- 1Institute of Environment and Sustainable Development in Agriculture, Chinese Academy of Agricultural Sciences, Beijing, China
- 2Shandong Agro-tech Extension Center, Jinan, China
- 3Institute of Dongying Shengli Salt Alkali Agriculture Industrialization and Technology Research, Dongying, China
Introduction: Salt stress in seed germination and early seedling growth is the greatest cause of crop loss in saline-alkali soils. Microbial seed coating is an effective way to promote plant growth and salt resistance, but these coatings suffer from poor seed adhesion and low survival rates under typical storage conditions.
Methods: In this study, the marine bacterium Pontibacter actiniarum DSM 19842 from kelp was isolated and microencapsulated with calcium alginate using the emulsion and internal gelation method.
Results: Compared to unencapsulated seeds, the spherical microcapsules demonstrated a bacterial encapsulation rate of 65.4% and survival rate increased by 22.4% at 25°C for 60 days. Under salt stress conditions, the seed germination percentage of microcapsule-embedded bacteria (M-Embed) was 90%, which was significantly increased by 17% compared to the germination percentage (73%) of no coating treatment (CK). Root growth was also significantly increased by coating with M-Embed. Chlorophyll, peroxidase, superoxide dismutase, catalase, proline, hydrogen peroxide and malondialdehyde levels indicated that the M-Embed had the best positive effects under salt stress conditions.
Discussion: Therefore, embedding microorganisms in suitable capsule materials provides effective protection for the survival of the microorganism and this seed coating can alleviate salt stress in wheat. This process will benefit the development of sustainable agriculture in coastal regions with saline soils.
Introduction
Biotic and abiotic stressors in the environment have become major factors limiting global crop growth and production and increased soil salinization has become a primary factor that decreases growth and production (Tavakkoli et al., 2011; Abideen et al., 2020). Saline environments harm plants through physiological drought (Dantas et al., 2019), ion dysregulation (Habib et al., 2016) and, oxidative stress (Yang and Guo, 2018) and leads to a generated metabolic disorder (Zhang et al., 2021). Seed germination and seedling growth are the most vulnerable periods in the seed life process and germination is easily disturbed by numerous stressors that in turn, affect seedling growth and final yield and quality (Smolikova and Medvedev, 2022). Therefore, successful seed germination and healthy seedling growth is a necessity for sustained crop production.
Under salt stress, plants reduce the toxic effects of salt on plants through numerous mechanisms including activation of antioxidant enzymes and synthesis of antioxidant compounds, ion homeostasis, biosynthesis of osmoprotectants and hormonal regulation (Sharaya et al., 2023). Microorganisms in the soil can also improve plant growth under salt stress via of the action of phytohormones, osmotic regulators, antioxidant enzymes and exopolysaccharide production (Choudhary et al., 2022). Additionally, seeds can be coated with microorganisms to improve performance and reduce production costs. This has been an effective method to reduce damage from abiotic stressors (Maity et al., 2019; Soumare et al., 2020). Microbial seed coating can ensure healthy seeds, improve germination, provide beneficial microorganisms that contributes to sustainable agriculture (Pedrini et al., 2017; Rocha et al., 2019; Zvinavashe et al., 2021). For instance, rhizobacteria such as Rhizobium tropici CIAT 899 have been used with the bean (Phaseolus vulgaris) as a model system to demonstrate that rhizobacteria delivered to the soil after coating dissolution are able to infect seedling roots, form root nodules, enhance yields, boost germination, and mitigate the effects of soil salinity (Zvinavashe et al., 2019). However, these coatings are insufficient to ensure microbial survival, are not stable for long term storage and are susceptible to environmental influences (Ma et al., 2010).
Microencapsulation is a novel technology to encapsulate microorganisms on a microscopic scale for their immobilization and protection. These provide a microenvironment that is less disturbed by adverse external environmental factors and can preserve viability (Kim et al., 2012). One such capsule material is the non-toxic and biocompatible alginate (Krasaekoopt et al., 2003) and improved microbial survival under salt stress was enhanced when applied as a seed coating agent to encapsulate Pseudomonas fluorescens VUPF506. This combination also successfully improved disease resistance and yield of potatoes (Fathi et al., 2021). Cotton seeds encapsulated with Bacillus subtilis SL-13 significantly improved cotton growth indicators including germination rate, fresh and dry weights and increased levels of the antioxidant enzymes peroxidase (POD) and superoxide dismutase (SOD) and accordingly, significantly decreased malondialdehyde (MDA) content (Tu et al., 2016).
The wheat (Triticum aestivum L) is one of the three major food crops in the world and is sensitive to salt levels. High saline soils retard its growth and delay development so that quality and yield suffer (Huang et al., 2023). The marine kelp (Laminaria japonica) is a widely distributed oceanic brown alga that is fast growing and highly adaptable to changing environmental conditions. Microorganisms associated with kelp are highly salt tolerant and can adapt to saline areas in coastal regions. Kelp-associated bacteria were able to mitigate the effects of salt stress on the growth and yield of rice (Rima et al., 2018). Therefore, microorganisms from kelp have potential uses as microbial seed coatings.
The objective of this study was to extract bacteria from kelp, encapsulate them by endogenous emulsification and prepare them as microbial seed coating. We then evaluated germination, biomass, chlorophyll content and indicators of oxidative stress in plants cultivated in the presence of 100 mM/L NaCl. This study provides information for the successful application of microbial microencapsulated seed coatings.
Materials and methods
Microorganisms and culture medium
Bacteria were isolated from fresh kelp as previously reported with some modifications (Li et al., 2022). In brief, 10 g of fresh kelp were cut into 0.5 cm2 pieces and added to 90 mL enrichment medium (5 g (NH4)2SO4, 15 g NaCl/L pH 7.5) in a 500 mL triangular bottle and incubated at 30°C with shaking at 180 rpm for 3 d until the plant pieces were visibly degraded. Samples (10 mL) were then transferred to another 500 mL bottle containing 90 mL of the same medium and incubated under the same conditions. The transfer and incubation process were again repeated for a total of 3 times. Dilutions of the enriched bacterial broth were plated on 1.5% agar isolation plates containing 1 g peptone, 1 g yeast paste, 5 g (NH4)2SO4, 15 g NaCl, pH 7.5 and incubated at 30°C for 1-2 d. Colonies were picked and purified 3 × using streak-plating until a single pure colony was obtained. The isolates were preserved and submitted to the commercial company Allwegene (Beijing, China) for 16S rDNA sequencing to identify the bacterial species.
