
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 29 September 2023
Sec. Plant Biotechnology
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1255819
This article is part of the Research Topic Applications of Novel Gene Editing Tools and Approaches in Plants View all 10 articles
The soybean (Glycine max L.) is an important crop with high agronomic value. The improvement of agronomic traits through gene editing techniques has broad application prospects in soybean. The polyethylene glycol (PEG)-mediated cell transfection has been successfully used to deliver the CRISPR/Cas9-based ribonucleoprotein (RNP) into soybean protoplasts. However, several downstream analyses or further cell regeneration protocols might be hampered by PEG contamination within the samples. Here in this study, we attempted to transfect CRISPR/Cas9 RNPs into trifoliate leaf-derived soybean protoplasts using Neon electroporation to overcome the need for PEG transfection for the first time. We investigated different electroporation parameters including pulsing voltage (V), strength and duration of pulses regarding protoplast morphology, viability, and delivery of CRISPR/Cas9. Electroporation at various pulsing voltages with 3 pulses and 10 ms per pulse was found optimal for protoplast electro-transfection. Following electro-transfection at various pulsing voltages (500 V, 700 V, 1,000 V, and 1,300 V), intact protoplasts were observed at all treatments. However, the relative frequency of cell viability and initial cell divisions decreased with increasing voltages. Confocal laser scanning microscopy (CLSM) confirmed that the green fluorescent protein (GFP)-tagged Cas9 was successfully internalized into the protoplasts. Targeted deep sequencing results revealed that on-target insertion/deletion (InDel) frequencies were increased with increasing voltages in protoplasts electro-transfected with CRISPR/Cas9 RNPs targeting constitutive pathogen response 5 (CPR5). InDel patterns ranged from +1 bp to −6 bp at three different target sites in CPR5 locus with frequencies ranging from 3.8% to 8.1% following electro-transfection at 1,300 V and 2.1% to 3.8% for 700 V and 1,000 V, respectively. Taken together, our results demonstrate that the CRISPR/Cas9 RNP system can be delivered into soybean protoplasts by the Neon electroporation system for efficient and effective gene editing. The electro-transfection system developed in this study would also further facilitate and serve as an alternative delivery method for DNA-free genome editing of soybean and other related species for genetic screens and potential trait improvement.
The CRISPR/Cas9 system has become a versatile technology in plant breeding and functional genomics due to its design simplicity and high efficiency in genome editing (Jiang and Doudna, 2017; Liu et al., 2022). Genome editing via CRISPR/Cas9 system uses single guide RNA (sgRNA) and Cas9 protein to make mutation events including insertions and deletions (InDels) at desired locations in the host genome through non-homologous end-joining (NHEJ) or homology-directed repair (HDR) pathways (Jinek et al., 2013). The CRISPR/Cas9 system enables targeted modifications in the genome by gene knock-in/out, base editing, prime editing, and guided transcriptional regulation (Feng et al., 2013; Zong et al., 2017; Mao et al., 2018; Lin et al., 2020; Rezazade Bazaz and Dehghani, 2022). Therefore, an optimized CRISPR/Cas9 cell system would facilitate efficient genetic screens for target genes and off-target activity in the plant genomes, thereby accelerating breeding and biosafety research.
The DNA expression cassettes of the CRISPR/Cas9 system can be transformed into plant cells using the most common delivery methods including Agrobacterium-mediated (Zhang et al., 2019), virus-mediated (Lei et al., 2022), particle bombardment (Hamada et al., 2018), and polyethylene glycol (PEG)-mediated transfection (Wu et al., 2020). Despite the fact that Agrobacterium and biolistic methods have been widely used in many organisms to deliver components for gene editing (Sandhya et al., 2020; Ghogare et al., 2021), their inadvertent incorporation of transposable elements (T-DNA) into the host genomes results in continued expression of CRISPR machinery, often resulting in unintended off-target mutation and genomic rearrangement (Zhang et al., 2018; Jupe et al., 2019; Chu and Agapito-Tenfen, 2022). Although virus-induced gene editing systems have many advantages including high editing efficiency and without integration of exogenous DNA into the host genome, their capacity to deliver the entire CRISPR/Cas9 system into plant cells is lower, which limits their application as a delivery method (Liu and Zhang, 2020; Zhang et al., 2022). Developing gene-edited lines without CRISPR T-DNA remnants in the host genome is gaining importance in the global genetically modified (GM) regulatory landscapes. Therefore, the DNA-free Cas9 ribonucleoprotein (RNP) (gRNA precomplexed to Cas9 nuclease) delivery into protoplasts has been adopted as a versatile method for genome editing of a diverse range of plants.
The direct delivery of RNPs into plant cells can be achieved by PEG-mediated as well as electroporation-mediated transfection or electro-transfection. PEG-mediated transfection has been widely used to deliver RNPs into plant protoplasts including Arabidopsis, tobacco and rice (Woo et al., 2015), petunia (Subburaj et al., 2016), apple and grape (Malnoy et al., 2016), wheat (Liang et al., 2017), cabbage (Murovec et al., 2018), pepper (Kim et al., 2020), maize (Sant’Ana et al., 2020), tomato (Nicolia et al., 2021), and soybean (Subburaj et al., 2022). PEG might exhibit incompatibility to several downstream analyses (e.g., proteomic analysis) and may cause some degree of cell cytotoxicity, with toxic effects occasionally seen in protoplasts when transfection with PEG is performed (Tyagi et al., 1989; Masani et al., 2014). Electro-transfection is another direct delivery method for the efficient transfection of the CRISPR/Cas9 system to living cells, and it has been widely used in the transformation of human and mouse primary T cells (Rupp et al., 2017; Seki and Rutz, 2018). Electro-transfection of RNPs has been demonstrated successfully in the microalga model Chlamydomonas reinhardtii (Baek et al., 2016; Shin et al., 2016). In addition, electroporation was also used for the delivery of RNPs (cabbage protoplast) and plasmid DNA containing CRISPR reagents (oil palm protoplast and wheat microspores) to plant cells (Bhowmik et al., 2018; Lee et al., 2020; Yeap W. et al., 2021). In soybean protoplasts, the transient expression of electroporated DNA has been reported by several studies (Dhir et al., 1991; Christou et al., 1987; Lin et al., 1987), but these available methods have not been adopted and or updated for targeted genome editing through transient introduction of RNA-guided endonuclease (RGEN) RNPs into soybean protoplasts.
Soybean is an economically important agronomic crop with high protein and oil, and several genetic engineering approaches have been made to improve the soybean traits (Rahman et al., 2023). Very recently, an efficient DNA-free genome editing platform for soybean protoplast systems using direct delivery of Cas9-RNP through PEG-mediated transfection was established by our research group (Subburaj et al., 2022). To date, no standardized protocols exist to transfect RNPs to soybean protoplasts through electroporation with reasonable mutation efficiency, which would greatly facilitate the CRISPR/Cas9 system to soybean protoplasts of different genetic backgrounds and further downstream analysis such as the impact of CRISPR exposure to soybean proteome.
In the present study, we report the establishment of a CRISPR/Cas9 RNP delivery system that facilitates efficient RNA-based genome editing in soybean protoplasts using electro−transfection. Using the Neon electroporation system (Thermo Fisher Scientific, Waltham, MA, USA), we investigated the important electrical factors including voltage strength and pulse duration on protoplasts, and we analyzed protoplasts of post-electroporation. With the established electrical parameters, we successfully demonstrated the delivery of exogenous green fluorescent protein (GFP)-Cas9 into the nucleus of soybean protoplasts using electro-transfection. By targeting the Glycine max CPR5 locus (GmCPR5) that is associated with soybean trichome growth, we validated the mutations at three different sgRNA targeted sites and determined the mutagenesis efficiency of CRISPR/Cas9 in soybean protoplasts by targeted deep sequencing.
