore, there are no reports on the effects of their application methods on the cold-resistance of rice. In the first year of this study, we examined the effects of different cold-resistant agents and their spraying times on the early rice yield and seedling cold resistance under machine-transplanted conditions to identify the most suitable cold-resistant agent. In the second year, we analyzed the techniques used to apply a selected cold-resistant agent to determine the optimal spraying amount and time for early rice. By identifying appropriate cold-resistant agents and application methods, we provide theoretical and technical support for improving the cultivation of stress-resistant and stable-yielding rice plants.
2 Materials and methods
2.1 Test site and materials
This study was conducted at the Field Experiment Station of the Agriculture and Rural Affairs Bureau of Hengyang county, Hunan province, China (26° 97′ N, 111° 37′ E) in 2018 and 2019. The soil nutrient conditions were as follows: 1.46 g kg-1 total N, 0.61 g kg-1 total phosphorus, 18.87 g kg-1 total potassium, 158.44 mg kg-1 alkaline hydrolysis N, 8.93 mg kg-1 available phosphorus, 114.07 mg kg-1 available potassium, and 26.01 g kg-1 organic matter in 2018, and correspondingly 1.53 g kg-1, 0.57 g kg-1, 19.05 g kg-1, 169.12 mg kg-1, 9.73 mg kg-1, 109.95 mg kg-1 and 25.68 g kg-1 in 2019. Meteorological data of daily average temperature in Hengyang County, Hengyang City, Hunan Province, China were collected from March to July in 2018 and 2019 (Supplementary Figure S1). The data comes from the National Meteorological Science Data Center (http://data.cma.cn). For some missing data, the average value of the meteorological element in the adjacent 2 days on that day is used as a replacement.
Zhongjiazao 17, which is an indica type conventional early rice variety with a growth period of 109 days, was used in both years. The following cold-resistant agents were analyzed: uniconazole aqueous agent (180 mg L−1) produced by Chongqing Tuyouta Company (China), abscisic acid aqueous agent (15 mg L−1) produced by Henan Zhifu Company (China), and zinc-amino acids chelate (ZAC; i.e., compound foliar fertilizer) powder (amino acids ≥100 g kg−1; Zn ≥50 g kg−1) produced by Hunan Guonong Company (China).
2.2 Experimental design
A two-factor experiment involving different cold-resistant agents and spraying times was conducted in 2018. The cold-resistant agents were tested in the main area, whereas the spraying times were tested in the secondary area. The cold-resistant agents were uniconazole (C1: 75 ml m−2), abscisic acid (C2: 75 ml m−2), and ZAC (C3: 650 g ha−1). The spraying times included the seed soaking stage (D1), one leaf and one heart stage (D2), two leaves and one heart stage (D3), 7 days before the transplanting stage (D4), and regreening stage (D5; 7 days after the transplanting stage). Fifteen treatments were included in the experiment, with each treatment conducted three times for a total of 45 plots.
In 2019, a two-factor experiment with different spraying amounts and spraying times was conducted using ZAC. The spraying amounts were tested in the main area, whereas the spraying times were tested in the secondary area. The spraying amounts were 750 g ha−1 (B1) and 1125 g ha−1 (B2). The spraying times were 7 days before the transplanting stage (A1), transplanting stage (A2), regreening stage (A3), and transplanting stage and regreening stage (A4). For each spraying amount, plants were sprayed twice. A control treatment (CK) group was also included. The experiment comprised nine treatments, with each treatment conducted three times for a total of 27 plots.
In both years, early rice seeds were soaked on March 24 and sown on March 28, with the resulting seedlings transplanted on April 20. The size of the test seedling tray was 58 cm × 28 cm. The machine planting density was 30 cm × 11 cm. The study field area was 20 m2, and the surrounding area was separated by protective rows covered with polyethylene plastic films (0.3 m width and 0.3 m height). Independent irrigation and drainage outlets were provided. Other management measures were in accordance with local practices.
2.3 Measurement items and methods
Starting on day 5 after transplanting, 20 consecutive and representative rice plants with consistent growth were selected in each plot. The number of rice tillers was recorded every 5 days. To examine the leaf area and dry weight during critical growth stages (tillering, booting, heading, milky, and maturity), three plants were sampled from each plot. The leaf area was measured using a length–width coefficient method (Park et al., 2004). The plant samples at each stage were divided into the leaf, stem, and panicle and bagged, cured at 105°C for 30 min, and dried to a constant weight at 80°C. The dry matter weight of each plant part was recorded.
At maturity, 80 rice plants in each plot were examined to calculate the effective panicle number per plant. On the basis of the average effective panicle number, five plants per plot were sampled and examined in the laboratory to determine the number of grains per panicle, seed setting rate, and thousand-grain weight. Next, in each plot, 80 randomly selected rice plants were harvested, but not from the outer three rows. After threshing, the straw and empty grains were removed before grains were weighed and the moisture content was measured according to the drying method. The actual yield was calculated using a moisture content of 13.5%. The following formula was used: actual output = harvested output × (1 − moisture content)/0.865.
In 2018, rice leaves were collected at 7 days after the regreening stage, whereas in 2019, rice leaves were collected at the 7 days after the regreening stage, peak tillering stage, booting stage, and milky stage. Extract the test solution according to the method of Xie et al. (2008). Weigh a small amount of fresh rice leaves, cut them into pieces and put them in a mortar. Add liquid nitrogen to cover the leaves, let it stand for a few minutes and then quickly grind it into powder. Then use the One ten thousandth of the balance scale(AB135-S, Mettler Toledo, Zurich, Switzerland) to weigh 0.1 grams (± 0.0005g) of the sample and put it in a centrifuge tube. Add 1.5 ml of phosphate buffer (pH 7.8) and centrifuge at 10,000 r·min-1 at 4°C for 20 min. Then transfer the supernatant to a 20 ml graduated test tube and dilute to 15 ml with 62.5 mmol·L-1 phosphate buffer and mix well for later use. Repeat each treatment 3 times. The enzyme activities of CAT, SOD, and POD were determined according to the method of Munir et al. (2021). That is, a UV-visible spectrophotometer (UV2700, Shimadzu, Kyoto, Japan) was used to measure the absorbance values of the corresponding indicators (SOD, POD, and CAT) of the extracted enzyme solution at different wavelengths, and then the enzyme activity was calculated.
2.4 Statistical analysis
One-way analysis of variance (ANOVA) was used for Duncan’s multiple comparisons (P<0.05) of different treatments using SPSS 24 for Windows (IBM, Armonk, NY, USA). Graphs were plotted by Origin 2023 (OriginLab, Northampton, MA, USA).
3 Results
3.1 Screening of suitable cold-resistant agents for machine-transplanted early rice
3.1.1 Rice tillering dynamics
The number of tillers initially increased up to the peak tillering stage and then decreased (Figure 1). In terms of the effects of cold-resistant agents, the number of tillers per plant was highest for C3 at each growth stage. For C3, C2, and C1, the number of tillers per plant in the peak tillering stage was 16.22, 15.47, and 15.08, respectively. There were no major differences in the number of tillers at each growth stage among the spraying times. In the peak tillering stage, the number of tillers was highest for D5 and D2 (16.01 and 15.66 tillers per plant, respectively), followed by D4 and D3 (15.60 and 15.50 tillers per plant, respectively). The D1 treatment resulted in the fewest tillers (15.17 tillers per plant). Among the combined treatments, the number of tillers at each growth stage was highest for C3D5 (16.57 tillers per plant). Considered together, these results suggest an ZAC treatment during the regreening stage may be ideal for maximizing the number of tillers.
Figure 1
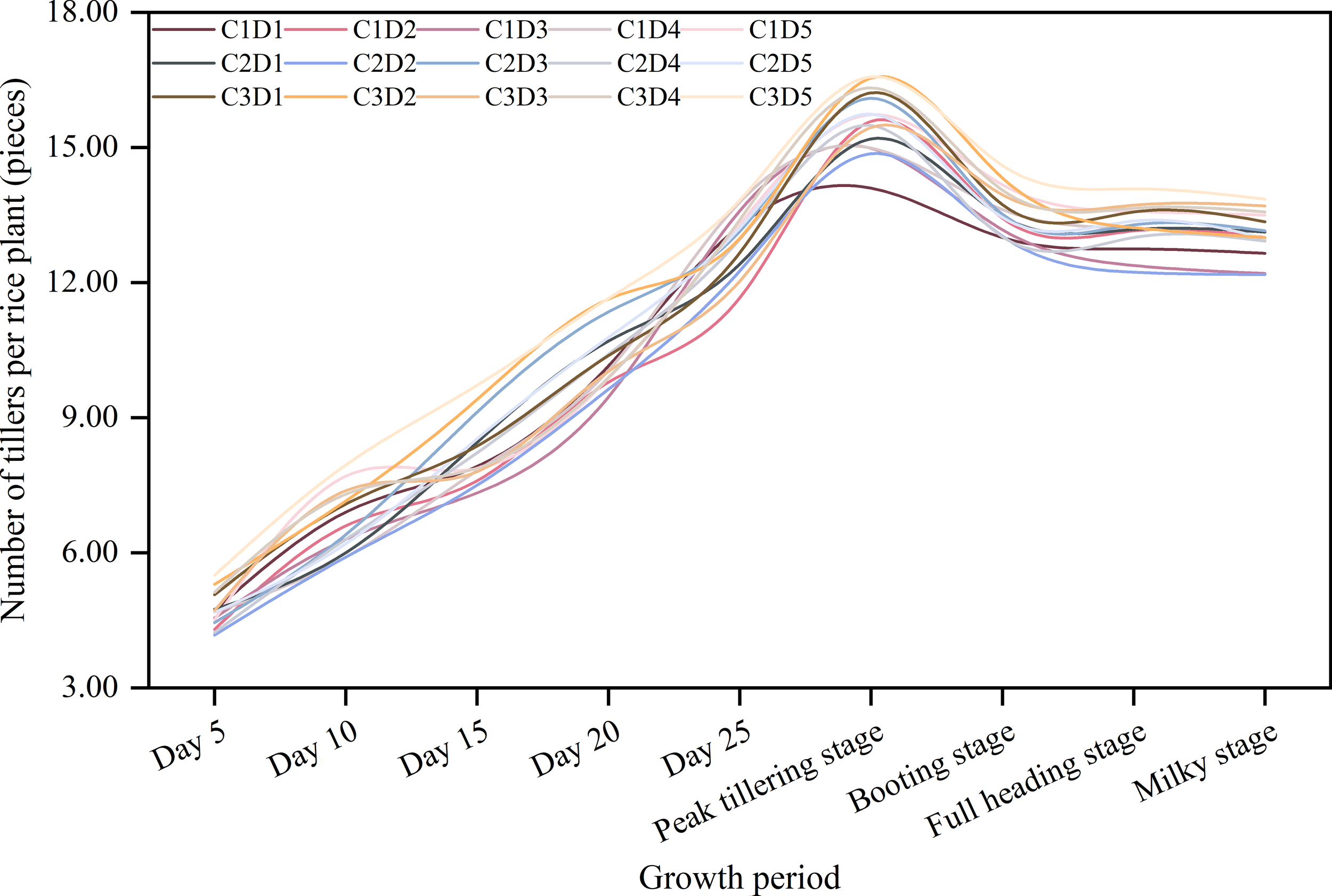
Effects of different cold-resistant agents and their spraying times on rice tillering dynamics. C1: Uniconazole; C2: Abscisic acid; C3: Zinc-amino acids chelate; D1: Seed soaking stage; D2: One leaf and one heart stage; D3: Two leaves and one heart stage; D4: 7 days before the transplanting stage; D5: regreening stage.
