- 1Crop Physiology Laboratory, Utah State University, Logan, UT, United States
- 2Department of Horticultural Sciences, Texas A&M University, College Station, TX, United States
Rhizosphere pH determines nutrient bioavailability, but this pH is difficult to measure. Standard pH tests require adding water to growth media. This dilutes hydrogen ion activity and increases pH. We used a novel, in situ, pointed-tip electrode to estimate rhizosphere pH without dilution. Measurements from this electrode matched a research-grade pH meter in hydroponic nutrient solutions. We then compared measurements from this electrode to saturated paste and pour-through methods in peat moss, coconut coir, and pine bark. The pointed-tip electrode was unable to accurately measure pH in the highly-porous pine bark media. Adding deionized water to the other media at container capacity using the saturated paste method resulted in a pH that was 0.59 ± 0.30 units higher than the initial in situ measurement at the top of the container. This increase aligns with established solution chemistry principles. Measurements of pH using the pour-through method were 0.38 ± 0.24 pH units higher than in situ measurements at the bottom of the container. We conclude that in situ pH measurements are not subject to dilution and are thus more representative of the rhizosphere pH than the saturated paste and pour-through techniques.
1 Introduction
Nutrient bioavailability depends on rhizosphere pH, which is the pH directly adjacent to root surfaces. The rhizosphere pH is difficult to measure and is often estimated from pH measurements of the bulk substrate. Smiley (1974) found that the rhizosphere pH varied from the bulk pH by 1.2 pH units for wheat (Triticum aestivum) plants depending on the nitrogen source (nitrate or ammonium). Nye (1981) further modelled that the bulk pH could vary from the rhizosphere pH by 1 to 2 pH units depending on distance from the root surface and nitrogen source. Methods to better estimate rhizosphere pH from bulk pH are needed.
Ion exchange principles are the same between field soils and soilless media substrates. The lower bulk density and higher water holding capacity of soilless media for horticultural crops often increases growth compared to field soils for agronomic crops. Container-grown plants can have more pH management challenges and experience more rapid pH changes than field-grown plants due to the confined root-zone with reduced media buffering capacity.
The saturated paste method is widely used to measure the bulk pH of field soils and container media (Miller and Kissel, 2010; Thomas, 2018). A substrate sample is removed from the container, deionized water is added until it is saturated and visibly glistens, and the pH of the saturated paste is measured (Kalra, 1995). Alternatively, the media solution may be vacuum-extracted and subsequently measured with a pH electrode. Media to water dilutions of 1:2 and 1:5 are also commonly used to determine bulk pH.
The pour-through method measures the pH of container leachate and does not require removal of the media (Wright, 1986). A tray is placed beneath the container and water, or a fertigation solution, is slowly poured through the container to collect a liquid sample in the tray (generally 50 to 100 mL). Yao et al. (2008) found no significant effect on pH when the leachate volume ranged from 40 mL to 120 mL in sphagnum moss planted with moth orchids (Phalaenopsis spp.). Torres et al. (2010) found no consistent differences between the pour-through pH of a 50 mL leachate and a 2.5% leachate of a peat moss, perlite, and bark mix planted with boxwood (Buxus x koreana). Small leachate volumes may not capture a representative pH, especially if root-zone stratifications exist. Altland and Owen (2024) analyzed the pH of pine bark media that had been intentionally stratified between fertilizer-amended and non-amended fractions within a container. They concluded that pH measurements using the pour-through method were always more similar to the pH measurement of a 1:1 saturated paste extraction from the bottom half of the container, regardless of stratification or particle size.
Saturated paste and pour-through methods have been compared in several studies. Yeage et al. (1983), Wright et al. (1990), and Cavins et al. (2008) found no difference in pH using saturated paste or pour-through methods with distilled water. A recent review of several studies indicated minimal differences between saturated paste and pour-through methods (Altland, 2021). However, both approaches require dilution of the root-zone solution.
The addition of deionized water to nutrient solutions dilutes hydrogen ion activity and increases pH. Sumner (1994) found an increase of 0.5 pH units when comparing saturated paste measurements to in situ measurements in soils. Schofield and Taylor (1955) reduced the dilution effect by measuring soil pH in a solution of 0.01 M calcium chloride. Matthiesen (2004) reported that saturated paste measurements were higher than in situ measurements by up to 0.4 pH units when using a pointed-tip pH electrode for pH measurements of soils, but the addition of calcium chloride to the samples minimized this increase. The dilution effect therefore increases the error in estimating rhizosphere pH.
The in situ method utilizes direct insertion of a pH electrode into the media and is faster than other methods. The electrode tip must contact the media solution, but electrodes with pointed tips facilitate contact with the media. In situ pH measurements remove the dilution effect experienced from saturated paste and pour-through pH measurements. There is no rhizosphere in unplanted containers, so the dilution effect simply makes bulk pH measurements inaccurate. The in situ method could help researchers and growers more closely estimate pH values and nutrient availabilities for plants grown in soilless media.
