- 1Department of Pharmacy, University of Genova, Genova, Italy
- 2Department of Pharmacy, University of Salerno, Fisciano, Italy
- 3Plant Pathology Laboratory, Section Microbiology and Molecular Biology, Centro di Sperimentazione e Assistenza Agricola (CeRSAA), Albenga, Italy
- 4Department of Informatics, Bioengineering, Robotics and System Science, University of Genova, Genova, Italy
- 5Department of Pharmaceutical Sciences, Division of Pharmaceutical Chemistry, University of Vienna, Vienna, Austria
- 6Research Centre For Vegetable and Ornamental Crops (CREA), Sanremo, Italy
Introduction: The development of agriculture in terms of sustainability and low environmental impact is, at present, a great challenge, mainly in underdeveloped and marginal geographical areas. The Salvia rosmarinus “Eretto Liguria” ecotype is widespread in Liguria (Northwest Italy), and farmers commonly use it by for cuttings and for marketing. In the present study, this ecotype was characterized in comparison with other cultivars from the same geographical region and Campania (Southern Italy), with a view to application and registration processes for the designation of protected geographical indications. Moreover, the possibility of using the resulting biomass after removing cuttings or fronds as a source of extracts and pure compounds to be used as phytosanitary products in organic farming was evaluated. Specifically, the potential of rosemary extracts and pure compounds to prevent soft rot damage was then tested.
Methods: A targeted NMR metabolomic approach was employed, followed by multivariate analysis, to characterize the rosemary accessions. Bacterial soft rot assay and disk diffusion test were carried out to evaluate the activity of extracts and isolated compounds against Pectobacterium carotovorum subsp. carotovorum. Enzymatic assay was performed to measure the in vitro inhibition of the pectinase activity produced by the selected pathogen. Molecular docking simulations were used to explore the possible interaction of the selected compounds with the pectinase enzymes.
Results and Discussion: The targeted metabolomic analysis highlighted those different geographical locations can influence the composition and abundance of bioactive metabolites in rosemary extracts. At the same time, genetic factors are important when a single geographical area is considered. Self-organizing maps (SOMs) showed that the accessions of “Eretto Liguria” appeared well characterized when compared to the others and had a good content in specialized metabolites, particularly carnosic acid. Soft rotting Enterobacteriaceae belonging to the Pectobacterium genus represent a serious problem in potato culture. Even though rosemary methanolic extracts showed a low antibacterial activity against a strain of Pectobacterium carotovorum subsp. carotovorum in the disk diffusion test, they showed ability in reducing the soft rot damage induced by the bacterium on potato tissue. 7-O-methylrosmanol, carnosol and isorosmanol appeared to be the most active components. In silico studies indicated that these abietane diterpenoids may interact with P. carotovorum subsp. carotovorum pectate lyase 1 and endo-polygalacturonase, thus highlighting these rosemary components as starting points for the development of agents able to prevent soft rot progression.
1 Introduction
Salvia rosmarinus Spenn. (Roma-Marzio and Galasso, 2019; POWO, 2023) is a Mediterranean aromatic, evergreen shrub widely cultivated for culinary, medicinal, and ornamental purposes. This species grows in open areas in a large diversity of dry, sunny, and calcareous habitats, from sea level up to 1,500 m (Rosselló et al., 2006). It is mainly distributed in the western half of the Mediterranean area, in Europe, and North Africa. It is rare in the eastern part of the Mediterranean and is regarded as introduced in the eastern most part of Europe. Based on this distribution, it is assumed that the center of diversification of the species is located in the South-Eastern Iberian Peninsula (Mateu-Andrés et al., 2013). S. rosmarinus is very polymorphic with high morphological variation in growth habit, leaf width, and flower color (Rosúa, 1986), and hybridization and introgression among subgeneric taxa (Drew et al., 2017) have been reported (Rosúa, 1986; Rosselló et al., 2006). Infra-specific taxa are maintained in the range of variability of the species, as the diversification reflects the existence of local ecotypes differing in adaptative traits (Rosselló et al., 2006). The systematics of varieties, subspecies, forms, races, biotypes, and ecotypes appear often uncertain and confused. Many of these variation patterns are available, with differences also in growth habits (upright, twisting, and creeping), and several of them grow wild in the Mediterranean countries (De Mastro et al., 2004). Currently, the distribution of biotypes, ecotypes, and landraces have been studied only in a few Italian areas (Mulas et al., 1998; Flamini et al., 2002; De Mastro et al., 2004), and the selection of cultivars and varieties adapted to local environmental conditions from spontaneous populations (Mulas and Mulas, 2005). The variability of the phytochemical profile of rosemary has been described as related to its phenological stage, geographic location, seasonal variation, environmental factors, abiotic stresses, and to genetic characteristics (Hidalgo et al., 1998; Tounekti and Munné-Bosch, 2012; Li et al., 2016). The chemical diversity of S. rosmarinus has been extensively studied, both as relates to the essential oil’s composition (Flamini et al., 2002; Gurbuz et al., 2016; Satyal et al., 2017) and to the antioxidant secondary metabolites (Wellwood and Cole, 2004). The abietane diterpenoids carnosic acid and carnosol, along with other diterpenoids, polyphenolic acids, and flavonoids, are considered rosemary extracts’ most relevant bioactive compounds (Cuvelier et al., 1996; Xiao et al., 2008; Birtić et al., 2015). Specifically, carnosic acid displays significant antioxidant properties, and it is of great interest in food, pharmaceutics, and cosmetic industries for antimicrobial, anti-inflammatory, anti-carcinogenic, anti-adipogenic (Birtić et al., 2015), and phytotoxic activities (Appiah et al., 2018). Carnosic acid has commercial interest as an approved food additive (Birtić et al., 2015), and the antioxidant activity of rosemary extracts is primarily associated with the amount of this compound. On this basis, the growers are rewarded based on the abundance of carnosic acid in the harvested biomass (Sahoo et al., 2022), and this compound is used to characterize rosemary accessions (Wellwood and Cole, 2004; Sahoo et al., 2022).
In Northwest Italy, wild populations of S. rosmarinus are characterized by upright habitus, elliptical light green leaves, and pale purplish-blue corolla. This ecotype, named ‘Eretto Liguria’, is distributed in the regional area, and farmers use it to collect germplasm as cuttings for the cultivation of marketed potted plants.
The present study’s first aim was to discriminate the ‘Eretto Liguria’ rosemary ecotype from other cultivars and varieties cultivated in the same area and another Italian geographical location, in different climatic zones, highlighting its phytochemical traits. NMR spectroscopy coupled with multivariate data analysis was applied to characterize the rosemary accessions, focusing on the main metabolites selected according to literature data (Xiao et al., 2008).
The second aim of this study was to point out new possibilities for the use of waste of rosemary chain. No official data on global rosemary production waste are available. At local level (Liguria region, Italy), considering an annual production of 37 million potted plants of rosemary (CeRSAA estimation, 2023) that are pruned two times during the cropping period, the produced pruning waste (vegetative tips and young branches 5–7 cm long) is estimated to be 370 t (CeRSAA GEP Testing Centre, 2022). To produce potted rosemary plants, growers apply integrated pest management regulations (DIR EU 128/2009) using low-toxicity chemicals characterized by a good residual profile and low environmental impact. At present, residual biomass from non-utilized parts of medicinal and aromatic plants is not regarded as waste, since it can be recycled and converted into value-added products (Saha and Basak, 2020) according to the principles of circular economy (Kirchherr et al., 2017). Specifically, the European Circular Economy Action Plan (European Union, 2020) focuses on the valorization of agricultural residues as a potential source of bioactive compounds (Skendi et al., 2022). Moreover, the Farm to Fork Strategy, within the European Green Deal, envisages a significant reduction of chemicals, whose target, by 2030, provides for an average decrease of 50% of chemicals compared to the current quantities. This target will be achieved through the revision of DIR EU 128/2009, which will impose an increased use of low-toxicity products, the rise in soil cultivation according to organic methods, and the increase in integrated pest management. Moreover, the use of natural-based products of natural origin and of botanical extracts, both used as plant protection products (EU REG 1107/2009) and as substances capable of increasing the defenses of plants against abiotic and biotic agents (Biostimulants—EU REG 1009/2019) will be strengthened. At present, the available products include natural-based products (i.e., Strobilurins and Azadiractins), natural compounds or plant extracts (i.e., eugenol, geraniol, orange oil, and spearmint oil) formulated as plant protection products and biostimulants extracts from plant biomass (i.e., sweet orange essential oil, blends of tannins, olive oil, cotton oil, propolis, Marseille soft soap, and wine/fruit vinegar). The market, in the latter case, is rapidly expanding in Europe (1.5 Bln € turnover; more than 2,000 employees; 10% of annual growth) and in Italy (50 Million € turnover; 10% of annual growth) (National Association of Fertilizer Manufacturers, 2023), and therefore, this sector appears to be extremely promising. S. rosmarinus extracts are used by some chemical companies (Benuzzi M. CBC (Europe) srl - division BIOGARD, 2023, personal communication) as a pathogen and pest control (REG. EU 1107/2009) or as biostimulants (REG. EU 1009/2019).
Considering the relevant production of waste biomass of S. rosmarinus in Liguria, finding a possibility of using extracts obtained from it in local agriculture could improve local, sustainable exploitation of the ‘Eretto Liguria’ ecotype, sold at fruit and vegetable markets as an aromatic plant for culinary use or for making cuttings. To this purpose, the activity against soft rot disease caused by Pectobacterium carotovorum subsp. carotovorum (syn. Erwinia carotovora) of the methanolic extracts was evaluated. P. carotovorum subsp. carotovorum is an enterobacterium infective to more than a hundred species of Brassicaceae, Solanaceae, Cucurbitaceae, and others, many of which are cultivated in the same Ligurian area. The bacterial soft rot induced by this microorganism during cultivation and storage is the cause of product loss in many vegetables chain. To our knowledge, there is no existing practical or effective chemical control for bacterial soft rot caused by P. carotovorum subsp. carotovorum (Mantsebo et al., 2014).
Many features of disease management caused by Pectobacterium spp. as cultivation measures, physical, chemical, and biological treatments for controlling soft rot were evaluated (van der Wolf et al., 2021). Analytical techniques for monitoring P. carotovorum were applied (Yang et al., 2021). Studies on various aspects of P. carotovorum virulence, ranging from quorum-sensing signal molecules, biofilm formation, regulation of expression of virulence genes by plant extracts and compounds, sensitivity to phage cocktails, and plant cell-wall-degrading enzymes (PCWDEs) production have been carried out (Põllumaa et al., 2012; van der Wolf et al., 2017; Gutierrez-Pacheco et al., 2018; Agyemang et al., 2020; Van Gijsegem et al., 2021a; Van Gijsegem et al., 2021b; Kim et al., 2022; Su et al., 2022). Pectobacterium spp. use pectin as a carbon source for growth and secrete multiple pectic enzymes, including pectate lyases (Pel), polygalacturonases (Peh), proteases (Prt), and cellulases (Cel), involved in the maceration of plant tissues (Hugouvieux-Cotte-Pattat et al., 2014; Agyemang et al., 2020; Mallick et al., 2022; Han et al., 2023). These enzymes were classified into various classes and subclasses depending on the substrate specificity and mode of action. According to the cleavage site, pectinases are divided into three groups: (i) hydrolases consisting of polygalacturonase, PG (EC 3.2.1.15); (ii) lyase/trans-eliminases comprising pectin lyase, PNL (EC 4.2.2.10), and pectate lyase, PL (EC 4.2.2.2); and (iii) pectin esterase, PE (EC 3.1.1.11) (Yadav et al., 2009). Plant signals and various transcriptional and post-transcriptional regulators coregulate the production of PCWDE (De Boer, 2003; Andresen et al., 2007; Agyemang et al., 2020). The PCWDE secretion is considered to be the main virulence factor employed by the pathogens to promote tissue maceration and rotting by disrupting the integrity of their host cells (De Boer, 2003; Põllumaa et al., 2012; Pun et al., 2021).
Plant extracts and compounds with the potential to prevent soft rot damage were reported (Yangui et al., 2013; Ashmawy et al., 2014; Sánchez-Hernández et al., 2023; Suaza-Gaviria et al., 2023). Among them, essential oils are well-known for their antimicrobial activity, and their possible application as coatings in storage and as preventive treatments against soft rot was evaluated (Zheng et al., 2013; Purnavab et al., 2015; Hajian-Maleki et al., 2019). Several pure plant constituents, including phenolic acids, quinones, flavonoids, terpenoids, and alkaloids, were examined for their efficacy in inhibiting bacterial growth, and their effect on several virulence factors, such as motility, biofilm formation, and exoenzyme activity (Joshi et al., 2015; Joshi et al., 2016; Gutierrez-Pacheco et al., 2018; Shaheen and Issa, 2020; Pun et al., 2021; Li et al., 2022).
Since the most abundant abietane diterpenoids of rosemary extracts (i.e., carnosic acid, carnosol, 7-O-methylrosmanol, 12-O-methylcarnosic acid, and isorosmanol) (Nieto et al., 2018) are characterized by a well-known antimicrobial activity (González, 2015), the present study also aimed to test these compounds against P. carotovorum. In this study, we focused on the depolymerizing enzymes of P. carotovorum subsp. carotovorum responsible for the random cleavage of the α-(1→4)-glycosidic bonds in the D-galacturonic acid moieties of the pectic substances: polygalacturonate lyase (pectate lyase 1) (PelA) and endo-polygalacturonase (PehA). The potential interactions of the selected abietanes with P. carotovorum subsp. carotovorum pectinase enzymes were explored using molecular docking simulations.
2 Materials and methods
2.1 Chemicals
Solvents, deuterium oxide (D2O, 99.9% D), CD3OD (99.8% D), and 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt (TSP) were purchased from Sigma-Aldrich Chemical Company (Sigma-Aldrich, Milano, Italy).
2.2 Plant material
A total of 37 accessions of S. rosmarinus ecotypes and cultivars grown in field conditions in two Italian regions, Liguria (Northern Italy) and Campania (Southern Italy), all collected in the same period (June 2021), were used for the study (Supplementary Tables S1–S4). The identification of all the rosemary accessions was performed by Dr. Claudio Cervelli, according to the literature (Rosúa, 1986; Cervelli and Masselli, 2013; Drew et al., 2017). The vouchers of all the accessions were deposited at the Herbarium of Giardini Botanici Hanbury (La Mortola, Ventimiglia, Italy) (Supplementary Table S2).
2.3 Bacterial strain
P. carotovorum subsp. carotovorum was obtained from the collection of the Plant Pathology Laboratory of CeRSAA (accession number: CeRSAA Rdp 665/14). The strain satisfied Koch’s postulates, and its identity was confirmed by a specific molecular method (Khoshkdaman et al., 2008) and sequencing.
