
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 17 October 2023
Sec. Plant Metabolism and Chemodiversity
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1249456
Gastrodia elata Blume (Tianma in Chinese), a myco-heterotrophic orchid, is widely distributed in China. Tubers derived from this orchid are traditionally used as both medicinal and edible materials. At present, five primary varieties of G. elata are recorded in the “Flora of China.” Among them, the three main varieties currently in artificial cultivation are G. elata f. elata (GR, red stem), G. elata f. glauca (GB, black stem), and G. elata f. viridis (GG, green stem). In our study, the metabolic profiles and chemical composition of these three varieties were determined via UPLC-MS/MS and HPLC-UV. In total, 11,132 metabolites were detected, from which multiple phytometabolites were identified as aromatic compounds, heteroatomic compounds, furans, carbohydrates, organic acids, and their derivatives. A number of differentially expressed metabolites (DEMs) were annotated as bioactive ingredients. Overall, parishins, vanilloloside, and gastrodin A/B in the GB group were markedly higher, whereas gastrodin, gastrol, and syringic acid were more enriched in the GG or GR groups. Moreover, HPLC fingerprint analysis also found six metabolites used as markers for the identification of Gastrodiae Rhizoma in the Chinese Pharmacopoeia, which were also typical DEMs in metabolomics. Of these, gastrodin, 4-hydroxybenzyl alcohol, citric acid, and adenosine were quantitatively detected, showing a similar result with the metabolomic data. In summary, our findings provide novel insights into the phytochemical ingredients of different G. elata varieties, highlighting diverse biological activities and healthcare value.
Gastrodia elata Blume (Tianma in Chinese) is a species of myco-heterotrophic herb in Orchidaceae. This orchid is a valuable traditional Chinese medicine that has been recorded in the ancient Chinese books “Shen Nong’s Classic of Materia Medica”. In this decade, the fresh tuber of G. elata has been considered an important healthcare food. This medicinal orchid has various pharmacological activities such as sedation, hypnosis, intelligence enhancement, anti-epileptics, analgesics, neuroprotective drugs, anti-depressants, cardiovascular protection, and immune enhancement (Zhan et al., 2016; Liu et al., 2018). A number of studies have exhibited that it had a strong potential for combating Alzheimer’s and Parkinson’s disease (Jang et al., 2015; Heese, 2020). Accordingly, G. elata is believed to possess several of the active components.
Records from “Flora of China” indicate that there are primarily five varieties of G. elata, which are widely distributed in Wumeng, Qinling, and Dabie mountains (Wu et al., 2009). They are G. elata f. elata (red stem, GR), G. elata f. glauca (black stem, GB), G. elata f. viridis (green stem, GG), G. elata f. flavid (yellow stem, GY), and G. elata f. alba (yellow-white stem, GYW). The numbers of wild G. elata have declined dramatically in recent years. Unique biological property, the damage of ecological environment, and over-exploitation were the main cause of wild resource reduction. Fortunately, artificial cultivation of G. elata has been developing in China, Japan, South Korea, and India for several decades (Guo et al., 2016). At present, there are three main cultivars in artificial cultivation: red Tianma (GR), black Tianma (GB), and green Tianma (GG). In general, G. elata varieties are primarily divided according to the characteristics in the stem and flower color. Of these, GR has the highest annual production, and GB has the highest price on market. That is mainly because GB was not only good in quality but also sweet in taste (Yu E. et al., 2022).
Over the past decade, numerous studies have reported on the major pharmaceutical application of a single variety of G. elata (Wu et al., 2023). Changes in compositional content have been related to cultivation area, fungal partner, and medicinal material processing, such as gastrodin and 4-hydroxybenzyl alcohol. Differences in chemical properties and biological activities among different varieties have not been widely investigated. From a recent study, polysaccharide characteristics were investigated among four different varieties of G. elata (Ji et al., 2022). Unfortunately, the multiple metabolites present in different G. elata are currently not described. Metabolomics is a rapidly developing technology that has effectively promoted plant sciences and drugs discovery. In this study, the global metabolic profile of three major G. elata varieties are investigated by UPLC-MS/MS-based metabolomics. Analyses and reviews of phytometabolites and their pharmacological effects were performed. In addition, HPLC was used for chromatographic fingerprint analysis and component examination. Our study has served as a foundation for the further development and use of different G. elata varieties.
The samples of red Tianma (GR), black Tianma (GB), and green Tianma (GG) varieties (Figure 1A) were collected from the planting area (104.29492°E and 27.76122°N, and the altitude was 1,870 mm), Xiaocaoba town, Yiliang County, Yunnan Province, China. Subsequently, the fresh tubers were washed and cut into slices. After freeze drying, samples from each group were stored at −80°C.