The soybean (G. max) seeds cv. OAC Bayfield was planted and grown in soil pots under a photoperiod of 8-h light and 16-h dark at 25°C in a growth chamber (Enviro Plant®) for 3 weeks. The newly expanded trifoliate leaves from 14–16-day- old soybean seedlings were used for protoplast isolation. The extraction of protoplasts was carried out according to our previous study (Subburaj et al., 2022) with minor modifications. Briefly, 12–18 trifoliate leaves were sliced into 0.2–0.4- mM strips and were agitated in 20 ml of VCP (Viscozyme® L, Celluclast® 1.5L, and Pectinex® Μltra SPL) (Sigma-Aldrich, Darmstadt, Germany) cell wall digesting enzyme solution for 4–5 h at 25°C in the dark to isolate protoplasts. After digestion, the solution was filtered through 0.75- µm nylon mesh, and the filtrate was pelleted at 600 rpm for 5 min. Harvested protoplasts were rinsed thrice with 10 ml of 9M cell and protoplast washing (CPW) solution, followed by resuspending and centrifugation. The washed and purified protoplasts were kept in ice for 1 h prior to further use.
In this study, we chose GmCPR5 as the target gene, as it was already attempted to make site-directed mutations in their coding region using CRISPR/Cas9 RNP through the PEG-mediated delivery method; to accomplish this, we designed five candidate gRNAs (denoted as T1–T5) against CPR5 coding region in our previous study (Subburaj et al., 2022). In the present study, we selected and used best gRNAs of T1 (5′-AGGCTGCGGCGTTCAAACGACGG-3′), T3 (5′-GTCTCCCAGTCATCTTTCGATGG-3′), and T5 (5′-AGCTTTAGTAATCCGCTCGTAGG-3′) due to their higher mutation frequency at the CPR5 locus (Subburaj et al., 2022). We carried out the in vitro transcript synthesis and purification of these gRNAs as reported previously (Subburaj et al., 2022).
The purified protoplasts were centrifuged and resuspended in 9M CPW solution and then counted using a hemocytometer. CPW solution with a volume of 100 µl containing approximately 4 × 105 protoplasts was transferred to a 1.5-ml Eppendorf tube as needed for each transfection. After brief centrifugation, CPW solution was removed, and protoplasts were resuspended in 80 µl of transfection buffer, which included R buffer (Neon suspension buffer), MMG solution (4 mM of MES, 0.4 M of mannitol, and 15 mM of MgCl), and HEPES electroporation buffer (10 mM of Hepes (pH 7.2), 0.2 M of mannitol, 5 mm of CaCl2, and 150 mM of NaCl). Next, 20 µl of RNP complex was added to 80 µl of protoplast resuspension to bring a final volume of 100 µl. Then, the electroporation was carried out using the Neon™ Transfection System (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions in 100-µl volumes. Protoplasts were electroporated with various voltages (500 V, 700 V, 1,000 V, and 1,300 V), pulse (1 and 3), and width (10 ms, 20 ms, and 30 ms) to optimize and obtain efficient transfection conditions as described in the Results section. With the optimized electro-transfection conditions, 20 µl of RNP complexes (1:3 molar ratio) consisted of 10 µg of ready-to-use GFP-tagged Cas9 from Sigma-Aldrich (CAS9GFPPRO), and 30 µg of sgRNA was electrophoretically introduced into 80 µl of resuspended protoplasts. Control and electroporated cells were immediately transferred in a 12-well poly-d-lysine-coated cell plate with 0.5 ml of precooled KP8 liquid medium (Kao, 1977) (supplemented with 9% mannitol and 3% sucrose). Then, the plates were kept on ice for 1–2 h and shifted to 25°C in darkness for 16–24 h prior to DNA extraction. Control and transfected cells were further cultured in 1 ml of KP8 liquid medium for 4 weeks at 25°C in the dark.
The bright-field images of isolated and transfected cells were analyzed using Motic AE2000 inverted microscope and captured with Moticam (Motic Hong Kong Limited, Hong Kong). The viability of control and electro-transfected cells was assessed by 0.5% fluorescein diacetate (FDA) staining, observed under reflected light with Axio Vert.A1 (FL-LED Stand) inverted light microscope, and captured via Axiocam 202 mono (Carl Zeiss MicroImaging GmbH, Oberkochen, Germany). The fluorescence images of GFP-tagged Cas9 were acquired under bright field and electronically switchable illumination and detection module (ESID) using confocal laser scanning microscopy (CLSM) (LSM 800; Carl Zeiss) using a diode laser (488-nm line) with a 40× objective lens. The viability and transfection efficiency were calculated as the number of fluorescent protoplasts divided by the total number of protoplasts in one representative microscope field (Adedeji et al., 2020; Subburaj et al., 2022).
The genomic DNA from control and electro-transfected samples was prepared using Plant DNAzol™ Reagent (Invitrogen Co., Carlsbad, CA, USA). For each sgRNA (T1, T3, and T5), the target-specific nested PCR primers were designed in GmCPR5 loci (Supplementary Table 1), and the extracted DNA was PCR amplified using designed primers. Targeted mutagenesis in PCR products of both control and electro-transfected protoplasts was detected by T7 endonuclease I (T7E1) assay. Further, the InDels at the targeted locations were also analyzed by targeted deep sequencing using the Illumina NovaSeq™ 6000 platform at Novogene Europe (Cambridge Science Park, UK) as described previously (Subburaj et al., 2022). CRISPR/Cas9 RNP induced InDels at GmCPR5 loci for each sgRNA were determined using Cas-Analyzer from the CRISPR RGEN tools (http://www.rgenome.net/cas-analyzer/) (Subburaj et al., 2016; Subburaj et al., 2022). Briefly, the raw data FASTQ files along with basic information about query sequences were submitted in the Cas-Analyzer software with the following analysis parameters: comparison range of 40 and minimum frequency of 1. Following submission, the total number of reads, the number of reads with InDels, and the calculated InDel frequency (defined as the percentage by dividing the count of reads that contained InDels at the target site by the total number of reads) were obtained from the output of a summarized result table.
The initial electroporation experiments showed that trifoliate protoplasts are the most suitable for electro-transfection compared to unifoliate cells, as they were severely damaged after electroporation. In this study, we efficiently isolated the protoplast from 15- day-old trifoliate leaves with a duration of 5–6 h of enzyme digestion (Figures 1A, B). The yield of protoplasts was approximately 2 × 106 cells per gram fresh weight. Isolated protoplasts were 10 μm to 50 μm in size, and most were spherical in shape (Figure 1C). Following FDA staining, it was determined that 70% ± 2.1% of the protoplasts were alive (Figures 1D, E). The isolated protoplasts were cultured in KM medium, and first cell divisions were noted after 4–6 days of culture initiation (Figure 1F), indicating that the trifoliate leaf-derived protoplasts could be suitable for the electroporation-mediated transformation.
