- 1Department of Horticulture Science, Faculty of Agriculture, Shahed University, Tehran, Iran
- 2Department of Agronomy and Plant Breeding, Faculty of Agriculture, Shahed University, Tehran, Iran
- 3Department of Plant and Wildlife Sciences, Brigham Young University, Provo, UT, United States
- 4Department of Horticulture Science, Shahed University, Tehran, Iran
The native populations of Iris hymenospatha and Iris histrio, two endangered bulbous species within the large Iris genus in Iridaceae, are threatened with extinction due to mining and other industrial activities in their natural habitats in Central Asia, including Iran. These species not only have a significant economic impact on the global horticultural industry due to their versatility and attractive phenotypic traits, but also have significant ecological value that necessitates their conservation. In this study, we examined the morphological and functional diversity between individuals within these two species, which exhibit high tolerance to environmental stresses. Our study examined 10 populations of I. hymenospatha and two populations of I. histrio based on bulb, flower, and leaf characteristics throughout Iran. We recognized a gradation of five different leaf shapes among I. hymenospatha populations with significant differences between some populations, including “Arak-Khomain” and “Arak-Gerdo”. The “Jaro”, “Natanz-Karkas”, “Ardestan-Taleghan”, “Arak-Rahjerd”, “Arak-Gerdo”, “Ganjnameh”, and “Abas-Abad” populations of I. hymenospatha displayed maximal values in leaf width, stem diameter under flower, crown diameter, flower number, leaf number, and bulb diameter. The I. histrio “Velian” population had a significantly larger flower size, a longer stem length, a larger style width, a longer flowering date, and a higher plant height compared to the “Ganjnameh” population of I. histrio. Such characteristics of both species make them remarkable ornamental plants. Our study also revealed that I. hymenospatha populations grow on different soils and elevations and have the ability to adapt to different growing conditions. Given the threats they face, conservation through horticultural selection and propagation offers a viable conservation strategy for both species. This approach not only preserves the genetic diversity of these species, but also enables their further contribution to the horticultural industry.
1 Introduction
Iris sensu lato, a large genus within Iridaceae, as delineated by Mavrodiev et al. (2014), comprises 260–300 species worldwide, which are distributed throughout the Northern Hemisphere (Lawrence, 1953; Mathew, 1989, 2000; Weber et al., 2020). While some Iris species naturally occur in mesic and wetland environments, the majority of species grow in arid, semi-arid, or dry montane environments (Roguz et al., 2020). Species within the genus exhibit a wide variety of flower color, including a range of hues of blue, violet, yellow, orange, and even black (Roguz et al., 2020). Such characteristics make Iris widely popular as a perennial garden plant. Taxonomists consider between 21 and 26 Iris species to be native to Iran (Azimi et al., 2018). Indeed, few reports exist regarding the Iris species native to Iran. Iris hymenospatha B. Mathew & Wendelbo and Iris histrio Rchb.f. naturally occur throughout Iran on dry mountain slopes (Ghahreman, 1984). The native range of I. hymenospatha extends through Iran and the Kurdistan region of Iraq (Wendelbo, 1977), whereas that of Iris histrio stretches from Iran to Turkey (RBG, 2024).
Iris histrio and I. hymenospatha have long been recognized as members of two different groups of Iris, although the taxonomic levels and names given to these two groups have been disputed (C. Wilson, personal communication). We are following the Iris classification scheme of Wilson (2011) in recognizing I. histrio in Iris section Reticulata Dykes, housed in Iris subgenus Xiphium Spach, and Iris hymenospatha in the Scorpiris Spach. section of the Iris subgenus Scorpiris Spach.
Species within the Iris genus have a sizable economic impact on the global horticulture industry (Khatib et al., 2022). Several Iris species and cultivars can be commonly found in gardens and landscapes throughout the world due to their large, colorful flowers and visually appealing linear leaves (Xu et al., 2019). The fragrant violet-like nature of the floral blooms contributes to their ornamental value (Amin et al., 2021). Beyond their horticultural value, several species are utilized in different traditional medicines to treat inflammation, cancer, and bacterial and viral infections (Amin et al., 2018). Essential oils extracted from I. hymenospatha flowers and leaves exhibited relatively high antifungal activity against three human-pathogenic fungal strains (Aspergillus niger 2CA936, Aspergillus flavus NRRL3357, and Candida albicans ATCC1024) (Khatib et al., 2022).
Plant breeding often involves phenotypic and genotypic characterization to capitalize upon genetic variation to select for desirable traits (Xu et al., 2019; Abenavoli et al., 2021). Understanding the genetic diversity and relationships among plant species, cultivars, and varieties underscores the importance of availability of wild genetic resources for breeding improvement efforts (Azimi et al., 2019), especially for those species that are rare or at risk of extinction (Perrino and Calabrese, 2018; Perrino and Wagensommer, 2022), or belonging to problematic taxonomic groups (Wagensommer et al., 2016), which are not favored by intensive agriculture (Calabrese et al., 2015). In theory, when high genotypic diversity occurs in plant populations, one or more individuals will likely be well adapted to various forms of environmental stress (Reynolds et al., 2012). Microenvironments, which act as powerful selective forces, are more likely to be found in close proximity rather than at larger distances (Reynolds et al., 2012).
Iris hymenospatha and I. histrio populate various habitats, including dry, rocky areas; semi-deserts; and wetlands (Hussain et al., 2020). Azimi et al. (2016) studied diversity within native Iranian Iris species using morphological traits and identified economically significant quantitative traits, including flower size, outer and inner tepal width, and leaf width. These traits may be considered in breeding programs, with the outer and inner tepal width and inner tepal length being important components of the flower (Azimi et al., 2016). An analysis of Iris × germanica L. hybrids found that the highest coefficient of variation among Iris species is linked to several traits, including flower height, diameter, filament length, seed diameter, and leaf number, with high heritability values for plant height, fall width, fall length, flower size, and leaf width (Azimi et al., 2018). Asgari et al. (2022) studied Iran’s endemic Iris species and identified four ecotypes of I. hymenospatha species in west-central Iran as highly suitable accessions for further selection due to their height, large flower size, and high durability.
These bulbous species generally bloom in late winter and early spring. Iris hymenospatha and I. histrio typically bloom at the end of March and the beginning of April. From a horticultural perspective, the springtime flowering habit of these species is ideal for commercial growers to market and sell this species. Moreover, these bulbous Iris species can be easily propagated through vegetative divisions (Kamenetsky and Okubo, 2012). According to Hassani et al. (2008), climatic conditions, grazing, and mining activities (Gholamzade Natanzi, 2023) are key elements that influence species composition and rangeland biodiversity in semi-arid ecosystems in Iran. In their native localities, I. hymenospatha and I. histrio are among the first plant species to bloom in early spring. This makes them particularly vulnerable to overgrazing by livestock, which can lead to a significant decline in the growth vigor and reproductive ability of these species. Overgrazing is a major problem for the conservation of these species, as it can lead to the destruction of their habitat and the loss of biodiversity (Farahmand and Farzad Nazari, 2015). Moreover, owing to the narrow ecological habit of I. hymenospatha and I. histrio, specimens of both species appear to naturally occur singly or in small populations (I. Rohollali, personal observation).
Horticulture plays an important role in conserving biodiversity and promoting sustainable development (Kumar, 2014). Little, however, appears to be known regarding the morphological diversity of I. hymenospatha and I. histrio within their native range. Therefore, 100 native specimens of I. hymenospatha and 20 accessions of I. histrio from native populations were identified, collected, and characterized from 12 sites (Table 1). In this study, we tested the hypothesis that accessions from both species from different habitats exhibit significant diversity in their leaf, bulb, and flower characteristics. Consequently, we examined morphological variability among populations to identify the most useful phenotypic variables for discrimination within populations that could serve as a genetic resource for breeding programs. This study appears to be the first to characterize I. hymenospatha and I. histrio populations based on morphological traits within their native range in Iran.
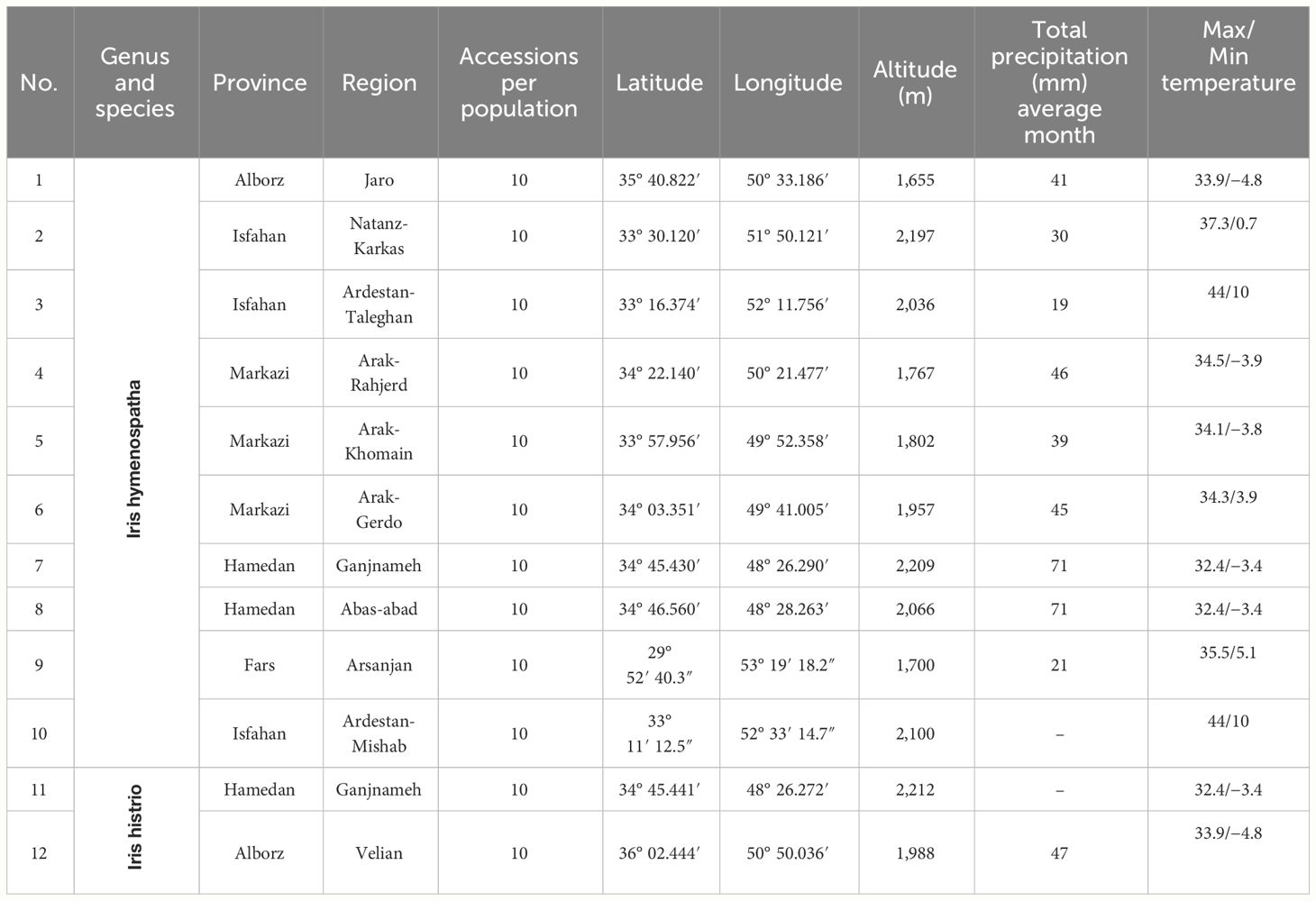
Table 1 Distribution and identification of native populations of Iris hymenospatha and Iris histrio across Iran: detailed geographical data and corresponding species found in each area.