3.1.2 Dry matter weight
The comparison of the effects of cold-resistant agents revealed the dry matter weight at each growth stage was highest for C3, followed by C2 and then C1 (Table 1). More specifically, the dry matter weight was significantly higher for C3 than for the other two treatments from the tillering stage to the full heading stage. Moreover, there was a significant difference in the dry matter weight between C3 and C1 in the milky stage and maturity stage. In terms of the spraying times, with the exception of the tillering stage, the dry matter weight was highest for D5, followed by D2. The differences in the dry matter weight between D5 and the other treatments were significant. For the combined treatments, C3D1 resulted in the highest dry matter weight in the tillering stage, whereas C3D5 produced the highest dry matter weight from the booting stage to the maturity stage.
Table 1
| Treatment | Peak tillering stage | Booting stage | Full-heading stage | Milky stage | Maturity stage | |
|---|---|---|---|---|---|---|
| Cold-resistant | C1 | 5.15 ± 0.52c | 16.18 ± 0.44b | 24.99 ± 0.64b | 33.38 ± 0.94b | 40.59 ± 1.25b |
| C2 | 5.50 ± 0.38b | 16.35 ± 0.53b | 25.84 ± 0.50b | 35.37 ± 1.37ab | 42.56 ± 1.17ab | |
| C3 | 5.90 ± 0.69a | 17.42 ± 0.48a | 27.62 ± 1.13a | 37.32 ± 1.22a | 43.87 ± 1.40a | |
| Spraying time | D1 | 5.77 ± 0.34a | 15.95 ± 0.75b | 25.62 ± 0.65b | 34.70 ± 0.93b | 39.72 ± 1.26b |
| D2 | 5.11 ± 0.21b | 17.76 ± 0.66a | 26.76 ± 0.71ab | 36.09 ± 0.97ab | 44.81 ± 1.57a | |
| D3 | 5.75 ± 0.37a | 15.43 ± 0.73b | 26.60 ± 0.57ab | 34.42 ± 1.21b | 40.53 ± 1.18b | |
| D4 | 5.45 ± 0.29ab | 15.76 ± 0.52b | 24.57 ± 0.40c | 33.94 ± 1.14b | 40.74 ± 1.09b | |
| D5 | 5.60 ± 0.26a | 18.36 ± 0.91a | 27.21 ± 0.83a | 38.12 ± 1.05a | 45.06 ± 1.49a | |
| Cold-resistant× Spraying time |
C1D1 | 5.40 ± 0.18cd | 16.00 ± 0.51cd | 24.32 ± 0.38de | 32.04 ± 0.95e | 33.66 ± 0.91f |
| C1D2 | 4.32 ± 0.17f | 18.65 ± 0.77a | 25.42 ± 0.59cd | 30.06 ± 0.62f | 43.96 ± 1.13bc | |
| C1D3 | 5.75 ± 0.25c | 13.81 ± 0.48e | 26.61 ± 0.51bc | 34.24 ± 0.83cd | 42.78 ± 1.03bc | |
| C1D4 | 5.02 ± 0.15e | 13.83 ± 0.32e | 23.87 ± 0.55e | 35.77 ± 1.07bc | 39.27 ± 0.89e | |
| C1D5 | 5.36 ± 0.21d | 18.20 ± 0.65ab | 24.75 ± 0.67de | 34.79 ± 1.09bcd | 42.91 ± 1.07bc | |
| C2D1 | 4.31 ± 0.12f | 16.76 ± 0.42bc | 24.89 ± 0.31d | 36.35 ± 1.14b | 39.03 ± 0.94e | |
| C2D2 | 5.45 ± 0.17cd | 15.83 ± 0.38d | 26.53 ± 0.47bc | 35.90 ± 1.03bc | 44.19 ± 1.18b | |
| C2D3 | 5.70 ± 0.22c | 15.15 ± 0.35d | 25.76 ± 0.44cd | 33.55 ± 0.89de | 40.95 ± 0.81d | |
| C2D4 | 6.25 ± 0.31b | 15.68 ± 0.40d | 23.80 ± 0.56e | 30.56 ± 0.47f | 40.94 ± 0.75d | |
| C2D5 | 5.92 ± 0.27bc | 18.35 ± 0.83ab | 28.03 ± 0.91a | 35.87 ± 1.31bc | 46.19 ± 1.14a | |
| C3D1 | 7.61 ± 0.43a | 15.09 ± 0.51d | 27.65 ± 0.65ab | 35.02 ± 1.24bcd | 45.67 ± 1.03ab | |
| C3D2 | 5.57 ± 0.19c | 18.41 ± 0.61a | 28.22 ± 0.88a | 43.01 ± 2.37a | 46.52 ± 1.37a | |
| C3D3 | 5.80 ± 0.20bc | 17.35 ± 0.39b | 27.63 ± 0.73ab | 35.46 ± 1.12bc | 37.85 ± 0.99e | |
| C3D4 | 5.10 ± 0.10de | 17.75 ± 0.52ab | 26.03 ± 0.61c | 35.81 ± 1.18bc | 42.01 ± 0.93cd | |
| C3D5 | 5.54 ± 0.14cd | 18.91 ± 0.82a | 28.77 ± 1.06a | 43.40 ± 3.02a | 46.60 ± 1.29a |
Effects of different cold-resistant agents and their spraying times on the early rice dry matter weight (g plant-1).
C1: Uniconazole; C2: Abscisic acid; C3: Zinc-amino acids chelate; D1: Seed soaking stage; D2: One leaf and one heart stage; D3: Two leaves and one heart stage; D4: 7 days before the transplanting stage; D5: regreening stage. For each treatment (C, D, or C × D), different letters in the same column indicate significant differences (P < 0.05).
3.1.3 Leaf area index
The early rice leaf area index (LAI) was significantly higher for C3 than for the other two treatments in each growth stage (Table 2). The comparison of the spraying times indicated there were no significant differences in LAI among treatments in the tillering stage, but LAI was highest and lowest for D5 and D4, respectively, from the booting stage to the milky stage; this difference was significant. For the combined treatments, C3D1 resulted in the highest LAI in the tillering stage, but C3D5 produced the highest LAI from the booting stage to the milky stage. Accordingly, the early rice LAI was highest following the application of ZAC in the regreening stage.
Table 2
| Treatment | Peak tillering stage | Booting stage | Full-heading stage | Milky stage | |
|---|---|---|---|---|---|
| Cold-resistant | C1 | 2.09 ± 0.17b | 5.56 ± 0.15b | 4.54 ± 0.18b | 3.82 ± 0.22b |
| C2 | 2.24 ± 0.20b | 5.62 ± 0.18b | 4.74 ± 0.15b | 3.76 ± 0.26b | |
| C3 | 2.76 ± 0.29a | 6.13 ± 0.22a | 5.27 ± 0.23a | 4.47 ± 0.31a | |
| Spraying time | D1 | 2.42 ± 0.22a | 5.63 ± 0.28bc | 5.10 ± 0.23a | 3.81 ± 0.16c |
| D2 | 2.49 ± 0.19a | 5.53 ± 0.33bc | 4.83 ± 0.19ab | 4.26 ± 0.19ab | |
| D3 | 2.60 ± 0.27a | 5.79 ± 0.25b | 4.98 ± 0.20a | 4.17 ± 0.11b | |
| D4 | 2.35 ± 0.21a | 5.26 ± 0.19c | 4.60 ± 0.16b | 3.77 ± 0.20c | |
| D5 | 2.25 ± 0.24a | 6.55 ± 0.41a | 5.04 ± 0.25a | 4.46 ± 0.17a | |
| Cold-resistant × Spraying time |
C1D1 | 2.34 ± 0.19de | 6.04 ± 0.14b | 4.83 ± 0.20cd | 3.46 ± 0.14d |
| C1D2 | 1.91 ± 0.15e | 4.97 ± 0.13e | 4.73 ± 0.11de | 3.81 ± 0.19c | |
| C1D3 | 2.27 ± 0.23de | 5.32 ± 0.20d | 4.68 ± 0.15de | 4.22 ± 0.18b | |
| C1D4 | 2.04 ± 0.14e | 5.39 ± 0.11d | 4.26 ± 0.17f | 3.72 ± 0.12c | |
| C1D5 | 2.10 ± 0.20e | 6.10 ± 0.15b | 4.47 ± 0.18ef | 4.30 ± 0.15ab | |
| C2D1 | 1.64 ± 0.11f | 5.27 ± 0.15d | 4.42 ± 0.13ef | 3.51 ± 0.17d | |
| C2D2 | 2.78 ± 0.21bc | 5.28 ± 0.15d | 4.80 ± 0.22d | 4.33 ± 0.20ab | |
| C2D3 | 2.51 ± 0.21cd | 5.57 ± 0.12cd | 5.10 ± 0.16bc | 3.71 ± 0.25c | |
| C2D4 | 2.47 ± 0.17cd | 5.50 ± 0.17cd | 4.48 ± 0.12ef | 3.54 ± 0.19c | |
| C2D5 | 2.10 ± 0.22e | 6.48 ± 0.21a | 5.22 ± 0.17b | 4.10 ± 0.14b | |
| C3D1 | 3.28 ± 0.26a | 5.57 ± 0.22cd | 5.25 ± 0.19b | 4.46 ± 0.19a | |
| C3D2 | 2.68 ± 0.15cd | 6.49 ± 0.19a | 5.55 ± 0.25ab | 4.64 ± 0.24a | |
| C3D3 | 3.03 ± 0.16ab | 5.85 ± 0.26bc | 5.18 ± 0.15b | 4.57 ± 0.17a | |
| C3D4 | 2.56 ± 0.26cd | 6.08 ± 0.17b | 5.06 ± 0.18bc | 4.06 ± 0.22b | |
| C3D5 | 2.55 ± 0.24cd | 6.65 ± 0.29a | 5.72 ± 0.26a | 4.89 ± 0.23a |
Effects of different cold-resistant agents and their spraying times on the early rice LAI.