Preparation of the microcapsules
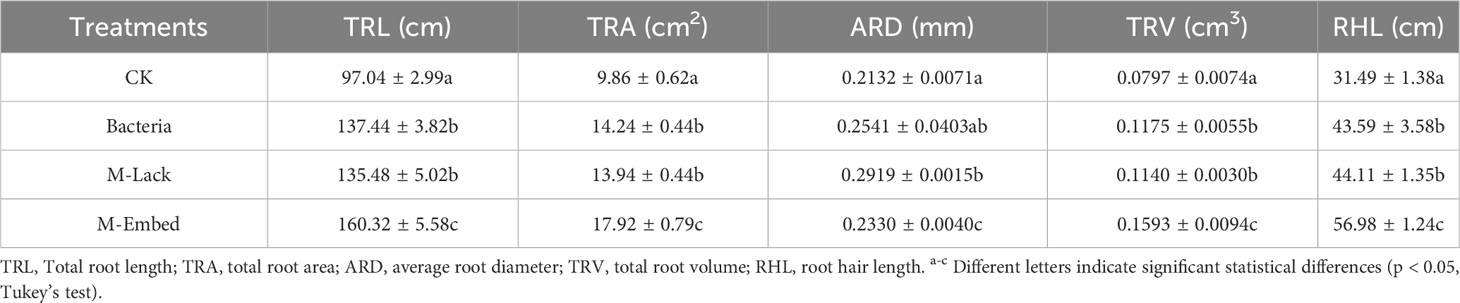
Bacterial microencapsulation was performed by a modification of the emulsion method as reported previously (Qi et al., 2019). Briefly, 50 mL of sterile 2% (w/v) potassium alginate and 5 mL of bacterial suspension (108 colony-forming units (CFU) mL-1) were mixed with 0.5 g calcium carbonate and homogenized. The mixture was dispersed into a 100 mL soybean oil phase that contained 1% (w/v) Span 80 and then was emulsified by stirring at 400 rpm for 5 min. Glacial acetic acid (0.25 mL) in 50 mL soybean oil was then added and stirring was continued for 10 min. After standing and stratifying, the aqueous (lower) phase was removed and centrifuged, washed with phosphate buffer and dried to obtain microcapsules. The oil layer on the top phase was harvested by aspiration and centrifuged for the next use. Hollow microcapsules lacking bacteria were harvested and dried in the same way and used as a control (Figure 1A).
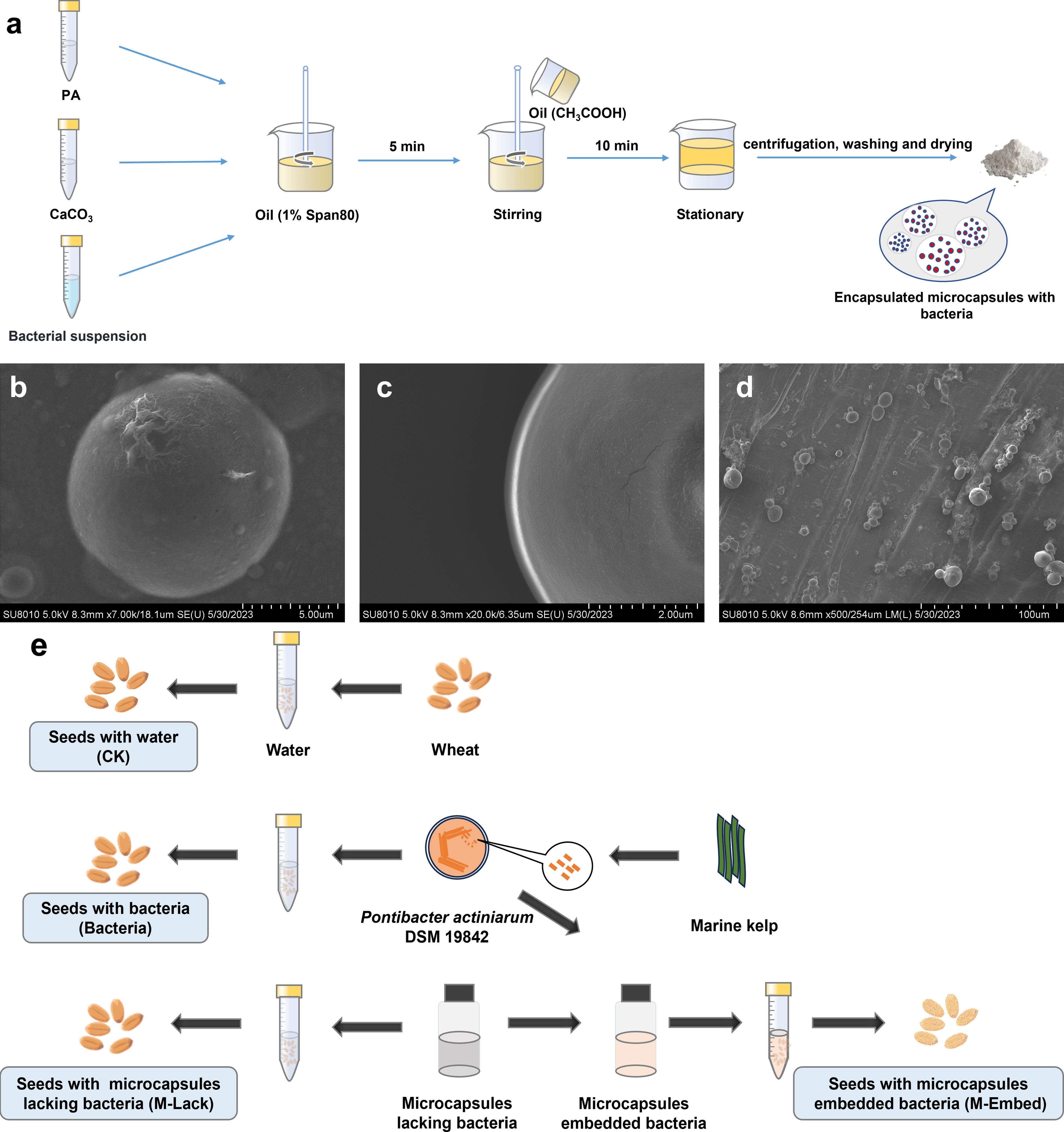
Figure 1 (A) Schematic of the process of preparing microcapsules by emulsion and internal gelation. (B, C) SEM of microbeads loaded with P. actiniarum DSM 19842. (D) SEM of wheat seeds dipped in microcapsules. (E) Illustration of the seed coating protocol.
