
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 15 April 2024
Sec. Plant Abiotic Stress
Volume 15 - 2024 | https://doi.org/10.3389/fpls.2024.1376427
This article is part of the Research Topic Plant Responses to Salt Stress View all 12 articles
 Yifan Duan1†
Yifan Duan1† Liqiong Jiang2†
Liqiong Jiang2† Ting Lei1
Ting Lei1 Keyu Ouyang1
Keyu Ouyang1 Cailei Liu1
Cailei Liu1 Zi’an Zhao1
Zi’an Zhao1 Yirui Li1
Yirui Li1 Lijuan Yang1
Lijuan Yang1 Jiani Li1
Jiani Li1 Shouli Yi3
Shouli Yi3 Suping Gao1*
Suping Gao1*Under salt stress, recretohalophyte Plumbago auriculata tetraploids enhance salt tolerance by increasing selective secretion of Na+ compared with that in diploids, although the mechanism is unclear. Using non-invasive micro-test technology, the effect of salt gland Ca2+ content on Na+ and K+ secretion were investigated in diploid and tetraploid P. auriculata under salt stress. Salt gland Ca2+ content and secretion rates of Na+ and K+ were higher in tetraploids than in diploids under salt stress. Addition of exogenous Ca2+ increased the Ca2+ content of the salt gland in diploids and is accompanied by an increase in the rate of Na+ and K+ secretion. With addition of a Ca2+ channel inhibitor, diploid salt glands retained large amounts of Ca2+, leading to higher Ca2+ content and Na+ secretion rate than those of tetraploids. Inhibiting H2O2 generation and H+-ATPase activity altered Na+ and K+ secretion rates in diploids and tetraploids under salt stress, indicating involvement in regulating Na+ and K+ secretion. Our results indicate that the increased Na+ secretion rate of salt gland in tetraploids under salt stress was associated with elevated Ca2+ content in salt gland.
● The Ca2+ content in salt glands and the rate of Na+ secretion were analyzed in diploid and tetraploid P. auriculata under salt stress, and high tetraploid rate of Na+ secretion was strongly correlated with high salt gland Ca2+ accumulation.
Salt stress is a major abiotic stress that affects plant growth (Ruan et al., 2010). In the early stages of salt stress, high salt concentrations limit plant water uptake, resulting in osmotic stress. With prolonged salt stress, accumulation of excessive salt ions can cause oxidative stress, ion toxicity, and nutrient deficiencies in plants (Munns and Tester, 2008). Plants increase salt tolerance by various mechanisms. In nonsucculent halophytes, one particular adaptation is the secretion of excess salt ions from stem and leaf surfaces, and plants that increase salt tolerance using this pathway are called recretohalophytes (Flowers et al., 2008; Guo et al., 2023). Recretohalophytes can secrete multiple ions, and secretion rates are influenced by rhizosphere ion concentrations (Ding et al., 2010).
The mechanism of salt secretion by the salt glands is still unclear, and only possible paths have been suggested. There are three hypotheses of secretion include (1) the role of the osmotic potential in salt secretion; (2) a transfer system that is similar to liquid flow in animals; (3) salt solution excretion by vesicles in the plasma membrane (Yuan et al., 2016). Secretion rates of Na+ and Cl– are usually higher than those of other ions (Yuan et al., 2016), which may be due to the many Na+ and Cl– transport channels on the plasma membrane of salt gland cells. However, there are some recretohalophytes, such as those in the family Plumbaginaceae, in which Ca2+ is the predominant ion secreted (Faraday and Thomson, 1986; Duan et al., 2023).
The Ca2+ ion can be a signaling molecule in plants in response to salt stress. Exogenous addition of Ca2+ inhibits Na+ uptake by roots under salt stress and reduces K+ loss, thereby maintaining plant Na+/K+ ratio (Jin et al., 2017). The ion K+ is an essential activator of various enzymes in plant metabolic processes, and therefore, maintaining K+ homeostasis under salt stress is crucial to increase salt tolerance (Horie et al., 2009). The plasma membrane Na+/H+ antiporter SOS1 plays a key role in Na+ efflux from cells and is activated by Ca2+ signaling. Salt stress increases cytosolic Ca2+ levels, leading to the activation of SOS3, which binds to SOS2 to form the SOS3–SOS2 complex, ultimately phosphorylating and activating SOS1 for Na+ efflux (Qiu et al., 2002; Zhu, 2016). The SOS1 relies on the activity of plasma membrane H+-ATPase for ion translocation across the membrane (Chen J. A. et al., 2010; Sanadhya et al., 2015; Che et al., 2019), whereas Ca2+ may regulate H+-ATPase activity (Sun et al., 2010). Hydrogen peroxide (H2O2) can be an important signaling molecule in regulating the Na+/K+ balance. Under salt stress, H2O2 promotes Na+ efflux by stabilizing the mRNA of SOS1, and inhibiting H2O2 production leads to increased K+ efflux under salt stress (Chung et al., 2008). The Ca2+ ion acts as an upstream signal for H2O2, suggesting that Ca2+ is involved in regulating the Na+/K+ balance by modulating H2O2 production.
In recretohalophytes, Ca2+ is involved in regulating ion secretion from salt glands, and exogenous addition of Ca2+ significantly increases Na+ secretion under salt stress (Ding et al., 2010). Salt gland excretion of ions in recretohalophytes depends on ion transport systems and vesicular transport (Li et al., 2020). The plasma membrane Na+/H+ antiporter SOS1 is a crucial pathway for salt gland Na+ secretion (Guo et al., 2020), and Ca2+ acts as an activating signal upstream of SOS1, potentially increasing Na+ secretion. Addition of Ca2+ also increases vesicular transport, promoting Na+ secretion (Ding et al., 2010).
Research on the ion channels involved in K+ secretion of recretohalophytes is limited, and currently, K+ secretion is proposed to primarily occur by a Na+-K+-Cl– cotransporter (Yuan et al., 2016). Under salt stress, plasma membrane depolarization in plant cells leads to activation of outward-rectifying K+ channels (DA-KORCs) and nonselective cation channels (DA-NSCCs), resulting in K+ efflux from cells (Shabala and Pottosin, 2014). However, whether DA-KORCs and DA-NSCCs are involved in K+ secretion in salt glands of recretohalophytes remains unclear. When exogenous Ca2+ channel inhibitors are added, which reduce plant Ca2+ levels, only the Na+ secretion rate is inhibited, without significant effects on K+ secretion rate (Kobayashi et al., 2007). Therefore, in addition to a Na+-K+-Cl– cotransporter, K+ secretion may also occur through other ion channels.
Plumbago auriculata (Plumbaginaceae) is a typical calcium-secreting plant that has important medicinal value. Under normal growth conditions, Ca2+ is the primary ion secreted by P. auriculata salt glands. Under NaCl stress in our previous study, tetraploid P. auriculata are more salt tolerant than diploids with better ion homeostasis and less morphology damage under saline conditions. The Na+ secretion rate in whole leaves of tetraploid P. auriculata was higher than that in whole leaves of diploids. However, the Ca2+ content in tetraploid leaves is significantly lower than that in diploid leaves (Duan et al., 2023). Those results contradict the results of previous studies that suggest Ca2+ promotes Na+ secretion under salt stress. In animals, salt gland secretions indicate that sustained elevation of Ca2+ content in salt glands serves as a primary signal for secretion activity (Shuttleworth and Thompson, 1989; Shuttleworth and Hildebrandt, 1999). Therefore, it was hypothesized that P. auriculata Na+ and K+ secretion rates are regulated by salt gland Ca2+ content, not by the overall Ca2+ content in leaves. Thus, in this study, changes in Ca2+ content in the salt glands of diploid and autotetraploid P. auriculata were investigated by adding exogeneous Ca2+, inhibiting Ca2+ transport channels, and suppressing the generation of H2O2 regulated by Ca2+ signaling and H+-ATPase activity under NaCl stress. Effects of Ca2+ and downstream substances on salt gland Na+ and K+ secretion rates were also examined. The results provide a new perspective for exploring the reasons behind increased salt tolerance in polyploid recretohalophytes.
Autotetraploid Plumbago auriculata Lam. (2n = 24) was induced by colchicine treatment of stem segments from diploid P. auriculata (2n = 12) in the laboratory of the Landscape Architecture Institute at Sichuan Agricultural University (Jiang et al., 2020). Both diploid and tetraploid cytotypes were obtained by tissue culture and were subcultured for 25 d. After root formation, all seedlings were transferred to a climate-controlled growth chamber. A total of 72 plants (36 diploids and 36 tetraploids) of similar size were grown for 3 months in a nutrient substrate composed of soil, vermiculite, and perlite in a 1:1:1 ratio (by volume). The plants were placed in an intelligent biochemical incubator (Ningbo Jiangnan Instrument Factory, Zhejiang, China) under controlled conditions: 25 °C/20 °C (day/night) temperature, 12-h day/12-h night photoperiod, 6,600 Lx light intensity, and 70% relative humidity. Observations on the structure and secretory components of the salt glands of diploids and tetraploids under normal growth conditions prior to salt stress provide a basic reference for analyzing ion secretion from diploid and tetraploid salt glands The sixth mature leaf, counted from the top, was selected from diploid and tetraploid plants for structural observation and analysis of salt gland secretory components.
