
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 11 June 2024
Sec. Plant Metabolism and Chemodiversity
Volume 15 - 2024 | https://doi.org/10.3389/fpls.2024.1419392
This article is part of the Research TopicPlant Natural Products: Biosynthesis, Regulation, and FunctionView all 15 articles
 Yanlin Wang1,2,3
Yanlin Wang1,2,3 Huanchu Liu1,3
Huanchu Liu1,3 Shuai Yu1,3*
Shuai Yu1,3* Yue Zhang1,3,4
Yue Zhang1,3,4 Yanqing Huang1,3,4
Yanqing Huang1,3,4 Xingyuan He1,2,3,4
Xingyuan He1,2,3,4 Wei Chen1,2,3,4*
Wei Chen1,2,3,4*The growth and quality of medicinal plants depend heavily on environmental variables. The quality of Rubia cordifolia, an important medicinal plant, is determined by the two main secondary metabolites of the root, purpurin and mollugin. However, their relationship with environmental factors has not been studied. In this study, the purpurin and mollugin contents of R. cordifolia roots from different sampling sites in China were measured using ultra-high-performance liquid chromatography, and the correlations between the two secondary metabolites and environmental variables were analyzed. The results showed that there were significant differences in the contents of purpurin and mollugin in the roots of R. cordifolia at different sampling points. The content of purpurin ranged from 0.00 to 3.03 mg g-1, while the content of mollugin ranged from 0.03 to 10.09 mg g-1. The quality of R. cordifolia in Shanxi, Shaanxi and Henan border areas and southeastern Liaoning was higher. Liaoning is expected to become a R. cordifolia planting area in Northeast China. Correlation and regression analysis revealed that the two secondary metabolites were affected by different environmental factors, the two secondary metabolites contents were positively correlated with longitude and latitude, and negatively correlated with soil nutrients. In addition, higher temperature and shorter sunshine duration facilitated the synthesis of purpurin. Annual precipitation might be the main factor limiting the quality of R. cordifolia because it had opposite effects on the synthesis of two major secondary metabolites. Therefore, this study is of great significance for the selection of R. cordifolia planting areas and the improvement of field planting quality.
Traditional Chinese Medicine (TCM) has been extensively used to treat various illnesses in China for thousands of years (Wang et al., 2021a). TCM continues to play a key role in safeguarding the health of Chinese people and is attracting increasing global attention (Xue et al., 2012). The idea of integrating Chinese and Western medicines to treat complex disorders has also been adopted by the modern medical community, underscoring the significance of TCM. Since the outbreak of the novel coronavirus pneumonia in 2019, TCM has demonstrated significant promise for the management of COVID-19 (Du et al., 2020; Lyu et al., 2021; Zhao et al., 2021).
The continual development of TCM requires support for further studies on medicinal plants. Only high-quality medicinal plants can be used to treat various diseases. The quality of medicinal plants is not only related to the clinical effect of Chinese medicine but also plays a key role in developing the Chinese medicine industry (Qin et al., 2018). The active components of Chinese medicine are the basis for the efficacy of Chinese medicine, and these innovative drugs are mainly extracted from medicinal plants (Ma et al., 2022). For example, the well-known antimalarial drug artemisinin is extracted from the medicinal plant Artemisia annua L (Tu, 1999).
Rubia cordifolia L. is a medicinal plant whose roots can be used to treat various diseases owing to its anti-inflammatory, anticancer, and anti-platelet aggregation properties as various secondary metabolites, including anthraquinones and naphthoquinones, are present in its roots (Figure 1) (Wen et al., 2022). The Chinese Pharmacopoeia usually specifies standards for Chinese herbal medicines, which stipulate that the two main compounds in the roots of R. cordifolia, that is, the purpurin content is not less than 0.1% and the mollugin content is not less than 0.4% (Chinese Pharmacopeia Commission, 2020). Purpurin has anticancer, antibacterial, and neuromodulatory effects owing to its strong antioxidant activity (Singh et al., 2021). Similarly, mollugin has a variety of pharmacological effects, including neuroprotective, anti-inflammatory, anticancer, and antiviral effects (Kim et al., 2013; Wang et al., 2017; Li et al., 2020). Therefore, the contents of both compounds are essential for the quality of R. cordifolia.
Figure 1 Photographs of R. cordifolia. (A) Leaf; (B) Flower; (C) Fruit; (D) Root. (A–C) were obtained from iNaturalist (https://www.inaturalist.org/), and (D) was obtained from iPlant (http://www.iplant.cn/).
Plant secondary metabolites are not only important for humans but also vital for themselves. Plants synthesize and accumulate various secondary metabolites to resist possible stresses caused by the external environment (Selmar and Kleinwächter, 2013; Loreto et al., 2014). For example, psoralen, the main secondary metabolite in Psoralea corylifolia L., increases under chromium and UV-B stress (Pandey et al., 2023). By contrast, a short drought stress period increased the amount of saponins in the roots of Bupleurum chinense DC (Yang et al., 2019a). However, an unfavorable growth environment may contribute to the synthesis and accumulation of secondary metabolites but may not be conducive to plant growth, resulting in reduced yield. Further research has shown that medicinal plants grow more effectively and to a higher quality in their appropriate habitats, which is strongly related to the environment in the habitat (Zhan et al., 2022; Zheng et al., 2022). The synthesis and accumulation of secondary metabolites in medicinal plants are affected by a combination of factors.
Although R. cordifolia has long been used as a medicinal plant, research on R. cordifolia has mainly focused on the discovery of its secondary metabolites and associated pharmacological effects (Shan et al., 2016; Wen et al., 2022). The quality and productivity of medicinal plants can be improved by understanding the relationship between environmental conditions and secondary metabolite content. Environmental factors may also influence the content of secondary metabolites in R. cordifolia; however, little work has been conducted on R. cordifolia in this area. Therefore, the objectives of this study were to (1) collect R. cordifolia throughout China and determine the content of two major secondary metabolites in the roots at each location; (2) analyze the environmental conditions of the sampling sites, including soil, climate, and topography; and (3) analyze the relationship between secondary metabolite content and environmental factors at each location to explore the primary environmental variables affecting the synthesis and accumulation of R. cordifolia root secondary metabolites.