No previous studies have compared these pH measurement methods in soilless media – this was the objective of our novel work. We hypothesized that in situ measurements would be lower than saturated paste and pour-through measurements due to the absence of the dilution effect.
2 Methods
2.1 pH electrode
A pointed-tip pH electrode (model HALO2 GroLine, Hanna Instruments, Woonsocket, RI, USA) was used to measure pH in all parts of the study (Supplementary Figure 1A). Preliminary studies (not shown) concluded identical measurements between the pointed-tip electrode and a research-grade electrode, which was evidence for its accuracy. The pH meter was paired with the Hanna Lab app on a smartphone through Bluetooth® for calibration. The gelled electrolyte inside the electrode needed to be refilled after about every 200 measurements.
2.2 Media types and composition
Containers with a volume of 1.7-L were filled with one of three media types: peat moss (Premier Pro-Moss TBK; Premier Horticulture, Inc., Quakertown, PA, USA), coconut coir (Black Gold Just Coir; Sun Gro Horticulture, Agawam, MA, USA), or pine bark (from Pinus taeda, particle size less than 2 cm). Each media type was then amended with 0%, 25%, 50%, or 75% perlite (Expanded Perlite; Hess Pumice, Malad City, ID, USA) by volume (Figure 1). Wetting agent (AquaGro® 2000 G; Aquatrols, Paulsboro, NJ, USA) was added at one gram per liter of media. Hydrated lime (calcium hydroxide) was added as needed to adjust pH of the peat from pH 5 to 7. Lime was not added to coconut coir or pine bark. Each treatment included three replicate containers. The containers were then saturated to container capacity with a nutrient solution containing 120 ppm nitrogen (72 ppm nitrate nitrogen, 42 ppm ammonium nitrogen, and 6 ppm urea nitrogen), 31 ppm phosphorus, 177 ppm potassium, 45 ppm calcium, 19 ppm magnesium, 25 ppm sulfur, 17 ppm silicon, 0.9 ppm iron, 0.3 ppm manganese, 0.3 ppm zinc, 0.41 ppm boron, 0.85 ppm copper, and 0.06 ppm molybdenum. The nutrient solution pH was 6.7 and electrical conductivity was 1.4 mS per cm. Containers drained for 1 hour before making pour-through and saturated paste pH measurements.
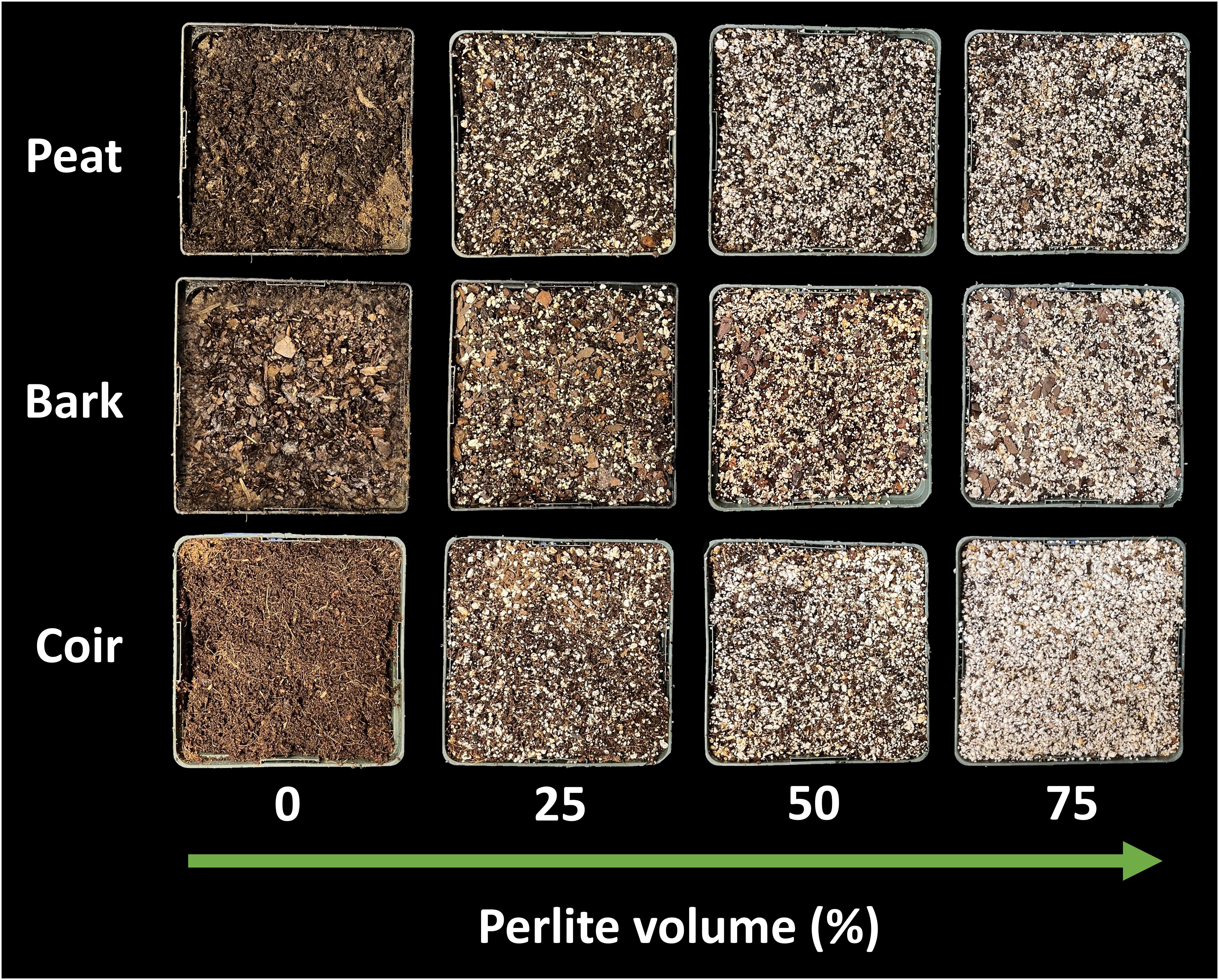
Figure 1 Peat moss, pine bark, and coconut coir mixed with increasing levels of perlite (0% to 75% by volume).