Calculation of bacterial embedding levels
1.0 g of microcapsules was weighed into 9 mL of NaH2PO4 solution (0.1 mol/L, NaH2PO4, pH 7.0) and shaken at a constant temperature of 37°C for 1 h to dissolve the microcapsules and release the encapsulated bacteria. After a series of 10-fold gradient dilutions, the appropriate dilution of the bacterial suspension was spread on the agar growth medium and incubated at 37°C for 48 h to determine the number of colony-forming units (CFU). The embedding levels were calculated as follows (Fareez et al., 2015):
where Nu represented CFU following capsule breakage and Nt the CFU of the bacterial solution prior to encapsulation.
Scanning electron microscopy
Microcapsule morphology was observed using a JSM-7401F scanning electron microscope (SEM) (JEOL, Tokyo, Japan). Aqueous dispersions of the samples were dropped onto clean silicon wafers and air-dried and plated with platinum using an ETD-800 sputter coater (Beijing, China). The sample area not covered by the carrier was selected to observe the morphology of the nanoparticles. Statistical particle sizes based on SEM images were calculated using Nano Measurer (https://nano-measurer.updatestar.com/en) software.
Determination of microcapsule particle size
Microcapsule particle sizes were measured using a Mastersizer 2000 laser particle size analyzer (Malvern Panalytical, Malvern, UK). The magnitude of the span value indicated the degree of dispersion of the particle size and was determined as follows (Lemos et al., 2017):
where D10, D50 and D90 represented the particle size values corresponding to 10%, 50% and 90% on the cumulative percent particle size distribution curve.
Microcapsule storage stability
The microbial microcapsules prepared by emulsion and internal gelation were placed at room temperature (20-25°C) for storage and samples were taken every 10 d for CFU counting as per above.
Wheat planting experiments
The wheat (Triticum aestivum L., Jimai 22) seeds were sterilized with 1% NaClO for 10 min and then rinsed 3 × with dH2O. Full-grained seeds were planted in pots with 50 seeds per pot (diameter 15cm, height 13cm). The salt stress experiment utilized the same concentration gradient of salt stress as that used in (Wahid et al., 2021). In the experiment, one third height of the pots with substrate were put into a solution of 100 mM/L NaCl (11.2 dS/m), through capillary movement, the NaCl uniformly distributed in the pots. The seeds were then planted. Seedlings were grown at 25°C or 20°C (day or night) with a 12 h photoperiod at a light intensity of 240 μmol m-2 s-1.
The seed coating experiments included (i) Wheat seeds with water (CK), (ii) Wheat seeds with bacteria (Bacteria), (iii) Wheat seeds with microcapsules lacking bacteria (M-Lack) and (iv) Wheat seeds with microcapsules embedded bacteria (M-Embed) (Figure 1E). Four replicates were used for each treatment, all of which were randomly arranged and rotated periodically to minimize the effects of environmental heterogeneity.
Plant growth indicators
Wheat germination rate (%) was recorded after 7 d treatment under salt stress. The germination rate was calculated as:
where G is the germination rate (%), parameter a is the number of germinating seeds, and b is the total number of seeds from the germination test.
4 plants were randomly selected from each treatment. The plants were divided into above-ground and below-ground parts from the rootstock union and the above-ground parts were measured for fresh weight. Above-ground dry weight was measured after drying the samples in an oven at 70°C for 48 h. The intact wheat roots were scanned and analyzed using WinRHIZO (Regent Instruments, Québec, Canada) to quantify root morphology using the protocol of the manufacturer.
Plant physiological indicators
Following 7 d under salt stress, 4 plants were randomly selected from each treatment to determine the physiological indicators. The content of physiological indicators is calculated by measuring the absorbance at the corresponding wavelength. Absorbance was measured three times per sample. SOD (absorbance at 450 nm), POD (absorbance at 470 nm), CAT (absorbance at 510 nm), PRO (absorbance at 520 nm), MDA (difference between 600 and 520 absorbance) and H2O2 (absorbance at 415 nm) levels were determined using commercial kits (Suzhou Grace Biotechnology, Suzhou, China) (He et al., 2022). Seven days after planting, wheat samples were collected, rapidly frozen in liquid nitrogen and ground into fine powders (40 Hz, 1 min). A total of 100 mg of each sample was dissolved in Kit-provided PBS. The mixture was then vortexed for 10 min and centrifuged for 5 min (12000 rpm, at 4°C). The supernatants were used for enzyme activity assessments using a 96-well Microplate Reader (Thermo Scientific, Pittsburg, PA, USA) at specific wavelengths as described in the kit instructions. Chlorophyll (Chl) was determined using a model TYS-B portable chlorophyll meter (Zhejiang TOP Cloud-Agri Technology, Zhejiang, China). Three replicates were carried out for each treatment.
Statistical analysis
All the data were analyzed using SPSS 21 (IBM. Armonk, NY, USA). The data were analyzed using two-tailed Student’s t test for single comparisons. The differences between experimental treatments were evaluated by the least significant difference test (LSD) at p < 0.05. GraphPad (GraphPad, Software, USA) was used to create the graphs. Mean values and standard errors (SE) are presented in figures and tables. Structural equation modeling was used to explore possible pathways for plant stress tolerance, plant root growth, H2O2 and MDA effects (shoot/root ratios) on fresh and dry weight. We chose the model with the smallest AIC value for comparisons.
Results
Strain identification and characterization
Bacteria associated with kelp plants were isolated by enrichment and then purified on agar plates. One species was isolated and selected and identified as the type strain (Pontibacter actiniarum DSM 19842) originally identified in the Pacific Ocean in Rudnaya Bay, Russia. P. actiniarum is an aerobic Gram-negative, pink-pigmented marine bacterium with gliding mobility of the phylum Bacteroides (https://doi.org/10.1099/ijs.0.63819-0).
Microencapsulation analysis
P. actiniarum microcapsules that were prepared using the emulsion and internal gelation method were present as smooth, spherical and individual microcapsules with no apparent pores (Figures 1B, C). The microcapsule average particle size was 10.6 µm and concentrated in the range of 5.5-15.9 µm. The particle size distribution range span was 1.04 and indicated a uniform particle size distribution. The microcapsules were applied to wheat seeds by dipping and adherence to the seeds was apparent in SEM photomicrographs (Figure 1D).
Bacteria microcapsule embedding and stability
The bacterial embedding score was examined by comparing bacterial CFUs of solutions used for encapsulation with those following bacteria release from the microcapsules. The encapsulation rate reached 65.4%. Survival rate of P. actiniarum in the microcapsules following 60 d of storage at room temperature (20-25°C) was 73.1%, while survival rate of those not microencapsulated were 49.3%, and increased by 22.4%.