Based on the distribution of R. cordifolia and its origin, as described in the literature (Xue et al., 2009; Yu et al., 2017; Yan, 2021), Liaoning, Hebei, Henan, Shanxi, Shaanxi, Gansu, and Sichuan were selected for our sampling (Figure 2). Samples were collected from July to August in 2021 and 2022, and 3–7 whole underground parts of R. cordifolia were collected from each sample point, while trying to ensure that the samples had the same plant age. Soil at 0–30 cm depth in the roots was collected for subsequent analysis. The coordinates of each sampling point were recorded for the subsequent acquisition of meteorological and topographical factors, as shown in Table 1.
The R. cordifolia root powder (0.5 g) was precisely weighed and placed in a stoppered conical flask. Methanol (100 mL) was added to the flask and stored it overnight. Secondary metabolites were extracted using ultrasound (power 250W, frequency 40kHz, 30 minutes). After the ultrasound was completed, the sample was cooled to room temperature, shaken well, and filtered. The filtrate (50 mL) was measured precisely, and evaporate to dryness in a 60°C water bath. The residue was dissolved by adding 20 mL methanol:25% hydrochloric acid (4:1). The solution was heated and hydrolyzed in an 80°C water bath for 30 minutes. The solution obtained after hydrolysis was cooled immediately, 3 ml of triethylamine was added, mixed, transferred to a 25 ml measuring flask, and diluted to volume with methanol. The solution was filtered through a microporous membrane and used for detection (Chinese Pharmacopeia Commission, 2020).
Ultra-high-performance liquid chromatography (UHPLC) (Ultimate 3000 RSLC, Thermo Fisher Scientific, MA, USA) was used to determine the mollugin and purpurin contents in the extract. Octadecylsilane bonded silica gel was used as the filler of chromatographic column, and the 25:50:25 mixture of methanol, acetonitrile, and 0.2% phosphoric acid was used as the mobile phase The detection wavelength is 250 nm. According to the calculations of mollugin and purpurin peaks, the theoretical plate number should not be less than 4000.
Soil samples were taken back to the laboratory and sieved to remove stones, fine roots, and other debris. The soil was naturally dried and ground, and part of the ground samples were passed through a 0.25 mm sieve to determine the organic matter content of the soil. The remaining samples were sieved through a 1 mm sieve, and the soil pH, available nitrogen, available phosphorus, and available potassium were determined using the methods described by Hu and Wang (2020). Soil pH was determined by the potentiometric method, organic matter content was determined by the potassium dichromate volumetric method, available nitrogen content was determined by the alkaline hydrolysis diffusion method, available phosphorus content was determined by the NaHCO3 extraction-colorimetric method, and available potassium content was determined by the ammonium acetate extraction-flame photometric method.
The annual mean temperature (TEM), annual precipitation (PRE), and annual sunshine hours (SSD) for 2010–2020 were obtained from the Resource and Environment Science and Data Center (https://www.resdc.cn/) (Xu, 2017). Elevation data were obtained from WorldClim (https://www.worldclim.org/). Finally, the climatic and geographic factors of each sampling site were extracted using the extraction tool in ArcGIS.
The data were pre-processed using Microsoft Excel 2019. The Spearman correlation analysis was conducted between environmental variables and secondary metabolites using the corrplot package in R 4.2.2. The stepwise regression was analyzed using the car package in R 4.4.2. Corresponding plots were constructed using ggplot2 package in R 4.2.2 and ArcGIS 10.7.
Sampling sites were covered from the east to the west and from the north to the south of China (Figure 2). The longitude spanned 23.24°, and the latitude spanned 13.34°. The elevation in the study area varied from 5 to 3395 m, rising from east to west (Table 1).
Soil properties varied considerably between the sampling sites (Table 1). The average soil pH varied between 6.13 and 8.54, the average organic matter varied between 10.02 and 334.23 g kg-1, the average available nitrogen content ranged from 41.18 to 1,168.00 mg kg-1, the average available phosphorus content ranged from 5.87 to 100.23 mg kg-1, and the average available potassium ranged from 71.76 to 1,038.73 mg kg-1 (Table 1).
The study area spans multiple climate zones in China, including temperate, warm temperate, subtropical, and alpine. The annual mean temperature of each sampling site varied between 3.12 and 17.31°C, annual precipitation varied between 446.45 and 1,206.15 mm, and annual sunshine hours varied between 1,037.23 and 2,690.20 h (Table 1).
The contents of purpurin and mollugin at each sampling point were calculated according to the UHPLC chromatogram (Table 2). The chromatograms of standard and sample solutions are shown in Figure 3. The purpurin and mollugin contents in R. cordifolia roots varied greatly among sampling sites in the study area. Purpurin content ranged from 0.00 to 3.03 mg g-1, the minimum and maximum values were found at sampling points in Sichuan. The content of mollugin varied from 0.03 to 10.09 mg g-1. The minimum value was found in Sichuan sampling site, while the maximum value was found in Liaoning sampling site. The contents of the two secondary metabolites in R. cordifolia roots varied greatly in different provinces. For example, the content of purpurin in more than half of the samples in Gansu was less than 1.00 mg g-1, and the highest was 1.34 mg g-1. The content of purpurin in in the samples in Hebei was higher than 1.00 mg g-1, and the highest content was 2.05 mg g-1. However, the content of mollugin in the samples in Hebei was less than 4.00 mg g-1, and the highest content was 5.10 mg g-1. The content of purpurin in the roots of R. cordifolia in nearly half of the sampling sites in Sichuan was less than 1.00 mg g-1, and the highest was 3.03 mg g-1. The content of mollugin in the roots in almost all sampling sites was not up to standard, indicating that the quality of R. cordifolia in each sampling site in Sichuan was uneven. The content of secondary metabolites in the roots of R. cordifolia in Shanxi, Shaanxi and Henan provinces can basically reach the standard. In addition, the content of secondary metabolites in the roots of R. cordifolia in a small number of sampling points in Liaoning was not up to standard, mainly distributed in the western part of Liaoning, while the quality of R. cordifolia in the southeastern part of Liaoning is higher (Figure 4).