C1: Uniconazole; C2: Abscisic acid; C3: ZincAmino Acids Chelate; D1: Seed soaking stage; D2: One leaf and one heart stage; D3: Two leaves and one heart stage; D4: 7 days before the transplanting stage; D5: regreening stage. For each treatment (C, D, or C × D), different letters in the same column indicate significant differences (P < 0.05).
3.1.4 Yield and related components
The rice yield was significantly higher for C3 than for C1 and C2 (i.e., 9.22% and 7.14% higher, respectively; Table 3). In terms of the yield-related components, the number of grains per panicle and seed setting rate were significantly higher for C3 than for C1 and C2. For the spraying time treatments, D5 produced the highest rice yield, followed by D2 and D1. The rice yield was lowest for D3. Notably, the rice yields for D2 and D5 were significantly higher than those for D1 and D3. In terms of the yield-related components, there were no significant differences in the effective panicle number and thousand-grain weight between treatments. The number of grains per panicle was significantly higher for D2, D4, and D5 than for D1 and D3. The seed setting rate was significantly lower for D3 than for the other treatments. Among the combined treatments, C3D5 and C1D1 resulted in the highest and lowest early rice yields, respectively. The number of grains per panicle and seed setting rate were significantly higher for C3D5 than for the other treatments, except for C3D2.
Table 3
| Treatment | Effective panicle (×104 ha-1) |
Grains per panicle |
Seed setting rate (%) | Thousand -grain weight (g) |
Theoretical Yield (t ha-1) |
Actual yield (t ha-1) | |
|---|---|---|---|---|---|---|---|
| Cold-resistant | C1 | 251.84 ± 9.22a | 109.16 ± 1.87b | 66.52 ± 2.04b | 24.06 ± 1.67a | 4.40 ± 0.21b | 4.12 ± 0.15b |
| C2 | 263.36 ± 11.76a | 107.02 ± 2.05b | 64.84 ± 2.27b | 24.15 ± 2.33a | 4.43 ± 0.19b | 4.20 ± 0.11b | |
| C3 | 250.84 ± 7.31a | 113.46 ± 2.27a | 70.93 ± 2.35a | 24.17 ± 1.73a | 4.89 ± 0.24a | 4.50 ± 0.17a | |
| Spraying time | D1 | 252.10 ± 8.65a | 104.80 ± 2.46b | 68.12 ± 1.87a | 24.22 ± 1.93a | 4.36 ± 0.17b | 4.07 ± 0.12b |
| D2 | 254.60 ± 10.09a | 111.83 ± 2.86a | 67.47 ± 1.29a | 24.06 ± 2.08a | 4.78 ± 0.21a | 4.41 ± 0.21a | |
| D3 | 259.50 ± 10.59a | 107.13 ± 2.05b | 64.88 ± 1.15b | 24.16 ± 2.31a | 4.37 ± 0.20b | 4.07 ± 0.10b | |
| D4 | 249.60 ± 7.91a | 113.13 ± 3.38a | 67.71 ± 1.44a | 24.20 ± 1.87a | 4.63 ± 0.24ab | 4.35 ± 0.17ab | |
| D5 | 260.93 ± 11.48a | 112.50 ± 3.10a | 68.97 ± 2.21a | 23.99 ± 2.15a | 4.85 ± 0.27a | 4.51 ± 0.26a | |
| Cold-resistant× Spraying time |
C1D1 | 231.80 ± 11.24c | 106.20 ± 4.31cd | 62.09 ± 3.17def | 24.38 ± 1.68a | 3.73 ± 0.13f | 3.42 ± 0.27e |
| C1D2 | 266.43 ± 10.93ab | 111.48 ± 3.66bc | 70.62 ± 4.75bc | 23.75 ± 2.04a | 4.97 ± 0.22b | 4.81 ± 0.19b | |
| C1D3 | 254.48 ± 7.15bc | 115.58 ± 3.04b | 66.83 ± 4.07cde | 24.10 ± 1.47a | 4.73 ± 0.20bc | 4.50 ± 0.15bc | |
| C1D4 | 232.40 ± 7.09c | 111.34 ± 5.84bc | 64.10 ± 2.89de | 23.33 ± 3.08a | 4.03 ± 0.17e | 3.73 ± 0.17de | |
| C1D5 | 275.19 ± 12.16a | 101.40 ± 5.04de | 68.95 ± 2.24cd | 23.75 ± 2.59a | 4.57 ± 0.11c | 4.18 ± 0.16d | |
| C2D1 | 266.46 ± 11.57ab | 111.96 ± 4.61bc | 69.62 ± 3.46bc | 23.63 ± 1.61a | 4.90 ± 0.30b | 4.73 ± 0.20b | |
| C2D2 | 243.28 ± 10.54c | 101.57 ± 4.49de | 57.10 ± 3.12f | 24.08 ± 1.38a | 3.40 ± 0.14g | 3.19 ± 0.10f | |
| C2D3 | 272.65 ± 9.05a | 104.56 ± 6.10cde | 66.25 ± 3.81cde | 23.54 ± 1.95a | 4.62 ± 0.18c | 4.31 ± 0.11c | |
| C2D4 | 272.13 ± 10.27a | 106.20 ± 5.04cd | 70.71 ± 2.59bc | 24.41 ± 2.23a | 4.99 ± 0.25b | 4.75 ± 0.17b | |
| C2D5 | 263.77 ± 7.35ab | 111.05 ± 4.65bc | 60.52 ± 4.09ef | 24.10 ± 3.20a | 4.26 ± 0.24de | 4.05 ± 0.14d | |
| C3D1 | 258.50 ± 8.17abc | 96.36 ± 2.81e | 72.66 ± 4.20abc | 24.65 ± 2.81a | 4.46 ± 0.23cd | 3.96 ± 0.18d | |
| C3D2 | 254.27 ± 9.68bc | 122.69 ± 4.28a | 74.69 ± 4.14ab | 24.36 ± 3.05a | 5.67 ± 0.38a | 5.22 ± 0.21a | |
| C3D3 | 252.53 ± 11.31bc | 101.40 ± 5.04de | 61.57 ± 2.76ef | 23.84 ± 1.43a | 3.76 ± 0.10f | 3.49 ± 0.20e | |
| C3D4 | 244.41 ± 9.25c | 121.93 ± 3.35a | 68.31 ± 2.42cd | 23.86 ± 1.96a | 4.86 ± 0.21b | 4.57 ± 0.14b | |
| C3D5 | 244.83 ± 10.40c | 125.10 ± 6.44a | 77.44 ± 3.60a | 24.13 ± 3.21a | 5.72 ± 0.32a | 5.29 ± 0.27a |
Effects of different cold-resistant agents and their spraying times on the early rice yield and related components.
C1: Uniconazole; C2: Abscisic acid; C3: Zinc-amino acids chelate; D1: Seed soaking stage; D2: One leaf and one heart stage; D3: Two leaves and one heart stage; D4: 7 days before the transplanting stage; D5: regreening stage. For each treatment (C, D, or C × D), different letters in the same column indicate significant differences (P < 0.05).
3.1.5 Rice leaf cold resistance characteristics
The CAT, SOD, and POD activities were highest for C3, followed by C2 and then C1, with significant differences between treatments (Table 4). Additionally, the CAT, SOD, and POD activities were highest for D5, followed by D2. The activities of these three enzymes were significantly lower for the other treatments. For the combined treatments, the CAT, SOD, and POD activities were highest and lowest for C3D5 and C1D3, respectively, with significant differences among treatments. Thus, the antioxidant capacity of leaves peaked when ZAC was applied at the regreening stage.