2.3 Dilution effect on pH
The pH of the above nutrient solution was measured after diluting with deionized water from a dilution factor of 1 to 10. This was repeated with a nutrient solution without phosphorus (P) to represent root-zone conditions with low buffering capacity (P concentrations are typically low in the root-zone as P is actively taken up by plants). This amended nutrient solution contained 84 ppm nitrogen, 141 ppm potassium, 60 ppm calcium, 19 ppm magnesium, 26 ppm sulfur, 17 ppm silicon, 0.39 ppm iron, 0.16 ppm manganese, 0.2 ppm zinc, 0.43 ppm boron, 0.25 ppm copper, 0.001 ppm molybdenum and 0.0003 ppm nickel.
2.4 Pour-through method
Pour-through measurements were undertaken first to ensure all replicates were near container capacity. The 1.7-L containers were slowly watered with 1 L of the nutrient solution described in the previous section to minimize channeling of the solution through the container. The leachate was collected in a tray and pH was measured. The 1 L leachate volume was selected to ensure displacement of the root-zone solution while not being large enough to contribute to the displaced solution.
2.5 Moist vs. wet in situ measurements
Moist in situ measurements were made in three locations on the media at the top of the container following pour-through measurements (water content just below container capacity). The pH meter was inserted at a depth of 4 cm and a reading was recorded after stabilization (about 5 s, Supplementary Figure 1B). A wet in situ measurement was then taken in the same location after adding three to five mL of deionized water onto the area and reinserting the probe. Bottom in situ measurements were made 4 cm deep into three locations at the bottom of the container through drainage holes.
2.6 Saturated paste method
The pH was measured by removing media around the three locations previously sampled on the top of the container to loosely fill a 30-mL beaker. Deionized water (electrical conductivity of less than 0.005 mS per cm) was added to the beaker to create a saturated paste as in Kalra (1995). The pH was then measured directly in the beaker using the in situ pH meter.
2.7 Unplanted vs. planted media
The replicate media measured in the above tests were then seeded with lettuce (Lactuca sativa cv. Grand Rapids). The same tests were repeated in these planted containers 28 days after seeding.
2.8 Statistical analysis
Measurement and planting methods were compared with T-tests and ANOVA using RStudio (Posit Software, PBC, Boston, MA). Replicate measurements using the same method in the same container were averaged to eliminate pseudo replicates.
3 Results
3.1 pH dilution from deionized water
The pH increased 0.16 pH units as the dilution factor of the complete nutrient solution increased from 1 to 10 (Figure 2). Removing phosphorus from the nutrient solution increased the pH 0.42 units as the dilution factor increased from 1 to 10. The pH would be expected to increase 0.72 units with only deionized water and hydrogen ions in equilibrium with ambient CO2 (3.4 x 10-4 atm; 400 ppm CO2 at 86 kPa atm) as the dilution factor increased from 1 to 10. Dissolved carbon dioxide produces carbonic acid, which buffers the solution and reduces the dilution effect.