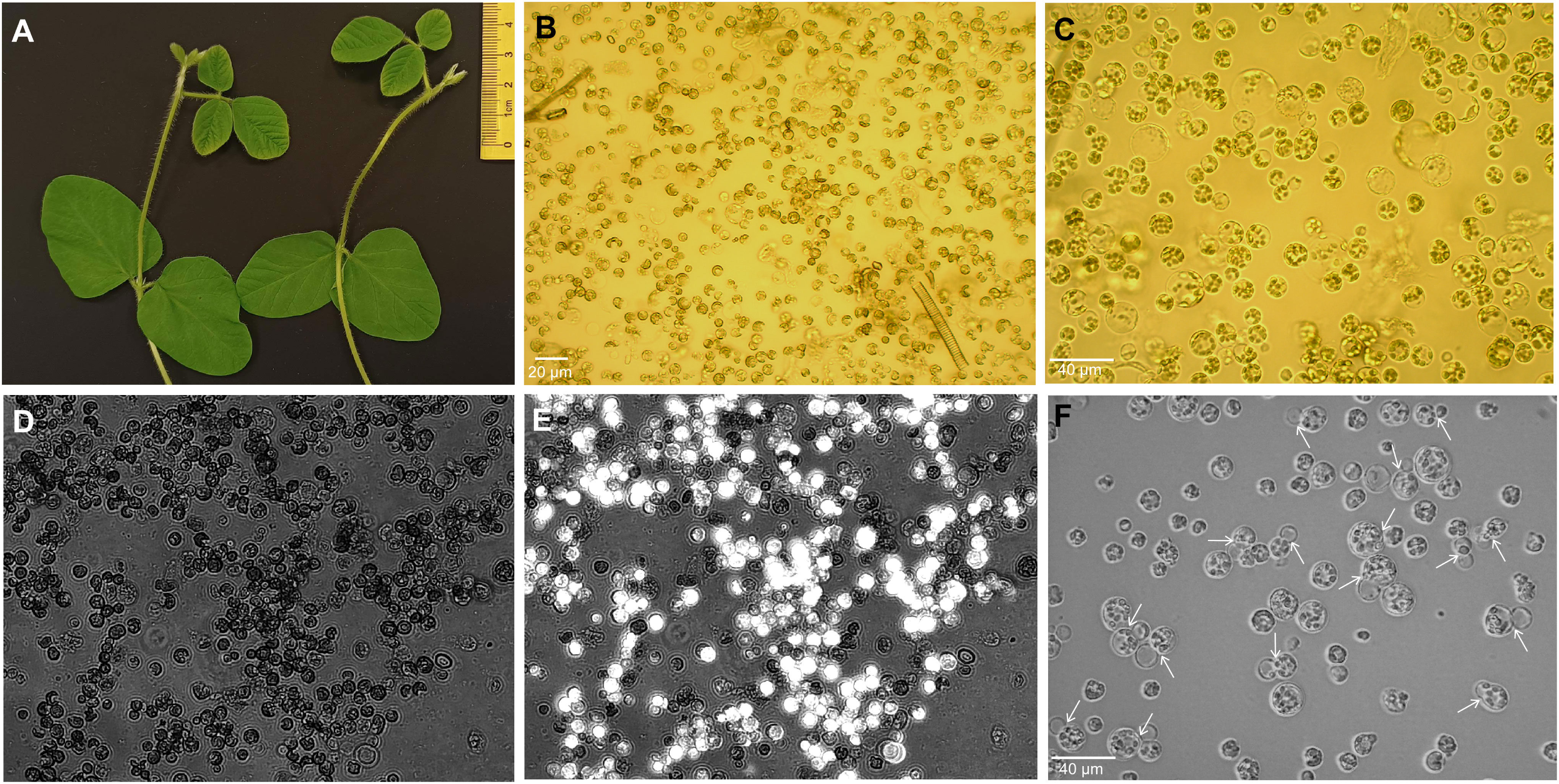
Figure 1 Isolation of protoplasts from trifoliate leaves of soybean plantlets. (A) Fifteen-day-old plants showing trifoliate leaves of suitable size. (B, C) Protoplasts of freshly extracted (B) and purified cells (C) under the Motic AE2000 inverted microscope with × 20 and × 40 objectives, respectively. Black scale bar, 30 µm. (D, E) The protoplast viability was assessed by FDA staining and observed under both bright field (D) and fluorescence channel, and simultaneously merged images are depicted (E) using Axio Vert.A1 inverted microscope with a × 20 objective. (F) Division of protoplasts (shown by white arrows) at 4 days after isolation in culture medium. FDA, fluorescein diacetate.
To establish an electroporation-mediated soybean protoplast transformation using the Neon system, the electro-transfection method was optimized based on cabbage and wheat protocols where transfection efficiency reaches 3.4% (1 pulse of 1,000 V, 20 seconds each) and 2.2% (3 pulses of 500 V, 30 seconds each), respectively (Bhowmik et al., 2018; Lee et al., 2020). Following cabbage and wheat electroporation conditions, initial soybean testing failed, as we could not detect or could only detect a few GFP fluorescent spots in electroporated cells. Subsequently, the various pulse strengths (1–3) along with voltages (500–1,300 V) and time duration (10–30 ms) were optimized. It was found that 3 pulses of 500 V and 10 ms per pulse were sufficient to transport the exogenous GFP-Cas9 with lower cell death rates. The effects of various ranges of pulsing voltages were further evaluated with optimized 3 pluses and 10 ms per pulse in the protoplasts (100 μl of 4 × 105 cells) for 500 V, 700 V, 1,000 V, and 1,300 V. As shown in Figure 2, the protoplasts’ morphology, viability, and cell division efficiency were examined after electroporation. It was noted that intact protoplasts with a large and round shape were observed at all the applied voltages. However, at 1,000 and 1,300 V, a certain proportion of broken and debris of dead cells was also noted.
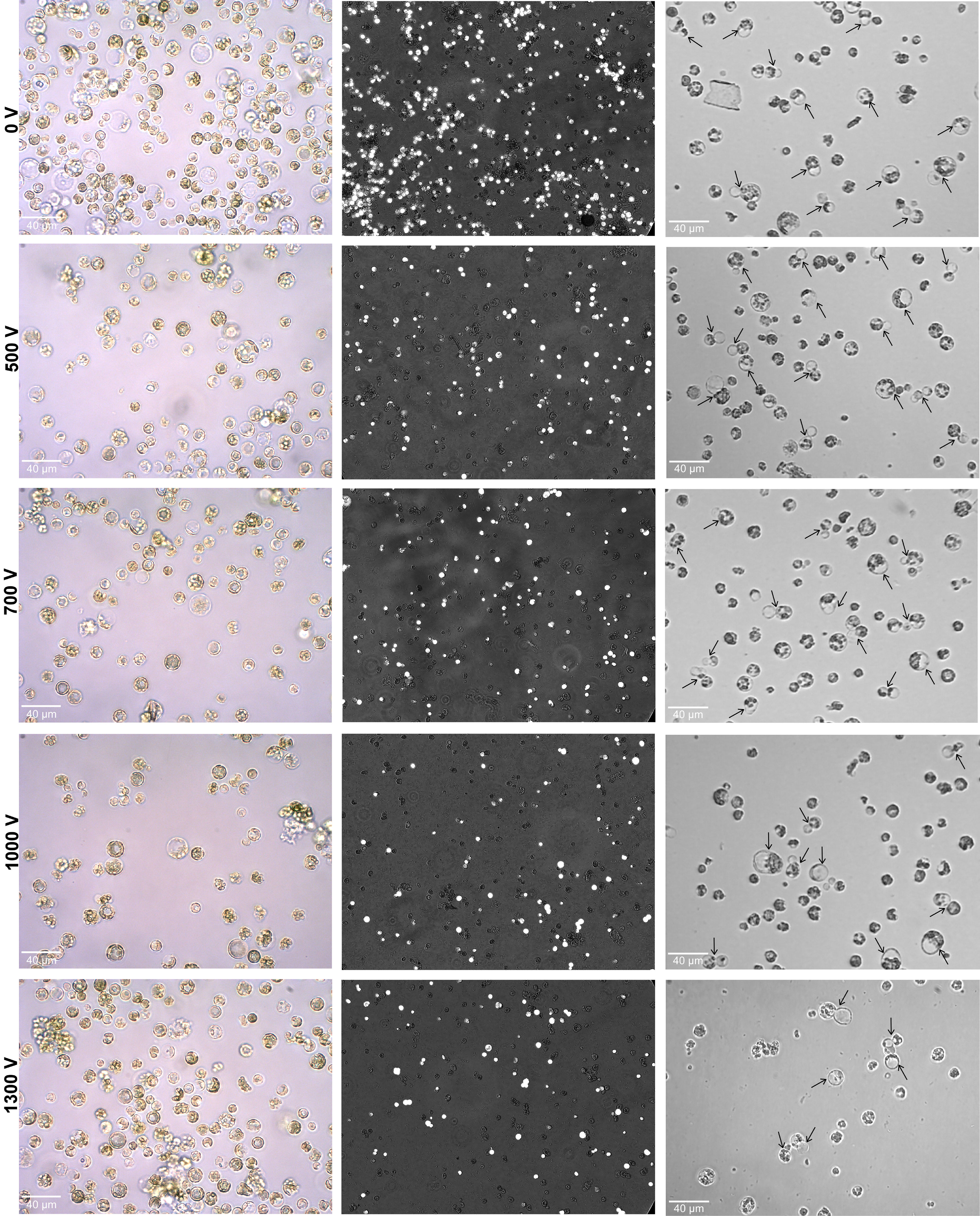
Figure 2 Effect of various pulsing voltage on the protoplasts morphology, viability, and cell division efficiency following electro-transfection. Left panel: representative images of electroporated protoplasts after 24 h are shown. Middle panel: the merged fluorescence images (under bright and fluorescence field using Axio Vert.A1 inverted microscope with a × 20 objective) showing the FDA-stained viable cells after 24 h of electroporation. Right panel: images showing primary divisions (shown by black arrows) of electroporated protoplasts at 4 days after culture initiation. FDA, fluorescein diacetate.