Table 4
| Treatment | CAT activity [U·(g·min)-1] |
SOD activity (U g-1) |
POD activity [U·(g·min)-1] |
|
|---|---|---|---|---|
| Cold-resistant | C1 | 103.25 ± 5.97c | 105.19 ± 5.01c | 123.64 ± 7.46c |
| C2 | 115.68 ± 4.57b | 146.10 ± 5.74b | 156.17 ± 8.09b | |
| C3 | 128.49 ± 7.13a | 158.54 ± 7.51a | 181.41 ± 11.17a | |
| Spraying time | D1 | 103.37 ± 6.70c | 116.10 ± 4.03c | 143.19 ± 8.22b |
| D2 | 129.54 ± 4.94a | 157.68 ± 10.26a | 160.59 ± 5.21a | |
| D3 | 93.66 ± 3.72d | 123.50 ± 3.15b | 138.83 ± 9.07b | |
| D4 | 119.59 ± 5.65b | 125.69 ± 5.28b | 146.26 ± 8.39b | |
| D5 | 132.91 ± 7.28a | 160.06 ± 8.17a | 179.84 ± 10.11a | |
| Cold-resistant× Spraying time |
C1D1 | 90.79 ± 4.68g | 98.67 ± 5.27f | 112.40 ± 5.57f |
| C1D2 | 119.19 ± 4.66d | 117.02 ± 9.12e | 129.13 ± 9.03e | |
| C1D3 | 87.89 ± 5.65g | 93.24 ± 6.08f | 106.03 ± 7.13f | |
| C1D4 | 107.19 ± 7.35ef | 95.87 ± 4.16f | 115.88 ± 4.21f | |
| C1D5 | 111.19 ± 4.46def | 121.14 ± 7.37e | 154.78 ± 8.72d | |
| C2D1 | 107.29 ± 5.65ef | 119.19 ± 8.41e | 138.78 ± 7.83e | |
| C2D2 | 128.90 ± 6.41c | 176.97 ± 7.24ab | 164.72 ± 9.86cd | |
| C2D3 | 89.13 ± 4.73g | 132.48 ± 7.10de | 133.51 ± 9.29e | |
| C2D4 | 115.83 ± 6.34de | 128.74 ± 5.04de | 161.12 ± 7.61cd | |
| C2D5 | 137.29 ± 5.26bc | 173.10 ± 4.66b | 182.72 ± 8.17b | |
| C3D1 | 112.02 ± 4.52de | 130.43 ± 5.78de | 178.38 ± 9.87bc | |
| C3D2 | 140.53 ± 5.78ab | 179.05 ± 5.62ab | 191.51 ± 10.36ab | |
| C3D3 | 103.95 ± 3.79f | 144.78 ± 6.68cd | 176.94 ± 7.06bc | |
| C3D4 | 135.74 ± 7.61bc | 152.47 ± 8.79c | 158.19 ± 10.41d | |
| C3D5 | 150.25 ± 7.40a | 185.95 ± 8.57a | 202.01 ± 11.09a |
Effects of different cold-resistant agents and their spraying times on the early rice leaf antioxidant system.
C1: Uniconazole; C2: Abscisic acid; C3: Zinc-amino acids chelate; D1: Seed soaking stage; D2: One leaf and one heart stage; D3: Two leaves and one heart stage; D4: 7 days before the transplanting stage; D5: regreening stage. For each treatment (C, D, or C × D), different letters in the same column indicate significant differences (P < 0.05).
3.2 Analysis of zinc-amino acids chelate application methods
3.2.1 Rice tillering dynamics
The number of tillers increased up to the peak tillering stage and then decreased (Figure 2). Compared with CK, the ZAC treatment increased the number of tillers. More specifically, in the peak tillering period, the number of tillers was higher for B1 and B2 (16.39 and 16.40 tillers per plant, respectively) than for CK (14.40 tillers per plant). The comparison of spraying times indicated A4 resulted in the highest number of tillers in each stage. In the peak tillering stage, the number of tillers was higher for A4 (16.93 tillers per plant) than for A2, A1, and A3 (16.72, 16.32, and 15.62 tillers per plant, respectively). In response to the combined treatments, the number of tillers in each stage was highest for A4B2, followed by A4B1, with up to 17.30 and 16.59 tillers per plant, respectively, in the peak tillering stage. Thus, spraying once with ZAC in the transplanting and regreening stages may be optimal for increasing the number of tillers, but there were no significant differences in the number of tillers between the tested dosages.
Figure 2
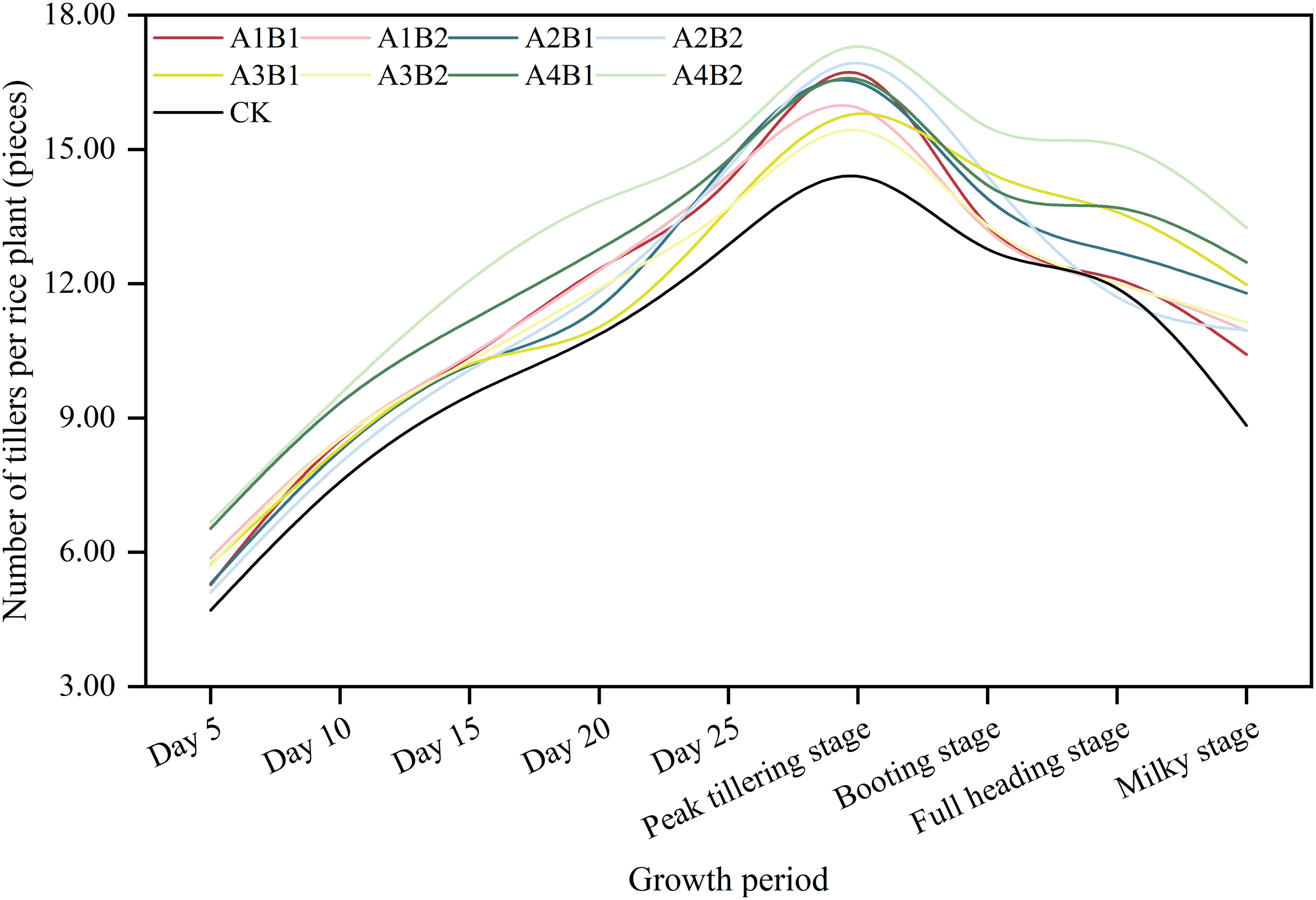
Effects of different zinc-amino acids chelate spraying amounts and times on early rice tillering dynamics. CK: control treatment; A1: 7 days before the transplanting stage; A2: Transplanting stage; A3: Regreening stage; A4: Transplanting stage and regreening stage; B1: 750 g ha−1; B2: 1125 g ha−1.
3.2.2 Dry matter weight
Rice yield is influenced by the production and accumulation of specific substances. Spraying with ZAC increased the dry matter weight in the later stages (Table 5). In the milky and maturity stages, the dry matter weight was higher for B1 and B2 than for CK, but there was no significant difference between B1 and B2. In terms of the spraying time, there were no significant differences in the dry matter weight between the treatments at the peak tillering stage and booting stage. The dry matter weights from the full-heading stage to the maturity stage were highest for A4, followed by A3, A2, and A1. The dry matter weight for A4 was significantly higher than that for A2 and A1. For the combined treatments, there were no significant differences in the dry matter weight between treatments at the peak tillering stage and booting stage, but the differences between treatments increased significantly from the full-heading stage to the later growth stages. The dry matter weight from the full-heading stage to the maturity stage was highest for A4B2.
