- 1College of Animal Science and Technology, Anhui Agricultural University, Hefei, China
- 2College of Resources and Environment, Anhui Agricultural University, Hefei, China
Plant essential oils have played an important role in the field of antibiotic alternatives because of their efficient bacteriostatic and fungistatic activity. As plant essential oils are widely used, their activity to improve the quality of plant silage has also been explored. This review expounds on the active ingredients of essential oils, their bacteriostatic and fungistatic activity, and mechanisms, as well as discusses the application of plant essential oils in plant silage fermentation, to provide a reference for the development and application of plant essential oils as silage additives in plant silage fermentation feed.
1 Introduction
Considerable attention has been paid to the application of plant extracts in livestock and poultry production as alternatives to banned additives such as antibiotics. Plant extracts is a mixture of natural compounds or components extracted from plant materials. Due to the presence of numerous bioactive compounds with pharmacological properties, they have great potential for research. Moreover, they are considered a sustainable and eco-friendly choice due to their natural, biodegradable nature, and their ability to reduce reliance on synthetic chemicals. Huge scientific studies regarding the application of plant extracts in silage preservation have reported the potential antifungal agents from this enriched flora (Cock and Van Vuuren, 2015), Aloe vera extract has a wide range of microbial growth inhibition activities, and it has been reported to have a significant inhibitory effect on the mycelial growth and spore germination of Penicillium italiana (Zapata et al., 2013). The organic easy extract of tea plant contains a variety of natural non-ionic surfactants, which can cooperate with some antibacterial agents to antagonize fungi (Hao et al., 2010). Some studies have reported that the ethanol extract of Ficus hirta Vahl exhibits a fungistatic effect against Penicillium tilikum (Wan et al., 2017). As an important class of plant extracts, an increasing number of studies have shown that plant essential oils have significant antibacterial activity, which makes them more attractive to researchers. Commonly used plant essential oils include thyme essential oil, clove essential oil, cinnamon essential oil, oregano essential oil, mint essential oil, and curcumin essential oil. At present, plant essential oils are primarily used to maintain animal health, improve animal performance, and enhance the quality of livestock products in the breeding industry. With the deepening of research on essential oils, the function of plant essential oils in improving the fermentation quality of feed silage has been explored (Matté et al., 2023).
2 Main active component of plant essential oils
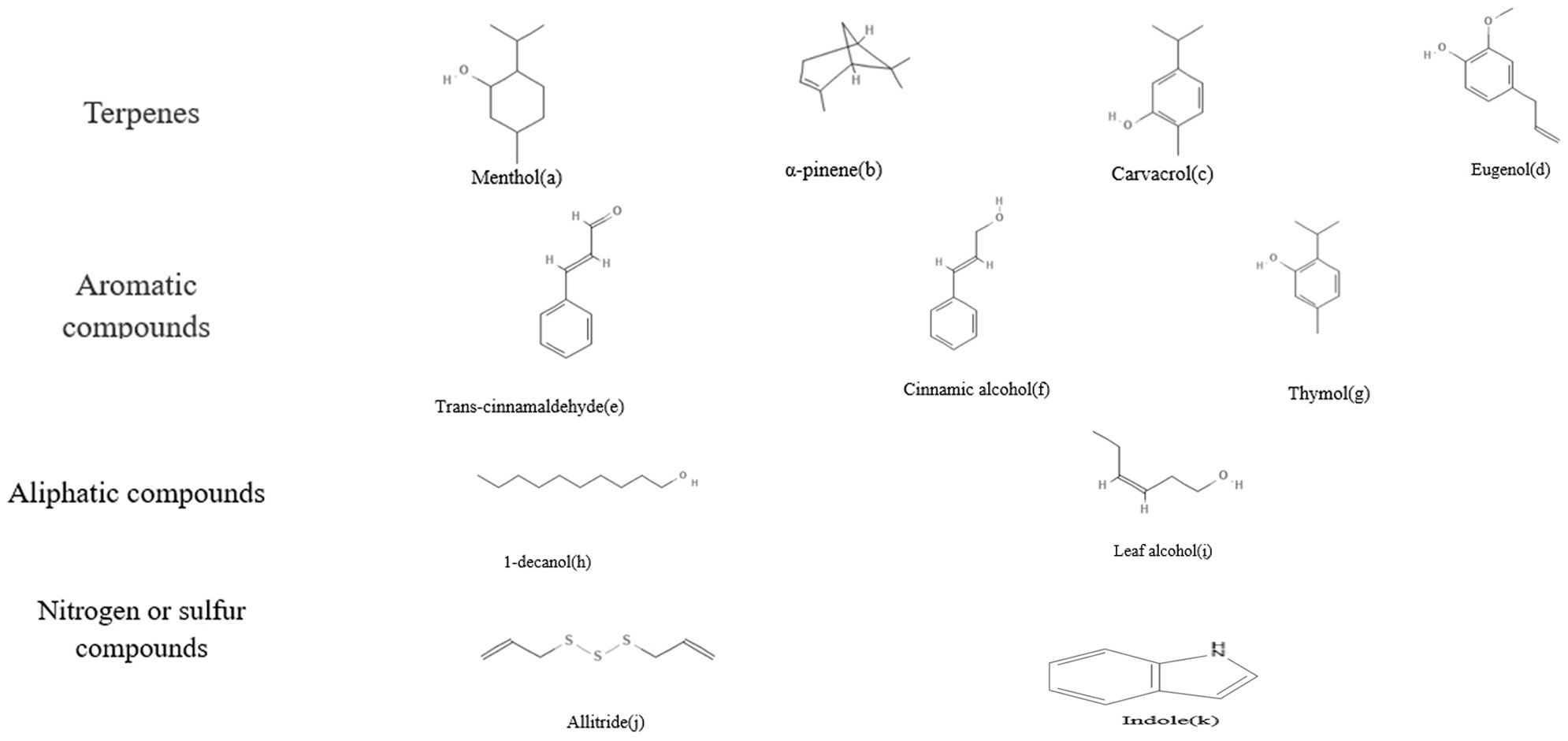
Plant essential oil is a chemical substance extracted from the bark, peel, leaves, buds, seeds, flowers, and other parts of plants by steam distillation, solvent-assisted extraction, hydrogenation distillation, ultrasonic-assisted extraction, supercritical fluid extraction, and solvent-free microwave extraction (Kant and Kumar, 2022). The active components of plant essential oil are divided into four categories in accordance with their structure: terpene compounds, aromatic compounds, aliphatic compounds, and sulfur-containing and nitrogen-containing compounds (Shao et al., 2020).
Terpene compounds are the most common chemical components in essential oils. They are generally chain or cyclic olefins with the general formula (C5H8)n Figure 1; PubChem, such as menthol (a), menthone, neomenthol, and isomenthone in peppermint essential oil (Zhao et al., 2022); α-terpinene (b) and myrcene in rosin essential oil (Couladis et al., 2003); carvacrol (c) and eugenol (d) in oregano and clove essential oil (Milovanović et al., 2009); and eucalyptol in rosemary essential oil (Jiang et al., 2011). The second common chemical component is aromatic compounds, which are a class of compounds with a benzene ring structure related to the fragrance of essential oils, such as cinnamaldehyde (e) and cinnamic alcohol (f) in cinnamon essential oil (Vasconcelos et al., 2018), as well as thymol (g) in thyme essential oil (Kim et al., 2022). Aliphatic compounds, which have the smallest relative molecular mass but are widely present in plant essential oils, are organic compounds composed of hydrocarbon chains or their derivatives, such as n-decyl alcohol (h) and leaf alcohol (i). Sulfur and nitrogen-containing compounds, such as allitride (j) and indole (k), are present in small amounts, but t hese compounds have extremely strong odors and characteristic aromas (Saad et al., 2013).
3 Bacteriostatic and fungistatic activity and mechanism of plant essential oils
The bacteriostatic and fungistatic effect of common plant essential oils such as thyme, clove, cinnamon, oregano, peppermint, and curcumin is related to their active ingredients. Each active ingredient exerts its bacteriostatic and fungistatic effect through its functional groups independently or synergistically. Therefore, the bacteriostatic and fungistatic mechanisms of different types of essential oils are distinct (Evangelista et al., 2022).
3.1 Bacteriostatic and fungistatic activity of common essential oils
3.1.1 Main bacteriostatic and fungistatic components and activities of essential oils
The common bacteriostatic and fungistatic active ingredient in thyme essential oil is thymol, chemically known as 5-methyl-2-isopropylphenol, which is a natural monoterpene. Thymol has the strongest inhibitory effect against molds, and 0.01–0.05 mg/mL of thymol is found to be effective against molds such as Aspergillus niger, Aspergillus flavus, and Aspergillus fumigatus. In addition, thymol can effectively inhibit Gram-positive pathogens such as Bacillus subtilis and Staphylococcus aureus between 0.06 and 0.2 mg/mL, while the inhibitory concentration for lactic acid bacteria must reach 0.1–0.5 mg/mL (Marchese et al., 2016). Another highly effective bacteriostatic and fungistatic active substance in thyme essential oil is terpinene, which can exhibit an bacteriostatic and fungistatic effect equivalent to 70% of streptomycin at a concentration of 0.07 mg/mL against Phytophthora capsici (Yang et al., 2022). Thyme essential oil (Thymus kotschyanus), as a mixture of multiple bacteriostatic and fungistatic active substances, has a minimum inhibitory concentration of 0.0625 mg/mL against common molds such as A. niger, A. flavus, and Fusarium oxysporum in silage feed or raw materials (Ownagh et al., 2010), and the minimum inhibitory concentration for yeast is 0.5 mg/mL. The inhibitory concentration for some lactic acid bacteria such as Lactobacillus brevis, Lactobacillus plantarum, and Lactococcus lactis must reach at least 3–10 mg/mL (Thymus vulgaris ct.linalol, Thymus serrulatus and Thymus schimperi Ronniger) (Gutierrez et al., 2008; Damtie and Mekonnen, 2020; de Oliveira Carvalho et al., 2020). Thus, whether the active ingredient is single or a mixture in thyme essential oil, it shows low inhibitory concentration against pathogens and high inhibitory concentration against lactic acid bacteria. This selective inhibition laid the foundation for its use in silage feed (Damtie and Mekonnen, 2020).