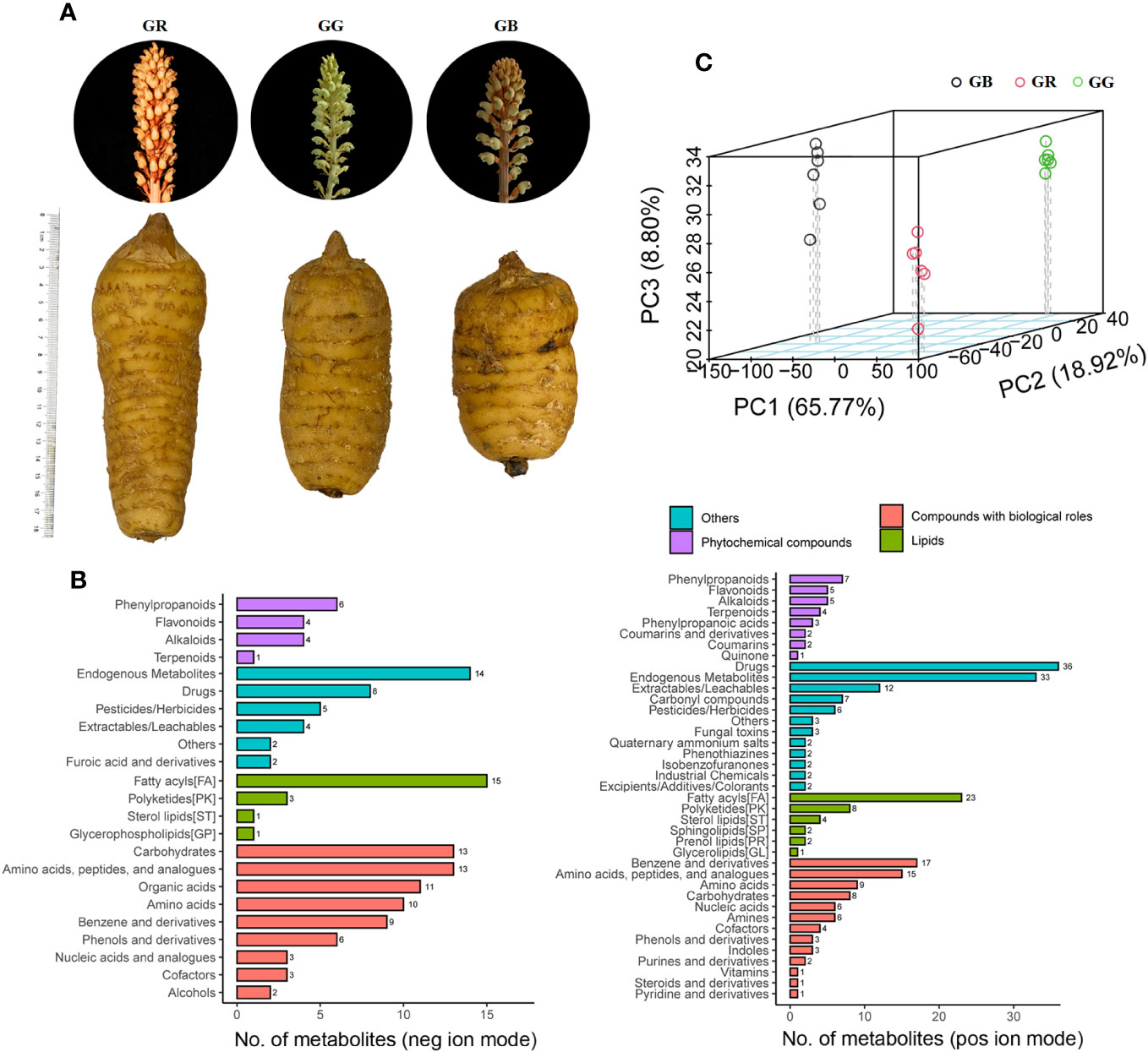
Figure 1 The tubers, stems, and flowers of three G. elata varieties (A), classification of metabolites (B), and PCA 3D score scatter plots (C).
Sample treatment and metabolomics analyses were performed on six biological replicates from the GR, GB, and GG groups. BGI Genomics Co., Ltd. (Shenzhen, China) performed metabolite extraction and UPLC-MS/MS as described in the prior studies (Dunn et al., 2011; Di Guida et al., 2016). Briefly, 50 mg of freeze-dried powder was accurately weighed for extraction by ultrasonication using a precooling buffer for 30 min. Buffer formulation was as follows: 800 μL of 70% methanol and 20 μL of internal standard (0.3 mg/ml d3-Leucine, 13C9-Phenylalanine, d5-Tryptophan, and 13C3-Progesterone). Then, the mixture was maintained at −20°C for 60 min and centrifuged at 14,000 rpm for 15 min. Next, 600 μL of supernatant was collected for untargeted metabolomics. Moreover, quality control (QC) sample was composed of 20 μL of supernatant from each samples. During experimental process, the QC sample was included in the queue every six test samples to evaluate stability and repeatability.
The samples were separated on a UPLC system (Waters 2D, Milford, MA, USA) with hypersil GOLD aQ column (100 mm × 2.1 mm i.d., 1.9 μm; Thermo Fisher Scientific, USA). Mobile phase A and B were 0.1% aqueous formic acid and acetonitrile containing 0.1% formic acid, respectively. Chromatographic parameters were as follows: injection volume, 5.00 μL; column temperature, 40°C; flowrate, 0.35 mL/min; and elution gradient, 95% A (0 min–2 min), 95%–5% A (2 min–22 min), 5% A (22 min–27 min), and 95% A (27.1 min–30 min). The Thermo Fisher Scientific Q Exactive mass spectrometer was used for the identification of metabolites, which were ionized by electrospray ionization (ESI) in both positive and negative ion mode. Mass spectrometry parameters were as follows: stepped NCE, 20–40–60 eV; sheath gas flowrate, 40 arb; aux gas flowrate, 10 arb; spray voltage(|KV|) 3.80 (+) and 3.20 (−); capillary temperature, 320°C; aux gas heater temperature, 350°C; and scanning range, 100–1,500 m/z.
The raw data from UPLC-MS/MS were analyzed by Compound Discoverer 3.1 software (Thermo Fisher Scientific, USA). Subsequently, the metaX from BGI’s metabolomics software package was used for metabolite annotation, classification, and statistical analysis (Wen et al., 2017). In order to analyze the overall metabolic differences between groups, principal component analysis (PCA) was performed. Using VIP >1, p < 0.05 and fold change (FC) > 2 or < 0.5 value as threshold to identify differentially expressed metabolites (DEMs) based on the OPLS-DA and Student’s t-test. Metabolites were annotated by BGI High-Resolution Plant Metabolome Database, mzCloud database, Human Metabolome (http://www.hmdb.ca/), KEGG databases (using MetaboAnalyst, https://www.metaboanalyst.ca/), and 211 phytometabolites isolated from G. elata described in previous studies.
The reference medicine materials of G. elata (purchased from National Institutes for Food and Drug Control) and freeze-dried samples were used for HPLC fingerprints analysis, as described in Chinese Pharmacopoeia 2020 edition and previous studies (Li et al., 2019; Commission, 2020). Briefly, 0.5 g of powder was ultrasonic extracted with 25 mL of 50% methanol for 30 min at 500 W and 40 kHz. After cooling, the filtrate was used for HPLC fingerprints analysis.
The HPLC system was composed of a 1260 HPLC system (Agilent Co., Milford, MA, United States) with Agela venusil ASB-C18 column (4.6 mm × 250 mm, 5 µm). Mobile phase A and B were acetonitrile and 0.1% phosphoric acid solution, respectively. Chromatographic parameters were as follows: injection volume, 3.00 μL; column temperature, 30°C; flowrate, 0.8 mL/min; detection wavelength, 220 nm; and elution gradient 3%–10% A (0 min–10 min), 10%–12% A (10 min–15 min), 12%–18% A (15 min–25 min), 18% A (25 min–40 min), and 18%–95% A (40 min–42 min). Chemstation software (Agilent) was used for data processing. Subsequently, Similarity Evaluation System for TCM chromatographic fingerprinting (2012 edition) were applied for evaluating the chromatographic profiles of the G. elata tuber.