To determine the effect of salt gland Ca2+ content on Na+ and K+ secretion in diploid and tetraploid plants under salt stress, plants were transferred to a hydroponic system containing 1/6th-strength Hoagland’s solution (pH 6.0 ± 0.2), with the nutrient solution refreshed at 5-d intervals. After 15 d of cultivation in the hydroponic system, diploid and tetraploid plants with similar growth were selected for six different treatments (Table 1). 300 mM NaCl was selected for salt stress treatment based on our preliminary experiments that it is the highest concentration at which both diploids and tetraploids will be stressed, but diploid salt gland secretion will not be inhibited because the stress is too severe. Treatments included LaCl3, used to inhibit Ca2+ channels; Na3VO4, used to inhibit H+-ATPase activity; and Diphenyleneiodonium chloride (DPI), used to inhibit plasma membrane NADPH oxidase. The CaCl2 and the inhibitors LaCl3, DPI, and Na3VO4 were pre-treated 20 min before the salt stress treatment. Salt stress treatment duration was 2 d, with six plants per treatment for each ploidy level.
Leaf samples were prepared following the method described by Ding et al. (2010) with slight modifications. Fresh leaves of diploid and tetraploid P. auriculata were cut into small pieces of 0.5 cm × 0.5 cm from the leaf margin to the middle position of the leaf vein using a razor blade. Leaf pieces were fixed in 2.5% glutaraldehyde solution at room temperature for 24 h. After fixation, leaf samples were washed with 0.05 mM HNO3 to remove salt crystals on leaf surfaces, followed by rinsing with distilled water. Leaves were gently dried with absorbent paper and then dehydrated in a graded ethanol series (30%, 50%, 70%, 80%, 90%, and 95% ethanol). Dehydration time for the 30% to 90% ethanol series was 20 min, whereas it was 1 h for 95% ethanol. After dehydration, samples were dried using a critical point dryer (Leica, Nussloch, Germany). Dried samples were observed and photographed using a scanning electron microscope (SEM; Carl Zeiss, Oberkochen, Germany), and the area of salt glands was measured using ImageJ software. Twenty salt glands from six individual plants were measured for both diploid and tetraploid P. auriculata.
Leaf samples were prepared following the method described by Yuan et al. (2013). Fresh leaves of diploid and tetraploid P. auriculata were cut into small pieces as previously described. Leaf pieces (0.5 cm × 0.5 cm) were fixed in Carnoy’s fixative (ethanol:acetone = 3:1) and then washed with 0.05 mM HNO3 to remove salt crystals on leaf surfaces. Leaves were rinsed with distilled water, gently dried with absorbent paper, and then decolorized in 70% ethanol. Decolorized leaves were mounted with Hoyer’s solution, making temporary slides. To measure density and area of salt glands, slides were observed and photographed using a fluorescence microscope (Olympus, Tokyo, Japan) under UV excitation light (330–380 nm) at 20× magnification. Twenty different fields of view from six leaves were measured for both diploid and tetraploid P. auriculata.
Fresh leaves of diploid and tetraploid P. auriculata were cross-sectioned in the middle using a sharp blade. Leaf pieces (1.0 cm × 1.0 cm) were quickly fixed in formalin-aceto-alcohol (FAA) fixative at room temperature for 24 h. After fixation, leaf tissues were rinsed with distilled water for 10 min, dried with absorbent paper, and then sequentially dehydrated in a gradient series of ethanol (70%, 80%, 90%, 95%, 100%, and 100% ethanol for two times). After dehydration, tissues were cleared with xylene and then embedded in paraffin in a constant-temperature oven at 60 °C. Leaf-containing paraffin blocks were sectioned using a microtome (Leica, Nussloch, Germany), producing 4 μm-thick sections. Sections were placed on glass slides coated with glycerol mounting medium and dried in a constant-temperature oven at 50 °C. Following dewaxing with xylene, rehydration with a gradient series of ethanol, and staining with SafraninO/Fast green, sections were dehydrated with ethanol and cleared with xylene. Last, sections were mounted with Canada balsam and air-dried at low temperature. Salt gland anatomical structures were observed and photographed using a fluorescence microscope (Olympus), and the external cuticle layer of salt glands was observed under UV excitation light (330–380 nm) using fluorescence mode. Twenty salt glands from six individual plants were observed for both diploid and tetraploid P. auriculata.
Salt gland secretory crystals were observed and photographed using an SEM (Carl Zeiss) equipped with an energy dispersive X-ray (EDX) system. Cross sections of mature leaf portions (0.5 cm × 0.5 cm) from the lower part of P. auriculata diploid and tetraploid leaves were cut with a sharp blade. Leaf sections were placed in a vacuum dryer for 1 h, and then, sections were attached to conductive adhesive tape on the SEM stage for EDX analysis of crystals secreted on leaf surfaces. Twenty salt glands from six leaves were analyzed for both diploid and tetraploid P. auriculata.
After 2-d treatment of diploid and tetraploid P. auriculata, mature leaves from the same position were collected and washed with 0.05 mM HNO3 to remove salt crystals on leaf surfaces. Leaves were rinsed with double-distilled water and quickly dried with absorbent paper to remove surface water. The lower epidermis of leaves was carefully torn off and placed in 0.4 mM Floura-8 Ca fluorescence probe dye solution, with addition of Pluronic F-127 to enhance fluorescence. To ensure sufficient contact between dye and leaf epidermis, a vacuum pump was used for 10 min, followed by incubation at room temperature for 40 min (avoiding light). After incubation, leaf epidermis tissues were thoroughly rinsed with double-distilled water to remove residual dye. Rinsed epidermis tissue was mounted with a fluorescence decay-resistant medium, making temporary slides. The fluorescence of Ca2+ in salt glands was observed with a laser scanning confocal microscope (Olympus LSCM) in the FITC channel, and fluorescence intensity was analyzed using ImageJ software. Twenty salt glands from six plants were observed for each treatment.
Instantaneous secretion rates of Na+ and K+ from individual salt glands on the abaxial side surface were measured using an NMT system (NMT-100SIM-YG; Younger USA LLC, Amherst, MA, USA) as described by Chen Z. et al. (2010). Leaves from the lower part of the same position of both diploid and tetraploid P. auriculata were collected following 2 d of salt stress treatment. The abaxial side epidermis of leaves was gently peeled off using forceps and fixed in a culture dish with the outer surface facing up. Test solutions (Na+: 1.0 mM NaCl, 0.1 mM KCl, 0.2 mM MES, pH 5.8; K+: 30 mM NaCl, 0.5 mM KCl, 0.2 mM MES, pH 5.8) were added to the culture dish, and samples were left undisturbed for 30 min before measurement. Under a microscope, a target salt gland was located, and a selective microelectrode (Na+: XY-SJ-Na; K+: XY-SJ-K; Xuyue, Beijing, China) was positioned approximately 5 μm above the outer surface of the salt gland without touching it. Each sample was measured for 5 min. Each group included nine replicates. The Na+ and K+ flux data were read directly and outputted using imFluxes v3.0 software (Xuyue, Beijing, China). Positive values represented Na+ and K+ secretion from a salt gland to the external environment, whereas negative values represented absorption from the external environment into a salt gland.
Data were analyzed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) for Windows, and all values are reported as the mean ± standard deviation (SD). Two-way ANOVA and Student’s t-test were used to compare means of different treatments for each data set at the significance level of P < 0.05.
Polyploidization significantly increased salt gland area of P. auriculata, with the area of tetraploid salt glands 41.1% larger than that of diploid salt glands. However, salt gland density decreased significantly in tetraploid plants (Figure 1). There were no significant differences in salt gland structure between diploid and tetraploid P. auriculata (Figures 2, 3). Salt glands in both cytotypes consisted of 16 salt gland cells, with four collecting cells, four accessory cells, four cup cells, and four secretory cells, each with a secretion pore in the center.
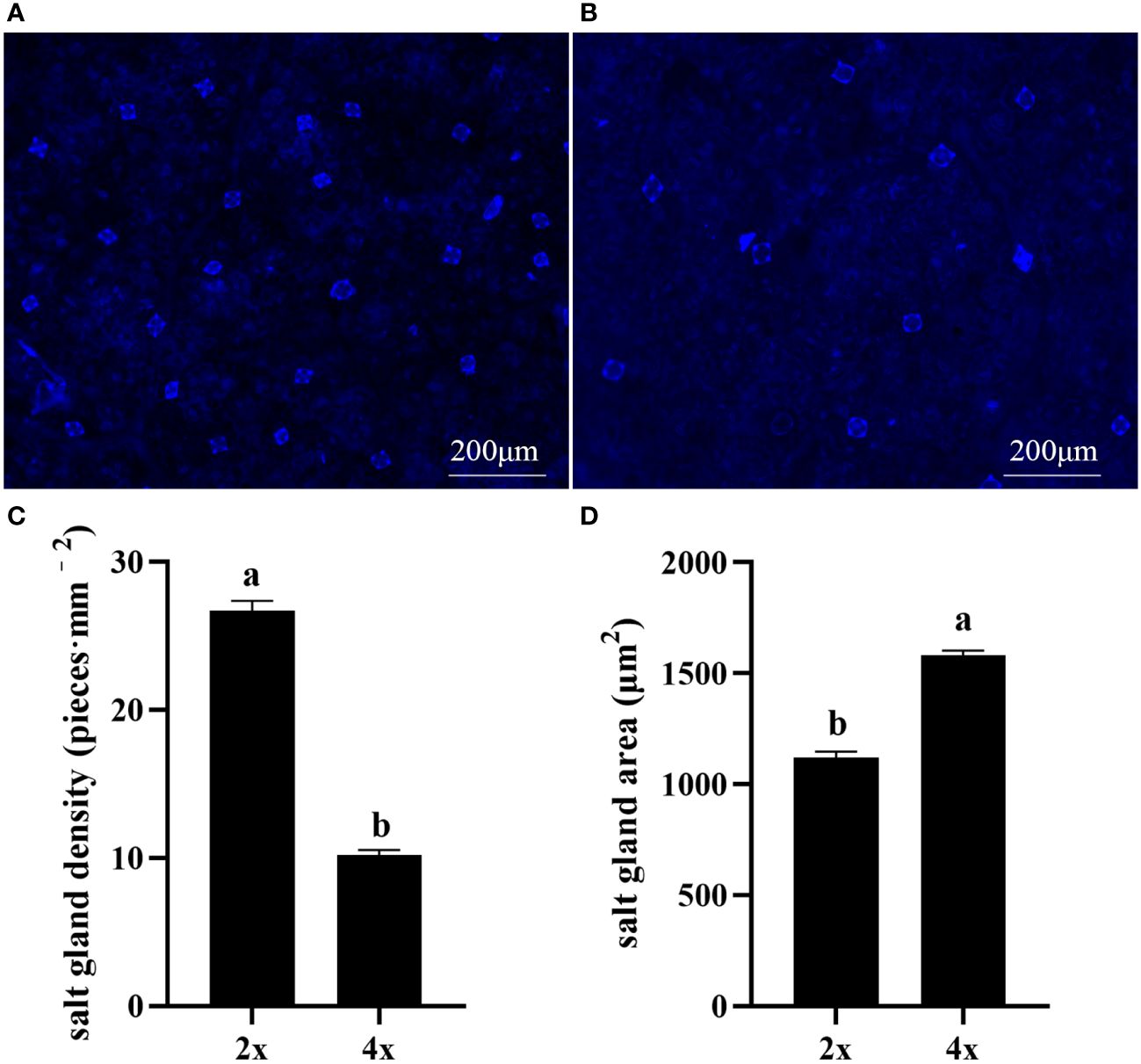
Figure 1 Salt gland density and salt gland area in diploid (2x) and tetraploid (4x) Plumbago auriculata. (A) Diploid salt glands and (B) tetraploid salt glands. (C) Salt gland density and (D) salt gland area of single salt gland in diploids and tetraploids. Different lowercase letters indicate significant differences (P < 0.05) between diploids and tetraploids.