2 Materials and methods
To determine the morphological diversity of I. hymenospatha and I. histrio populations within their native range, we evaluated 30 morphological traits (Table 2). We collected 10 accessions per population from each of the 10 populations of I. hymenospatha and from 2 populations of I. histrio, totaling 120 accessions, along the eastern edge of the Zagros Mountains in western Iran (Table 1). To clarify, there were 100 accessions of I. hymenospatha and 20 accessions of I. histrio. Although native to the region, I. histrio populations appear to be few and infrequent in number. Accessions were identified using morphological keys and taxonomic descriptions of the species (Wendelbo, 1977; Azimi et al., 2019). The geographical data of the study area are presented in Table 1. We analyzed the morphological variance of I. hymenospatha and I. histrio populations to characterize their phenotypic trait diversity (Table 3).
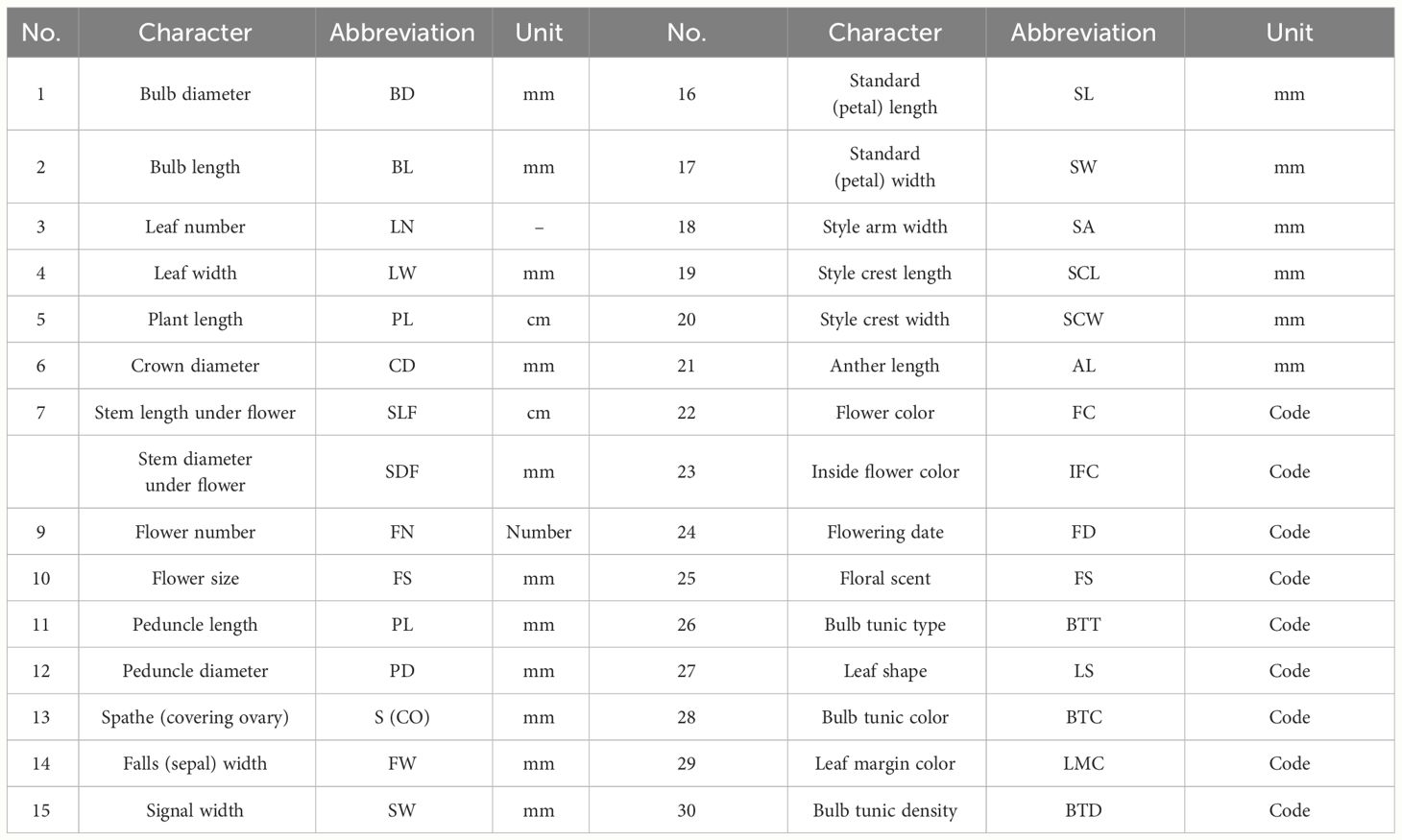
Table 2 List of morphological characters of Iris hymenospatha and Iris histrio studied and their corresponding abbreviations.

Table 3 Comprehensive analysis of morphological variance (AMOVA) in Iris hymenospatha and Iris histrio populations.
2.1 Morphological evaluation
Thirty floral and morphological traits, such as quantitative and qualitative characteristics of leaves, bulb, and flower, were measured (Tables 2, 4). Among the I. hymenospatha and I. histrio populations, flower characteristics were recorded for only 6 of the 10 populations of I. hymenospatha and the 2 populations of I. histrio due to differences in their respective flowering times in the wild (Table 5). Measurements of all morphological traits were taken in the field during the peak of flowering season (mid-February to early March) in 2022. The populations are relatively clustered, with up to two accessions per square meter, with there being several dozen plants per population. When sampling in the field, population boundaries were clearly recognizable. Accessions were defined as clumps (ramets) of leaf fans spaced more than 30–100 m apart. All accessions were evaluated in each population. Quantitative traits such as leaf width, plant length, flower size, fall width, standard length, anther length, bulb length, bulb diameter, and stem length were evaluated using a digital caliper (Table 2). We also measured leaf number and flower number. In addition, qualitative characteristics, such as flower scent, flower color, inner flower color, flowering date, bulb tunic type, leaf shape, bulb tunic color, leaf margin color, and bulb tunic density, were surveyed based on rating, scoring, and coding (Table 4).
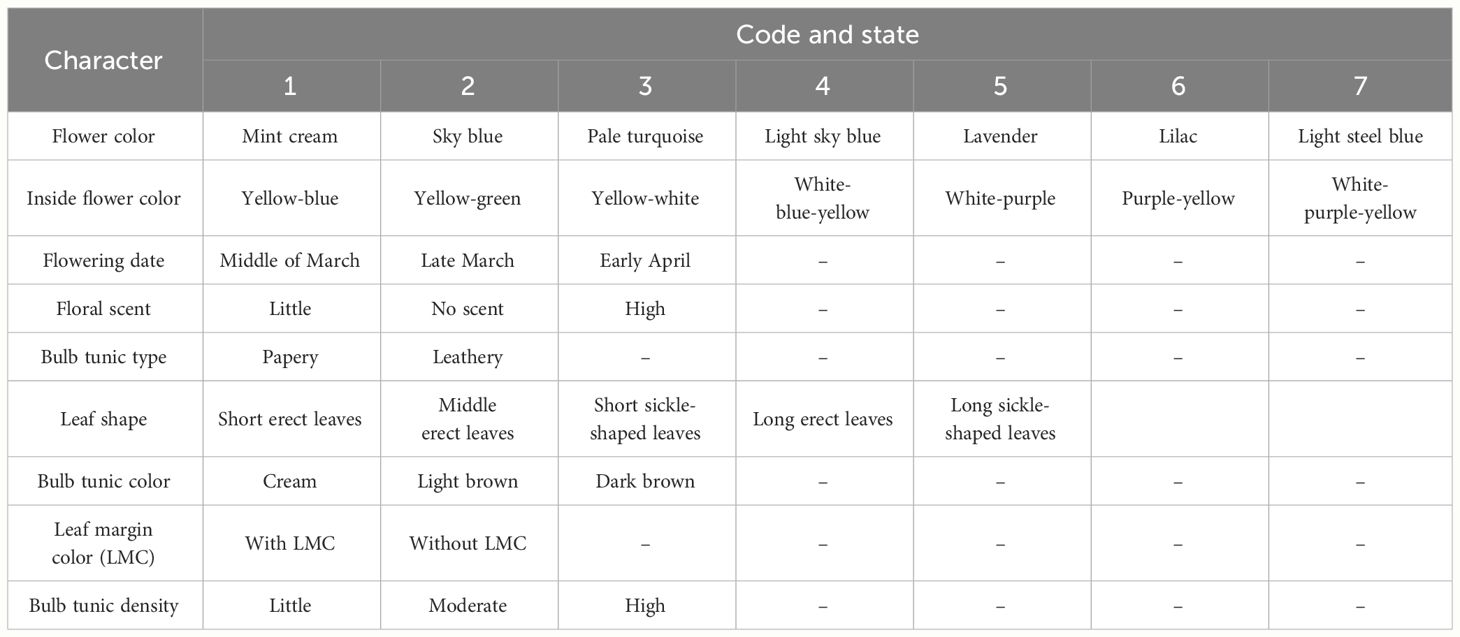
Table 4 Qualitative trait descriptors used in the study of Iris hymenospatha and Iris histrio populations.