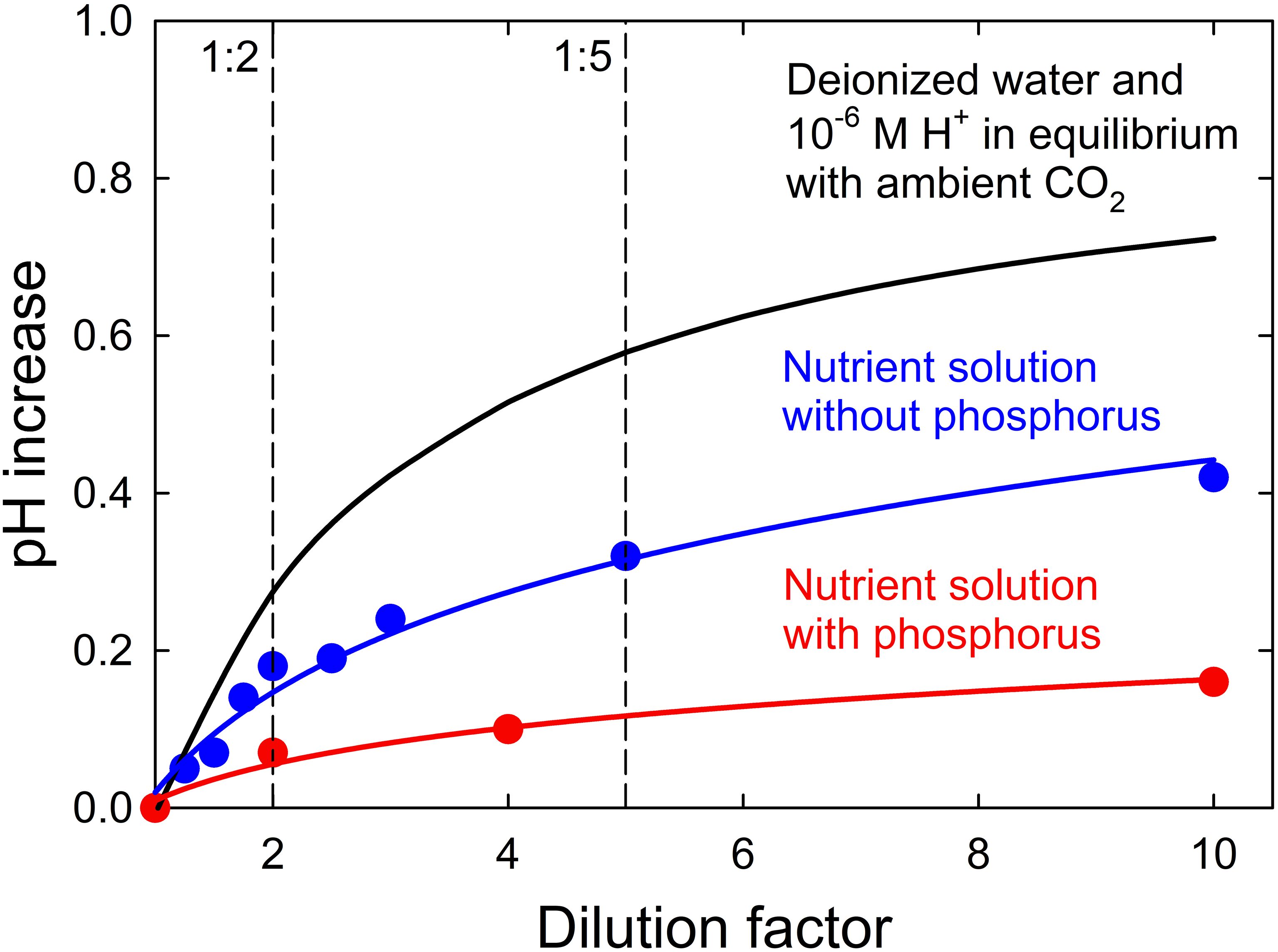
Figure 2 The pH increase of a solution with deionized water and 10-6 M hydrogen ions in equilibrium with ambient CO2 (3.4 x 10-4 atm), a nutrient solution without phosphorus, and a nutrient solution with phosphorus as the dilution factor with deionized water increases from 1 to 10.
3.2 Pre-wetting the measurement area
Wetting the measurement area with deionized water prior to pH measurement (wet in situ) did not change the pH (Supplementary Figure 2) if the media moisture content was greater than 3 on a 5-point moisture scale (Huang and Fisher, 2013). Measurements were erratic if the media was at moisture level 1 or 2 on the scale (data not shown).
3.3 Comparing in situ with saturated paste and pour-through
Saturated paste pH averaged 0.57 ± 0.22 (p = 0.05, n = 30) pH units higher than top in situ pH in unplanted containers (Figure 3A) and 0.61 ± 0.33 (p = 0.02, n = 30) pH units higher in planted containers (Figure 3B). Pour-through pH averaged 0.31 ± 0.18 (p = 0.36, n = 30) pH units higher than bottom in situ pH in unplanted containers (Figure 3C) and 0.45 ± 0.19 pH (p = 0.22, n = 30) units higher in planted containers (Figure 3D). Treatments with pine bark and 75% perlite-based media had highly variable in situ pH measurements with reduced plant growth from low water retention. They were not included in the above data and were not analyzed further.