Germination and seedling development
Under salt stress, seed germination is considered the key point throughout the plant growth period and after that, plants can cope with salt stress in the environment through other strategies. Wheat seeds treated with Bacteria, M-Lack and M-Embed all had higher germination rates (84%, 82% and 90% respectively) than the controls (average of 73%). We measured the response ratios for the 4 experimental treatments (Figure 2). Bacteria, M-Lack and M-Embed all displayed significant increases in germination compared to the CK while M-Embed displayed a significant increase in germination compared to group Bacteria. In addition, Bacteria and M-Lack displayed a significant increase in dry and fresh weight as well as in the root/shoot ratio compared to CK while M-Embed possessed a significant increase in the dry weight and root/shoot ratio compared with Bacteria (Figure 3).

Figure 2 Photographs of wheat seedlings grown for 7 days. Wheat seeds with water (CK); Wheat seeds with bacteria (Bacteria); Wheat seeds with microcapsules lacking bacteria (M-Lack); Wheat seeds with microcapsules embedded bacteria (M-Embed). (Scale bar is 5 cm).

Figure 3 Response ratio calculations for root/shoot, germination (%), dry/fresh weights for plants for the groups (A) Bacteria, (B) M-Lack and (C) M-Embed. Response ratios for Chl, POD, SOD, CAT, PRO H2O2 and MDA for (D) Bacteria, (E) M-Lack and (F) M-Embed. Superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), proline (PRO), malondialdehyde (MDA), hydrogen peroxide (H2O2) and Chlorophyll (Chl). TRL, Total root length; TRA, total root area; ARD, average root diameter; TRV, total root volume; RHL, root hair length. *p<0.05, ** p<0.01, *** p<0.001.
The increases in the root/shoot ratio were correlated with the presence of bacteria and microcapsules on the wheat root system. M-Lack and Bacteria displayed significant increases in total root length (TRL), total root area (TRA), total root volume (TRV) and root hair length (RHL) compared to CK. In addition, M-Embed plants displayed significant increases in TRL, TRA, TRV and RHL and a significant decrease in mean root stem compared to Bacteria (Table 1).
Physiological activity
Our response ratio analysis indicated a significant increase in the physiological indices Chl, POD, CAT and SOD in Bacteria and M-Lack compared to CK as well as a significant increase in PRO in Bacteria. There was also a significant decrease in MDA and H2O2 in both Bacteria and M-Lack compared with CK. The physiological index levels of Chl, POD, SOD and PRO were significantly higher in M-Embed than in Bacteria while MDA and H2O2 were significantly lower. (Figure 3). In addition, two-factor ANOVA tests indicated significant interactions between M-Lack and Bacteria for SOD (Table 2).
The increased levels of POD, CAT, SOD and PRO indicated that the plants were coping with adversity in a favorable way and the root system indicators displayed a similar pattern. We therefore used these factors to determine the plant stress tolerance using principal component analysis. Structural equation modeling indicated that bacteria and microcapsules had no direct effect on shoot and root fresh weights. Bacteria addition increased plant stress tolerance (0.84) and decreased oxidative makers content (0.56) and microcapsules increased plant stress tolerance (0.50) and decreased oxidative makers content (0.76). The plant stress tolerance increased shoot fresh weight (0.86) (Figure 4A). The standardized total effects of bacteria and microcapsules on shoot fresh weight were 0.72 and 0.42, respectively (Figure 4B). Overall, the indirect effects of bacteria and microcapsules worked by altering plant stress tolerance that explained 73% of shoot fresh weight (Figure 4A). Bacteria increased plant root growth (0.68) and plant stress tolerance (0.84) while microcapsules increased plant root growth (0.70) and plant stress tolerance (0.50). The plant stress tolerance increased root fresh weight (0.66) (Figure 4C). The standardized total effects for bacteria and microcapsules on root fresh weight were 0.75 and 0.54, respectively (Figure 4D). Overall, the indirect effects of bacteria and microcapsules through altered plant stress tolerance explained 85% of the root fresh weight (Figure 4C). The inferences of this model were that bacteria and microcapsules increased the fresh weight of wheat seedlings by indirectly increasing the plant resistance via elevated levels of POD, SOD, CAT and PRO.
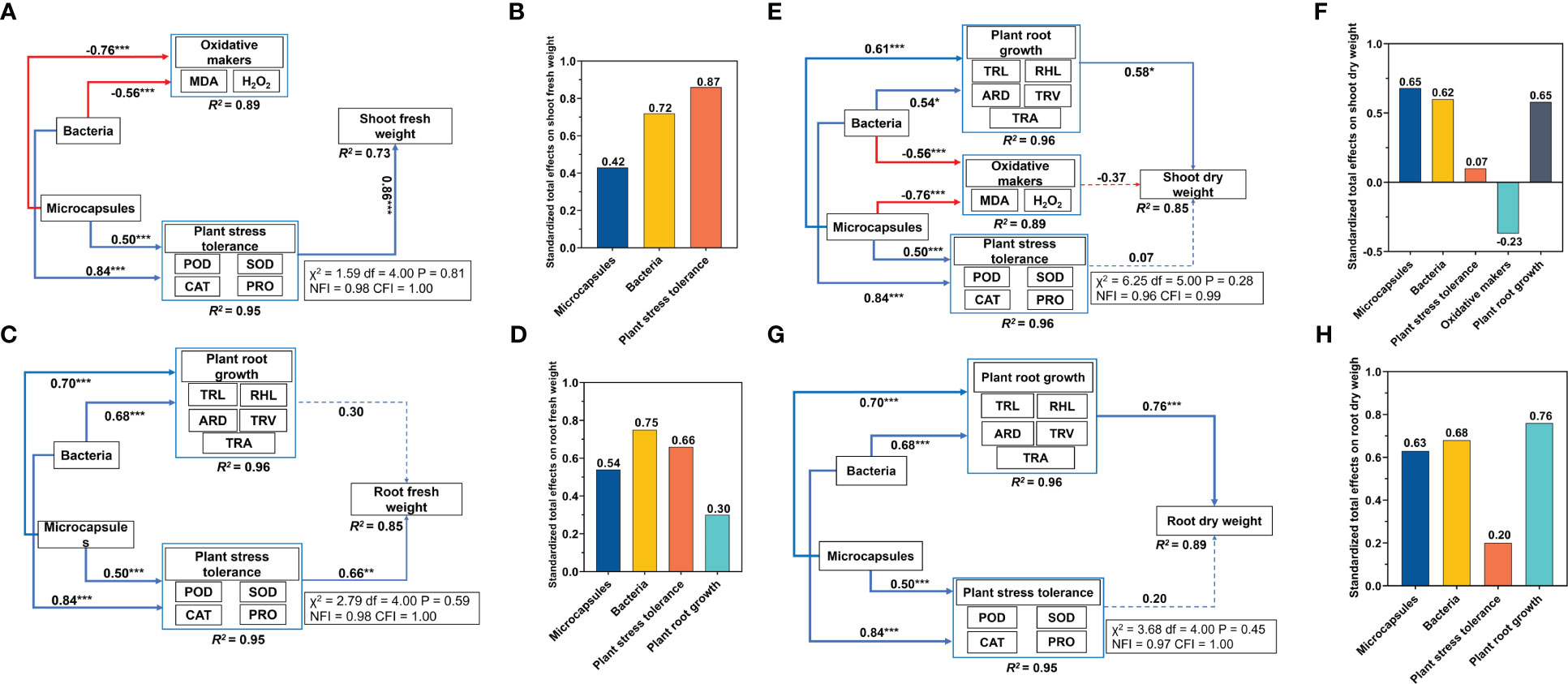
Figure 4 Results of the structural equation modeling. (A, C, E, G) standardized total effects of bacteria and (B, D, F, H) microcapsules on shoot/root fresh and dry weights. See Figure 3 for abbreviations. Red lines, negative correlations; blue lines, positive correlations. Standardized path coefficients are described by the numbers on the lines. The proportions of variance explained for each dependent variable in the model are shown at the bottom and details evaluating the models are shown in the boxes beside each figure. * p < 0.05, **p < 0.01, *** p < 0.001.