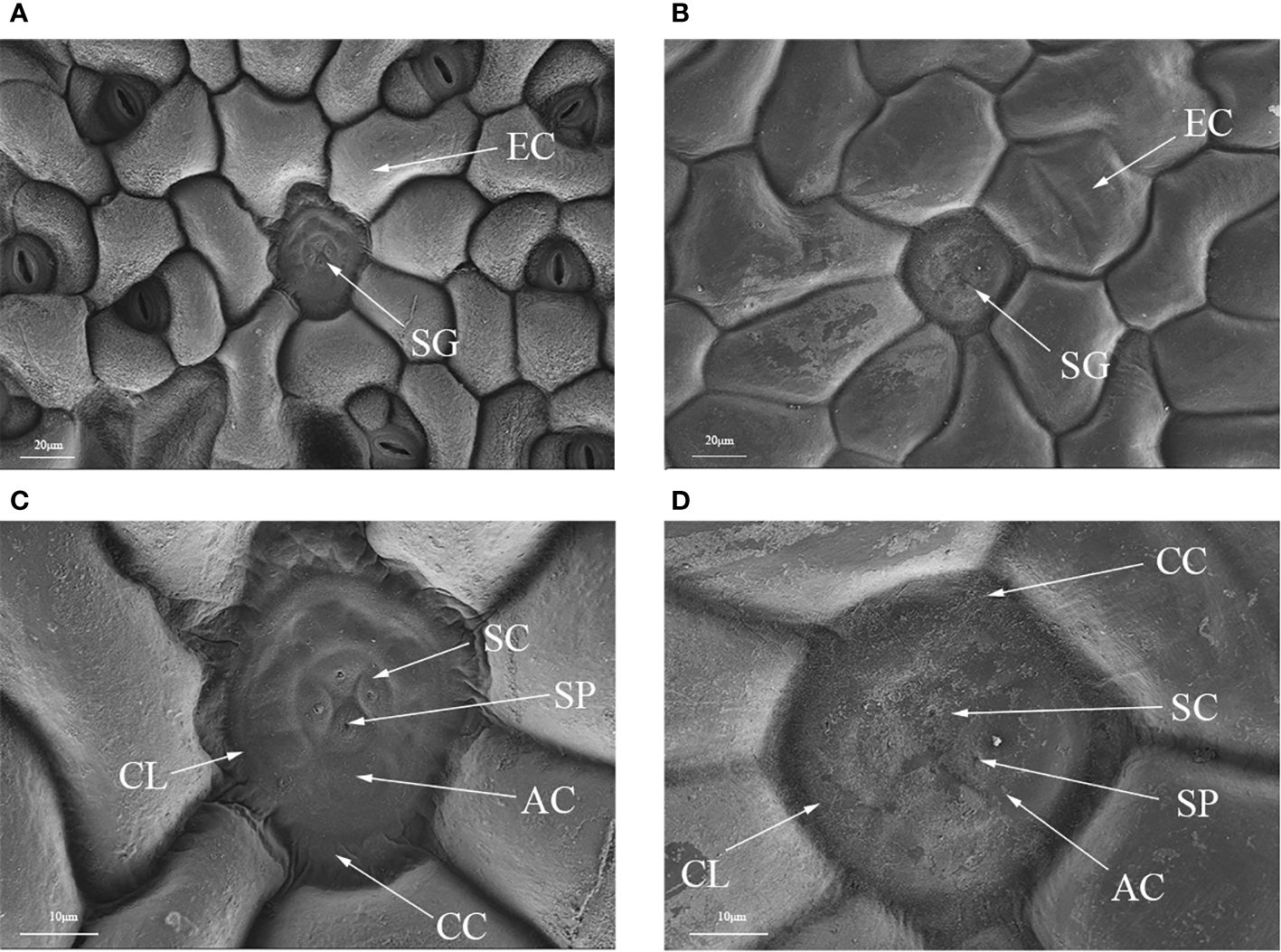
Figure 2 Optical microscope and scanning electron microscope images of salt glands in diploid and tetraploid Plumbago auriculata. (A) Diploid salt gland and (B) tetraploid salt gland observed under an optical microscope. (C) Diploid salt gland and (D) tetraploid salt gland observed under a scanning electron microscope. SG, salt gland; EP, epidermal cell; SC, secretory cell; AC, accessory cell; SP, secretion pore; CC, collecting cell; CL, cuticle layer.
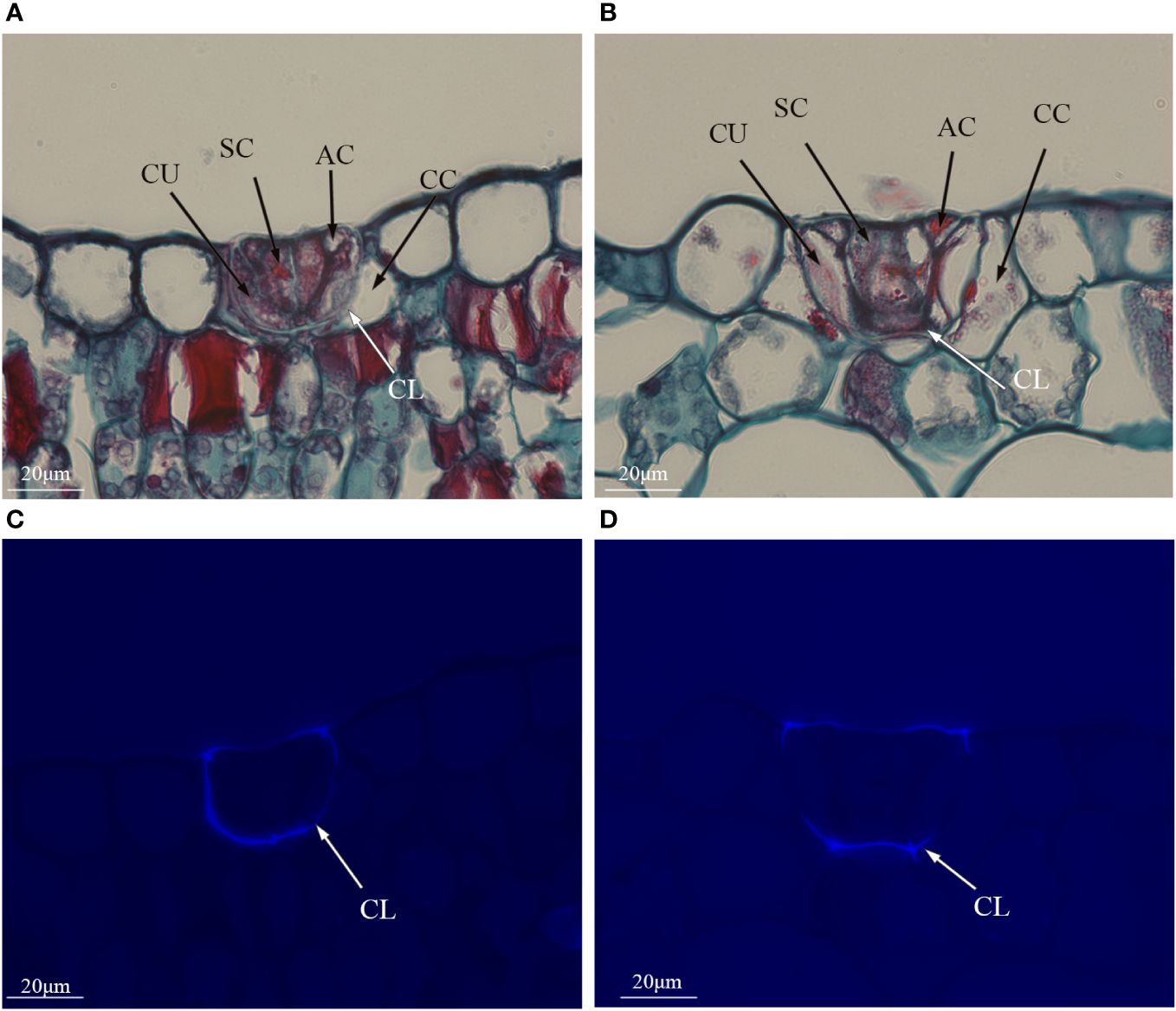
Figure 3 Histological cross sections of salt glands in diploid and tetraploid Plumbago auriculata. Cross section of (A) diploid salt gland and (B) tetraploid salt gland. Fluorescence of the cuticle layer in (C) diploid salt gland and (D) tetraploid salt gland. SC, secretory cell; AC, accessory cell; CC, collecting cell; CU, cup cell; CL, cuticle layer.