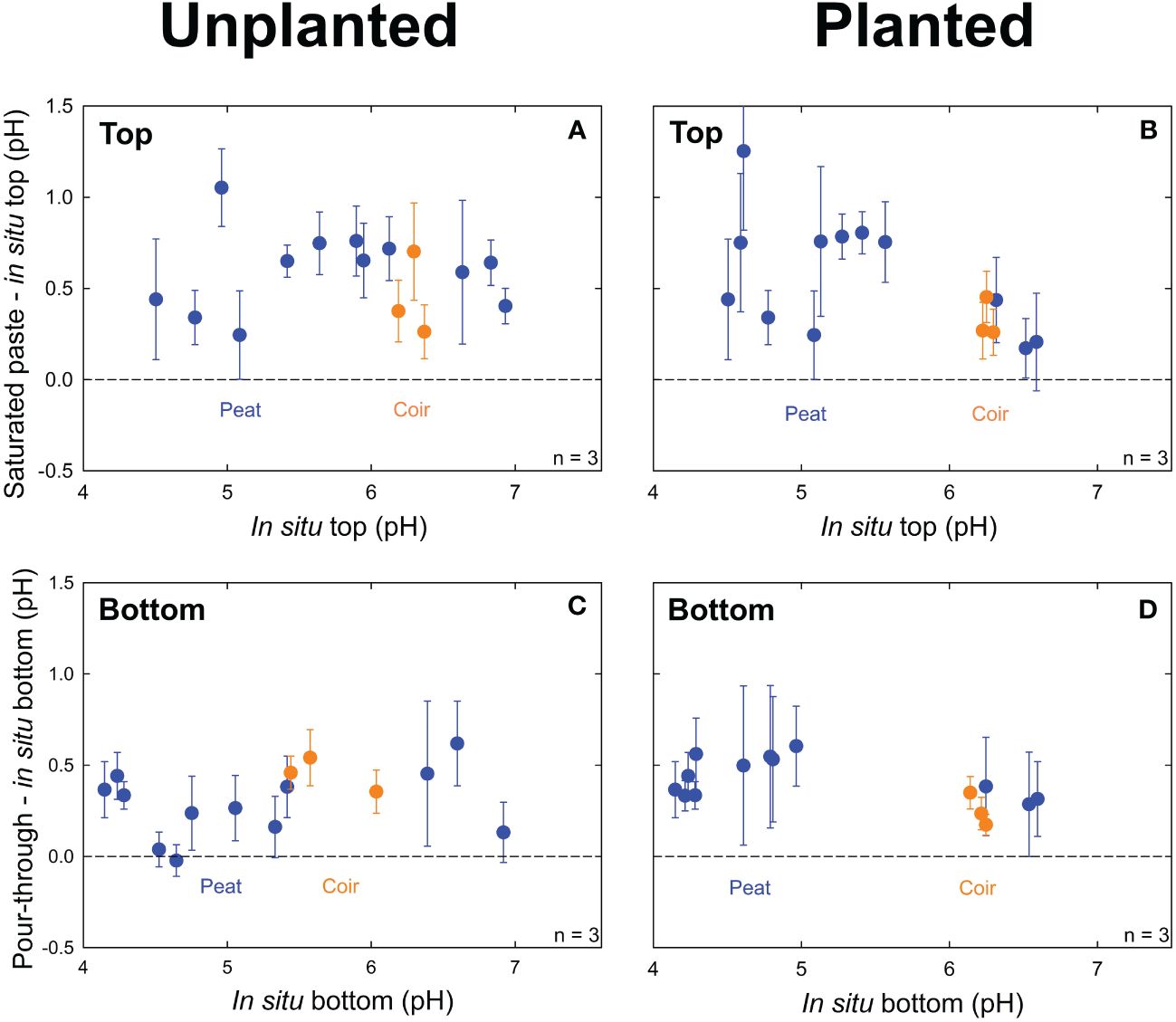
Figure 3 pH measurements in unplanted containers and containers planted with lettuce (Lactuca sativa). The average difference between pH measurements from the saturated paste and in situ methods at the top of the container versus in situ pH at the top of the container in unplanted containers (A) and planted containers (B). The difference between pH measurements from the pour-through and in situ pH methods at the bottom of the container versus in situ pH at the bottom of the container in unplanted containers (C) and planted containers (D). Peat moss or coconut coir were mixed with 0%, 25%, or 50% perlite to obtain 15 treatments with 3 containers per treatment. Error bars represent standard deviation of the container measurements.
3.4 Saturated paste vs. pour-through pH
We found saturated paste pH measurements at the top of the container to be significantly higher (0.66 ± 0.47, p = 0.003, n = 60) than pour-through pH measurements (Figure 4), but there was no significant difference between unplanted and planted containers (p = 0.54, n = 60).