Freeze-dried powder of test samples were used for the extraction of gastrodin and 4-hydroxybenzyl alcohol by the ultrasound-assisted method, as described in the Chinese Pharmacopoeia 2020 edition (Commission, 2020). In brief, 2 g of powder was weighed for extraction by ultrasonication using 50 mL ethyl alcohol for 30 min at 120 W and 40 kHz. After cooling, additional ethyl alcohol was added to compensate for the weight loss. Next, 10 mL of the supernatant fluid was concentrated, and ethanol was removed. The residue was further immersed with 25 mL of 3% acetonitrile. A 0.22-µm membrane was used to filter all the solution. Gastrodin and 4-hydroxybenzyl alcohol were determined by an external standard one-point method (50 and 25 μg/mL, respectively). The HPLC system consisted of a 1260 HPLC system (Agilent Co., Milford, MA, United States) with Agela venusil ASB-C18 column (4.6 mm × 250 mm, 5 µm). Mobile phase was acetonitrile–0.05% phosphoric acid solution (3:97, v:v). Chromatographic parameters were as follows: injection volume, 5.00 μL; column temperature, room temperature; flowrate, 0.8 mL/min; and detection wavelength, 220 nm.
Citric acid was extracted with by ultrasonics, as described in a previous study (Zhang et al., 2022). In brief, 1 g of freeze-dried powder of the test sample was extracted with 10 mL of 60% methanol for 30 min. After centrifugalizing, additional 60% methanol was added to compensate for the weight loss and filtered through a 0.22-µm membrane. The standard substance of citric acid was dissolved in 60% methanol to a concentration of 9 mg/mL. After diluting, the final concentrations of 0.1, 0.6, 0.9, 2, 3, 6, and 9 mg/mL were prepared (Supplementary Figure S1). The HPLC system consisted of a 1260 HPLC system (Agilent Co., Milford, MA, United States) with Akzo Nobel Kromasil 100-3.5-C18 column (4.6 mm × 150 mm, 3.5 µm). Mobile phase A and B were acetonitrile and 0.1% phosphoric acid solution, respectively. Chromatographic parameters were as follows: injection volume, 10.00 μL; column temperature, 30°C; flowrate, 1.0 mL/min; detection wavelength, 220 nm; and elution gradient, 15%–60% A (0 min–25 min).
The extract method of adenosine was similar with citric acid. The only difference between them was solvate. Adenosine was extracted using 10% methanol as solvate. The standard substance of adenosine was dissolved in water to a concentration of 500 μg/mL. After diluting, the final concentrations of 0.5, 1, 10, 25, 50, and 100 μg/mL were prepared (Supplementary Figure S1). The 1260 HPLC system (Agilent Co., Milford, MA, United States) with Akzo Nobel Kromasil 100-3.5-C18 column (4.6 mm × 150 mm, 3.5 µm) was used. Mobile phase A and B were acetonitrile and water, respectively. Chromatographic parameters were as follows: injection volume, 10.00 μL; column temperature, 30°C; flowrate, 1.0 mL/min; detection wavelength, 260 nm; and elution gradient, 10% A (0 min–5 min) and 90% A (5 min–10 min).
Chemstatio software (Agilent) was used for data processing. Student’s t-test for unpaired data by SPSS 19.0 was used for statistical analysis. A p-value < 0.05 was taken as significant.
Plant secondary metabolites offer broad perspectives for application in the food and pharmaceutical industry. The metabolomics data set primarily consisted of secondary metabolites, which indicated differences in phytometabolites among the G. elata varieties. In the present study of fresh tubers from red Tianma (GR), black Tianma (GB), and green Tianma (GG), untargeted metabolomics was performed using UPLC-MS/MS approach. The MS1 intensity chromatograms of QC samples are presented in Supplementary Figure S2. After removing those that are redundant and noisy, a total of 11,132 spectral signals were detected by positive and negative ion modes (6,767 and 4,365, respectively, Supplementary Table S1). Metabolites were annotated by comparing MS1 molecular weight, MS2 fragment spectra, retention time, and whether reference standards existed. In total, there were 990 metabolites annotated in our metabolomics data from the G. elata varieties samples (Supplementary Table S2). Based on KEGG database, 173 and 110 metabolites were classified into 20 categories based on positive and negative ion modes, respectively (Figure 1B, Supplementary Table S1). Most phytometabolites were enriched in fatty phenylpropanoids, flavonoids, alkaloids, and terpenoids. The major lipid metabolites were grouped into fatty acyls, polyketides, and sterol lipids. Moreover, numerous metabolites were identified as carbohydrates, phenols, benzenes, amino acids, and peptides in the metabolomics data set.
In addition to being a famous unsupervised pattern recognition method, PCA is also an important approach for reducing multi-dimensional data. Figure 1C shows the 3D score scatter plot of metabolomics profiles derived from G. elata varieties samples, with PC1 (x-axis), PC2 (y-axis), and PC3 (z-axis) accounting for 93.94% of the total variance. This result suggested that three G. elata varieties groups had large metabolic differences overall. In contrast, all of the GR (red dots), GB (black dots), and GG samples (green dots) were relatively concentrated, indicating small differences within the group. Meanwhile, OPLS-DA model, a supervised pattern recognition method, was used to further identify metabolite differences between groups. The score plot of three G. elata varieties is shown in Figure 2. The OPLS-DA score plot for each group showed strong clustering without overlap. The repeatability of OPLS-DA model was measured by permutation test (Figure 2). All of the goodness-of-fit parameter (R2) and the predictive ability parameter (Q2) were more than 0.9, indicating the repeatability of the model.