In SEM images of secreted salt gland crystals, the volume of salt crystals secreted on leaf surfaces was significantly larger in tetraploids than in diploids, indicating higher secretion capacity of individual salt glands in tetraploids under normal growth conditions. According to the EDX analysis of secreted crystals in diploids and tetraploids, the main ion secreted was Ca2+, followed by Mg2+, whereas the secretion of Na+ and Cl– was minimal (Figure 4). This result suggested that polyploidization of P. auriculata did not significantly affect composition of salt gland secretions.
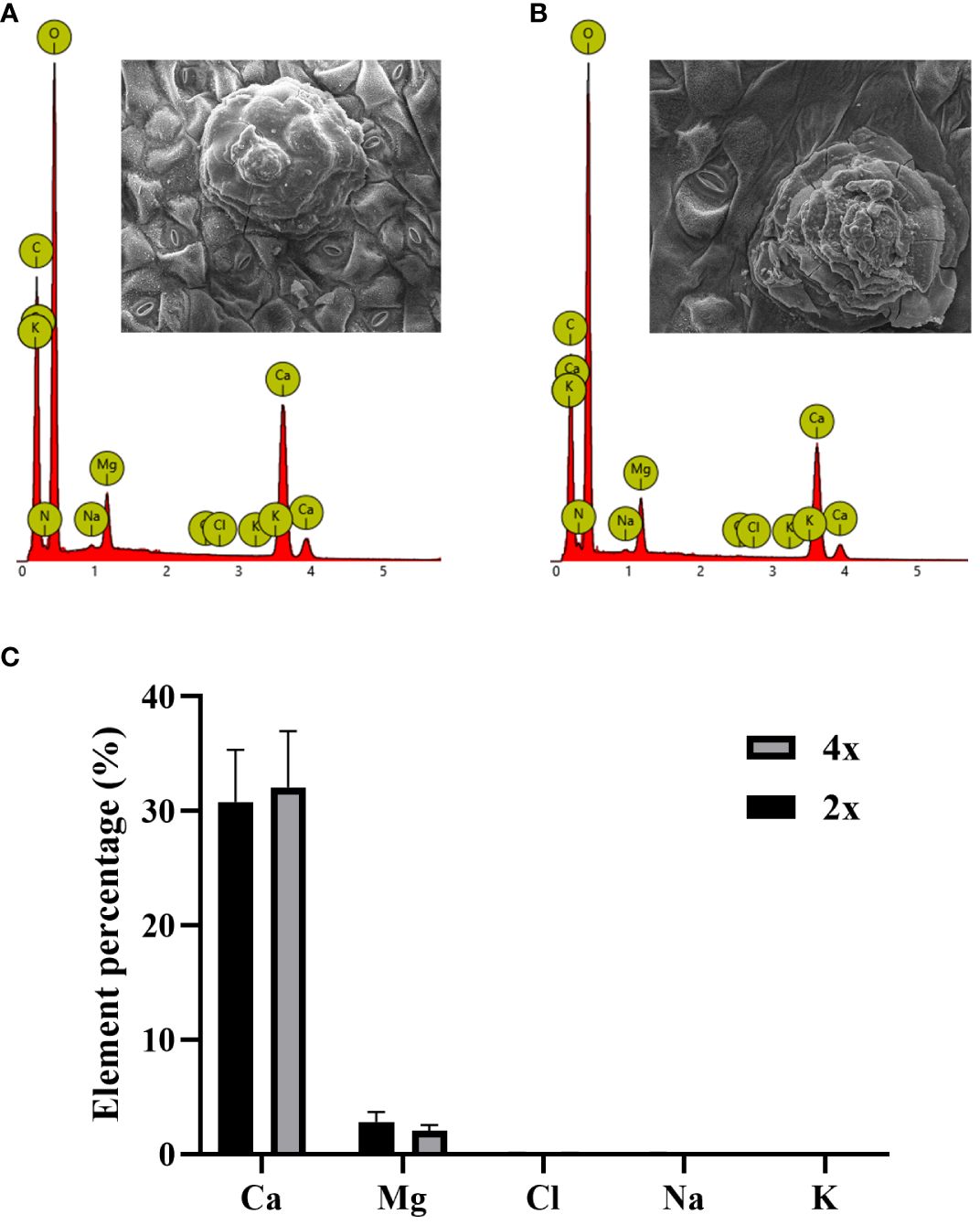
Figure 4 Ion composition of crystals secreted by salt glands in diploid and tetraploid Plumbago auriculata. Energy dispersive spectroscopy scan of secreted crystals from (A) diploid and (B) tetraploid salt glands. Element percentage of crystals secreted from the salt glands of (C) diploids (2x) and tetraploids (4x).
Under control conditions, Ca2+ content in salt glands of diploids was significantly higher than that in salt glands of tetraploids, with content in diploids 4.5 times higher (Figure 5). Compared with the control, NaCl stress significantly increased Ca2+ accumulation in the salt glands of tetraploid plants, whereas it did not significantly affect Ca2+ accumulation in diploid salt glands. After adding CaCl2, Ca2+ content in the salt glands of diploids under NaCl stress increased significantly compared with that in the NaCl treatment alone, whereas no significant change was observed in tetraploids, and then, there was no significant difference in Ca2+ content between diploid and tetraploid salt glands. The addition of the Ca2+ inhibitor LaCl3 suppressed NaCl-induced accumulation of Ca2+ in tetraploid salt glands, resulting in no significant difference in Ca2+ accumulation compared with the control. Notably, Ca2+ content in the salt glands of diploids increased significantly with LaCl3 treatment, surpassing that of both the NaCl treatment alone and tetraploid salt glands treated with LaCl3. When the H2O2 inhibitor DPI or the H+-ATPase inhibitor Na3VO4 was added, Ca2+ content in the salt glands of both diploids and tetraploids decreased significantly compared with that in the NaCl treatment alone.
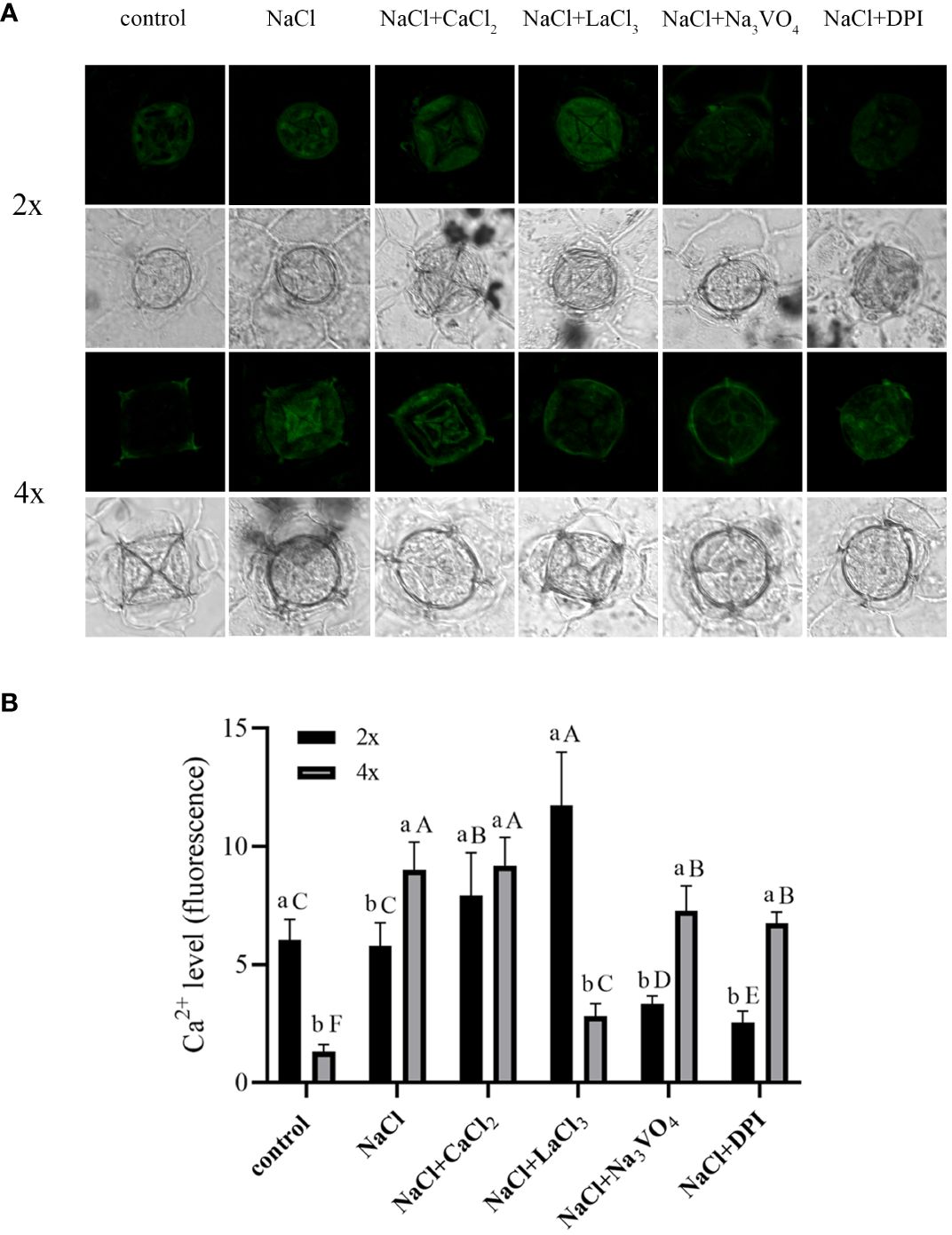
Figure 5 Changes in Ca2+ accumulation in salt glands of diploid (2x) and tetraploid (4x) Plumbago auriculata induced by NaCl stress and effects of LaCl3, Na3VO4, and DPI on Ca2+ accumulation under NaCl stress. (A) Representative graphs showing changes in Ca2+ accumulation in (top) diploid and (bottom) tetraploid salt glands before and after salt stress, as well as effects under LaCl3, Na3VO4, and DPI treatments. (B) Fluorescence intensity values from (A) measured by Image J software. For each treatment, nine salt glands from six individual plants were observed and quantified. In (B), different capital letters indicate significant differences (P < 0.05) between different treatments of the same ploidy level, whereas different lowercase letters indicate significant differences (P < 0.05) between ploidy levels of the same treatment.