The calculated relative frequency of cell viability was 86.5% ± 2.4% under 0 V compared to the non-treatment control of 100%. Under various voltage treatments, the viability of cells was decreased with the increase in pulsing voltage. The observed relative frequency of viable rates for treatments under 500 V, 700 V, 1,000 V, and 1,300 V were 76.7% ± 2.8%, 66% ± 1.4%, 59.1% ± 1.2%, and 57.6% ± 1.1%, respectively (Figure 2). Upon culturing of electroporated protoplasts, the primary cell divisions were noted in all treatments. However, a higher proportion of cell divisions was only observed at 0 V and 500 V when compared to the higher voltages of 700 V, 1,000 V, and 1,300 V (Figure 2).
To further optimize and validate the delivery of Cas9 protein, a fixed amount of 10 µg of GFP-conjugated Cas9 was electro-transfected into protoplasts with the abovementioned pulsing voltages. After 24 h of electroporation, the internalization of GFP-tagged Cas9 inside the protoplasts was confirmed under the CLSM microscope (Figure 3). Regardless of voltage, the GFP-Cas9 was successfully localized inside of cells. However, the calculated internalization efficiency of GFP-Cas9 with an unsupervised eye was ⪸ 40% under 1,300 V compared to other treatments such as 500 V, 700 V, and 1,000 V where there was only ∼ 20%–23% noted. These results suggest that the established soybean protoplast transfection using the Neon electroporation system can be suitable for the delivery of RNPs into protoplasts.
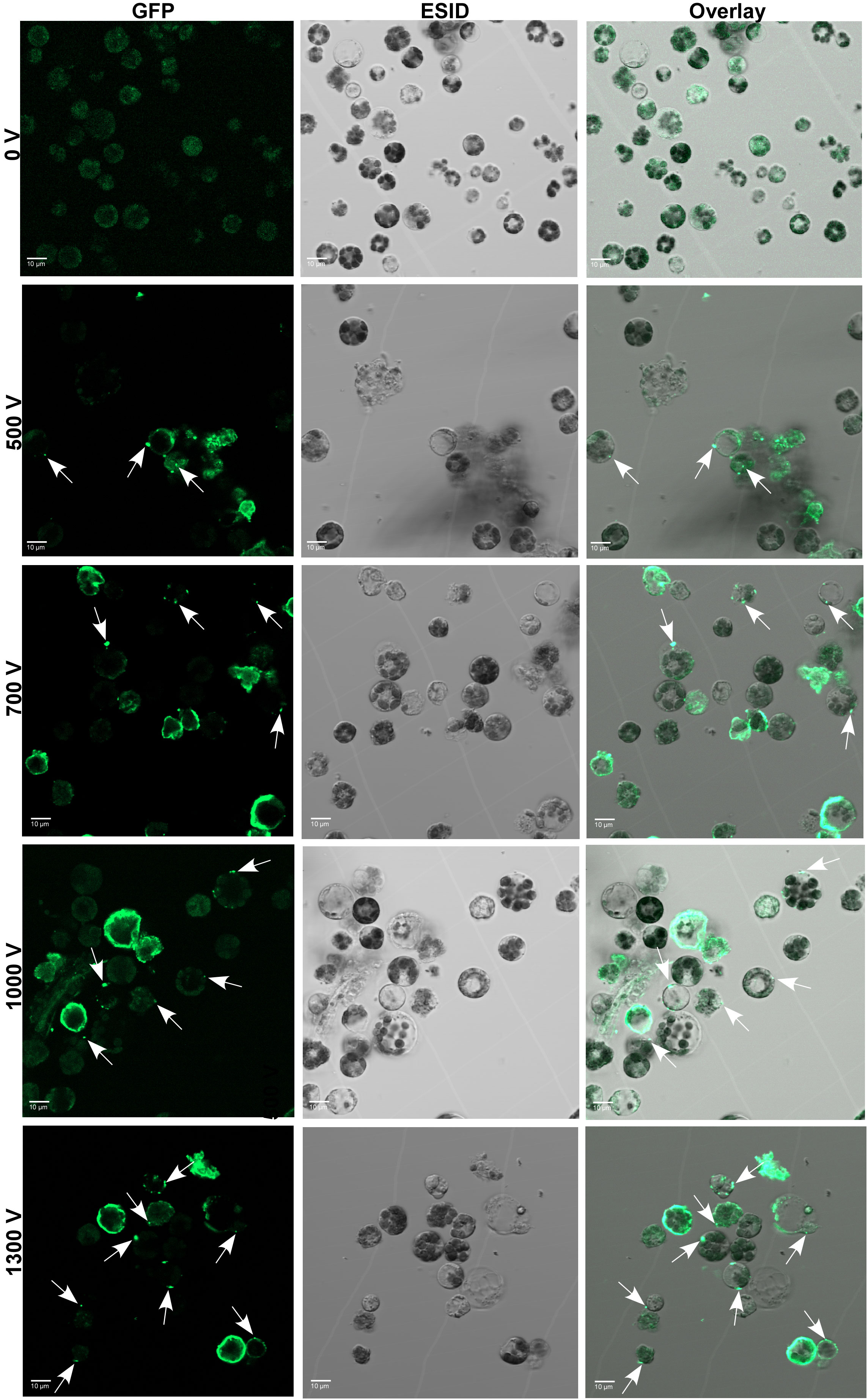
Figure 3 Delivery and cellular localization of Cas9-GFP to soybean protoplasts through electro-transfection. GFP-Cas9 in electro-transfected (0 V to 1,300 V) protoplasts at 24 h after electroporation was seen using a laser scanning confocal microscope under GFP (left panel) and bright field of ESID channel (middle panel). Right panel: representative overlay images of GFP and bright field are shown. White arrows show the location of internalized GFP-Cas9. GFP, green fluorescent protein; ESID, electronically switchable illumination and detection module.
To demonstrate whether the established electro-transfection system can be employed for RGEN RNP-mediated genome editing in soybean, three sgRNAs were chosen and prepared based on our previous study (Subburaj et al., 2022), namely, T1, T3, and T5 to target exons 1, 2, and 4 of CPR5 gene, respectively (Figure 4A). RNP complex consisting of a 1:3 molar ratio of Cas9 (10 µg) and synthesized sgRNAs (30 µg) were electro-transfected into protoplasts using the Neon system at different pulsing voltages as mentioned earlier. Following transfection, the genomic DNA was extracted from control and transfected samples after 24 h of incubation. The targeted sites were PCR amplified using designed nested primers (Supplementary Table 1), and a T7E1 assay was carried out for preliminary detection of RNP-induced mutations for electro-transfected samples under different pulsing voltages. Upon agarose gel electrophoresis of T7E1-digested PCR products, cleaved PCR fragments at expected sizes were noted for all the RNP electro-transfected samples under 1,000 V and 1,300 V (Figures 4B–D). In addition, there were no cleaved PCR products observed for all the target sites under 500 V and or 700 V, except for T1 under 700 V; the same was observed for the wild type and the Cas9 alone transfected samples. This suggests that RNPs successfully induced site-specific double-strand breaks followed by DNA repair mechanisms within the GmCPR5 locus in soybean.

Figure 4 CRISPR/Cas9-mediated editing of the exogenous GmCPR5 gene in soybean protoplasts using electro-transfection. (A) GmCPR5 locus, location of target sites (T1, T3, and T5), and their gRNA sequences. (B–D) Results of T7E1 endonuclease assay for target sites T1, T3 and T5. Lane M: a DNA ladder. Lane WT: non- transfected wild type (control). Lane Cas9: transfected with SpCas9 only. Lanes T1–T5: electro-transfected with RNPs at 700 V, 1,000 V, and 1,300 V. Red arrows indicate the T7E1-mediated cleaved bands. The mutation patterns observed by targeted deep sequencing for the corresponding target sites of T1–T5 at GmCPR5 loci by electro-transfection at 1,300 V are shown on the right panel. Wild-type (WT) nuclease target sequences are in bold and underlined. PAM sites are denoted in red. RNPs, ribonucleoproteins; gRNA, guide RNA.