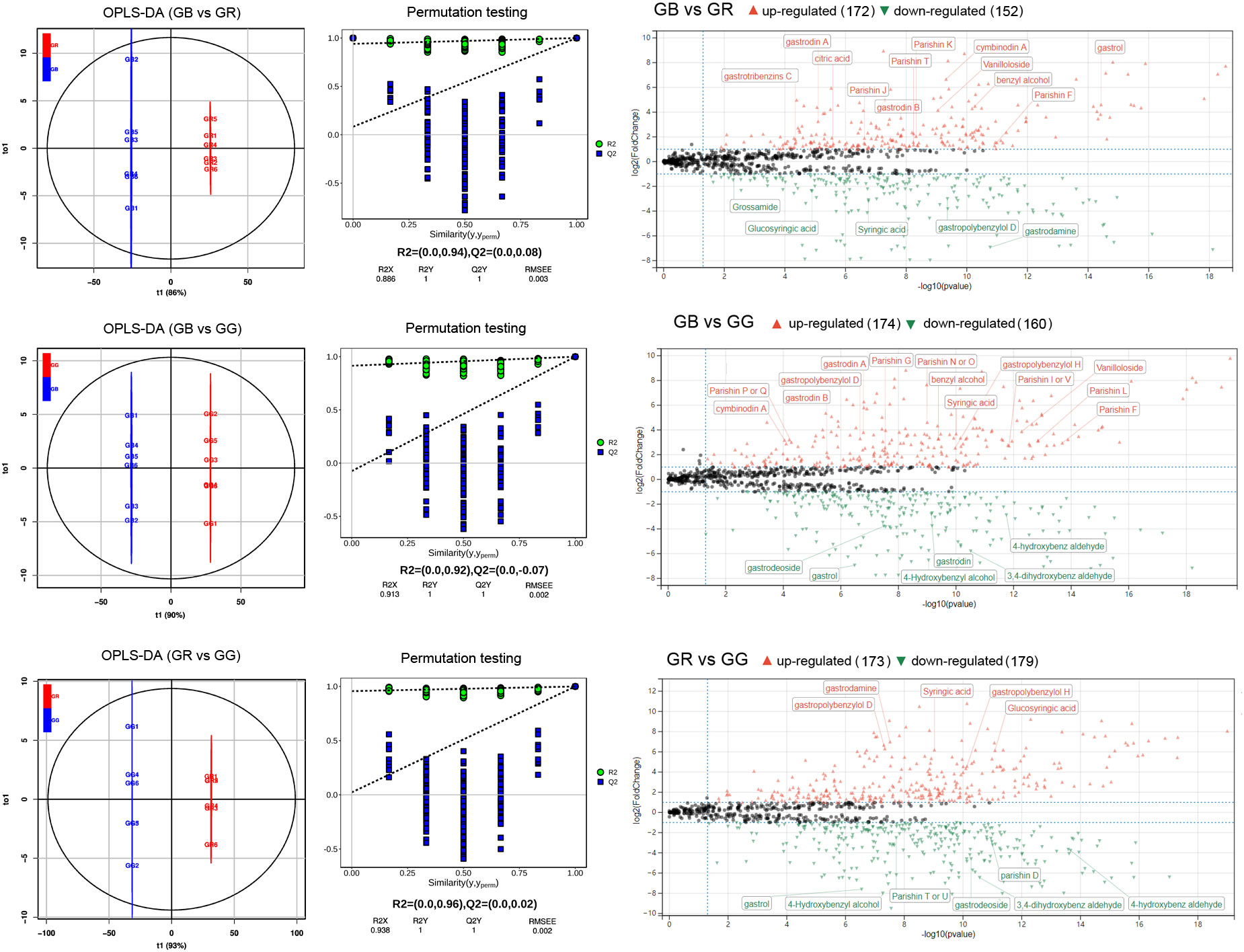
Figure 2 OPLS-DA score plots, permutation test, and volcano plot of differential metabolites between different G. elata varieties.
There was good separation of significantly differentially expressed metabolites (DEMs) between groups of G. elata varieties based on our criteria. When GB vs. GR, GB vs. GG, and GR vs. GG groups were compared, the 172, 174, and 173, and 152, 160, and 179 annotated metabolites (Figure 2) were identified as up- and down-accumulated metabolites, respectively. Taken together, numerous parishins (F, J, T, K, G, etc.), gastrodin A/B, and vanilloloside were up-accumulated metabolites in the GB group; gastropolybenzylol D, syringic acid, glucosyringic acid, and gastrodamine were more abundant in the GR group; and 4-hydroxybenzly alcohol, 3,4-dihydroxyben aldehyde, and gastrol were upregulated metabolites in the GG group.
To date, there are more than 210 phytometabolites reported by the previous research on G. elata (Su et al., 2023), including aromatic compounds, furans, steroids, carbohydrates, organic acids and their esters, N-containing compounds, S-containing compounds, and their glycosides (Yu H. et al., 2022). Here, most of them were identified in the metabolomics data set from three G. elata varieties (Supplementary Table S3). Furthermore, aromatic compounds, the most abundant ingredients in G. elata, were composed of monobenzyl compounds, polyaromatic substituted glycosides, polybenzyl ethers, polybenzyl compounds, parishins, etc. As shown in Supplementary Table S3, 104 aromatic compounds, 2 furans, 4 carbohydrates, 11 organic acids and their esters, 11 N-containing compounds, 8 S-containing compounds, and 3 other metabolites were detected.
Numerous reviews on G. elata have summarized the phytochemistry, compound structure, and quality control methods. Phenolics compounds and polysaccharides have been widely regarded as the typical and bio-active ingredients of G. elata (Shan et al., 2021). Among them, aromatic compounds are the main category. Recently, these aromatic compounds were further divided into eight subgroups on the basis of parent nucleus structure, connection modes, and subgroups function (Yu H. et al., 2022). DEMs annotated as aromatic compounds are shown in Figure 3A.
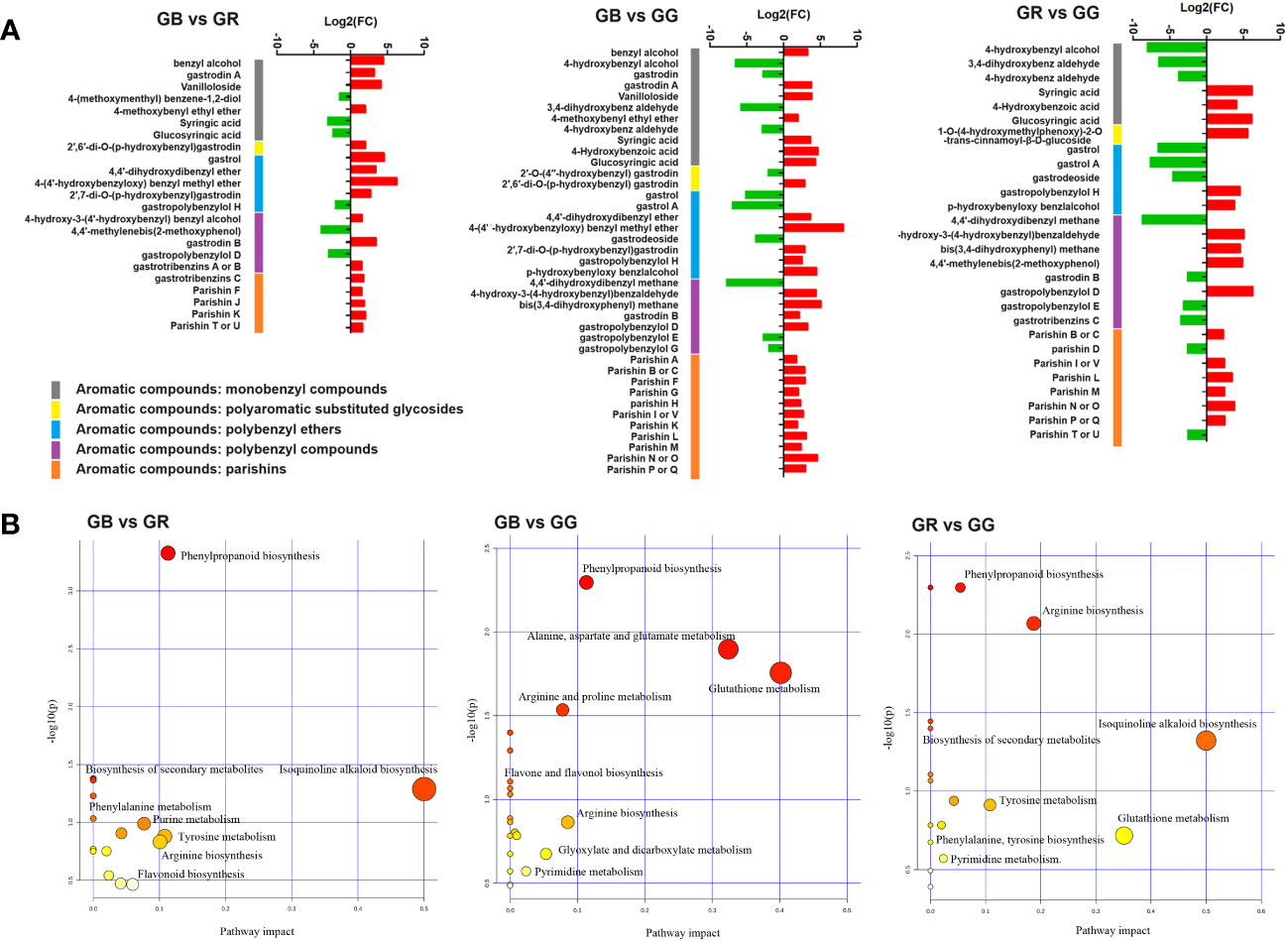
Figure 3 Fold change of differential metabolites annotated as aromatic compounds between different G. elata varieties (A) and KEGG pathway of DEMs between different G. elata varieties (B).
Monobenzyl compounds contain only one benzyl in the parent nucleus. It was widely reported that these constituents had neuroprotective effects, anti-inflammatory activity, anti-oxidant actions, and numerous other pharmaceutical functions. As shown in Figure 3A and Supplementary Table S3, there were 7, 11, and 6 monobenzyl compounds detected as DEMs between GB vs. GR, GB vs. GG, and GR vs. GG group, including gastrodin, 4-hydroxybenzyl alcohol, syringic acid, 4-hydroxybenz aldehyde, gastrodin A, and 6′-O-acetylgastrodin. In detail, gastrodin and 4-hydroxybenzyl alcohol, which are major bioactive constituents and two markers for the HPLC identification of Gastrodiae Rhizoma in the Chinese Pharmacopeia 2020 edition, were found to be up-accumulated in the GB group. The effects and mechanisms of gastrodin on the central nervous system disorders from preclinical models and the clinical evidence supporting pharmacological activities have been reviewed in a recent study (Liu et al., 2018). For example, an earlier study showed that gastrodin and 4-hydroxybenzyl alcohol exhibited