Salt stress induces strong and stable Na+ secretion. After salt stress, Na+ secretion rate increased significantly in both diploids and tetraploids and was 212.11 and 50.92 times higher, respectively, than that in the control. The Na+ secretion rate was 5.28 times higher in tetraploids than in diploids (Figures 6A, B). Thus, salt stress activated Na+ secretion in P. auriculata, and Na+ secretion capacity in individual salt glands under salt stress was greater in tetraploids than in diploids. Addition of CaCl2 significantly increased Na+ secretion in diploid salt glands under salt stress, with secretion 1.8 times higher than that in the NaCl treatment alone. However, CaCl2 addition significantly inhibited Na+ secretion in tetraploid salt glands, reaching 67.56% of the NaCl treatment alone. Nevertheless, the Na+ secretion rate in tetraploid salt glands remained significantly higher than that in diploid salt glands (Figure 6C). After addition of the Ca2+ channel inhibitor LaCl3, Na+ secretion in tetraploids was severely inhibited, reaching only 6.5% and 9.68% of that in NaCl and CaCl2 treatments, respectively, and with secretion only 3.26 times higher than that in the control. By contrast, the Na+ secretion rate in diploid salt glands increased significantly and was 2.60 and 1.44 times higher than that in NaCl and CaCl2 treatments, respectively, and was 7.25 times higher than that in tetraploid salt glands under the same conditions (Figure 6D). Pretreatment with the H+-ATPase inhibitor (Na3VO4) or the H2O2 inhibitor (DPI) significantly decreased Na+ secretion rate under salt stress in both diploids and tetraploids, indicating the important roles of endogenous H+-ATPase and H2O2 in increasing Na+ secretion rate under salt stress (Figures 6E, F). Overall, the Na+ secretion rate in tetraploids was lower than that in diploids only in the LaCl3+NaCl treatment, whereas in other treatments, the Na+ secretion rate was higher in tetraploids than in diploids (Figure 6G).
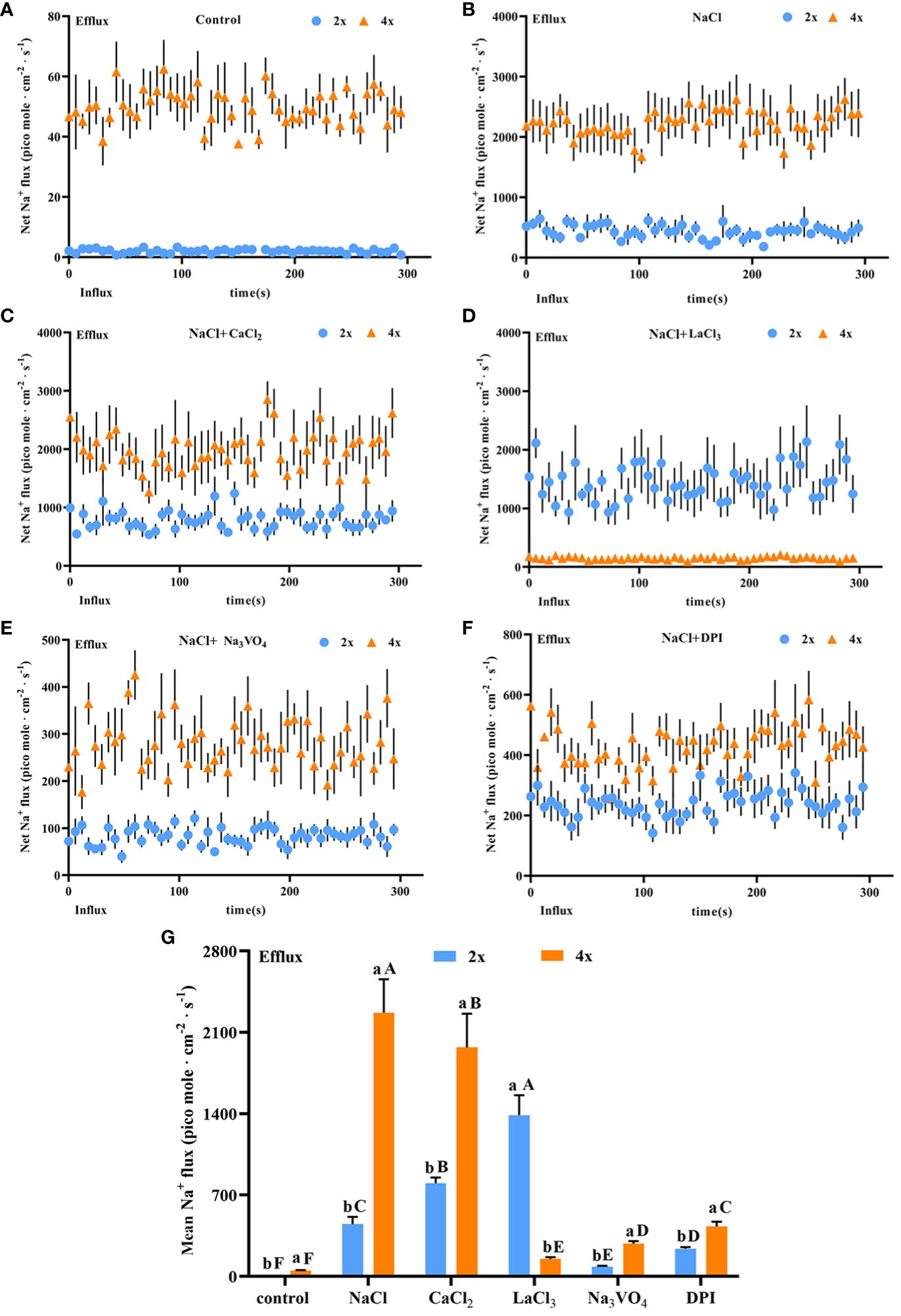
Figure 6 Changes in Na+ instantaneous and average secretion rates in salt glands of diploid (2x) and tetraploid (4x) Plumbago auriculata under NaCl stress and effects of LaCl3, Na3VO4, and DPI on Na+ secretion rate under NaCl stress. (A) Instantaneous Na+ secretion rate in salt glands under control conditions. (B) Instantaneous Na+ secretion rate in salt glands under NaCl stress. (C) Instantaneous Na+ secretion rate in salt glands under NaCl stress with exogenous CaCl2 addition. (D) Instantaneous Na+ secretion rate in salt glands under NaCl stress with LaCl3 addition. (E) Instantaneous Na+ secretion rate in salt glands under NaCl stress with Na3VO4 addition. (F) Instantaneous Na+ secretion rate in salt glands under NaCl stress with DPI addition. (G) Average Na+ secretion rate (~300 s) in different treatments. Each point represents the mean of the nine salt glands collected from six individual plants.
Salt stress induced a significant increase in K+ secretion rate in individual salt glands of both diploids and tetraploids, with the K+ secretion rate in tetraploids significantly higher than that in diploids by 3.20 times. However, the Na+/K+ secretion rate ratio was also higher in tetraploids, indicating stronger selective secretion of Na+ in tetraploids than in diploids under salt stress (Figures 7A, B). After addition of CaCl2, K+ secretion in tetraploid salt glands under salt stress was inhibited, reaching 60.52% of that in the NaCl treatment, whereas K+ secretion in diploid salt glands increased significantly and was 3.51 times higher than that in the NaCl treatment (Figure 7C).