To estimate InDel frequency and characterization of mutation patterns, targeted deep sequencing was performed on the DNA samples with positive results from the T7E1 assay. The raw data from targeted deep sequencing were submitted to the National Center for Biotechnology Information (NCBI) Bioproject (http://www.ncbi.nlm.nih.gov/bioproject/) under the accession number PRJNA983990. Targeted deep sequencing results confirmed that the InDels present at all three target sites including T1, T3, and T5 under different pulsing voltages, similar to that in the T7E1 cleavage assay. As shown in Table 1, the percentage of InDel (mutation) frequency of each target site was estimated by dividing the number of mutated InDel sequences by the number of total sequences in the CPR5 target sequences using the Cas-Analyzer tool. The target sites of T1, T3, and T5 have InDel frequencies that ranged from a minimum of 2.1% to a maximum of 8.1% with an average frequency of 4.21% ± 0.73% in the GmCPR5 locus. It was found that the estimated InDel frequencies were increased with the increase in pulsing voltage. For the T1 target site, InDel frequency following electro-transfection at 700 V, 1,000 V, and 1,300 V were 2.1%, 3.7%, and 8.1%, respectively. Similarly, in T3 and T5, the frequency was increased from 2.1% and 3.8% (at 1,000 V) to 3.8% and 5.7% at 1,300 V, respectively (Table 1).
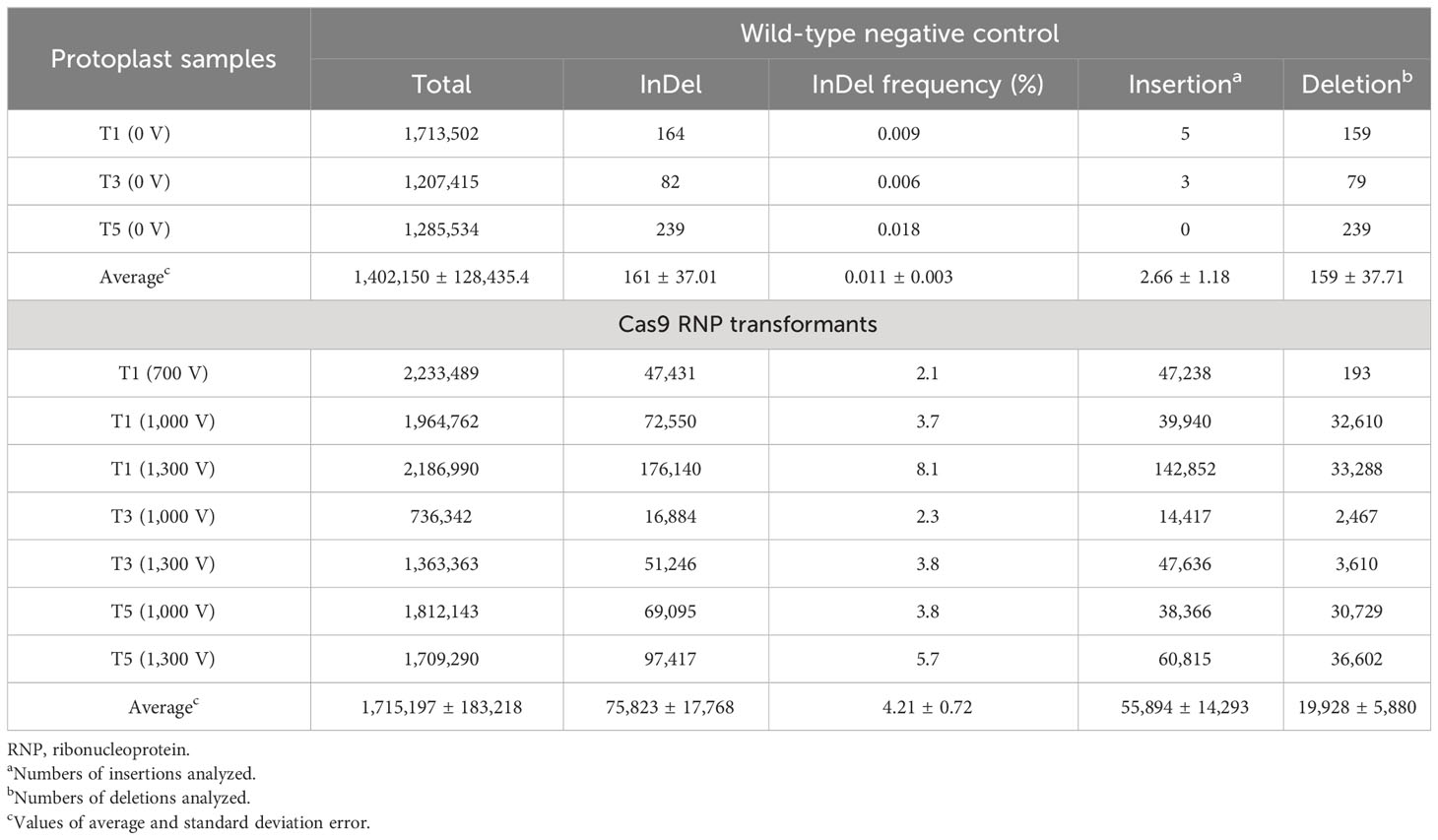
Table 1 Estimation of mutation rates at GmCPR5 locus following electro-transfection of Soybean protoplasts.
The mutation pattern for each target site was further characterized. The distribution of the five most frequent alleles observed around the cleavage site in GmCPR5 loci after electro-transfection under different pulsing voltages is presented in Supplementary Figure 1 (700 V and 1,000 V) and Figure 4 (1,300 V). gRNAs produced InDels at corresponding target sites, which ranged from +1 to −6 nucleotide (nt) in length, and all induced mutations were observed prevalently at 4th nt upstream of the PAM site except for target sites with few alleles, which were the T1 (1,000 V and 1,300 V), T3 (1,300 V), and T5 (1,000 V) (Supplementary Figures 1B, C; Figures 4B–D). As shown in Table 1, the highest mutation rate was observed only in protoplast samples under 1,300 V, in which the five most frequent alleles were responsible for 6%, 3.14%, and 4% of the total mutation rates for T1 (8.1%), T3 (3.8%), and T5 (5.7%) (Figures 4B–D). Further, +1 nt insertion of adenine or thiamine was found prevalent among the observed InDels in the frequent alleles for all the target sites (Supplementary Figure 1; Figure 4). Apart from this, some of the target sites possessed +1 nt insertion of guanine (T3 under 1,000 V and 1,300 V) and cytosine (T5 under 1,300 V) (Supplementary Figure 1C; Figures 4C, D). A +2 nt insertion (thiamine and adenine) was also noted for T1 under 700 V (Supplementary Figure 1A). A maximum of −5- and −6-bp deletions were observed for T1 (1,000 V) and T5 (1,000 and 1,300 V) following treatments, respectively (Supplementary Figures 1B, D; Figure 4D). In summary, all the mutant alleles have frameshift mutations, which would result in a complete loss of CPR5 protein function compared to the wild-type alleles of GmCPR5. The result analysis from targeted deep sequencing demonstrated that the electro-transfection of RGEN RNPs using the Neon system can be used for site-directed mutagenesis in soybean protoplasts.
The CRISPR/Cas9 technology has emerged as a powerful tool for genome editing and crop improvement. Transient gene expression in leaf mesophyll-derived protoplasts is an excellent resource for genetic manipulation and genome editing using CRISPR/Cas9, which enables the high-throughput analysis of gene functions (Zhu et al., 2016; Xu et al., 2020; Yu et al., 2021). The efficiency of genome editing highly relies on the transfection system and delivery methods including PEG-mediated, electroporation, and microinjection, which have been utilized to introduce DNA, RNA, or protein into plant cells (Masani et al., 2014; Subburaj et al., 2016; Lee et al., 2020; Yeap W. et al., 2021). In addition to electroporation and microinjection, PEG-mediated transient expression technology has been predominantly applied in both model and non-model plants due to their high transfection efficiency as reviewed by Zhang et al. (2021). To date, there have been no reports of CRISPR/Cas9-mediated genome editing via electroporation in soybean species, although several studies have utilized it for transfecting plasmid DNA into soybean protoplasts (Christou et al., 1987; Lin et al., 1987; Dhir et al., 1991). In this study, we standardized the protocols for the delivery of CRISPR/Cas9 RNPs into soybean protoplasts using the Neon electroporation system for the first time. The detection of site-directed mutations in endogenous targeted genes in this work would provide an additional and alternative methodology to the PEG-mediated transient expression technology-based genome editing of the soybean and their related species.