2.4 Sample collection and preparation
Fresh biomass (5–10 cm at the top of plant shoots), including leaves and stems (100 g each sample) were collected in May 2021, frozen and lyophilized in a freeze-dryer (Super Modulyo, Edwards, UK) for 48 h. Three biological replicates for each accession were used, with a total of 111 samples. All samples were sealed in plastic bags and stored dry in the dark until analysis. The dried material (approximately 35 g per sample) was then grounded. The powder samples for NMR analysis were prepared following the method reported by Tajidin et al. (2019). For each sample, 50 mg of dried leaves were extracted with a mixture of deuterated solvents made up of 250 µL of 90 mM H2KPO4 buffer in D2O (pH 6) and 650 µL of CD3OD, containing 0.01% 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid sodium salt (TSP), using a vortex mixer. The ultrasound-assisted extraction was performed for 30 min at room temperature using an ultrasound bath (Branson 2510E-MTH, Bransonic®, Milano, Italy), and centrifuged for 10 min at 13,000 rpm. The supernatant was then transferred into an NMR tube.
Methanolic extracts for antimicrobial and bacterial soft rot assays were prepared by stirring 5 g of dried biomass with 100 mL CH3OH ≥ 99.9% at 25°C at 150 rpm for 24 h and filtered through Whatman No. 4 paper (Sigma-Aldrich, Milano, Italy). The extraction was repeated three times. The combined methanolic extracts were evaporated to dryness under reduced pressure and stored at 4°C for further use.
2.5 NMR spectroscopy and processing
NMR data were acquired on a Bruker DRX-500 NMR spectrometer (Bruker BioSpin GmBH, Rheinstetten, Germany). The temperature was maintained at 30°C, and CD3OD was used as internal lock. Each 1H NMR spectrum consisted of 64 scans, 6.14 s acquisition time, relaxation delay (RD) of 4 s, mixing time of 0.01 s, and spectral width of 15.99 ppm. A presaturation sequence (NOESY-presat sequence, Bruker: noesygppr1d) was used to suppress the residual signal of water (Vignoli et al., 2019; Monzón Daza et al., 2021). A Chenomx 500 MHz custom library (CCL) (Chenomx NMR Suite 8.6, Chenomx Inc., Edmonton, 252 Canada) was set up using pure compounds both previously isolated in our laboratories from S. rosmarinus and from other plant sources and obtained from commercial sources (Sigma-Aldrich, Milano, Italy) (Xiao et al., 2008) (MSI level of identification according to Sumner et al. (2007) (Supplementary Table S5). A Chenomx Compound Builder tool was used. The custom library contained 27 metabolites. The CCL metabolites included abietane diterpenoids [carnosic acid (Pukalskas et al., 2005), carnosol (Pukalskas et al., 2005), isorosmanol (Topçu et al., 2013), epiisorosmanol (Pukalskas et al., 2005), 7-O-methylrosmanol (Richheimer et al., 1996), and 12-O-methylcarnosic acid (Richheimer et al., 1996; Pukalskas et al., 2005)], flavonoids [luteolin (Lin et al., 2015), scutellarein (Li et al., 2006), acacetin (King-Díaz et al., 2015), rutin (Napolitano et al., 2012), isorhamnetin-3-O-β-D-rutinoside (narcissin) (Rastrelli et al., 1995), quercetin (Napolitano et al., 2012), apigenin (Shen et al., 1993), apigenin-7-O-β-D-glycoside (Liu et al., 2012; Peng et al., 2016), catechin hydrate (Kiehlmann and Tracey, 1986), isorhamnetin-7-O-rutinoside (Mohamed et al., 2020), genkwanin (Gomes et al., 2011), diosmetin (Park et al., 2007), epicatechin (Abd El-Razek, 2007), and kaempferol (Napolitano et al., 2012)], and phenolic acids [rosmarinic acid (Lecomte et al., 2010), rosmarinate (Kuo et al., 2000; Correia et al., 2020), gallic acid (López-Martínez et al., 2015), ferulic acid (López-Martínez et al., 2015), caffeic acid (López-Martínez et al., 2015), p-coumaric acid (López-Martínez et al., 2015), and chlorogenic acid (Pauli et al., 1999)]. Additionally, 11 metabolites of the Chenomx 500 MHz version 11 library (CL), selected based on literature data (Xiao et al., 2008), were used (Supplementary Table S6). The CL metabolites included amino acids (alanine, valine, proline, and asparagine), carboxylic acids (acetate, malate, malonate, and fumarate), carbohydrates (fructose, sucrose), and choline (Xiao et al., 2008; Deborde et al., 2021). Each 1H NMR spectrum was acquired using the 1D. All spectra were acquired in triplicate. The metabolites were identified by comparing their 1H NMR spectra to those of the reference compounds in both the CCL and the CL libraries.
2.6 NMR data analysis
Quantitative analysis of NMR spectra was performed using NMRProcFlow 1.4.14 (INRA UMR 1332 BFP, Bordeaux Metabolomics Facility, Villenave d’Ornon, France) (Jacob et al., 2017) following the method reported by Grimaldi et al. (Grimaldi et al., 2021). Briefly, corrections of phasing and baseline were performed manually for all spectra using TOPSPIN version 3.2. All spectra were calibrated by using the internal standard at 0 ppm. Spectral area integration was made by variably sized bucketing using the online server NMRProcFlow. Buckets with a signal-to-noise ratio above 3 were selected for further analysis. The residual solvent regions of water (δH 4.65–4.75) were removed. Ppm ranges for each characterizing metabolite peak (Supplementary Tables S5, S6) were selected for quantification, and all the data needed were exported into a spreadsheet workbook using the “qHNMR” template. The data matrices generated by NMRProcFlow, one of 27 buckets (CCL compounds) and one of 11 buckets (CL compounds), were then subjected to multivariate analysis.
2.7 Multivariate data analysis
Classical statistical analysis was performed using Systat software for Windows Version 13 (Systat Software Inc., Chicago, IL, USA). The correlations among variables were calculated with the Pearson correlation coefficient. Hierarchical clustering (HC) analysis (single linkage as agglomeration method) was applied to detect groupings in variables (based on Pearson correlation coefficient as distance metric) and in samples (based on Euclidean distance) (Anderberg, 1973). Different data pre-treatment methods were tested, i.e., autoscaling, log transformation, and vast scaling (van den Berg et al., 2006). For explorative analysis, principal component analysis (PCA) with Varimax rotation was applied to the matrix of autoscaled data (Jackson, 2004). Statistical significance was set at p < 0.05. Self-organizing maps (SOMs) were then applied using Matlab R2022a and SOM toolbox 2.1 (The MathWorks, Inc., Natick, MA, USA) (Vatanen et al., 2015). SOMs belong to the category of unsupervised models and are a type of network organization of information processes. The log-transformed (van den Berg et al., 2006) data were then pre-processed before sending them to the algorithm, to prevent variables with a higher range from dominating the map due to their greater impact on the distances involved. A variance-based normalization of the data was performed. Subsequently, linear initialization and batch training were performed. The training took place in two steps: first, the rough phase with a larger radius and learning rate that also considers the most distant nodes is performed, and then, a refinement phase with a smaller radius and learning rate was done. After these steps, the U-matrix was generated, visualizing the distances between neighboring map units. Uniform areas identify clusters, while higher values indicate a cluster edge. The other maps represent the component plan (single compounds), and highly correlated variables show similar maps. Depending on the component values, hits are associated with a single unit of the map. Hits are the number of times a single map unit responds to inputs and indicate how input information is collected in each neuron.
2.8 Bacterial soft rot assay
Potato tubers, var. ‘Colomba’ were obtained from the production field of CeRSAA, in Albenga, SV, Italy. P. carotovorum subsp. carotovorum was obtained from the collection of the Plant Pathology Laboratory of CeRSAA (Accession number: CeRSAA Rdp 665/14). P. carotovorum subsp. carotovorum was cultured at 28°C on a nutrient agar medium [NYDA, made up of 8.0 g nutrient broth, 1.5 g glucose, 20.0 g agar, and 4.0 g yeast extract (Sigma-Aldrich, Milano, Italy), and 1 L deionized water] in Petri dishes for 2 days at 28°C. The bacterial inoculum was prepared from the 2-day-old cultured bacteria in a vial containing Buffered Pepton Water (Generon. Italy). The bacterial inoculum (1 × 108 CFU/mL) was quantified by serial dilution plating method and stored at 4°C.
The assay on potato tuber slices was carried out following the methods reported in the literature (Mikiciński et al., 2010; Osei et al., 2022). Briefly, the tubers were first washed under tap water, dried with cleaning paper cloth, dipped for 5 s into 96% ethanol, air dried, and then flamed and cut into 10-mm-thick slices. The slices were placed on wet filter paper in Petri dishes. The bacterial inoculum was prepared from 2-day-old cultured bacteria in a vial containing Buffered Pepton Water (Generon, Modena, Italy) at a 108 CFU/mL concentration. DMSO working solution was obtained by dissolving the extract in the solvent DMSO with the later addition of deionized water. Extracts and pure compounds were dissolved separately in a solution of sterile distilled water and DMSO working solution (1:1 v/v) (Sigma-Aldrich, Milano, Italy) to obtain, after inoculation, a concentration of 1,000 ppm (the preliminary test carried out to define the working concentration is reported in Supplementary Table S9). This solution was then inoculated with bacterial suspension to obtain a final concentration of 107 CFU/mL of P. carotovorum subsp. carotovorum. Five minutes after the inoculation, filter paper disks 10 mm in diameter were soaked with 0.05 mL of this suspension. The filter paper disks were then placed at the center of the upper surface of each potato slice. After incubation at 25°C for 5 days, tissue rotting was determined, and the development of rot radius was measured. Each sample was tested on three potato slices. Control tubers were treated in the same way, but paper disks were soaked with a solution of inoculated sterile distilled water and DMSO working solution 1:1 v/v (positive control, PC) or with a solution of sterile distilled water and DMSO working solution 1:1 v/v (negative control, NC).
2.9 Disk diffusion test
A disk diffusion test was carried out to evaluate the extracts antimicrobial activity. Pure colonies of P. carotovorum subsp. carotovorum were suspended in a sterile saline solution with 0.9% sodium chloride (Generon, Modena, Italy) until a turbidity matched McFarland tube number of 0.5 (108 CFU/mL). A loopful from the adjusted suspension was swabbed onto Muller Hinton agar (MH 2.0 g meat extract, 17.5 g hydrolyzed casein, 1.5 g starch, 17.0 g agar, and 1 L deionized water). Sterile paper disks (Whatman No. 40; 6 mm in diameter) were soaked in 10 μL of each diluted extract and pure compound (Supplementary Table S10), then dried at ambient temperature for 15 min, and positioned onto the surface of the inoculated agar plate. The plates were then incubated at 35°C for 24 h (Hemeg et al., 2020). Each extract and pure compound were tested in triplicates at 2,000 ppm, 1,000 ppm, 500 ppm, 250 ppm, 50 ppm, 16 ppm, and 0 ppm, respectively. The working solution (2,000 ppm) of each extract and pure compound was diluted in 10% DMSO; then, a parallel test with 10%, 5%, 2.5%, 1.25%, 0.25%, and 0.08% was conducted to verify the absence of sensitivity of the strain to DMSO. Ampicillin (Sigma Aldrich) at 1 ppm, 8 ppm, and 16 ppm were tested as positive control. The sensitivity of the tested microorganism to the different concentrations of extracts and pure compounds was assessed and classified by the diameter of the inhibition halos (growth inhibition zone including the 6 mm disk), as not sensitive for the diameters ≤ 8 mm, sensitive for diameters 9–15 mm, and very sensitive for diameters > 15 mm (Ponce et al., 2003). The extract and pure compounds were then classified into inactive, active, and very active substances (Steglińska et al., 2022). The results were expressed as the average of three independent repetitions with a standard deviation value.
2.10 Enzymatic activity assay
In vitro inhibition of the pectinase activity was measured using a commercially available enzymatic activity assay (QuantiChromTM Pectinase Assay Kit (DPEC-100), Bioassay Systems, Hayward, CA, USA). The turbidity, measured at OD 600 nm, is proportional to the amount of unhydrolyzed pectin, thus inversely proportional to the pectinase activity. The experiment was performed according to the manufacturer’s instructions, and all samples were assayed in triplicate. The bacterial inoculum of P. carotovorum subsp. carotovorum was prepared from 2-day-old cultured bacteria in a vial containing Buffered Pepton Water (Generon, Modena, Italy) at a 108 CFU/mL concentration. Pure compounds (carnosic acid, 12-O-methylcarnosic, 7-O-methylrosmanol, carnosol, and isorosmanol) were dissolved separately in a solution of sterile distilled water and DMSO working solution (1:1 v/v) to obtain, after the addition of the inoculum of P. carotovorum subsp. carotovorum, a concentration of 1,000 ppm and a final concentration of P. carotovorum subsp. carotovorum of 107 CFU/mL. Five minutes after the inoculation, 0.02 mL of these suspensions and 0.02 mL of the pure inoculum at 107 CFU/mL were transferred separately in a single well of a 96-well plate for the pectinase enzyme activity assay. The assay was then performed as recommended by the manufacturer’s instructions. Optical density (OD) was measured at 600 nm using the microplate reader ChroMate 4300 (Awareness Technology Inc., Palm City, FL, USA). The pectinase activity was then calculated according to the standard protocol of the manufacturer’s instructions, considering specific background readings of each solution.
2.11 Molecular docking
For P. carotovorum endo-polygalacturonase (PehA), Protein Data Bank (Berman et al., 2000) entry 1BHE was used to represent the structure of the enzyme (Pickersgill et al., 1998). For P. carotovorum pectate lyase 1 (UniProt ID: P0C1C0) (The UniProt Consortium, 2020), a homology model was derived from PDB entry 2EWE (Scavetta et al., 1999) with Prime, which is part of the Schrödinger Suite 2020-4 (Schrödinger, 2021). More specifically, the template protein and the target protein sequences were aligned with Prime (amino acid sequence identity of 68%). Next, a homology model was generated with Prime using default settings.
To evaluate and validate the modeled 3D structure, a Ramachandran plot was constructed using PROCHECK, on the SAVE server (http://services.mbi.ucla.edu/SAVES). The ProSA web server (https://prosa.services.came.sbg.ac.at/prosa.pHp) (Wiederstein and Sippl, 2007) was employed to calculate the Z-score and evaluate the consistency between the crystal structure of the template and modeled protein.
The protein structures were processed according to the following protocol: the protonation states were optimized at pH 8.0 ± 0.5 and pH 7.0 ± 2.0 for P. carotovorum pectate lyase 1 (PelA) and P. carotovorum endo-polygalacturonase (PehA), respectively. Disulfide-bond formation was carried out, and all water molecules were removed. The protein structures were then subjected to energy minimization with the OPLS3e force field (Harder et al., 2016) to converge heavy atoms to an RMSD of 0.3. The receptor grid generation tool of the Glide module (Schrödinger, 2021) (also part of the Schrödinger Suite) was used to define the grid boxes. As deduced from sequence similarity and site-directed mutagenesis studies, the protruding loops on one side of the parallel β helix form the pectolytic active site. A 20-Å3 grid box was centered on Arg239, which is reported as the catalytic moiety of P. carotovorum pectate lyase 1. In the case of P. carotovorum endo-polygalacturonase (PehA), the 20-Å3 grid box was centered on centroid formed between the conserved residues Asn201-Thr202-Asp203, Gly222-Asp223-Asp224, Gly250-His251-Gly252, and Arg280-Ile281-Lys282 (Pickersgill et al., 1998). The molecular structures of carnosic acid, carnosol, 7-O-methylrosmanol, 12-O-methylcarnosic acid, and isorosmanol were energetically minimized with LigPrep (Schrödinger, 2021) (also part of the Schrödinger Suite) using the OPLS3e force field. All possible tautomers and protonation states at a pH of 7.0 ± 1.0 were enumerated with LigPrep for each ligand. Docking simulations were carried out with Glide in SP and in XP mode.