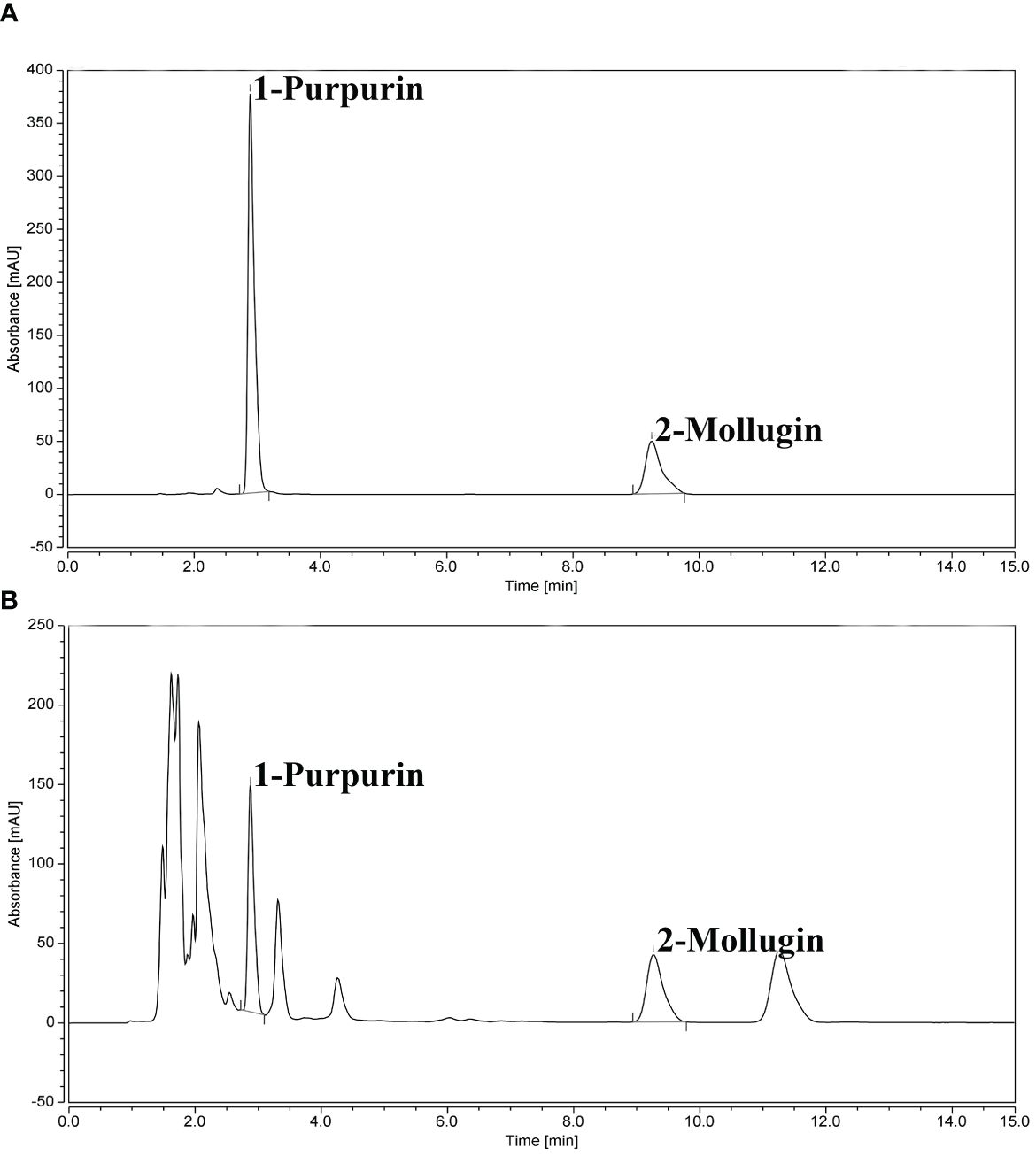
Figure 3 Chromatographic diagram of standard and sample solution. (A) Standard solution; (B) Sample solution.
Purpurin and mollugin contents showed different relationships with soil, climate, and topographic factors (Figure 5). Longitude showed a significant positive correlation with purpurin content (p < 0.05) (Figure 5A), while elevation (p < 0.001) showed a significant negative correlation (Figure 5C). Annual mean temperature (p < 0.001) and annual precipitation (p < 0.001) showed highly significant positive correlations with purpurin content (Figures 5D, E), whereas annual sunshine hours showed a significant negative correlation with purpurin content (p < 0.05) (Figure 5F). Organic matter content (p < 0.05), available phosphorus content (p < 0.05), and available potassium content (p < 0.01) were significantly negatively correlated with purpurin content (Figures 5H, J, K). For mollugin, longitude (p < 0.05) and latitude (p < 0.05) were significantly positively correlated with mollugin content (Figures 5A, B). Organic matter content (p < 0.05) showed a significant and negative correlation with mollugin content (p < 0.05) (Figure 5H). There was no significant correlation between mollugin content and climatic factors (Figures 5D–F), however, there was a negative correlation between annual precipitation and mollugin content (p = 0.052) (Figure 5E).