Table 5
| Treatment | Peak tillering stage | Booting stage | Full-heading stage | Milky stage | Maturity stage | |
|---|---|---|---|---|---|---|
| Spraying time | A1 | 8.80 ± 1.41a | 19.88 ± 1.17a | 28.29 ± 0.81b | 34.28 ± 1.55b | 42.54 ± 1.62c |
| A2 | 7.89 ± 1.27a | 18.81 ± 1.27a | 29.82 ± 0.69b | 36.85 ± 1.78b | 46.21 ± 2.02b | |
| A3 | 7.37 ± 0.83a | 19.55 ± 0.94a | 30.26 ± 1.13ab | 41.15 ± 2.12a | 48.47 ± 2.14ab | |
| A4 | 7.70 ± 1.30a | 20.33 ± 2.41a | 31.54 ± 1.02a | 41.26 ± 2.04a | 51.10 ± 2.59a | |
| Spraying dosage | B1 | 7.74 ± 0.76a | 20.10 ± 1.61a | 29.72 ± 0.57a | 38.19 ± 1.79a | 46.30 ± 2.38a |
| B2 | 8.14 ± 1.68a | 19.19 ± 1.07a | 29.96 ± 1.21a | 38.98 ± 2.26a | 47.85 ± 2.11a | |
| Spraying time × Spraying dosage |
CK | 7.03 ± 0.97a | 18.15 ± 0.89a | 28.13 ± 1.33b | 31.31 ± 1.49c | 41.93 ± 2.05c |
| A1B1 | 9.28 ± 1.43a | 20.84 ± 1.68a | 28.22 ± 1.06b | 34.03 ± 2.16bc | 42.45 ± 1.57bc | |
| A1B2 | 8.31 ± 1.55a | 18.91 ± 0.72a | 28.36 ± 0.89b | 34.52 ± 1.73bc | 42.63 ± 1.69bc | |
| A2B1 | 6.88 ± 2.19a | 19.54 ± 0.80a | 28.84 ± 1.12b | 36.44 ± 2.01b | 45.66 ± 2.31b | |
| A2B2 | 8.90 ± 1.36a | 18.08 ± 1.10a | 30.83 ± 1.45ab | 37.25 ± 1.67b | 46.75 ± 2.15b | |
| A3B1 | 7.45 ± 0.82a | 20.54 ± 1.08a | 30.49 ± 1.26ab | 41.28 ± 1.93a | 48.62 ± 1.93ab | |
| A3B2 | 7.28 ± 1.40a | 18.56 ± 2.23a | 30.03 ± 0.91ab | 41.02 ± 2.06a | 48.31 ± 2.10ab | |
| A4B1 | 7.34 ± 2.14a | 19.47 ± 2.52a | 31.04 ± 1.05a | 41.21 ± 1.70a | 48.48 ± 1.87ab | |
| A4B2 | 8.06 ± 2.25a | 21.19 ± 1.58a | 32.03 ± 1.83a | 41.31 ± 2.12a | 53.72 ± 3.04a |
Effects of different ZAC spraying amounts and times on the dry matter weight of early rice (g plant-1).
CK: control treatment; A1: 7 days before the transplanting stage; A2: Transplanting stage; A3: Regreening stage; A4: Transplanting stage and regreening stage; B1: 750 g ha−1; B2: 1125 g ha−1. For each treatment (A, B, or A × B), different letters in the same column indicate significant differences (P < 0.05).
3.2.3 Leaf area index
Spraying with ZAC resulted in an increase in the LAI early rice, but there was no significant difference between B1 and B2 (Table 6). There were also no significant differences in LAI in the peak tillering stage among the spraying times. From the booting stage to the maturity stage, LAI was highest for A4, followed by A2, A3, and A1. Of these treatments, A4 and A2 resulted in a significantly higher LAI than A3 and A1. Among the combined treatments, A4B2 and A4B1 resulted in the highest LAI in each stage (the CK LAI was significantly lower).
Table 6
| Treatment | Peak tillering stage | Booting stage | Full-heading stage | Milky stage | |
|---|---|---|---|---|---|
| Spraying time | A1 | 0.99 ± 0.16a | 3.74 ± 0.22b | 3.25 ± 0.18c | 2.63 ± 0.17b |
| A2 | 1.03 ± 0.10a | 4.58 ± 0.29a | 4.24 ± 0.31a | 3.32 ± 0.28a | |
| A3 | 0.97 ± 0.08a | 3.87 ± 0.40b | 3.67 ± 0.22b | 2.83 ± 0.20b | |
| A4 | 1.08 ± 0.13a | 4.89 ± 0.37a | 4.43 ± 0.39a | 3.56 ± 0.24a | |
| Spraying dosage | B1 | 0.99 ± 0.11a | 4.21 ± 0.31a | 3.80 ± 0.28a | 3.02 ± 0.19a |
| B2 | 1.04 ± 0.14a | 4.33 ± 0.28a | 4.00 ± 0.20a | 3.15 ± 0.26a | |
| Spraying time× Spraying dosage |
CK | 0.89 ± 0.09b | 3.58 ± 0.15d | 3.25 ± 0.15e | 2.62 ± 0.16cd |
| A1B1 | 0.97 ± 0.11ab | 3.64 ± 0.12d | 3.28 ± 0.12e | 2.40 ± 0.13d | |
| A1B2 | 1.01 ± 0.13ab | 3.83 ± 0.27cd | 3.21 ± 0.11e | 2.78 ± 0.16c | |
| A2B1 | 0.98 ± 0.08ab | 4.71 ± 0.30ab | 4.03 ± 0.16c | 3.26 ± 0.18ab | |
| A2B2 | 1.08 ± 0.09a | 4.43 ± 0.21b | 4.44 ± 0.20ab | 3.37 ± 0.26a | |
| A3B1 | 0.94 ± 0.12ab | 3.72 ± 0.17cd | 3.72 ± 0.14d | 2.76 ± 0.22c | |
| A3B2 | 0.99 ± 0.10ab | 4.02 ± 0.19c | 3.61 ± 0.20d | 2.90 ± 0.21bc | |
| A4B1 | 1.08 ± 0.07a | 4.76 ± 0.26ab | 4.15 ± 0.19bc | 3.53 ± 0.33a | |
| A4B2 | 1.09 ± 0.12a | 5.02 ± 0.34a | 4.71 ± 0.33a | 3.56 ± 0.25a |
Effects of different ZAC spraying amounts and times on the early rice LAI.
CK: control treatment; A1: 7 days before the transplanting stage; A2: Transplanting stage; A3: Regreening stage; A4: Transplanting stage and regreening stage; B1: 750 g ha−1; B2: 1125 g ha−1. For each treatment (A, B, or A × B), different letters in the same column indicate significant differences (P < 0.05).
3.2.4 Yield and related components
The ZAC spray treatments increased the rice yield to some extent (Table 7), but the different dosages had no significant effect on yield. Compared with CK, B1 and B2 resulted in yield increases of 4.28% and 5.99%, respectively. Analyses of the yield components indicated the number of grains per panicle was slightly higher for B1 and B2 than for CK. Among the spraying times, A4 resulted in the highest rice yield, followed by A2, A3, and A1; the difference between A4 and A1 was significant. The rice yield for A4 was 9.54%, 3.67%, and 5.53% higher than that for A1, A2, and A3, respectively. In terms of the yield-related components, there were no significant differences in the number of grains per panicle, seed setting rate, and thousand-grain weight among treatments. The effective panicle number was highest for A4, followed by A2, A1, and A3; the difference between A4 and A1 was significant. For the combined treatments, the actual yield of rice was the highest in A4B2 and A4B1, and the lowest in A1B1, which were significantly higher than A1B1 by 11.40% and 9.89% respectively. The rice yields for A4B1 and A4B2 were 11.82% and 13.35% higher than that for CK, respectively. Both A4B1 and A4B2 mainly increased the effective panicle number (compared with the other treatments).
Table 7
| Treatment | Effective panicle (×104 ha-1) |
Grains per panicle |
Seed setting rate (%) | Thousand-grain weight (g) |
Theoretical Yield (t ha-1) |
Actual yield (t ha-1) |
|
|---|---|---|---|---|---|---|---|
| Spraying time | A1 | 249.17 ± 10.59b | 129.46 ± 5.59a | 64.02 ± 3.86a | 23.88 ± 1.41a | 4.90 ± 0.19b | 4.70 ± 0.14b |
| A2 | 267.51 ± 13.36ab | 122.23 ± 3.47a | 68.27 ± 2.69a | 23.40 ± 2.83a | 5.20 ± 0.22ab | 4.91 ± 0.19ab | |
| A3 | 254.46 ± 11.28ab | 126.78 ± 4.10a | 67.00 ± 4.11a | 23.73 ± 1.39a | 5.13 ± 0.15ab | 4.88 ± 0.25ab | |
| A4 | 281.25 ± 15.12a | 121.55 ± 4.16a | 66.39 ± 3.36a | 23.84 ± 3.07a | 5.40 ± 0.28a | 5.15 ± 0.31a | |
| Spraying dosage | B1 | 265.17 ± 9.13a | 123.25 ± 5.04a | 65.69 ± 3.24a | 23.79 ± 2.80a | 5.08 ± 0.17a | 4.87 ± 0.23a |
| B2 | 260.92 ± 12.81a | 126.76 ± 4.29a | 67.15 ± 5.08a | 23.63 ± 1.45a | 5.23 ± 0.26a | 4.95 ± 0.30a | |
| Spraying time × Spraying dosage |
CK | 257.29 ± 11.14bc | 116.99 ± 3.46c | 66.24 ± 2.45abc | 23.60 ± 2.74a | 4.72 ± 0.20c | 4.57 ± 0.17c |
| A1B1 | 267.46 ± 9.52ab | 124.47 ± 3.51bc | 61.23 ± 2.52c | 23.97 ± 1.24a | 4.85 ± 0.17c | 4.65 ± 0.21c | |
| A1B2 | 231.37 ± 12.09c | 134.45 ± 5.32a | 66.80 ± 2.69ab | 23.78 ± 2.55a | 4.94 ± 0.21bc | 4.75 ± 0.16bc | |
| A2B1 | 261.06 ± 10.31b | 119.81 ± 3.77bc | 70.01 ± 3.61a | 23.41 ± 2.43a | 5.11 ± 0.15abc | 4.87 ± 0.22abc | |
| A2B2 | 274.93 ± 13.28ab | 124.64 ± 4.24bc | 66.52 ± 3.19abc | 23.39 ± 1.25a | 5.29 ± 0.23abc | 4.96 ± 0.30abc | |
| A3B1 | 260.45 ± 9.18b | 127.16 ± 5.56ab | 64.33 ± 2.04bc | 23.88 ± 3.36a | 5.06 ± 0.19abc | 4.81 ± 0.24abc | |
| A3B2 | 249.68 ± 13.56bc | 126.40 ± 4.26ab | 69.67 ± 3.27a | 23.57 ± 2.61a | 5.20 ± 0.24abc | 4.93 ± 0.19abc | |
| A4B1 | 272.57 ± 15.26ab | 121.56 ± 5.27bc | 67.18 ± 3.03ab | 23.89 ± 1.77a | 5.31 ± 0.26ab | 5.11 ± 0.21ab | |
| A4B2 | 289.23 ± 17.35a | 121.53 ± 4.69bc | 65.59 ± 2.28abc | 23.78 ± 1.95a | 5.49 ± 0.34a | 5.18 ± 0.25a |
Effects of different ZAC spraying amounts and times on the early rice yield and related components.