The main active ingredient in clove essential oil is eugenol, chemically known as 4-allyl-2-methoxyphenol, which belongs to the terpene class of compounds and has a broad-spectrum antibacterial activity against Gram-negative and Gram-positive bacteria (Khalil et al., 2017). The minimum inhibitory concentration of eugenol for Gram-positive bacteria such as S. mutans and S. aureus is generally between 0.1 and 0.2 mg/mL. Its minimal inhibitory concentrations against Fusarium species commonly found in silage, such as Fusarium avenaceum, Fusarium graminearum and Aspergillus nidulans, ranged from 0.1 mg/mL to 0.14 mg/mL. The minimum inhibitory concentration against Saccharomyces cerevisiae is 0.25 mg/mL, whereas the minimum inhibitory concentration for lactic acid bacteria such as Lactobacillus casei must reach 1 mg/mL (Marchese et al., 2017). Low levels of eugenol (below the minimum inhibitory concentration) show strong inhibitory activity against the expression of the physiological functions of microorganisms and the production of toxins such as ochratoxin (Jiang et al., 2022). Clove essential oil also exhibits a remarkable bacteriostatic and fungistatic activity, with a minimum inhibitory concentration of 0.2–0.3 mg/mL for yeast and a minimum inhibitory concentration of 0.031, 0.031, and 0.25 mg/mL for fungi such as F. graminearum, Rhizopus stolonifer, and Penicillium crustosum, respectively, which is lower than its minimum inhibitory concentration for lactic acid bacteria such as Lactobacillus fermentum and Lactobacillus lactis (Syzygium aromaticum (L.) Merr. and L. M. Perry) (Sameza et al., 2016; Sharma et al., 2017; Chan et al., 2018). The low inhibitory concentration of clove essential oil against harmful microorganisms and high inhibitory concentration against lactic acid bacteria determines its use in later production.
The bacteriostatic and fungistatic activity of cinnamon essential oil is derived from cinnamaldehyde, which has the strongest bacteriostatic and fungistatic activity among other secondary metabolites. Cinnamaldehyde has shown antifungal activity against Candida (Choonharuangdej et al., 2021), A. flavus (Achar et al., 2020), F. graminearum (Gwiazdowska et al., 2022), Aspergillus ochraceus (Wang et al., 2018), and other fungi, as well as broad-spectrum antifungal activity against Escherichia coli and S. aureus (Zhang et al., 2016). Compared with other active components of plant essential oil, cinnamaldehyde has the strongest inhibitory activity on the synthesis of aflatoxin and ochratoxin. The minimal inhibitory concentration of cinnamaldehyde against F. oxysporum and F. gramineum is 0.8 mg/mL, and its minimal inhibitory concentration against spoilage yeast is 0.31–1.25 mg/mL (Muñoz‐González et al., 2022). However, its minimal inhibitory concentration against lactic acid bacteria varies, with a minimal inhibitory concentration of 5 mg/mL against Lactobacillus sakei and a minimum inhibitory concentration of 50 mg/mL against Lactobacillus brucei (Sameza et al., 2016; Sharma et al., 2017; Muñoz-González et al., 2022). Cinnamon essential oil also shows good application effects. Cinnamon essential oil inhibits E. coli, S. aureus, and Listeria at a concentration of 0.3–0.5 mg/mL (Cinnamomum zeylanicum) (Nematollahi et al., 2020), and its minimum inhibitory concentration against Penicillium citrinosus and Penicillium expandatum is between 0.4 and 0.5 mg/mL (Cinnamomum cassia) (Lucas-Gonzalez et al., 2023). However, the minimum inhibitory concentration against lactic acid bacteria is approximately 10 mg/mL (C. zeylanicum) (de Oliveira Carvalho et al., 2020).
The main active ingredients in oregano essential oil are carvacrol and thymol. Thymol has been elaborated in the previous section, and carvacrol is also a class of phenolic compounds with strong bacteriostatic and fungistatic activity. The minimum inhibitory concentration of carvacrol against S. aureus is 0.3 mg/mL; the minimum inhibitory concentration of carvacrol against Streptococcus is 2.5 mg/mL, and its minimum inhibitory concentration against Gram-negative bacteria such as E. coli and Salmonella typhimurium is less than 0.25 mg/mL (Marinelli et al., 2018; Rathod et al., 2021). The minimum inhibitory concentration against a variety of yeasts is approximately 0.2 mg/mL. On the contrary, oregano essential oil containing a mixture of carvacrol and thymol shows considerable synergistic bacteriostatic and fungistatic effects. Its minimum inhibitory concentration against a variety of Fusarium species is less than 0.8 mg/mL, and its antifungal activity is mostly between 0.2 and 0.3 mg/mL, whereas its minimum inhibitory concentration against a variety of lactic acid bacteria is between 5.5 and 13 mg/mL (Origanum vulgare L.) (Gutierrez et al., 2008; Konuk and Ergüden, 2017; Sharma et al., 2017). The bacteriostatic and fungistatic activity of oregano essential oil against lactic acid bacteria is lower than that against pathogenic fungi. Furthermore, oregano essential oil can inhibit the biosynthesis of aflatoxin after it acts on aflatoxin (Origanum majorana L.) (Chaudhari et al., 2020).
Peppermint essential oil has been used as a preservative in the food industry for a long time, and its bioactive substances have good effects on inhibiting pathogenic bacteria and spoilage microorganisms, such as menthol, menthone, and neomenthol (Kazem et al., 2011). A number of reports have shown that peppermint essential oil inhibits the cell activity of S. aureus (Kang et al., 2019), Candida albicans, Pseudomonas aeruginosa (Mahboubi and Kazempour, 2014), Helicobacter pylori, and Salmonella enteritidis (Imai et al., 2001). The in vitro anti-aflatoxin concentration of menthol is 1.0 mg/mL, while menthol stereoisomers and menthone do not show evident antitoxin function, which is related to their structural types (Ownagh et al., 2010; Muñoz-González et al., 2022). Peppermint essential oil has weaker bacteriostatic and fungistatic activity than menthol. Peppermint essential oil has a minimum inhibitory concentration of 62 mg/mL against a variety of mold species and more than 150 mg/mL against lactic acid bacteria, but it has considerable bacteriostatic and fungistatic activity against S. cerevisiae, with a minimum inhibitory concentration of 1 mg/mL. The results show that antifungal sensitivity to mold and yeast is higher than sensitivity to lactic acid bacteria. (Mentha Piperita) (de Oliveira Carvalho et al., 2020).
Turmerone is a glycosides active substance derived from turmeric essential oil. It mainly includes ar-turmerone and β-turmerone. Given its special structure, it has strong antibacterial and antifungal activity. At a concentration of 0.1% ar-turmerone, this purified compound reduced the growth of Fusarium semitectum, Aspergillus ochraceous, and Calletotrichum musae by 70%, 55%, and 68%, respectively (Dhingra et al., 2007). At 1 mg/disk, ar-turmerone strongly inhibited the growthl of C perfringens and moderately inhibited the growth of E. coli without any adverse effects on the growth of four lactic acid bacteria(Lee, 2006). Given the synergistic effect of various bacteriostatic and fungistatic active substances in turmeric essential oil, the bacteriostatic and fungistatic activity of the mixture is remarkably enhanced. The minimum inhibitory concentration of turmeric essential oil to various yeasts is between 0.01 and 0.03 mg/mL (Curcuma longa L.) (Konuk and Ergüden, 2017), and the concentration of turmeric essential oil has a remarkable inhibitory effect on Fusarium verticillium at 0.072 mg/mL (C. longa) (Avanço et al., 2017). However, the minimum inhibitory concentration against Lactobacillus is as high as 11–14.5 mg/mL (C. longa) (Khorsandi et al., 2018).
All of the above mentioned essential oils show strong inhibition against yeast, fusarium, and other pathogenic bacteria but relatively weak inhibition against lactic acid bacteria. This unique bacteriostatic and fungistatic property of essential oil contributes to the inhibition of spoilage bacteria in silage and to the improvement of silage quality.
3.1.2 Synergistic bacteriostatic and fungistatic activity of essential oils
The synergistic bacteriostatic and fungistatic effects of plant oils have been gradually recognized with the exploration of their bacteriostatic and fungistatic properties. The bacteriostatic and fungistatic activity of plant essential oils results from interactions among different components, often by terpenoid compounds with the strongest internal bacteriostatic and fungistatic properties, as well as by a variety of compounds of different classes,For instance, there are interactions between compounds such as citral and thujone, camphor, ethyl acetate of borneol, and citronellal. Similarly, interactions between α-pinene and thujone, camphor, citral, citronellal, and geraniol occur. Moreover, the interaction of cineole oxide with compounds like thujone, hexanal, and hinokitiol also demonstrates a synergistic enhancement of cytotoxicity(Wright et al., 2007), and the fractional inhibitory concentration of each component against bacteria is markedly reduced. The mixed use of eugenol and linalool in basil essential oil shows higher bacteriostatic, fungistatic and antioxidant activity to some fungi and bacteria than each component itself (Juliani et al., 2009). The bacteriostatic and fungistatic active components of cinnamon essential oil, cinnamaldehyde and cinnamic acid, also show bacteriostatic and fungistatic properties when combined. The combination of components derived from different essential oils also shows synergistic effects, such as the chimerism of cinnamaldehyde and carotenoids to form a mixture of solid and liquid fats to cause defects in the structure of the lipid matrix and improve the bioavailability and retention ability of active ingredients (Procopio et al., 2022). The combination of cinnamon essential oil and clove essential oil also has synergistic effects, and the enhanced essential oil remarkably inhibits biofilm formation, destroying the cell wall structure and scavenging free radicals (Yen and Chang, 2008). The same study found that the combination of thyme and rosemary as well as the triple combination of thyme, rosemary, and cinnamon show synergistic effects on Bacillus cinesulata and Pseudomonas ectrosp (Nikkhah et al., 2017). The combination of oregano and thyme essential oils, bay and almond essential oils, and basil and thyme essential oils all showed synergistic bacteriostatic and fungistatic effects, with minimum inhibitory concentrations reduced by up to 128 times.