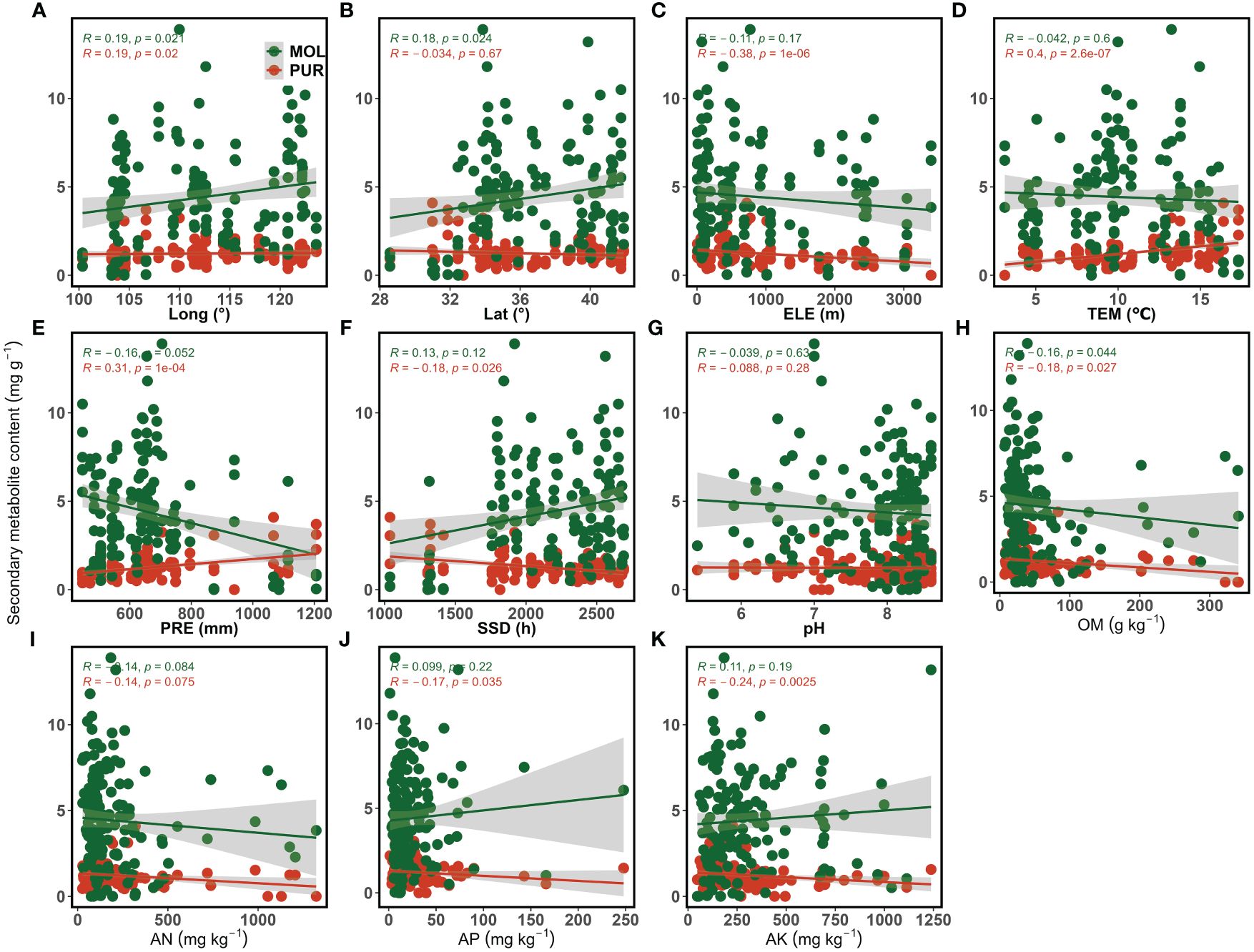
Figure 5 Correlation between purpurin, mollugin content, and environmental variables. (A) The relationship between secondary metabolite content and longitude; (B) The relationship between secondary metabolite content and latitude; (C) The relationship between secondary metabolite content and elevation; (D) The relationship between secondary metabolite content and annual mean temperature; (E) The relationship between secondary metabolite content and annual precipitation; (F) The relationship between secondary metabolite content and annual sunshine hours; (G) The relationship between secondary metabolite content and soil pH; (H) The relationship between secondary metabolite content and organic matter content; (I) The relationship between secondary metabolite content and available nitrogen content; (J) The relationship between secondary metabolite content and available phosphorus content; (K) The relationship between secondary metabolite content and available potassium content. PUR, purpurin; MOL, mollugin; Long, Longitude; Lat, Latitude; ELE, Elevation; TEM, Annual mean temperature; PRE, Annual precipitation; SSD, Annual sunshine hours; OM, Organic matter; AN, Available nitrogen; AP, Available phosphorus; AK, Available potassium.
In order to further explore the relationship between the two secondary metabolites and environmental factors, a stepwise regression model between purpurin, mollugin and environmental factors was established (Table 3). Model results showed that purpurin and mollugin were affected by different factors. Purpurin in R. cordifolia roots was affected by latitude, annual mean temperature and annual precipitation, and the three factors had a positive impact on purpurin. Otherwise, the model results showed that root mollugin content was only negatively affected by annual precipitation.
R. cordifolia is widely distributed in China; therefore, there are several places of origin recorded in the literature, including Shanxi, Sichuan, Henan, Shaanxi, Jiangsu, Hubei, Shandong, Anhui, and Hebei (Xue et al., 2009; Yan, 2021). Several studies have investigated the medicinal components in the roots of R. cordifolia from different origins and found that R. cordifolia from Shanxi, Shaanxi, Henan, Hebei, Jiangsu, Shandong, and Anhui met the Chinese Pharmacopoeia standards (Xue et al., 2009; Yu et al., 2017). Henan and Shaanxi are recognized R. cordifolia producing areas (Wen et al., 2022). The purpurin and mollugin contents of R. cordifolia roots from eastern and central regions in this study investigated met the requirements of the Chinese Pharmacopoeia, especially in central regions. Therefore, the study confirmed that R. cordifolia produced in Henan, Shaanxi, and Shanxi contained higher levels of purpurin and mollugin. However, R. cordifolia resources in Northeastern China have not yet been investigated. In this study, R. cordifolia from Liaoning contained higher purpurin and mollugin contents, which met the requirements of the Chinese Pharmacopoeia, suggesting that Liaoning could be a choice for R. cordifolia cultivation.