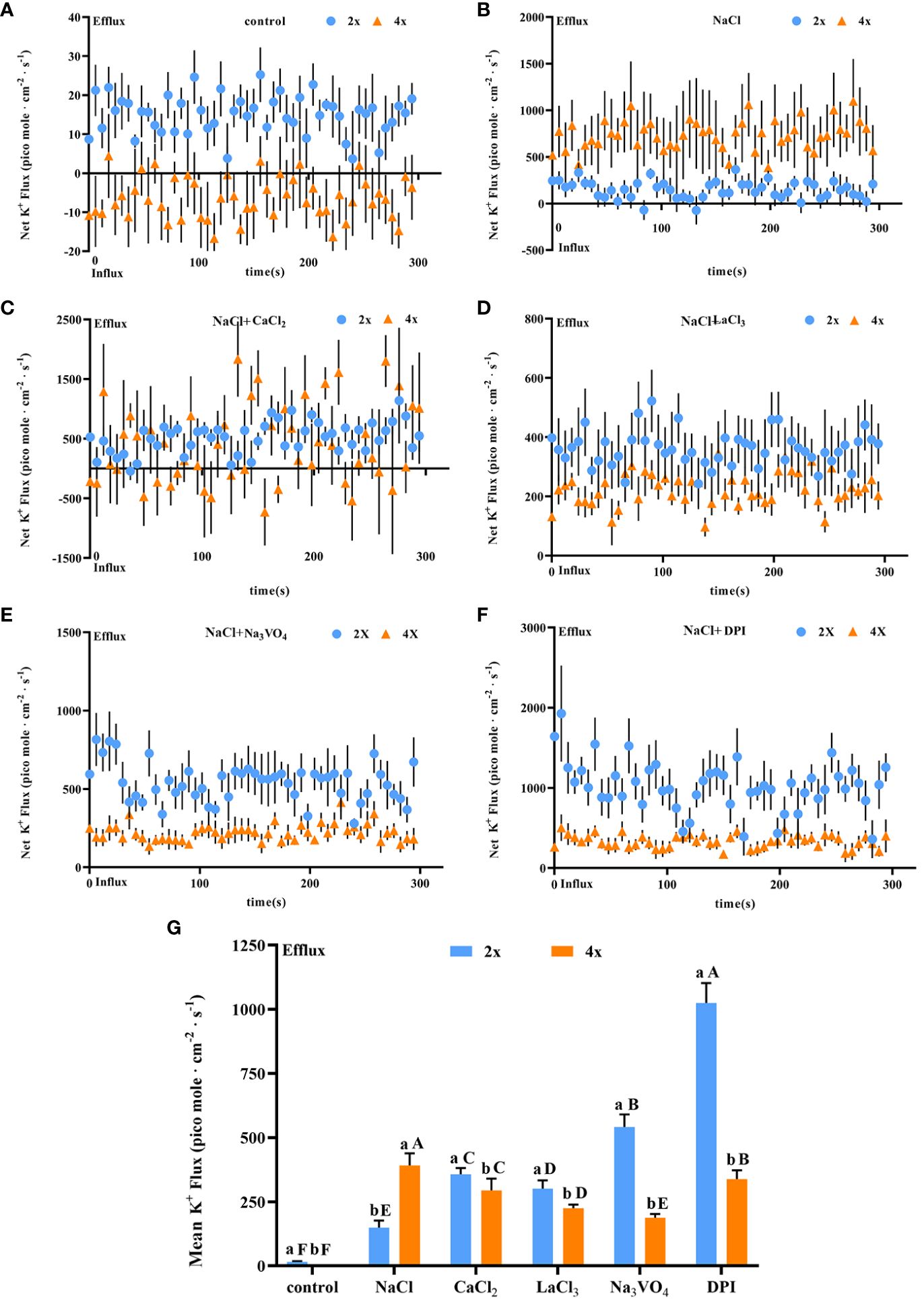
Figure 7 Changes in K+ instantaneous and average secretion rates in salt glands of diploid (2x) and tetraploid (4x) Plumbago auriculata induced by NaCl stress and effects of LaCl3, Na3VO4, and DPI on K+ secretion rate under NaCl stress. (A) Instantaneous K+ secretion rate in salt glands under control conditions. (B) Instantaneous K+ secretion rate in salt glands under NaCl stress. (C) Instantaneous K+ secretion rate in salt glands under NaCl stress with exogenous CaCl2 addition. (D) Instantaneous K+ secretion rate in salt glands under NaCl stress with LaCl3 addition. (E) Instantaneous K+ secretion rate in salt glands under NaCl stress with Na3VO4 addition. (F) Instantaneous K+ secretion rate in salt glands under NaCl stress with DPI addition. (G) Average K+ secretion rate (~300 s) in different treatments. Each point represents the mean of the nine salt glands collected from six individual plants.
The K+ secretion rate was inhibited in tetraploid plants by the addition of LaCl3 compared with NaCl alone due to the inhibitory effect of LaCl3 on K+ channels (Wegner et al., 1994). However, it increased in diploid plants. This may be due to the fact that in addition to the inhibitory effect of LaCl3 on K+ channels the presence of large amounts of retained Ca2+ in the salt glands increased the overall secretion level including the K+ secretion rate (Figure 7D). Notably, the addition of the H+-ATPase inhibitor (Na3VO4) or the H2O2 inhibitor (DPI) inhibited K+ secretion in tetraploid salt glands under salt stress, whereas in diploid salt glands, secretion increased significantly (Figures 7E, F). Overall, the K+ secretion rate in tetraploid salt glands was higher than that in diploid salt glands only in the NaCl treatment, and in other treatments, the rate was lower in tetraploids than in diploids (Figure 7G).
There may be a positive correlation between salt gland size and secretion rate (Feng et al., 2014; Mi et al., 2021). Polyploidization in P. auriculata significantly increased the size of salt glands, and under normal growth conditions, the volume of secreted crystals also increased significantly. It is hypothesized that polyploidization enhances the secretory capacity of individual salt glands of P. auriculata under normal growth conditions.
Under control conditions, Ca2+ content in tetraploid salt glands was only 22.22% of that in diploid glands, indicating that tetraploids decreased the allocation of Ca2+ to salt glands and increased that to leaf mesophyll cells for plant growth and metabolism (Dayod et al., 2010; Gilliham et al., 2011; Nomura and Shiina, 2014). In a previous study, under control conditions, P. auriculata had significantly lower Ca2+ secretion rates in tetraploid leaves than in diploid leaves, only reaching 33% of the diploid secretion (Duan et al., 2023). Therefore, the decrease in Ca2+ allocation to salt glands might be an important factor contributing to the decrease in Ca2+ secretion rate in tetraploids compared with that in diploids under control conditions. Salt stress stimulates Ca2+ influx into cells (Dong et al., 2022) but also inhibits root Ca2+ uptake (Guo et al., 2022), leading to a decrease in overall Ca2+ content in plants (Guo et al., 2015). However, although Ca2+ content showed no significant change in diploid salt glands under salt stress, it increased significantly in tetraploid salt glands, indicating that tetraploids significantly increased Ca2+ transport to salt glands.
With loss of Ca2+, selective transport of Ca2+ to salt glands requires increases in energy consumption (Dassanayake and Larkin, 2017). Under control conditions, tetraploids decreased the allocation of Ca2+ to salt glands, thereby reducing the energy expenditure for transport and retaining more Ca2+ in leaf mesophyll cells as essential nutrients and signaling molecules for normal plant growth and metabolism (Dayod et al., 2010). Under salt stress, Ca2+ promotes Na+ secretion (Mahajan et al., 2008; Guo et al., 2009), and increased allocation of Ca2+ to salt glands might be the reason for higher Na+ secretion rate in tetraploids (Duan et al., 2023).
Ca2+ content in salt glands is affected by both Ca2+ transport from the chloroplasts and Ca2+ secretion from the salt glands. No direct evidence has been obtained to confirm the pathway of salt transport into the salt gland, but ion carriers or channels, plasmodesmata and vesicles may play an important role in transporting (Yuan et al., 2016). Ca2+ influx in plant cells is predominantly mediated by NSCC, and it has been shown that Ca2+ channel inhibitors do not only block Ca2+ influx but also Ca2+ efflux (Zherelova et al., 1994), and La3+ can also block outward current (Terry et al., 1992). The diploid salt glands secreted large amounts of Ca2+ under control conditions, and the addition of LaCl3 resulted in a significant increase in the Ca2+ content of the salt glands, probably due to the inhibition of Ca2+ secretion leading to its accumulation in the salt glands. At this time, we found that the Ca2+ content in diploid salt glands was significantly higher than that in tetraploids, and the rate of Na+ secretion from salt glands was also significantly higher than that in tetraploids, so we hypothesized that the Ca2+ content of salt glands was one of the important factors affecting the rate of Na+ secretion.
It was shown that Ca2+ promotes the secretion of Na+ from salt glands of recretohalophytes (Ding et al., 2010; Maeda, 2019). In our previous study on whole-leaf secretion rates in P. auriculata under salt stress, Ca2+ content in diploid leaves was higher than that in tetraploid leaves, but the Na+ secretion rate was lower in diploids than in tetraploids (Duan et al., 2023). Besides, it has been suggested in the other research that K+ in the salt gland promotes salt gland NaCl secretion (Feng et al., 2015). After salt stress, Ca2+ content in diploid salt glands was significantly lower than that in tetraploid salt glands, and the Na+ secretion rate in individual diploid salt glands was also significantly lower. Therefore, it was hypothesized that Ca2+ content in salt glands is a key factor determining salt gland Na+ secretion rate, not the overall Ca2+ content in leaves. Besides, increasing Na+ secrete rate is companied with increased Ca2+ content in salt gland of tetraploid. The plasma membrane Na+/H+ antiporter (SOS1) been shown to be involved in Na+ secretion in recretohalophyte with SOS1 genes up-regulated after salt treatment and Na+ secretion rate was decreased when Silencing SOS1 (Guo et al., 2020; Zhang et al., 2017). The higher secretion rate in tetraploid in salt glands under salt stress might suggested higher SOS1gene expression, and the molecular mechanisms require further research.