3.2 Bacteriostatic and fungistatic mechanism of plant essential oils
Most of the current studies have shown that the main bacteriostatic and fungistatic mechanism of plant essential oils is achieved by destroying the integrity of biofilm, destroying and inhibiting cell wall biosynthesis, damaging membrane proteins, and inhibiting mitochondrial function.
3.2.1 Destruction of biofilm integrity
The bacteriostatic and fungistatic effects of essential oils are directly related to their lipophilicity. The six-carbon aromatic phenol group of cinnamaldehyde allows it to cross the phospholipid bilayer of the bacterial cell wall and bind to the inner and outer membrane proteins, thereby preventing them from performing their normal function. Altered membrane permeability and loss of functional proteins that transport molecules and ions perturb microbial cells, which leads to cytoplasmic coagulation, enzymatic denaturation, and loss of proteins, as well as loss of metabolites and ions (Suxia Shen et al., 2015). Cell membrane damage by essential oil is usually manifested as the change in cell membrane surface structure (usually wrinkled or irregular), the increase of relative electrical conductivity, the decrease of membrane potential, the increase of extracellular nucleic acid and protein concentration, the change of membrane potential and conductivity, and the accumulation of intracellular active components of essential oil, which leads to the acidification of the cell membrane and the damage of the cell membrane caused by protein denaturation (Trinh et al., 2015).
Cellular ion leakage is also an important bacteriostatic and fungistatic mechanism of plant essential oils. The α- and β-unsaturated bonds of various cinnamaldehydes can be conjugated to the plasma membrane calcium ATPase on the fungal plasma membrane, which opens the ion pathway, induces Ca2+ efflux, and inhibits fungal activity (Hu et al., 2013). Under ergosterol inhibition, celery essential oil acts on the cell membrane of A. flavus to take part in the α-demethylation of lanosterol, and Ca2+ leakage is observed. Mg2+ influx is enhanced, leading to the depletion of nutrient uptake, inhibition of nucleic acid synthesis, and inhibition of ATPase-dependent respiratory activity, thereby leading to cell lysis (Das et al., 2019). P. capsici Leonian and A. flavus, which have no ergosterol production ability, and ethylene glycol bis (2-aminoethyl ether) tetraacetic acid elution treatment were used to exclude the influence of exogenous Ca2+. The cells showed Ca2+ outflow and ergosterol reduction after treatment with cinnamon essential oil, fenestra essential, oil and peppermint essential oil (Dwivedy et al., 2017). Ca2+ is the most prevalent regulator in the whole living system, which is involved in the regulation of the proliferation, differentiation, and apoptosis of organisms. In fungi, Ca2+ regulates spore formation, spore germination, hyphal branching, apical growth, and structural differentiation (Shreaz et al., 2016). The disorder of Ca2+ may interfere with the amount of circulating calcium ions in the mitochondria and activate the mitochondrial permeability transition pore, thereby leading to the initiation of the fungal apoptosis program (Berridge et al., 2000).
3.2.2 Reduction of quorum sensing
Quorum sensing (QS) is a feedback intercellular communication system of bacteria based on the secretion and sensing of external signaling molecules. The formation of biofilms is highly correlated with density-dependent QS propagation, which affects the production of bacterial secondary metabolites and regulates the secretion of virulence factors. Bacteria in the form of biofilms are different from planktic bacteria because their association with abiotic surfaces generates a three-dimensional organizational structure that is protected from the threat of being killed by fungicides, disinfectants, and antibiotics. Bacterial cells produce extracellular polymeric substances, proteins, and extracellular DNA, which support structural stability and improve substrate exchange and nutrient cycling in biofilms (Tan et al., 2019). The inhibition of intercellular communication between bacteria and biofilm formation is considered as an bacteriostatic and fungistatic pathway of plant essential oils (Bo et al., 2023). After applying cinnamaldehyde to the biofilm, the hydrophobicity of the cell membrane decreases, resulting in the reduced adhesion of the hydrophobic surface to the biofilm surface. Aggregation also decreased, whereas self-aggregation formed the complete biological structure of several different biofilm strains, which played an important role in biofilm stability (Yu et al., 2020). Molecular docking analysis showed that cinnamaldehyde downregulated the expression of cellulose synthase (BcsA) and transcription activator protein (luxR) receptor genes, thereby inhibiting the synthesis of signaling molecules in QS (Liu et al., 2021). Diallyl disulfide in garlic essential oil inhibits the virulence factors of P. aeruginosa at MIC concentrations by affecting the transcription of key genes in three different QS systems (Li W.-R. et al., 2018). Carvacrol binds to homoserine lactone synthase (Expl) and transcriptional regulators (Khan et al., 2017), which in turn inhibits the production of QS signaling molecules and the expression of QS-controlled genes (Joshi et al., 2016). Eugenol inhibits protease, pyocyanin, pyranan biosynthesis, extracellular polysaccharide, and rhamnolipid and closely binds to the synthesis of carbonyl N-coa acylation regulatory protein (LasR) by P. aeruginosa, thereby leading to the inhibition of QS (Rathinam et al., 2017). Cyclic diguanosine monophosphate (C-di-GMP) is considered as a key cytoplasmic signal and second messenger that controls bacterial virulence, cell cycle reproduction, motility, and other behaviors, such as the biofilm life cycle in several bacteria. Cinnamaldehyde carbon can affect the level of nitric oxide by regulating C-di-GMP, thereby inducing biofilm dispersion (Topa et al., 2018), whereas the ginger essential oil test found that cinnamaldehyde carbon promoted the degradation of proteins with EAL (Glu-Ala-Leu) or HD-GUP (His-Asp-Gly-Tyr-Pro) domains. This domain is directly associated with C-di-GMP levels, thereby inhibiting biofilm formation (Kim and Park, 2013).
3.2.3 Inhibition of cell wall formation
The cell wall is an important structure in fungi, indispensable for maintaining shape integrity and fluidity, for interacting with its surroundings, and for regulating the fungal membrane. Chitin, as a scaffold for the cell wall, imparts integrity and strength to the cell wall, thereby offers protection against external stresses, mechanical damage, and immune responses from hosts (Baker et al., 2007). Glucans attached to chitin serve as attachment points for other structures, constituting amorphous outer and intermediate fillers of the fungal cell wall (Shenghui Shen et al., 2019). Glycosylated proteins anchor β (1,6)-glucan chains through glycosylphosphatidylinositol (GPI). Mannosyl proteins account for 40% of the cell wall structure. Damage to mannosyl proteins will lead to the decreased activity of proteins synthesizing the cell wall, thereby affecting the strength and integrity of the cell wall, which is of great importance to the dynamic system of fungal cell wall (Schmidt et al., 2005). The chitin–glucan complex or chitosan complex is the main component of fungal cell wall, accounting for 60% of the dry weight of the cell (Araújo et al., 2020).
The active substances in many plant essential oils are mixed inhibitors of 1, 3-β-glucan synthase (FKS-1) and chitin synthase in fungi. Diallyl disulfide downregulates the expression of the chitin synthase gene, and the treated cells have reduced chitin, damaged epidermis, and changed morphology and physiological activity (Shah et al., 2020). Previous studies found that cinnamaldehyde and eugenol could bind to the active sites of these two enzyme proteins through different amino acid residues (Ju et al., 2022). Artemisia monosperma Del., Callistemon viminals G. Don, Citrus aurantifolia Swingle, and Cupressus macrocarpa Hartw. ex Gordon essential oils also inhibited chitin production (Abdelgaleil and El-Sabrout, 2018). Some experiments have found that a sub-inhibitory concentration of cinnamaldehyde can directly or indirectly attack the target of azole, polyene, and echinocandin through the accumulation of reactive oxygen species to induce moderate downregulation of the transcription of ERG-2, ERG-3, ERG-4, and ERG-11, which is consistent with the change trend of ergosterol (Parks and Casey, 1995). The upregulation of sterol influx transporters (such as AUS-1 and TIR-3) and sterol metabolism regulators (such as SUT-1 and UPC-2) inhibits ergosterol in a dose-dependent manner and then affects cell membrane integrity (Li Q. Q. et al., 2018). Estragole and linalool were found to inhibit ergosterol in a similar way as azole antifungal agents, both of which showed an inhibitory effect on 14α-demethylase, a key enzyme in ergosterol synthesis (Khan et al., 2010).
3.2.4 Damage to mitochondrial function
ATPases are involved in most biological and physiological activities in the cell through energy coupling. When plant oils disrupt the permeability and fluidity of plasma membranes, the membrane potential of organelles is reduced; proton pumping is disrupted, and the synthesis of H-ATPase, the key enzyme in ATP production, is inhibited. Some polyphenolic compounds block the activity of ATP synthase by binding to some chemical binding cavities of the enzyme, and the functional groups of inhibitors have established interactions with key amino acid residues of the enzyme through hydrogen bonding, hydrophobic interaction, etc. (Issa et al., 2019). After the inhibition of ATPase, related dependent enzymes are also affected. The decrease of protease and phospholipase activities can be attributed to the degradation of ATPase mediated by cinnamaldehyde (Pootong et al., 2017), and the expression level of fatty acid synthases such as acetyl-CoA carboxylase and fatty acid biosynthetic enzymes (fasI, fasH, and fasF) is inhibited. In addition, glycerophospho acyltransferase (plsX, plsY, plsC), cytidine diphosphate-diacylglycerol synthase A (cdsA), phosphatidylglycerol synthase A (pgsA), cardiolipin synthase (cls), and polypeptide resistance factor (mprF) glycerophospholipid biosynthesis pathways are inhibited; thus, the degree of damage of bacterial biofilm is aggravated (Pang et al., 2021). After cinnamaldehyde treatment, the ATP permeability of biofilms was increased; the ATP level in biofilms was remarkable decreased, and the intracellular ATP was rapidly consumed to maintain pH. After eugenol and citral treatment, Pseudomonas roqueforti was found to have an altered mitochondrial morphology and membrane potential, which further damaged the metabolic pathways of cellular energy and finally induced apoptosis (Ju et al., 2020).