Bacteria and microcapsules had no direct effect on the shoot dry weight and root dry weight. Bacteria increased plant root growth (0.54) and plant stress tolerance (0.84) and decreased oxidative makers content (0.56). Microcapsules increased plant root growth (0.61) and plant stress tolerance (0.50) and decreased oxidative makers content (0.76) while plant root growth increased shoot dry weight (0.58) (Figure 4E). The standardized total effects of bacteria and microcapsules on shoot dry weight were 0.62 and 0.65, respectively (Figure 4F). Overall, the indirect effects of bacteria and microcapsules through altered plant root growth explained 85% of the shoot dry weight (Figure 4E). Bacteria increased plant root growth (0.68) and plant stress tolerance (0.84) while microcapsules increased plant root growth (0.70) and plant stress tolerance (0.50). The standardized total effects of bacteria and microcapsules on the root dry weight were 0.68 and 0.63, respectively (Figure 4H). Overall, the indirect effects of bacteria and microcapsules that acted via altering plant root growth explained 89% of the root dry weight (Figure 4D). The bacteria and microcapsules treatments indirectly increased plant root growth that included TRL, RHL, ARD, TRV and TRA as well as plant dry weight.
Discussion
Germination and seedling stages are critical points in the crop growth cycle and are vulnerable to environmental stressors (Nakhla et al., 2021; Tarchoun et al., 2022). Seed coating is a technology that covers the surface of seeds with a material that improves germination, crop growth and yield and has become an effective way to reduce production costs in precision agriculture by requiring only a small amount of microbial coating (Sohail et al., 2022). Bacteria seed coating can form a protective film on the surface of seeds and enhances plant tolerance and promote plant growth (Rocha et al., 2019). However, traditional microbial coating agents suffer from poor adhesion, low survival and storage stability resulting in reductions in bacteria numbers and activity (Afzal et al., 2020).
In the current work, we found that seeds embedded with microencapsulated bacteria under salt stress conditions performed better than control counterparts in terms of growth and physiological indices. The encapsulated bacteria were thus present in a more favorable microenvironment and a protective effect was achieved using an alginate and bacterial coating. Microencapsulation of the bacteria improved germination and conferred significant seedling viability. The primary capsule material calcium alginate worked with the bacteria for the best effect on plant growth although individual action of each also promoted plant growth.
The bacterium (P. actiniarum DSM 19842) used in these experiments was obtained from fermentation of kelp collected from the ocean. The closely related Pontibacter actiniarum KMM 6156T used in previous studies possessed peroxidase and oxidase activities that could minimize oxidative damage caused by salt stress (Chhetri et al., 2019). It has been shown that alginate is a major compound of macroalgae and as such an important carbon and energy source for marine bacteria (Jouanneau et al., 2021).
Calcium alginate has a mitigating effect on plants grown in salt-stressed environments. This compound can promote root development and growth and increase root absorption area. These effects increase water and nutrient absorption counteracting the effects of salt stress (Xu et al., 2003). Calcium ions in calcium alginate also can act to regulate ionic balance in plants. Salt stress will lead to an increase in Na+ content, thus reducing the absorption of K+, Mg2+ and Ca2+. The addition of Ca2+ can attenuate the cytotoxicity caused by Na+ ions under salt stress and promote ionic homeostasis (Guo et al., 2021). In addition, calcium alginate has antioxidant capacities. Salt stress can lead to excessive production of reactive oxygen radicals in plants triggering oxidative damage (Mangal et al., 2023). The natural oxidizing substance of calcium alginate can assist plants in free radical scavenging to reduce oxidative damage (Kumari and Bhatla, 2021). Alginate may also contain small amounts of potassium alginate and K+ has equally positive effects in maintaining ionic balance (Kumari and Bhatla, 2021), improving the antioxidant capacity of plants (Rady et al., 2022) and promoting plant root development (Bojórquez-Quintal et al., 2016).
Salt stress affects plant survival and crop yield by inhibiting seed water uptake (Zhu et al., 2019), interfering with metabolic activities within the seed (Zou et al., 2020) and inhibiting seed enzyme activities (Gou et al., 2020) leading to decreased levels of germination. In our experiments, M-Embed had significant increases in germination under salt stress and these plants displayed significantly increased values for TRL, TRA, TRV and RHL compared to the other treatments. Salt stress can damage the osmotic balance of plant cells and lead to water loss while these significant changes in root-to-crown ratios and root structure can increase the ability of plants to access available water and nutrients (Li et al., 2021). Therefore, in terms of growth indicators, M-Embed reduced the damage caused by salt stress on plant growth by increasing germination and promoting root growth. The structural equation modeling demonstrated that the use of bacteria and microcapsules can play an important role in improving plant root growth and increasing plant dry weight (Figure 4).
The protection of plant photosynthetic mechanisms contributes to the ability of plants to resist the generation of reactive oxygen species (ROS) radicals under salt stress as well as to participate in the scavenging of preexisting ROS (Tian et al., 2022). The experiments demonstrated leaf chlorophyll content in M-Embed under salt stress was greater than for the other treatments and would assist plants in maintaining a normal metabolism. Previous studies have indicated that bacteria promote photosynthetic activity in plants under salt stress possibly due to increased levels of the plant growth regulators indole acetic acid (IAA) (Cheng et al., 2022) and 1-aminocyclopropane-1-carboxylate (ACC) deaminase involved in ethylene regulation (Misra and Chauhan, 2020) as well as with extracellular polymeric substances (EPS) (Amna et al., 2019). These compounds can also increase chlorophyll levels. Alginate therefore can play an important role in plant-microbe interactions (Fathi et al., 2021).