Notably, although addition of CaCl2 did not significantly affect Ca2+ content in tetraploid salt glands, the Na+ secretion rate in tetraploids was significantly lower than that in the NaCl treatment alone. It was presumed that because Ca2+ content of salt glands did not increase, the decrease in Na+ secretion from salt glands was due to CaCl2 reducing the activation of Na+ secretion by NaCl stress (Mulaudzi et al., 2020).
The Ca2+ ion is an important secondary messenger that regulates H+-ATPase activity and H2O2 generation (Demidchik and Shabala, 2018; Shabala, 2019). In the present study, we found that inhibition of H+-ATPase activity or H2O2 production inhibited both diploid and tetraploid Na+ secretion rates in P. auriculata. Ca2+ may also indirectly regulate Na+ secretion through H+-ATPase and H2O2 (Kong et al., 2016; Wang et al., 2020), and the mechanisms need to be further investigated.
Although the mechanism of salt secretion in recretohalophytes remains not fully understood, salt secretion is an active transport process that requires H+-ATPase to provide energy (Chen JA et al., 2010). Therefore, inhibiting H+-ATPase activity significantly suppresses ion secretion in salt glands. In this study, inhibiting H+-ATPase significantly inhibited Na+ secretion in both diploids and tetraploids. Notably, under salt stress, inhibiting H+-ATPase activity suppressed K+ secretion in tetraploids but significantly increased it in diploids. Therefore, it was hypothesized that the K+ efflux channels DA-KORCs and DA-NSCCs, activated by plasma membrane depolarization, were involved in K+ secretion in P. auriculata salt glands (Shabala et al., 2006; Sun et al., 2009). Therefore, inhibiting H+-ATPase activity induced severe depolarization of the plasma membrane in diploid salt gland cells, activating DA-KORCs and DA-NSCCs efflux channels (Goncalves et al., 2000) and increasing the rate of K+ secretion. Additionally, Ca2+ inhibits the activation of the K+ efflux channels DA-KORCs and DA-NSCCs by depolarization, and the decrease in Ca2+ content in salt glands after H+-ATPase inhibition contributed to the increase in K+ efflux and increased K+ secretion. Hydrogen peroxide is a signaling molecule that increases H+-ATPase activity (Lu et al., 2013), and the addition of the NADPH oxidase inhibitor DPI, which inhibits H2O2 production, increases the loss of K+ caused by depolarization of the plasma membrane (Ma et al., 2012). By contrast, the secretion of K+ was inhibited in tetraploids following inhibition of H+-ATPase or H2O2, suggesting that the effect of depolarization-induced K+ efflux on tetraploids was relatively minor than diploids.
Rates of Na+ and K+ secretion in salt glands of the recretohalophyte P. auriculata were regulated by Ca2+ content in the glands. The high Ca2+ content in salt glands was an important factor contributing to higher Na+ secretion rates in tetraploids than in diploids under salt stress. The downstream signals of Ca2+, H2O2 and H+-ATPase, were also involved in regulating Na+ and K+ secretion rates. In addition to directly promoting ion secretion, Ca2+ might have indirectly regulated Na+ and K+ secretion by modulating H2O2 and H+-ATPase. Inhibiting H2O2 production or H+-ATPase activity significantly increased the rate of K+ secretion in diploids under salt stress, suggesting the involvement of depolarization-activated K+ efflux channels (DA-KORCs and DA-NSCCs) in salt stress-induced salt gland K+ secretion. Overall, polyploidization in P. auriculata led to an increase in utilization efficiency of Ca2+, resulting in increased accumulation of Ca2+ in salt glands under salt stress. This, in turn, increased Na+ secretion from salt glands and decreased Na+ accumulation in plant tissues, thereby mitigating the damage caused by salt stress.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
YD: Writing – original draft, Writing – review & editing. LJ: Funding acquisition, Writing – review & editing. TL: Writing – review & editing. KO: Writing – review & editing. CL: Writing – review & editing. ZZ: Writing – review & editing. YL: Writing – review & editing. LY: Writing – review & editing. JL: Writing – review & editing. SY: Writing – review & editing. SG: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Sichuan Province (project no. 2022NSFSC0099), the Sichuan Province Science and Technology Support Program (project no. 2021YFYZ0006), the Science and Technology Department of Sichuan Province (project no. 2021YJ0497), the Doctoral Fund of Chongqing Industry Polytechnic College (project no. 2023GZYBSZK2-02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Che, B., Cheng, C., Fang, J., Liu, Y., Yu, B. (2019). The recretohalophyte Tamarix TrSOS1 gene confers enhanced salt tolerance to transgenic hairy root composite cotton seedlings exhibiting virus-induced gene silencing of GhSOS1. Int. J. Mol. Sci. 20, 2930. doi: 10.3390/ijms20122930
Chen, Z., Newman, I., Zhou, M., Mendham, N., Zhang, G., Shabala, S. (2010). Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ. 28, 1230–1246. doi: 10.1111/j.1365-3040.2005.01364.x
Chen, J. A., Xiao, Q. A., Wu, F. H., Dong, X. J., He, J. X., Pei, Z. M., et al. (2010). Nitric oxide enhances salt secretion and Na+ sequestration in a mangrove plant, Avicennia marina, through increasing the expression of H+-ATPase and Na+/H+ antiporter under high salinity. Tree Physiol. 30, 1570–1585. doi: 10.1093/treephys/tpq086
Chung, J. S., Zhu, J. K., Bressan, R. A., Hasegawa, P. M., Shi, H. H. (2008). Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J. 53, 554–565. doi: 10.1111/j.1365-313X.2007.03364.x
Dassanayake, M., Larkin, J. C. (2017). Making plants break a sweat: the structure, function, and evolution of plant salt glands. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00406
Dayod, M., Tyerman, S. D., Leigh, R. A., Gilliham, M. (2010). Calcium storage in plants and the implications for calcium biofortification. Protoplasma 247, 215–231. doi: 10.1007/s00709-010-0182-0
Demidchik, V., Shabala, S. (2018). Mechanisms of cytosolic calcium elevation in plants: the role of ion channels, calcium extrusion systems and NADPH oxidase-mediated ‘ROS-Ca2+ Hub’. Funct. Plant Biol. 45, 9–27. doi: 10.1071/FP16420
Ding, F., Chen, M., Sui, N., Wang, B. S. (2010). Ca2+ significantly enhanced development and salt-secretion rate of salt glands of Limonium bicolor under NaCl treatment. South Afr. J. Botany. 76, 95–101. doi: 10.1016/j.sajb.2009.09.001
Dong, Q. Y., Wallrad, L., Almutairi, B. O., Kudla, J. (2022). Ca2+ signaling in plant responses to abiotic stresses. J. Integr. Plant Biol. 64, 287–300. doi: 10.1111/jipb.13228
Duan, Y. F., Lei, T., Li, W. J., Jiang, M. Y., Zhao, Z. A., Yu, X. F., et al. (2023). Enhanced Na+ and Cl- sequestration and secretion selectivity contribute to high salt tolerance in the tetraploid recretohalophyte Plumbago auriculata Lam. Planta 257 (3), 52. doi: 10.1007/s00425-023-04082-7
Faraday, C. D., Thomson, W. W. (1986). Functional aspects of the salt glands of the Plumbaginaceae. J. Exp. Botany. 37, 1129–1135. doi: 10.1093/jxb/37.8.1129
Feng, Z. T., Sun, Q. J., Deng, Y. Q., Sun, S. F., Zhang, J. G., Wang, B. S. (2014). Study on pathway and characteristics of ion secretion of salt glands of Limonium bicolor. Acta Physiologiae Plantarum 36, 2729–2741. doi: 10.1007/s11738-014-1644-3
Feng, Z. T., Deng, Y. Q., Zhang, S. C., Liang, X., Yuan, F., Hao, J. L., et al. (2015). K+ accumulation in the cytoplasm and nucleus of the salt gland cells of Limonium bicolor accompanies increased rates of salt secretion under NaCl treatment using NanoSIMS. Plant Science 238, 286–296. doi: 10.1016/j.plantsci.2015.06.021
Flowers, T. J., Colmer, T. D. (2008). Salinity tolerance in halophytes. New Phytol. 179, 945–963. doi: 10.1111/j.1469-8137.2008.02531.x
Gilliham, M., Dayod, M., Hocking, B. J., Xu, B., Conn, S. J., Kaiser, B. N., et al. (2011). Calcium delivery and storage in plant leaves: exploring the link with water flow. J. Exp. Botany. 62, 22–50. doi: 10.1093/jxb/err111
Goncalves, P. P., Meireles, S. M., Neves, P., Vale, M. G. (2000). Distinction between Ca2+ pump and Ca2+/H+ antiport activities in synaptic vesicles of sheep brain cortex. Neurochemist. Int. 37, 387–396. doi: 10.1016/S0197-0186(00)00009-7
Guo, K. M., Babourina, O., Rengel, Z. (2009). Na+/H+ antiporter activity of the SOS1 gene: lifetime imaging analysis and electrophysiological studies on Arabidopsis seedlings. Plant Physiol. 137, 155–165. doi: 10.1111/j.1399-3054.2009.01274.x