3.2.5 Inhibition of filamentous temperature-sensitive proteins
The filamentous temperature-sensitive protein Z (FtsZ) is a GTPase with weak a sequence homology with tubulin, which plays an active role in guiding the binary division of bacteria. The FtsZ is assembled into a Z-ring structure at the future cell division site. The Z ring can promote cytodivision and recruit more than a dozen other division proteins into the Z ring. As the Z ring contraction leads to membrane closure, the cell divides to form two daughter cells (Osawa et al., 2008). The inhibition of the FtsZ by most plant oils is related to GTPase activity, FtsZ binding capacity, Z-ring assembly, and contraction. The majority of FtsZ inhibitory compounds in essential oils are phenylpropanoids and polyphenols. Cinnamaldehyde can bind to the T7 loop in the C-terminal region of the FtsZ monomer, which interferes with the formation of the Z ring and disrupts its morphology in vivo. The formation of the cell membrane is incomplete, and it is in a filamentous structure that is not completely divided (Domadia et al., 2007). By interacting with the GTPase binding cavity, curcumin enhanced the GTPase activity and interfered with the assembly and polymerization of the FtsZ, thereby shortening the steady-state duration of polymer assembly (Duggirala et al., 2014). Totarol is an bacteriostatic and fungistatic active ingredient derived from the plant Rhamnosus pinus. After its treatment, the cell GTPase activity and FtsZ polymerization are inhibited; the cells are filamentous, and the Z-ring assembly is misaligned (Jaiswal et al., 2007). Germarene and gemmarene D-4ol in pine needle essential oil can also bind to the hydrophobic cavity of the FtsZ (Anderson et al., 2012).
3.2.6 Interference with microbial gene expression
Nucleic acid is the material in which genetic information is stored, copied, and transmitted. The expression of genetic material controls the synthesis and metabolism of cellular proteins. Therefore, nucleic acid and gene expression are also important antimicrobial pathways. Listeria monocytogenes were treated with lemongrass essential oil to observe their transcriptome response, and the virulence genes hly and inlj were downregulated in a dose-dependent manner, whereas the fatty acid biosynthesis gene accP was upregulated (Hadjilouka et al., 2017). Fennel essential oil downregulates genes related to ochratoxin A (Nowotarska et al., 2017) biosynthesis in A. niger, thereby reducing OTA levels, but no changes in fungal spore morphology were observed, so fennel essential oil may only inhibit OTA production by reducing the expression of virulence-related genes (El Khoury et al., 2016). The test of citrus essential oil on S. aureus also showed that the expression of cytotoxic genes (comC, comD, gtfB, gffC, and gbpB) was remarkably downregulated, and its main active components, namely, linalool and limonene, indirectly inhibited the production of glucans required for biofilm formation (Benzaid et al., 2021). Transcriptome analysis showed that the indirect exposure of essential oil could affect the expression of Penicillium rubens genes, and essential oil could inhibit the activity of P. rubens by affecting polysaccharide, carbohydrate, fatty acid, nucleotide, and nucleoside metabolism (Kisová et al., 2020).
4 Plant essential oil regulates the fermentation quality of feed silage
The quality of silage feed largely depends on the ability to preserve the nutritional components of the silage raw material. After the plant has been harvested, microbial and plant cell respiration are the main sources of nutrient loss. Therefore, silage should be carried out as soon as possible after the plant is harvested, using lactic acid bacteria to produce organic acids, lower the pH of the silage environment, inhibit the growth of harmful microorganisms, and reduce the loss of raw material nutrients. However, in the early and late stages of plant silage and after the silage feed is opened, the quality of silage feed is often affected by harmful microorganisms such as mold. The characteristic of essential oils that have strong inhibitory effects on mold at equal concentrations but are not remarkably inhibitory to lactic acid bacteria meets the needs of silage. Therefore, plant essential oils can be applied to silage feed to improve silage quality. (Bolsen et al., 1996).
4.1 Inhibition of growth and reproduction of adverse microorganisms in silage
The quality of silage depends on the microbial community of the silage raw material and the succession of microbial colonies during fermentation. However, adverse factors such as loose sealing measures and low sugar content in silage production may lead to changes in microbial community structure in silage affected by Clostridium, yeast, mold and other spoilage microorganisms, thereby reducing the quality of silage (Dunière et al., 2013; Xin et al., 2021). Clostridia can grow in an anaerobic environment, especially in high-moisture forage, and can compete with LAB. It has been reported that Bacteroidetes such as Palidibacter propionicigenes and Prevotella ruminicola have better ability to degrade small molecular substances than Firmicutes. It is an important factor in the loss and degradation of small molecular substances such as monosaccharides, disaccharides and non-cellulosic polysaccharides in silage (Klang et al., 2015). Spoilage microorganisms, such as mold and yeast, usually multiply in large numbers during the secondary fermentation of silage, degrading the nutritional quality of silage, affecting the palatability and producing a series of harmful substances: aflatoxins, ochratoxins, trichothecenes, fumonisins, and mycophenolic acids (Haq et al., 2021). In reducing the growth and reproduction of harmful microorganisms in early silage, adding appropriate amount of plant essential oil to inhibit the growth and reproduction of harmful microorganisms can improve the quality of silage. Several experiments have shown that cinnamon essential oil (C. zeylanicum), thyme essential oil (Thymus mongolicus), oregano essential oil (Origanum minutiflorum) (Foskolos et al., 2016; Çayıroğlu et al., 2020), cumin essential oil (Cuminum cyminum) (Turan and Önenç, 2018), lemon seed essential oil(Citrus limon) (Besharati and Niazifar, 2020), lemongrass essential oil (C. citratus) (Júnior et al., 2020), flaxseed essential oil (Linum usitatissimum), Amomum villosum Lour essential oil (Li et al., 2022), and sweet orange essential oil (Amomum villosum Lour.) (Hodjatpanah-Montazeri et al., 2016; Chaves et al., 2021) significantly inhibited the growth of Proteobacteria, Bacteroidetes and Actinobacteria, which provided a favorable environment for the growth of Firmicutes. The addition of cinnamon essential oil in silage reduced the relative abundance of unfavorable silage bacteria, such as Proteobacteria, Bacteroidetes and Actinobacteria, and increased the relative abundance of Firmicutes (Sheng-tan et al., 2011). After the silage was opened and fermented, the number of fungus and yeast colonies in the silage supplemented with cumin essential oil (Turan and Önenç, 2018), cinnamon essential oil (C. zeylanicum), oregano essential oil (Origanum minutiflorum) and sweet orange essential oil (Citrus sinensis) (Chaves et al., 2012) decreased significantly. The results showed that the addition of plant essential oil inhibited the proliferation of mold and yeast and prevented aerobic decomposition.
4.2 Inhibition of mycotoxin production in silage
Mycotoxins are a group of secondary metabolites secreted by fungal organisms belonging to the genera Aspergillus, Fusarium, Alternaria, and Penicillium. The ingestion of mycotoxins by animals affects feed intake, livestock product production, neurological activity, hormone levels, and immune capacity (Ogunade et al., 2018). Although ruminal microflora of ruminants has a certain mycotoxin degradation capacity, intake of feed containing high levels of mycotoxins will also have adverse effects on animal health and livestock product production because of the saturation of ruminal detoxification capacity (Cheli et al., 2013). Studies have shown that plant essential oils can reduce the content of mycotoxins in silage, and the use of lemongrass (C. citratus), turmeric (C. longa), mint (Mentha canadensis Linnaeus), rosemary (Rosmarinus officinalis), rose grass (Cymbopogon martini), and other essential oil extracts can inhibit the production of a variety of mycotoxins at a certain concentration (Bryła et al., 2022). Hedychium spicatum L. essential oil can remarkably reduce the enol concentration (DON) and zearalenone (ZEA) content of F. deoxynivalene (Kalagatur et al., 2018a). Ylang-ylang essential oil (Cananga odorata (Lam.) Hook. F. and Thomson) at 3.9 mg/g can completely inhibit the production of DON and ZEA mycotoxins in corn containing F. graminearum (Kalagatur et al., 2018b). Further research has found that some plant essential oils can directly reduce mycotoxins and destroy their toxicity. Of these, Cinnamomum cassia is the most efficient at degrading Fumonisin B1, followed by citral (C. limon), S. aromaticum, eucalyptus (Eucalyptus spp.), and camphor (Cinnamomum camphora L.) essential oils. However, lemon, grapefruit, eucalyptus, and palm essential oils had the highest degradation efficiency of ZEA (Perczak et al., 2019). The levels of ochratoxin A (Nowotarska et al., 2017) were below the limit of detection and the levels of ZEA, DON and T-2 toxins (Trichothecenes) were significantly reduced in corn silage treated with 3 mg/kg of oregano (O. vulgare.) ethanol extract and 6 mg/kg of thyme (T. vulgaris) ethanol extract, respectively (Vaičiulienė et al., 2020). When the ethanolic extract mixture of oregano (O. vulgare) and thyme (T. vulgaris) was involved in the silage of whole corn, the levels of ZEA and DON mycotoxins, as well as T-2 toxins, in the two above mentioned plant extract mixtures were remarkably lower than those in the other experimental groups (Vaičiulienė et al., 2022).