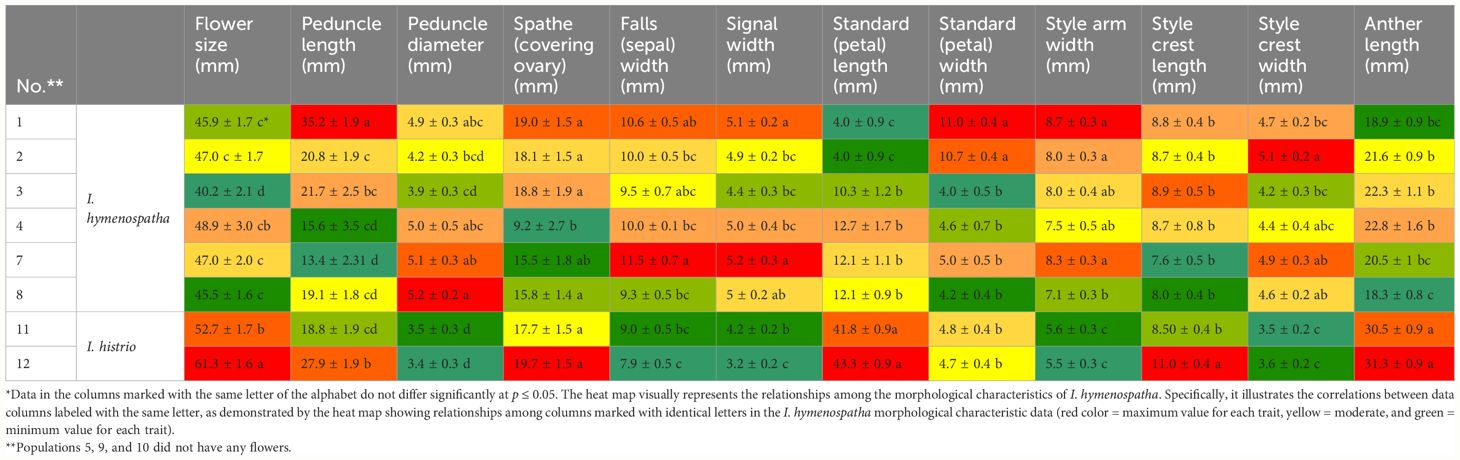
Table 5 Detailed morphological characteristics of Iris hymenospatha and Iris histrio flowers: analysis of flower size, spathe, standard (petal), style crest, and anther length across populations.
2.2 Statistical analysis
The morphological properties were statistically analyzed by JMP Pro software version 13 (SAS Institute Inc. 2017. JMP® 13 Consumer Research, Second Edition). Analyses of variance were performed to detect significant differences between populations with the means being compared using the Duncan’s multiple range tests. Cluster analysis was performed using the unweighted pair group method with an arithmetic average (UPGMA) using five qualitative and nine quantitative traits that we collected for every population (Figures 1A, B, 2A, B). For qualitative traits, the statistical significance was assessed using a two-tailed non-parametric Kruskal–Wallis test. Relationships among the accessions were investigated by principal component analysis (PCA) using JMP Pro. Additionally, the scatter plot was made utilizing the first and second principal components (PC1/PC2) with JMP Pro. The biplot for the first two PCs showed consistent results for 100 accessions of I. hymenospatha (Figure 3A) and 20 accessions of I. histrio (Figure 3C) based on morphological traits. In addition, the most effective morphological traits were determined based on the first two PCs and are shown in Figures 3B, D.
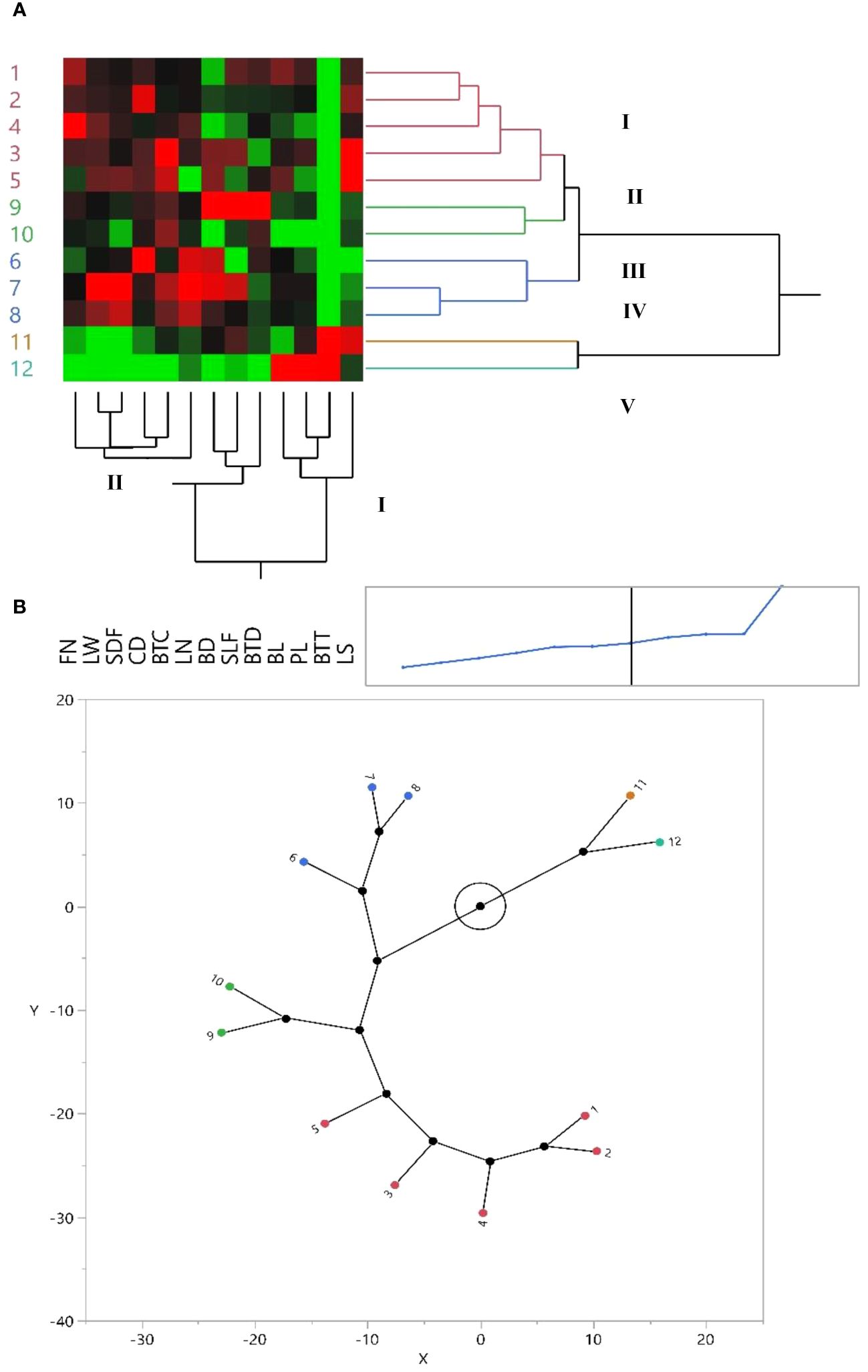
Figure 1 (A) Dendrogram and heat map derived from hierarchical clustered analysis of Iris hymenospatha in 10 populations and Iris histrio in 2 populations based on morphological traits. Red color = maximum value, black = moderate, and green = minimum value for each trait. See Table 2 for the definition of trait abbreviations. Similarity: Euclidean distance. Method: Ward’s linkage. (B) Constellation plot of I. hymenospatha and I. histrio populations.
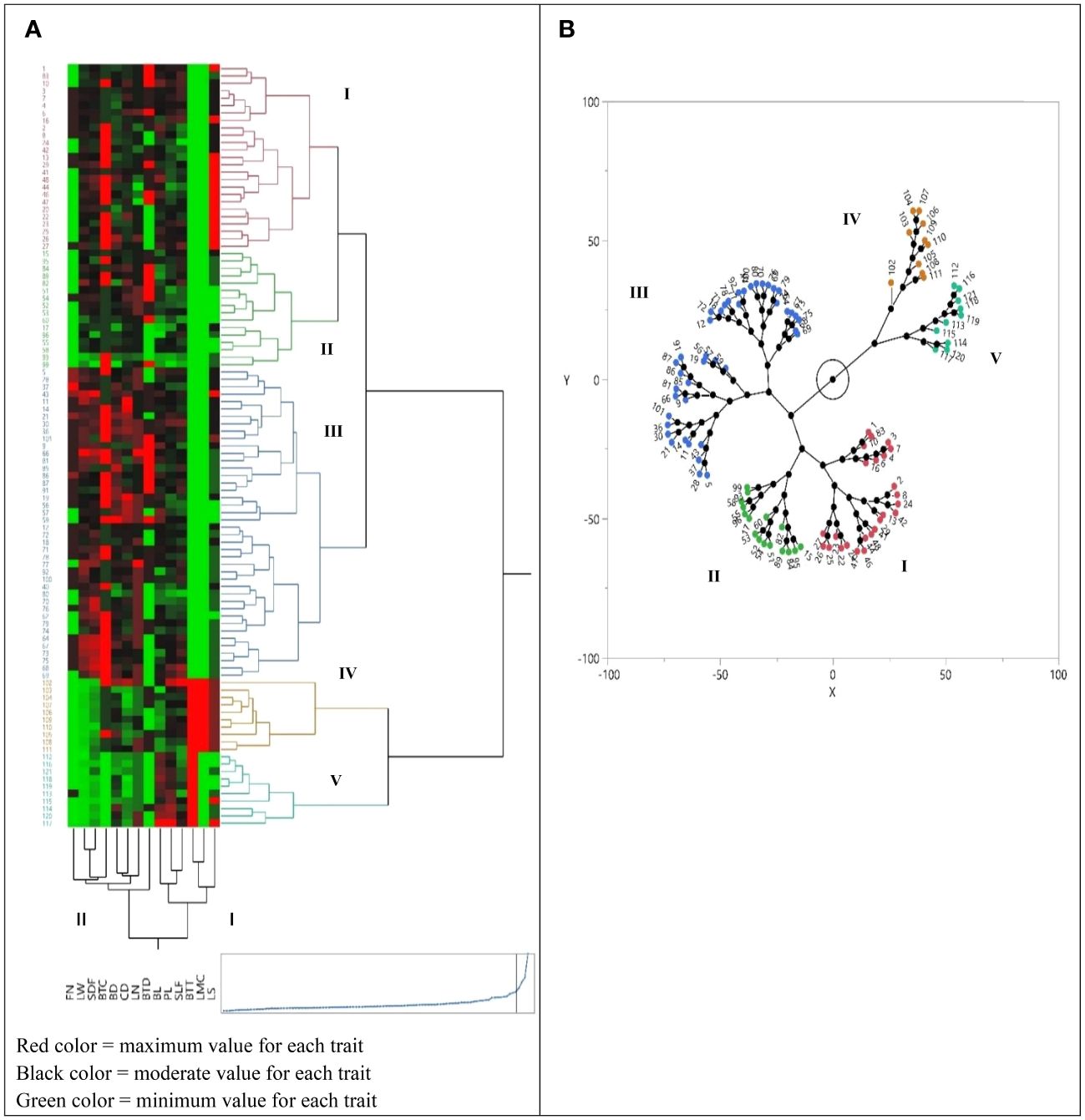
Figure 2 (A) Cluster analysis of 100 accessions of Iris hymenospatha belonging to 10 populations and 20 accessions of Iris histrio belonging to 2 populations based on morphological traits using Euclidean distances. Heat map shows the relationship between cluster analysis of morphological characteristic data. See Table 2 for the definition of trait abbreviations. (B) Cluster analysis depicting the constellation lot of I. hymenospatha and I. histrio accessions.