Environmental factors, including soil, climate, and topography, showed different degrees of correlation with the two secondary metabolites in the roots. The effects of geographic factors on plant secondary metabolite content have been extensively reported. The content of proanthocyanidins in Ribes nigrum L. was positively correlated with latitude (Yang et al., 2019b), whereas harpagoside content in S. ningpoensis was negatively correlated with latitude (Yang et al., 2011). Different essential oil contents in H. italicum showed different correlations with elevation (Melito et al., 2016). In this study, purpurin and mollugin contents were significantly and positively correlated with longitude and latitude (Figure 5; Table 2), suggesting that when the environment was closer to northern and eastern China, the purpurin and mollugin contents in R. cordifolia roots were higher, which is similar to the findings of Weston et al. (2013). The two secondary metabolites can cause oxidative stress in cells and therefore act as natural biocides (Robertson et al., 2009; Hook et al., 2018). The higher purpurin and mollugin content found in the eastern and central regions of this study might be due to the fact that the environmental conditions in these regions cause some kind of biotic stress, which in turn leads to the synthesis of more purpurin and mollugin. Previous studies have shown that anthraquinone content in medicinal plants Rheum officinale Baill. (Wang et al., 2013; Yan et al., 2017; Ge et al., 2018) and Rumex nepalensis Spreng (Farooq et al., 2013) increased with elevation, but in the present study elevation negatively affected purpurin content in R. cordifolia roots, and a similar result was found in Rheum australe D. Don (Pandith et al., 2014). The negative correlation between secondary metabolite content and elevation may be related to factors such as ultraviolet radiation or temperature (Spitaler et al., 2006). The positive correlation between purpurin content and annual mean temperature and the negative correlation between elevation and temperature just proved this point.
Soil nutrients play a crucial role in supporting plant growth. In the case of medicinal plants, the presence of appropriate soil nutrients not only enhances their yield but also improves their overall quality. Changes in soil nutrition will inevitably lead to changes in secondary metabolites, understanding the relationship between the content of secondary metabolites and soil nutrients can guide the cultivation of medicinal plants to improve the effectiveness of medicinal plants. However, specific soil factors that cause an increase in secondary metabolite levels in medicinal plants are not the same. Soil pH may be an important factor determining the secondary metabolite content of Echinacea purpurea (L.) Moench (Xu et al., 2022). The available phosphorus in the soil is thought to be a key factor influencing the secondary metabolite content in Salvia miltiorrhiza Bunge (Liang et al., 2021). For Rhodiola sachalinensis A. Bor, available phosphorus and available potassium are the main factors limiting the synthesis of salidroside (Yan et al., 2004). In this study, the findings found that higher soil nutrients were not necessarily beneficial to the synthesis of purpurin and mollugin, which is similar to the study by Shen et al. (2017), where the content of anthraquinone compounds in the roots of Rheum tanguticum Maxim. ex Balf was higher in nutrient-poor soil. This phenomenon might be due to the fact that under nutrient-sufficient conditions R. cordifolia mainly carries out primary metabolism for growth, while under poor soil conditions the plants are stressed and then carry out secondary metabolism and produce secondary metabolites to alleviate the stress.
The climate is crucial for the growth and quality of medicinal plants. Studies have shown that different medicinal plants have different requirements for climatic conditions, and secondary metabolite production can be affected by climatic conditions (Liu et al., 2020). Temperature has multiple effects on the synthesis of secondary metabolites in medicinal plants. On the one hand, temperature stress may cause medicinal plants to produce secondary metabolites for defense (Dong et al., 2011); on the other hand, high or low temperatures may increase the expression of genes involved in the synthesis of secondary metabolites (Tao et al., 2017; Yang et al., 2019b). The harpagoside content in Scrophularia ningpoensis Hemsl (Yang et al., 2011), and the nerolidol content in Helichrysum italicum (Roth) G. Don (Melito et al., 2016) are correlated positively with temperature, while there is a negative correlation between high temperature and total proanthocyanidins in R. nigrum fruits (Yang et al., 2019b). Temperature promoted the synthesis of purpurin in the roots of R. cordifolia, which may be due to high temperature stress or increased expression of related genes. Precipitation also has an impact on the synthesis of secondary metabolites in plants. Typically, drought conditions stimulate the synthesis and accumulation of secondary metabolites in plants (Humbal and Pathak, 2023). However, chlorogenic acid, choline, 3,5-o-dicaffeoylquinic acid, and coumaric acid in E. purpurea are positively correlated with annual precipitation (Xu et al., 2022). Two secondary metabolites in the roots of R. cordifolia were oppositely affected by precipitation, which promoted the synthesis of purpurin but limited the synthesis of mollugin. The impact of precipitation on the secondary metabolism of medicinal plants is complex. This complexity arises due to the potential changes in soil properties, microbial fungi, and root activity that can occur as a result of precipitation (Wang et al., 2021b; Xu et al., 2022). Sunshine hours also affect the production of plant secondary metabolites. For instance, a longer duration of sunshine can increase the content of geniposidic acid in Eucommia ulmoides Oliv. (Dong et al., 2011) and tanshinones in S. miltiorrhiza (Zhang et al., 2018). However, in the present study, correlation analysis suggested that sunshine duration might have an inhibitory effect on purpurin synthesis in R. cordifolia root.
The cultivation of medicinal plants needs to ensure both yield and quality, so Daodi medicinal materials are recognized as high-quality Chinese medicinal products. However, as wild medicinal plants have been over-exploited, they can no longer meet market demand. Therefore, field cultivation has become the main way to ensure the market supply of medicinal plants (Kling, 2016). In the case of field planting, many factors need to be considered comprehensively, including geographical location, soil, climate, etc. First of all, it is necessary to select the appropriate planting area, which needs to consider geographical and climatic factors. Therefore, eastern and northern China can be used as a choice when planting R. cordifolia. Higher temperatures facilitate the synthesis of purpurin, so the temperature in the suitable area needs to be considered. Precipitation has opposite effects on the two secondary metabolites in the roots of R. cordifolia. When planting R. cordifolia, the precipitation situation needs to be weighed, and the amount of watering in the field must also be considered. In order to ensure the yield of medicinal plants, fertilization is a necessary measure. However, the synthesis of two major secondary metabolites in R. cordifolia roots is inhibited by soil nutrients, therefore necessary fertilization experiments need to be performed to determine the appropriate fertilization ratio.