4.3 Improving the feeding quality of silage
Reducing the loss of nutrients from plant raw materials is an important role of silage. During silage fermentation, plant nutrients such as protein and starch are partially degraded by plant enzymes into soluble nitrogen and soluble carbohydrates, and this reaction continues until the pH drops below 4.0. Enzymes are key factors in the degradation of nutrients such as protein and starch during silage, among which bacterial enzymes are the main cause of proteolysis (60%), followed by plant proteases (30%); fungal enzymes and fermentation products contribute about 5% of proteolysis during fermentation (Junges et al., 2017). The use of essential oils as a silage additive can indirectly reduce nutrient loss during silage. After adding 200 mg/kg of cumin (C. cyminum) essential oil, the colony structure of wild oat silage changes considerably, and the lactic acid bacteria multiply in large quantities to produce acid, reduce the pH value, and then inhibit the activity of protein degradation enzymes, thereby reducing the degradation of proteins (Akinci and Önenç, 2021). In addition, the results showed that plant oils directly inhibit the activity of bacterial amylases and proteases. Oregano essential oil shows strong inhibitory properties on enzymes that degrade nutrients, such as tyrosinase, α-amylase, and α-glucosidase (Sarikurkcu et al., 2015). Phenylalanine ammonia-lyase can also catalyze the deamination of L-phenylalanine, and this enzyme is an important factor in plant browning. The research results show that phenolic essential oils such as cinnamaldehyde and carvacrol can inhibit the activity of phenylalanine ammonia lyase through hydrogen bridges and ionic or hydrophobic interactions, thereby reducing the loss of feed nutrients (Foskolos et al., 2016). The silage with 50 mL/kg of cinnamon (C. cassia) essential oil showed the lowest ammonia nitrogen level (Hodjatpanah-Montazeri et al., 2016). Cumin (C. cyminum) essential oil, lemongrass (Cymbopogon citratus) essential oil with a low concentration level, lemon seed (C. limon) essential oil, oregano (O. vulgare) essential oil, and flaxseed (L. usitatissimum) essential oil inhibited the production of ammonia nitrogen, propionic acid, and butyric acid during silage (Turan and Önenç, 2018; Çayıroğlu et al., 2020; Besharati and Niazifar, 2020; Besharati et al., 2020; Júnior et al., 2020). Plant essential oil extracts such as cinnamon (Cinnamomum cassia L.) essential oil, lemongrass (C. citratus) essential oil, flaxseed (L. usitatissimum) essential oil, clove (E. caryophyllata Thunb.) essential oil, thyme (T. mongolicus) essential oil, A. villosum Lour essential oil, lemon (C. limon) seed essential oil, and flaxseed (L. usitatissimum) essential oil with appropriate content as silage additives improved the evaluation level of silage and reduced the loss of crude protein, crude fat, and soluble sugar (Hodjatpanah-Montazeri et al., 2016; Turan and Önenç, 2018; Çayıroğlu et al., 2020; Besharati and Niazifar, 2020; Besharati et al., 2020; Júnior et al., 2020; Chaves et al., 2021; Li et al., 2022).
4.4 Improving the aerobic stability of silage
Given its wide application, silage can easily produce secondary fermentation in an aerobic environment after opening the bag (open the cellar), and aerobic microorganisms multiply in large numbers, consume nutrients in the feed, and produce a lot of heat. Therefore, the aerobic stability of silage after prolonged opening of the bag (open the cellar) is important (Wilkinson and Davies, 2013). Plant essential oil remarkably extended the aerobic stability of silage because of its excellent bacteriostatic and fungistatic activity. Studies showed that 0.13 mL/cm2 of oregano(O. vulgare) essential oil was sprayed in beet pulp (Çayıroğlu et al., 2020). Alfalfa was supplemented with 60 mg/kg of lemon seed(C. limon) essential oil or 60 mg/kg of flaxseed(L. usitatissimum), cinnamon(C. cassia) seed, and lemon seed(C. limon) mixed essential oil (Besharati and Niazifar, 2020). The addition of 120 mg/kg of cinnamon essential oil(C. cassia), thyme essential oil(T. vulgaris), oregano essential oil(O. vulgare), or cumin essential oil(C. cyminum) to corn silage can improve the aerobic stability of silage (Hodjatpanah-Montazeri et al., 2016). Cinnamon(C. cassia) essential oil, sweet orange(Citrus sinensis) essential oil, oregano(O. vulgare) essential oil, and thyme(T. vulgaris) essential oil at a level of 120 mg/kg can maintain aerobic stability for 2 weeks (Chaves et al., 2021), whereas the blank control group without added essential oil can only maintain aerobic stability for 72 h. Peppermint(Mentra piperita) essential oil has also been proven to have an inhibitory effect against F. oxysporum and Costomyces in vitro, and the aerobic stability time of silage was prolonged by 50 h after adding peppermint essential oil to silage (Moghaddam et al., 2013).
5 Summary
Plant essential oils have strong inhibitory activity against a variety of adverse microorganisms in silage. Plant essential oil achieves its bacteriostatic and fungistatic ability by inhibiting biofilm formation, changing cell membrane permeability, and interfering with cell division and ATPase activity. The difference in bacteriostatic and fungistatic activity of plant essential oil against some fungi and lactic acid bacteria lays a theoretical foundation for its application as feed additives in silage. The use of appropriate levels of plant essential oils in silage production can control changes in colony structure by inhibiting the growth of some adverse microorganisms such as Clostridium, Fusarium and yeast in silage, and indirectly promote lactic acid bacteria to become dominant microorganisms. Reduce nutrient loss in silage, improve fermentation quality, and improve the aerobic stability of silage after secondary fermentation in the later stage.
Author contributions
LC: Writing – original draft, Writing – review & editing. XL: Writing – original draft, Writing – review & editing. YW: Writing – review & editing. ZG: Writing – review & editing. GW: Writing – review & editing. YZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (32172769).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelgaleil, S. A., El-Sabrout, A. M. (2018). Anti-nutritional, antifeedant, growth-disrupting and insecticidal effects of four plant essential oils on Spodoptera littoralis (Lepidoptera: Noctuidae). J. Crop Prot. 7 (2), 135–150.
Achar, P. N., Quyen, P., Adukwu, E. C., Sharma, A., Msimanga, H. Z., Nagaraja, H., et al. (2020). Investigation of the antifungal and anti-aflatoxigenic potential of plant-based essential oils against Aspergillus flavus in peanuts. J. Fungi. 6 (4), 383. doi: 10.3390/jof6040383
Akinci, Y., Önenç, S. S. (2021). The effect of cumin essential oil on the fermentation quality, aerobic stability, and in vitro digestibility of vetch-oat silages. Ege. Üniversitesi. Ziraat. Fakültesi. Dergisi. 58 (2), 217–228. doi: 10.20289/zfdergi.694965
Anderson, D. E., Kim, M. B., Moore, J. T., O’Brien, T. E., Sorto, N. A., Grove, C. I., et al. (2012). Comparison of small molecule inhibitors of the bacterial cell division protein FtsZ and identification of a reliable cross-species inhibitor. ACS Chem. Biol. 7 (11), 1918–1928. doi: 10.1021/cb300340j
Araújo, D., Ferreira, I. C., Torres, C. A., Neves, L., Freitas, F. (2020). Chitinous polymers: extraction from fungal sources, characterization and processing towards value-added applications. J. Chem. Technol. Biotechnol. 95 (5), 1277–1289. doi: 10.1002/jctb.6325
Avanço, G. B., Ferreira, F. D., Bomfim, N. S., Peralta, R. M., Brugnari, T., Mallmann, C. A., et al. (2017). Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 73, 806–813. doi: 10.1016/j.foodcont.2016.09.032
Baker, L. G., Specht, C. A., Donlin, M. J., Lodge, J. K. (2007). Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryotic. Cell 6 (5), 855–867. doi: 10.1128/EC.00399-06
Benzaid, C., Belmadani, A., Tichati, L., Djeribi, R., Rouabhia, M. (2021). Effect of Citrus aurantium L. Essential oil on Streptococcus mutans growth, biofilm formation and virulent genes expression. Antibiotics 10 (1), 54. doi: 10.3390/antibiotics100
Berridge, M. J., Lipp, P., Bootman, M. D. (2000). The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1 (1), 11–21. doi: 10.1038/35036035
Besharati, M., Niazifar, M.J. (2020). The effect of lemon seed essential oil on composition, chemical characteristics, and gas production parameters of alfalfa silage. J. Anim. Sci. 30 (1), 93–104. doi: 10.22034/AS.2020.11018
Besharati, M., Palangi, V., Niazifar, M., Nemati, Z. (2020). Comparison study of flaxseed, cinnamon and lemon seed essential oils additives on quality and fermentation characteristics of lucerne silage. Acta Agricult. Slovenica. 115 (2), 455–462. doi: 10.14720/aas.2020.115.2.1483
Bo, R., Zhan, Y., Wei, S., Xu, S., Huang, Y., Liu, M., et al. (2023). Tea tree oil nanoliposomes: optimization, characterization, and antibacterial activity against Escherichia coli in vitro and in vivo. Poultry. Sci. 102 (1), 102238. doi: 10.1016/j.psj.2022.102238
Bolsen, K., Ashbell, G., Weinberg, Z. J. (1996). Silage fermentation and silage additives-Review. J. Anim. Sci. 9 (5), 483–494. doi: 10.5713/ajas.1996.483
Bryła, M., Pierzgalski, A., Zapaśnik, A., Uwineza, P. A., Ksieniewicz-Woźniak, E., Modrzewska, M., et al. (2022). Recent research on fusarium mycotoxins in maize—A review. Foods 11 (21), 3465. doi: 10.3390/foods11213465
Çayıroğlu, H., Filik, G., Coşkun, İ., Filik, A. G., Çayan, H., Şahin, A. (2020). Spraying opened sugar beet pulp silage with oregano essential oil helps to sustain quality and stability. South Afr. J. Anim. Sci. 50 (1), 9–16. doi: 10.4314/sajas.v50i1.2
Chan, C.-L., Gan, R.-Y., Shah, N. P., Corke, H. (2018). Polyphenols from selected dietary spices and medicinal herbs differentially affect common food-borne pathogenic bacteria and lactic acid bacteria. Food Control. 92, 437–443. doi: 10.1016/j.foodcont.2018.05.032
Chaudhari, A. K., Singh, V. K., Das, S., Prasad, J., Dwivedy, A. K., Dubey, N. K. (2020). Improvement of in vitro and in situ antifungal, AFB1 inhibitory and antioxidant activity of Origanum majorana L. essential oil through nanoemulsion and recommending as novel food preservative. Food Chem. Toxicol. 143, 111536. doi: 10.1016/j.fct.2020.111536
Chaves, A. V., Baah, J., Wang, Y., McAllister, T. A., Benchaar, C. (2012). Effects of cinnamon leaf, oregano and sweet orange essential oils on fermentation and aerobic stability of barley silage. J. Sci. Food and Agricul. 92 (4), 906–915. doi: 10.1002/jsfa.4669
Chaves, A., Baah, J., Yang, W., McAllister, T., Benchaar, C. (2021). Effect of essential oils used as inoculants on the nutritive value of barley silage. XXI International Grassland Congress and VII International Rangeland Congress: Multifunctional Grassland in Changing World. Inner Mogolia, China, p. 654.