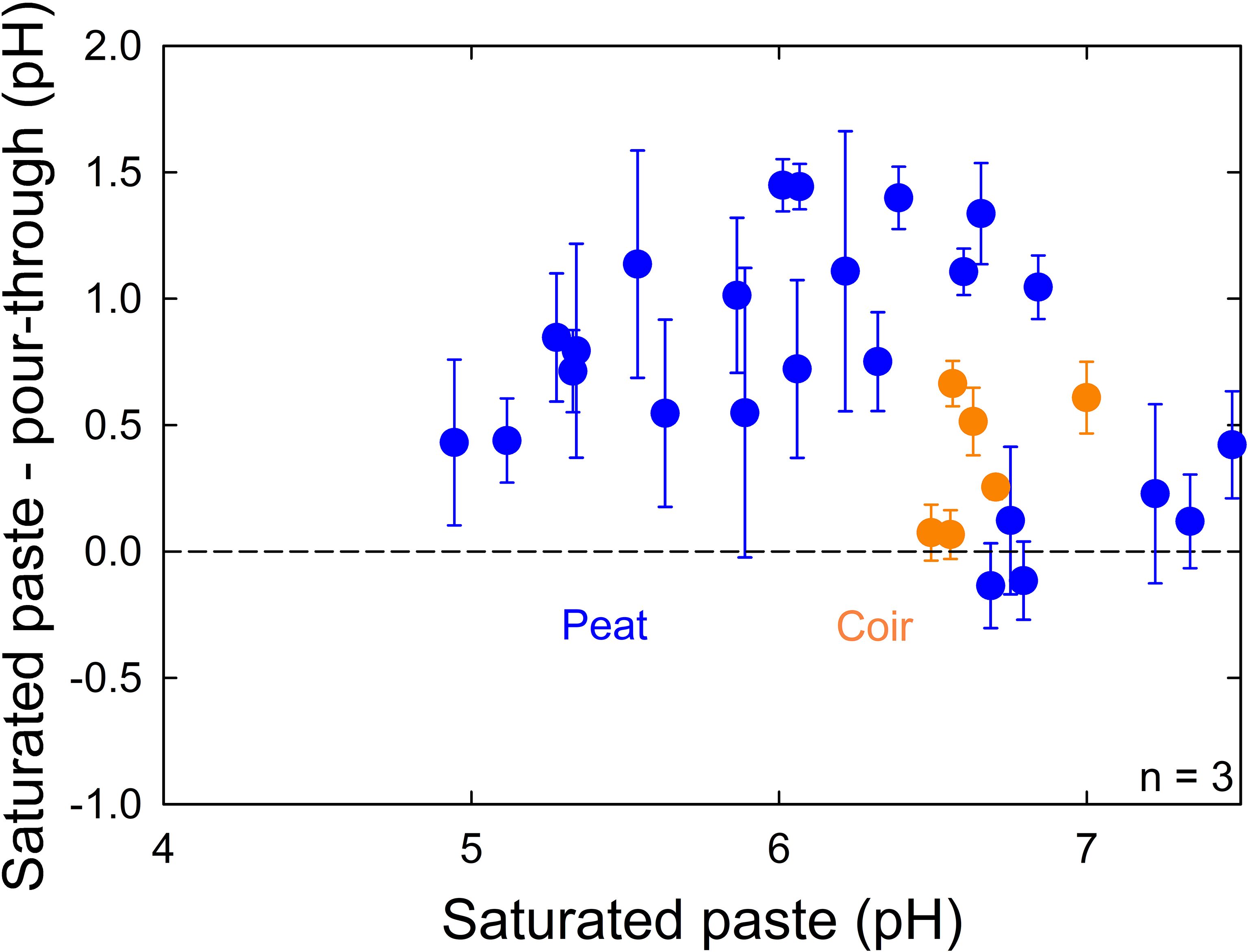
Figure 4 The difference between saturated paste and pour-through pH measurements compared to saturated paste pH measurements in both unplanted containers and containers planted with lettuce (Lactuca sativa). The difference between saturated paste and pour-through measurements was significant (p = 0.003), but there was no significant difference between unplanted and planted containers (p = 0.54). Peat moss or coconut coir were mixed with 0%, 25%, or 50% perlite to obtain 15 treatments with 3 containers per treatment. Error bars represent standard deviation of the container measurements.
3.5 pH meter stability
The meters we used (n = 2) displayed stabilized measurements within 5 seconds throughout their more than 20 months of use. The only periodic maintenance that was required was refilling the electrolyte gel after about every 200 samples and removing humus materials using an acidic cleaning solution from the manufacturer every few months.
4 Discussion
Similar to our results, Matthiesen (2004) found a deviation of in situ pH compared to saturated paste pH in wet soils up to 1 pH unit. Keaton (1938) measured the pH of soil samples from across the United States using increasing ratios of water to soil. He demonstrated a general increase in pH for soils as the water to soil ratio increased. His measurements were 0.5 pH units higher for a 1:1 dilution and 0.9 pH units higher for a 10:1 dilution than for samples at the original soil moisture content, which is in a similar range to our results. Keaton hypothesized this discrepancy was due to cation exchange and differential base saturation, which he confirmed by observing little change in pH measurements among soil moistures when soils were completely desaturated of metal ions.
Increasing the dilution factor from in situ to saturated paste measurements reduces H+ activity and increases pH (Davis, 1943; Peech, 1965). In a pure solution, dilution increases pH in acidic solutions and decreases pH in alkaline solutions. However, in soil or media suspensions, pH always increases with dilution. The increasing base saturation in alkaline suspensions (Havlin, 2005) leads to base cation hydrolysis with increasing dilution, which buffers out any expected decrease in pH (Thomas, 2018) and continues to make it susceptible to the dilution effect (Conyers and Davey, 1988). Keaton (1938) demonstrated that the pH increased from 6.6 to 7.5 in alkaline soils when diluting 1:1. Higher dilutions can also increase the mobility of potassium ions from pH electrodes leading to higher pH readings (Sumner, 1994).