A stable protoplast isolation method and choice of protoplast source with high-quality cells are required for efficient transient gene expression studies (Huang et al., 2013; Li et al., 2018; Lee et al., 2020). Recently, we developed a successful protoplast isolation method for unifoliate leaves of soybean (Subburaj et al., 2022). Following this method, we isolated the protoplasts from trifoliate leaves and obtained a high quantity (2 × 106 cells) and viability of protoplasts (70% ± 2.1%) in this study. While ensuring high transfection efficiency in protoplast cells, and at the same time retaining viability and ability to differentiate (cell division), we have tested the protoplasts of both unifoliate and trifoliate to the different pulsing voltages of 500 V to 1,000 V in a pilot study. It was found that trifoliate cells endure and survive under electrophoretic conditions compared to the unifoliate cells, as they were greatly damaged after electroporation, even at 500 V. In addition, the isolated trifoliate cells had exhibited first protoplast divisions at 5 days after culture initiation as noted for the unifoliate cells in our previous study (Subburaj et al., 2022). Cotyledon and zygotic embryo-derived protoplasts were used in earlier soybean studies for the electroporation-mediated transient expression of introduced DNA molecules (Christou et al., 1987; Lin et al., 1987; Dhir et al., 1991). In this study, we suggest that trifoliate leaf-derived protoplasts of Neon electro-transfection would facilitate DNA-free gene editing using the CRISPR/Cas9 system because of its easy isolation and manipulation.
Electroporation experiments often need an appropriate buffer to provide conductivity as well as cell survival. Therefore, we have investigated the impact of electroporation buffers on protoplasts survival by electroporating them with three different buffers including MMG solution, Neon R, and HEPES buffer as those reported in earlier studies (Dhir et al., 1992; Bhowmik et al., 2018; Lee et al., 2020). Soybean protoplasts did not survive Neon R and HEPES buffers but only survived in MMG solution following electro-transfection, similar to the observed results in cabbage protoplasts (Lee et al., 2020). Upon electroporation with optimized protocol, our results revealed that cell survival and division efficiency decreased with increased pulse voltage. At the same time, the transfection efficiency was increased when the protoplasts were subjected to increasing pulse voltage. This difference might be due to the high electrical pulse applied to protoplast membranes, but more likely, the cellular damage could be induced by increasing voltage, which results in cell death. Many transient pores could also be created on the protoplast membranes under high voltage, which allows the uptake of exogenous GFP-Cas9, thereby increasing the efficiency of transfection. The relative frequency of cell viability was the highest at 500 V (76%) and the lowest at 1,300 V (57%), suggesting that high voltages could decrease the survival rate of cells. Similarly, Bhowmik et al. (2018) also reported that the microspore survival increased with decreasing voltage, with the highest microspore survival of 50% noted at 500 V compared to 1,000 V. Moreover, the efficiency of cell division following 0 V and 500 V was higher than that of other treatments such as 700 V, 1,000 V, and 1,300 V, indicating that the pulsing voltages did not affect the process of mitotic divisions of protoplasts, as they have undergone primary cell divisions at 5 days after culture initiation. In wheat microspores, the transfection efficiency has been noted to increase with decreasing voltage (Bhowmik et al., 2018). In contrast, in cabbage protoplasts, InDel frequencies increased with increasing voltage (Lee et al., 2020). GFP-tagged Cas9 was electro-transfected into soybean protoplasts, the GFP-Cas9 signal was successfully detected in intracellular compartments of electroporated protoplasts, and the highest transfection efficiency was noted under 1,300 V (∼ 40%) compared to other treatments (∼ 20%–23%), indicating that electro-transfection efficiency increased with increasing pulse voltage in soybean protoplasts. This has also corresponded well with the observed InDel frequency rates from targeted deep sequencing results. The InDel frequency of GmCPR5 following sgRNA (T1, T3, and T5) transfection was increased with increasing pulse voltage from 700 V or 1,000 V to 1,300 V. In this study, the calculated InDel frequencies of T1 (700 V) and T5 (1,000 V) were slightly higher than the observed InDel frequencies of 1.2% (750 V) and 3.4% (1,000 V) for PDS1 gene in cabbage protoplasts following electro-transfection (Lee et al., 2020). By PEG-mediated transfection of RNPs, a very low editing efficiency has been achieved in protoplasts of several plant species including cabbage (0.09%–2.25%) (Murovec et al., 2018), Cavendish banana (0.19%– 0.92%) (Wu et al., 2020), wild tobacco (0.01–0.9%) (Kim et al., 2017), and grapevine (0.1%) (Malnoy et al., 2016). Further, the targeted deep sequencing results showed that three sgRNAs (T1, T3, and T5) were induced with various mutation pattern sizes ranging from +1 to −6 nt in length at targeted sites, which were found similar to our previous mutagenic CRISPR/Cas9 study in soybean using PEG-mediated delivery method (Subburaj et al., 2022). Taken together, in this study, by electro-transfection, we achieved much higher mutation rates (2.1%–8.1%), suggesting that electro-transfection may potentially be helpful and applicable to the abovementioned plant species to improve editing efficiency compared with the PEG-mediated method.
The high pulse voltage of 1,400 V or 1,250 V has been reported to be lethal to wheat microspores and cabbage protoplasts (Bhowmik et al., 2018; Lee et al., 2020). In this study, we attempted to electro-transfect the soybean protoplasts at 1,300 V and found that trifoliate leaf-derived protoplasts could survive well in post-electroporation, as it had shown 57% viability and ability to divide in culture. However, this study expects further improvements to increase transfection efficiency with a progressive decrease in pulsing voltage within 1,000 V. The regeneration of protoplasts is highly necessary for DNA-free genome editing systems. In this study, after 12 days in culture, dividing protoplasts could not form colonies regardless of treatment of pulsing voltages. This is probably due to the constraining factors including the recalcitrant nature of soybean, genotype-dependent response to culture conditions, type of explant, and age (Wei and Xu, 1988; Cutler et al., 1991; Dhir et al., 1992; Eeckhaut et al., 2013). As demonstrated in previous studies (Wei and Xu, 1988; Dhir et al., 1991), well-established protocols exist for the regeneration of soybean protoplasts. Perhaps a genotype of the perfect donor species or cultivar of soybean is advisable to improve the chances of regeneration of gene-edited protoplasts. In addition, the reduction of non-transfected cells by enriching transfected cells through the application of a fluorescence-activated cell sorting (FACS) system could also have a positive effect on the regeneration of successfully edited cells. Altogether, the protoplast-based gene editing through the Neon electro-transfection system described in this study provides an alternative gene editing platform to the PEG-mediated system for evaluating the efficacy of CRISPR systems as well as gene functional validations in soybean and other related species.
Our study demonstrated that the CRISPR/Cas9 DNA-free genome editing is effective and efficient in editing soybean genes using the Neon electroporation system. In this study, we demonstrated a time- and cost-efficient in vitro electro-transfection assay that provides a rapid assessment and evaluation of gRNA efficiency in soybean protoplasts. The balance between higher voltages and higher targeted mutagenesis will be the challenge for future applications of this method. Nevertheless, a DNA-free transformation system in soybeans to generate non-transgenic gene-edited mutants is highly desired to reduce the occurrence of vector backbone spurious introgressions. This enabling platform for genome editing may accelerate the exploration of gene function for trait improvement in soybean lines. In addition, our study offers new insights into other related species, such as the pinto bean and other Phaseolus species, that share similar limitations in genetic transformation and inefficient tissue culture propagation and regeneration processes using other non-transgenic approaches.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI Bioproject accession PRJNA983990.