Cheli, F., Campagnoli, A., Dell’Orto, V. (2013). Fungal populations and mycotoxins in silages: From occurrence to analysis. Anim. Feed. Sci. Technol. 183 (1-2), 1–16. doi: 10.1016/j.anifeedsci.2013.01.013
Choonharuangdej, S., Srithavaj, T., Thummawanit, S. (2021). Fungicidal and inhibitory efficacy of cinnamon and lemongrass essential oils on Candida albicans biofilm established on acrylic resin: An in vitro study. J. Prosthetic. Dentistry. 125 (4), 707. e701–707. e706. doi: 10.1016/j.prosdent.2020.12.017
Cock, I., Van Vuuren, S. (2015). South African food and medicinal plant extracts as potential antimicrobial food agents. J. Food Sci. Technol. 52, 6879–6899. doi: 10.1007/s13197-015-1806-3
Couladis, M., Özcan, M., Tzakou, O., Akgül, A. (2003). Comparative essential oil composition of various parts of the turpentine tree (Pistacia terebinthus L) growing wild in Turkey. J. Sci. Food Agriculture 83 (2), 136–138. doi: 10.1002/jsfa.1295
Damtie, D., Mekonnen, Y. (2020). Antibacterial activity of essential oils from Ethiopian thyme (Thymus serrulatus and Thymus schimperi) against tooth decay bacteria. PloS One 15 (10), e0239775. doi: 10.1371/journal.pone.0239775
Das, S., Singh, V. K., Dwivedy, A. K., Chaudhari, A. K., Upadhyay, N., Singh, A., et al. (2019). Antimicrobial activity, antiaflatoxigenic potential and in situ efficacy of novel formulation comprising of Apium graveolens essential oil and its major component. Pesticide. Biochem. Physiol. 160, 102–111. doi: 10.1016/j.pestbp.2019.07.013
de Oliveira Carvalho, I., Purgato, G. A., Píccolo, M. S., Pizziolo, V. R., Coelho, R. R., Diaz-Muñoz, G., et al. (2020). In vitro anticariogenic and antibiofilm activities of toothpastes formulated with essential oils. Arch. Oral. Biol. 117, 104834. doi: 10.1016/j.archoralbio.2020.104834
Dhingra, O. D., Jham, G. N., Barcelos, R. C., Mendonça, F. A., Ghiviriga, I. (2007). Isolation and identification of the principal fungitoxic component of turmeric essential oil. J. Essential Oil Res. 19 (4), 387–391. doi: 10.1080/10412905.2007.9699312
Domadia, P., Swarup, S., Bhunia, A., Sivaraman, J., Dasgupta, D. (2007). Inhibition of bacterial cell division protein FtsZ by cinnamaldehyde. Biochem. Pharmacol. 74 (6), 831–840. doi: 10.1016/j.bcp.2007.06.029
Duggirala, S., Nankar, R. P., Rajendran, S., Doble, M. (2014). Phytochemicals as inhibitors of bacterial cell division protein FtsZ: coumarins are promising candidates. Appl. Biochem. Biotechnol. 174, 283–296. doi: 10.1007/s12010-014-1056-2
Dunière, L., Sindou, J., Chaucheyras-Durand, F., Chevallier, I., Thévenot-Sergentet, D. (2013). Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Technol. 182 (1-4), 1–15. doi: 10.1016/j.anifeedsci.2013.04.006
Dwivedy, A. K., Prakash, B., Chanotiya, C. S., Bisht, D., Dubey, N. K. (2017). Chemically characterized Mentha cardiaca L. essential oil as plant based preservative in view of efficacy against biodeteriorating fungi of dry fruits, aflatoxin secretion, lipid peroxidation and safety profile assessment. Food Chem. Toxicol. 106, 175–184. doi: 10.1016/j.fct.2017.05.043
El Khoury, R., Atoui, A., Verheecke, C., Maroun, R., El Khoury, A., Mathieu, F. (2016). Essential oils modulate gene expression and ochratoxin A production in Aspergillus carbonarius. Toxins 8 (8), 242. doi: 10.3390/toxins8080242
Evangelista, A. G., Corrêa, J. A. F., Pinto, A. C. S. M., Luciano, F. B. (2022). The impact of essential oils on antibiotic use in animal production regarding antimicrobial resistance–a review. Critic. Rev. Food Sci. Nutrit. 62 (19), 5267–5283.
Foskolos, A., Cavini, S., Ferret, A., Calsamiglia, S. (2016). Effects of essential oil compounds addition on ryegrass silage protein degradation. Can. J. Anim. Sci. 96 (2), 100–103. doi: 10.1139/cjas-2015-0025
Gutierrez, J., Rodriguez, G., Barry-Ryan, C., Bourke, P. (2008). Efficacy of plant essential oils against foodborne pathogens and spoilage bacteria associated with ready-to-eat vegetables: antimicrobial and sensory screening. J. Food Prot. 71 (9), 1846–1854. doi: 10.4315/0362-028x-71.9.1846
Gwiazdowska, D., Marchwińska, K., Juś, K., Uwineza, P. A., Gwiazdowski, R., Waśkiewicz, A., et al. (2022). The concentration-dependent effects of essential oils on the growth of Fusarium graminearum and mycotoxins biosynthesis in wheat and maize grain. Appl. Sci. 12 (1), 473. doi: 10.3390/app12010473
Hadjilouka, A., Mavrogiannis, G., Mallouchos, A., Paramithiotis, S., Mataragas, M., Drosinos, E. H. (2017). Effect of lemongrass essential oil on Listeria monocytogenes gene expression. Lwt 77, 510–516. doi: 10.1016/j.lwt.2016.11.080
Hao, W., Zhong, G., Hu, M., Luo, J., Weng, Q., Rizwan-ul-Haq, M. (2010). Control of citrus postharvest green and blue mold and sour rot by tea saponin combined with imazalil and prochloraz. Postharvest Biol. Technol. 56 (1), 39–43. doi: 10.1016/j.postharvbio.2009.10.003
Haq, I. U., Sarwar, M. K., Mohyuddin, Z. (2021). Microbial determinants in silage rotting: A challenge in winter fodders. In Sustainable Winter Fodder (CRC Press LLC, 301–329.
Hodjatpanah-Montazeri, A., Danesh Mesgaran, M., Vakili, A., Tahmasebi, A. (2016). Effect of essential oils of various plants as microbial modifier to alter corn silage fermentation and in vitro methane production. Iranian. J. Appl. Anim. Sci. 6 (2), 269–276.
Hu, L., Wang, D., Liu, L., Chen, J., Xue, Y., Shi, Z. (2013). Ca2+ efflux is involved in cinnamaldehyde-induced growth inhibition of Phytophthora capsici. PloS One 8 (10), e76264. doi: 10.1371/journal.pone.0076264
Imai, H., Osawa, K., Yasuda, H., Hamashima, H., Arai, T., Sasatsu, M. (2001). Inhibition by the essential oils of peppermint and spearmint of the growth of pathogenic bacteria. Microbios 106, 31–39.
Issa, D., Najjar, A., Greige-Gerges, H., Nehme, H. (2019). Screening of some essential oil constituents as potential inhibitors of the ATP synthase of Escherichia coli. J. Food Sci. 84 (1), 138–146. doi: 10.1111/1750-3841.14421
Jaiswal, R., Beuria, T. K., Mohan, R., Mahajan, S. K., Panda, D. (2007). Totarol inhibits bacterial cytokinesis by perturbing the assembly dynamics of FtsZ. Biochemistry 46 (14), 4211–4220. doi: 10.1021/bi602573e
Jiang, N., Wang, L., Jiang, D., Wang, M., Liu, H., Yu, H., et al. (2022). Transcriptomic analysis of inhibition by eugenol of ochratoxin A biosynthesis and growth of Aspergillus carbonarius. Food Control. 135, 108788. doi: 10.1016/j.foodcont.2021.108788
Jiang, Y., Wu, N., Fu, Y.-J., Wang, W., Luo, M., Zhao, C.-J. (2011). Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environment. Toxicol. Pharmacol. 32 (1), 63–68. doi: 10.1016/j.etap.2011.03.011
Joshi, J. R., Khazanov, N., Senderowitz, H., Burdman, S., Lipsky, A., Yedidia, I. (2016). Plant phenolic volatiles inhibit quorum sensing in pectobacteria and reduce their virulence by potential binding to ExpI and ExpR proteins. Sci. Rep. 6 (1), 1–15. doi: 10.1038/srep38126
Ju, J., Guo, Y., Cheng, Y., Yaoc, W. (2022). Analysis of the synergistic antifungal mechanism of small molecular combinations of essential oils at the molecular level. Ind. Crops Products. 188, 115612. doi: 10.1016/j.indcrop.2022.115612
Ju, J., Xie, Y., Yu, H., Guo, Y., Cheng, Y., Zhang, R., et al. (2020). Major components in Lilac and Litsea cubeba essential oils kill Penicillium roqueforti through mitochondrial apoptosis pathway. Ind. Crops Products. 149, 112349. doi: 10.1016/j.indcrop.2020.112349
Juliani, H., Koroch, A., Simon, J. (2009). Chemical diversity of essential oils of Ocimum species and their associated antioxidant and antimicrobial activity. Essential. Oils. Aromas.: Green Extractions. Applications 68, 756–762.
Junges, D., Morais, G., Spoto, M., Santos, P., Adesogan, A., Nussio, L., et al. (2017). Influence of various proteolytic sources during fermentation of reconstituted corn grain silages. J. Dairy. Sci. 100 (11), 9048–9051. doi: 10.3168/jds.2017-12943
Júnior, R. C., Capucho, E., Garcia, T. M., Del Valle, T. A., Campana, M., Zilio, E. M., et al. (2020). Lemongrass essential oil in sugarcane silage: Fermentative profile, losses, chemical composition, and aerobic stability. Anim. Feed. Sci. Technol. 260, 114371. doi: 10.1016/j.anifeedsci.2019.114371
Kalagatur, N. K., Kamasani, J. R., Siddaiah, C., Gupta, V. K., Krishna, K., Mudili, V. (2018a). Combinational inhibitory action of Hedychium spicatum L. essential oil and γ-radiation on growth rate and mycotoxins content of Fusarium graminearum in maize: response surface methodology. Front. Microbiol. 9, 1511. doi: 10.3389/fmicb.2018.01511
Kalagatur, N. K., Mudili, V., Kamasani, J. R., Siddaiah, C. (2018b). Discrete and combined effects of Ylang-Ylang (Cananga odorata) essential oil and gamma irradiation on growth and mycotoxins production by Fusarium graminearum in maize. Food Control. 94, 276–283. doi: 10.1016/j.foodcont.2018.07.030
Kang, J., Jin, W., Wang, J., Sun, Y., Wu, X., Liu, L. (2019). Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. Lwt 101, 639–645. doi: 10.1016/j.lwt.2018.11.093
Kant, R., Kumar, A. (2022). Review on essential oil extraction from aromatic and medicinal plants: Techniques, performance and economic analysis. Sustain. Chem. Pharm. 30, 100829. doi: 10.1016/j.scp.2022.100829
Kazem, A. R., Sharifan, A., Aghazadeh, M. M. (2011). Study of chemical composition and antimicrobial activity of peppermint essential oil. J. Comparat. Pathobiol. Iran 7 (4), 355–63.