The two main secondary metabolites in the root of R. cordifolia belong to quinone compounds, among which purpurin belongs to anthraquinone compounds (Singh et al., 2021), and mollugin belongs to naphthoquinone compounds (Morita et al., 2015). The Chinese Pharmacopoeia requires that the contents of the two secondary metabolites in the roots of R. cordifolia should be up to standard before they can be used as medicines (Chinese Pharmacopeia Commission, 2020), so the products of R. cordifolia on the market are uneven. How to improve the quality when planting R. cordifolia has become a problem. At present, some researchers have carried out transcriptome studies on R. cordifolia, and found that the synthesis of anthraquinones in the roots involves the synthesis of A, B, and C rings. Among them, A and B rings are completed by the shikimic acid pathway, while the synthesis of C ring comes from isopentenyl diphosphate (Peng et al., 2018). Previous studies have confirmed that isopentenyl diphosphate is synthesized through the 2-C-methyl-D-erythritol 4-phosphate pathway in the Rubiaceae. Isochorismate synthase gene, o-succinylbenzoic acid gene, 1-deoxy-D-xylulose 5-phosphate reductoisomerase gene, and 1-deoxy-D-xylulose 5-phosphate synthase gene are all key genes for the synthesis of anthraquinones (Han et al., 2001). However, the synthetic pathway of naphthoquinone compounds in the roots of R. cordifolia has not been reported. Therefore, in the future, on the one hand, the biosynthesis pathway of anthraquinones will be explored by molecular biology methods, and key genes will be screened. On the other hand, the relationship between the key genes in the synthesis pathway of anthraquinones and naphthoquinones and the ecological environment factors should be analyzed to find the key factors, and then measures can be taken to promote the synthesis of two secondary metabolites.
This study found that the quality of R. cordifolia varied considerably depending on the region; however, in general, the quality of R. cordifolia from southeastern Liaoning and the border of Shanxi, Shaanxi and Henan met the Chinese Pharmacopoeia standards, whereas Liaoning proved to be a potential source of R. cordifolia in northeastern China. Environmental factors have different effects on the two secondary metabolites. The purpurin and mollugin contents of R. cordifolia roots were significantly and positively correlated with longitude and latitude, while were negatively correlated with soil nutrients. Temperature contributed to the synthesis of purpurin, but sunshine duration inhibited the synthesis. Precipitation is a key factor limiting the quality of R. cordifolia because it has opposite effects on the synthesis of purpurin and mollugin. To meet the requirements of the Chinese Pharmacopoeia, the limiting factors affecting the two secondary metabolites should be considered when planting R. cordifolia in field. In the future, it is necessary to combine the key genes in the synthesis pathway of the two secondary metabolites, further analyze the key factors affecting the synthesis of the two compounds, and then promote the synthesis of the two secondary metabolites by controlling the environmental factors to improve the quality of R. cordifolia.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
YW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HL: Formal analysis, Resources, Writing – review & editing. SY: Data curation, Writing – review & editing. YZ: Resources, Writing – review & editing. YH: Resources, Writing – review & editing. XH: Conceptualization, Writing – review & editing. WC: Conceptualization, Investigation, Methodology, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R&D Program of China (No. 2022YFF1300500) and the Youth Innovation Promotion Association CAS (2022195).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Chinese Pharmacopeia Commission (2020). Pharmacopoeia of the People’s Republic of China: Pharmacopoeia I (Beijing, China: Chinese Medical Science Press).
Dong, J. E., Ma, X. H., Wei, Q., Peng, S. B., Zhang, S. C. (2011). Effects of growing location on the contents of secondary metabolites in the leaves of four selected superior clones of Eucommia ulmoides. Ind. Crops Prod. 34, 1607–1614. doi: 10.1016/j.indcrop.2011.06.007
Du, H. Z., Hou, X. Y., Miao, Y. H., Huang, B. S., Liu, D. H. (2020). Traditional Chinese Medicine: an effective treatment for 2019 novel coronavirus pneumonia (NCP). Chin. J. Nat. Med. 18, 206–210. doi: 10.1016/S1875-5364(20)30022-4
Farooq, U., Pandith, S. A., Singh Saggoo, M. I., Lattoo, S. K. (2013). Altitudinal variability in anthraquinone constituents from novel cytotypes of Rumex Nepalensis Spreng—a high value medicinal herb of North Western Himalayas. Ind. Crops Prod. 50, 112–117. doi: 10.1016/j.indcrop.2013.06.044
Ge, Y. H., Sun, M. M., Salome-Abarca, L. F., Wang, M., Choi, Y. H. (2018). Investigation of species and environmental effects on rhubarb roots metabolome using H-1 NMR combined with high performance thin layer chromatography. Metabolomics 14, 137. doi: 10.1007/s11306-018-1421-1
Han, Y. S., van der Heijden, R., Verpoorte, R. (2001). Biosynthesis of anthraquinones in cell cultures of the Rubiaceae. Plant Cell Tissue Organ Cult. 67, 201–220. doi: 10.1023/A:1012758922713
Hook, I., Sheridan, H., Reid, C. (2018). Trichomes and naphthoquinones protect Streptocarpus dunnii Hook.f. against environmental stresses. South Afr. J. Bot. 119, 193–202. doi: 10.1016/j.sajb.2018.09.016
Hu, H. R., Wang, Y. X. (2020). Experimental tutorial of soil science. 2nd Edition (Beijing, China: China Forestry Publishing House).