3 Results
3.1 1H-NMR spectra of rosemary accessions and metabolite identification
1H-NMR spectra were obtained for all the 111 samples. Figures 1, 2 show a 1H-NMR representative spectrum of one rosemary extract (accession 2, Supplementary Table S2). The complexity of the spectra can be visualized in some regions due to the overlapping of signals (Supplementary Figure S5). The spectral resonances were assigned based on the custom (CCL) and the 500 MHz version 11 (CL) Chenomx libraries. The identity of several metabolites was further confirmed through 2D NMR spectroscopic data (Supplementary Figures S1–S5). 1H-NMR spectra revealed the presence of the 38 major metabolites comprising primary and secondary ones mostly belonging to the chemical classes of amino acids, sugars, phenolic derivatives, and terpenoids (Supplementary Tables S5, S6). The NMR spectra showed signals at aliphatic (δH 0.7–3.0), carbohydrate (δH 3.0–5.5), and aromatic (δH 5.5–8.0) regions. The upfield (aliphatic) spectrum region from 0.4 to 3.0 ppm contained several overlapped signals mainly derived from amino acids (valine and alanine), acetate, and the methyl signals C16–C19 of diterpenoids. The mid-region from 3.0 ppm to 5.5 ppm was crowded with signals from amino acids (proline and asparagine), carbohydrates (fructose and sucrose), organic acids (malonate and malate), and choline. This spectral area showed an intense signal overlapping. The signals due to the anomeric protons of sugars were quite easily identifiable. The signals of methoxy groups of both diterpenoids [7-O-methylrosmanol δH 3.67 (s), 12-O-methylcarnosic acid δH 3.69 (s)] and polyphenolic compounds [acacetin δH 3.91 (s), ferulic acid δH 3.89 (s), diosmetin δH 3.94 (s), genkwanin δH 3.92 (s) isorhamnetin-7-O-rutinoside δH 3.95 (s), isorhamnetin-3-O-β-D-rutinoside (narcissin) δH 3.95 (s), methylrosmarinate δH 3.73 (s)), and the signals of choline methyls (δH 3.21(s)] were also present and mainly overlapped. The downfield region from 6.0 ppm to 8.5 ppm exhibited signals due to the aromatic resonances, mainly of phenolic acids (caffeic, chlorogenic, p-coumaric, gallic, and ferulic acids), rosmarinic acid and methylrosmarinate, flavonoids (acacetin, apigenin, apigenin-7-O-β-D-glycoside, catechin hydrate, diosmetin, epicatechin, genkwanin, isorhamnetin-3-O-β-D-rutinoside (narcissin), kaempferol, luteolin, quercetin, rutin, and scutellarein), the trans-olefinic protons of phenylpropanoids (rosmarinic acid δH 7.50 and δH 6.29, methylrosmarinate δH 7.56 and δH 7.29, ferulic acid δH 7.46 and δH 6.34, caffeic acid δH 7.42 and δH 6.27, coumaric acid δH 7.52 and δH 6.32, and chlorogenic acid δH 7.59 and δH 6.34) all doublets and with coupling constants J = 15.9–16.0 Hz), and the aromatic protons of diterpenoids [isorosmanol δH 6.98 (s), epiisorosmanol δH 6.81 (s), 7-O-methylrosmanol δH 6.79 (s), 12-O-methylcarnosic acid δH 6.48 (s), carnosol δH 6.73 (s), and carnosic acid δH 6.44 (s)].

Figure 1 Representative 1H NMR spectrum of one extract of S. rosmarinus (accession 2, Supplementary Table S1), from δH 0.0 to 8.5 ppm, with annotation of the metabolites of the Chenomx 500 MHz custom library (CCL). Regions δH 0.9–2.1, δH 3.6–5.2, δH 6.0–8.1 (dotted boxes) were expanded. The peaks used for the quantification of the identified CCL metabolites are annotated with numbers. Keys: 1, 12-O-methylcarnosic acid; 2, 7-O-methylrosmanol; 3, methylrosmarinate; 4, genkwanin; 5, epicatechin; 6, isorhamnetin-3-O-β-D-rutinoside; 7, carnosic acid; 8, diosmetin; 9, luteolin; 10, scutellarein; 11, acacetin; 12, carnosol; 13, catechin hydrate; 14, gallic acid; 15, rosmarinic acid; 16, ferulic acid; 17, caffeic acid; 18, coumaric acid; 19, chlorogenic acid; 20, rutin; 21, quercetin; 22, apigenin; 23, isorosmanol; 24, apigenin-7-O-β-D-glycoside; 25, kaempferol; 26, isorhamnetin-3-O-rutinoside (narcissin); 27, epiisorosmanol.
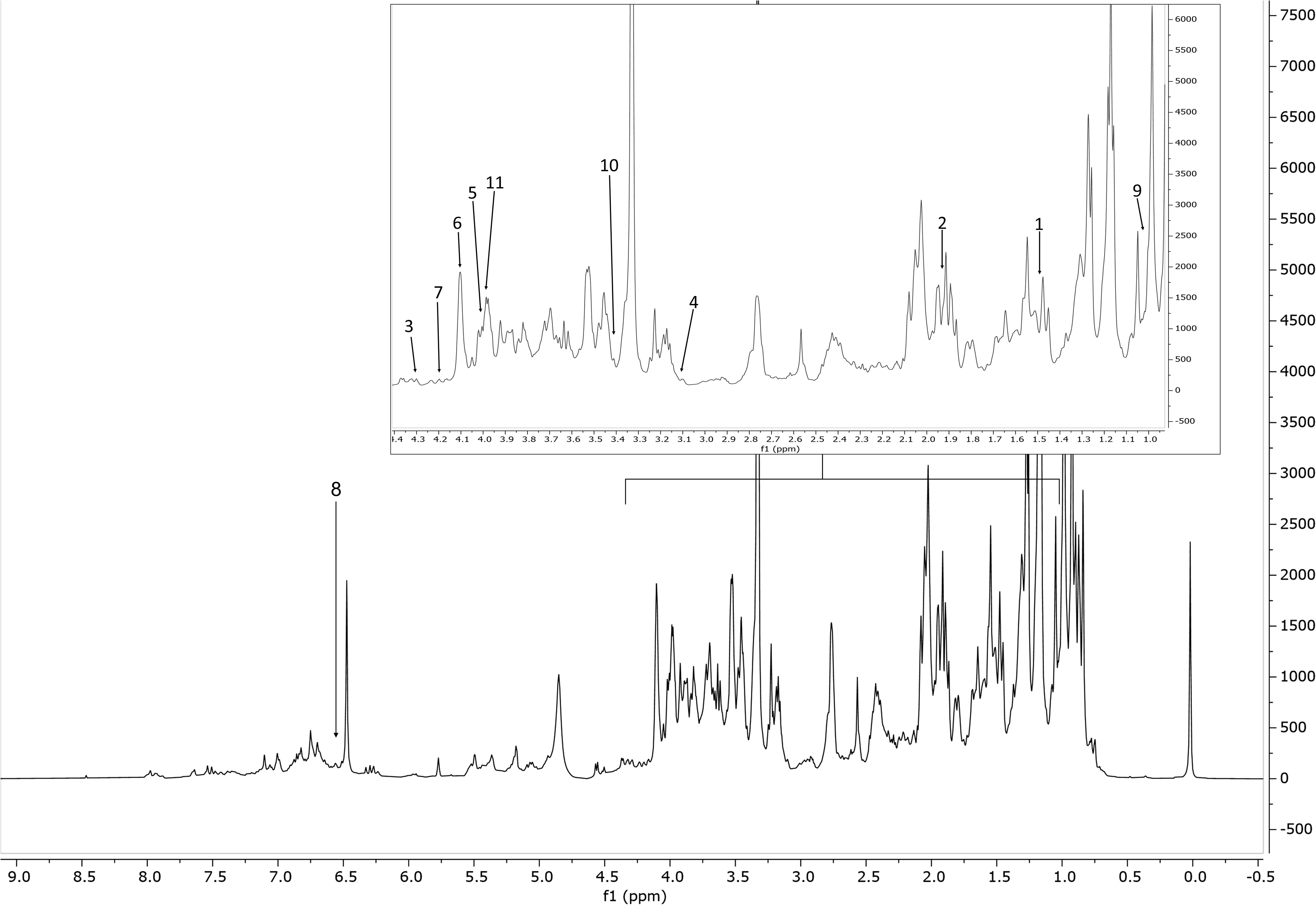
Figure 2 Representative 1H NMR spectrum of the extract of S. rosmarinus (accession 2, Supplementary Table S1), from δH 0.0 to 8.5 ppm with annotation of the metabolites of the Chenomx 500 MHz version 11 library (CL). Region δH 0.9–4.4 (dotted box) was expanded. The peaks used for quantification of the identified metabolites are annotated with numbers. Keys: 1, alanine; 2, acetate; 3, malate; 4, malonate; 5, choline; 6, fructose; 7, sucrose; 8, fumarate; 9, valine; 10, proline; 11, asparagine.
3.2 Multivariate data analysis
To characterize the ‘Eretto Liguria’ ecotype, both accessions (ecotypes and cultivars) from the same geographical area, in North Italy, and accessions from another area, in South Italy, were considered. The relationships among the compounds detected in the rosemary accessions were investigated by correlation analysis of the selected metabolites comprised in the custom (CCL) and the 500 MHz version 11 (CL) Chenomx libraries, considered both jointly and separately. Only a few metabolites of the CL showed significant correlations (Supplementary Table S7). Cluster analysis allowed us to visualize them as a similarity dendrogram, pointing out a cluster formed by asparagine, proline, and choline (Supplementary Figure S6A). Several significant correlations were observed among the CCL metabolites (Supplementary Table S8), as shown in Supplementary Figure S1B: nine metabolites [methylrosmarinate, acacetin, carnosol, rosmarinic acid, ferulic acid, coumaric acid, chlorogenic acid, rutin, and isorhamnetin-3-O-rutinoside (narcissin)] joined into a cluster. Other two groups of correlated variables were formed by the apigenins and kaempferol, and by genkwanin, 7-O-methylrosmanol, diosmetin, and 12-O-methylcarnosic acid, respectively. No relevant information was added when CL and CCL metabolites were studied all together (Supplementary Figure S7). When cluster analysis was applied to the dataset of the CL metabolites to detect groupings among the samples, almost all the accessions from the Ligurian geographical area joined into a large group except for a few outliers (e.g., 9 and 22, and one of the three samples of 18, 27, and 32 accessions). Among the samples from Campania, only one sample of the 37 accession was included in the large cluster of Ligurian samples (Supplementary Figure S8). This grouping was substantially confirmed for CCL metabolites, with similar patterns of outliers; only two samples of 33 and 34 accessions from Campania showed similarity with Ligurian samples, as shown in Supplementary Figures S9, S10, where all the 38 variables (CL and CCL metabolites) were jointly considered.
Explorative analysis of the dataset was performed by PCA, which represents in a few plots the grouping of the accessions, the correlations among the metabolites, and the relationships between accessions and metabolites. Supplementary Figure S11 shows the biplot of the 37 accessions described by the 11 CL metabolites on the plane of the first two PCs, explaining the 52% of the total variance. Accessions 36 and 37 (six samples), corresponding to cultivars not represented among the Ligurian samples, were characterized by low content of alanine, valine, and fumarate and high content of proline, choline, and asparagine. On the plane of the third and fourth PCs (29% of variance), low contents of fructose and sucrose characterized all the accessions from Campania (Supplementary Figure S12).
PCA performed on the 37 accessions described by the 27 CCL metabolites was also strongly affected by the difference between the two geographical regions. The presence of outliers (accessions 36 and 37 from Campania) was evident on the plane of the first two PCs, explaining the 51% of the total variance (Supplementary Figure S13). On the plane of the third and fourth PCs (17% of the variance), one accession from Campania at high content of luteolin and isorosmanol had a very strong leverage effect, making the visualization of the information contained in the data difficult (Supplementary Figure S14). The biplot on the fifth and sixth PCs (14% of the variance) showed that the accessions from Campania formed a separated group, at low content of carnosic acid, isorhamnetin-7-O-rutinoside, and epicatechin, and high content of epiisorosmanol and isorosmanol (Supplementary Figure S15). For these reasons, the data analysis was repeated excluding the accessions from Campania, to improve the analysis of the accessions from Ligurian region.
In this case, despite some degree of overlapping among the accessions, the ‘Eretto Liguria’ appeared characterized, as shown by the dendrogram in Figure 3, where these accessions (green color) were grouped in one cluster including only a few other accessions, and by the biplots of PCA (Figures 4, 5) on the plane of the first four PCs. ‘Eretto Liguria’ was characterized by low content of the variables at high loading on PC1, i.e., methylrosmarinate; acacetin; catechin hydrate; rosmarinic, ferulic, coumaric, and chlorogenic acids; rutin; and isorhamnetin-3-O-rutinoside. The variables at high loading on PC2 (12-O-methylcarnosic acid, 7-O-methylrosmanol, genkwanin, and diosmetin) were not discriminant among the accessions, and the high content of these metabolites allowed the detection of a few anomalous samples. Three CCL metabolites showed high loading on PC4, i.e., carnosic acid, isorhamnetin-7-O-rutinoside, and isorosmanol. Most of the ‘Eretto Liguria’ samples showed a higher content of carnosic acid with respect to other accessions such as ‘Santa Barbara Blue’, ‘Boule’, and ‘Joyce DeBaggio’.