The dilution effect can often be minimized by fixing the ionic strength using calcium chloride. Miller and Kissel (2010) found that the pH measured with the saturated paste method using water was consistently about 0.5 pH units higher than when measured with dilute calcium chloride. Sumner (1994) additionally found that measurement in calcium chloride reduced pH measurement variability. Although using calcium chloride can counteract the effect of ion activity, it is an additional step, and was not analyzed in this paper.
The significantly higher pH measurements of the saturated paste method compared to the pour-through method that we found are in contrast to the results of Altland (2021) and Cavins et al. (2008) discussed earlier. The media, lime, and nutrient solution may not have been at equilibrium in unplanted pots measured after 2 h, but we found the same discrepancy in planted pots after 28 days. This suggests that this effect is independent of stage in the crop life cycle. While Yeage et al. (1983), Wright et al. (1990), and Cavins et al. (2008) used distilled water in the pour-through method, we used nutrient solution, which could have displaced more H+ ions and decreased pH. Our leachate volume was higher than others, but we observed no bias in diluting pH with increasing leachate volumes.
We did not include treatments that had stunted and variable lettuce growth among replicates. This occurred with pine bark and treatments with 75% perlite by volume. The pine bark had larger particle sizes and was more hydrophobic than the peat moss and coconut coir even after addition of the wetting agent. These characteristics made moisture retention more difficult in pine bark leading to uneven moisture distribution and variable in situ pH measurements. The high air-filled porosity with 75% perlite similarly led to variable in situ measurements. Pour-through pH measurements were more consistent than the in situ measurements in these circumstances as they captured larger areas.
We selected a common pH range from 5 to 7 because metal toxicities, such as those from aluminium (Imadi et al., 2016) and manganese (El-Jaoual and Cox, 1998), are more common below pH 5 and iron precipitates and becomes unavailable above pH 7 (Colombo et al., 2014). The peat moss, coconut coir, and pine bark were representative of media components used in research and commercial production. The peat moss was more acidic (Elliott, 1996) than coconut coir and pine bark and required the addition of lime to increase the pH.
Increased solution contact from increased media moisture should lead to increased pH measurement accuracy, but we observed no difference in pH measurements between moist (no pre-wetting, moisture level 3 to 4) and wet (pre-wetting, moisture level 4) in situ insertion techniques. This was likely because we only added 3 to 5 mL of deionized water and the media we tested was already near container capacity. We observed increasing measurement variability in preliminary studies when the media was visually dry (moisture level 1 to 2). This was presumably because contact between the electrode and media surface was incomplete.
5 Conclusion
Our measurements of the pH of soilless media using the saturated paste and pour-through methods were consistently 0.4 to 0.6 pH units higher than in situ measurements. Measuring pH using saturated paste and pour-through methods dilutes hydrogen ion activity and results in a higher pH that may not be representative of rhizosphere conditions.
We found no bias between peat moss or coconut coir media, but in situ measurements were more variable in media with pine bark and perlite levels of 75%.
In situ measurements are not subject to dilution effects experienced by saturated paste and pour-through measurements and may provide a more accurate indication of rhizosphere pH.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
NL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. HS: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. RH: Conceptualization, Writing – review & editing. BB: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Utah Agricultural Experiment Station, Utah State University, and approved as journal paper number 9611; NASA, Center for the Utilization of Biological Engineering in Space (grant number NNX17AJ31G).
Acknowledgments
We gratefully acknowledge the insightful review comments from Daniel Fernandez Pinto. We also acknowledge Young’s Plant Farm for their donation of the pine bark used in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1334328/full#supplementary-material
References
Altland, J. E. (2021). The pour-through procedure for monitoring container substrate chemical properties: A review. Horticulturae 7, 536. doi: 10.3390/horticulturae7120536
Altland, J. E., Owen, J. S. (2024). The pour-through procedure preferentially extracts substrate solution from the bottom of the container in conventional and stratified substrates. horts 59, 201–208. doi: 10.21273/HORTSCI17425-23
Cavins, T. J., Whipker, B. E., Fonteno, W. C. (2008). PourThru: A method for monitoring nutrition in the greenhouse. Acta Hortic. 779, 289–298. doi: 10.17660/ActaHortic.2008.779.35
Colombo, C., Palumbo, G., He, J.-Z., Pinton, R., Cesco, S. (2014). Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J. Soils Sediments 14, 538–548. doi: 10.1007/s11368-013-0814-z
Conyers, M. K., Davey, B. G. (1988). Observations on some routine methods for soil pH determination. Soil Sci. 145, 29–36. doi: 10.1097/00010694-198801000-00004
Davis, L. E. (1943). Measurements of pH with the glass electrode as affected by soil moisture. Soil Sci. 56, 405–422. doi: 10.1097/00010694-194312000-00002
El-Jaoual, T., Cox, D. A. (1998). Manganese toxicity in plants. J. Plant Nutr. 21, 353–386. doi: 10.1080/01904169809365409
Elliott, G. C. (1996). pH management in container media. Commun. Soil Sci. Plant Anal. 27, 635–649. doi: 10.1080/00103629609369584
Havlin, J. L. (2005). “Fertility,” in Encyclopedia of Soils in the Environment (Amsterdam, Netherlands: Elsevier), 10–19. doi: 10.1016/B0-12-348530-4/00228-9
Huang, J., Fisher, P. (2013) The 1 to 5 Plug Tray Moisture Scale. Available online at: https://www.backpocketgrower.org/archive/S10_The_1_to_5_Plug_Tray_Moisture_Scale.pdf.