Khalil, A. A., ur Rahman, U., Khan, M. R., Sahar, A., Mehmood, T., Khan, M. (2017). Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC. Adv. 7 (52), 32669–32681. doi: 10.1039/C7RA04803C
Khan, A., Ahmad, A., Akhtar, F., Yousuf, S., Xess, I., Khan, L. A., et al. (2010). Ocimum sanctum essential oil and its active principles exert their antifungal activity by disrupting ergosterol biosynthesis and membrane integrity. Res. Microbiol. 161 (10), 816–823. doi: 10.1016/j.resmic.2010.09.008
Khan, S. T., Khan, M., Ahmad, J., Wahab, R., Abd-Elkader, O. H., Musarrat, J., et al. (2017). Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. Amb Express 7 (1), 1–11. doi: 10.1186/s13568-017-0344-y
Khorsandi, A., Ziaee, E., Shad, E., Razmjooei, M., Eskandari, M. H., Aminlari, M., et al. (2018). Antibacterial effect of essential oils against spoilage bacteria from vacuum-packed cooked cured sausages. J. Food Protect. 81 (8), 1386–1393. doi: 10.4315/0362-028x.jfp-17-474
Kim, H.-S., Park, H.-D. (2013). Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14. PloS One 8 (9), e76106. doi: 10.1371/journal.pone.0076106
Kim, M., Sowndhararajan, K., Kim, S. J. M. (2022). The chemical composition and biological activities of essential oil from Korean native thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.). Molecules 27 (13), 4251. doi: 10.3390/molecules27134251
Kisová, Z., Šoltýsová, A., Bučková, M., Beke, G., Puškárová, A., Pangallo, D. (2020). Studying the gene expression of Penicillium rubens under the effect of eight essential oils. Antibiotics 9 (6), 343. doi: 10.3390/antibiotics9060343
Klang, J., Theuerl, S., Szewzyk, U., Huth, M., Tölle, R., Klocke, M. (2015). Dynamic variation of the microbial community structure during the long-time mono-fermentation of maize and sugar beet silage. Microbial. Biotechnol. 8 (5), 764–775. doi: 10.1111/1751-7915.12263
Konuk, H., Ergüden, B. (2017). Antifungal activity of various essential oils against Saccharomyces cerevisiae depends on disruption of cell membrane integrity. Biocell 41 (1), 13. doi: 10.32604/biocell.2017.41.013
Lee, H.-S. (2006). Antimicrobial properties of turmeric (Curcuma longa L.) rhizome-derived ar-turmerone and curcumin. Food Sci. Biotechnol. 15 (4), 559–563.
Li, M., Fan, X., Cheng, Q., Chen, Y., Long, J., Lei, Y., et al. (2022). Effect of Amomum villosum essential oil as an additive on the chemical composition, fermentation quality, and bacterial community of paper mulberry silage. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.951958
Li, W.-R., Ma, Y.-K., Shi, Q.-S., Xie, X.-B., Sun, T.-L., Peng, H., et al. (2018b). Diallyl disulfide from garlic oil inhibits Pseudomonas aeruginosa virulence factors by inactivating key quorum sensing genes. Appl. Microbiol. Biotechnol. 102, 7555–7564. doi: 10.1007/s00253-018-9175-2
Li, Q. Q., Tsai, H. F., Mandal, A., Walker, B. A., Noble, J. A., Fukuda, Y., et al. (2018a). Sterol uptake and sterol biosynthesis act coordinately to mediate antifungal resistance in Candida glabrata under azole and hypoxic stress. Mol. Med. Rep. 17 (5), 6585–6597. doi: 10.3892/mmr.2018.8716
Liu, Y., Wu, L., Han, J., Dong, P., Luo, X., Zhang, Y., et al. (2021). Inhibition of biofilm formation and related gene expression of Listeria monocytogenes in response to four natural antimicrobial compounds and sodium hypochlorite. Front. Microbiol. 11, 617473. doi: 10.3389/fmicb.2020.617473
Lucas-Gonzalez, R., Yilmaz, B., Khaneghah, A. M., Hano, C., Shariati, M. A., Bangar, S. P., et al. (2023). Cinnamon: An antimicrobial ingredient for active packaging. Food Packaging. Shelf. Life 35, 101026. doi: 10.1016/j.fpsl.2023.101026
Mahboubi, M., Kazempour, N. (2014). Chemical composition and antimicrobial activity of peppermint (Mentha piperita L.) Essential oil. Songklanakarin. J. Sci. Technol. 36 (1), 83–87.
Marchese, A., Barbieri, R., Coppo, E., Orhan, I. E., Daglia, M., Nabavi, S. F., et al. (2017). Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 43 (6), 668–689. doi: 10.1080/1040841X.2017.1295225
Marchese, A., Orhan, I. E., Daglia, M., Barbieri, R., Di Lorenzo, A., Nabavi, S. F., et al. (2016). Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 210, 402–414. doi: 10.1016/j.foodchem.2016.04.111
Marinelli, L., Di Stefano, A., Cacciatore, I. (2018). Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 17, 903–921. doi: 10.1007/s11101-018-9569-x
Matté, E. H. C., Luciano, F. B., Evangelista, A. G. (2023). Essential oils and essential oil compounds in animal production as antimicrobials and anthelmintics: an updated review. Anim. Health Res. Rev. 24 (1), 1–11. doi: 10.1017/S1466252322000093
Milovanović, I. L., Mišan, A.Č., Sakač, M. B., Čabarkapa, I. S., Šarić, B. M., Matić, J. J. (2009). Evaluation of a GC-MS method for the analysis of oregano essential oil composition. Food Feed Res. 36 (3-4), 75–79.
Moghaddam, M., Pourbaige, M., Tabar, H. K., Farhadi, N., Hosseini, S. M. A. (2013). Composition and antifungal activity of peppermint (Mentha piperita) essential oil from Iran. J. Essential. Oil Bearing. Plants 16 (4), 506–512. doi: 10.1080/0972060X.2013.813265
Muñoz-González, R., Pino, C., Henríquez, H., Villanueva, F., Riquelme, A., Montealegre, R., et al. (2022). Elucidation of antimicrobial and antioxidant activities of selected plant-based mayonnaise-derived essential oils against lactic acid bacteria. J. Food Process. Preservation. 46 (3), e16339. doi: 10.1111/jfpp.16339
Nematollahi, Z., Ebrahimi, M., Raeisi, M., Dadban Shahamat, Y., Ghodsi Moghadam, M., Hashemi, M., et al. (2020). The antibacterial activity of cinnamon essential oil against foodborne bacteria: A mini-review. J. Hum. Environ. Health Promotion. 6 (3), 101–105. doi: 10.29252/jhehp.6.3.1
Nikkhah, M., Hashemi, M., Najafi, M. B. H., Farhoosh, R. (2017). Synergistic effects of some essential oils against fungal spoilage on pear fruit. Int. J. Food Microbiol. 257, 285–294. doi: 10.1016/j.ijfoodmicro.2017.06.021
Nowotarska, S. W., Nowotarski, K., Grant, I. R., Elliott, C. T., Friedman, M., Situ, C. J. F. (2017). Mechanisms of antimicrobial action of cinnamon and oregano oils, cinnamaldehyde, carvacrol, 2, 5-dihydroxybenzaldehyde, and 2-hydroxy-5-methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map). Foods 6 (9), 72. doi: 10.3390/foods6090072
Ogunade, I., Martinez-Tuppia, C., Queiroz, O., Jiang, Y., Drouin, P., Wu, F., et al. (2018). Silage review: Mycotoxins in silage: Occurrence, effects, prevention, and mitigation. J. Dairy Sci. 101 (5), 4034–4059. doi: 10.3168/jds.2017-13788
Osawa, M., Anderson, D. E., Erickson, H. P. (2008). Reconstitution of contractile FtsZ rings in liposomes. Science 320 (5877), 792–794. doi: 10.1126/science.1154520
Ownagh, A. O., Hasani, A., Mardani, K., Ebrahimzadeh, S. (2010). Antifungal effects of thyme, agastache and satureja essential oils on Aspergillus fumigatus, Aspergillus flavus and Fusarium solani. In Veterinary Research Forum.Faculty of Veterinary Medicine, Urmia University. 1 (2), 99–105.
Pang, D., Huang, Z., Li, Q., Wang, E., Liao, S., Li, E., et al. (2021). Antibacterial mechanism of cinnamaldehyde: modulation of biosynthesis of phosphatidylethanolamine and phosphatidylglycerol in Staphylococcus aureus and Escherichia coli. J. Agric. Food Chem. 69 (45), 13628–13636. doi: 10.1021/acs.jafc.1c04977
Parks, L. W., Casey, W. M. (1995). Physiological implications of sterol biosynthesis in yeast. Annu. Rev. Microbiol. 49 (1), 95–116. doi: 10.1146/annurev.mi.49.100195.000523
Perczak, A., Juś, K., Gwiazdowska, D., Marchwińska, K., Waśkiewicz, A. (2019). The efficiency of deoxynivalenol degradation by essential oils under in vitro conditions. Foods 8 (9), 403. doi: 10.3390/foods8090403
Pootong, A., Norrapong, B., Cowawintaweewat, S. (2017). Antifungal activity of cinnamaldehyde against Candida albicans. Southeast. Asian J. Trop. Med. Public Health 48 (1), 150–158.