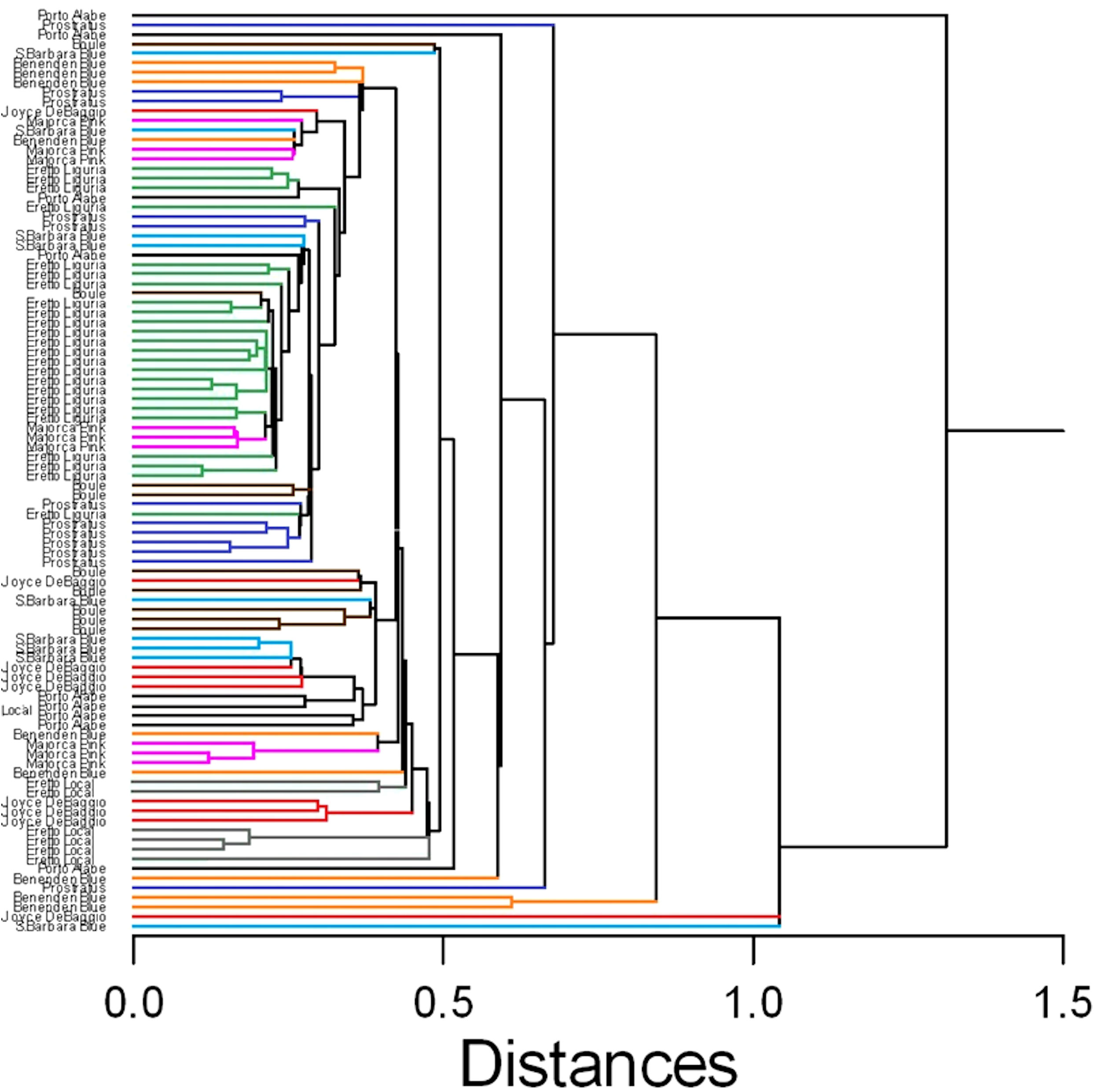
Figure 3 Similarity dendrogram of the 96 rosemary samples collected in Liguria. Hierarchical cluster analysis was applied to the 27 Chenomx 500 MHz custom library metabolites (CCL), using single linkage method based on Euclidean distance. The different ecotypes/cultivars are indicated by the name and shown with different color.

Figure 4 Results of PCA of the 27 Chenomx 500 MHz custom library metabolites (CCL) measured in 96 samples: biplot of Principal Components 1 and 2 (53% of explained variance).  = “Eretto Liguria”; × = other ecotypes/cultivars.
= “Eretto Liguria”; × = other ecotypes/cultivars.
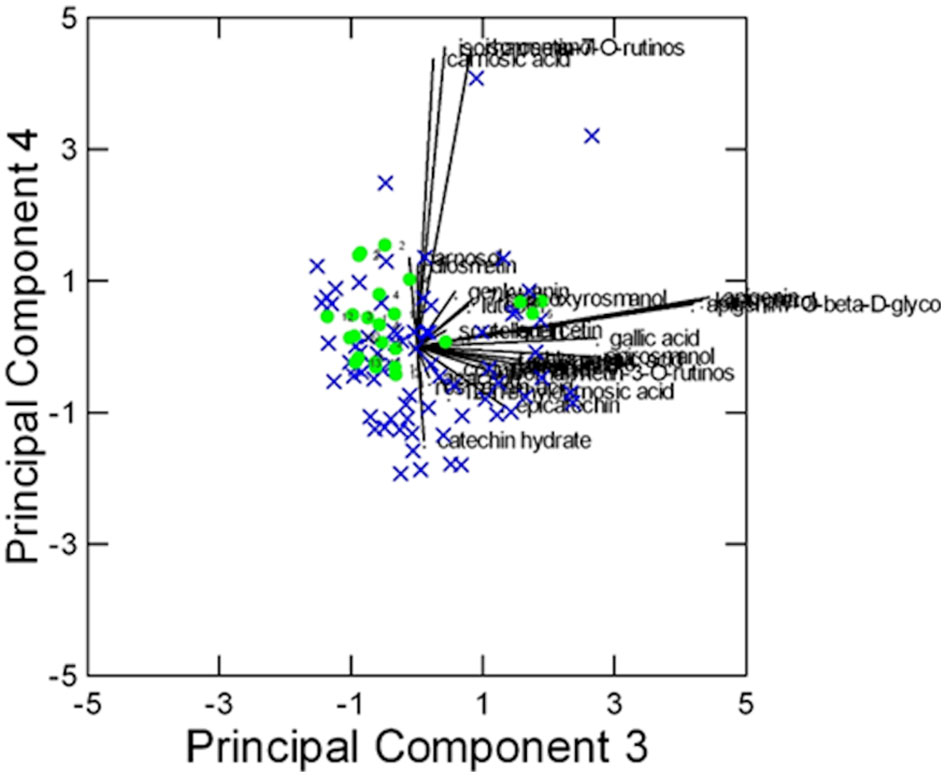
Figure 5 Results of PCA of the 27 Chenomx 500 MHz custom library metabolites (CCL) measured in 96 samples: biplot of Principal Components 3 and 4 (24% of explained variance).  = “Eretto Liguria”; × = other ecotypes/cultivars.
= “Eretto Liguria”; × = other ecotypes/cultivars.
The results obtained by SOMs were in agreement with those of PCA (Supplementary Figures S16–S21). The number of clusters was assessed based on the k-means algorithm and on the Davies–Bouldin index (DBI) (Davies and Bouldin, 1979). This index allows the identification of the most reliable number of clusters that corresponds to a minimum value of DBI (Supplementary Figures S18A, S21A). CL metabolites appeared less important to characterize the ecotype ‘Eretto Liguria’, as it was present in five of the six clusters, mainly characterized by acetate, sucrose, and fumarate (Supplementary Figures S17, S18). As for CCL metabolites, all the accessions of ‘Eretto Liguria’ were in the bottom cluster of the map, except for only one accession (5) (Supplementary Figures S20, S21). A higher content of carnosic acid characterized the neurons containing these accessions compared with the other clusters (Supplementary Figures S19, S20). In addition, accession 5 showed a high carnosic acid content (Supplementary Figures S19, C7, Figure S20). The other ecotypes/cultivars occupied the top part of the map at high content of methylrosmarinate, acacetin, carnosol, rosmarinic acid, ferulic acid, coumaric acid, chlorogenic acid, rutin, and isorhamnetin-3-O-rutinoside (narcissin), i.e., the group of highly correlated variables (Supplementary Figure S6B). The Ligurian accessions did not show significantly different relative amounts of abietane diterpenoids. Carnosic acid was the only compound that appeared to characterize the ‘Eretto Liguria’ ecotype.
3.3 Activity against bacterial soft rot
A total of 12 representative Ligurian rosemary accessions (i.e., ‘Eretto Liguria’ (1, 3, and 5), ‘Prostratus’ (Prostrata Group) (6 and 9), ‘Eretto’ (local ecotype) (7), ‘Boule’ (Prostrata Group, ‘Rampant Boule’) (16), ‘Joyce DeBaggio’ (20), ‘Benenden Blue’ (22), ‘Majorca Pink’ (26), ‘Porto Alabe’ (28), and ‘Santa Barbara Blue’ (32) (Supplementary Table S1), were selected for bacterial soft rot assay. The exposure for 5 min of a liquid suspension of P. carotovorum subsp. carotovorum (107 CFU/mL) to a solution of the methanolic extracts of the selected rosemary accessions at the concentration of 1,000 ppm inhibited the bacterial soft rot at values ranging from 20.8% to 100%, compared to the untreated control (PC). The extracts 1, 6, 16, 20, 22, 26, 28, and 32 showed efficacy values (Abbott index) ranging from 76.9% to 100% (Table 1). These values were significantly different from those obtained with extracts 3, 5, 7, and 9, although 7 and 9 showed efficacy values in a medium range (63.7%). Pure abietane diterpenoids, i.e., isorosmanol, 12-O-methylcarnosic acid, carnosic acid, carnosol, and 7-O-methylrosmanol, characteristic of rosemary extracts, were then tested. The exposure for 5 min of a liquid suspension of P. carotovorum subsp. carotovorum (107 CFU/mL) to a solution of isorosmanol, carnosol, and 7-O-methylrosmanol, at the concentration of 1,000 ppm, completely inhibited the bacterial soft rot. At the same test conditions, 12-O-methylcarnosic acid inhibited the bacterial soft rot by 70.3%, and carnosic acid by 30%, compared to the untreated control (PC) (Table 2) (Supplementary Figure S22).
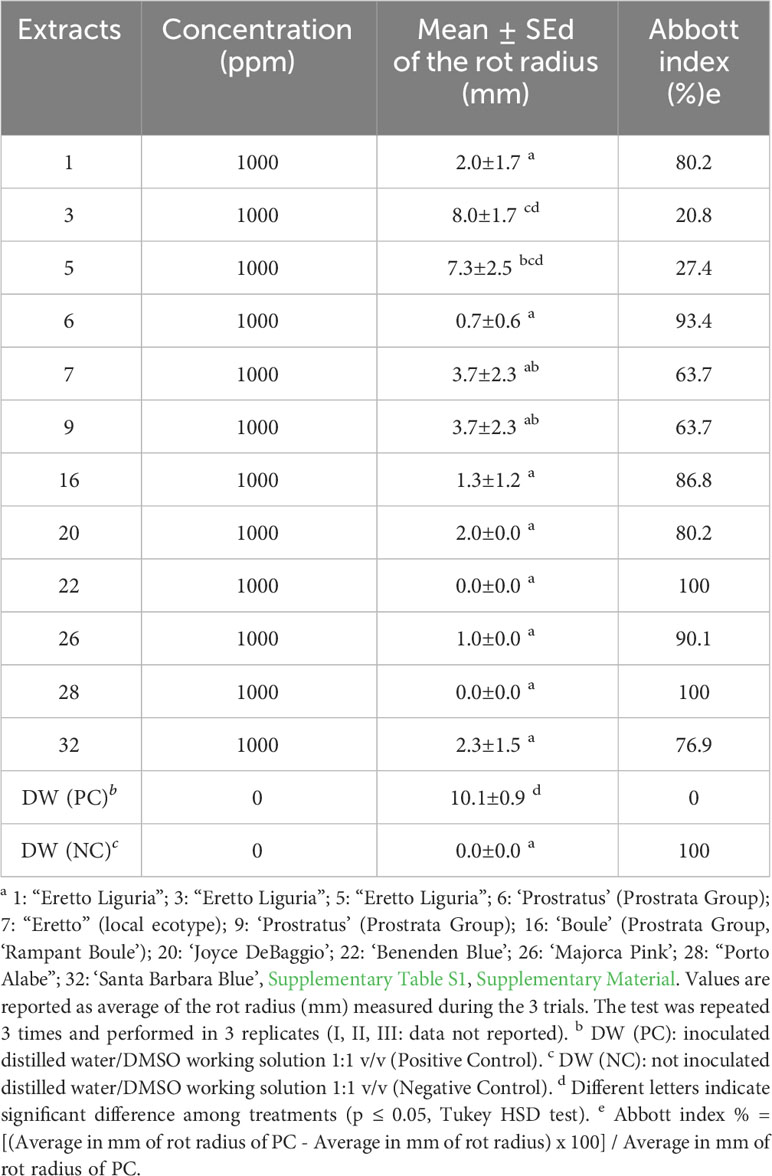
Table 1 Evaluation of the activity against bacterial soft rot induced by Pectobacterium carotovorum subsp. carotovorum after treatments with methanolic extracts of rosemary accessions a.
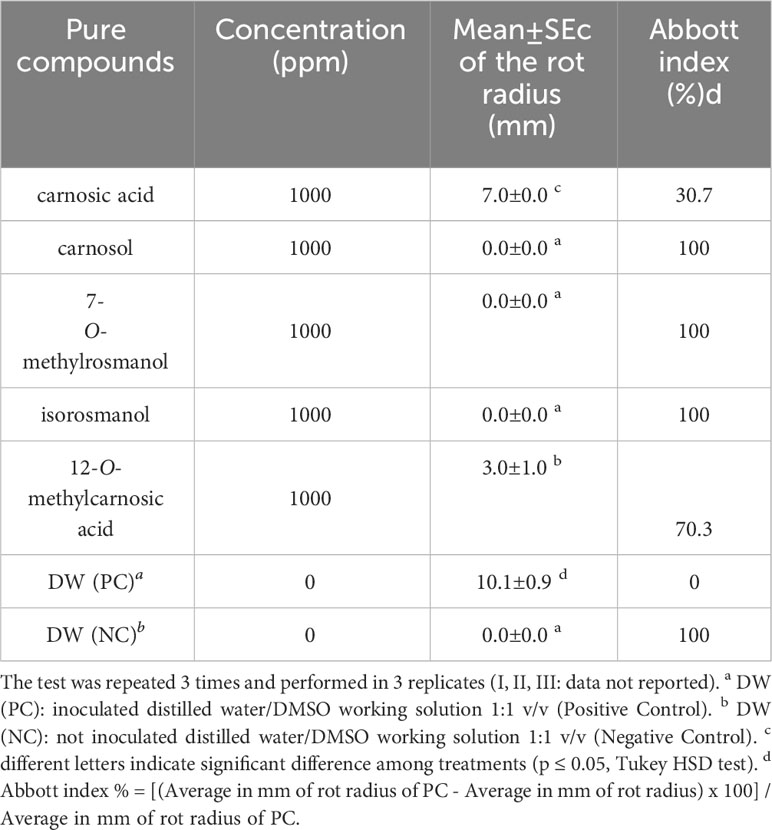
Table 2 Evaluation of the activity against bacterial soft rot induced by Pectobacterium carotovorum subsp. carotovorum after treatments with rosemary abietane diterpenoids.
3.4 In vitro evaluation of antimicrobial activity against Pectobacterium carotovorum subsp. carotovorum
Although the effect of the selected extracts and pure compounds on the bacterial growth was previously evaluated in the antimicrobial preliminary assay (Supplementary Table S9), they were further tested by a disk diffusion method to deeply investigate their antimicrobial activity against the strain of P. carotovorum subsp. carotovorum. As observed in the preliminary test, extracts and pure compounds showed a mild ability to inhibit the growth of the bacterium. In the disk diffusion test, the extract 26 was active against the strain of P. carotovorum subsp. carotovorum at concentrations higher than 250 μg/mL; the extracts 1, 7, 16, 20, 22, and 28 at concentrations higher than 500 μg/mL; the extract 6 at concentrations higher than 1,000 μg/mL; and extracts 5, 9, and 32 at concentrations higher than 2,000 μg/mL. Isorosmanol, carnosic acid, and carnosol showed to be active against the strain of P. carotovorum subsp. carotovorum at concentrations higher than 250 μg/mL, and 12-O-methylcarnosic acid and 7-O-methylrosmanol at concentrations higher than 1,000 μg/mL (Supplementary Table S10). The activity of the tested antibiotic ampicillin against the same strain was several orders of magnitude higher than extracts and pure compounds with a concentration activity value below 1 μg/mL. This result, compared with literature data (Ashmawy et al., 2014; Shaheen and Issa, 2020; Suaza-Gaviria et al., 2023), led to the assessment of a low antimicrobial activity of the selected extracts and pure compounds against the strain of P. carotovorum subsp. carotovorum.