Under salt stress, ROS accumulates and produces oxidative damage to plant cells. H2O2 is one of the most abundant reactive ROS in cells and H2O2 content plays an important role in plant salt tolerance (Wu et al., 2018). SOD, CAT and POD improve plant adaptation by reducing the oxidative damage to cells by participating in ROS scavenging (Santos et al., 2018). We found that antioxidant enzyme levels in wheat seedlings for M-Embed were higher than for the other treatments while the H2O2 content was lower than in the other treatments. Thus, wheat with microbial microcapsules coating showed better performance than other treatments in terms of antioxidant defense mechanisms.
Osmoregulation is a central part of the physiological mechanism of plant response to salt stress. Under salt stress, osmoprotectants such as free proline, glycine betaine and other amino acids are formed (Suprasanna et al., 2016). These assist cells to maintain normal osmoregulation and cell membrane stability, ultimately improving plant growth and development (Suprasanna et al., 2016). Therefore, PRO was one of the key factors in the osmoregulatory function of plants. We found that M-Embed had the highest PRO content compared to the other treatments. This could be a mechanism to alleviate the problem of osmotic accumulation caused by salt stress. Structural equation modeling indicated that bacteria and microcapsules affect plant stress tolerance and indirectly increase plant dry weight. Thus, the changes in antioxidant enzymes and proline induced by M-Embed were important factors that increase plant dry weight (Figure 4).
One of the main consequences of salt stress is excess ROS in plant cells leading to lipid peroxidation that damages the cell membrane and increases MDA in leaves (Sun et al., 2010). The presence of bacteria can inhibit oxidative damage thereby enhancing plant cell stability decreased levels of ROS under salt stress conditions (Bharti et al., 2013). The lowest levels of the oxidative markers (MDA and H2O2) in M-Embed were also an indicator of decreased oxidative damage.
Conclusions
This promising seed coating improved germination, promoted root growth, increased levels of enzymatic antioxidants and osmoprotectants and reduced oxidative stress markers. Based on these results, microencapsulation of P. actiniarum for use as seed coatings can reduce the negative effects of salt stress on plant growth. This study provides new insights into the application of microcapsules in functional seed coating. Moreover, future research is needed to confirm the interaction process between the microbe and plant relationship.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
MG: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Writing – original draft. JH: Formal Analysis, Methodology, Writing – review & editing. MK: Formal Analysis, Methodology, Writing – review & editing. QH: Formal Analysis, Methodology, Project administration, Writing – review & editing. YJ: Formal Analysis, Methodology, Project administration, Writing – review & editing. JS: Writing – review & editing, Methodology. WH: Funding acquisition, Supervision, Validation, Writing – review & editing. GL: Funding acquisition, Supervision, Validation, Writing – review & editing, Investigation, Methodology, Resources.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Key Research and Development Program of Shandong Province (2022TZXT0045-04), the Agricultural Science and Technology Innovation Program [ASTIP No. CAAS-ZDRW202201], and the National Key Research and Development Program of China (2019YFE0197100).
Acknowledgments
We thank the Allwegene Technology Technology Co., Ltd. (Beijing, China) for providing guidance and technical assistance in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abideen, Z., Koyro, H., Huchzermeyer, B., Ahmed, M., Zulfiqar, F., Egan, T., et al. (2020). Phragmites karka plants adopt different strategies to regulate photosynthesis and ion flux in saline and water deficit conditions. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology 155 (3), 524–534. doi: 10.1080/11263504.2020.1762783
Afzal, I., Javed, T., Amirkhani, M., Taylor, A. G. (2020). Modern seed technology: seed coating delivery systems for enhancing seed and crop performance. Agriculture 10 (11), 526. doi: 10.3390/agriculture10110526
Amna, N., Ud Din, B., Sarfraz, S., Xia, Y., Kamran, M. A., Javed, M.T., et al. (2019). Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and acc- deaminase producing bacillus strains under induced salinity stress. Ecotoxicology Environ. Saf. 183, 109466. doi: 10.1016/j.ecoenv.2019.109466
Bharti, N., Barnawal, D., Awasthi, A., Yadav, A., Kalra, A. (2013). Plant growth promoting rhizobacteria alleviate salinity induced negative effects on growth, oil content and physiological status in mentha arvensis. Acta Physiologiae Plantarum 36 (1), 45–60. doi: 10.1007/s11738-013-1385-8
Bojórquez-Quintal, E., Ruiz-Lau, N., Velarde-Buendía, A., Echevarría-MaChado, I., Pottosin, I., Martínez-Estévez, M. (2016). Natural variation in primary root growth and k+ retention in roots of habanero pepper (Capsicum chinense) under salt stress. Funct. Plant Biol. 43 (12), 1114–1125. doi: 10.1071/fp15391
Cheng, S., Jiang, J., Tan, L., Deng, J., Liang, P., Su, H., et al. (2022). Plant growth-promoting ability of mycorrhizal fusarium strain kb-3 enhanced by its iaa producing endohyphal bacterium, klebsiella aerogenes. Front. Microbiol. 13, 855239. doi: 10.3389/fmicb.2022.855399
Chhetri, G., Kim, J., Kim, H., Kim, I., Seo, T. (2019). Pontibacter oryzae sp. Nov., A carotenoid-producing species isolated from a rice paddy field. Antonie Van Leeuwenhoek 112 (11), 1705–1713. doi: 10.1007/s10482-019-01298-0