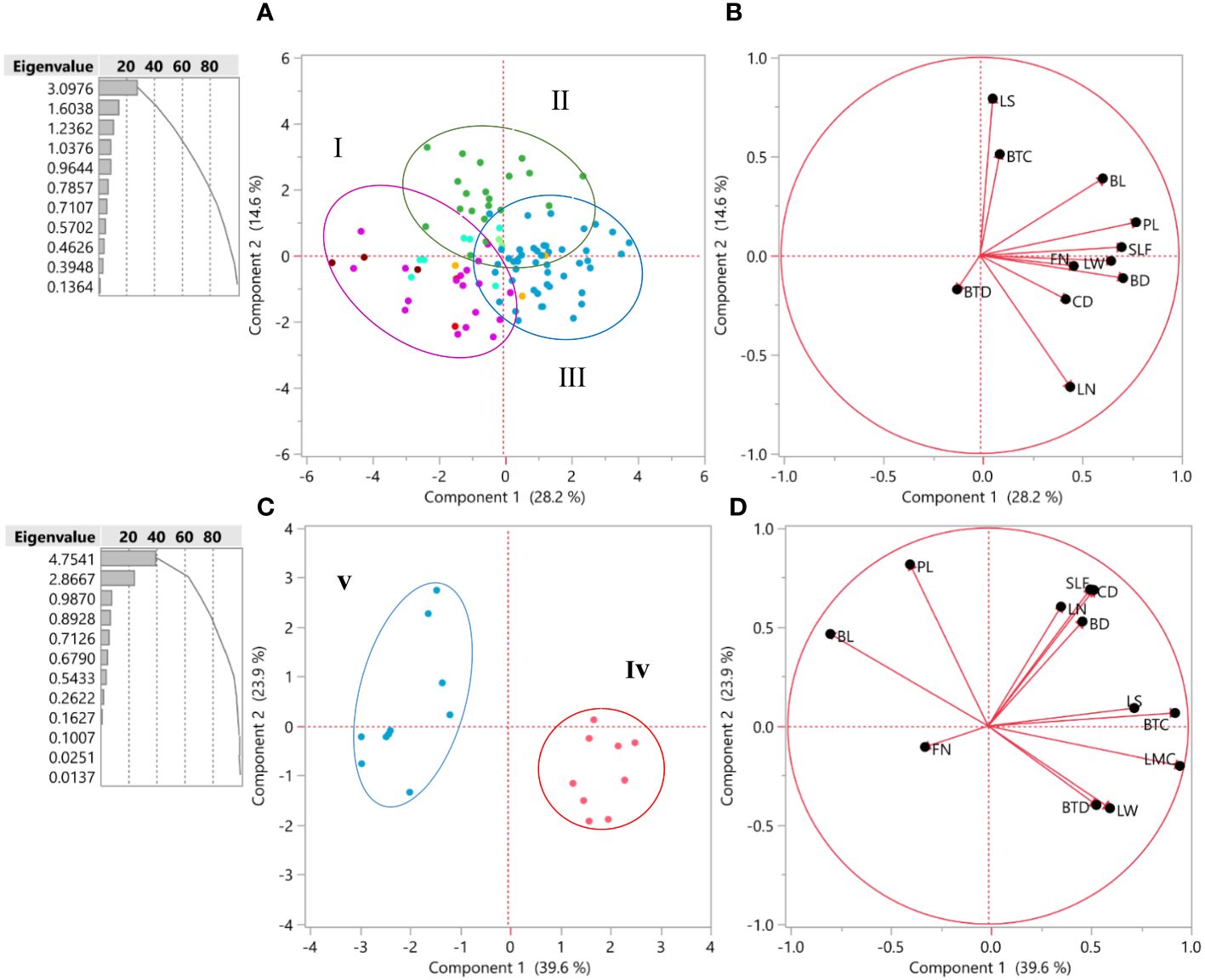
Figure 3 (A) Biplot for the first two principal components’ 100 accessions of Iris hymenospatha based on morphological traits. Population colors indicate the three groups identified through the PCA [I = purple, II = green, and III = blue]. (B) The most effective morphological traits based on the first two principal components. (C) Biplot for the first two principal components’ 20 accessions of Iris histrio based on morphological traits. Population colors indicate the two groups identified through the PCA [iv = red, v = blue]. (D) The most effective morphological traits based on the first two principal components. leaf number, flower number, crown diameter, stem diameter under flower, leaf width, and bulb tunic color.
Both species have been included in a single analysis to obtain a single figure for the cluster analysis for the specific purpose of visually summarizing the data. This approach was taken to simplify the presentation of the data and to avoid any potential confusion that could arise from presenting the data separately for each species. Additionally, I. hymenospatha and I. histrio were analyzed separately in PCA to better understand the differences between the two species.
3 Results
The use of morphological descriptors to characterize wild populations of I. hymenospatha and I. histrio reveals wide variation among most flower, bulb, and leaf morphological traits, such as flower size, peduncle length, flower number, bulb length, leaf number, leaf width, plant length, crown diameter, flower color, and leaf shape (Tables 5–7; Figure 4).
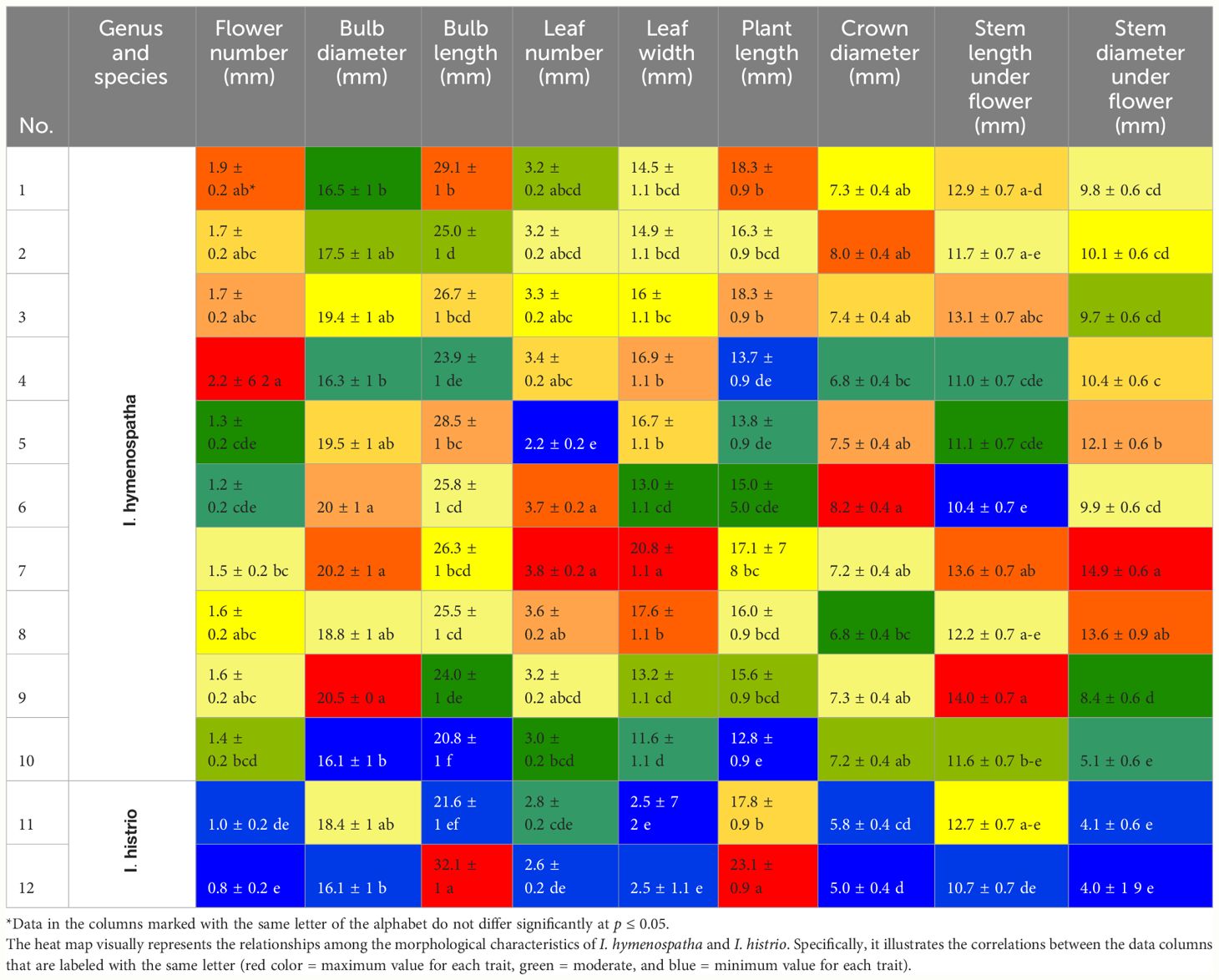
Table 6 Detailed examination of bulb and leaf morphological characteristics in Iris hymenospatha and Iris histrio populations.