3.5 Enzymatic activity
In vitro inhibition of pectinase activity by rosemary diterpenes was investigated using an enzymatic assay. The untreated bacterial suspension of P. carotovorum subsp. carotovorum at the concentration of 107 CFU/mL showed a pectinase activity of 234.5 U/L corresponding to 234.5 μmol of galacturonic acid hydrolyzed from polygalacturonic acid per minute at 25°C and pH 4 (test conditions). The pectinase activity of the treated bacterial inoculum of P. carotovorum subsp. carotovorum was 0 U/L for carnosic acid, 20.4 U/L for 12-O-methylcarnosic, 0 U/L for 7-O-methylrosmanol, 2.15 U/L for carnosol, and 0 U/L for isorosmanol. Carnosic acid, 7-O-methylrosmanol, and isorosmanol was shown to be able to inhibit the pectinase activity of bacterial suspension of P. carotovorum subsp. carotovorum at the concentration of 1,000 ppm. Carnosol and 12-O-methylcarnosic applied at the same concentration were shown to partially inhibit the pectinase activity.
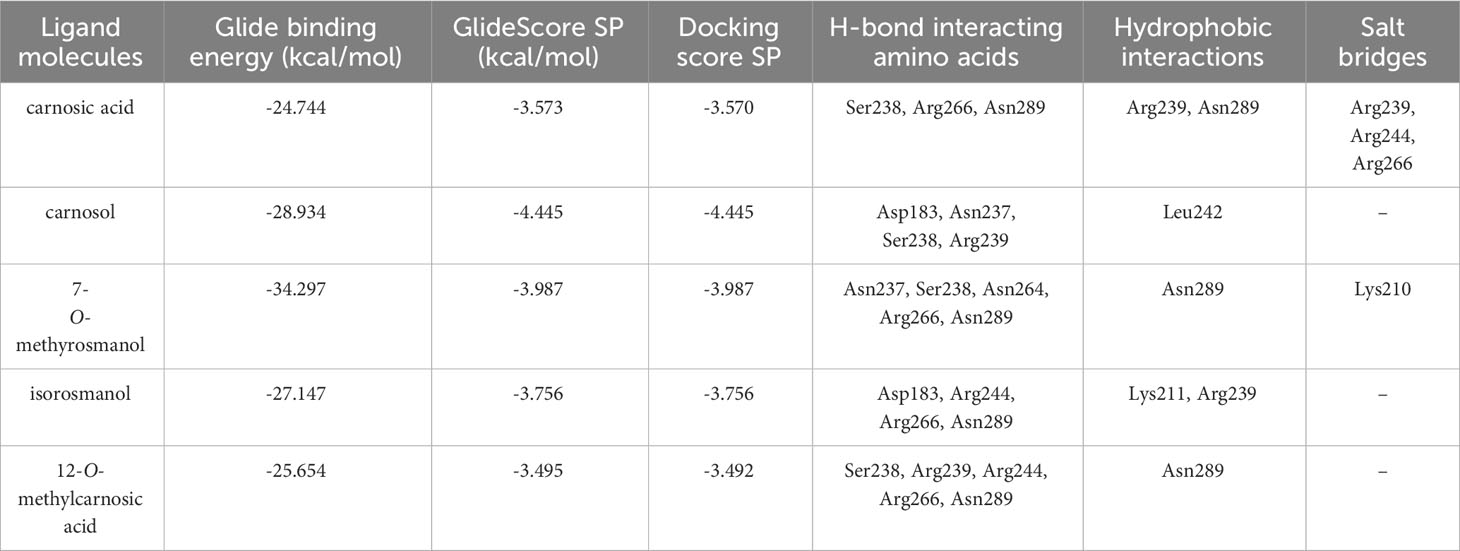
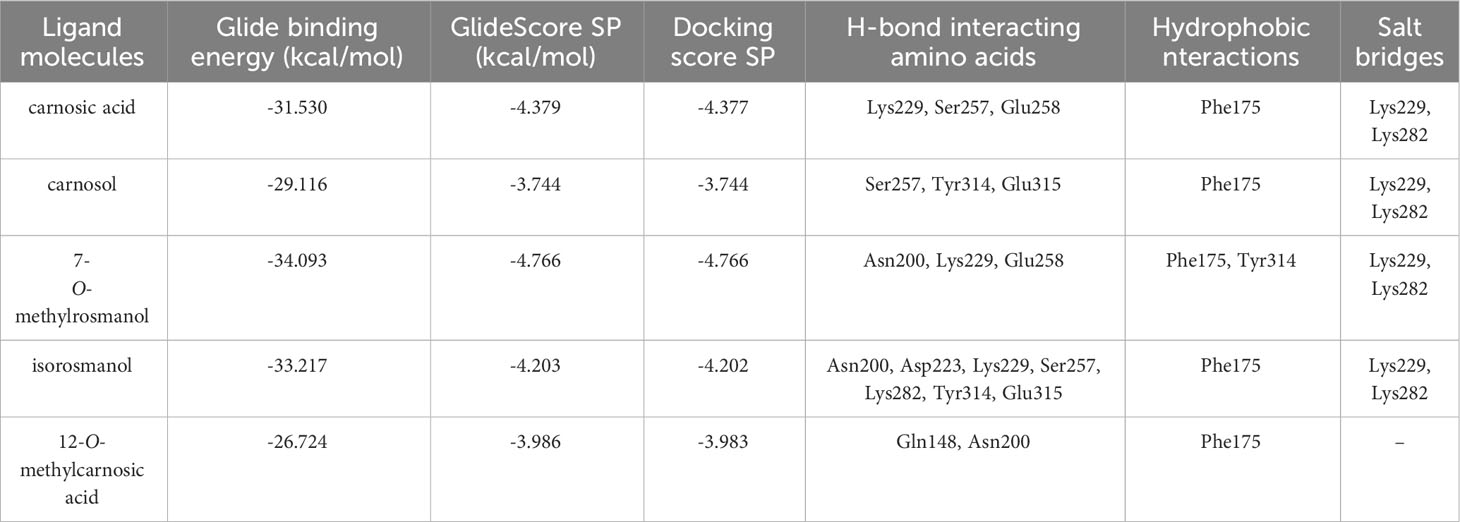
3.6 Molecular docking
The predicted molecular interactions of carnosic acid, carnosol, 7-O-methyl-rosmanol, 12-O-methyl-carnosic acid, and isorosmanol with the binding site residues of P. carotovorum pectate lyase 1 (PelA) and P. carotovorum endo-polygalacturonase (PehA) were studied. The Ramachandran plot of the P. carotovorum pectate lyase 1 (PelA) modeled structure showed that 80.5% of residues were in the most favored region, 19.1% were in additional allowed regions, and 0.3% of residues were in disallowed regions. These results confirmed the high quality of the homology model. The ProSA-web has shown a Z-score of −7.66 that falls in the range of scores commonly found in the case of a similar native protein (Supplementary Figure S23). In the P. carotovorum pectate lyase 1 active site, all compounds showed the catechol moiety bound with H-bonds (Supplementary Figure S24). 12-O-Methylcarnosic acid and carnosol showed a common H-bond interaction with Arg239. Carnosic acid, 7-O-methylrosmanol, isorosmanol, and 12-O-methylcarnosic acid were bound through H-bonds to Arg266 and Asn289 in the active site cleft. The docking poses generated for the selected compounds were similar, except for carnosic acid, for which a “head–tail arrangement” was observed (Figure 6). Concerning docking scores, the best result was obtained for carnosol (−4.445 kcal/mol), although no substantial differences to the other compounds were apparent (Table 3). In the P. carotovorum endo-polygalacturonase (PehA) active site, 7-O-methylrosmanol and carnosic acid achieved the best docking score values of −4.766 kcal/mol and −4.377 kcal/mol, respectively (Table 4). These two compounds also reported a similar binding pose and two common interactions. 7-O-Methylrosmanol was predicted to form two H-bonds with Glu258, one H-bond with Lys229, and one additional H-bond with Asn200. The same interaction pattern with Glu258 and Lys229 was also predicted for carnosic acid (Figure 6). Isorosmanol and carnosol showed consistent binding poses, with the catechol moiety bound to Glu315 and Tyr314 (Supplementary Figure S25). All the selected compounds showed a hydrophobic interaction with the Phe175 planar ring, reported as an important residue in the binding cleft (Pickersgill et al., 1998).

Figure 6 Binding pose and interaction of carnosic acid docked to ligand binding site of P. carotovorum pectate lyase 1 (PelA) (A) and endo-polygalacturonase (PehA) (B). Left: the protein is reported as light-brown ribbons; the ligand is reported as capped sticks; H-bonds are presented as cyan dotted lines. Right, the ligand is surrounded by the protein residues represented as follows: the negatively charged residues are indicated in red, polar residues are in cyan, hydrophobic residues are shown in green, and H-bonds are depicted as purple arrows.
4 Resource identification initiative
Chenomx NMR Suite 8.6 (Chenomx Inc., Edmonton, 252 Canada) was used to identify the metabolites. NMRProcFlow 1.4.14 (INRA UMR 1332 BFP, Bordeaux Metabolomics Facility, Villenave d’Ornon, France) was used for quantitative analysis of NMR spectra. Systat software for Windows Version 13 (Systat Software Inc., Chicago, IL, USA) and Matlab R2022a and SOM toolbox 2.1 (The MathWorks, Inc., Natick, MA, USA) were used for multivariate data analysis. Schrödinger Suite Schrödinger Release 2020-4: Maestro version 12.6.144, Glide, Ligprep, Prime (Schrödinger, LLC: New York, NY, 2021) was used for in silico studies.
5 Discussion
Plant bio-residues were identified as an excellent source of high-added value products potentially useful as beneficial food constituents and supplements, cosmetics, chemopreventive agents, and drug adjuvants. Hence, it is of growing interest to develop sustainable strategy to fully valorize agri-food by-products to obtain bioactive components and preparations (Lemes et al., 2021). The aim of the present study was to characterize the qualitative profile of a local rosemary ecotype for comparison with other cultivars and ecotypes and to identify new possible applications in the agro-food chain. In the current study, targeted NMR-based metabolite analysis was used to study local rosemary accessions compared with commercial cultivars grown in both the same and different climatic conditions and geographical areas. All samples were collected in summer when the content of abietane diterpenoids is at its maximum (Hidalgo et al., 1998). Unsupervised techniques, i.e., principal component analysis (PCA), clustering, and self-organizing maps (SOMs), were applied (Corsaro et al., 2022). Multivariate analysis showed that accessions from the Campania region differed from the Ligurian ones, thus leading to the hypothesis of the influence of geographical location in the composition and abundance of metabolites in rosemary extracts. The evident differences in the metabolite profiles of the samples collected in the two Italian regions (Liguria and Campania) seemed to confirm the relevance of the local environmental conditions in the characterization of the rosemary samples. Carnosic acid characterized northern accessions, consistent with the fact that in drought conditions typical of Southern Italy, it undergoes oxidation to other abietane diterpenes such as isorosmanol and other related compounds (Munné-Bosch et al., 2000; Zhang et al., 2012). However, genetic factors became more important when a more restricted geographical area was considered, such as the Ligurian region. This result is in accordance with the literature (Hidalgo et al., 1998) and for volatile constituents of rosemary essential oil (Li et al., 2016), where genetic and origin are the key factors in chemotype variation. The accessions of ‘Eretto Liguria’ analyzed in the present study, even though they were collected in different locations of the same region (i.e., coastal and inland, different producers, different soils, etc.), appeared well characterized, while accessions of other ecotypes/cultivars, although grown in the same location in the same conditions, were not so similar to each other. The concentrations of carnosic acid, carnosol, and rosmarinic acid were considered decisive for the ranking of rosemary accessions (Wellwood and Cole, 2004). In our study, maps of CCL compounds (Supplementary Figures S19, S20) showed that the most abundant abietane diterpenes in Ligurian rosemary extracts (Supplementary Table S1) were 12-O-methylcarnosic acid (CCL1), 7-O-methylrosmanol (CCL2), carnosic acid (CCL7), carnosol (CCL12), and isorosmanol (CCL23). 12-O-Methylcarnosic acid (CCL1) characterized ‘Benenden Blue’ (21, 22), ‘Prostratus’ (Prostrata Group) (9), ‘Joyce DeBaggio’ (18), ‘Majorca Pink’ (25), ‘Porto Alabe’ (27 and 28), and ‘Santa Barbara Blue’ (30, 31, and 32). 7-O-methylrosmanol (CCL2) characterized ‘Eretto Liguria’ (12), ‘Prostratus’ (Prostrata Group) (9), 18 ‘Joyce DeBaggio’ (18), and ‘Santa Barbara Blue’ (32). Higher amounts of carnosic acid (CCL7) were shown by ‘Eretto Liguria’ (1, 2, 4, 12, 13, and 14), ‘Eretto’ (local ecotype) (7), ‘Prostratus’ (Prostrata Group) (6 and 11), and ‘Boule’ (Prostrata Group, ‘Rampant Boule’) (17). Carnosol (C12) characterized ‘Eretto’ (local ecotype) (7), ‘Prostratus’ (Prostrata Group) (9), ‘Joyce DeBaggio’ (18, 19), ‘Santa Barbara Blue’ (31 and 32), ‘Benenden Blue’ (22), and ‘Porto Alabe’ (28). Isorosmanol (CCL23) characterized ‘Prostratus’ (Prostrata Group) (9), ‘Joyce DeBaggio’ (18), and ‘Santa Barbara Blue’ (32). Epiisorosmanol (CCL27) was widespread among accessions but in very small amount. Abietane diterpenoids were distributed among the Ligurian accessions with no relatively different quantities. The ‘Eretto Liguria’ ecotype appeared well characterized by a high carnosic acid content, which can be considered a good source of abietane diterpenes.