CK: control treatment; A1: 7 days before the transplanting stage; A2: Transplanting stage; A3: Regreening stage; A4: Transplanting stage and regreening stage; B1: 750 g ha−1; B2: 1125 g ha−1. For each treatment (A, B, or A × B), different letters in the same column indicate significant differences (P < 0.05).
3.2.5 Rice leaf cold resistance characteristics
Compared with the CK treatment, spraying with ZAC significantly increased the rice leaf antioxidant capacity in each stage (Table 8). For the combined treatments, the activities of SOD, POD and CAT in leaf at each stage were highest in A4B2 and A4B1, and lowest in A1B2 and A1B1. There were no significant differences between the spray dosages. Except for CAT activity, there were significant differences in SOD and POD activities from peak tillering stage to milky stage among the spraying times (A1, A2, A3, and A4).
Table 8
| Enzyme | Treatment | 7 days after the regreening stage |
Peak tillering stage | Booting stage | Milky stage |
|---|---|---|---|---|---|
| SOD (U g-1) |
CK | 74.73 ± 5.57d | 81.32 ± 4.93e | 92.82 ± 6.83e | 59.11 ± 4.34d |
| A1B1 | 103.28 ± 7.26c | 116.01 ± 6.19d | 121.41 ± 10.56d | 83.63 ± 8.05c | |
| A1B2 | 107.29 ± 9.28c | 125.43 ± 7.55d | 126.43 ± 8.10d | 80.20 ± 7.59c | |
| A2B1 | 150.59 ± 12.90b | 180.43 ± 10.11b | 206.42 ± 10.60b | 111.13 ± 8.32b | |
| A2B2 | 179.11 ± 10.26b | 185.21 ± 11.13b | 211.31 ± 9.49b | 113.70 ± 9.46b | |
| A3B1 | 160.64 ± 9.06b | 157.79 ± 9.87c | 174.43 ± 10.25c | 98.50 ± 10.20b | |
| A3B2 | 157.75 ± 12.39b | 155.63 ± 7.41c | 180.41 ± 8.21c | 101.30 ± 8.81b | |
| A4B1 | 204.01 ± 14.17a | 215.18 ± 18.38a | 232.32 ± 13.18a | 143.53 ± 14.12a | |
| A4B2 | 215.42 ± 18.42a | 220.77 ± 15.79a | 236.42 ± 11.64a | 157.35 ± 10.44a | |
| POD [U·(g·min)-1] |
CK | 83.32 ± 8.41c | 98.32 ± 9.46e | 143.73 ± 6.14e | 84.68 ± 5.13e |
| A1B1 | 150.05 ± 10.39b | 160.03 ± 11.84d | 188.22 ± 5.58d | 109.61 ± 7.51d | |
| A1B2 | 155.78 ± 11.09b | 167.68 ± 8.75d | 184.58 ± 8.20d | 106.72 ± 7.26d | |
| A2B1 | 166.03 ± 13.36b | 204.10 ± 9.89b | 229.55 ± 9.78b | 145.23 ± 9.68b | |
| A2B2 | 176.25 ± 10.74b | 209.60 ± 7.96b | 231.94 ± 8.90b | 150.03 ± 8.76b | |
| A3B1 | 171.09 ± 12.34b | 184.00 ± 7.38c | 208.11 ± 10.32c | 122.27 ± 7.98c | |
| A3B2 | 160.17 ± 16.29b | 185.91 ± 8.12c | 209.88 ± 7.05c | 125.67 ± 10.06c | |
| A4B1 | 208.47 ± 15.90a | 226.88 ± 9.07a | 250.68 ± 9.27a | 168.49 ± 11.25a | |
| A4B2 | 200.69 ± 13.61a | 229.21 ± 12.70a | 253.99 ± 12.85a | 167.34 ± 9.33a | |
| CAT [U·(g·min)-1] |
CK | 90.64 ± 6.48d | 95.63 ± 6.11d | 125.63 ± 7.26c | 62.39 ± 4.31e |
| A1B1 | 112.02 ± 4.35c | 128.75 ± 6.93c | 189.75 ± 10.79b | 78.30 ± 3.09d | |
| A1B2 | 103.82 ± 5.18c | 129.52 ± 7.42c | 189.52 ± 9.21b | 80.22 ± 6.11d | |
| A2B1 | 127.05 ± 7.63b | 146.20 ± 8.95b | 196.00 ± 6.08b | 108.36 ± 6.17b | |
| A2B2 | 125.69 ± 6.10b | 148.67 ± 6.76b | 198.67 ± 9.26b | 112.10 ± 5.64b | |
| A3B1 | 133.62 ± 7.54b | 146.37 ± 4.16b | 196.37 ± 10.15b | 87.54 ± 4.52c | |
| A3B2 | 131.42 ± 9.09b | 147.70 ± 8.71b | 197.70 ± 8.43b | 90.61 ± 5.26c | |
| A4B1 | 153.43 ± 8.23a | 163.11 ± 7.60a | 240.17 ± 13.06a | 127.85 ± 7.47a | |
| A4B2 | 151.69 ± 10.58a | 165.27 ± 10.53a | 245.09 ± 11.55a | 129.23 ± 7.02a |
Effects of different ZAC spraying amounts and times on the early rice antioxidant enzyme activities.
CK: control treatment; A1: 7 days before the transplanting stage; A2: Transplanting stage; A3: Regreening stage; A4: Transplanting stage and regreening stage; B1: 750 g ha−1; B2: 1125 g ha−1. For each antioxidant enzyme (SOD, POD, and CAT), different letters in the same column indicate significant differences (P < 0.05).
4 Discussion
4.1 Screening of suitable cold-resistant agents for machine-transplanted early rice
Low temperatures seriously affect plant growth and threaten global food security. The application of foliar fertilizers can regulate various plant physiological processes and alleviate the harmful effects of adverse environmental conditions. Foliar fertilizers are quickly absorbed by plants, even at low dosages (i.e., high efficiency). Hence, they are commonly applied to increase crop quality and stress resistance. Compared with simple foliar fertilizers, compound foliar fertilizers provide plants with more diverse nutrients and plant growth-regulating substances (Niu et al., 2021). In the current study, the rice yield following the ZAC treatment was 9.22% and 7.14% higher than the rice yield resulting from the uniconazole and abscisic acid treatments, respectively. The comparison of spraying times revealed the spray application of foliar fertilizer during the regreening stage produced the highest early rice yield. Analyzing the yield related components, the ZAC treatment during the regreening stage significantly increased the number of grains per panicle and the seed setting rate of early rice, ultimately leading to the largest increase in yield.
Rice yield is mainly influenced by rice tillering, coordinated sink–source relationships, and the accumulation of dry matter. In this study, the number of tillers and dry matter weight were highest in each stage following the ZAC treatment. Moreover, the application of ZAC resulted in the highest LAI. In terms of the spraying time, the regreening stage was best for enhancing various yield-related parameters. Recent research showed that zinc fertilizers primarily improve photosynthetic activities in leaves and the absorption and utilization of nutrients (Prakash et al., 2022). Amino acids are essential nutrients for plant growth and development. It can convert inorganic substances to their organic chelated states, thereby significantly improving nutrient absorption, accumulation, and utilization rates of rice, while also promoting robust plant growth (Xie et al., 2015), ultimately enhancing the grain filling process and decreasing the number of empty grains (Navizaga et al., 2017). Therefore, compared with simple foliar fertilizers, compound foliar fertilizers are more effective for optimizing plant growth and development and increasing rice yields.
Antioxidant enzymes (i.e., CAT, SOD, and POD) are critical for balancing reactive oxygen species accumulation and removal when crops are subjected to stress (Shah and Nahakpam, 2012). They can inhibit membrane lipid peroxidation, minimize the damages to cell membranes caused by active oxygen, and delay cell aging (Xiao et al., 2011). Therefore, antioxidant enzyme activities can reflect the ability of crops to tolerate diverse stresses. Previous study showed that the application of various compounds, including paclobutrazol, abscisic acid, and uniconazole, can increase antioxidant enzyme activities and the production of secondary metabolites in rice (Wang et al., 2023). Additionally, cell membranes may be stabilized and the activities of intracellular substances may increase; these changes can increase the cold stress resistance of rice plants (Baninasab, 2009; Chen et al., 2017; Wu et al., 2023). In the present study, antioxidant enzyme activities were higher in the leaves treated with ZAC than in the leaves treated with the other cold-resistant agents. Accordingly, compared with abscisic acid and uniconazole, ZAC can more effectively maintain antioxidant enzyme activities, with beneficial effects on early rice performance under low-temperature stress conditions. Of the spraying times selected for this study, the treatments during the regreening stage resulted in the highest antioxidant enzyme activities.