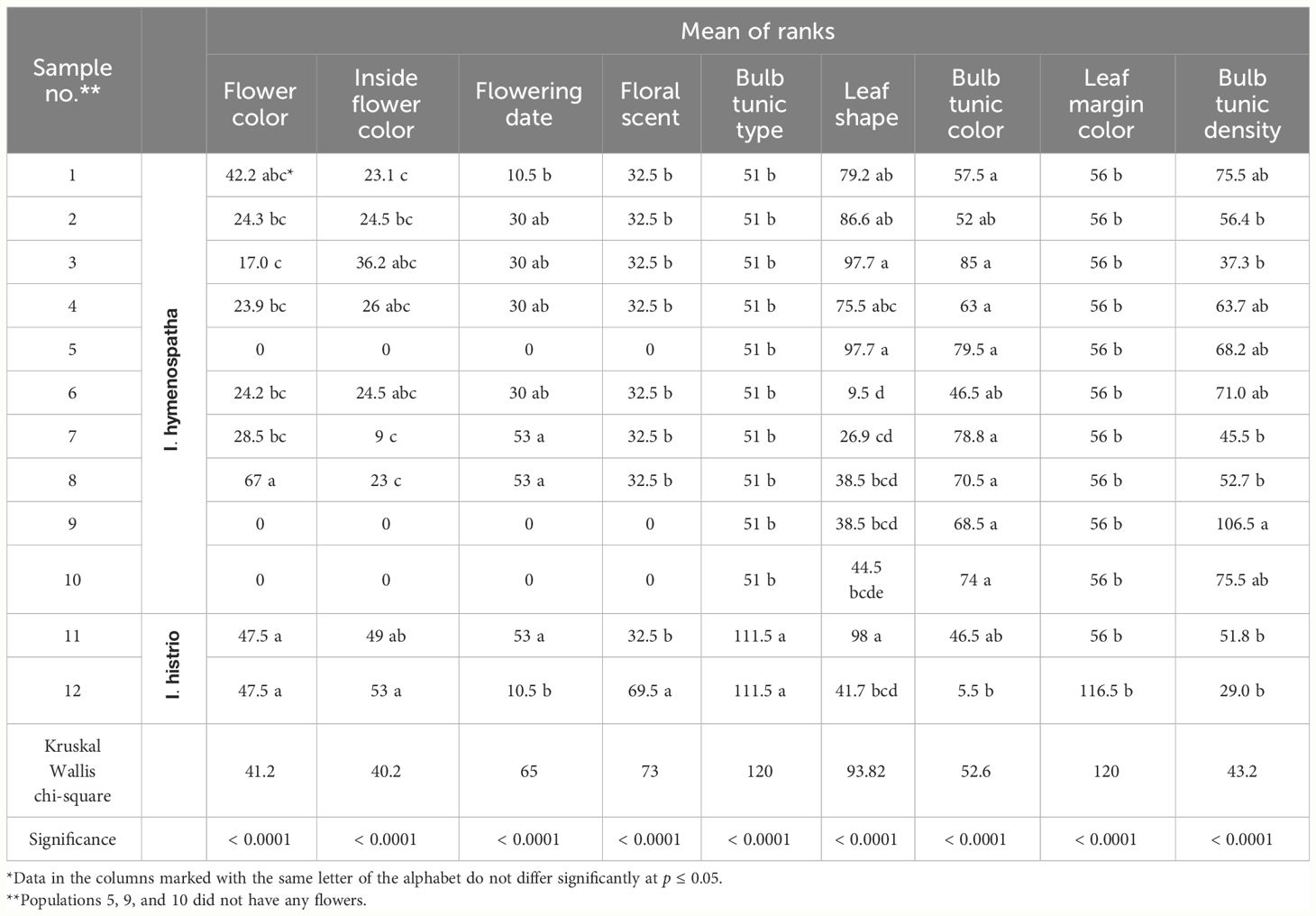
Table 7 Qualitative trait morphology and Kruskal–Wallis test results: A comparative study of Iris hymenospatha and Iris histrio populations.
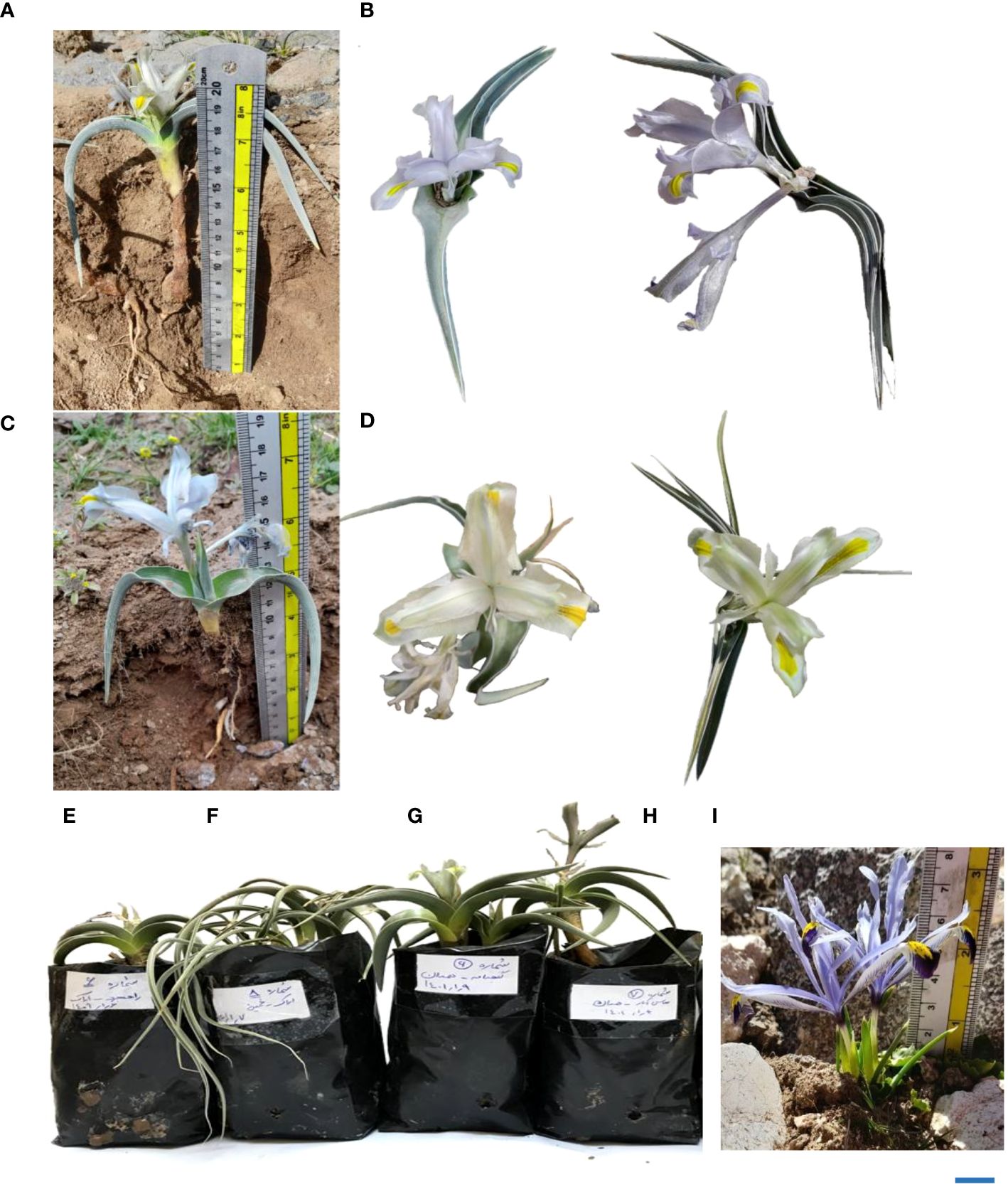
Figure 4 (A) Comparative display of flower and leaf shapes in various Iris hymenospatha and Iris histrio populations: detailed morphological differences in I. hymenospatha “Natanz-Karkas”, (B, E) I. hymenospatha “Arak-Rahjerd” (long erect leaf shape), (C) I. hymenospatha “Jaro”, (D, H) I. hymenospatha “Abas-abad” (middle erect leaf shape), (F) I. hymenospatha “Arak-khomain” (long sickle-shaped leaf shape), (G) I. hymenospatha “Ganjnameh” (long erect leaf shape), and (I) I. histrio (bar = 5 cm).
3.1 Morphological characteristics of flowers
Differences were found to exist among populations in flower size, peduncle diameter, peduncle length, spathe (covering ovary), falls (sepal width), signal width, petal length and width, style arm, style crest length and width, and anther length (Table 5).
Among I. hymenospatha populations, “Arak-Rahjerd” had the largest flower size at 48.9 ± 3.0 mm, while “Ardestan-Taleghan” had the smallest at 40.2 ± 2.1 mm (Table 5). Among I. hymenospatha populations, the average peduncle length was found to be significantly higher in the “Jaro” population at 35.2 ± 1.9 mm, while the largest peduncle diameter was found in the “Abas-abad” population at 5.2 ± 0.2 mm. “Arak-Rahjerd”, “Jaro”, and “Ganjnameh” also had significant values of peduncle diameter (Table 5). The shortest peduncle length was found in the I. hymenospatha “Ganjnameh” population at 13.4 ± 2.31 mm, while the smallest peduncle diameter was found in the “Ardestan-Taleghan” population at 3.9 ± 0.3 mm (Table 5). Among I. hymenospatha populations, there was no significant difference in the spathe (covering ovary) among the “Jaro”, “Natanz-Karkas”, “Ardestan-Taleghan”, “Ganjnameh”, and “Abas-abad” populations (Table 5). The “Arak-Rahjerd” population had a significantly shorter spathe (9.2 ± 2.7 mm) compared to other I. hymenospatha populations (Table 5). I. hymenospatha “Ganjnameh”, “Jaro”, and “Abas-abad” populations had a significantly wider fall (sepal) width (11.5 ± 0.7 mm), compared to “Natanz-Karkas”, “Arak-Rahjerd”, and “Arak-Taleghan” populations (Table 5). In I. hymenospatha populations, the largest standard (petal) width was found in “Jaro” at 11.0 ± 0.4 mm and “Natanz-Karkas” at 10.7 ± 0.4 mm (Table 5). I. hymenospatha “Abas-Abad” indicated a significantly smaller style arm width and anther length among the I. hymenospatha population (Table 5). The I. histrio population “Velian” had a significantly larger flower size (61.3 ± 1.6 mm), plant length (27.9 ± 1.9 cm), and style crest length (11.0 ± 0.4 mm) compared to the I. histrio population “Ganjnameh”. The I. histrio population “Ganjnameh” had a significantly larger signal width between two I. histrio populations.
3.2 Morphological characteristics of bulb and leaf
Analysis of morphological variance revealed significant bulb and leaf trait diversity among I. hymenospatha and I. histrio populations (Table 8). Populations statistically differed in flower number, bulb diameter, bulb length, leaf number, leaf width, plant length, crown diameter, stem length under flower, and stem diameter under flower (Table 6). The highest number of flower among I. hymenospatha populations occurred in the “Arak-Rahjerd” population with approximately two flowers per accessions (Table 6). With regard to number of flowers, there were no significant differences among “Jaro”, “Natanz-Karkas”, “Ardestan-Taleghan”, and “Arak-Rahjerd” populations of I. hymenospatha (Table 6). The lowest number of flowers was found in the I. histrio “Velian” accession compared to I. histrio “Ganjnameh” populations (Table 6).