Abietane diterpenoids are reported as potent antimicrobials and in phytopatological defense (Birtić et al., 2015; González, 2015; Bellumori et al., 2021). In the present work, the activity against bacterial soft rot induced by P. carotovorum subsp. carotovorum of the methanolic extracts of the rosemary accessions, and of selected abietane diterpenoids, was tested. P. carotovorum subsp. carotovorum causes severe soft rots in the field and in storage, leading to extensive harvest losses. In the field, P. carotovorum subsp. carotovorum can survive in the soil for up to 6 months, even in the absence of plant debris, and it can also be present in aerosols and water irrigation. Wet conditions increase the effects of its spreading; therefore, some seasons have a high level of damage (OEPP/EPPO, 1994). In addition, airborne insects can carry bacteria from one plant to another (Rossmann et al., 2018). The most distinguishing feature of the pathogenicity of soft rot bacteria as P. carotovorum subsp. carotovorum produces several plant cell-wall-degrading enzymes, such as pectinases, cellulases, and proteases, leading to tissue decomposition and the release of nutrients for bacterial growth (Pritchard et al., 2016). The bacterium enters potato tubers through lenticels and fresh wounds, roots, and above-ground plant parts. Tubers harvested from plants infected during the growing season may develop a soft rot in storage, which results in considerable postharvest losses (OEPP/EPPO, 1994; Hadizadeh et al., 2019). Currently, the control of soft rot pathogens relies on integrated pest management because no chemical treatments, such as synthetic bactericides, are currently recommended to control this disease (Mantsebo et al., 2014). Due to the ease of developing soft rot rapidly, the potato soft rot test is usually performed to define the ability of an inoculum to induce pectolytic activity (Lelliott et al., 1966). In our experiment, potato tuber assay was used to test the methanolic extracts’ ability to reduce the soft rot induced by a strain of P. carotovorum subsp. carotovorum applied on potato tissue after treatment and measuring tissue maceration. The best results in the inhibition of bacterial soft rot were attributable to ‘Eretto Liguria’ (1), ‘Prostratus’ (Prostrata Group) (6), ‘Boule’ (Prostrata Group, ‘Rampant Boule’) (16), ‘Joyce DeBaggio’ (20), ‘Benenden Blue’ (22) ‘Majorca Pink’ (26), ‘Porto Alabe’ (28), and ‘Santa Barbara Blue’ (32). The inhibition induced by various extracts appeared then not linked to the distribution of the different accessions in the clusters (Supplementary Figures S17, S18, S20, S21), thus suggesting that the differences in relative abundance of individual compounds within the phytocomplex are almost negligible. The bacterial soft rot assay performed with the pure compounds showed the best results for 7-O-methylrosmanol, carnosol and isorosmanol, while 12-O-methylcarnosic acid and carnosic acid were less active. The low inhibition activity of carnosic acid is probably related to the phytotoxicity effect observed in vivo when the compound is applied at concentration higher than 500 ppm (unpublished data). The mild antimicrobial activity showed by the extracts and pure compounds in the disk diffusion test suggested that the ability in inhibiting the progression of bacterial soft rot on potato tissue could be only partially affected by the activity on the bacterial growth, and different mechanisms could probably lead it.
Based on these results and considering the relevant role of the pectinase enzymes in the soft rot infections and the increasing interest in their biotechnological application, a computational approach was employed to derive the potential binding mode of the abietane diterpenoids to selected pectinase enzymes. Pathogens secrete plant cell-wall-degrading enzymes as virulence factors. Pectinases are mainly produced by plant pathogens, and they are involved in plant cell wall degradation (Davidsson et al., 2013). Pectic enzymes are considered a central factor in plant tissue maceration during soft rot infection (Abbott and Boraston, 2008). Pectinases are classified into three major groups according to the substrate and the mechanism of action. Pectobacterium spp. produces polygalacturonase (PG, EC 3.2.1.15), polygalacturonate lyase (PGL, EC 4.2.2.2), and oligogalacturonide lyase (OGL, EC 4.2.2.6) (Sakai et al., 1993). Pectin degradation by endopolygalacturonase lyase (PGL), also called pectic transeliminase, involves a β-elimination mechanism and a basic residue acting as a Brønsted base to abstract the proton at the C-5 position of the galacturonate residue (Hugouvieux-Cotte-Pattat et al., 2014). These extra cellular enzymes are implicated in the degradation of plant tissue, in particular in the cleavage of (1-4)-α-D-galacturonan to give oligosaccharides with 4-deoxy-α-D-galact-4-enuronosyl groups at their non-reducing ends (Collmer and Keen, 1986; Marín-Rodríguez et al., 2002). At present, pectate lyases are under investigation for their biotechnological and industrial applications (Wu et al., 2020). Most of the polygalacturonate lyase activity results from the cluster activity of five major isoenzymes, PelA to PelE, which were differentiated by an increasing pI from PelA to PelE (Collmer and Keen, 1986). A more alkaline pH than the hydrolytic enzymes and divalent cations were found to be essentials for the activity of pectate lyases (Tardy et al., 1997). PelA has been defined as critical for developing symptoms (Barras et al., 1994), and its production could be the first step of an infectious process (Nachin and Barras, 2000). The peculiar role of this isoenzyme in the infection process has been underlined (Beaulieu and Van Gijsegem, 1993). Moreover, the optimum pH for its enzymatic activity is reported to be 8–8.5 (Hugouvieux-Cotte-Pattat et al., 2014). Endo-polygalacturonase (endoPG), also known as pectic hydrolase, cleaves the α-(1–4) linkages between D-galacturonic acid residues of homogalacturonan, the main component of pectin, by hydrolysis (Sakai et al., 1993). The strict similarity in the whole electrostatic properties of polygalacturonase and polygalacturonate lyase (pectate lyase) substrate-binding clefts, which bind and cleave the same substrate, polygalacturonic acid, has been revealed (Pickersgill et al., 1998).
Among the several enzymes produced by P. carotovorum, only the crystal structure of endo-polygalacturonase (PehA) from P. carotovorum (Pickersgill et al., 1998) has been reported in the Protein Data Bank (Berman et al., 2000). Moreover, since the three-dimensional structure of the P. carotovorum pectate lyase 1 (PelA) has not yet been solved, a new homology model of PelA was built using the crystal structure of pectate lyase C (Scavetta et al., 1999) as the template, due to the high amount of sequence similarity. A detailed analysis of the active site revealed a high degree of conservation of the residues of the ligand binding site of the family 1 Pels (PelA) and PelC (PDB ID, 2EWE) (Abbott and Boraston, 2008). The docking simulations suggested that the selected compounds may interact with both modeled PelA and PehA binding sites. As expected, no substantial differences in docking scores were observed given the structural similarity between the compounds. The hypothetical bond of the abietane diterpenoids at the PelA and PehA active sites may prevent the pectolytic reactions on potato tubers. Further studies of the pectinase enzymes 3D-structures will be needed to derive the pectolytic activity inhibition exerted by these natural compounds. The ability to inhibit the pectinase activity by the tested compounds was then assayed in vitro. Quantitative pectinase activity determination confirmed that carnosic acid, 7-O-methylrosmanol, and isorosmanol can inhibit the pectinase activity of bacterial suspension of P. carotovorum subsp. carotovorum. The obtained results suggest that treatments with methanolic extracts of rosemary can also reduce the severity of soft rot disease during storage, and these extracts could be further studied as an alternative strategy for bacterial soft rot management. The NMR-based metabolomic software used in the present study required a specific sample preparation based on the use of deuterated methanol. Therefore, the methanolic extracts for antimicrobial and bacterial soft rot assay were prepared. Additional research is needed involving less-impacting binary solvent mixtures (e.g., water and organic solvent) (Plaskova and Mlcek, 2023). Based on these results, a further study will provide various bacterial strains acting on the pectinase enzymes. Moreover, considering the possible phytopathological application, the use of dried powder material could be considered.
6 Conclusion
Targeted NMR-based metabolite analysis was employed to analyze local rosemary accessions compared with commercial cultivars grown in the same/or in different climatic conditions and geographical areas. Unsupervised techniques were applied, i.e., PCA, clustering, and SOMs (Corsaro et al., 2022). Multivariate data analysis showed that accessions from the Campania region differed from the Ligurian ones, thus leading to the hypothesis of the influence of geographical location in the composition and abundance of metabolites in rosemary extracts. Moreover, cluster analysis reported the accessions of ‘Eretto Liguria’ to be well characterized among the other Ligurian cultivars and ecotypes, showing high content of carnosic acid. The methanolic extracts of the rosemary accessions and all the selected abietane diterpenoids exhibited activity against soft rot induced by P. carotovorum subsp. carotovorum on potato tuber slices. ‘Eretto Liguria’ achieved one of the best results in inhibiting bacterial soft rot. Isorosmanol, carnosol, and 7-O-methylrosmanol completely inhibited the bacterial soft rot. In addition, the in silico study suggested possible molecular interactions of the selected abietane diterpenoids with P. carotovorum subsp. carotovorum pectolytic enzymes. Finally, the in vitro enzymatic assay confirmed the possibility of inhibition of pectinase activity. The possible use of rosemary methanolic extracts and pure abietane diterpenoids in preventing and reducing the soft rot disease strictness has been underlined.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
VI, VP, and GDo performed phytochemical investigations. VI performed the metabolite identification. VI, NM, MG, and GDr performed the multivariate analysis. AL and GM performed the antibacterial investigation. VI and JK performed the in silico study. AB and ND performed the conception and design of the study and wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the funds provided by Interreg V-A Francia-Italia (ALCOTRA) (2014 - 2020) ALCOTRA Progetto-Ponte “ANTES - Fiori eduli e piante aromatiche: attività capitalizzazione dei progetti ANTEA ed ESSICA” n° 8336, and by ‘RGV-FAO’ project funded by the Italian Ministry of Agriculture, Food and Forestry (http://planta-res.politicheagricole.it/pages/index.php).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1164859/full#supplementary-material
References
Abbott, D. W., Boraston, A. B. (2008). Structural biology of pectin degradation by Enterobacteriaceae. Microbiol. Mol. Biol. Rev. 72 (2), 301–316. doi: 10.1128/mmbr.00038-07
Abd El-Razek, M. H. (2007). NMR assignments of four catechin epimers. Asian J. Chem. 19 (6), 4867–4872.
Agyemang, P. A., Kabir, M. N., Kersey, C. M., Dumenyo, C. K. (2020). The bacterial soft rot pathogens, Pectobacterium carotovorum and P. atrosepticum, respond to different classes of virulence-inducing host chemical signals. Horticulturae 6 (1), 13. doi: 10.3390/horticulturae6010013
Andresen, L., Kõiv, V., Alamäe, T., Mäe, A. (2007). The Rcs phosphorelay modulates the expression of plant cell wall degrading enzymes and virulence in Pectobacterium carotovorum ssp. carotovorum. FEMS Microbiol. Lett. 273 (2), 229–238. doi: 10.1111/j.1574-6968.2007.00794.x
Appiah, K. S., Mardani, H. K., Omari, R. A., Eziah, V. Y., Ofosu-Anim, J., Onwona-Agyeman, S., et al. (2018). Involvement of carnosic acid in the phytotoxicity of Rosmarinus officinalis leaves. Toxins (Basel) 10 (12), 1–16. doi: 10.3390/toxins10120498
Ashmawy, N., Behiry, S., Ali, H., Salem, M. (2014). Evaluation of Tecoma stans and Callistemon viminalis extracts against potato soft rot bacteria in vitro. J. Pure Appl. Microbiol. 8, 667–673.
Barras, F., Van Gijsegem, F., Chatterjee, A. K. (1994). Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu. Rev. Phytopathol. 32, 201–234. doi: 10.1146/annurev.py.32.090194.001221
Beaulieu, C., Van Gijsegem, F. (1993). Pathogenic behavior of pectinase-defective Erwinia chrysanthemi mutants on different plants. Mol. Plant-Microbe Interact. 6, 197. doi: 10.1094/MPMI-6-197
Bellumori, M., Innocenti, M., Congiu, F., Cencetti, G., Raio, A., Menicucci, F., et al. (2021). Within-plant variation in Rosmarinus officinalis L. terpenes and phenols and their antimicrobial activity against the rosemary phytopathogens Alternaria alternata and Pseudomonas viridiflava. Molecules 26 (11), 1–19. doi: 10.3390/molecules26113425
Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., et al. (2000). The protein data bank. Nucleic Acids Res. 28, 235–242. doi: 10.1093/nar/28.1.235
Birtić, S., Dussort, P., Pierre, F.-X., Bily, A. C., Roller, M. (2015). Carnosic acid. Phytochemistry 115, 9–19. doi: 10.1016/j.phytochem.2014.12.026
Cervelli, C., Masselli, L. (2013). “Characterization of rosemary cultivars for ornamental purposes,” in VII International Symposium on New Floricultural Crops. Eds. Facciuto, G., Sánchez, M. I. (Leuven, Belgium: ISHS Acta Horticulturae), 107–114.
Collmer, A., Keen, N. T. (1986). The role of pectic enzymes in plant pathogenesis. Annu. Rev. Phytopathol. 24 (1), 383–409. doi: 10.1146/annurev.py.24.090186.002123
Correia, F. C. S., Targanski, S. K., D., B. T. R., da Silva, Y. S. A. D., Violante, I. M. P., de Carvalho, M. G., et al. (2020). Chemical constituents and antimicrobial activity of branches and leaves of Cordia insignis (Boraginaceae). Rev. virtual Quimica 12 (3), 809–816. doi: 10.21577/1984-6835.20200063
Corsaro, C., Vasi, S., Neri, F., Mezzasalma, A. M., Neri, G., Fazio, E. (2022). NMR in Metabolomics: from conventional statistics to machine learning and neural network approaches. Appl. Sci. 12 (6), 1–39. doi: 10.3390/app12062824
Cuvelier, M.-E., Richard, H., Berset, C. (1996). Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chemists’ Soc. 73 (5), 645–652. doi: 10.1007/BF02518121
Davidsson, P. R., Kariola, T., Niemi, O., Palva, E. T. (2013). Pathogenicity of and plant immunity to soft rot pectobacteria. Front. Plant Sci. 4. doi: 10.3389/fpls.2013.00191
Davies, D. L., Bouldin, D. W. (1979). A cluster separation measure. IEEE Trans. Pattern Anal. Mach. Intell. PAMI-1 (2), 224–227. doi: 10.1109/TPAMI.1979.4766909
De Boer, S. H. (2003). Characterization of pectolytic Erwinias as highly sophisticated pathogens of plants. Eur. J. Plant Pathol. 109 (9), 893–899. doi: 10.1023/B:EJPP.0000003967.34041.65
Deborde, C., Hounoum, B. M., Moing, A., Maucourt, M., Jacob, D., Corraze, G., et al. (2021). Putative imbalanced amino acid metabolism in rainbow trout long term fed a plant-based diet as revealed by (1)H-NMR metabolomics. J. Nutr. Sci. 10, e13. doi: 10.1017/jns.2021.3
De Mastro, G., Ruta, C., Mincione, A., Poiana, M. (2004). Bio-morphological and chemical characterization of rosemary (Rosmarinus officinalis L.) biotypes. Acta Hortic. 629, 471–482. doi: 10.17660/ActaHortic.2004.629.61
Drew, B. T., González-Gallegos, J. G., Xiang, C. L., Kriebel, R., Drummond, C. P., Walked, J. B., et al. (2017). Salvia united: the greatest good for the greatest number. Taxon 66, 133–145. doi: 10.12705/661.7
European Union (2020). “A new Circular Economy Action Plan For a cleaner and more competitive Europe,” in COM/2020/98 final. (ed.) t.C. Commission to the European Parliament, the European Economic and Social Committee and the Committee of the Regions. (Brussels: European Commission).