Choudhary, M., Chandra, P., Dixit, B., Nehra, V., Choudhary, U., Choudhary, S. (2022). Plant growth-promoting microbes: role and prospective in amelioration of salt stress. Commun. Soil Sci. Plant Anal. 53 (13), 1692–1711. doi: 10.1080/00103624.2022.2063316
Dantas, B. F., Matias, J. R., Aragão, C. A., Lopes, A. P. (2019). Physiological changes in seeds and seedlings of pumpkins submitted to salt stress. Bioscience J. 35, 763–774. doi: 10.14393/bj-v35n3a2019-41887
Fareez, I. M., Lim, S. M., Mishra, R. K., Ramasamy, K. (2015). Chitosan coated alginate-xanthan gum bead enhanced ph and thermotolerance of lactobacillus plantarum lab12. Int. J. Biol. Macromolecules 72, 1419–1428. doi: 10.1016/j.ijbiomac.2014.10.054
Fathi, F., Saberi-Riseh, R., Khodaygan, P. (2021). Survivability and controlled release of alginate-microencapsulated pseudomonas fluorescens vupf506 and their effects on biocontrol of rhizoctonia solani on potato. Int. J. Biol. Macromolecules 183, 627–634. doi: 10.1016/j.ijbiomac.2021.04.159
Gou, T., Chen, X., Han, R., Liu, J., Zhu, Y., Gong, H. (2020). Silicon can improve seed germination and ameliorate oxidative damage of bud seedlings in cucumber under salt stress. Acta Physiologiae Plantarum. 42 (1), 12. doi: 10.1007/s11738-019-3007-6
Guo, Y., Liu, Y., Zhang, Y., Liu, J., Gul, Z., Guo, X.-R., et al. (2021). Effects of exogenous calcium on adaptive growth, photosynthesis, ion homeostasis and phenolics of gleditsia sinensis lam. Plants under salt stress. Agriculture 11 (10), 978. doi: 10.3390/agriculture11100978
Habib, S. H., Kausar, H., Saud, H. M. (2016). Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ros-scavenging enzymes. BioMed. Res. Int. 6284547. doi: 10.1155/2016/6284547
He, J., Kong, M., Qian, Y., Gong, M., Lv, G., Song, J. (2022). Cellobiose elicits immunity in lettuce conferring resistance against. J. Exp. Bot. 74 (3), 1022–1038. doi: 10.1093/jxb/erac448
Huang, S., Gill, S., Ramzan, M., Ahmad, M. Z., Danish, S., Huang, P., et al. (2023). Uncovering the impact of am fungi on wheat nutrient uptake, ion homeostasis, oxidative stress, and antioxidant defense under salinity stress. Sci. Rep. 13 (1), 8249. doi: 10.1038/s41598-023-35148-x
Jouanneau, D., Klau, L. J., Larocque, R., Jaffrennou, A., Duval, G., Le Duff, N., et al. (2021). Structure–function analysis of a new pl17 oligoalginate lyase from the marine bacterium zobellia galactanivorans dsijt. Glycobiology 31 (10), 1364–1377. doi: 10.1093/glycob/cwab058
Kim, I., Pusey, P. L., Zhao, Y., Korban, S. S., Choi, H., Kim, K. K. (2012). Controlled release of pantoea agglomerans e325 for biocontrol of fire blight disease of apple. J. Controlled Release 161 (1), 109–115. doi: 10.1016/j.jconrel.2012.03.028
Krasaekoopt, W., Bhandari, B., Deeth, H. (2003). Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 13 (1), 3–13. doi: 10.1016/s0958-6946(02)00155-3
Kumari, A., Bhatla, S. C. (2021). Regulation of salt-stressed sunflower (helianthus annuus) seedling. Funct. Plant Biol. 48 (6), 573–587. doi: 10.1071/fp20334
Lemos, Y. P., Mariano Marfil, P. H., Nicoletti, V. R. (2017). Particle size characteristics of buriti oil microcapsules produced by gelatin-sodium alginate complex coacervation: effect of stirring speed. Int. J. Food Properties 20 (2),1438–1447. doi: 10.1080/10942912.2017.1349139
Li, H., Yu, T., Ning, Y., Li, H., Zhang, W., Yang, H.-Q. (2021). Hydrogen sulfide alleviates alkaline salt stress by regulating the expression of micrornas in malus hupehensis rehd. Roots. Front. Plant Sci. 12, 663519. doi: 10.3389/fpls.2021.663519
Li, Z., Du, Z., Li, H., Chen, Y., Zheng, M., Jiang, Z., et al. (2022). Characterisation of marine bacterium microbulbifer sp. Alw1 with laminaria japonica degradation capability. Amb Express 12 (1), 139. doi: 10.1186/s13568-022-01482-y
Ma, Y., Prasad, M. N. V., Rajkumar, M., Freitas, H. (2010). Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Advances 29 (2), 248–258. doi: 10.1016/j.bioteChadv.2010.12.001
Maity, A., Sharma, J., Pal, R. K. (2019). Novel potassium solubilizing bio-formulation improves nutrient availability, fruit yield and quality of pomegranate (punica granatum l.) In semi-arid ecosystem. Scientia Horticulturae 255, 14–20. doi: 10.1016/j.scienta.2019.05.009
Mangal, V., Lal, M.K., Tiwari, R.K., Altaf, M.A., Sood, S., Kumar, D., et al. (2023). Molecular insights into the role of reactive oxygen, nitrogen and sulphur species in conferring salinity stress tolerance in plants. J. Plant Growth Regul. 42 (2), 554–574. doi: 10.1007/s00344-022-10591-8
Misra, S., Chauhan, P. S. (2020). Acc deaminase-producing rhizosphere competent bacillus spp. Mitigate salt stress and promote zea mays growth by modulating ethylene metabolism. 3 Biotech. 10 (3), 119. doi: 10.1007/s13205-020-2104-y
Nakhla, W. R., Sun, W., Fan, K., Yang, K., Zhang, C., Yu, S. (2021). Identification of qtls for salt tolerance at the germination and seedling stages in rice. Plants 10 (3), 428. doi: 10.3390/plants10030428
Pedrini, S., Merritt, D. J., Stevens, J., Dixon, K. (2017). Seed coating: science or marketing spin? Trends Plant Science 22 (2), 106–116. doi: 10.1016/j.tplants.2016.11.002
Qi, W., Liang, X., Yun, T., Guo, W. (2019). Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation. J. Food Sci. Technology 56 (3), 1398–1404. doi: 10.1007/s13197-019-03616-w
Rady, M.M., Mossa, A.-T.H., Youssof, A.M.A., Sh. Osman, A., Ahmed, S.M.A., Mohamed, I.a.A., et al. (2022). Exploring the reinforcing effect of nano-potassium on the antioxidant defense system reflecting the increased yield and quality of salt-stressed squash plants. Scientia Hortic. 308, 111609. doi: 10.1016/j.scienta.2022.111609
Rima, F. S., Biswas, S., Sarker, P. K., Islam, M. R., Seraj, Z. I. (2018). Bacteria endemic to saline coastal belt and their ability to mitigate the effects of salt stress on rice growth and yields. Ann. Microbiol. 68 (9), 525–535. doi: 10.1007/s13213-018-1358-7