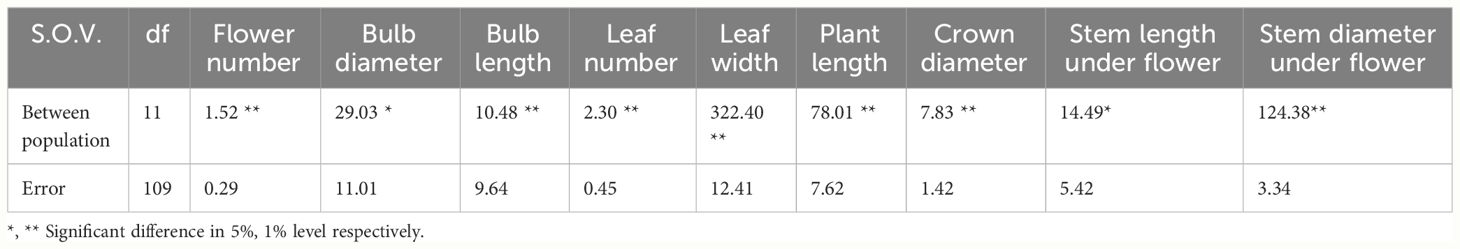
Table 8 Comprehensive analysis of morphological variance (AMOVA) in Iris hymenospatha and Iris histrio populations.
The bulb diameter of I. hymenospatha populations ranged from 16.5 ± 1 to 20.5 ± 1 mm. The “Arsanjan” (20.5 ± 1 mm) had the highest bulb diameter and “Jaro” (29.1 ± 1 mm) had the longest bulb lengths among I. hymenospatha populations (Table 6). The bulb diameter of I. histrio populations ranged from 16.1 ± 1 to 18.4 ± 1 mm, but there were no significant differences among I. histrio populations. The I. histrio “Velian” population (32.1 ± 1 mm) had significantly longer bulb lengths relative to the I. histrio “Ganjnameh” population (21.6 ± 1 mm) (Table 6).
The “Arak-Gerdo”, “Abas-abad”, “Arsanjan”, and “Ganjnameh” populations had the largest number of leaves, with approximately four leaves per accession relative to other I. hymenospatha populations (Table 6). The “Ganjnameh”, “Arak-Gerdo”, and “Arsanjan” populations had the largest leaf width (20.8 ± 1.1 mm), crown diameter (8.2 ± 0.4 mm), and stem length under flower (14.0 ± 0.7 mm) among I. hymenospatha populations, respectively. The shortest plant length was found in the “Ardestan-Mishab” population (12.8 ± 0.9 mm) among I. hymenospatha populations (Table 6). The plant length of the I. histrio “Velian” population (23.1 ± 0.9 cm) was significantly longer compared to that of the I. histrio “Ganjnamneh” population (17.8 ± 0.9 mm) (Table 6).
3.3 Morphological characteristics of flower color and bulb tunic traits
Differences existed among I. hymenospatha populations in flower color, inner flower color, floral scent, bulb tunic type, leaf shape, bulb tunic color, leaf margin color, and bulb tunic density (Kruskal–Wallis, p < 0.0001; Table 7). The lavender flower color of the I. histrio population differed from the flower color of I. hymenospatha, although there was no significant difference between I. histrio populations (Table 7). In addition, large variations were observed among I. hymenospatha populations in flower traits, such as flower color, inner flower color, and floral scent (Table 7). For instance, the flower color of the I. hymenospatha “Abas-abad” population was significantly different among I. hymenospatha populations (Table 7). Within I. hymenospatha populations, flower color varied from mint cream in the “Abas-abad” population to light steel blue in the “Arak-Rahjerd” population (Table 7; Figure 4). We identified seven different inner flower colors among I. hymenospatha populations (Tables 2, 7). I. hymenospatha populations “Jaro”, “Ganjnameh”, and “Abas-abad” showed substantial differences in inner flower color, with yellow-blue, yellow-white, and yellow-green colors, respectively. On the other hand, populations of I. histrio displayed differences inside flower color, ranging from white to purple (Table 7).
The flowering period of I. hymenospatha and I. histrio populations ranged from the middle of March to early April. The blooming date for the I. hymenospatha populations “Abas-abad” and “Ganjnameh” occurred on 29 March and 1 April 2022, respectively, which could be due to colder temperatures in the region where these accessions were located (Table 7). The “Velain” population of I. histrio had a significantly shorter flowering date compared to I. histrio “Ganjnameh” (Table 7). I. histrio “Velain” bloomed before March (Table 7). There was significant diversity in flower color, inner flower color, and tunic traits among I. hymenospatha and I. histrio populations (Table 3). The “Velain” population of I. histrio was significantly more fragrant compared to the I. histrio “Ganjnameh” (Table 7). Interestingly, the bulb tunic density of the I. hymenospatha “Arsanjan” was significantly higher among I. hymenospatha accessions (Table 7). Five different leaf shapes were identified among I. hymenospatha populations (Table 2; Figure 4). The “Ardestan-Taleghan” and “Arak-Khomain” populations of I. hymenospatha (long sickle-shaped leaf) showed significantly different leaf shapes compared to the I. hymenospatha “Arak-Gerdo” population (short erect leaf shape) (Figure 4F). In summary, we detected the largest flower size and number in the “Arak-Rahjerd” population of I. hymenospatha (Table 5). I. hymenospatha “Jaro” had the longest peduncle length, widest standard (petal) width, and longest bulb length among I. hymenospatha populations (Table 5). Moreover, the longest peduncle diameter among I. hymenospatha populations was in the “Abas-abad” population (5.2 ± 0.2 mm) (Table 5). There were no significant differences among I. hymenospatha populations “Abas-abad”, “Arak-Rahjerd”, and “Ganjnameh” (Table 5).
3.4 Hierarchical cluster analysis
Our use of hierarchical cluster analysis (HCA), using Ward’s method, organized I. hymenospatha and I. histrio populations (Figure 1A) and 120 accessions (Figure 2A) into five main clusters on the y-axis of each dendrogram. The first cluster (I) included five I. hymenospatha populations, which were collected from Alborz, Isfahan, and Markazi provinces, while the second cluster (II) comprised two I. hymenospatha populations, which were collected from Isfahan and Shiraz provinces. The third cluster (III) involved one and two populations collected from the Markazi and Hamedan provinces, respectively (Figure 1A). The fourth (IV) and fifth (V) clusters each included one I. histrio population from the Alborz and Hamedan provinces, respectively (Figure 1A).
The morphological traits were placed into two major clusters on the x-axis of each dendrogram (Figures 1A, 2A). The first cluster (I) included four morphological traits: bulb length, plant length, bulb tunic type, and leaf shape (Figure 1A). The second cluster (II) included stem length under flower, bulb length, plant length, bulb tunic type, and leaf shape (Figure 2A). Morphological traits in the first cluster (I) showed minimum to moderate values in I. hymenospatha populations compared to the traits in the second cluster (II) (Figures 1A, 2A). Most of the traits, such as flower number, leaf width, stem diameter under flower, crown diameter, bulb tunic color, leaf number, bulb diameter, stem length under flower, and bulb tunic density in the second cluster (II) indicated moderate to maximum values for I. hymenospatha populations compared to the first cluster (I) traits (Figures 1A, 2A). Some representative morphological differences in I. hymenospatha, such as flower color, leaf shape, and leaf number, are shown in Figure 4. On the other hand, the heat map traits in the first cluster (I) for I. histrio populations indicated maximum values compared to the traits in the second cluster (II) on the x-axis of each dendrogram (Figures 1A, 2A).
A constellation plot provided the best outcomes for differentiating population diversity based on the morphological traits of I. hymenospatha and I. histrio (Figure 1B). The constellation plot of I. hymenospatha and I. histrio populations indicated five different groups, with the same result appearing in the cluster analysis (Figure 1A).
The cluster analysis of morphological traits of 120 accessions demonstrated that those belonging to different populations clustered together (Figure 2B). The cluster analysis separated the 100 accessions of I. hymenospatha and 20 accessions of I. histrio into five groups (Figure 2B). Cluster I was mainly composed of I. hymenospatha accessions from the populations “Jaro” (32% of the population), “Arak-Khomain” (24% of the population), “Ardestan-Taleghan” (28% of the population), “Natanz-Karkas” (12% of the population), and “Ardestan-Mishab” (4% of the population) (Figure 2B). Cluster II consisted of I. hymenospatha accessions from the populations “Arak-Gerdo” (44% of the population), “Ardestan-Mishab” (25% of the population), “Arsanjan” (19% of the population), and “Natanz-Karkas” (13% of the population). Cluster III represents a combination of I. hymenospatha accessions from the populations of “Abas-Abad” (22.5% of the population), “Ganjnameh” (15% of the population), “Natanz-Karkas” (13% of the population), “Arsanjan” (12.5% of the population), “Arak-Gerdo” (7.5% of the population), “Ardestan-Mishab” (7.5% of the population), “Arak-Rahjerd” (5% of the population), and “Jaro” (5% of the population). Cluster IV includes accessions from the I. histrio population “Ganjnameh”, and cluster V consisted of the I. histrio population “Velian” (Figure 2B).
3.5 Principal component analysis
The arrangement of I. hymenospatha and I. histrio accessions among 10 and 2 populations, respectively, was analyzed using PCA based on morphological data (Figure 3). The population color and cluster number in Figures 3A, C are consistent with the cluster analysis shown in Figures 2A, B. The biplot, which visualizes the first two PCs, consistently displayed the results for 100 accessions of I. hymenospatha and 20 accessions of I. histrio (Figures 3A, C). These results were derived from the morphological traits that were most influential in differentiating between the accessions (Figures 3B, D). For I. hymenospatha accessions, these influential traits include stem length under flower, bulb length, plant length, leaf number, flower number, crown diameter, leaf width, and bulb diameter (Figure 3B).
For I. histrio accessions, these influential traits include leaf shape, bulb tunic color, leaf margin color, leaf width, and bulb tunic density (Figure 3D). The population color and cluster number are consistent with the cluster analysis shown in Figures 2A, B. Using the Kaiser’s criterion (“Eigenvalue” > 1) (Kaiser, 1958), two significant components were achieved, which explained 42.8% of the total variation in 100 I. hymenospatha accessions and 63.5% of the total variation in 20 I. histrio accessions (Figure 3). For I. hymenospatha accessions, PCA revealed that some traits, such as bulb length, plant length, stem length under flower, flower number, leaf width, bulb diameter, and crown diameter, had the highest scores (28.2%) relative to the total variance in the first components (Figure 3B). Leaf shape, bulb tunic color, bulb tunic density, and leaf number were the major contributors to the second component (PC2), which explained 16.5% of the total variance (Figure 3B). For I. histrio accessions, PCA revealed that some traits, such as leaf shape, bulb tunic color, leaf margin color, leaf width, and bulb tunic density, had the highest scores (39.6%) relative to the total variance in the first components (Figure 3D). Stem length under flower, crown diameter, leaf number, bulb diameter, and plant length were the major contributors to the second component (PC2), which explained 23.9% of the total variance (Figure 3D).