Imadi, S. R., Waseem, S., Kazi, A. G., Azooz, M. M., Ahmad, P. (2016). “Aluminum toxicity in plants,” in Plant Metal Interaction (Amsterdam, Netherlands: Elsevier), 1–20. doi: 10.1016/B978-0-12-803158-2.00001-1
Kalra, Y. P. (1995). Determination of pH of soils by different methods: collaborative study. J. AOAC Int. 78, 310–324. doi: 10.1093/jaoac/78.2.310
Keaton, C. (1938). A theory explaining the relation of soil-water ratios to pH values. Soil Sci. 46, 259–266. doi: 10.1097/00010694-193809000-00006
Matthiesen, H. (2004). In situ measurement of soil pH. J. Archaeological Sci. 31, 1373–1381. doi: 10.1016/j.jas.2004.03.005
Miller, R. O., Kissel, D. E. (2010). Comparison of soil pH methods on soils of North America. Soil Sci. Soc Am. J. 74, 310–316. doi: 10.2136/sssaj2008.0047
Nye, P. H. (1981). Changes of pH across the rhizosphere induced by roots. Plant Soil 61, 7–26. doi: 10.1007/BF02277359
Peech, M. (1965). “Hydrogen-ion activity,” in Agronomy Monographs. Ed. Norman, A. G. (American Society of Agronomy, Soil Science Society of America, Madison, WI, USA), 914–926. doi: 10.2134/agronmonogr9.2.c9
Schofield, R. K., Taylor, A. W. (1955). The measurement of soil pH. Soil Sci. Soc. America J. 19, 164–167. doi: 10.2136/sssaj1955.03615995001900020013x
Smiley, R. W. (1974). Rhizosphere pH as influenced by plants, soils, and nitrogen fertilizers. Soil Sci. Soc. Amer J. 38, 795–799. doi: 10.2136/sssaj1974.03615995003800050030x
Sumner, M. E. (1994). Measurement of soil pH: Problems and solutions. Commun. Soil Sci. Plant Anal. 25, 859–879. doi: 10.1080/00103629409369085
Thomas, G. W. (2018). “Soil pH and soil acidity,” in SSSA Book Series. Eds. Sparks, D. L., Page, A. L., Helmke, P. A., Loeppert, R. H., Soltanpour, P. N., Tabatabai, M. A., et al (Soil Science Society of America, American Society of Agronomy, Madison, WI, USA), 475–490. doi: 10.2136/sssabookser5.3.c16
Torres, A. P., Mickelbart, M. V., Lopez, R. G. (2010). Leachate volume effects on pH and electrical conductivity measurements in containers obtained using the pour-through method. hortte 20, 608–611. doi: 10.21273/HORTTECH.20.3.608
Wright, R. D. (1986). The pour-through nutrient extraction procedure. horts 21, 227–229. doi: 10.21273/HORTSCI.21.2.227
Wright, R. D., Grueber, K. L., Leda, C. (1990). Medium nutrient extraction with the pour-through and saturated medium extract procedures for poinsettia. HortSci 25, 658–660. doi: 10.21273/HORTSCI.25.6.658
Yao, H.-Y., Chung, R.-S., Ho, S.-B., Alex Chang, Y.-C. (2008). Adapting the pour-through medium extraction method to phalaenopsis grown in sphagnum moss. horts 43, 2167–2170. doi: 10.21273/HORTSCI.43.7.2167
Keywords: pH electrode, controlled environments, peat moss, coconut coir, pine bark, perlite, saturated paste, pour-through
Citation: Langenfeld NJ, Skabelund HA, Heins R and Bugbee B (2024) Advantages of a novel in situ pH measurement for soilless media. Front. Plant Sci. 15:1334328. doi: 10.3389/fpls.2024.1334328
Received: 07 November 2023; Accepted: 18 March 2024;
Published: 27 March 2024.
Edited by:
Tae In Ahn, Seoul National University, Republic of KoreaReviewed by:
Youbin Zheng, University of Guelph, CanadaBuruiana Daniela, Dunarea de Jos University, Romania
Copyright © 2024 Langenfeld, Skabelund, Heins and Bugbee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noah James Langenfeld, bm9haGpsYW5nZW5mZWxkQGdtYWlsLmNvbQ==
 Noah James Langenfeld
Noah James Langenfeld Hikari Ai Skabelund
Hikari Ai Skabelund Royal Heins1
Royal Heins1 Bruce Bugbee
Bruce Bugbee