SS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SA-T: Conceptualization, Formal Analysis, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
This project was funded by The Research Council of Norway (Project Number: 301911 - FOODPRINT: Traceability and labelling of gene-edited products in the food chain).
We would like to thank Jennifer A. L. Nunn for helping to take the confocal microscopy images.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1255819/full#supplementary-material
Supplementary Figure 1 | CRSIPR/Cas9-mediated editing of the exogenous GmCPR5 gene in Soybean protoplasts using electro-transfection and characterization of five most frequent mutation patterns. (A) Target sites of T1 at GmCPR5 loci by electro-transfection at 700 V. (B) T1 at GmCPR5 loci by electro-transfection at 1000 V. (C) T3 at GmCPR5 loci by electro-transfection at 1000 V. (D) T5 at GmCPR5 loci by electro-transfection at 1000 V. Wild type (WT) nuclease target sequences were in bold and underlined. PAM sites denoted by red.
9M CPW, 9% mannitol with CPW salts; CLSM, confocal laser scanning microscope; CPR5, constitutive pathogen response 5; CPW, cell and protoplast washing; ESID, electronically switchable illumination and detection module; FDA, fluorescein diacetate; GFP, green fluorescent protein; HDR, homology-directed repair; InDels, insertions/deletions; NCBI, National Center for Biotechnology Information; NHEJ, non-homologous end-joining; PEG, polyethylene glycol; RGEN, RNA-guided endonuclease; RNP, ribonucleoprotein; sgRNA, single guide RNA; SRA, Sequence Read Archive; T7E1, T7 endonuclease I; V, voltage.
Adedeji, O. S., Naing, A. H., Kim, C. K. (2020). Protoplast isolation and shoot regeneration from protoplast-derived calli of Chrysanthemum cv. White ND. Plant Cell Tiss. Organ. Cult. 141, 571–581. doi: 10.1007/s11240-020-01816-3
Baek, K., Kim, D. H., Jeong, J., Sim, S. J., Melis, A., Kim, J. S., et al. (2016). DNA-free two-gene knockout in Chlamydomonas reinhardtii via CRISPR-Cas9 ribonucleoproteins. Sci. Rep. 6, 30620. doi: 10.1038/srep30620
Bhowmik, P., Ellison, E., Polley, B., Bollina, V., Kulkarni, M., Ghanbarnia, K., et al. (2018). Targeted mutagenesis in wheat microspores using CRISPR/Cas9. Sci. Rep. 8, 6502. doi: 10.1038/s41598-018-24690-8
Christou, P., Murphy, J. E., Swain, W. F. (1987). Stable transformation of soybean by electroporation and root formation from transformed callus. Proc. Natl. Acad. Sci. U.S.A. 84, 3962–3966. doi: 10.1073/pnas.84.12.3962
Chu, P., Agapito-Tenfen, S. Z. (2022). Unintended genomic outcomes in current and next generation GM techniques: A systematic review. Plants (Basel). 11, 2997. doi: 10.3390/plants11212997
Cutler, A. J., Saleem, M., Wang., H. (1991). Cereal protoplast recalcitrance. In Vitro Cell. Dev. Biol. Plant 27, 104–111. doi: 10.1007/BF02632192
Dhir, S. K., Dhir, S., Widholm, J. M. (1991). Plantlet regeneration from immature cotyledon protoplasts of soybean (Glycine max L.). Plant Cell Rep. 10, 39–43. doi: 10.1007/BF00233030
Dhir, S. K., Dhir, S., Widholm, J. M. (1992). Regeneration of fertile plants from protoplasts of soybean (Glycine max L. Merr.): genotypic differences in culture response. Plant Cell Rep. 11, 285–289. doi: 10.1007/BF00235083
Eeckhaut, T., Lakshmanan, P. S., Deryckere, D., Van Bockstaele, E., Van Huylenbroeck, J. (2013). Progress in plant protoplast research. Planta. 238, 991–1003. doi: 10.1007/s00425-013-1936-7
Feng, Z., Zhang, B., Ding, W., Liu, X., Yang, D., Wei, P., et al. (2013). Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 23, 1229–1232. doi: 10.1038/cr.2013.114
Ghogare, R., Ludwig, Y., Bueno, G. M., Slamet-Loedin, I. H., Dhingra, A. (2021). Genome editing reagent delivery in plants. Transgenic Res. 30, 321–335. doi: 10.1007/S11248-021-00239-W
Hamada, H., Liu, Y., Nagira, Y., Miki, R., Taoka, N., Imai, R. (2018). Biolistic-delivery-based transient CRISPR/Cas9 expression enables in planta genome editing in wheat. Sci. Rep. 8, 14422. doi: 10.1038/s41598-018-32714-6
Huang, H., Wang, Z., Cheng, J., Zhao, W., Li, W., Wang, H., et al. (2013). An efficient cucumber (Cucumis sativus L.) protoplast isolation and transient expression system. Sci. Hortic. Amst. 150, 206–212. doi: 10.1016/j.scienta.2012.11.011
Jiang, F., Doudna, J. A. (2017). CRISPR-cas9 structures and mechanisms. Annu. Rev. Biophys. 46, 505–529. doi: 10.1146/annurev-biophys-062215-010822
Jinek, M., East, A., Cheng, A., Lin, S., Ma, E., Doudna, J. (2013). RNA programmed genome editing in human cells. eLife 2, e00471. doi: 10.7554/eLife
Jupe, F., Rivkin, A. C., Michael, T. P., Zander, M., Motley, S. T., Sandoval, J. P., et al. (2019). The complex architecture and epigenomic impact of plant T-DNA insertions. PloS Genet. 15, e1007819. doi: 10.1371/journal.pgen.1007819
Kao, K. N. (1977). Chromosomal behaviour in somatic hybrids of soybean- Nicotiana glauca. Molec. Gen. Genet. 150, 225–230. doi: 10.1007/BF00268120
Kim, H., Choi, J., Won, K. H. (2020). A stable DNA-free screening system for CRISPR/RNPs-mediated gene editing in hot and sweet cultivars of Capsicum annuum. BMC Plant Biol. 20, 449. doi: 10.1186/s12870-020-02665-0
Kim, H., Kim, S. T., Ryu, J., Kang, B. C., Kim, J. S., Kim, S. G. (2017). CRISPR/Cpf1-mediated DNA-free plant genome editing. Nat. Commun. 8, 14406. doi: 10.1038/ncomms14406
Lee, M. H., Lee, J., Choi, S. A., Kim, Y.-S., Koo, O., Choi, S. H., et al. (2020). Efficient genome editing using CRISPR-Cas9 RNP delivery into cabbage protoplasts via electrotransfection. Plant Biotechnol. Rep. 14, 695–702. doi: 10.1007/s11816-020-00645-2
Lei, J., Li, Y., Dai, P., Liu, C., Zhao, Y., You, Y., et al. (2022). Efficient virus-mediated genome editing in cotton using the CRISPR/Cas9 system. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1032799
Li, J., Liao, X., Zhou, S., Liu, S., Jiang, L., Wang, G. (2018). Efficient protoplast isolation and transient gene expression system for Phalaenopsis hybrid cultivar ‘Ruili Beauty’. In Vitro Cell. Dev. Biol. Plant 54, 87–93. doi: 10.1007/s11627-017-9872-z
Liang, Z., Chen, K., Li, T., Zhang, Y., Wang, Y., Zhao, Q., et al. (2017). Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 8, 14261. doi: 10.1038/ncomms14261
Lin, W., Odell, J. T., Schreiner, R. M. (1987). Soybean protoplast culture and direct gene uptake and expression by cultured soybean protoplasts. Plant Physiol. 84, 856–861. doi: 10.1104/pp.84.3.856
Lin, Q., Zong, Y., Xue, C., Wang, S., Jin, S., Zhu, Z., et al. (2020). Prime genome editing in rice and wheat. Nat. Biotechnol. 38, 582–585. doi: 10.1038/s41587-020-0455-x
Liu, H., Chen, W., Li, Y., Sun, L., Chai, Y., Chen, H., et al. (2022). CRISPR/cas9 technology and its utility for crop improvement. Int. J. Mol. Sci. 23, 10442. doi: 10.3390/ijms231810442