Procopio, F. R., Ferraz, M. C., do Prado-Silva, L., Paulino, B. N., Sant’Ana, A. S., Pastore, G. M., et al. (2022). Antifungal synergistic effect of paprika and cinnamon oleoresins and their coencapsulation by spray chilling technique to produce a carotenoid-cinnamaldehyde-rich food powder. Food Bioprocess. Technol. 15 (12), 2826–2838. doi: 10.1007/s11947-022-02918-w
PubChem. Available at: https://pubchem.ncbi.nlm.nih.gov/.
Rathinam, P., Vijay Kumar, H., Viswanathan, P. (2017). Eugenol exhibits anti-virulence properties by competitively binding to quorum sensing receptors. Biofouling 33 (8), 624–639. doi: 10.1080/08927014.2017.1350655
Rathod, N. B., Kulawik, P., Ozogul, F., Regenstein, J. M., Ozogul, Y. (2021). Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends Food Sci. Technol. 116, 733–748. doi: 10.1016/j.tifs.2021.08.023
Saad, N. Y., Muller, C. D., Lobstein, A. J. F., Journal, F. (2013). Major bioactivities and mechanism of action of essential oils and their components. Flavour Fragrance J. 28 (5), 269–279. doi: 10.1002/ffj.3165
Sameza, M. L., Nguemnang Mabou, L. C., Tchameni, S. N., Boat Bedine, M. A., Tchoumbougnang, F., Jazet Dongmo, P. M., et al. (2016). Evaluation of clove essential oil as a mycobiocide against Rhizopus stolonifer and Fusarium solani, tuber rot causing fungi in yam (Dioscorea rotundata Poir.). J. Phytopathol. 164 (7-8), 433–440. doi: 10.1111/jph.12468
Sarikurkcu, C., Zengin, G., Oskay, M., Uysal, S., Ceylan, R., Aktumsek, A. (2015). Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crops Products. 70, 178–184. doi: 10.1016/j.indcrop.2015.03.030
Schmidt, M., Strenk, M. E., Boyer, M. P., Fritsch, B. J. (2005). Importance of cell wall mannoproteins for septum formation in Saccharomyces cerevisiae. Yeast 22 (9), 715–723. doi: 10.1002/yea.1242
Shah, S., Hafeez, M., Wu, M.-Y., Zhang, S.-S., Ilyas, M., Wu, G., et al. (2020). Downregulation of chitin synthase A gene by diallyl trisulfide, an active substance from garlic essential oil, inhibits oviposition and alters the morphology of adult Sitotroga cerealella. J. Pest Sci. 93, 1097–1106. doi: 10.1007/s10340-020-01226-6
Shao, F., Chen, J., Li, H. D., Liu, X. J., Li, W., Zhen, J. (2020). Research progress on extraction methods, biological functions and applications of plant essential oils. Feed. Res. (in Chinese). 45 (15), 119–123. doi: 10.13557/j.cnki.issn1002-2813.2022.15.026
Sharma, A., Rajendran, S., Srivastava, A., Sharma, S., Kundu, B. (2017). Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioengineer. 123 (3), 308–313. doi: 10.1016/j.jbiosc.2016.09.011
Shen, S., Zhang, T., Yuan, Y., Lin, S., Xu, J., Ye, H. (2015). Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control. 47, 196–202. doi: 10.1016/j.foodcont.2014.07.003
Shen, S., Zhou, R., Li, Y., Liu, B., Pan, G., Liu, Q., et al. (2019). Bacterium, fungus, and virus microorganisms for energy storage and conversion. Small. Methods 3 (12), 1900596. doi: 10.1002/smtd.201900596
Sheng-tan, Z., Zhao-yu, W., Tie-shan, W., Miao-Xia, L., Jing-ming, L. (2011). Composition and antimicrobial activities of essential oil of fructus Amomi. Natural Product. Res. Dev. 23 (3), 464–472.
Shreaz, S., Wani, W. A., Behbehani, J. M., Raja, V., Irshad, M., Karched, M., et al. (2016). Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 112, 116–131. doi: 10.1016/j.fitote.2016.05.016
Tan, Y., Leonhard, M., Moser, D., Ma, S., Schneider-Stickler, B. (2019). Antibiofilm efficacy of curcumin in combination with 2-aminobenzimidazole against single-and mixed-species biofilms of Candida albicans and Staphylococcus aureus. Colloids. And. Surfaces. B.: Biointerfaces. 174, 28–34. doi: 10.1016/j.colsurfb.2018.10.079
Topa, S. H., Subramoni, S., Palombo, E. A., Kingshott, P., Rice, S. A., Blackall, L. L. (2018). Cinnamaldehyde disrupts biofilm formation and swarming motility of Pseudomonas aeruginosa. Microbiology 164 (9), 1087–1097. doi: 10.1099/mic.0.000692
Trinh, N.-T.-T., Dumas, E., Thanh, M. L., Degraeve, P., Amara, C. B., Gharsallaoui, A., et al. (2015). Effect of a Vietnamese Cinnamomum cassia essential oil and its major component trans-cinnamaldehyde on the cell viability, membrane integrity, membrane fluidity, and proton motive force of Listeria innocua. Can. J. Microbiol. 61 (4), 263–271. doi: 10.1139/cjm-2014-0481
Turan, A., Önenç, S. S. (2018). Effect of cumin essential oil usage on fermentation quality, aerobic stability and in vitro digetibility of alfalfa silage. Asian-Australasian. J. Anim. Sci. 31 (8), 1252. doi: 10.5713/ajas.17.0834
Vaičiulienė, G., Bakutis, B., Jovaišienė, J., Falkauskas, R., Gerulis, G., Baliukonienė, V. (2020). Origanum vulgare and thymus vulgaris extract usability to improve silage hygienic quality and reduce mycotoxin concentrations. doi: 10.4014/jmb.2003.03010
Vaičiulienė, G., Bakutis, B., Jovaišienė, J., Falkauskas, R., Gerulis, G., Bartkienė, E., et al. (2022). Effects of ethanol extracts of origanum vulgare and thymus vulgaris on the mycotoxin concentrations and the hygienic quality of maize (Zea mays L.) Silage. Toxins 14 (5), 298. doi: 10.3390/toxins140502
Vasconcelos, N., Croda, J., Simionatto, S.J.M.p. (2018). Antibacterial mechanisms of cinnamon and its constituents: A review. Microbial Pathogenesis 120, 198–203. doi: 10.1016/j.micpath.2018.04.036
Wan, C., Chen, C., Li, M., Yang, Y., Chen, M., Chen, J. J. P. (2017). Chemical constituents and antifungal activity of Ficus hirta Vahl. fruits. Plants 6 (4), 44. doi: 10.3390/plants6040044
Wang, L., Jin, J., Liu, X., Wang, Y., Liu, Y., Zhao, Y., et al. (2018). Effect of cinnamaldehyde on morphological alterations of Aspergillus ochraceus and expression of key genes involved in ochratoxin a biosynthesis. Toxins 10 (9), 340. doi: 10.3390/toxins10090340
Wilkinson, J., Davies, D. (2013). The aerobic stability of silage: key findings and recent developments. Grass. Forage. Sci. 68 (1), 1–19. doi: 10.1111/j.1365-2494.2012.00891.x
Wright, B. S., Bansal, A., Moriarity, D. M., Takaku, S., Setzer, W. N. (2007). Cytotoxic leaf essential oils from Neotropical Lauraceae: Synergistic effects of essential oil components. Natural Product. Commun. 2 (12), 1934578X0700201210. doi: 10.1177/1934578X0700201210
Xin, Y.-F., Chen, C., Zeng, T.-R., Du Zhao-Chang, N., Zhong, Y.-H., Tan, X.-P., et al. (2021). Research progress in the effects of additives to silage on microbial diversity. Biotechnol. Bull. 37 (9), 24.
Yang, Y., Li, Y., Mei, X., Yang, M., Huang, H., Du, F., et al. (2022). Antimicrobial terpenes suppressed the infection process of phytophthora in fennel-pepper intercropping system. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.890534
Yen, T.-B., Chang, S.-T. (2008). Synergistic effects of cinnamaldehyde in combination with eugenol against wood decay fungi. Bioresource. Technol. 99 (1), 232–236. doi: 10.1016/j.biortech.2006.11.022
Yu, H. H., Song, Y. J., Yu, H. S., Lee, N. K., Paik, H. D. (2020). Investigating the antimicrobial and antibiofilm effects of cinnamaldehyde against Campylobacter spp. using cell surface characteristics. J. Food Sci. 85 (1), 157–164. doi: 10.1111/1750-3841.14989
Zapata, P., Navarro, D., Guillén, F., Castillo, S., Martínez-Romero, D., Valero, D. (2013). Characterisation of gels from different Aloe spp. as antifungal treatment: Potential crops for industrial applications. Industrial Crops Products 42, 223–230. doi: 10.1016/j.indcrop.2012.06.002
Zhang, Y., Liu, X., Wang, Y., Jiang, P., Quek, S. (2016). Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 59, 282–289. doi: 10.1016/j.foodcont.2015.05.032
Keywords: essential oil, active ingredient, bacteriostatic, fungistatic, silage
Citation: Chen L, Li X, Wang Y, Guo Z, Wang G and Zhang Y (2023) The performance of plant essential oils against lactic acid bacteria and adverse microorganisms in silage production. Front. Plant Sci. 14:1285722. doi: 10.3389/fpls.2023.1285722
Received: 30 August 2023; Accepted: 25 October 2023;
Published: 10 November 2023.
Edited by:
Siran Wang, Nanjing Agricultural University, ChinaReviewed by:
Margarita Terentjeva, Latvia University of Agriculture, LatviaSeverino Zara, University of Sassari, Italy
Adnane Remmal, Sidi Mohamed Ben Abdellah University, Morocco
Copyright © 2023 Chen, Li, Wang, Guo, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunhua Zhang, eXVuaHVhOTY4MUAxNjMuY29t
 Lijuan Chen
Lijuan Chen Xi Li
Xi Li Yili Wang
Yili Wang Zelin Guo
Zelin Guo Guoming Wang
Guoming Wang Yunhua Zhang
Yunhua Zhang