Zinc is an activator and co-factor of many enzymes. During plant growth and development, CO2 fixation, biofilm maintenance, and auxin synthesis are all regulated by zinc-containing enzymes (Chantal et al., 2013). In plants, phytic acid and phosphoric acid combine with zinc ions to form insoluble chelates. The adsorption of zinc ions by the cell wall substantially decreases zinc transport and bioavailability in plants (Liu et al., 2023). As a better type of organic chelating agent, amino acids can convert inorganic zinc into its organic chelated state, significantly improving the zinc utilization rate in rice. In addition, amino acid foliar fertilizers are easily absorbed by crops. Notably, they can increase disease resistance and crop quality, which may be related to their positive effects on protective enzyme activities and cell stability (Kitir et al., 2019). Earlier research indicated that spraying plants with amino acid foliar fertilizers can promote leaf physiological activities and root growth and development, increase chlorophyll contents, and significantly improve the quality of vegetative tissues (Shehata et al., 2011). Hence, compared with simple foliar fertilizers, such as abscisic acid and uniconazole, amino acid-based compound foliar fertilizers contain more major nutrients, which can improve crop growth and development and increase stress resistance.
4.2 Zinc-amino acids chelate application methods
The application of cold-resistant agents is important for increasing early rice cold resistance and yield. However, different spraying times and dosages have diverse effects on rice cold resistance and yield. This study preliminarily demonstrated that compared with abscisic acid and uniconazole, ZAC had a better effect on early rice cold resistance and yield. Nevertheless, the ZAC dosage and spraying time may need to be further optimized for commercial rice production. In this study, compared with CK, the foliar spray application of ZAC increased the rice yield. More specifically, the actual yields after the B1 and B2 treatments increased by 4.28% and 5.99%, respectively. These results are consistent with those of earlier studies by Tian et al. (2015) and Song et al. (2008). According to the examination of specific yield-related components, the increase in the rice yield was mainly due to increases in the number of grains per panicle. There were no significant differences in rice yield between the tested dosages. Therefore, to minimize the cost of fertilizer applications, early rice plants should be treated with 750 g ha−1 ZAC (B1). There were significant differences in the early rice yield among ZAC spraying times. The rice yield was highest for A4, with an actual yield that was 9.54%, 3.67%, and 5.53% higher than those of A1, A2, and A3, respectively. The A4 treatment mainly increased the effective panicle number.
These results suggest that multiple applications of ZAC in the early rice growth stage may increase the final rice yield by increasing the effective panicle number. As one of the yield-related components, the effective panicle number depends on the tillering process during the vegetative growth phase, which may be related to the effects of arginine and glutamine on plant growth and development. One of the most significant characteristics of rice in the early vegetative growth stage is its strong tillering ability. Arginine is an essential amino acid for plants, but it also considerably promotes rice cell division (Itoh et al., 2005). Glutamine can effectively induce the accumulation of photosynthetic products in rice leaves, while also converting inorganic carbon to required organic carbohydrates, thereby ensuring the early nutritional needs of rice are met and inhibiting the formation of ineffective tillers (Ohashi et al., 2018).
In this study, compared with CK, the ZAC treatment positively modulated rice tillering and led to increases in LAI and the aboveground dry matter weight. Zinc is an essential element for rice growth. The application of zinc fertilizers will promote the growth and development of rice plants, improve stress resistance, increase the number of tillers, and increase the grain yield (Raza et al., 2023). In the present study, different ZAC application amounts had no significant effects on early rice yield-related characteristics. In contrast, there were differences in specific yield-related traits among the different application times, with A4 revealed as the best treatment. The tiller number, LAI, and dry matter weight at each growth stage increased most significantly. Furthermore, compared with CK, the ZAC treatment significantly increased CAT, SOD, and POD activities. There were no differences in the antioxidant enzyme activities following the B1 and B2 treatments (different dosages), but of the analyzed application times, the A4 treatment resulted in the highest antioxidant enzyme activities.
In the middle and lower reaches of the Yangtze River in China as well as in the South China region, low temperatures in spring (i.e., until the end of April) can affect early rice seedlings, which are transplanted during this period. Therefore, spraying early rice seedlings with cold-resistant agents may positively affect the subsequent plant growth and development. In this study, there were no significant differences in the early rice yield and antioxidant enzyme activities between the ZAC treatment dosages, implying dosages exceeding 750 g ha−1 have no additional beneficial effects. To maximize the effects of ZAC, plants should be sprayed once during the transplanting stage and the regreening stage. Spraying cold-resistant agents during the seedling stage can improve seedling quality by activating stress response mechanisms, leading to increased cold tolerance (Phutdhawong et al., 2014). Because rice seedlings have relatively small leaves, it may be difficult for the seedlings to fully absorb the cold-resistant agent. Hence, excessive or single spray applications may not be ideal. Alternatively, the application of a small amount of compound foliar fertilizer before and after the rice transplanting stage may be more effective for improving cold resistance than a single application of a relatively high fertilizer dosage, but this will need to be experimentally verified.
5 Conclusions
Among the three analyzed cold-resistant agents, ZAC was best for promoting rice tillering and increasing the plant LAI, dry matter weight, antioxidant enzyme activities, cold resistance, and yield. Although different amounts of ZAC had no significant effect on the early rice yield and cold resistance, there were differences among the effects of the tested spraying times. For each dosage, the early rice yield and cold resistance was highest when samples were treated with ZAC once during the transplanting stage and the regreening stage. Therefore, spraying early rice plants with 750 g ha−1 ZAC once during the transplanting stage and the regreening stage may be the ideal treatment for optimizing cold resistance and yield.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The author states that the rice involved in this study do not involve ethical relations. Experimental research on plants, including the collection of plant material, complies with relevant institutional, national, and international guidelines and legislation.
Author contributions
SY: Data curation, Formal analysis, Methodology, Writing – original draft. SQ: Data curation, Formal analysis, Writing – original draft. QS: Data curation, Methodology, Writing – original draft. PC: Methodology, Writing – review & editing. NT: Funding acquisition, Project administration, Resources, Writing – review & editing. WZ: Funding acquisition, Project administration, Supervision, Writing – review & editing. ZY: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was financially supported by the National Key R&D Program of China (2018YFD0301005, 2017YFD0301501, 2023YFD2301400), the Hunan Provincial Natural Science Foundation Project (2022JJ30303, 2023JJ60227), and the Hunan Provincial Department of Agricultural and Rural Affairs Project grant number “XIANG CAI JIAN ZHI” (2023, No. 98).
Acknowledgments
Special thanks to reviewers for their valuable comments. In addition, the authors gratefully acknowledge every teacher, classmate, and friend who helped the authors with their experiment and writing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1422374/full#supplementary-material
References
1
Ai X. Xiong R. Tan X. Wang H. Zhang J. Zeng Y. et al . (2022). Effects of high temperature and strong light combine stress on yield and quality of early indica rice with different amylose content during grout filling. Phyton-International J. Exp. Bot.91, 1257–1267. doi: 10.32604/phyton.2022.018328
2
Baninasab B. (2009). Amelioration of chilling stress by paclobutrazol in watermelon seedlings. Scientia Hortic.121, 144–148. doi: 10.1016/j.scienta.2009.01.028
3
Chantal K. Shao X. Jing B. Yuan Y. Hou M. Liao L. (2013). Effects of effective microorganisms (EM) and bio-organic fertilizers on growth parameters and yield quality of flue-cured tobacco. J. Food Agric. Environ.11, 1212–1215. doi: 10.4067/S0718-58392020000300422
4
Chen J. Chen C. Tian Y. Zhang X. Dong W. Zhang B. et al . (2017). Differences in the impacts of nighttime warming on crop growth of rice-based cropping systems under field conditions. Eur. J. Agron.82, 80–92. doi: 10.1016/j.eja.2016.10.006
5
Crofts N. Hareyama K. Miura S. Hosaka Y. Oitome N. F. Fujita N. (2022). Effect of heading date on the starch structure and grain yield of rice lines with low gelatinization temperature. Int. J. Mol. Sci.23, 10783. doi: 10.3390/ijms231810783
6
Gao H. Li Y. Zhou Y. Guo H. Chen L. Yang Q. et al . (2022). Influence of mechanical transplanting methods and planting geometry on grain yield and lodging resistance of indica rice taoyouxiangzhan under rice-crayfish rotation system. Agronomy-Basel12, 1029. doi: 10.3390/agronomy12051029
7
Ge L. Cang L. Liu H. Zhou D. (2015). Effects of different warming patterns on the translocations of cadmium and copper in a soil-rice seedling system. Environ. Sci. pollut. Res.22, 15835–15843. doi: 10.1007/s11356-015-4760-8
8
Gurav P. P. Ray S. K. Choudhari P. L. Shirale A. O. Meena B. P. Biswas A. K. et al . (2019). Potassium in shrink-swell soils of India. Curr. Sci.117, 587–596. doi: 10.18520/cs/v117/i4/587-596
9
Huang M. Jiang L. Zou Y. Zhang W. (2013). On-farm assessment of effect of low temperature at seedling stage on early-season rice quality. Field Crops Res.141, 63–68. doi: 10.1016/j.fcr.2012.10.019
10
Huang C. Wang D. Sun. L. Wei L. (2016). Effects of exogenous salicylic acid on the physiological characteristics of Dendrobium officinale under chilling stress. Plant Growth Regul.79, 199–208. doi: 10.1007/s10725-015-0125-z
11
Itoh J.-I. Nonomura K.-I. Ikeda K. Yamaki S. Inukai Y. Yamagishi H. et al . (2005). Rice plant development: from zygote to spikelet. Plant Cell Physiol.46, 23–47. doi: 10.1093/pcp/pci501
12
Jia Y. Liu H. Wang H. Zou D. Qu Z. Wang J. et al . (2022). Effects of root characteristics on panicle formation in japonica rice under low temperature water stress at the reproductive stage. Field Crops Res.277, 108395. doi: 10.1016/j.fcr.2021.108395
13
Kitir N. Gunes A. Turan M. Yildirim E. Topcuoglu B. Turker M. et al . (2019). Bio-boron fertilizer applications affect amino acid and organic acid content and physiological properties of strawberry plant. Erwerbs-Obstbau61, 129–137. doi: 10.1007/s10341-018-0409-3
14
Kwak J.-E. Lee J.-S. Won Y.-J. Park H.-M. Kim M. Lee C.-K. et al . (2018). Effects of ripening temperature on starch structure and storage protein characteristics of early maturing rice varieties during grain filling. Korean J. Crop Sci.63, 77–85. doi: 10.7740/kjcs.2018.63.2.077
15
Lee J. Kwon S.-W. (2015). Analysis of quantitative trait loci associated with seed germination and coleoptile length under low temperature condition. J. Crop Sci. Biotechnol.18, 273–278. doi: 10.1007/s12892-015-0114-9
16
Li Y. Dou Z. Guo H. Xu Q. Jiang J. Che Y. et al . (2022). Effects of mechanical transplanting methods and planting geometry on yield formation and canopy structure of indica rice under rice-crayfish rotation. Agriculture-Basel12, 1817. doi: 10.3390/agriculture12111817
17
Li H. Han K. Wang Q. Lu C. (2015). Pyrolysis of rice straw with ammonium dihydrogen phosphate: Properties and gaseous potassium release characteristics during combustion of the products. Bioresource Technol.197, 193–200. doi: 10.1016/j.biortech.2015.08.070
18
Liu J. Feng X. Qiu G. Li H. Wang Y. Chen X. et al . (2023). Inhibition roles of calcium in cadmium uptake and translocation in rice: A review. Int. J. Mol. Sci.24, 11587. doi: 10.3390/ijms241411587
19
Liu S.-L. Wang X. Ma S.-T. Zhao X. Chen F. Xiao X.-P. et al . (2019). Extreme stress threatened double rice production in Southern China during 1981-2010. Theor. Appl. Climatology137, 1987–1996. doi: 10.1007/s00704-018-2719-7
20
Munir S. Qureshi M. K. Shahzad A. N. (2021). Antioxidant response of brassica plants in protection against alternaria brassicicola. Pakistan J. Bot.53, 749–754. doi: 10.30848/PJB2021-2(39)
21
Navizaga C. Lenzo C. Zhang H. Braziene Z. Paltanavicius V. Petrauskiene J. et al . (2017). Efficiency evaluation of dairy wastewater derived zinc micronutrient containing sustainable fertilizers. ACS Sustain. Chem. Eng.5, 6692–6699. doi: 10.1021/acssuschemeng.7b00933
22
Niu J. Liu C. Huang M. Liu K. Yan D. (2021). Effects of foliar fertilization: a review of current status and future perspectives. J Soil Sci Plant Nutrition. 21, 104–118. doi: 10.1007/s42729-020-00346-3
23
Ohashi M. Ishiyama K. Kojima S. Konishi N. Sasaki K. Miyao M. et al . (2018). Outgrowth of rice tillers requires availability of glutamine in the basal portions of shoots. Rice11, 31. doi: 10.1186/s12284-018-0225-2
24
Park H. K. Choi W. Y. Back N. H. Kim S. S. Kim B. K. Kim K. K. (2004). Estimation of leaf area index by plant canopy analyzer in rice. Korean J. Crop Sci.49 (9), 463–467.