The biplot was created based on the first two PCs and mainly categorized individual accessions of I. hymenospatha into three independent groups (Figure 3A) and I. histrio into two independent groups (Figure 3C) consistent with HCA and the constellation plot (Figures 2A, B), with some admixture in I. hymenospatha populations (Figure 3A). Among I. hymenospatha populations, the second (II) and third (III) groups were plotted on the right side of PC1 (Figure 3A). These accessions were distinguished by bulb length, plant length, stem length under flower, flower number, leaf width, bulb diameter, and crown diameter (Figure 3B). Group two (II) among I. histrio populations was located on the right side of the PC1 (Figure 3D) and correlated with leaf shape, bulb tunic color, leaf margin color, leaf width, and bulb tunic density (Figure 3D).
4 Discussion
Morphological characterization is a crucial step in breeding programs as it enables the monitoring of genetic quality, allowing for the selection of the most suitable accessions for use in breeding programs. Our study shows that there are significant floral, bulb, leaf, and qualitative characteristics in I. hymenospatha and I. histrio populations collected from different regions of Iran, such as the variety of onion tunic color and onion tunic diameter. The I. hymenospatha populations “Arak-Rahjerd” and “Jaro” had the largest flower size (48.9 ± 3 mm) and greatest peduncle length (35.2 ± 1.9 mm), respectively (Table 5), which could be of value from a horticultural perspective, making these populations attractive for cultivation and conservation. Similarly, “Abas-Abad” had the largest peduncle diameter (5.2 ± 0.2 mm) among I. hymenospatha populations. There was no significant difference in peduncle diameter among I. hymenospatha “Abas-Abad”, “Arak-Rahjerd”, “Jaro”, and “Ganjnameh” populations. In our study, we observed that the populations of “Abas-Abad”, “Ganjnameh”, and “Arak-Gerdo” exhibited a similar average leaf number per accession, which was approximately 3.7. The I. hymenospatha “Jaro” (1.9 ± 0.2), “Natanz-Karkas” (1.7 ± 0.2), “Ardestan-Taleghan” (1.7 ± 0.2), and “Arak-Rahjerd” (2.2 ± 0.2) populations had the greatest number of flowers among the I. hymenospatha populations. The bulbous Iris inflorescence typically features two flowers, each enclosed in a spathe, which bloom in sequence (Kamenetsky and Okubo, 2012). The I. histrio “Velian” population had a significantly higher flower size and peduncle length, relative to the I. histrio “Ganjnameh” population. These findings highlight the potential of horticulture in conserving these species. By selecting for these traits in cultivation, we can help in the conservation of these species.
Morphological characterization is a crucial step in breeding programs as it enables the monitoring of genetic quality, allowing for the selection of the most suitable accessions for use in breeding programs. This is particularly important for species like I. hymenospatha and I. histrio, which are facing threats in their native habitats. Horticulture can play a significant role in the conservation of such species by preserving their genetic diversity and promoting their propagation. Our study shows that there is significant floral, bulb, leaf, and qualitative characteristics in I. hymenospatha and I. histrio populations collected from different regions of Iran, such as the variety of onion tunic color and onion tunic diameter. These traits not only have horticultural value, but also contribute to the species adaptability and survival.
Our results are consistent with those of Moradi et al. (2023), who investigated morphological variability in wild-growing, but threatened Fritillaria imperialis populations (Moradi et al., 2023). They displayed several key ornamental traits that are highly suitable for breeding initiatives, such as plant length, peduncle length, peduncle diameter, flower number, flower size, and leaf number (Moradi et al., 2023). Wild-growing populations of F. imperialis are rapidly declining due to overgrazing, overharvesting, and climate change. Similar to our results, Khaleghi and Khadivi (2022) noted that flower characteristics, such as inflorescence length, diameter, and color; floral scent; and peduncle length, are of great ornamental and commercial importance.
In this study, we found that the I. hymenospatha population “Arak-Rahjerd” has a light steel blue flower color, which is significantly different from the mint cream color found in the “Abas-abad” population (Table 7; Figure 4). Flower color is one of the most important concerns for breeders due to its influence on ornamental and commercial value (Nakamura et al., 2015). Members of the Iris genus display a wide variety of flower colors, including dark purple, violet, pink, yellow, and white flowers (Roguz et al., 2020). Owing to distinctive features, such as the fragrance and color variety of I. hymenospatha, they can be used as breeding stock for new cultivars (Asgari et al., 2022). Given the aesthetic quality of the flowers of I. hymenospatha, they exhibit potential to be introduced and further developed as ornamental flowers (Asgari et al., 2022). Various traits in this bulbous species underscore how it can be used to select desired attributes for development, efficiency, and commercial development.
The I. histrio “Velian” population had a significantly higher flower size, floral scent, peduncle length, style arm width, flowering date, and plant length compared to the “Ganjnameh” population of I. histrio. From an ornamental and conservation perspective, the scent of a flower is of great importance. In addition, flower scent and color may be derived from the same biosynthesis pathways (Roguz et al., 2020). Morphological characteristics of qualitative traits showed significantly shorter flowering date of the I. hymenospatha “Ganjnameh” and “Abas-Abad” populations, which bloomed on 1 April and 29 March, respectively (Table 5). The I. hymenospatha “Jaro” (11.0 ± 0.4 mm) and “Natanz-Karkas” (10.7 ± 0.4 mm) populations had the widest standard (petal) width among populations (Table 3). A significantly higher signal width was reported in I. hymenospatha “Jaro” (5.1 ± 0.2 mm) and “Ganjnameh” (5.2 ± 0.3 mm) among I. hymenospatha populations (Table 5). Similar to our results, Azimi et al. (2016) collected 14 Iris species from different provinces of Iran and classified them into three clusters based on quantitative traits and four groups based on qualitative traits. According to Azimi et al. (2016), the components of Iris flowers that have significant economic implications for breeding programs include the quantitative traits of flower size, outer and inner tepal width, inner tepal length, and leaf width.
In our study, characteristics such as bulb length, plant length, stem length under flower, flower number, leaf width, bulb diameter, and crown diameter contributed to the first component (PC1) and leaf shape, bulb tunic color, bulb tunic density, and leaf number were the major contributors to the second component (PC2) in the biplot analysis of the two Iris species. With 42.8%, the first two components had the highest scores relative to the total variance in I. hymenospatha (Figures 3A, B). Our PCA findings show that morphological characteristics, such as the stem length under flower, bulb length, plant length, leaf number, flower number, crown diameter, leaf width, and bulb diameter, are the most relevant morphological traits in I. hymenospatha (PC1) (Figure 3B). To summarize, the accessions of I. hymenospatha from the “Arak-Rahjerd”, “Ganjnameh”, “Arak-Gerdo”, and “Abas-abad” populations have the highest number of these traits (PC1) (Figure 3A). Out of all the I. hymenospatha populations, they appear to have the greatest potential for selection in breeding programs (Tables 5, 6).
Leaf shape, bulb tunic color, leaf margin color, leaf width, stem length under flower, crown diameter, leaf number, and bulb diameter emerge as primary contributors to the first component (PC1) in the populations of I. histrio we analyzed. Plant length, bulb length, and flower number are identified as major contributors to the second component (PC2) (Figures 3C, D). These components collectively explain 63.5% of the total variance (Figures 3C, D). In summary, the accessions of I. histrio from the “Ganjnameh” populations have the highest number of these relevant traits (PC1) (Figures 3C, D). I. histrio “Ganjnameh” populations collected from the province of Hamedan (Table 1) appear to have the greatest potential for selection as an ornamental bulbous crop.
PCA has been previously used to evaluate the genetic relationship of other plant species, such as Muscari (Labbaf et al., 2020) and Tulipa (Khaleghi and Khadivi, 2022). Azimi et al. (2018) investigated the morphological characteristics of Iris germanica hybrids and found a 97% heritability value for plant height, fall width and length, flower size, and leaf width. They concluded that these values can be used as useful traits in Iris breeding and hybrid selection. Asgari et al. (2022) studied five Iris species and derived four factors from multivariate morphological analysis that explained 84.79% of total variance. Consistent with our results, Asgari et al. (2022) reported that some variables, such as petal length, flower height, seedling number, number of flowers, bulb length, leaf number, bulb diameter, leaf width, and stem diameter, were significant in explaining variation among accessions.
5 Conclusion
Populations of I. hymenospatha from “Jaro”, “Natanz-Karkas”, “Ardestan-Taleghan”, “Arak-Rahjerd”, “Arak-Gerdo”, “Ganjnameh”, and “Abas-Abad”, which belong to clusters I and III (Figures 1, 2, and 3A), exhibited moderate to maximum values in selected traits such as flower number, leaf width, stem diameter under flower, crown diameter, bulb tunic color, leaf number, bulb diameter, stem length under flower, and bulb tunic density (Figure 3B). These traits make these populations not only valuable for horticultural purposes but also crucial for conservation efforts. The use of horticulture as a tool for conservation is particularly important for these populations, as it allows for the preservation of their genetic diversity and resilience. These traits appear useful for further selection of I. hymenospatha accessions for ornamental purposes. Moreover, the I. histrio “Velian” population had a significantly higher flower size, peduncle length, style arm width, flowering date, and plant and bulb length compared to the “Ganjnameh” population of I. histrio. Variation in such traits suggests that this species should be further evaluated for horticultural purposes (Figures 1A, 2A).
Our results indicate that the five populations of I. hymenospatha (“Arak-Rahjerd”, “Arak-Gerdo”, “Jaro”, “Abas-Abad”, and “Ganjnameh”), which we collected from Alborz, Markazi, and Hamedan provinces, showed wide variation in morphological traits, such as flower size, peduncle diameter, peduncle length, flower number, leaf number, flower color, and flower scent. As such, they can be used in breeding programs to improve commercial cultivars (Tables 5–7). In addition, some accessions of “Ganjnameh” and “Abas-abad” had contractile roots that are responsible for the movement of the underground portion of the species (I. Rohollali, personal observation). On the other hand, from an ornamental point of view, the “Arak-Rahjerd”, “Ganjnameh”, and “Abas-Abad” populations of I. hymenospatha seem to be the most promising for conservation breeding programs since they were characterized by a wide variation of horticulturally important qualitative traits such as flower color and flowering date (Table 7 and Figure 4).