Flamini, G., Cioni, P. L., Morelli, I., Macchia, M., Ceccarini, L. (2002). Main agronomic–productive characteristics of two ecotypes of Rosmarinus officinalis L. and chemical composition of their essential oils. J. Agric. Food Chem. 50 (12), 3512–3517. doi: 10.1021/jf011138j
Gomes, R., Ramirez, R., Maciel, J., Agra, M., Souza, M., Falcão-Silva, V., et al. (2011). Phenolic compounds from Sidastrum micranthum (A. St.-Hil.) Fryxell and evaluation of acacetin and 7,4’-di-O-methylisoscutellarein as motulator of bacterial drug resistence. Química Nova 34, 1385–1388. doi: 10.1590/S0100-40422011000800016
González, M. A. (2015). Aromatic abietane diterpenoids: their biological activity and synthesis. Natural Product Rep. 32 (5), 684–704. doi: 10.1039/c4np00110a
Grimaldi, M., Marino, C., Buonocore, M., Santoro, A., Sommella, E., Merciai, F., et al. (2021). Prenatal and early postnatal cerebral D-aspartate depletion influences L-amino acid pathways, bioenergetic processes, and developmental brain metabolism. J. Proteome Res. 20 (1), 727–739. doi: 10.1021/acs.jproteome.0c00622
Gurbuz, B., Bagdat, R. B., Uyanik, M., Rezaeieh, K. A. P. (2016). Rosemary (Rosmarinus officinalis L.) cultivation studies under Ankara ecological conditions. Ind. Crops Products 88, 12–16. doi: 10.1016/j.indcrop.2015.12.028
Gutierrez-Pacheco, M. M., Gonzalez-Aguilar, G. A., Martinez-Tellez, M. A., Lizardi-Mendoza, J., Madera-Santana, T. J., Bernal-Mercado, A. T., et al. (2018). Carvacrol inhibits biofilm formation and production of extracellular polymeric substances of Pectobacterium carotovorum subsp. carotovorum. Food Control 89, 210–218. doi: 10.1016/j.foodcont.2018.02.007
Hadizadeh, I., Peivastegan, B., Hannukkala, A., van der Wolf, J. M., Nissinen, R., Pirhonen, M. (2019). Biological control of potato soft rot caused by Dickeya solani and the survival of bacterial antagonists under cold storage conditions. Plant Pathol. 68 (2), 297–311. doi: 10.1111/ppa.12956
Hajian-Maleki, H., Baghaee-Ravari, S., Moghaddam, M. (2019). Efficiency of essential oils against Pectobacterium carotovorum subsp. carotovorum causing potato soft rot and their possible application as coatings in storage. Postharvest Biol. Technol. 156, 110928. doi: 10.1016/j.postharvbio.2019.06.002
Han, W., Wang, J., Pirhonen, M., Pan, Y., Qin, J., Zhang, S., et al. (2023). Identification and characterization of opportunistic pathogen Pectobacterium polonicum causing potato blackleg in China. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1097741
Harder, E., Damm, W., Maple, J., Wu, C., Reboul, M., Xiang, J. Y., et al. (2016). OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput. 12 (1), 281–296. doi: 10.1021/acs.jctc.5b00864
Hemeg, H. A., Moussa, I. M., Ibrahim, S., Dawoud, T. M., Alhaji, J. H., Mubarak, A. S., et al. (2020). Antimicrobial effect of different herbal plant extracts against different microbial population. Saudi J. Biol. Sci. 27 (12), 3221–3227. doi: 10.1016/j.sjbs.2020.08.015
Hidalgo, P. J., Ubera, J. L., Tena, M. T., Valcárcel, M. (1998). Determination of the carnosic acid content in wild and cultivated Rosmarinus officinalis. J. Agric. Food Chem. 46 (7), 2624–2627. doi: 10.1021/jf970974j
Hugouvieux-Cotte-Pattat, N., Condemine, G., Shevchik, V. E. (2014). Bacterial pectate lyases, structural and functional diversity. Environ. Microbiol. Rep. 6 (5), 427–440. doi: 10.1111/1758-2229.12166
Jackson, J. E. (2004). A User’s Guide to Principal Components (Hoboken, New Jersey, U.S.A: John Wiley & Sons, Inc).
Jacob, D., Deborde, C., Lefebvre, M., Maucourt, M., Moing, A. (2017). NMRProcFlow: a graphical and interactive tool dedicated to 1D spectra processing for NMR-based metabolomics. Metabolomics 13 (4), 36. doi: 10.1007/s11306-017-1178-y
Joshi, J. R., Burdman, S., Lipsky, A., Yedidia, I. (2015). Effects of plant antimicrobial phenolic compounds on virulence of the genus Pectobacterium. Res. Microbiol. 166 (6), 535–545. doi: 10.1016/j.resmic.2015.04.004
Joshi, J. R., Khazanov, N., Senderowitz, H., Burdman, S., Lipsky, A., Yedidia, I. (2016). Plant phenolic volatiles inhibit quorum sensing in pectobacteria and reduce their virulence by potential binding to ExpI and ExpR proteins. Sci. Rep. 6, 38126. doi: 10.1038/srep38126
Khoshkdaman, M., Mostafa Niknejad Kazmpour, M. N., Ali Akbar Ebadi, A. A., Mossanejad, S., Pedramfar, H. (2008). Identification of causal agent of foot and sheath rot of rice in the fields of guilan province of Iran. Agricultura Tropica Subtropica 41 (1), 17–20.
Kiehlmann, E., Tracey, A. S. (1986). Proton magnetic resonance spectra of catechin and bromocatechin derivatives: C6- vs. C8-substitution. Can. J. Chem. 64 (10), 1998–2005. doi: 10.1139/v86-330
Kim, H., Kim, M., Jee, S. N., Heu, S., Ryu, S. (2022). Development of a bacteriophage cocktail against Pectobacterium carotovorum subsp. carotovorum and its effects on pectobacterium virulence. Appl. Environ. Microbiol. 88 (19), e0076122. doi: 10.1128/aem.00761-22
King-Díaz, B., Granados-Pineda, J., Bah, M., Rivero-Cruz, J. F., Lotina-Hennsen, B. (2015). Mexican propolis flavonoids affect photosynthesis and seedling growth. J. Photochem. Photobiol. B: Biol. 151, 213–220. doi: 10.1016/j.jphotobiol.2015.08.019
Kirchherr, J., Reike, D., Hekkert, M. (2017). Conceptualizing the circular economy: an analysis of 114 definitions. Resources Conserv. Recycling 127, 221–232. doi: 10.1016/j.resconrec.2017.09.005
Kuo, Y.-H., Lee, S.-M., Lai, J.-S. (2000). Constituents of the whole herb of Clinoponium laxiflorum. J. Chin. Chem. Soc. 47 (1), 241–246. doi: 10.1002/jccs.200000028
Lecomte, J., Giraldo, L. J. L., Laguerre, M., Baréa, B., Villeneuve, P. (2010). Synthesis, characterization and free radical scavenging properties of rosmarinic acid fatty esters. J. Am. Oil Chemists’ Soc. 87 (6), 615–620. doi: 10.1007/s11746-010-1543-8
Lelliott, R. A., Billing, E., Hayward, A. C. (1966). A determinative scheme for the fluorescent plant pathogenic pseudomonads. J. Appl. Bacteriology 29 (3), 470–489. doi: 10.1111/j.1365-2672.1966.tb03499.x
Lemes, A. C., Egea, M. B., de Oliveira Filho, J. G., Gautério, G. V., Ribeiro, B. D., Coelho, M. A. Z. (2021). Biological approaches for extraction of bioactive compounds from agro-industrial by-products: a review. Front. Bioengineering Biotechnol. 9. doi: 10.3389/fbioe.2021.802543
Li, G., Cervelli, C., Ruffoni, B., Shachter, A., Dudai, N. (2016). Volatile diversity in wild populations of rosemary (Rosmarinus officinalis L.) from the Tyrrhenian Sea vicinity cultivated under homogeneous environmental conditions. Ind. Crops Products 84, 381–390. doi: 10.1016/j.indcrop.2016.02.029
Li, N., Huang, J., Weng, W., Su, S., Yu, Y. (2006). Preparation and identification of scutellarein by HPLC with an enzymolysis reaction. J. Liquid Chromatogr. Related Technol. 29 (9), 1297–1305. doi: 10.1080/10826070600598878
Li, B., Huang, J., Yi, Y., Liu, S., Liu, R., Xiao, Z., et al. (2022). Effects of rhapontigenin as a novel quorum-sensing inhibitor on exoenzymes and biofilm formation of Pectobacterium carotovorum subsp. carotovorum and its application in vegetables. Molecules 27 (24), 8878. doi: 10.3390/molecules27248878
Lin, L.-C., Pai, Y.-F., Tsai, T.-H. (2015). Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. J. Agric. Food Chem. 63 (35), 7700–7706. doi: 10.1021/jf505848z
Liu, J., Chen, L., Cai, S., Wang, Q. (2012). Semisynthesis of apigenin and acacetin-7-O-β-D-glycosides from naringin and their cytotoxic activities. Carbohydr. Res. 357, 41–46. doi: 10.1016/j.carres.2012.05.013
López-Martínez, L., Santacruz, H., Navarro, R., Sotelo-Mundo, R., Aguilar, G. (2015). A 1H NMR Investigation of the interaction between phenolic acids found in mango (Manguifera indica cv Ataulfo) and papaya (Carica papaya cv Maradol) and 1,1-diphenyl- 2-picrylhydrazyl (DPPH) Free Radicals. PloS One 10, e0140242. doi: 10.1371/journal.pone.0140242
Mallick, T., Mishra, R., Mohanty, S., Joshi, R. K. (2022). Genome wide analysis of the potato soft rot pathogen Pectobacterium carotovorum strain ICMP 5702 to predict novel insights into its genetic features. Plant Pathol. J. 38 (2), 102–114. doi: 10.5423/ppj.Oa.12.2021.0190
Mantsebo, C. C., Mazarura, U., Goss, M., Ngadze, E. (2014). The epidemiology of Pectobacterium and Dickeya species and the role of calcium in postharvest soft rot infection of potato (Solanum tuberosum) caused by the pathogens: A review. Afr. J. Agric. Res. 9 (19), 1509–1515. doi: 10.5897/AJAR2013.8558
Marín-Rodríguez, M. C., Orchard, J., Seymour, G. B. (2002). Pectate lyases, cell wall degradation and fruit softening. J. Exp. Bot. 53 (377), 2115–2119. doi: 10.1093/jxb/erf089
Mateu-Andrés, I., Aguilella, A., Boisset, F., Currás, R., Guara, M., Laguna, E., et al. (2013). Geographical patterns of genetic variation in rosemary (Rosmarinus officinalis) in the Mediterranean basin. Botanical J. Linn. Soc. 171 (4), 700–712. doi: 10.1111/boj.12017
Mikiciński, A., Sobiczewski, P., Sulikowska, M., Puławska, J., Treder, J. (2010). Pectolytic bacteria associated with soft rot of calla lily (Zantedeschia spp.) tubers. J. Phytopathol. 158 (4), 201–209. doi: 10.1111/j.1439-0434.2009.01597.x
Mohamed, E. M., Hetta, M. H., Rateb, M. E., Selim, M. A., M. AboulMagd, A. A., Badria, A. F., et al. (2020). Bioassay-guided isolation, metabolic profiling, and docking studies of hyaluronidase inhibitors from Ravenala Madagascariensis. Molecules 25 (7), 1–12. doi: 10.3390/molecules25071714
Monzón Daza, G., Meneses Macías, C., Forero, A. M., Rodríguez, J., Aragón, M., Jiménez, C., et al. (2021). Identification of α-amylase and α-glucosidase inhibitors and ligularoside A, a new triterpenoid saponin from Passiflora ligularis Juss (Sweet Granadilla) leaves, by a nuclear magnetic resonance-based metabolomic study. J. Agric. Food Chem. 69 (9), 2919–2931. doi: 10.1021/acs.jafc.0c07850
Mulas, M., Brigaglia, N., Cani, M. R. (1998). Clone selection from spontaneous germplasm to improve Rosmarinus officinalis L. crop. Acta Hortic. 457, 287–294. doi: 10.17660/ActaHortic.1998.457.36
Mulas, M., Mulas, G. (2005). “Cultivar selection from rosemary (Rosmarinus officinalis L.) spontaneous populations in the Mediterranean area,” in III WOCMAP Congress on Medicinal and Aromatic Plants - Volume 2: Conservation, Cultivation and Sustainable Use of Medicinal and Aromatic Plants. Eds. Jatisatienr, A., Paratasilpin, T., Elliott, S., Anusarnsunthorn, V., Wedge, D., Craker, L. E., Gardner, Z. E. (Leuven, Belgium: ISHS Acta Horticulturae), 127–133.
Munné-Bosch, S., Alegre, L., Schwarz, K. (2000). The formation of phenolic diterpenes in Rosmarinus officinalis L. under Mediterranean climate. Eur. Food Res. Technol. 210 (4), 263–267. doi: 10.1007/s002179900108
Nachin, L., Barras, F. (2000). External pH: an environmental signal that helps to rationalize pel gene duplication in Erwinia chrysanthemi. Mol. Plant-Microbe Interact. 13 (8), 882–8866. doi: 10.1094/mpmi.2000.13.8.882
Napolitano, J. G., Lankin, D. C., Chen, S.-N., Pauli, G. F. (2012). Complete 1H NMR spectral analysis of ten chemical markers of Ginkgo biloba. Magnetic Resonance Chem. 50 (8), 569–575. doi: 10.1002/mrc.3829
National Association of Fertilizer Manufacturers (2023) Fertilizers (National Federation of Chemical Industry). Available at: https://assofertilizzanti.federchimica.it/en/who-we-are-group/about-us (Accessed 28_12_23).
Nieto, G., Ros, G., Castillo, J. (2018). Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis L.): a review. Medicines (Basel) 5 (3), 1–13. doi: 10.3390/medicines5030098
OEPP/EPPO (1994). EPPO Standard PP 2/1(1) Guideline on good plant protection practice: principles of good plant protection practice. Bull. OEPP/EPPO 24, 233–240.
Osei, R., Yang, C., Wei, L., Jin, M., Boamah, S. (2022). Effects of combined application of salicylic acid and proline on the defense response of potato tubers to newly emerging soft rot bacteria (Lelliottia amnigena) infection. Sustainability 14 (14), 8870. doi: 10.3390/su14148870
Park, Y., Moon, B.-H., Yang, H., Lee, Y., Lee, E., Lim, Y. (2007). Complete assignments of NMR data of 13-hydroxymethoxyflavones. Magnetic Resonance Chem. 45 (12), 1072–1075. doi: 10.1002/mrc.2063
Pauli, G. F., Kuczkowiak, U., Nahrstedt, A. (1999). Solvent effects in the structure dereplication of caffeoyl quinic acids. Magnetic Resonance Chem. 37 (11), 827–836. doi: 10.1002/(SICI)1097-458X(199911)37:11<827::AID-MRC568>3.0.CO;2-W
Peng, H. Y., Zhang, X. H., Xu, J. Z. (2016). Apigenin-7-O-β-D-glycoside isolation from the highly copper-tolerant plant Elsholtzia splendens. J. Zhejiang University-SCIENCE B (Biomedicine Biotechnology) 17 (6), 447–454. doi: 10.1631/jzus.B1500242
Pickersgill, R., Smith, D., Worboys, K., Jenkins, J. (1998). Crystal structure of Polygalacturonase from Erwinia carotovora ssp. carotovora. J. Biol. Chem. 273 (38), 24660–24664. doi: 10.1074/jbc.273.38.24660
Plaskova, A., Mlcek, J. (2023). New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 10. doi: 10.3389/fnut.2023.1118761
Põllumaa, L., Alamäe, T., Mäe, A. (2012). Quorum sensing and expression of virulence in Pectobacteria. Sensors 12 (3), 3327–3349. doi: 10.3390/s120303327
Ponce, A. G., Fritz, R., del Valle, C., Roura, S. I. (2003). Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. LWT - Food Sci. Technol. 36 (7), 679–684. doi: 10.1016/S0023-6438(03)00088-4
POWO (2023) Plants of the World Online (Kew (UK: Royal Botanic Gardens). Available at: https://powo.science.kew.org (Accessed 06 February 2023).