25
Phutdhawong W. Winyakul C. Phutdhawong W. S. (2014). Synthesis of 3-indolylacetamide derivatives and evaluation of their plant growth regulator activity. Maejo Int. J. Sci. Technol.8, 181–189. doi: 10.14456/mijst.2014.15
26
Prakash V. Rai P. Sharma N. C. Singh V. P. Tripathi D. K. Sharma S. et al . (2022). Application of zinc oxide nanoparticles as fertilizer boosts growth in rice plant and alleviates chromium stress by regulating genes involved in oxidative stress. Chemosphere303, 134554. doi: 10.1016/j.chemosphere.2022.134554
27
Qian W.-H. Zhang Z.-J. (2012). Precursors to predict low-temperature freezing-rain events in southern China. Chin. J. Geophysics-Chinese Edition55, 1501–1512. doi: 10.6038/j.issn.0001-5733.2012.05.007
28
Raza S. Zia-ur-Rehman M. Alghamdi S. A. Alghanem S. M. S. Usman M. Ahmed R. et al . (2023). Effects of zinc-enriched amino acids on rice plants (Oryza sativa L.) for adaptation in saline-sodic soil conditions: Growth, nutrient uptake and biofortification of zinc. South Afr. J. Bot.162, 370–380. doi: 10.1016/j.sajb.2023.09.011
29
Shah K. Nahakpam S. (2012). Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars. Plant Physiol. Biochem.57, 106–113. doi: 10.1016/j.plaphy.2012.05.007
30
Shehata S. A. Gharib A. A. El-Mogy M. M. Abdel G. K. F. Shalaby E. A. (2011). Influence of compost, amino and humic acids on the growth, yield and chemical parameters of strawberries. J. Medicinal Plants Res.5, 2304–2308. doi: 10.5897/JMPR.9000789
31
Song L. Ding W. Shen J. Zhang Z. Bi Y. Zhang L. (2008). Nitric oxide mediates abscisic acid induced thermotolerance in the calluses from two ecotypes of reed under heat stress. Plant Sci.175, 826–832. doi: 10.1016/j.plantsci.2008.08.005
32
Theocharis A. Clement C. Barka E. A. (2012). Physiological and molecular changes in plants grown at low temperatures. Planta235, 1091–1105. doi: 10.1007/s00425-012-1641-y
33
Tian X. Wang Z. Li X. Lv T. Liu H. Wang L. et al . (2015). Characterization and functional analysis of pyrabactin resistance-like abscisic acid receptor family in rice. Rice8, 28. doi: 10.1186/s12284-015-0061-6
34
Wang W. Chen L. Liu Y. Zeng Y. Wu Z. Tan X. et al . (2021b). Effects of humidity-cold combined stress at the seedling stage in direct-seeded indica rice. Environ. Exp. Bot.191, 104617. doi: 10.1016/j.envexpbot.2021.104617
35
Wang R. Mi K. Yuan X. Chen J. Pu J. Shi X. et al . (2023). Zinc oxide nanoparticles foliar application effectively enhanced zinc and aroma content in rice (Oryza sativa L.) grains. Rice16, 36. doi: 10.1186/s12284-023-00653-0
36
Wang Q. Song X. Wang Y. Yang X. Qin D. Xie T. (2021a). Exogenous salicylic acid improves chilling tolerance in maize seedlings by improving plant growth and physiological characteristics. Agronomy11, 1341. doi: 10.3390/agronomy11071341
37
Wu W. Li Z. Xi M. Tu D. Xu Y. Zhou Y. et al . (2023). Ratoon rice system of production: A rapid growth pattern of multiple cropping in China: A review. Plants (Basel Switzerland)12, 3446. doi: 10.3390/plants12193446
38
Xiao C. Wei H. Neng X. Jun L. Lan H. Fa C. (2011). Effects of elevated CO2 and transgenic Bt rice on yeast-like endosymbiote and its host brown planthopper. J. Appl. Entomology135, 333–342. doi: 10.1111/j.1439-0418.2010.01558.x
39
Xie Z. Duan L. Tian X. Wang B. Eneji A. E. Li Z. (2008). Coronatine alleviates salinity stress in cotton by improving the antioxidative defense system and radical-scavenging activity. J. Plant Physiol.165, 375–384. doi: 10.1016/j.jplph.2007.06.001
40
Xie Y. Mao Y. Xu S. Zhou H. Duan X. Cui W. et al . (2015). Heme-heme oxygenase 1 system is involved in ammonium tolerance by regulating antioxidant defence in Oryza sativa. Plant Cell Environ.38, 129–143. doi: 10.1111/pce.2015.38.issue-1
41
Xu J. Henry A. Sreenivasulu N. (2020). Rice yield formation under high day and night temperatures-A prerequisite to ensure future food security. Plant Cell Environ.43, 1595–1608. doi: 10.1111/pce.13748
42
Yang A. Dai X. Zhang W.-H. (2012). A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J. Exp. Bot.63, 2541–2556. doi: 10.1093/jxb/err431
43
Zhang Z. Lou Y. Li J. Li R. Ma L. (2020). Impacts of nighttime warming on rice growth, physiological properties and yield under water saving irrigation. Int. J. Global Warming21, 105–119. doi: 10.1504/IJGW.2020.108182
44
Zhang M. Zhao R. Huang K. Huang S. Wang H. Wei Z. et al . (2022). The OsWRKY63-OsWRKY76-OsDREB1B module regulates chilling tolerance in rice. Plant J.112, 383–398. doi: 10.1111/tpj.v112.2
45
Zhou Y. Li X. Cao J. Li Y. Huang J. Peng S. (2018). High nitrogen input reduces yield loss from low temperature during the seedling stage in early-season rice. Field Crops Res.228, 68–75. doi: 10.1016/j.fcr.2018.08.018
46
Zsiros O. Nagy V. Parducz A. Nagy G. Unnep R. El-Ramady H. et al . (2019). Effects of selenate and red Se-nanoparticles on the photosynthetic apparatus of Nicotiana tabacum. Photosynthesis Res.139, 449–460. doi: 10.1007/s11120-018-0599-4
Summary
Keywords
rice yield, cold resistance characteristics, yield formation, application of cold-resistant agent, early rice
Citation
Yuan S, Qin S, Shi Q, Chen P, Tu N, Zhou W and Yi Z (2024) Effects of different cold-resistant agents and application methods on yield and cold-resistance of machine-transplanted early rice. Front. Plant Sci. 15:1422374. doi: 10.3389/fpls.2024.1422374
Received
23 April 2024
Accepted
18 September 2024
Published
02 October 2024
Volume
15 - 2024
Edited by
Dongliang Xiong, Huazhong Agricultural University, China
Reviewed by
Peng Jiang, Sichuan Academy of Agricultural Sciences, China
Jing Xiang, Chinese Academy of Agricultural Sciences, China
Baohua Feng, Chinese Academy of Agricultural Sciences, China
Updates
Copyright
© 2024 Yuan, Qin, Shi, Chen, Tu, Zhou and Yi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxin Zhou, zwxok@hunau.net; Zhenxie Yi, yizhenxie@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.