potential anti-depressant effects in rats by downregulating monoamine metabolism and directly decreasing Slit1 expression (Chen et al., 2016). In addition, another study showed that 4-hydroxybenz aldehyde and vanilline (both more abundant in GG) derived from G. elata water extract reduced insulin resistance in diet-induced obese rats via inhibiting fat accumulation in adipocytes, promoting fat oxidation and increasing leptin signaling (Park et al., 2011). Moreover, benzyl alcohol, 4-methoxybenyl ethyl ether, and gastrodin A were more accumulated in GB. In summary, the results suggested that numerous monobenzyl compounds identified as DEMs were accumulated higher in the GG or GB groups.
Parishins are important ingredients in G. elata, which show several biological and pharmacologic effects. In the structure, the chemical bonds connecting the moieties of citric acid and gastrodin are ester linkages. The connections are easily broken by strong acidic or alkaline environment, enzymatic reaction, and continuous light (Tang et al., 2015). Our metabolomic analysis identified nearly all of the parishins recorded in the previous studies of G. elata (Supplementary Table S3). Among them, the relative contents of seven members, including parishin F, J, K, A, B, and H, was higher in the GB than in the GR or GG groups, whereas the relative content of parishin B/C, L, and M was lower in GG compared with GR group (Figure 3A). Parishins have been reported to possess unique bioactivity for the treatment of brain disorders such as anti-epileptic, anti-convulsive, sedative, and neuroprotective effects. A recent study reported that parishins exhibited the prevention of gut aging and the improvement of “leaky gut” in aged mice (Gong et al., 2023). Another study has shown that parishin J in G. elata could have protective effects on hypoxia/reoxygenation injury in H9c2 cardiomyocytes (Wang et al., 2020). Taken together, our results implied that various parishins were much more abundant in the GB than in the GR or GG groups.
Polyaromatic-substituted glycosides are novel compounds that have been detected in the past few years (Wang et al., 2019; Xu et al., 2019). In their structure, two or more hydroxy groups in a monosaccharide are substituted to aromatic groups. At present, only nine polyaromatic-substituted glycosides from G. elata have been studied for structural properties. All of them were identified in our metabolomics. Noteworthy, 1-O-(4-hydroxymethylphenoxy)-2-O-trans-cinnamoyl-β-D-glucoside, a new derivative of gastrodin with a trans-cinnamoyl unit (pos_3330) was up-accumulated in the GR group. Moreover, 2′-O-(4″-hydroxybenzyl) gastrodin was found to be more abundant in the GG than GB or GR groups. Unfortunately, there have been no pharmacological studies of these compounds to date.
Polybenzyl ethers were a class of organic compounds with at least one ether group that contained an oxygen atom connected to two benzyl groups. In 1981, bis-(4-hydroxybenzyl) ether was identified as the first polybenzyl ether in G. elata (Taguchi et al., 1981). To date, 21 polybenzyl ethers have been reported in the prior research (Yu H. et al., 2022). Among them, 17 members appeared in our metabolomics data (Supplementary Table S3). Most noteworthy, gastrols were more accumulated in the GB or GG compared with GR group. A prior study has exhibited that gastrol had relaxant effects on smooth muscle preparations isolated from the guinea pig ileum (Hayashi et al., 2002). In addition to that study, there has been few pharmacological research on polybenzyl ethers in G. elata.