Global biodiversity is declining at an unprecedented rate (12.5% of the estimated world flora), and immediate conservation action is required to protect many of these species (Walter and Gillett, 1998). The collection of plant material in this study was carried out in accordance with the rules of the Genetic Resources Protection and Exploitation Law of the Government of Iran. We stored the collected seeds of both Iris species in the gene bank of Shahed University in Tehran, Iran. All relevant information regarding the preservation of the living germplasm of both species will be donated to the Iranian Biological Resource Center.
Taken together, we confirmed the hypothesis that there is broad morphological variation among I. hymenospatha and I. histrio accessions in Iran at the population scale. We determined several morphological characters that can be used to introduce I. hymenospatha and I. histrio into conservation and breeding programs for further development as horticultural selections. Protection of scattered habitats, even those relatively small in size, including small populations of I. hymenospatha and I. histrio, which naturally thrive in such semi-arid and arid regions, is of great importance. I. hymenospatha populations naturally grow in regions with low rainfall such as Isfahan, where the “Ardestan-Taleghan” population was located, and in regions with high rainfall, such as Hamedan, where the “Ganjnameh” and “Abas-abad” populations occurred (Table 2). The species also grows in a variety of soils, from rocky to sandy clay, between altitudes of 1,655 and 2,212 m with reasonable adaptability to a variety of natural conditions. Conserving these species while allowing for sustainable ornamental use of these species should continue to be pursued.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.try-db.org/TryWeb/Data.php#101.
Author contributions
IR: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing – original draft. AN: Writing – review & editing, Data curation, Formal analysis, Supervision. JS: Writing – review & editing, Funding acquisition. RK: Investigation, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We express our gratitude to Mojdeh and Shadi Abedini for their assistance in the field and data collection. We also express our gratitude to Dr. Carol Wilson for providing us insightful information on the taxonomy of Iris hymenospatha and Iris histrio.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1305240/full#supplementary-material
References
Abenavoli, L., Milanovic, M., Procopio, A. C., Spamoinato, G., Maruca, G., Perrino, E. V., et al. (2021). Ancient wheats: beneficial effects on insulin resistance. Minerva. Med. 112, 641–650. doi: 10.23736/S0026-4806.20.06873-1
Amin, H. I. M., Hussain, F. H. S., Maggiolini, M., Vidari, G. (2018). Bioactive constituents from the traditional Kurdish plant Iris persica. Natural Product. Commun. 13, 1127–1128. doi: 10.1177/1934578x1801300907
Amin, H. I. M., Hussain, F. H. S., Najmaldin, S. K., Thu, Z. M., Ibrahim, M. F., Gilardoni, G., et al. (2021). Phytochemistry and biological activities of iris species growing in Iraqi kurdistan and phenolic constituents of the traditional plant iris postii. Molecules 26, 2. doi: 10.3390/molecules26020264
Asgari, E., Taghizadeh, M., Abbasifar, A. (2022). Exploration and morphologic variation of Iris wild species with ornamental potential. Ornamental. Horticult. 28, 36–48. doi: 10.1590/2447-536X.V28I1.2409
Azimi, M. H., Jozghasemi, S., Barba-Gonzalez, R. (2018). Multivariate analysis of morphological characteristics in Iris germanica hybrids. Euphytica 214, 1–11. doi: 10.1007/s10681-018-2239-7
Azimi, M. H., Jozghasemi, S., Hasanzadeh Davarani, F., Aliabadi, H. M. (2019). A review of Iranian Iris germplasm. Acta Hortic. 1240, 51–56. doi: 10.17660/ActaHortic.2019.1240.8
Azimi, M. H., Tahernezhad, Z., Zamani, M. J. (2016). Genetic variation within Iranian iris species using morphological traits. Int. J. Hortic. Sci. Technol. 3, 89–98.
Calabrese, G., Perrino, E. V., Ladisa, G., Aly, A., Tesfmichael Solomon, M., Mazdaric, S., et al. (2015). Short-term effects of different soil management practices on biodiversity and soil quality of Mediterranean ancient olive orchards. Organic. Agric. 5, 209–223. doi: 10.1007/s13165-015-0120-8
Farahmand, H., Farzad Nazari (2015). Environmental and anthropogenic pressures on geophytes of Iran and the possible protection strategies: A review homayoun. Int. J. Hortic. Sci. Technol. 2, 111–132.
Ghahreman, A. (1984). Color Atlas of Iranian Plants (5th ed.) (Botany Division: Institute of Forestries and Grasslands).
Gholamzade Natanzi, A. (2023). Destruction of the Karkas Mountain and the stones that come out of the cemetery (Isfahan: IRNA). Available at: https://irna.ir/xjHZ2j.
Hassani, N., Asghari, H. R., Frid, A. S., Nurberdief, M. (2008). Impacts of overgrazing in a long term traditional grazing ecosystem on vegetation around watering points in a semi-arid rangeland of North-Eastern Iran. Pakistan J. Biol. Sci. 11, 1733–1737. doi: 10.3923/pjbs.2008.1733.1737
Hussain, F. H. S., Amin, H. I. M., Patel, D. K., Porwal, O. (2020). An overview of the therapeutic potential of iris persica. Curr. Traditional. Med. 7, 152–160. doi: 10.2174/2215083806666200117111320
Kaiser, H. F. (1958). The varimax criterion for analytic rotation in factor analysis. Psychometrika 23, 187–200. doi: 10.1007/BF02289233
Khaleghi, A., Khadivi, A. (2022). Genetic diversity of wild grape hyacinth (Muscari neglectum Guss. ex Ten.) germplasm with ornamental potential in the central region of Iran. S. Afr. J. Bot. 148, 307–314. doi: 10.1016/j.sajb.2022.05.001
Khatib, S., Faraloni, C., Bouissane, L. (2022). Exploring the use of iris species: antioxidant properties, phytochemistry, medicinal and industrial applications. Antioxidants 11, 526. doi: 10.3390/antiox11030526
Kumar, A. (2014). “Role of Horticulture in Biodiversity Conservation,” in Sustainable Development and Biodiversity Issues, Technology and Innovation (Switzerland: Springer), 143–155.
Labbaf, N., Rohollahi, I., Naji, A. M. (2020). Genetic diversity and population structure of wild Persian grape hyacinths (Muscari neglectum Guss. ex Ten.) assessed by morphological and molecular markers. Genet. Resour. Crop Evol. 67, 1481–1492. doi: 10.1007/s10722-020-00922-7
Mavrodiev, E. V., Martínez-Azorín, M., Dranishnikov, P., Crespo, M. B. (2014). At least 23 genera instead of one: The case of Iris L. s.l. (Iridaceae). PloS One 9, 2. doi: 10.1371/journal.pone.0106459
Moradi, M., Khaleghi, A., Khadivi, A. (2023). Morphological variability of wild-growing crown imperial (Fritillaria imperialis L.) germplasm in central region of Iran—implications for in-situ conservation initiatives. BMC Plant Biol. 23, 9. doi: 10.1186/s12870-022-04032-7
Nakamura, N., Katsumoto, Y., Brugliera, F., Demelis, L., Nakajima, D., Suzuki, H., et al. (2015). Flower color modification in Rosa hybrida by expressing the S-adenosylmethionine: Anthocyanin 3′,5′-O-methyltransferase gene from Torenia hybrida. Plant Biotechnol. 32, 109–117. doi: 10.5511/plantbiotechnology.15.0205a
Perrino, E. V., Calabrese, G. (2018). Endangered segetal species in southern Italy: distribution, conservation status, trends, actions and ethnobotanical notes. Genet. Resour. Crop Evol. 65, 2107–2134. doi: 10.1007/s10722-018-0678-6
Perrino, E. V., Wagensommer, R. P. (2022). Crop wild relatives (CWRs) threatened and endemic to Italy: urgent actions for protection and use. Biology 11, 2. doi: 10.3390/biology11020193
RBG (2024) Royal botanic gardens Kew. Iris histrio Rchb.f. Available at: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:438704-1.
Reynolds, L. K., McGlathery, K. J., Waycott, M. (2012). Genetic diversity enhances restoration success by augmenting ecosystem services. PloS One 7 (6), 2. doi: 10.1371/journal.pone.0038397
Roguz, K., Gallagher, M. K., Senden, E., Bar-Lev, Y., Lebel, M., Heliczer, R., et al. (2020). All the colors of the rainbow: diversification of flower color and intraspecific color variation in the genus iris. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.569811
Walter, K. S., Gillett, H. J. (1998). “1997 IUCN Red list of threatened plants,” in IUCN. Available at: http://biodiversitylibrary.org/page/31085360.
Wagensommer, R. P., Perrino, E. V., Albano, A., Medagli, P., Passalacqua, N. G. (2016). Lectotypification of four lacaita’s names in the genus centaurea (Asteraceae). Phytotaxa 269, 54–58. doi: 10.11646/phytotaxa.269.1.7
Weber, T., Jakše, J., Sladonja, B., Hruševar, D., Landeka, N., Brana, S., et al. (2020). Molecular study of selected taxonomically critical taxa of the genus iris L. From the broader alpine-dinaric area. Plants 9, 1–16. doi: 10.3390/plants9091229
Wendelbo, P. (1977). “Tulips and Irises of Iran and their relatives,” in Tehran: Botanical Institute of Iran, Ariamer Botanical Garden 83p.-Illus., col. illus., map. Icones, Maps. Geog (Vol. 2). Tehran: Botanical Institute of Iran.
Wilson, C. A. (2011). Subgeneric classification in Iris re-examined using chloroplast sequence data. TAXON 60, 27–35. doi: 10.1002/tax.601004
Keywords: Iris, morphological, natural conditions, native, populations, variability
Citation: Rohollahi I, Naji AM, Stewart JR and Kamrani R (2024) Morphological characterization of Iris hymenospatha and Iris histrio populations in Iran: implications for conservation and breeding. Front. Plant Sci. 15:1305240. doi: 10.3389/fpls.2024.1305240
Received: 01 October 2023; Accepted: 18 April 2024;
Published: 28 May 2024.
Edited by:
Robert Philipp Wagensommer, Free University of Bozen-Bolzano, ItalyReviewed by:
Emanuele Del Guacchio, University of Naples Federico II, ItalyEnrico Vito Perrino, International Centre for Advanced Mediterranean Agronomic Studies, Italy
Copyright © 2024 Rohollahi, Naji, Stewart and Kamrani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iman Rohollahi, aW1hbnJvb2hvbGxhaGlAZ21haWwuY29t
 Iman Rohollahi
Iman Rohollahi Amir Mohammad Naji2
Amir Mohammad Naji2 J. Ryan Stewart
J. Ryan Stewart Rozita Kamrani
Rozita Kamrani