Pritchard, L., Glover, R. H., Humphris, S., Elphinstone, J. G., Toth, I. K. (2016). Genomics and taxonomy in diagnostics for food security: soft-rotting enterobacterial plant pathogens. Analytical Methods 8 (1), 12–24. doi: 10.1039/C5AY02550H
Pukalskas, A., van Beek, T. A., de Waard, P. (2005). Development of a triple hyphenated HPLC–radical scavenging detection–DAD–SPE–NMR system for the rapid identification of antioxidants in complex plant extracts. J. Chromatogr. A 1074 (1), 81–88. doi: 10.1016/j.chroma.2005.03.089
Pun, M., Khazanov, N., Galsurker, O., Weitman, M., Kerem, Z., Senderowitz, H., et al. (2021). Phloretin, an apple phytoalexin, affects the virulence and fitness of Pectobacterium brasiliense by interfering with quorum-sensing. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.671807
Purnavab, S., Ketabchi, S., Rowshan, V. (2015). Chemical composition and antibacterial activity of methanolic extract and essential oil of Iranian Teucrium polium against some of phytobacteria. Natural Product Res. 29 (14), 1376–1379. doi: 10.1080/14786419.2014.1000320
Rastrelli, L., Saturnino, P., Schettino, O., Dini, A. (1995). Studies on the constituents of Chenopodium pallidicaule (Canihua) seeds. Isolation and characterization of two new flavonol glycosides. J. Agric. Food Chem. 43 (8), 2020–2024. doi: 10.1021/jf00056a012
Richheimer, S. L., Bernart, M. W., King, G. A., Kent, M. C., Beiley, D. T. (1996). Antioxidant activity of lipid-soluble phenolic diterpenes from rosemary. J. Am. Oil Chemists’ Soc. 73 (4), 507–514. doi: 10.1007/BF02523927
Roma-Marzio, F., Galasso, G. (2019). New combinations for two hybrids in Salvia subg. Rosmarinus (Lamiaceae). Ital. Botanist 7, 31–34. doi: 10.3897/italianbotanist.7.34379
Rosselló, J. A., Cosín, R., Boscaiu, M., Vicente, O., Martínez, I., Soriano, P. (2006). Intragenomic diversity and phylogenetic systematics of wild rosemaries (Rosmarinus officinalis L. s.l., Lamiaceae) assessed by nuclear ribosomal DNA sequences (ITS). Plant Systematics Evol. 262 (1), 1–12. doi: 10.1007/s00606-006-0454-5
Rossmann, S., Dees, M. W., Perminow, J., Meadow, R., Brurberg, M. B. (2018). Soft rot Enterobacteriaceae are carried by a large range of insect species in potato fields. Appl. Environ. Microbiol. 84 (12), 1–11. doi: 10.1128/aem.00281-18
Rosúa, J. L. (1986). Contribución al estudio del género Rosmarinus L. en el Mediterráneo Occidental. Lagascalia 14 (2), 179–188.
Saha, A., Basak, B. B. (2020). Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind. Crops Products 145, 111979. doi: 10.1016/j.indcrop.2019.111979
Sahoo, M. M., Perach, O., Shachter, A., Gonda, I., Porwal, A., Dudai, N., et al. (2022). Spectral estimation of carnosic acid content in in vivo rosemary plants. Ind. Crops Products 187, 115292. doi: 10.1016/j.indcrop.2022.115292
Sakai, T., Sakamoto, T., Hallaert, J., Vandamme, E. J. (1993). Pectin, pectinase and protopectinase: production, properties, and applications. Adv. Appl. Microbiol. 39, 213–294. doi: 10.1016/s0065-2164(08)70597-5
Sánchez-Hernández, E., González-García, V., Martín-Gil, J., Lorenzo-Vidal, B., Palacio-Bielsa, A., Martín-Ramos, P. (2023). Phytochemical screening and antibacterial activity of Taxus baccata L. against Pectobacterium spp. and Dickeya chrysanthemi. Horticulturae 9 (2), 201. doi: 10.3390/horticulturae9020201
Satyal, P., Jones, T. H., Lopez, E. M., McFeeters, R. L., Ali, N. A., Mansi, I., et al. (2017). Chemotypic characterization and biological activity of Rosmarinus officinalis. Foods 6 (3), 1–15. doi: 10.3390/foods6030020
Scavetta, R. D., Herron, S. R., Hotchkiss, A. T., Kita, N., Keen, N. T., Benen, J. A., et al. (1999). Structure of a plant cell wall fragment complexed to pectate lyase C. Plant Cell 11 (6), 1081–1092. doi: 10.1105/tpc.11.6.1081
Schrödinger (2021). Schrödinger Release 2020-4: Maestro version 12.6.144, Glide, Ligprep, Prime (New York, NY: Schrödinger, LLC).
Shaheen, H. A., Issa, M. Y. (2020). In vitro and in vivo activity of Peganum harmala L. alkaloids against phytopathogenic bacteria. Scientia Hortic. 264, 108940. doi: 10.1016/j.scienta.2019.108940
Shen, C.-C., Chang, Y.-S., Hott, L.-K. (1993). Nuclear magnetic resonance studies of 5,7-dihydroxyflavonoids. Phytochemistry 34 (3), 843–845. doi: 10.1016/0031-9422(93)85370-7
Skendi, A., Irakli, M., Chatzopoulou, P., Bouloumpasi, E., Biliaderis, C. G. (2022). Phenolic extracts from solid wastes of the aromatic plant essential oil industry: potential uses in food applications. Food Chem. Adv. 1, 100065. doi: 10.1016/j.focha.2022.100065
Steglińska, A., Bekhter, A., Wawrzyniak, P., Kunicka-Styczyńska, A., Jastrząbek, K., Fidler, M., et al. (2022). Antimicrobial activities of plant extracts against Solanum tuberosum L. phytopathogens. Molecules 27 (5), 1–20. doi: 10.3390/molecules27051579
Su, Z., Liu, X., Guo, Q., Xuan, L., Lu, X., Dong, L., et al. (2022). Insights into complex infection by two Pectobacterium species causing potato blackleg and soft rot. Microbiological Res. 261, 127072. doi: 10.1016/j.micres.2022.127072
Suaza-Gaviria, V., Mesa-Vanegas, A. M., Ocampo-Jiménez, O., Monsalve-Fonnegra, Z. I. (2023). Antioxidant activity and phytopathogenic control of extracts and fraction from Struthanthus calophyllus A.C.Sm. (Loranthaceae). Chem. Biodiversity 20 (2), e202200830. doi: 10.1002/cbdv.202200830
Sumner, L. W., Amberg, A., Barrett, D., Beale, M. H., Beger, R., Daykin, C. A., et al. (2007). Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3 (3), 211–221. doi: 10.1007/s11306-007-0082-2
Tajidin, N. E., Shaari, K., Maulidiani, M., Salleh, N. S., Ketaren, B. R., Mohamad, M. (2019). Metabolite profiling of Andrographis paniculata (Burm. f.) Nees. young and mature leaves at different harvest ages using 1H NMR-based metabolomics approach. Sci. Rep. 9 (1), 16766. doi: 10.1038/s41598-019-52905-z
Tardy, F., Nasser, W., Robert-Baudouy, J., Hugouvieux-Cotte-Pattat, N. (1997). Comparative analysis of the five major Erwinia chrysanthemi pectate lyases: enzyme characteristics and potential inhibitors. J. Bacteriology 179 (8), 2503–2511. doi: 10.1128/jb.179.8.2503-2511.1997
The UniProt Consortium (2020). UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 49 (D1), D480–D489. doi: 10.1093/nar/gkaa1100
Topçu, G., Öztürk, M., Kusman, T., Barla Demirkoz, A., Kolak, U., Ulubelen, A. (2013). Terpenoids, essential oil composition, fatty acid profle, and biological activities of Anatolian Salvia fruticosa Mill. Turkish J. Chem. 37, 619–632. doi: 10.3906/kim-1303-25
Tounekti, T., Munné-Bosch, S. (2012). Enhanced phenolic diterpenes antioxidant levels through non-transgenic approaches. Crit. Rev. Plant Sci. 31 (6), 505–519. doi: 10.1080/07352689.2012.696457
van den Berg, R. A., Hoefsloot, H. C., Westerhuis, J. A., Smilde, A. K., van der Werf, M. J. (2006). Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics 7, 142. doi: 10.1186/1471-2164-7-142
van der Wolf, J. M., de Boer, S. H., Czajkowski, R. L., Cahill, G., van Gijsegem, F., Davey, T., et al. (2021). “Management of diseases caused by Pectobacterium and Dickeya species,” in Plant diseases caused by Pectobacterium and Dickeya species. Eds. van Gijsegem, F., van der Wolf, J. M., Toth, J. K. (Berlin, Germany: Springer), 175–214.
van der Wolf, J. M., de Haan, E. G., Kastelein, P., Krijger, M., de Haas, B. H., Velvis, H., et al. (2017). Virulence of Pectobacterium carotovorum subsp. brasiliense on potato compared with that of other Pectobacterium and Dickeya species under climatic conditions prevailing in the Netherlands. Plant Pathol. 66 (4), 571–583. doi: 10.1111/ppa.12600
Van Gijsegem, F., Hugouvieux-Cotte-Pattat, N., Kraepiel, Y., Lojkowska, E., Moleleki, L. N., Gorshkov, V., et al. (2021a). “Molecular nteractions of Pectobacterium and Dickeya with plants,” in Plant Diseases Caused by Dickeya and Pectobacterium Species. Eds. Van Gijsegem, F., van der Wolf, J. M., Toth, I. K. (Cham, Switzerland: Springer International Publishing), 85–147.
Van Gijsegem, F., Toth, I. K., van der Wolf, J. M. (2021b). “Soft rot pectobacteriaceae: A brief overview,” in Plant Diseases Caused by Dickeya and Pectobacterium Species. Eds. Van Gijsegem, F., van der Wolf, J. M., Toth, I. K. (Cham, Switzerland: Springer International Publishing), 1–11.
Vatanen, T., Osmala, M., Raiko, T., Lagus, K., Sysi-Aho, M., Orešič, M., et al. (2015). Self-organization and missing values in SOM and GTM. Neurocomputing 147, 60–70. doi: 10.1016/j.neucom.2014.02.061
Vignoli, A., Ghini, V., Meoni, G., Licari, C., Takis, P. G., Tenori, L., et al. (2019). High-throughput metabolomics by 1D NMR. Angewandte Chemie 58 (4), 968–994. doi: 10.1002/anie.201804736
Wellwood, C. R. L., Cole, R. A. (2004). Relevance of carnosic acid concentrations to the selection of rosemary, Rosmarinus officinalis (L.), accessions for optimization of antioxidant yield. J. Agric. Food Chem. 52 (20), 6101–6107. doi: 10.1021/jf035335p
Wiederstein, M., Sippl, M. J. (2007). ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 35 (Web Server issue), W407–W410. doi: 10.1093/nar/gkm290
Wu, P., Yang, S., Zhan, Z., Zhang, G. (2020). Origins and features of pectate lyases and their applications in industry. Appl. Microbiol. Biotechnol. 104 (17), 7247–7260. doi: 10.1007/s00253-020-10769-8
Xiao, C., Dai, H., Liu, H., Wang, Y., Tang, H. (2008). Revealing the metabonomic variation of rosemary extracts using 1H NMR spectroscopy and multivariate data analysis. J. Agric. Food Chem. 56 (21), 10142–10153. doi: 10.1021/jf8016833
Yadav, P. K., Singh, V. K., Yadav, S., Yadav, K. D. S., Yadav, D. (2009). In silico analysis of pectin lyase and pectinase sequences. Biochemistry 74 (9), 1049–1055. doi: 10.1134/S0006297909090144
Yang, J. S., Lee, H. W., Song, H., Ha, J. H. (2021). Volatile metabolic markers for monitoring Pectobacterium carotovorum subsp. Carotovorum using headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry. J. Microbiol. Biotechnol. 31 (1), 70–78. doi: 10.4014/jmb.2009.09028
Yangui, T., Sayadi, S., Dhouib, A. (2013). Sensitivity of Pectobacterium carotovorum to hydroxytyrosol-rich extracts and their effect on the development of soft rot in potato tubers during storage. Crop Prot. 53, 52–57. doi: 10.1016/j.cropro.2013.06.014
Zhang, Y., Smuts, J. P., Dodbiba, E., Rangarajan, R., Lang, J. C., Armstrong, D. W. (2012). Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J. Agric. Food Chem. 60 (36), 9305–9314. doi: 10.1021/jf302179c
Keywords: Salvia rosmarinus, NMR spectroscopy, multivariate data analysis, rosemary ecotypes, Pectobacterium carotovorum subsp. carotovorum
Citation: Iobbi V, Donadio G, Lanteri AP, Maggi N, Kirchmair J, Parisi V, Minuto G, Copetta A, Giacomini M, Bisio A, De Tommasi N and Drava G (2024) Targeted metabolite profiling of Salvia rosmarinus Italian local ecotypes and cultivars and inhibitory activity against Pectobacterium carotovorum subsp. carotovorum. Front. Plant Sci. 15:1164859. doi: 10.3389/fpls.2024.1164859
Received: 13 February 2023; Accepted: 12 January 2024;
Published: 02 February 2024.
Edited by:
Hui Xie, Fudan University, ChinaReviewed by:
Fabio Boylan, Trinity College Dublin, IrelandMina Rastgou, Urmia University, Iran
Younes Rezaee Danesh, Urmia University, Iran
Copyright © 2024 Iobbi, Donadio, Lanteri, Maggi, Kirchmair, Parisi, Minuto, Copetta, Giacomini, Bisio, De Tommasi and Drava. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela Bisio, YW5nZWxhLmJpc2lvQHVuaWdlLml0; Nunziatina De Tommasi, ZGV0b21tYXNpQHVuaXNhLml0
†These authors have contributed equally to this work and share first authorship
 Valeria Iobbi
Valeria Iobbi Giuliana Donadio
Giuliana Donadio Anna Paola Lanteri
Anna Paola Lanteri Norbert Maggi
Norbert Maggi Johannes Kirchmair
Johannes Kirchmair Valentina Parisi
Valentina Parisi Giovanni Minuto
Giovanni Minuto Andrea Copetta
Andrea Copetta Mauro Giacomini
Mauro Giacomini Angela Bisio
Angela Bisio Nunziatina De Tommasi
Nunziatina De Tommasi Giuliana Drava
Giuliana Drava