Polybenzyl compounds are a kind of specific phenolic derivatives containing biphenylyl group, which have been widely studied in G. elata. In total, 31 polybenzyl compounds have been reported in previous studies, including 4,4′-dihydroxydibenzyl methane, gastrodin B, gastrol B, gastropolybenzylols, gastrodibenzins, and gastrotribenzins (Supplementary Table S3). One of these was 4,4′-dihydroxydibenzyl methane, a metabolite more abundant in the GG group, which was found to activate melatonin receptors (Yu H. et al., 2022). Similarly, gastropolybenzylol G, an upregulated metabolite in the GG, was shown to have agonist effects on melatonin receptors in vitro (Chen et al., 2019). Nevertheless, gastrodin B, an upregulated compounds in the GB and GG, has been suggested to have neuroprotective effects on H2O2-induced PC12 cell damage (Zhang et al., 2013). Plenty of studies have suggested that polybenzyl compounds from G. elata showed potential efficacy in improving sleep and enhancing immunity.
To date, the research on other aromatic compounds in G. elata is limited (Supplementary Table S3). Overall, eight furans, five phenylpropanoids, and one fused ring compound were isolated from this orchid (Yu H. et al., 2022). However, only a few metabolites were identified in our data, including cymbinodin A, 5-hydroxymethyl fural, 5-hydroxymethyl fural, and 5-(4-hydroxylbenzyloxymethyl)-furan-2-carbaldehyde.
Based on previous studies on G. elata, a variety of heteroatomic compounds such as nitrogen and sulfur compounds were isolated and detected. In the case of N-containing compounds, derivatives of amino acids and nucleosides were the main constituents reported by the previous studies (shown in Table 1. Supplementary Table S3). In more detail, gastrodamine (Zhan et al., 2016), di-(p-hydroxyl benzyl) hydroxylamine, and gastrodamine were upregulated heteroatomic aroma compounds in the GR than in the GB and GG groups. However, grossamide was not a DEM between the G. elata varieties groups, which was a representative lignanamide in hemp seed and possessed potential anti-neuroinflammatory actions (Luo et al., 2017). Moreover, there is a large number of nutritional and pharmacological research on nucleosides, amino acids, and their derivatives; therefore, we have not provided further information here.
The second type of heteroatomic compounds was the S-containing compounds, including thioester, sulfonamide, sulfone, sulfoxide, and sulfonic acid (Yu H. et al., 2022). In total, more than 20 S-containing compounds were isolated from G. elata. Here, there were eight metabolites that were detected by UPLC-MS/MS analysis (Table 1; Supplementary Table S3). Of these, S-(4-hydroxybenzyl)-glutathione was obviously accumulated in the GB group. There was much evidence of the connection between S-containing aroma compounds and anti-cancer or anti-bacterial activities. A pharmacological study showed that (−)-(SS)-γ-L-glutamyl-L-[S-(4-hydroxybenzyl)] cysteinylglycine sulfoxide could increase the cell viability and show protective effects on serum deprivation-induced PC12 cell injury (Guo et al., 2015). Therefore, heteroatomic compounds from G. elata show a promising potential for further development and application.
Organic acids and their esters were a major constituent in G. elata. In the present study, 11 of the 14 known organic acids were identified by our metabolomics analysis. Similar with most parishins, citric acid was more abundant in the GB than in the GR group, which was the basic element of parishins. The broad pharmacological activities of citric acid have been elaborated upon in numerous studies. It finds its application in almost all the food and pharmaceutical industry as a flavoring, acidifier, and chelating agent (Lambros et al., 2022). Additionally, trans-3-phenylacrylic acid, an upregulated metabolite in the GB or GG groups, demonstrated therapeutic effects in cancer, bacterial infections, diabetes, and neurological disorders (Ruwizhi and Aderibigbe, 2020).
In recent years, some studies have focused on carbohydrates, steroids, flavonoids, and their glycosides (Sun et al., 2023). Currently, there are nine steroids isolated from G. elata. Unfortunately, none of them appeared in our metabonimics data. In contrast, systematic research on flavonoids in G. elata has not been carried out previously, while more than 40 flavonoids have been annotated in our metabonomics analysis (Supplementary Table S1). Therefore, the need for extensive research on carbohydrates, steroids, flavonoids, and their glycosides in G. elata is highlighted.
As shown in Figure 3B, all DEMs were mapped to KEGG pathways, most of which were enriched in phenylpropanoid biosynthesis, isoquinoline alkaloid biosynthesis, arginine biosynthesis, flavonoid biosynthesis, phenylalanine metabolism, purine metabolism, glutathione metabolism, and pyrimidine metabolism.
Gastrodin and 4-hydroxybenzyl alcohol are widely considered to be the primary phytometabolites with medicinal functions of G. elata tubers. Both of them are chemical markers for quality control of Gastrodiae Rhizoma. As described in Chinese Pharmacopeia 2020 edition, the total content of gastrodin and 4-hydroxybenzyl alcohol must be higher than 0.25% in the medicinal materials of G. elata. In our study (Figure 4), the content of gastrodin in GR and GG samples were 0.02301% and 0.02039%, respectively, which were significantly higher than 0.00496% in GB samples. However, the content of 4-hydroxybenzyl alcohol in the GG, GR, and GG group were 0.3186%, 0.2456%, and 0.3203%, with no differences among three groups.

Figure 4 The content of gastrodin (A) and 4-hydroxybenzyl alcohol (B), parishins structures (C), and the characteristic fingerprint chromatography (D). * statistically significant, p < 0.05.
Citric acid and adenosine were differentially expressed metabolites described in our metabolomics data. Here, the determination of citric acid and adenosine with HPLC reported similar results. In GB and GB samples, the content of citric acid was 50.16 ± 6.05 mg/g and 53.72 ± 1.19 mg/g, respectively, which was significantly higher than 40.75 ± 1.79 mg/g in GR. In addition, the content of adenosine in GG (29.02 ± 0.69 μg/g) was significantly higher than that in GB (23.09 ± 1.72 μg/g) and GR (24.12 ± 1.06 μg/g). Overall, the results from HPLC analysis reported similar findings in the previous metabolomics data.
The Chinese Pharmacopeia 2020 edition and several studies have provided several practical methods for evaluating G. elata tubers. Among them, HPLC fingerprints is an efficient technique for the analysis of complex substances. In a previous study, HPLC fingerprint was used for analyzing the similarity and difference among Gastrodiae Rhizoma from different producing areas (Sun et al., 2019). In another study, this technique has been applied to determine the content of each active component in Gastrodiae Rhizoma (Li et al., 2019). Nevertheless, previous studies were primarily focused on the quality control of Gastrodiae Rhizoma (cut crude drugs). In this study, application of freeze-dried powder from fresh tubers in HPLC fingerprint analysis was beneficial for investigating the characteristic constituents of different G. elata varieties comprehensively.