Humbal, A., Pathak, B. (2023). Influence of exogenous elicitors on the production of secondary metabolite in plants: A review (“VSI: secondary metabolites”). Plant Stress. 8, 100166. doi: 10.1016/j.stress.2023.100166
Kim, H., Choi, H. K., Jeong, T. C., Jahng, Y., Kim, D. H., Lee, S. H., et al. (2013). Selective inhibitory effects of mollugin on CYP1A2 in human liver microsomes. Food Chem. Toxicol. 51, 33–37. doi: 10.1016/j.fct.2012.09.013
Kling, J. (2016). Protecting medicine’s wild pharmacy. Nat. Plants. 2, 16064. doi: 10.1038/nplants.2016.64
Li, J., Zhang, J. L., Gong, X. P., Xiao, M., Song, Y. Y., Pi, H. F., et al. (2020). Anti-inflammatory activity of mollugin on DSS-induced colitis in mice. Curr. Med. Sci. 40, 910–916. doi: 10.1007/s11596-020-2262-5
Liang, H. Y., Kong, Y. H., Chen, W., Wang, X. G., Jia, Z. F., Dai, Y. H., et al. (2021). The quality of wild Salvia miltiorrhiza from Dao Di area in China and its correlation with soil parameters and climate factors. Phytochem. Anal. 32, 318–325. doi: 10.1002/pca.2978
Liu, X. D., Zhang, Y., Wu, M. H., Ma, Z. G., Huang, Z. H., Tian, F., et al. (2020). The scientific elucidation of daodi medicinal materials. Chin. Med. 15, 86. doi: 10.1186/s13020-020-00367-1
Loreto, F., Dicke, M., Schnitzler, J. P., Turlings, T. C. J. (2014). Plant volatiles and the environment. Plant Cell Environ. 37, 1905–1908. doi: 10.1111/pce.12369
Lyu, M., Fan, G. W., Xiao, G. X., Wang, T. Y., Xu, D., Gao, J., et al. (2021). Traditional chinese medicine in COVID-19. Acta Pharm. Sin. B. 11, 3337–3363. doi: 10.1016/j.apsb.2021.09.008
Ma, Y.g., Zhao, Y. J., Ma, X. J., Guo, J., Huang, L. Q. (2022). Biosynthetic pathway of active components in Traditional Chinese Medicine and its application. Scientia Sin. Vitae. 52, 894–907. doi: 10.1360/SSV-2021-0401
Melito, S., Petretto, G. L., Podani, J., Foddai, M., Maldini, M., Chessa, M., et al. (2016). Altitude and climate influence Helichrysum italicum subsp microphyllum essential oils composition. Ind. Crops Prod. 80, 242–250. doi: 10.1016/j.indcrop.2015.11.014
Morita, H., Nishino, H., Nakajima, Y., Kakubari, Y., Nakata, A., Deguchi, J., et al. (2015). Oxomollugin, a potential inhibitor of lipopolysaccharide-induced nitric oxide production including nuclear factor kappa B signals. J. Nat. Med. 69, 608–611. doi: 10.1007/s11418-015-0927-3
Pandey, A., Agrawal, M., Agrawal, S. B. (2023). Individual and combined effects of chromium and ultraviolet-B radiation on defense system, ultrastructural changes, and production of secondary metabolite psoralen in a medicinal plant Psoralea corylifolia L. Environ. Sci. pollut. Res. 30, 4372–4385. doi: 10.1007/s11356-022-22480-4
Pandith, S. A., Hussain, A., Bhat, W. W., Dhar, N., Qazi, A. K., Rana, S., et al. (2014). Evaluation of anthraquinones from Himalayan rhubarb (Rheum emodi Wall. ex Meissn.) as antiproliferative agents. South Afr. J. Bot. 95, 1–8. doi: 10.1016/j.sajb.2014.07.012
Peng, L., Zhang, G., Yan, Y. G., Sun, T., Wang, M., Li, J., et al. (2018). Analysis of transcriptomes and exploring of anthraquinones biosynthetic pathway genes in Rubia cordifolia L. Chin. Tradition. Herb. Drugs 49, 1890–1896. doi: 10.7501/j.issn.0253–2670.2018.08.024
Qin, K. M., Cai, H., Li, W. D., Lu, T. L., Jin, J. J., Liu, X., et al. (2018). Research on construction of quality control system for high quality Chinese herbal medicine and industrialization application. Modern. Tradition. Chin. Med. Materia Medica-World Sci. Technol. 20, 383–389.
Robertson, P. K. J., Black, K. D., Adams, M., Willis, K., Buchan, F., Orr, H., et al. (2009). A new generation of biocides for control of crustacea in fish farms. J. Photochem. Photobiol. B: Biol. 95, 58–63. doi: 10.1016/j.jphotobiol.2008.12.009
Selmar, D., Kleinwächter, M. (2013). Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crops Prod. 42, 558–566. doi: 10.1016/j.indcrop.2012.06.020
Shan, M. Q., Yu, S., Yan, H., Chen, P. D., Zhang, L., Ding, A. W. (2016). A review of the botany, phytochemistry, pharmacology and toxicology of Rubiae Radix et Rhizoma. Molecules 21, 1747. doi: 10.3390/molecules21121747
Shen, N., Cui, Y. L., Xu, W. H., Zhao, X. H., Yang, L. C. (2017). Impact of phosphorus and potassium fertilizers on growth and anthraquinone content in Rheum tanguticum Maxim. ex Balf. Ind. Crops Prod. 107, 312–319. doi: 10.1016/j.indcrop.2017.05.044
Singh, J., Hussain, Y., Luqman, S., Meena, A. (2021). Purpurin: A natural anthraquinone with multifaceted pharmacological activities. Phytother. Res. 35, 2418–2428. doi: 10.1002/ptr.6965
Spitaler, R., Schlorhaufer, P. D., Ellmerer, E. P., Merfort, I., Bortenschlager, S., Stuppner, H., et al. (2006). Altitudinal variation of secondary metabolite profiles in flowering heads of Arnica montana cv. ARBO. Phytochem. 67, 409–417. doi: 10.1016/j.phytochem.2005.11.018
Tao, X., Wang, M. X., Dai, Y., Wang, Y., Fan, Y. F., Mao, P., et al. (2017). Identification and expression profile of CYPome in perennial ryegrass and tall fescue in response to temperature stress. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01519
Tu, Y. Y. (1999). The development of new antimalarial drugs: Qinghaosu and dihydro-qinghaosu. Chin. Med. J. 112, 976–977.