Liu, H., Zhang, B. (2020). Virus-based CRISPR/cas9 genome editing in plants. Trends Genet. 36, 810–813. doi: 10.1016/j.tig.2020.08.002
Malnoy, M., Viola, R., Jung, M. H., Koo, O. J., Kim, S., Kim, J. S., et al. (2016). DNAFree genetically edited grapevine and apple protoplast using CRISPR/cas9 ribonucleoproteins. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01904
Mao, Y., Yang, X., Zhou, Y., Zhang, Z., Botella, J. R., Zhu, J.-K. (2018). Manipulating plant RNA-silencing pathways to improve the gene editing efficiency of CRISPR/Cas9 systems. Genome Biol. 19, 1–15. doi: 10.1186/s13059-018-1529-7
Masani, M. Y., Noll, G. A., Parveez, G. K., Sambanthamurthi, R., Prüfer, D. (2014). Efficient transformation of oil palm protoplasts by PEG-mediated transfection and DNA microinjection. PloS One 9, e96831. doi: 10.1371/journal.pone.0096831
Murovec, J., Guˇcek, K., Bohanec, B., Avbelj, M., Jerala, R. (2018). DNA-Free Genome Editing of Brassica oleracea and B. rapa Protoplasts Using CRISPRCas9 Ribonucleoprotein Complexes. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01594
Nicolia, A., Andersson, M., Hofvander, P., Festa, G., Cardi, T. (2021). Tomato protoplasts as cell target for ribonucleoprotein (RNP)- mediated multiplexed genome editing. Plant Cell Tiss. Organ. Cult. 144, 463–467. doi: 10.1007/s11240-020-01954-8
Rahman, S. U., McCoy, E., Raza, G., Ali, Z., Mansoor, S., Amin, I. (2023). Improvement of soybean; A way forward transition from genetic engineering to new plant breeding technologies. Mol. Biotechnol. 65, 162–180. doi: 10.1007/s12033-022-00456-6
Rezazade Bazaz, M., Dehghani, H. (2022). From DNA break repair pathways to CRISPR/Cas-mediated gene knock-in methods. Life Sci. 295, 120409. doi: 10.1016/j.lfs.2022.120409
Rupp, L. J., Schumann, K., Roybal, K. T., Gate, R. E., Ye, C. J., Lim, W. A., et al. (2017). CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 7, 737. doi: 10.1038/s41598-017-00462-8
Sandhya, D., Jogam, P., Allini, V. R., Abbagani, S., Alok, A. (2020). The present and potential future methods for delivering CRISPR/cas9 components in plants. J. Genet. Eng. Biotechnol. 18, 25. doi: 10.1186/S43141-020-00036-8
Sant’Ana, R., Caprestano, C. A., Nodari, R. O., Agapito-Tenfen, S. Z. (2020). PEG-delivered CRISPR-cas9 ribonucleoproteins system for gene-editing screening of maize protoplasts. Genes. 11, 1029. doi: 10.3390/genes11091029
Seki, A., Rutz, S. (2018). Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T cells. J. Exp. Med. 215, 985–997. doi: 10.1084/jem.20171626
Shin, S. E., Lim, J. M., Koh, H. G., Kim, E. K., Kang, N. K., Jeon, S., et al. (2016). CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Sci. Rep. 6, 27810. doi: 10.1038/srep27810
Subburaj, S., Chung, S. J., Lee, C., Ryu, S. M., Kim, D. H., Kim, J. S., et al. (2016). Site-directed mutagenesis in Petunia × hybrida protoplast system using direct delivery of purified recombinant Cas9 ribonucleoproteins. Plant Cell Rep. 35, 1535–1544. doi: 10.1007/s00299-016-1937-7
Subburaj, S., Zanatta, C. B., Nunn, J. A. L., Hoepers, A. M., Nodari, R. O., Agapito-Tenfen, S. Z. (2022). A DNA-free editing platform for genetic screens in soybean via CRISPR/cas9 ribonucleoprotein delivery. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.939997
Tyagi, S., Spörlein, B., Tyagi, A. K., Herrmann, R. G., Koop, H. U. (1989). PEG- and electroporation-induced transformation in Nicotiana tabacum: influence of genotype on transformation frequencies. Theor. Appl. Genet. 78, 287–292. doi: 10.1007/BF00288813
Wei, Z. M., Xu, Z. H. (1988). Plant regeneration from protoplasts of soybean (Glycine max L.). Plant Cell Rep. 7, 348–351. doi: 10.1007/BF00269935
Woo, J. W., Kim, J., Kwon, S. I., Corvalán, C., Cho, S. W., Kim, H., et al. (2015). DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 33, 1162–1164. doi: 10.1038/nbt.3389
Wu, S., Zhu, H., Liu, J., Yang, Q., Shao, X., Bi, F., et al. (2020). Establishment of a PEG-mediated protoplast transformation system based on DNA and CRISPR/Cas9 ribonucleoprotein complexes for banana. BMC Plant Biol. 20, 425. doi: 10.1186/s12870-020-02609-8
Xu, J., Kang, B. C., Naing, A. H., Bae, S. J., Kim, J. S., Kim, H., et al. (2020). CRISPR/Cas9-mediated editing of 1-aminocyclopropane-1-carboxylate oxidase1 enhances Petunia flower longevity. Plant Biotechnol. J. 18, 287–297. doi: 10.1111/pbi.13197
Yeap W., -C., Norkhairunnisa, C. M. K., Norfadzilah, J., Muhammad Rashdan, M., Appleton, D. R., Harikrishna, K. (2021). An efficient clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 mutagenesis system for oil palm (Elaeis guineensis). Front. Plant Sci. 12. doi: 10.3389/fpls.2021.773656
Yu, J., Tu, L., Subburaj, S., Bae, S., Lee, G. J. (2021). Simultaneous targeting of duplicated genes in Petunia protoplasts for flower color modification via CRISPR-Cas9 ribonucleoproteins. Plant Cell Rep. 40, 1037–1045. doi: 10.1007/s00299-020-02593-1
Zhang, Z., Hua, L., Gupta, A., Tricoli, D., Edwards, K. J., Yang, B., et al. (2019). Development of an Agrobacterium-delivered CRISPR/Cas9 system for wheat genome editing. Plant Biotechnol. J. 17, 1623–1635. doi: 10.1111/pbi.13088
Zhang, Y., Iaffaldano, B., Qi., Y. (2021). CRISPR ribonucleoprotein-mediated genetic engineering in plants. Plant Commun. 2, 100168. doi: 10.1016/j.xplc.2021.100168
Zhang, C., Liu, S., Li, X., Zhang, R., Li, J. (2022). Virus-induced gene editing and its applications in plants. Int. J. Mol. Sci. 23, 10202. doi: 10.3390/ijms231810202
Zhang, Q., Xing, H. L., Wang, Z. P., Zhang, H. Y., Yang, F., Wang, X. C., et al. (2018). Potential high-frequency off-target mutagenesis induced by CRISPR/Cas9 in Arabidopsis and its prevention. Plant Mol. Biol. 96, 445–456. doi: 10.1007/s11103-018-0709-x
Zhu, J., Song, N., Sun, S., Yang, W., Zhao, H., Song, W., et al. (2016). Efficiency and inheritance of targeted mutagenesis in maize using CRISPR-cas9. J. Genet. Genomics 43, 25–36. doi: 10.1016/j.jgg.2015.10.006
Keywords: CRISPR/Cas9 RNPs, gene-editing, neon electroporation system, soybean, protoplasts, target-deep sequencing, trait improvement
Citation: Subburaj S and Agapito-Tenfen SZ (2023) Establishment of targeted mutagenesis in soybean protoplasts using CRISPR/Cas9 RNP delivery via electro−transfection. Front. Plant Sci. 14:1255819. doi: 10.3389/fpls.2023.1255819
Received: 09 July 2023; Accepted: 08 September 2023;
Published: 29 September 2023.
Edited by:
Tong Zhang, South China Agricultural University, ChinaReviewed by:
Phetole Mangena, University of Limpopo, South AfricaCopyright © 2023 Subburaj and Agapito-Tenfen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah Zanon Agapito-Tenfen, c2FhZ0Bub3JjZXJlc2VhcmNoLm5v
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.