In our result (Figure 4D), six peaks were unambiguously detected as gastrodin, 4-hydroxybenzyl alcohol, parishin E, B, C, and A, respectively, based on comparison of their chromatographic retention with that of reference standards. All of them were detected by our metabolomics data. Significantly, it has been shown that reference medicinal materials (R line, cut crude drugs) are higher in gastrodin and lower in 4-hydroxybenzyl alcohol compared with our freeze-dried samples. According to the preparation methods of Rhizoma Gastrodiae, fresh tubers are washed in water, boiled or steamed, cut into slices, and then dried, such as reference medicinal materials. Previous studies have shown that high-temperature processing such as boiling or steaming treatment could significantly increase gastrodin content (Xie et al., 2023). Thus, gastrodin content in fresh tubers is obviously lower than in cut crude drugs. As shown in our results, HPLC fingerprint technique can also be used for the quality evaluation of fresh tubers from different G. elata varieties. In addition, fresh samples could accurately reflect the composition difference between plant varieties.
In this study, we analyzed the global metabolic profiles of three G. elata varieties in China. Our integrated bioinformatics pipeline enabled metabolite classification and identification. Based on UPLC-MS/MS-based metabolomics, there were a total of 990 metabolites identified and annotated. The majority of DEMs were classified as aromatic compounds, heteroatomic compounds, furans, carbohydrates, organic acids, and their derivatives. In detail, parishins, vanilloloside, and gastrodin A/B were significantly higher in the GB samples, whereas gastrodin, gasrtrol, and 3,4-dihydroxybenz aldehyde were more enriched in the GR or GG. In light of our findings, numerous differential metabolites from different G. elata varieties implied that this orchid could have diverse biological activities and healthcare values.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
XZ and SG discussed and planned the work. JL and TC conducted the experiments. XZ and YL carried out the data analysis. XZ and JL wrote the manuscript. SG revised parts of the manuscript. All authors contributed to manuscript revision and approved the submitted version.
This work was supported by grants from CAMS Innovation Fund for medical Sciences (Grant No. 2021-I2M-1-031).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JD declared a shared affiliation with the authors to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1249456/full#supplementary-material
Supplementary Table 1 | Raw data from metabolomics of G. elata.
Supplementary Table 2 | Overview of annotated metabolites.
Supplementary Table 3 | Phytometabolites in G. elata.
Supplementary Figure 1 | The standard curve of citric acid and adenosine.
Supplementary Figure 2 | Typical base peak intensity chromatograms of QC samples.
Chen, S. Y., Geng, C. A., Ma, Y. B., Chen, J. J. (2019). Melatonin receptors agonistic activities of phenols from Gastrodia elata. Nat. Prod. Bioprospect. 9, 297–302. doi: 10.1007/s13659-019-0213-2
Chen, W. C., Lai, Y. S., Lin, S. H., Lu, K. H., Lin, Y. E., Panyod, S., et al. (2016). Anti-depressant effects of Gastrodia elata Blume and its compounds gastrodin and 4-hydroxybenzyl alcohol, via the monoaminergic system and neuronal cytoskeletal remodeling. J. Ethnopharmacol. 182, 190–199. doi: 10.1016/j.jep.2016.02.001
Commission, C. P. (2020). Pharmacopoeia of the People’s Republic of China Vol. Vol. I (Beijing: China Medical Science and Technology Press), 59.
Di Guida, R., Engel, J., Allwood, J. W., Weber, R. J., Jones, M. R., Sommer, U., et al. (2016). Non-targeted UHPLC-MS metabolomic data processing methods: a comparative investigation of normalisation, missing value imputation, transformation and scaling. Metabolomics 12, 93. doi: 10.1007/s11306-016-1030-9
Dunn, W. B., Broadhurst, D., Begley, P., Zelena, E., Francis-Mcintyre, S., Anderson, N., et al. (2011). Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 6, 1060–1083. doi: 10.1038/nprot.2011.335
Gong, C. X., Ma, C., Irge, D. D., Li, S. M., Chen, S. M., Zhou, S. X., et al. (2023). Gastrodia elata and parishin ameliorate aging induced ‘leaky gut’ in mice: correlation with gut microbiota. Biomed. J. 46 (4), 100547. doi: 10.1016/j.bj.2022.07.001
Guo, Q. L., Wang, Y. N., Zhu, C. G., Chen, M. H., Jiang, Z. B., Chen, N. H., et al. (2015). 4-Hydroxybenzyl-substituted glutathione derivatives from Gastrodia elata. J. Asian Nat. Prod. Res. 17, 439–454. doi: 10.1080/10286020.2015.1040000
Guo, T., Wang, H. C., Xue, W. Q., Zhao, J., Yang, Z. L. (2016). Phylogenetic analyses of Armillaria reveal at least 15 phylogenetic lineages in China, seven of which are associated with cultivated Gastrodia elata. PloS One 11, e0154794. doi: 10.1371/journal.pone.0154794
Hayashi, J., Sekine, T., Deguchi, S., Lin, Q., Horie, S., Tsuchiya, S., et al. (2002). Phenolic compounds from Gastrodia rhizome and relaxant effects of related compounds on isolated smooth muscle preparation. Phytochemistry 59, 513–519. doi: 10.1016/S0031-9422(02)00008-0
Heese, K. (2020). Gastrodia elata Blume (Tianma): Hope for brain aging and dementia. Evid. Based Complement. Alternat. Med. 8870148, 1–7. doi: 10.1155/2020/8870148
Jang, J. H., Son, Y., Kang, S. S., Bae, C. S., Kim, J. C., Kim, S. H., et al. (2015). Neuropharmacological potential of Gastrodia elata Blume and its components. Evid. Based Complement. Alternat. Med. 309261, 1–14. doi: 10.1155/2015/309261
Ji, N., Liu, P., Zhang, N., Yang, S., Zhang, M. (2022). Comparison on bioactivities and characteristics of polysaccharides from four varieties of Gastrodia elata Blume. Front. Chem. 10, 956724. doi: 10.3389/fchem.2022.956724