Guo, J. X., Lu, X. Y., Tao, Y. F., Guo, H. J., Min, W. (2022). Comparative ionomics and metabolic responses and adaptive strategies of cotton to salt and alkali stress. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.871387
Guo, Q., Meng, L., Han, J. W., Mao, P. C., Tian, X. X., Zheng, M. L., et al. (2020). SOS1 is a key systemic regulator of salt secretion and K+/Na+ homeostasis in the recretohalophyte Karelinia caspia. Environ. Exp. Bot. 177, 104098. doi: 10.1016/j.envexpbot.2020.104098
Guo, Z., Wei, M. Y., Zhong, Y. H., Wu, X., Chi, B. J., Li, J., et al. (2023). Leaf sodium homeostasis controlled by salt gland is associated with salt tolerance in mangrove plant Avicennia marina. BMC Plant Biol. 43, 817–831. doi: 10.1093/treephys/tpad002
Guo, R., Yang, Z. Z., Li, F., Yan, C. R., Zhong, X. L., Liu, Q., et al. (2015). Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 15, 170. doi: 10.1186/s12870-015-0546-x
Horie, T., Hauser, F., Schroeder, J. I. (2009). HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 12, 660–668. doi: 10.1016/j.tplants.2009.08.009
Jiang, Y. L., Liu, S. L., Hu, J., He, G. T., Liu, Y. Q., Chen, X., et al. (2020). Polyploidization of Plumbago auriculata Lam. in vitro and its characterization including cold tolerance. Plant Cell Tissue Organ Culture. 140, 315–325. doi: 10.1007/s11240-019-01729-w
Jin, J., Cui, H. M., Lv, X. M., Yang, Y. F., Wang, Y., Lu, X. Y. (2017). Exogenous CaCl2 reduces salt stress in sour jujube by reducing Na+ and increasing K+, Ca2+, and Mg2+ in different plant organs. J. Hortic. Sci. Biotechnol. 92, 98–106. doi: 10.1080/14620316.2016.1228435
Kobayashi, H., Masaoka, Y., Takahashi, Y. (2007). Ability of salt glands in Rhodes grass (Chloris gayana Kunth) to secrete Na+ and K+. Soil Sci. Plant Nutr. 53, 764–771. doi: 10.1111/j.1747-0765.2007.00192.x
Kong, X. Q., Luo, Z., Dong, H. Z., Eneji, A. E., Li, W. J. (2016). H2O2 and ABA signaling are responsible for the increased Na+ efflux and water uptake in Gossypium hirsutum L. roots in the non-saline side under non-uniform root zone salinity. J. Exp. Botany. 67, 2247–2261. doi: 10.1093/jxb/erw026
Li, J. P., Fang, Y., Liu, Y. L., Zhang, M. J., Liu, Y., Zhao, Y., et al. (2020). Exogenous melatonin enhances salt secretion from salt glands by upregulating the expression of ion transporter and vesicle transport genes in Limonium bicolor. BMC Plant Biol. 20, 493–493. doi: 10.1186/s12870-020-02703-x
Lu, Y. J., Li, N. Y., Sun, J., Hou, P. C., Jing, X. S., Zhu, H. P., et al. (2013). Exogenous hydrogen peroxide, nitric oxide and calcium mediate root ion fluxes in two non-secretor mangrove species subjected to NaCl stress. Tree Physiol. 33, 81–95. doi: 10.1093/treephys/tps119
Ma, L. Y., Zhang, H., Sun, L. R., Jiao, Y. H., Zhang, G. Z., Miao, C., et al. (2012). NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J. Exp. Botany. 63, 305–317. doi: 10.1093/jxb/err280
Maeda, Y. (2019). Effects of calcium application on the salt tolerance and sodium excretion from salt glands in zoysiagrass (Zoysia japonica). Grassland Sci. 65, 189–196. doi: 10.1111/grs.12234
Mahajan, S., Pandey, G. K., Tuteja, N. (2008). Calcium- and salt-stress signaling in plants: shedding light on SOS pathway. Arch. Biochem. Biophys. 471, 146–158. doi: 10.1016/j.abb.2008.01.010
Mi, P., Yuan, F., Guo, J. R., Han, G. L., Wang, B. S. (2021). Salt glands play a pivotal role in the salt resistance of four recretohalophyte Limonium Mill. Species. Plant Biol. 23, 1063–1073. doi: 10.1111/plb.13284
Mulaudzi, T., Hendricks, K., Mabiya, T., Muthevhuli, M., Ajayi, R. F., Mayedwa, N., et al. (2020). Calcium improves germination and growth of Sorghum bicolor seedlings under salt stress. Plants-Basel 9, 9060730. doi: 10.3390/plants9060730
Munns, R., Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Nomura, H., Shiina, T. (2014). Calcium signaling in plant endosymbiotic organelles: mechanism and role in physiology. Mol. Plant 7, 1094–1104. doi: 10.1093/mp/ssu020
Qiu, Q. S., Guo, Y., Dietrich, M. A., Schumaker, K. S., Zhu, J. K. (2002). Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. United States Am. 99, 8436–8441. doi: 10.1073/pnas.122224699
Ruan, C. J., da Silva, J. A. T., Mopper, S., Qin, P., Lutts, S. (2010). Halophyte improvement for a salinized world. Crit. Rev. Plant Sci. 29, 329–359. doi: 10.1080/07352689.2010.524517
Sanadhya, P., Agarwal, P., Khedia, J., Agarwal, P. K. (2015). A low-affinity K+ transporter AlHKT2; 1 from recretohalophyte Aeluropus lagopoides confers salt tolerance in yeast. Mol. Biotechnol. 57, 489–498. doi: 10.1007/s12033-015-9842-9
Shabala, S. (2019). Linking ploidy level with salinity tolerance: NADPH-dependent ‘ROS-Ca2+ Hub’ in the spotlight. J. Exp. Botany. 70, 1063–1067. doi: 10.1093/jxb/erz042
Shabala, S., Demidchik, V., Shabala, L., Cuin, T. A., Smith, S. J., Miller, A. J., et al. (2006). Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. J. Plant Physiol. 141, 1653–1665. doi: 10.1104/pp.106.082388
Shabala, S., Pottosin, I. (2014). Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. J. Plant Physiol. 151, 257–279. doi: 10.1111/ppl.12165
Shuttleworth, T. J., Hildebrandt, J. P. (1999). Vertebrate salt glands: short- and long-term regulation of function. J. Exp. Zool. 183, 689–701. doi: 10.1002/(SICI)1097-010X(19990601)283:7<689::AID-JEZ7>3.0.CO;2-T
Shuttleworth, T. J., Thompson, J. L. (1989). Intracellular Ca2+ and inositol phosphates in avian nasal gland cells. Am. J. Physiol. 257, C1020–C1029. doi: 10.1152/ajpcell.1989.257.5.C1020
Sun, J., Dai, S. X., Wang, R. G., Chen, S. L., Li, N. Y., Zhou, X. Y., et al. (2009). Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiol. 29, 1175–1186. doi: 10.1093/treephys/tpp048
Sun, J., Wang, M. J., Ding, M. Q., Deng, S. R., Liu, M. Q., Lu, C. F., et al. (2010). H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ. 33, 943–958. doi: 10.1111/j.1365-3040.2010.02118.x
Terry, B. R., Findlay, G. P., Tyerman, S. D. (1992). Direct effects of Ca2+-channel blockers on plasma membrane cation channels of Amaranthus tricolor protoplasts. J. Exp. Botany. 43, 7–1473. doi: 10.1093/jxb/43.11.1457
Wang, W. L., Xing, L., Xu, K., Ji, D. H., Xu, Y., Chen, C. S., et al. (2020). Salt stress-induced H2O2 and Ca2+ mediate K+/Na+ homeostasis in Pyropia haitanensis. J. Appl. Phycol. 32, 4199–4210. doi: 10.1007/s10811-020-02284-0
Wegner, L. H., De Boer, A. H., Raschke, K. (1994). Properties of the K+ inward rectifier in the plasma membrane of xylem parenchyma cells from barley roots: Effects of TEA+, Ca2+, Ba2+ and La3+. J. Membrane Biol. 142, 363–379. doi: 10.1007/BF00233442
Yuan, F., Chen, M., Leng, B. Y., Wang, B. S. (2013). An efficient autofluorescence method for screening Limonium bicolor mutants for abnormal salt gland density and salt secretion. South Afr. J. Botany. 88, 110–117. doi: 10.1016/j.sajb.2013.06.007
Yuan, F., Leng, B. Y., Wang, B. S. (2016). Progress in studying salt secretion from the salt glands in recretohalophytes: how do plants secrete salt? Frontiers in Plant Science 7, 977. doi: 10.3389/fpls.2016.00977
Zhang, W. D., Wang, P., Bao, Z. L. T., Ma, Q., Duan, L. J., Bao, A. K., et al. (2017). SOS1, HKT1; 5, and NHX1 synergistically modulate Na+ homeostasis in the halophytic grass Puccinellia tenuiflora. Frontiers in Plant Science 8, 576. doi: 10.3389/fpls.2017.00576
Zherelova, O. M., Grishchenko, V. M., Chaylakhyan, L. M. (1994). Blockers of Ca2+ channels in the plasmalemma of perfused Characeae cells. Comp. Biochem. Physiol. c-Toxicol. Pharmacol. 107, 475–480. doi: 10.1016/1367-8280(94)90079-5
Keywords: recretohalophyte, salt gland, salt stress, selective secretion, tetraploid
Citation: Duan Y, Jiang L, Lei T, Ouyang K, Liu C, Zhao Z, Li Y, Yang L, Li J, Yi S and Gao S (2024) Increasing Ca2+ accumulation in salt glands under salt stress increases stronger selective secretion of Na+ in Plumbago auriculata tetraploids. Front. Plant Sci. 15:1376427. doi: 10.3389/fpls.2024.1376427
Received: 25 January 2024; Accepted: 29 March 2024;
Published: 15 April 2024.
Edited by:
Zulfiqar Ali Sahito, Zhejiang University, ChinaReviewed by:
Lars Hendrik Wegner, Foshan University, ChinaCopyright © 2024 Duan, Jiang, Lei, Ouyang, Liu, Zhao, Li, Yang, Li, Yi and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suping Gao, Z2FvX3N1cGluZ0BzaWNhdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.