Rocha, I., Ma, Y., Souza-Alonso, P., Vosátka, M., Freitas, H., Oliveira, R. S. (2019). Seed coating: a tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 10, 1357. doi: 10.3389/fpls.2019.01357
Santos, A. D. A., Silveira, J. A. G. D., Bonifacio, A., Rodrigues, A. C., Figueiredo, M. D. V. B. (2018). Antioxidant response of cowpea co-inoculated with plant growth-promoting bacteria under salt stress. Braz. J. Microbiol. 49 (3), 513–521. doi: 10.1016/j.bjm.2017.12.003
Sharaya, R., Gill, R., Kalwan, G., Naeem, M., Tuteja, N., Gill, S. S. (2023). Plant-microbe interaction mediated salinity stress tolerance for sustainable crop production. South African Journal of Botany 161, 454–471. doi: 10.1016/j.sajb.2023.08.043
Smolikova, G., Medvedev, S. (2022). Seed-to-seedling transition: novel aspects. Plants. 11 (15), 1988. doi: 10.3390/plants11151988
Sohail, M., Pirzada, T., Opperman, C., Khan, S. (2022). Recent advances in seed coating technologies: transitioning toward sustainable agriculture. Green Chem. 24 (16), 6052–6085. doi: 10.1039/d2gc02389j
Soumare, A., Boubekri, K., Lyamlouli, K., Hafidi, M., Ouhdouch, Y., Kouisni, L. (2020). From isolation of phosphate solubilizing microbes to their formulation and use as biofertilizers: status and needs. Front. Bioengineering Biotechnol. 7, 425. doi: 10.3389/fbioe.2019.00425
Sun, C., Johnson, J. M., Cai, D., Sherameti, I., Oelmüller, R., Lou, B. (2010). Piriformospora indica confers drought tolerance in chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized cas protein. J. Plant Physiol. 167 (12), 1009–1017. doi: 10.1016/j.jplph.2010.02.013
Suprasanna, P., Nikalje, G. C., Rai, A. N. (2016). Osmolyte accumulation and implications in plant abiotic stress tolerance. In: Iqbal, N., Nazar, R., Khan, A. N. (eds) osmolytes and plants acclimation to changing environment: Emerging omics technologies (New Delhi: Springer), 1–12. doi: 10.1007/978-81-322-2616-1_1
Tarchoun, N., Saadaoui, W., Mezghani, N., Pavli, O. I., Falleh, H., Petropoulos, S. A. (2022). The effects of salt stress on germination, seedling growth and biochemical responses of Tunisian squash (cucurbita maxima duchesne) germplasm. Plants 11 (6), 800. doi: 10.3390/plants11060800
Tavakkoli, E., Fatehi, F., Coventry, S., Rengasamy, P., McDonald, G. K. (2011). Additive effects of na+ and cl- ions on barley growth under salinity stress. J. Exp. Botany. 62 (6), 2189–2203. doi: 10.1093/jxb/erq422
Tian, T., Wang, J., Wang, H., Cui, J., Shi, X., Song, J., et al. (2022). Nitrogen application alleviates salt stress by enhancing osmotic balance, ros scavenging, and photosynthesis of rapeseed seedlings (brassica napus). Plant Signaling Behav. 17 (1), 2081419. doi: 10.1080/15592324.2022.2081419
Tu, L., He, Y., Shan, C., Wu, Z. (2016). Preparation of microencapsulated bacillus subtilis sl-13 seed coating agents and their effects on the growth of cotton seedlings. BioMed. Res. Int. 3251357. doi: 10.1155/2016/3251357
Wahid, I., Rani, P., Kumari, S., Ahmad, R., Hussain, S.J., Alamri, S., et al. (2021). Biosynthesized gold nanoparticles maintained nitrogen metabolism, nitric oxide synthesis, ions balance, and stabilizes the defense systems to improve salt stress tolerance in wheat. Chemosphere 287, 132142. doi: 10.1016/j.chemosphere.2021.132142
Wu, J., Shu, S., Li, C., Sun, J., Guo, S. (2018). Spermidine-mediated hydrogen peroxide signaling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant Physiol. Biochem. 128, 152–162. doi: 10.1016/j.plaphy.2018.05.002
Xu, X., Iwamoto, Y., Kitamura, Y., Oda, T., Muramatsu, T. (2003). Root growth-promoting activity of unsaturated oligomeric uronates from alginate on carrot and rice plants. Bioscience Biotechnology Biochem. 67 (9), 2022–2025. doi: 10.1271/bbb.67.2022
Yang, Y., Guo, Y. (2018). Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 60 (9), 796–804. doi: 10.1111/jipb.12689
Zhang, P., Duo, T., Wang, F., Zhang, X., Yang, Z., Hu, G. (2021). De novo transcriptome in roots of switchgrass (panicum virgatum l.) Reveals gene expression dynamic and act network under alkaline salt stress. BMC Genomics 22 (1), 82. doi: 10.1186/s12864-021-07368-w
Zhu, G., An, L., Jiao, X., Chen, X., Zhou, G., Mclaughlin, N. (2019). Effects of gibberellic acid on water uptake and germination of sweet sorghum seeds under salinity stress. Chilean J. Agric. Res. 79, 415–424. doi: 10.4067/s0718-58392019000300415
Zou, H., Zhao, D., Wen, H., Li, N., Qian, W., Yan, X. (2020). Salt stress induced differential metabolic responses in the sprouting tubers of jerusalem artichoke (helianthus tuberosus l.). PloS One 15 (6), e0235415. doi: 10.1371/journal.pone.0235415
Zvinavashe, A. T., Laurent, J., Mhada, M., Sun, H., Fouda, H. M. E., Kim, D., et al. (2021). Programmable design of seed coating function induces water-stress tolerance in semi-arid regions. Nat. Food. 2 (7), 485–493. doi: 10.1038/s43016-021-00315-8
Keywords: bacteria, microcapsules, salt stress, seed coating, wheat
Citation: Gong M, He J, Kong M, Huo Q, Jiang Y, Song J, Han W and Lv G (2023) A microencapsulation approach to design microbial seed coatings to boost wheat seed germination and seedling growth under salt stress. Front. Plant Sci. 14:1283590. doi: 10.3389/fpls.2023.1283590
Received: 26 August 2023; Accepted: 03 November 2023;
Published: 21 November 2023.
Edited by:
Zamin Shaheed Siddiqui, University of Karachi, PakistanReviewed by:
Mohammad Saidur Rhaman, Bangladesh Agricultural University, BangladeshMuhammad Umar, Government College for Men Nazimabad, Pakistan
Copyright © 2023 Gong, He, Kong, Huo, Jiang, Song, Han and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Han, d2hhbjAxQDE2My5jb20=; Guohua Lv, bHZndW9odWFAY2Fhcy5jbg==
 Min Gong
Min Gong Jiuxing He1
Jiuxing He1 Jiqing Song
Jiqing Song Wei Han
Wei Han Guohua Lv
Guohua Lv