Wang, W. Y., Zhou, H., Wang, Y. F., Sang, B. S., Liu, L. (2021a). Current policies and measures on the development of Traditional Chinese Medicine in China. Pharmacol. Res. 163, 105187. doi: 10.1016/j.phrs.2020.105187
Wang, Y. T., Xie, Y. Z., Rapson, G., Ma, H. B., Jing, L., Zhang, Y., et al. (2021b). Increased precipitation enhances soil respiration in a semi-arid grassland on the Loess Plateau, China. PeerJ 9, e10729. doi: 10.7717/peerj.10729
Wang, Z., Li, M. Y., Mi, C. L., Wang, K. S., Ma, J., Jin, X. J. (2017). Mollugin has an anti-cancer therapeutic effect by inhibiting TNF-alpha-Induced NF-kappa B activation. Int. J. Mol. Sci. 18, 1619. doi: 10.3390/ijms18081619
Wang, Z., Ma, P., Xu, L. J., He, C. N., Peng, Y., Xiao, P. G. (2013). Evaluation of the content variation of anthraquinone glycosides in rhubarb by UPLC-PDA. Chem. Cent. J. 7, 170. doi: 10.1186/1752–153X-7–170
Wen, M., Chen, Q., Chen, W., Yang, J., Zhou, X. G., Zhang, C. X., et al. (2022). A comprehensive review of Rubia cordifolia L.: Traditional uses, phytochemistry, pharmacological activities, and clinical applications. Front. Pharmacol. 13. doi: 10.3389/fphar.2022.965390
Weston, P. A., Weston, L. A., Hildebrand, S. (2013). Metabolic profiling in Echium plantagineum: presence of bioactive pyrrolizidine alkaloids and napthoquinones from accessions across southeastern Australia. Phytochem. Rev. 12, 831–837. doi: 10.1007/s11101-013-9306-4
Xu, X. L. (2017). “Spatial interpolation dataset of average condition of meteorological elements in China,” (System, Resource and Environmental Science Data Registration and Publishing System, Beijing, China).
Xu, W. Q., Cheng, Y. L., Guo, Y. H., Yao, W. R., Qian, H. (2022). Effects of geographical location and environmental factors on metabolite content and immune activity of EChinacea purpurea in China based on metabolomics analysis. Ind. Crops Prod. 189, 115782. doi: 10.1016/j.indcrop.2022.115782
Xue, L., Chen, S. Z., Suo, F. Y., Yang, B. (2009). HPLC determination of rubimaillin in Chinese herbal pieces of Radix et Rhizoma Rubiae. Chin. J. Pharm. Anal. 29, 363–366.
Xue, R. C., Fang, Z., Zhang, M. X., Yi, Z. H., Wen, C. P., Shi, T. L. (2012). TCMID: Traditional Chinese Medicine integrative database for herb molecular mechanism analysis. Nucleic Acids Res. 41, D1089–D1095. doi: 10.1093/nar/gks1100
Yan, J. F. (2021). Study on market investigation and quality evaluation of Rubia cordifolia (Beijing: Beijing University of Chinese Medicine).
Yan, X. F., Wu, S. X., Wang, Y., Shang, X. H., Dai, S. J. (2004). Soil nutrient factors related to salidroside production of Rhodiola sachalinensis distributed in Chang Bai Mountain. Environ. Exp. Bot. 52, 267–276. doi: 10.1016/j.envexpbot.2004.02.005
Yan, Y. G., Wang, Y. H., Deng, C., Zhang, G., Chen, Y., Shen, X., et al. (2017). Effects of growth years, altitude, and light factors on contents of eight components in Rheum officinale. Chin. Tradition. Herb. Drugs 48, 2285–2291.
Yang, L. L., Zhao, Y. J., Zhang, Q., Cheng, L., Han, M., Ren, Y. Y., et al. (2019a). Effects of drought–re-watering–drought on the photosynthesis physiology and secondary metabolite production of Bupleurum chinense DC. Plant Cell Rep. 38, 1181–1197. doi: 10.1007/s00299-019-02436-8
Yang, S. T., Li, J. H., Zhao, Y. P., Chen, B. L., Fu, C. X. (2011). Harpagoside variation is positively correlated with temperature in Scrophularia ningpoensis Hemsl. J. Agric. Food Chem. 59, 1612–1621. doi: 10.1021/jf104702u
Yang, W., Ma, X. Y., Laaksonen, O., He, W. J., Kallio, H., Yang, B. R. (2019b). Effects of latitude and weather conditions on proanthocyanidins in blackcurrant (Ribes nigrum) of Finnish commercial cultivars. J. Agric. Food Chem. 67, 14038–14047. doi: 10.1021/acs.jafc.9b06031
Yu, R., Gao, M. J., Cui, B. B., Zhao, Y. S., Jin, M. Y., Meng, X. Y., et al. (2017). Determination of alizarin, purpurin and rubimaillin in Rubia cordifolia L. by HPLC-DAD. J. Harbin Med. University. 51, 195–199.
Zhan, P., Wang, F. Y., Xia, P. G., Zhao, G. H., Wei, M. T., Wei, F. G., et al. (2022). Assessment of suitable cultivation region for Panax notoginseng under different climatic conditions using MaxEnt model and high-performance liquid chromatography in China. Ind. Crops Prod. 176, 114416. doi: 10.1016/j.indcrop.2021.114416
Zhang, X. D., Yu, Y. G., Yang, D. F., Qi, Z. C., Liu, R. Z., Deng, F. T., et al. (2018). Chemotaxonomic variation in secondary metabolites contents and their correlation between environmental factors in Salvia miltiorrhiza Bunge from natural habitat of China. Ind. Crops Prod. 113, 335–347. doi: 10.1016/j.indcrop.2018.01.043
Zhao, Z. Y., Li, Y. D., Zhou, L. Y., Zhou, X. T., Xie, B. W., Zhang, W. J., et al. (2021). Prevention and treatment of COVID-19 using Traditional Chinese Medicine: A review. Phytomedicine 85, 153308. doi: 10.1016/j.phymed.2020.153308
Keywords: Rubia cordifolia L., purpurin, mollugin, environmental factors, planting
Citation: Wang Y, Liu H, Yu S, Zhang Y, Huang Y, He X and Chen W (2024) Effects of geographical, soil and climatic factors on the two marker secondary metabolites contents in the roots of Rubia cordifolia L. Front. Plant Sci. 15:1419392. doi: 10.3389/fpls.2024.1419392
Received: 18 April 2024; Accepted: 27 May 2024;
Published: 11 June 2024.
Edited by:
Zongxia Yu, Lushan Botanical Garden (CAS), ChinaReviewed by:
Jingang Wang, Northeast Agricultural University, ChinaCopyright © 2024 Wang, Liu, Yu, Zhang, Huang, He and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Chen, Y2hlbndlaUBpYWUuYWMuY24=; Shuai Yu, eXVzaHVhaUBpYWUuYWMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.