Lambros, M., Tran, T. H., Fei, Q., Nicolaou, M. (2022). Citric acid: a multifunctional pharmaceutical excipient. Pharmaceutics 14 (5), 972. doi: 10.3390/pharmaceutics14050972
Li, Y., Zhang, Y., Zhang, Z., Hu, Y., Cui, X., Xiong, Y. (2019). Quality evaluation of Gastrodia elata tubers based on HPLC fingerprint analyses and quantitative analysis of multi-components by single marker. Molecules 24 (8), 1521. doi: 10.3390/molecules24081521
Liu, Y., Gao, J., Peng, M., Meng, H., Ma, H., Cai, P., et al. (2018). A review on central nervous system effects of gastrodin. Front. Pharmacol. 9, 24. doi: 10.3389/fphar.2018.00024
Luo, Q., Yan, X., Bobrovskaya, L., Ji, M., Yuan, H., Lou, H., et al. (2017). Anti-neuroinflammatory effects of grossamide from hemp seed via suppression of TLR-4-mediated NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. Mol. Cell Biochem. 428, 129–137. doi: 10.1007/s11010-016-2923-7
Park, S., Kim, D. S., Kang, S. (2011). Gastrodia elata Blume water extracts improve insulin resistance by decreasing body fat in diet-induced obese rats: vanillin and 4-hydroxybenzaldehyde are the bioactive candidates. Eur. J. Nutr. 50, 107–118. doi: 10.1007/s00394-010-0120-0
Ruwizhi, N., Aderibigbe, B. A. (2020). Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 21 (16), 5712. doi: 10.3390/ijms21165712
Shan, T., Yin, M., Wu, J., Yu, H., Liu, M., Xu, R., et al. (2021). Comparative transcriptome analysis of tubers, stems, and flowers of Gastrodia elata Blume reveals potential genes involved in the biosynthesis of phenolics. Fitoterapia 153, 104988. doi: 10.1016/j.fitote.2021.104988
Su, Z., Yang, Y., Chen, S., Tang, Z., Xu, H. (2023). The processing methods, phytochemistry and pharmacology of Gastrodia elata Bl.: A comprehensive review. J. Ethnopharmacol. 314, 116467. doi: 10.1016/j.jep.2023.116467
Sun, S., Li, Y., Zhu, L., Ma, H., Li, L., Liu, Y. (2019). Accurate discrimination of Gastrodia elata from different geographical origins using high-performance liquid chromatography fingerprint combined with boosting partial least-squares discriminant analysis. J. Sep. Sci. 42, 2875–2882. doi: 10.1002/jssc.201900073
Sun, X., Jia, B., Sun, J., Lin, J., Lu, B., Duan, J., et al. (2023). Gastrodia elata Blume: a review of its mechanisms and functions on cardiovascular systems. Fitoterapia 167, 105511. doi: 10.1016/j.fitote.2023.105511
Taguchi, H., Yosioka, I., Yamasaki, K., Kim, I. (1981). Studies on the constituents of Gastrodia elata Blume. Chem. Pharm. Bull. 29, 55–62. doi: 10.1248/cpb.29.55
Tang, C., Wang, L., Li, J., Liu, X., Cheng, M., Xiao, H. (2015). Analysis of the metabolic profile of parishin by ultra-performance liquid chromatography/quadrupole-time of flight mass spectrometry. Biomed. Chromatogr. 29, 1913–1920. doi: 10.1002/bmc.3516
Wang, Z. W., Li, Y., Liu, D. H., Mu, Y., Dong, H. J., Zhou, H. L., et al. (2019). Four new phenolic constituents from the rhizomes of Gastrodia elata Blume. Nat. Prod. Res. 33, 1140–1146. doi: 10.1080/14786419.2018.1460836
Wang, Q., Li, Z., Wang, D., Yang, S., Feng, Y. (2020). Myocardial protection properties of parishins from the roots of Gastrodia elata Bl. Biomed. Pharmacother. 121, 109645. doi: 10.1016/j.biopha.2019.109645
Wen, B., Mei, Z., Zeng, C., Liu, S. (2017). metaX: a flexible and comprehensive software for processing metabolomics data. BMC Bioinf. 18, 183. doi: 10.1186/s12859-017-1579-y
Wu, Y. N., Wen, S. H., Zhang, W., Yu, S. S., Yang, K., Liu, D., et al. (2023). Gastrodia elata Bl.: A comprehensive review of its traditional use, botany, phytochemistry, pharmacology, and pharmacokinetics. Evid. Based Complement. Alternat. Med. 2023, 5606021. doi: 10.1155/2023/5606021
Xie, Y. K., Li, X. Y., Chen, C., Zhang, W. P., Yu, X. L., Xiao, H. W., et al. (2023). Effects of steam and water blanching on drying characteristics, water distribution, microstructure, and bioactive components of Gastrodia elata. Plants (Basel) 12 (6), 1372. doi: 10.3390/plants12061372
Xu, C. B., Guo, Q. L., Wang, Y. N., Lin, S., Zhu, C. G., Shi, J. G. (2019). Gastrodin derivatives from Gastrodia elata. Nat. Prod. Bioprospect. 9, 393–404. doi: 10.1007/s13659-019-00224-1
Yu, E., Gao, Y., Li, Y., Zang, P., Zhao, Y., He, Z. (2022). An exploration of mechanism of high quality and yield of Gastrodia elata Bl. f. glauca by the isolation, identification and evaluation of Armillaria. BMC Plant Biol. 22, 621. doi: 10.1186/s12870-022-04007-8
Yu, H., Zang, J., Chen, B. Q., Huang, H., Li, S. H., Huang, S. W., et al. (2022). Research progress on classification of chemical constituents from Gastrodia elata and their pharmacological effects. Chin. Tradit. Herbal Drugs 53, 5553–5562. doi: 10.7501/j.issn.0253-2670.2022.17.033
Zhan, H. D., Zhou, H. Y., Sui, Y. P., Du, X. L., Wang, W. H., Dai, L., et al. (2016). The rhizome of Gastrodia elata Blume - an ethnopharmacological review. J. Ethnopharmacol. 189, 361–385. doi: 10.1016/j.jep.2016.06.057
Zhang, C. H., Shi, P., Yang, Y. Q., Chang, M. L. (2022). Determination of the content of 2 kind of organic acids in Schisandra Chinensis by HPLC. Shanxi Forestry Sci. Tech. 51, 13–15. doi: 10.19989/j.cnki.1007-726X.20220405
Keywords: Gastrodia elata, variety, phytometabolite, aromatic compounds, HPLC fingerprints
Citation: Zeng X, Li J, Chen T, Li Y and Guo S (2023) Global metabolic profile and multiple phytometabolites in the different varieties of Gastrodia elata Blume. Front. Plant Sci. 14:1249456. doi: 10.3389/fpls.2023.1249456
Received: 28 June 2023; Accepted: 26 September 2023;
Published: 17 October 2023.
Edited by:
Wei Sun, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Jungui Dai, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaCopyright © 2023 Zeng, Li, Chen, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shunxing Guo, c3hndW8xOTg2QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.